
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 22 December 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1250509
This article is part of the Research Topic Dietary Patterns Affecting Cardiovascular Health View all 23 articles
 Qingming Fu1†
Qingming Fu1† Rumeng Chen2†
Rumeng Chen2† Yining Ding3†
Yining Ding3† Shuling Xu4
Shuling Xu4 Chunxia Huang1
Chunxia Huang1 Binsheng He5
Binsheng He5 Ting Jiang1
Ting Jiang1 Bin Zeng1
Bin Zeng1 Meihua Bao5,6*
Meihua Bao5,6* Sen Li3*
Sen Li3*Background: The existing literature on the link between sodium intake and cardiovascular disease (CVD) largely consists of observational studies that have yielded inconsistent conclusions. In this study, our objective is to assess the causal relationship between sodium intake and 50 CVDs using two-sample Mendelian randomization (MR) analysis.
Methods: MR analyses were performed to investigate the associations between urinary sodium/creatinine ratio (UNa/UCr), an indicator of sodium intake, and 50 CVDs. The genome-wide association study (GWAS) for UNa/UCr was from the UK Biobank (UKBB), and the GWASs for CVDs were from FinnGen. A false discovery rate (FDR) threshold of 5% was applied for multiple comparison correction.
Results: The inverse-variance weighted method indicated that the genetically predicted UNa/UCr was significantly associated with 7 of 50 CVDs, including “Coronary atherosclerosis” (OR = 2.01; 95% CI: 1.37, 2.95), “Diseases of arteries, arterioles and capillaries” (OR = 1.88; 95% CI: 1.20, 2.94), “Hard cardiovascular diseases” (OR = 1.71; 95% CI: 1.24, 2.35), “Ischemic heart diseases” (OR = 2.06; 95% CI: 1.46, 2.93), “Major coronary heart disease event” (OR = 1.99; 95% CI: 1.36, 2.91), “Myocardial infarction” (OR = 2.03; 95% CI: 1.29, 3.19), and “Peripheral artery disease” (OR = 2.50; 95% CI: 1.35, 4.63). Similar results were obtained with the MR-Egger and weighted median methods. No significant heterogeneity or horizontal pleiotropy was found in this analysis.
Conclusion: Our study has uncovered a significant positive causal relationship between UNa/UCr and various CVDs. These results offer a new theoretical foundation for advocating the restriction of sodium intake as a preventive measure against CVD.
Cardiovascular disease (CVD) ranks as the primary global cause of mortality (1–3), accounting for approximately 32% of all mortality cases (4). Since 1990, the number of individuals affected by CVD has doubled, and as of 2019, it is estimated that approximately 523 million individuals have suffered from CVD worldwide (5). Furthermore, CVD is associated with a high global disability rate, placing a significant burden on both patients and their families (6, 7).
The observational studies have indeed shown that both excessive and insufficient salt intake can elevate the risk of cardiovascular disease and mortality (8–10). Accurate assessment of sodium intake is crucial, but direct measurement of sodium intake levels in clinical settings is challenging. Several studies have shown that the urinary sodium/creatinine ratio (UNa/UCr) is a reliable indicator and is a simpler, more accessible approach for sodium intake assessment (11–13). However, previous observational research on the association between UNa/UCr and the risk of CVD has yielded inconsistent results. A cross-sectional study carried out in South Korea found a correlation between UNa/UCr and hypertension (14), whereas two other studies in Chinese populations did not find any association between UNa/UCr and hypertension (15, 16). Observational studies are limited in their ability to establish causal relationships, and causal studies are needed to investigate the relationship between UNa/UCr and CVDs.
Mendelian randomization (MR) analysis is an epidemiological approach that employs genetic variation closely linked to the exposure of interest as an instrumental variable (IV). The methodologies used in MR analysis are based on Mendel's second law, which states that alleles follow a principle of random allocation. This property assists in mitigating biases arising from confounding factors and reverses causation. In order to investigate if high levels of UNa/UCr can result in numerous cardiovascular diseases (including 50 types of CVDs), this study will apply MR analyses.
Single-nucleotide polymorphisms (SNPs) are the most common genetic variations used as IVs in MR, which are employed to calculate the causal associations of traits with diseases. In this study, we performed MR analyses employing data from GWAS datasets for both exposure (UNa/UCr) and outcome (50 CVDs).
In the MR analysis, we used a variety of publicly available GWAS summary data (Supplementary Table 1). To fulfill the requirements of the two-sample MR design, the exposure and outcome were obtained from two different European populations, as described previously (17).
Previous studies have shown that estimates obtained from spotted urine samples are effective in assessing 24-h urinary sodium excretion. This effectiveness can be achieved by establishing equations that take into account the UNa/UCr. Because sodium intake and excretion are correlated, UNa/UCr was used as a measure of sodium intake. This approach corrected for variations in urinary concentration due to differences in fluid intake levels, making it a more precise indicator of sodium intake. The GWAS for UNa/UCr (sample size = 327,616) was obtained from the UK Biobank (UKBB) (18). The UKBB enlisted half a million individuals, aged 40–69, from various regions of the country to participate in this initiative between 2006 and 2010. These participants have undergone assessments, submitted samples of blood, urine, and saliva for future analysis, provided comprehensive personal information, and consented to continuous health monitoring.
