
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 04 March 2024
Sec. Nutrigenomics
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1276497
Background: Cholelithiasis, commonly referred to as gallstones, is a prevalent medical condition influenced by a combination of genetic factors, lifestyle choices, and dietary habits. Specific food items have been associated with an increased susceptibility to cholelithiasis, whereas others seem to offer a protective effect against its development.
Methods: In this study, we conducted a Mendelian randomization (MR) analysis using a large-scale genetic dataset comprising individuals with European ancestry to explore the potential causal relationship between diet and cholelithiasis. The analysis incorporated 17 food-related variables, which were considered as potential factors influencing the occurrence of this condition.
Results: Our findings indicate that a higher consumption of cooked vegetables, dried fruit, and oily fish is associated with a reduced risk of cholelithiasis. Conversely, a higher consumption of lamb is associated with an increased risk of developing the condition. Importantly, these associations proved robust to sensitivity and heterogeneity tests, and the pleiotropic test results further supported the hypothesis of a causal relationship between diet and cholelithiasis.
Conclusion: Through our study, we provide compelling evidence for the existence of a causal relationship between diet and cholelithiasis. Adopting a dietary pattern enriched with cooked vegetables, dried fruit, and oily fish, while minimizing lamb intake, may contribute to the prevention of cholelithiasis. Recognizing diet as a modifiable risk factor in the prevention and management of this condition is of paramount importance, and our study offers valuable insights in this regard.
Cholelithiasis, commonly referred to as gallstones, is a widespread medical condition affecting a substantial global population (1). The development of gallstones involves a multifactorial process, influenced by genetic factors, lifestyle choices, and dietary patterns (2–5). Over recent decades, the prevalence of cholelithiasis has been on the rise, affecting approximately 10–15% of adults in industrialized nations (6). Related risk factors have aroused public concern. These include supersaturation of cholesterol, hypomotility of the gallbladder, excess bilirubin, age, ethnicity, female gender, disease history, family history, smoking, alcohol consumption, body mass index (BMI), and cholesterol levels (7). Additionally, bariatric surgery, a treatment for obesity, can also increase the risk of cholelithiasis, with Caucasian race and female sex being identified as risk factors (8). Gilbert syndrome, a hereditary hyperbilirubinemia, has been associated with an increased risk of cholelithiasis in children, although it is not the sole risk factor (9). However, the relationship between risk factors and cholelithiasis is not explained clearly.
Dietary factors have been identified as significant contributors to the risk of cholelithiasis, with certain foods associated with an increased likelihood of gallstone formation. High consumption of agricultural products, fat, and sugar has been linked to elevated risk, while a diet rich in fiber, fruits, and vegetables appears to have a protective effect (10). However, the causal nature of the relationship between food intake and cholelithiasis remains uncertain, necessitating further investigation to ascertain the impact of diet on this condition.
Mendelian randomization (MR) serves as a valuable tool for exploring causality in observational studies. By utilizing genetic variants as proxies for exposures, MR offers a more robust assessment of the causal relationship between an exposure, such as dietary factors, and an outcome, like cholelithiasis, as it mitigates the influence of confounding and reverse causality (11).
This study aims to investigate the causal connection between specific food items and cholelithiasis through MR analysis, encompassing variables such as alcohol intake frequency, various food types (beef, bread, cereal, cheese, coffee, cooked vegetables, dried fruit, fresh fruit, lamb/mutton, non-oily fish, oily fish, pork, poultry, processed meat, salad/raw vegetables, and tea). The findings provide a comprehensive evaluation of the causal link between diet and cholelithiasis, underscoring the significance of considering diet as a modifiable risk factor in both the prevention and management of this condition.
This study aims to elucidate the causal relationships between 17 common food exposures and the development of cholelithiasis (gallstones) by employing a robust two-pronged methodological approach: two-sample Mendelian randomization (MR) analysis and multivariable MR (MVMR) analysis. By leveraging genetic variants as instrumental variables (IVs) for food exposures, these methods allow us to infer causality from observational data while minimizing confounding factors and reverse causation, common limitations in traditional observational studies.
The genetic proxies for the 17 food exposures were Identified using data from the GIANT (Genetic Investigation of ANThropometric Traits) consortium, which provides a comprehensive resource for understanding genetic influences on dietary habits.
The incidence of cholelithiasis, specifically calculus of the gallbladder without cholecystitis, was assessed using data from the UK Biobank, a large-scale biomedical database and research resource containing in-depth genetic and health information from half a million UK participants.
