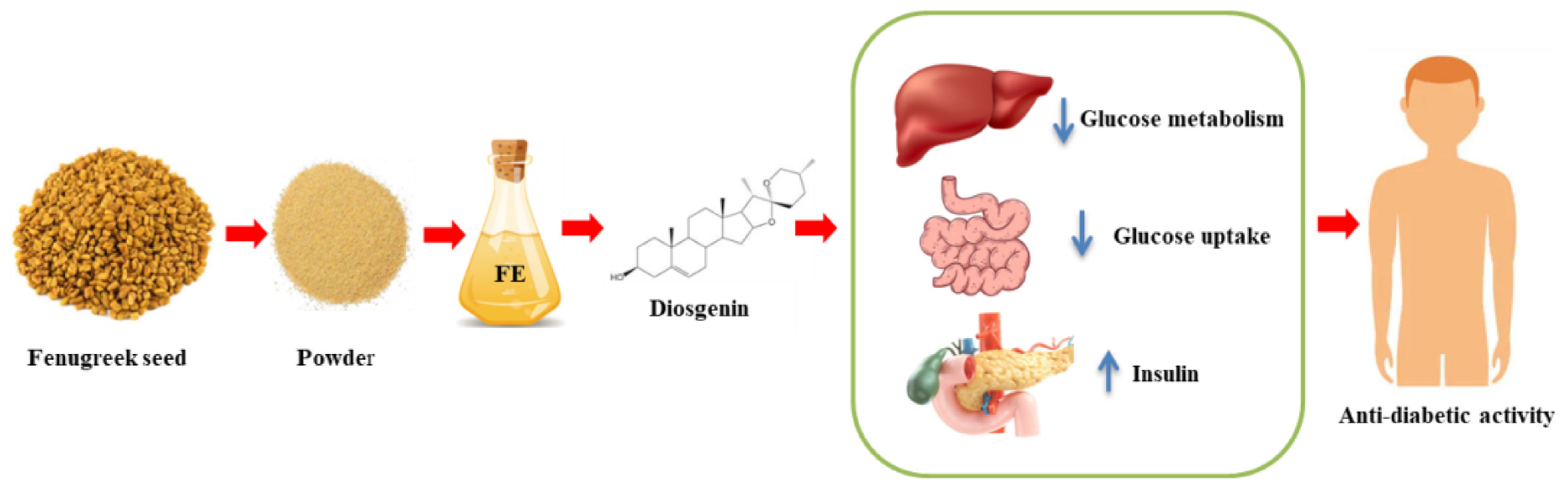
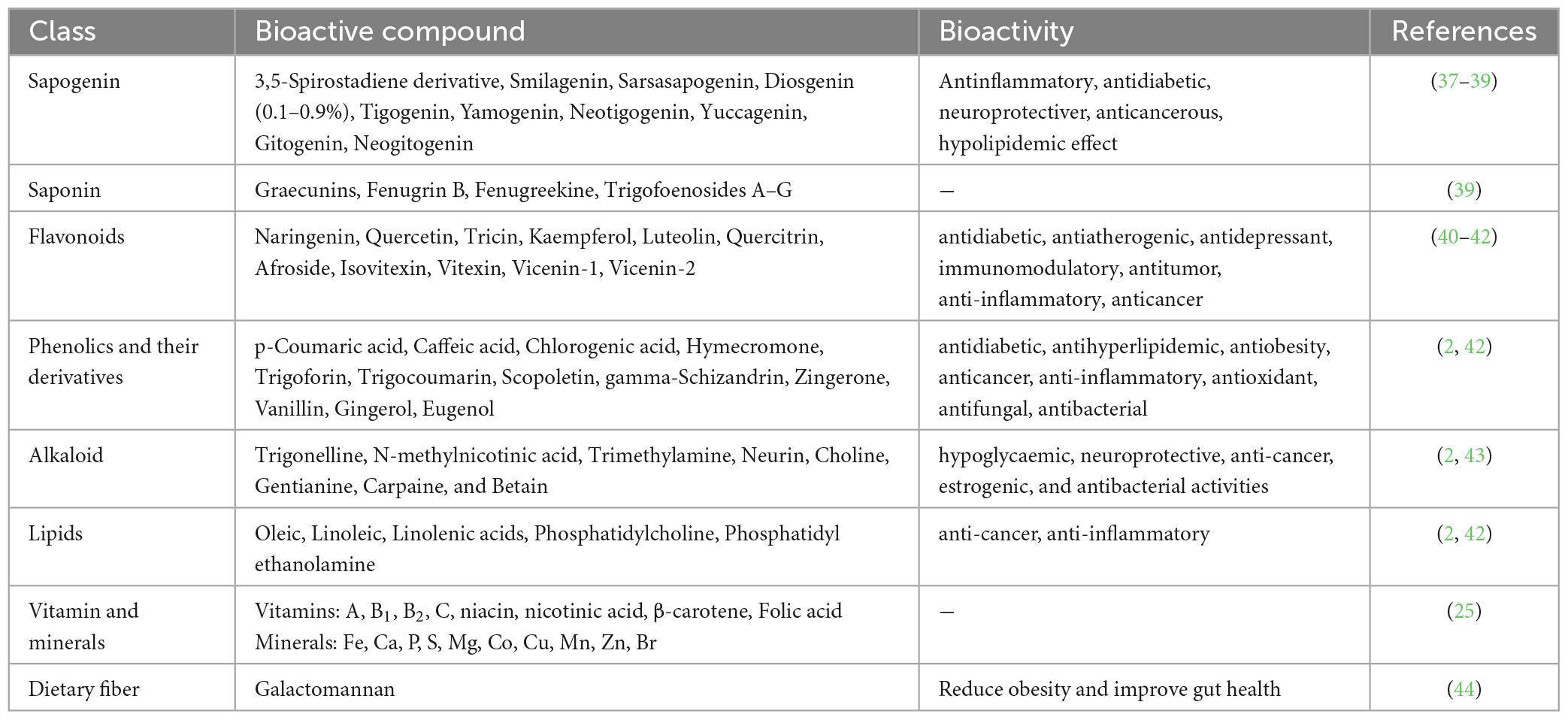
- 1Agricultural Research Station, Agriculture University, Kota, India
- 2Department of Biochemistry, Punjab Agricultural University, Ludhiana, India
- 3Division of Biochemistry, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 4ICAR-Central Institute of Post-Harvest Engineering & Technology, Ludhiana, India
- 5School of Sciences, Rayat Bahra University, Mohali, India
- 6Sri Karan Narendra Agriculture University, Jaipur, India
Diabetes is a chronic metabolic disease that endangers the entire body’s tissues and organs. Diabetes impairs glucose and insulin regulation in the human body by causing pancreatic cell damage. Diabetes modifies pathways such as serine/threonine protein kinase (Akt) and Protein kinase C (PKC)/- glucose transporter 4 (GLUT4), peroxisome proliferator-activated receptor (PPAR) glucose absorption, and inhibits α-amylase and α-glucosidase, Sodium/glucose cotransporter 1 (SGLT-1), and Na+-K+-ATPase activity. Diabetes may also be caused by a decrease in the expression of sterol regulatory element binding protein 1 (SREBP-1) and its target genes, fatty acid synthase (FAS), stearoyl-CoA desaturase-1 (SCD-1), and acetyl-CoA carboxylase α (ACC), as well as a decrease in the levels of C/EBP homologous protein (CHOP), Caspase12, and Caspase3 proteins. Diabetes has long been linked to diseases of the cardiovascular, nervous, skeletal, reproductive, hepatic, ocular, and renal systems. Diosgenin, a steroidal compound derived from fenugreek, aids in the prevention of diabetes by altering cellular pathways in favor of healthy bodily functions. Diosgenin is a new nutraceutical on the market that claims to cure diabetes in particular. This article focuses on diosgenin extraction and purification, fenugreek bioactive compounds, pharmacological properties of diosgenin, mode of action of diosgenin to cure diabetes, and dosages.
Introduction
Due to promising results and rare side effects, medicinal herbs have recently received a lot of attention across the world for their use in the treatment of various ailments. Phytochemicals found in herbal medicines, such as phenolic acids, saponins, flavonoids, tannins, alkaloids, and terpenoids, aid in the treatment of human disease. Fenugreek (Trigonella foenum-graecum L.) is an ancient or traditional remedial plant is one of the evidence-based herbal treatments (1). Fenugreek, also known as Methi, Chandrika, Alholva, Bird’s Foot, Bockshornsame, and Greek Clover, is a member of the Fabaceae family that originated in India and Northern Africa and is now commercially grown in
Mediterranean Europe, China, Southeast Asia, Australia, the United States, Argentina, and Canada. India is one of the world’s greatest fenugreek growers, yet it does not have a significant proportion of the worldwide fenugreek trade due to high internal consumption. Fenugreek seeds and leaves have been used for millennia in Indian, Tibetan, and Chinese medicine to treat a variety of ailments, including diabetes, obesity, polycystic ovarian syndrome, atherosclerosis, cancer, inflammation, and blood cholesterol (2, 3). The excellent nutritional profile and bioactive components in fenugreek give it medicinal and pharmacological properties including antibacterial, anticholesterolemic, carminative, restorative, uterine tonic, anti-carcinogenic, anti-inflammatory, antiviral, antioxidant, and hypotensive effects (4). Essential oil, coumarins, alkaloids (trigonelline, isoorientin), polyphenols (rhaponticin, isovitexin), and steroidal saponins (diosgenin) are all found in fenugreek seed extract (5, 6). The most active antidiabetic bioactive found in fenugreek is diosgenin, which possesses antioxidative properties (7).
According to recent data from the United States, prediabetes affects 34.6% of the adult population, whereas impaired fasting glucose (IFG) affects 19.4%, impaired glucose tolerance (IGT) affects 5.4%, and IFG plus IGT affect 9.8% (8). In India, according to ICMR 11.4% population living with diabetes whereas, 15.3% of the population is prediabetic. IGT is believed to have 316 million users worldwide, with that number expected to climb to 471 million by 2035 (9). Persons with diabetes have a 7-year reduction in life expectancy than the general non-diabetic population, an effect that is intimately linked to the most serious diabetic outcomes, which include heart disease, limb amputations, End-Stage Renal Disease, and blindness (10). Currently used chemotherapeutic mediators have shown to be helpful in the treatment of diabetes, but they come with a slew of unpleasant side effects, including appetite loss, stomach discomfort, muscle cramps, and weakness. Diosgenin has antioxidative properties and aids in the treatment of diabetes through a variety of mechanisms, including β-cell renewal and insulin secretion stimulation by increasing CCAAT/enhancer-binding protein (C/EBP δ) and peroxisome proliferator-activated receptor- γ (PPAR- γ) mRNA transcription levels (11). Chemotherapeutic mediators used today have shown to be quite helpful in the treatment of diabetes; however, they have a number of unfavorable side effects such as loss of appetite, abdominal discomfort, muscle cramping, and weakness. This review article seeks to make the most of the information available to highlight the topic’s scientific standing and the requirement for new research in order to improve current knowledge since more study is currently needed in this area.