We selected the GWASs for CVDs from the FinnGen datasets, a large-scale biomedical research project based in Finland (19). The FinnGen dataset consisted of genomic data collected from 500,000 individuals of Finnish ancestry. This dataset was then merged with data obtained from the National Healthcare Register of Finland. During the selection process, we excluded a series of phenotypes, such as (1) phenotypes that are not related to the circulation system; (2) similar phenotypes but with a smaller sample size or particular population; (3) broad-defined phenotypes that cannot be specifically categorized as a certain disease; and (4) phenotypes that involved interventions such as operations and medications. As a result, we retained 50 CVDs as outcomes for this study. The corresponding sample sizes for each CVD are provided in Supplementary Table 1.
In the MR analysis, IVs were selected according to a set of criteria, including: (1) IVs and exposure were significantly associated at the genomic level (P < 5.00E-08); (2) independent IVs identified by clumping within a 10 Mb window and linkage disequilibrium (LD) of R2 < 0.001; and (3) the minor allele frequency (MAF) > 0.01. Additionally, we excluded palindromic SNPs with an intermediate allele frequency, as previously reported (20). The F-statistics were calculated for the strength of IVs, and a value over 10 indicates a lower risk of weak IV bias (Supplementary Table 2) (21).
The primary strategy utilized in the MR analysis was the inverse-variance weighted (IVW) method. Additionally, we used the weighted median (WM) and MR-Egger methods in sensitivity analyses. The WM method remains unbiased under the condition that no more than 50% of the weight comes from invalid instruments. The pleiotropy-corrected data from MR-PRESSO were used to eliminate probable outliers. The Cochrane Q-value was employed to assess heterogeneity. With the leave-one-out strategy, which examined how each included IV affected the causal associations, the robustness of the results was assessed. Causal estimations were presented as odds ratios (ORs) and 95% confidence intervals (CIs). To address multiple comparisons, a false discovery rate (FDR) threshold of 5% was applied for correction. The two-sample MR package was used to perform all the MR analyses in R.
