- 1Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Clinical Nutrition, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: The intricate role of oxidative stress (OS) in colorectal cancer (CRC) initiation is underscored by an imbalance between pro-oxidants and antioxidants. Utilizing the Oxidative Balance Score (OBS) as a metric, this study aims to investigate the association between OS exposure and CRC risk, while also examining potential sex-specific differences in a large U.S. cohort.
Methods: The study included 98,395 adults from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. To construct the OBS, 14 dietary and lifestyle factors intricately associated with oxidative stress were quantified. A higher OBS value indicated a more favorable oxidative balance pattern or diminished OS exposure. Due to sex-specific differences in OBS, associations were evaluated separately for men and women based on Cox regression analysis. Subgroup analyses were conducted to elucidate potential modifiers.
Results: During 867,963.4 person-years of follow-up, 1,054 CRCs occurred. The mean (SD) age and OBS were 65.52 (5.73) years and 14.09 (3.95) points, respectively. In the fully adjusted Cox model, we observed an inverse association between OBS and CRC incidence in women (HRQ5vsQ1: 0.72; 95% CI: 0.52, 0.99; P for trend = 0.018) but not men. Subgroup analyses revealed the inverse association was more pronounced among women without versus with a family history of CRC (HRQ5 vsQ1: 0.66, 95% CI: 0.47–0.93; P for trend = 0.001; P for interaction = 0.001). The results remained robust after several sensitivity analyses.
Conclusion: Higher OBS was associated with lower CRC risk in women but not men; this inverse association was stronger among women without a family history of CRC. These findings suggest exposure to OS may confer sex-specific CRC risk effects, especially for women without a family history of CRC.
Introduction
Colorectal cancer (CRC) is a multifaceted disease that ranks third in new cancer cases yet second in cancer mortality. In 2020, over 1.9 million incident CRCs and 935,000 deaths were estimated globally (1). The majority of CRC malignancies arise sporadically, with modifiable lifestyle factors including obesity, physical inactivity, poor diet, alcohol use, and smoking constituting the primary environmental risk factors (2). Numerous investigations have demonstrated that dietary and lifestyle factors play a significant role in the development and progression of CRC, underscoring opportunities for prevention through diet or lifestyle modifications (3, 4). Research into diet and health has shown that nutrients rarely operate in isolation; rather, the combined effects of various dietary and lifestyle factors on CRC risk may be greater than any single element considered individually (5, 6).
Oxidative stress (OS), defined as an imbalance between pro-oxidants and antioxidants favoring the former, is the primary cause of reactive oxygen species and is hypothesized to be involved in colorectal carcinogenesis (7, 8). The oxidative balance score (OBS) allows assessment of an individual’s antioxidant status by accounting for both pro-oxidant and antioxidant components of dietary and lifestyle factors (9–11). As a key metric of cellular metabolism, OBS has been linked to several major human diseases related to health, including cardiovascular disease (9), diabetes (12, 13), and cancer (14). However, current evidence regarding the association between OBS and CRC risk remains inconclusive, with conflicting findings reported. One previous study in 80,063 participants found an increased risk of CRC associated with higher oxidative stress levels (15), while another study using the Health Professionals Follow-up Cohort showed no clear association between overall antioxidant capacity and CRC risk (16). Notably, the components comprising OBS differ between males and females; however, previous investigations have not considered potential sex differences (15). To provide additional epidemiological evidence clarifying these controversial associations while accounting for potential sex disparities, we performed a retrospective analysis stratified by sex in a large U.S. population.
Materials and methods
Study design and cohort
The present analysis utilized data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. The PLCO trial enrolled 154,887 participants aged 55–74 years at 10 U.S. centers from 1993 to 2001. The trial primarily aimed to evaluate whether screening could reduce mortality for the aforementioned cancers (17). Questionnaires completed by participants included a baseline questionnaire (BQ) capturing demographics and medical history, a dietary history questionnaire (DHQ) using a 137-item food frequency questionnaire (FFQ) to assess dietary intake, and a supplemental questionnaire (SQX). Prior studies have validated the FFQ as a nutritional evaluation tool (18, 19). The PLCO trial was approved by institutional review boards at National Cancer Institute (NCI) and participating centers, and all participants provided informed consent. Specific trial details are published elsewhere (17).
Our present study aimed to examine the association between OS exposure and CRC risk based on PLCO trial. OS exposure was assessed using the OBS, composed of 14 dietary and lifestyle factors closely related to OS (9). The outcome was incidence of CRC. Follow-up time was defined as the interval between completion of the dietary questionnaire and the date of CRC diagnosis, death, loss to follow-up, or end of follow-up (i.e., December 31, 2009) (Figure 1). Exclusion criteria eliminated the following participants: (I) unreturned BQ (n = 4,918); (II) invalid DHQ, defined as ≥ 8 missing responses, extreme caloric intake (gender-specific 1st and 99th percentiles), and DHQ completion date preceding death (n = 38,463); (III) personal cancer history before DHQ (n = 9,683); (IV) missing smoking status (n = 20); (V) outcome event between randomization and DHQ completion (n = 114); and (VI) potentially unreliable caloric intake (<800 or > 4,200 kcal/day for males; < 600 or > 3,500 kcal/day for females) (20) (n = 3,294). After applying exclusions, 98,395 participants remained eligible (Figure 2).