Diabetes types and issues
Diabetes is a chronic disease in which the pancreas is unable to produce insulin or the human body is unable to use the insulin produced effectively, resulting in an imbalance in glucose metabolism or an increase in blood sugar levels. According to American Diabetes Association 2009 (12), there are mainly diabetes types are: Type 1 diabetes, type 2 diabetes, type 3 diabetes, and gestational diabetes. Diabetes also causes other serious health problems and is a significant financial burden for a vast number of people around the world. Poor nutritional diets and unhealthy or contemporary lifestyles contribute to weight gain and obesity, which can be a contributing factor to diabetes around the world. According to the World Health Organization (WHO), diabetes affects around 422 million people globally, the majority of whom live in low- and middle-income countries. Diabetes or higher-than-optimal blood glucose caused 1.5 million individuals to die directly from cardiovascular disease, chronic renal disease, or tuberculosis in 2019. According to the American Diabetes Association (ADA) (13), almost 1.9 million Americans, including roughly 2.44 lakh children and adolescents, have type 1 diabetes. In 2017, the entire cost of diabetes was $327 billion, with $237 billion in direct medical costs and $90 billion in indirect costs due to lost productivity. Type 1 diabetes is a polygenic inherited disease caused by a complicated interplay between the pancreatic β-cell and the innate and adaptive immune systems. Insulin is not generated as a result of destructed- β cell and the human body is unable to store excess glucose, resulting in an increase in blood sugar levels. Hypoglycemia is a complication of Type 1 diabetes that causes confusion, convulsions, or coma in the patient. Individuals with Type 1 diabetes can benefit from continuous subcutaneous insulin infusions, oral medication, and a nutritious diet combined with a healthy lifestyle, thyroid dysfunction treatment, and glucose self-monitoring (14, 15).
Globally, Diabetes mellitus affects approximately one in every eleven adults, with Type 2 diabetes accounting for 90% of cases. Asia is a chief region of the world’s rapidly spreading Type 2 diabetes mellitus epidemic, with China and India serving as the top two epicenters (16). Type 2 diabetes is caused by high blood sugar levels. Blood glucose is your primary source of energy, and it is derived primarily from the foods we consume. In Type 2 diabetes, the body either does not produce enough insulin or does not use insulin effectively. Type 2 diabetes affects more than 90% of diabetic patients, causing microvascular and macrovascular complications that cause profound psychological and physical distress in patients, as well as a significant burden on health-care systems. Overweight and obesity, a lack of physical activity, insulin resistance, and genetic factors all contribute to Type 2 diabetes (17). Insulin resistance is linked to a slew of metabolic issues, including glucose intolerance, hypertension, a distinct dyslipidemia, a procoagulant state, and an upsurge in macrovascular disease (18). Cardiovascular disease is the leading cause of illness and death in Type 2 diabetes and necessitates close monitoring of glucose and lipid levels, as well as blood pressure, to reduce the risk of complications and disease progression (19). Increased thirst, frequent urination, increased hunger, weight loss, blurred vision, slow-healing sores, and frequent infections are common symptoms of Type 2 diabetes.
Gestational diabetes is a type of glucose intolerance that develops during pregnancy as a result of insufficient insulin supply to meet tissue demands for standard blood glucose regulation (20). Gestational diabetes appears to be caused by the same wide range of physiological and genetic abnormalities that characterize diabetes in general. Maternal overweight and obesity, later childbearing age, previous history of Gestational diabetes, family history of type 2 diabetes mellitus, and ethnicity are all major risk factors for Gestational diabetes (21). In the United States, African American, Hispanic American, Native American, Pacific Islander, and South or East Asian women have a higher prevalence than Caucasian women (22). According to a recent International Diabetes Federation report, 16% of live births worldwide in 2013 were complicated by hyperglycemia during pregnancy (23). According to the most recent meta-analysis by Saeedi et al. (24), the global prevalence of GDM is 14.7% based on the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria, which is the most widely used screening method worldwide. Pregnancy diabetes can result in problems such as the baby growing larger than usual, polyhydramnios, premature birth, pre-eclampsia, and infant jaundice.
Fenugreek’s cultural and historical importance
Fenugreek is culturally significant Ayurvedic medicine around the world, including India, Egypt, and the Middle East. Fenugreek is a spice that is widely used in India and the Mediterranean region and is recognized to have a variety of medicinal properties. Fenugreek seeds are widely used in Indian cuisine and are an essential ingredient in curry powders. They are also used to enhance the flavour of pickles and chutneys (25). Fenugreek leaves are commonly used in salads and traditional Egyptian dishes such as Ful medames. Fenugreek seeds are used as a spice in many Middle Eastern dishes, including meat and vegetable dishes. In recent years, selected genotypes of this species have developed a niche crop that produces high yields of bloat-free forage, which can boost both beef and milk production in semiarid regions of western Canada. Fenugreek has cultural significance in Jewish tradition, where it is used during the Sukkot festival. The herb is one of four plant species that are gathered and waved during prayers. Fenugreek is also important in Islamic culture, where it is used in cooking and medicine.
Fenugreek has a long history dating back to antiquity. The herb was used for its medicinal properties in ancient Egypt and was thought to have been used by Cleopatra to enhance her beauty. Fenugreek was used to treat a variety of ailments in ancient Greece, including digestive issues, respiratory problems, and skin inflammation. Fenugreek was also used extensively in ancient Ayurvedic medicine in India. The herb was thought to have healing properties that could be used to treat a variety of health issues, including diabetes, inflammation, and digestive disorders (26). Fenugreek was also used in traditional Chinese medicine to treat various conditions, including asthma and digestive problems. In addition to its medicinal uses, Fenugreek was also used for religious and cultural purposes in ancient times. In ancient Egypt, Fenugreek was used in religious ceremonies and was believed to have healing properties. In ancient Rome, Fenugreek was used as a flavouring agent in food and was also used in perfumes and cosmetics. Fenugreek is still used for its medicinal and culinary properties today. It is thought to have a variety of health benefits, including lowering blood sugar levels, reducing inflammation (27), and increasing milk production in breastfeeding mothers National Institute of Child Health and Human Development (28). Fenugreek is also a popular ingredient in bodybuilding supplements because it is thought to boost testosterone levels (29).
Bioactive compounds of fenugreek seeds and their pharmacological property
Fenugreek seeds are high in bioactive compounds such as saponins, alkaloids, flavonoids, and phenolic compounds. At this point, we will look at fenugreek’s bioactive compounds and their potential health benefits (Table 1). Secondary metabolites found in fenugreek seed include saponin (4.8%), flavonoids (100°mg/gm), alkaloids (35%), and diosgenin (0.2−0.9%) (30). Among these, alkaloids are primarily responsible for the distinctive taste and aroma. Saponins, which are glycosides with strong foam-forming properties, are abundant in fenugreek seeds. Saponins are thought to have a variety of health benefits, including cholesterol-lowering and anticancer properties. Saponins have been shown in studies to bind to bile acids in the intestine and prevent their reabsorption, resulting in lower cholesterol levels. Saponins have also been shown in vitro to inhibit cancer cell growth and induce apoptosis (programmed cell death) (31).
Trigonelline, gentianine, and carpaine are among the alkaloids found in fenugreek seeds. Trigonelline is an extremely powerful antioxidant that has been shown to protect against oxidative stress and DNA damage (32). Gentianine has anti-inflammatory and analgesic properties, whereas carpaine has hypotensive (blood pressure-lowering) properties. Flavonoids, which are polyphenolic compounds with antioxidant properties, are abundant in fenugreek seeds (33). The flavonoids vitexin and isovitexin are the most abundant in fenugreek seeds. Vitexin has been shown to have anti-diabetic properties by increasing insulin sensitivity and decreasing blood glucose levels. Isovitexin has been shown to have neuroprotective properties and may aid in the prevention of cognitive decline (34). Fenugreek seeds contain phenolic compounds such as coumarins and lignans. Coumarins have anticoagulant properties and may aid in the prevention of blood clots. Lignans have anticancer properties and may help reduce the risk of breast cancer (35).
Other bioactive compounds found in fenugreek seeds include galactomannans, mucilages, and phytosterols. Galactomannans are complex carbohydrates that have been shown to lower cholesterol levels. Mucilages are water-soluble fibres that have been shown to have prebiotic properties and may aid in the improvement of digestive health (36). Phytosterols are plant-based compounds that have been shown to lower cholesterol levels. It contains a lot of dietary fibre, protein, amino acids, iron, silica, and vitamin B1. Furthermore, fenugreek seed contains an unusual amino acid called hydroxy isoleucine, which increases insulin secretion and helps to prevent diabetes (11).
Diosgenin extraction, purification, and characterization
The most active bioactive ingredient in fenugreek seed extract is diosgenin [0.113−0.135% (w/w)], which has anti-diabetic properties. The melavonate route, which comprises of a hydrophilic sugar moiety attached to a hydrophobic steroid aglycone, is used to produce diosgenin. When fenugreek seed was extracted using 70% (v/v) 2-propanol in water and sulphuric acid, the extract included a mixture of diosgenin with steroidal saponins, and other slight sapogenins (11). Diosgenin can be extracted from Dioscorea nipponica Makino using a magnetic sulfonated solid composite and hydrolyzing it with 2.5°M hydrochloric acid at 110°C for 5°h (45). Green extraction technologies such as ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) can extract the most diosgenin from fenugreek seeds, which can then be used to treat diabetes patients. For diosgenin extraction, the MAE method used a sample-to-solvent ratio is 1:5 (w/v) with solvents (acetone, ethanol, hexane, and petroleum ether) and extraction time (1.5, 3.0, 4.5, and 6.0°min) at 180 W power to yield 7.83% diosgenin content. In the UAE approach, 21.48% diosgenin was obtained when the sample-to-solvent ratio was 1:5 (w/v) with different solvents, treatment times were varied (30, 40, 50, and 60 min), and the ultrasonic bath temperature was prolonged at 30°C throughout the procedure (46). Likewise, deoiled fenugreek seed powder (20 g) was extracted with 100 ml ethanol (20−100%,v/v), extraction time 40, 50, and 60 min, and the temperature of the ultrasonic bath throughout the extraction process was 35°C to detect diosgenin (0.041−1.294 geq/100 g) (47). UAE method showed highest diosgenin from fenugreek seeds with 80% ethanol for 5 min, with α-amylase inhibition (IC50 crude = 371.7°μg/ml, IC50 defat = 370.5°μg/ml) and pancreatic lipase (IC50 crude = 550.0°μg/ml and IC50 defat = 497.6°μg/ml). For purification dehydrated fenugreek seed extract was dissolved in distilled water (50 ml), flushed twice with diethyl ether (50 mL), and extract liquid layer was extracted with water-saturated n-butanol (50 mL). Diosgenin profiles were constructed for seed samples of various fenugreek varieties, and the results revealed 200 and 480 mg/100 g FW diosgenin concentrations (48).