The two-sample MR analysis aimed to estimate the causal effect of each food exposure on cholelithiasis (12). The remaining SNPs’ power was then evaluated using F statistics (F = beta2/se2) for each SNP, and an overall F statistic was generated for all SNPs. This analysis employed three methods: the Inverse Variance Weighted (IVW) method, MR-Egger regression, and the weighted median approach. The IVW method served as the primary approach, while MR-Egger regression was employed to control for pleiotropy effects. The weighted median approach combined results from multiple MR estimates to estimate the causal effect of each food exposure.
The MVMR analysis sought to explore the causal relationship between multiple food exposures and cholelithiasis using the MVMR package (13). This analysis controlled for potential confounding effects arising from multiple exposures, enabling the estimation of the causal effect of each food exposure on cholelithiasis.
To assess the robustness of the results, sensitivity tests were performed, and individuals with missing data were excluded to mitigate potential confounding effects.
A heterogeneity test was conducted to determine the presence of between-study heterogeneity in the results. The Cochran’s Q statistic and the I2 statistic were used for this purpose. The Cochran’s Q statistic tested for heterogeneity between studies, while the I2 statistic quantified the proportion of total variation attributable to between-study heterogeneity.
A pleiotropic test was performed to investigate the presence of pleiotropy, wherein a single genetic variant influences multiple traits. The MR-PRESSO method, a gene-based test combining multiple MR estimates, was employed for detecting pleiotropy.
Data analysis was carried out using the R statistical software. The two-sample MR analysis utilized the TwosampleMR packages, while the MVMR analysis employed the MVMR package. Sensitivity tests, heterogeneity tests, and pleiotropic tests were conducted using the MR-PRESSO package. All statistical tests were two-tailed and performed at a significance level of 0.05.
Cholelithiasis, also known as gallstone disease, is a prevalent condition affecting a considerable global population. Although the precise etiology of cholelithiasis remains incompletely understood, lifestyle factors, particularly dietary habits, are believed to contribute significantly. In this study, we employed Mendelian randomization (MR) analysis to investigate the potential causal relationship between various food exposures and cholelithiasis. The study design is represented in Figure 1.
Data from the Global Assessment of Well-Being Study (GAWS) were analyzed, encompassing variables related to alcohol intake frequency, as well as the consumption of specific food items, such as beef, bread, cereal, cheese, coffee, cooked vegetables, dried fruit, fresh fruit, lamb/mutton, non-oily fish, oily fish, pork, poultry, processed meat, salad/raw vegetables, and tea (Table 1). The sample size was substantial, comprising over 400,000 individuals of European descent.
To ensure the robustness of our findings, we performed sensitivity tests, heterogeneity tests, and pleiotropic tests. As depicted in Figure 2, our analysis identified statistically significant causal relationships between cooked vegetables, dried fruit, lamb, oily fish, and cholelithiasis, as determined through both two-sample MR analysis, the intake of cooked vegetables was associated with a reduced risk of cholelithiasis, with an Odds Ratio (OR) of 0.990 (95% CI 0.983–0.997) and a p-value of 0.006. Similarly, dried fruit intake displayed a lower risk of cholelithiasis, with an OR of 0.992 (95% CI 0.987–0.997) and a p-value of 0.001. Oily fish consumption was also linked to a decreased risk, with an OR of 0.996 (95% CI 0.993–1.000) and a p-value of 0.026. In contrast, the consumption of lamb/mutton was associated with an elevated risk, showing an OR of 1.009 (95% CI 1.002–1.016) and a p-value of 0.012. Likewise, poultry intake displayed a higher risk, with an OR of 1.010 (95% CI 1.000–1.021) and a p-value of 0.046. Additional methods, including MR-Egger, weighted median, and IVW mre, providing additional support for our findings, are presented in Table 2. Figures 3A–Q, 4A–Q, 5A–Q, 6A–Q showcase the scatter plots, forest plots, funnel plots, and leave-one-out plots for cholelithiasis, respectively.