A total of 20 distinct SNPs were used as the genetic IVs for UNa/UCr, and the F-statistics for the IVs ranged from 30.53 to 125.48, suggesting good instrument strength (Supplementary Table 2). FDR correction for multiple testing was used to guide the interpretation of the findings. The genetically predicted UNa/UCr was found to be strongly linked with 7 of 50 CVDs, according to the IVW approach in MR, including “Coronary atherosclerosis” (OR = 2.01; 95% CI: 1.37, 2.95), “Diseases of arteries, arterioles and capillaries” (OR = 1.88; 95% CI: 1.20, 2.94), “Hard cardiovascular diseases” (OR = 1.71; 95% CI: 1.24, 2.35), “Ischemic heart diseases” (OR = 2.06; 95% CI: 1.46, 2.93), “Major coronary heart disease event” (OR = 1.99; 95% CI: 1.36, 2.91), “Myocardial infarction” (OR = 2.03; 95% CI: 1.29, 3.19), and “Peripheral artery disease” (OR = 2.50; 95% CI: 1.35, 4.63) (Figures 1, 2; Supplementary Table 3). Using the MR-Egger and WM approaches, the relationships between UNa/UCr and seven CVDs had the same direction (Figure 2; Supplementary Table 3). The scatter plot visually showed causal associations between UNa/UCr and seven diseases of the circulation system (Figure 3). This analysis revealed no discernible heterogeneity (Figure 4; Supplementary Table 4). According to the intercept term of the MR-Egger technique (Supplementary Table 5), horizontal pleiotropy was not significant in the causality analysis, which is similar to the findings of MR-PRESSO, where no outlier IV was found. The leave-one-out analysis revealed that the exclusion of one SNP could not significantly change the observed outcomes (Figure 5).
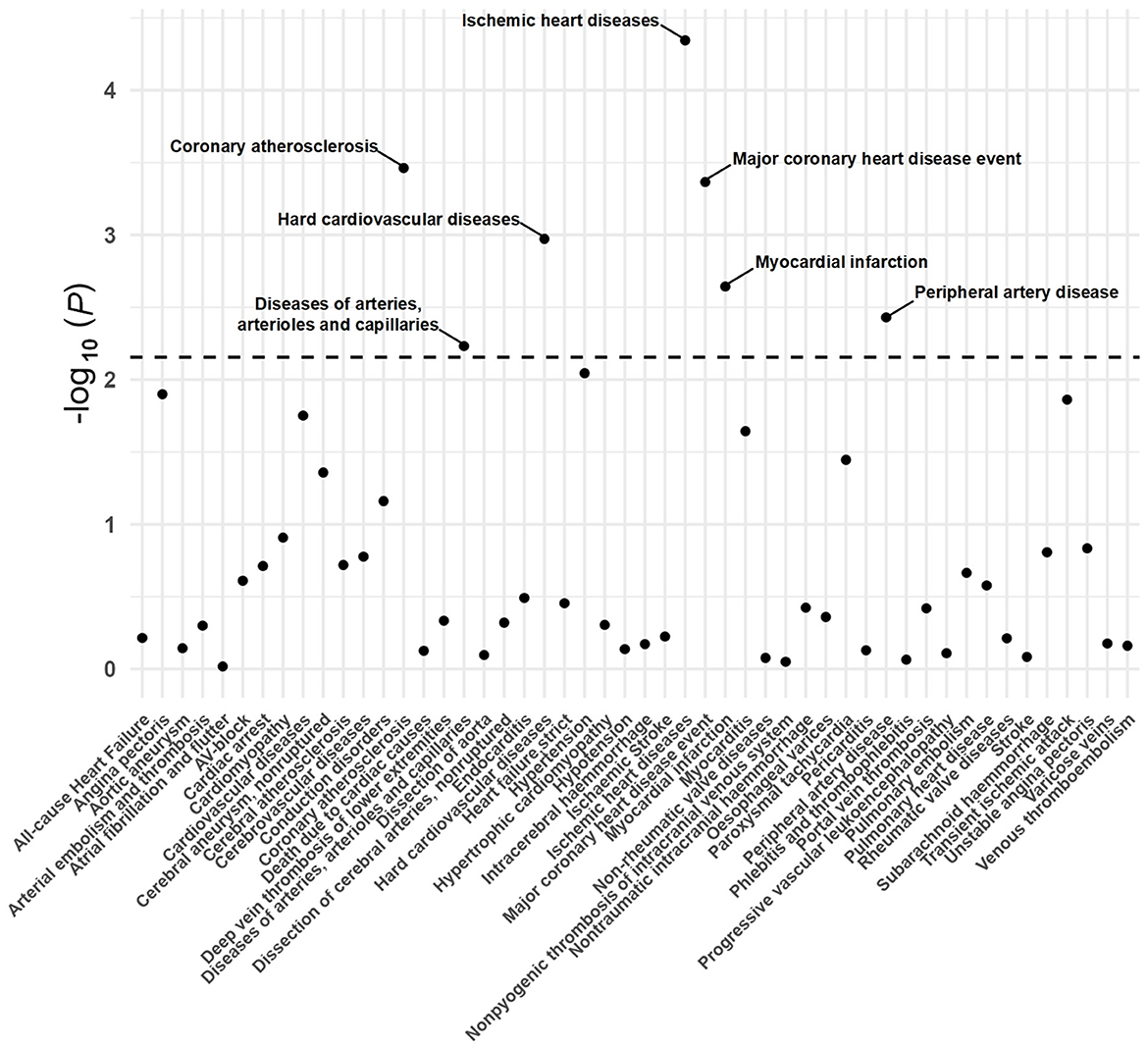
Figure 1. P-value distribution of associations between urinary sodium/creatinine ratio (UNa/UCr) and 50 CVDs in the Mendelian randomization analysis. The dashed line represents the significance threshold adjusted by the false discovery rate.
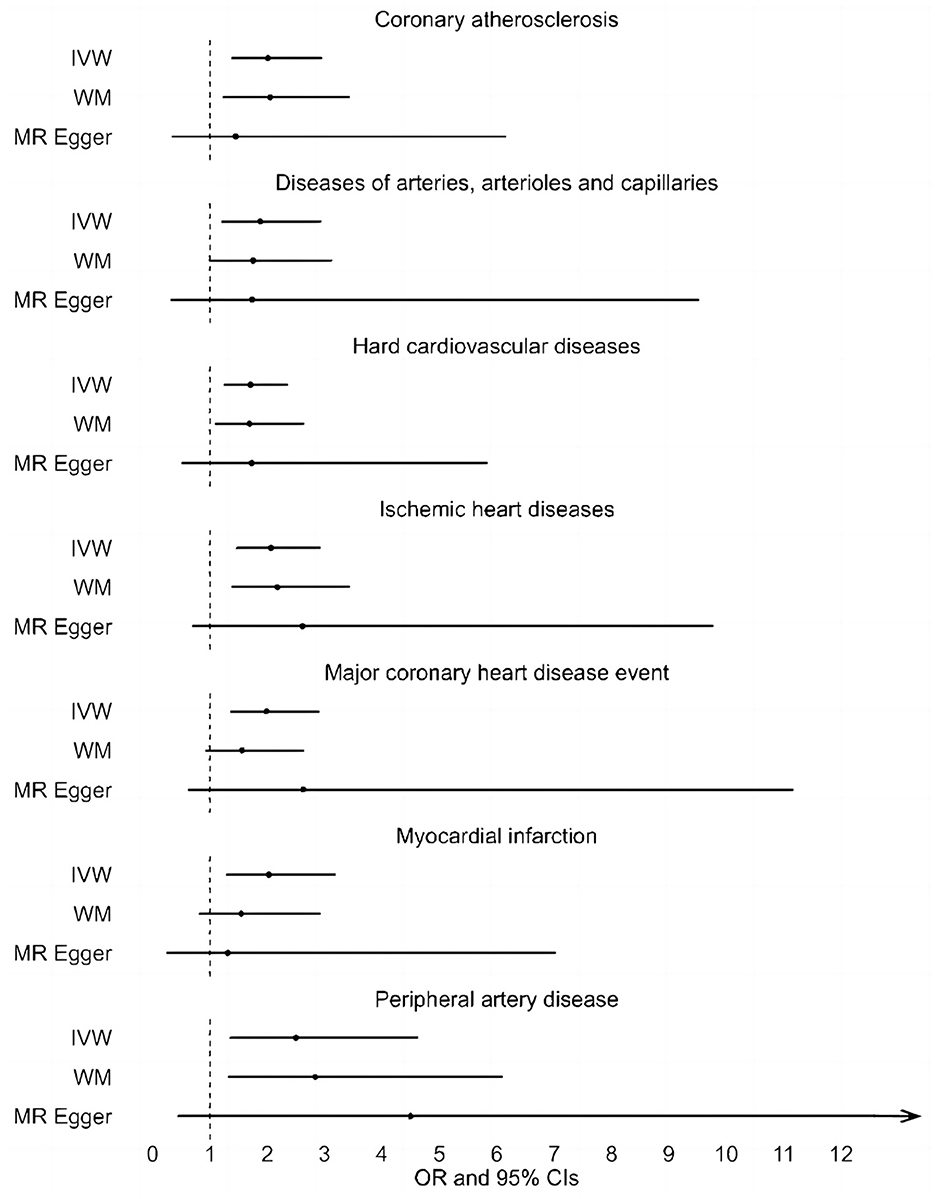
Figure 2. Associations between genetically predicted UNa/UCr and seven CVDs examined by three MR methods. MR, Mendelian randomization; UNa/UCr, urinary sodium/creatinine ratio; IVW, inverse-variance weighted; WM, weighted median; OR, odds ratio; CI, confidence interval.
