
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 14 November 2023
Sec. Nutritional Immunology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1254681
This article is part of the Research TopicFrom Sea to Fork: Novel Seafood and Effects on Human HealthView all 7 articles
Seafood is highly enriched in n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFAs), particularly eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3), in contrast to the ultra-processed foods included in the modern Western diet that have high levels of n-6 linoleic acid (LA, 18:2 n-6), precursor for the pro-inflammatory n-6 arachidonic acid (ARA, 20:4 n-6). The capacity of marine lipids to reduce plasmatic triglycerides and blood pressure have been well-described. Moreover, recent studies have also raised evidence of a potential regulatory action of marine lipids on inflammation, the immune system, and food allergy (FA). FA is considered one of the main concerns to become life threatening in food safety. The prevalence of this emerging global problem has been increasing during the last two decades, especially in industrialized countries. About a 6-8% of young children and 2-4% of adults is estimated to be affected by FA. The main objective of the current study is to update the existing knowledge, but also the limitations, on the potential impact of marine lipids and their lipid mediators in regulating immunity, inflammation, and ultimately, food allergies. In particular, the focus is on the effect of marine lipids in modulating the key factors that control the sensitization and effector phases of FA, including gut microbiota (GM), inflammation, and immune system response. Results in animal models highlight the positive effect that consuming marine lipids, whether as a supplement or through seafood consumption, may have a relevant role in improving gut dysbiosis and inflammation, and preventing or reducing the severity of FA. However, more systematic studies in humans are needed to optimize such beneficial actions to each particular FA, age, and medical condition to reach an effective clinical application of marine lipids to improve FAs and their outcomes.
Lipids contained in seafood are characterized by higher proportions of n-3, long-chain (with 20 carbon atoms or more) polyunsaturated fatty acids (n-3 LCPUFAs), particularly eicosapentaenoic (EPA, 20:5 n-3) and docosahexaenoic acids (DHA, 22:6 n-3), in contrast to the lipids found in edible terrestrial animals and plants. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are a class of fatty acids with more than one double bound, or unsaturation, in which the first double bound is located between the third and the fourth carbon atom, starting from the terminal methyl group. It is well-accepted that EPA and DHA are endogenously biosynthesized at very low rates by humans and fish species (1, 2); therefore, diet constitutes an essential source to incorporate those marine lipids either for humans or fish species.
Traditionally, marine lipids have been incorporated into our diets with the intake of seafood. The content of marine lipids is highly dependent on the fish species, feeding, and season, although in general, the flesh of fatty fish is endowed with a more elevated absolute content of marine lipids (EPA + DHA) compared to that of lean fish, such as cod, hake, and pollock. This fact is due to the capacity of fatty fish, such as salmon, sardine, mackerel, or tuna, to store lipids in the flesh; meanwhile, the liver is the main lipid storage reservoir in lean fish. Thus, as an example, the content of EPA + DHA per 100 g of the flesh ranged 1.79–1.84 g for mackerel, 1.59–2.14 g for salmon, 0.98 g for sardine, levels approximately 4–10 times higher than those found in the flesh of the lean fish species cod (0.15–0.24 g of EPA + DHA), catfish (0.17–0.28 g of EPA + DHA) and haddock (0.15 g of EPA + DHA) (2).
Additionally, the consumption of nutraceutical supplements enriched in fish oil or non-fish sources, such as oil from microalgae and marine invertebrates, specifically copepods and krill, has becoming nowadays very relevant to compensate for possible deficiencies of marine lipids in our diets (2). Microalgae are the prime producers of EPA and DHA in the aquatic habitat and are essential to transfer these fatty acids to the rest of the food chain. However, it should be noted that not all microalgae can produce EPA and DHA, and among those with this capacity, the content of total lipids and EPA/DHA fatty acids may be highly different between species but also within the same species. Several environmental factors highly influence the microalgae lipid metabolism and, consequently, the production of EPA and DHA, such as salinity stress, pH, temperature, light, and the availability of nutrients, vitamins, and hormones (3). The content of total lipids has been reported to represent up to 50-77% in Schizochytrium and 30-50% in Crypthecodinium, two relevant microalgae in human nutrition, although both species are essentially producers of DHA (up to 97 % of total lipids) rather than EPA (0-2 %) (2). However, new investigations have shown an important improvement in EPA and DHA co-production with Schizochytrium by using a specific strain (4), and optimizing nutrients carbon sources (5), so attempting to match EPA/DHA ratios found in fish species. It is relevant to mention that EPA/DHA ratios may have a key relevance in the physiological activity of marine lipids. For instance, marine lipids with different proportions of EPA and DHA have shown differences in the cardiovascular effect (6–8) and the protection against inflammation and oxidative stress (9, 10).
Antarctic krill (Euphausia superba) has also received important attention as a source of marine lipids due to its abundant availability, high content in n-3 LCPUFAs (with reported values as elevated as 60% of total fatty acids), and relatively large size compared to copepods and other zooplankton (2).
Additionally, marine lipids from krill are mainly constituted by phospholipids (reaching up to 80% of total lipids), a fact that induces higher bioavailability compared to marine lipids derived from fish, mainly composed of triglycerides.
An important number of the physiological effects of marine lipids are induced by the integration of EPA and DHA into the cell membranes. The incorporation of marine lipids into the phospholipid membranes of the cell provides a specific environment that modulates physical properties, such as fluidity, and the function of membrane proteins like transporters, signaling enzymes, and receptors, but also induces that EPA and DHA can be used as substrates for the generation of effective lipid mediators with the capacity to modulate the immune system and resolve inflammation (i.e., eicosanoids, resolvins…) (1). Marine lipids can also alter membranes of different cells, including immune cells, neurons, hepatocytes, adipocytes, and cancer cells, so affecting intracellular signaling pathways and cell response. On the other hand, other investigations have shown that EPA and DHA can directly interact with specific G-protein coupled receptors, such as GPR120, which is extremely high present in inflammatory macrophages and on adipocytes, and whose interaction with marine lipids can induce effective anti-inflammatory and insulin sensitizer effects (11).
Regarding their physiological activity, clinical evidence supports that EPA and DHA have an effect in lowering the risk of cardiovascular disease, particularly coronary heart disease. Increasing evidence from human and animal investigations also suggests that marine lipids may have an effect in reducing risk of developing some cancers, particularly breast and colorectal, and protecting against different inflammatory conditions and the development of childhood allergic diseases (1, 12). The following sections will be focused on the description of the effect of marine lipids and their lipid mediators in the immune system, inflammation, gut dysbiosis, and other factors with potential impact on the development of food allergy (FA).
The two principal PUFA families, n-6 and n-3 series, are the main substrates for the formation of the bioactive lipid metabolites oxylipins. Based on the number of carbon atoms, oxylipins can be grouped in octadecanoids, metabolites derived from 18-carbon PUFAs; eicosanoids, metabolites derived from 20-carbon PUFAs; and docosanoids, metabolites derived from 22-carbon PUFAs. Oxylipins are synthesized during normal cell regulation, but even more importantly, after cell activation in pathological conditions such as stress, allergy, fever, and inflammation, functioning as signaling molecules for the control of different processes implicated in cell metabolism and immune response. Some of the biological processes regulated by oxylipins include inflammation, blood coagulation, pain response, apoptosis, cell growth, and blood vessel permeability (13).
Figure 1 summarizes the metabolization of dietary marine lipids into bioactive oxylipins relevant for the regulation of inflammation, immunity, and allergy. Oxylipin formation, including those EPA-and DHA-derived, is cell activated by the activity of a phospholipase, generally the cytosolic phospholipase A2 (cPLA2), that releases fatty acids from the phospholipid bilayer of cell membranes, which constitute the main pool of bioavailable precursors of oxylipins (13). Then, the free PUFAs are metabolized into oxylipins via non-enzymatic or enzymatic oxidation processes. Cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) epoxygenase enzymes are the main catalyzers for the production of bioactive lipid mediators via enzymatic pathways (14). It is well-recognized a competition between n-6 and n-3 PUFAs in the production of oxylipins, so the incorporation of EPA and DHA into the phospholipid membranes induces a higher generation of lipid mediators derived from EPA and DHA-, meanwhile lowering the production of n-6 arachidonic acid (ARA)-derived eicosanoids, which are generally categorized as proinflammatory mediators (15).
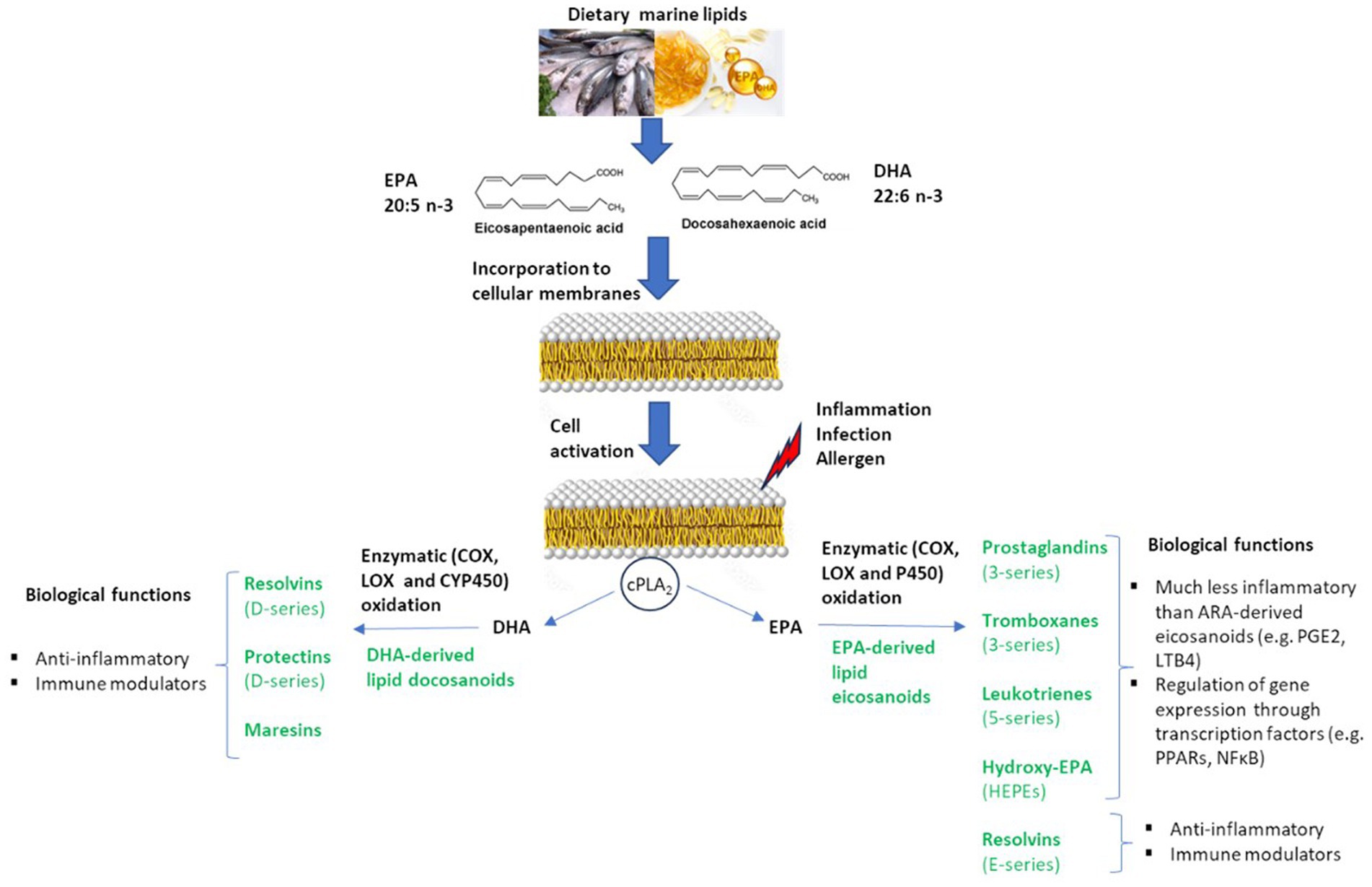
Figure 1. Biosynthesis of marine lipid-derived bioactive lipid mediators with biological capacity to modulate inflammation, immunity and food allergy.