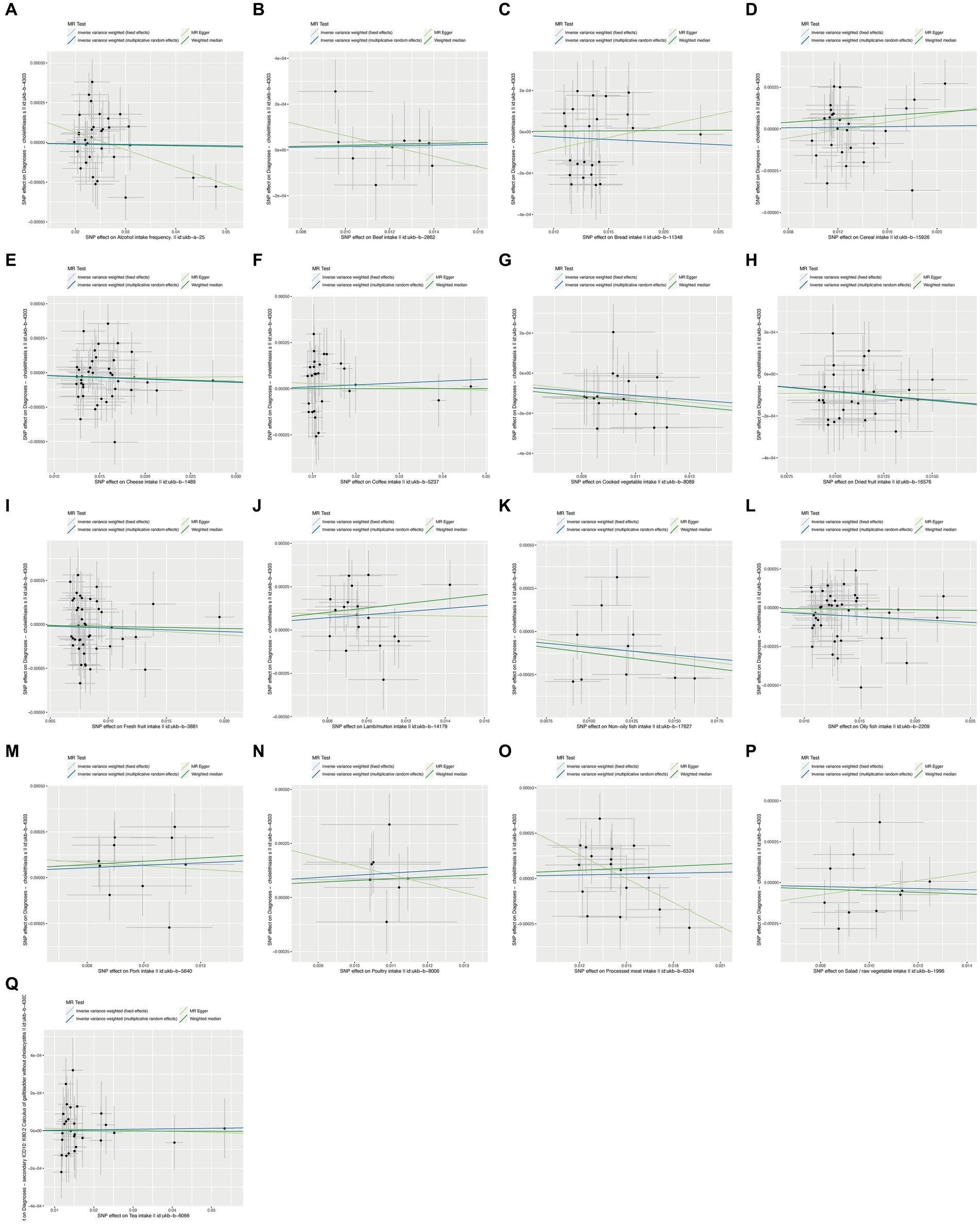
Figure 3. Scatter plots of food on cholelithiasis. (A) Alcohol; (B) Beef; (C) Bread; (D) Cereal; (E) Cheese; (F) Coffee; (G) Cooked vegetable; (H) Dried fruit; (I) Fresh fruit; (J) Lamb/mutton; (K) Non-oily fish; (L) Oily fish; (M) Pork; (N) Poultry; (O) Processed meat; (P) Salad/raw vegetable; (Q) Tea.
