- Department of International Nursing School, Hainan Medical University, Haikou, Hainan, China
Background: The pan-immune-inflammation value (PIV) has been reported as a promising prognostic biomarker in multiple cancers but still remains inconclusive. The objective of this study is to systematically investigate the association of the pretreatment PIV with survival outcomes in cancer patients, based on available literature.
Methods: Online databases including PubMed, Embase and the Web of Science were thoroughly searched for studies evaluating the prognostic role of the pretreatment PIV in cancers from the inception to June 2023. Hazard ratios (HRs) with 95% confidence intervals (CIs) were always assessed using a random-effects model. Statistical analyses were performed using Stata 12.0.
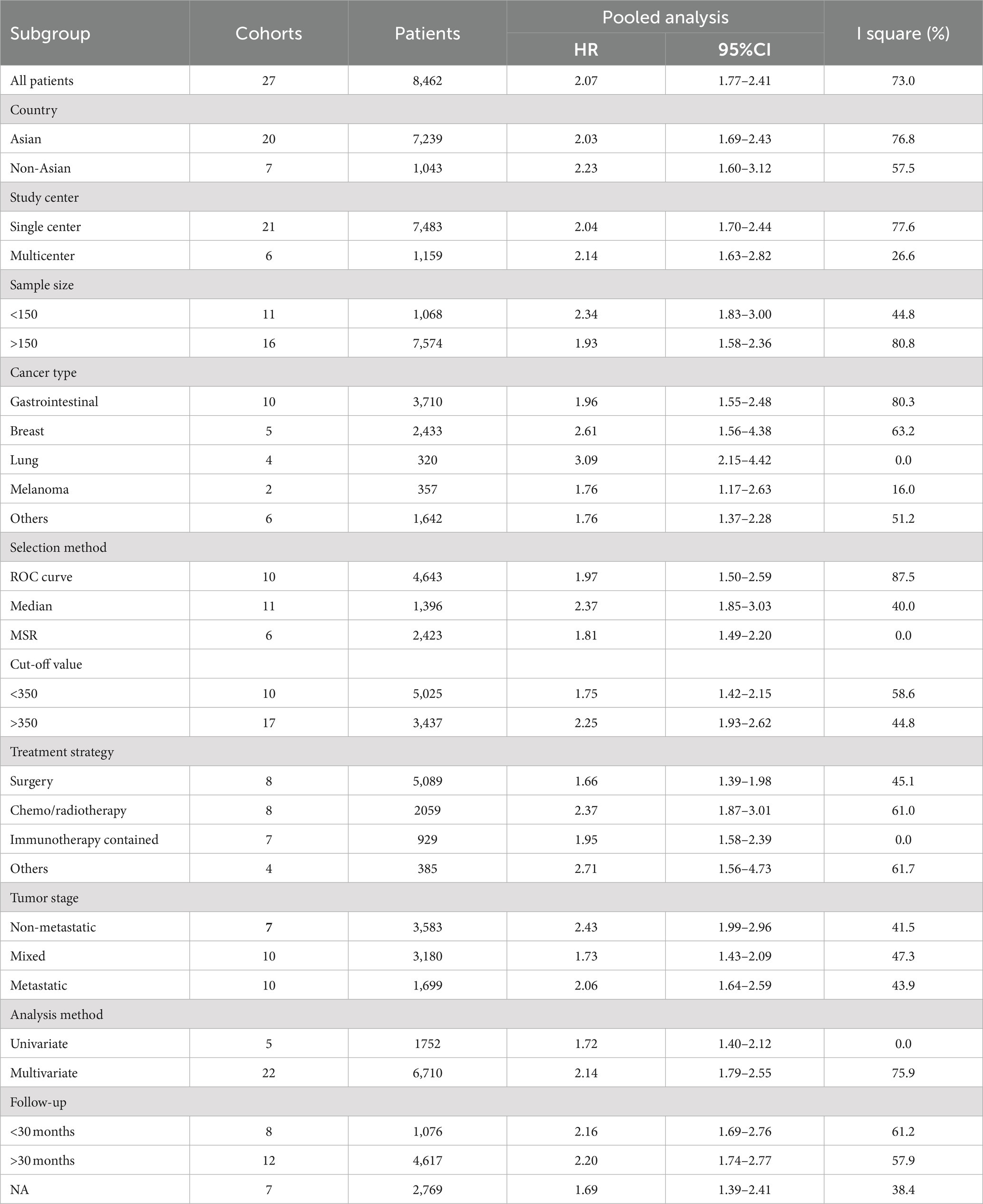
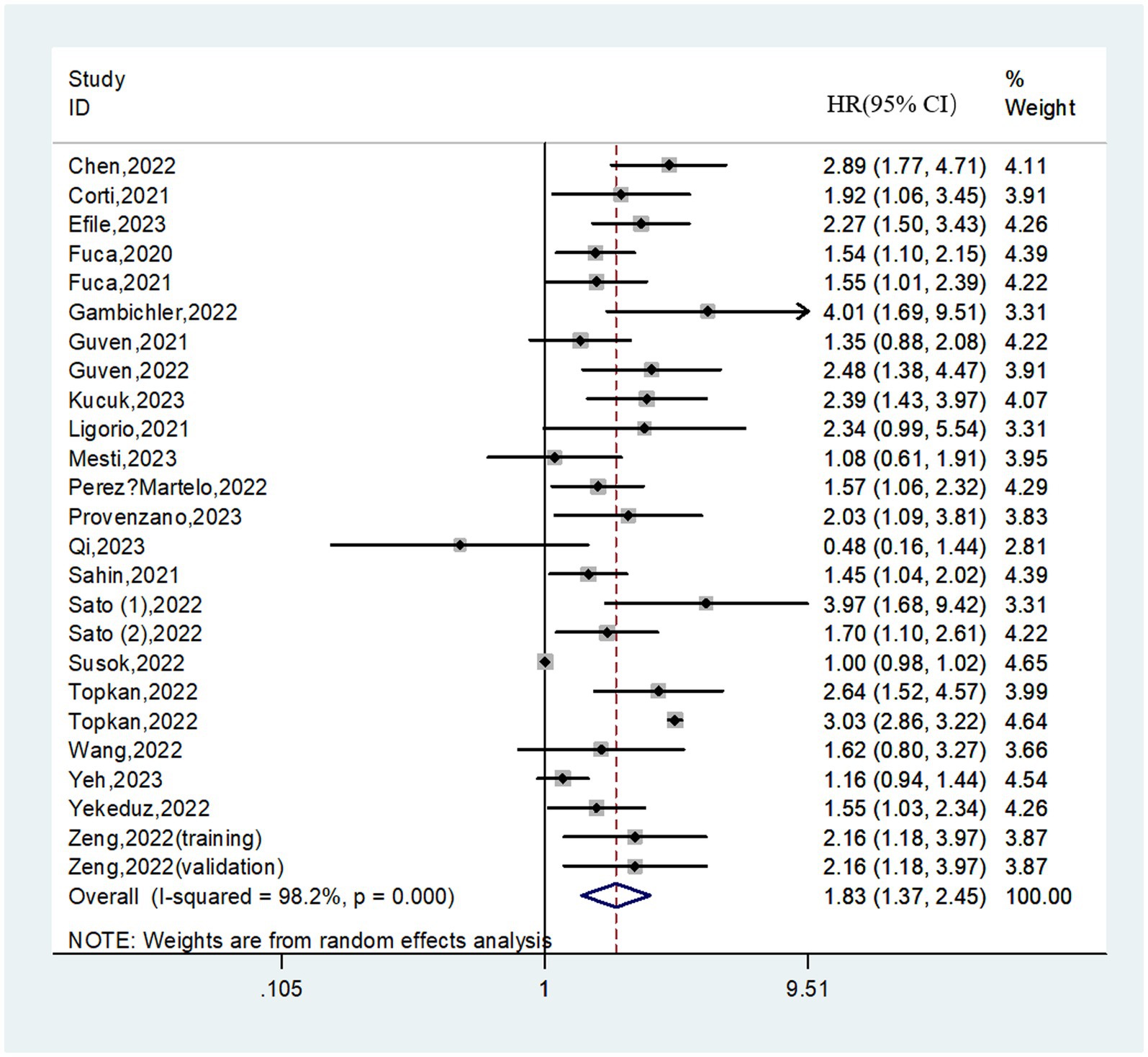
Results: Thirty studies were finally included after comprehensively study searching. In total, 8,799 cancer patients were enrolled in this meta-analysis. The pooled results demonstrated that patients in the high PIV group had a significantly poorer overall survival (HR = 2.07; 95%CI: 1.77–2.41; I2 = 73.0%) and progression-free survival (HR = 1.83; 95%CI: 1.37–2.45; I2 = 98.2%) than patients in the low PIV group. The prognostic significance of the PIV score on overall survival and progression-free survival was observed across various geographical regions, tumor stages and treatment strategies. Sensitivity analyses supported the stability of the above combined results.
Conclusion: This meta-analysis demonstrated that the pretreatment PIV could be a non-invasive and efficacious prognostic biomarker for cancer patients.
1. Introduction
With the global population and the proportion of elderly people growing, cancer has become one of the leading causes of death worldwide (1). Although the development of surgery and medical treatment has made great progress in cancer patients, the prognosis for these patients remains not yet satisfactory (2). Therefore, based on the estimated survival time of cancer patients, it is essential to develop individualized and effective treatment strategies to improve their chances of survival. Currently, anti-tumor therapy relies primarily on a conventional staging system. Nevertheless, in clinical practice, the staging system alone is not able to support treatment decision-making as well as prognosis assessment well (3, 4). It is therefore urgent to construct novel prognostic markers to guide more precise treatment for cancer patients.
The accumulating evidence suggests that host inflammation and immune status play an important role in the progression, treatment response and survival outcomes of cancer patients (5, 6). Based on this insight, several inflammation/immune-related biomarkers have been developed to predict the clinical outcomes of cancer patients, such as neutrophil to lymphocyte ratio (NLR) (7), platelet to lymphocyte ratio (PLR) (8) and monocyte to lymphocyte ratio (MLR) (9). Recently, a newly developed prognostic biomarker- the pan-immune-inflammation value (PIV), has garnered significant interest of clinicians (10). PIV integrates neutrophils, platelets, monocytes and lymphocyte together, and has been reported to be a better prognostic predictor than these simple biomarkers, including NLR, PLR and MLR (11, 12). To be specific, PIV is calculated using serum neutrophil, platelet, monocyte