Prostaglandins (PGs) from 3-series, thromboxanes (TXs) from 3-series, leukotrienes (LTs) from 5-series, resolvins (Rvs) from E-series, and hydroxy-EPAs (HEPEs) are principal EPA-derived eicosanoids; meanwhile, resolvins (Rvs) from D-series, protectins (PDs) and maresins (MaRs) are main DHA-derived bioactive oxylipins (Figure 1). The eicosanoids derived from EPA and DHA are considered to be less inflammatory compared to eicosanoids from ARA, or even with anti-inflammatory properties. Thus, the marine lipid-derived mediators Rvs, PDs, and MaRs have shown capacity as lipid mediators in the resolution of inflammation and the regulation of immunity, either in vitro or in vivo (14). Marine lipids, and their lipid mediators, also exert anti-inflammatory actions via regulation of inflammatory gene expression through their effects on transcription factors such as peroxisome proliferator-activated receptors (PPARs) and nuclear factor kappa B [NFκB (14, 16)].
The principal eicosanoids from ARA are the PGs and TXs from 2-series generated by COX pathways; LTs from 4-series, hydroxyeicosatetraenoic acids (HETEs) and lipoxins generated by LOX pathways; and HETEs and epoxyeicosatrienic (EETs) acids generated by cytochrome P450 epoxygenase pathways (14, 17). The incidence of inflammatory episodes is associated with excessive production of the n-6 ARA-derived leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) (15). In the modern Western diet, essentially enriched in ultra-processed foods, high levels of ARA can be maintained in cellular membranes due to its low content of n-3 PUFAs and high levels of the n-6 linoleic acid (LA, C18:2n-6), that is the main precursor for n-6 ARA (15, 17). On the contrary, dietary marine lipids have shown an effect on modulating the profile of oxylipins and decreasing the levels of proinflammatory cytokines. Previous investigations in animal models demonstrate that the consumption of marine lipids causes a replacement of the n-6 ARA by the n-3 EPA and DHA in membranes from erythrocytes and tissues, and that accordingly increased the content of EPA-and DHA-derived oxylipins, while reducing the production of n-6 arachidonic-derived eicosanoids (9, 18, 19). This modification of the profile of oxylipins toward the production of EPA-and DHA-derived mediators, instead of the generation of the pro-inflammatory ARA-derived eicosanoids, agreed with an improvement for parameters of inflammation, oxidative stress, and protein oxidation (9, 10). The consumption of marine lipids also increased the content of EPA and DHA in inflammatory cell membranes and subsequently reduced the generation of ARA-derived eicosanoids that induce the production of proinflammatory cytokines in macrophages and cause pain and vasodilation (15).
In allergic diseases, ARA-derived eicosanoids are generated from cells during allergic responses and clinical symptoms, including bronchial spasms, blood pressure variations, and diarrhea (20). In the immune system, eicosanoids are produced predominately by antigen-presenting cells of the immune system and have specific effects on dendritic cells. EPA-and DHA-derived lipid mediators have shown pro-resolving functions by inhibiting neutrophil accumulation into inflammatory sites and promoters of apoptotic cell clearance by macrophages (20). These mediators can regulate many types of inflammatory and immune cells, including T cells, dendritic cells, eosinophils, and mast cells. Particularly, the EPA-derived resolvin E1 (RvE1) and DHA-derived protectin D1 (PD1) promote phagocyte removal during acute inflammation by regulating leukocyte infiltration, increasing macrophage phagocytosis of apoptotic polymorphonuclear neutrophils, and enhancing the appearance of phagocytes carrying zymosan in lymph nodes and spleen (21). DHA-derived maresins (MaR) are macrophage mediators in resolving inflammation, being MaR1 identified first in self-resolving inflammatory exudates with human macrophages via 12-LOX-initiated mechanisms (22). This investigation suggested the beneficial actions of maresins in tissue homeostasis, inflammation resolution, wound healing, and host defense. Dyall et al. (17) have recently published a study about PUFA-related lipid mediators, including those derived from the EPA and DHA, that provides updated information about their biosynthesis, structures, and biological function.
Several studies have shown that n-3 marine PUFAs play a vital role in reducing the risk of infants developing FA when pregnant and lactating women are supplemented with fish oil (23). Accordingly, Kunisawa et al. (24) reported that n-3 fatty acids play a role in alleviating FA by converting EPA into 17,18-epoxyeicosa-pentaenoic acid, in the gut of a murine FA model. This EPA-derived metabolite showed an in vivo anti-allergic effect by decreasing the incidence of allergic diarrhea due to impairment of mast cell degranulation without affecting allergen-specific serum IgE. In the next sections, factors and mechanisms responsible for the immune response in FA, as well as the potential role of marine lipids to mitigate hypersensitivity to food allergens, will be deeply described.
FA is a relevant and communal health concern according to the World Health Organization (WHO). In the European Union (EU), the European Food Safety Authority (EFSA) has indeed identified 14 foods as major allergens: wheat, fish, mollusks, crustaceans, milk, eggs, peanut, nuts, soybean, sesame, mustard, celery, lupin, and sulfur dioxide/sulfites. Up to the present time, the unique demonstrated and successful remedy for this class of sensitivity is to follow a regimen restricted in the allergenic food and the products. The clinical symptoms of FA emerge within 60 min of consumption and involve nausea, vomiting, acute urticaria, diarrhea, asthma, and wheezing. In the gravest situations, potentially life-threatening anaphylactic shock can occur (hypotension, trouble breathing, and weak pulse) (25, 26).
FAs are attributable to a distorted reaction of oral tolerance to food antigens. Early works on allergic sensitization indicated that this process is initiated because of the alteration of the regular oral tolerance in the gastrointestinal (GI) tract by the regulatory T cells (Tregs), that generate the preservation of tolerance to foodstuff antigens, which are distorted and substituted by the initiation of an effector immunological reaction diverged toward an allergenic T cell reaction headed by Th2 cells. Then, the effector Th2 cells are proposed to control the IgE response through the secretion of IL4, IL5, and IL13 cytokines. These specific cytokines are essential for B cell class converting, production of specific IgE, extension of allergic effector cells, and occurrence of clinical manifestations (27). Figure 2 shows a summary of the main factors and triggers that induces a FA immune response.
However, current progress in high-throughput methodologies and data analysis is moving this simplistic vision to a more complex model in which different T-cell lineages, such as T-cell receptor (TCR)αβ, TCRγδ, regulatory T cells (Tregs), and follicular T helper cells (Tfhs) are involved (28). Interestingly, the subset Tfh13 has also been demonstrated to be required to produce high-affinity IgE, which is responsible for the anaphylactic reactions (29).
Concerning the possible origin of tolerance rupture, external damages and innate triggers have been indicated as in charge of the cytokine liberation by intestinal epithelial cells (IECs) of thymic stromal lymphopoietin, IL-33 and IL-25, that perform a crucial task in the initiation of allergic reactions at the mucosa line (30). The combined emission by IEC has been exposed critical for the sensitization to food antigens, while the liberation of some of these cytokines can continue a determined FA (31). Additionally, IECs have described as a main resource of eotaxin in the intestine that controls the release of eosinophils, which are correlated to the gravity of the intestinal symptoms against food allergens (32).
The immune mechanisms of FA maintenance continue to be unclear. In this concern, the main task executed by the allergen-Th2 cells in starting the allergic reply has newly been defined (33). The authors found a subgroup of allergen-specific memory Th2 cells distinguished by a specific signature (CD45RB-CD27-) and the secretion of CD161, CD49d, CRTH2. Such cells present several operational characteristics different from conservative Th2 cells, involving the mutual liberation of different Th2-associated cytokines (IL13, IL4, IL9, IL5). Additionally, this affected T cell subgroup has demonstrated an established allergic-associated phenotype.
Animal studies demonstrated that the ingestion of antigens via oral treatment is operative in the generation of oral tolerance of food. Therefore, mice consuming oral proteins prompts peripheral allergen-mediated Foxp3+ CD25 + - Tregs and provokes modulatory reactions dependent on TGF-b secretion (34). More Treg subgroups that have been related to the stimulation of oral tolerance are Th3 and Tregs type 1 (Tr1). The Tr1 cells are categorized by exhibition of the CD49b and the LAG-3 in the expression of absent CD25 and Foxp3 manifestation (35). Then, Th3 cells are recognized by the superficial production of latent-related peptides, and they besides have restrictive actions due to TGF-b production (36). In both cases, their responsibility in oral tolerance stimulation has been associated with the defeat of immunological reactions via IL-10 liberation (37). While Tregs specificity appears to be tolerance contrasted with allergy to aero-specific Tregs to foodstuff antigens have not ocurred exposed changed in allergic patients contrasted to healthful persons (38). These details limit the current comprehension of the function of Tregs in FA in persons.
Furthermore, accumulative confirmation proposes that sensitization in FA can be established by non-oral ways, such as through the skin. Thus, primary cutaneous contact with food antigens across a disturbed skin wall endorses allergic sensitization before the primary digestion of food, as divergent to the tolerogenic character of oral contact. Thus, double interaction indicates that contact with food allergens across disturbed skin endorses sensitization, while primary experience with food allergens across oral via stimulates tolerance (39). In effect, there is a robust connection between sensitization to food allergens and eczematous skin or atopic dermatitis (40). However, while topical contact has been suggested as a way of sensitization in FA, investigational data have established that skin is not integrally sensitizing, as skin presentation of food allergens in the deficiency of peripheral adjuvants (41). Additionally, cutaneous sensitization to food allergens may necessitate the influence of supplementary elements, such as skin wall alterations (42) and the incidence of adjuvants like toxins created by microorganisms growing in the skin (43). These confirmations sustenance the premise that, in several circumstances of skin wall inflammation or alteration, food allergens sensitization can be provoked by the skin.
Regarding the other T-cell subsets (TCR)αβ, TCRγδ, and follicular helper other T (Tfh) cells studies from food-allergic patients and murine models indicate that Th2 and some Tfh cells induce the production of IgE by B cells. γδ T cells in healthy and allergic subjects harbor both IgE-enhancing and suppressive subsets, positioning them as master regulators of oral tolerance (44–46). Recently, Merino et al. (47) have achieved the allergic sensitization of BALB/c mice with a systemic specific IgE and a perturbed TCRγ chain repertoire in Peyer’s patches using supplementation with the major fish allergen beta-parvalbumin and alum as an adjuvant. Preliminary mRNA massive sequencing experiments have linked the sensitization to an increase in TCR-Vγ1 expression in Peyer’s patches of the sensitized mice. The involvement of these TCR-Vγ1+ cells in asthma and airway inflammation has also been reported (44). All these data suggest a role of this T-cell subset in allergic IgE-mediated response. However, the molecular mechanisms underlying the control of IgE-mediated immune response by γδ T-cells and the role of each γδ T-cell subset in allergic disease remain elusive. It must be stressed that opposite to the FA studies in humans limiting the study to immune cells in peripheral blood, the use of mouse models allows to target the mucosal and compare it with the systemic level (48).