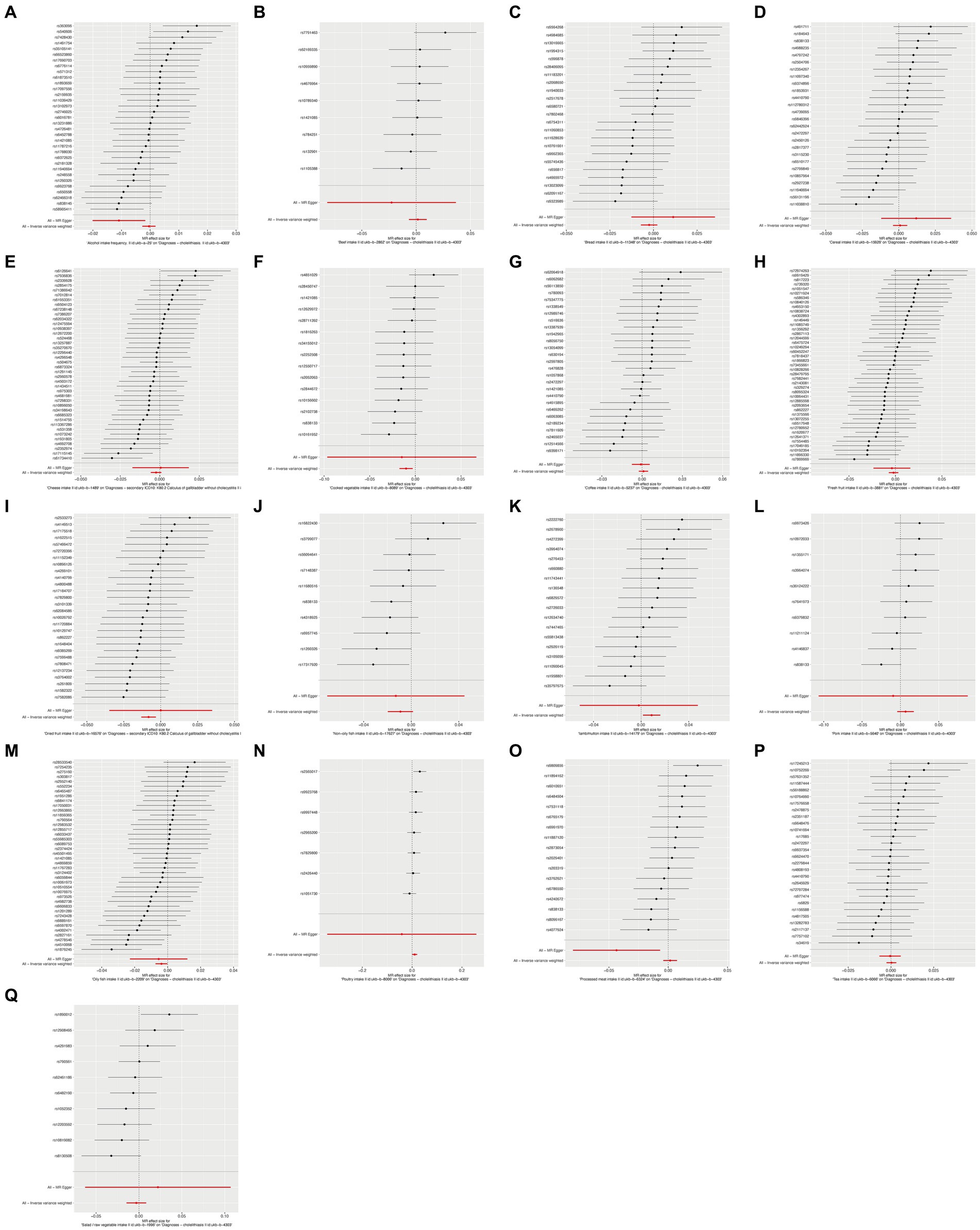
Figure 4. Forest plots of food on cholelithiasis. (A) Alcohol; (B) Beef; (C) Bread; (D) Cereal; (E) Cheese; (F) Cooked vegetable; (G) Coffee; (H) Fresh fruit; (I) Dried fruit; (J) Non-oily fish; (K) Lamb/mutton; (L) Pork; (M) Oily fish; (N) Poultry; (O) Processed meat; (P) Tea; (Q) Salad/raw vegetable.
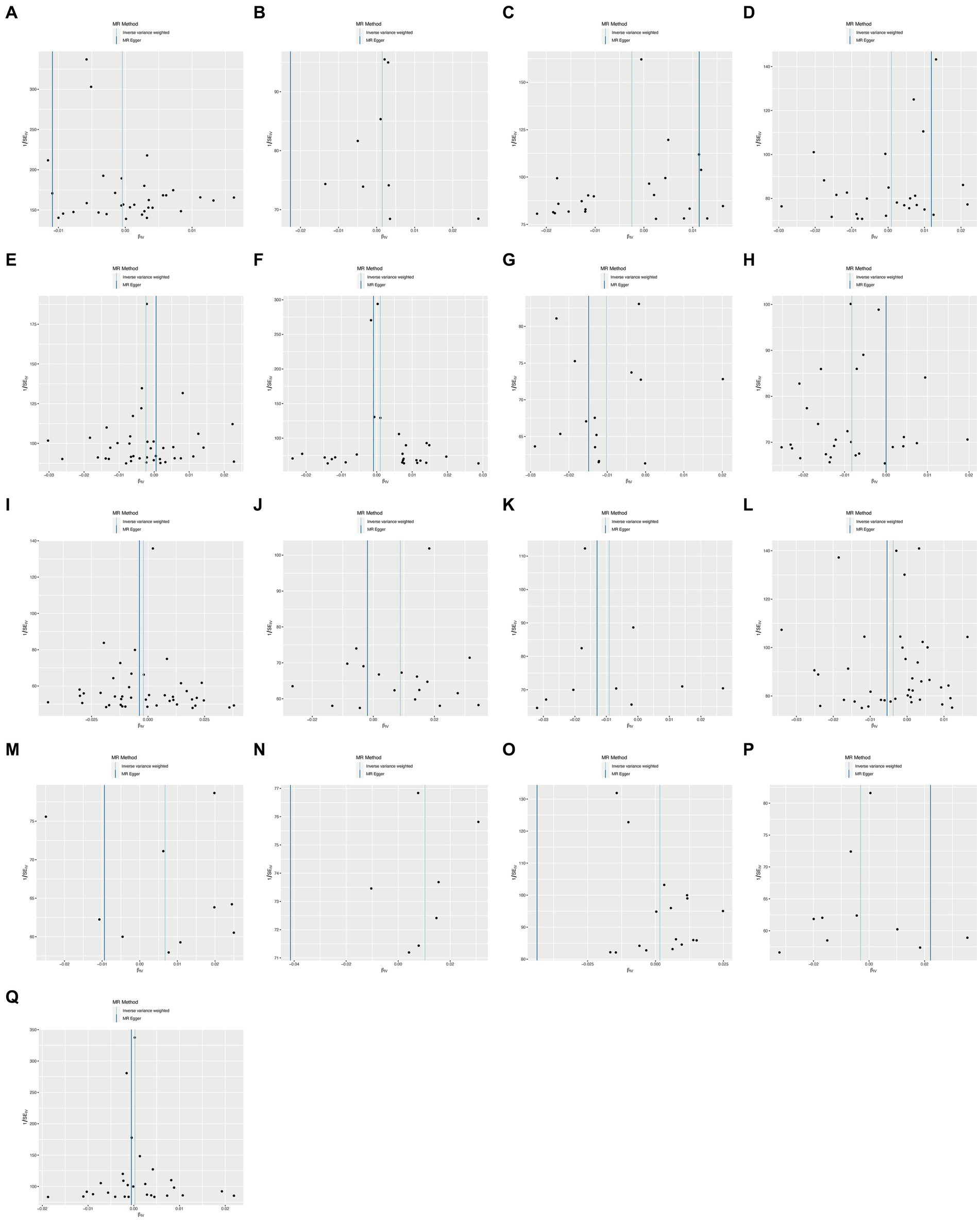
Figure 5. Funnel plots of food on cholelithiasis. (A) Alcohol; (B) Beef; (C) Bread; (D) Cereal; (E) Cheese; (F) Coffee; (G) Cooked vegetable; (H) Dried fruit; (I) Fresh fruit; (J) Lamb/mutton; (K) Non-oily fish; (L) Oily fish; (M) Pork; (N) Poultry; (O) Processed meat; (P) Salad/raw vegetable; (Q) Tea.
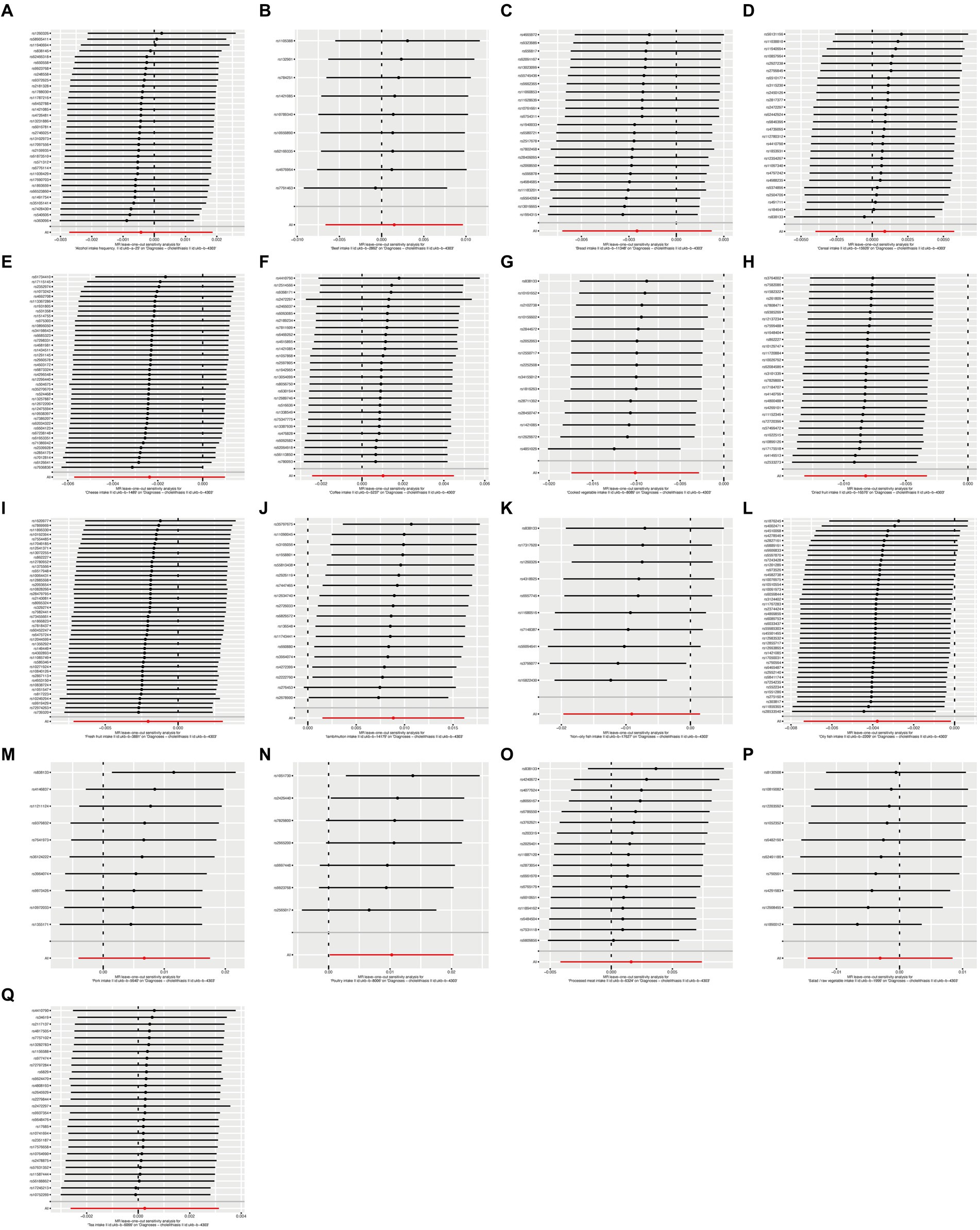
Figure 6. Leave-one-out plots of food on cholelithiasis. (A) Alcohol; (B) Beef; (C) Bread; (D) Cereal; (E) Cheese; (F) Coffee; (G) Cooked vegetable; (H) Dried fruit; (I) Fresh fruit; (J) Lamb/mutton; (K) Non-oily fish; (L) Oily fish; (M) Pork; (N) Poultry; (O) Processed meat; (P) Salad/raw vegetable; (Q) Tea.
Ultimately, our analysis identified statistically significant causal relationships between cooked vegetables, dried fruit, lamb, oily fish, and cholelithiasis, as determined through multivariable MR (MVMR) analysis (14). The harmonized data in the MVMR analysis was shown in Supplementary Table S1. Furthermore, multivariable MR analysis confirmed that Cooked Vegetable, Dried Fruit, and Oily Fish were significantly associated with a reduced risk of cholelithiasis, while lamb exhibited an increased risk (Figure 7).
