- Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
Nonalcoholic fatty liver disease (NAFLD) represents a significant global health concern. Numerous investigations have explored the implications of time-restricted eating (TRE) in the management of NAFLD. Therefore, the objective of our study was to conduct a systematic review to summarize and analyze all randomized controlled trials (RCTs) of TRE for patients with NAFLD. A thorough literature search was executed across Embase, Cochrane Library, and PubMed databases, covering all records from their inception until 1 September 2023. All clinical studies of TRE for NAFLD were summarized and analyzed. Our systematic review included four RCTs, encompassing a total of 443 NAFLD patients. These studies varied in sample size from 32 to 271 participants. The TRE intervention was consistently applied in an 8-h window, over durations ranging from 4 weeks to 12 months. The findings suggest that TRE could offer several health benefits for NAFLD patients, such as improved liver health indicators like liver stiffness and intrahepatic triglyceride (IHTG) levels. Consequently, TRE appears to be a promising dietary intervention for NAFLD patients. However, it is premature to recommend TRE for patients with NAFLD. The existing body of research on the effects of TRE in NAFLD contexts is limited, underscoring the need for further high-quality studies to expand our understanding of TRE’s benefits in treating NAFLD. Ongoing clinical trials may provide more insights into the effects of TRE in NAFLD.
1 Introduction
Nonalcoholic fatty liver disease (NAFLD) is a prevalent global health concern, affecting approximately 20 to 30% of individuals worldwide, thus representing a significant public health challenge (1–3). Defined by the accumulation of fat in over 5% of hepatocytes, and unrelated to viral hepatitis, excessive alcohol consumption, or other causes such as steatogenic medications, NAFLD is a multifaceted condition influenced by a combination of genetic predispositions and environmental factors (2, 3). Recent research indicates a global incidence rate of NAFLD of 4,613 cases per 100,000 person-years, with higher rates observed in individuals who are obese or overweight and in men (3). The disease poses a substantial risk for liver-related morbidity and mortality, along with metabolic comorbidities, underscoring the need for increased attention from health policymakers, primary care physicians, and specialists (2). Notably, NAFLD is comorbid in over 70% of individuals with obesity or diabetes and is commonly associated with type 2 diabetes mellitus (T2DM), hyperlipidemia, obesity, and certain cardiovascular diseases (4–7). Recent research has elucidated the potential of specific dietary interventions and supplements in ameliorating liver and metabolic parameters in patients with NAFLD (8–13).
In recent years, intermittent fasting (IF) diets have gained significant popularity due to their clinical benefits, including improvements in metabolic factors and the facilitation of weight loss (14–16). These diets are primarily categorized into three types: time-restricted eating (TRE), which involves consuming meals within a consistent daily window (typically 8 h); alternate-day fasting (ADF), characterized by alternating fasting and feasting days; and the 5:2 diet, which involves 5 days of normal eating and two fasting days per week (17–22). These dietary approaches have demonstrated benefits in managing hyperglycemia (23) and nonalcoholic fatty liver disease (NAFLD) (24). TRE may have some benefits for healthy individuals, especially early TRE (25–27). Additionally, specific forms of IF, such as Ramadan fasting and the fasting-mimicking diet, have been identified as potentially beneficial in managing obesity (28, 29), NAFLD (30, 31), and even in cancer therapy (32, 33). Among these, TRE has been increasingly emphasized for its potential to enhance adherence, improve various cardiometabolic parameters, and contribute to overall health throughout the lifespan (34–40). TRE is a dietary strategy within the broader intermittent fasting paradigm, focusing on limiting food intake to a specific time window, typically 8 h each day. This approach contrasts with traditional dietary methods that concentrate on caloric or food type restrictions. The primary premise of TRE is that aligning eating patterns with circadian rhythms can optimize physiological processes, potentially leading to improved metabolic health, weight management, and reduced risk of chronic diseases. A systematic scoping review explored the feasibility and safety of TRE for individuals with T2DM, prediabetes, obesity, and who were overweight, and found that TRE was acceptable, implementable, and safe (41). Previous studies have demonstrated that TRE may be beneficial for some disorders, including diabetes (42, 43), prediabetes (44, 45), polycystic ovary syndrome (PCOS) (46, 47), metabolic syndrome (48, 49), and multiple sclerosis (50). In addition, several studies have explored the effects of TRE in NAFLD. Therefore, the objective of our study was to conduct a systematic review to summarize and analyze all clinical studies on the effects of TRE for patients with NAFLD on body composition, cardiometabolic and inflammatory biomarkers, and NAFLD parameters.
2 Methods
2.1 Search strategy
The methodology for this systematic review was meticulously predefined and registered on the INPLASY platform (Registration ID: 202380036). This review was executed in accordance with the methodologies recommended by the Cochrane Collaboration and adhered to the PRISMA guidelines for reporting outcomes (51). A thorough literature search was conducted across the Embase, PubMed, and Cochrane databases, spanning from their inception to 1 September 2023. The search incorporated the following terms: (“Nonalcoholic Fatty Liver” OR “Nonalcoholic Fatty Liver Disease” OR “Fatty Liver, Nonalcoholic” OR “Liver, Nonalcoholic Fatty” OR NAFLD OR “Non-alcoholic Fatty Liver Disease” OR “Nonalcoholic Fatty Livers” OR “Nonalcoholic Steatohepatitis” OR “Steatohepatitis, Nonalcoholic” OR “metabolic dysfunction-associated fatty liver disease” OR MAFLD) AND (“time restricted” or “time restricted feeding” or “time restricted eating” or time-restricted or “time-restricted feeding” or “time-restricted eating” OR “Eating, Time Restricted” OR “Time Restricted Feedings” OR TRE OR intermittent OR “Intermittent Fasting” OR “Fasting, Intermittent” OR “Fasting, Time Restricted” OR “Feeding, Time Restricted” OR “Time Restricted Eating” OR “Time Restricted Fasting” OR “Time Restricted Feeding”). The references in relevant reviews were also searched. In cases where there was uncertainty, the initial literature search and screening were conducted by two authors (LXX and WS), and any ambiguities were resolved through consultation with a third author (HJY).
2.2 Inclusion criteria
In accordance with the PICOS framework (52), the inclusion criteria for this systematic review were meticulously established as follows: (P) patients, encompassing individuals diagnosed with NAFLD; (I) intervention, specifically focusing on time-restricted eating (TRE); (C) control, which included several comparative conditions such as calorie restriction (CR), habitual meal timing, alternate-day fasting (ADF), or a standard diet; (O) outcomes, with primary endpoints being the effects of TRE on liver parameters in NAFLD patients and secondary outcomes including alterations in body weight, plasma lipid levels, body composition, insulin resistance, glucoregulatory factors, inflammation parameters (cytokeratin and high-sensitivity C-reactive protein), and the safety profile of TRE; and (S) study type, which was RCTs. Exclusions were made for editorials, duplicates, comments, conference abstracts, and case reports.