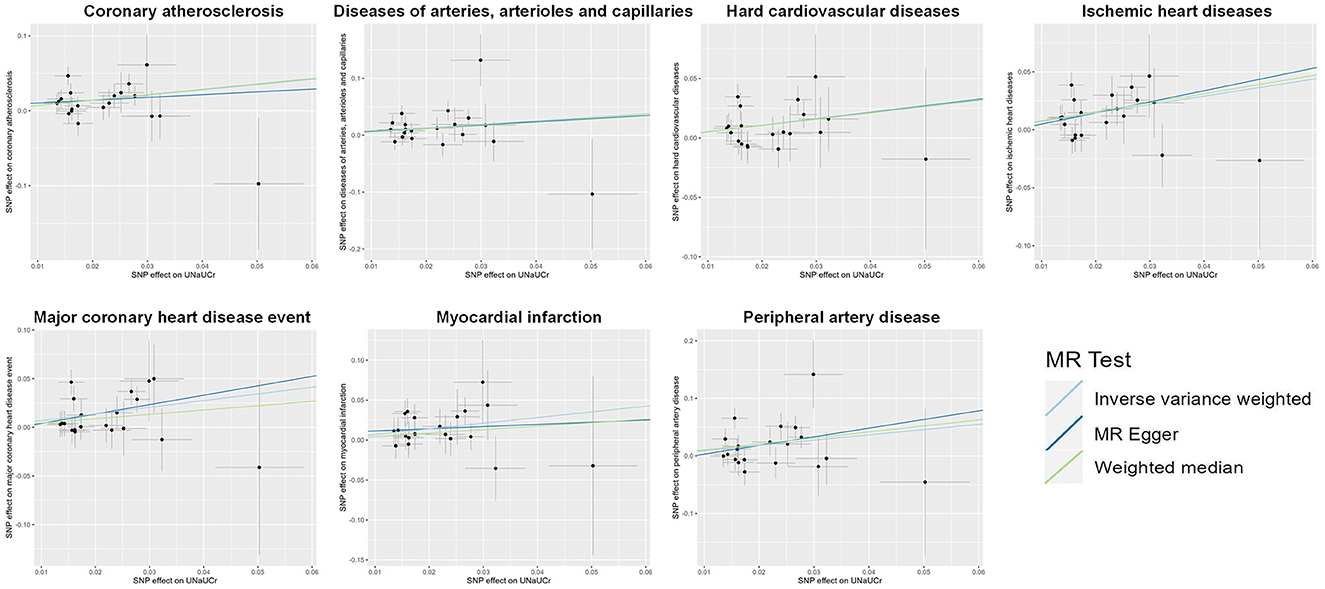
Figure 3. Scatter plot showing the causal effects of UNa/UCr on seven CVDs. SNP, single-nucleotide polymorphism; UNa/UCr, urinary sodium/creatinine ratio.

Figure 4. Funnel plot indicating the causal associations of UNa/UCr on seven CVDs. SNP, single-nucleotide polymorphism; UNa/UCr, urinary sodium/creatinine ratio; IV, instrumental variable; SE, standard error.
