- 1Alliance for Research in Exercise, Nutrition and Activity (ARENA), University of South Australia, Adelaide, SA, Australia
- 2Clinical and Health Sciences, University of South Australia, Adelaide, SA, Australia
- 3Allied Health and Human Performance, University of South Australia, Adelaide, SA, Australia
- 4Innovation, IMPlementation and Clinical Translation (IIMPACT), University of South Australia, Adelaide, SA, Australia
- 5Persistent Pain Research Group, Hopwood Centre for Neurobiology, South Australian Health and Medical Research Institute (SAHMRI), Adelaide, SA, Australia
- 6Institute for Physical Activity and Nutrition (IPAN), Deakin University, Burwood, VIC, Australia
- 7Microbiome and Host Health, South Australian Health and Medical Research Institute (SAHMRI), Adelaide, SA, Australia
Background: The relationship between adiposity and pain is complex. Excess weight increases the risk for chronic musculoskeletal pain (CMP), driven by increased biomechanical load and low-grade systemic inflammation. Pain limits physical function, impacting energy balance contributing to weight gain. The primary aims of this study were to profile pain characteristics in participants with overweight or obesity and determine if weight loss through dietary-induced energy restriction, and presence of CMP, or magnitude of weight loss, was associated with changes in adiposity, pain, functional mobility, and inflammation.
Methods: This was a secondary analysis of data from adults (25–65 years) with overweight or obesity (BMI 27.5–34.9 kg/m2) enrolled in a 3-month, 30% energy-restricted dietary intervention to induce weight loss (January 2019–March 2021). Anthropometric measures (weight, waist circumference and fat mass), pain prevalence, pain severity (McGill Pain Questionnaire, MPQ), pain intensity (Visual Analog Scale, VAS), functional mobility (timed up and go, TUG) and inflammation (high sensitivity C-Reactive Protein, hsCRP) were assessed at baseline and 3-months.
Results: One hundred and ten participants completed the intervention and had weight and pain assessed at both baseline and 3-months. Participants lost 7.0 ± 0.3 kg, representing 7.9% ± 3.7% of body mass. At 3-months, functional mobility improved (TUG −0.2 ± 0.1 s, 95% CI −0.3, −0.1), but there was no change in hsCRP. Compared to baseline, fewer participants reported CMP at 3-months (n = 56, 51% to n = 27, 25%, p < 0.001) and presence of multisite pain decreased from 22.7% to 10.9% (p < 0.001). Improvements in anthropometric measures and functional mobility did not differ between those presenting with or without CMP at baseline. Improvements in pain were not related to the magnitude of weight loss.
Conclusion: Weight loss was effective in reducing pain prevalence and improving functional mobility, emphasizing the importance of considering weight-loss as a key component of pain management.
Clinical trial registration: identifier, ACTRN12618001861246.
1 Introduction
Chronic musculoskeletal pain (CMP) is the leading contributor to the burden of disease and disability worldwide, affecting 20%–33% of the world’s population (1). The body of evidence for the coexistence of obesity and CMP is growing (2, 3), with both obesity and CMP adversely impacting an individual’s health, functioning, and quality of life (3–5).
The prevalence of pain complaints rises incrementally across body mass index (BMI) categories (6–8) and with longer exposure to excess weight (9). To date, research has focused primarily on the relationship between BMI status and chronic pain, rather than adiposity (body fatness) (10). However, several population studies have documented that higher body fat mass is associated with musculoskeletal pain (9, 11), and is a risk factor for the development of chronic widespread, multi-site and site-specific (lower back, foot, and knee) pain (10, 12–17). Adiposity is of interest because it may be associated with increased systemic inflammation, and there is emerging evidence that inflammation may promote the progression of acute to chronic pain by altering the neurophysiological properties of peripheral pathways and central neurons of the pain system (neuroinflammation), contributing to central sensitization, and pain hypersensitivity (18–21).
Various studies have sought to evaluate the relationship between weight, inflammation and pain through mediation analyses or interventions designed to reduce body weight. Some studies suggest inflammation may mediate the link between higher body mass and osteoarthritis (OA) (22–24), while others have found no clear evidence that inflammatory factors influence pain (25), indicating a more direct impact of weight status on pain (26). However, an earlier meta-regression analysis of 11 randomized-controlled trials by Cooper et al. (27) failed to establish a significant relationship between pain reduction and weight loss in patients with musculoskeletal conditions. Nevertheless, the effectiveness of weight-loss interventions (diet and/or exercise) for reducing chronic (non-cancer) pain was recently recognized in a report by the Canadian Agency for Drugs and Technologies which evaluated three systematic reviews (specific to knee osteoarthritis) and two non-randomized studies (from weight management services) (28). More recent systematic reviews (totaling 33 studies) have also reported favorable effects of weight loss on pain and disability in CMP populations, however the breadth of pain measures used in these analyses were limited, and quality of included studies was low (29, 30). These reviews have predominantly included studies that focused on the link between obesity and excess mechanical load on weight bearing joints (knee and back) (27–30). Many of these studies included a physical activity or exercise component, precluding the ability to isolate changes in pain and function due to dietary-induced weight loss. Further, previous studies have been conducted predominantly in people with a pre-existing pain diagnosis, but it is common for people with overweight/obesity to have undiagnosed pain.
Therefore, the primary aims of this study were to: (1) profile sociodemographic and pain characteristics in free-living participants enrolled in a dietary intervention weight-loss trial, (2) determine if weight loss through dietary-induced energy-restriction, and presence of CMP, is associated with changes in weight, adiposity, functional mobility, inflammation, and pain, and (3) determine if magnitude of weight loss (clinically significant ≥ 5% weight loss or not) is associated with adiposity, pain, functional mobility, and inflammation. An exploratory aim was to quantify pair-wise change relationships between primary analysis outcome variables (adiposity, inflammation, and pain).
2 Materials and methods
2.1 Participants and study design
This was a secondary analysis of data from a dietary intervention trial conducted at the University of South Australia between January 2019 and March 2021. A protocol paper detailing the primary study design, participant eligibility criteria and details of outcomes has been published (31).
In brief, men and women from Adelaide and surrounding areas, aged 25–65 years with overweight and obesity (BMI 27.5–34.9 kg/m2) were recruited for a 9-month randomized controlled parallel arm intervention study. Participants were required to be non-smokers, consuming ≤14 standard alcohol drinks per week, not taking supplements or medication that might have potentially interfered with study measurements, and free from chronic health conditions such as diabetes, cardiovascular disease, thyroid, kidney or liver disease, or gastrointestinal disorders. Eligible participants provided written informed consent at screening visits. Ethical approval for the primary study was obtained from the University of South Australia Human Research Ethics Committee (Application ID: 201436) and was registered on the Australian New Zealand Clinical Trials Registry (ACTRN12618001861246).
2.2 Exposure: weight loss through dietary-induced energy-restriction
The dietary intervention was designed to investigate the effects of different snack foods (15% of energy from almonds or carbohydrate-rich snack foods) within a 30% energy-restricted diet on weight loss (3-months) and weight maintenance (6-months) (31). The effect of the different diets on the primary outcomes has been published (32). However, for this study, diet groups were combined as there were no differences in weight loss between groups (32), and we were interested in the overall effect of weight loss at 3-months (through energy restriction) on adiposity, pain, functional mobility, and inflammation. Weight loss was characterized as a continuous variable for assessing baseline and 3-month differences, and as a binary variable for assessing the magnitude of weight loss (<5% or ≥ 5% of baseline body weight over 3 months).
2.3 Exposure: chronic musculoskeletal pain
Body charts captured the site of pain experienced in the previous 24 h. Pain duration > 3 months at any site was used to define CMP, and considered as a binary variable to assess the effect of CMP presence.