and lymphocyte (neutrophil x platelet x monocyte/ lymphocyte), which was first introduced by Fuca et al. (13) in 2020 as a prognostic index for metastatic colorectal cancer receiving chemotherapy combined with target therapy. After that, the prognostic role of the PIV has been explored in various cancers (14–16). A recent meta-analysis of 15 studies demonstrated that a high PIV was associated with a poor prognosis in cancer patients (17). Nonetheless, it is important to note that some common tumor types, such as pancreatic cancer and hepatic cancer, were not available in this meta-analysis. Besides, abstract without sufficient data was also included for analysis. These factors undoubtedly have a certain impact on the universality and reliability of the results.
As growing body of additional research has been addressed to further explore the prognostic value of PIV in cancer patients. We therefore performed an updated pooled analysis to systematically explore the relationship between the pretreatment PIV and survival outcomes in cancer patients.
2. Methods
2.1. Search strategy
This meta-analysis was conducted as per the PRISMA guidelines (18) (see PRISMA checklist in the Supplementary Information) to identify literature evaluating the association of pretreatment PIV with survival outcomes in cancer patients. Related studies from the Web of Science, PubMed, and Embase were thoroughly examined from the inception to June 30, 2023. The key word “pan-immune-inflammation value” was applied to search potential studies. During the search process, studies published in any language were included. In addition, references to enrolled studies and related reviews were prudently scanned for additional reporting. The search was performed by two investigators (Y-HJ and RS) independently.
2.2. Study selection
The inclusion criteria were as follows: (1) patients were pathologically diagnosed as cancer; (2) patients were divided into two groups according to the pretreatment PIV cut-off value; (3) studies investigated the relationship between the pretreatment PIV and survival outcomes of cancer patients. The exclusion criteria were: (1) letters, case reports, abstracts or reviews; (2) duplicated studies.
2.3. Data extraction and quality assessment
Data extraction and subsequent cross-checks were performed by two independent reviewers (YH-J and RS). Information extracted from included studies was as follows: first author, year of publication, country, study interval, sample size, cancer type, selection method, cut-off value, period of blood collection, information on exclusion of diseases affecting blood parameters, age, sex, tumor stage, treatment strategy, survival data and follow-up time. The quality assessment of included literature was evaluated via the method by Lin et al. (19). After careful evaluation from 9 domains, a study could get a total score ranging from 0 to 9. Quality assessment was not used as exclusion criterion for included studies.
2.4. Outcome assessment
In this study, the primary endpoint was to explore the relationship between the pretreatment PIV and survival outcomes in cancer patients. Long-term survival outcomes included overall survival (OS), progression-free survival (PFS), disease-free survival (DFS) and recurrence-free survival (RFS). Since DFS, RFS and PFS share the similar endpoints, they were analyzed together as one outcome, PFS, as previously suggested (20, 21).
