
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 17 July 2023
Sec. Nutrition and Food Science Technology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1230204
This article is part of the Research Topic Nutritional Components in Woody Oil Crops View all 5 articles
Avocado oil has gained a lot of favor in foods and cosmetics because of its high-quality fatty acid composition and bioactive components. This study aimed to compare the effect of various predry-treatments on the yield and quality of avocado oil from three Chinese avocado (Persea americana Mill.) varieties (Hass, Reed, and Pinkerton). The results showed that drying methods had significant effect on the avocado oil yield and its composition. Among the three drying methods the highest yield was obtained by freeze drying, and Hass showed the highest yield in the three avocado varieties with its oil owning the lowest peroxide and anisidine value. Reed oil owned the highest levels of functional micronutrients (e.g., tocopherols, phenolics, squalene). Vacuum drying resulted in higher concentrations of tocopherols, phytosterols, phenolics, squalene, and thus rendered greater DPPH and ABTS scavenging activity. These results are important to improve the quality of Chinese avocado oil.
Avocado (Persea Americana Mill.) is nutritious fruit widely distributed in tropical and subtropical regions. Its cultivation and processing are gaining popularity due to the multi-faceted salutary benefits associated with its consumption. The last several years have witnessed the growing demand for its oil because of its great potential applications in human nutrition, cosmetic and pharmaceutical industries (1–3). One remarkable fact about avocado oil (AO) is that it contains essential fatty acids (~13%), such as linoleic and linolenic acid, and monounsaturated fatty acids (~60%) that are salutary to the human cardiovascular system (2, 4). Moreover, AO has a higher phenolic content than other tropical and subtropical oils, and its high antioxidant, antibacterial properties and other health-promoting effects, such as lower risk of cataracts, diabetes, coronary heart disease, chemoprevention, age-related macular disease and prostate cancer, have been reported (1, 5).
Interest in developing and improving extraction technology that preserves the nutritional and physicochemical characteristics of AO as much as possible has been increasing. Many factors, including climatic conditions, growing region, variety and degree of fruit ripeness, can influence the chemical composition of AO, and extraction as well as processing techniques have been reported to significantly influence the yield and fatty acid composition of AO from Chile, Peru and Spain varieties (6, 7). With the large-scale cultivation of avocado fruit in China (e.g., Hainan, Guangxi, Yunnan and etc.), the development of avocado varieties with high-quality AO has attracted more and more attention. Hass, Reed, and Pinkerton are the three most profitable avocado varieties in China, which represent as many as two-thirds of the domestic avocado production. Moreover, extraction of AO is significantly affected by the drying of avocado pulp as it affects the physiochemical properties of oil (2, 8, 9). Mostly in modern industry, AO is extracted through vacuum drying in low temperature (10) or air drying with microwaves (8, 11, 12). However, these methods only slightly improve the extraction and produce AO with little quality improvement compared to those obtained by conventional drying methods such as oven drying with forced air circulation as well as traditional solvent extraction techniques. Additionally, oil quality is impacted by the oil composition per se and could be influenced by many processing factors like pulp drying (7, 13). However, there is little investigation on the influence of Chinese avocado varieties and avocado fruit processing (for instance pulp drying) on the quality of AO.
Therefore, the objective of this study was to evaluate the quality of AO extracted from three avocado cultivars (Hass, Reed, and Pinkerton) growing in China whose pulp was subjected to three drying pretreatments (freeze drying, vacuum drying and oven drying). The impact of drying treatments on the extraction efficiency and quality of AO were investigated. Moreover, the physicochemical properties and the functional components of AO were systematically analyzed. The combination of drying pretreatment and avocado variety in China may produce AO with various characteristics to better fit individual preferences.
6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (trolox), tocopherols (α-, β-, γ-, and δ-), phytosterols, squalene, fatty acid methyl esters (37 FAME mix), 2,2′-azinobis (3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich (Bellefonte, PA, USA). All other solvents were of chromatography grade and ultrapure water (Milli-Q system, Millipore, Bedford, MA, USA) was used throughout the experiments.
Avocado fruits (P. americana Mill. cv. Hass, Pinkerton, and Reed) were harvested from Hainan, Chain in February 2020 and stored at room temperature until full maturity before manually separated into seeds, pulp and peel. The weight of the whole fruit, pits, peel and pulp of more than 50 avocado fruits were evaluated. The pulp was immersed into citric acid solution (1%, w/v) for 15 min to avoid possible enzymatic darkening (7).
Avocado pulp was pulped into puree in a beater with a creamy consistency and then either oven-dried or vacuum-dried or freeze-dried as detailed below. The puree was spread onto stainless steel trays (80 × 100 cm) and then either dried in an oven (Fanen 320 SE, Brazil) with forced air circulation at 60°C or in a vacuum drier (Memmert Inc. VO 500 model, Germany) at 40°C and 600 mbar for 24 h. The temperatures were chosen according to the results of dos Santos et al. (8). For freeze drying, pulp was lyophilized at-40°C for 24 h with a freeze-drier (Edwards Pirani 501, West Sussex, United Kingdom). All drying was performed in triplicate and the dried pulp was vacuum packaged with light-proof bags and stored at-50°C for further use. Oil extraction was performed by Soxhlet extraction with petroleum ether, as described by AOAC 920.39. Yield was calculated as the ratio of the weight of oil extract to the dry weight of puree.
The moisture content, dry weight, total sugars, crude fat, crude fiber, protein and ash contents were determined according to AOAC, 2000. Fat content was determined as the method described by Rodríguez-Carpena et al. (14). The slip melting point, iodine value, free fatty acids content, saponification value and unsaponifiable matter content were analyzed as described in AOCS, 1999.
