
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 24 August 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1250305
This article is part of the Research TopicIntensive Care Unit Acquired Weakness: Potential Role of Medical Nutrition Treatment Quantity, Timing, and CompositionView all 9 articles
 Juan Carlos Lopez-Delgado1,2*
Juan Carlos Lopez-Delgado1,2* Lluís Servia-Goixart3,4
Lluís Servia-Goixart3,4 Teodoro Grau-Carmona5,6
Teodoro Grau-Carmona5,6 Luisa Bordeje-Laguna7
Luisa Bordeje-Laguna7 Esther Portugal-Rodriguez8
Esther Portugal-Rodriguez8 Carolina Lorencio-Cardenas9
Carolina Lorencio-Cardenas9 Paula Vera-Artazcoz10
Paula Vera-Artazcoz10 Laura Macaya-Redin11
Laura Macaya-Redin11 Juan Francisco Martinez-Carmona12
Juan Francisco Martinez-Carmona12 Judith Marin Corral13
Judith Marin Corral13 Jose Luis Flordelís-Lasierra5
Jose Luis Flordelís-Lasierra5 Carlos Seron-Arbeloa14
Carlos Seron-Arbeloa14 Maravillas de las Nieves Alcazar-Espin15
Maravillas de las Nieves Alcazar-Espin15 Elisabeth Navas-Moya16
Elisabeth Navas-Moya16 Sara Aldunate-Calvo11
Sara Aldunate-Calvo11 Beatriz Nieto Martino17
Beatriz Nieto Martino17 Itziar Martinez de Lagran18
Itziar Martinez de Lagran18Background and aims: Despite enteral nutrition (EN) is the preferred route of nutrition in patients with critical illness, EN is not always able to provide optimal nutrient provision and parenteral nutrition (PN) is needed. This is strongly associated with gastrointestinal (GI) complications, a feature of gastrointestinal dysfunction and disease severity. The aim of the present study was to investigate factors associated with the need of PN after start of EN, together with the use and complications associated with EN.
Methods: Adult patients admitted to 38 Spanish intensive care units (ICUs) between April and July 2018, who needed EN therapy were included in a prospective observational study. The characteristics of EN-treated patients and those who required PN after start EN were analyzed (i.e., clinical, laboratory and scores).
Results: Of a total of 443 patients, 43 (9.7%) received PN. One-third (29.3%) of patients presented GI complications, which were more frequent among those needing PN (26% vs. 60%, p = 0.001). No differences regarding mean energy and protein delivery were found between patients treated only with EN (n = 400) and those needing supplementary or total PN (n = 43). Abnormalities in lipid profile, blood proteins, and inflammatory markers, such as C-Reactive Protein, were shown in those patients needing PN. Sequential Organ Failure Assessment (SOFA) on ICU admission (Hazard ratio [HR]:1.161, 95% confidence interval [CI]:1.053–1.281, p = 0.003) and modified Nutrition Risk in Critically Ill (mNUTRIC) score (HR:1.311, 95% CI:1.098–1.565, p = 0.003) were higher among those who needed PN. In the multivariate analysis, higher SOFA score (HR:1.221, 95% CI:1.057–1.410, p = 0.007) and higher triglyceride levels on ICU admission (HR:1.004, 95% CI:1.001–1.007, p = 0.003) were associated with an increased risk for the need of PN, whereas higher albumin levels on ICU admission (HR:0.424, 95% CI:0.210–0.687, p = 0.016) was associated with lower need of PN.
Conclusion: A higher SOFA and nutrition-related laboratory parameters on ICU admission may be associated with the need of PN after starting EN therapy. This may be related with a higher occurrence of GI complications, a feature of GI dysfunction.
Clinical trial registration: ClinicalTrials.gov: NCT03634943.
Appropriate nutrition therapy of severely ill patients admitted to intensive care units (ICUs) aims at avoiding malnutrition of primarily well-nourished patients and preventing further deterioration of preexisting malnutrition. Nutrition therapy with optimal delivery of nutrients provides the substrates to preserve organ function, helps to modulate the inflammatory response to surgery, trauma, or any severe disease, and optimizes the metabolic status, all of which may ultimately contribute to improvement in clinical patient outcomes (1, 2). Malnutrition has been shown to be a significant prognostic factor for mortality, length of stay, infection rates, and duration of mechanical ventilation (3, 4).
However, performing an adequate nutrition therapy remains difficult because critically ill suffer from pathophysiological alterations in metabolism. During the acute phase of critical illness (i.e., first 48–72 h of ICU admission), there is an increased demand of metabolic substrates, especially glucose, caused by higher resting energy expenditure needs, which are mainly obtained from the stimulation of gluconeogenesis (5–8). Furthermore, exogenous substrates no longer inhibit the production of glucose by gluconeogenic organs (e.g., liver), and nutrition therapy should be progressive during the acute phase to avoid the detrimental effects of overfeeding (i.e., >110% of energy demands) over morbidity (e.g., longer length of mechanical ventilation) and even mortality (7, 8). At the same time, glycogen reserves in the liver do not last long (i.e., <24 h) and patients suffer from muscle catabolism since amino acids are main source to preserve gluconeogenesis, leading to protein depletion and sarcopenia, which is linked with worst outcomes (9).
Although nutritional therapy is an integral part in the care of ICU patients and clinical practice recommendations for nutrition in critically ill patients have been published by different guidelines (10–13), macronutrient nutrition targets (i.e., caloric and protein) are frequently not achieved in routine daily conditions (14, 15). For example, a recent large observational study showed that almost 20 and 35% of ICU patients do not receive adequate delivery of protein targeted needs (16). These findings are also quite similar to previous studies (17, 18). Indeed, the benefits of nutrition therapy in ICUs are related to several factors, including the delivery route, initiation time, macronutrient dose, patient’s nutritional risk, duration of ICU stay, or severity of critical illness.
Enteral nutrition (EN) is the preferred nutritional route for the majority of critically ill patients, with advantages over parenteral nutrition (PN), such as cost-effectiveness, efficient use of nutrients, maintenance of gastrointestinal barrier or support of intestinal immunological function, but there is a large variation in how nutritional therapy is provided clinically, which ultimately difficult macronutrient targets (10, 11). Moreover, barrier factors influencing enteral feeding include delay in the start of EN, low infusion rate, lack of standardized and failure to follow EN protocols, disruptions to EN (e.g., diagnostic testing, accidental pull-out of nasogastric tubing, gastrointestinal intolerance), insufficient dietitian coverage, and prioritize other aspects of patient care over nutrition (19–22). Despite these factors can be overcome by improving adherence to guidelines, there are some ICU patients not able to tolerate prescribed EN due to the occurrence of gastrointestinal (GI) complications (e.g., high gastric residual volumes, diarrhea, vomiting), leading to the lack of optimal nutrient provision (23, 24). In consequence, this suboptimal EN intake may worsen nutritional status, which is associated with higher complications (e.g., infections) and higher mortality (25). At the same time, it is important to remark that the occurrence of GI complications and EN intolerance are both features of GI dysfunction (25).
The use of PN after the initiation of EN could help to improve nutritional intake and mitigate the development of malnutrition in ICU patients who do not tolerate prescribed EN (26). Moreover, the use of PN has also been associated with disease severity and higher mortality, suggesting that it is a surrogate marker of GI dysfunction (27, 28). In a previous nationwide evaluation of nutritional practices in ICU patients admitted to 38 Spanish ICUs and focused on ICU mortality, patients who needed PN after starting EN were found to be a high-risk group for mortality (23).
The main aim of the present study was to explore the potential of different factors (i.e., clinical variables, laboratory markers, and nutritional and ICU scores) to be associated with the need of PN after starting EN, especially those recorded on ICU admission or during the early stage. We hypothesized that this may be helpful to identify early those patients that may need PN after starting EN and to prescribe earlier PN, avoiding suboptimal nutrition intake, and the development of malnutrition during ICU stay, which ultimately may prevent negative effects of GI dysfunction. The present research may be also helpful to reflect the difficulty of achieving macronutrient targets only with enteral route (i.e., EN) under certain clinical conditions in critically ill. We also aimed to describe the use and GI complications, especially those associated with EN, together with differences in nutrition therapy between patients receiving EN only and those who need PN after starting EN were examined.
