- 1Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, United States
- 2Department of Molecular Biology and Microbiology, Tufts University, Boston, MA, United States
- 3Department of Developmental, Molecular and Chemical Biology, Tufts University, Boston, MA, United States
- 4Department of Pathology, University of Cambridge, Cambridge, United Kingdom
- 5Department of Food Science and Human Nutrition, Iowa State University, Ames, IA, United States
Introduction: The safety of novel forms of iron in healthy, iron-replete adults as might occur if used in population-based iron supplementation programs was examined. We tested the hypotheses that supplementation with nanoparticulate iron hydroxide adipate tartrate (IHAT), an iron-enriched Aspergillus oryzae product (ASP), or ferrous sulphate heptahydrate (FS) are safe as indicated by erythrocyte susceptibility to malarial infection, bacterial proliferation, and gut inflammation. Responses to FS administered daily or weekly, and with or without other micronutrients were compared.
Methods: Two phases of randomized, double-blinded trials were conducted in Boston, MA. Phase I randomized 160 volunteers to six treatments: placebo, IHAT, ASP, FS, and FS plus a micronutrient powder (MNP) administrated daily at 60 mg Fe/day; and FS administered as a single weekly dose of 420 mg Fe. Phase II randomized 86 volunteers to IHAT, ASP, or FS administered at 120 mg Fe/day. Completing these phases were 151 and 77 participants, respectively. The study was powered to detect effects on primary endpoints: susceptibility of participant erythrocytes to infection by Plasmodium falciparum, the proliferation potential of selected pathogenic bacteria in sera, and markers of gut inflammation. Secondary endpoints for which the study was not powered included indicators of iron status and gastrointestinal symptoms.
Results: Supplementation with any form of iron did not affect any primary endpoint. Regarding secondary endpoints, in Phase I participants taking IHAT more frequently reported abdominal pain (27%, p = 0.008) than other iron forms; those taking the weekly FS dose more frequently reported nausea (20%, p = 0.009) than the other forms and modes of administration. In phase II, no such differences were observed.
Discussion: With respect to the primary endpoints, few differences were found when comparing these forms of iron, indicating that 28 days of 60 or 120 mg/day of IHAT, ASP, or FS may be safe for healthy, iron-replete adults. With respect to other endpoints, subjects receiving IHAT more frequently reported abdominal pain and nausea, suggesting the need for further study.
Clinical Trial Registration: ClinicalTrials.gov, NCT03212677; registered: 11 July 2017.
1. Introduction
Iron-deficiency affects more than a billion people worldwide, mostly children and female adults in resource-poor countries comprising a persistent global disease burden. Addressing iron deficiency through population-based iron supplementation programs has been frustrated by serious side effects of inorganic forms of iron which, due to low enteric absorption, must be given in relatively high levels (1). Those effects include gut inflammation (2–5), changes in the gut microbiome (4, 5), bloody diarrhea (2, 5–7); among iron-replete children in malaria-endemic areas increased serious morbidity has been reported (8–15). These effects are thought to involve stress on the gut by unabsorbed iron, which can be pro-oxidative and pro-inflammatory, favoring the proliferation of pathogenic enteric bacteria (14),and contributing to inflammatory responses (2, 5, 16, 17) that lead to down-regulation of iron absorption (18). Non-transferrin-bound iron (NTBI) formed from high supplements of rapidly absorbed forms of iron (e.g., ferrous sulphate, FS) has been proposed to increase malaria infection severity (19, 20) by increasing capillary sequestration of infected erythrocytes (21). Iron-replete individuals with inflammation may, thus, have increased risk of adverse effects of supplemental iron, particularly in malaria-endemic regions. Adverse effects associated with currently available forms of iron in addressing prevalent iron deficiency have effectively halted population-based iron supplementation in malaria-endemic areas.
The present study was conducted to inform the development of modalities of providing bioavailable iron with minimal adverse effects on iron-replete individuals. Three forms of iron were used: ferrous sulphate heptahydrate (FeSO4·7H2O, FS), nanoparticulate iron hydroxide adipate tartrate (IHAT), and an iron-enriched Aspergillus oryzae product (ASP). The novel forms of iron have been reported to have apparent bioavailability’s less than FS, as assessed by different methods (22–25). The study sought to determine whether IHAT and ASP produce fewer adverse effects than FS in iron-replete participants. The primary outcomes were: infectivity of malarial parasites (Plasmodium falciparum) on host erythrocytes (assessed ex vivo), proliferation of selected bacterial species in host plasma (assessed ex vivo), gut inflammation (assessed in vivo). Effects on iron status were also assessed. The study was conducted in two phases, each comprised of a clinical intervention trials described previously (26, 27).