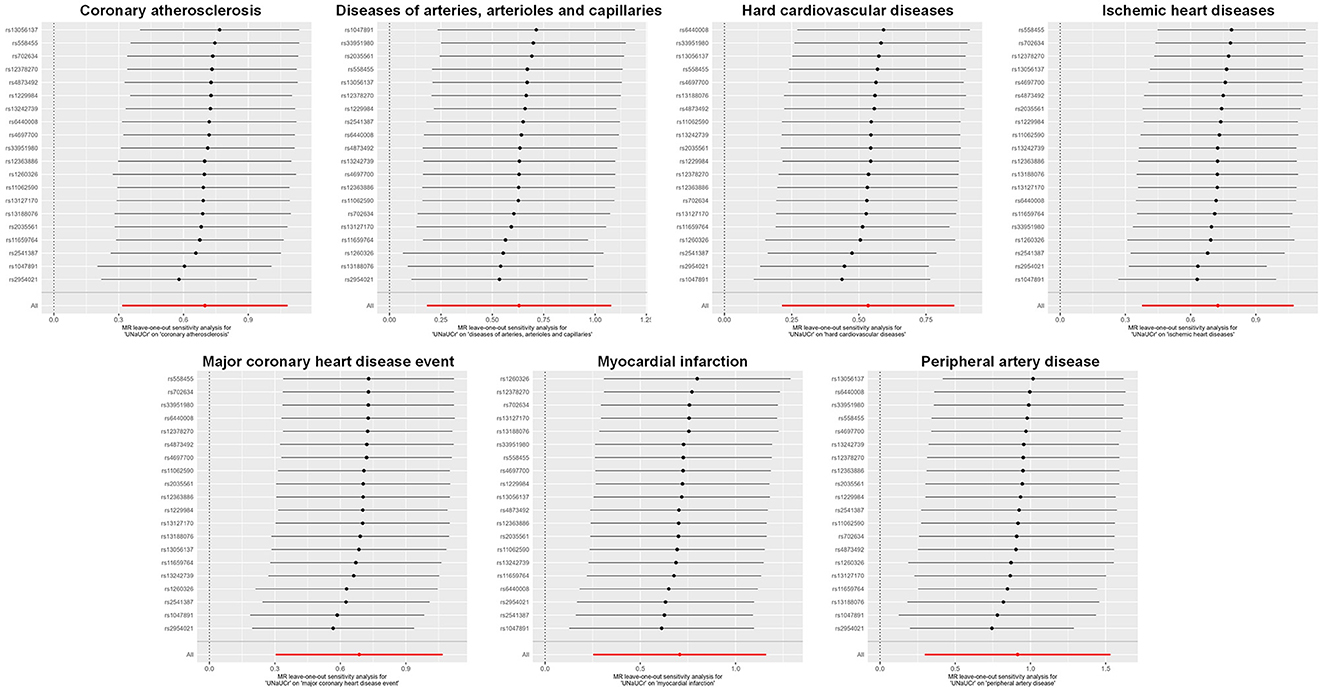
Figure 5. Leave-one-out sensitivity analysis examining the causal estimates of UNa/UCr on seven CVDs by the IVW method after excluding a specific SNP from the analysis. MR, Mendelian randomization; SNP, single-nucleotide polymorphism; UNa/UCr, urinary sodium/creatinine ratio; IVW, inverse-variance weighted.
This study employed a two-sample MR analysis to evaluate the causal association between sodium intake and a wide range of 50 CVDs, leveraging data from public databases and comprehensive GWAS. The results showed a positive causal relationship between UNa/UCr and seven types of CVDs. To account for the overlap among some of the seven CVDs, we have categorized and discussed them separately into the following two categories.
Ischemic heart disease (IHD) is an umbrella term used to describe various heart problems resulting from reduced blood flow to the heart. IHD is the predominant manifestation of coronary heart diseases (CHDs). It arises due to arterial stenosis, or the narrowing of the blood vessels responsible for supplying oxygenated blood to the myocardium, also known as the heart muscle (22). A model study conducted in China demonstrated that reducing salt intake by 1 gram per day could potentially lower the risk of IHD by approximately 4% (23). The findings of our study are consistent with prior research. IHD is most commonly caused by atherosclerosis (24, 25). A Swedish cohort study found a correlation between a higher estimated 24-h sodium excretion and coronary atherosclerosis (26). This finding provides additional support for the conclusions drawn in our study. Major coronary heart disease (CHD) events consist of both fatal and non-fatal events, with the former defined as death resulting from CHD and the latter indicating myocardial infarction or heart attack (27). Several observational studies have found that high urinary sodium excretion is linked to an increased risk of myocardial infarction. For example, a study carried out in China revealed a significant correlation between excessive excretion of urinary sodium and increased susceptibility to cardiovascular diseases. This connection was noticed concerning instances of non-fatal heart attacks and fatalities attributed to coronary heart disease (28). Additionally, a meta-analysis indicated that elevated sodium levels, detected through multiple 24-h urine collections, were associated with a heightened risk of cardiovascular events. These occurrences encompass both lethal and non-lethal instances of heart attack, stroke, or the requirement for coronary revascularization (29).
Arterial stiffness is a well-known contributor to CVD. Studies have found that excessive sodium intake in the diet can induce changes in the extracellular matrix of the arterial wall, thereby facilitating the process of arteriosclerosis (30). Arterial stiffness is characterized by an imbalance between elastin and collagen fibers, which are key components of arterial elasticity. This process is regulated by matrix metalloproteinases (MMPs) (31). It has been discovered that a high sodium intake leads to the activation of extracellular matrix metalloproteinases, specifically MMP-2 and MMP-9, which subsequently stimulate the production of transforming growth factor-beta 1 (TGF-β1) (31, 32). TGF-β1 is a fibrotic growth factor that, when overexpressed, can cause thickening of the arterial intima, thinning and fracturing of elastin fibers, and a reduction in the ratio of elastin to collagen. These changes ultimately contribute to the development of arterial stiffness (33).
Furthermore, an MR study investigating the impact of urinary sodium on cardiovascular risk factors, ischemic stroke, and heart failure (HF) demonstrated that higher levels of urinary sodium were associated with an elevated risk of both HF and global ischemic stroke (34). Consistent with our own findings, a cohort study conducted in Taiwan revealed a significant association between increased urine sodium excretion and a higher risk of CVD, particularly stroke (35). However, a Finnish cohort study reported contrasting results, finding no significant correlation between urinary sodium levels and major adverse coronary events in men with HF (36). This discrepancy may be attributed to the population selection in the study, which excluded female patients with chronic HF and included only male patients.