2.4 Data collection
Participants attended the Clinical Trials Facility at the University of South Australia for screening, baseline, and 3-month assessments. Anthropometric measures [weight, height, waist circumference (WC), body composition], dietary intake, pain, functional mobility, and inflammation were assessed at baseline and 3 months. Prior to clinic assessments, participants were required to be fasted (>10 h) and have refrained from alcohol (>24 h). South Australian Government enforced Covid-19 restrictions interrupted 3-month clinic visits between April–June 2020. During this period participants measured their body mass at home using Bluetooth-enabled scales (Withings/Nokia WBS06, Nokia), but other anthropometric data (WC, body composition), blood samples, and functional mobility measures were not obtained. Weight captured by Bluetooth-enabled scales were used in analyses after determining there was no difference in the magnitude of weight loss achieved by participants assessed using Bluetooth-enabled scales compared to those whose weight was measured in the Clinical Trial Facility.
2.4.1 Outcome measures
2.4.1.1 Anthropometry
Height and weight measures were used to determine BMI [weight (kg)/height (m)2], classified according to WHO criteria (33). Waist circumference measurements were conducted as per the International Society for the Advancement of Kinanthropometry protocol (34). Whole-body dual-energy X-ray absorptiometry (DEXA) scans (Lunar Prodigy Model, GE Healthcare, Madison, Wisconsin, United States) were used to determine body composition (fat and lean mass, g, %) using enCORE 2015 software (V.13.31).
2.4.1.2 Pain
Body charts captured the location of pain experienced in the previous 24 h (pain prevalence). In those reporting pain, the number of pain sites were recorded, along with pain duration. Participants also ranked pain sites from most troublesome to least troublesome. In participants reporting CMP, the Short Form McGill Pain Questionnaire (MPQ) measured the nature and severity of pain experienced at each pain site through 11 sensory and 4 affective words (35). Participants ranked each word on a Likert intensity scale from 0 (none), 1 (mild), 2 (moderate) to 3 (severe), to provide a total pain severity score out of 45. Current pain level at the site ranked most troublesome was captured via visual analog scale (VAS) in a subset of participants (n = 48, as VAS assessment began after study commencement). Participants selected a point along a 10 cm VAS from 0 representing ‘no pain’ and 10 ‘worst possible pain’, accordingly a higher score indicated greater pain intensity. The MPQ and VAS are suitable for the evaluation of pain complaints, and to measure the effects of interventions or pain relief in individuals, with both pain measures shown to be acceptable, reliable, and valid in adults (36).
The following measures were used to assess the effect of weight loss: pain prevalence, changes in number of pain sites, changes in the nature and severity of pain (MPQ), and changes in pain intensity (VAS). As pain locations were ranked from most to least troublesome, we determined pain severity and intensity scores from the pain site that was identified as the most troublesome at each time point. Sites of pain were additionally matched to the same location to gauge changes at the same location (for instance, comparing shoulder pain) for both time points. To calculate differences in pain intensity and severity from baseline, we assigned a score of 0 to any instance where CMP was absent at 3-months. If acute pain was reported at the 3-month time point it was not scored (and change scores were not determined).
2.4.1.3 Functional mobility
Functional mobility was assessed by the timed up and go (TUG) test. Participants were timed getting up from a seated position in a chair, walking 3 meters, turning, returning to the chair, and sitting down again (37). Four trials were attempted with the average time (seconds) of the final three scored.
2.4.1.4 Inflammatory markers
Following an overnight fast (>10 h), venous blood samples were collected into serum separator gel tubes. Samples were immediately transferred to a local pathology laboratory where high-sensitivity C-reactive protein [hsCRP (mg/L)] was measured. To account for acute inflammatory processes or infection, cut offs for high hsCRP were applied and values >10 mg/L excluded (38).
2.4.2 Other assessments
Screening questionnaires captured age, gender, medication and supplement use, and socio-economic status (Socio-Economic Indices for Areas (SEIFA) deciles of advantage and disadvantage) (39). The diagnoses of pain provoking condition(s), analgesia usage, and past injuries and/or surgeries were documented for people with CMP.
Dietary intake was assessed with 4-day weighed food records captured in the week preceding baseline and 3-month visits. Weighed food records were analyzed using FoodWorks Nutritional Analysis Software Version 10 (Xyris Software, Brisbane) to provide estimates of daily energy intake. To exclude participants suspected of under or overestimating daily intake, established cut-offs of <2,090 kJ or >16,720 kJ/day (500–4,000 kcal) were applied to total energy intake (40). The dietary data for one participant was excluded due to kJ intake falling below the lower cut-off, which reduced the sample size to 135 for energy intake (Table 1).
3 Sample size
The sample size of this study was determined by the availability of weight and pain data in participants enrolled in the primary study (31, 32). Thus, 136 participants with weight and pain data at baseline determined the sample size for profiling sociodemographic and pain characteristics (primary aim 1). Participants with complete weight and pain data at both baseline and 3-months (n = 110) governed the sample size for investigating whether weight loss and presence of CMP, was associated with change in adiposity, functional mobility, inflammation, and pain (primary aims 2 and 3).
4 Statistical analysis
Statistical analyses were undertaken using SPSS version 28.0 (SPSS, Chicago, IL, United States). For all analyses, a statistical significance of p < 0.05 was set.
4.1 Sociodemographic and pain characteristics
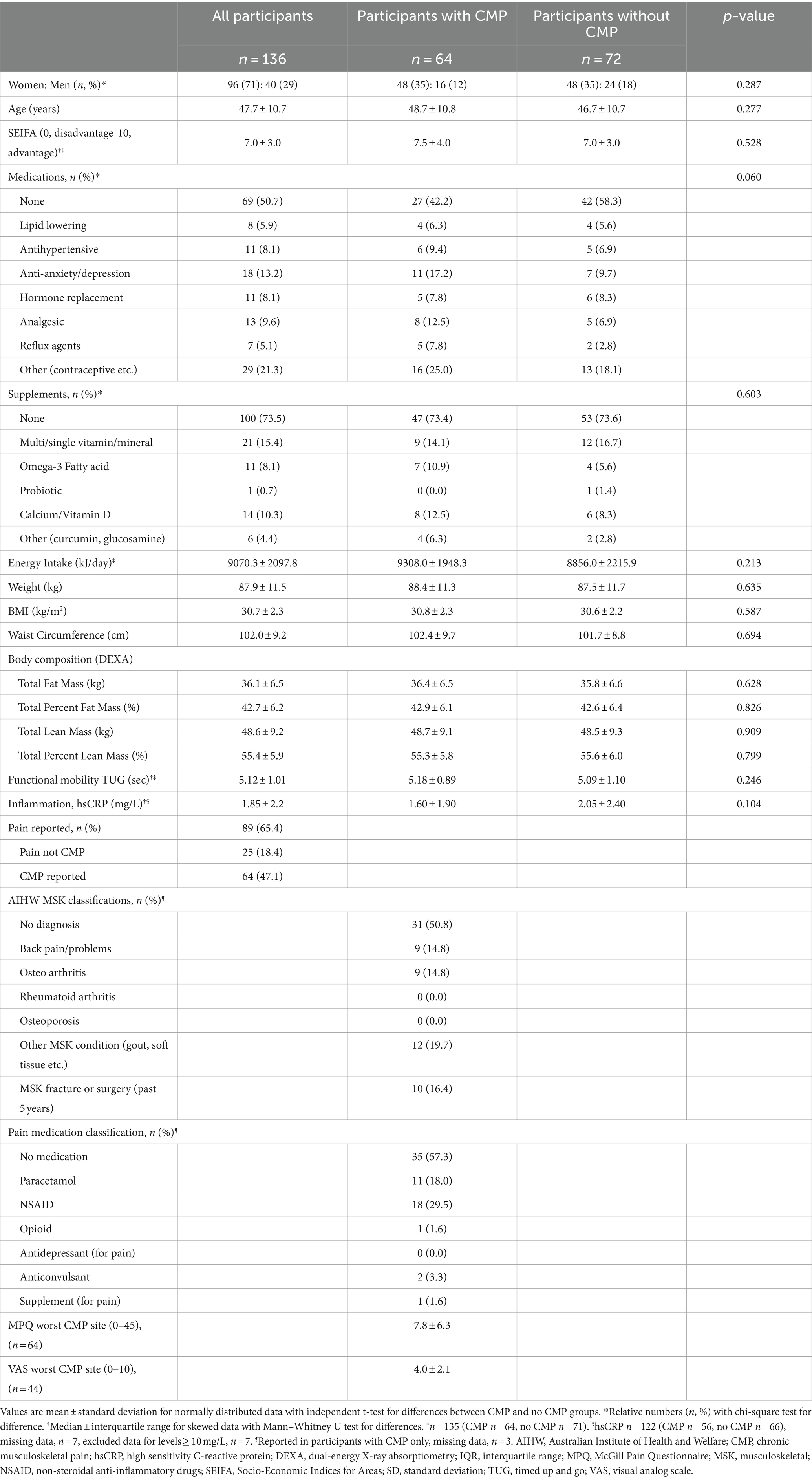
Participant demographics and pain characteristics were documented for all participants with weight and pain data at baseline. Sample means and standard deviations (SD), and medians and interquartile ranges (IQR) for continuous variables, and counts and proportions, calculated for discrete variables, were descriptively reported overall, and for those with and without CMP at baseline (i.e., reporting pain of ≥3 months duration or reporting no pain/pain of <3 months duration, respectively). Following normality testing using histogram plots and Shapiro–Wilk test, differences between those with and without CMP at baseline were assessed using independent t-test and Mann Whitney-U tests as appropriate. Chi-square test for homogeneity was used to test the difference between proportion of men and women reporting CMP at baseline.
