- Department of Nutrition and Dietetics, School of Health Science and Education, Harokopio University, Athens, Greece
Introduction: Phase angle (PA) is derived from bioelectrical impedance analysis (BIA). It reflects cell membrane function and decreases in disease. It is affected by inflammation, oxidative stress, and diet. Platelet-activating factor (PAF) is a potent inflammatory lipid mediator. Its levels, along with the activity of its metabolic enzymes, including CDP-choline:1-alkyl-2-acetyl-sn-glycerol-cholinephosphotransferase, acetyl-CoA:lyso-PAF-acetyltransferases, and PAF-AH/Lp-PLA2 are also related to dietary factors, such as the dietary antioxidant capacity (DAC). The aim of the study was to estimate whether the PAF metabolic circuit and related dietary factors are associated with PA in healthy volunteers.
Methods: In healthy subjects, PAF, its metabolic enzyme activity, and erythrocyte fatty acids were measured, while desaturases were estimated. Food-frequency questionnaires and recalls were used, and food groups, macronutrient intake, MedDietScore, and DAC were assessed. Lifestyle and biochemical variables were collected. DXA and BIA measurements were performed.
Results: Lp-PLA2 activity was positively associated with PA (rho = 0.651, p < 0.001, total population; rho = 0.780, p < 0.001, women), while PAF levels were negatively associated with PA only in men (partial rho = −0.627, p = 0.012) and inversely related to DAC. Estimated desaturase 6 was inversely associated with PA (rho = −0.404, p = 0.01, total sample). Moreover, the DAC correlated positively with PA (rho = 0.513, p = 0.03, women). All correlations were adjusted for age, body mass index, and sex (if applicable).
Conclusion: PA is associated with PAF levels and Lp-PLA2 activity in a gender-dependent fashion, indicating the involvement of PAF in cell membrane impairment. The relationship of PA with DAC suggests a protective effect of antioxidants on cellular health, considering that antioxidants may inhibit PAF generation.
Introduction
Bioelectrical impedance analysis (BIA) is a simple technique to assess body composition (1). Phase angle (PA) is calculated from the reactance (Xc) and resistance (R) ratios (BIA raw parameters) (1). Although its biological meaning in health and disease is not completely understood, PA is considered a measure of cell membrane function (1, 2). High values suggest intact or healthy cell membranes, while low values suggest cell death and impaired cell integrity/permeability and are documented in various diseases, such as malnutrition and cancer (1). Although cell membrane function and integrity can be altered by fatty acids (3), choline-based phospholipids (4, 5), and other dietary constituents, such as polyphenols (6) and antioxidants (7), scarce data exist on the relationship of the above constituents with PA. In addition, PA has been negatively related to inflammatory indices (such as C-reactive protein), cytokines (such as interleukin-6) (1, 8), and markers of oxidative stress (9, 10), and it has been positively related to the Mediterranean diet (11), to a pattern rich in animal proteins and potatoes in cancer patients (12), as well as to erythrocyte n-3 fatty acids (13, 14).
Platelet-activating factor (PAF) is an ether-linked glyceryl-phospholipid containing choline with an acetyl group in the sn-2 position that is responsible for its characteristic biological action. PAF was initially implicated in immediate hypersensitivity-type allergic reactions and platelet activation, while now it is considered the most potent inflammatory lipid mediator orchestrating inflammation, thrombosis, and disease pathophysiology (15–20). PAF is produced by various cells, among which endothelial cells, platelets, macrophages, monocytes, neutrophils, and mast cells are unique tissue immune cells that can be activated by numerous triggers. PAF also functions as an immunoregulatory mediator (21, 22) by modulating T- and B-cell activation and proliferation (23–25). It acts by binding to its specific receptor, namely PAFR, expressed by many cells and tissues (20), including mast cells, granulocytes, B-lymphocytes, dendritic cells, and macrophages (20, 21). The binding of PAF to its receptor stimulates complex cell signaling through the activation of G-proteins (19). Moreover, components of the bacterial wall, such as lipoteichoic acid and lipopolysaccharides, bind to PAFR, while the PAF-PAFR complex activates toll-like receptor 4 (TLR4), all supporting the idea that PAFR acts as an alternative system for innate immunity (26, 27). PAF is biosynthesized by the de novo and the remodeling pathway (20), key enzymes of which are CDP-choline: 1-alkyl-2-acetyl-sn-glycerol cholinephosphotransferase (PAF-CPT) and acetyl-CoA: lyso-PAF acetyltransferases (Lyso-PAF-AT), respectively (20). The remodeling pathway starts with the action of cytoplasmic phospholipase A2 (cPLA2) on the existing membrane ether-linked choline-containing phospholipids, resulting in the formation of lyso-PAF and the release of the fatty acids in the sn-2 glyceryl backbone position. The catabolism of PAF is mainly attributed to PAF acetylhydrolases (PAF-AH), which hydrolyze PAF, yielding lyso-PAF (20). The circulating isoform of PAF-AH is also known as lipoprotein-associated phospholipase A2 (Lp-PLA2) due to its binding to lipoproteins (28).
Studies with model membranes support the idea that ether phospholipid analogs, including PAF, can be inserted into membranes, but they may also perturb and disorder membrane bilayers at high concentrations (5). In addition, 1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphocholine, a synthetic anti-tumor ether analog of PAF, has been reported to affect the properties of lipid rafts (29). PAF is related to membrane integrity (and possibly PA) either directly as a lipophilic membrane lipid constituent (5, 30, 31) or indirectly by modifying the antioxidant/inflammatory cellular milieu or the produced fatty acids (as a result of its metabolism) (20). For example, damage to cellular membranes results in the generation of PAF and PAF agonists, all binding to PAFR (32). In addition, PAFR stimulation induces the production of microvesicle particles (MVP), which are small membrane-bound particles, by activating the translocation of acid sphingomyelinase (aSMase) to the plasma membrane. PAFR stimulation activates the translocation of aSMase to the plasma membrane. This results in the hydrolysis of sphingomyelin (SM) and elevated ceramide production, both altering membrane integrity and fluidity (33–35).
Moreover, PAF and its metabolic enzymes are related to oxidative stress, inflammation, and dietary factors, all of which may affect PA (8–11, 13, 14). More specifically, oxidative stress activates Lyso-PAF-AT, leading to increased PAF levels (36). PAF can also be formed non-enzymatically during the oxidative modification of LDL, which reduces the activity of Lp-PLA2 (37). Additionally, PAF has been implicated in the production of reactive oxygen species (ROS) in neutrophils, eosinophils, endothelial cells, and monocytes (38–41). Recent data suggest that a healthy diet rich in antioxidants is associated with improved levels of PAF and Lp-PLA2 (42, 43), modulating PAF's pro-inflammatory actions and its metabolism (42). According to a systematic review of our group, several components of the Mediterranean diet, such as cereals, legumes, plant foods, fish, and wine, may modulate PAF actions and/or the activity of its enzymes in humans (44). PAF and its metabolic enzymes have also been associated with erythrocyte fatty acids (45).
