- 1Abode Biotec India Private Ltd., Hyderabad, India
- 2Process Design and Engineering Cell, CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar, India
Introduction: Irritable bowel syndrome (IBS) is a common gastrointestinal condition. Some studies have shown the efficacy of probiotics in the treatment of irritable bowel syndrome (IBS). Weizmannia (Bacillus) coagulans LMG S-31876 has been marketed as a dietary ingredient, but to date, its efficacy in diarrhea-predominant irritable bowel syndrome (IBS) condition has not been clinically elucidated. Thus, a double-blind placebo-controlled multi-centered trial was planned to evaluate the safety and efficacy of Weizmannia (Bacillus) coagulans LMG S-31876 in diarrhea-predominant IBS patients.
Experimental design: Study participants (n = 50) diagnosed with IBS prominent symptoms that include abdominal pain and other GI-related symptoms were treated with ProBC Plus (2 billion CFU) along with a placebo capsule once daily for approximately 8 weeks. Study participants were evaluated for the treatment success determined by the differences in stool consistency and frequency per day between the intervention and placebo groups over the study period.
Results: The vital signs and the biochemistry parameters were under the normal range; the other parameters showed a significant result as compared to the placebo during the study period.
Conclusion: This study depicts a significant decline in the clinical symptoms such as abdominal pain, bloating, diarrhea, and frequency of the stool as compared to the placebo. All the parameters such as hematology, lipid profile, and vital signs were in the normal range during the supplementation of ProBC Plus for a period of 8 weeks. Furthermore, the study verified that Weizmannia (Bacillus) coagulans LMG S-31876 and its probiotic product ProBC Plus at a dose of 2 billion/CFU/day has a prominent action in the relief from the clinical symptoms of IBS-D. Therefore, the product is intended safe to utilize for IBS-related symptoms.
Clinical trial registration: The clinical study has been registered with CTRI/2023/01/048644 with https://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=77708&EncHid=24313.96864&userName=CTRI/2023/01/048644 [CTRI/2023/01/048644].
1 Introduction
IBS (irritable bowel syndrome) with predominant diarrhea (IBS-D) is a frequent gastrointestinal disorder that crucially impairs the quality of life for the affected individuals. These are characterized by abdominal pain, changes in bowel habits, and frequent diarrhea. IBS-D represents a multiplex challenge for the patients seeking relief and for the clinicians who strive to provide treatment options. In recent years, the utilization of probiotics has been emerging to alleviate the symptoms and improve the wellbeing of individuals suffering from IBS. IBS (irritable bowel syndrome) is a common and chronic gastrointestinal (GI) disorder that constitutes the largest group in gastroenterology (1). The symptoms can be stimulated by changes in GI functions caused by bacterial infections, an altered diet, or stress. Subsequently, it also impacts immune activation and inflammation. The major symptoms of the disorder manifest as bloating, reflux, constipation, vomiting, diarrhea, abdominal pain, and cramping (2). Diarrhea is one of the major problems defined as the commotion of the water content and deviation from the normal functioning of the physiologic processes. It is categorized as acute, persistent, and chronic (3). Diarrheal disease is the second leading cause of death in children under the age of 5 and has also become a major gastrointestinal problem in adults in recent times (4).
This disease exhibits altered gut flora that may lead to disordered GI function. The fecal flora of IBS patients differs from that of normal patients. Some individuals with IBS may harbor bacterial overgrowth, and their symptoms may be ameliorated by its eradication. GI disorders are not life-threatening, but they do impact general contentment and quality of life (QOL) (5). Clinical intervention typically includes patient education, reassurance, and dietary modification to alleviate symptoms (1), all of which can be unsatisfactory for patients who are facing this lifelong disease.
There are many readily available solutions for the treatment of this problem, which include the utilization of ORS (oral rehydration solutions), antibiotics, and gut-suppressing agents. Probiotics are live microorganisms that maintain immunologic equilibrium and impart health benefits to the host. The major mechanisms include the exclusion of pathogenic microbes (competitively), a hindrance to the pathogenic microbes and antimicrobial substance production, and also modulation of the immune system (6).
Various species of bacteria are categorized into lactic acid producers, i.e., Lactobacillus, Bifidobacterium, Peptostreptococcus, Akkermansia, and Bacillus coagulans. To maintain the conventional microflora within the gut, these species are important to retain (7). Bacillus (Weizmannia) coagulans are naturally distributed in various fermented foods. These strains have been established as major clinical symptoms, such as IBS and other disturbances in bowel movements, and have also improved the microbiome.
The prevalence of IBS varies by geographic region but has been reported in 1–18% of the general population (4, 8). The British Society of Gastroenterology published their 2021 guidelines for the treatment of IBS and recommended probiotics, in general, as a first-line treatment, but as their analysis pooled dissimilar strains of probiotics, they could not identify which specific probiotics might be useful (9). Choosing an appropriate probiotic for patients can be challenging due to the diversity of different types of available probiotics, efficacy is strain-specific and the paucity of randomized clinical trials for some probiotic strains (10, 11). Unfortunately, many meta-analyses have not followed these recommendations and have not been able to determine which specific probiotic strains might be effective for IBS patients (12–15).
Kumar et al. (16) reported the potential of probiotics in alleviating symptoms and improving overall gut health. Specifically, these studies underscore the importance of understanding the intricate interplay between the microbial community and the symptomatic manifestations of IBS (16). Dale et al. (17) reported the role of probiotic interventions in ameliorating specific symptoms commonly associated with IBS, contributing to the growing body of evidence supporting probiotics as a viable therapeutic option. Wang et al. (18) conducted a randomized controlled trial investigating the efficacy of probiotics in alleviating diarrhea and improving stool frequency in IBS patients. Barraza-Ortiz et al. (19) delve into the intricate relationship between probiotics and gut microbiota composition, providing insights into the mechanisms through which probiotics may exert their beneficial effects. This understanding is crucial for unraveling the complex dynamics that influence the efficacy of probiotic interventions in managing IBS (19). Furthermore, Selvaraj et al. (20) reported the long-term effects of probiotic supplementation on the quality of life in IBS patients. Their findings suggest sustained improvements, highlighting the potential of probiotics not only in symptom relief but also in enhancing overall wellbeing for individuals grappling with the challenges of IBS (20).