2.3 Quality assessment and data extraction
For the assessment of randomized controlled trials (RCTs) (53), we employed the Cochrane Collaboration’s Risk of Bias 2 tool to evaluate the quality. Data extraction was carried out independently by two authors (LXX and WS) and was organized into two predefined templates: (1) study characteristics, encompassing details of the first author, publication year, study design, sample size, intervention and control groups, TRE window, main outcomes measured, and principal findings and (2) patient characteristics, detailing the first author, publication year, mean age, body mass index (BMI) in kg/m2, waist circumference, percentage of female participants, weight, fat mass, and lean body mass. Additionally, an exploration for ongoing TRE studies involving patients with NAFLD was conducted on the ClinicalTrials.gov website.1 The extracted data from these ongoing studies were organized into a predefined format, including study title, NCT number, conditions, interventions, study type, study commencement, locations, and expected completion dates.
3 Results
3.1 Literature search
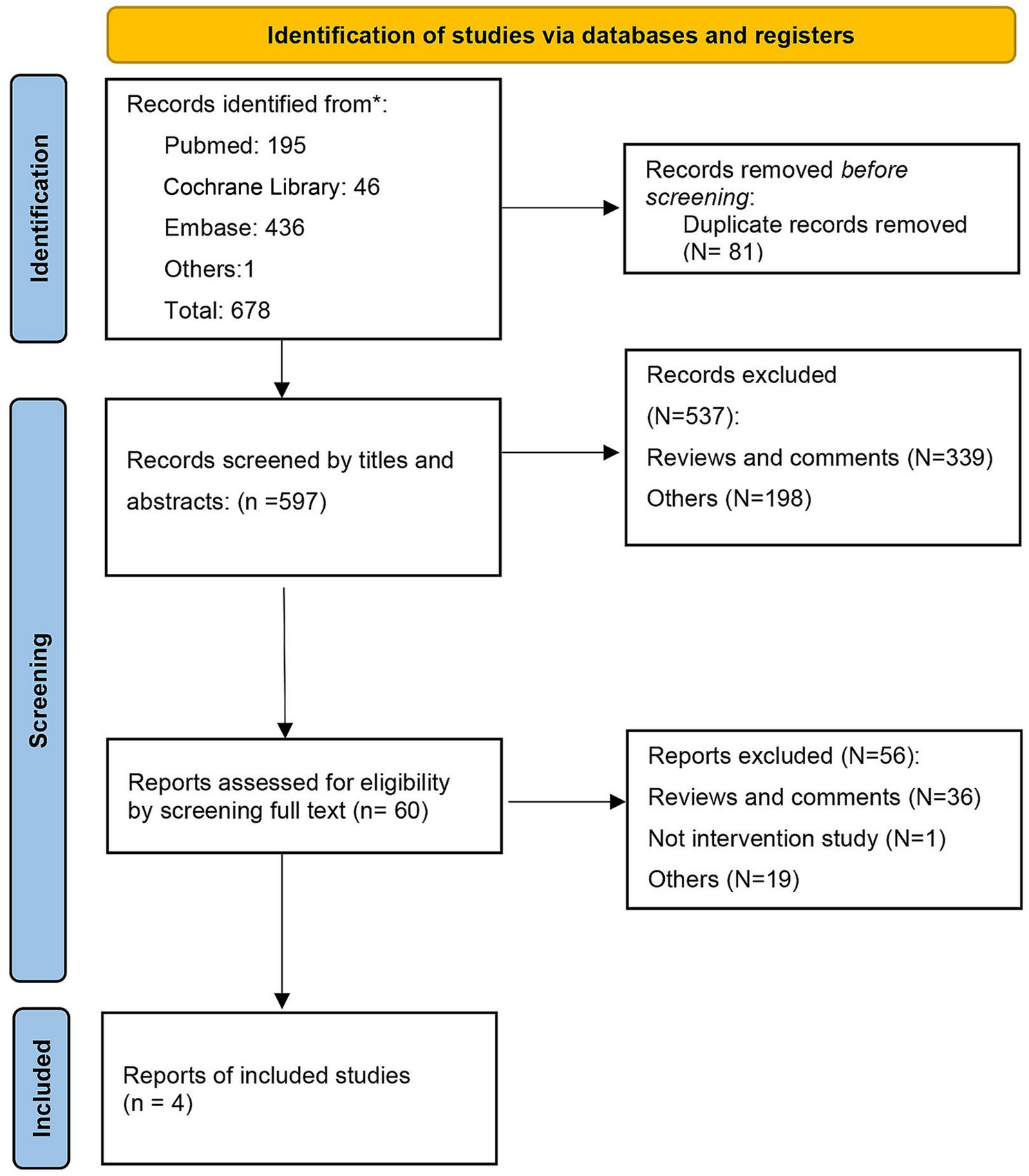
A total of 678 records were found in our primary database search. After excluding duplications, 596 records were screened by title, abstracts, and full text. Finally, 4 RCTs were included in the systematic review (54–57). The search flow diagram is displayed in Figure 1.