Chemical structure, health benefits, and worldwide status of diosgenin
Diosgenin is a major bioactive constituent of many edible pulses and roots, particularly in the seeds of fenugreek and the root tubers of wild yams. Diosgenin is found in 137 different Dioscorea species. A total of 41 of them contain more than 1% diosgenin. It is derived from the roots of the Dioscorea wild yam that is commonly used as a precursor in the production of synthetic steroid chemicals such as progesterone and cortisol (49). According to the National Center for Biotechnology Information 2023 (50), Diosgenin (25R-spirost-en-3β-ol) is a C27 triterpenoid spiroketal steroid sapogenin with a molecular weight of 414.62 and its formula is C27H42O3 (Figure 1). It is described as a spirostan with a hydroxyl group at the β position in terms of molecular structure. It also contains a double bond at 5,6 position and has an R configuration at position 25. It has a hydroxyl group in the third position; hydroxyl groups are typically found in combination with sugars, making the compounds water-soluble and highly saponaceous (51). In aqueous medium, it has a solubility of about 0.7°ng/ml. It is a white crystalline powder that dissolves in organic solvents such as ethanol, DMSA, and dimethylformamide. Cholesterol is a precursor in the biosynthesis of diosgenin, which is catalysed by two P450 enzymes: C-16,22-dihydroxylase and C-26 hydroxylase (52).
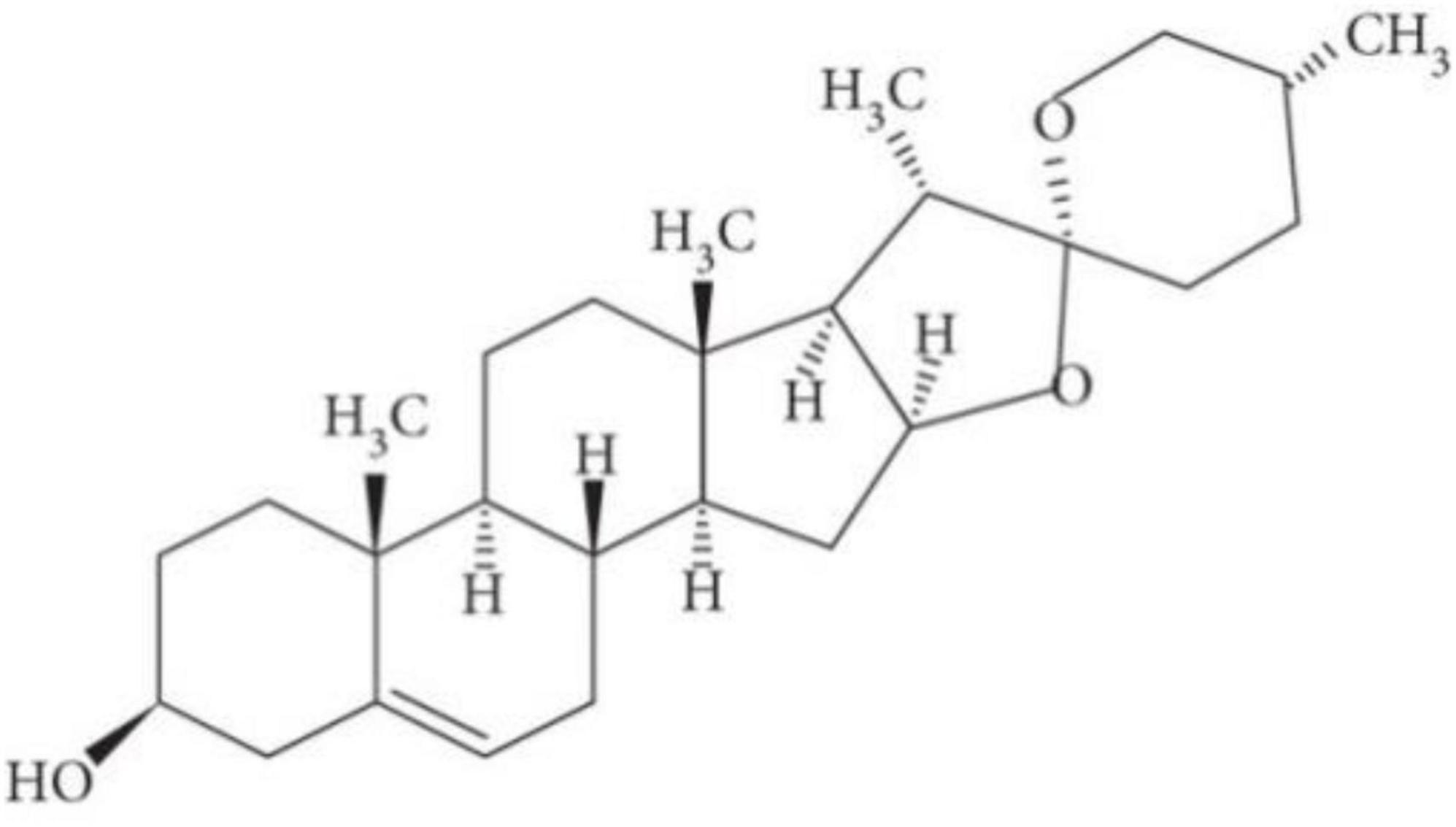
Figure 1. Chemical structure of diosgenin (53).
In addition to being an important starting material for the preparation of several steroidal drugs in the pharmaceutical industry, diosgenin has shown high potential and interest in the treatment of various disorders such as cancer, diabetes, arthritis, asthma, and cardiovascular disease (54). Diosgenin supplementation is thought to be an excellent way to promote women’s health because it slows the decline of estrogen and progesterone levels and aids in the prevention of hormonal imbalances. In essence, it may reduce the risk of osteoporosis, mood swings, irritability, and other symptoms associated with fluctuating hormone levels (55). Furthermore, research suggests that Diosgenin supplementation may help gastroprotection against stomach mucosa damage by inhibiting certain enzyme activity (56).
Because of the COVID-19 pandemic, the global Diosgenin market is estimated to be worth USD 99.5 million in 2022 and is expected to grow to USD 142.3 million by 2030, with a CAGR of 6.1% from 2022 to 2030. According to a Press Release of Diosgenin Market Size by 2030, Diosgenin accounts for 60% of the world’s steroidal products out of all steroid drug precursors. With a market share of roughly 60%, China is the world’s largest diosgenin consumer market. Sabinsa, Himachal Pharmaceuticals, Namiex Chemicals, Zhenhua Biology, and Shaanxi Jiahe Biotechnology are the global top five diosgenin manufacturers, with a combined market share of more than 85%, with Zhenhua Bio being the largest manufacturer, with a market share of more than 55%.
Modes of action of fenugreek derived diosgenin in diabetes
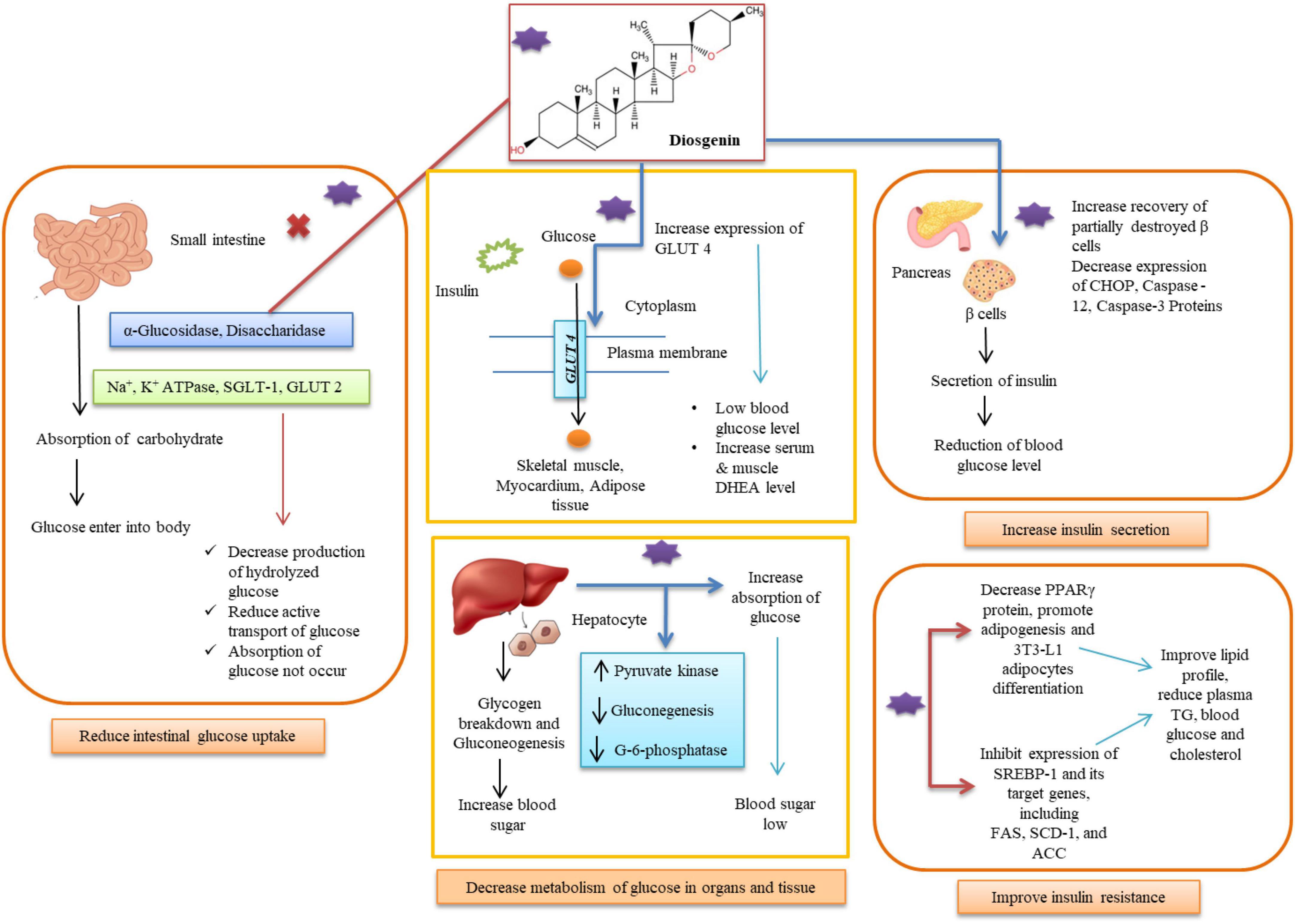
Numerous researches have looked into the effects of fenugreek extracts and diosgenin in the treatment of diabetes and their mechanisms of action with possible advantages for diabetics (Table 2). These consist of clinical trials, in vitro and in vivo studies (Figure 2). Further explanations of the processes, outcomes, and diosgenin’s mode of action are provided in this section.
