
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 21 December 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1263804
This article is part of the Research Topic Body Composition and Cardiovascular Health View all 6 articles
 Jian Huang1
Jian Huang1 Yubo Xie2,3*
Yubo Xie2,3*Background: Basal metabolic rate (BMR) is the minimum amount of energy needed by the body to carry out essential physiological functions. The goal of this study was to evaluate whether BMR causally influences venous thromboembolism (VTE) and its subtypes in European individuals.
Methods: A two-sample Mendelian randomization (MR) was performed. Within a genome-wide association study (GWAS) involving 454,874 people, genetic variants were chosen as instrumental variables based on their significant associations (p < 5 × 10−8) with BMR and their limited linkage disequilibrium (r2 < 0.001). The FinnGen project served as sources for summary statistics of VTE, encompassing different subtypes.
Results: Using the multiplicative random-effect inverse variance weighted method, our investigation revealed that one standard deviation higher BMR was associated with VTE (odds ratio [OR] = 1.684, 95% confidence interval [CI]: 1.465–1.936, p = 2.339 × 10−13), PE (OR = 1.824, 95% CI: 1.512–2.200, p = 3.399 × 10−10), and DVT of lower extremities (OR = 1.887, 95% CI: 1.562–2.280, p = 4.778 × 10−11). The consistency of these associations was observed in sensitivity analyses using various MR techniques like Mendelian randomization pleiotropy residual sum and outlier, MR-Egger, weighted median, and contamination mixture method. In addition, multivariable MR revealed direct effects of BMR on VTE and its subtypes when taking body mass index and current tobacco smoking into account.
Conclusion: Higher BMR may increase the risk of VTE and its subtypes including PE and DVT of lower extremities.
Venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is the third most prevalent cardiovascular disorder and represents a significant public health challenge worldwide (1). With a yearly incidence of 1–2 cases per 1,000 adults, the global burden of VTE has been on a steady rise in recent years (2). Through epidemiologic studies, many risk factors have been identified as being related to VTE, such as advanced age, inflammation, cancer, major surgery, and trauma (2). Additionally, VTE risk can be influenced by several lifestyle factors such as smoking and body mass index (BMI).
Basal metabolic rate (BMR) is the minimum energy the body needs to perform basic physiological functions including breathing, circulation, heart rate, cellular growth, and brain function when at rest. Major factors affecting an individual’s BMR include age, genetics, body weight, environmental temperature, and health status (3). So far, limited studies assessed the association between BMR and cardiovascular diseases. The thickening of myointimal layer was observed in high-BMR mice compared with their counterparts (4). On the other hand, in humans, the level of BMR was found to be correlated with cardiovascular diseases such as aortic aneurysm, atrial fibrillation, and heart failure (5–8). Moreover, it is known that there is a close link between BMR and proinflammatory status, a critical promoter for VTE (9). These lines of evidence raise the question of whether there is a relationship between BMR and VTE. However, the unethical nature of attempting to control an individual’s basal metabolic rate and the long interval between exposure and outcome make it difficult to conduct randomized controlled trials (RCTs).
Mendelian randomization (MR) is a research technique using genetic information to study causal associations between phenotypes. It provides a means for investigating causality in the absence of RCTs, while also addressing key limitations of observational studies such as small sample sizes, unmeasured confounding, and reverse causation (10). We aimed to perform a two-sample MR to estimate whether genetically predicted BMR was causally associated with VTE and its subtypes (PE and DVT of lower extremities). As far as we know, this is the inaugural MR investigation focused on this matter.
Three fundamental assumptions underpinned the MR study: (1) the SNPs possess a close relationship with BMR, (2) the SNPs lack any association with confounders, and (3) the SNPs act on VTE solely via BMR. The research methodology is depicted in Figure 1. The STROBE-MR checklist was followed in reporting our methods and findings (Supplementary Table 1). Both the exposure and outcome summary statistics are available from the IEU GWAS database1 (11). Through a GWAS conducted by the University of Bristol’s MRC-IEU consortium (12), instrumental SNPs for BMR were obtained from up to 454,874 European individuals. BMR was estimated by impedance measurement at assessment centres of the UK Biobank. Kilojoules (kJ) were the units used to measure BMR. According to UK biobank data,2 the mean BMR level was 6597.38 kJ (SD 1357.83 kJ). Data concerning the associations of BMR-associated SNPs with VTE overall or its subtypes were generated by FinnGen (13). The cases and controls were restricted to those of European ancestry. Specifically, these datasets included 9,176 cases and 209,616 controls for VTE, 4,185 cases and 214,228 controls for PE, and 4,576 cases and 190,028 controls for DVT of lower extremities. The relevant information on the GWAS for BMR and VTE are summarized in Supplementary Table 2.