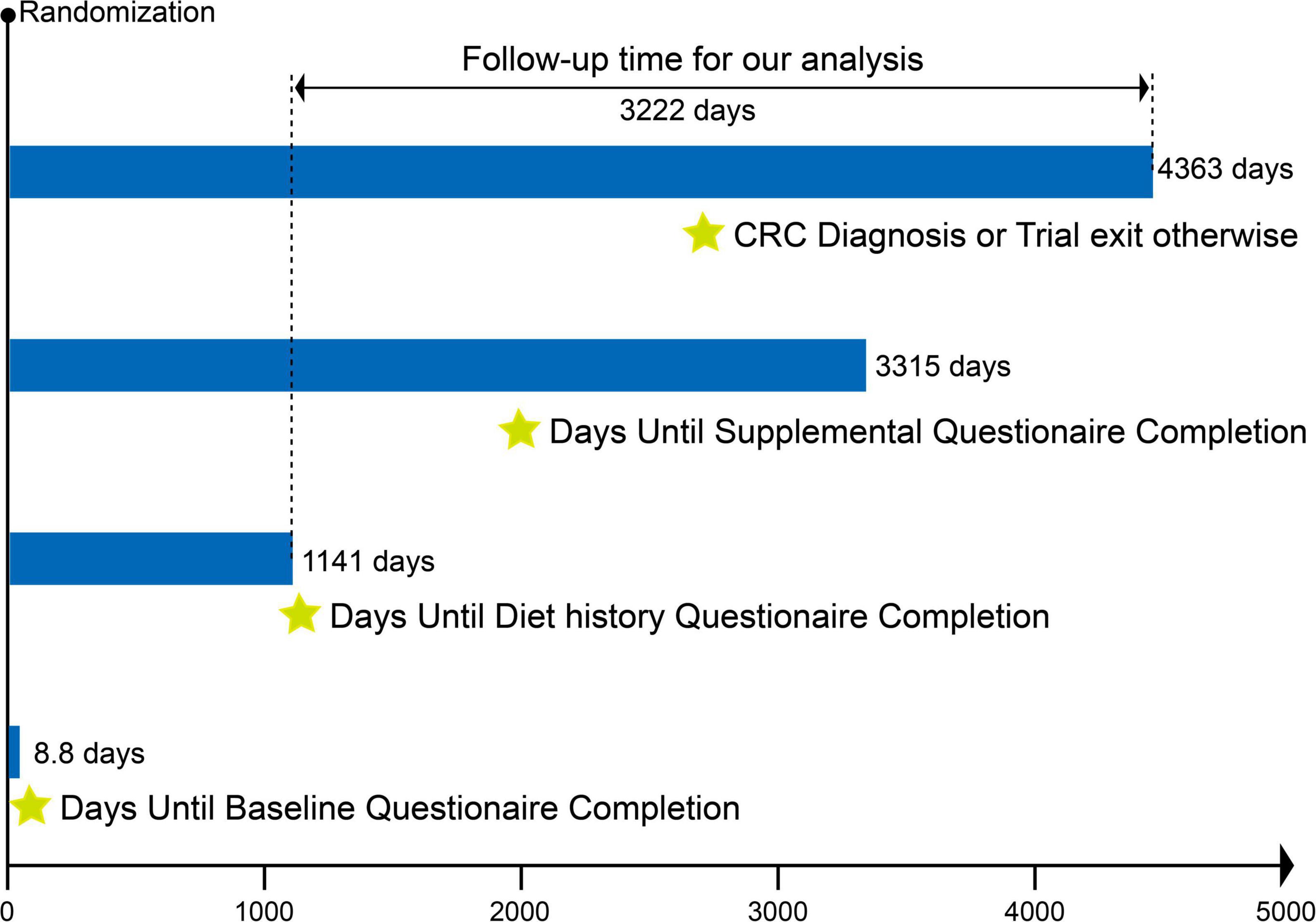
Figure 1. Timeline and follow-up scheme of the study. The mean time from randomization in the PLCO trial to completion of the baseline questionnaire, diet history questionnaire, and supplementary questionnaire was 8.8 days, 1,141 days, and 3,315 days, respectively. The follow-up period for our study was defined as the interval between completion of the diet history questionnaire and the date of CRC diagnosis, death, loss to follow-up, or end of follow-up, whichever came first. The mean follow-up time for our study was 3,222 days.
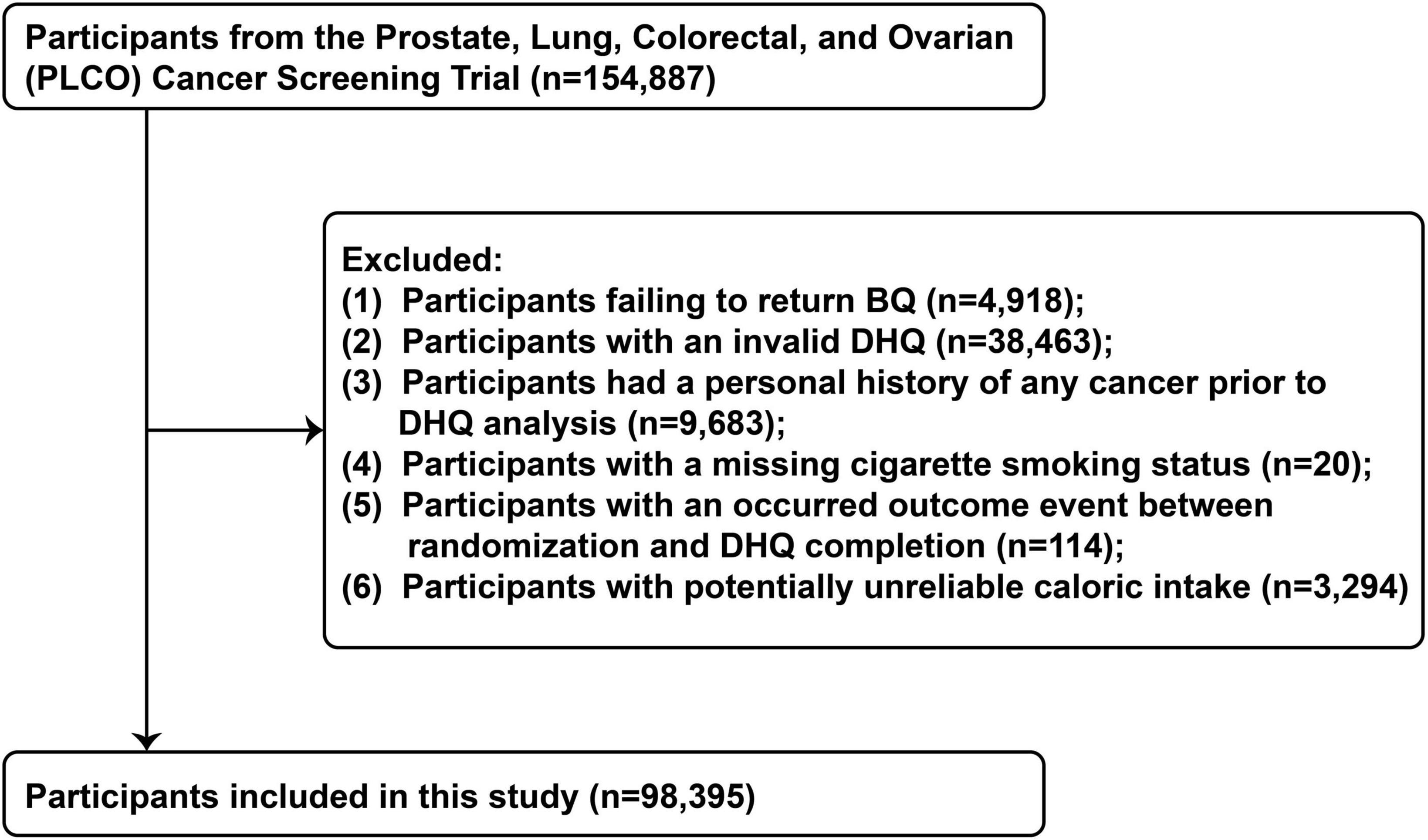
Figure 2. The flow chart of identifying eligible participants. PLCO, Prostate, Lung, Colorectal, and Ovarian; BQ, baseline questionnaire; DHQ, diet history questionnaire.