Between April and July 2018, a nationwide prospective observational study was conducted in which 38 ICUs throughout Spain participated (We shown in Supplementary Table S1 location of participating hospitals). All adult patients aged 18 years or older were consecutively included in the study providing that they need artificial nutrition support (EN or PN or both) for more than 48 h and a length of stay in the ICU of at least 72 h. Patients able to feed orally were excluded. Patients admitted to the ICU for postoperative recovery or ICU monitoring without the needing specific treatment for organ support (e.g., non-invasive mechanical ventilation or vasopressors) were also excluded. Only patients who required initial EN on ICU admission were included in the present analysis. Results of the present research corresponds to a planned subanalysis about the insights of the use of EN in the ENPIC study (ClinicalTrials.gov: NCT03634943). Despite the observational nature of the study, participating ICUs were required to have at least clinical nutrition practices that included the use of a local nutritional protocol in compliance with current guidelines or in the absence of this, the involvement of a health care professional specialized in artificial nutrition therapy (15). Participating sites and investigators were encouraged to follow recommendations for the feeding route delivery and management for giving optimal nutrition therapy in ICU patients (see Supplementary Figure S1) (13, 15). It is important to highlight that all participants were interested in clinical nutrition and are members of the Metabolism & Nutrition Working Group the Spanish Society of Intensive Care Medicine (SEMICYUC) and/ or Critical Care Working Group from the Spanish Society of Metabolism & Clinical Nutrition (SENPE).
The study was approved by the Clinical Research Ethics Committee of Hospital Universitari de Bellvitge (Barcelona, Spain) as a central institutional review board (code PR401/17). The need for informed consent was waived due to the observational design of the study and collection of data from an anonymous centralized database.
For each patient, the following data were collected: demographics; body mass index; type of patient (medical, trauma, surgery); comorbidities; Acute Physiology and Chronic Health Evaluation (APACHE) II score; Simplified Acute Physiology Score (SAPS) II score; Sequential Organ Failure Assessment (SOFA) score on ICU admission; modified Nutrition Risk in the Critically II (mNUTRIC) score; details of nutritional therapy including time of initiation of EN, mean energy and protein intakes until ICU discharge or for a maximum of 14 days; laboratory data; GI (or EN-related) complications (i.e., high gastric residual volume, vomiting, aspiration, diarrhea, mesenteric ischemia); and outcomes during ICU stay, which included mechanical ventilation and days on mechanical ventilation, vasoactive drug support, renal replacement therapy (RRT), respiratory tract infection, catheter-related bloodstream infection, length of stay in the ICU and in the hospital, and 28-day mortality. We registered mNUTRIC score as dichotomous variable to evaluate nutritional risk: a mNUTRIC score ≥ 5 on ICU admission was considered a higher nutritional risk when compared with a score 0–5. The type of nutritional therapy included two subgroups: EN only and EN-PN (i.e., patients who received EN initially followed by PN).
The study outcomes included the difference between EN and EN-PN groups in GI complications, mechanical ventilation, duration of mechanical ventilation, use of vasoactive drugs, RRT, respiratory and catheter-related infections, length of ICU and hospital stay, and 28-mortality.
Categorical data are expressed as frequencies and percentages, and continuous data as mean and standard deviation (SD) or median an interquartile range (IQR) (25th-75th percentile). Categorical data were compared with the chi-square test or the Fisher’s exact test, and quantitative data with the Student’s t test or the Mann–Whitney U test according to the conditions of application. Survival analysis was performed using the Kaplan–Meier method with the Log-Rank test to assess the need of PN over time.
Variables independently associated with the need of supplemental PN were assessed using an adjusted multiple stepwise Cox regression analysis. Variables were included in the model if they had a p value of <0.2 in the univariate analysis. Subsequent multivariate analysis was conducted using an adjusted multiple stepwise Cox regression analysis adjusted by age, patient type (e.g., medical, surgical, or trauma), illness severity (e.g., APACHE score), length of nutritional therapy, and data for which there were significant differences in baseline characteristics between both subgroups. The size difference between EN and EN-PN groups (i.e., number of patients) and time of initiation of PN was also considered for adjusting. Statistical significance was set at p < 0.05. The IBM SPSS version 20.0 (IMB Corp., Armonk, NY, United States) was used for the analysis of data.
During the study period, 644 ICU patients received artificial nutrition support, but 201 patients were excluded because of incomplete data collection (n = 13) or use of initial PN (n = 189). Of the remaining 443 patients treated with EN, 400 (90.3%) received EN exclusively and 43 (9.7%) were treated with EN and PN. The study flow chart is shown in Figure 1.
Most of the patients (n = 426, 96.2%) received EN by means of nasogastric tube, whereas a few patients received EN by the transpyloric route (n = 6, 1.3%) or via a surgical ostomy (n = 11, 2.5%). Almost half of the patients (n = 216, 48.8%) made a switch from EN to oral nutrition before ICU discharge. No patient included in this analysis received any oral supplements.
In the group of 43 patients with EN-PN, the indication of PN was related to difficulties in achieving sufficient nutritional requirements (n = 38, 88.4%) (i.e., <60% of macronutrient target (2, 13)), mainly due to paralytic ileus (n = 23, 53.5%). Very few patients received PN due to severe acute pancreatitis (n = 3, 6.9%) and small bowel fistula (n = 2, 4.6%). PN was usually administered through a central venous line, except in 4 (9.3%) patients in which a peripherally inserted central catheter was used. All patients from EN-PN received prokinetics (i.e., metoclopramide, erythromycin or both) before the indication of PN. As shown in Figure 2, a total of 23 (53.5%) patients received supplemental PN in addition to EN and 20 (46.5%) received PN following discontinuation of EN. Nutritional therapy was given for a median duration of 15 days (range from 6 to 15 days), and PN was given between day 3 and 10 after ICU admission in all cases.
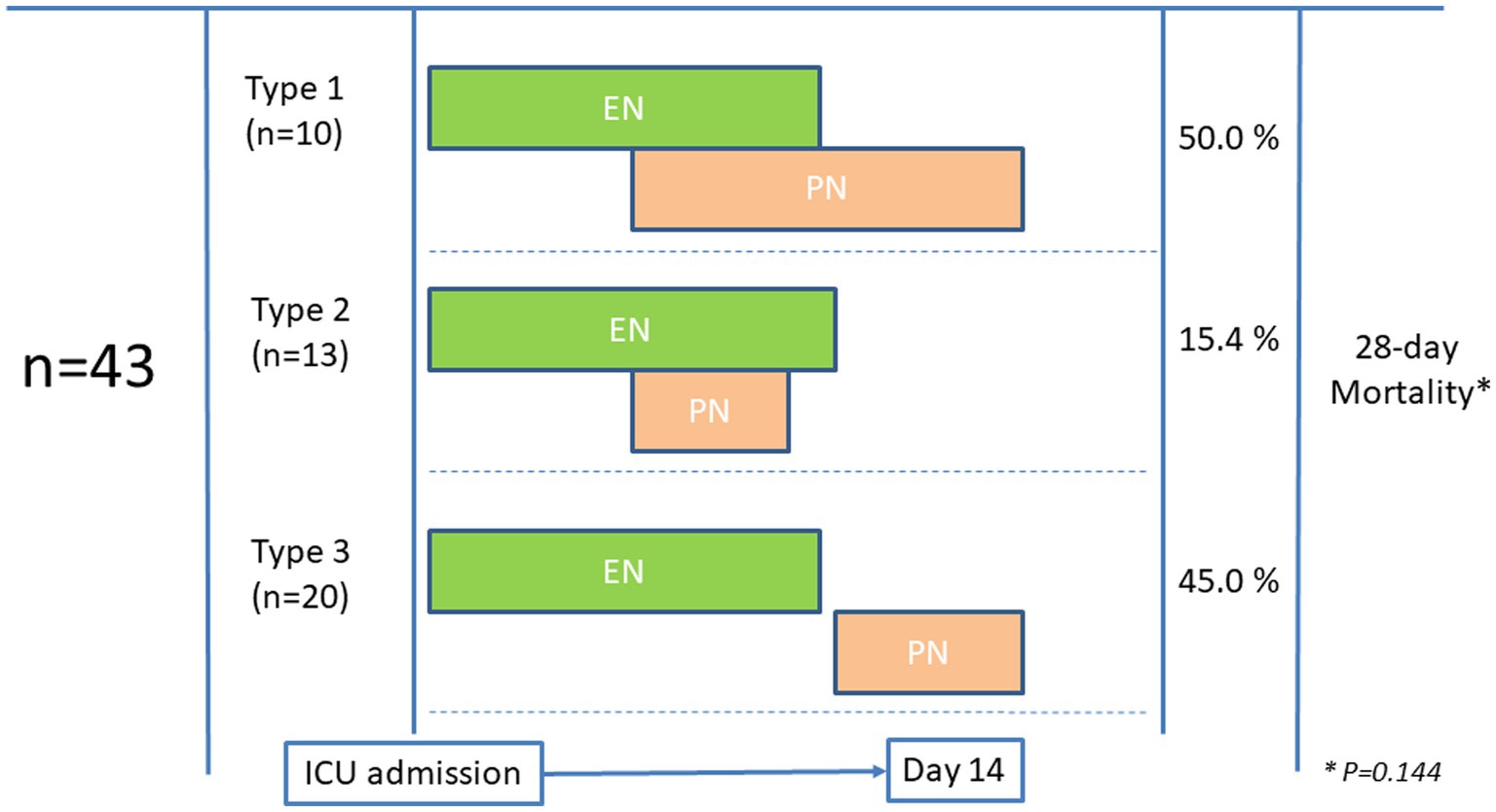
Figure 2. Patterns of administration of parenteral nutrition (PN) in patients who initially received enteral nutrition (EN).