Besides the dual assumption, the hygiene hypothesis furthermore increases attention. Industrialization and urbanization, together with an intensification of primary contact with cleaning products and antimicrobials, increase exposure to chemical diversity, reduce microbial outdoor exposure, and alter human microbiota (49). Primary microbial contact has repercussions with FA, as confirmed with germ-free mice, because these animals are extremely predisposed to anaphylactic reactions to food. The contact of neonatal babies with the maternal vaginal area, the outer bacteria and breast milk, produces the induction of microbial receptors and the expansion in the mucosa of tolerogenic immunological nets that defend of allergic responses. Augmented ratios in birth antibiotic use and cesarean deliveries have been decreasing the neonate microbial contact to the maternal microbiota and the soil pets’ microbiotas and farming animals’. Such as circumstances have been related to human health with the amplified transition from infectious to non-communicable illnesses, including FAs. Moreover, nutrition is the major powerful modulator of microbiota, prompting variations in microbiome and bacteria-resulting metabolites. Truthfully, short-chain fatty acids (SCFAs) and dietary fiber endorse regulatory immune reactions and protein breaks by the intestinal microbiome give rise to amino acid-resulting metabolites with immunological modulatory features (50). All these aspects are discussed in detail in the next section.
It is currently well-established that individuals suffering from FAs display an intestinal microbiota with a different microbial composition than in healthy conditions. The GI tract is composed of thousands of microorganism species, including bacteria, protists, fungi, and viruses as bacteriophages, each one with a specific direct or indirect role in the prevention of the development of allergies. The gut microbiome is modified from birth to death, and many factors are involved in these changes. Factors associated with GI microbial composition modifications that are involved in the development of allergies are the diet, the manner of birth, environmental interactions and pet exposition, and administration of medical therapies such as the use of antibiotics (51). Strict maintenance of microbiota composition is required to avoid food intolerances as the alterations can lead to dysbiosis, consequently producing inflammation and pathogenesis in the gut resulting in the development of FA (52). Dysbiosis in the gut produces GI tract malfunction resulting in occasions in a breach in the intestinal barrier, modifying GI permeability. This can cause that gut antigen may reach the bloodstream producing allergic reactions in other organs (53). The modulation of gut microbiota (GM) seems to be a good strategy for treating allergies (54, 55). Different studies of microbial transplants in mice and humans have demonstrated the implication of dysbiosis in FAs. Fecal transplants and other bacterial therapeutic approaches such as probiotics, prebiotics, and symbiotics can provide prevention and treatment of FAs (52).
Those factors affecting intestinal microbiota are involved in microbiome development during life. In healthy conditions, Bifidobacterium and Lactobacillus genera predominate in the post-natal gut microbiome, which provides a healthy immune regulatory response involved in gut T effectors (helper T cells) and secretion of IgAs. With the introduction of solid food in infants, appear new species of the orders Clostridiales and Bacteroidetes, which play a role in the suppression of IgE typically found in FA (56). The diminution of Clostridiales, and more specifically, species of the genera Leuconostoc, Weissella, and Veillonella during the first year of life, make babies can suffer FA (51, 56–58). Moreover, different bacteria, such as Enterobacteriaceae and Parabacteroides, and families, such as Lachnospiraceae and Ruminococcaceae, can improve these FA conditions (59). Lacto-dysbiosis also influence the development of FA with the reduction of lactate-utilizing bacteria, such as Eubacterium and Anaerostipes, that produce butyrate (57).
Several studies have evaluated the association between GM and the increase of allergies. It was demonstrated that species of Clostridiales and Bacteroidales taxa inhibit in mice the development of FAs via the induction of the retinoic orphan receptor gamma T (RORγt+) in T regulatory cells (Tregs) decreasing GATA-31 (GATA binding protein 31) Tregs and IgE while enhancing IgA production (56, 60). Moreover, as mentioned above, fecal microbial transplantation of beneficial commensal bacteria can be used to prevent and treat allergies by inducing RORγt+, promoting tolerance to dietary antigens (57, 58, 61).
Species of Lachnospiraceae, Streptococcaceae, and Leuconostocaceae are abundant in the GI of children with egg allergies. It was studied how having older siblings or a pet dog reduced the possibility of developing egg allergies in infants (62). Another study evaluated the importance of acquiring commensal microorganisms from mothers during the process of birth by vagina delivery, while cesarean delivery leads to the development of FAs in children (51).
A study was performed on 18 pairs of twins from 6 months to 58 years of age. In 13 of these pairs of twins, one of them suffered from a FA, and in the remaining 5 pairs, both twins suffered from FA. Results showed a higher number of differences in bacterial composition in fecal samples between the first set of twins with and without FA individuals. This research has demonstrated the protective effect of some microbial families preventing the development of FA and their role in the GI (59). Moreover, there were found higher concentrations of specific commensal-produced metabolites, such as diacylglycerol, in healthy twins. The main responsible for diacylglycerol production in GI was Phascolarctobacterium faecium, while Ruminococcus bromii plays a role in both starch digestion and the metabolism of amino acids and sterol (59).
Studies in germ-free (GF) mice have also shown the importance of microbial GM in the prevention and development of FAs (63). GF mice received fecal microbiota transplants from animals with FAs and developed the same type of FA as the donor. Other analyses were performed to study the influence of Anaerostipes caccae in GF mice. The animals were protected from the development of FA. In addition, orally administered Citrobacter koseri increased systemic allergic symptoms in allergic mice by inducing IL-33 release (64). Furthermore, a murine model (Il4raF709; C.129×1-Il4ratm3.1Tch) that is prone to FA and harbors a gain-of-function mutation in the Interleukin-4 receptor-alpha chain (IL-4Ra) is susceptible to FA associated with changes in the GM (65). This mouse model was the tool of several studies to evaluate the relationship between FA, microbiome composition, and the corresponding immune responses produced (57, 58, 66).
GM dysbiosis is directly related to the development of inflammatory disorders such as allergies. At this point, the consumption of specific food supplements may modulate the GM affecting human health. In this sense, the marine environment is an important source of bioactive compounds, including polysaccharides such as algae-compounds (alginate, fucoidan) and animal-derived polysaccharides (chitin, chitosan) active peptides and PUFAs (67).
Dietary lipids display different results on GM dysbiosis depending on the type of fatty acid (68–70). Some microorganisms use n-3 PUFAs to produce secondary metabolites. Changes in GM have been observed after diet supplementation with marine lipids. Lactic acid-producing bacteria, among others, can produce PUFA-derived intermediate metabolites, and these PUFA-derived bacterial molecules provide beneficial anti-obesity and anti-inflammatory effects (71). Particularly, marine n-3 EPA and DHA have an important role in the modulation of the diversity and abundance of GM (72–74), and such impact on GM has been related to an attenuation of the metabolic dysfunction associated with obesity when supplemented with marine lipids (75). Moreover, marine lipids improve the barrier function of the intestinal mucosa and intestinal microenvironment, and also increase intestinal mucosal thickness. In addition, marine lipids are involved in weight loss by modulating fat metabolism genes (76). The metabolism and absorption of n-3 PUFAs are influenced by GM; otherwise, the information about the direct implication of these lipids in GM is limited. The composition and diversity of GM is modulated by marine lipids, modifying the present proinflammatory mediators and balancing the levels of SCFAs (77).
Studies using animal models (Table 1) have demonstrated a clear association between dietary fatty acids and changes in GM (97). Dietary intake of fish oil can significantly affect the diversity of the intestinal flora compared to sunflower oil. This effect is due to the high levels of n-3 PUFAs present in fish oil, particularly EPA and DHA, which can modify the GM (98, 99). In addition, n-3 PUFA intake increases the growth of Bifidobacteria while decreasing the growth of Enterobacteria, providing the inhibition of inflammatory mediators linked to metabolic endotoxemia (100). Bifidobacteria and Lactobacilli, among other anaerobic bacteria, partially metabolize n-3 PUFAs in the distal intestine. This metabolism can affect the distribution of the intestinal flora, as it promotes the growth of beneficial bacteria, such as Bifidobacterium, while inhibiting the growth of potentially harmful bacteria (101, 102). It has been elucidated how marine lipids increase the richness of Bifidobacteria in the gut of male rats (103) and can prevent GM dysregulation in mice (104), increasing the abundance of commensal bacteria that produce lactic acid and Bifidobacteria in the gut of the mice fed a high-fat diet (84, 105).
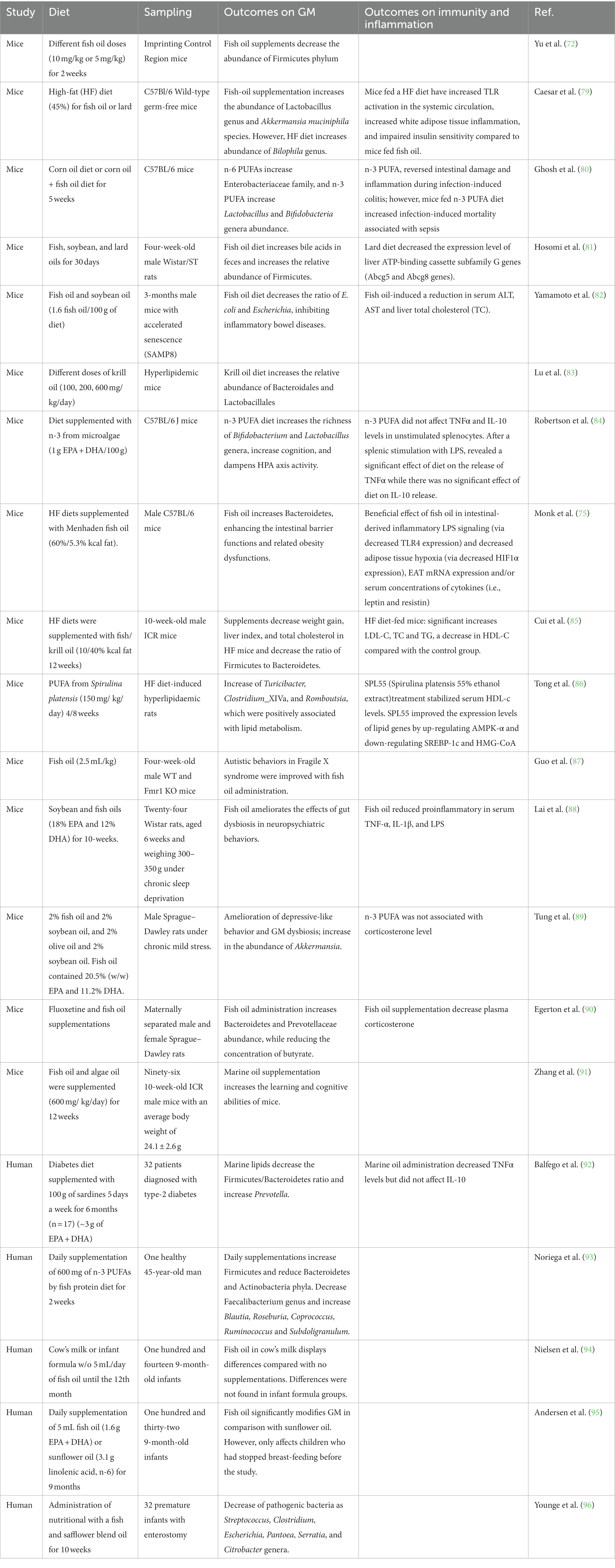
Table 1. Summarized studies that evaluate the influence of marine lipids on gut microbiome (GM), immunity and inflammation [Modified from Costantini et al. (78) and Wang et al. (67)].