Assessment of OBS
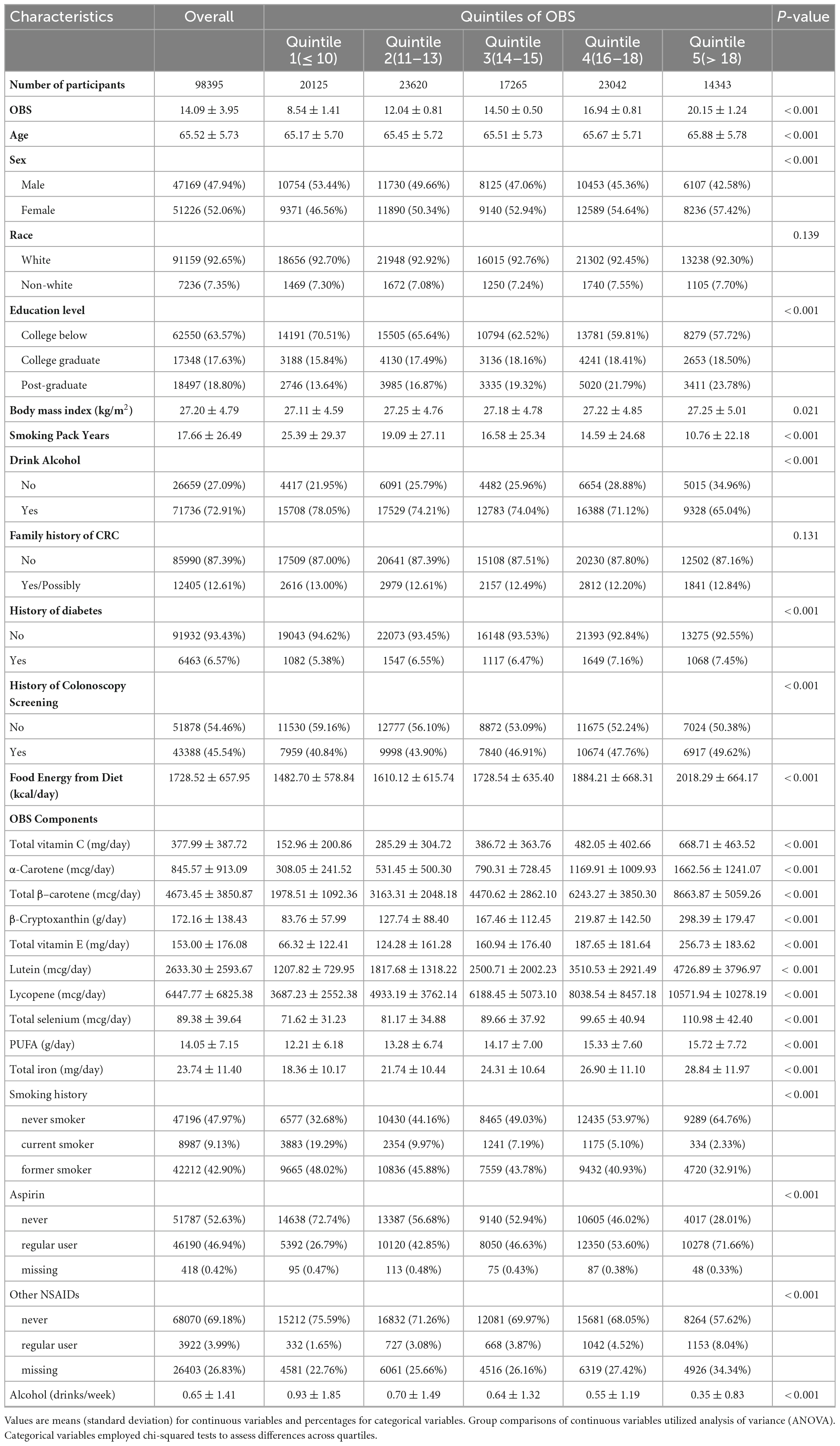
We calculated the OBS based on the computational method proposed by Lakkur et al. (9) in 2015. The OBS comprised 14 components selected based on their known associations with OS. In brief, we categorized continuous dietary factors related to antioxidant exposure according to sex-specific tertile cutpoints. Individuals with lower antioxidant exposure (first tertile) for particular dietary antioxidants (e.g., vitamin C, α-carotene, β–carotene, β-Cryptoxanthin, vitamin E, Lutein, Lycopene, selenium) received 0 points, while those with moderate (second tertile) or higher (third tertile) exposure were assigned 1 or 2 points, respectively. Similarly, we classified continuous dietary factors associated with pro-oxidant exposure (polyunsaturated fats and iron) into tertiles, awarding 2 points for low exposure, 1 point for moderate exposure, and 0 points for high exposure. Smoking status was coded as 2 points for never smokers, 1 point for former smokers, and 0 points for current smokers. For aspirin and other NSAIDs use, non-regular use received 0 points, missing responses received 1 point, and regular use received 2 points. Due to sex differences in alcohol intake, we used separate cutoffs to categorize alcohol intake for men versus women. For men, alcohol intake > 14 drinks/week was classified as heavy drinking (0 points), 1–14 drinks/week as moderate drinking (1 point), and no alcohol intake (2 points). For women, alcohol intake > 7 drinks/week was considered heavy drinking (0 points), 1–7 drinks/week as moderate drinking (1 point), and no alcohol intake (2 points). Summing the points for each component produced the total OBS (range 0–28), which we divided into quintiles. A higher OBS indicates a potentially favorable balance between pro-oxidants and antioxidants or lower OS exposure. Supplementary Table 1 summarizes the components comprising the OBS and displays the detailed scoring pattern.
Identification of CRC cases
In the PLCO trial, all participants were sent an annual health update questionnaire asking them to disclose any new cancer diagnoses, including the cancer site and diagnosis date. Research staff followed-up via phone or email with non-respondents to complete the questionnaire. Separately, death certificates and reports from family members were reviewed to capture additional cancer cases. For self-reported CRC cases, diagnostic verification involved retrieving the medical records to confirm the diagnosis and collect relevant clinical details about CRC. CRC cases were defined using the International Classification of Diseases for Oncology (ICD-O) codes for colon cancer (C18) and rectal cancer (C19–C20).
Assessment of covariates
Participant demographics, health behaviors, and medical history were obtained at baseline using self-administered questionnaires (including BQ, DHQ and SQX). Demographic variables included race and education level. Lifestyle factors encompassed smoking history and physical activity. Disease history covered family history of CRC, personal history of diabetes, colorectal polyps, colon-related comorbidities, diverticulitis/diverticulosis, and colonoscopy screening. Age at DHQ completion, drinking status and food energy from diet were assessed with the DHQ. Physical activity level was collected through the SQX and defined as the total minutes per week of self-reported moderate to vigorous physical activity. Body mass index (BMI) calculated as weight in kg divided by height squared in m2.
Statistical analysis
For categorical and continuous covariates missing < 5% of data, modal and median imputation was utilized, respectively. Due to a large proportion (up to 25.3%) of missing data, the physical activity level covariate was imputed using multiple imputation methods (21). Further details regarding the imputed datasets are provided in Supplementary Table 2. All statistical analyses were conducted using the imputed datasets.