3.2 Study characteristics and quality assessment
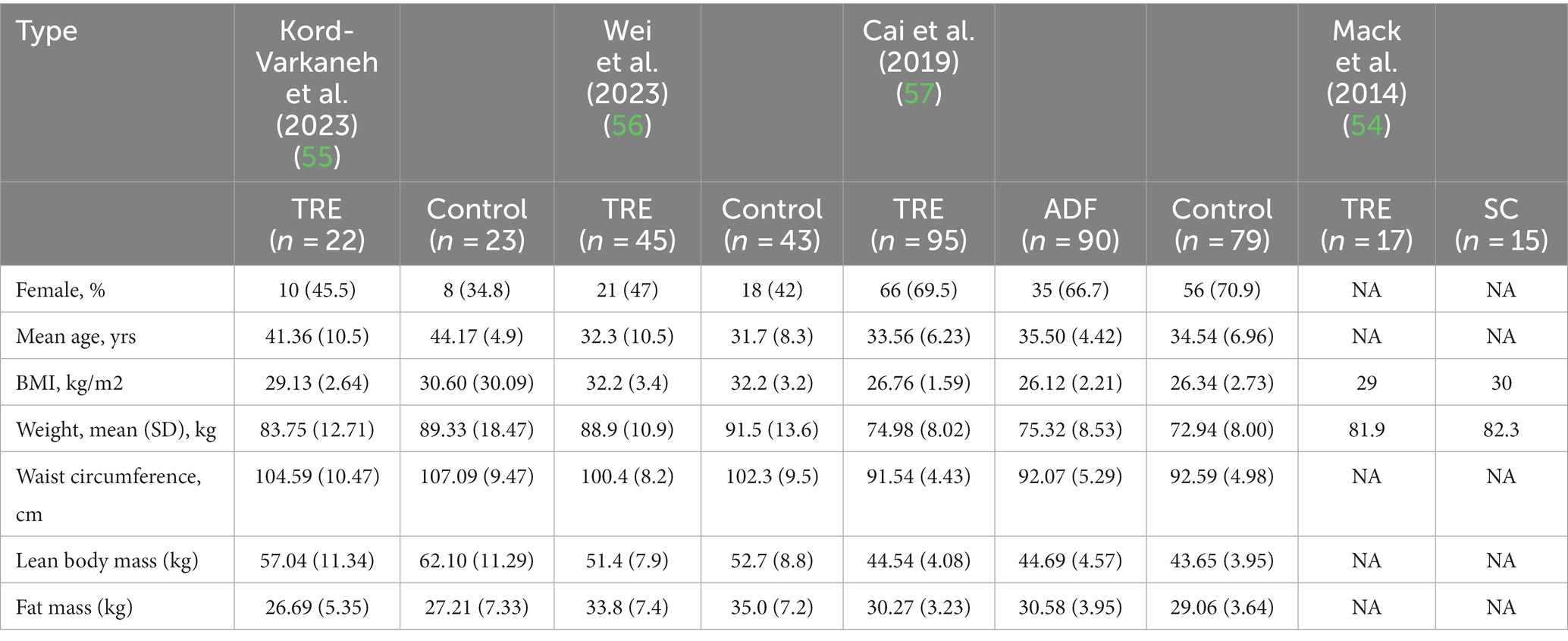
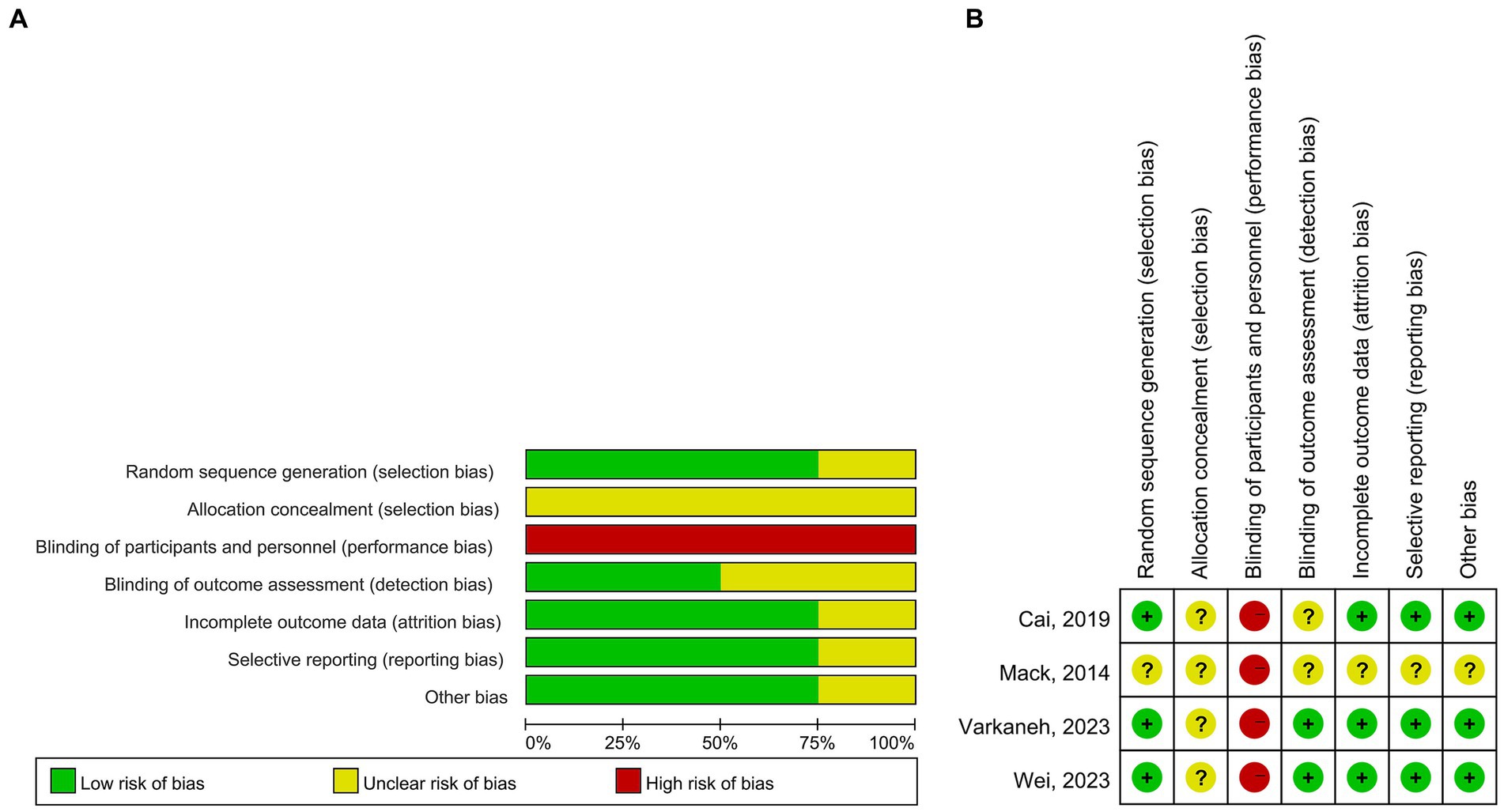
A total of 443 patients with NAFLD were included. The sample size ranged from 32 to 271. The TRE windows in all studies were 8 h. The interventions were TRE without CR in two studies, TRE with CR in one study, and TRE with a low-sugar diet in one study. The time of interventions ranged from 4 weeks to 12 months. One study included patients with obesity only (56) and two studies did not (55, 57). The mean age ranged from 32.3 to 41.36 years, the ratios of females ranged from 45.5 to 69.5%, and the mean BMI ranged from 26.76 to 32.2 in TRE groups. Study and patient characteristics are summarized in Tables 1, 2. For quality assessment, three studies reported sequence generation methods, the computer-generated random number sequence was used in two studies, and a block size of six was used in one study. The results of the quality assessment for RCTs are shown in Figure 2.
3.3 The effects of TRE for NAFLD
Cai et al. (57) performed a 4-week study to investigate the effects of TRE and ADF on dyslipidemia, body composition, and body weight in patients with NAFLD. A total of 271 adults were included, with 95 individuals in the TRF group, 90 individuals in the ADF group, and 79 individuals in the control group. The results demonstrated that both TRE and ADF can reduce body weight, fat mass, total cholesterol, and serum triglycerides. There were no significant changes in HDL, fasting insulin, fat-free mass, LDL, liver stiffness, glucose, and systolic or diastolic blood pressure between the TRE and control groups. The authors found that TRE, as well as ADF, were effective diet interventions for patients with NAFLD, with an improvement in dyslipidemia and a reduction in body weight achieved.