Among the various strains of probiotics, Weizmannia (Bacillus) coagulans LMG S-31876 has gained attention because of its ability to survive in harsh gastric conditions due to its ability to form spores and to provide protection to the host. Weizmannia (Bacillus) coagulans LMG S-31876 is well characterized; it is a member of the subspecies Bacillus species and is a spore-forming bacterium isolated from fermented rice samples. The strain is safely deposited with the reference code ProBC Plus. The accession numbers obtained from the Belgian Coordinated Collections of Microorganisms/Laboratorium voor Microbiologie (BCCM/LMG) and Microbial Type Culture Collection-International Depositary Authority (MTCC-IDA) under the Budapest Treaty are LMG S-31876 and MTCC 25396, respectively. It is also deposited with the American Type Culture Collection (ATCC); the assigned number is ATCC SD-7789 (21). In addition to these preliminary findings, evaluation of the efficacy and safety of Weizmannia (Bacillus) coagulans LMG S-31876-based interference remains indispensable.
To address this gap, this clinical trial study evaluates the effect of ProBC Plus in patients diagnosed with IBS-D and, subsequently, provides robust and substantial information in the management of IBS-D, thereby enhancing the quality of life for the affected individuals. Gut microbiota plays a crucial role in gastrointestinal health, and its disruption leads to imbalances in IBS-D.
2 Materials and methods
The study was conducted at Medstar Speciality Hospital, Bengaluru, Karnataka.
2.1 Study design and Its criteria
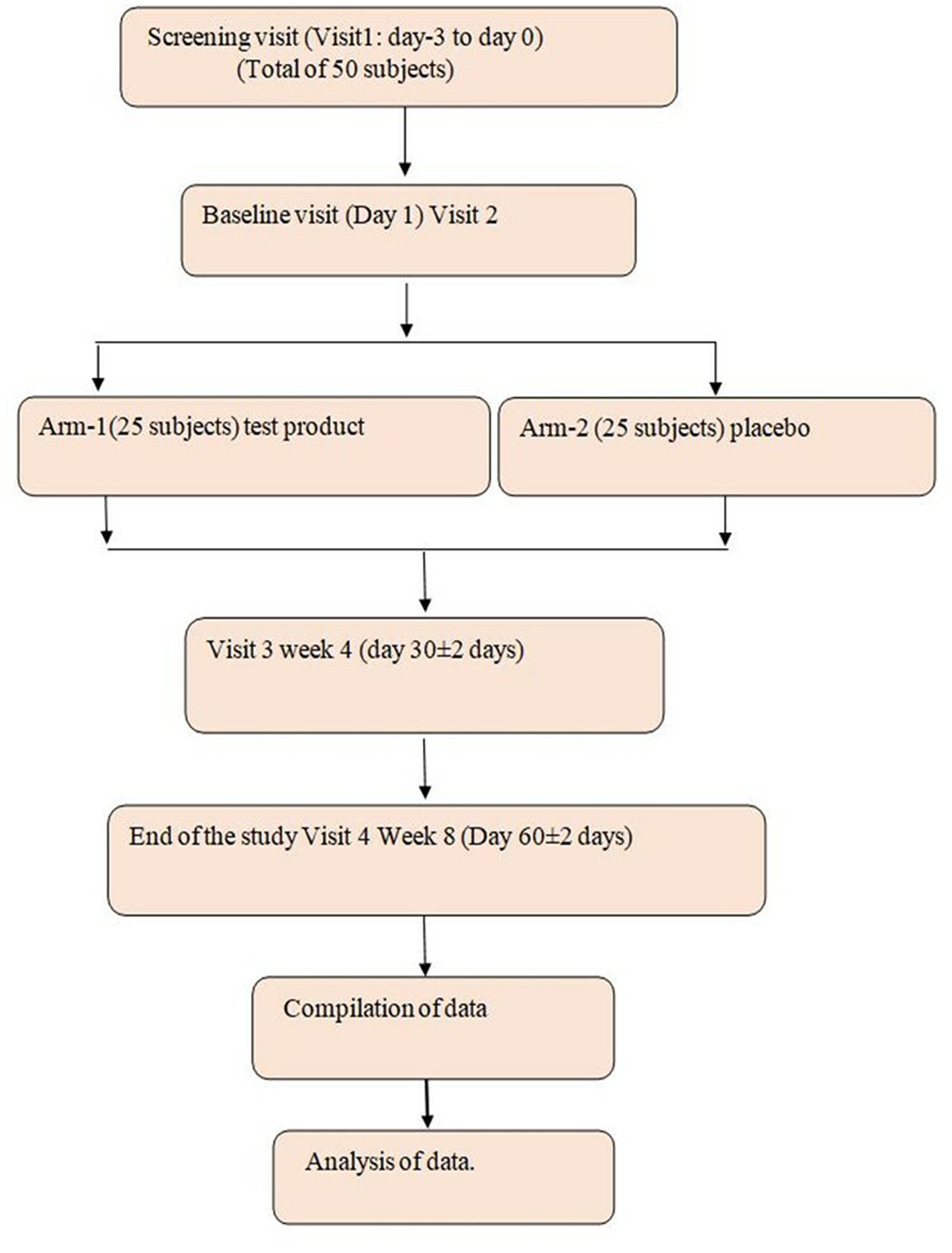
This study is a randomized, double-blind, placebo-controlled parallel group to evaluate the efficacy and safety of the investigational product (IP) B. coagulans LMG S-31876 (106–108 billion CFU) in the treatment of IBS. The study duration for each subject is up to 8 weeks. A total of 50 study participants will be included in the study. This consisted of a total of 4 visits including screening visits: visit 1: screening visit: (−5 to −2 days); visit 2: baseline (Day 1); visit 3: week 4; and visit 4: week 8 (Figure 1).
2.1.1 Inclusion criteria
The inclusion criteria were as follows: The study participants should be (i) either male or female; (ii) aged between 18 and 65 years; (iii) signed an informed consent; (iv) IBS-Diarrhea (IBS-D) patients satisfying Rome III criteria. Symptoms such as recurrent abdominal pain or discomfort, 3 days per month in the last 3 months (12 weeks). The criteria are fulfilled with symptoms onset 6 months before diagnosis—improvement with defecation, onset associated with a change in stool frequency, and onset associated with a change in stool form (appearance).