2. Participants and methods
The Safe Iron Study design has been described previously (26). Briefly, it was a randomized, double-blind, placebo-controlled, two-phase study with iron supplementation in adults who were iron-replete. For both phases, each arm was comprised of an intervention period of 4 weeks, after which time the outcome parameters were compared between the baseline (wk 0) and post-intervention (wk 4) times.
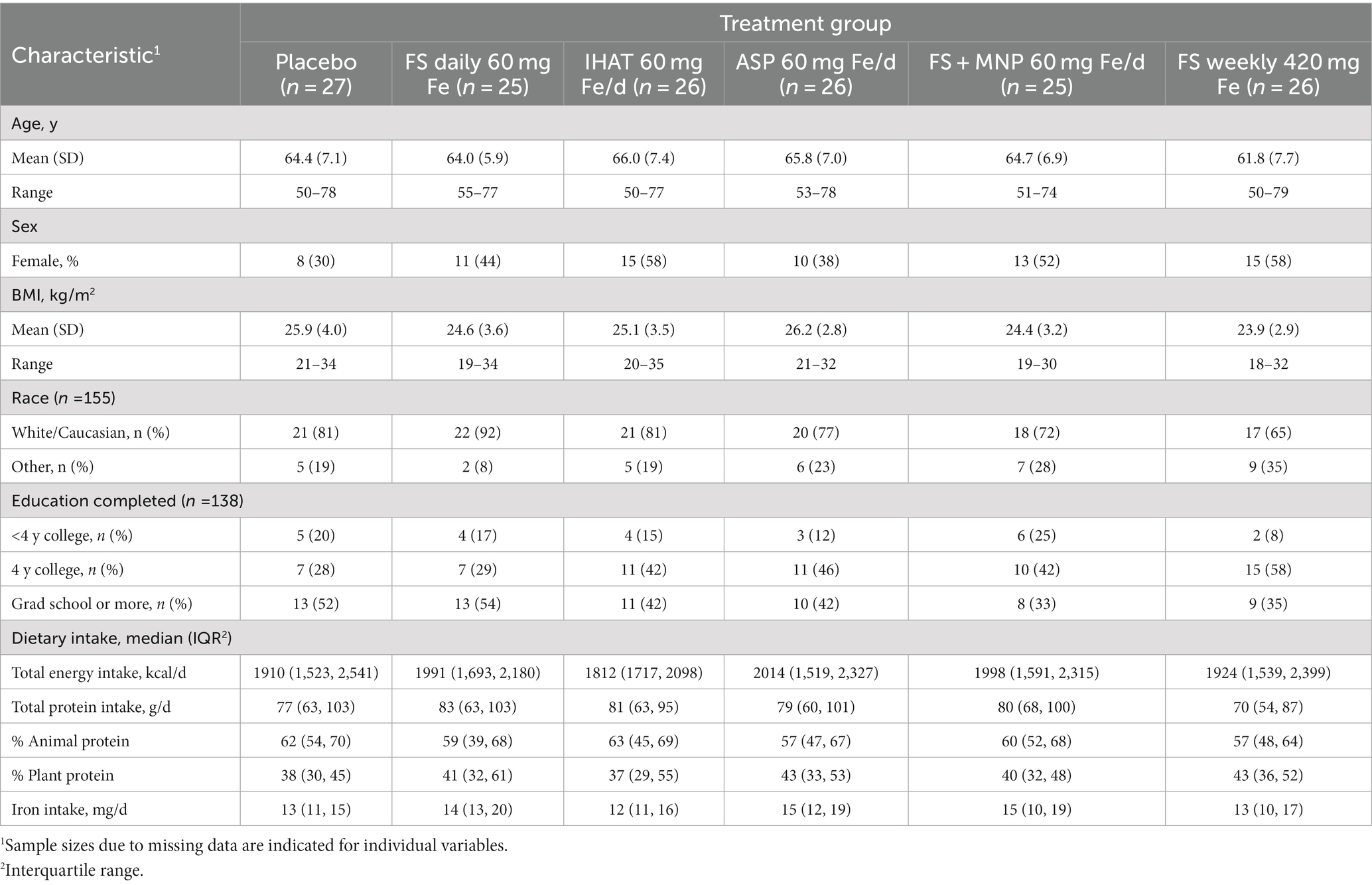
Volunteers were recruited from the greater Boston area using advertisements in print and electronic media, flyers posted in public places, and mailings to participants in previous studies at this Center. Those who responded and gave consent were pre-screened by telephone; eligible, pre-screened individuals were invited to the Center for screening. Interested adults were asked to sign a screening consent form (28). The screening process was conducted by an experienced research study nurse. It consisted of taking a blood sample (7 ml), reviewing participant medical history and general health (including gastrointestinal health history), administering a gut irritation questionnaire, and inquiring about the use of medications and nutritional supplements including iron. Volunteers were eligible on the basis of the criteria previously described (26). Briefly, those included: Inclusion - apparently healthy males and post-menopausal females (no menses for ≥1 year); 50–80 years of age; BMI 18–35 kg/m2; typical bowel pattern of at least one stool every other day; willing to comply with study procedures; Exclusion - taking an iron supplement; any major illness or condition that may interfere with study outcomes at the discretion of the study physician (JBM). Volunteers deemed eligible were invited to enroll in the study at which time each was asked to complete a questionnaire recording age, body weight, height, educational level, and self-identified sex and race.