4.2 Effect of weight loss and presence of CMP on anthropometry, functional mobility, inflammation, and pain
Pre-post-intervention analyses were conducted in participants with complete weight and pain data (pain prevalence) at baseline and 3-months. This excluded seven participants who reported CMP at 3-months that was not present at baseline because it led to uncertainty about the presence of CMP. McNemar’s test was used to determine if there was a difference in the proportion of participants reporting pain at baseline vs. 3-months (pain prevalence). Effects of weight loss through dietary-induced energy-restriction (time effect) on outcomes (anthropometry, functional mobility, and inflammation), and differences in outcomes between those with and without CMP at baseline (group effect), were determined using mixed effects models, with time and group as fixed effects, group by time as an interaction, and participant random intercepts to account for repeated measures. Covariate importance was assessed by Akaike information criterion (AIC) and Bayesian information criterion (BIC) values. Estimated marginal means (EMM) and standard error of the mean (SEM) are reported for the mixed effects models.
A mixed model including time as a fixed effect and participant random intercepts to account for repeated measures was used to evaluate changes in pain severity and intensity over time in the CMP group only. AIC and BIC values determined covariate inclusion in this model. McNemar’s test was used to test the differences in proportions reporting pain at the different sites in the body.
4.3 Effect of magnitude of weight loss on adiposity, functional mobility, inflammation, and pain
We evaluated whether changes in outcomes (body composition, functional mobility, inflammation, and pain) after weight loss through dietary-induced energy-restriction (time effect) differed between those who achieved weight loss considered clinically meaningful due to associations with improved health outcomes, compared to those who did not reach this level of weight loss (group effect; i.e., ≥ 5% weight loss and <5% weight loss) (41). The same model structure and covariate selection as described in 4.2 was used, except with the magnitude of weight loss as the fixed group effect of interest. Where a group by time interaction was identified, Bonferroni post hoc tests were applied.
4.3.1 Exploratory associations
Regression analyses were conducted in (1) participants completing the intervention to understand the relationship between changes in adiposity and changes in inflammation (hsCRP; n = 70–75), and (2) in those with CMP at baseline, to determine the relationship between change in inflammation and change in pain characteristics (n = 19–39). Age, gender, and baseline BMI were included in the linear regression models.
5 Results
Of 174 people assessed for eligibility, 140 participants completed baseline assessments with both weight and pain (prevalence) data obtained from 136 participants at baseline, and 110 participants had weight and pain data at both baseline and 3-months (Figure 1).
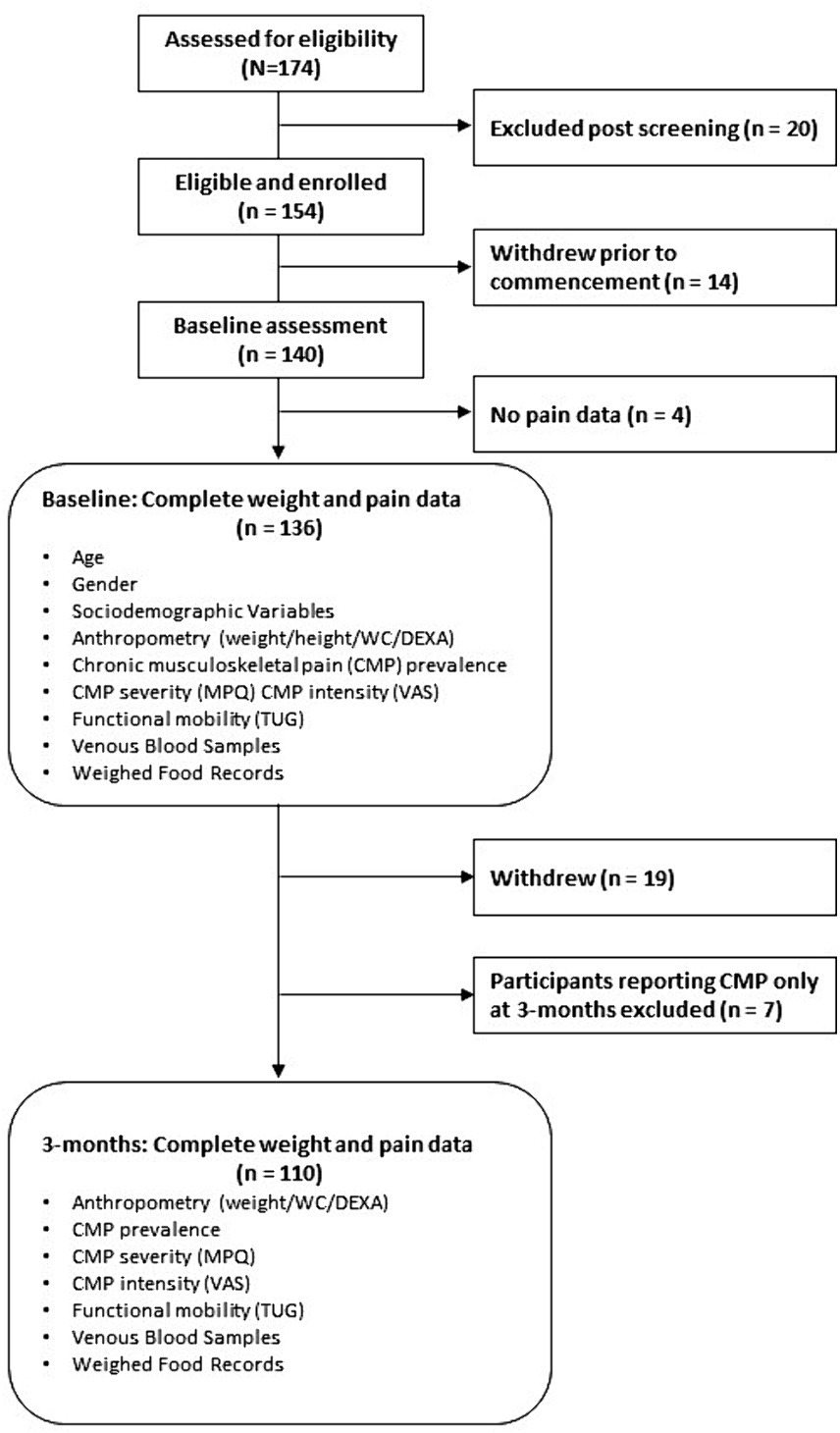
Figure 1. Flow of participants through the study and sample sizes for analyses. BMI, body mass index; CMP, chronic musculoskeletal pain; DEXA, dual-energy X-ray absorptiometry; MPQ, McGill Pain Questionnaire; SES, socio-economic status; TUG, timed up and go; VAS, visual analog scale; WC, waist circumference.