2.1.2 Exclusion criteria
The study participants were excluded from the study if they were on antibiotics, utilization of complementary and alternative medicines for IBS symptoms, and pregnant or lactating women. Study participants will be excluded if the treatment limits of permitted medication are more than 2 days/week during the study period, requiring treatments with non-permitted medication. IBS study participants with untreated lactose intolerance, regular utilization of probiotics, study participants with rectal bleeding, and allergies to active ingredients are excluded from the study.
2.1.3 Ethical considerations
The study is designed, conducted, analyzed, and reported by regulatory and ethical guidelines (ICH GCP, Indian GCP, and Schedule-Y). The clinical study is registered in CTRI (Clinical Trial Registry India) with CTRI number CTRI/2023/01/048644.
2.2 Investigational product
There were two groups for the study. Group 1: Probiotic containing B. coagulans LMG S-31876 strain (ProBC Plus) (106–108 CFU), 2 billion CFU/Capsule/day; Group II: Placebo.
2.3 Method of analysis and formulation
All the study participants were treated with 2 billion CFU/Capsule/day to be re-constituted in water to be consumed in the morning and evening (one each).
2.3.1 Study tools
The study consisted of an assessment of Abdominal pain VAS (0–10 scale), IBS Symptom Severity Scale (IBS-SSS), Bristol Stool Form Scale (BSFS), stool frequency, assessment of blood hematological parameters, and lipid profile and vital signs were analyzed.
2.3.2 Efficacy and safety assessment
The primary outcomes include the difference in the overall symptom relief between the intervention group and placebo group as assessed by IBS-SSS (time frame: 8 weeks). Secondary outcomes include the measurement of (a) differences in stool consistency and frequency/day between the intervention group and the placebo group over the study period and (b) differences in hematological parameters and lipid profile between the intervention and placebo groups over the study period (time frame: 8 weeks).
2.3.3 Statistical significance
The baseline values for VAS, Bristol Stool Form, hematology, and biochemical parameters were compared to the treatment groups by appropriate statistical tools (SPSS).
The statistical data obtained for the study were analyzed utilizing two sample T-tests for the comparative analysis of the results between the baseline and the end of the treatment. p-values are based on the analysis of the covariance (ANCOVA) model with the change from the baseline values. p-values (drug vs. placebo) at week 8 are based on the ANCOVA model.
3 Results
A total of 50 study participants were enrolled in the study. The mean age was between 18 and 65, and study participants were treated with ProBC plus. There was no medical history of the study participants utilized for the study. The study participants were divided equally into placebo and treatment groups (Supplementary Table S1).
3.1 Vital signs and physical examination
Vital signs such as oral temperature, blood pressure, and pulse rate were found to be statistically significant from the baseline. Figure 2 represents the vital signs for the treatment and placebo groups.
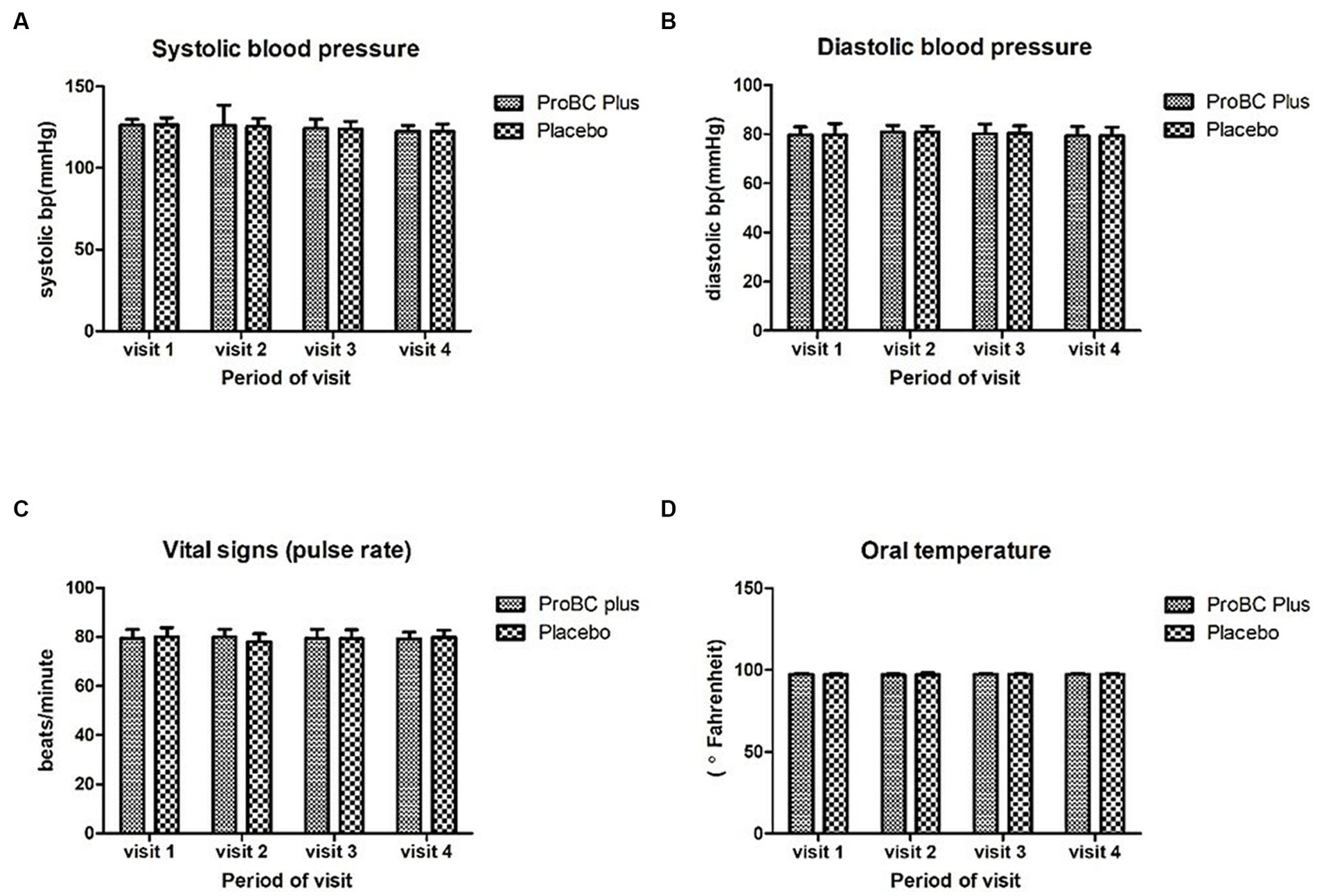
Figure 2. Measurements of vital signs: (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse rate, and (D) oral temperature.