Each was free to withdraw at any time by writing, calling, or emailing the study PI (GFC). Each could be terminated if they no longer met any of the study eligibility, failed to comply with study requirements, or had adverse reaction that were considered severe in the judgment of the study physician and the PI. Each was offered a modest honorarium for participating. If a participant failed to complete the study, the honorarium was prorated accordingly, and data or specimens collected before withdrawal were used in the study.
The study protocol was approved by the Institutional Review Board of Tufts Medical Center and Tufts University Health Sciences Campus (Phase I IRB #12455, Phase II IRB#13341) which found that, because it presented minimal risk for participants, safety monitoring could be conducted by the PI (GFC) and study Physician (JBM). Nevertheless, we impaneled an external advisory board to review de-identified data provided to them periodically and assess both harms and benefits.
2.1. Intervention agents
The intervention agents included a placebo (Melojel® corn starch) and three forms of iron: FeSO4·7H2O (FS), IHAT, and ASP. Reagent grade FS was used. In Phase I, one arm also included a multiple micronutrient powder [MNP; MixMe™ Vitamin and Mineral Powder (DSM Nutritional Products, Geneva, Switzerland) (29)] the contents of which are based on recommendations by UNICEF/WHO/WFP for one RDA of 15 vitamins and minerals.
IHAT, developed by the Medical Research Council Elsie Widdowson Laboratory, Cambridge, UK, is composed of three GRAS substances: iron hydroxide, tartaric acid and adipic acid. It is a nano-particulate iron supplement, recently approved by the European Commission as a novel food (2022/1373; Aug. 5, 2022). IHAT is a tartrate-modified, nano-dispersed Fe(III) oxo-hydroxide with similar functional properties and small primary particle size (~2 nm) to the iron form found in the ferritin core (i.e., ferrihydrite) (30), and has been designed to have a benign side-effect profile by withholding any unabsorbed iron from redox activity and the gut flora (22, 30–33). Insoluble in the gut lumen (31, 32), IHAT appears poorly utilized by enteric bacteria (22); thus, it may be less irritating to the gut and less pro-inflammatory than FS. IHAT-iron appears to enter the metabolic iron pool more slowly than Fe-iron (33), suggesting a lesser post-absorptive surge in pro-oxidative NTBI. In female adults who were iron-deficient, the bioavailability of IHAT 75% that of FS (33). The European Food Safety Authority found the no observed adverse effect level of IHAT to be 231 mg (77 mg Fe) per kg body weight per day (34).
ASP (Aspiron™ Natural Koji Iron), a dried, iron-enriched Koji biomass containing 8–10% iron, was developed by Cura Global Health Inc. (Ames, IA). Koji is a GRAS constituent of food considered safe by the FAO/WHO Committee on Food Additives and (23, 24). Its bioavailability for the rat is of 60% of FS (25). Studies with female adults who were healthy and non-anemic with marginal iron stores (serum ferritin <40 μg/L) showed the absorption of ASP-Fe to be comparable to that of FS-Fe, but slower to appear in the plasma where it persisted longer, produced less NTBI, and produced fewer side effects (27, 35, 36).