Most of the research on marine lipids in microbiota focuses on Bacteroidetes and Firmicutes phyla in animal models as they are the two principal phyla of bacteria in the human gut microbiota (GM). n-3 PUFAs from fish oil decrease the population of Firmicutes (72). Moreover, an increase in the Firmicutes/Bacteroidetes ratio (F/B ratio) is related to metabolic conditions such as obesity, insulin resistance, and non-alcoholic fatty liver disease because of the synthesis of SCFAs. Marine lipids exert a positive effect by increasing the production of anti-inflammatory compounds, such as SCFAs (106). Butyric acid-producing bacteria degrade nonfermentable dietary fibers into SCFAs, such as butyrate (107). The addition of marine lipids to Salmonella-infected mice substantially rises the production of SCFAs by increasing the abundance of SCFA (butyrate)-producing genera (108, 109), such as Blautia, Bacterioides, Roseburia, and Coprococcus (110) and Lactobacillus in the mouse intestinal tract (111). In addition, it has been studied how an imbalanced n-3/n-6 PUFA intake provides dysbiosis (112, 113). n-3 PUFAs modify the content of commensal gut bacteria, particularly Akkermansia (112). Caesar et al. (79) demonstrated that the type of dietary fat can significantly impact the GM. The study found that rats fed a fish oil diet had a higher abundance of Lactobacillus and Akkermansia muciniphila, while those fed a lard diet had higher levels of Bilophila. These findings suggest that fish oil may have anti-inflammatory effects through its impact on the GM. These results are consistent with other studies showing that marine lipids can modulate GM and improve metabolic health (79).
Ghosh et al. (80) investigated the effects of dietary fat on GM in mice. The researchers found that diets supplemented with fish oil decreased Enterobacteriaceae and Clostridia abundance. In addition, Monk et al. oil (75) evaluated the consequences of the administration of fish oil in GM content and their effects on the epithelial barrier. Male mice were daily fed a high-fat diet based on meat or a high-fat diet based on fish oil. The supplementation with fish oil provided an increase in the content of Firmicutes and Bacteroidetes phylum. Moreover, oral glucose tolerance was improved, providing intestinal health. Furthermore, n-3 PUFAs can improve symptoms of Inflammatory bowel disease (IBD) by reverting the microbiota composition, increasing the abundance of the Escherichia, Faecalibacterium, Streptococcus, Sutterella, and Veillonella genera (114) and inhibiting the growth of the Bacteroides, Flavobacterium, and Oscillospira genera resulting in the decrease of Firmicutes/Bacteroidetes (F/B) ratio (115). Moreover, Yamamoto and colleagues (82) have evaluated the effect of fish oil administration on aging. It is known that intestinal inflammation is affected by aging; otherwise, it was decreased by a moderate-fat diet supplemented with fish oil in mice. In addition, it was shown a decrease in the bacteria that are involved in energy consumption with aging. Other studies evaluated different fat sources, such as soybean oil, lard, menhaden oil, or tuna oil (81). The results showed that dietary fats affected differently the relative composition of fecal microbiota and bile acid metabolism, and in particular, menhaden oil increased fecal bile acids excretion compared with soybean oil and lard diets. Fecal BA excretion was found to be directly associated with the relative abundance of Firmicutes, and negatively associated with the relative abundance Bacteroidetes. This investigation also suggested that the impact of fish oils on the fecal microbiota may vary greatly correlated to the ratio of EPA to DHA and the composition of fatty acids other than n-3 PUFA. Guo and colleagues (87) administrated fish oil to four-week-old male WT and Fmr1 KO mice and demonstrated that fish oil ameliorates autistic behaviors and gut dysbiosis in fragile X protein (FMRP)-deficient mice (87). Moreover, dietary marine lipids have also been involved in inhibiting the production of proinflammatory mediators and in the restoration of GM, which is produced by life stress (90). Tung and coworkers (89) have studied how fish and olive oils ameliorate dysbiosis and depressive-like symptoms. Results suggest that fish oil, but not olive oil, improves depressive-like behavior and GM homeostasis in rats under chronic mild stress. In addition, Lai et al. (88) have evaluated how fish oil ameliorates the effects of gut dysbiosis in neuropsychiatric behaviors in rats under chronic sleep deprivation, accordingly with a reduction of proinflammatory markers in serum.
Another study has been performed to investigate the effects of fish and krill oils as supplements on a high-fat diet to reduce weight in mice (85). Results showed that dietary fish and krill oils were effective in the modulation of GM and the reduction of weight, liver index, and total cholesterol in high-fat diet-induced obesity mice. Lu et al. (83) also evaluated the role of different doses of dietary krill oil in modulating microbial communities at different gut locations (ileum and colon). Robertson et al. (84) investigated the effect of dietary marine lipids on pregnant mice and their male offspring. The study’s findings indicate that the addition of marine lipids from microalgae led to slight changes in the behavior of the male offspring and showed a strong ability to modulate cognitive function. The study found that the F/B ratio increased, indicating a shift in the overall composition of the GM. Additionally, the study found an increase in the abundance of the genera Bifidobacterium (Actinobacteria phyla) and Lactobacillus (Firmicutes phyla) (84). The effect of lipids from the microalgae Spirulina, as a dietary supplement, was investigated in rats fed a high-fat diet (86). The study found that this dietary intervention led to a reduction in hepatic lipid accumulation and steatosis, which are markers of non-alcoholic fatty liver disease (NAFLD). Additionally, the study found a decrease in the relative abundance of three bacterial genera Turicibacter, Clostridium_XIVa, and Romboutsia, that were positively associated with lipid metabolism.
Several studies (Table 1) have evaluated how a diet rich in marine n-3 PUFA leads to an increase in the presence of lactic acid bacteria and Bifidobacterium spp. in the human gut while saturated fatty acids disrupt the homeostasis of the GM components, promoting inflammation genera such as Bilophila or Bacteroides (93, 116). A high monounsaturated fatty acids diet during pregnancy can impact GM richness and diversity and potentially have negative effects, such as a relative increase of Salmonella spp. in feces. This highlights the importance of considering dietary fat quality and its effects on GM when designing interventions for pregnant women (117). In addition, Balfego et al. (92) studied how a diet rich in sardine affects type-2 diabetes, and the ratio of Phytoplankton/Bacteroidetes and TNFα levels were reduced after administration. Another study showed that enteral supplementation of a fish and safflower blend oil in premature infants with enterostomy led to greater bacterial diversity and a decrease in the abundance of some pathogenic bacteria, such as Streptococcus, Clostridium, Escherichia, Pantoea, Serratia, and Citrobacter (96). Noriega and colleagues (93) conducted a study in 2016 to analyze the effect of the supplementation of marine lipids for 2 weeks on the GM of a 45-year-old man. Results showed that fish lipid supplementation enhanced the abundance of the Firmicutes phylum while reducing the abundance of Bacteroidetes and Actinobacteria. Additionally, the Faecalibacterium genus was reduced, and the Blautia, Roseburia, Coprococcus, Ruminococcus, and Subdoligranulum genera abundance was increased; some of these genera are linked to the synthesis of the SCFA butyrate (93). Overall, this study is new evidence that marine lipids may have some effect on the composition of human GM, particularly on the content of specific beneficial bacterial taxa.
Nielsen and colleagues (94) investigated the implication of n-3 PUFAs in infant microbiota. Infants were fed with cow’s milk or infant formula w/o fish oil from 9 to 12th months of age. The results showed that fish oil supplementation in cow’s milk groups developed a different GM composition than in the infant formula groups. The same research group performed a similar study to evaluate microbiota composition in nine-month-old infants fed daily with fish oil or sunflower oil for 9 months (95). Results showed that fish oil could beneficially modify microbiota in comparison to sunflower oil; however, these changes were only visualized in infants who stopped breastfeeding before supplementations, suggesting that breastfeeding is also involved in microbiota composition. The authors suggested that cessation of breastfeeding allows the introduction of new bacteria to the infant gut microbiota, and thus supplementation with fish oil may have a greater impact on gut microbial composition in non-breastfed infants. In another study, in elderly population w/o HIV was administered fish oil supplements for 12 weeks. Gut barrier function and inflammatory factors related to the tract were evaluated, and the results showed fish oil supplements were able to reduce inflammation and intestinal permeability in this elderly population (118).
Therefore, in general, marine lipids have demonstrated capacity to modulate GM and improve gut dysbiosis by increasing the abundance and diversity of beneficial bacteria in animal models and in human studies, although more clinical studies are required to evaluate how factors such as the protocol for supplementation (time, doses), marine lipid compositions (ratio EPA/DHA), age (children vs. adult/elderly people), or even if infants are breastfed or formula fed, are modulating effect of marine lipids on the GM.
Several investigations support that marine lipids have the potential for anti-allergic activity by decreasing allergic symptoms. An enhancement in n-6/n-3 PUFA ratio cause disruption in the homeostasis of Th1/Th2; however, dietary marine n-3 LC-PUFAs are related to the reduction of Th2 and Th1 reactions, Treg cells frequency increases and IgE levels are decreased (119, 120).
Several animal models have been applied to investigate the effect of marine lipids (fish oils, EPA, DHA, or mixtures) on the development of FAs and related inflammatory processes. A study conducted on mice investigated the effects of feeding mice with EPA, DHA, or EPA + DHA, compared to corn oil (121). Results found that feeding mice with fish oil reduced the activation and differentiation of T cells in the spleen into pro-inflammatory Th17 cells compared to corn oil. This research also found that the expression of the pro-inflammatory cytokine IL-17 and the transcription factor RORγt were reduced without affecting Treg cell polarization in mice fed fish oil. Globally, these results show that marine lipids have a direct impact on the development of Th17 cells, which can be considered an anti-inflammatory mechanism via the suppression of this inflammatory T-cell subset (121). In addition, other studies have reported that fish oil reduces signs of FA in ovalbumin-sensitized mice (122) and prevents allergy sensitization to cow’s milk protein in mice (123). In this last investigation, the anti-allergy effect of marine lipids to cow’s milk allergy was related to an improvement of local intestinal and systemic Treg and a reduction of acute allergic indicators (123). Differences in the effect of EPA and DHA on FA have also been described. In particular, the supplementation of a DHA-rich fish oil was found to be more effective in suppressing allergic symptoms in mice allergic to whey and peanut compared to an EPA-rich fish oil (124).
Several clinical studies have been performed to evaluate the effects of marine lipids on allergies (Table 2). Results of multiple prospective studies found that lactating mothers who were fed diets rich in fish oils, such as tuna, salmon, or sardines, tend to have a higher EPA concentration in their breast milk. Furthermore, infants whose mothers consume these foods may have a lower risk of developing allergic diseases (133). Ellul et al. (134) found that the supplementation of fish oil in antenatal stages was related to higher levels of DHA and other n-3-related metabolites in infants at 1 year of age. This finding suggests that the intake of marine lipids during pregnancy may have benefits for infant health and development. On the contrary, higher levels of n-6 PUFAs were associated with an increased risk of developing FA (134). However, other studies that evaluated maternal supplementation with fish oil have found that offspring protection against food allergen sensitization was not related to maternal fish oil supplementation, although sensitization was lower than in the control group (127, 129). Similarly, another study performed on 657 infants breastfed from mothers administrated with tuna oil or soy oil supplements did not report a significant effect of fish oil supplementation on the incidence of FA (126).