The clinical characteristics, nutritional support, and outcomes of the patients receiving EN and EN-PN are shown in Table 1. Most patients were admitted due to medical illnesses (71.3%), received early EN (i.e., <48 h) support (75.4%) and a higher proportion of patients were at risk of malnutrition based on mNUTRIC score (43.34%). SOFA (on ICU admission) was higher in those who needed PN (Hazard ratio [HR]:1.161, 95% confidence interval [CI]:1.053–1.281, p = 0.003) and those patients with low SOFA (from 0 to 6) showed lower need of PN (HR:0.283, 95% CI:0.120–0.668, p = 0.004). The mNUTRIC score was higher in those who need PN (HR:1.311, 95% CI:1.098–1.565, p = 0.003).
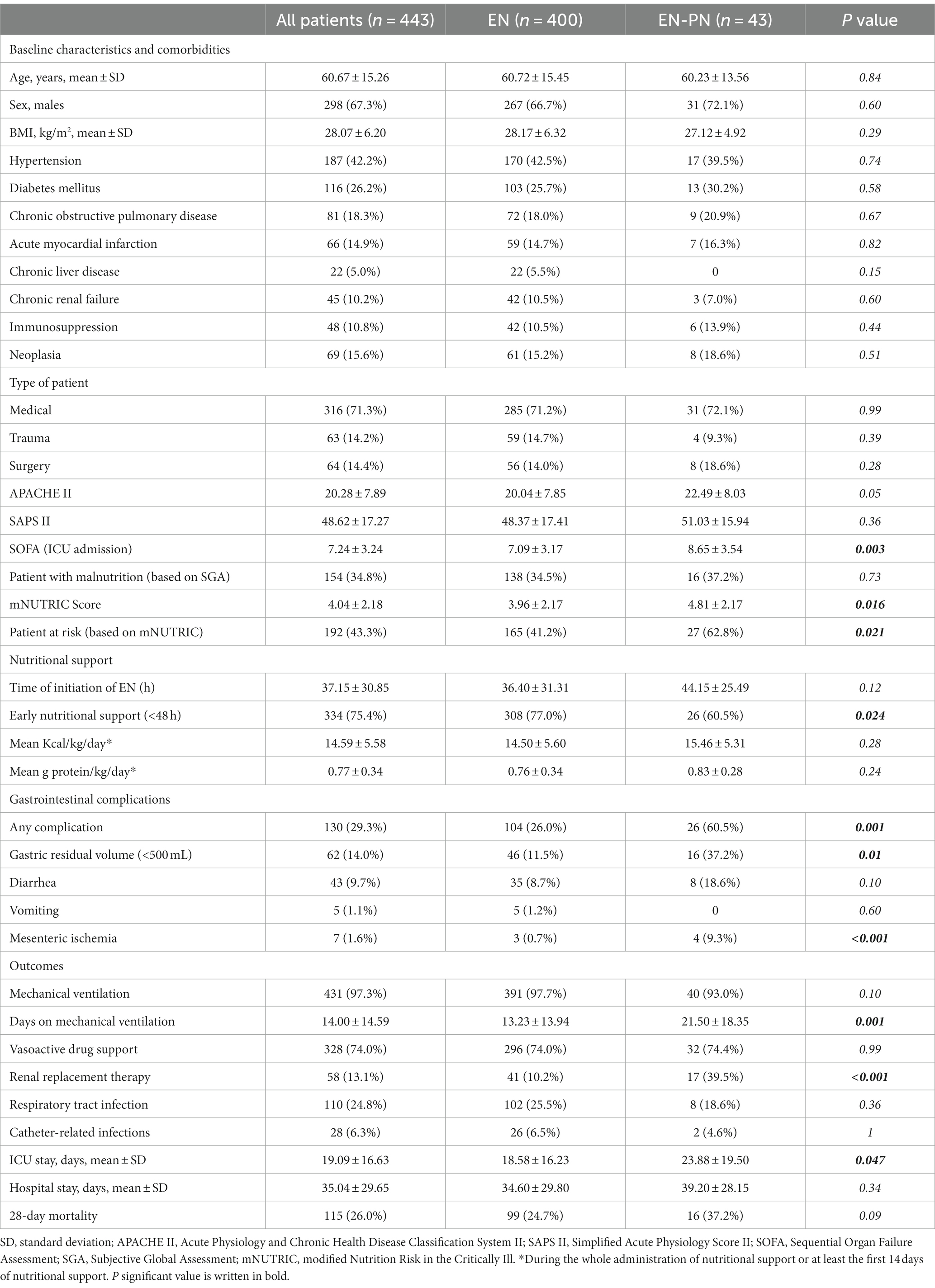
Table 1. Clinical characteristics, nutritional support, and outcomes of ICU patients receiving enteral nutrition (EN) and enteral nutrition with parenteral nutrition (EN-PN) during ICU stay.
The percentage of patients who needed PN after initiation of EN varied significantly according to SOFA score on ICU admission (5.5% for SOFA score 0–6, 11.4% for SOFA score 7–9, and 16.3% for SOFA score ≥ 10, p = 0.013). Also, the percentage of patients requiring PN were higher for higher nutritional risk, which is a mNUTRIC score ≥ 5 (14.1%) as compared with a score 0–5 (6.3%) (p = 0.021). Kaplan–Meier survival analysis also showed higher need of PN with higher SOFA and mNUTRIC scores during ICU admission (Figure 3).