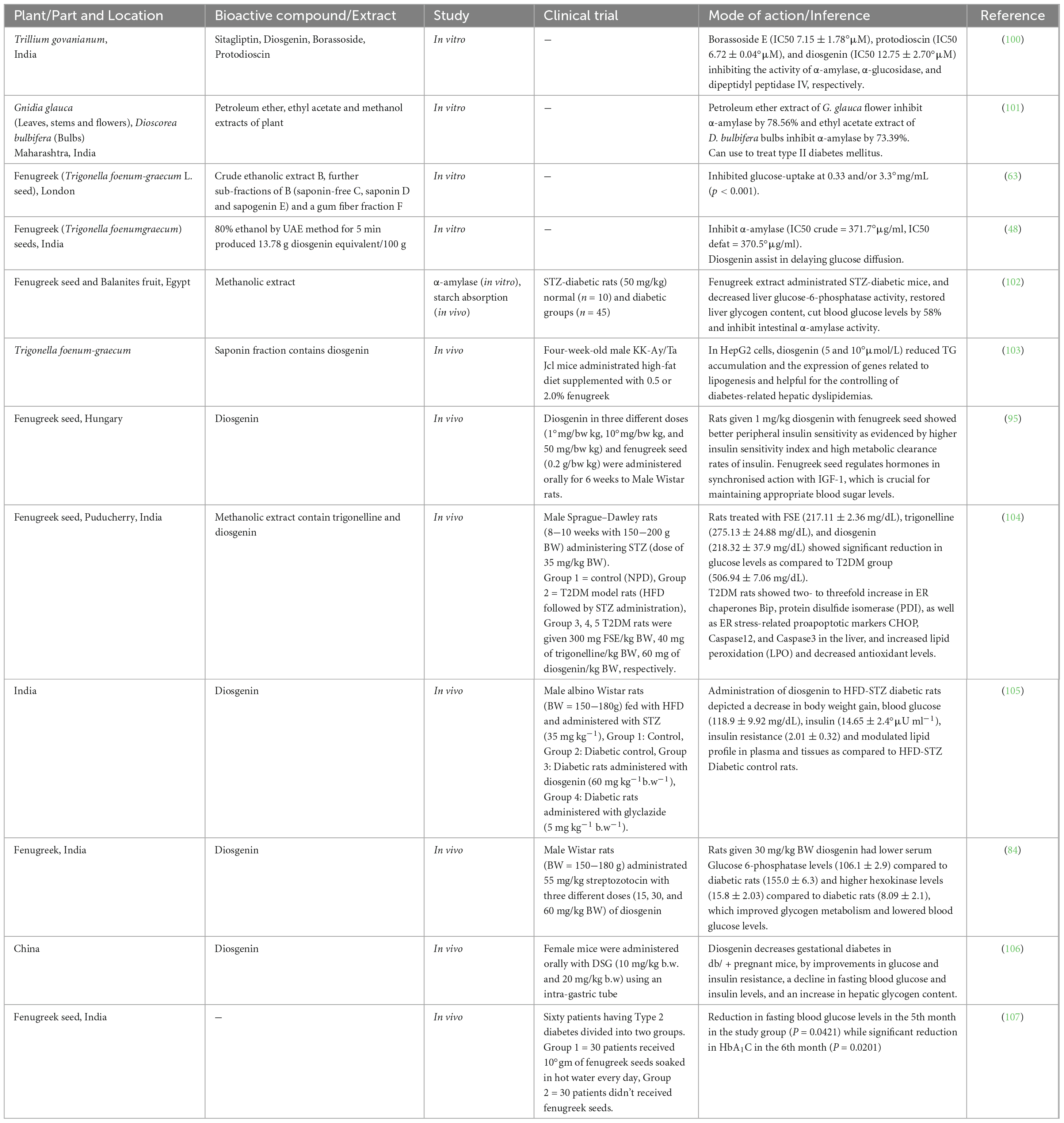
Table 2. In vitro, in vivo studies and mechanism of action for treating diabetes with fenugreek and diosgenin.
Diminish glucose absorption in intestine
The pancreatic β-cells’ ability to secrete insulin is reduced by hyperglycemia in the toxicity cycle, which is then followed by a rise in insulin resistance, which worsens hyperglycemia and renders β -cells completely ineffective. The long-lasting and serious health repercussions of hyperglycemia take time to manifest (57). The small intestine has the ability to absorb carbohydrates, which allows glucose to enter the bloodstream and easily raises postprandial hyperglycemia in diabetics. Polysaccharides are primarily broken down into oligosaccharides in the luminal bulk fluid by secreted enzymes such as α-Glucosidase, disaccharidases, Na+-K+-ATPase, and additional hydrolysis is carried out by an array of carbohydrases in the brush border of the mature enterocytes (58). The Na+-glucose cotransporter SGLT1 actively transports glucose and galactose into the enterocyte via the transmembrane electrochemical Na+ gradient, whereas the glucose transporter GLUT2 actively transports glucose and galactose out across the basolateral membrane (59). Diosgenin showed a considerable amount of α -amylase and α -glucosidase inhibitory impact, supporting its involvement in lowering high blood glucose levels. Only one catalytic residue from α-amylase is involved in hydrogen bonding contact with diosgenin, while diosgenin interacts with two catalytic residues from α-glucosidase (Asp352 and Glu411) to generate the lowest energy inhibitor complex (60). Other hydrophobic interactions, in addition to hydrogen bonding interactions, contributed to the maximum binding affinity of diosgenin toward α-glucosidase. 33 steroidal saponins and sapogenins were extracted from fenugreek, and their in vitro α -glucosidase inhibitory action was assessed (61). There were five 25R and 25S isomer combinations of spirostanol saponins or sapogenins among them, and these were compounds 10 (25R/S)-5α-spirostane-2α,3β-diol 3-O-α-L-rhamnopyranosyl- (1 → 2)-β-D-glucopyranoside, 12 diosgenin/yamogenin, 17 (25R/S)-5-en-spirostane-3β-ol 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D-glucopyranoside, 22 (25R/S)-5-enspirostane-2α,3β-diol 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D-glucopyranoside, and 29 sarsasapogeninn/smilagenin. Saponins 18 (25R)-5-en-spirostane-3β-ol 3-O-β-Dglucopyranosyl-(1 → 4)-β-D-glucopyranoside, 23 (25R)-5-en-spirostane-2α,3β-diol 3-O-α-L-rhamnopyranosyl-(1 → 2)-[α-L-rhamnopyranosyl-(1 → 4)]-β-D-glucopyranoside, 26 soyasapogenol B, 27 3-O-β-D-glucuronopyranosyl soyasapogenol B methyl ester, and 14 isonarthogenin significantly inhibited α-glucosidase at IC50 values of 15.16, 8.98, 7.26, 5.49, and 14.01°M, respectively, as compared to the positive control. Lactase and maltase activity in the gut of diabetic rats was significantly reduced by sapogenin extract or diosgenin supplementation. Diosgenin consumption revealed hypoglycemic qualities that are advantageous in diabetes by lowering intestinal disaccharidases activity. Diabetic rats’ Na+-K+-ATPase activity was shown to be drastically decreased when diosgenin was added to their diet. Supplementation of the diet with the 1% commercial diosgenin significantly reduced Na+-K+-ATPase activity in all three regions proximal (12.8 ± 0.2°nmol Pi/min/mg protein), mid (9.5 ± 0.1°nmol Pi/min/mg protein), distal (7.3 ± 0.1°nmol Pi/min/mg protein) of the intestine of the diabetic control rats when compared to the normal control group compared to the diabetic control group (62). Comparing the diabetes control to the diosgenin-supplemented group, the proximal area of the body showed a significantly higher level of Ca2+ ATPase activity. Sapogenin found in fenugreek extract reduced glucose absorption at concentrations of 0.33 and/or 3.3 mg/mL (p < 0.001). A total of 1 kg Fenugreek seed was ground and continuously extracted with light petroleum for 16 h and dried by rotary evaporation to yield an oil fraction (A; 45°g). Fenugreek seed powder (895°g), which had been defatted, was dried at room temperature and subjected to a 24-hour continuous extraction with 100% ethanol. The crude ethanolic extract (B; 65°g) was obtained after drying as before. A portion of B was redissolved in water and further extracted by n-butanol (C; 16.9°g) by evaporating the n-butanol layer, leaving the water layer containing saponin-free extract (D; 13.7°g on freeze drying). Diosgenin and trigonelline both prevented the absorption of glucose, with IC50 values of nearly 8 and 19 mM, respectively. Diosgenin (1.65 mg/ml) was more efficient than trigonelline (9.4% non-significant inhibition at 1.65 mg/ml) in inhibiting glucagon-induced HGPa activity (63). Diosgenin, which shares structural similarities with dehydroepiandrosterone (DHEA), reduces hyperglycemia in streptozotocin (STZ)-induced type 1 diabetes mellitus mice through increasing muscle GLUT4 signalling. After receiving diosgenin injection for 120 min, serum DHEA levels dramatically increased; concurrently, blood glucose levels significantly dropped (64).