To the best of our knowledge, there is no data connecting PAF levels or its metabolism with PA, while an association of platelet count with PA has been reported in patients with dengue fever (46). It can be hypothesized that PAF levels may affect cellular health and thus PA. Given the prognostic role of PA in diseases (47–51) where PAF is implicated (16, 52–54), the identification of potential modulators of PA is crucial. Thus, the aim of the present work was to study the associations of PA with PAF, its metabolic enzymes, and related dietary parameters in healthy participants.
Materials and methods
Subjects
The present study is a sub-analysis of a previous study investigating PAF and its enzymes in 106 participants (55). In the present analysis, all subjects from the above population with available data on BIA were considered (n = 39, 19 women). All participants gave written consent to participate. The protocol was approved by the Bioethics Committee of the university. The study was in accordance with the Declaration of Helsinki (1989) of the World Medical Association, as revised in 2013.
Anthropometry, body composition, and PA
Weight, height, waist, and hip circumferences were measured as previously described (55). Body composition was assessed by DXA (Lunar Corporation, Brussels, Belgium) following the manufacturer's instructions. For each subject, the standard body composition analysis was performed (software version 4.6), and a region of interest (ROI) was manually defined as a quadrilateral box around the L1–L4 area, as previously described (55).
Whole-body BIA was performed with BODYSTAT (BODYSTAT, 1500) at a frequency of 50 kHz. After an overnight fast, the volunteers lied down on a bed with their arms and legs extended apart. The skin of hands and feet was disinfected with alcohol (70%) using disposable tissue paper. The electrodes were placed on the right hand (between the knuckles of the wrist) and on the right foot (on the ankle between the knuckles of the ankle). The subjects were at least 9–12 h without food (since they were in a fasted state to facilitate biochemical measurements) and 3 h without water. Regarding the menstrual cycle, women were neither in the middle of menses nor ±3 days, so as to avoid edemas.
The minimal distance between the electrodes was 5 cm. A single measurement was taken. PA was calculated from the Xc and R ratios as follows:
Dietary assessment and lifestyle variables assessment
Two non-consecutive multiple-pass 24-h recalls were collected and assessed with the Nutritionist Pro™ software (Axxya Systems, Stafford, TX) expanded with local foods (56). Moreover, a semi-quantitative food frequency questionnaire (FFQ) was used (42) and Mediterranean diet adherence was assessed with the MedDietScore (57). The estimation of dietary antioxidant capacity (DAC) was based on previously published databases for raw Italian foods (58, 59), in which three different antioxidant assays were considered: the total radical-trapping antioxidant parameters (TRAP) (60), the ferric-reducing antioxidant power (FRAP) (61), and the Trolox-equivalent antioxidant capacity (TEAC) (62). More particularly, in the present study, 14 fruits, 17 vegetables, five types of legumes, six beverages, 17 composite foods, chocolate, honey, marmalade, nuts, and four cereal-based products were assigned respective values for FRAP, TRAP, and TEAC according to published values (58, 59). Greek tables for the composition of Greek foods were used to analyze composite foods (56). It is noted that the estimation of DAC with a similar methodology has been previously reported in the ATTICA study (63). Smoking and physical activity status were assessed as previously described (55).
Basic biochemical measurements
Basic biochemical measurements (serum glucose, triacylglycerols, total cholesterol, and HDL cholesterol) were performed as described elsewhere (55).
Determination of PAF levels in blood
PAF was isolated from blood, purified, and determined as previously reported (64). Whole blood with ethanol was centrifuged at 500 × g for 20 min, and the resulting supernatant (containing free PAF) and pellet (containing bound PAF) were extracted according to the Bligh–Dyer method. Subsequently, purification was followed by chromatography, and PAF levels were determined by a biological assay (64). Total PAF represents the sum of free PAF and bound PAF.
Determination of lyso-PAF-AT, PAF-CPT, and PAF-AH activities in leukocyte homogenates and lp-PLA2 in serum
Leukocyte homogenate isolation is described elsewhere (18). For the determination of Lyso-PAF-AT activity, leukocyte homogenates were incubated with lyso-PAF and acetyl-CoA (18). For the determination of PAF-CPT activity, CDP-choline and 1-O-hexadecyl-2-acetyl-sn-glycerol were used (18). After stopping the reactions, the produced PAF was measured (64). Enzymatic activity was expressed as a specific activity in pmol/min/mg protein.
PAF-AH and Lp-PLA2 activities in leukocyte homogenates and serum, respectively, were determined by the trichloroacetic acid precipitation method using [3H] PAF as a substrate (18). The enzyme activity was expressed as nmol of PAF degraded per minute per milligram of leukocyte homogenate protein or per milliliter of serum. All assays were performed in duplicate.
Erythrocyte fatty acid determination and estimation of desaturase indices
The erythrocyte fatty acid profile was determined by gas chromatography (Agilent HP-6890, Avondale, PA, USA) with a flame ionization detector as described elsewhere (45). Peak identification was accomplished by means of a standard mixture of 37 FAME (Sigma L9405, St Louis, MO, USA). Of the 46 fatty acids identified, 34 that comprised >0.01% of total fatty acids were considered (45).
Desaturases were indirectly estimated by fatty acid product/precursor ratios. Desaturase 5 (D5) was calculated as the ratio 20:4n6/20:3n6, D6 as the ratio 18:3n6/18:2n6, desaturase 9 (D9) C16 as the ratio 16:1n−7/16:0, and desaturase 9 (D9) C18 as the ratio 18:1n−9/18:0 (45).
Statistical analysis
Normality was tested with the Kolmogorov–Smirnoff criterion. Normally distributed continuous variables are presented as mean ± standard deviation, while skewed variables are presented as medians and 25th−75th quartiles. Categorical variables are presented as relative frequencies (%). A t-test or Mann–Whitney test was applied for comparisons of parametric/log-transformed or non-parametric variables, respectively. A chi-square test was used to compare categorical variables between groups. Pearson partial correlation coefficients (after ranking variables) were evaluated to test correlations after adjustments for age, body mass index (BMI), and sex (if applicable). Given the strong differentiation between men and women in PAF metabolism (45) and PA (65), sex-specific analysis was also performed.
All reported p-values were two-sided (significance level 5%). The SPSS v22 software was used for statistical analysis (IBM Corp., Released 2013, Armonk, NY).