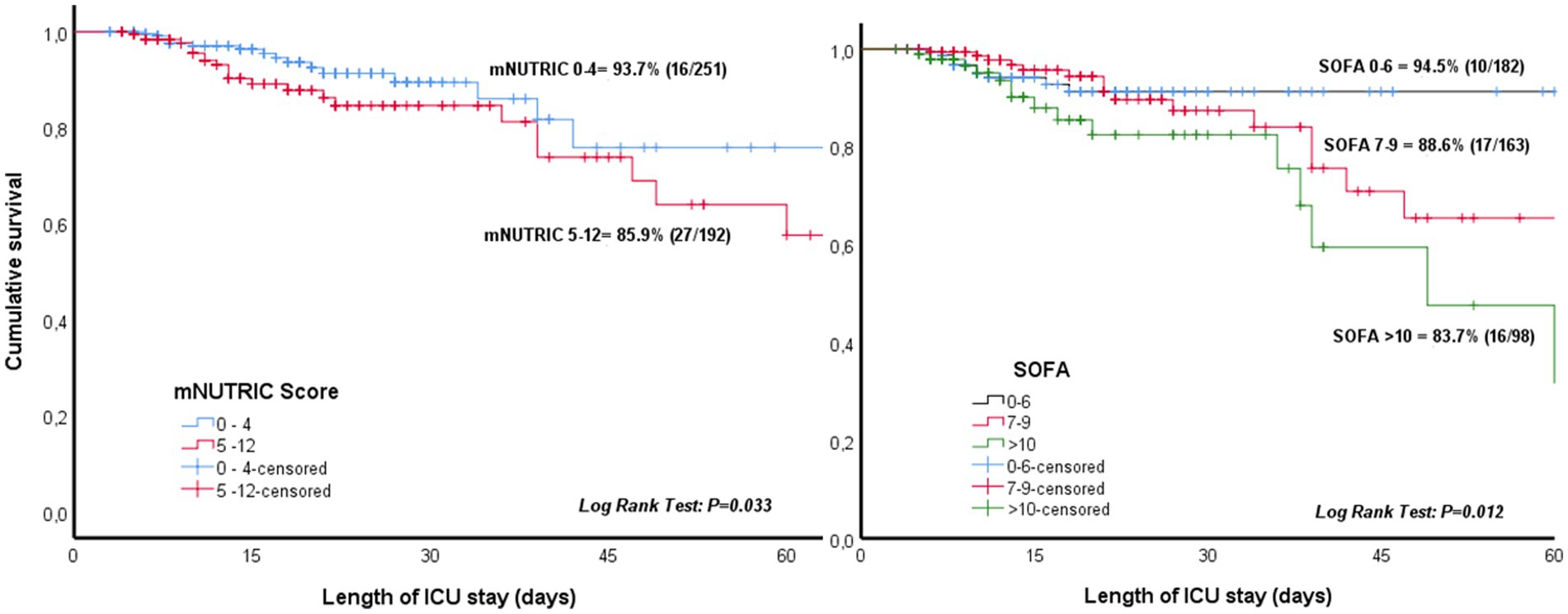
Figure 3. Need of parenteral nutrition during ICU admission according to the sequential organ failure assessment (SOFA) score on ICU admission and modified nutrition risk in the critically Ill (mNUTRIC) score.
Patients in the EN group received early nutritional therapy (i.e., <48 h) more frequently than those in the EN-PN group (77% vs. 60.5%, p = 0.024). Almost one-third of the patients suffered from GI complications, which were more frequent in those patients needing PN, with significant differences in elevated gastric residual volume and mesenteric ischemia (Table 1). In relation to outcomes, more days on mechanical ventilation, more need of RRT, longer ICU stay and a trend toward lower mortality were found in patients treated with PN (Table 1). Also, patients who need PN required RRT more frequently (HR:1.316, 95% CI:1.160–1.626, p = 0.001) and experienced a higher mortality during ICU admission (HR:1.460, 95% CI:0.22–0.954, p = 0.037). Differences in 28-day mortality according to the pattern of feeding (as shown in Figure 2) were not observed (p = 0.144).
Even though patients who needed EN exclusively performed better during early nutritional therapy, there were no statistical differences in mean energy (Figure 4A) and protein (Figure 4B) delivery as compared to the EN-PN group (Table 2). In relation to laboratory analyses, almost all patients presented some type of electrolyte disbalance during ICU admission, with abnormalities in lipid profile (especially triglycerides), lower levels of blood proteins (albumin and prealbumin), and worst parameters of renal function (urea and creatinine) among patients needing PN. Laboratory values with statistically significant differences between the groups of EN and EN-PN are shown in Table 3. Patients did not develop significant increase in liver enzymes and bilirubin during PN administration. The complete list of laboratory results is included in the Supplementary Table S2.
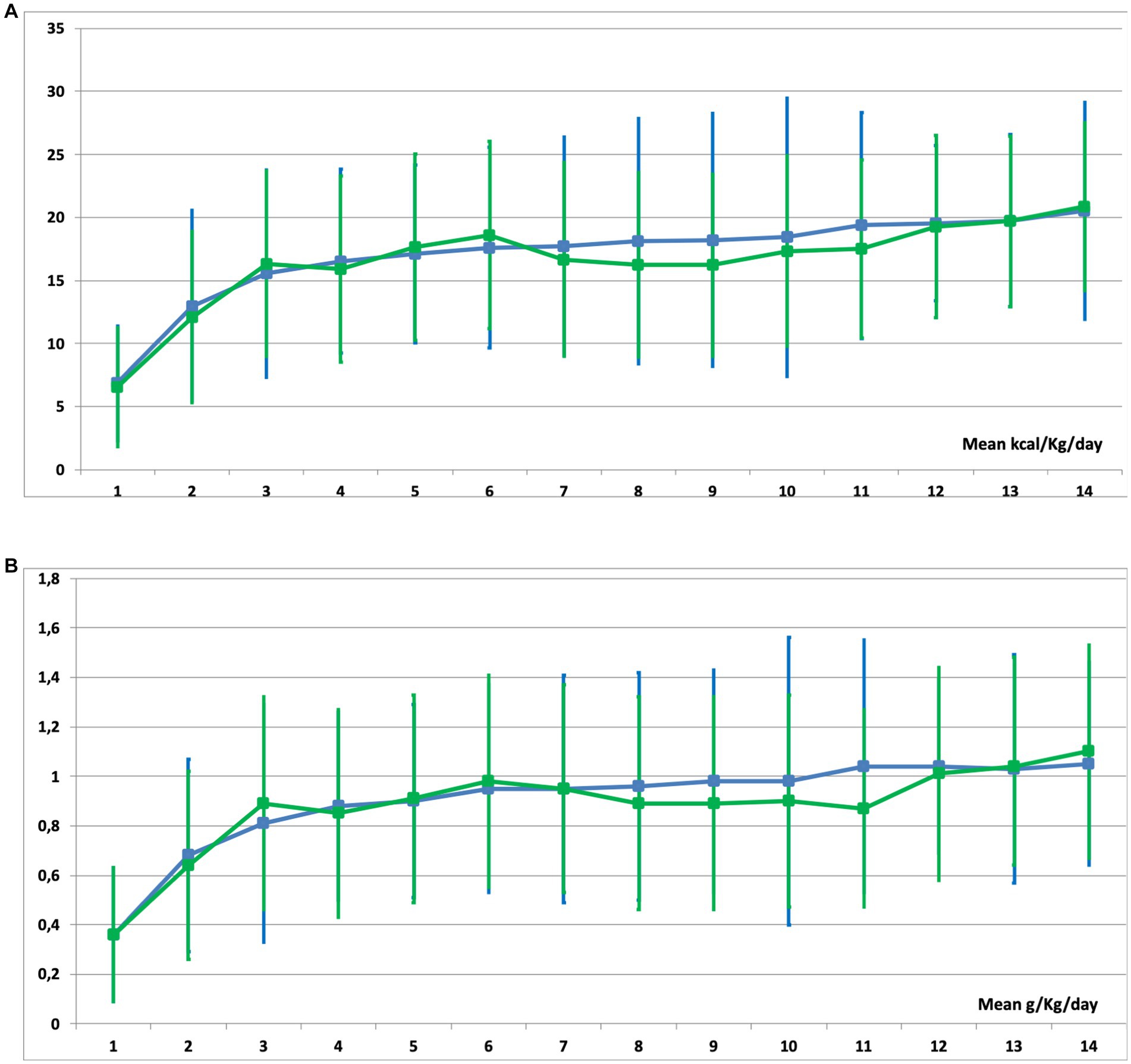
Figure 4. Mean caloric (A) and protein (B) delivery in patients receiving enteral nutrition (EN) (green line) and those receiving EN with parenteral nutrition (PN) (blue line) during their stay in the ICU.