The original GWAS received ethical approval from their respective ethics committees, and prior to their involvement, each participant gave their written consent. Our analysis proceeded without the need for additional approval from the institutional review board, as only publicly available summary statistics were analyzed.
Instrumental variables linked to BMR were extracted based on a rigorous significance threshold of 5 × 10−8. The TwoSampleMR package’s clump function was utilized to evaluate the extracted SNPs’ linkage disequilibrium (LD) (11), with data from the 1,000 Genomes LD reference panel comprising solely European participants. Using a 10,000 kb window and restricting pairwise LD r2 to <0.001 allowed us to ensure the independence of instrumental variables. Variant harmonization involved aligning betas from diverse GWAS examining the same effect allele. If it was unable to find a genetic variant within the outcome GWAS data, we chose proxy variants linked by LD with a minimum r2 value of 0.8 (14). Quantification of instrument strength involved the use of the F-statistic, while r-squared served to quantify the variance explained (15).
The main approach was the inverse variance weighted method (multiplicative random-effect). This involved meta-analyzing the Wald estimates computed for each genetic variant’s effect, which were obtained as the ratio between the genetic variant’s associations with VTE and BMR. Under the premise of the inverse variance weighted method, it is expected that every instrument is deemed valid (16). The findings were presented as the odds ratio (OR) of VTE risk for a one standard deviation (SD) increase in genetically predicted BMR along with its respective 95% confidence interval (CI). Moreover, more robust techniques, comprising Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO), weighted median, MR-Egger, and contamination mixture method were utilized to overcome deviations from assumptions of MR arising owing to pleiotropy (17–21). The presence of pleiotropy can be inferred if the MR-Egger intercept value significantly deviates from zero. The existence of pleiotropy was also estimated applying MR-PRESSO which involves the exclusion of outlying SNPs from the instruments and re-assessment of effect estimates. Through the application of Cochran’s Q-statistic, the disparities in effects between instruments were examined. BMI and smoking are two important risk factors for VTE. To account for their possible effects modifying the causality between BMR and VTE, multivariable MR was performed as a supplementary analysis using the mv_multiple () function of the TwoSampleMR package. The meta-analysis conducted by Yengo and colleagues, which involved a total of 681,275 European individuals, yielded summary statistics pertaining to BMI (22). In regards to current tobacco smoking, we employed the results of a GWAS executed by the MRC-IEU consortium involving 462,434 European individuals (12). Supplementary Table 3 presents the relevant information on these datasets. Finally, the individual contributions of the instrumental SNPs to the IVW MR assessments were assessed using a leave-one-out analysis.
R software version 4.1.0 were employed to conduct all analyses, with the analysis involving the utilization of TwoSampleMR,3 MendelianRandomization,4 and MR-PRESSO5 packages (default settings). Taking into consideration multiple testing, a significance threshold of p < 0.017 (0.05/3) was established for the IVW after applying the Bonferroni correction. By following the methodology proposed by Brion et al. (23), the statistical power was determined.
Descriptive information on the instruments for BMR and their association with VTE and its subtypes is presented in Supplementary Tables 4–6. The particular features of these instrumental SNPs included the value of beta, standard error (SE), effect allele, effect allele frequency, other allele, F-statistic, etc. The F-statistics for each instrumental variable were observed to be higher than 16, surpassing the conventional limit of >10, which indicated satisfactory instrumental strength.
With regards to the primary MR analysis, our study had 80% power to detect the smallest OR ranging from 1.160 to 1.234 for the effects of genetically predicted BMR on VTE and its subtypes (Supplementary Table 7).