The fatty acid composition was analyzed as the method stated by Tan et al. (15) with minor modifications. FAME solution was prepared by adding 0.1 g of oil into hexane solution (5 ml) and sodium methoxide reagent (250 μl). Then, 5 ml of sodium chloride solution (26.5%, w/v) was added. After vigorous shaking for 15 s, the solution was stood at room temperature for 10 min to allow stratification. The top layer (1 μl) was analyzed with a gas chromatograph (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled with a polar capillary column (30 m × 0.25 mm, 0.25 μm, Austin, Texas, USA) and a flame ionization detector. The initial column temperature was maintained at 100°C for 2 min and then increased to 230°C at a rate of 5°C/min and was held there for 10 min. The temperatures of the injector and the detector were fixed at 250°C throughout the analysis. FAME standards were employed to identify the peaks on the obtained chromatograms.
Solid fat content (SFC) was measured using a Bruker Minispec (Model Mq 20) pulse nuclear magnetic resonance (NMR) spectrometer (Karlsruhe, Germany). Samples in the NMR tubes were heated at 80°C for 60 min, and then held at the corresponding measuring temperatures. After 30 min, SFC measurements were taken at 5°C intervals over the range of 0–40°C.
The contents of tocopherols were determined by HPLC as previously described (16). Folin–Ciocalteu reagent was employed to determine the total phenolic content according to the method of Rubilar et al. (17) with some modification. Phytosterols and squalene were identified and quantified with GC–MS. (18)
Antioxidant activity was assessed via DPPH assay as the method described by Sun et al. (19) with some changes. Briefly, 50 ml of various concentrations of AO solution (25, 50, 100, 200 or 250 mg/ml, dissolved in methanol) was blended with 2 ml of DPPH solution (24 mg/ml) and then incubated in the dark at room temperature. After 30 min, absorbance was then measured at 517 nm using a UV–visible spectrophotometer (Varian Cary 50, Mulgrave, Australia). Tert-butyl hydroquinone was recruited as a reference. Radical scavenging activity was calculated as follows: radical scavenging activity = (ADPPH − AAO)/ADPPH × 100%. ADPPH and AAO are the absorbance of the DPPH solution and the test solution, respectively.
All experiments were carried out in triplicate. Results are presented as mean ± standard deviations. Analysis of variance and multiple comparison was performed with Duncan’s tests. p values less than 0.05 were considered statistically significant.
An avocado fruit includes three anatomical parts: peel, pulp and seed. The morphometric measurement results of the whole fruit, peel, pulp and seed from the three avocado varieties are demonstrated in Table 1. In the light of all significantly different measurements among these avocado varieties, a clear differentiation among them was indicated. Reed showed the largest fruit and seed. Hass presented the lowest pulp percentage (60.59%), which agreed with the data reported by Costagli and Betti (20). Moisture content in the fresh pulp from Hass was 73.35 ± 0.45%, and its crude fat content was 16.53 ± 0.13%, which was significantly higher than that of Pinkerton (12.55 ± 1.43%) and Reed (14.25 ± 0.99%). This data was similar to a previous study that reported the lipids content in avocado pulp to range from 10 to 15%, showing its dependence on genotype as well as other factors (e.g., cultivar., season and growing conditions) (13, 21). Unlike oil extracted from other fruits such as palm kernel oil, AO is extracted from pulp rather than seeds as they contain very little oil (<2%) and have hepatoxic substances (22). Furthermore, compared to Reed and Pinkerton varieties, Hass variety is rich in edible oils (23). Therefore, the pulp of Hass would be a better candidate for obtaining AO.
AOs were extracted from the three avocado varieties that were subjected to different predry-treatments (freeze drying, oven drying and vacuum drying). The yields of the five oils are demonstrated in Figure 1A. It was found that drying methods had significant effect on AO yield with the highest yield obtained by freeze drying. Comparatively, vacuum drying pretreatment produced the lowest oil yield (51.13 ± 4.33%). This result was in accordance with other studies (2, 24). The changes of AO yield resulted from different drying treatments could result from drying temperature and drying method (9). Besides, the results also indicated that under the same kind of drying method (freeze drying) significant different AO yields could be observed from varieties (Figure 1A). The highest oil yield (65.30 ± 0.10%) was observed for Hass variety, which was 12.10 and 8.23% higher than those of Pinkerton and Reed varieties, respectively. Therefore, freeze drying treatment could increase oil yield and Hass showed the highest extraction efficiency among the three varieties investigated in the present study. This agreed well with some earlier works (7, 24).

Figure 1. Oil yields (A), solid fat content (B) and viscosity (C) of the AOs extracted from avocado pulp treated with different drying methods. OHO: oven-dried Hass oil, VHO: vacuum-dried Hass oil, FHO: freeze-dried Hass oil, FPO: freeze-dried Pinkerton oil, FRO: freeze-dried Reed oil. Experiments were carried out in triplicate. Results are presented as mean ± standard deviations.
Figure 1B shows the SFC profile of the AOs. The SFCs of freeze-dried Hass oil (FHO), freeze-dried Pinkerton oil (FPO) and freeze-dried Reed oil (FRO) at 0°C were 13.36, 7.30 and 7.25%, respectively, and decreased almost to 0% at 40°C, showing consistency to previous findings (25, 26). Notably, FHO stood out from the other two oils (FPO and FRO) due to its higher SFC in a wide temperature range. This is contrary to the results reported by Yanty et al. (27), who detected low SFC for Australian Hass oil. However, the Hass oil from West Malaysia displayed high SFC from 13.57 to 18.00% at 0°C. This difference could result from the variance in fatty acids and triglycerides composition (25). In terms of the influence of drying treatments, the SFC curves for oven-dried Hass oil (OHO), vacuum-dried Hass oil (VHO) and FHO intertwined with each other in a small range, suggesting no obvious difference was induced from drying treatments. Therefore, Hass can be a promising base material in making cream and lotion for skin nourishment. Reed and Pinkerton, on the other hand, could be used for making sauce, marinade and salad dressing. In general, these results illustrate that the variety but not predry-treatment affects the SFC in AO. With respect to the viscosity profile (Figure 1C), FHO is remarkably different from others, which may be due to its unique physicochemical properties (2, 28).