2.5. Statistical analysis
Stata 12.0 statistical software was used to perform all the statistical analyses. Hazard ratios (HRs) with 95% confidence intervals (CIs) reported from multivariate analyses were preferentially used to incorporate survival outcomes. Otherwise, univariate assessments were the sources of effect sizes. In addition, for studies whose survival data were not directly available, corresponding HRs with 95% CIs were extracted from the survival curves through the methods reported by Tierney et al. (22). In the present study, I2 statistics were utilized to evaluate inter-study heterogeneity, and a random-effects model was always performed, which accounts for variance across included studies (23, 24). Subgroup analyses and meta-regression analyses were applied to explore the sources of heterogeneity. Leave-one-out sensitivity analyses were utilized to assess the reliability of pooled results. Possible publication bias was evaluated using Begg’s test. If there was a significant publication bias, a trim and fill analysis was employed to assess the impact of it on the pooled result. p values <0.05 were considered statistically significant.
3. Results
3.1. Study characteristics
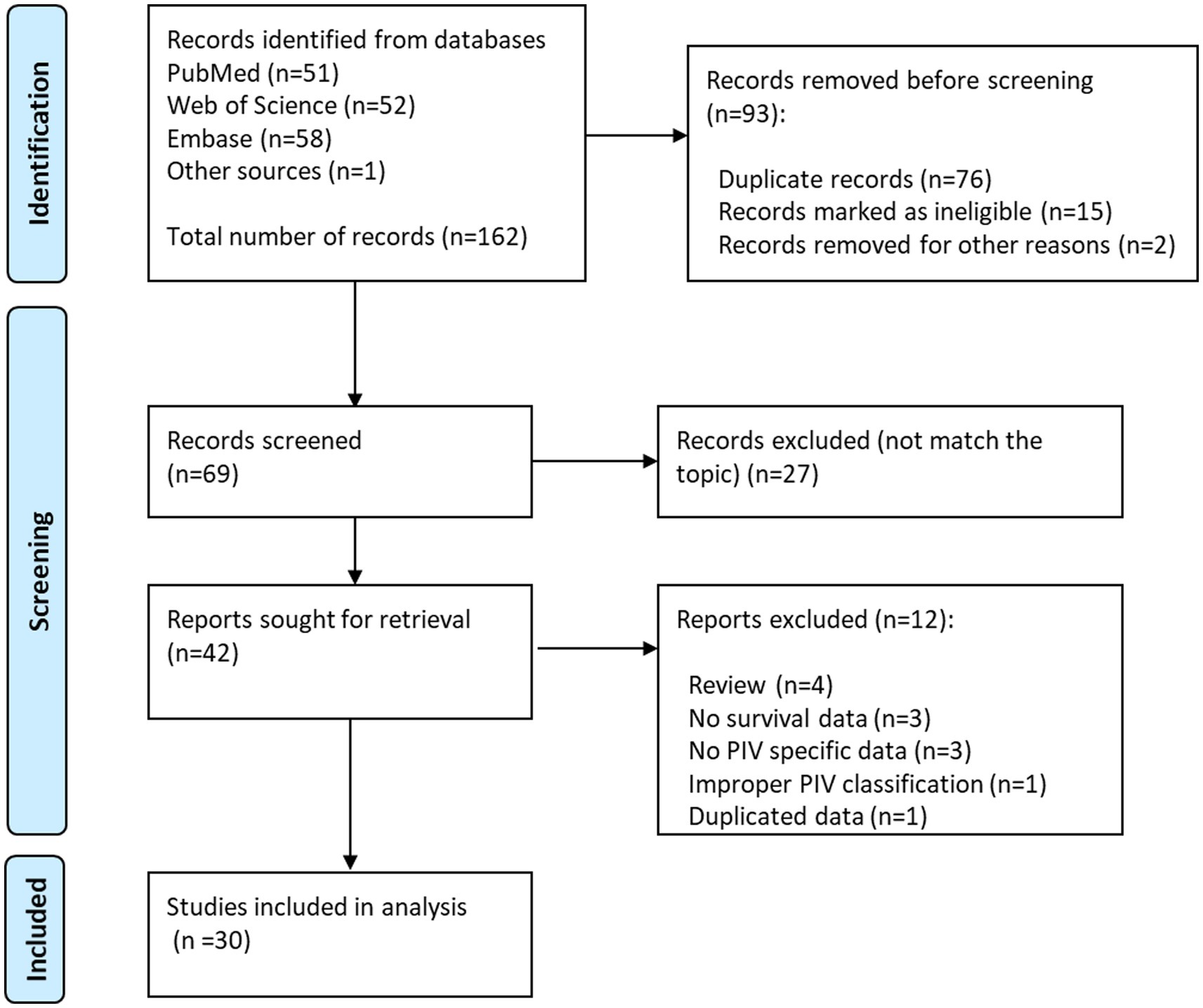
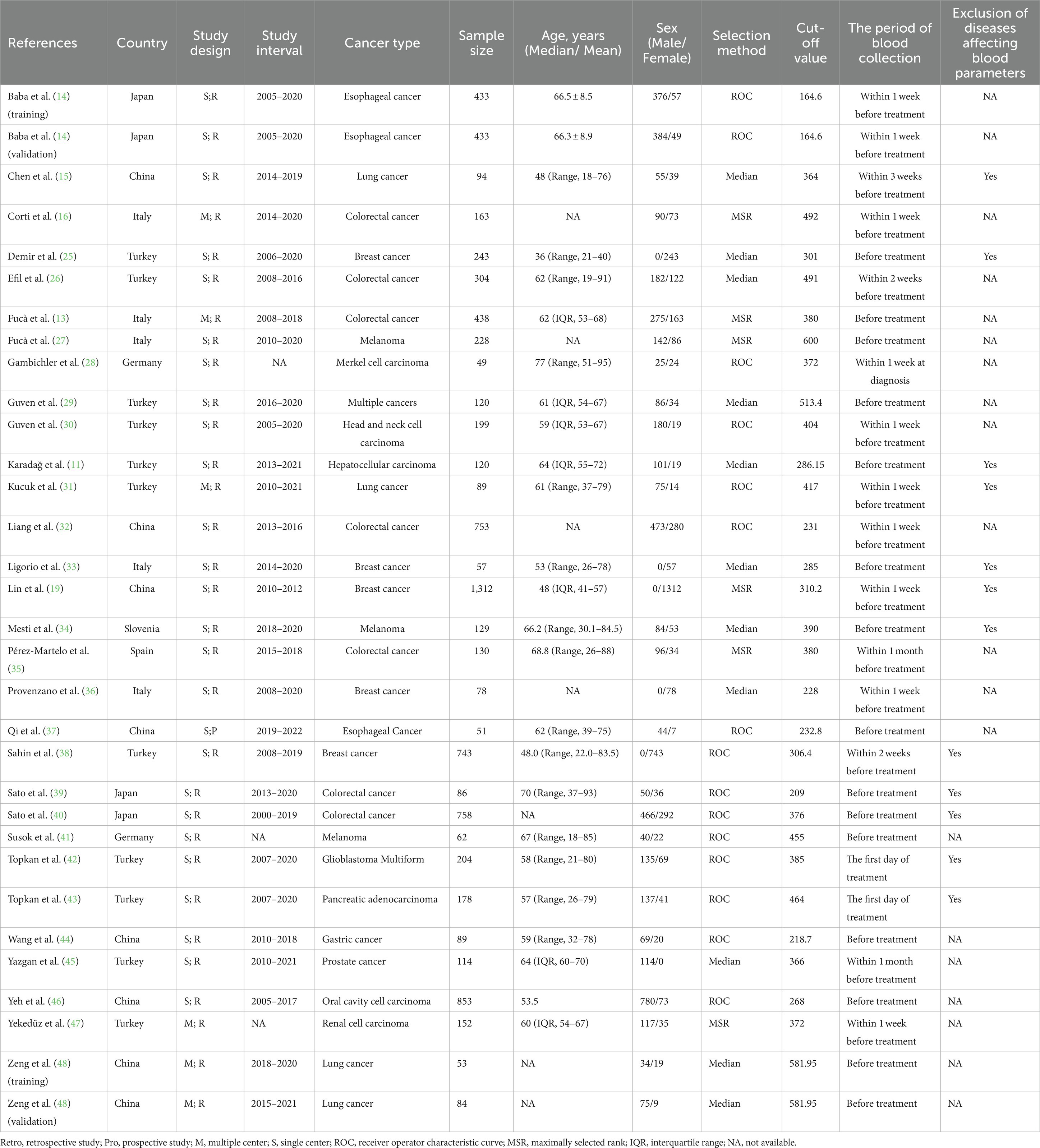