3.2 Evaluation of laboratory parameters
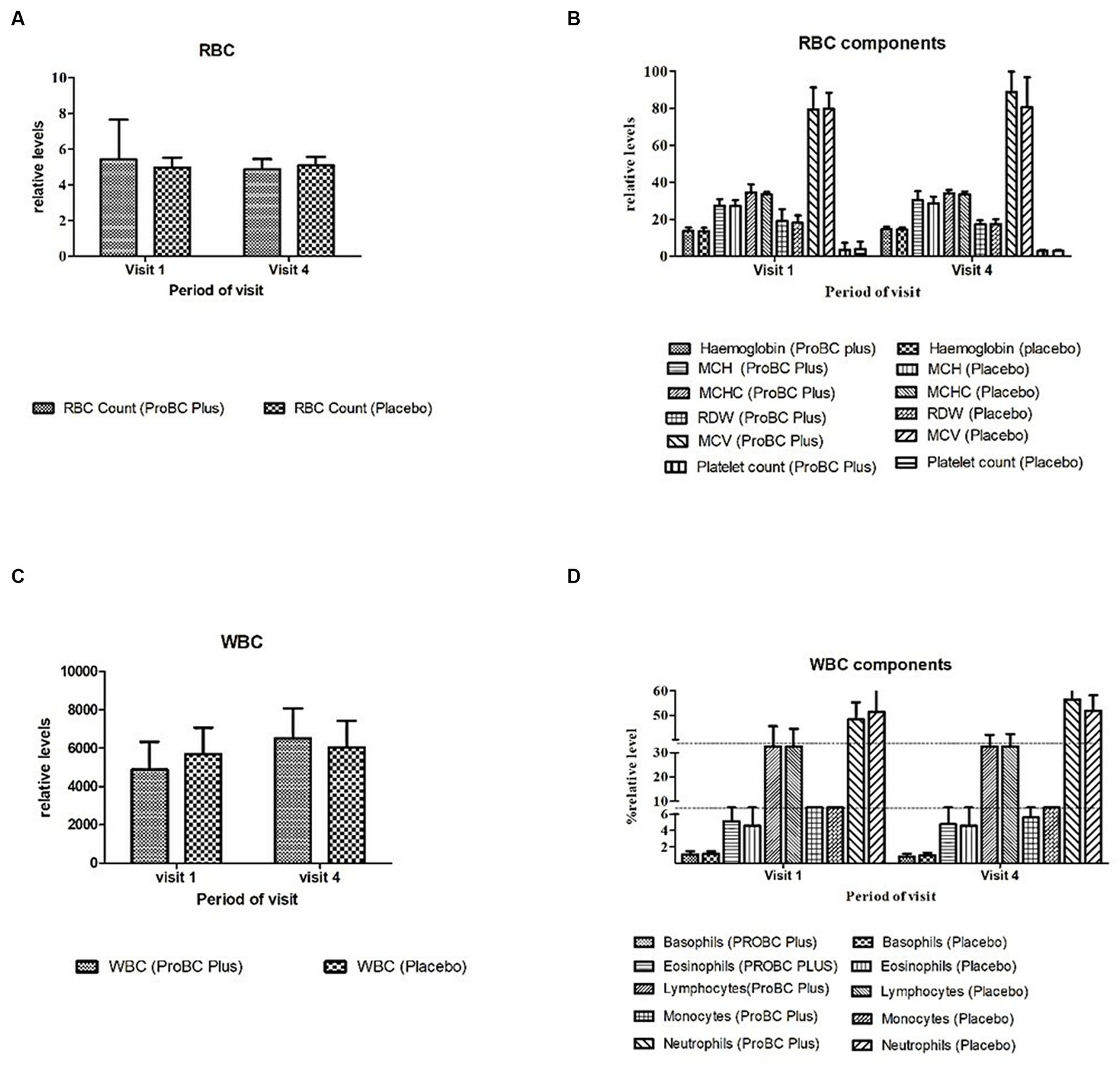
3.2.1 Analysis of hematology parameters
The hematology parameters indicated in Figure 3 depict a statistical significance concerning the baseline and revealed no statistical difference between the placebo and test product, and the ranges were within the reference levels.
3.2.2 Estimation of lipid profiling
The lipid profiling parameters were found to be in the normal range (Figure 4). HDL (high-density lipoproteins), LDL (low-density lipoproteins), total cholesterol and triglycerides, and VLDL (very low-density lipoproteins) were found to be non-significant as compared to the placebo.