Kord-Varkaneh et al. (55) conducted a 12-week study to explore the effects of TRF combined with a low-sugar diet versus a traditional diet for NAFLD. In their study, 52 patients were included, and 3 and 4 patients dropped out of the control group and intervention group, respectively; eventually, 45 patients were included in the analysis. Compared with the traditional diet, TRF combined with a low-sugar diet could significantly reduce the γ-glutamyl transpeptidase, alanine aminotransferase, fibrosis score, aspartate aminotransferase, inflammatory markers of cytokeratin, high-sensitivity C-reactive protein, triacylglycerols, and total cholesterol statistically. The authors concluded that TRF combined with a low-sugar diet can improve lipid, liver, and inflammatory markers, and reduce adiposity. Therefore, TRF combined with a low-sugar diet could be regarded as a promising non-pharmacologic therapy for NAFLD, and long-term adherence should be considered in further studies.
In their 12-month study, Wei et al. (56) compared the effect of TRE with calorie restriction for patients with NAFLD and obesity; 88 patients were included. In total, 81 (92%) and 74 (84%) patients completed the 6-month intervention and entire 12-month intervention, respectively. TRE and CR reduced the intrahepatic triglyceride (IHTG) content by 8.3 and 8.1%, respectively, at the 6-month assessment. TRE and DCR can significantly reduce body weight, metabolic risk factors, and liver stiffness; however, there was no significant difference between the two groups regarding these factors. In addition, regarding the safety of TRE, the authors found that there were no significant differences between the TRE plus CR group and the CR group for adverse events, including appetite change, dyspepsia, constipation, hunger, fatigue, decreased appetite, discomfort in the stomach, and dizziness. Their findings supported that caloric restriction should be added to TRE.
Mack et al. (54) investigated the effects of TRE on patients with NAFLD, comparing it to the standard care (SC) of diet and exercise. The study enrolled 32 NAFLD patients, who were randomly assigned to either the SC or TRE group. The results indicated improvements in both groups in terms of weight, BMI, and total body fat mass reduction. However, the TRE group showed more significant improvements in liver stiffness, liver steatosis, waist circumference, and visceral fat volume compared to the SC group. Additionally, insulin resistance, measured by HOMA-IR, decreased more in the TRE group. Notably, there were no significant differences in dietary energy consumption, activity levels, hunger, or quality of life scores between the two groups. The study concluded that TRE is a viable and more effective approach than standard diet and exercise for treating NAFLD and central adiposity, offering significant improvements in liver health and insulin resistance.
In addition, there were six ongoing studies on TRE for patients with NAFLD that were registered in ClinicalTrials.gov (58). Among them, two trials will explore the effects of TRE on insulin sensitivity, metabolic inflammation, liver steatosis (NCT05220956), and the amount of fat in the liver (NCT04899102). Two trials will investigate the effects of the combination of TRE and a balanced mediterranean diet for glucose metabolism (NCT05866744) and metabolism and inflammation (NCT05968378), and one trial (NCT05332613) will assess the impact of TRE with standard of care lifestyle recommendations (30 min of exercise at least 5 days/week and a mediterranean, hypocaloric diet) on the degree of fat in the liver, measured by magnetic resonance imaging (MRI). One study aims to conduct a prospective study to determine the effects of TRE on 10-year cardiovascular disease risk and the amount of intrahepatic fat in adults with NAFLD via a mobile application (NCT05579158). An overview of the relevant ongoing trials registered at ClinicalTrials.gov is displayed in Table 3.
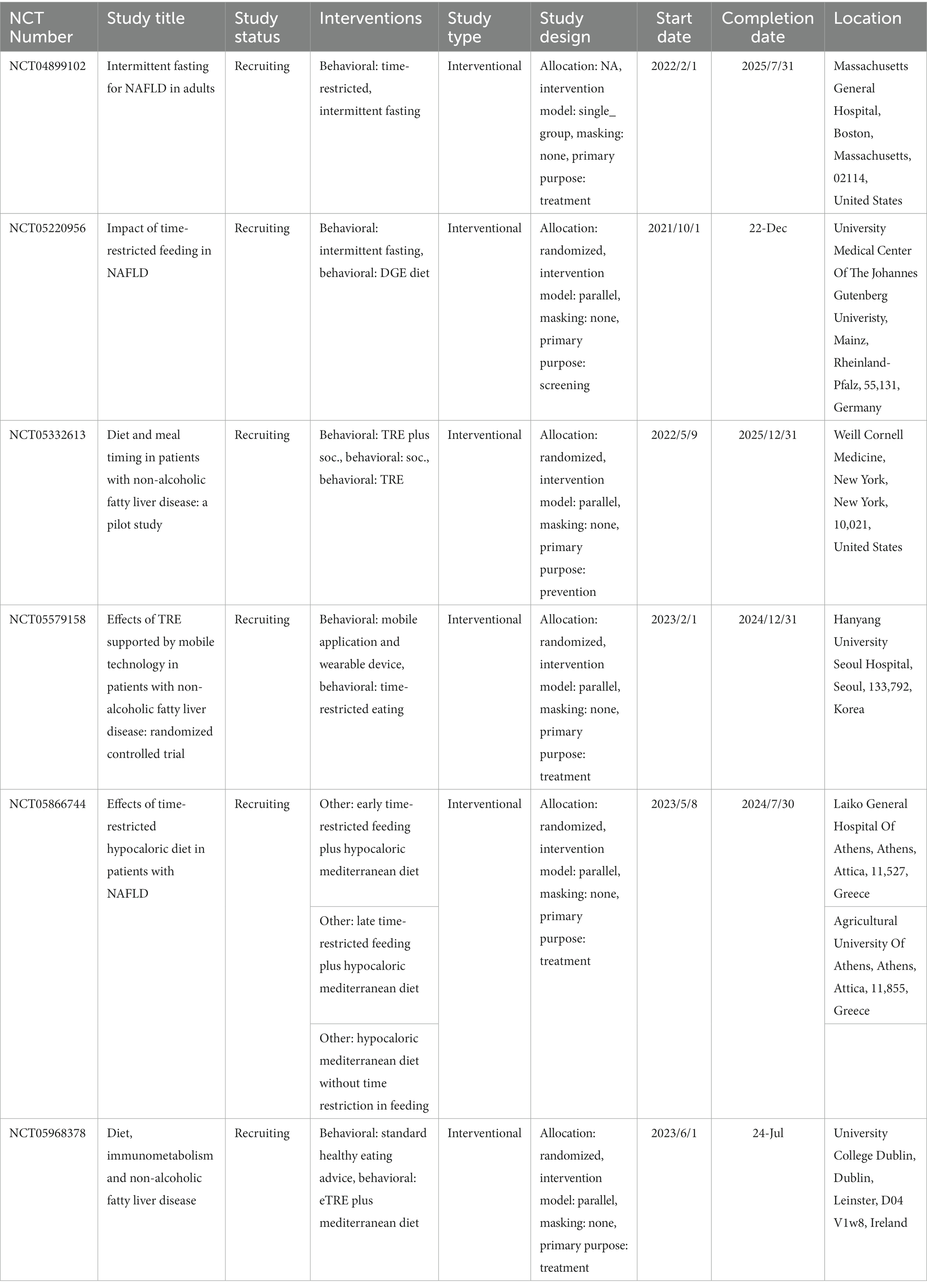
Table 3. Overview of ongoing trials registered at ClinicalTrials.gov.