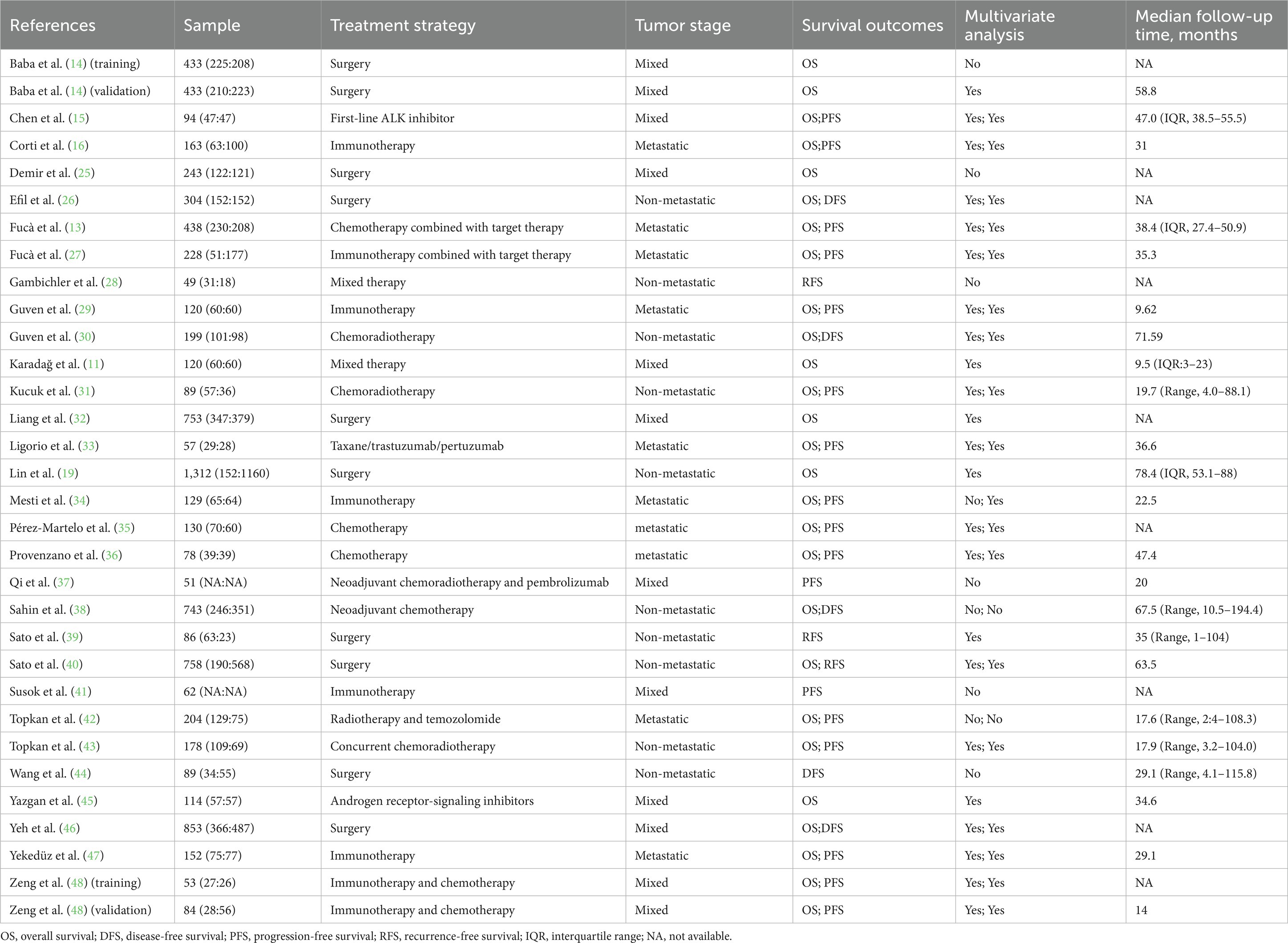
The initial search of online databases yielded a total of 162 records. By removing duplicated studies, and reviewing titles, abstracts and full-text studies, 30 studies (11–16, 25–48) with 32 cohorts were ultimately incorporated in our meta-analysis (Figure 1), The main characteristics of these studies were shown in Tables 1, 2. In total, 8,799 participants from China, Germany, Italy, Japan, Slovenia, Spain and Turkey were enrolled in the present study. These studies were published from 2020 to 2023, with a sample size ranging from 49 to 1,312. The most common cancer type was gastrointestinal cancer, followed by breast cancer and lung cancer. As regards blood parameters, the period of blood collection before treatment ranged from 1 day to 1 month, and most of the included studies did not mention the exclusion of diseases affecting hematological parameters. The cut-off value of PIV ranged from 164.6 to 600.0. In terms of main primary treatments, surgery was performed in 8 cohorts, chemo/radiotherapy was performed in 8 cohorts and immunotherapy contained treatment was performed in 7 cohorts. The median follow-up time ranged from 9.5 to 78.4 months. The literature quality of these studies was good with a median score of 8 (range: 7–9, Supplementary Table S1).