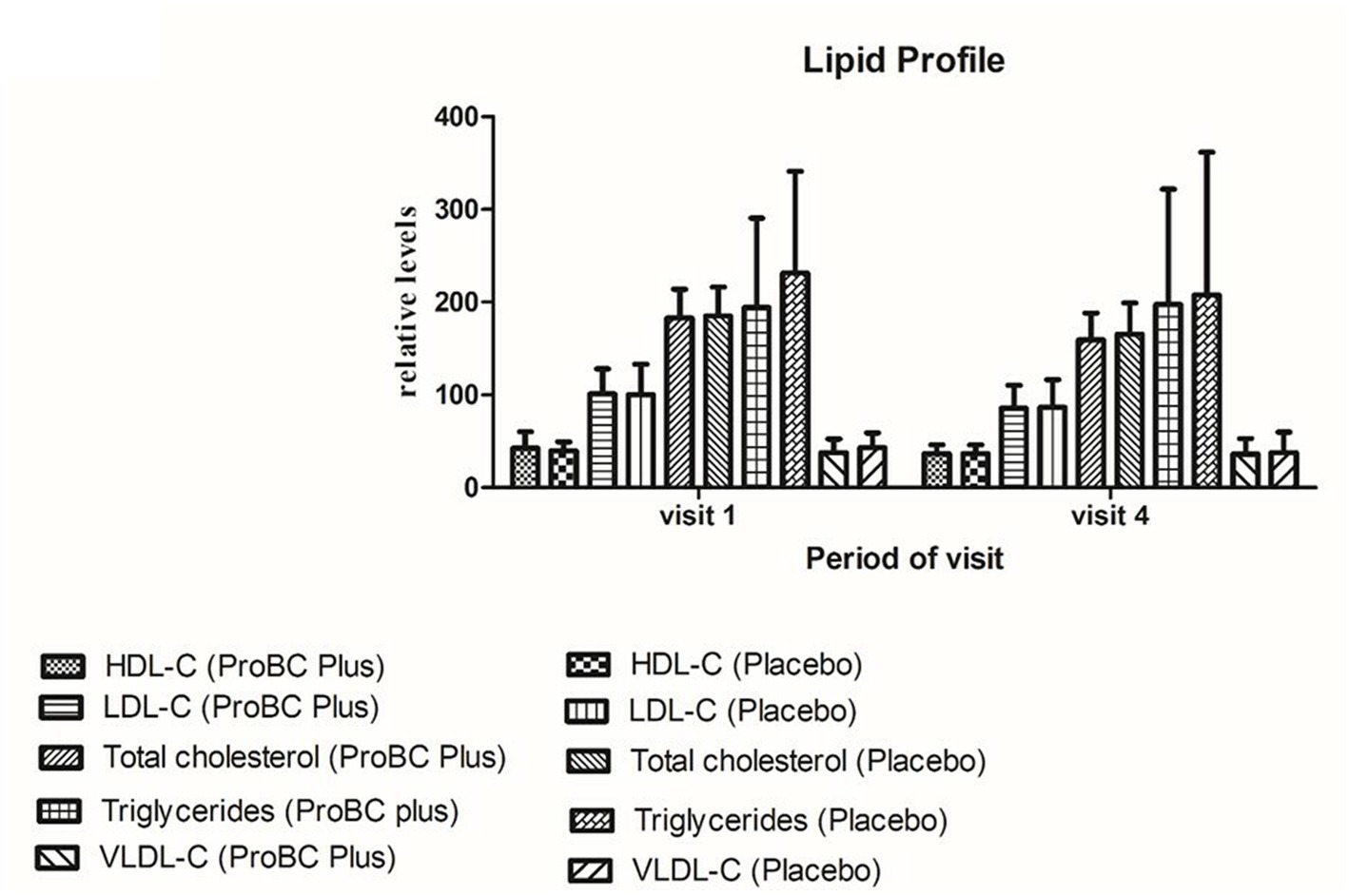
Figure 4. Analysis of lipid parameters (HDL—high-density lipoprotein; LDL—low-density lipoprotein; total cholesterol and triglycerides; VLDL—very low-density lipoproteins).
3.3 Analyzing the efficacy of the product
The common symptoms of IBS involve bloating, vomiting, and diarrhea. Changes in these symptoms were noticed throughout the visit.
VAS (Visual Analogue Scale), a value of 0 indicates “no pain,” while 10 represents “extreme pain.” A significant decline (p < 0.0001) from visit 1 to visit 4 depicts the efficacy of the test product as compared to the placebo. The change from baseline during visits 3 and 4 was estimated to be from −0.80 to −2.16 depicting the efficacy of the ProBC plus as compared to the placebo.
The other parameter, i.e., IBS-SSS (IBS Symptom Severity Scale) is a score that evaluates the abdominal pain/bloating (Figure 5). Supplementary Table S2 represents the significant change (decline) in the score as compared to the placebo (p < 0.0001). The change in the baseline from weeks 3 to 5 in the case of the test product ranged from −15.2 to −68.2 representing the efficacy of the test product as compared to the placebo.
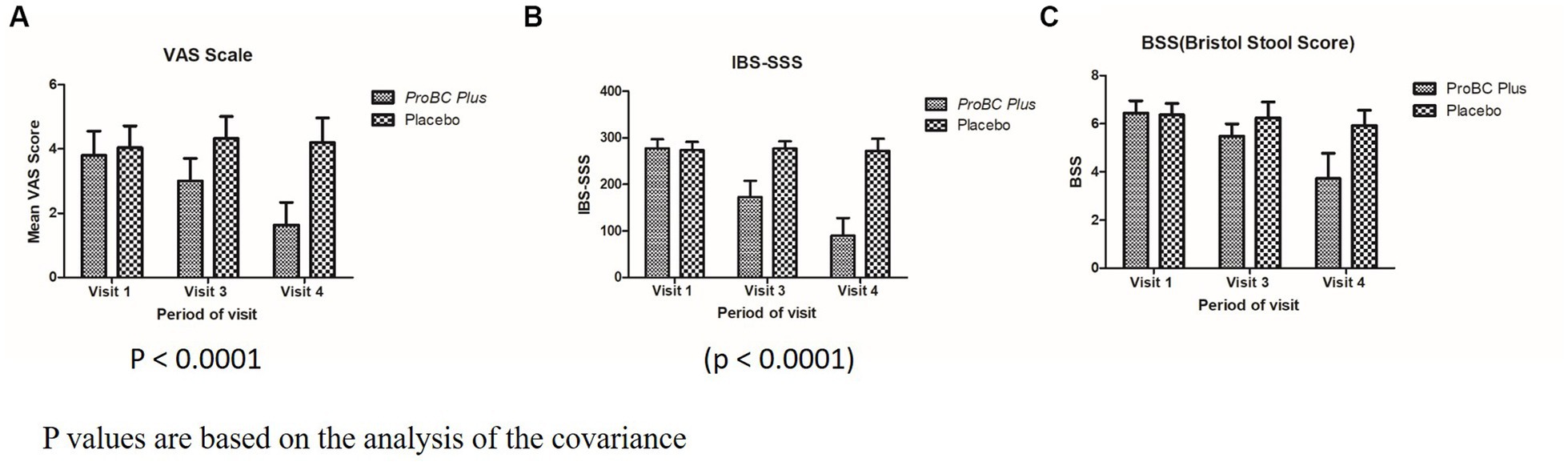
Figure 5. Analysis of covariance (p-value) (A) Mean VAS score with respect to period of visit (B) Mean IBS-SSS with respect to period of visit (C) BSS with respect to period of visit.