We utilized Cox proportional hazards regression to evaluate the sex-specific association between the OBS and CRC risk, with follow-up time as the time metric. To test for linear trends, participants were assigned the median OBS value of their quintile, treated as a continuous predictor with the lowest quintile as reference. In the Cox regression analyses, two multivariable Cox models were constructed based on potential confounding variables. Model 1 adjusted for age and race. Model 2 further adjusted for education level, BMI, smoking pack-years, alcohol intake, food energy intake, physical activity level, family history of CRC, history of diabetes, colorectal polyps, colon-related comorbidities, diverticulitis/diverticulosis, and colonoscopy screening.
We conducted stratified analyses across categories of age, BMI, diabetes history, family history of CRC, smoking pack-years, alcohol use, physical activity level, energy intake, and colonoscopy screening history. For continuous subgroup variables, subgroups were defined by dichotomizing at the median based on clinical relevance. Interaction tests were conducted by incorporating OBS-by-subgroup product terms in multivariate Cox models, comparing models with and without the interaction terms.
We performed several sensitivity analyses to evaluate the robustness of the findings: (1) participants with a family history of CRC were excluded, as they may be predisposed to develop CRC. (2) participants with a history of diabetes were excluded, as they may have been required to follow stricter dietary control (22). (3) CRC cases diagnosed within the first 2 and 4 years of follow-up were excluded to minimize reverse causation.
All statistical analyses were performed using R software version 4.3.1. A two-tailed P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
The detailed baseline characteristics of the study population across OBS quintiles are presented in Table 1. Among the 98,395 included participants, the mean (standard deviation) OBS was 14.09 (3.95). Participants were categorized into quintiles based on OBS [Quintile 1 (OBS ≤ 10), n = 20,125; Quintile 2 (OBS 11–13), n = 23,620; Quintile 3 (OBS 14–15), n = 17,265; Quintile 4 (OBS 16–18), n = 23,042; Quintile 5 (OBS > 18), n = 14,343]. Higher quintiles indicated adherence to an antioxidant pattern or lower OS exposure. Compared to the lowest quintile (Quintile 1), those in the highest quintile (Quintile 5) were more likely to be female, have higher educational level, undergo colonoscopy screening, and have greater total energy intake. In the gender baseline characteristics (Supplementary Table 3), compared to males, females had lower BMI, smoking history and intensity, alcohol drinking history and intensity, aspirin use, and higher intakes of dietary antioxidants (e.g., vitamin C, α-carotene, β–carotene, β-cryptoxanthin, vitamin E, lutein). Females also had lower intakes of pro-oxidant dietary components (polyunsaturated fats and iron). Overall, females had higher OBS scores than males.
Association between OBS and CRC incidence
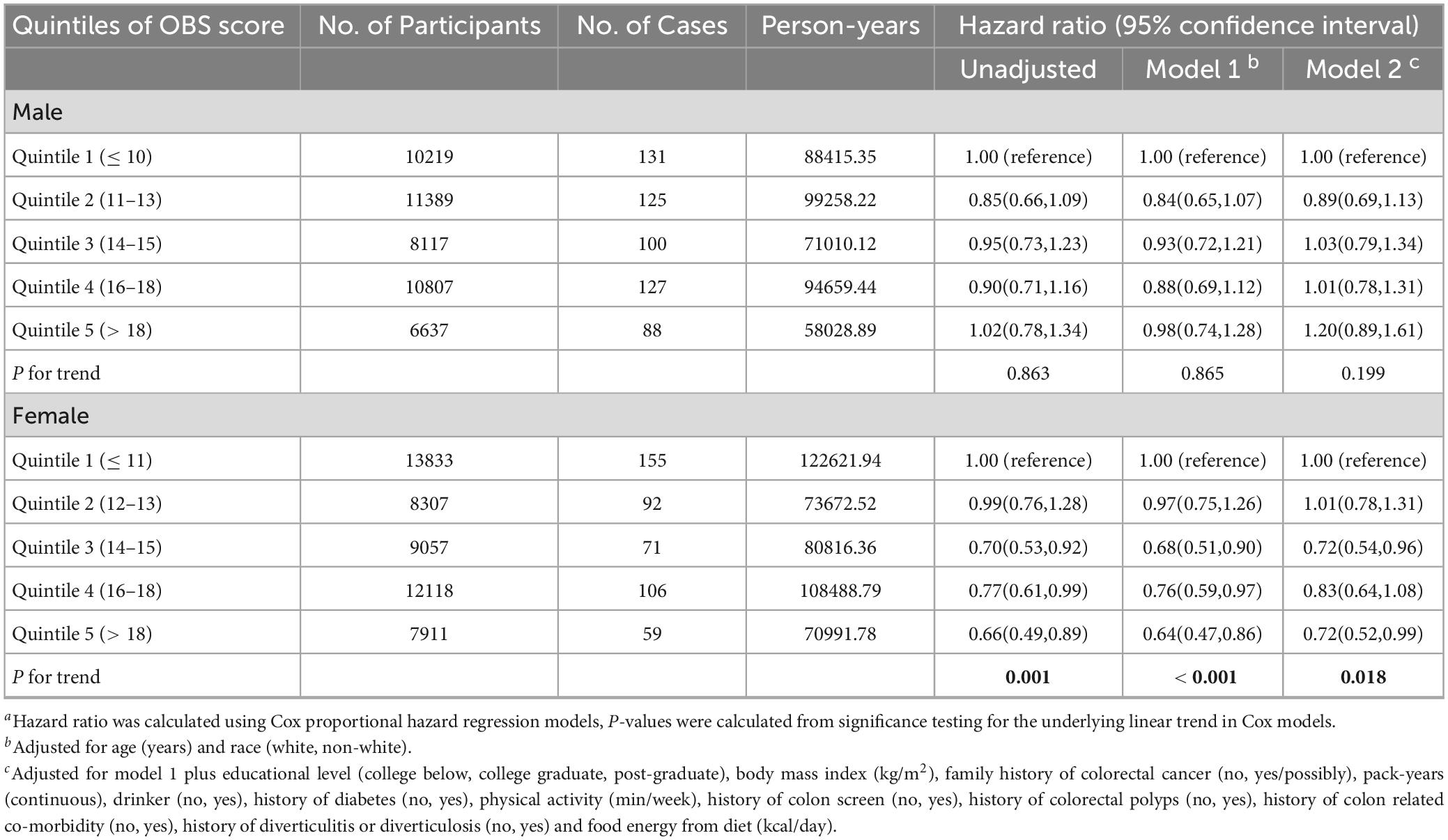
Over a mean (SD) follow-up time of 8.82 (1.95) years totaling 867,963.4 person-years, 1054 CRC cases (571 female and 483 male) were documented among 98,395 participants (51,226 women and 47,169 men), reflecting an overall CRC incidence rate of 12 cases per 10,000 person-years, with incidence rates of 10.6 and 13.9 cases per 10,000 person-years in women and men, respectively. Compared to women, the CRC incidence rate in men was 31.1% higher (95% CI: 30.1% to 32.2%). In this study, we identified an inverse association between OBS and CRC incidence in women but not men. In women, the unadjusted model showed a lower CRC risk for those in the highest OBS quintile compared to the lowest quintile (HRQ5vs.Q1: 0.66; 95% CI: 0.49, 0.89; P for trend = 0.001) (Table 2). This inverse association persisted after full adjustment for potential confounders (HRQ5vsQ1: 0.72; 95% CI: 0.52, 0.99; P for trend = 0.018) (Table 2). However, no significant association was observed in men in either the crude or fully adjusted models (Table 2).