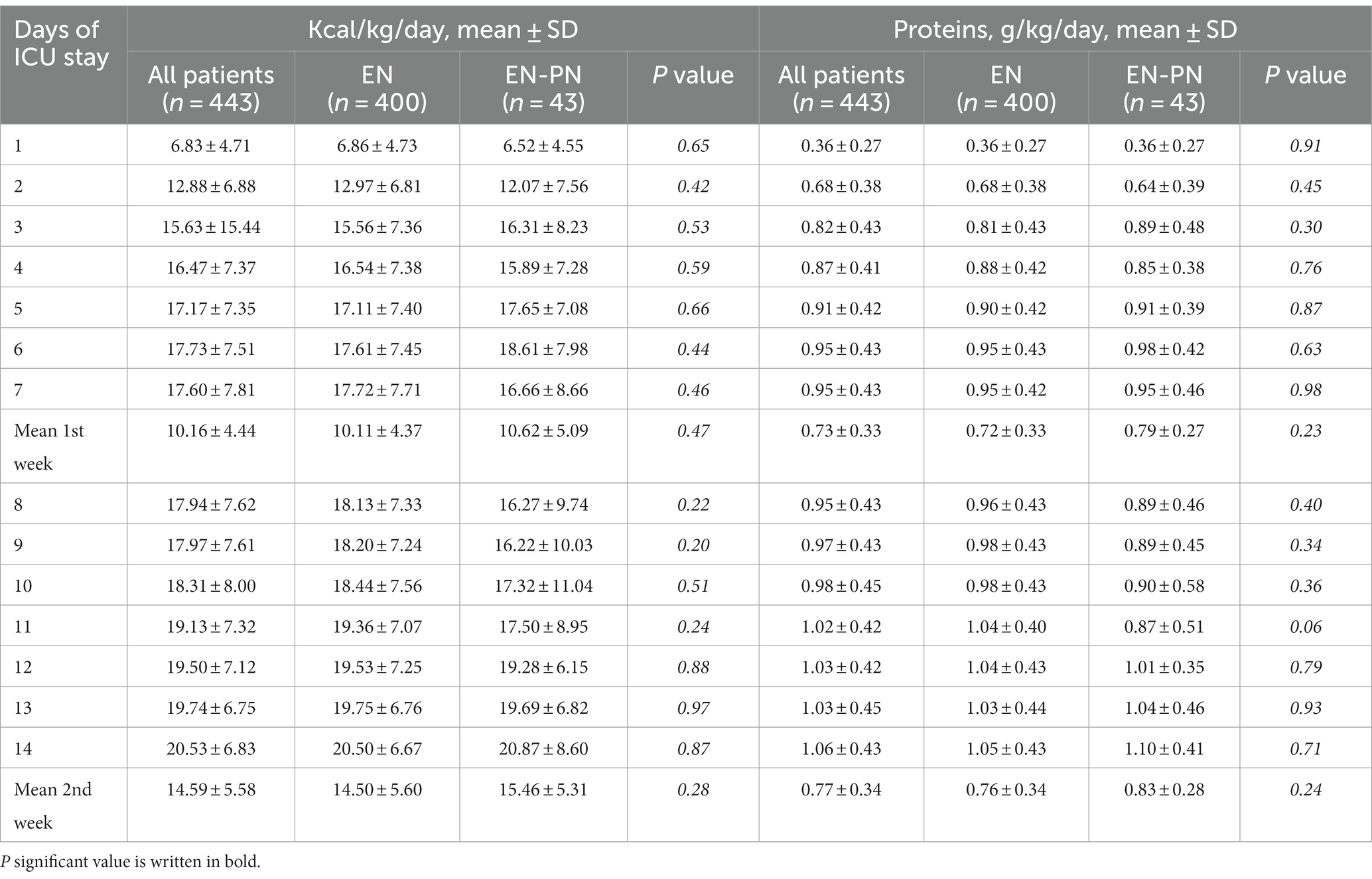
Table 2. Mean caloric and protein requirements in ICU patients receiving enteral nutrition (EN) and enteral nutrition with parenteral nutrition (EN-PN) during ICU stay.
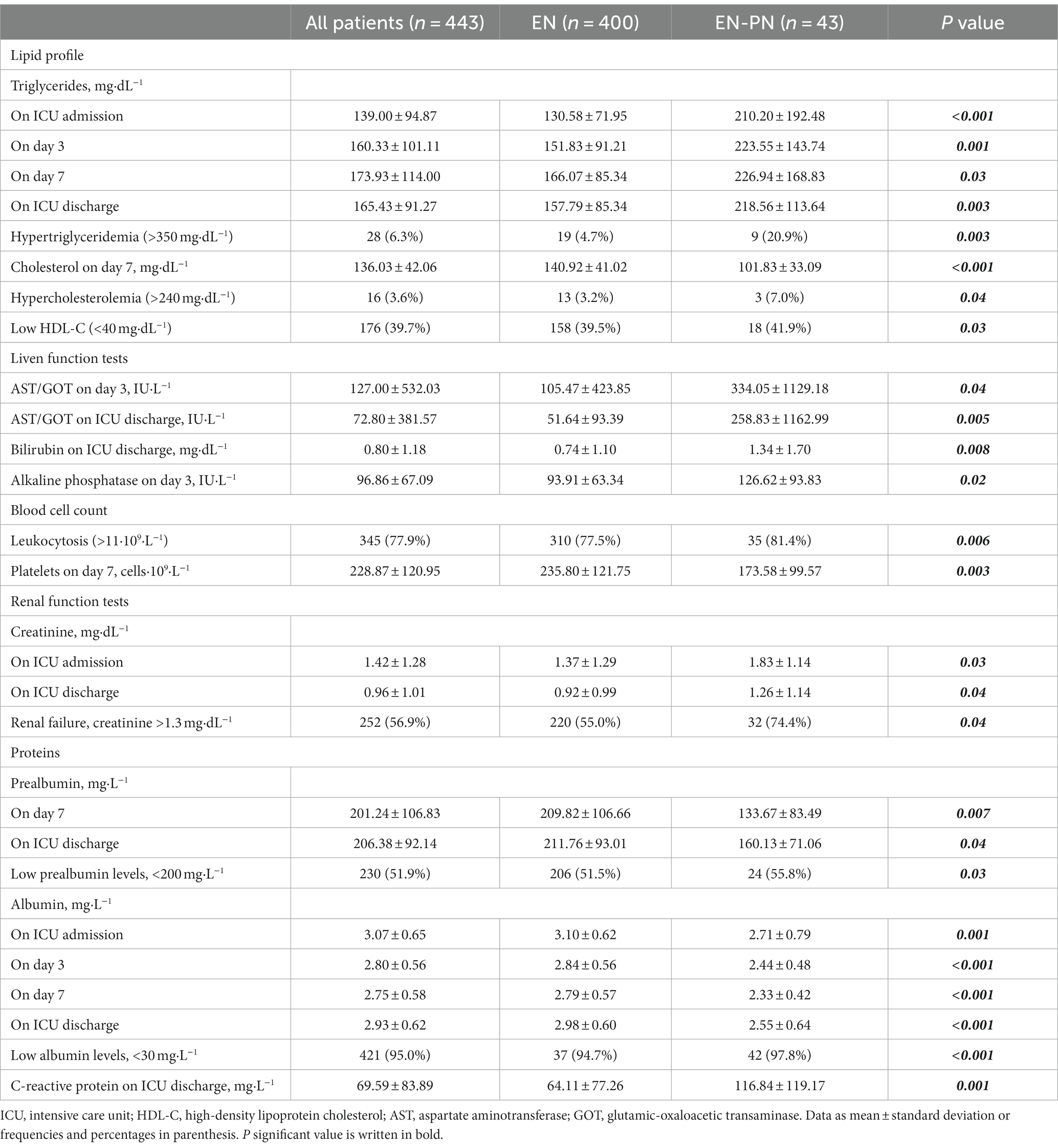
Table 3. Significant differences in laboratory values between receiving enteral nutrition (EN) and enteral nutrition with parenteral nutrition (EN-PN) during ICU stay.
Once adjusted by confounding factors, the multivariate analysis showed that initial higher SOFA score and serum triglycerides on ICU admission were independently associated with the need of PN after initiation of EN, whereas higher serum albumin levels on ICU admission were inversely associated with the need of PN (Table 4).
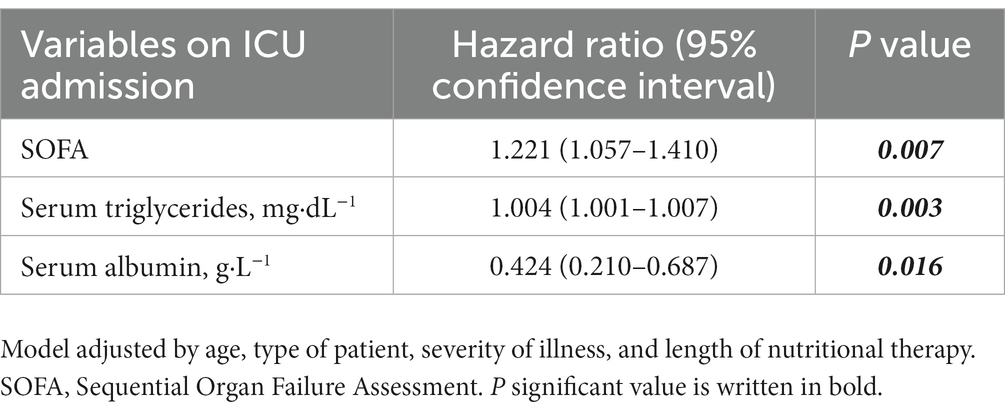
Table 4. Results of multivariate analysis of factors associated with the need of parenteral nutrition (PN) in patients receiving enteral nutrition (EN) during ICU stay.