Results
Basic characteristics of participants
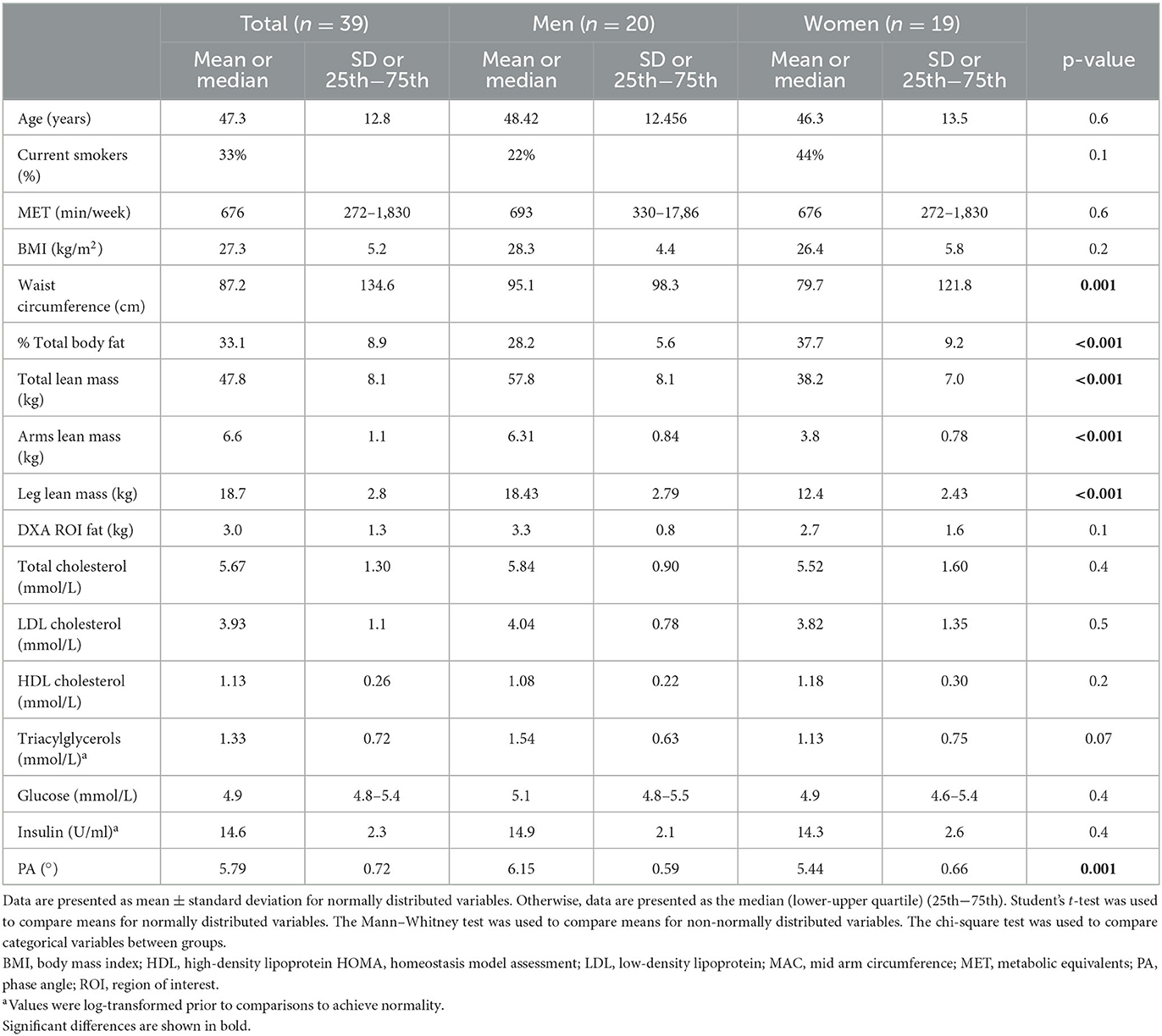
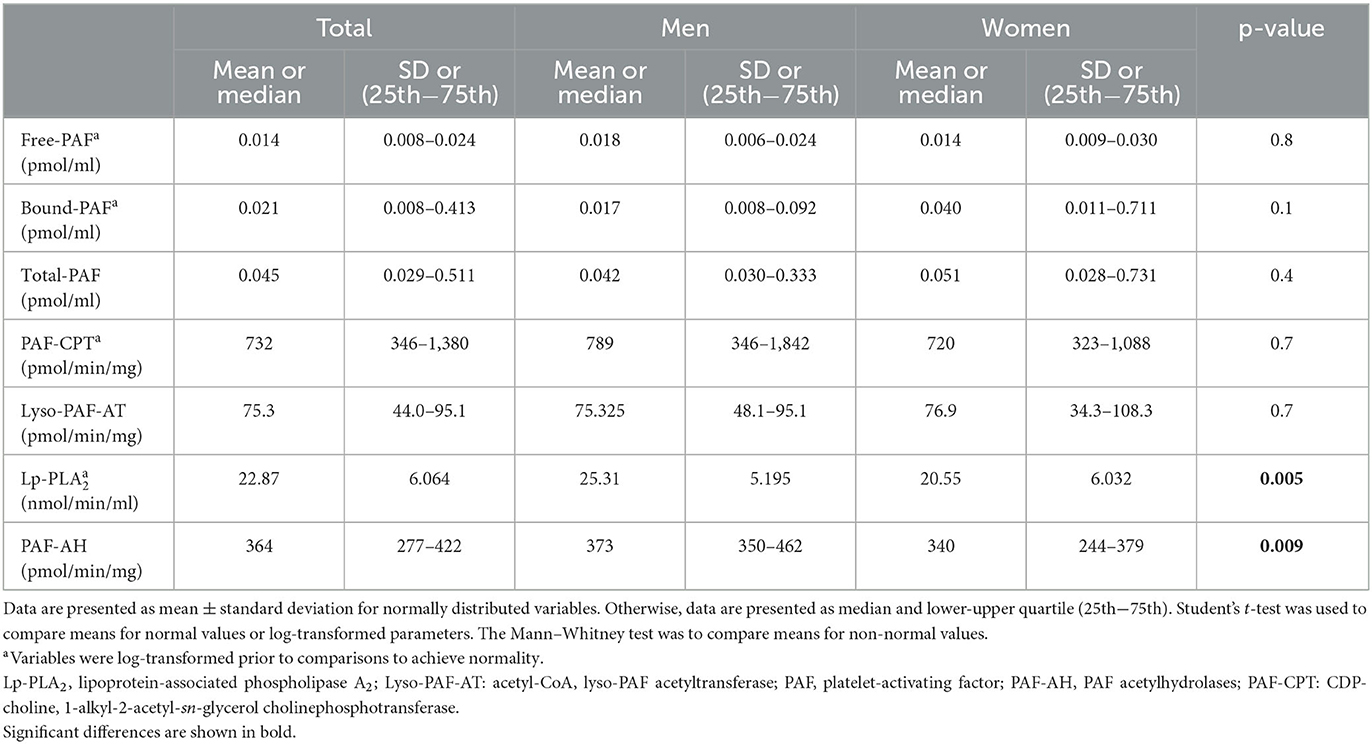
The basic clinical, anthropometric, and biochemical characteristics of the volunteers are shown in Table 1. Men had a higher waist circumference and a higher lean mass compared to women. Women had higher total body fat (%) and leg fat, as well as lower R and PA compared to men. The levels of PAF and the specific activities of its metabolic enzymes are shown in Table 2. In this group, PAF levels and its biosynthetic enzymes did not differ between sexes. The specific activities of both catabolic enzymes, namely, Lp-PLA2 and leukocyte PAF-AH, were higher in men.