Additional analyses
As shown in Table 3, subgroup analyses revealed no modification by age, BMI, history of diabetes, pack-years of smoking, drinking status, physical activity level, food energy from diet or history of colonoscopy screening on the OBS-CRC risk association in women (all P for interaction > 0.05). Interestingly, the inverse OBS-CRC risk association was more pronounced among women without a family history of CRC (HRQ5vsQ1: 0.66, 95% CI: 0.47–0.93; P for trend = 0.001), with a significant interaction by family history of CRC (P for interaction = 0.001). In sensitivity analyses excluding those with family history of CRC, history of diabetes, or early follow-up, the inverse OBS-CRC risk association persisted among women (Table 4).
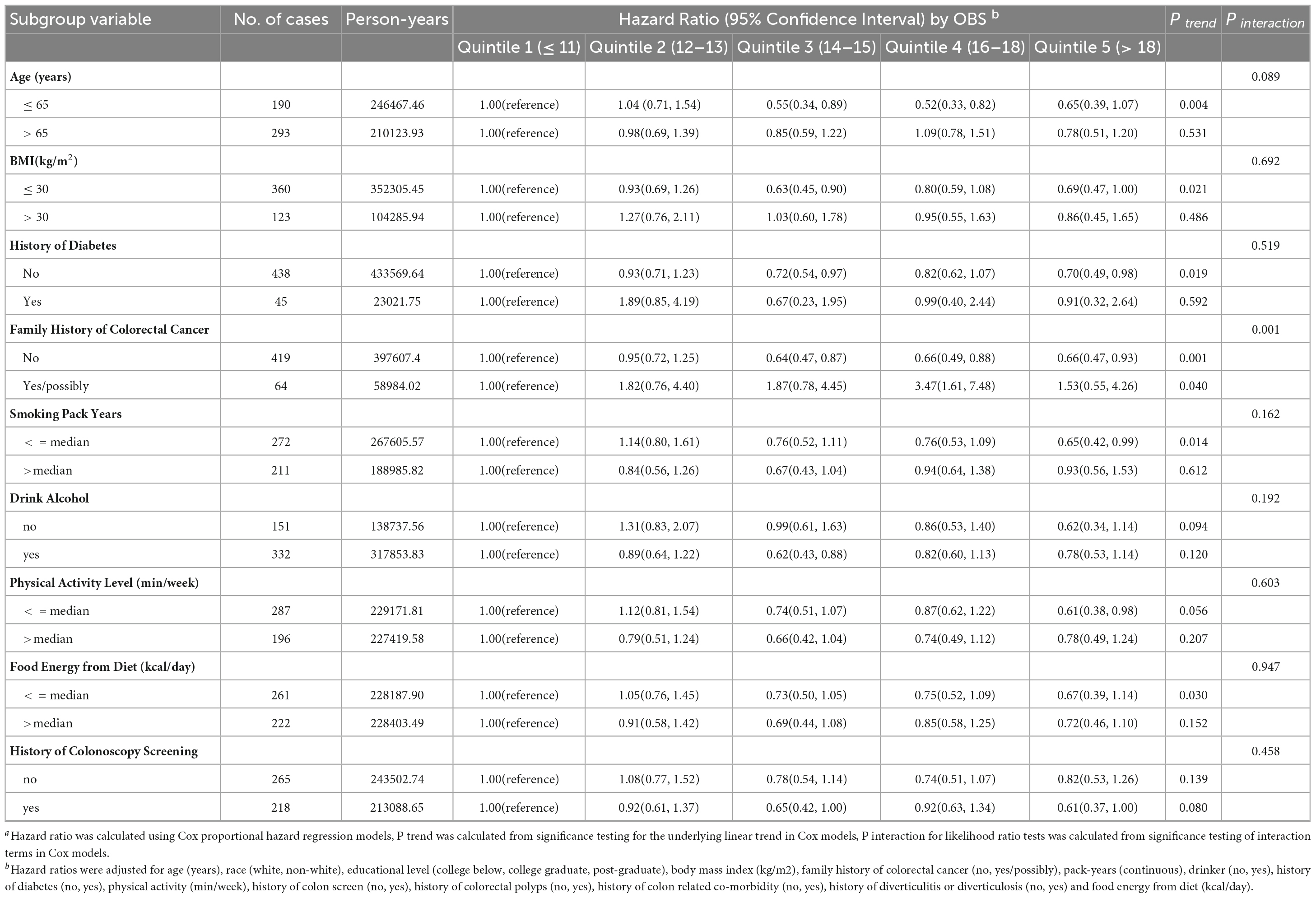
Table 3. Subgroup analyses on the association of OBS with the risk of colorectal cancer in femalesa.
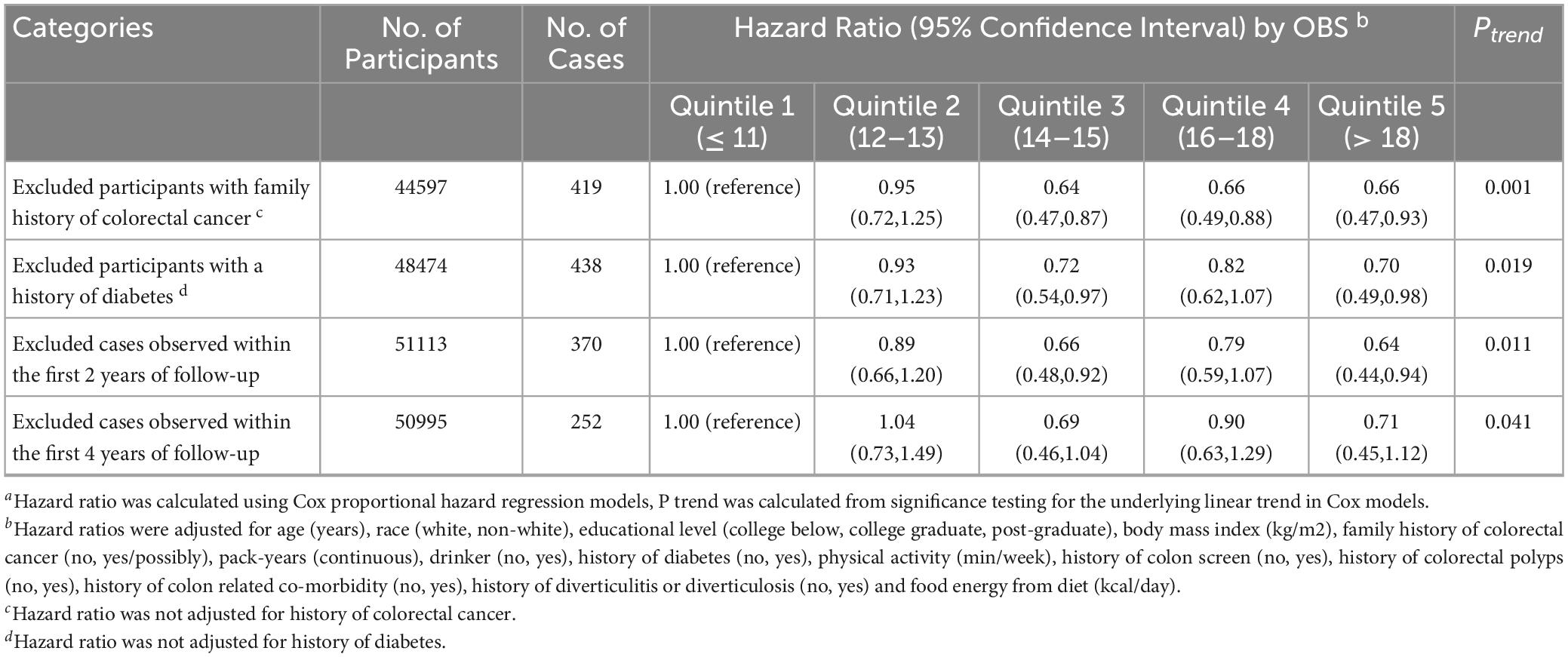
Table 4. Sensitivity analyses on the association of OBS with the risk of colorectal cancer in female a.