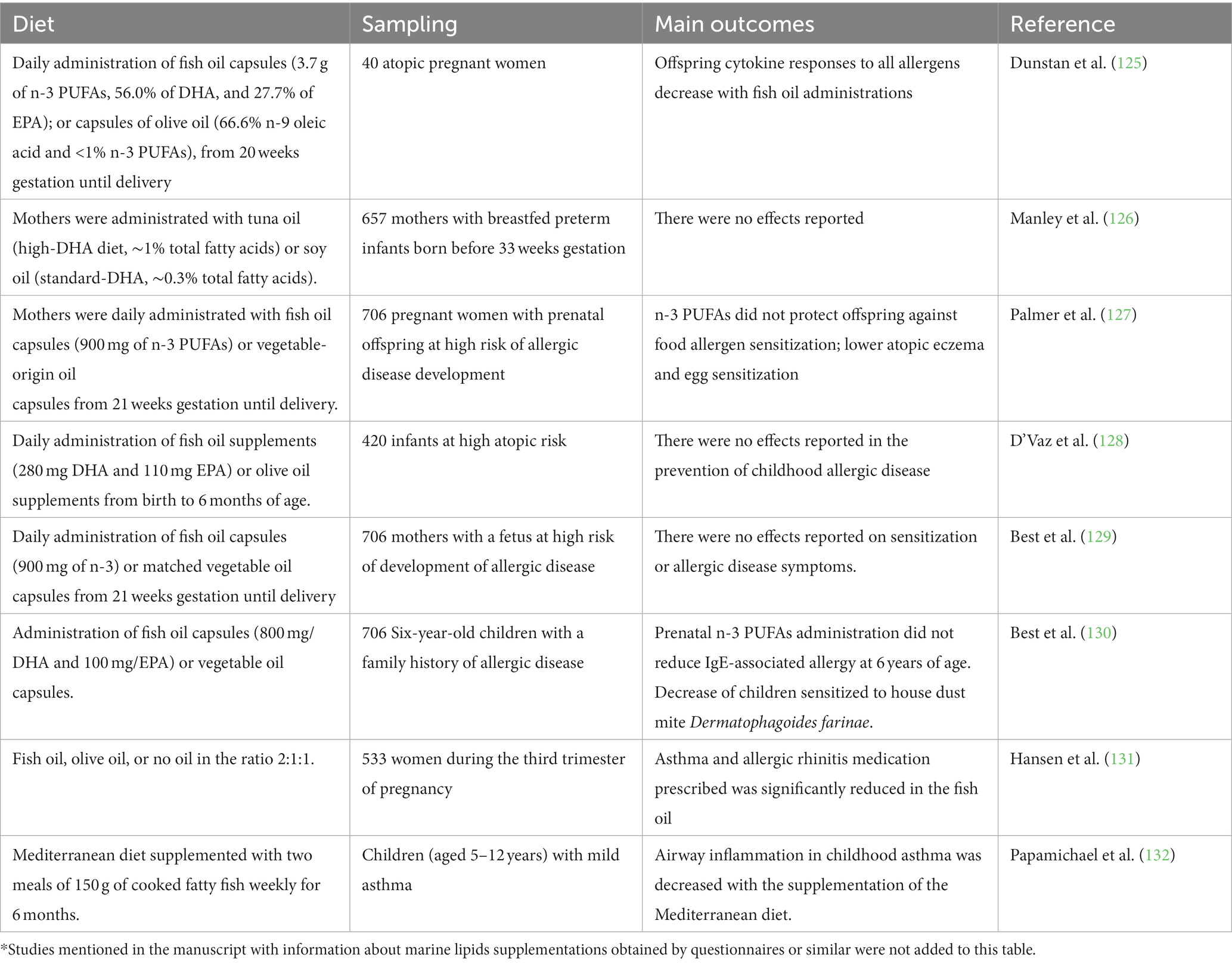
Table 2. Summarized clinical studies investigating how the marine lipids administration and complement influence allergies*.
Aldámiz-Echevarría et al. (135) evaluated the content of fatty acids in the plasma of children with multiple FAs, reporting significantly lower levels for the n-3 EPA and DHA compared to healthy children. This deficiency in n-3 EPA and DHA can be more dramatic in children with fish allergy (136), depending on how many fish species this individual is allergic to (137) since fish consumption is a major source of dietary EPA and DHA. Dunstan et al. (125) have reported in infants sensitized to hen’s egg through the skin, that allergy can be reduced by about one-half in infants of mothers who were previously administrated fish oil (3.7 g n-3 PUFAs per day) (125). D’Vaz et al. (128) found that daily fish oil supplementation, which contained 280 mg of DHA and 110 mg of EPA, improved the n-3 PUFA status of infants at high atopic risk. However, the study also found that fish oil administration did not protect against the occurrence of allergic outcomes, including sensitization, eczema, asthma, or FA, in infants at high atopic risk (128).
Although there is evidence suggesting that consuming fish during infancy and childhood may reduce the risk of developing allergic diseases, results from epidemiological studies are not entirely consistent (138). Different meta-studies have analyzed the effects of n-3 PUFA content of fish oil supplementation in pregnancy, lactation, infancy, or childhood, and discrepant results have been obtained. Fish oil administration is related to modifications in cytokine levels in cord blood and peripheral blood. These modifications provide the reduction of Th2 cytokine production; however there is less evidence about the effects on the Th1 response (71, 128, 138–143). The latest meta-analyses involved in the evaluation of the potential supplements of PUFAs given to infants or pregnant or breastfeeding mothers did not reveal any evidence of preventing the progress of asthma, dermatitis, or FA in infants or childhood (130, 144, 145). These results should be due to the use of different durations for supplementation and intakes, variable sources of PUFAs, and different n-3 to n-6 ratios (145, 146). Moreover, available results of antenatal fish oil administration on allergic respiratory diseases have not yet provided conclusive results (130, 131). Moreover, adults with diets rich in fish are suggested to prevent the development of inflammatory processes, such as rheumatoid arthritis (147), and enhance pulmonary function in asthma (132).
In summary, studies in animal models showed that the intake of marine lipids is able to decrease the outcomes for several FAs, according with a reduction on the activation and differentiation of T cells into pro-inflammatory variants, as well as the expression of pro-inflammatory cytokines. However, different clinical studies have produced inconclusive results regarding the potential benefits of marine lipids on fatty acids.
The present investigation provides the most recent information available about the impact that dietary marine lipids, essentially the n-3 EPA and DHA fatty acids consumed as supplements or directly in seafood, may have on modulating FAs and their immunological outcomes. FA is a biological response of the immune system to specific food components (allergens) with an important inflammatory component. Marine lipids can be physiologically metabolized to the bioactive lipid metabolites oxylipins that can regulate inflammation and immunity. In general, EPA-and DHA-derived lipid mediators are less inflammatory than those mediators related to n-6 ARA, and even some of them may act to resolve inflammation, reporting, in general, an anti-inflammatory effect. Dietary marine can also modulate GM structure and composition with potential human health benefits, such as in the case of FAs. Moreover, the intake of marine lipids is found to be effective in reducing activation and differentiation of T cells into pro-inflammatory T cell variants and expression of pro-inflammatory cytokines. Thus, abundant studies in animal models report the beneficial effects of dietary marine lipids to reduce outcomes for several FAs, although different clinical studies were found to be inconclusive about the potential beneficial effect of marine lipids on FA. To our best knowledge, these discrepancies in the results could be explained by a different mechanism of action of marine lipids depending on the food allergen, the use of diverse protocols for supplementation (time, doses), and fish oils with different compositions in terms of the content of EPA, DHA and, n-3 to n-6 ratios. In conclusion, the current study highlights the potential of marine lipids, whether consumed as a supplement or through seafood, to improve food allergies by reducing inflammation and regulating the gut microbiome and immune system. However, further systematic clinical investigations are required to tailor these beneficial effects to specific food allergies and individual circumstances. In the future, successful clinical applications of marine lipids can be expected to enhance the management of food allergies.
AGA: Conceptualization, Writing – original draft, Writing – review & editing. MC: Conceptualization, Writing – original draft, Writing – review & editing. MP: Conceptualization, Writing – original draft, Writing – review & editing.
AGA thanks USC for the “Convocatoria de Recualificación do Sistema Universitario Español-Margarita Salas” postdoc grant under the “Plan de Recuperación Transformación” pro-gram funded by the Spanish Ministry of Universities with European Union’s NextGeneration EU funds. The study was financed by the GAIN-Xunta de Galicia Project (IN607D 2017/01) and the Spanish AEI/EU-FEDER PID2019-103845RB-C21 project. The study was also supported by the Plan Complementario en Ciencias Marinas (PCCM), funded by the Ministry of Science and Innovation (Activity 3.6.B. NANOSEAOMICS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Calder, PC. Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc. Nutr. Soc. (2018) 77:52–72. doi: 10.1017/S0029665117003950
2. Demets, R, and Foubert, I. Traditional and novel sources of long-chain omega-3 fatty acids. Omega-3 Deliv Syst. (2021):3–23. doi: 10.1016/B978-0-12-821391-9.00013-2
3. Perdana, BA, Chaidir, Z, Kusnanda, AJ, Dharma, A, Zakaria, IJ, Syafrizayanti, BA, et al. Omega-3 fatty acids of microalgae as a food supplement: a review of exogenous factors for production enhancement. Algal Res. (2021) 60:102542. doi: 10.1016/j.algal.2021.102542
4. Winwood, RJ. Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. OCL. (2013) 20:D604. doi: 10.1051/ocl/2013030
5. Ma, W, Liu, M, Zhang, Z, Xu, Y, Huang, P, Guo, D, et al. Efficient co-production of EPA and DHA by Schizochytrium sp. via regulation of the polyketide synthase pathway. Commun Biol. (2022) 5:1356. doi: 10.1038/s42003-022-04334-4
6. Yang, ZH, Amar, M, Sampson, M, Courville, AB, Sorokin, AV, Gordon, SM, et al. Comparison of omega-3 eicosapentaenoic acid versus docosahexaenoic acid-rich fish oil supplementation on plasma lipids and lipoproteins in normolipidemic adults. Nutrients. (2020) 12:749. doi: 10.3390/nu12030749
7. Liu, L, Hu, Q, Wu, H, Xue, Y, Cai, L, Fang, M, et al. Protective role of n6/n3 PUFA supplementation with varying DHA/EPA ratios against atherosclerosis in mice. J Nutr Biochem. (2016) 32:171–80. doi: 10.1016/j.jnutbio.2016.02.010