The IVW MR analyses demonstrated that one standard deviation (SD) increase in genetically predicted BMR was associated with an increased risk of VTE (OR = 1.684, 95% CI: 1.465–1.936, p = 2.339 × 10−13), PE (OR = 1.824, 95% CI: 1.512–2.200, p = 3.399 × 10−10), and DVT of lower extremities (OR = 1.887, 95% CI: 1.562–2.280, p = 4.778 × 10−11) (Figure 2 and Table 1). A leave-one-out analysis using the IVW method found that no individual instrumental SNPs significantly influenced the MR estimates (Supplementary Tables 8–10).
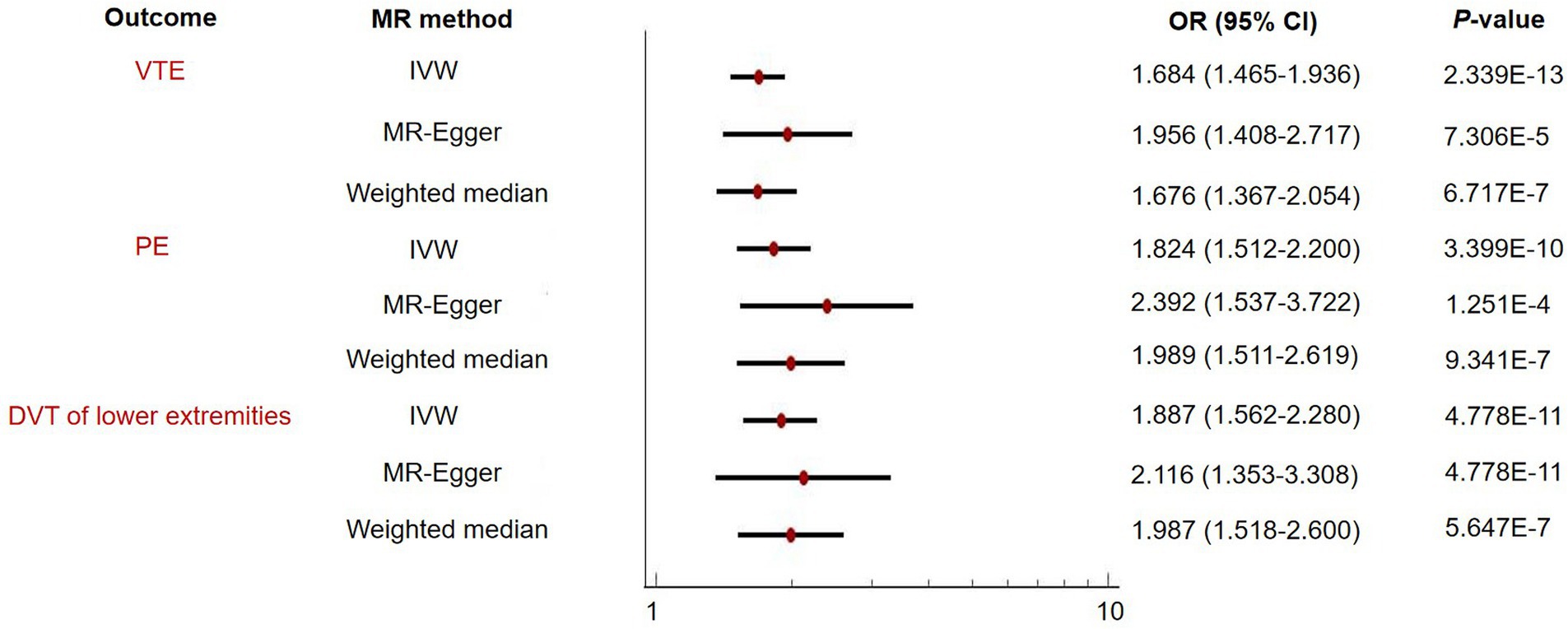
Figure 2. Two-sample Mendelian randomization (MR) analysis evaluating the relationship of basal metabolic rate with venous thromboembolism (VTE) and its subtypes including pulmonary embolism (PE) and deep vein thrombosis (DVT) of lower extremities.