3.2. Relationship between the PIV and OS
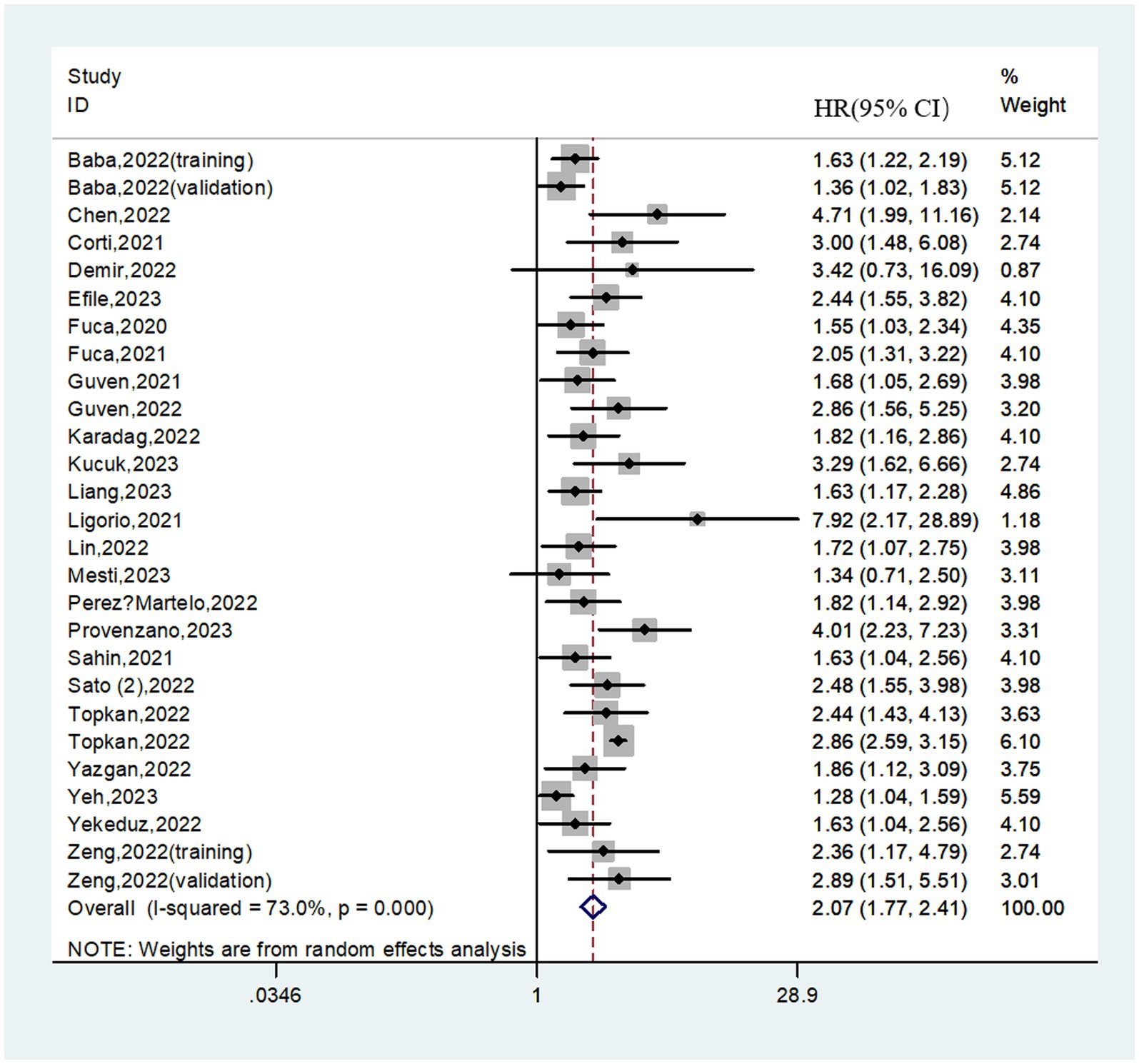
A total of 8,462 patients from 27 cohorts were included in the pooled analysis of OS. The pooled result revealed that higher PIV predicted poorer OS (HR = 2.07; 95%CI:1.77–2.41; I2 = 73.0%; Figure 2). Furthermore, subgroup analyses based on country, study center, sample size, cancer type, selection method, cut-off value, treatment strategy, tumor stage, analysis method and follow-up time were performed. As shown in Table 3 and Supplementary Figure S1, the pooled outcomes from all subgroup analyses consistently revealed that patients in the high PIV group had a significantly worse OS compared to those in the low PIV group. In addition, a meta-regression analysis based on these variables was performed to investigate the source of heterogeneity. As shown in Supplementary Table S2, none of these covariates had a significant effect on the hazard ratios of OS (all p values>0.05).
3.3. Relationship between the PIV and PFS
In total, 25 cohorts involving 5,391 patients reported on PFS. The pooled HR was 1.83 (95%CI: 1.37–2.45; I2 = 98.2%), suggesting that higher PIV was associated with a significantly worse PFS (Figure 3). Similarly, subgroup analyses based on above variables were performed due to the significant heterogeneity existed. We found that in almost all subgroups analyses, patients in the high PIV group has an inferior PFS, except for the pooled results from melanoma (HR = 1.13; 95% CI: 0.86–1.47) and univariate analysis (HR = 1.53; 95% CI: 0.99–2.35) (Table 4 and Supplementary Figure S2). Additionally, meta-regression analysis revealed that none of these factors was the source of heterogeneity (all p values>0.05; Supplementary Table S2).
3.4. Sensitivity analyses and publication bias
Sensitivity analyses were conducted to assess the robustness of the pooled OS and PFS. After omitting any individual study, pooled HRs with 95% CIs for both OS and DFS were not significantly altered (Supplementary Figure S3).
The Begg’s funnel plots were applied to evaluate the potential publication bias. As shown in Supplementary Figure S4, the funnel plot for PFS was bilaterally symmetric with a Begg’s p value of 0.691, indicating that there was no significant publication bias for PFS. While for OS, the Begg’s funnel plot was asymmetric with the p value <0.0001, which suggested a high risk of publication bias for this outcome. Trim-and-fill analysis was therefore applied, supplementing a total of 8 unpublished cohorts to balance the funnel plot. Finally, PIV was still associated with inferior OS (HR = 1.82; 95%CI: 1.56–2.13), indicating the robustness of the pooled result.
4. Discussion
Cancer-related inflammation is prevalent in patients with malignant diseases, which has been confirmed to promote cancer progression and advancement (6). Traditionally, host inflammation status can be detected through several blood biomarkers, such as neutrophil count, platelet count, and lymphocyte count. Additionally, evidence from numerous studies has demonstrated that their ratios can be applied to predict patient’s short-term and long-term outcomes, especially in cancer patients (7, 8). Importantly, these markers have the natural advantage of being non-invasive, objective, and cost-effective, which provides great potential for their wide clinical applications.