Inhibit glucose uptake
It’s crucial to comprehend the mechanism through which organs and tissues absorb glucose. Inhibiting hyperglycemia and its associated consequences can be prevented in the ideal way thanks to this information. Since most live cells in the body require the transportation of glucose, it is a primary source of energy for mammalian cells. Every cell’s internal structure is protected by a phospholipid bilayer (65). Glucose molecules must cross lipid bilayers in the cell membrane as part of the transportation mechanism. Passive diffusion is used to allow hydrophobic species to travel across the hydrophobic lipid bilayers, which are permeable to them. Contrarily, glucose is hydrophilic and travels through the bilayer via enhanced diffusion, which is made possible by the career protein. Although the precise structure of the career protein for glucose update is unclear, conformational changes are known to trigger transport (66). The direction of glucose molecule migration during facilitated diffusion is governed by the relative concentration on the two sides of the lipid bilayer. In facilitated diffusion, the molecules are moved across the membrane with the aid of channel proteins and carrier proteins rather than dissolving in the lipid bilayer (67). When the concentration of glucose outside the cell declines, such as in liver cells where glucose is synthesized when blood sugar levels are low, the transportation of glucose via the lipid bilayer can also occur in the opposite direction. The largest superfamily of membrane transporters, which includes 74 families and more than 10,000 members, includes glucose transporters. The facilitative glucose transporters (GLUTs) and sodium glucose co-transporters (SGLTs) are two possible types of glucose transporters (68). The rate of pancreatic insulin production is inversely correlated with the rate of glucose diffusion. Glucose-6-phosphate is produced after diffusion into the cell. By doing this, a cell is equipped with an energy reserve that may be tapped into as needed by the body (69).
HepG2 cells were treated in media containing insulin in a study to determine the action and mechanism of diosgenin and 5-methoxypsorlen (5-MOP). The findings showed that glucose consumption decreased at high insulin concentrations (10–6°μmol/L) (70). However, the intake of glucose increased in a group of cells treated with diosgenin. The intracellular glycogen content also rose, showing that diosgenin was having a positive effect. During study related to action mechanism of diosgenin, it was revealed that the action of diosgenin significantly increased the phosphorylated expression of estrogen receptor-α (ERα), sarcoma (Src), Akt/protein kinase B, glycogen synthase kinase-3β (GSK3β), and the p85 regulatory subunit of phosphatidylinositol 3-kinase p85 (PI3Kp85x). In a group of cells that had received diosgenin treatment, the expression of GLUT-4 had significantly increased. Thus, it was possible to draw the following conclusions from the studies: diosgenin could reduce insulin resistance, increase glucose uptake, and hasten intracellular glycogen production. By encouraging the expression of adipocyte development in 3T3-L1 cells, diosgenin improves glucose uptake. The increased expression of mRNA also demonstrated its therapeutic potential (11, 49, 71). Diosgenin’s therapeutic potential was also examined in groups of 10 male Wister rats that had been given STZ. Sesame oil, diosgenin (10 mg), and diosgenin combined with a 5α-reductase (1 mg) inhibitor were administered to each diabetic group. Within 90−180 min, it was seen that the diosgenin-injected group’s blood glucose level had dropped. Diosgenin elevated serum dehydroepiandrosterone, which caused hyperglycemia in type 1 diabetic individuals. Diosgenin caused a 28% drop in glucose levels. The process is happening through the activation of AkT and PKC ζ/λ - GLUT4 signalling pathways. Both muscular AkT and PKC ζ/λ phosphorylation and GLUT4 translocation increased by 30% as compared to the untreated type 1 diabetic mice (64, 72).
Hepatic functions including gluconeogenesis and decomposition of glycogens also contribute to high blood sugar levels. These hepatic processes have been successfully controlled by diosgenin dose (73). The activity of glucose-6-phosphate dehydrogenase was dramatically decreased in the diet supplemented with 1% steroidal saponins from bitter yam or commercial diosgenin (74). Steroidal saponins possess a hexacyclic aglycone, such as diosgenin or tigogenin, in which the 3-OH group is decorated with an oligosaccharide chain. Also, diosgenin is a steroid saponin that is present in many plant species and is thought to provide a variety of essential therapeutic qualities. It is well recognized that glucose-6-phosphate plays a crucial function in controlling blood sugar levels while fasting. In glycolysis, glucose-6-phosphate is used to generate energy in place of ATP and NADH. Hyperglycemia has also been connected to a deficit in glucose-6-phosphate dehydrogenase (75).
Insulin, insulin analogues, improved delivery, and insulin resistance
Insulin is a natural hormone playing a critical role in blood sugar level regulation and is formed by beta cells of Islets of Langerhans in the pancreas. Though the main function of insulin is the regulation of blood sugar levels, besides it is essential for certain metabolic regulations in the body including glucose storage control, protein and fat metabolism, and appetite regulation. In persons suffering from diabetes, either insulin is not produced by the body which is Type 1 diabetes, or the produced insulin is not used effectively by the body which is Type 2 diabetes (76). Insulin helps in the regulation of blood sugar levels by permitting sugar (glucose) from the blood to pass into the cells, where it is utilized as an energy source. During diabetes, the absence of insulin or its inability consequences in raised sugar levels in the bloodstream.
The artificial sorts of insulin being altered to enhance their pharmacokinetic activities are called Insulin analogs. They are produced to impersonate the normal insulin release from the pancreas resulting in improved glucose control in the blood in diabetic patients (77). Insulin analogs are produced to have advantages than traditional insulins including better absorption, extended action-time, and better flexibility. Insulin analogs are of a number of types including rapid-acting, short-acting, intermediate-acting, and long-acting analogs (78). Insulin which is rapid-acting works swiftly and should primarily be taken prior to meals in order to resistor sugar spikes post-meal blood. These insulins work within 15 min post administration and showed a crowning effect between 0.5−3°h and last for around 3−5 h. On the other hand, short-acting insulin also known as regular insulin (such as human insulin) works slowly comparatively and likewise is also given formerly meals. This starts working within 0.5°h after administration, peaks in 2−4 h, and lasts for 6−8 h. Insulin that is intermediate-acting shows an extended interval and is taken once or twice a day, having a listless action onset, typically 1−2 h after taking. Its peak effect is between 4−12 h which lasts for 12−18 h. Neutral Protamine Hagedorn is this type of insulin analogue (79). While long-lasting insulin delivers a sturdy insulin release for a prolonged time and is frequently administered as basal insulin. These insulins provide a basal level of insulin release over an extended period, usually covering 24 h or longer. They have a relatively stable effect with no pronounced peaks. However, considering an individual’s requirements, an amalgamation of diverse types of insulin might be given.
Maintaining sugar (glucose) levels in the blood, insulin therapy lessens the menace of severe snags and recovers long-term consequences in diabetic patients. By providing exogenic insulin, it recompenses for the insufficient formation or insulin utilization in the body. This assistance averts hyperglycemia and its related problems. Type 1 diabetes, a disorder identified as diabetic ketoacidosis may happen during severe insulin lack (80). Insulin therapy improves essential in diabetic ketoacidosis prevention. Giving sufficient insulin, energy, or fat breakdown can be prevented, which is usually responsible for ketones accumulation and blood acidification. Insulin enables glucose uptake by cells, letting it be cast off for the production of energy.
There are numerous approaches to the improvement of insulin delivery that aim to augment the absorption, administration, and glycemic control of insulin. Insulin pens are expedient devices using one-use insulin-filled cartridges and deliver insulin better than outdated vials and syringes. Insulin pumps deliver insulin continuously by using a catheter located underneath the skin, provide a stable basal insulin rate, and permit bolus dosages prior to meals (81). Jet injectors distribute insulin by using a high-pressure stream that infiltrates the skin, eradicating the use of needles. Inhalable insulin is a newfangled insulin delivery method using the device to deliver insulin in a powdered form and inhaled it into the lungs. Insulin patches stick to the skin and deliver insulin over microneedles or a permeable membrane and offer continuous insulin delivery (82). Although the abdomen is the furthermost site for the injection of insulin, alternate injection sites viz., thighs, upper arms, and buttocks. Insulin resistance is a disorder where the cells of the body especially fat, muscle and liver turn out to be less receptive to the insulin effects (83). As an outcome, the pancreas produces more insulin is being produced to recompense for the diminished sensitivity which leads to advanced insulin levels in the bloodstream called hyperinsulinemia.
Diosgenin has been explored for its anti-diabetic activities and its latent to advance insulin sensitivity. Some reports have indicated that diosgenin enhances the uptake of glucose in the cells, improves insulin signaling, and ameliorates diabetes effects. It has been reported that administration of different diosgenin doses (15, 30, and 60 mg/kg body weight) daily to diabetic rats for 45 days caused a significant (p < 0.05) decrease in glucose levels in the bloodstream and an upsurge in plasma insulin. The transformed actions of key enzymes of carbohydrate metabolism in the muscle and kidneys were regressed significantly (p < 0.05) to almost normal levels (84) and the found outcomes were related to a standard oral hypoglycaemic drug. Diosgenin effect on the skeletal disarrays persuaded by experimental type 1 diabetes in 3-month-old female rats induced by single streptozotocin injection (60 mg/kg i.p.) was studied (85). Diosgenin (50 mg/kg/day) was given after 2 weeks till 4 weeks and found that diosgenin countered the diabetes effect on the growth and cancellate bone in the distal femur signifying positive impact on the skeleton. Recently, it has been studied that diosgenin attenuates non-alcoholic fatty liver disease in type 2 diabetes by regulating SIRT6-related fatty acid uptake in spontaneous diabetic db/db mice in vitro and in vivo (86). Fenugreek extract and diosgenin protected the liver against Non-alcoholic steatohepatitis, and diosgenin showed a dose-dependent impact. Though, the activation of the AMPK cascade was believed to be the mechanism for hepatoprotective effects (87). However, most of the investigation of diosgenin’s effect on insulin role is led in animal models or cell cultures. Partial clinical research is done in humans, and the consequences are not yet decisive. Additional examination is required to elucidate the exact actions or mechanisms and therapeutic potential.
Promote adipocyte differentiation
Adipocyte differentiation is a process in which preadipocytes (precursor cells) progress into mature adipocytes having a tendency to store and release fat. In the milieu of diabetes, lessened differentiation of adipocytes may result in insulin resistance, a key indicator of type 2 diabetes, and led to raised sugar levels in the bloodstream and the pancreas generating more insulin in an effort to reimburse, ultimately causing dysfunction of pancreatic beta-cells. Numerous aspects including chronic inflammation, obesity, hormonal imbalance, and genetic malfunctioning can interrupt the normal adipocyte process differentiation leading to insulin resistance during diabetes (88). Visceral fat in the abdominal cavity is further active metabolically and related to a sophisticated risk of insulin resistance and diabetes related to subcutaneous fat under the skin. In diabetes, the study of molecular mechanisms and dysregulation of adipocyte differentiation is an active extent of research. By detecting vital aspects intricated in the process, investigators aim to advance new beneficial approaches for controlling diabetes and its linked difficulties. Despite the fact that diminished differentiation of adipocytes and insulin resistance are strictly associated with type 2 diabetes (89), type 1 diabetes is chiefly an autoimmune disorder when the immune system erroneously outbreaks and abolishes insulin-generating beta cells. Differentiation of adipocytes is not as much directly linked to type 1 diabetes, nonetheless, metabolic dysregulation and resistance of insulin may still happen in persons with this illness.