The MR-Egger and weighted median sensitivity analyses validated the IVW analyses, confirming the consistency of the results (Figure 2 and Table 1). We then proceeded to use MR-PRESSO to identify and exclude any possible instrumental variable outliers. Our MR-PRESSO outlier corrected method found genetically predicted BMR causally associated with VTE (OR = 1.669, 95% CI: 1.455–1.914, p = 1.656 × 10−12, removal of one SNP), PE (OR = 1.822, 95% CI: 1.516–2.190, p = 3.524 × 10−10, removal of two SNPs), and DVT of lower extremities (OR = 1.827, 95% CI: 1.515–2.204, p = 6.789 × 10−10, removal of two SNPs) (Figure 2 and Table 1), further supporting the IVW analyses. Additionally, the contamination mixture method was utilized for sensitivity analysis, allowing us to conduct MR efficiently and robustly even in the presence of invalid instrumental SNPs (20). The results also suggested that BMR was causally associated with VTE (OR = 1.572, 95% CI: 1.294–1.908, p = 1.53 × 10−5), PE (OR = 1.706, 95% CI: 1.378–2.095, p = 1.59 × 10−5), and DVT of lower extremities (OR = 2.132, 95% CI: 1.679–2.708, p = 3.08 × 10−8). Heterogeneity was observed (p-value<0.001) in our analysis; however, the MR-Egger intercept did not reveal any signs of directional pleiotropy (Table 2).
BMI and smoking are recognized as significant contributing factors to VTE. To account for the influence of BMI and current tobacco smoking, we employed multivariable MR analysis in our study. Multivariable MR analysis demonstrated independent positive effects of BMR on VTE, PE, and DVT of lower extremities (Table 3).
Finally, we evaluated the causal effects of VTE and its subtypes on BMR. The number of instrument SNPs predicting VTE, PE and DVT of lower extremities were 13, 6, and 9, respectively. Supplementary Table 11 shows the characteristics of these instrument SNPs. Our MR results did not uncover any indications of reverse causation (Supplementary Table 12). No evidence of pleiotropy was revealed using the MR-Egger intercepts (Supplementary Table 12).
Utilizing GWAS summary-level data, we conducted a MR analysis to investigate the causal relationship between genetically predicted BMR and venous thromboembolism (VTE), including its subtypes. Our results were supportive of BMR having positive effects on VTE, PE, and DVT of lower extremities. A series of sensitivity analyses reinforced the validity of the results. As far as we know, the current analysis represents the inaugural exploration of the causal relationship between BMR and VTE, providing novel insights into the predisposition to VTE.
Our findings coincided with the outcomes of prior animal studies, observational investigations and an MR research, all of which indicated a significant relationship between BMR and cardiovascular diseases (4–8, 24). In their research using a mouse model, Sawicka et al. observed that mice with a high BMR displayed a notably thicker middle layer of the aorta in comparison to those with a low BMR; this change contributed to arterial stiffness and the development of cardiovascular diseases (4). In addition, some research groups revealed an association between BMR and human cardiovascular diseases, including heart failure, aortic aneurysm, and atrial fibrillation, through a cross-sectional study design (5–8). A recent MR investigation performed by Li and colleagues reported a causal link between BMR and common cardiovascular diseases including atrial fibrillation and flutter, myocardial infarction, heart failure, and aortic aneurysm (24). Our research provided important supplementary evidence to the existing relationship between BMR and cardiovascular diseases. It confirmed that in addition to the above-mentioned cardiovascular diseases, BMR was also a risk factor for VTE and its subtypes.
An elevated risk of VTE may be attributed to some potential mechanisms associated with an increased BMR. Firstly, reactive oxygen species (ROS) are produced as a detrimental byproduct during cellular metabolism. Elevated BMR can be indicative of a high intrinsic metabolic activity, potentially resulting in an augmented production of ROS. Excessive ROS production can directly impair endothelial cell function by disrupting normal endothelial cell signaling and gene expression and damaging cellular components, such as lipids, proteins, and DNA (25). ROS can also stimulate the formation of oxidative stress, enhance the production and secretion of vasoconstrictor molecules, and promote inflammation within the endothelium (25). These processes ultimately result in endothelial dysfunction, thereby promoting a pro-inflammatory and pro-coagulant milieu in the vasculature, consequently heightening the predisposition to VTE. Secondly, high BMR may promote platelet activation and aggregation which are important in determining a prothrombotic state. Studies have reported an association between a high metabolic rate and changes in different hormones and metabolites levels in the body, such as higher levels of adrenaline and catecholamines (26, 27). These substances can enhance platelet function and promote aggregation. The increased metabolic rate may also induce alterations in blood flow and shear stress within the vasculature (28, 29), which can activate platelets and facilitate their aggregation at sites of vascular injury or dysfunction. In addition, high BMR-induced excessive ROS production can play a crucial role in platelet activation and aggregation. Third, among individuals of normal weight and those who are overweight, there is a positive link between BMR and proinflammatory status (30, 31). Elevated BMR can contribute to the generation of inflammatory cytokines such as interleukin (IL)-6 and interferon (IFN)-γ through inducing ROS production (32). Moreover, alterations in metabolic pathways and hormonal imbalances associated with high BMR, such as insulin resistance and dyslipidemia, may also contribute to a proinflammatory state (33–35). It is known that a proinflammatory state plays an important role in VTE development (36). Further investigation is necessary to fully understand the underlying pathways by which an elevated BMR influences the susceptibility to VTE.