Dietary intake of participants
The dietary intake of the participants is shown in Supplementary Table 1. The median energy intake was 2, 216 kcal. The median fat, protein, and carbohydrate intake was 39, 12.7, and 33.7%, respectively. The adherence to the Mediterranean diet assessed with the MedDietScore was 33.6 ± 5.9, and the estimation of DAC was 20.8 ± 6.5 mmol/day for FRAP, 8.0 ± 2.7 mmol/day for TRAP, and 7.9 ± 2.5 mmol/day for TEAC (means ± SD). No sex differences were documented. It is noted that PAF levels were inversely related to DAC, as shown in Supplementary Table 2.
Erythrocyte fatty acid composition
The erythrocyte fatty acid composition is presented in Supplementary Table 3. The most abundant fatty acids were SFA and polyunsaturated fatty acids (PUFA), followed by MUFA with a median content of 36.2, 34.2, and 18.5%, respectively. The mean n−6 and n−3 content of erythrocytes was 26.4 and 8.0%, respectively. Mean D5 was 8.8, median D6 was 0.000, and median D9 was 0.014. Women had higher estimated activities in D5 and D9.
Relationship of PA with PAF and activity of PAF metabolic enzymes
PAF levels were negatively correlated with PA in men (rho = −0.627, p = 0.01), while the activity of its catabolic enzyme Lp-PLA2 was positively correlated with PA in the total sample (rho = 0.651, p < 0.001) and women (rho = 0.780, p < 0.001). All the associations were adjusted for age, sex, and BMI. It is noted that the relationship between Lp-PLA2 and PA remained significant after further adjustment for LDL-cholesterol, which is the main carrier of LpPLA2 (rho = 0.450, p = 0.07 in the total sample, and rho = 0.613, p = 0.009 in women). In alternative models adjusted for ROI fat instead of BMI, the observed relationships were similar.
Relationship of PA to anthropometric characteristics, erythrocyte fatty acid composition, and dietary intake
As far as anthropometric characteristics are concerned, PA was inversely correlated with fat indices in age/BMI/sex adjusted analysis for the total population and in particular with hip circumference (rho = −0.405, p = 0.01), %body fat (rho = −0.361, p = 0.03), central fat as expressed with a ROI area (rho = −0.463, p = 0.004), legs fat (rho = −0.382, p = 0.02), and arms fat (rho = −0.358, p = 0.03) (total sample).
As far as erythrocyte fatty acid content is concerned, PA was negatively associated with DHA (rho = −0.510, p = 0.03), total n-3 (rho = −0.488, p = 0.04), and the omega-3 index (rho = −0.509, p = 0.03) in men after adjustment for age and BMI. Moreover, D6 was negatively associated with PA in the total sample (rho = −0.404, p = 0.01) and both sexes (rho = −0.651, p = 0.005 in men and rho = −0.413, p = 0.09 in women).
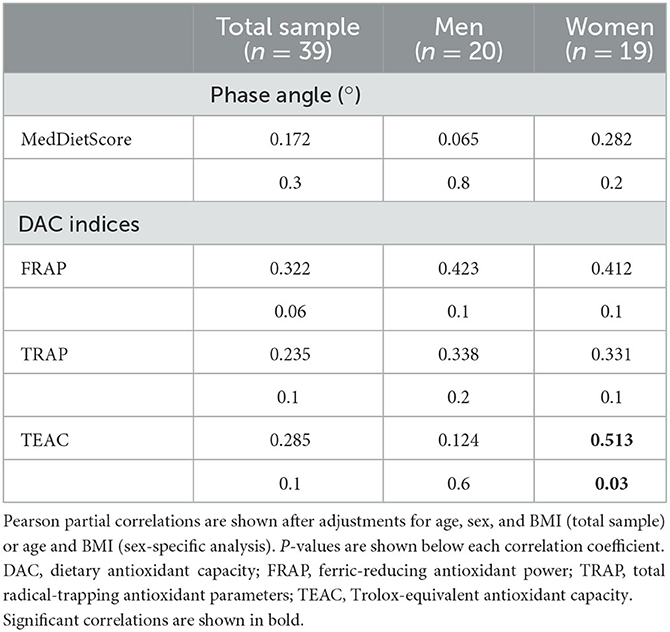
Energy intake was positively correlated with PA (rho = 0.410, p = 0.02), and negatively associated with % PUFA intake (rho = −0.412, p = 0.01) after adjustment for BMI, age, and sex, while positive associations were documented for the DAC in women (Table 3). The MedDietScore was not associated with PA.
Discussion
The present study documented first-time associations of the PA with PAF and its metabolic enzymes, erythrocyte fatty acid content, and DAC in healthy participants. More particularly, a gender-dependent nature was observed since PAF levels were negatively associated with PA in men, while its catabolic enzyme, Lp-PLA2, was positively associated with PA, especially in women. Moreover, the DAC based on FRAP had a borderline association with PA values in the whole population (p = 0.06), while the DAC based on TEAC showed a significant relationship with PA in women, suggesting a role of exogenous antioxidants in cellular health. Bound PAF levels, reflecting PAF that is strongly bound to cellular structures, were inversely related to FRAP, TRAP, and TEAC in the whole population.
In the present study, PA was 6.15° for men and 5.44° for women, which is lower than values recently suggested for healthy subjects (7.3° for men and 6.4° for women aged 18–48 years) (65). The PA was higher in men and was negatively associated with adiposity measures of total and localized fat, as previously reported (1, 65).
Several inflammatory markers, such as C-reactive protein, interleukin-6, and tumor necrosis factor-a (TNF-a), have been negatively associated with PA (8, 9, 66). In the present study, PAF, a potent lipid inflammatory mediator, was negatively associated with PA in men. It has been reported that pro-oxidative environmental stressors resulting in cell membrane damage lead to the production of PAF and PAF agonists (32). It should be noted that during the biosynthesis of PAF through the remodeling pathway, arachidonic acid is released and converted into a variety of eicosanoid mediators. The generation of inflammatory mediators, including PAF, metalloproteinases, etc., may further disrupt membrane integrity (67) and lead to lower PA values. PAF may also be related to membrane integrity either directly (30, 31) as one of the ether lipids that participates in membrane structure (68) or indirectly by modifying the antioxidant/inflammatory cellular milieu and/or the availability of fatty acids (20). Specifically, PAF and PAF agonists modulate the redox status by the production of ROS (38–41) and trigger the excretion of other pro-inflammatory mediators (69). PAF also mediates NLRP3-NEK7 inflammasome induction (70), which in turn results in cell death (71). Finally, PAFR stimulation activates the translocation of acid sphingomyelinase (aSMase) to the plasma membrane, resulting in alterations in membrane integrity and fluidity (33–35). In the same context, Lp-PLA2 was positively associated with PA in the whole population and in women. Lp-PLA2 hydrolyzes oxidatively modified phospholipids and other pro-inflammatory molecules, such as PAF (20), and it is inactivated upon LDL oxidation (37). Thus, a potentially oxidative environment, which relates to low PA, could be combined with low Lp-PLA2 activity. The sex-dependent nature of the observed associations of PAF metabolism with PA may be partially attributed to the already reported different PAF metabolic patterns in both sexes (72), while the role of fat distribution and related dietary habits cannot be excluded (73). It is also noted that in a pathological context, such as cancer, PA and Lp-PLA2 are differentially affected: PA is reduced in cases of high inflammatory burden, such as cancer and sarcopenia (49, 74), while Lp-PLA2 is increased (75). In addition, in a recent meta-analysis, the values of PA were associated with cardiovascular diseases (76), and Lp-PLA2 has been proposed as a marker (77). This observation is in line with the negative association between PA and Lp-PLA2 in the present study.