The two factors influencing the functionality of oil are physicochemical properties and bioactive constituents. Therefore, the appearance, microstructure, fatty acid composition, quality indices as well as the tocopherol, phytosterol, squalene and polyphenol contents of the AOs obtained from the three avocado varieties that were dried with different methods were examined systematically.
The AOs appeared olive drab or olive color (Figure 2), which was partly due to the existence of chlorophylls and carotenoids. Comparatively, the color of FHO was lighter than that of OHO and VHO, which might because lower temperature in lyophilization prevented polyphenols from oxidation (24). A homologous result was reported by Tan et al. (15) that described darker color in tray-dried AO. This indicated AO extracted by freeze-dried avocado pulp was lighter-colored. Moreover, FHO and FRO exhibited lighter color than FPO, alluding that the variety also has an influence on the oil appearance. The microstructure of these AOs was also investigated (Figure 2). For the oil from Hass avocado, spherulites resulted from the aggregation of needle-like crystals that grown from nucleation centers with several branches were observed. Meanwhile, sparse plate crystals were revealed in FRO and very few crystals were observed in FPO. These results show that there are obvious differences in the physical properties of the oils extracted from the three avocado varieties.
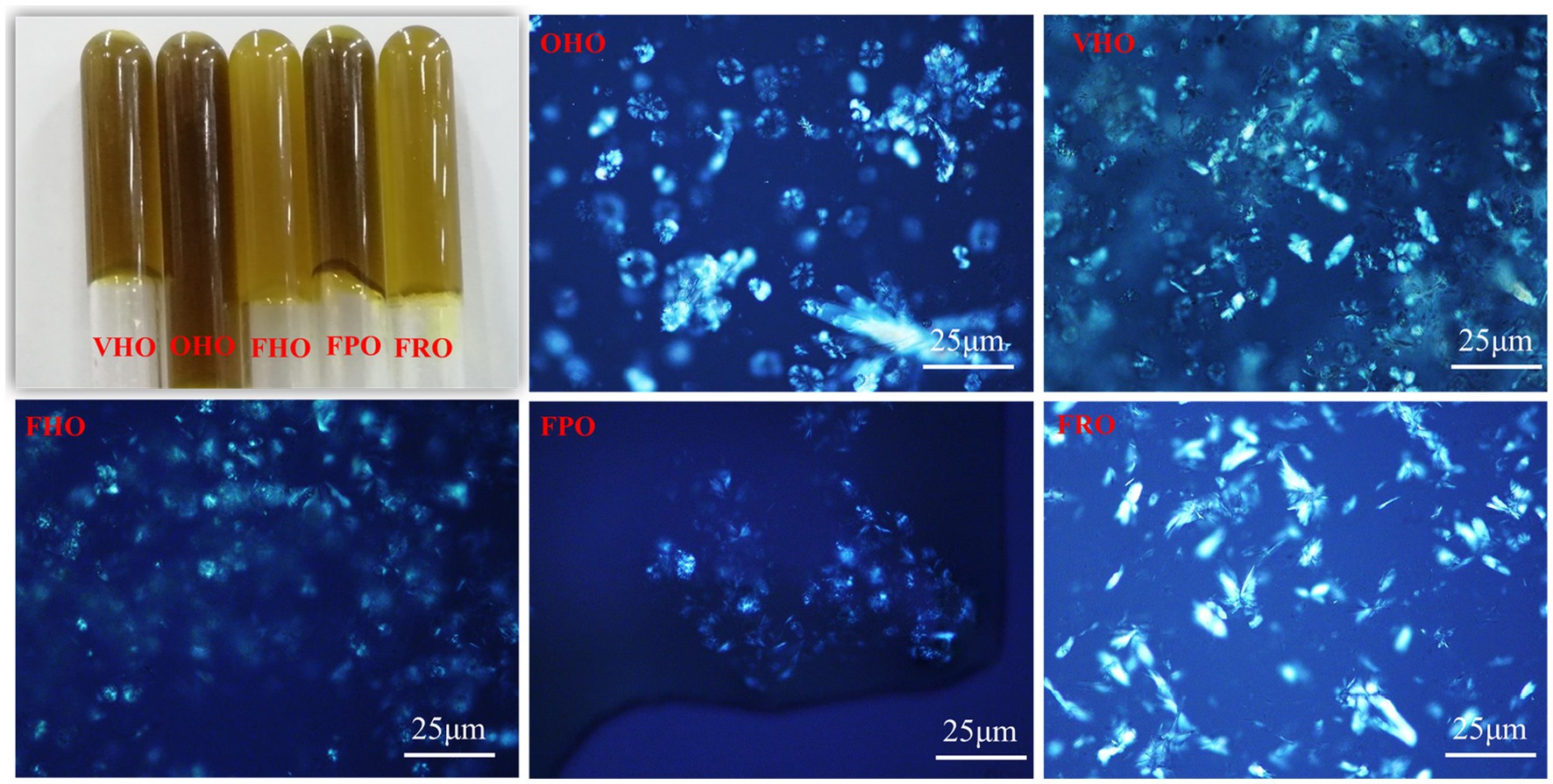
Figure 2. Appearance and polarized photomicrograph of the AOs extracted from avocado pulp treated with different drying methods. OHO: oven-dried Hass oil, VHO: vacuum-dried Hass oil, FHO: freeze-dried Hass oil, FPO: freeze-dried Pinkerton oil, FRO: freeze-dried Reed oil.