The implications of this study could extend to clinical practice, particularly in the context of preventing and managing VTE. Current strategies for the prevention of VTE include pharmacological prophylaxis, mechanical prophylaxis, and early mobilization, among others. However, these strategies may not be effective for all patients and may also be associated with adverse effects. For example, the use of anticoagulant medications, such as heparin or low molecular weight heparin, can raise the bleeding probability (37). Our MR findings implied that reducing BMR might be a promising strategy for preventing VTE. This could be achieved through lifestyle modifications, such as weight management and caloric restriction. Future studies could investigate the interactions of these interventions with BMR in reducing the risk of VTE.
There are several limitations to this study. Firstly, horizontal pleiotropy poses a major constraint in MR design, as it indicates that instrumental variables employed for MR investigations have an impact on outcomes through alternative pathways instead of the intended exposure. However, it is unlikely that pleiotropic effects would significantly impact the validity of this study, as the sensitivity analysis using alternative MR methods produced congruent results. Additionally, the MR-Egger intercept did not reveal any evidence of horizontal pleiotropy, and the multivariable MR identified notable relationships. Secondly, it should be noted that our MR study only evaluated the long-term impact of BMR on VTE and its subtypes. This is because MR serves as a statistical method to investigate how long-term differences in exposures can influence the risk of developing diseases. We did not evaluate the short-term contribution of BMR to VTE and its subtypes. Thirdly, in our study, the assessment of the association between BMR and VTE, including its subtypes, was limited to the linear relationship and relied exclusively on GWAS summary statistics. The lack of access to individual-level data prohibited us from evaluating a nonlinear association. Fourthly, we focused on the association of BMR with VTE, PE, and DVT of lower extremities; other subtypes of VTE, such as cerebral venous thrombosis, were not included in our analysis. Fifthly, considering that our analyses solely included individuals of European descent, it is important to interpret these findings with caution regarding their applicability to other populations. Additional research is required to evaluate this aspect.
In conclusion, our MR study has provided the first-ever evidence that genetically predicted BMR has a positive causal impact on VTE and its subtypes, including PE and DVT of lower extremities. Modulating BMR might hold promise as a clinically relevant intervention to prevent VTE and its subtypes.
The IEU Open GWAS Project database (https://gwas.mrcieu.ac.uk/) provides public access to all datasets analyzed in the present study. Further inquiries can be directed to the corresponding author.
JH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YX: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by Special Fund of Neurotoxicity of General Anesthetics and Its Prevention and Treatment Innovation Team of the First Affiliated Hospital of Guangxi Medical University (No. YYZS2022001), Guangxi Clinical Research Center for Anesthesiology (No. GK AD22035214), and the Key Project of Natural Science Foundation of Guangxi (No. 2020GXNSFDA238025).