Baseline characteristics of participants with weight and pain data (n = 136) are presented in Table 1. Most participants were women (71%), body weight ranged from 64.5–118.7 kg, and 62.5% of participants fell into the obese BMI category (mean ± SD; 30.7 ± 2.3 kg/m2). Nearly half (47%) of participants enrolled in the study reported CMP at baseline. Considering participants with or without CMP at baseline, there were no differences in age, gender, SEIFA, use of medications or supplements, energy intake (kJ/day), anthropometric measurements (weight, WC, or body composition), functional mobility (TUG), or inflammation (hsCRP) (Table 1).
In participants with CMP, most reported non-specific chronic pain (n = 31, 50.8%), while similar numbers reported a diagnosis of osteoarthritis (n = 9, 14.8%) or non-specific back pain (n = 9, 14.8%), and other musculoskeletal conditions (capturing soft tissue injuries, gout etc.) was reported in 12 (19.7%). The use of pain medication for CMP was reported in 43 and 16% reported a musculoskeletal fracture or surgical procedure in the previous 5 years (Table 1).
5.1 Impact of weight loss and presence of CMP
By 3-months, all participants (n = 110) reduced their total daily energy intake (mean change ± SEM −3,250 ± 171 kJ/day, 95% CI −3,588, −2,911) with accompanying reductions in weight (−7.0 ± 0.3 kg, 95% CI −7.6, −6.3), WC (−7.2 ± 0.5 cm, 95% CI −8.2, −6.1), percent fat mass (−3.9 ± 0.2, 95% CI −4.3, −3.4), and an increase in percent lean mass (3.5 ± 0.2, 95% CI 3.1, 3.9). Table 2 presents the effect of weight loss (time) and the interaction with presence of CMP (group by time). See Supplementary Table S1 for baseline characteristics of this sub-group. Models were adjusted for age, gender, SEIFA (tertiles), analgesic medication use, and anti-inflammatory/analgesic supplement use. Baseline BMI was also included for non-weight outcomes (AIC and BIC values presented in Supplementary Table S2). A significant improvement in functional mobility was observed (TUG change −0.2 ± 0.1 s, 95% CI −0.3, −0.1) but there was no change in levels of circulating hsCRP (−0.1 ± 0.2 mg/L, 95% CI −0.5, 0.3). At 3-months, CMP prevalence reduced from 50.9% to 24.6% (p < 0.001) and multisite pain (CMP in two or more sites) decreased from 22.7% to 10.9% (p < 0.001). There were no group by time interactions indicating that the presence of CMP did not influence the anthropometric, physical functioning (TUG) or inflammation (hsCRP) outcomes.
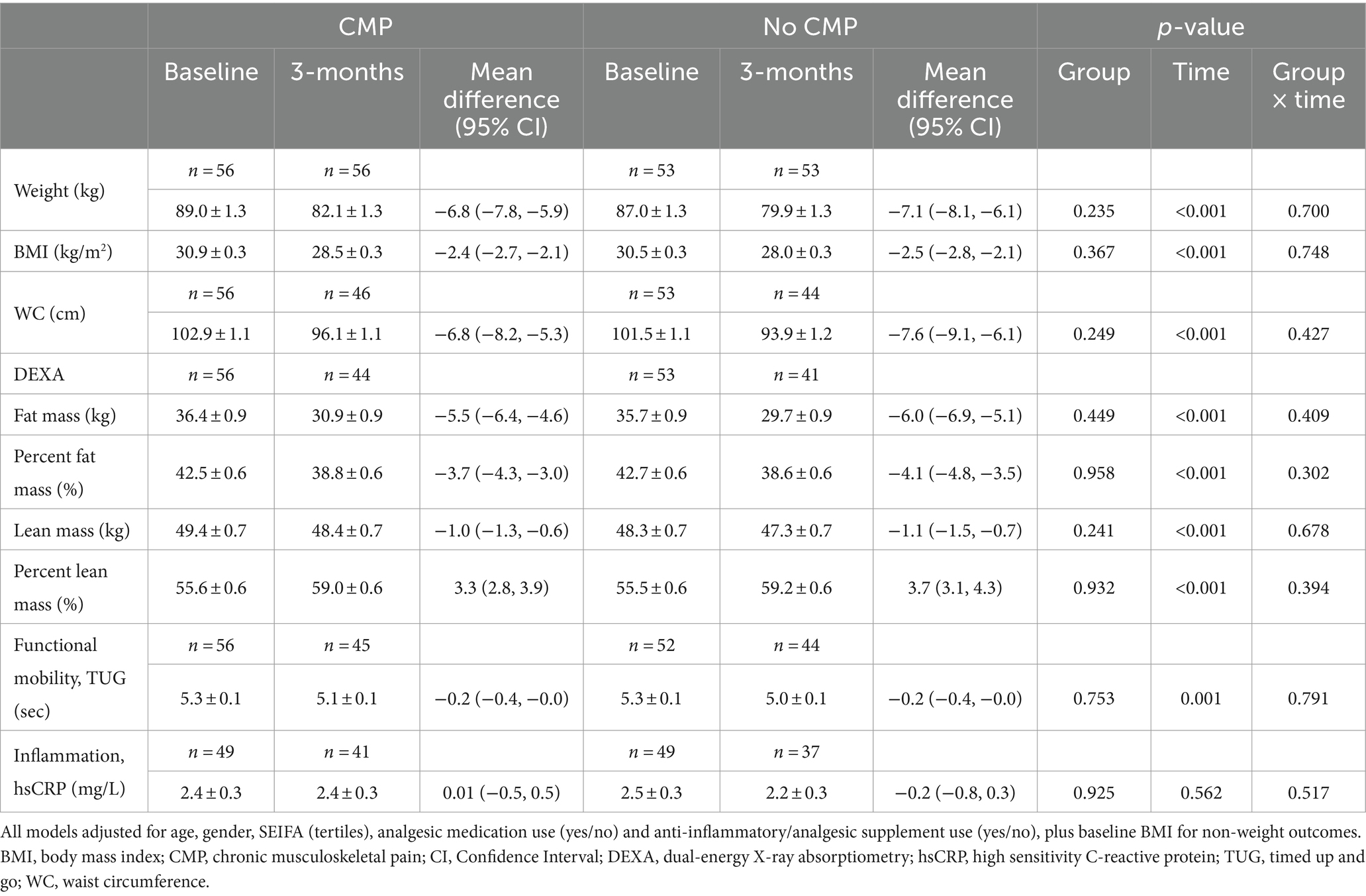
Table 2. Model estimated marginal means (± standard error) and mean difference (95% CI) for weight, adiposity, functional mobility, and inflammation by presence of CMP over time.
Changes in pain variables in participants with CMP following intervention are shown in Supplementary Table S3 (see Supplementary Table S2 for AIC and BIC values). There was a reduction in the average number of pain sites (mean change ± SEM, −0.8 ± 0.1, 95% CI −1.1, −0.6) by 3-months compared to baseline. There were significant reductions in pain severity (MPQ change −3.2 ± 0.9, 95% CI −4.8, −1.6) and pain intensity (VAS change −1.8 ± 0.5, 95% CI −2.8, −0.9) at the site of pain that participants identified as being most troublesome. When areas of pain were matched for site, there were again significant reductions in pain severity (MPQ change −3.1 ± 0.9, 95% CI −4.8, −1.4) and pain intensity (VAS change −2.6 ± 0.5 (26%), 95% CI −3.6, −1.5). A 20% change in VAS has been defined as a clinically significant reduction in pain intensity (42). Figure 2 provides a visual representation of the specific sites of CMP at baseline compared to 3-months. Collectively, spinal (neck, thoracic, lower back) pain reduced in prevalence from 31.8% in all participants to 13.6% (p < 0.001), upper limb (shoulder and hand) pain reduced from 3.6% to 0.9% (p = 0.250), and lower limb (hip, knee, foot) pain from 14.5% to 9.0% (p = 0.210). By sites, significant improvements were observed for some [lower back (p = 0.011), neck (p = 0.021) and foot (p = 0.016)], but not in all (no significant differences in thoracic, shoulder, hand, hip, or knee pain).