8. Taltavull, N, Muñoz-Cortés, M, Lluís, L, Jové, M, Fortuño, A, Molinar-Toribio, E, et al. Eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio improves histological alterations in obese rats with metabolic syndrome. Lipids Health Dis. (2014) 13:31. doi: 10.1186/1476-511X-13-31
9. Dasilva, G, Pazos, M, García-Egido, E, Gallardo, JM, Rodríguez, I, Cela, R, et al. Healthy effect of different proportions of marine ω-3 PUFAs EPA and DHA supplementation in Wistar rats: Lipidomic biomarkers of oxidative stress and inflammation. J Nutr Biochem. (2015) 26:1385–92. doi: 10.1016/j.jnutbio.2015.07.007
10. Méndez, L, Pazos, M, Gallardo, JM, Torres, JL, Pérez-Jiménez, J, Nogués, R, et al. Reduced protein oxidation in Wistar rats supplemented with marine ω3 PUFAs. Free Radic Biol Med. (2013) 55:8–20. doi: 10.1016/j.freeradbiomed.2012.11.004
11. Oh, DY, Talukdar, S, Bae, EJ, Imamura, T, Morinaga, H, Fan, WQ, et al. GPR120 is an Omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cells. (2010) 142:687–98. doi: 10.1016/j.cell.2010.07.041
12. Calder, PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. (2012) 56:1073–80. doi: 10.1002/mnfr.201100710
13. Chavan-Gautam, P, Rani, A, and Freeman, DJ. Distribution of fatty acids and lipids during pregnancy. Adv Clin Chem. (2018) 84:209–39. doi: 10.1016/BS.ACC.2017.12.006
14. Stables, MJ, and Gilroy, DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. (2011) 50:35–51. doi: 10.1016/j.plipres.2010.07.005
15. Wall, R, Ross, RP, Fitzgerald, GF, and Stanton, C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. (2010) 68:280–9. doi: 10.1111/j.1753-4887.2010.00287.x
16. Lucarelli, R, Gorrochotegui-Escalante, N, Taddeo, J, Buttaro, B, Beld, J, and Tam, V. Eicosanoid-activated PPARα inhibits NFκB-dependent bacterial clearance during post-influenza superinfection. Front Cell Infect Microbiol. (2022) 12:881462. doi: 10.3389/fcimb.2022.881462
17. Dyall, SC, Balas, L, Bazan, NG, Brenna, JT, Chiang, N, da Costa, SF, et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: recent advances in the understanding of their biosynthesis, structures, and functions. Prog Lipid Res. (2022) 86:101165. doi: 10.1016/j.plipres.2022.101165
18. Dasilva, G, Pazos, M, García-Egido, E, Gallardo, JM, Ramos-Romero, S, Torres, JL, et al. A lipidomic study on the regulation of inflammation and oxidative stress targeted by marine ω-3 PUFA and polyphenols in high-fat high-sucrose diets. J Nutr Biochem. (2017) 43:53–67. doi: 10.1016/j.jnutbio.2017.02.007
19. Dasilva, G, Pazos, M, García-Egido, E, Pérez-Jiménez, J, Torres, JL, Giralt, M, et al. Lipidomics to analyze the influence of diets with different EPA:DHA ratios in the progression of metabolic syndrome using SHROB rats as a model. Food Chem. (2016) 205:196–203. doi: 10.1016/j.foodchem.2016.03.020
20. Harizi, H, Corcuff, JB, and Gualde, N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. (2008) 14:461–9. doi: 10.1016/j.molmed.2008.08.005
21. Schwab, JM, Chiang, N, Arita, M, and Serhan, CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. (2007) 447:869–74. doi: 10.1038/nature05877
22. Serhan, CN, Yang, R, Martinod, K, Kasuga, K, Pillai, PS, Porter, TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. (2009) 206:15–23. doi: 10.1084/jem.20081880
23. Wang, K, Pramod, SN, Pavase, TR, Ahmed, I, Lin, H, Liu, L, et al. An overview on marine anti-allergic active substances for alleviating food-induced allergy. Crit Rev Food Sci Nutr. (2020) 60:2549–63. doi: 10.1080/10408398.2019.1650716
24. Kunisawa, J, Arita, M, Hayasaka, T, Harada, T, Iwamoto, R, Nagasawa, R, et al. Dietary ω3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci Rep. (2015) 5:9750. doi: 10.1038/srep09750
25. Ben-Shoshan, M, Turnbull, E, and Clarke, A. Food allergy: temporal trends and determinants. Curr Allergy Asthma Rep. (2012) 12:346–72. doi: 10.1007/s11882-012-0274-3
26. Jeebhay, MF, Robins, TG, Lehrer, SB, and Lopata, AL. Occupational seafood allergy: a review (2001) 58:553–62. doi: 10.1136/oem.58.9.553
27. Yu, W, Freeland, D, and Nadeau, K. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. (2016) 16:751–65. doi: 10.1038/nri.2016.111
28. Walsh, KP, Brady, MT, Finlay, CM, Boon, L, and Mills, KHG. Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J Immunol. (2009) 183:1577–86. doi: 10.4049/jimmunol.0803803
30. Roan, F, Obata-Ninomiya, K, and Ziegler, SF. Epithelial cell–derived cytokines: more than just signaling the alarm. J Clin Investig. (2019) 129:1441–51. doi: 10.1172/JCI124606
31. Khodoun, MV, Tomar, S, Tocker, JE, Wang, YH, and Finkelman, FD. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J Allergy Clin Immunol. (2018) 141:171–179.e1. doi: 10.1016/j.jaci.2017.02.046
32. Kim, E, Lembert, M, Fallata, GM, Rowe, JC, Martin, TL, Satoskar, AR, et al. Intestinal epithelial cells regulate gut eotaxin responses and severity of allergy. Front Immunol. (2018) 9:1692. doi: 10.3389/fimmu.2018.01692
33. Wambre, E, Bajzik, V, DeLong, JH, O’Brien, K, Nguyen, QA, Speake, C, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. (2017) 9:eaam9171. doi: 10.1126/scitranslmed.aam9171
34. Curotto de Lafaille, MA, Kutchukhidze, N, Shen, S, Ding, Y, Yee, H, and Lafaille, JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. (2008) 29:114–26. doi: 10.1016/j.immuni.2008.05.010
35. Gagliani, N, Magnani, CF, Huber, S, Gianolini, ME, Pala, M, Licona-Limon, P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. (2013) 19:739–46. doi: 10.1038/nm.3179
36. Carrier, Y, Yuan, J, Kuchroo, VK, and Weiner, HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-β T cell-transgenic mice. J Immunol. (2007) 178:179–85. doi: 10.4049/jimmunol.178.1.179
37. Zhou, Y, Kawasaki, H, Hsu, SC, Lee, RT, Yao, X, Plunkett, B, et al. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. (2010) 16:1128–33. doi: 10.1038/nm.2201
38. Bacher, P, Heinrich, F, Stervbo, U, Nienen, M, Valhdieck, M, Iwert, C, et al. Regulatory T cell specificity directs tolerance versus allergy against aeroantigens in humans. Cells. (2016) 167:1067–1078.e16. doi: 10.1016/j.cell.2016.09.050
39. Lack, G. Update on risk factors for food allergy. J Allergy Clin Immunol. (2012) 129:1187–97. doi: 10.1016/j.jaci.2012.02.036
40. Tsakok, T, Marrs, T, Mohsin, M, Baron, S, Du Toit, G, Till, S, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. (2016) 137:1071–8. doi: 10.1016/j.jaci.2015.10.049
41. Dunkin, D, Berin, MC, and Mayer, L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. (2011) 128:1251–1258.e2. doi: 10.1016/j.jaci.2011.06.007
42. Oyoshi, MK, Larson, RP, Ziegler, SF, and Geha, RS. Mechanical injury polarizes skin dendritic cells to elicit a TH2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. (2010) 126:976-84, 984.e1-5. doi: 10.1016/j.jaci.2010.08.041
43. Tordesillas, L, Mondoulet, L, Blazquez, AB, Benhamou, PH, Sampson, HA, and Berin, MC. Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol. (2017) 139:189–201.e4. doi: 10.1016/j.jaci.2016.03.057
44. Huang, Y, Jin, N, Roark, CL, Aydintug, MK, Wands, JM, Huang, H, et al. The influence of IgE-enhancing and IgE-suppressive γδ T cells changes with exposure to inhaled ovalbumin. J Immunol. (2009) 183:849–55. doi: 10.4049/jimmunol.0804104
45. Bol-Schoenmakers, M, Marcondes Rezende, M, Bleumink, R, Boon, L, Man, S, Hassing, I, et al. Regulation by intestinal γδ T cells during establishment of food allergic sensitization in mice. Allergy. (2011) 66:331–40. doi: 10.1111/j.1398-9995.2010.02479.x
46. Frossard, CP, Asigbetse, KE, Burger, D, and Eigenmann, PA. Gut T cell receptor-γδ+ intraepithelial lymphocytes are activated selectively by cholera toxin to break oral tolerance in mice. Clin Exp Immunol. (2015) 180:118–30. doi: 10.1111/cei.12561