The fatty acid composition of the five AOs was determined and quantified by gas chromatography. As shown in Table 2, seven fatty acids were identified, and the content of palmitic, palmitoleic, oleic and linolenic acids differed obviously. Specifically, the predominant fatty acid was oleic acid (C18:1, n-9, cis), followed by palmitic (C16:0), linoleic (C18:2, n-6, cis) and palmitoleic (C16:1, n-7, cis) acids. Correspondingly, stearic (C18:0) and α-linolenic (C18:3, n-3, cis) acids were barely found, which agreed with the observation of Yanty et al. (27) who found that both stearic acid and α-linolenic acid contents were below 1%. The distribution of these fatty acids in the AOs from Hass, Reed and Pinkerton is homothetic to many past studies, such as that in palm oil and lard (29, 30). Lignoceric (24:0), arachidic (20:0) and cis-11-eicosenoic (20:1 n-9) were found to have a rather low content (< 0.3%), for which their results are not shown here. Interestingly, the content of the monounsaturated oleic acid (C18:1, from 33.25 to 39.10%) was lower than that in the oils from avocado harvested from other parts of the world (45.9–54.5%) (21, 31). This discordance could result from climate and geographical variations. In particular, monounsaturated palmitoleic acid (1, 16), with pharmaceutical applications in anti-inflammation and triglycerides-lowering effect, was the most abundant fatty acid (9–14%). The major saturated fatty acid (palmitic acid), with the content ranging from 26 to 29%, was the second most abundant fatty acid. These fatty acid contents in the five AOs were as expected for avocado and showed general consistency with a previous report on avocado fruit (11, 32). The fatty acid profiles of the five oils by different predry-treatments were very similar (Table 2). However, the Hass variety had higher abundance of palmitoleic acid than many other varieties (3–10%) (33), which reflected the potential of avocado as a dietary supplement to help lower blood cholesterol level and prevent cardiovascular diseases as well as dermatosis. Besides, a high UFAs/SFAs ratio (>2.0) is considered beneficial to human health; the higher the ratio, the higher the nutritional quality of a vegetable oil (34).
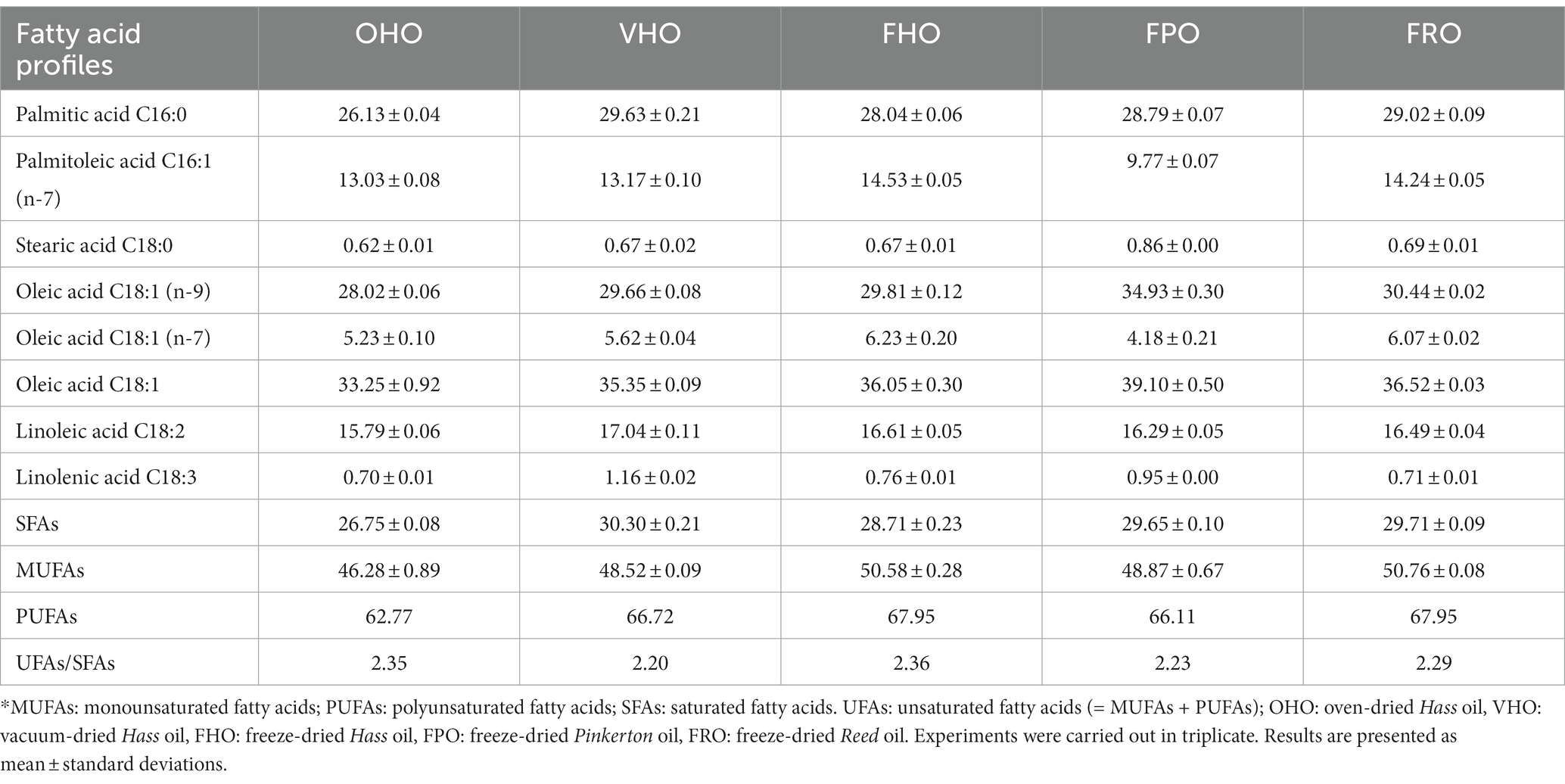
Table 2. Fatty acid composition of the five AOs extracted from avocado pulp treated with different predry-treatments.*
The predry-treatment had significantly influenced the peroxide, anisidine and acid value of the AOs. The main products of primary degradation of lipids autoxidation are peroxides. FHO presented the lowest peroxide value than other oils (Figure 3A), which could originate from the low temperature in freeze drying that provides considerable protection against lipid oxidation. This result suggests that the peroxide value of AO is dependent on the predry-treatment. Moreover, under the same predry-treatment, peroxide value of AO varied significantly with avocado varieties, where FRO showed the highest peroxide value. The anisidine content stands for the degree of secondary degradation of lipids. Correspondingly, the anisidine value of FHO was also significantly lower than other oils (Figure 3B). The free fatty acid content indicates the presence of hydrolytic degradation that is associated with off flavor and oil changes (35). The acid value of OHO was significantly lower than that of VHO and FHO (Figure 3C), suggesting that the free fatty acid content in AO was affected by predry-treatment. Further, FHO showed the lowest acidity level (0.37 mg KOH/g). Even so, the acidity level of Hass oil either by oven or vacuum drying pretreatment and Pinkerton as well as Reed oil exceeded the threshold of 4 mg KOH/g oil, which abided by the international standards of crude, cold-pressed oil and indicated that there was no considerable hydrolysis or oxidation of lipids during pulp processing and oil extraction. This agreed with the results of Krumreich et al. (7). Taken together, all the findings purported that the AO extracted from Hass by freeze drying was of good quality.