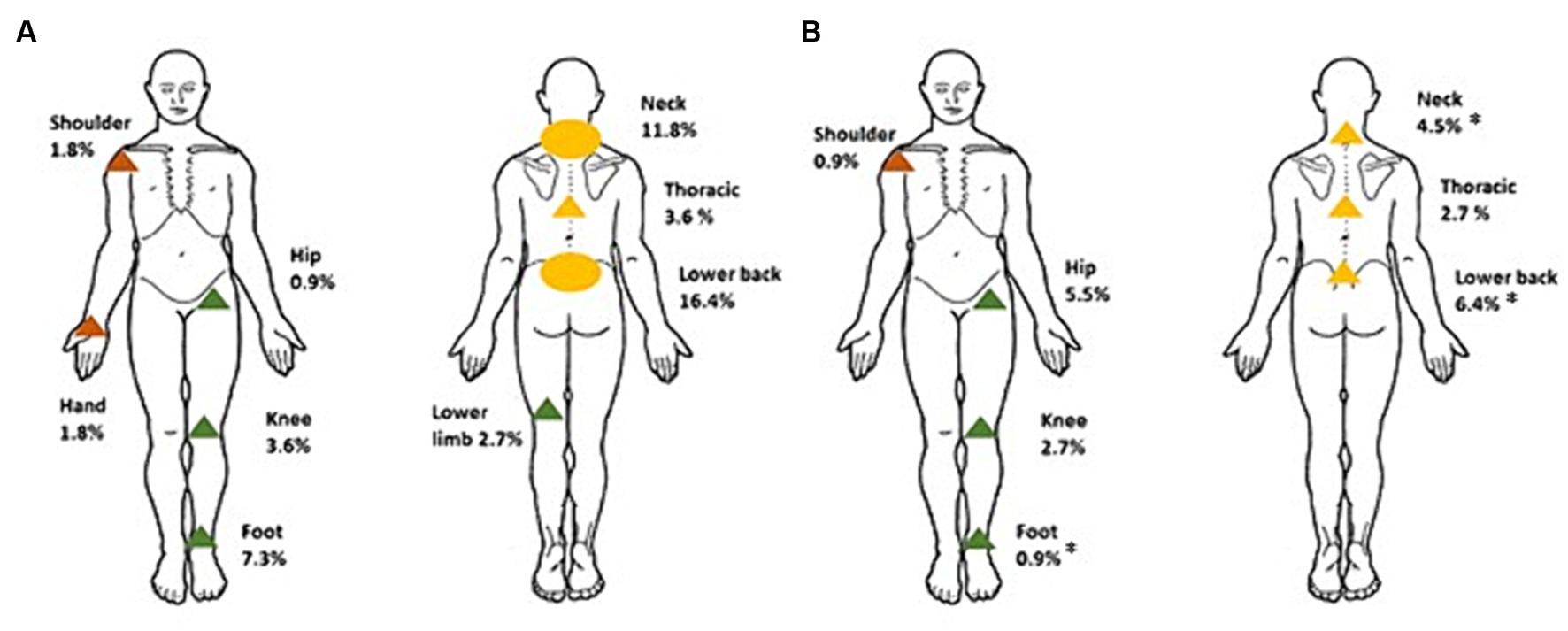
Figure 2. Distribution of most troublesome site of CMP reported in participants at baseline (A) and 3-months (B). *Significantly different to baseline, p < 0.05. Circles represent where >10% of participants reported CMP, and triangles where <10% of participants reported CMP. Green corresponds to pain complaints in the lower limb, orange, the upper limb, and yellow, spinal pain.
5.2 Impact of magnitude of weight loss
Clinically significant weight loss (≥5% of body weight) was achieved in 80% of participants, with an average 7.9 ± 3.7% reduction in body weight for all participants (n = 110). Changes in outcomes by weight loss categories (clinically significant weight loss ≥ 5% of body weight compared to <5% loss of body weight) showed a near significant group by time improvement in physical functioning (TUG; p = 0.055). However, magnitude of weight loss was not related to changes in inflammation (hsCRP) or any pain measures (Table 3). See Supplementary Table S2 for model AIC and BIC values.

Table 3. Model estimated marginal means (± standard error) and mean difference (95% CI) for adiposity (WC, body composition), functional mobility, and pain by magnitude of weight loss over time.
5.3 Relationship between adiposity, inflammation, and pain
Changes in adiposity were associated with change in hsCRP (weight, β = 0.273, 95% CI 0.024, 0.244; WC, β = 0.240, 95% CI 0.003, 0.160; fat mass (kg), β = 0.263, 95% CI 0.00002, 0.0003; percent fat mass (%), β = 0.268, 95% CI 0.005, 0.104). In participants reporting CMP, there was a significant association between change in hsCRP and pain severity scores, but only when identical pain sites were compared between visits (Supplementary Table S4).
6 Discussion
This study investigated pain characteristics in adults with overweight and obesity participating in a dietary intervention for weight loss. In this population, baseline pain prevalence was high with pain determined to be chronic (extending beyond 3 months) in 47% of participants, and multisite (>1 site) pain present in 21% of participants (~45% of those presenting with CMP). Capturing sites of pain showed the distribution of pain was highest in areas of weight bearing (spine and lower limbs). The main finding was that participants achieved significant weight loss, resulting in improved prevalence, severity and intensity of CMP, and functional mobility.
Pain complaints were high in areas of mechanical load. The distribution of pain is not commonly reported, as interventions for weight loss are usually directed at specific pain populations, such as hip or knee osteoarthritis, rather than in community samples. Though a retrospective study of pain presentation in a weight-management service (mean BMI 50.8 ± 8.1 kg/m2) similarly described high pain prevalence with multisite pain reported in two-thirds of participants (43). As with previous studies, also performed in weight management services (43, 44), a reduction in pain prevalence in several weight bearing joints along with a reduction in multisite pain was seen with weight loss. The change in pain distribution with weight loss was varied, with a reduction in lower back, neck, and foot pain, and, although not reaching significance, hip pain complaints increased. Future studies should consider whether this shift in pain presentation is due to alterations in posture along with changes in mechanical loading associated with increased movement after weight loss.
The potential for weight loss to be impacted by the presence of severe pain prior to intervention has been investigated (45–47). A retrospective study of 386 adults in the United States, determined that joint pain was predictive of weight loss in women but not men (mean BMI 40.7 ± 10.12 kg/m2) (45), and in a weight-loss intervention in United States veterans (85% men, mean BMI 36.4 ± 6.2 kg/m2), those with coexisting severe pain and overweight/obesity lost significantly less weight than those reporting mild or moderate pain (46). Similarly, obese individuals (mean BMI 46.3 ± 7.2 kg/m2) reporting severe pain, upon entry to a weight management service, lost less weight than those reporting moderate to no pain (48). Furthermore, after similar initial weight loss in the first 6-months, weight loss at 2 years was less likely to be sustained in participants presenting with CMP, compared to those without, in a behavioral weight-loss program (47). However, we saw no difference in improvements in adiposity between pain free participants and those reporting CMP at baseline. These differences may be due to the BMI of enrolled participants in the aforementioned studies, which was above 35 kg/m2, the upper limit set for our study; pain may be a barrier to weight loss in those with higher BMIs where associated functional impairments and impacts to physical activity are also higher (45, 48).
Elevated levels of proinflammatory cytokines interleukin-6 (IL-6) and CRP have been linked to central sensitization and chronic pain, suggesting involvement in these processes (49, 50). These cytokines are indicative of low-grade inflammation in obesity, where IL-6 is produced by adipose tissue, and CRP (whose production is induced by Il-6) serves as a biomarker (51). Improvements in pain characteristics following weight loss could be the result of lowering of obesity mediated inflammation alongside the lessening of mechanical load (52–54). However, most participants (73% at baseline and 71% at 3-months) presented with hsCRP levels below 3 mg/L, considered within the normal range in previous dietary studies (55), and we saw a non-significant reduction in hsCRP in the no CMP group compared to a trivial rise in the CMP group post-intervention. Interestingly, despite the sample having low levels of inflammation at baseline, the exploratory regression analysis showed reductions in adiposity were associated with reductions in hsCRP. Furthermore, significant relationships between reductions in hsCRP levels and pain severity change scores were only observed for subgroups where identical pain sites were matched. It remains unclear as to what level of CRP is important in the association with CMP (56–58). While some studies suggest a mediating role for inflammatory biomarkers (particularly leptin) in the link between excess weight and OA (22–24), others have found no clear indirect effect through measured inflammatory parameters on back and hand pain suggesting direct mechanical loading effects have a greater influence (25, 26). Again, the limited BMI range for this study may also have impacted our ability to determine shifts in inflammatory status with weight loss.