Diosgenin improves the metabolism of glucose by endorsing the differentiation of adipocytes and hindering inflammation in adipose tissues as reported in the literature. Diosgenin reduced the adipocytes and improved the expression levels of mRNA differentiation-related genes in adipose tissues, repressed penetration of macrophage into adipose tissues, and hindered expressions of numerous molecular components linked with inflammation in 3T3-L1 cells (90). Diosgenin weakened metabolic dysfunction in high-fat diet-fed mice, as demonstrated by declined glucose levels in the blood and improvement of glucose and insulin intolerance. Diosgenin repressed 3T3-L1 adipocyte differentiation, declined the size of adipocytes, inhibited PPARγ, and increased nuclear expression of Erβ which significantly suppressed diosgenin-exerted suppression of adipocyte differentiation and PPARγ expression indicating the repressive effect of diosgenin on adipocyte differentiation and validated that ERβ-exerted regulation of PPARγ expression and action is critical for diosgenin-inhibited adipocyte differentiation (91). 3T3-L1 adipocytes and RAW 264 macrophages evidently heightened tumor necrosis factor-α production, chemoattractant protein-1 monocyte, and nitric oxide, however, diosgenin treatment repressed the formation of these proinflammatory mediators and also blocked the inflammation macrophages persuaded from 3T3-L1 adipocytes. Also, diosgenin repressed the degradation of inhibitor κB and c-jun N-terminal kinase phosphorylation in macrophages and might be useful for amending the seditious changes in obese adipose tissues (92). Type 2 diabetes was induced in experimental animals by feeding high-fat diet (HFD) for 8 weeks followed by streptozotocin (STZ) injection (sub-diabetogenic dose; 35 mg/kg body weight). Oral administration of diosgenin at two doses (40 and 80 mg/kg body weight) for 14 days abridged hyperglycemia, hypercholesterolemia and hypertriglyceridemia and lipid accumulation in 3T3-L1 preadipocytes inveterate its adipogenic activity prejudiced by PPAR γ and PPAR α (93). Diosgenin lowers the damage of diabetes by altering cellular pathways for pancreatic β cell renewal for improved secretion of insulin and modifying ER-α-mediated PI3K/Akt pathways (94).
Safety dosage of diosgenin
Diosgenin is the main component of fenugreek saponins and a secondary metabolite. At dosages of 1125 mg/kg and higher, steroidal saponins, which include diosgenin, exhibited deleterious effects and even death. Interestingly, the traditional steroidal saponins dosage is 510 mg/kg/day, implying that steroidal saponins, in combination with diosgenin, have no significant toxicity at this dosage. Male Wistar rats were given diosgenin in three different doses (1, 10, and 50 mg/bw kg, respectively) and fenugreek seed (0.2 g/bwkg) orally for 6 weeks. The rats given 1 mg/kg diosgenin and fenugreek seed had a higher insulin sensitivity index and a higher metabolic clearance rate (95). For 1°week, male F344 rats were fed 0 or 1% fenugreek seed powder (FSP) or 0.05% or 0.1% diosgenin before receiving azoxymethane (15 mg/kg body weight). Bioactive substances found in fenugreek seeds, including protodioscin, trigoneoside, diosgenin, and yamogenin, affect a number of enzymes, including those involved in glucose and lipid metabolism. Dietary FSP at 1% and diosgenin at 0.1% inhibited total aberrant crypt foci by up to 33 and 39%, respectively, during the promotional stage (96). The viability and growth of HCT-116 cells were reduced by diosgenin in a dose-dependent manner. After 24 h, the IC50 cytotoxic dose of diosgenin in HCT-116 was 35°M, while concentrations of 32°M or higher reduced the percentage viable cells by 50%. The viability and growth of HCT-116 cells were reduced by diosgenin in a dose-dependent manner. After 24 h, the IC50 cytotoxic dose of diosgenin in HCT-116 was ∼35°μM, while concentrations of ∼32°μM or higher reduced the percentage viable cells by 50%. Increasing diosgenin concentrations reduced HMG-CoA reductase expression at both the mRNA and protein levels. Food saponin, diosgenin, is a powerful inhibitor of HCT-116 human colon carcinoma cells, inhibiting growth and inducing apoptosis (97). In rats, a dose of diosgenin (40 mg/kg) protected against changes in liver markers and the antioxidant system of red blood cells without causing any side effects (98). Diabetic rats were given diosgenin (40 mg/kg bw) orally, which significantly reduced plasma glucose while increasing insulin levels. Diosgenin may play a protective role against aortic damage caused by oxidative stress in diabetics by modulating antioxidant defence and reducing lipid peroxidation in the aorta (99). These studies demonstrated that diosgenin and its derivatives are non-toxic, highlighting their utility in the treatment of chronic diseases.
Conclusion and future prospective
Recent years have seen an increase in interest in using herbal medicine to supplement conventional therapies or treat a variety of conditions. The growing diabetic population, the demand for new medications from patients, and the potential market for new medications will all continue to drive fresh and creative research into all parts of diabetes therapy. The foundation for the subsequent generation of targeted drug development programmes has been laid by the mainstay medicines, which have been intensively researched throughout the years. Diosgenin, the primary active component of fenugreek, has been the subject of numerous tests by researchers looking at its role in the treatment of diabetes. Additionally, it is well recognized that the majority of diabetic problems are intimately tied to inflammation and oxidative stress in addition to impaired glucose and lipid metabolism. In addition to having an effective anti-inflammatory and antioxidant action, diosgenin also has a positive therapeutic effect on diabetes complications. Numerous studies have demonstrated the pharmacological advantages of diosgenin and its derivatives in the treatment of cancer, diabetes, osteoporosis, Alzheimer’s disease, and stroke. It has been demonstrated that diosgenin interacts with a number of molecular targets that are crucial actors in the occurrence and incidence of many major illnesses. Additionally, a multitarget medication strategy targeting various risk factors is a crucial paradigm and a cutting-edge technique for treating neurological diseases with complicated pathophysiology. To treat the various pathogenic characteristics of these disorders, combination therapies of diosgenin with substances exhibiting numerous modes of action are anticipated to be more effective than single medications. Therefore, it is strongly advised that future research use a systematic experimental design to evaluate the long-term effects of DG and/or its derivatives for the treatment of neurodegenerative illnesses and the management of associated symptoms. In-depth research must also be done on the risk assessment and safety evaluation of the pharmaceutical use of diosgenin or its derivatives in the treatment of neurodegenerative diseases.
Author contributions
YT: Conceptualization, Writing – original draft. MK: Conceptualization, Writing – review & editing. AC: Writing – review & editing. MS: Supervision, Writing – review & editing. PV: Writing – review & editing. MB: Conceptualization, Writing – review & editing. CK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mujeeb F, Bajpai P, Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res Int. (2014) 2014:497606. doi: 10.1155/2014/497606
2. Wani S, Kumar P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci. (2018) 17:97–106. doi: 10.1016/j.jssas.2016.01.007
3. Rana P, Kumar A, Choudhary A, Kaur H, Singh R. The wisdom of prevention: Holistic, preventive herb approach for healing of the globe. J Pharm Innov. (2021) 10:29–46.
4. Ranade M, Mudgalkar N. A simple dietary addition of fenugreek seed leads to the reduction in blood glucose levels: A parallel group, randomized single-blind trial. Ayu. (2017) 38(1–2):24–7. doi: 10.4103/ayu.AYU_209_15
5. Syed Q, Rashid Z, Ahmad M, Shukat R, Ishaq A, Muhammad N, et al. Nutritional and therapeutic properties of fenugreek (Trigonella foenum-graecum): a review. Int J Food Prop. (2020) 23:1777–91. doi: 10.1080/10942912.2020.1825482
6. Paramesha M, Priyanka N, Crassina K. Evaluation of diosgenin content from eleven different Indian varieties of fenugreek and fenugreek leaf powder fortified bread. J Food Sci Technol. (2021) 58:4746–54. doi: 10.1007/s13197-021-04967-z
7. Baset M, Ali T, Elshamy H, El Sadek A, Sami D, Badawy M, et al. Anti-diabetic effects of fenugreek (Trigonella foenum-graecum): A comparison between oral and intraperitoneal administration - an animal study. Int J Fun Nut. (2020) 1:2. doi: 10.3892/ijfn.2020.2
8. Karve A, Hayward R. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care. (2010) 33:2355–9. doi: 10.2337/dc09-1957
9. Gaddam A, Galla C, Thummisetti S, Marikanty R, Palanisamy U, Rao B. Role of Fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. J Diabetes Metab Disord. (2015) 14:74. doi: 10.1186/s40200-015-0208-4
10. Deshpande A, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Physical therapy (2008) 88:1254–64. doi: 10.2522/ptj.20080020
11. Fuller S, Stephens J. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: mechanisms of actions and potential effects on metabolic syndrome. Adv Nutr. (2015) 6:189–97. doi: 10.3945/an.114.007807
12. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2009) 32:S62–7. doi: 10.2337/dc09-S062
13. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. (2019) 1:S46–60. doi: 10.2337/dc19-S005
14. Atkinson M. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb Perspect Med. (2012) 2:a007641. doi: 10.1101/cshperspect.a007641
15. Haque M, Das J, Xiong X, Song J. Targeting stem cell-derived tissue-associated regulatory t cells for type 1 diabetes immunotherapy. Curr Diab Rep. (2019) 19:89. doi: 10.1007/s11892-019-1213-7
16. Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. (2021) 69:2932–8. doi: 10.4103/ijo.IJO_1627_21
17. Wondmkun Y. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab Syndr Obes. (2020) 13:3611–6. doi: 10.2147/DMSO.S275898
18. Deedwania P. Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: benefits of vasodilating β-blockers. J Clin Hypertens. (2011) 13:52–9. doi: 10.1111/j.1751-7176.2010.00386.x
19. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Ca?izo-Gómez F. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. (2014) 5:444–70. doi: 10.4239/wjd.v5.i4.444
20. Quintanilla Rodriguez B, Mahdy H. Gestational Diabetes. [Updated 2022 Sep 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
21. Buchanan T, Xiang A. Gestational diabetes mellitus. J Clin Invest. (2005) 115:485–91. doi: 10.1172/JCI24531
22. Spanakis E, Golden S. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. (2013) 13:814–23. doi: 10.1007/s11892-013-0421-9
23. Kampmann U, Madsen L, Skajaa G, Iversen D, Moeller N, Ovesen P. Gestational diabetes: A clinical update. World J Diabetes. (2015) 6:1065–72. doi: 10.4239/wjd.v6.i8.1065
24. Saeedi M, Cao Y, Fadl H, Gustafson H, Simmons D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2021) 172:108642. doi: 10.1016/j.diabres.2020.108642
25. Ahmad A, Alghamdi S, Mahmood K, Afzal M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J Biol Sci. (2016) 23:300–10. doi: 10.1016/j.sjbs.2015.09.015
26. Cortez-Navarrete M, Pérez-Rubio K, Escobedo-Gutiérrez M. Role of Fenugreek, Cinnamon, Curcuma longa, Berberine and Momordica charantia in Type 2 Diabetes Mellitus Treatment: A Review. Pharmaceuticals (Basel). (2023) 16:515. doi: 10.3390/ph16040515
27. Hassani S, Fallahi Arezodar F, Esmaeili S, Gholami-Fesharaki M. Effect of Fenugreek Use on Fasting Blood Glucose, Glycosylated Hemoglobin, Body Mass Index, Waist Circumference, Blood Pressure and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trials. Galen Med J. (2019) 8:e1432. doi: 10.31661/gmj.v8i0.1432
28. National Institute of Child Health and Human Development.Drugs and Lactation Database (LactMed§) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development (2006).