This study has shown a positive causal association between UNa/UCr and peripheral arterial disease, as well as diseases affecting arteries, arterioles, and capillaries. These findings are in line with our study and support the conclusions of previous investigations. Two cross-sectional studies conducted in China demonstrated a robust positive association between urine sodium excretion and the presence of carotid atherosclerosis (37, 38). In a Korean cohort study, it was found that dietary sodium intake in adults aged 40 years and older may be positively associated with subsequent levels of carotid intima-media thickness (39). However, another cross-sectional study and systematic evaluation reported a limited correlation between small vessel disease and current salt intake, or UNa/UCr (40).
The underlying biological mechanism may be due to oxidative stress and endothelial dysfunction. The endothelium serves as a dynamic monolayer that critically regulates vascular tone and inflammation in the blood vessel wall (41, 42). Endothelial cells synthesize nitric oxide (NO), which acts as a protective molecule crucial for maintaining optimal vascular function (43, 44). Several enzymatic systems, such as NADPH oxidase and xanthine oxidase, are responsible for deactivating NO while simultaneously increasing levels of superoxide anions () (45), contributing to the development of endothelial dysfunction. Previous studies have demonstrated that salt loading impairs vascular endothelial function (46, 47). In a study on middle-aged hypertensive individuals, restricted sodium intake was found to reverse microvascular endothelial dysfunction through a reduction in oxidative stress (48). Additionally, administration of the antioxidant ascorbic acid improved microvascular function after another sodium-induced impairment, further supporting the role of oxidative stress in this process (49).
This study has several noteworthy advantages. First, a two-sample MR strategy was used in the study to reduce the influence of confounding factors and reverse causality on the outcomes. Second, this study is the largest investigation to date on the causal relationship between UNa/UCr and a broad type of CVD. Finally, we achieved relatively robust results by using GWAS summary statistics and conducting various sensitivity analyses to minimize the possibility of horizontal pleiotropy.
Our study also has limitations. First, our study only included individuals from Europe, which limits the generalizability of our findings to other ethnic groups because there may be variations in the association between UNa/UCr and CVD across different ethnicities. Further research should encompass large-scale GWAS across diverse geographical regions. Second, selection bias may have affected our results, as individuals who died due to outcome competing risk might have been missed in GWAS. Third, stratification is necessary when populations demonstrate different disease rates, trait distributions, and allele frequencies. However, we encountered limitations in conducting population stratification based on additional factors such as age and gender due to using GWAS summary data instead of individual-level data in our study. Finally, previous observational studies have shown a U-shaped or J-shaped relationship between sodium intake and diverse cardiovascular diseases (50, 51), and this study neglects assessing possible non-linear connections between sodium consumption and outcomes. Future research utilizing extensive biobanks may provide further insight into the potential existence of such a relationship.
Our research has identified a significant and positive causal relationship between UNa/UCr and seven CVDs. This provides evidence that elevated levels of sodium intake may play a contributing role in the onset or advancement of these CVDs. Therefore, it underscores the importance of closely monitoring and effectively managing sodium intake to mitigate the risk of developing CVD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
SL designed the manuscript. QF, RC, YD, SX, CH, BH, TJ, and BZ performed the statistical analyses and drafted the manuscript. MB critically reviewed the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Grant No. 81973698 and 81703942), the Young Elite Scientists Sponsorship Program by CACM (Grant No. 2019-QNRC2-B08), the BUCM Precision Cultivation Program (Grant No. JZPY-202205), the Science Fund for Distinguished Young Scholars in BUCM (Grant No. BUCM-2019-JCRC004), and the BUCM Research Development Fund (Grant No. 2021-ZXFZJJ-052).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1250509/full#supplementary-material
CVD, cardiovascular disease; MR, Mendelian randomization; UNa/UCr, urinary sodium/creatinine ratio; GWAS, genome-wide association study; UKBB, UK Biobank; IVW, inverse-variance weighted; WM, weighted median; FDR, false discovery rate; OR, odds ratio; CI, confidence interval; RCTs, randomized controlled trials; SNP, single-nucleotide polymorphism; IV, instrumental variable; LD, linkage disequilibrium; MAF, minor allele frequency; IHD, ischemic heart disease; CHD, coronary heart disease; MMPs, matrix metalloproteinases; TGF-β1, transforming growth factor-beta 1; HF, heart failure.
1. Amin V, Bowes DA, Halden RU. Systematic scoping review evaluating the potential of wastewater-based epidemiology for monitoring cardiovascular disease and cancer. Sci Total Environ. (2023) 858:160103. doi: 10.1016/j.scitotenv.2022.160103
2. Gao X, Huang D, Hu Y, Chen Y, Zhang H, Liu F, et al. Direct oral anticoagulants vs. Vitamin K antagonists in atrial fibrillation patients at risk of falling: a meta-analysis. Front Cardiovasc Med. (2022) 9:833329. doi: 10.3389/fcvm.2022.833329
3. Yu T, Xu B, Bao M, Gao Y, Zhang Q, Zhang X, et al. Identification of potential biomarkers and pathways associated with carotid atherosclerotic plaques in type 2 diabetes mellitus: A transcriptomics study. Front Endocrinol. (2022) 13:981100. doi: 10.3389/fendo.2022.981100
4. World Health Organization. Noncommunicable Diseases. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed September 16, 2022).
5. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD (2019). Study J Am College Cardiol. (2020) 76:2982–3021.