Consistent with previous single-arm interventions (low energy diet, multi-disciplinary weight-loss program, weight-management services) reporting on chronic pain in people with overweight and obesity, we observed significant improvements in pain prevalence and intensity (43, 46, 47, 59, 60). Although a critical review, primarily of studies involving knee osteoarthritis, recognized the effectiveness of weight loss for chronic pain, the findings were varied for pain prevalence and pain severity, with favorable or null effects (28). Subsequent systematic reviews also reported limited and low-quality evidence to support weight-loss interventions for chronic osteoarthritis-related, and low back pain (29, 30), indicating a need for further high-quality interventions. Similar improvements in pain measures were observed in participants who achieved ≥5% weight loss, and those who achieved only a small degree of weight loss or remained weight stable. Dose response relationships in a weight-management service have previously been reported for weight loss and pain prevalence but not pain intensity (43, 44). Also, favorable dose–response relationships between the percentage weight loss and improvements in osteoarthritic pain of the knee have been reported in a meta-analysis (61), and in a large diet and exercise intervention (53). The 4% improvement in TUG times was lower than the 9% considered by Claes et al. (60) as a minimal clinical improvement for patients with knee osteoarthritis, but times were within normative limits for adults (62). While some studies have reported improvements in objective measures of physical function with dietary weight-loss interventions (60), others have reported no significant differences (53, 63) which might be related to the magnitude of weight lost.
6.1 Limitations
In addressing the complex relationship between obesity and CMP there are several limitations to consider in this study. Firstly, the primary study governed the study design, and as such, the lack of a weight-stable control group limited our interpretation of pain outcomes as a function of time. Further, the predetermined sample restricted our analyses, as less than half of enrolled participants had CMP. Pain outcomes were limited by how pain data were captured. Participants were asked to report any bodily pain in the previous 24 h meaning that some participants with CMP were not captured at baseline if they had a pain-free day preceding assessment. This created difficulty establishing CMP in participants reporting pain of a chronic nature at 3-months that was not present at baseline. Participants also ranked their pain from most to least troublesome, meaning analyses were primarily made from pain at the most troublesome site, rather than being able to assess changes in pain at the same site. Further, VAS was not captured in all participants, being introduced into data collection 3-months post study commencement. Considering the study population were not a pain population, pain experienced by most participants was rated (via VAS) as mild (64) constraining the ability to establish relationships between those with and without CMP.
We did not measure levels of IL-6 in our study, hsCRP is an indicator of IL-6, given CRP’s production in the liver is largely in response to Il-6 (65). Additionally, CRP has more defined reference values than IL-6 (66), making it a potential clinical marker for chronic inflammatory states in musculoskeletal disorders (57).
The scanning limits of the DEXA meant eligibility was restricted to an upper BMI limit of 34.9 kg/m2, additionally, history (duration) of overweight/obesity was not captured. The BMI range therefore may not be high enough or weight status not sufficiently long-term to establish inflammatory patterns (9, 67). In our study ~40% of participants had overweight and ~ 60% obesity, and BMI range (27.5–34.9 kg/m2) was lower than reported in systematic reviews examining weight-loss interventions for pain, where the average BMIs ranged from ≥25–51 kg/m2 (27–29). Finally, most participants achieved at least a 5% weight reduction compared to a smaller weight stable group, potentially impacting the ability to infer statistical significance between these groups.
7 Conclusion
Chronic musculoskeletal pain was common in participants with overweight or obesity enrolled in a weight-loss dietary intervention trial. Weight loss, even in small amounts, resulted in a reduction in pain prevalence, severity, and intensity. Improvements in measures of adiposity and functional mobility were observed irrespective of whether participants had CMP at the beginning of the study, indicating that weight loss can be beneficial for both pain-related and general health outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the University of South Australia Human Research Ethics Committee (Application ID: 201436). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SW: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. AC: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. SC: Data curation, Methodology, Project administration, Writing – review & editing. KB: Investigation, Methodology, Writing – review & editing, Supervision. CB: Methodology, Writing – review & editing. TS: Methodology, Writing – review & editing. CY: Methodology, Writing – review & editing, Data curation, Project administration. JB: Methodology, Project administration, Writing – review & editing, Conceptualization, Funding acquisition. S-YT: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. GR: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. AH: Conceptualization, Funding acquisition, Methodology, Writing – review & editing, Data curation, Investigation, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Almond Board of California. SW was supported by a University of South Australia Research Training Program domestic (RTPd) Fee Offset Scholarship and Australian Government Research Training Program (RTP) scholarship. The funder had no role in the design of the study, in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
The authors would like to thank the study participants for their willingness to be part of the study. We gratefully acknowledge the following for their assistance in screening participants, data collection and/or data entry: Louise Massie, Emma Tregoweth, and Elizabeth Connole, and Ty Stanford for statistical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1274356/full#supplementary-material
References
1. World Health Organisation. Musculoskeletal Conditions. (2022) Available at: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (Accessed 12 March 2021).
2. Chin, SH, Huang, WL, Akter, S, and Binks, M. Obesity and pain: a systematic review. Int J Obes. (2020) 44:969–79. doi: 10.1038/s41366-019-0505-y
3. Okifuji, A, and Hare, BD. The association between chronic pain and obesity. J Pain Res. (2015) 8:399–408. doi: 10.2147/JPR.S55598
4. Australian Institute of Health and Welfare. Chronic Pain in Australia. Cat. No. Phe 267. Canberra: AIHW, (2020). Available at: https://www.aihw.gov.au/reports/chronic-disease/chronic-pain-in-australia. (Accessed 20 June 2021).