Discussion
In this study, higher OBS were associated with a lower risk of CRC in women. However, no significant association was observed in men. Subgroup analyses showed that the inverse association was stronger in women with no family history compared to those with a family history of CRC. The inverse association remained robust in sensitivity analyses excluding participants with potential confounding characteristics, lending strength to the conclusions.
CRC has been demonstrated to be closely related to dietary and lifestyle factors. For example, adherence to the Mediterranean diet (MD) and Dietary Approaches to Stop Hypertension (DASH) dietary patterns have been associated with lower incidence of colorectal cancer in several studies (23, 24). The OBS constructed in this study incorporated 14 dietary and lifestyle indicators with established links to OS exposures. While the OBS has been linked with several major chronic human diseases related to health, including cardiovascular disease (9), diabetes (12, 13), and cancer (14). It should be noted that although prior studies have explored OBS in relation to CRC (15), the overall linkage between OS exposure and CRC risk remains ambiguous with inconsistent literature findings (16). The pathogenesis of CRC is intimately connected with factors that heighten OS and impair antioxidant defenses. For instance, lifestyle factors of OBS like smoking and alcohol enlarge reactive oxygen species production, whereas reduced antioxidant enzyme activity and DNA repair capacity attenuate antioxidant protection (25, 26). Conversely, sufficient intakes of antioxidant nutrients such as vitamins E and carotenoids can remove excess reactive oxygen species, boost antioxidant enzyme activity, safeguard DNA from oxidative damage, and thus mitigate CRC occurrence (27–30). In addition, it has been demonstrated that reactive oxygen species (ROS) generated by OS can disrupt critical cellular functions by interacting with cellular macromolecules, including proteins, nucleic acids, and lipids (31). For instance, oxidative damage to DNA may result in base oxidation, single- and double-strand breaks, or the creation of non-basic sites (32). Furthermore, unrepaired oxidative DNA damage enhances the risk of mutagenesis. If these mutations occur in genes imperative for regulating cell growth, such as tumor suppressor genes and proto-oncogenes, they may engender CRC (33). The body’s response to injury of intestinal mucosal cells exposed to oxidative stress is inflammation. Repeated exposure to inflammatory sites can elicit chronic inflammation and activation of autoimmune processes (34). Inflammation instigates epigenetic alterations that promote colorectal carcinogenesis through increased production of growth factors and proinflammatory cytokines (35). Animal and clinical investigations have delineated the primary mechanism by which free radicals contribute to colorectal carcinogenesis; specifically, free radicals intercede in inflammation and carcinogenesis via the transcription factor NRF2 (36–38). Therefore, OBS as an integrative indicator of in vivo redox balance exhibits clear biological relevance to CRC risk, although the exact associations and gender differences warrant further investigation.
A unique finding of this study was the effect modification by sex on the association between OBS and CRC risk. The potential reasons may relate to the following points: (I) Several studies indicate lower NADPH oxidase activity and function in females, attributable to direct estrogen-mediated reduction of NADPH oxidase activity as well as lower expression of the essential assembly factor p47 and superoxide production, independent of estrogen effects, culminating in lower superoxide levels in females with lower oxidative stress (39–42). (II) Clinical and experimental studies have indicated that women have stronger antioxidant potential than men (43). This may be because estrogen has antioxidant qualities, making women less vulnerable to oxidative stress (44). (III) In our present analysis, the CRC incidence rate is lower in women and the gender baseline characteristics showed that relative to men, women often adopt healthier lifestyles, such as limited smoking and alcohol consumption, that may reduce oxidative damage and inflammation (45). Additionally, Supplementary Table 3 also showed that women had higher intakes of antioxidant nutrients and higher OBS score, which may minimize oxidative damage and preserve oxidative balance, thereby lowering CRC risk (46).
This study has several notable strengths. It was a well-designed observational study in a large population, and the extensive follow-up period of up to 8 years allowed sufficient time for outcome events to occur. Moreover, we extensively adjusted for potential demographic, lifestyle, and disease history confounders, thereby minimizing residual confounding of the observed associations. Importantly, we identified a gender difference in the association between OBS and colorectal cancer risk. Furthermore, the inverse association demonstrated good robustness across multiple sensitivity analyses.
Some limitations should be acknowledged. Firstly, multiple variants of the OBS scoring system have been developed in prior studies (11, 47, 48). The present study developed an OBS scoring system using the framework proposed by Lakkur et al. (9), which integrated 14 dietary and lifestyle factors into the score calculation. Given the controversial role of PUFAs, aspirin, and NSAIDs on OS (49, 50), we reconstructed the OBS score after removing these 3 components. This reconstructed score was associated with the occurrence of CRC in the unadjusted and demographic-adjusted models, but no statistically significant association was observed in the fully adjusted model (Supplementary Table 4). Therefore, caution must be taken when examining the relationship between OS and CRC, as differing OBS scoring systems may lead to disparate results. In addition, incorporating direct biomarkers of OS, such as markers of DNA damage or lipid peroxidation, could have provided more objective measures of OBS. Unfortunately, such biomarkers were not available in the database used for this analysis. Secondly, dietary data was solely gathered at the baseline. Any shifts in dietary habits during the follow-up period could introduce non-differential misclassification bias. Thirdly, our study cohort exclusively comprised American adults aged 55–74. Therefore, the generalizability of the conclusions remains subject to further investigation. Fourthly, as detailed genetic data and important blood markers (e.g., hormones and estrogen) were not available in the PLCO cohort, which limits our ability to explore the impacts of genetic predispositions, molecular subtypes, and familial predispositions on the association between OBS and CRC risk as well as its gender difference. This is an important limitation of our study. In future research, we will utilize data from databases with genomic information and biological blood markers (e.g., UK Biobank) to further explore these factors in OBS-related CRC development and distinguish the associations in different sex and CRC subtypes. Finally, while this large, observational study with lengthy follow-up identified an association between OBS and CRC in female, the lack of genomic data is a limitation to establish causal relationships through Mendelian randomization approaches.