In case of BSS (Bristol Stool Score) that is analyzed for stool frequency, the score reduction from 6.44 to 3.72 from visit 1 to visit 4 and the variation in baseline values from −0.96 to −2.72 depict the efficacy of the treatment with ProBC Plus as compared to the placebo.
4 Discussion
The current study was carried out to investigate the effects of ProBC Plus in study participants treated for IBS and its related symptoms. Imbalances in the microbiota of the gastrointestinal tract could exert significant alterations. IBS symptoms such as bloating, diarrhea, vomiting, and abdominal pain could be one of the major symptoms of gastrointestinal discomfort (22, 23).
Probiotics are very often defined as live microorganisms which when administered in optimum quantity possess a health benefit to humans which attracted a lot of attention during subsequent years (24–27). There have been quite a few studies that involve the utilization of probiotics and the delivery of probiotics in the form of capsules (28). Kank et al. (29) reported usage of Weizmannia (Bacillus) coagulans as a dietary supplement has significantly improved bowel movements (29). There is a continuous increase in demand for functional foods containing probiotic strains due to the awareness of the benefits for gut health and disease prevention and therapy, which also includes the potential of these strains to treat various forms of gastroenteritis (30, 31). However, health benefits provided by probiotics are strain specific, no probiotic strain will provide all proposed benefits, not even strains of the same species, and not all strains of the same species will be effective against defined health conditions (32–35).
In the present study, the study participants were divided equally into test and placebo. The parameters such as vital signs, hematology, and biochemical exhibited a normal range, and there were no significant differences between the baseline to the last visit, i.e., visit 8, and also placebo. The other important parameters such as VAS (Visual Analogue Scale) depicted a significant result as compared to the placebo; subsequently, BSS (Bristol Stool Scale) and SSS (Symptom Severity Scale) determined the efficacy of the product as compared to the placebo. This important observation establishes that the product ProBC Plus is safe to use and has no adverse events (AEs) or serious adverse events (SAEs). A similar type of result was obtained from the Weizmannia (Bacillus) coagulans LBSC and MTCC 5856 (22). Vaclav and Jana (36) reported that the probiotic Bacillus coagulans MTCC 5856 in combination with an aqueous extract of cinnamon has strong synergetic effects on phagocytosis and regulation of cholesterol and blood sugar levels and also confirmed that the combination reduced intestinal damage in a mouse model of colitis (37). There are quite a few studies indicating the utilization of Weizmannia (Bacillus) coagulans in reducing the symptoms of irregular bowel movements; according to one of the studies, the strain GBI-30, 6,086 is reported for the decline in baseline intensity score for 8 weeks as compared to the placebo (36). Similarly, in the present study, ProBC plus was found to be significant as compared to the placebo, and also, the baseline intensity score was reduced in the case of the VAS scale; in the treatment group, the baseline range was approximately 1.36, whereas, in the placebo group, it was approximately 0.12; similarly in IBS-SSS, the baseline range in the treatment group was approximately 52.8 and in the placebo was approximately 5.0 depicting high significant results. With BSS, in the treatment group, the baseline range was approximately 1.76, whereas in the placebo was approximately 0.32, which signifies the efficacy of ProBC Plus concerning the placebo.
Furthermore, it was also noticed that the mechanistic approach of Weizmannia (Bacillus) coagulans LMG S-31876 in the treatment of IBS-related issues can be a beneficial effect as it helps in suppressing the binding and further growth of pathogenic bacteria and improving the functions of the barrier and tight junctions (38). However, the possible mechanism could be suppression of the growth and binding of pathogenic bacteria, improvement in the barrier function of the epithelium, and alteration of the immune activity of the host as reported by Majeed et al. (39) wherein animal studies with B. coagulans MTCC 5856 elicited anti-diarrhoeal activity and inhibited the gastrointestinal motility in fasted Rats (39–41). Also, these types of probiotics are known to produce the short-chain fatty acids, an action that results in decreased luminal pH and production of bactericidal proteins (42–45).
Furthermore, according to Skrzydło-Radomańska et al. (46), in a prospective double-blind placebo-controlled clinical trial, Bacillus coagulans BC 300 NORDBIOTIC signifies that the IBS-SSS score significantly decreased in the study group. Madempudi et al. (47) exhibited that Bacillus coagulans Unique IS2 was efficacious in lowering abdominal pain, bloating, and stool consistency in IBS patients. Similarly, Bacillus coagulans LBS has improved the IBS symptoms that include abdominal pain in the intervention group as compared to the placebo (48). ProBC Plus had a similar impact of reducing symptoms such as abdominal pain, bloating, diarrhea, and stool frequency and had marked a remarkable difference as compared to the placebo.
5 Conclusion
The present study depicts a substantial reduction in clinical symptoms associated with irritable bowel syndrome and diarrhea (IBS-D). In particular, symptoms such as abdominal pain, bloating, diarrhea, and stool frequency depicted a significant decline and exhibited a marked improvement as compared to the placebo. Furthermore, the comprehensive analysis of the parameters such as hematology, vital signs, and lipid profile were found to be in the normal range with the supplementation of ProBC Plus throughout the period of 8 weeks. Therefore, this steadfastly reinforces the safety of the probiotic product ProBC Plus. Subsequently, the results indicate the notion that Weizmannia (Bacillus) coagulans LMG S-31876, administered in the form of ProBC Plus at a dosage of 2 billion CFU/day, exhibits therapeutic influence in managing clinical symptoms associated with IBS-D prominently and provides a great promise in the enhancement of the quality of the life for the individual grappling with the IBS-D issues.
5.1 Recommendation
Individuals experiencing symptoms of irritable bowel syndrome and diarrhea (IBS-D) consider the incorporation of ProBC Plus, containing Weizmannia (Bacillus) coagulans LMG S-31876 at a dosage of 2 billion CFU/day, as a potential therapeutic intervention.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by Medstar Specialty Hospital, Bengaluru. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RK: Conceptualization, Formal Analysis, Supervision, Writing – original draft. SM: Project administration, Resources, Supervision, Writing – review & editing. AM: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft. SB: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was carried out from in-house funds of Abode Biotec Pvt. Ltd.