6. Huo R, Liu Y, Xu H, Li J, Xin R, Xing Z, et al. Associations between carotid atherosclerotic plaque characteristics determined by magnetic resonance imaging and improvement of cognition in patients undergoing carotid endarterectomy. Quant Imaging Med Surg. (2022) 12:2891–903. doi: 10.21037/qims-21-981
7. Jiang X, Yan M. Comparing the impact on the prognosis of acute myocardial infarction critical patients of using midazolam, propofol, and dexmedetomidine for sedation. BMC Cardiovasc Disord. (2021) 21:584. doi: 10.1186/s12872-021-02385-9
8. O'Donnell M, Mente A, Alderman MH, Brady AJB, Diaz R, Gupta R, et al. Salt and cardiovascular disease: insufficient evidence to recommend low sodium intake. Eur Heart J. (2020) 41:3363–73. doi: 10.1093/eurheartj/ehaa586
9. Mente A, O'Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. (2016) 388:465–75. doi: 10.1016/S0140-6736(16)30467-6
10. Alderman MH. Presidential Address: 21st Scientific Meeting of the International Society of Hypertension: dietary sodium and cardiovascular disease: the 'J'-shaped relation. J Hypertens. (2007) 25:903–7. doi: 10.1097/HJH.0b013e3280c14394
11. Choi S, Casey L, Albersheim S, Van Oerle R, Irvine MA, Piper HG. Urine sodium to urine creatinine ratio as a marker of total body sodium in infants with intestinal failure. J Pediatr Surg. (2022) 57:937–40. doi: 10.1016/j.jpedsurg.2021.12.035
12. Huang F, Yu P, Yuan Y, Li Q, Lin F, Gao Z, et al. The relationship between sodium excretion and blood pressure, urine albumin, central retinal arteriolar equivalent. BMC Cardiovasc Disord. (2016) 16:194. doi: 10.1186/s12872-016-0369-1
13. Mann SJ, Gerber LM. Estimation of 24-hour sodium excretion from spot urine samples. J Clin Hypertens. (2010) 12:174–80. doi: 10.1111/j.1751-7176.2009.00241.x
14. Lee S-G, Lee W, Kwon OH, Kim J-H. Association of urinary sodium/creatinine ratio and urinary sodium/specific gravity unit ratio with blood pressure and hypertension: KNHANES 2009-2010. Clin Chim Acta. (2013) 424:168–73. doi: 10.1016/j.cca.2013.05.027
15. Woo J, Ho SC, Donnan S, Swaminathan R. Nutritional correlates of blood pressure in elderly Chinese. J Hum Hypertens. (1988) 1:287–91.
16. Woo J, Lau E, Chan A, Cockram C, Swaminathan R. Blood pressure and urinary cations in a Chinese population. J Hum Hypertens. (1992) 6:299–304.
17. Chen R, Xu S, Ding Y, Li L, Huang C, Bao M, et al. Dissecting causal associations of type 2 diabetes with 111 types of ocular conditions: a Mendelian randomization study. Front Endocrinol. (2023). doi: 10.3389/fendo.2023.1307468
18. Zanetti D, Rao A, Gustafsson S, Assimes TL, Montgomery SB, Ingelsson E. Identification of 22 novel loci associated with urinary biomarkers of albumin, sodium, and potassium excretion. Kidney Int. (2019) 95:1197–208. doi: 10.1016/j.kint.2018.12.017
19. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
20. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. ELife. (2018) 7:e34408. doi: 10.7554/eLife.34408
21. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
22. Institute of Medicine (US) Committee on Social Security Cardiovascular Disability Criteria. Cardiovascular Disability: Updating the Social Security Listings. (2010). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK209964/ (accessed December 9, 2023).
23. Tan M, He F, Morris JK, MacGregor G. Reducing daily salt intake in China by 1 g could prevent almost 9 million cardiovascular events by 2030: a modelling study. BMJ Nutr Prev Health. (2022) 5:164–70. doi: 10.1136/bmjnph-2021-000408
24. Xu BF, Liu R, Huang CX, He BS Li GY, Sun HS, et al. Identification of key genes in ruptured atherosclerotic plaques by weighted gene correlation network analysis. Sci Rep. (2020) 10:10847. doi: 10.1038/s41598-020-67114-2
25. Bao MH, Luo HQ, Chen LH, Tang L, Ma KF, Xiang J, et al. Impact of high fat diet on long non-coding RNAs and messenger RNAs expression in the aortas of ApoE(-/-) mice. Sci Rep. (2016) 6:34161. doi: 10.1038/srep34161
26. Wuopio J, Ling Y-T, Orho-Melander M, Engström G, Ärnlöv J. The association between sodium intake and coronary and carotid atherosclerosis in the general Swedish population. Eur Heart J Open. (2023) 3:oead024. doi: 10.1093/ehjopen/oead024
27. Wannamethee SG, Shaper AG, Whincup PH, Walker M. Role of risk factors for major coronary heart disease events with increasing length of follow up. Heart. (1999) 81:374–9. doi: 10.1136/hrt.81.4.374
28. Liu H, Gao X, Zhou L, Wu Y, Li Y, Mai J, et al. Urinary sodium excretion and risk of cardiovascular disease in the Chinese population: a prospective study. Hypertens Res. (2018) 41:849–55. doi: 10.1038/s41440-018-0091-8
29. Ma Y, He FJ, Sun Q, Yuan C, Kieneker LM, Curhan GC, et al. 24-hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. (2022) 386:252–63. doi: 10.1056/NEJMoa2109794
30. Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G. Sodium intake and hypertension. Nutrients. (2019) 11:1970. doi: 10.3390/nu11091970
31. Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. (2016) 32:659–68. doi: 10.1016/j.cjca.2016.02.070
32. Wang M, Zhao D, Spinetti G, Zhang J, Jiang L-Q, Pintus G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. (2006) 26:1503–9. doi: 10.1161/01.ATV.0000225777.58488.f2
33. Schulick AH, Taylor AJ, Zuo W, Qiu CB, Dong G, Woodward RN, et al. Overexpression of transforming growth factor beta1 in arterial endothelium causes hyperplasia, apoptosis, cartilaginous metaplasia. Proc Natl Acad Sci U S A. (1998) 95:6983–8. doi: 10.1073/pnas.95.12.6983
34. Au Yeung SL, Schooling CM. Impact of urinary sodium on cardiovascular disease and risk factors: A 2 sample Mendelian randomization study. Clinical Nutrition. (2021) 40:1990–6. doi: 10.1016/j.clnu.2020.09.018
35. Wang Y-J, Chien K-L, Hsu H-C, Lin H-J, Su T-C, Chen M-F, et al. Urinary sodium excretion and the risk of CVD: a community-based cohort study in Taiwan. Br J Nutr. (2022) 127:1086–97. doi: 10.1017/S0007114521001768
36. Ganes A, Davis JA, Virtanen JK, Voutilainen A, Tuomainen T-P, Atherton JJ, et al. Urinary sodium concentration predicts time to major adverse coronary events and all-cause mortality in men with heart failure over a 28-33-year period: a prospective cohort study. BMC Cardiovasc Disord. (2022) 22:391. doi: 10.1186/s12872-022-02830-3
37. Peng S, Wang J, Xiao Y, Yin L, Peng Y, Yang L, et al. The association of carotid artery atherosclerosis with the estimated excretion levels of urinary sodium and potassium and their ratio in Chinese adults. Nutr J. (2021) 20:50. doi: 10.1186/s12937-021-00710-8
38. Dai X-W, Wang C, Xu Y, Guan K, Su Y-X, Chen Y-M. Urinary sodium and potassium excretion and carotid atherosclerosis in chinese men and women. Nutrients. (2016) 8:612. doi: 10.3390/nu8100612
39. Jung S, Kim MK, Shin J, Choi BY, Lee Y-H, Shin DH, et al. High sodium intake and sodium to potassium ratio may be linked to subsequent increase in vascular damage in adults aged 40 years and older: the Korean multi-rural communities cohort (MRCohort). Eur J Nutr. (2019) 58:1659–71. doi: 10.1007/s00394-018-1712-3
40. Makin SDJ, Mubki GF, Doubal FN, Shuler K, Staals J, Dennis MS, et al. Small vessel disease and dietary salt intake: cross-sectional study and systematic review. J Stroke Cerebrovasc Dis. (2017) 26:3020–8. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.004
41. Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. (2019) 11:2090. doi: 10.3390/nu11092090
42. Bao MH Li JM, Zhou QL Li GY, Zeng J, Zhao J, et al. Effects of miR-590 on oxLDL-induced endothelial cell apoptosis: Roles of p53 and NF-κB. Mol Med Rep. (2016) 13:867–73. doi: 10.3892/mmr.2015.4606
43. Bao MH, Zhang YW, Lou XY, Cheng Y, Zhou HH. Protective effects of let-7a and let-7b on oxidized low-density lipoprotein induced endothelial cell injuries. PLoS One. (2014) 9:e106540. doi: 10.1371/journal.pone.0106540
44. Yang YY, Shi LX Li JH, Yao LY, Xiang DX. Piperazine ferulate ameliorates the development of diabetic nephropathy by regulating endothelial nitric oxide synthase. Mol Med Rep. (2019) 19:2245–53. doi: 10.3892/mmr.2019.9875
45. Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. (2017) 24:50. doi: 10.1186/s12929-017-0357-5
46. Cavka A, Jukic I, Ali M, Goslawski M, Bian J-T, Wang E, et al. Short-term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. J Hypertens. (2016) 34:676–84. doi: 10.1097/HJH.0000000000000852
47. Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. (2008) 51:1525–30. doi: 10.1161/HYPERTENSIONAHA.108.109868
48. Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. (2013) 61:335–43. doi: 10.1016/j.jacc.2012.09.010
49. Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. (2012) 590:5519–28. doi: 10.1113/jphysiol.2012.236992
50. Graudal N, Jürgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. (2014) 27:1129–37. doi: 10.1093/ajh/hpu028
Keywords: sodium intake, cardiovascular disease, Mendelian randomization, UK Biobank, FinnGen
Citation: Fu Q, Chen R, Ding Y, Xu S, Huang C, He B, Jiang T, Zeng B, Bao M and Li S (2023) Sodium intake and the risk of various types of cardiovascular diseases: a Mendelian randomization study. Front. Nutr. 10:1250509. doi: 10.3389/fnut.2023.1250509
Received: 07 July 2023; Accepted: 27 November 2023;
Published: 22 December 2023.
Edited by:
Iain Brownlee, Northumbria University, United KingdomReviewed by:
Longlong Hu, Second Affiliated Hospital of Nanchang University, ChinaCopyright © 2023 Fu, Chen, Ding, Xu, Huang, He, Jiang, Zeng, Bao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meihua Bao, bWhiYW83OEAxNjMuY29t; Sen Li, c2VubGlAY29ubmVjdC5oa3UuaGs=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.