4 Discussion
To the best of our knowledge, this is the first systematic review to summarize and analyze all RCTs on the effects of TRE in patients with NAFLD. The findings suggest that TRE may be an effective diet therapy for patients with NAFLD, which can improve liver, lipid, and inflammatory markers.
NAFLD is a heterogeneous disease involving complex interplay between inflammatory mediators, immune cells, and metabolic target tissues of skeletal muscle and adipose (59, 60). It is a huge challenge to develop effective drugs for NAFLD. To date, no effective drugs have gone through late-phase trials and into licensing (4, 6). The main interventions for patients with NAFLD are weight loss and lifestyle intervention (61). Traditionally, diets include a balanced mediterranean diet and/or limiting consumption of saturated fats, fructose, and ultra-processed foods, which focus on the dietary macronutrient composition and have been proven to improve hepatic steatosis and liver biochemistry (62). Recently, accumulating evidence has demonstrated that the timing of energy intake may play a key role in the risk of NAFLD, including night-time eating and irregular meal patterns (63–66). The link between NAFLD and eating habits may be mediated through disruption to circadian rhythms. The benefits of TRE for patients with NAFLD should be investigated.
In our systematic review, Cai et al. (57) found that TRE was well tolerated and related to significantly decreased fat mass, weight loss, and triglycerides. In this study, the parameters of circadian markers, inflammation, steatosis, and liver biochemistry at the baseline and follow-up were not measured, and no change of liver stiffness may be on account of the short intervention period (only 4 weeks). Kord-Varkaneh et al. (55) found that TRF combined with a low-sugar diet can improve lipid, inflammatory, and liver markers, and reduce adiposity for NAFLD. The intervention period was still short (3 months), and a long-term intervention (more than 1 year) may provide a better understanding of the adherence and the chronic effects of the intervention. Wei et al. (56) found that both TRE plus CR and CR were effective for weight loss, with marked reductions in both liver stiffness and IHTG; however, there was no significant difference between the two groups. In this study, the stringent calorie restriction targets might mask the relatively subtle benefits of TRE. Mack et al. (54) demonstrated in their pilot study that TRE is a favorable approach for managing NAFLD and central obesity, showing notably better outcomes in terms of improving liver health, reducing waist size, decreasing visceral fat, and enhancing insulin sensitivity when compared to conventional diet and exercise recommendations. In total, limited evidence demonstrated that TRE may have potential benefits for patients with NAFLD, including weight loss, the reduction of cardiometabolic parameters such as triacylglycerols and total cholesterol, insulin resistance, the reduction of inflammation parameters such as cytokeratin and high-sensitivity C-reactive protein, and an improvement in the live parameters of liver stiffness and IHTG. In addition, in terms of the feasibility and safety of TRE, all four RCTs demonstrated that TRE had good feasibility. Although short intervention can provide relevant results, a longer-term study (e.g., more than 1 year) would be superior for understanding certain factors, including safety and efficacy. However, the intervention time is relatively short (no more than 1 year) in these studies, and a long-term study of TRE should be conducted in further studies. In addition, a cross-sectional study found that TRE may be associated with a lower risk of NAFLD. However, the time of TRE was derived from recall, which may cause bias; the cross-sectional design could not demonstrate causality (67). For the safety of TRE, only one included study reported the AE of TRE, and there were no significant differences between the TRE group and control group for AEs, discomfort in the stomach, appetite change, dyspepsia, constipation, hunger, fatigue, decreased appetite, and dizziness. It seems that TRE is a safe dietary intervention and many previous studies have also demonstrated that TRE is safe. Nevertheless, it is imperative to note that TRE may pose potential side effects, including the risk of orthorexia and sarcopenia. These concerns warrant careful consideration and monitoring during the implementation of TRE interventions (68, 69).
The mechanisms of the benefits of TRE for patients with NAFLD are multiple (16, 19, 70), including: (1) TRE activates pathways in the liver (70, 71). During the long period of daily fasting, the expression and functional states of liver-based sirtuins, ketone bodies (ketogenesis), and adeno-sine monophosphate–activated protein kinase C (AMPK) are increased, which can activate pathways implicated in mediating the benefits of CR, including weight loss, and the improvement in cardiometabolic parameters. (2) TRE improves circadian rhythms (72). TRE can change the temporal pattern activity of the important mediator of the insulin signaling pathway within the liver. (3) The effects of TRE on adipose tissue (73). TRE can increase lipolysis and β-oxidation in adipose tissue. In addition, the overall reduction in free fatty acid in the liver as a result of TRE can reduce inflammation. (4) The effects of TRE on gut microbiota (74). TRE can restore the microbiota-related molecular pathways, including hormonal signaling, neural responses, metabolic regulators, the circadian system, and immune-inflammatory pathways. (5) The effects of TRE on endocrine regulators of metabolism and physiology (75). TRE, particularly early TRE, is related to the most marked improvements in appetite reduction and endocrine regulators, considering the setting of isocaloric intake.
One included study (55) investigated the combination of eating window (TRE) and dietary macronutrient composition (a low-sugar diet) for patients with NAFLD. Whether this combination is more effective should be explored in further studies, including but not limited to the combination of TRE and a low-sugar diet, TRE and a balanced mediterranean diet, and TRE and a low and very low carbohydrate diet. In addition, previous studies have demonstrated that exercise-based interventions have benefits for patients with NAFLD and suggested their use in the management of NAFLD. However, there is no study investigating the effects of TRE combined with exercise for patients with NAFLD. The combined interventions should be explored in further studies.