47. Marino, A. Lymphocytes T gamma delta in food allergy to fish parvalbumin. Vigo, Spain: University of Vigo (2019).
48. Schülke, S, and Albrecht, M. Mouse models for food allergies: where do we stand? Cells. (2019) 8:546. doi: 10.3390/cells8060546
49. Dominguez-Bello, MG, Godoy-Vitorino, F, Knight, R, and Blaser, MJ. Role of the microbiome in human development. Gut. (2019) 68:1108–14. doi: 10.1136/gutjnl-2018-317503
50. Lozano-Ojalvo, D, Martínez-Blanco, M, Pérez-Rodríguez, L, Molina, E, Peláez, C, Requena, T, et al. Egg white peptide-based immunotherapy enhances vitamin A metabolism and induces RORγt+ regulatory T cells. J Funct Foods. (2019) 52:204–11. doi: 10.1016/j.jff.2018.11.012
51. Bunyavanich, S, and Berin, MC. Food allergy and the microbiome: current understandings and future directions. J Allergy Clin Immunol. (2019) 144:1468–77. doi: 10.1016/J.JACI.2019.10.019
52. Abril, AG, Carrera, M, Sánchez-Pérez, Á, and Villa, TG. Gut microbiome proteomics in food allergies. Int J Mol Sci. (2023) 24:2234. doi: 10.3390/ijms24032234
53. Salameh, M, Burney, Z, Mhaimeed, N, Laswi, I, Yousri, NA, Bendriss, G, et al. The role of gut microbiota in atopic asthma and allergy, implications in the understanding of disease pathogenesis. Scand J Immunol. (2020) 91:e12855. doi: 10.1111/sji.12855
54. Di Costanzo, M, Paparo, L, Cosenza, L, Di Scala, C, Nocerino, R, Aitoro, R, et al. Food allergies: novel mechanisms and therapeutic perspectives. Methods Mol Biol. (2016) 1371:215–21. doi: 10.1007/978-1-4939-3139-2_14/COVER
55. Varela, Z, Tc, B-T, Villaseñor, A, and Pérez-Gordo, M. Microbiome and allergy: new insights and perspectives. J Investig Allergol Clin Immunol. (2022) 32:327–44. doi: 10.18176/jiaci.0852
56. Rachid, R, Stephen-Victor, E, and Chatila, TA. The microbial origins of food allergy. J Allergy Clin Immunol. (2021) 147:808–13. doi: 10.1016/j.jaci.2020.12.624
57. Stephen-Victor, E, and Chatila, TA. Regulation of oral immune tolerance by the microbiome in food allergy. Curr Opin Immunol. (2019) 60:141–7. doi: 10.1016/j.coi.2019.06.001
58. Abdel-Gadir, A, Stephen-Victor, E, Gerber, GK, Noval Rivas, M, Wang, S, Harb, H, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat Med. (2019) 25:1164–74. doi: 10.1038/s41591-019-0461-z
59. Bao, R, Hesser, LA, He, Z, Zhou, X, Nadeau, KC, and Nagler, CR. Fecal microbiome and metabolome differ in healthy and food-allergic twins. J Clin Invest. (2021) 131:e141935. doi: 10.1172/JCI141935
60. Abril, AG, Carrera, M, Sánchez-Pérez, Á, and Villa, TG. Gut microbiome proteomics in food allergies. Int J Mol Sci. (2023, 2023) 24, 24:2234. doi: 10.3390/IJMS24032234
61. Wang, S, Charbonnier, LM, Noval Rivas, M, Georgiev, P, Li, N, Gerber, G, et al. MyD88 adaptor-dependent microbial sensing by regulatory T cells promotes mucosal tolerance and enforces commensalism. Immunity. (2015) 43:289–303. doi: 10.1016/j.immuni.2015.06.014
62. Fazlollahi, M, Chun, Y, Grishin, A, Wood, RA, Burks, AW, Dawson, P, et al. Early-life gut microbiome and egg allergy. Allergy. (2018) 73:1515–24. doi: 10.1111/all.13389
63. Rodriguez, B, Prioult, G, Hacini-Rachinel, F, Moine, D, Bruttin, A, Ngom-Bru, C, et al. Infant gut microbiota is protective against cow’s milk allergy in mice despite immature ileal T-cell response. FEMS Microbiol Ecol. (2012) 79:192–202. doi: 10.1111/j.1574-6941.2011.01207.x
64. Matsui, S, Kataoka, H, Tanaka, JI, Kikuchi, M, Fukamachi, H, Morisaki, H, et al. Dysregulation of intestinal microbiota elicited by food allergy induces IgA-mediated oral dysbiosis. Infect Immun. (2020) 88:e00741-19. doi: 10.1128/IAI.00741-19
65. Noval Rivas, M, Burton, OT, Wise, P, Zhang, YQ, Hobson, SA, Garcia Lloret, M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. (2013) 131:201–12. doi: 10.1016/j.jaci.2012.10.026
66. Goguyer-Deschaumes, R, Waeckel, L, Killian, M, Rochereau, N, and Paul, S. Metabolites and secretory immunoglobulins: messengers and effectors of the host–microbiota intestinal equilibrium. Trends Immunol. (2022) 43:63–77. doi: 10.1016/j.it.2021.11.005
67. Wang, M, Zhou, J, Selma-Royo, M, Simal-Gandara, J, Collado, MC, and Barba, FJ. Potential benefits of high-added-value compounds from aquaculture and fish side streams on human gut microbiota. Trends Food Sci Technol. (2021) 112:484–94. doi: 10.1016/j.tifs.2021.04.017
68. Imamura, F, Micha, R, Wu, JHY, de Oliveira Otto, MC, Otite, FO, Abioye, AI, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. (2016) 13:e1002087. doi: 10.1371/journal.pmed.1002087
69. Zhang, N, Ju, Z, and Zuo, T. Time for food: the impact of diet on gut microbiota and human health. Nutrition. (2018) 51–52:80–5. doi: 10.1016/j.nut.2017.12.005
70. Wolters, M, Ahrens, J, Romaní-Pérez, M, Watkins, C, Sanz, Y, Benítez-Páez, A, et al. Dietary fat, the gut microbiota, and metabolic health – A systematic review conducted within the MyNewGut project. Clin Nutr. (2019) 38:2504–20. doi: 10.1016/j.clnu.2018.12.024
71. Noakes, PS, Vlachava, M, Kremmyda, LS, Diaper, ND, Miles, EA, Erlewyn-Lajeunesse, M, et al. Increased intake of oily fish in pregnancy: effects on neonatal immune responses and on clinical outcomes in infants at 6 mo. Am J Clin Nutr. (2012) 95:395–404. doi: 10.3945/ajcn.111.022954
72. Yu, HN, Zhu, J, Pan, WS, Shen, SR, Shan, WG, and Das, UN. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch Med Res. (2014) 45:195–202. doi: 10.1016/j.arcmed.2014.03.008
73. Cao, ZJ, Yu, JC, Kang, WM, Ma, ZQ, Ye, X, and Tian, SB. Effect of n-3 polyunsaturated fatty acids on gut microbiota and endotoxin levels in portal vein of rats fed with high-fat diet. Acta Acad Med Sin. (2014) 36:496–500. doi: 10.3881/j.issn.1000-503X.2014.05.007
74. Wang, G, Huang, S, Wang, Y, Cai, S, Yu, H, Liu, H, et al. Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol Life Sci. (2019) 76:3917–37. doi: 10.1007/s00018-019-03190-6
75. Monk, JM, Liddle, DM, Hutchinson, AL, Wu, W, Lepp, D, Ma, DWL, et al. Fish oil supplementation to a high-fat diet improves both intestinal health and the systemic obese phenotype. J Nutr Biochem. (2019) 72:108216. doi: 10.1016/j.jnutbio.2019.07.007
76. Warner, DR, Warner, JB, Hardesty, JE, Song, YL, King, TN, Kang, JX, et al. Decreased ω-6:ω-3 PUFA ratio attenuates ethanol-induced alterations in intestinal homeostasis, microbiota, and liver injury. J Lipid Res. (2019) 60:2034–49. doi: 10.1194/jlr.RA119000200
77. Fu, Y, Wang, Y, Gao, H, Li, D, Jiang, R, Ge, L, et al. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediat Inflamm. (2021) 2021:8879227. doi: 10.1155/2021/8879227
78. Costantini, L, Molinari, R, Farinon, B, and Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. (2017) 18:2645. doi: 10.3390/ijms18122645
79. Caesar, R, Tremaroli, V, Kovatcheva-Datchary, P, Cani, PD, and Bäckhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. (2015) 22:658–68. doi: 10.1016/j.cmet.2015.07.026
80. Ghosh, S, DeCoffe, D, Brown, K, Rajendiran, E, Estaki, M, Dai, C, et al. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing Sepsis. PLoS One. (2013) 8:e55468. doi: 10.1371/journal.pone.0055468
81. Hosomi, R, Matsudo, A, Sugimoto, K, Shimono, T, Kanda, S, Nishiyama, T, et al. Dietary fat influences the expression of genes related to sterol metabolism and the composition of cecal microbiota and its metabolites in rats. J Oleo Sci. (2019) 68:1133–47. doi: 10.5650/jos.ess19183
82. Yamamoto, K, Kushida, M, and Tsuduki, T. The effect of dietary lipid on gut microbiota in a senescence-accelerated prone mouse model (SAMP8). Biogerontology. (2018) 19:367–83. doi: 10.1007/s10522-018-9764-6
83. Lu, C, Sun, T, Li, Y, Zhang, D, Zhou, J, and Su, X. Microbial diversity and composition in different gut locations of hyperlipidemic mice receiving krill oil. Appl Microbiol Biotechnol. (2018) 102:355–66. doi: 10.1007/s00253-017-8601-1
84. Robertson, RC, Seira Oriach, C, Murphy, K, Moloney, GM, Cryan, JF, Dinan, TG, et al. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. (2017) 59:21–37. doi: 10.1016/j.bbi.2016.07.145
85. Cui, C, Li, Y, Gao, H, Zhang, H, Han, J, Zhang, D, et al. Modulation of the gut microbiota by the mixture of fish oil and krill oil in high-fat diet-induced obesity mice. PLoS One. (2017) 12:e0186216. doi: 10.1371/journal.pone.0186216
86. Li, TT, Tong, AJ, Liu, YY, Huang, ZR, Wan, XZ, Pan, YY, et al. Polyunsaturated fatty acids from microalgae Spirulina platensis modulates lipid metabolism disorders and gut microbiota in high-fat diet rats. Food Chem Toxicol. (2019) 131:110558. doi: 10.1016/j.fct.2019.06.005
87. Guo, P, Yang, X, Guo, X, Yang, H, Pan, J, and Li, Y. Dietary fish oil improves autistic behaviors and gut homeostasis by altering the gut microbial composition in a mouse model of fragile X syndrome. Brain Behav Immun. (2023) 110:140–51. doi: 10.1016/j.bbi.2023.02.019
88. De, LW, Tung, TH, Teng, CY, Chang, CH, Chen, YC, Huang, HY, et al. Fish oil ameliorates neuropsychiatric behaviors and gut dysbiosis by elevating selected microbiota-derived metabolites and tissue tight junctions in rats under chronic sleep deprivation. Food Funct. (2022) 13:2662–80. doi: 10.1039/d2fo00181k
89. Tung, TH, Tung, YT, Lin, IH, Shih, CK, Nguyen, NTK, Shabrina, A, et al. Fish oil, but not olive oil, ameliorates depressive-like behavior and gut microbiota dysbiosis in rats under chronic mild stress. Biomol Ther. (2019) 9:516. doi: 10.3390/BIOM9100516
90. Egerton, S, Donoso, F, Fitzgerald, P, Gite, S, Fouhy, F, Whooley, J, et al. Investigating the potential of fish oil as a nutraceutical in an animal model of early life stress. Nutr Neurosci. (2020) 25:356–78. doi: 10.1080/1028415X.2020.1753322
91. Zhang, H, Li, Y, Cui, C, Sun, T, Han, J, Zhang, D, et al. Modulation of gut microbiota by dietary supplementation with tuna oil and algae oil alleviates the effects of D-galactose-induced ageing. Appl Microbiol Biotechnol. (2018) 102:2791–801. doi: 10.1007/s00253-018-9421-7
92. Balfegò, M, Canivell, S, Hanzu, FA, Sala-Vila, A, Martínez-Medina, M, Murillo, S, et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. (2016) 15:78. doi: 10.1186/s12944-016-0245-0
93. Noriega, BS, Sanchez-Gonzalez, MA, Salyakina, D, and Coffman, J. Understanding the impact of Omega-3 rich diet on the gut microbiota. Case Rep Med. (2016) 2016:3089303. doi: 10.1155/2016/3089303
94. Nielsen, S, Nielsen, DS, Lauritzen, L, Jakobsen, M, and Michaelsen, KF. Impact of diet on the intestinal microbiota in 10-month-old infants. J Pediatr Gastroenterol Nutr. (2007) 44:613–8. doi: 10.1097/MPG.0b013e3180406a11
95. Andersen, AD, Mølbak, L, Michaelsen, KF, and Lauritzen, L. Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J Pediatr Gastroenterol Nutr. (2011) 53:303–9. doi: 10.1097/MPG.0b013e31821d298f
96. Younge, N, Yang, Q, and Seed, PC. Enteral high fat-polyunsaturated fatty acid blend alters the pathogen composition of the intestinal microbiome in premature infants with an enterostomy. J Pediatr. (2017) 181:93-101.e6. doi: 10.1016/j.jpeds.2016.10.053
97. Druart, C, Neyrinck, AM, Vlaeminck, B, Fievez, V, Cani, PD, and Delzenne, NM. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS One. (2014) 9:e87560. doi: 10.1371/journal.pone.0087560
98. Wijekoon, MPA, Parrish, CC, and Mansour, A. Reprint of effect of dietary substitution of fish oil with flaxseed or sunflower oil on muscle fatty acid composition in juvenile steelhead trout (Oncorhynchus mykiss) reared at varying temperatures. Aquaculture. (2015) 447:108–15. doi: 10.1016/j.aquaculture.2015.06.022
99. Quin, C, Vollman, DM, Ghosh, S, Haskey, N, Estaki, M, Pither, J, et al. Fish oil supplementation reduces maternal defensive inflammation and predicts a gut bacteriome with reduced immune priming capacity in infants. ISME J. (2020) 14:2090–104. doi: 10.1038/s41396-020-0672-9
100. Cao, W, Wang, C, Chin, Y, Chen, X, Gao, Y, Yuan, S, et al. DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL) prevent intestinal dysfunction induced by chronic stress. Food Funct. (2019) 10:277–88. doi: 10.1039/C8FO01404C
101. Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre-and post-weaning. J Anim Sci. (2020) 98, 1–12. doi: 10.1093/jas/skaa086
102. Kaliannan, K, Wang, B, Li, XY, Kim, KJ, and Kang, JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep. (2015) 5:11276. doi: 10.1038/srep11276
103. Guinane, CM, and Cotter, PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol. (2013) 6:295–308. doi: 10.1177/1756283X13482996
104. Feng, R, Ma, LJ, Wang, M, Liu, C, Yang, R, Su, H, et al. Oxidation of fish oil exacerbates alcoholic liver disease by enhancing intestinal dysbiosis in mice. Commun Biol. (2020) 3:481. doi: 10.1038/s42003-020-01213-8
105. Mujico, JR, Baccan, GC, Gheorghe, A, Díaz, LE, and Marcos, A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. (2013) 110:711–20. doi: 10.1017/S0007114512005612
106. Awoyemi, A, Trøseid, M, Arnesen, H, Solheim, S, and Seljeflot, I. Effects of dietary intervention and n-3 PUFA supplementation on markers of gut-related inflammation and their association with cardiovascular events in a high-risk population. Atherosclerosis. (2019) 286:53–9. doi: 10.1016/j.atherosclerosis.2019.05.004
107. Fang, S, Chen, X, Ye, X, Zhou, L, Xue, S, and Gan, Q. Effects of gut microbiome and short-chain fatty acids (SCFAs) on finishing weight of meat rabbits. Front Microbiol. (2020) 11:1835. doi: 10.3389/FMICB.2020.01835/BIBTEX
108. Hofmanová, J, Vaculová, A, Koubková, Z, Hýžd’alová, M, and Kozubík, A. Human fetal colon cells and colon cancer cells respond differently to butyrate and PUFAs. Mol Nutr Food Res. (2009) 53:S102–13. doi: 10.1002/mnfr.200800175
109. Chen, J, Xu, Q, Li, Y, Tang, Z, Sun, W, Zhang, X, et al. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J Anim Sci. (2019) 97:4256–67. doi: 10.1093/jas/skz284
110. Ochoa-Repáraz, J, and Kasper, LH. The second brain: is the gut microbiota a link between obesity and central nervous system disorders? Curr Obes Rep. (2016) 5:51–64. doi: 10.1007/s13679-016-0191-1
111. Watson, H, Mitra, S, Croden, FC, Taylor, M, Wood, HM, Perry, SL, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. (2018) 67:1974–83. doi: 10.1136/gutjnl-2017-314968
112. Liu, HQ, Qiu, Y, Mu, Y, Zhang, XJ, Liu, L, Hou, XH, et al. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr Res. (2013) 33:849–58. doi: 10.1016/j.nutres.2013.07.004
113. Onishi, JC, Campbell, S, Moreau, M, Patel, F, Brooks, AI, Xiuzhou, Y, et al. Bacterial communities in the small intestine respond differently to those in the caecum and colon in mice fed low-and high-fat diets. Microbiology (United Kingdom). (2017) 163:1189–97. doi: 10.1099/mic.0.000496
114. Belluzzi, A. Polyunsaturated fatty acids (n-3 PUFAs) and inflammatory bowel disease (IBD): pathogenesis and treatment. Eur Rev Med Pharmacol Sci. (2004) 8:225–9.