This multicenter study shows the higher need of PN associated with an initial higher organ failure (i.e., higher SOFA score) on ICU admission in those patients in whom EN had been chosen as initial route of nutrition therapy in ICU patients. We have also shown the association of nutrition-related laboratory parameters, such as triglyceride and albumin levels, with this phenomenon. This is of special importance since there are not validated tools to assess and predict the lack of efficacy of EN to provide adequate nutrition therapy during ICU stay (29). Despite this study was observational, it was performed by participating investigators interested in nutrition, and we may assume that the indication of PN was not related to barrier factors influencing enteral feeding (e.g., delay in the start of EN) but with difficulties in achieving sufficient nutritional requirements based on the clinical condition of the patient (e.g., severity of critical illness and GI complications). The novelty of this research also remains in the analysis of objective and measurable variables, and the combination of all these observations in a single multivariable analysis, which may add new knowledge related to the quality of in-data in observational studies. Finally, we have described the characteristics of EN in a large ICU population, examining differences in clinical features, energy and protein intake, GI complications, and outcome between EN and EN-PN subgroups.
In our cohort, we showed that patients with a SOFA score > 6 on ICU admission were more likely to need PN after initiation of EN. The association of higher SOFA score on ICU admission with higher need of PN during ICU stay is probably related to GI dysfunction. GI dysfunction, which is defined as a transient state (e.g., gastroparesis or ileus) secondary to or in the context of severe illness (i.e., major surgery, sepsis, malnutrition, etc.) has been recognized as a main cause for the use of PN in ICU patients (24). Moreover, impaired gastric motility in critically ill patients is associated with an increased gastric bacterial colonization, pulmonary aspiration and progressive malnutrition leading to adverse outcomes. This is reflected by higher rates of GI complications, which were more frequent in the EN-PN subgroup in our population (27–29). This is not surprising since some of the complications (e.g., high gastric residual volume) are related with the inability of providing adequate nutrition delivery and the need of PN.
It is estimated that at least 60% of ICU patients are affected by some form of GI dysfunction, and that in 30% of patients in whom EN is attempted the feeding route needs to be modified to PN route because of not achieving prescribed nutrient provision or the occurrence of GI complications or both (25). In our study, the use of PN was related to paralytic ileus or the inability to fully tolerate EN, accounted for the impossibility of achieving adequate and sufficient nutritional requirements with EN alone in 10% of the patients. The reasons behind this lower incidence may be probably related with a little presence of barrier factors influencing enteral feeding based on the nature of participating sites. In addition, we did not find differences regarding caloric and protein delivery when EN and EN-PN subgroups where compared. Thus, our findings may also reflect the importance of PN for optimal nutrition delivery in those patients with initial higher disease severity.
Nutrition-related laboratory parameters, such as higher triglycerides on ICU admission, were associated with the need of PN in patients receiving EN, whereas high albumin levels appeared to be a protective factor. Hypertriglyceridemia has been reported to be associated with high-grade inflammation increasing the risk of different conditions, such as acute pancreatitis (30). Indeed, elevated triglyceride concentrations have been reported in patients with systemic inflammatory response (i.e., stress response) due to sepsis or other diseases (31). Hypoalbuminemia, one of the most prevalent abnormalities in ICU patients, is associated with inflammation in a part as a reflection of the extent of physiologic stress resulting from critical illness (32, 33). During critical illness hepatic reprioritization of protein synthesis and an increase in capillary permeability occurs, resulting in lower albumin production and redistribution of serum proteins, respectively (34). Hypertriglyceridemia and hypoalbuminemia, together with C-reactive protein, are probably metabolic factors associated to systemic inflammation and not only to poor or altered metabolic and nutritional status. The association between systemic inflammation and malnutrition is closely linked with the occurrence of GI failure, and it may help to explain our finding regarding nutrition-related laboratory markers (34).
Hypertriglyceridemia is related with metabolic response during acute phase (i.e., first 48–72 h of ICU admission) since inflammatory mediators (i.e., hormones, cytokines, lymphokines) released during the stress response stimulates lipolysis (5). Lipolysis triggers hydrolysis of triglycerides in adipose tissue, producing fatty acids and glycerol. Glycerol is used for gluconeogenesis in the liver, contributing to glucose production, and fatty acids are used by the liver and muscles, converted to ketone bodies, or re-esterified (6). However, lipid metabolism, and more specifically lipid oxidation, is limited due to the lower availability of oxygen during acute phase (i.e., tissular hypoxia), which is also related with the occurrence of mitochondrial dysfunction during critical illness (7, 8). In addition, a higher delivery of lipid metabolites is not associated with an effective inhibition of lipolysis (similar to other metabolic pathways), resulting in an even higher concentration of lipid metabolites when patients received nutrition therapy and some lipid drugs (e.g., propofol) (7). The rate of fatty acid released may also exceed energy needs, and those that are not oxidized may be re-esterified to triglyceride (6). Thus, the occurrence of hypertriglyceridemia may be related with the degree of stress response in critically ill on the basis of an ineffective lipid metabolism.
The nutritional status of ICU patients deteriorates rapidly after admission due to severe catabolism caused by proinflammatory cytokines and hormones, even when patients are well nourished (35, 36). Also, half of the patients showed high nutritional risk (i.e., mNUTRIC score ≥ 5) in our population and in the univariate analysis, high nutritional risk was associated with the need of PN. However, this may be more linked with disease severity rather than the relationship of higher nutritional risk with GI dysfunction, since mNUTRIC score includes ICU prognosis scores, such as SOFA and APACHE II.
The duration of mechanical ventilation, the need of RRT, and the length of stay in the ICU were higher in the EN-PN subgroup, as well as a trend toward higher 28-day mortality. Despite PN avoids caloric debt and it may improve outcome of patients who do not tolerate fully EN, the benefit over mortality is not entirely elucidated (26, 37). This trend toward higher mortality in the EN-PN subgroup may reflect the severity disease of those patients, which also may be a feature of additional organ dysfunction, such as GI dysfunction, more than a lack of benefit of PN per se. As we underlined above, we showed no difference in terms of caloric or protein delivery between EN and EN-PN subgroups of patients, which may reflect the adequacy of PN in providing appropriate nutrition therapy when EN is not completely or partially feasible.
Even though our results are debatable, they are clinically relevant to provide a basis for future trials, especially regarding the strategy in giving nutrition therapy in the most critically ill with high organ failure on admission or during ICU stay. It is important to anticipate and identify ICU patients who are not able to tolerate adequate EN for supplying energy and protein needs, and those would benefit from supplemental PN (38, 39). We have hypothesized that those patients needing PN may benefit from a different nutritional strategy, such as the early use of trophic nutrition together with supplemental PN, in order to avoid the deleterious effects of undernutrition, especially in patients with established malnutrition or at high nutritional risk. This would minimize the risks that GI or EN-related complications may entail for severe critically ill (e.g., invasive ventilated patients with shock) (40). On the basis of our findings and the present discussion, we would like to suggest a proposal of a modified algorithm to select the feeding route for giving nutrition therapy (Figure 5) (13, 38, 39).