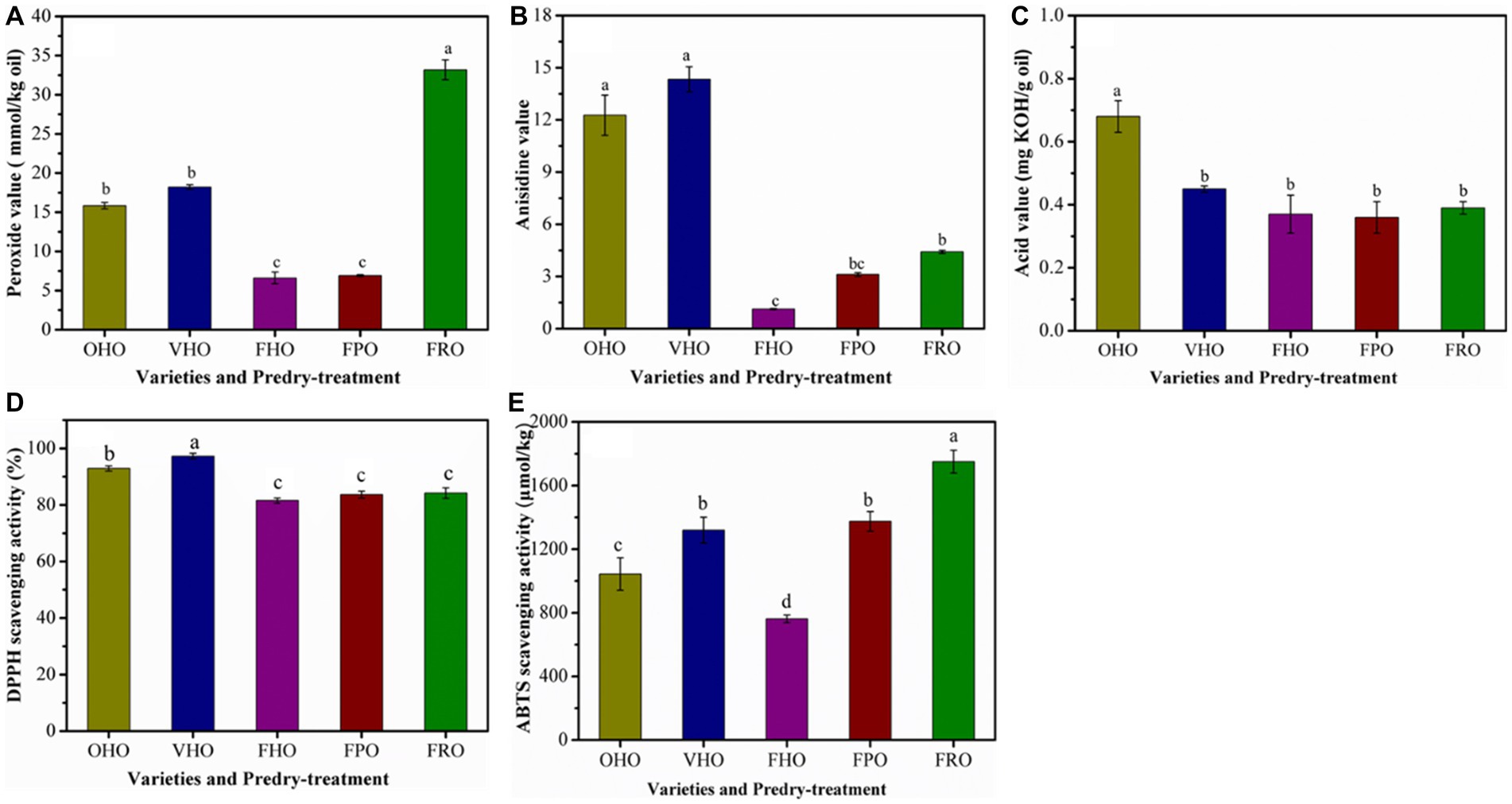
Figure 3. Peroxide value (A), anisidine value (B), acid value (C), DPPH radical scavenging activity (D) and ABTS radical scavenging activity (E) of the AOs extracted from avocado pulp treated with different drying methods. OHO: oven-dried Hass oil, VHO: vacuum-dried Hass oil, FHO: freeze-dried Hass oil, FPO: freeze-dried Pinkerton oil, FRO: freeze-dried Reed oil. Experiments were carried out in triplicate. Results are presented as mean ± standard deviations.