The data regarding the relationship of PA with fatty acids are limited, and there is no study investigating the relationship between desaturases and PA. N-3 PUFA has been positively associated with PA in patients (13, 14) and increases membrane fluidity (78). However, EPA supplements did not change PA in cancer patients (79). In our study, n-3 and the omega-3 index were negatively associated with PA, but when smoking status (rho = −0.501, p = 0.057 and rho = −0.476, p = 0.07, respectively) or circulating thiobarbituric acid reactive substances (TBARS) were considered (rho = −0.338, p = 0.2 and rho = −0.342, p = 0.1, respectively), the correlations were no longer significant, suggesting an effect of oxidative stress. It is possible that phospholipids with polyunsaturated fatty acyl residues, such as n−3, may be more easily oxidized (80). Such oxidatively truncated phospholipids can serve as mediators of TNF-a-induced cell death (80) and may damage mitochondrial integrity (81). Thus, in cases of high oxidative stress, a high erythrocyte PUFA content may disrupt cell integrity (81). The association of PA with estimated desaturase activity has not been previously assessed. In our study, D6 was negatively associated with PA, which is in line with the role of D6 in cardiometabolic diseases (82, 83). The recently reported positive associations of PAF biosynthetic enzymes with D6 may also partially explain the observed finding (45).
The relationship of diet with PA has not been thoroughly investigated. Pilot results have shown a positive association of PA with Mediterranean diet adherence (11). In the present study, macronutrient intake was not related to PA similarly to others (84), while only the DAC was positively associated with PA, suggesting a protective role of antioxidants in cell integrity. Indeed, PA has been negatively related to inflammatory and oxidative stress markers in health and disease (8, 9). Moreover, a significant positive association between PA and plasma antioxidant capacity has been reported (9), which corroborates our findings.
Limitations of our study include its cross-sectional nature, which cannot prove causality. Pitfalls in dietary assessment may exist since subjects may not have appropriately estimated their intake. For this reason, multiple dietary recalls were used, along with the FFQ and erythrocyte PUFA. As far as the DAC is concerned, published values on raw Italian foods were used (58, 59), which may differ from the actual values of Greek foods or the in vivo activity of cooked foods. It is also noted that PAF has a short half-life, and its fluctuations at the time of measurement may not reveal fully its biological correlates. In the present study, its metabolic enzymes were also measured to serve as an “index” of the circulating PAF trend. Finally, a relatively small number of apparently healthy volunteers had available data on BIA and was included, since the present study was a sub-analysis of a previous study investigating PAF and its enzymes in 106 participants (55). Indeed, in a power analysis using the G-Power software (version 3.1.9.7, Kiel University), the achieved power was high for significant associations with relatively high correlation coefficients (rho = 0.4–0.6) (>90%) (such as the association of PA with DAC in woman), but low for non-significant associations with lower correlation coefficients (rho = 0.2) (~40%) (such as the association of PA with DAC in the total sample), meaning that the present study may be underpowered for several associations. The healthy status of the volunteers also implies that the observed relationships may be different in pathological conditions.
Conclusion
PA is inversely associated with PAF levels and positively associated with Lp-PLA2 activity in a gender-dependent manner, indicating the involvement of PAF in the impairment of cell membrane. Moreover, PA was positively associated with high dietary antioxidant intake in apparently healthy participants. The associations of PAF and diet with PA can be explained by the fact that PAF may impair cell membranes, while antioxidants inhibit PAF generation and have a beneficial effect on cell integrity. The fact that those associations were observed in the context of an apparently healthy population emphasized the role of PA as a novel, sensitive, non-invasive, and easily determined index of PAF-mediated subclinical inflammation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation upon request.
Ethics statement
The studies involving humans were approved by Bioethics Committee of Harokopio University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PD and SA: conceptualization. PD: data curation, formal analysis, and roles/writing—original draft. SA: funding acquisition, resources, and supervision. PD, EF, and TN: investigation. EF, TN, and SA: methodology and writing—review and editing. PD, EF, TN, and SA: project administration. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Dr. M. Xanthopoulou and Mrs. S. Letsiou for their assistance in blood handling, Mrs. O. Kounari for her support in data collection, as well as Mrs. M. Christea for her technical support in blood collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1237086/full#supplementary-material
Abbreviations
AA, arachidonic acid; BIA, bioelectrical impedance analysis; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FAME, fatty acids methyl esters; FFQ, food frequency questionnaire; GA, gondoic acid; HOMA, homeostatic model assessment; LA, linoleic acid; Lp-PLA2, lipoprotein-associated phospholipase A2; Lyso-PAF-AT, acetyl-CoA, lyso-PAF acetyltransferase; MET, metabolic equivalents; MUFA, monounsaturated fatty acids; PA, phase angle; PAF, platelet-activating factor; PAF-AH, PAF acetylhydrolases; PAF-CPT, CDP-choline, 1-alkyl-2-acetyl-sn-glycerol cholinephosphotransferase; PUFA, polyunsaturated fatty acids; RBC, red blood cell; SFA, saturated fatty acids; TNF-a, tumor necrosis factor-a.