5. Dong, H-J, Larsson, B, Levin, L-Å, Bernfort, L, and Gerdle, B. Is excess weight a burden for older adults who suffer chronic pain? BMC Geriatr. (2018) 18:270. doi: 10.1186/s12877-018-0963-4
6. Hitt, HC, McMillen, RC, Thornton-Neaves, T, Koch, K, and Cosby, AG. Comorbidity of obesity and pain in a general population: results from the southern pain prevalence study. J Pain. (2007) 8:430–6. doi: 10.1016/j.jpain.2006.12.003
7. Stone, AA, and Broderick, JE. Obesity and pain are associated in the United States. Obesity. (2012) 20:1491–5. doi: 10.1038/oby.2011.397
8. Wright, LJ, Schur, E, Noonan, C, Ahumada, S, Buchwald, D, and Afari, N. Chronic pain, overweight, and obesity: findings from a community-based twin registry. J Pain. (2010) 11:628–35. doi: 10.1016/j.jpain.2009.10.004
9. Costa, ABP, Machado, LAC, Telles, RW, and Barreto, SM. Dose-response associations of clinical markers of obesity and duration of exposure to excess weight with chronic musculoskeletal pain: cross-sectional analysis at baseline of Elsa-Brasil musculoskeletal cohort. Rheumatol Int. (2020) 40:881–91. doi: 10.1007/s00296-020-04557-w
10. Brady, SRE, Urquhart, DM, Hussain, SM, Teichtahl, A, Wang, Y, Wluka, AE, et al. High baseline fat mass, but not lean tissue mass, is associated with high intensity low Back pain and disability in community-based adults. Arthritis Res Ther. (2019) 21:4. doi: 10.1186/s13075-019-1953-4
11. Yoo, JJ, Cho, NH, Lim, SH, and Kim, HA. Relationships between body mass index, fat mass, muscle mass, and musculoskeletal pain in community residents. Arthritis Rheumatol. (2014) 66:3511–20. doi: 10.1002/art.38861
12. Radojčić, MR, Perera, RS, Chen, L, Spector, TD, Hart, DJ, Ferreira, ML, et al. Specific body mass index trajectories were related to musculoskeletal pain and mortality: 19-year follow-up cohort. J Clin Epidemiol. (2022) 141:54–63. doi: 10.1016/j.jclinepi.2021.09.020
13. Heuch, I, Heuch, I, Hagen, K, and Zwart, J-A. Body mass index as a risk factor for developing chronic low Back pain: a follow-up in the Nord-Trøndelag health study. Spine. (2013) 38:133–9. doi: 10.1097/BRS.0b013e3182647af2
14. Jin, X, Ding, C, Wang, X, Antony, B, Laslett, LL, Blizzard, L, et al. Editors. Longitudinal associations between adiposity and change in knee pain: Tasmanian older adult cohort study. Semin Arthritis Rheum. (2016) 45:564–9. doi: 10.1016/j.semarthrit.2015.10.006
15. Pan, F, Laslett, L, Blizzard, L, Cicuttini, F, Winzenberg, T, Ding, C, et al. Associations between fat mass and multisite pain: a five-year longitudinal study. Arthritis Care Res. (2017) 69:509–16. doi: 10.1002/acr.22963
16. Walsh, TP, Gill, TK, Evans, AM, Yaxley, A, Shanahan, EM, and Hill, CL. Association of fat Mass and Adipokines with foot pain in a community cohort. Arthritis Care Res. (2016) 68:526–33. doi: 10.1002/acr.22719
17. Shiri, R, Falah-Hassani, K, Heliövaara, M, Solovieva, S, Amiri, S, Lallukka, T, et al. Risk factors for low Back pain: a population-based longitudinal study. Arthritis Care Res. (2019) 71:290–9. doi: 10.1002/acr.23710
18. Eichwald, T, and Talbot, S. Neuro-immunity controls obesity-induced pain. Front Hum Neurosci. (2020) 14:181. doi: 10.3389/fnhum.2020.00181
19. DiSabato, DJ, Quan, N, and Godbout, JP. Neuroinflammation: the devil is in the details. J Neurochem. (2016) 139:136–53. doi: 10.1111/jnc.13607
20. Nijs, J, Elma, O, Yilmaz, ST, Mullie, P, Vanderweeen, L, Clarys, P, et al. Nutritional neurobiology and central nervous system sensitisation: missing link in a comprehensive treatment for chronic pain? Br J Anaesth. (2019) 123:539–43. doi: 10.1016/j.bja.2019.07.016
21. Kieskamp, SC, Paap, D, Carbo, MJG, Wink, F, Bos, R, Bootsma, H, et al. Central sensitization, illness perception and obesity should be considered when interpreting disease activity in axial Spondyloarthritis. Rheumatology. (2021) 60:4476–85. doi: 10.1093/rheumatology/keab019
22. Fowler-Brown, A, Kim, DH, Shi, L, Marcantonio, E, Wee, CC, Shmerling, RH, et al. The mediating effect of leptin on the relationship between body weight and knee osteoarthritis in older adults. Arthritis Rheumatol. (2015) 67:169–75. doi: 10.1002/art.38913
23. Kroon, F, Veenbrink, A, de Mutsert, R, Visser, A, van Dijk, K, le Cessie, S, et al. The role of leptin and adiponectin as mediators in the relationship between adiposity and hand and knee osteoarthritis. Osteoarthr Cartil. (2019) 27:1761–7. doi: 10.1016/j.joca.2019.08.003
24. Gløersen, M, Steen Pettersen, P, Neogi, T, Jafarzadeh, SR, Vistnes, M, Thudium, CS, et al. Associations of body mass index with pain and the mediating role of inflammatory biomarkers in people with hand osteoarthritis. Arthritis Rheumatol. (2022) 74:810–7. doi: 10.1002/art.42056
25. Perera, RS, Chen, L, Hart, DJ, Spector, TD, Arden, NK, Ferreira, ML, et al. Effects of body weight and fat mass on Back pain–direct mechanical or indirect through inflammatory and metabolic parameters? Semin Arthritis Rheum. (2022) 52:151935. doi: 10.1016/j.semarthrit.2021.11.007
26. Perera, R, Gulati, M, Shah, K, Hart, D, Spector, T, Arden, N, et al. The effect of body mass index on hand pain was not mediated via leptin: the Chingford 1000 women study. Arthritis Rheumatol. (2022) 74:1452–4. doi: 10.1002/art.42132
27. Cooper, L, Ryan, CG, Ells, LJ, Hamilton, S, Atkinson, G, Cooper, K, et al. Weight loss interventions for adults with overweight/obesity and chronic musculoskeletal pain: a mixed methods systematic review. Obes Rev. (2018) 19:989–1007. doi: 10.1111/obr.12686
28. Li, Y, and Argáez, C. Cadth rapid response reports. Body weight modification interventions for chronic non-Cancer pain: a review of clinical effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health (2020).
29. Robson, EK, Hodder, RK, Kamper, SJ, O’Brien, KM, Williams, A, Lee, H, et al. Effectiveness of weight-loss interventions for reducing pain and disability in people with common musculoskeletal disorders: a systematic review with Meta-analysis. J Orthop Sports Phys Ther. (2020) 50:319–33. doi: 10.2519/jospt.2020.9041
30. Chen, LH, Weber, K, Mehrabkhani, S, Baskaran, S, Abbass, T, and Macedo, LG. The effectiveness of weight loss programs for low Back pain: a systematic review. BMC Musculoskelet Disord. (2022) 23:1–14. doi: 10.1186/s12891-022-05391-w
31. Carter, S, Hill, AM, Yandell, C, Buckley, JD, Tan, SY, Rogers, GB, et al. Study protocol for a 9-month randomised controlled trial assessing the effects of almonds versus carbohydrate-rich snack foods on weight loss and weight maintenance. BMJ Open. (2020) 10:e036542. doi: 10.1136/bmjopen-2019-036542
32. Carter, S, Hill, AM, Mead, LC, Wong, HY, Yandell, C, Buckley, JD, et al. Almonds vs. carbohydrate snacks in an energy-restricted diet: weight and Cardiometabolic outcomes from a randomized trial. Obesity. (2023) 31:2467–81. doi: 10.1002/oby.23860
33. World Health Organization. Obesity: preventing and managing the global epidemic: report of a who consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253.
34. Norton, K, and Olds, T. Anthropometrica: A textbook of body measurement for sports and health courses. Sydney, Australia: UNSW press (1996).
35. Melzack, R. The short-form Mcgill pain questionnaire. Pain. (1987) 30:191–7. doi: 10.1016/0304-3959(87)91074-8
36. Hawker, GA, Mian, S, Kendzerska, T, and French, M. Visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), Mcgill pain questionnaire (MPQ), short-form Mcgill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short Form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (Icoap). Arthritic Care Res. (2011) 63:S240–52. doi: 10.1002/acr.20543
37. Podsiadlo, D, and Richardson, S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x
38. Pearson, TA, Mensah, GA, Alexander, RW, Anderson, JL, Cannon, RO III, Criqui, M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. (2003) 107:499–511. doi: 10.1161/01.cir.0000052939.59093.45
39. Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016, Cat. No. 2033.0.55.001: Australian Bureau of Statistics (2018). Available at: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2033.0.55.001~2016~Main%20Features~SOCIO-ECONOMIC%20INDEXES%20FOR%20AREAS%20(SEIFA)%202016~1. (Accessed 5 May 2022).