The authors would like to thank the MRC-IEU, FinnGen, and Neale lab for providing publicly available GWAS summary statistics.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1263804/full#supplementary-material
1. ^ https://gwas.mrcieu.ac.uk/
2. ^ https://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=23105
3. ^ https://mrcieu.github.io/TwoSampleMR/index.html
1. Khan, F, Tritschler, T, Kahn, SR, and Rodger, MA. Venous thromboembolism. Lancet. (2021) 398:64–77. doi: 10.1016/S0140-6736(20)32658-1
2. Pastori, D, Cormaci, VM, Marucci, S, Franchino, G, Del Sole, F, Capozza, A, et al. A comprehensive review of risk factors for venous thromboembolism: from epidemiology to pathophysiology. Int J Mol Sci. (2023) 24:3169. doi: 10.3390/ijms24043169
3. Taousani, E, Savvaki, D, Tsirou, E, Poulakos, P, Mintziori, G, Zafrakas, M, et al. Regulation of basal metabolic rate in uncomplicated pregnancy and in gestational diabetes mellitus. Hormones (Athens). (2017) 13:235–50. doi: 10.14310/horm.2002.1743
4. Sawicka, D, Maciak, S, Kozłowska, H, Kasacka, I, Kloza, M, Sadowska, A, et al. Functional and structural changes in aorta of mice divergently selected for basal metabolic rate. J Comp Physiol B. (2020) 190:101–12. doi: 10.1007/s00360-019-01252-6
5. Riley, M, Elborn, JS, McKane, WR, Bell, N, Stanford, CF, and Nicholls, DP. Resting energy expenditure in chronic cardiac failure. Clin Sci (Lond). (1991) 80:633–9. doi: 10.1042/cs0800633
6. Poehlman, ET, Scheffers, J, Gottlieb, SS, Fisher, ML, and Vaitekevicius, P. Increased resting metabolic rate in patients with congestive heart failure. Ann Intern Med. (1994) 121:860–2. doi: 10.7326/0003-4819-121-11-199412010-00006
7. Delaney, CL, Miller, MD, Allan, RB, and Spark, JI. The impact of abdominal aortic aneurysm on muscle mass and energy expenditure: a novel preliminary investigation. Vascular. (2015) 23:602–6. doi: 10.1177/1708538114566849
8. Anaszewicz, M, Banaś, W, Wawrzeńczyk, A, and Budzyński, J. Body composition in patients with atrial fibrillation. Acta Cardiol Sin. (2019) 35:484–92. doi: 10.6515/ACS.201909_35(5).20190323A
9. Yuan, S, Bruzelius, M, Damrauer, SM, Håkansson, N, Wolk, A, Åkesson, A, et al. Anti-inflammatory diet and venous thromboembolism: two prospective cohort studies. Nutr Metab Cardiovasc Dis. (2021) 31:2831–8. doi: 10.1016/j.numecd.2021.06.021
10. Smith, GD, and Ebrahim, S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. (2004) 33:30–42. doi: 10.1093/ije/dyh132
11. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-base platform supports systematic causal inference across the human phenome. elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
12. Mitchell, R., Elsworth, B.L., Mitchell, R., Raistrick, C.A., Paternoster, L., and Hemani, G.. MRC IEU UK biobank GWAS pipeline version 2. (2019). doi: 10.5523/bris.pnoat8cxo0u52p6ynfaekeigi
13. Kurki, MI, Karjalainen, J, Palta, P, Sipilä, TP, Kristiansson, K, Donner, KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
14. Gagnon, E, Mitchell, PL, Manikpurage, HD, Abner, E, Taba, N, Esko, T, et al. Impact of the gut microbiota and associated metabolites on cardiometabolic traits, chronic diseases and human longevity: a Mendelian randomization study. J Transl Med. (2023) 21:60. doi: 10.1186/s12967-022-03799-5
15. Pierce, BL, Ahsan, H, and Vanderweele, TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
16. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
17. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
18. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
19. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
20. Burgess, S, Foley, CN, Allara, E, Staley, JR, and Howson, JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. (2020) 11:376. doi: 10.1038/s41467-019-14156-4
21. Zhang, Y, Zhao, M, Guo, P, Wang, Y, Liu, L, Zhao, J, et al. Mendelian randomisation highlights hypothyroidism as a causal determinant of idiopathic pulmonary fibrosis. EBioMedicine. (2021) 73:103669. doi: 10.1016/j.ebiom.2021