This systematic review presents several strengths. It is the inaugural comprehensive analysis that collates and evaluates all clinical studies on the effects of TRE in patients with NAFLD. Our findings indicate the potential health benefits of TRE for NAFLD patients, including weight reduction, decreased cardiometabolic parameters such as triacylglycerols and total cholesterol levels, and improvements in liver health indicators, notably liver stiffness and IHTG levels. There are some limitations in this systematic review. First, although meta-analysis was initially planned, the types of interventions (TRE with and without CR, TRE plus a low-sugar diet), the interventions time (from 4 weeks to 1 year), the primary measured outcomes, and statistical heterogeneity make meta-analysis inappropriate. Second, there are only four RCTs on the effects of TRE in NAFLD, and none of the RCTs compared “TRE without additional dietary intervention” to control conditions. Therefore, the superiority of TRE itself cannot be deduced. Notably, several ongoing trials, including those investigating the synergistic effects of TRE with a balanced mediterranean diet, promise to yield novel insights into this domain, potentially highlighting augmented benefits for NAFLD patients.
In conclusion, TRE might be an effective dietary intervention for improving liver parameters and reducing the cardiometabolic risk in patients with NAFLD. However, it is premature to recommend TRE for patients with NAFLD, given the current limitations in evidence. Thus, further high-quality research is imperative to broaden our understanding of TRE’s benefits in the context of NAFLD. The results from ongoing clinical trials are anticipated to offer additional insights into the efficacy of TRE in managing NAFLD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XL: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. SW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JH: Formal analysis, Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TRE, time-restricted eating; NAFLD, nonalcoholic fatty liver disease; IHTG, intra-hepatic triglyceride; CR, calorie restriction; T2DM, type 2 diabetes; ADF, alternate day fasting; AMPK, adenosine monophosphate–activated protein kinase C; CSO, camelina sativa oil.
Footnotes
References
1. Abdelmalek, MF. Nonalcoholic fatty liver disease: another leap forward. Nat Rev Gastroenterol Hepatol. (2021) 18:85–6. doi: 10.1038/s41575-020-00406-0
2. Younossi, Z, Anstee, QM, Marietti, M, Hardy, T, Henry, L, Eslam, M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
3. le, MH, le, DM, Baez, TC, Wu, Y, Ito, T, Lee, EY, et al. Global incidence of non-alcoholic fatty liver disease: a systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol. (2023) 79:287–95. doi: 10.1016/j.jhep.2023.03.040
4. Mir, BA, Majeed, T, and Chauhan, A. Nonalcoholic fatty liver disease. N Engl J Med. (2022) 386:295. doi: 10.1056/NEJMc2118255
5. Rinella, ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
6. Wang, XJ, and Malhi, H. Nonalcoholic fatty liver disease. Ann Intern Med. (2018) 169:ITC65. doi: 10.7326/AITC201811060
7. Stahl, EP, Dhindsa, DS, Lee, SK, Sandesara, PB, Chalasani, NP, and Sperling, LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:948–63. doi: 10.1016/j.jacc.2018.11.050
8. Pan, L, Sui, J, Ying, X, and Zhao, Q. Effect of nut consumption on nonalcoholic fatty liver disease: a systematic review and Meta-analysis. Nutrients. (2023) 15:2394. doi: 10.3390/nu15102394
9. Del Bo, C, Perna, S, Allehdan, S, Rafique, A, Saad, S, AlGhareeb, F, et al. Does the Mediterranean diet have any effect on lipid profile, central obesity and liver enzymes in non-alcoholic fatty liver disease (NAFLD) subjects? A systematic review and Meta-analysis of randomized control trials. Nutrients. (2023) 15:2250. doi: 10.3390/nu15102250
10. Musazadeh, V, Roshanravan, N, Dehghan, P, and Ahrabi, SS. Effect of probiotics on liver enzymes in patients with non-alcoholic fatty liver disease: an umbrella of systematic review and Meta-analysis. Front Nutr. (2022) 9:844242. doi: 10.3389/fnut.2022.844242
11. Musazadeh, V, Dehghan, P, and Khoshbaten, M. Efficacy of omega-3-rich Camelina sativa on the metabolic and clinical markers in nonalcoholic fatty liver disease: a randomized, controlled trial. Eur J Gastroenterol Hepatol. (2022) 34:537–45. doi: 10.1097/MEG.0000000000002297
12. Musazadeh, V, Dehghan, P, Saleh-Ghadimi, S, and Abbasalizad, FM. Omega 3-rich Camelina sativa oil in the context of a weight loss program improves glucose homeostasis, inflammation and oxidative stress in patients with NAFLD: a randomised placebo-controlled clinical trial. Int J Clin Pract. (2021) 75:e14744. doi: 10.1111/ijcp.14744
13. Farhangi, MA, Dehghan, P, Musazadeh, V, Kavyani, M, and Maleki, P. Effectiveness of omega-3 and prebiotics on adiponectin, leptin, liver enzymes lipid profile and anthropometric indices in patients with non-alcoholic fatty liver disease: a randomized controlled trial. J Funct Foods. (2022) 92:105074. doi: 10.1016/j.jff.2022.105074
14. Manoogian, ENC, Chow, LS, Taub, PR, Laferrere, B, and Panda, S. Time-restricted eating for the prevention and Management of Metabolic Diseases. Endocr Rev. (2022) 43:405–36. doi: 10.1210/endrev/bnab027
15. Sarri, KO, Tzanakis, NE, Linardakis, MK, Mamalakis, GD, and Kafatos, AG. Effects of Greek orthodox Christian Church fasting on serum lipids and obesity. BMC Public Health. (2003) 3:16. doi: 10.1186/1471-2458-3-16
16. Varady, KA, Cienfuegos, S, Ezpeleta, M, and Gabel, K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. (2022) 18:309–21. doi: 10.1038/s41574-022-00638-x
17. Park, J, Seo, YG, Paek, YJ, Song, HJ, Park, KH, and Noh, HM. Effect of alternate-day fasting on obesity and cardiometabolic risk: a systematic review and meta-analysis. Metabolism. (2020) 111:154336. doi: 10.1016/j.metabol.2020.154336
18. Patikorn, C, Roubal, K, Veettil, SK, Chandran, V, Pham, T, Lee, YY, et al. Intermittent fasting and obesity-related health outcomes an umbrella review of Meta-analyses of randomized clinical trials. JAMA Netw Open. (2021) 4:e2139558. doi: 10.1001/jamanetworkopen.2021.39558
19. Santos, HO, Genario, R, Tinsley, GM, Ribeiro, P, Carteri, RB, Coelho-Ravagnani, CF, et al. A scoping review of intermittent fasting, chronobiology, and metabolism. Am J Clin Nutr. (2022) 115:991–1004. doi: 10.1093/ajcn/nqab433
20. Carter, S, Clifton, PM, and Keogh, JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. (2018) 1:e180756. doi: 10.1001/jamanetworkopen.2018.0756
22. Stower, H. Intermittent fasting passes trial. Nat Med. (2020) 26:1170. doi: 10.1038/s41591-020-1029-7
23. Corley, BT, Carroll, RW, Hall, RM, Weatherall, M, Parry-Strong, A, and Krebs, JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med. (2018) 35:588–94. doi: 10.1111/dme.13595