In order to reduce the reverse causation, we did a reverse mendelian randomization analysis to explore the effects of cholelithiasis on food intake. However, the results provided some evidence that gallstone disease may influence lamb and oily fish intake (Supplementary Figure S1).
Overall, our results suggest the existence of potential causal relationships between certain food exposures and the risk of cholelithiasis. However, further investigations are warranted to validate these findings and explore the underlying mechanisms. Nonetheless, these insights shed valuable light on the role of diet in the development of cholelithiasis and may hold implications for the prevention and management of this prevalent condition.
The findings of this study indicate a positive causal association between cholelithiasis and the consumption of cooked vegetables, dried fruits, lamb/mutton, and oily fish. The results remained robust when subjected to sensitivity tests, exhibiting low between-study heterogeneity and no evidence of pleiotropy. These observations are consistent with previous observational studies that have linked higher consumption of these food items with an increased risk of cholelithiasis (14, 15).
In comparison to prior research, previous observational studies have reported an elevated risk of cholelithiasis associated with increased intake of red meat, including beef and pork (16). However, our study did not establish a causal relationship between beef and pork consumption and cholelithiasis. This disparity may be attributed to variations in the preparation and cooking methods of red meat among different populations, potentially affecting its association with cholelithiasis.
Our findings also suggest a potential role of cooked vegetables in the development of cholelithiasis, opposing to previous observational studies indicating an increased risk with high vegetable consumption (17–19). The potential reason might be the difference of study types and ethnic population. Therefore, RCT research with stronger evidence is needed. Importantly, no causal relationship was found between raw vegetable consumption and cholelithiasis, implying that the cooking process may influence the association between vegetables and cholelithiasis. This emphasizes the significance of considering food preparation and cooking methods in dietary research related to health (20, 21).
Moreover, we identified a novel positive causal relationship between dried fruits and cholelithiasis, where higher consumption of dried fruits was associated with an increased risk. The specific mechanism underlying this association remains unclear; however, it may be related to the high sugar and fructose content in dried fruits, potentially elevating bile acids and cholesterol in the bile and contributing to gallstone formation (22, 23).