AO is rich in bioactive compounds that can serve as antioxidants and improve human health. The contents of bioactive components in the five AOs are shown in Table 3. α-Tocopherol appeared as the dominant tocopherol in all AOs. In terms of the effect of drying techniques, oils from freeze-dried pulps (FHO) showed the highest contents of tocopherols. The main sterols in the AOs were campesterol, Δ5-avenasterol, β-sitosterol and stigmasterol, where β-sitosterol was the most predominant one. Variety, planting location and different test methods could induce the high variation in total sterols content. The results also revealed significant variation in the total phenolic content of the AOs with respect to both predry-treatment and variety. With respect to the unsaponifiable fraction, squalene was the major component (205.58–611.27 mg/kg), whose content was higher that of total tocopherols and phenolics. FRO had higher squalene content (611.27 mg/kg) than FPO (306.15 mg/kg) and FHO (218.93 mg/kg). These AOs present much greater squalene content in comparison to that in the oils from Brazilian, Mexican and Australian avocados (3, 28).
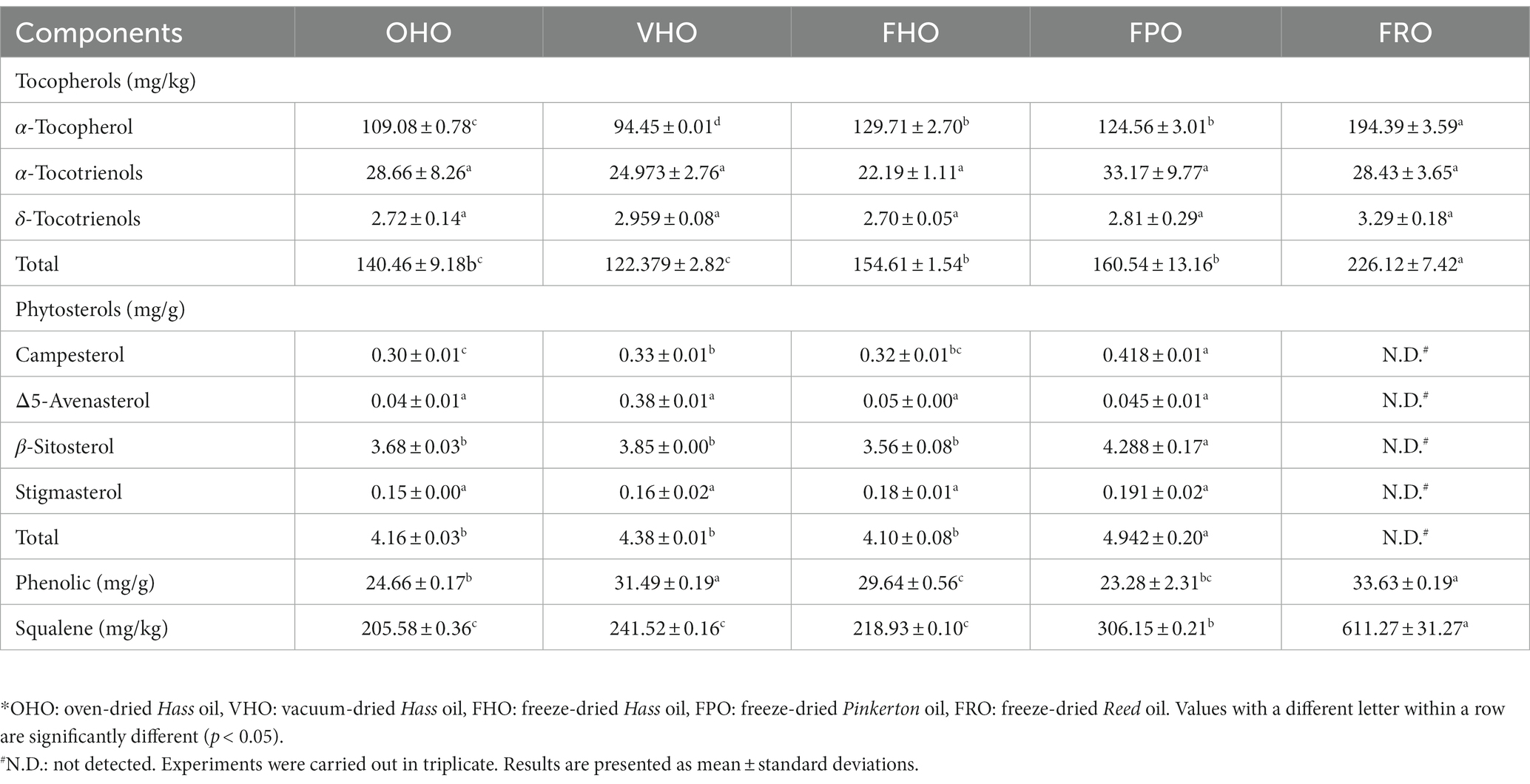
Table 3. Tocopherols, phytosterols, polyphenols and squalene contents of AOs extracted from avocado pulp treated with different predry-treatments.*
The antioxidant capacities of the AOs determined by DPPH and ABTS assay are presented in Figure 3. The DPPH scavenging capacity ranged from 81 to 97%, which indicated favorable radical scavenging activity of all the AOs (Figure 3D). In terms of the effect of predry-treatments, vacuum drying endowed AO with higher radical scavenging activity as DPPH scavenging activity of VHO was greater than that of OHO and FHO. In addition, Hass oil extracted from oven-dried and vacuum-dried avocado pulp exhibited significantly higher DPPH scavenging capability than that of Pinkerton and Reed ones. With respect to ABTS scavenging activity, the Reed oil exhibited the highest capacity, followed by Pinkerton oil and Hass oil (Figure 3E). The high ABTS scavenging activity of Pinkerton oil was probably associated with the content of tocopherols and phenolic (Table 3). Similar to the results obtained in the DPPH assays the Hass oil from vacuum-dried pulp showed higher radical scavenging activity than that of oils from other predry-treatments. Likewise, a great decrease in the antioxidant activity of phenolic compounds was observed after its subjection to oven-drying (14, 23, 36).