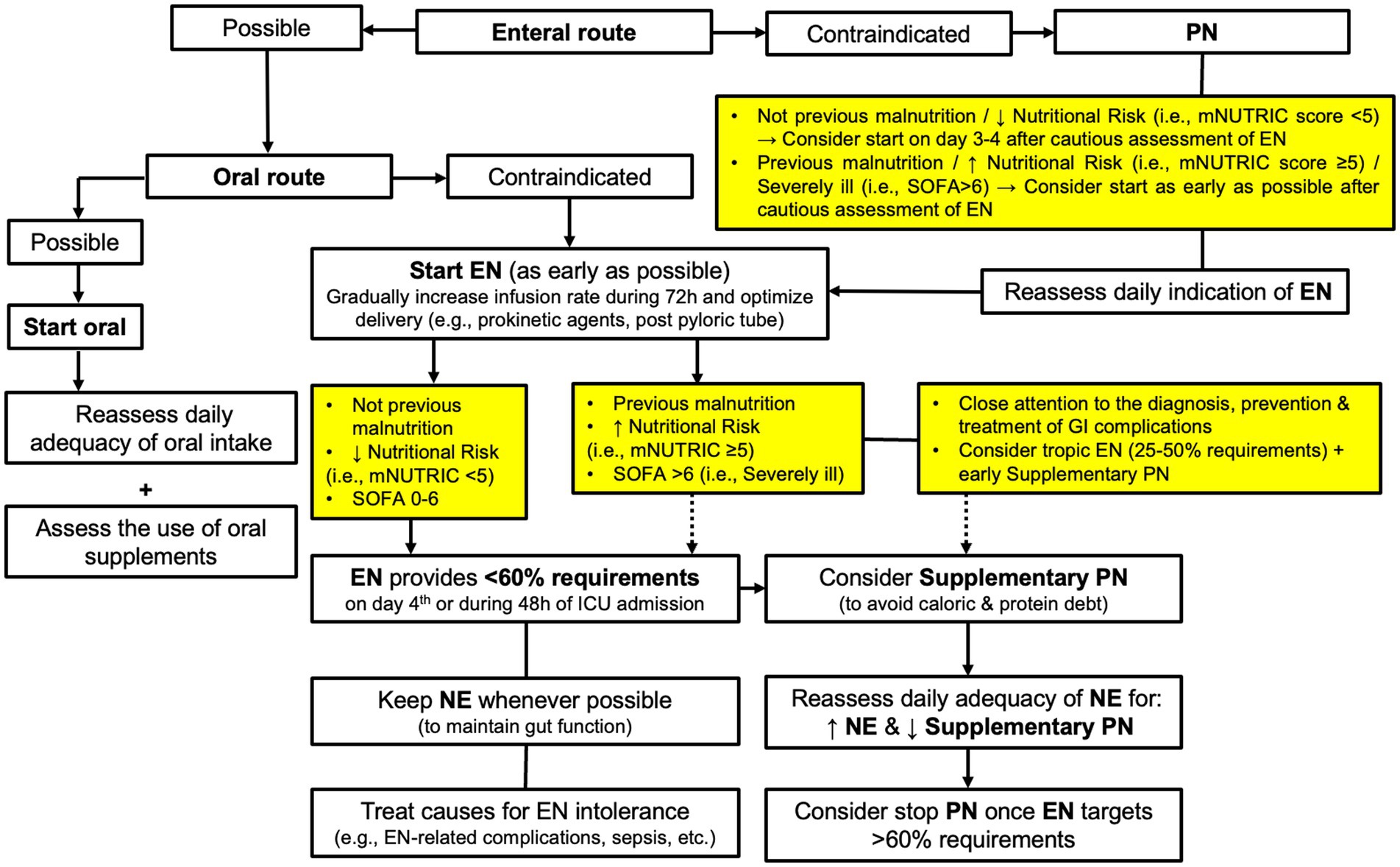
Figure 5. Proposal of a modified algorithm to select the feeding route for giving nutrition therapy in ICU patients. EN, Enteral Nutrition; PN, Parenteral Nutrition; mNUTRIC, modified Nutrition Risk in the Critically II score; SOFA, Sequential Organ Failure Assessment score.
Limitations of the study are mainly related to the observational nature, which only allows to assess associations, the heterogeneity of participants, and the low number of patients in the EN-PN group (n = 43). The latter did not allow subgroup analyses based on the type of patient (i.e., medical, surgical and trauma). However, these limitations have been counterbalanced by means of statistical analysis that have minimized the impact of confounders (e.g., confounding by indication), as well as their influence in our results (described in the Methods section). Strengths of the study also include the large sample size (n = 443), the prospective design with a high data quality, the participation of a high number of ICUs, and the follow-up of nutrition therapy during 14 days after ICU admission, as well as the fact that data here reported reflect real-world practice of intensivists delivering nutrition therapy (16).
In this study, patients admitted to the ICU who need EN may be frequently at nutritional risk and PN may provide adequate delivery of nutrition therapy when needed. A higher organ failure (i.e., higher SOFA) and nutrition-related laboratory parameters, such as albumin and triglycerides, on ICU admission may be associated with the need of PN after starting EN therapy. This may be related with a higher occurrence of GI complications, a feature of GI dysfunction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Clinical Research Ethics Committee of Hospital Universitari de Bellvitge (Barcelona, Spain). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because observational nature of the study & Compliance with legal requirements.
JL-D, TG-C, and LS-G conceived, designed, and coordinated the study. TG-C, JL-D, and JM performed the statistical analysis and developed the first draft of the manuscript. All authors collected the data during the study period and participated in the revision, involved in the interpretation of the results, critically revised the manuscript, and approved the final version.
This study was supported and received a grant from the Spanish Society of Nutrition and Metabolism (SENPE).
We thank all the members of the ENPIC Study Group who participated in the meeting held in Madrid on October 26-27, 2017 for their contribution to a consensus in drafting the database used in the present study. We also thank the Spanish Society of Nutrition and Metabolism (SENPE; Sociedad Espanola de Metabolismo y ~ Nutricion) for funding support and CERCA Programme/Generalitat de Catalunya for institutional support. Finally, we would like to thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1250305/full#supplementary-material
ICU, Intensive Care Unit; EN, Enteral Nutrition; PN, Parenteral Nutrition; GI, Gastrointestinal; APACHE, Acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; mNUTRIC, modified Nutrition Risk in the Critically II; RRT, Renal Replacement Therapy.
1. Elhassan, AO, Tran, LB, Clarke, RC, Singh, S, and Kaye, AD. Total parenteral and enteral nutrition in the ICU: evolving concepts. Anesthesiol Clin. (2017) 35:181–90. doi: 10.1016/j.anclin.2017.01.004
2. Lambell, KJ, Tatucu-Babet, OA, Chapple, LA, Gantner, D, and Ridley, EJ. Nutrition therapy in critical illness: a review of the literature for clinicians. Crit Care. (2020) 24:35. doi: 10.1186/s13054-020-2739-4
3. Lew, CCH, Yandell, R, Fraser, RJL, Chua, AP, Chong, MFF, and Miller, M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review. JPEN J Parenter Enteral Nutr. (2017) 41:744–58. doi: 10.1177/0148607115625638
4. Hill, A, Elke, G, and Weimann, A. Nutrition in the intensive care unit-a narrative review. Nutrients. (2021) 13:2851. doi: 10.3390/nu13082851
5. Patkova, A, Joskova, V, Havel, E, Kovarik, M, Kucharova, M, Zadak, Z, et al. Energy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: a systematic review. Adv Nutr. (2017) 8:624–34. doi: 10.3945/an.117.015172
6. Langin, D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C R Biol. (2006) 329:598–607. doi: 10.1016/j.crvi.2005.10.008
7. de Lorenzo, G, and Mateos, A. Seventh Jesús Culebras lecture. Systemic inflammatory response and multi organic dysfunction/failure following aggression: metabolic implications. Nutr Hosp. (2017) 34:244–50. doi: 10.20960/nh.1001
8. Preiser, JC, Ichai, C, Orban, JC, and Groeneveld, AB. Metabolic response to the stress of critical illness. Br J Anaesth. (2014) 113:945–54. doi: 10.1093/bja/aeu187
9. Muscaritoli, M, Lucia, S, and Molfino, A. Sarcopenia in critically ill patients: the new pandemia. Minerva Anestesiol. (2013) 79:771–7.