Conclusion
In this study of U.S. adults, higher OBS were associated with lower CRC risk among women but not men. This suggests that adherence to an antioxidant diet and lifestyle pattern may aid in CRC prevention, particularly for women without a family history of CRC. Further research is warranted to confirm these findings and should consider potential sex-specific mechanisms.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://cdas.cancer.gov/.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the National Cancer Institute. The patients/participants provided their written informed consent to participate in the PLCO study.
Author contributions
LX: Conceptualization, Formal analysis, Resources, Visualization, Writing-original draft. BL: Writing-original draft, Data curation, Methodology. HG: Methodology, Funding acquisition, Writing-review and editing. ZX: Methodology, Writing-review and editing. YT: Methodology, Writing-review and editing. ZZ: Methodology, Writing-review and editing. YJ: Methodology, Writing-review and editing. LP: Methodology, Formal analysis, Funding acquisition, Writing-review and editing. HH: Writing-review and editing, Conceptualization, Supervision. YW: Conceptualization, Supervision, Writing-review and editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of the article. This work was supported by the Natural Science Foundation Project of Chongqing, Chongqing Science and Technology Commission, China [cstc2021jcyj-msxmX0153 (LP)], [cstc2021jcyj-msxmX0112 (YW)], and [CSTB2022NSCQ-MSX1005 (HG)], and Kuanren Talents Project of the Second Affiliated Hospital of Chongqing Medical University in China [kryc-yq-2110 (HG)].
Acknowledgments
We sincerely appreciate the PLCO study group and PLCO participants. This research has been conducted using the PLCO resource (https://cdas.cancer.gov/plco/) under application number PLCO-1296.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1284066/full#supplementary-material
Abbreviations
BMI, body mass index; BQ, baseline questionnaire; CIs, confidence intervals; CRC, colorectal cancer; DHQ, dietary history questionnaire; FFQ, food frequency questionnaire; HRs, hazard ratios; ICD-O, International Classification of Diseases for Oncology; NCI, national cancer institute; OBS, oxidative balance score; OS, oxidative stress; PLCO, prostate lung colorectal and ovarian; SD, standard deviation; SQX, supplemental questionnaire.
References
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
3. Zhou E, Rifkin S. Colorectal cancer and diet. Gastroenterol Clin North Am. (2021) 50:101–11. doi: 10.1016/j.gtc.2020.10.01
4. Botteri E, Peveri G, Berstad P, Bagnardi V, Chen S, Sandanger T, et al. Changes in lifestyle and risk of colorectal cancer in the european prospective investigation into cancer and nutrition. Am J Gastroenterol. (2023) 118:702–11. doi: 10.14309/ajg.0000000000002065
5. Slattery M, Boucher K, Caan B, Potter J, Ma K. Eating patterns and risk of colon cancer. Am J Epidemiol. (1998) 148:4–16. doi: 10.1093/aje/148.1.4-a
6. Huijbregts P, Feskens E, Rasanen L, Fidanza F, Nissinen A, Menotti A, et al. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and the Netherlands: longitudinal cohort study. BMJ. (1997) 315:13–7. doi: 10.1136/bmj.315.7099.13
7. Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. (1985) 311:617–31. doi: 10.1098/rstb.1985.0168
8. Basak D, Uddin M, Hancock J. The role of oxidative stress and its counteractive utility in Colorectal Cancer (CRC). Cancers. (2020) 12:3336. doi: 10.3390/cancers12113336
9. Lakkur S, Judd S, Bostick R, McClellan W, Flanders W, Stevens V, et al. Oxidative stress, inflammation, and markers of cardiovascular health. Atherosclerosis. (2015) 243:38–43. doi: 10.1016/j.atherosclerosis.2015.08.032
10. Ilori T, Sun Ro Y, Kong S, Gutierrez O, Ojo A, Judd S, et al. Oxidative balance score and chronic kidney disease. Am J Nephrol. (2015) 42:320–7. doi: 10.1159/000441623
11. Hernández-Ruiz Á, García-Villanova B, Guerra-Hernández E, Amiano P, Ruiz-Canela M, Molina-Montes E. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients. (2019) 11:774. doi: 10.3390/nu11040774
12. Wu C, Ren C, Song Y, Gao H, Pang X, Zhang L. Gender-specific effects of oxidative balance score on the prevalence of diabetes in the US population from NHANES. Front Endocrinol. (2023) 14:1148417. doi: 10.3389/fendo.2023.1148417
13. Kwon Y, Park H, Lee J. Inverse association between oxidative balance score and incident type 2 diabetes mellitus. Nutrients. (2023) 15:2497. doi: 10.3390/nu15112497
14. Lakkur S, Goodman M, Bostick R, Citronberg J, McClellan W, Flanders W, et al. Oxidative balance score and risk for incident prostate cancer in a prospective U.S. cohort study. Ann Epidemiol. (2014) 24:475–8.e4. doi: 10.1016/j.annepidem.2014.02.015
15. Dash C, Bostick R, Goodman M, Flanders W, Patel R, Shah R, et al. Oxidative balance scores and risk of incident colorectal cancer in a US Prospective Cohort Study. Am J Epidemiol. (2015) 181:584–94. doi: 10.1093/aje/kwu318
16. Mekary R, Wu K, Giovannucci E, Sampson L, Fuchs C, Spiegelman D, et al. Total antioxidant capacity intake and colorectal cancer risk in the Health Professionals Follow-up Study. Cancer Causes Control. (2010) 21:1315–21. doi: 10.1007/s10552-010-9559-9
17. Prorok P, Andriole G, Bresalier R, Buys S, Chia D, David Crawford E, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. (2000) 21:273S–309S. doi: 10.1016/S0197-2456(00)00098-2
18. Thompson F, Kipnis V, Midthune D, Freedman L, Carroll R, Subar A, et al. Performance of a food-frequency questionnaire in the US NIH–AARP (National Institutes of Health–American Association of Retired Persons) Diet and Health Study. Public Health Nutr. (2008) 11:183–95. doi: 10.1017/S1368980007000419
19. Subar A, Thompson F, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the block, willett, and national cancer institute food frequency questionnaires. Am J Epidemiol. (2001) 154:1089–99. doi: 10.1093/aje/154.12.1089
20. Shan Z, Guo Y, Hu F, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. (2020) 180:513. doi: 10.1001/jamainternmed.2019.6980
21. Brancato G, Pezzotti P, Rapiti E, Perucci C, Abeni D, Babbalacchio A, et al. Multiple imputation method for estimating incidence of HIV infection. The Multicenter Prospective HIV Study. Int J Epidemiol. (1997) 26:1107–14. doi: 10.1093/ije/26.5.1107
22. Aas A, Axelsen M, Churuangsuk C, Hermansen K, Kendall C, Kahleova H, et al. Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. (2023) 66:965–85. doi: 10.1007/s00125-023-05894-8
23. Mahmod A, Haif S, Kamal A, Al-ataby I, Talib W. Chemoprevention effect of the Mediterranean diet on colorectal cancer: current studies and future prospects. Front Nutr. (2022) 9:924192. doi: 10.3389/fnut.2022.924192
24. Tangestani H, Salari-Moghaddam A, Ghalandari H, Emamat H. Adherence to the Dietary Approaches to Stop Hypertension (DASH) dietary pattern reduces the risk of colorectal cancer: a systematic review and meta-analysis. Clin Nutr. (2020) 39:2975–81. doi: 10.1016/j.clnu.2020.02.002