40. Banna, JC, McCrory, MA, Fialkowski, MK, and Boushey, C. Examining plausibility of self-reported energy intake data: considerations for method selection. Front Nutr. (2017) 4:45. doi: 10.3389/fnut.2017.00045
41. Williamson, DA, Bray, GA, and Ryan, DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity. (2015) 23:2319–20. doi: 10.1002/oby.21358
42. Haefeli, M, and Elfering, A. Pain Assessment. Eur Spine J. (2006) 15:S17–24. doi: 10.1007/s00586-005-1044-x
43. Dunlevy, C, MacLellan, GA, O’Malley, E, Blake, C, Breen, C, Gaynor, K, et al. Does changing weight change pain? Retrospective data analysis from a National Multidisciplinary Weight Management Service. Eur J Pain. (2019) 23:1403–15. doi: 10.1002/ejp.1397
44. Schrepf, A, Harte, SE, Miller, N, Fowler, C, Nay, C, Williams, DA, et al. Improvement in the spatial distribution of pain, somatic symptoms, and depression after a weight loss intervention. J Pain. (2017) 18:1542–50. doi: 10.1016/j.jpain.2017.08.004
45. Wachholtz, A, Binks, M, Eisenson, H, Kolotkin, R, and Suzuki, A. Does pain predict interference with daily functioning and weight loss in an obese residential treatment-seeking population? Int J Behav Med. (2010) 17:118–24. doi: 10.1007/s12529-010-9088-7
46. Masheb, RM, Lutes, LD, Kim, HM, Holleman, RG, Goodrich, DE, Janney, CA, et al. Weight loss outcomes in patients with pain. Obesity. (2015) 23:1778–84. doi: 10.1002/oby.21160
47. Goessl, CL, Befort, CA, Pathak, RD, Ellerbeck, EF, and VanWormer, JJ. Chronic pain and weight regain in a lifestyle modification trial. Obes Sci Pract. (2021) 7:192–8. doi: 10.1002/osp4.471
48. Ryan, CG, Vijayaraman, A, Denny, V, Ogier, A, Ells, L, Wellburn, S, et al. The association between baseline persistent pain and weight change in patients attending a specialist weight management service. PLoS One. (2017) 12:e0179227. doi: 10.1371/journal.pone.0179227
49. Ronchetti, S, Migliorati, G, and Delfino, DV. Association of Inflammatory Mediators with pain perception. Biomed Pharmacother. (2017) 96:1445–52. doi: 10.1016/j.biopha.2017.12.001
50. Crespo-Pardo, L, and Taboada-Iglesias, Y. Inflammatory mediators: its connection with chronic pain and associated problems: Review. Rev Soc Esp del Dolor. (2021) 28:20. doi: 10.20986/resed.2021.3841/2020
51. Del Giudice, M, and Gangestad, SW. Rethinking Il-6 and Crp: why they are more than inflammatory biomarkers, and why it Matters. Brain Behav Immun. (2018) 70:61–75. doi: 10.1016/j.bbi.2018.02.013
52. Beavers, KM, Beavers, DP, Newman, JJ, Anderson, AM, Loeser, RF Jr, Nicklas, BJ, et al. Effects of Total and regional fat loss on plasma Crp and Il-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthr Cartil. (2015) 23:249–56. doi: 10.1016/j.joca.2014.11.005
53. Messier, SP, Mihalko, SL, Legault, C, Miller, GD, Nicklas, BJ, DeVita, P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the idea randomized clinical trial. JAMA. (2013) 310:1263–73. doi: 10.1001/jama.2013.277669
54. Runhaar, J, Beavers, D, Miller, G, Nicklas, B, Loeser, R, Bierma-Zeinstra, S, et al. Inflammatory cytokines mediate the effects of diet and exercise on pain and function in knee osteoarthritis independent of BMI. Osteoarthr Cartil. (2019) 27:1118–23. doi: 10.1016/j.joca.2019.04.009
55. Silveira, BKS, Oliveira, TMS, Andrade, PA, Hermsdorff, HHM, Rosa, COB, and Franceschini, SCC. Dietary pattern and macronutrients profile on the variation of inflammatory biomarkers: scientific update. Cardiol Res Pract. (2018) 2018:4762575–18. doi: 10.1155/2018/4762575
56. Generaal, E, Vogelzangs, N, Macfarlane, GJ, Geenen, R, Smit, JH, Dekker, J, et al. Basal inflammation and innate immune response in chronic multisite musculoskeletal pain. Pain. (2014) 155:1605–12. doi: 10.1016/j.pain.2014.05.007
57. Macphail, K. C-reactive protein, chronic low Back pain and, diet and lifestyle. Int Musculoskel Med. (2015) 37:29–32. doi: 10.1179/1753615415Y.0000000001
58. Morris, P, Ali, K, Merritt, M, Pelletier, J, and Macedo, LG. A systematic review of the role of inflammatory biomarkers in acute, subacute and chronic non-specific low Back pain. BMC Musculoskelet Disord. (2020) 21:1–12. doi: 10.1186/s12891-020-3154-3
59. Bartels, EM, Christensen, R, Christensen, P, Henriksen, M, Bennett, A, Gudbergsen, H, et al. Effect of a 16 weeks weight loss program on osteoarthritis biomarkers in obese patients with knee osteoarthritis: a prospective cohort study. Osteoarthr Cartil. (2014) 22:1817–25. doi: 10.1016/j.joca.2014.07.027
60. Claes, B-EA, Leung, HWC, Matters, K, Williams, MJ, and Hunter, DJ. Interim analysis: an interdisciplinary team approach in facilitating weight reduction and improving function for people with knee or hip osteoarthritis. The osteoarthritis chronic care program at Royal North Shore Hospital. Nutr Diet. (2015) 72:232–9. doi: 10.1111/1747-0080.12166
61. Christensen, R, Bartels, EM, Astrup, A, and Bliddal, H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and Meta-analysis. Ann Rheum Dis. (2007) 66:433–9. doi: 10.1136/ard.2006.065904
62. Kear, BM, Guck, TP, and McGaha, AL. Timed up and go (tug) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. (2017) 8:9–13. doi: 10.1177/2150131916659282
63. Christensen, R, Henriksen, M, Leeds, AR, Gudbergsen, H, Christensen, P, Sørensen, TJ, et al. Effect of weight maintenance on symptoms of knee osteoarthritis in obese patients: a twelve-month randomized controlled trial. Arthritis Care Res. (2015) 67:640–50. doi: 10.1002/acr.22504
64. Boonstra, AM, Stewart, RE, Köke, AJ, Oosterwijk, RF, Swaan, JL, Schreurs, KM, et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol. (2016) 7:1466. doi: 10.3389/fpsyg.2016.01466
65. Ridker, PM, Koenig, W, Kastelein, JJ, Mach, F, and Lüscher, TF. Has the time finally come to measure Hscrp universally in primary and secondary cardiovascular prevention? Eur Heart J. (2018) 39:4109–11. doi: 10.1093/eurheartj/ehy723
66. Naylor, R, Hayes, C, and Egger, G. The relationship between lifestyle, Metaflammation, and chronic pain: a systematic review. Am J Lifestyle Med. (2013) 7:130–7. doi: 10.1177/1559827612451710
67. Dong, HJ, Larsson, B, Rivano Fischer, M, and Gerdle, B. Maintenance of quality of life improvement for patients with chronic pain and obesity after interdisciplinary multimodal pain rehabilitation—a study using the Swedish quality registry for pain rehabilitation. Eur J Pain. (2019) 23:1839–49. doi: 10.1002/ejp.1457
Keywords: diet, energy restriction, chronic pain, musculoskeletal pain, inflammation, overweight, obesity
Citation: Ward SJ, Coates AM, Carter S, Baldock KL, Berryman C, Stanton TR, Yandell C, Buckley JD, Tan S-Y, Rogers GB and Hill AM (2024) Effects of weight loss through dietary intervention on pain characteristics, functional mobility, and inflammation in adults with elevated adiposity. Front. Nutr. 11:1274356. doi: 10.3389/fnut.2024.1274356
Edited by:
Kieron Bernard Rooney, The University of Sydney, AustraliaReviewed by:
Hosam Alzahrani, Taif University, Saudi ArabiaClaire Joanne Stocker, Aston University, United Kingdom
Filipa Pinto-Ribeiro, University of Minho, Portugal
Copyright © 2024 Ward, Coates, Carter, Baldock, Berryman, Stanton, Yandell, Buckley, Tan, Rogers and Hill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison M. Hill, YWxpc29uLmhpbGxAdW5pc2EuZWR1LmF1
 Susan J. Ward
Susan J. Ward Alison M. Coates1,3
Alison M. Coates1,3 Carolyn Berryman
Carolyn Berryman Tasha R. Stanton
Tasha R. Stanton Geraint B. Rogers
Geraint B. Rogers Alison M. Hill
Alison M. Hill