Acknowledgments
We would like to thank Bio Agile Therapeutics Pvt. Ltd., Bengaluru, for the clinical research services, and Medstar Specialty Hospital, Bengaluru, for the conduction of the trials.
Conflict of interest
The authors declare that this study received funding from Abode Biotec India Private Ltd. The funder had the following involvement in the study: data collection, analysis, decision to publish.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer HS declared a past co-authorship with the author SB to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1310462/full#supplementary-material
References
1. Drossman, DA, Whitehead, WE, and Camilleri, M. American Gastroenterological Association medical position statement: irritable bowel syndrome. J Gastroenterol. (1997) 112:2118–9. doi: 10.1053/gast.1997.1122118
2. Nemeth, V, and Pfleghaar, N. Diarrhea. 2022 Nov 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. (2023).
3. Randel, A. Infectious diarrhea: IDSA updates guidelines for diagnosis and management. Am Fam Physician. (2018) 97:676–7.
4. Sperber, AD, Bangdiwala, SI, Drossman, DA, Ghoshal, UC, Simren, M, Tack, J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. J Gastroenterol. (2021) 160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014
5. Wauters, L, Talley, NJ, Walker, MM, Tack, J, and Vanuytsel, T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. (2020) 69:591–600. doi: 10.1136/gutjnl-2019-318536
6. Bron, PA, Kleerebezem, M, Brummer, RJ, Cani, PD, Mercenier, A, TT, MD, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. (2017) 117:93–107. doi: 10.1017/S0007114516004037
7. Wang, X, Zhang, P, and Zhang, X. Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules. (2021) 26:6076. doi: 10.3390/molecules26196076
8. Van den Houte, K, Carbone, F, Pannemans, J, Corsetti, M, Fischler, B, Piessevaux, H, et al. Prevalence and impact of self-reported irritable bowel symptoms in the general population. United European Gastroenterol J. (2019) 7:307–15. doi: 10.1177/2050640618821804
9. Vasant, DH, Paine, PA, Black, CJ, Houghton, LA, Everitt, HA, Corsetti, M, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. (2021) 70:1214–40. doi: 10.1136/gutjnl-2021-324598
10. Sniffen, JC, LV, MF, Evans, CT, and EJC, G. Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One. (2018) 13:e0209205. doi: 10.1371/journal.pone.0209205
11. LV, MF, Evans, CT, and EJC, G. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med. (2018) 5:1–14. doi: 10.3389/fmed.2018.00124
12. Szajewska, H, Canani, RB, Guarino, A, Hojsak, I, Indrio, F, Kolacek, S, et al. ESPGHAN working Group for Probiotics Prebiotics. Probiotics for the prevention of antibiotic-associated. Diarrhea in children. J Pediatr Gastroenterol Nutr. (2016) 62:495–506. doi: 10.1097/MPG.0000000000001081
13. Su, GL, Ko, CW, Bercik, P, Falck-Ytter, Y, Sultan, S, Weizman, AV, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. (2020) 159:697–705. doi: 10.1053/j.gastro.2020.05.059
14. Ford, AC, Harris, LA, Lacy, BE, Quigley, EM, and Moayyedi, P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. (2018) 48:1044–60. doi: 10.1111/apt.15001
15. Wen, Y, Li, J, Long, Q, Yue, CC, He, B, and Tang, XG. The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: a systematic review and meta-analysis based on seventeen randomized controlled trials. Int J Surg. (2020) 79:111–9. doi: 10.1016/j.ijsu.2020.04.063
16. Kumar, LS, Pugalenthi, LS, Ahmad, M, Reddy, S, Barkhane, Z, and Elmadi, J. Probiotics in irritable bowel syndrome: a review of their therapeutic role. Cureus. (2022) 14:e24240. doi: 10.7759/cureus.24240
17. Dale, HF, Rasmussen, SH, Asiller, ÖÖ, and Lied, GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. (2019) 11:2048. doi: 10.3390/nu11092048
18. Wang, J, Ke, H, Liu, KX, and Qu, JM. Effects of exogenous probiotics on the gut microbiota and clinical outcomes in critically ill patients: a randomized controlled trial. Ann Palliat Med. (2021) 10:1180–90. doi: 10.21037/apm-20-202
19. Barraza-Ortiz, DA, Pérez-López, N, Medina-López, VM, Minero-Alfaro, JI, Zamarripa-Dorsey, F, Fernández-Martínez, ND, et al. Combination of a probiotic and an antispasmodic increases quality of life and reduces symptoms in patients with irritable bowel syndrome: a pilot study. Dig Dis. (2021) 39:294–300. doi: 10.1159/000510950
20. Selvaraj, SM, Wong, SH, Ser, HL, and Lee, LH. Role of low FODMAP diet and probiotics on gut microbiome in irritable bowel syndrome (IBS). Prog Microb Mol Biol. (2020) 3:853011. doi: 10.36877/pmmb.a0000069
21. Sreenadh, M, Kumar, KR, and Nath, S. In vitro evaluation of Weizmannia coagulans strain LMG S-31876 isolated from fermented rice for potential probiotic properties, safety assessment and technological properties. Life. (2022) 12:1388. doi: 10.3390/life12091388
22. King, CK, Glass, R, Bresee, JS, and Duggan, C. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. (2003) 52:1–16.
23. Tuohy, KM, Probert, HM, Smejkal, CW, and Gibson, GR. Using probiotics and prebiotics to improve gut health. Drug Discov Today. (2003) 8:692–700. doi: 10.1016/S1359-6446(03)02746-6
25. FAO/WHO. Working Group. Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada, April 30 and may 1, 2002. Available at: ftp://ftp.fao.org/es/esn/food/wgreport2.pdf (Accessed October 4, 2023).
26. Gill, H, and Prasad, J. Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol. (2008) 606:423–54. doi: 10.1007/978-0-387-74087-4_17
27. Shida, K, and Nomoto, K. Probiotics as efficient immunopotentiators: translational role in cancer 1 prevention. Indian J Med Res. (2013) 138:808–14.