22. Yengo, L, Sidorenko, J, Kemper, KE, Zheng, Z, Wood, AR, Weedon, MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. (2018) 27:3641–9. doi: 10.1093/hmg/ddy271
23. Brion, MJ, Shakhbazov, K, and Visscher, PM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
24. Li, Y, Zhai, H, Kang, L, Chu, Q, Zhao, X, and Li, R. Causal association between basal metabolic rate and risk of cardiovascular diseases: a univariable and multivariable Mendelian randomization study. Sci Rep. (2023) 13:12487. doi: 10.1038/s41598-023-39551-2
25. Lindström, S, Germain, M, Crous-Bou, M, Smith, EN, Morange, PE, van Hylckama Vlieg, A, et al. Assessing the causal relationship between obesity and venous thromboembolism through a Mendelian randomization study. Hum Genet. (2017) 136:897–902. doi: 10.1007/s00439-017-1811-x
26. Bloomer, RJ, Canale, RE, Blankenship, MM, Hammond, KG, Fisher-Wellman, KH, and Schilling, BK. Effect of the dietary supplement meltdown on catecholamine secretion, markers of lipolysis, and metabolic rate in men and women: a randomized, placebo controlled, cross-over study. Lipids Health Dis. (2009) 8:32. doi: 10.1186/1476-511X-8-32
27. Hollstein, T, Basolo, A, Ando, T, Votruba, SB, Krakoff, J, and Piaggi, P. Urinary norepinephrine is a metabolic determinant of 24-hour energy expenditure and sleeping metabolic rate in adult humans. J Clin Endocrinol Metab. (2020) 105:1145–56. doi: 10.1210/clinem/dgaa047
28. Maeda, T, Fukushima, T, Ishibashi, K, and Higuchi, S. Involvement of basal metabolic rate in determination of type of cold tolerance. J Physiol Anthropol. (2007) 26:415–8. doi: 10.2114/jpa2.26.415
29. Mammadova-Bach, E, Gudermann, T, and Braun, A. Platelet Mechanotransduction: regulatory cross talk between mechanosensitive receptors and calcium channels. Arterioscler Thromb Vasc Biol. (2023) 43:1339–48. doi: 10.1161/ATVBAHA.123.318341
30. Drabsch, T, Holzapfel, C, Stecher, L, Petzold, J, Skurk, T, and Hauner, H. Associations between C-reactive protein, insulin sensitivity, and resting metabolic rate in adults: a mediator analysis. Front Endocrinol. (2018) 9:556. doi: 10.3389/fendo.2018.00556
31. Liu, C, Lu, Y, Chen, J, Qiu, W, Zhan, Y, and Liu, Z. Basal metabolic rate and risk of multiple sclerosis: a Mendelian randomization study. Metab Brain Dis. (2022) 37:1855–61. doi: 10.1007/s11011-022-00973-y
32. Wu, E, Ni, J, Tao, L, and Xie, T. A bidirectional Mendelian randomization study supports the causal effects of a high basal metabolic rate on colorectal cancer risk. PLoS One. (2022) 17:e0273452. doi: 10.1371/journal.pone.0273452
33. Compher, C, and Badellino, KO. Obesity and inflammation: lessons from bariatric surgery. JPEN J Parenter Enteral Nutr. (2008) 32:645–7. doi: 10.1177/0148607108326070
34. Montecucco, F, Steffens, S, and Mach, F. Insulin resistance: a proinflammatory state mediated by lipid-induced signaling dysfunction and involved in atherosclerotic plaque instability. Mediat Inflamm. (2008) 2008:767623. doi: 10.1155/2008/767623
35. Hong, N, Lin, Y, Ye, Z, Yang, C, Huang, Y, Duan, Q, et al. The relationship between dyslipidemia and inflammation among adults in east coast China: a cross-sectional study. Front Immunol. (2022) 13:937201. doi: 10.3389/fimmu.2022.937201
36. Dix, C, Zeller, J, Stevens, H, Eisenhardt, SU, Shing, KSCT, Nero, TL, et al. C-reactive protein, immunothrombosis and venous thromboembolism. Front Immunol. (2022) 13:1002652. doi: 10.3389/fimmu.2022.1002652
Keywords: basal metabolic rate, venous thromboembolism, deep vein thrombosis of lower extremities, pulmonary embolism, Mendelian randomization
Citation: Huang J and Xie Y (2023) Genetically predicted basal metabolic rate and venous thromboembolism risk: a Mendelian randomization study. Front. Nutr. 10:1263804. doi: 10.3389/fnut.2023.1263804
Received: 20 July 2023; Accepted: 05 December 2023;
Published: 21 December 2023.
Edited by:
Ian Glynn Davies, Liverpool John Moores University, United KingdomReviewed by:
Mohsen Mazidi, University of Oxford, United KingdomCopyright © 2023 Huang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yubo Xie, MTE1NzgxNzc5MUBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.