References
1. Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis – Clinical relevance and applicability of impedance parameters. Clin Nutr. (2012) 31:854–61. doi: 10.1016/j.clnu.2012.05.008
2. Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero J-J, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. (2020) 76:S1–S107. doi: 10.1053/j.ajkd.2020.05.006
3. Demirbolat GM, Coskun GP, Erdogan O, Cevik O. Long chain fatty acids can form aggregates and affect the membrane integrity. Colloids Surf B Biointerf. (2021) 204:111795. doi: 10.1016/j.colsurfb.2021.111795
4. Tayebati S. Phospholipid and lipid derivatives as potential neuroprotective compounds. Molecules. (2018) 23:2257. doi: 10.3390/molecules23092257
5. Xie X-Q, Moring J, Makriyannis A. Differential scanning calorimetry and small angle x-ray diffraction study of the interaction of (R)-PAF, (R)-ET-18-OMe and (R)-Lyso-PAF with model membranes. Life Sci. (1997) 61:909–23. doi: 10.1016/S0024-3205(97)00593-6
6. Zhu H, Chen G, Chen S, Wang R, Chen L, Xue H, et al. Changes in cell membrane properties and phospholipid fatty acids of bacillus subtilis induced by polyphenolic extract of Sanguisorba officinalis L. J Food Sci. (2020) 85:2164–70. doi: 10.1111/1750-3841.15170
7. Alonge S, Melandri M, Leoci R, Lacalandra G, Caira M, Aiudi G. The effect of dietary supplementation of vitamin e, selenium, zinc, folic acid, and N-3 polyunsaturated fatty acids on sperm motility and membrane properties in dogs. Animals. (2019) 9:34. doi: 10.3390/ani9020034
8. Barrea L, Muscogiuri G, Pugliese G, Laudisio D, de Alteriis G, Graziadio C, et al. Phase angle as an easy diagnostic tool of meta-inflammation for the nutritionist. Nutrients. (2021) 13:1446. doi: 10.3390/nu13051446
9. Tomeleri CM, Cavaglieri CR, de Souza MF, Cavalcante EF, Antunes M, Nabbuco HCG, et al. Phase angle is related with inflammatory and oxidative stress biomarkers in older women. Exp Gerontol. (2018) 102:12–8. doi: 10.1016/j.exger.2017.11.019
10. Da Silva BR, Rufato S, Mialich MS, Cruz LP, Gozzo T, Jordão AA. Phase angle is related to oxidative stress and antioxidant biomarkers in breast cancer patients undergoing chemotherapy. PLoS ONE. (2023) 18:e0283235. doi: 10.1371/journal.pone.0283235
11. Barrea L, Muscogiuri G, Macchia P, Di Somma C, Falco A, Savanelli M, et al. Mediterranean diet and phase angle in a sample of adult population: results of a pilot study. Nutrients. (2017) 9:151. doi: 10.3390/nu9020151
12. Detopoulou P, Tsiouda T, Pilikidou M, Palyvou F, Mantzorou M, Perzirkianidou P, et al. Dietary habits are related to phase angle in male patients with non-small-cell lung cancer. Current Oncology. (2022) 29:8074–83. doi: 10.3390/curroncol29110637
13. VanderJagt DJ, Trujillo MR, Bode-Thomas F, Huang Y-S, Chuang L-T, Glew RH. Phase angle correlates with n-3 fatty acids and cholesterol in red cells of Nigerian children with sickle cell disease. Lipids Health Dis. (2003) 2:2. doi: 10.1186/1476-511X-2-2
14. VanderJagt DJ. Phase angle and n-3 polyunsaturated fatty acids in sickle cell disease. Arch Dis Child. (2002) 87:252–4. doi: 10.1136/adc.87.3.252
15. Detopoulou P, Demopoulos CA, Antonopoulou S. Micronutrients, phytochemicals and mediterranean diet: a potential protective role against COVID-19 through modulation of PAF actions and metabolism. Nutrients. (2021) 13:462. doi: 10.3390/nu13020462
16. Iatrou C, Moustakas G, Antonopoulou S, Demopoulos CA, Ziroyiannis P. Platelet-activating factor levels and PAF acetylhydrolase activities in patients with primary glomerulonephritis. Nephron. (1996) 72:611–6. doi: 10.1159/000188948
17. Kelesidis T, Papakonstantinou V, Detopoulou P, Fragopoulou E, Chini M, Lazanas MC, et al. The role of platelet-activating factor in chronic inflammation, immune activation, and comorbidities associated with HIV infection. AIDS Rev. (2015) 17:191–201.
18. Detopoulou P, Nomikos T, Fragopoulou E, Antonopoulou S, Kotroyiannis I, Vassiliadou C, et al. Platelet activating factor (PAF) and activity of its biosynthetic and catabolic enzymes in blood and leukocytes of male patients with newly diagnosed heart failure. Clin Biochem. (2009) 42:44–9. doi: 10.1016/j.clinbiochem.2008.09.113
19. Upton JEM, Grunebaum E, Sussman G, Vadas P. Platelet activating factor (PAF): a mediator of inflammation. BioFactors. (2022) 48:1189–202. doi: 10.1002/biof.1883
20. Lordan R, Tsoupras A, Zabetakis I, Demopoulos CA. Forty years since the structural elucidation of platelet-activating factor (PAF): historical, current, and future research perspectives. Molecules. (2019) 24:4414. doi: 10.3390/molecules24234414
21. Denizot Y, Dupuis F, Praloran V. Effects of platelet-activating factor on human T and B cells — an overview. Res Immunol. (1994) 145:109–16. doi: 10.1016/S0923-2494(94)80021-9
22. Travers JB, Rohan JG, Sahu RP. New insights into the pathologic roles of the platelet-activating factor system. Front Endocrinol. (2021) 12:624132. doi: 10.3389/fendo.2021.624132
23. Vivier E, Deryckx S, Wang J-L, Valentin H, Peronne C, Vries JED, et al. Immunoregulatory functions of paf-acether. VI Inhibition of T cell activation via CD3 and potentiation of T cell activation via CD2. Int Immunol. (1990) 2:545–53. doi: 10.1093/intimm/2.6.545
24. Elazzouzi B, Jurgens P, Benveniste J, Thomas Y. Immunoregulatory functions of paf-acether. IX. modulation of apoptosis in an immature T cell line. Biochem Biophys Res Commun. (1993) 190:320–4. doi: 10.1006/bbrc.1993.1050
25. Leprince C, Vivier E, Treton D, Galanaud P, Benveniste J, Richard Y, et al. Immunoregulatory functions of paf-acether. VI. Dual effect on human B cell proliferation. Lipids. (1991) 26:1204–8. doi: 10.1007/BF02536532
26. Fillon S, Soulis K, Rajasekaran S, Benedict-Hamilton H, Radin JN, Orihuela CJ, et al. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol. (2006) 177:6182–91. doi: 10.4049/jimmunol.177.9.6182
27. Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med. (2002) 8:41–6. doi: 10.1038/nm0102-41
28. Santoso A, Heriansyah T, Rohman MS. Phospholipase A2 is an inflammatory predictor in cardiovascular diseases: is there any spacious room to prove the causation? CCR. (2020) 16:3–10. doi: 10.2174/1573403X15666190531111932
29. Heczková B, Slotte JP. Effect of anti-tumor ether lipids on ordered domains in model membranes. FEBS Lett. (2006) 580:2471–6. doi: 10.1016/j.febslet.2006.03.079
30. Sawyer DB, Andersen OS. Platelet-activating factor is a general membrane perturbant. Biochim Biophys Acta Biomembr. (1989) 987:129–32. doi: 10.1016/0005-2736(89)90464-1
31. Flasiński M, Broniatowski M, Wydro P, Hac-Wydro K, Dynarowicz-Łatka P. Behavior of platelet activating factor in membrane-mimicking environment. Langmuir monolayer study complemented with grazing incidence X-ray diffraction and brewster angle microscopy. J Phys Chem B. (2012) 116:10842–55. doi: 10.1021/jp302907e
32. Travers JB. Platelet-activating factor as an effector for environmental stressors. In:Gomez-Cambronero J, Frohman MA, , editors. Lipid Signaling in Human Diseases. Handbook of Experimental Pharmacology. Cham: Springer International Publishing (2019). p. 185–203.