28. Govender, M, Choonara, YE, Kumar, P, Du Toit, LC, Van Vuuren, S, and Pillay, V. A review of the advancements in probiotic delivery: conventional vs. non-conventional formulations for intestinal flora supplementation. Aaps Pharm SciTech. (2014) 15:29–43. doi: 10.1208/s12249-013-0027-1
29. Kang, S, Park, MY, Brooks, I, Lee, J, Kim, SH, Kim, JY, et al. Spore-forming Weizmannia (Bacillus) coagulans SNZ 1969 improved intestinal motility and constipation perception mediated by microbial alterations in healthy adults with mild intermittent constipation: a randomized controlled trial. Food Res Int. (2021) 146:110428. doi: 10.1016/j.foodres.2021.110428
30. Figueroa-González, I, Quijano, G, Ramírez, G, and Cruz-Guerrero, A. Probiotics and prebiotics -perspectives and challenges. J Sci Food Agric. (2011) 91:1341–8. doi: 10.1002/jsfa.4367
31. Longstreth, GF, Thompson, WG, Chey, WD, Houghton, LA, Mearin, F, and Spiller, RC. Functional bowel disorders. Gastroenterology. (2006) 130:1480–91. doi: 10.1053/j.gastro.2005.11.061
32. Luyer, MD, Buurman, WA, Hadfoune, M, Speelmans, G, Knol, J, Jacobs, JA, et al. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun. (2005) 73:3686–92. doi: 10.1128/IAI.73.6.3686-3692.2005
33. Kekkonen, RA, Lummela, N, Karjalainen, H, Latvala, S, Tynkkynen, S, Jarvenpaa, S, et al. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol. (2008) 14:2029–36. doi: 10.3748/wjg.14.2029
34. Lee, IC, Tomita, S, Kleerebezem, M, and Bron, PA. The quest for probiotic effector molecules--unraveling strain specificity at the molecular level. Pharmacol Res. (2013) 69:61–74. doi: 10.1016/j.phrs.2012.09.010
35. Million, M, and Raoult, D. Species and strain specificity of Lactobacillus probiotics effect on weight regulation. Microb Pathog. (2013) 55:52–4. doi: 10.1016/j.micpath.2012.09.013
36. Vaclav, V, and Jana, V. Physiological effects of a combination of cinnulin with probiotics. Am J Immunol. (2013) 9:103–9. doi: 10.3844/ajisp.2013.103.109
37. Maity, C, and Gupta, AK. A prospective, interventional, randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Weizmannia (Bacillus) coagulans LBSC in the treatment of acute diarrhea with abdominal discomfort. Eur J Clin Pharmacol. (2019) 75:21–31. doi: 10.1007/s00228-018-2562-x
38. Hun, L. Weizmannia (Bacillus) coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med J. (2009) 121:119–24. doi: 10.3810/pgm.2009.03.1984
39. Majeed, M, Majeed, S, Nagabhushanam, K, Natarajan, S, Sivakumar, A, Ali, F, et al. Evaluation of anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 and its effect on gastrointestinal motility in wistar rats. IJPBS. (2016) 7:311–6.
40. Collins, SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. (2014) 11:497–505. doi: 10.1038/nrgastro.2014.40
41. Collins, SM. A case for an immunological basis for irritable bowel syndrome. J Gastroenterol. (2002) 122:2078–80. doi: 10.1053/gast.2002.34097
42. O’Mahony, L, Mccarthy, J, Kelly, P, Hurley, G, and Luo, F. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. J Gastroenterol. (2005) 128:541–51. doi: 10.1053/j.gastro.2004.11.050
43. Guyonnet, D, Chassany, O, Ducrotte, P, Picard, C, and Mouret, M. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. (2007) 26:475–86. doi: 10.1111/j.1365-2036.2007.03362.x
44. Moayyedi, P, Ford, AC, Talley, NJ, Cremonini, F, and Foxx-Orenstein, A. The efficacy of probiotics in the therapy of irritable bowel syndrome: a systematic review. Gut. (2008) 59:325–32. doi: 10.1136/gut.2008.167270
45. Majeed, M, Nagabhushanam, K, Arumugam, S, and Ali, F. Process for enhancing the viable counts of lactic acid Bacteria and useful compositions thereof. US application No. 14839923; PCT/US2015/47608. (2015)
46. Skrzydło-Radomańska, B, Prozorow-Król, B, Kurzeja-Mirosław, A, Cichoż-Lach, H, Laskowska, K, Majsiak, E, et al. The efficacy and safety of single-strain probiotic formulations containing Bifidobacterium lactis or Bacillus coagulans in adult patients with irritable bowel syndrome—a randomized double-blind placebo-controlled three-arm interventional trial. J Clin Med. (2023) 12:4838. doi: 10.3390/jcm12144838
47. Madempudi, RS, Ahire, JJ, Neelamraju, J, Tripathi, A, and Nanal, S. Randomized clinical trial: the effect of probiotic Bacillus coagulans unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci Rep. (2019) 9:12210. doi: 10.1038/s41598-019-48554-x
Keywords: Weizmannia (Bacillus) coagulans LMG S-31876, probiotics, irritable bowel syndrome, diarrhea, clinical trials
Citation: Kallur RK, Madapati S, Mathur A and Bhattacharya S (2024) The role of Weizmannia (Bacillus) coagulans LMG S-31876 in treating IBS-diarrhea. Front. Nutr. 10:1310462. doi: 10.3389/fnut.2023.1310462
Edited by:
Oana Lelia Pop, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Dhaneshwar Kumar, National Institute of Diabetes and Digestive and Kidney Diseases (NIH), United StatesHeba H. Salama, National Research Centre, Egypt
Saugata Hazra, Indian Institute of Technology Roorkee, India
Copyright © 2024 Kallur, Madapati, Mathur and Bhattacharya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranjith Kumar Kallur, a3JrQGFib2RlYmlvdGVjLmNvbQ==; Sreenadh Madapati, c3JlZW5hZGhAYWJvZGViaW90ZWMuY29t
 Ranjith Kumar Kallur
Ranjith Kumar Kallur Sreenadh Madapati
Sreenadh Madapati Ankita Mathur
Ankita Mathur Sourish Bhattacharya
Sourish Bhattacharya