In recent years, a new biomarker, the pan-immune-inflammation value, which consists of serum neutrophil, platelet, monocyte and lymphocyte, has attracted the attention of clinicians due to its promising prognostic significance in several malignancies (12, 30, 39). A recent meta-analysis by Guven et al. (17) has initially demonstrated that high PIV was associated with decreased survival outcomes in cancer patients. Nevertheless, this meta-analysis included only 15 studies (including an abstract) and several common cancer types (such as pancreatic cancer, hepatic cancer and prostate cancer) were not available, which made the prognostic value of PIV in cancer patients still inconclusive. To clarify this issue accurately, an updated meta-analysis including 30 studies with 8,799 cancer patients was performed. Through our quantitatively analyses, we convinced that an elevated PIV markedly predicted poorer OS (HR = 2.07; 95%CI: 1.77–2.41) and PFS (HR = 1.83; 95%CI: 1.37–2.45) in cancer patients. Additionally, benefiting from the inclusion of sufficient studies, we were able to perform detailed subgroup analyses, as well as sensitivity and publication bias analyses It can be seen that the PIV achieved reliable performance in predicting prognosis. Therefore, the PIV may be a valuable and effective inflammatory index to evaluate the oncological outcomes of patients with malignancies.
Dysregulation of inflammatory and immune cells in the tumor microenvironment has been identified as being involved in the tumor progression (49–51). Simultaneously, a higher PIV may result from higher neutrophils, monocytes, and platelets and/or lower lymphocytes. Although the detailed mechanisms of the PIV’s prognostic value in malignancies are unclear, they can be explained as follows: First, neutrophils, as the most common innate immune cells, have been reported to promote tumor invasion and metastasis by secreting VEGFA, MMPs, and other chemokines such as IL-6 and TGF-β (52, 53). At the same time, elevated neutrophils can also cause T cell activation disorders by largely releasing nitric oxide, arginase, and reactive oxygen species, ultimately inhibiting the body’s killing effect on cancer cells (54). Second, monocytes, especially those differentiated into tumor-associated macrophages (TAMs), can induce apoptosis of T cells with antitumor functions (55). In addition, TAM density has been shown to affect tumor tissue angiogenesis by stimulating the production and secretion of pro-angiogenic factors (56, 57). Third, platelets, are reported to induce epithelial–mesenchymal transition and angiogenesis by secreting TGF-β, VEGF and FGF. Moreover, platelets are also able to recruit neutrophils and monocytes, thereby promoting the distant metastasis of tumor cells. Finally, lymphocytes, especially cytotoxic T lymphocytes, play an essential role in cancer immune surveillance and defense (58). It has been reported that high lymphocyte levels in the tumor microenvironment are beneficial for inducing lysis and apoptosis of cancer cells, thereby inhibiting cancer cell proliferation and metastasis (59). On the contrary, lymphopenia has been shown to be associated with a poor prognosis in cancer patients (60).
Notably, the pooled outcomes from subgroup analyses demonstrated that the prognostic value of the PIV for both OS and PFS was consistent in treatment strategies, such as surgery (HR = 1.66 and 1.80), chemo/radiotherapy (HR = 2.37 and 2.06), immunotherapy (HR = 1.95 and 1.40). Given that patients with malignancies would receive one or more anti-tumor treatment strategies, these results showed that PIV could provide prognosis prediction for malignant patients receiving different treatments, especially for those receiving chemo/radiotherapy. In addition, PIV has been shown to have considerable prognostic value across different tumor species, particularly in lung cancer (HR = 3.09 and 2.43). Moreover, the prognostic value of PIV was not affected by the country of publication, cut-off value, and tumor stage, further confirming the clinical universality and efficacy of PIV in cancer patients.
This meta-analysis had several limitations must be acknowledged. First, all of the included studies except one by Qi et al. (37) were designed to be retrospective, which may increase the risk of selection bias. Second, the heterogeneities of pooled outcomes for both OS and PFS were remarkable, even though the subgroup analyses and sensitivity analyses showed consistent results, we failed to find the sources of heterogeneity. Third, significant inconsistencies in the measurement of blood parameters in the included studies, including but not limited to factors such as measurement time, may have contributed to the large variability in the cut-off values of PIV, and may also have had some impact on the confidence of our pooled results. Finally, the cut-off values of PIV varied widely due to various factors such as disease type, population differences, sample size, and detection method, which somewhat limits the clinical use of PIV.
5. Conclusion
In conclusion, the present meta-analysis demonstrates an association between elevated pre-treatment PIV and poor survival outcomes in cancer patients. PIV has the potential to be a noninvasive and effective prognostic biomarker for cancer patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YH-J: Funding acquisition, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. RS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing, Visualization. XJ-Q: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Hainan Provincial Natural Science Foundation (Nos. 822QN320 and 823RC495).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1259929/full#supplementary-material
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Liu, R, Zhao, K, Wang, K, Zhang, L, Ma, W, Qiu, Z, et al. Prognostic value of nectin-4 in human cancers: a meta-analysis. Front Oncol. (2023) 13:1081655. doi: 10.3389/fonc.2023.1081655
3. Pang, HY, Chen, XF, Yan, MH, Chen, LH, Chen, ZX, Zhang, SR, et al. Clinical significance of the advanced lung cancer inflammation index in gastrointestinal cancer patients: a systematic review and meta-analysis. Front Oncol. (2023) 13:1021672. doi: 10.3389/fonc.2023.1021672
4. Liu, H, Yang, XC, Liu, DC, Tong, C, Wen, W, and Chen, RH. Clinical significance of the controlling nutritional status (CONUT) score in gastric cancer patients: a meta-analysis of 9,764 participants. Front Nutr. (2023) 10:1156006. doi: 10.3389/fnut.2023.1156006