The changes in the bioactive constituents and quality parameters of AOs from three Chinese avocado varieties subjected to different predry-treatments (oven drying, vacuum drying and freeze drying) was investigated in this study. It was found that both avocado varieties and drying methods had significant influence on the oil composition and oil quality. The best extraction yield of AO was obtained by the combination of freeze drying and Hass variety. More importantly, it was also found that AO extracted from freeze-dried Hass pulp has high nutritional quality, as suggested by its fatty acid composition, peroxide value, anisidine value, acid value, bioactive compounds profile and antioxidant activity. The findings of the present study are important to improve the quality of Chinese AO and shed light on the future exploitation of Hass variety as a raw material to produce functional oils that can be applied to food or cosmetic industry.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
JW: conceptualization, methodology, writing – original draft, writing – review and editing, and funding acquisition. HY: formal analysis, investigation, visualization, and writing – original draft. PW, JZ, WM, YL, and JL: conceptualization, supervision, and writing – review and editing. All authors contributed to the article and approved the submitted version.
This work was financially supported by Key R&D Projects of Hainan Province (ZDYF2021XDNY162) and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 16300920210010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bhuyan, DJ, Alsherbiny, MA, Perera, S, Low, M, Basu, A, Devi, OA, et al. The odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidants. (2019) 8:426. doi: 10.3390/antiox8100426
2. Cervantes-Paz, B, and Yahia, EM. Avocado oil: production and market demand, bioactive components, implications in health, and tendencies and potential uses. Compr Rev Food Sci F. (2021) 20:4120–58. doi: 10.1111/1541-4337.12784
3. Flores, M, Saravia, C, Vergara, CE, Avila, F, Valdés, H, and Ortiz-Viedma, J. Avocado oil: characteristics, properties, and applications. Molecules. (2019) 24:2172. doi: 10.3390/molecules24112172
4. Satriana, S, Supardan, MD, Arpi, N, and Wan Mustapha, WA. Development of methods used in the extraction of avocado oil. Eur J Lipid Sci Technol. (2019) 121:800210. doi: 10.1002/ejlt.201800210
5. Unlu, NZ, Bohn, T, Clinton, SK, and Schwartz, SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. (2005) 135:431–6. doi: 10.1093/jn/135.3.431
6. Donetti, M, and Terry, LA. Biochemical markers defining growing area and ripening stage of imported avocado fruit cv. Hass. J Food Compos Anal. (2014) 34:90–8. doi: 10.1016/j.jfca.2013.11.011
7. Krumreich, FD, Borges, CD, Mendonça, CRB, Jansen-Alves, C, and Zambiazi, RC. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. (2018) 257:376–81. doi: 10.1016/j.foodchem.2018.03.048
8. dos Santos, MA, Alicieo, TV, Pereira, CM, Ramis-Ramos, G, and Mendonça, CR. Profile of bioactive compounds in avocado pulp oil: influence of the drying processes and extraction methods. J Am Oil Chem Soc. (2014) 91:19–27. doi: 10.1007/s11746-013-2289-x
9. Sena-Moreno, E, Pardo, JE, Catalán, L, Gómez, R, Pardo-Giménez, A, and Alvarez-Ortí, M. Drying temperature and extraction method influence physicochemical and sensory characteristics of pistachio oils. Eur J Lipid Sci Technol. (2015) 117:684–91. doi: 10.1002/ejlt.201400366
10. Hamzah, B. The effect of homogenization pressures on extraction of avocado oil by wet method. Adv J Food Sci Technol. (2013) 5:1666–8. doi: 10.19026/ajfst.5.3407
11. Moreno, AO, Dorantes, L, Galíndez, J, and Guzmán, RI. Effect of different extraction methods on fatty acids, volatile compounds, and physical and chemical properties of avocado (Persea americana mill.) oil. J Agric Food Chem. (2003) 51:2216–21. doi: 10.1021/jf0207934
12. Mostert, ME, Botha, BM, Plessis, LMD, and Duodu, KG. Effect of fruit ripeness and method of fruit drying on the extractability of avocado oil with hexane and supercritical carbon dioxide. J Sci Food Agric. (2007) 87:2880–5. doi: 10.1002/jsfa.3051
13. Santana, I, dos Reis, LM, Torres, AG, Cabral, LM, and Freitas, SP. Avocado (Persea americana Mill.) oil produced by microwave drying and expeller pressing exhibits low acidity and high oxidative stability. Eur J Lipid Sci Technol. (2015) 117:999–1007. doi: 10.1002/ejlt.201400172
14. Rodríguez-Carpena, JG, Morcuende, D, Andrade, MJ, Kylli, P, and Estévez, M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J Agric Food Chem. (2011) 59:5625–35. doi: 10.1021/jf1048832
15. Tan, CX, Chong, GH, Hamzah, H, and Ghazali, HM. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. J Supercrit Fluids. (2018) 135:45–51. doi: 10.1016/j.supflu.2017.12.036
16. Santana, I, Castelo-Branco, VN, Guimarães, BM, de Oliveira, SL, Peixoto, VODS, Cabral, LMC, et al. Hass avocado (Persea americana mill.) oil enriched in phenolic compounds and tocopherols by expeller-pressing the unpeeled microwave dried fruit. Food Chem. (2019) 286:354–61. doi: 10.1016/j.foodchem.2019.02.014