10. Compher, C, Bingham, AL, McCall, M, Patel, J, Rice, TW, Braunschweig, C, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. (2022) 46:12–41. doi: 10.1002/jpen.2267
11. Singer, P, Blaser, AR, Berger, MM, Alhazzani, W, Calder, PC, Casaer, MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38:48–79. doi: 10.1016/j.clnu.2018.08.037
12. Dhaliwal, R, Cahill, N, Lemieux, M, and Heyland, DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract. (2014) 29:29–43. doi: 10.1177/0884533613510948
13. Herrero Meseguer, JI, Lopez-Delgado, JC, and Martínez García, MP. Recommendations for specialized nutritional-metabolic management of the critical patient: indications, timing and access routes. Metabolism and nutrition working Group of the Spanish Society of intensive and critical care medicine and coronary units (SEMICYUC). Med Intensiva. (2020) 44:33–8. doi: 10.1016/j.medine.2019.12.009
14. Griffiths, RD, and Bongers, T. Nutrition support for patients in the intensive care unit. Postgrad Med J. (2005) 81:629–36. doi: 10.1136/pgmj.2005.033399
15. Serviá Goixart, L, López Delgado, JC, and Grau Carmona, T. Evaluation of the degree of adherence to the nutritional recommendations of the critical care patient. Nutr Hosp. (2019) 36:510–6. doi: 10.20960/nh.02323
16. Matejovic, M, Huet, O, Dams, K, Elke, G, Vaquerizo Alonso, C, Csomos, A, et al. Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit Care. (2022) 26:143. doi: 10.1186/s13054-022-03997-z
17. Heyland, DK, Dhaliwal, R, Wang, M, and Day, AG. The prevalence of iatrogenic underfeeding in the nutritionally 'at-risk' critically ill patient: results of an international, multicenter, prospective study. Clin Nutr. (2015) 34:659–66. doi: 10.1016/j.clnu.2014.07.008
18. Ridley, EJ, Peake, SL, Jarvis, M, Deane, AM, Lange, K, Davies, AR, et al. Nutrition therapy in Australia and New Zealand intensive care units: an international comparison study. JPEN J Parenter Enteral Nutr. (2018) 42:1349–57. doi: 10.1002/jpen.1163
19. Huang, J, Yang, L, Zhuang, Y, Qi, H, Chen, X, and Lv, K. Current status and influencing factors of barriers to enteral feeding of critically ill patients: a multicenter study. J Clin Nurs. (2019) 28:677–85. doi: 10.1111/jocn.14667
20. Cahill, NE, Murch, L, Cook, D, and Heyland, DK. Canadian critical care trials group. Barriers to feeding critically ill patients: a multicenter survey of critical care nurses. J Crit Care. (2012) 27:727–34. doi: 10.1016/j.jcrc.2012.07.006
21. Kim, H, Stotts, NA, Froelicher, ES, Engler, MM, and Porter, C. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care. (2012) 27:702–13. doi: 10.1016/j.jcrc.2012.07.019
22. Kozeniecki, M, McAndrew, N, and Patel, JJ. Process-related barriers to optimizing enteral nutrition in a tertiary medical intensive care unit. Nutr Clin Pract. (2016) 31:80–5. doi: 10.1177/0884533615611845
23. Servia-Goixart, L, Lopez-Delgado, JC, Grau-Carmona, T, Trujillano-Cabello, J, Bordeje-Laguna, ML, Mor-Marco, E, et al. Evaluation of nutritional practices in the critical care patient (the ENPIC study): does nutrition really affect ICU mortality? Clin Nutr ESPEN. (2022) 47:325–32. doi: 10.1016/j.clnesp.2021.11.018
24. Bielawska, B, and Allard, JP. Parenteral nutrition and intestinal failure. Nutrients. (2017) 9:466. doi: 10.3390/nu9050466
25. Stojek, M, and Jasiński, T. Gastroparesis in the intensive care unit. Anaesthesiol Intensive Ther. (2021) 53:450–5. doi: 10.5114/ait.2021.110959
26. Heidegger, CP, Berger, MM, Graf, S, Zingg, W, Darmon, P, Costanza, MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. (2013) 381:385–93. doi: 10.1016/S0140-6736(12)61351-8
27. Asrani, VM, Brown, A, Huang, W, Bissett, I, and Windsor, JA. Gastrointestinal dysfunction in critical illness: a review of scoring tools. J Parenter Enter Nutr. (2020) 44:182–96. doi: 10.1002/jpen.1679
28. Reintam, A, Parm, P, Kitus, R, Starkopf, J, and Kern, H. Gastrointestinal failure score in critically ill patients: a prospective observational study. Crit Care. (2008) 12:R90. doi: 10.1186/cc6958
29. Reintam Blaser, A, Padar, M, Mändul, M, Elke, G, Engel, C, Fischer, K, et al. Development of the gastrointestinal dysfunction score (GIDS) for critically ill patients - a prospective multicenter observational study (iSOFA study). Clin Nutr. (2021) 40:4932–40. doi: 10.1016/j.clnu.2021.07.015
30. Hansen, SEJ, Madsen, CM, Varbo, A, and Nordestgaard, BG. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem. (2019) 65:321–32. doi: 10.1373/clinchem.2018.294926
31. Golucci, APBS, Marson, FAL, Ribeiro, AF, and Nogueira, RJN. Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition. (2018) 55-56:7–14. doi: 10.1016/j.nut.2018.04.007
32. Ballmer, PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. (2001) 20:271–3. doi: 10.1054/clnu.2001.0439
33. Soeters, PB, Wolfe, RR, and Shenkin, A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43:181–93. doi: 10.1002/jpen.1451
34. Evans, DC, Corkins, MR, Malone, A, Miller, S, Mogensen, KM, Guenter, P, et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. (2021) 36:22–8. doi: 10.1002/ncp.10588
35. Koekkoek, KW, and van Zanten, AR. Nutrition in the critically ill patient. Curr Opin Anaesthesiol. (2017) 30:178–85. doi: 10.1097/ACO.0000000000000441
36. Webster, NR, and Galley, HF. Nutrition in the critically ill patient. J R Coll Surg Edinb. (2000) 45:373–9.
37. Lewis, SR, Schofield-Robinson, OJ, and Alderson Smith, AF. Enteral versus parenteral nutrition and enteral versus a combination of enteral and parenteral nutrition for adults in the intensive care unit. Cochrane Database System Rev. (2018) 6:CD012276. doi: 10.1002/14651858.CD012276.pub2
38. Weimann, A, and Singer, P. Avoiding underfeeding in severely ill patients. Lancet. (2013) 381:1811–2. doi: 10.1016/S0140-6736(13)61112-5
39. Vincent, JL, and Preiser, JC. When should we add parenteral to enteral nutrition? Lancet. (2013) 381:354–5. doi: 10.1016/S0140-6736(12)61893-5
40. Reignier, J, Boisramé-Helms, J, Brisard, L, Lascarrou, JB, Ait Hssain, A, Anguel, N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. (2018) 391:133–43. doi: 10.1016/S0140-6736(17)32146-3
Keywords: nutrition therapy, intensive care unit, enteral nutrition, parenteral nutrition, gastrointestinal dysfunction
Citation: Lopez-Delgado JC, Servia-Goixart L, Grau-Carmona T, Bordeje-Laguna L, Portugal-Rodriguez E, Lorencio-Cardenas C, Vera-Artazcoz P, Macaya-Redin L, Martinez-Carmona JF, Marin Corral J, Flordelís-Lasierra JL, Seron-Arbeloa C, Alcazar-Espin MdlN, Navas-Moya E, Aldunate-Calvo S, Nieto Martino B and Martinez de Lagran I (2023) Factors associated with the need of parenteral nutrition in critically ill patients after the initiation of enteral nutrition therapy. Front. Nutr. 10:1250305. doi: 10.3389/fnut.2023.1250305
Received: 29 June 2023; Accepted: 10 August 2023;
Published: 24 August 2023.
Edited by:
Mary S. McCarthy, Madigan Army Medical Center, United StatesReviewed by:
Miguel Angel Cuevas Budhart, Mexican Social Security Institute (IMSS), MexicoCopyright © 2023 Lopez-Delgado, Servia-Goixart, Grau-Carmona, Bordeje-Laguna, Portugal-Rodriguez, Lorencio-Cardenas, Vera-Artazcoz, Macaya-Redin, Martinez-Carmona, Marin Corral, Flordelís-Lasierra, Seron-Arbeloa, Alcazar-Espin, Navas-Moya, Aldunate-Calvo, Nieto Martino and Martinez de Lagran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Carlos Lopez-Delgado, anVhbmNhcmxvc2xvcGV6ZGVAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.