5. Diakos, CI, Charles, KA, McMillan, DC, and Clarke, SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
6. Singh, R, Mishra, MK, and Aggarwal, H. Inflammation, immunity, and Cancer. Mediat Inflamm. (2017) 2017:1. doi: 10.1155/2017/6027305
7. Diem, S, Schmid, S, Krapf, M, Flatz, L, Born, D, Jochum, W, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
8. Pang, Q, Zhang, LQ, Wang, RT, Bi, JB, Zhang, JY, Qu, K, et al. Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma. World J Gastroenterol. (2015) 21:6675–83. doi: 10.3748/wjg.v21.i21.6675
9. Mao, S, Yu, X, Shan, Y, Fan, R, Wu, S, and Lu, C. Albumin-bilirubin (ALBI) and monocyte to lymphocyte ratio (MLR)-based nomogram model to predict tumor recurrence of AFP-negative hepatocellular carcinoma. J Hepat Carcin. (2021) 8:1355–65. doi: 10.2147/JHC.S339707
10. Yang, XC, Liu, H, Liu, DC, Tong, C, Liang, XW, and Chen, RH. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: a systematic review and meta-analysis. Front Oncol. (2022) 12:1036890. doi: 10.3389/fonc.2022.1036890
11. Karadağ, I, Karakaya, S, Yılmaz, ME, and Çakmak Öksüzoğlu, ÖB. The potential prognostic novel markers PIV and PILE score to predict survival outcomes at hepatocellular cancer. Eur Rev Med Pharmacol Sci. (2022) 26:7679–86. doi: 10.26355/eurrev_202210_30044
12. Lin, F, Zhang, LP, Xie, SY, Huang, HY, Chen, XY, Jiang, TC, et al. Pan-immune-inflammation value: a new prognostic index in operative breast Cancer. Front Oncol. (2022) 12:830138. doi: 10.3389/fonc.2022.830138
13. Fucà, G, Guarini, V, Antoniotti, C, Morano, F, Moretto, R, Corallo, S, et al. The Pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. (2020) 123:403–9. doi: 10.1038/s41416-020-0894-7
14. Baba, Y, Nakagawa, S, Toihata, T, Harada, K, Iwatsuki, M, Hayashi, H, et al. Pan-immune-inflammation value and prognosis in patients with esophageal Cancer. Ann Surg Open. (2022) 3:e113. doi: 10.1097/AS9.0000000000000113
15. Chen, X, Hong, X, Chen, G, Xue, J, Huang, J, Wang, F, et al. The Pan-immune-inflammation value predicts the survival of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer treated with first-line ALK inhibitor. Transl Oncol. (2022) 17:101338. doi: 10.1016/j.tranon.2021.101338
16. Corti, F, Lonardi, S, Intini, R, Salati, M, Fenocchio, E, Belli, C, et al. The Pan-immune-inflammation value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. (2021) 150:155–67. doi: 10.1016/j.ejca.2021.03.043
17. Guven, DC, Sahin, TK, Erul, E, Kilickap, S, Gambichler, T, and Aksoy, S. The association between the Pan-immune-inflammation value and Cancer prognosis: a systematic review and Meta-analysis. Cancers. (2022) 14:14. doi: 10.3390/cancers14112675
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Lin, Y, Liu, Z, Qiu, Y, Zhang, J, Wu, H, Liang, R, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. (2018) 44:1494–503. doi: 10.1016/j.ejso.2018.07.052
20. Pang, HY, Yan, MH, Chen, LH, Chen, XF, Chen, ZX, Zhang, SR, et al. Detection of asymptomatic recurrence following curative surgery improves survival in patients with gastric cancer: a systematic review and meta-analysis. Front Oncol. (2022) 12:1011683. doi: 10.3389/fonc.2022.1011683
21. Papakonstantinou, A, Gonzalez, NS, Pimentel, I, Suñol, A, Zamora, E, Ortiz, C, et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: a systematic review and meta-analysis. Cancer Treat Rev. (2022) 104:102362. doi: 10.1016/j.ctrv.2022.102362
22. Tierney, JF, Stewart, LA, Ghersi, D, Burdett, S, and Sydes, MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
23. Pang, HY, Liang, XW, Chen, XL, Zhou, Q, Zhao, LY, Liu, K, et al. Assessment of indocyanine green fluorescence lymphography on lymphadenectomy during minimally invasive gastric cancer surgery: a systematic review and meta-analysis. Surg Endosc. (2022) 36:1726–38. doi: 10.1007/s00464-021-08830-2
24. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. Demir, H, Demirci, A, Eren, SK, Beypinar, I, Davarcı, SE, and Baykara, M. A new prognostic index in Young breast Cancer patients. J Coll Physicians Surg Pak. (2022) 32:86–91. doi: 10.29271/jcpsp.2022.01.86
26. Efil, SC, Guner, G, Guven, DC, Celikten, B, Celebiyev, E, Taban, H, et al. Prognostic and predictive value of tumor infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Clin Res Hepatol Gastroenterol. (2023) 47:102171. doi: 10.1016/j.clinre.2023.102171
27. Fucà, G, Beninato, T, Bini, M, Mazzeo, L, di Guardo, L, Cimminiello, C, et al. The Pan-immune-inflammation value in patients with metastatic melanoma receiving first-line therapy. Target Oncol. (2021) 16:529–36. doi: 10.1007/s11523-021-00819-0
28. Gambichler, T, Said, S, Abu Rached, N, Scheel, CH, Susok, L, Stranzenbach, R, et al. Pan-immune-inflammation value independently predicts disease recurrence in patients with Merkel cell carcinoma. J Cancer Res Clin Oncol. (2022) 148:3183–9. doi: 10.1007/s00432-022-03929-y
29. Guven, DC, Erul, E, Yilmaz, F, Yasar, S, Yildirim, HC, Ercan, F, et al. The association between pan-immune-inflammation value and survival in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. (2023) 280:2471–8. doi: 10.1007/s00405-022-07804-x
30. Guven, DC, Yildirim, HC, Bilgin, E, Aktepe, OH, Taban, H, Sahin, TK, et al. PILE: a candidate prognostic score in cancer patients treated with immunotherapy. Clin Transl Oncol. (2021) 23:1630–6. doi: 10.1007/s12094-021-02560-6
31. Kucuk, A, Topkan, E, Ozkan, EE, Ozturk, D, Pehlivan, B, and Selek, U. A high pan-immune-inflammation value before chemoradiotherapy indicates poor outcomes in patients with small-cell lung cancer. Int J Immunopathol Pharmacol. (2023) 37:3946320231187759. doi: 10.1177/03946320231187759
32. Liang, XL, and Wei, SZ. Predictive value of pan-immune-inflammation value for prognosis of patients with resectable colorectal cancer. Cancer Res Prev Treat. (2023) 50:5. doi: 10.3971/j.issn.1000-8578.2023.23.0150
33. Ligorio, F, Fucà, G, Zattarin, E, Lobefaro, R, Zambelli, L, Leporati, R, et al. The Pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast Cancer treated with first-line Taxane-Trastuzumab-Pertuzumab. Cancers. (2021) 13:13. doi: 10.3390/cancers13081964
34. Mesti, T, Grašič Kuhar, C, and Ocvirk, J. Biomarkers for outcome in metastatic melanoma in first line treatment with immune checkpoint inhibitors. Biomedicine. (2023) 11:11. doi: 10.3390/biomedicines11030749