115. Santoru, ML, Piras, C, Murgia, A, Palmas, V, Camboni, T, Liggi, S, et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci Rep. (2017) 7:9523. doi: 10.1038/s41598-017-10034-5
116. Shin, NR, Whon, TW, and Bae, JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. doi: 10.1016/j.tibtech.2015.06.011
117. Roÿtiö, H, Mokkala, K, Vahlberg, T, and Laitinen, K. Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women. Br J Nutr. (2017) 118:343–52. doi: 10.1017/S0007114517002100
118. Zhang, YG, Xia, Y, Lu, R, and Sun, J. Inflammation and intestinal leakiness in older HIV+ individuals with fish oil treatment. Genes Dis. (2018) 5:220–5. doi: 10.1016/j.gendis.2018.07.001
119. Willemsen, LEM. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur J Pharmacol. (2016) 785:174–86. doi: 10.1016/j.ejphar.2016.03.062
120. Yang, H, Qu, Y, Gao, Y, Sun, S, Ding, R, Cang, W, et al. Role of the dietary components in food allergy: A comprehensive review. Food Chem. (2022) 386:132762. doi: 10.1016/j.foodchem.2022.132762
121. Monk, JM, Hou, TY, Turk, HF, McMurray, DN, and Chapkin, RS. n3 PUFAs reduce mouse CD4+ T-cell ex vivo polarization into Th17 cells. J Nutr. (2013) 143:1501–8. doi: 10.3945/jn.113.178178
122. De Matos, OG, Amaral, SS, Pereira Da Silva, PEM, Perez, DA, Alvarenga, DM, Ferreira, AVM, et al. Dietary supplementation with omega-3-pufa-rich fish oil reduces signs of food allergy in ovalbumin-sensitized mice. Clin Dev Immunol. (2012):2012–236564. doi: 10.1155/2012/236564
123. Van den Elsen, LWJ, Van Esch, BCAM, Hofman, GA, Kant, J, Van de Heijning, BJM, Garssen, J, et al. Dietary long chain n-3 polyunsaturated fatty acids prevent allergic sensitization to cow’s milk protein in mice. Clin Exp Allergy. (2013) 43:798–810. doi: 10.1111/cea.12111
124. van den Elsen, LWJ, Bol-Schoenmakers, M, van Esch, BCAM, Hofman, GA, van de Heijning, BJM, Pieters, RH, et al. DHA-rich tuna oil effectively suppresses allergic symptoms in mice allergic to whey or peanut. J Nutr. (2014) 144:1970–6. doi: 10.3945/jn.114.198515
125. Dunstan, JA, Mori, TA, Barden, A, Beilin, LJ, Taylor, AL, Holt, PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. (2003) 112:1178–84. doi: 10.1016/j.jaci.2003.09.009
126. Manley, BJ, Makrides, M, Collins, CT, McPhee, AJ, Gibson, RA, Ryan, P, et al. High-dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics. (2011) 128:e71-7. doi: 10.1542/peds.2010-2405
127. Palmer, DJ, Sullivan, T, Gold, MS, Prescott, SL, Heddle, R, Gibson, RA, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. BMJ (Online). (2012) 344:e184. doi: 10.1136/bmj.e184
128. D’Vaz, N, Meldrum, SJ, Dunstan, JA, Martino, D, McCarthy, S, Metcalfe, J, et al. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics. (2012) 130:674–82. doi: 10.1542/peds.2011-3104
129. Best, KP, Sullivan, TR, Palmer, DJ, Gold, M, Martin, J, Kennedy, D, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood - A longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. (2018) 11:10. doi: 10.1186/s40413-018-0190-7
130. Best, KP, Gold, M, Kennedy, D, Martin, J, and Makrides, M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. (2016) 103:128–43. doi: 10.3945/ajcn.115.111104
131. Hansen, S, Strøm, M, Maslova, E, Dahl, R, Hoffmann, HJ, Rytter, D, et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J Allergy Clin Immunol. (2017) 139:104–111.e4. doi: 10.1016/j.jaci.2016.02.042
132. Papamichael, MM, Katsardis, C, Lambert, K, Tsoukalas, D, Koutsilieris, M, Erbas, B, et al. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: a randomised controlled trial. J Hum Nutr Diet. (2019) 32:185–97. doi: 10.1111/jhn.12609
133. Zhang, Y, Lin, J, Zhou, R, Zheng, X, and Dai, J. Effect of omega-3 fatty acids supplementation during childhood in preventing allergic disease: a systematic review and Meta-analysis. J Asthma. (2021) 58:523–36. doi: 10.1080/02770903.2019.1709866
134. Ellul, S, Marx, W, Collier, F, Saffery, R, Tang, M, Burgner, D, et al. Plasma metabolomic profiles associated with infant food allergy with further consideration of other early life factors. Prostaglandins Leukot Essent Fatty Acids. (2020) 159:102099. doi: 10.1016/j.plefa.2020.102099
135. Aldámiz-Echevarría, L, Bilbao, A, Andrade, F, Elorz, J, Prieto, JA, and Rodríguez-Soriano, J. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediat Int J Paediat. (2008) 97:1572–6. doi: 10.1111/j.1651-2227.2008.00963.x
136. D’Auria, E, Pendezza, E, and Zuccotti, GV. Personalized nutrition in food allergy: tips for clinical practice. Front Pediatr. (2020) 8:113. doi: 10.3389/fped.2020.00113
137. Mourad, AA, and Bahna, SL. Fish-allergic patients may be able to eat fish. Expert Rev Clin Immunol. (2015) 11:419–30. doi: 10.1586/1744666X.2015.1009896
138. Kremmyda, LS, Vlachava, M, Noakes, PS, Diaper, ND, Miles, EA, and Calder, PC. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: a systematic review. Clin Rev Allergy Immunol. (2011) 41:36–66. doi: 10.1007/s12016-009-8186-2
139. Krauss-Etschmann, S, Hartl, D, Rzehak, P, Heinrich, J, Shadid, R, del Carmen, R-TM, et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-β levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol. (2008) 121:464-470.e6. doi: 10.1016/j.jaci.2007.09.018
140. Furuhjelm, C, Jenmalm, MC, Fälth-Magnusson, K, and DuchIn, K. Th1 and Th2 chemokines, vaccine-induced immunity, and allergic disease in infants after maternal ω-3 fatty acid supplementation during pregnancy and lactation. Pediatr Res. (2011) 69:259–64. doi: 10.1203/PDR.0b013e3182072229
141. Klemens, CM, Berman, DR, and Mozurkewich, EL. The effect of perinatal omega-3 fatty acid supplementation on inflammatory markers and allergic diseases: a systematic review. BJOG. (2011). doi: 10.1111/j.1471-0528.2010.02846.x
142. Lee, HS, Barraza-Villarreal, A, Hernandez-Vargas, H, Sly, PD, Biessy, C, Ramakrishnan, U, et al. Modulation of DNA methylation states and infant immune system by dietary supplementation with v-3 PUFA during pregnancy in an intervention study. Am J Clin Nutr. (2013) 98:480–7. doi: 10.3945/ajcn.112.052241
143. D’Auria, E, Del Giudice, MM, Barberi, S, Mandelli, M, Verduci, E, Leonardi, S, et al. Omega-3 fatty acids and asthma in children. Allergy Asthma Proc. (2014) 35:233–40. doi: 10.2500/aap.2014.35.3736
144. Gunaratne, AW, Makrides, M, and Collins, CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. (2015) 2015:CD010085. doi: 10.1002/14651858.CD010085.pub2
145. Schindler, T, Sinn, JK, and Osborn, DA. Polyunsaturated fatty acid supplementation in infancy for the prevention of allergy. Cochrane Database Syst Rev. (2016) 10:CD010112. doi: 10.1002/14651858.CD010112.pub2
146. Barman, M. Effect of maternal supplementation with fish oil during pregnancy and lactation on allergy development in childhood. Acta Paediatr. (2016) 105:1348. doi: 10.1111/apa.13512
Keywords: marine lipids, EPA, DHA, immunity, inflammation, microbiota, dysbiosis
Citation: Abril AG, Carrera M and Pazos M (2023) Immunomodulatory effect of marine lipids on food allergy. Front. Nutr. 10:1254681. doi: 10.3389/fnut.2023.1254681
Received: 07 July 2023; Accepted: 24 October 2023;
Published: 14 November 2023.
Edited by:
Jose Manuel Miranda, University of Santiago de Compostela, SpainReviewed by:
Fahrul Nurkolis, State Islamic University of Sunan Kalijaga (UIN Sunan Kalijaga Yogyakarta), IndonesiaCopyright © 2023 Abril, Carrera and Pazos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel Pazos, bXBhem9zQGlpbS5jc2ljLmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.