33. Lang PA, Kempe DS, Tanneur V, Eisele K, Klarl BA, Myssina S, et al. Stimulation of erythrocyte ceramide formation by platelet-activating factor. J Cell Sci. (2005) 118:1233–43. doi: 10.1242/jcs.01730
34. Göggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, et al. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. (2004) 10:155–60. doi: 10.1038/nm977
35. Pollet H, Conrard L, Cloos A-S, Tyteca D. Plasma membrane lipid domains as platforms for vesicle biogenesis and shedding? Biomolecules. (2018) 8:94. doi: 10.3390/biom8030094
36. Balestrieri ML, Castaldo D, Balestrieri C, Quagliuolo L, Giovane A, Servillo L. Modulation by flavonoids of PAF and related phospholipids in endothelial cells during oxidative stress. J Lipid Res. (2003) 44:380–7. doi: 10.1194/jlr.M200292-JLR200
37. Liapikos TA, Antonopoulou S, Karabina S-AP, Tsoukatos DC, Demopoulos CA, Tselepis AD. Platelet-activating factor formation during oxidative modification of low-density lipoprotein when PAF-acetylhydrolase has been inactivated. Biochim Biophys Acta Lipids Lipid Metab. (1994) 1212:353–60. doi: 10.1016/0005-2760(94)90210-0
38. Niwa Y, Ozaki Y, Kanoh T, Akamatsu H, Kurisaka M. Role of Cytokines, Tyrosine kinase, and protein kinase C on production of superoxide and induction of scavenging enzymes in human leukocytes. Clin Immunol Immunopathol. (1996) 79:303–13. doi: 10.1006/clin.1996.0083
39. Chanez P, Dent G, Yukawa T, Barnes PJ, Chung KF. Generation of oxygen free radicals from blood eosinophils from asthma patients after stimulation with PAF or phorbol ester. Eur Respir J. (1990) 3:1002–7. doi: 10.1183/09031936.93.03091002
40. Childs EW, Udobi KF, Wood JG, Hunter FA, Smalley DM, Cheung LY. In vivo visualization of reactive oxidants and leukocyte-endothelial adherence following hemorrhagic shock. Shock. (2002) 18:423–7. doi: 10.1097/00024382-200211000-00006
41. Verouti SN, Fragopoulou E, Karantonis HC, Dimitriou AA, Tselepis AD, Antonopoulou S, et al. effects on MCP-1 and IL-6 secretion in U-937 monocytes in comparison with OxLDL and IL-1β effects. Atherosclerosis. (2011) 219:519–25. doi: 10.1016/j.atherosclerosis.2011.07.123
42. Detopoulou P, Fragopoulou E, Nomikos T, Yannakoulia M, Stamatakis G, Panagiotakos DB, et al. The relation of diet with PAF and its metabolic enzymes in healthy volunteers. Eur J Nutr. (2015) 54:25–34. doi: 10.1007/s00394-014-0682-3
43. English CJ, Mayr HL, Lohning AE, Reidlinger DP. The association between dietary patterns and the novel inflammatory markers platelet-activating factor and lipoprotein-associated phospholipase A2: a systematic review. Nutr Rev. (2022) 80:1371–91. doi: 10.1093/nutrit/nuab051
44. Nomikos T, Fragopoulou E, Antonopoulou S, Panagiotakos DB. Mediterranean diet and platelet-activating factor; a systematic review. Clin Biochem. (2018) 60:1–10. doi: 10.1016/j.clinbiochem.2018.08.004
45. Fragopoulou E, Detopoulou P, Alepoudea E, Nomikos T, Kalogeropoulos N, Antonopoulou S. Associations between red blood cells fatty acids, desaturases indices and metabolism of platelet activating factor in healthy volunteers. Prostaglandins Leukotrienes Essent Fatty Acids. (2021) 164:102234. doi: 10.1016/j.plefa.2020.102234
46. Devarasu N, Sudha GF. Dual-frequency bioelectrical phase angle to estimate the platelet count for the prognosis of dengue fever in Indian children. Biomed Eng. (2020) 65:417–28. doi: 10.1515/bmt-2018-0203
47. Popiolek-Kalisz J, Szczygiel K. Bioelectrical impedance analysis and body composition in cardiovascular diseases. Curr Probl Cardiol. (2023) 48:101911. doi: 10.1016/j.cpcardiol.2023.101911
48. Youjia Z, Yuanzhao X, Bing Z, Cai X, Jian C, Qian D, et al. Clinical prognostic role of bioimpedance phase angle in diabetic and non-diabetic hemodialysis patients. Asia Pac J Clin Nutr. (2022) 31:619–25. doi: 10.6133/apjcn.202212_31(4).0005
49. Detopoulou P, Voulgaridou G, Papadopoulou S. Cancer, phase angle and sarcopenia: the role of diet in connection with lung cancer prognosis. Lung. (2022) 200:347–79. doi: 10.1007/s00408-022-00536-z
50. Alves FD, Souza GC, Clausell N, Biolo A. Prognostic role of phase angle in hospitalized patients with acute decompensated heart failure. Clin Nutr. (2016) 35:1530–4. doi: 10.1016/j.clnu.2016.04.007
51. Lima J, Eckert I, Gonzalez MC, Silva FM. Prognostic value of phase angle and bioelectrical impedance vector in critically ill patients: a systematic review and meta-analysis of observational studies. Clin Nutr. (2022) 41:2801–16. doi: 10.1016/j.clnu.2022.10.010
52. Lordan R, Tsoupras A, Zabetakis I. The potential role of dietary platelet-activating factor inhibitors in cancer prevention and treatment. Adv Nutr. (2019) 10:148–64. doi: 10.1093/advances/nmy090
53. Detopoulou P, Nomikos T, Fragopoulou E, Chrysohoou C, Antonopoulou S. Platelet activating factor in heart failure: potential role in disease progression and novel target for therapy. Curr Heart Fail Rep. (2013) 10:122–9. doi: 10.1007/s11897-013-0131-2
54. Claus RA, Russwurm S, Dohrn B, Bauer M, Lösche W. Plasma platelet-activating factor acetylhydrolase activity in critically ill patients*. Crit Care Med. (2005) 33:1416–9. doi: 10.1097/01.CCM.0000165807.26485.ED
55. Detopoulou P, Nomikos T, Fragopoulou E, Panagiotakos DB, Pitsavos C, Stefanadis C, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) activity, platelet-activating factor acetylhydrolase (PAF-AH) in leukocytes and body composition in healthy adults. Lipids Health Dis. (2009) 8:19. doi: 10.1186/1476-511X-8-19