29. National Institute of Child Health and Human Development, NIH, DHHS.The NICHD Study of Early Child Care and Youth Development (SECCYD): Findings for Children up to Age 4 1/2 Years (Reference Only) (05-4318). Washington, DC: U.S. Government Printing Office (2006).
30. Wankhede S, Mohan V, Thakurdesai P. Beneficial effects of fenugreek glycoside supplementation in male subjects during resistance training: A randomized controlled pilot study. J Sport Health Sci. (2016) 5:176–82. doi: 10.1016/j.jshs.2014.09.005
31. Singh P, Bajpai V, Gond V, Kumar A, Tadigoppula N, Kumar B. Determination of Bioactive Compounds of Fenugreek (Trigonella foenum-graecum) Seeds Using LC-MS Techniques. Methods Mol Biol. (2020) 2107:377–93. doi: 10.1007/978-1-0716-0235-5_21
32. Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. (2004) 7:67–78. doi: 10.1089/109662004322984734
33. Visuvanathan T, Than L, Stanslas J, Chew S, Vellasamy S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants. (2022) 11:1450. doi: 10.3390/plants11111450
34. Salam S, Rashed M, Ibrahim N, Rahim E, Aly T, Al-Farga A. Phytochemical screening and in-vitro biological properties of unprocessed and household processed fenugreek (Trigonella foenum-graecum Linn.) seeds and leaves. Sci Rep. (2023) 13:7032. doi: 10.1038/s41598-023-31888-y
35. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. (2004) 74:2157–84. doi: 10.1016/j.lfs.2003.09.047
36. Goyal S, Gupta N, Chatterjee S. Investigating Therapeutic Potential of Trigonella foenum-graecum L. as Our Defense Mechanism against Several Human Diseases. J Toxicol. (2016) 2016:1250387. doi: 10.1155/2016/1250387
37. Sauvaire Y, Ribes G, Baccou J, Loubatieeres-Mariani M. Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids. (1991) 26:191–7.
38. Pang X, Huang H, Zhao Y, Xiong C. Conversion of furostanol saponins into spirostanol saponins improves the yield of diosgenin from Dioscorea zingiberensis by acid hydrolysis. RSC Adv. (2015) 5:4831–7. doi: 10.1039/C4RA12709A
39. Navarro del Hierro J, Reglero G, Martin D. Chemical Characterization and Bioaccessibility of Bioactive Compounds from Saponin-Rich Extracts and Their Acid-Hydrolysates Obtained from Fenugreek and Quinoa. Foods. (2020) 9:1159. doi: 10.3390/foods9091159
40. Wagner H, Iyengar M, Horhammer L. Vicenin-1 and -2 in the seeds of Trigonella foenum-graecum. Phytochem. (1973) 12:2548.
41. Rayyan S, Fossen T, Andersen Q. Flavone Cglycosides from seeds of fenugreek, Trigonella foenumgraecum L. J Agric Food Chem. (2010) 58:7211–7. doi: 10.1021/jf100848c
42. Sethi G, Shanmugam M, Warrier S, Merarchi M, Arfuso F, Kumar A, et al. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients. (2018) 10:645. doi: 10.3390/nu10050645
45. Zhang F, Shen B, Jiang W. Hydrolysis extraction of diosgenin from Dioscorea nipponica Makino by sulfonated magnetic solid composites. J Nanopart Res. (2019) 21:269. doi: 10.1007/s11051-019-4702-3
46. Arya P, Kumar P. Comparison of ultrasound and microwave assisted extraction of diosgenin from Trigonella foenum-graceum seed. Ultrason Sonochem. (2021) 74:105572. doi: 10.1016/j.ultsonch.2021.105572
47. Wani S, Bishnoi S, Kumar P. Ultrasound and microwave assisted extraction of diosgenin from fenugreek seed and fenugreek-supplemented cookies. J Food Meas Charact. (2016) 10:527–32. doi: 10.1007/s11694-016-9331-2
48. Dsouza M, Rufina K, Hana D. Extraction of Diosgenin from Fenugreek and evaluation of its pharmacological role in alleviating Metabolic Syndrome in vitro. Res J Biotechnol. (2018) 13:10–7.
49. Jesus M, Martins A, Gallardo E, Silvestre S. Diosgenin: Recent Highlights on Pharmacology and Analytical Methodology. J Anal Methods Chem. (2016) 2016:4156293. doi: 10.1155/2016/4156293
50. National Center for Biotechnology Information.PubChem Compound Summary for CID 99474, Diosgenin. Bethesda, MD: National Center for Biotechnology Information (2023).
51. D?browska-Balcerzak K, Nartowska J, Wawer I, Siudem P, Paradowska K. Spirostanol Sapogenins and Saponins from Convallaria majalis L. Structural Characterization by 2D NMR, Theoretical GIAO DFT Calculations and Molecular Modeling. Molecules. (2021) 26:2999. doi: 10.3390/molecules26102999
52. Zhou C, Yang Y, Tian J, Wu Y, An F, Li C, et al. 22R- but not 22S-hydroxycholesterol is recruited for diosgenin biosynthesis. Plant J. (2022) 109:940–51. doi: 10.1111/tpj.15604
53. Ghayur M, Abdalla M, Khalid A, Ahmad S, Gilani A. Trigonella foenum-graecum methanolic extract on isolated smooth muscles and acetylcholinesterase enzyme: an in vitro and mechanistic in silico investigation. Biomed Res Int. (2022) 5:4849464. doi: 10.1155/2022/4849464
54. Abdulai I, Kwofie S, Gbewonyo W, Boison D, Puplampu J, Adinortey M. Multitargeted effects of vitexin and isovitexin on diabetes mellitus and its complications. Sci World J. (2021) 2021:6641128. doi: 10.1155/2021/6641128
55. Semwal P, Painuli S, Abu-Izneid T, Rauf A, Sharma A, Da?tan S, et al. Diosgenin: an updated pharmacological review and therapeutic perspectives. Oxid Med Cell Longev. (2022) 29:1035441. doi: 10.1155/2022/1035441
56. Sirotkin A, Alexa R, Alwasel S, Harrath A. The phytoestrogen, diosgenin, directly stimulates ovarian cell functions in two farm animal species. Domest Anim Endocrinol. (2019) 69:35–41. doi: 10.1016/j.domaniend.2019.04.002
57. Zhao H, Zhang X, Zhang B, Qu X. Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities. Open Life Sci. (2021) 16:719–27. doi: 10.1515/biol-2021-0075
58. Kotani K, Peroni O, Minokoshi Y, Boss O, Kahn B. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest. (2004) 114:1666–75. doi: 10.1172/JCI21341
59. Gromova L, Fetissov S, Gruzdkov A. Mechanisms of Glucose Absorption in the Small Intestine in Health and Metabolic Diseases and Their Role in Appetite Regulation. Nutrients. (2021) 13:2474. doi: 10.3390/nu13072474
60. Poulsen S, Fenton R, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens. (2015) 24:463–9. doi: 10.1097/MNH.0000000000000152
61. Ghosh S, More P, Derle A, Patil A, Markad P, Asok A, et al. Diosgenin from Dioscorea bulbifera: novel hit for treatment of type II diabetes mellitus with inhibitory activity against α-amylase and α-glucosidase. PLoS One. (2014) 9:e106039. doi: 10.1371/journal.pone.0106039
62. Zhang H, Xu J, Wang M, Xia X, Dai R, Zhao Y. Steroidal saponins and sapogenins from fenugreek and their inhibitory activity against α-glucosidase. Steroids. (2020) 161:108690. doi: 10.1016/j.steroids.2020.108690
63. McAnuff M, Harding W, Omoruyi F, Jacobs H, Morrison E, Asemota H. Hypoglycemic effects of steroidal sapogenins isolated from Jamaican bitter yam, Dioscorea polygonoides. Food Chem Toxicol. (2005) 43:1667–72. doi: 10.1016/j.fct.2005.05.008
64. Al-Habori M, Raman A, Lawrence M, Skett P. In vitro effect of fenugreek extracts on intestinal sodium-dependent glucose uptake and hepatic glycogen phosphorylase A. Int J Exp Diabetes Res. (2001) 2:91–9. doi: 10.1155/edr.2001.91
65. Sato K, Fujita S, Iemitsu M. Acute administration of diosgenin or dioscorea improves hyperglycemia with increases muscular steroidogenesis in STZ-induced type 1 diabetic rats. J Steroid Biochem Mol Biol. (2014) 143:152–9. doi: 10.1016/j.jsbmb.2014.02.020
66. Nakrani M, Wineland R, Anjum F. Physiology, Glucose Metabolism. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
68. Upadhyay R. Transendothelial transport and its role in therapeutics. Int Sch Res Notices. (2014) 2014:309404. doi: 10.1155/2014/309404
69. Navale A, Paranjape A. Glucose transporters: physiological and pathological roles. Biophys Rev. (2016) 8:5–9. doi: 10.1007/s12551-015-0186-2