24. Holmer, M, Lindqvist, C, Petersson, S, Moshtaghi-Svensson, J, Tillander, V, Brismar, TB, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep. (2021) 3:100256. doi: 10.1016/j.jhepr.2021.100256
25. Xie, Z, Sun, Y, Ye, Y, Hu, D, Zhang, H, He, Z, et al. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat Commun. (2022) 13:1003. doi: 10.1038/s41467-022-28662-5
26. Manoogian, ENC, Zadourian, A, Lo, HC, Gutierrez, NR, Shoghi, A, Rosander, A, et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: the healthy heroes randomized control trial. Cell Metab. (2022) 34:1442–1456.e7. doi: 10.1016/j.cmet.2022.08.018
27. Jamshed, H, Beyl, RA, Della, DL, Manna, ES, Yang, ER, and Peterson, CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. (2019) 11:1234. doi: 10.3390/nu11061234
28. Zouhal, H, Bagheri, R, Ashtary-Larky, D, Wong, A, Triki, R, Hackney, AC, et al. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol Behav. (2020) 225:113090. doi: 10.1016/j.physbeh.2020.113090
29. Zouhal, H, Bagheri, R, Triki, R, Saeidi, A, Wong, A, Hackney, AC, et al. Effects of Ramadan intermittent fasting on gut hormones and body composition in males with obesity. Int J Environ Res Public Health. (2020) 17:5600. doi: 10.3390/ijerph17155600
30. Badran, H, Elsabaawy, M, Sakr, A, Eltahawy, M, Elsayed, M, Elsabaawy, D, et al. Impact of intermittent fasting on laboratory, radiological, and anthropometric parameters in NAFLD patients. Clin Exp Hepatol. (2022) 8:118–24. doi: 10.5114/ceh.2022.115056
31. Aliasghari, F, Izadi, A, Gargari, BP, and Ebrahimi, S. The effects of 33. Ramadan fasting on body composition, blood pressure, glucose metabolism, and markers of inflammation in NAFLD patients: an observational trial. J Am Coll Nutr. (2017) 36:640–5. doi: 10.1080/07315724.2017.1339644
32. Caffa, I, Spagnolo, V, Vernieri, C, Valdemarin, F, Becherini, P, Wei, M, et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. (2020) 583:620–4. doi: 10.1038/s41586-020-2502-7
33. Zhong, Z, Zhang, H, Nan, K, Zhong, J, Wu, Q, Lu, L, et al. Fasting-mimicking diet drives antitumor immunity against colorectal Cancer by reducing IgA-producing cells. Cancer Res. (2023) 83:3529–43. doi: 10.1158/0008-5472.CAN-23-0323
34. Chen, JH, Lu, LW, Ge, Q, Feng, D, Yu, J, Liu, B, et al. Missing puzzle pieces of time-restricted-eating (TRE) as a long-term weight-loss strategy in overweight and obese people? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2023) 63:2331–47. doi: 10.1080/10408398.2021.1974335
35. Kang, J, Ratamess, NA, Faigenbaum, AD, Bush, JA, Beller, N, Vargas, A, et al. Effect of time-restricted feeding on anthropometric, metabolic, and fitness parameters: a systematic review. J Am Nutr Assoc. (2022) 41:810–25. doi: 10.1080/07315724.2021.1958719
36. Marjot, T, Tomlinson, JW, Hodson, L, and Ray, DW. Timing of energy intake and the therapeutic potential of intermittent fasting and time-restricted eating in NAFLD. Gut. (2023) 72:1607–19. doi: 10.1136/gutjnl-2023-329998
37. Moon, S, Kang, J, Kim, SH, Chung, HS, Kim, YJ, Yu, JM, et al. Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and Meta-analysis. Nutrients. (2020) 12:1267. doi: 10.3390/nu12051267
38. Pureza, I, Macena, ML, da Silva Junior, AE, Praxedes, DRS, Vasconcelos, LGL, and Bueno, NB. Effect of early time-restricted feeding on the metabolic profile of adults with excess weight: a systematic review with meta-analysis. Clin Nutr. (2021) 40:1788–99. doi: 10.1016/j.clnu.2020.10.031
39. Stanek, A, Brozyna-Tkaczyk, K, Zolghadri, S, Cholewka, A, and Myslinski, W. The role of intermittent energy restriction diet on metabolic profile and weight loss among obese adults. Nutrients. (2022) 14:1509. doi: 10.3390/nu14071509
40. Tippairote, T, Janssen, S, and Chunhabundit, R. Restoration of metabolic tempo through time-restricted eating (TRE) as the preventive measure for metabolic diseases. Crit Rev Food Sci Nutr. (2021) 61:2444–53. doi: 10.1080/10408398.2020.1781050
41. Termannsen, AD, Varming, A, van Elst, C, Bjerre, N, Nørgaard, O, Hempler, NF, et al. Feasibility of time-restricted eating in individuals with overweight, obesity, prediabetes, or type 2 diabetes: a systematic scoping review. Obesity. (2023) 31:1463–85. doi: 10.1002/oby.23743
42. Andriessen, C, Fealy, CE, Veelen, A, van Beek, SMM, Roumans, KHM, Connell, NJ, et al. Three weeks of time-restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: a randomised crossover trial. Diabetologia. (2022) 65:1710–20. doi: 10.1007/s00125-022-05752-z
43. Che, T, Yan, C, Tian, D, Zhang, X, Liu, X, and Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab (Lond). (2021) 18:88. doi: 10.1186/s12986-021-00613-9
44. Chair, SY, Cai, H, Cao, X, Qin, Y, Cheng, HY, and Ng, MT. Intermittent fasting in weight loss and Cardiometabolic risk reduction: a randomized controlled trial. J Nurs Res. (2022) 30:e185. doi: 10.1097/jnr.0000000000000469
45. Sutton, EF, Beyl, R, Early, KS, Cefalu, WT, Ravussin, E, and Peterson, CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. (2018) 27:1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010
46. Feyzioglu, BS, Guven, CM, and Avul, Z. Eight-hour time-restricted feeding: a Strong candidate diet protocol for first-line therapy in polycystic ovary syndrome. Nutrients. (2023) 15:2260. doi: 10.3390/nu15102260
47. Li, C, Xing, C, Zhang, J, Zhao, H, Shi, W, and He, B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. (2021) 19:148. doi: 10.1186/s12967-021-02817-2
48. Henderson, GV, Verma, S, Rakhesh, A, and Brose, SW. Time restricted eating facilitates weight loss and improves cardiometabolic profile in a female veteran with multiple sclerosis: a case report. J Spinal Cord Med. (2023) 46:525–7. doi: 10.1080/10790268.2022.2163136
49. Świątkiewicz, I, Mila-Kierzenkowska, C, Woźniak, A, Szewczyk-Golec, K, Nuszkiewicz, J, Wróblewska, J, et al. Pilot clinical trial of time-restricted eating in patients with metabolic syndrome. Nutrients. (2021) 13:346. doi: 10.3390/nu13020346
50. He, M, Wang, J, Liang, Q, Li, M, Guo, H, Wang, Y, et al. Time-restricted eating with or without low-carbohydrate diet reduces visceral fat and improves metabolic syndrome: a randomized trial. Cell Rep Med. (2022) 3:100777. doi: 10.1016/j.xcrm.2022.100777
51. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
52. Lin, X, Guan, Y, Wu, G, Huang, J, and Wang, S. Time-restricted eating for patients with diabetes and prediabetes: a systematic review. Front Nutr. (2022) 9:1025919. doi: 10.3389/fnut.2022.1025919
53. Zeng, X, Zhang, Y, Kwong, JS, Zhang, C, Li, S, Sun, F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
54. Mack, A, Hodge, A, Tuck, C, Tchongue, J, Holt, D, Sievert, W, et al. Non-alcoholic fatty liver disease intermittent fasting time intervention (NIFTI): fasting without calorie restriction improves hepatic transient elastography, visceral adiposity and insulin resistance compared to standard care. J Gastroenterol Hepatol. (2014) 29:68–101.