25. Wu D, Cederbaum A. Alcohol, oxidative stress, and free radical damage. Oxid Stress. (2003) 27:277–84.
26. Barreiro E, Peinado V, Galdiz J, Ferrer E, Marin-Corral J, Sánchez F, et al. Cigarette smoke–induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med. (2010) 182:477–88. doi: 10.1164/rccm.200908-1220OC
27. Valko M, Leibfritz D, Moncol J, Cronin M, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
28. Burton G, Traber M. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr. (1990) 10:357–82. doi: 10.1146/annurev.nu.10.070190.002041
29. Chaudière J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. (1999) 37:949–62. doi: 10.1016/S0278-6915(99)00090-3
30. Mortensen A, Skibsted L, Truscott T. The interaction of dietary carotenoids with radical species. Arch Biochem Biophys. (2001) 385:13–9. doi: 10.1006/abbi.2000.2172
31. Caliri A, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Mutat Res. (2021) 787:108365. doi: 10.1016/j.mrrev.2021.108365
32. Hegde M, Izumi T, Mitra S. Oxidized base damage and single-strand break repair in mammalian genomes: role of disordered regions and posttranslational modifications in early enzymes. Prog Mol Biol Transl Sci. (2012) 110:123–53. doi: 10.1016/B978-0-12-387665-2.00006-7
33. Poetsch A. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput Struct Biotechnol J. (2020) 18:207–19. doi: 10.1016/j.csbj.2019.12.013
34. Lakatos P, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. (2008) 14:3937. doi: 10.3748/wjg.14.3937
35. Schmitt M, Greten F. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. (2021) 21:653–67. doi: 10.1038/s41577-021-00534-x
36. He F, Antonucci L, Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. (2020) 41:405–16. doi: 10.1093/carcin/bgaa039
37. He F, Antonucci L, Yamachika S, Zhang Z, Taniguchi K, Umemura A, et al. NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly. J Hepatol. (2020) 72:1182–95. doi: 10.1016/j.jhep.2020.01.023
38. He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. (2020) 21:4777. doi: 10.3390/ijms21134777
39. Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arteriosc Thromb Vasc Biol. (2002) 22:438–42. doi: 10.1161/hq0302.104515
40. Niveditha S, Deepashree S, Ramesh S, Shivanandappa T. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J Comp Physiol B. (2017) 187:899–909. doi: 10.1007/s00360-017-1061-1
41. Borrás C, Sastre J, García-Sala D, Lloret A, Pallardó F, Viña J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. (2003) 34:546–52. doi: 10.1016/S0891-5849(02)01356-4
42. Miller A, Drummond G, Mast A, Schmidt H, Sobey C. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. (2007) 38:2142–9. doi: 10.1161/STROKEAHA.106.477406
43. Mendoza-Núñez V, Beristain-Pérez A, Pérez-Vera S, Altamirano-Lozano M. Age-related sex differences in glutathione peroxidase and oxidative DNA damage in a healthy Mexican population. J Womens Health. (2010) 19:919–26. doi: 10.1089/jwh.2009.1684
44. Kendall B, Eston R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med. (2002) 32:103–23. doi: 10.2165/00007256-200232020-00003
45. Chang S, Chang Y, Wu L. Gender differences in lifestyle and risk factors of metabolic syndrome: do women have better health habits than men? J Clin Nurs. (2019) 28:2225–34. doi: 10.1111/jocn.14824
46. Bacchetti T, Turco I, Urbano A, Morresi C, Ferretti G. Relationship of fruit and vegetable intake to dietary antioxidant capacity and markers of oxidative stress: a sex-related study. Nutrition. (2019) 61:164–72. doi: 10.1016/j.nut.2018.10.034
47. Van Hoydonck P, Temme E, Schouten E. A dietary oxidative balance score of vitamin C, beta-carotene and iron intakes and mortality risk in male smoking Belgians. J Nutr. (2002) 132:756–61. doi: 10.1093/jn/132.4.756
48. Hernández-Ruiz Á, García-Villanova B, Guerra-Hernández E, Carrión-García C, Amiano P, Sánchez M, et al. Oxidative Balance Scores (OBSs) integrating nutrient, food and lifestyle dimensions: development of the NutrientL-OBS and FoodL-OBS. Antioxidants. (2022) 11:300. doi: 10.3390/antiox11020300
49. Stone W, Krishnan K, Campbell S, Palau V. The role of antioxidants and pro-oxidants in colon cancer. World J Gastrointest Oncol. (2014) 6:55–66. doi: 10.4251/wjgo.v6.i3.55
Keywords: oxidative stress exposure, colorectal cancer, epidemiology, sex-specific cohort studies, oxidative balance score
Citation: Gu H, Li B, Xiang L, Xu Z, Tang Y, Zhu Z, Jiang Y, Peng L, He H and Wang Y (2023) Association between oxidative stress exposure and colorectal cancer risk in 98,395 participants: results from a prospective study. Front. Nutr. 10:1284066. doi: 10.3389/fnut.2023.1284066
Received: 27 August 2023; Accepted: 20 November 2023;
Published: 15 December 2023.
Edited by:
Esther Molina-Montes, University of Granada, SpainReviewed by:
Raees Tonse, Baptist Hospital of Miami, United StatesDenisse Castro-Eguiluz, National Council of Science and Technology (CONACYT), Mexico
Venkataraghavan Ramamoorthy, Baptist Health South Florida, United States
Copyright © 2023 Gu, Li, Xiang, Xu, Tang, Zhu, Jiang, Peng, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei He, MTA2MTk1ODI5N0BxcS5jb20=; Yaxu Wang, MzAwODk3QGhvc3BpdGFsLmNxbXUuZWR1LmNu
†These authors have contributed equally to this work
 Haitao Gu1†
Haitao Gu1† Bo Li
Bo Li Ling Xiang
Ling Xiang Linglong Peng
Linglong Peng Yaxu Wang
Yaxu Wang