70. Fu Z, Gilbert E, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. (2013) 9:25–53.
71. Fang K, Dong H, Jiang S, Li F, Wang D, Yang D, et al. Diosgenin and 5-Methoxypsoralen Ameliorate Insulin Resistance through ER-α/PI3K/Akt-Signaling Pathways in HepG2 Cells. Evid Based Complement Alternat Med. (2016) 2016:7493694. doi: 10.1155/2016/7493694
72. Leng J, Li X, Tian H, Liu C, Guo Y, Zhang S, et al. Neuroprotective effect of diosgenin in a mouse model of diabetic peripheral neuropathy involves the Nrf2/HO-1 pathway. BMC Complement Med Ther. (2020) 20:126. doi: 10.1186/s12906-020-02930-7
73. Cai B, Zhang Y, Wang Z, Xu D, Jia Y, Guan Y, et al. Therapeutic Potential of Diosgenin and Its Major Derivatives against Neurological Diseases: Recent Advances. Oxid Med Cell Longev. (2020) 2020:3153082. doi: 10.1155/2020/3153082
74. Sharabi K, Tavares C, Rines A, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med. (2015) 46:21–33. doi: 10.1016/j.mam.2015.09.003
75. Mahmoud A, Nor El-Din AK. Glucose-6-Phosphate Dehydrogenase Activity and Protein Oxidative Modification in Patients with Type 2 Diabetes Mellitus. J Biomark. (2013) 2013:430813. doi: 10.1155/2013/430813
76. Rajas F, Gautier-Stein A, Mithieux G. Glucose-6 Phosphate. A Central Hub for Liver Carbohydrate Metabolism. Metabolites. (2019) 9:282. doi: 10.3390/metabo9120282
77. Rajput D, Basha S, Xin Q, Gadekallu T, Kaluri R, Lakshmanna K, et al. Providing diagnosis on diabetes using cloud computing environment to the people living in rural areas of India. J Ambient Intell Human Comput. (2022) 13:2829–40. doi: 10.1007/s12652-021-03154-4
78. Valla V. Therapeutics of diabetes mellitus: focus on insulin analogues and insulin pumps. Exp Diabetes Res. (2010) 2010:178372. doi: 10.1155/2010/178372
79. Sharma A, Taneja G, Kumar A, Sahu M, Sharma G, Kumar A, et al. Insulin analogs: Glimpse on contemporary facts and future prospective. Life Sci. (2019) 219:90–9. doi: 10.1016/j.lfs.2019.01.011
80. Brunetti V, Yu O, Platt R, Filion K. The association of long-acting insulin analogue use versus neutral protamine Hagedorn insulin use and the risk of major adverse cardiovascular events among individuals with type 2 diabetes: A population-based cohort study. Diabetes Obes Metab. (2022) 24:2169–81. doi: 10.1111/dom.14802
81. Calimag A, Chlebek S, Lerma E, Chaiban J. Diabetic ketoacidosis. Dis Mon. (2023) 69:101418. doi: 10.1016/j.disamonth.2022.101418
82. Thompson A, Lathan P, Fleeman L. Update on insulin treatment for dogs and cats: insulin dosing pens and more. Vet Med (Auckl). (2015) 6:129–42. doi: 10.2147/VMRR.S39984
83. Khafagy E, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Deliv Rev. (2007) 59:1521–46. doi: 10.1016/j.addr.2007.08.019
84. Lee W. MicroRNA, Insulin Resistance, and Metabolic Disorders. Int J Mol Sci. (2022) 23:16215. doi: 10.3390/ijms232416215
85. Saravanan G, Ponmurugan P, Deepa M, Senthilkumar B. Modulatory effects of diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in streptozotocin-induced diabetic rats. Can J Diabetes. (2014) 38:409–14. doi: 10.1016/j.jcjd.2014.02.004
86. Londzin P, Kisiel-Nawrot E, Kocik S, Janas A, Trawczy?ski M, Cegieła U, et al. Effects of diosgenin on the skeletal system in rats with experimental type 1 diabetes. Biomed Pharmacother. (2020) 129:110342. doi: 10.1016/j.biopha.2020.110342
87. Nie K, Gao Y, Chen S, Wang Z, Wang H, Tang Y, et al. Diosgenin attenuates non-alcoholic fatty liver disease in type 2 diabetes through regulating SIRT6-related fatty acid uptake. Phytomedicine. (2023) 111:154661. doi: 10.1016/j.phymed.2023.154661
88. Eltamalawy M, Abdel-Aziz A, Mohamed T, Khedr N. The prophylactic treatment of Egyptian, Trigonella foenum-graecum L., Extract in comparison to pure diosgenin on experimentally induced non-alcoholic steatohepatitis: New targets via AMPK, RAR, and FXR pathways. Phytomed Plus. (2023) 3:100421. doi: 10.1016/j.phyplu.2023.100421
89. Tong Y, Xu S, Huang L, Chen C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov Today. (2022) 27:822–30. doi: 10.1016/j.drudis.2021.11.001
90. Muscogiuri G, Sorice G, Mezza T, Prioletta A, Lassandro A, Pirronti T, et al. High-normal TSH values in obesity: is it insulin resistance or adipose tissue’s guilt? Obesity (Silver Spring). (2013) 21:101–6. doi: 10.1002/oby.20240
91. Uemura T, Hirai S, Mizoguchi N, Goto T, Lee J, Taketani K, et al. Diosgenin present in fenugreek improves glucose metabolism by promoting adipocyte differentiation and inhibiting inflammation in adipose tissues. Mol Nutr Food Res. (2010) 11:1596–608. doi: 10.1002/mnfr.200900609
92. Wang X, Liu J, Long Z, Sun Q, Liu Y, Wang L, et al. Effect of diosgenin on metabolic dysfunction: Role of ERβ in the regulation of PPARγ. Toxicol Appl Pharmacol. (2015) 289:286–96. doi: 10.1016/j.taap.2015.09.015
93. Hirai S, Uemura T, Mizoguchi N, Lee J, Taketani K, Nakano Y. Diosgenin attenuates inflammatory changes in the interaction between adipocytes and macrophages. Mol Nut Food Res. (2010) 54:797–804. doi: 10.1002/mnfr.200900208
94. Sangeetha M, ShriShri Mal N, Atmaja K, Sali V, Vasanthi H. PPAR’s and Diosgenin a chemico biological insight in NIDDM. Chem Biol Interact. (2013) 206:403–10. doi: 10.1016/j.cbi.2013.08.014
95. Arya P, Kumar P. Diosgenin: An ingress towards solving puzzle for diabetes treatment. J Food Biochem. (2022) 46:e14390. doi: 10.1111/jfbc.14390
96. Kiss R, Pesti-Asbóth G, Szarvas M, Stündl L, Cziáky Z, Hegedûs C, et al. Diosgenin and Its Fenugreek Based Biological Matrix Affect Insulin Resistance and Anabolic Hormones in a Rat Based Insulin Resistance Model. Biomed Res Int. (2019) 4:7213913. doi: 10.1155/2019/7213913
97. Raju J, Patlolla J, Swamy M, Rao C. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. (2004) 13: 1392–8.
98. Raju J, Bird R. Diosgenin, a naturally occurring steroid [corrected] saponin suppresses 3-hydroxy-3-methylglutaryl CoA reductase expression and induces apoptosis in HCT-116 human colon carcinoma cells. Cancer Letters. (2007) 255:194–204. doi: 10.1016/j.canlet.2007.04.011
99. Manivannan J, Barathkumar TR, Sivasubramanian J, Arunagiri P, Raja B, Balamurugan E. Diosgenin attenuates vascular calcification in chronic renal failure rats. Mol Cell Biochem. (2013) 378:9–18. doi: 10.1007/s11010-013-1588-8
100. Pari L, Monisha P, Mohamed Jalaludeen A. Beneficial role of diosgenin on oxidative stress in aorta of streptozotocin induced diabetic rats. Eur J Pharmacol. (2012) 691:143–50. doi: 10.1016/j.ejphar.2012.06.038
101. Suresh P, Singh P, Padwad Y, Sharma U. Steroidal saponins from Trillium govanianum as α-amylase, α-glucosidase, and dipeptidyl peptidase IV inhibitory agents. J Pharm Pharmacol. (2021) 73:487–95. doi: 10.1093/jpp/rgaa038
102. Ghosh S, Ahire M, Patil S, Jabgunde A, Bhat Dusane M, Joshi B, et al. Antidiabetic Activity of Gnidia glauca and Dioscorea bulbifera: Potent Amylase and Glucosidase Inhibitors. Evid Based Complement Alternat Med. (2012) 2012:929051. doi: 10.1155/2012/929051
103. Gad M, El-Sawalhi M, Ismail M, El-Tanbouly N. Biochemical study of the anti-diabetic action of the Egyptian plants fenugreek and balanites. Mol Cell Biochem. (2006) 281:173–83. doi: 10.1007/s11010-006-0996-4
104. Uemura T, Goto T, Kang M, Mizoguchi N, Hirai S, Lee J, et al. Diosgenin, the main aglycon of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J Nutr. (2011) 141:17–23. doi: 10.3945/jn.110.125591
105. Mayakrishnan T, Nakkala J, Jeepipalli S. Fenugreek seed extract and its phytocompounds- trigonelline and diosgenin arbitrate their hepatoprotective effects through attenuation of endoplasmic reticulum stress and oxidative stress in type 2 diabetic rats. Eur Food Res Technol. (2015) 240:223–32. doi: 10.1007/s00217-014-2322-9
106. Naidu P, Ponmurugan P, Begum M, Mohan K, Meriga B, Ravindar N, et al. Diosgenin reorganises hyperglycaemia and distorted tissue lipid profile in high-fat diet-streptozotocin-induced diabetic rats. J Sci Food Agric. (2015) 95:3177–82. doi: 10.1002/jsfa.7057
Keywords: bioactive, diabetes, diosgenin, extraction, fenugreek
Citation: Tak Y, Kaur M, Chitranashi A, Samota MK, Verma P, Bali M and Kumawat C (2024) Fenugreek derived diosgenin as an emerging source for diabetic therapy. Front. Nutr. 11:1280100. doi: 10.3389/fnut.2024.1280100
Received: 19 August 2023; Accepted: 08 January 2024;
Published: 02 February 2024.
Edited by:
Shaikh Jamal Uddin, Khulna University, BangladeshReviewed by:
Pukar Khanal, Emory University, United StatesMaria Maisto, University of Naples Federico II, Italy
Vincenzo Piccolo, University of Naples Federico II Naples, Italy, in collaboration with reviewer MM
Copyright © 2024 Tak, Kaur, Chitranashi, Samota, Verma, Bali and Kumawat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yamini Tak, eWFtaW5pdGFrMTk5MkBnbWFpbC5jb20=; Mahesh Kumar Samota, bWFoZXNoLmlhcmkxQGdtYWlsLmNvbQ==
 Yamini Tak
Yamini Tak Manpreet Kaur
Manpreet Kaur Abhishek Chitranashi
Abhishek Chitranashi Mahesh Kumar Samota
Mahesh Kumar Samota Preeti Verma1
Preeti Verma1 Manoj Bali
Manoj Bali