55. Kord-Varkaneh, H, Salehi-Sahlabadi, A, Tinsley, GM, Santos, HO, and Hekmatdoost, A. Effects of time-restricted feeding (16/8) combined with a low-sugar diet on the management of non-alcoholic fatty liver disease: a randomized controlled trial. Nutrition. (2023) 105:111847. doi: 10.1016/j.nut.2022.111847
56. Wei, X, Lin, B, Huang, Y, Yang, S, Huang, C, Shi, L, et al. Effects of time-restricted eating on nonalcoholic fatty liver disease: the TREATY-FLD randomized clinical trial. JAMA Netw Open. (2023) 6:e233513. doi: 10.1001/jamanetworkopen.2023.3513
57. Cai, H, Qin, Y-L, Shi, Z-Y, Chen, JH, Zeng, MJ, Zhou, W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. (2019) 19:219. doi: 10.1186/s12876-019-1132-8
58. de Cabo, R, and Mattson, MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. (2019) 381:2541–51. doi: 10.1056/NEJMra1905136
59. Targher, G, Byrne, CD, and Tilg, H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. (2020) 69:1691–705. doi: 10.1136/gutjnl-2020-320622
60. Yip, TC-F, Vilar-Gomez, E, Petta, S, Yilmaz, Y, Wong, GLH, Adams, LA, et al. Geographical similarity and differences in the burden and genetic predisposition of NAFLD. Hepatology. (2023) 77:1404–27. doi: 10.1002/hep.32774
61. Dufour, J-F, Anstee, QM, Bugianesi, E, Harrison, S, Loomba, R, Paradis, V, et al. Current therapies and new developments in NASH. Gut. (2022) 71:2123–34. doi: 10.1136/gutjnl-2021-326874
62. Younossi, ZM, Corey, KE, and Lim, JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the Management of Nonalcoholic Fatty Liver Disease: expert review. Gastroenterology. (2021) 160:912–8. doi: 10.1053/j.gastro.2020.11.051
63. Marjot, T, Ray, DW, and Tomlinson, JW. Is it time for chronopharmacology in NASH? J Hepatol. (2022) 76:1215–24. doi: 10.1016/j.jhep.2021.12.039
64. Saran, AR, Dave, S, and Zarrinpar, A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease. Gastroenterology. (2020) 158:1948-+. doi: 10.1053/j.gastro.2020.01.050
65. Stokkan, KA, Yamazaki, S, Tei, H, Sakaki, Y, and Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science. (2001) 291:490–3. doi: 10.1126/science.291.5503.490
66. Turek, FW, Joshu, C, Kohsaka, A, Lin, E, Ivanova, G, McDearmon, E, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. (2005) 308:1043–5. doi: 10.1126/science.1108750
67. Zeng, X, Xie, S, Jiang, F, Li, X, Li, M, Zhang, T, et al. Association between time-restricted eating and non-alcoholic fatty liver disease in a nationwide cross-sectional study. Br J Nutr. (2023) 130:1787–94. doi: 10.1017/S0007114523000818
68. Templeman, I, Gonzalez, JT, Thompson, D, and Betts, JA. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc Nutr Soc. (2020) 79:76–87. doi: 10.1017/S0029665119000636
69. Lowe, DA, Wu, N, Rohdin-Bibby, L, Moore, AH, Kelly, N, Liu, YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. (2020) 180:1491–9. doi: 10.1001/jamainternmed.2020.4153
70. Chaix, A, Manoogian, ENC, Melkani, GC, and Panda, S. Time-restricted eating to prevent and manage chronic metabolic diseases In: PJ Stover and R Balling, editors. Annual review of nutrition, vol. 39, 39. Palo Alto: Annual Reviews (2019). 291–315.
71. Hatori, M, Vollmers, C, Zarrinpar, A, DiTacchio, L, Bushong, EA, Gill, S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. (2012) 15:848–60. doi: 10.1016/j.cmet.2012.04.019
72. Petrenko, V, and Dibner, C. Circadian orchestration of insulin and glucagon release. Cell Cycle. (2017) 16:1141–2. doi: 10.1080/15384101.2017.1326768
73. Atshaves, BP, Martin, GG, Hostetler, HA, McIntosh, AL, Kier, AB, and Schroeder, F. Liver fatty acid-binding protein and obesity. J Nutr Biochem. (2010) 21:1015–32. doi: 10.1016/j.jnutbio.2010.01.005
74. Zeb, F, Osaili, T, Obaid, RS, Naja, F, Radwan, H, Cheikh Ismail, L, et al. Gut microbiota and time-restricted feeding/eating: a targeted biomarker and approach in precision nutrition. Nutrients. (2023) 15:259. doi: 10.3390/nu15020259
Keywords: nonalcoholic fatty liver disease, time-restricted eating, systematic review, efficacy, live parameters
Citation: Lin X, Wang S and Huang J (2024) The effects of time-restricted eating for patients with nonalcoholic fatty liver disease: a systematic review. Front. Nutr. 10:1307736. doi: 10.3389/fnut.2023.1307736
Edited by:
Valentini Konstantinidou, Dnanutricoach, GreeceReviewed by:
Vali Musazadeh, Tabriz University of Medical Sciences, IranStefan Kabisch, Charité University Medicine Berlin, Germany
Copyright © 2024 Lin, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuai Wang, ZHJ3YW5nc2h1YWlAemp1LmVkdS5jbg==; Jinyu Huang, ZHJodWFuZ2ppbnl1QDEyNi5jb20=
 Xiaoxiao Lin
Xiaoxiao Lin Shuai Wang
Shuai Wang Jinyu Huang
Jinyu Huang