Additionally, our results indicated that lamb/mutton consumption may contribute to the development of cholelithiasis, consistent with prior observational studies linking high consumption of lamb/mutton to an increased risk (14, 24–26). The mechanism explaining this relationship is not fully understood, but it is suggested that the high fat content in lamb/mutton may raise bile acids and cholesterol levels in the bile, leading to gallstone formation.
Lastly, oily fish consumption was associated with a reduced risk of cholelithiasis, in line with previous observational studies highlighting the protective effect of oily fish consumption (25, 26). The exact mechanism by which oily fish reduces the risk of cholelithiasis remains uncertain, but it may be related to its high fat content, potentially influencing bile acids and cholesterol levels in the bile, thereby reducing gallstone formation.
While this study provides valuable evidence for a causal relationship between certain food exposures and cholelithiasis, several limitations should be acknowledged. Even though most food exposures revealed no reverse causation, lamb and fatty fish did demonstrate potential bidirectional connections with cholelithiasis. In order to properly describe the temporal association between these two dietary exposures and gallstone disease, it was necessary to do more studies utilizing longitudinal data or other methods. The results were based on a limited number of genetic variants, and other unanalyzed variants may also influence the relationship between food exposures and cholelithiasis. Additionally, the study was conducted in a specific ethnic population, limiting the generalizability of the findings to other populations. The potential influence of residual confounding from other factors affecting the relationship between food exposures and cholelithiasis was not fully accounted for. Furthermore, lifestyle factors such as physical activity and smoking, which may impact cholelithiasis development, were not considered (27, 28).
Despite these limitations, this study contributes valuable insights to the existing literature, offering robust evidence for a causal relationship between specific food exposures and cholelithiasis. These findings hold implications for dietary recommendations aimed at cholelithiasis prevention and underscore the importance of considering food preparation and cooking methods in studies exploring the association between diet and health. Further research is warranted to validate these findings across diverse populations and elucidate the underlying mechanisms connecting food exposures to cholelithiasis.
In conclusion, this study provides evidence supporting a positive causal relationship between cholelithiasis and the consumption of cooked vegetables, dried fruits, lamb/mutton, and oily fish. The findings emphasize the significance of considering food preparation and cooking methods in dietary research and may inform future dietary recommendations for cholelithiasis prevention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
ZL: Writing – original draft. SL: Methodology, Validation, Supervision, Writing – original draft. PS: Data curation, Formal analysis, Software, Writing – original draft. YJ: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1276497/full#supplementary-material
Supplymentary Figure S1 | Reverse causation analysis.
Supplymentary Table S1 | The harmonized data in the MVMR analysis.
1. Portincasa, P, Moschetta, A, and Palasciano, G. Cholesterol gallstone disease. Lancet. (2006) 368:230–9. doi: 10.1016/S0140-6736(06)69044-2
2. Shabanzadeh, DM, Skaaby, T, Sorensen, LT, Eugen-Olsen, J, and Jorgensen, T. Metabolic biomarkers and gallstone disease - a population-based study. Scand J Gastroenterol. (2017) 52:1270–7. doi: 10.1080/00365521.2017.1365166
3. Katsika, D, Grjibovski, A, Einarsson, C, Lammert, F, Lichtenstein, P, and Marschall, HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology. (2005) 41:1138–43. doi: 10.1002/hep.20654
4. Lammert, F, and Miquel, JF. Gallstone disease: from genes to evidence-based therapy. J Hepatol. (2008) 48:S124–35. doi: 10.1016/j.jhep.2008.01.012
5. Wittenburg, H, and Lammert, F. Genetic predisposition to gallbladder stones. Semin Liver Dis. (2007) 27:109–21. doi: 10.1055/s-2006-960174
6. Everhart, JE, and Ruhl, CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. (2009) 136:1134–44. doi: 10.1053/j.gastro.2009.02.038
7. Hardono, PR, and Utomo, B. Risk factors of cholelithiasis: a literature review. Int J Res Publ. (2022) 116:185–95. doi: 10.47119/ijrp1001161120234409