56. Trichopoulou A, Georga K. Composition Tables of Food and Greek Dishes. Athens: Parisianos SA (2004).
57. Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. (2006) 16:559–68. doi: 10.1016/j.numecd.2005.08.006
58. Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, et al. Total Antioxidant capacity of plant foods, beverages and oils consumed in italy assessed by three different in vitro assays. J Nutr. (2003) 133:2812–9. doi: 10.1093/jn/133.9.2812
59. Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res. (2006) 50:1030–8. doi: 10.1002/mnfr.200600067
60. Ghiselli A, Serafini M, Maiani G, Azzini E, Ferro-Luzzi A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic Biol Med. (1995) 18:29–36. doi: 10.1016/0891-5849(94)00102-P
61. Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. (1999) 299:15–27. doi: 10.1016/S0076-6879(99)99005-5
62. Fellegrini N, Ke R, Yang M, Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis(3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. Methods Enzymol. (1999) 299:379–89. doi: 10.1016/S0076-6879(99)99037-7
63. Detopoulou P, Panagiotakos DB, Chrysohoou C, Fragopoulou E, Nomikos T, Antonopoulou S, et al. Dietary antioxidant capacity and concentration of adiponectin in apparently healthy adults: the ATTICA study. Eur J Clin Nutr. (2009) 64:161–8. doi: 10.1038/ejcn.2009.130
64. Demopoulos CA, Andrikopoulos NK, Antonopoulou S. A simple and precise method for the routine determination of platelet-activating factor in blood and urine. Lipids. (1994) 29:305–9. doi: 10.1007/BF02536336
65. Mattiello R, Amaral MA, Mundstock E, Ziegelmann PK. Reference values for the phase angle of the electrical bioimpedance: systematic review and meta-analysis involving more than 250,000 subjects. Clin Nutr. (2020) 39:1411–7. doi: 10.1016/j.clnu.2019.07.004
66. Moreto F, de França NAG, Gondo FF, Callegari A, Corrente JE, Burini RC, et al. High C-reactive protein instead of metabolic syndrome is associated with lower bioimpedance phase angle in individuals clinically screened for a lifestyle modification program. Nutrire. (2017) 42:15. doi: 10.1186/s41110-017-0043-0
67. Nissinen L, Kähäri V-M. Matrix metalloproteinases in inflammation. Biochim Biophys Acta Gen Sub. (2014) 1840:2571–80. doi: 10.1016/j.bbagen.2014.03.007
68. Tremblay M, Almsherqi ZA, Deng Y. Plasmalogens and platelet-activating factor roles in chronic inflammatory diseases. BioFactors. (2022) 48:1203–16. doi: 10.1002/biof.1916
69. Soares AC, Pinho VS, Souza DG, Shimizu T, Ishii S, Nicoli JR, et al. Role of the platelet-activating factor (PAF) receptor during pulmonary infection with gram negative bacteria: PAF receptor and lung infection. Br J Pharmacol. (2002) 137:621–8. doi: 10.1038/sj.bjp.0704918
70. Deng M, Guo H, Tam JW, Johnson BM, Brickey WJ, New JS, et al. Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J Exp Med. (2019) 216:2838–53. doi: 10.1084/jem.20190111
71. Kesavardhana S, Malireddi RKS, Kanneganti T-D. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. (2020) 38:567–95. doi: 10.1146/annurev-immunol-073119-095439
72. Detopoulou P, Nomikos T, Fragopoulou E, Stamatakis G, Panagiotakos DB, Antonopoulou S, et al. and its metabolic enzymes in healthy volunteers: Interrelations and correlations with basic characteristics. Prostaglandins Other Lipid Mediat. (2012) 97:43–9. doi: 10.1016/j.prostaglandins.2011.10.003
73. Spadaro O, Youm Y, Shchukina I, Ryu S, Sidorov S, Ravussin A, et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science. (2022) 375:671–7. doi: 10.1126/science.abg7292
74. Detopoulou P, Tsiouda T, Pilikidou M, Palyvou F, Tsekitsidi E, Mantzorou M, et al. Changes in body weight, body composition, phase angle, and resting metabolic rate in male patients with stage IV non-small-cell lung cancer undergoing therapy. Medicina. (2022) 58:1779. doi: 10.3390/medicina58121779
75. Morigny P, Kaltenecker D, Zuber J, Machado J, Mehr L, Tsokanos F, et al. Association of circulating PLA2G7 levels with cancer cachexia and assessment of darapladib as a therapy. J Cachexia Sarcopenia Muscle. (2021) 12:1333–51. doi: 10.1002/jcsm.12758
76. De Borba EL, Ceolin J, Ziegelmann PK, Bodanese LC, Gonçalves MR, Cañon-Montañez Cañon-Montañez W, et al. Phase angle of bioimpedance at 50 kHz is associated with cardiovascular diseases: systematic review and meta-analysis. Eur J Clin Nutr. (2022) 76:1366–73. doi: 10.1038/s41430-022-01131-4
77. Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res. (2012) 53:1767–82. doi: 10.1194/jlr.R024190
78. Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res. (1985) 26:1015–35. doi: 10.1016/S0022-2275(20)34276-0
79. dos Cruz BC, de Carvalho TC, da Saraiva CA, dos Santos A, dos Reis PF. Effect of nutritional supplement enriched with eicosapentaenoic acid in lean mass of subjects with oral cavity cancer in oncologic pretreatment: a clinical trial. Rev Bras Cancerol. (2021) 67:e-05868. doi: 10.32635/2176-9745.RBC.2021v67n1.868
80. McIntyre TM. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: Formation, targets, and inactivation. Biochim Biophys Acta Biomembr. (2012) 1818:2456–64. doi: 10.1016/j.bbamem.2012.03.004
81. Chen R, Yang L, McIntyre TM. cytotoxic phospholipid oxidation products. J Biol Chem. (2007) 282:24842–50. doi: 10.1074/jbc.M702865200
82. Jacobs S, Schiller K, Jansen EH, Boeing H, Schulze MB, Kröger J. Evaluation of various biomarkers as potential mediators of the association between Δ5 desaturase, Δ6 desaturase, and stearoyl-CoA desaturase activity and incident type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition–Potsdam Study. Am J Clin Nutr. (2015) 102:155–64. doi: 10.3945/ajcn.114.102707
83. Lamantia V, Bissonnette S, Provost V, Devaux M, Cyr Y, Daneault C, et al. The association of polyunsaturated fatty acid δ-5-desaturase activity with risk factors for type 2 diabetes is dependent on plasma ApoB-lipoproteins in overweight and obese adults. J Nutr. (2019) 149:57–67. doi: 10.1093/jn/nxy238
84. Koseoglu SZA, Dogrusoy M. Evaluation of phase angle measurements and nutrient consumption by bioelectrical impedance method of 20-65 years old women: Evaluation of Phase Angle Measurements and Nutrient Consumption by Bioelectrical Impedance Method. Progr Nutr. (2020) 22:e2020012. doi: 10.23751/pn.v22i3.8523
Keywords: phase angle, bioelectrical impedance analysis, erythrocyte fatty acids, platelet-activating factor, dietary antioxidant capacity, Mediterranean diet
Citation: Detopoulou P, Fragopoulou E, Nomikos T and Antonopoulou S (2023) Associations of phase angle with platelet-activating factor metabolism and related dietary factors in healthy volunteers. Front. Nutr. 10:1237086. doi: 10.3389/fnut.2023.1237086
Received: 08 June 2023; Accepted: 09 October 2023;
Published: 03 November 2023.
Edited by:
Marija Takic, University of Belgrade, SerbiaReviewed by:
Denisse Castro-Eguiluz, National Council of Science and Technology (CONACYT), MexicoIoannis Zabetakis, University of Limerick, Ireland
Copyright © 2023 Detopoulou, Fragopoulou, Nomikos and Antonopoulou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Smaragdi Antonopoulou, YW50b25vcEBodWEuZ3I=
 Paraskevi Detopoulou
Paraskevi Detopoulou Elizabeth Fragopoulou
Elizabeth Fragopoulou Tzortzis Nomikos
Tzortzis Nomikos Smaragdi Antonopoulou
Smaragdi Antonopoulou