35. Pérez-Martelo, M, González-García, A, Vidal-Ínsua, Y, Blanco-Freire, C, Brozos-Vázquez, EM, Abdulkader-Nallib, I, et al. Clinical significance of baseline Pan-immune-inflammation value and its dynamics in metastatic colorectal cancer patients under first-line chemotherapy. Sci Rep. (2022) 12:6893. doi: 10.1038/s41598-022-10884-8
36. Provenzano, L, Lobefaro, R, Ligorio, F, Zattarin, E, Zambelli, L, Sposetti, C, et al. The pan-immune-inflammation value is associated with clinical outcomes in patients with advanced TNBC treated with first-line, platinum-based chemotherapy: an institutional retrospective analysis. Ther Adv Med Oncol. (2023) 15:175883592311659. doi: 10.1177/17588359231165978
37. Qi, WX, Wang, X, Li, C, Li, S, Li, H, Xu, F, et al. Pretreatment absolute lymphocyte count is an independent predictor for survival outcomes for esophageal squamous cell carcinoma patients treated with neoadjuvant chemoradiotherapy and pembrolizumab: an analysis from a prospective cohort. Thorac Cancer. (2023) 14:1556–66. doi: 10.1111/1759-7714.14898
38. Şahin, AB, Cubukcu, E, Ocak, B, Deligonul, A, Oyucu Orhan, S, Tolunay, S, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep. (2021) 11:14662. doi: 10.1038/s41598-021-94184-7
39. Sato, R, Oikawa, M, Kakita, T, Okada, T, Abe, T, Tsuchiya, H, et al. A decreased preoperative platelet-to-lymphocyte ratio, systemic immune-inflammation index, and pan-immune-inflammation value are associated with the poorer survival of patients with a stent inserted as a bridge to curative surgery for obstructive colorectal cancer. Surg Today. (2023) 53:409–19. doi: 10.1007/s00595-022-02575-8
40. Sato, S, Shimizu, T, Ishizuka, M, Suda, K, Shibuya, N, Hachiya, H, et al. The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage I-III colorectal cancer patients undergoing surgery. Surg Today. (2022) 52:1160–9. doi: 10.1007/s00595-021-02448-6
41. Susok, L, Said, S, Reinert, D, Mansour, R, Scheel, CH, Becker, JC, et al. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J Cancer Res Clin Oncol. (2022) 148:3103–8. doi: 10.1007/s00432-021-03878-y
42. Topkan, E, Kucuk, A, and Selek, U. Pretreatment Pan-immune-inflammation value efficiently predicts survival outcomes in glioblastoma Multiforme patients receiving radiotherapy and Temozolomide. J Immunol Res. (2022) 2022:1–9. doi: 10.1155/2022/1346094
43. Topkan, E, Selek, U, Kucuk, A, and Pehlivan, B. Low pre-ChemoradiotherapyPan-immune-inflammation value (PIV) measures predict better survival outcomes in locally advanced pancreatic adenocarcinomas. J Inflamm Res. (2022) 15:5413–23. doi: 10.2147/JIR.S385328
44. Wang, SB, Chen, JY, Xu, C, Cao, WG, Cai, R, Cao, L, et al. Evaluation of systemic inflammatory and nutritional indexes in locally advanced gastric cancer treated with adjuvant chemoradiotherapy after D2 dissection. Front Oncol. (2022) 12:1040495. doi: 10.3389/fonc.2022.1040495
45. Yazgan, SC, Yekedüz, E, Utkan, G, and Ürün, Y. Prognostic role of pan-immune-inflammation value in patients with metastatic castration-resistant prostate cancer treated with androgen receptor-signaling inhibitors. Prostate. (2022) 82:1456–61. doi: 10.1002/pros.24419
46. Yeh, CC, Kao, HK, Huang, Y, Tsai, TY, Young, CK, Hung, SY, et al. Discovering the clinical and prognostic role of Pan-immune-inflammation values on Oral cavity squamous cell carcinoma. Cancers. (2023) 15:15. doi: 10.3390/cancers15010322
47. Yekedüz, E, Tural, D, Ertürk, İ, Karakaya, S, Erol, C, Ercelep, Ö, et al. The relationship between pan-immune-inflammation value and survival outcomes in patients with metastatic renal cell carcinoma treated with nivolumab in the second line and beyond: a Turkish oncology group kidney cancer consortium (TKCC) study. J Cancer Res Clin Oncol. (2022) 148:3537–46. doi: 10.1007/s00432-022-04055-5
48. Zeng, R, Liu, F, Fang, C, Yang, J, Luo, L, Yue, P, et al. PIV and PILE score at baseline predict clinical outcome of anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung Cancer patients. Front Immunol. (2021) 12:724443. doi: 10.3389/fimmu.2021.724443
49. Khandia, R, and Munjal, A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol. (2020) 119:199–245. doi: 10.1016/bs.apcsb.2019.09.004
50. Mantovani, A, Allavena, P, Sica, A, and Balkwill, F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
51. Tian, BW, Yang, YF, Yang, CC, Yan, LJ, Ding, ZN, Liu, H, et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy. (2022) 14:1481–96. doi: 10.2217/imt-2022-0133
52. Ocana, A, Nieto-Jiménez, C, Pandiella, A, and Templeton, AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. (2017) 16:137. doi: 10.1186/s12943-017-0707-7
53. Xiong, S, Dong, L, and Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. (2021) 14:173. doi: 10.1186/s13045-021-01187-y
54. Jaillon, S, Ponzetta, A, di Mitri, D, Santoni, A, Bonecchi, R, and Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
55. Mantovani, A, Schioppa, T, Porta, C, Allavena, P, and Sica, A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. (2006) 25:315–22. doi: 10.1007/s10555-006-9001-7
56. Huang, C, Li, Z, Li, N, Li, Y, Chang, A, Zhao, T, et al. Interleukin 35 expression correlates with microvessel density in pancreatic ductal adenocarcinoma, recruits monocytes, and promotes growth and angiogenesis of xenograft tumors in mice. Gastroenterology. (2018) 154:675–88. doi: 10.1053/j.gastro.2017.09.039
57. Ugel, S, Canè, S, De Sanctis, F, and Bronte, V. Monocytes in the tumor microenvironment. Annu Rev Pathol. (2021) 16:93–122. doi: 10.1146/annurev-pathmechdis-012418-013058
58. Gonzalez, H, Hagerling, C, and Werb, Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. (2018) 32:1267–84. doi: 10.1101/gad.314617.118
59. Pang, H, Zhang, W, Liang, X, Zhang, Z, Chen, X, Zhao, L, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric Cancer: a two-center cohort study. Adv Ther. (2021) 38:4917–34. doi: 10.1007/s12325-021-01870-z
Keywords: cancer, pan-immune-inflammation value, overall survival, progression-free survival, meta-analysis
Citation: Hai-Jing Y, Shan R and Jie-Qiong X (2023) Prognostic significance of the pretreatment pan-immune-inflammation value in cancer patients: an updated meta-analysis of 30 studies. Front. Nutr. 10:1259929. doi: 10.3389/fnut.2023.1259929
Edited by:
Gabriela Villaça Chaves, National Cancer Institute (INCA), BrazilReviewed by:
Amine Costa, National Cancer Institute (INCA), BrazilLeonardo Murad, National Cancer Institute (INCA), Brazil
Copyright © 2023 Hai-Jing, Shan and Jie-Qiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Jie-Qiong, eGlhanEwMjFAMTYzLmNvbQ==
†These authors share first authorship
 Yu Hai-Jing†
Yu Hai-Jing† Xia Jie-Qiong
Xia Jie-Qiong