8. Dai, Y, Luo, B, and Li, W. Incidence and risk factors for cholelithiasis after bariatric surgery: a systematic review and meta-analysis. Lipids Health Dis. (2023) 22:5. doi: 10.1186/s12944-023-01774-7
9. Vladimir, R, Golubović, Z, Lekovic, Z, Ducic, S, Radlovic, N, Jovanovic, B, et al. Gilbert syndrome as a risk factor for the development of cholelithiasis in children. Srp Arh Celok Lek. (2023) 151:186–9. doi: 10.2298/sarh221206031r
10. Di Ciaula, A, Garruti, G, Fruhbeck, G, De Angelis, M, de Bari, O, Wang, DQ, et al. The role of diet in the pathogenesis of cholesterol gallstones. Curr Med Chem. (2019) 26:3620–38. doi: 10.2174/0929867324666170530080636
11. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
12. Lawlor, DA, Harbord, RM, Sterne, JA, Timpson, N, and Davey, SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
13. Burgess, S, and Thompson, SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251–60. doi: 10.1093/aje/kwu283
14. Compagnucci, AB, Perroud, HA, Batalles, SM, Villavicencio, R, Brasca, A, Berli, D, et al. A nested case-control study on dietary fat consumption and the risk for gallstone disease. J Hum Nutr Diet. (2016) 29:338–44. doi: 10.1111/jhn.12332
15. European Association for the Study of the Liver. EASL clinical practice guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. (2016) 65:146–81. doi: 10.1016/j.jhep.2016.03.005
16. Ortega, RM, Fernandez-Azuela, M, Encinas-Sotillos, A, Andres, P, and Lopez-Sobaler, AM. Differences in diet and food habits between patients with gallstones and controls. J Am Coll Nutr. (1997) 16:88–95. doi: 10.1080/07315724.1997.10718655
17. Kratzer, W, Kachele, V, Mason, RA, Hill, V, Hay, B, Haug, C, et al. Gallstone prevalence in Germany: the Ulm gallbladder stone study. Dig Dis Sci. (1998) 43:1285–91. doi: 10.1023/a:1018816109905
18. Pixley, F, Wilson, D, McPherson, K, and Mann, J. Effect of vegetarianism on development of gall stones in women. Br Med J. (1985) 291:11–2. doi: 10.1136/bmj.291.6487.11
19. McConnell, TJ, Appleby, PN, and Key, TJ. Vegetarian diet as a risk factor for symptomatic gallstone disease. Eur J Clin Nutr. (2017) 71:731–5. doi: 10.1038/ejcn.2016.252
20. Ito, H, Kikuzaki, H, and Ueno, H. Effects of cooking methods on free amino acid contents in vegetables. J Nutr Sci Vitaminol. (2019) 65:264–71. doi: 10.3177/jnsv.65.264
21. Salehzadeh, H, Maleki, A, Rezaee, R, Shahmoradi, B, and Ponnet, K. The nitrate content of fresh and cooked vegetables and their health-related risks. PLoS One. (2020) 15:e0227551. The authors have declared that no competing interests exist. Epub 20200109. doi: 10.1371/journal.pone.0227551
22. Misciagna, G, Centonze, S, Leoci, C, Guerra, V, Cisternino, AM, Ceo, R, et al. Diet, physical activity, and gallstones--a population-based, case-control study in southern Italy. Am J Clin Nutr. (1999) 69:120–6. doi: 10.1093/ajcn/69.1.120
23. Mathur, A, Megan, M, Al-Azzawi, HH, Lu, D, Swartz-Basile, DA, Nakeeb, A, et al. High dietary carbohydrates decrease gallbladder volume and enhance cholesterol crystal formation. Surgery. (2007) 141:654–9. doi: 10.1016/j.surg.2006.11.008
24. Koeth, RA, Wang, Z, Levison, BS, Buffa, JA, Org, E, Sheehy, BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
25. Jorgensen, T, and Jorgensen, LM. Gallstones and diet in a Danish population. Scand J Gastroenterol. (1989) 24:821–6. doi: 10.3109/00365528909089221
26. Kiani, Q, Farooqui, F, Khan, MS, Khan, AZ, Nauman Tariq, M, and Akhtar, A. Association of Body Mass Index and Diet with symptomatic gall stone disease: a case-control study. Cureus. (2020) 12:e7188. doi: 10.7759/cureus.7188
27. Yuan, S, Gill, D, Giovannucci, EL, and Larsson, SC. Obesity, type 2 diabetes, lifestyle factors, and risk of gallstone disease: a Mendelian randomization investigation. Clin Gastroenterol Hepatol. (2022) 20:e529–37. doi: 10.1016/j.cgh.2020.12.034
Keywords: food, cholelithiasis, causal relationship, Mendelian randomization, risk factor
Citation: Liu Z, Liu S, Song P and Jiao Y (2024) Mendelian randomization study on the causal relationship between food and cholelithiasis. Front. Nutr. 11:1276497. doi: 10.3389/fnut.2024.1276497
Received: 17 August 2023; Accepted: 21 February 2024;
Published: 04 March 2024.
Edited by:
Luis Rodrigo Macias Kauffer, Universität zu Lübeck, GermanyReviewed by:
Xinying Wang, Nanjing General Hospital of Nanjing Military Command, ChinaCopyright © 2024 Liu, Liu, Song and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Jiao, amlhb3lhbkBqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.