17. Rubilar, M, Morales, E, Sáez, R, Acevedo, F, Palma, B, Villarroel, M, et al. Polyphenolic fractions improve the oxidative stability of microencapsulated linseed oil. Eur J Lipid Sci Technol. (2012) 114:760–71. doi: 10.1002/ejlt.201100230
18. Hu, QH, Ning, XY, Ma, CG, and Chen, XW. Comparative study on functional components, physicochemical properties and antioxidant activity of Amaranthus Caudatus L. oils obtained by different solvents extraction. J Oleo Sci. (2021) 70:155–64. doi: 10.5650/jos.ess20157
19. Sun, Z, Wang, H, Wang, J, Zhou, L, and Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS One. (2014) 9:e114767. doi: 10.1371/journal.pone.0114767
20. Costagli, G, and Betti, M. Avocado oil extraction processes: method for cold-pressed high-quality edible oil production versus traditional production. J Agric Eng. (2015) 46:115–22. doi: 10.4081/jae.2015.467
21. Meyer, MD, and Terry, LA. Development of a rapid method for the sequential extraction and subsequent quantification of fatty acids and sugars from avocado mesocarp tissue. J Agric Food Chem. (2008) 56:7439–45. doi: 10.1021/jf8011322
22. Qin, X, and Zhong, J. A review of extraction techniques for avocado oil. J Oleo Sci. (2016) 65:881–8. doi: 10.5650/jos.ess16063
23. Kosińska, A, Karamać, M, Estrella, I, Hernández, T, Bartolomé, B, and Dykes, GA. Phenolic compound profiles and antioxidant capacity of Persea americana mill. Peels and seeds of two varieties. J Agric Food Chem. (2012) 60:4613–9. doi: 10.1021/jf300090p
24. Gutiérrez, L, Ratti, C, and Belkacemi, K. Effects of drying method on the extraction yields and quality of oils from Quebec Sea buckthorn (Hippophae rhamnoides L.) seeds and pulp. Food Chem. (2008) 106:896–904. doi: 10.1016/j.foodchem.2007.06.058
25. Manaf, YN, Rahardjo, AP, Yusof, YA, Desa, MN, and Nusantoro, BP. Lipid characteristics and tocopherol content of the oils of native avocado cultivars grown in Indonesia. Int J Food Prop. (2018) 21:2758–71. doi: 10.1080/10942912.2018.1564761
26. Noorzyanna, Y, Marikkar, N, Mustafa, S, and Mat, SM. Composition and thermal analysis of ternary mixtures of avocado fat: palm stearin: cocoa butter (Avo: PS: CB). Int J Food Prop. (2017) 20:465–74. doi: 10.1080/10942912.2016.1166130
27. Yanty, N, Marikkar, J, and Long, K. Effect of varietal differences on composition and thermal characteristics of avocado oil. J Am Oil Chem Soc. (2011) 88:1997–2003. doi: 10.1007/s11746-011-1877-x
28. Jorge, TS, Polachini, TC, Dias, LS, Jorge, N, and Telis-Romero, J. Physicochemical and rheological characterization of avocado oils. Ciênc Agrotecnol. (2015) 39:390–400. doi: 10.1590/S1413-70542015000400010
29. Marikkar, J, Lai, O, Ghazali, H, and Che, MY. Detection of lard and randomized lard as adulterants in refined-bleached-deodorized palm oil by differential scanning calorimetry. J Am Oil Chem Soc. (2001) 78:1113–9. doi: 10.1007/s11746-001-0398-5
30. Nurjuliana, M, Che Man, Y, and Mat, HD. Analysis of lard’s aroma by an electronic nose for rapid halal authentication. J Am Oil Chem Soc. (2011) 88:75–82. doi: 10.1007/s11746-010-1655-1
31. Takenaga, F, Matsuyama, K, Abe, S, Torii, Y, and Itoh, S. Lipid and fatty acid composition of mesocarp and seed of avocado fruits harvested at northern range in Japan. J Oleo Sci. (2008) 57:591–7. doi: 10.5650/jos.57.591
32. Ozdemir, F, and Topuz, A. Changes in dry matter, oil content and fatty acids composition of avocado during harvesting time and post-harvesting ripening period. Food Chem. (2004) 86:79–83. doi: 10.1016/j.foodchem.2003.08.012
33. Villa-Rodríguez, JA, Molina-Corral, FJ, Ayala-Zavala, JF, Olivas, GI, and González-Aguilar, GA. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res Int. (2011) 44:1231–7. doi: 10.1016/j.foodres.2010.11.012
34. Pérez-Saucedo, M, Jiménez-Ruiz, E, Rodríguez-Carpena, J, Ragazzo-Sánchez, J, Ulloa, J, Ramírez-Ramírez, J, et al. Properties of the avocado oil extracted using centrifugation and ultrasound-assisted methods. Food Sci Biotechnol. (2021) 30:1051–61. doi: 10.1007/s10068-021-00940-w
35. Ghafoor, K, Özcan, MM, Fahad, A, Babiker, EE, and Fadimu, GJ. Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven-and microwave-roasted poppy seeds and oil. LWT. (2019) 99:490–6. doi: 10.1016/j.lwt.2018.10.017
Keywords: avocado oil, predry-treatment, avocado cultivar, bioactive constituents, quality assessment
Citation: Wang J, Yang H, Wu P, Zhang J, Ma W, Li Y and Liu J (2023) Effect of Predry-treatment on the bioactive constituents and quality of avocado (Persea americana Mill.) oil from three cultivars growing in China. Front. Nutr. 10:1230204. doi: 10.3389/fnut.2023.1230204
Received: 28 May 2023; Accepted: 03 July 2023;
Published: 17 July 2023.
Edited by:
Ningxiang Yu, Zhejiang University of Technology, ChinaReviewed by:
Xiao-Wei Chen, Henan University of Technology, ChinaCopyright © 2023 Wang, Yang, Wu, Zhang, Ma, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiashui Wang, amlhc2h1aXdhbmdAY2F0YXMuY24=; amlhc2h1aXdhbmdAMTI2LmNvbQ==; Jinping Liu, bGl1MzMwNTYwMkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.