Each of these agents was approved for human use. Each was encapsulated in opaque, double-zero gelatin capsules identical in size, color, and weight. This was done by the Richardson Centre for Functional Foods and Nutraceuticals, University of Manitoba (Winnipeg, Manitoba, Canada), which is licensed as a Health Canada Natural Health Product Site with the ability to produce good manufacturing practices and Hazard Analysis and Critical Control Points certified products.
2.2. Randomization
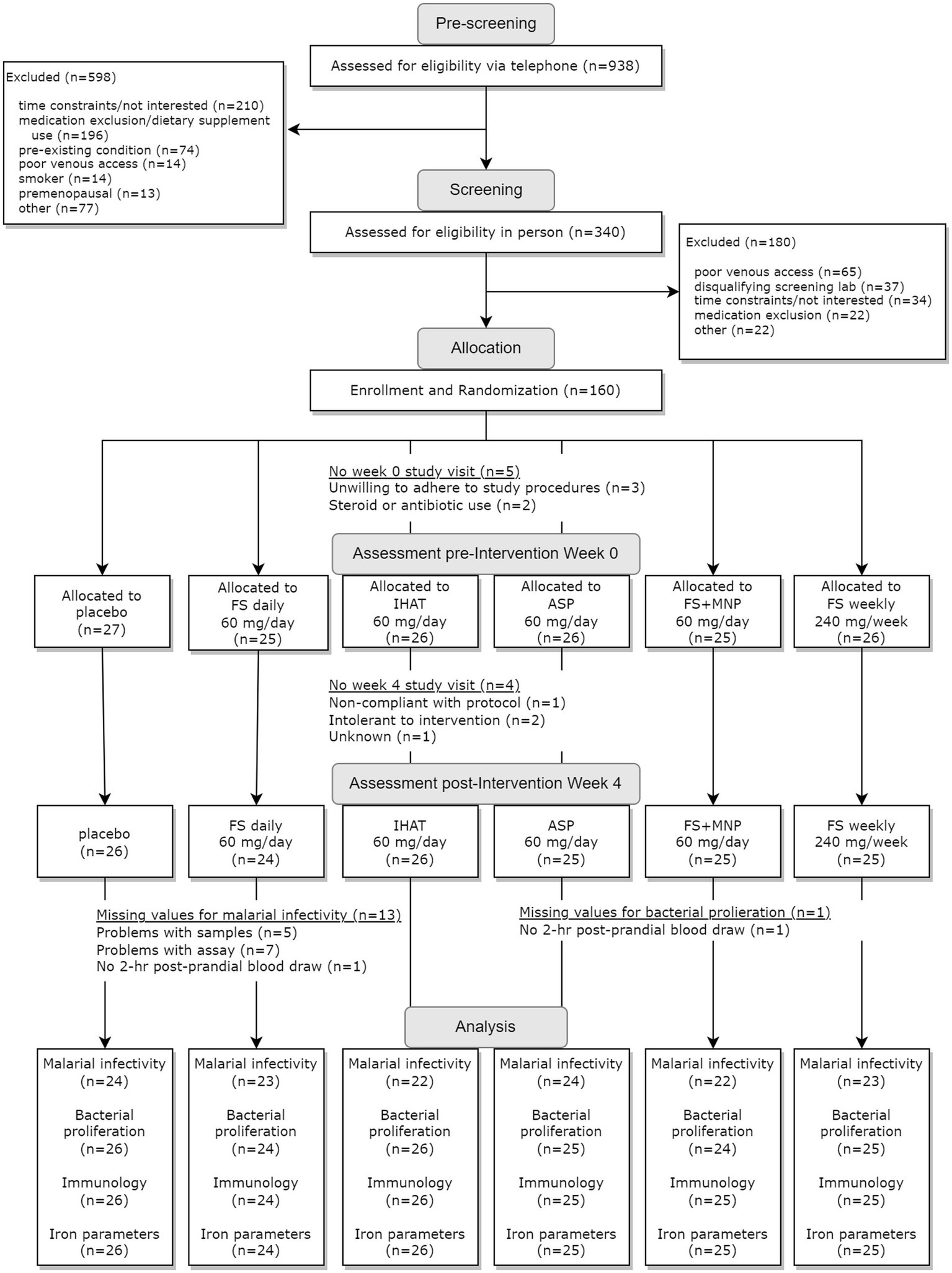
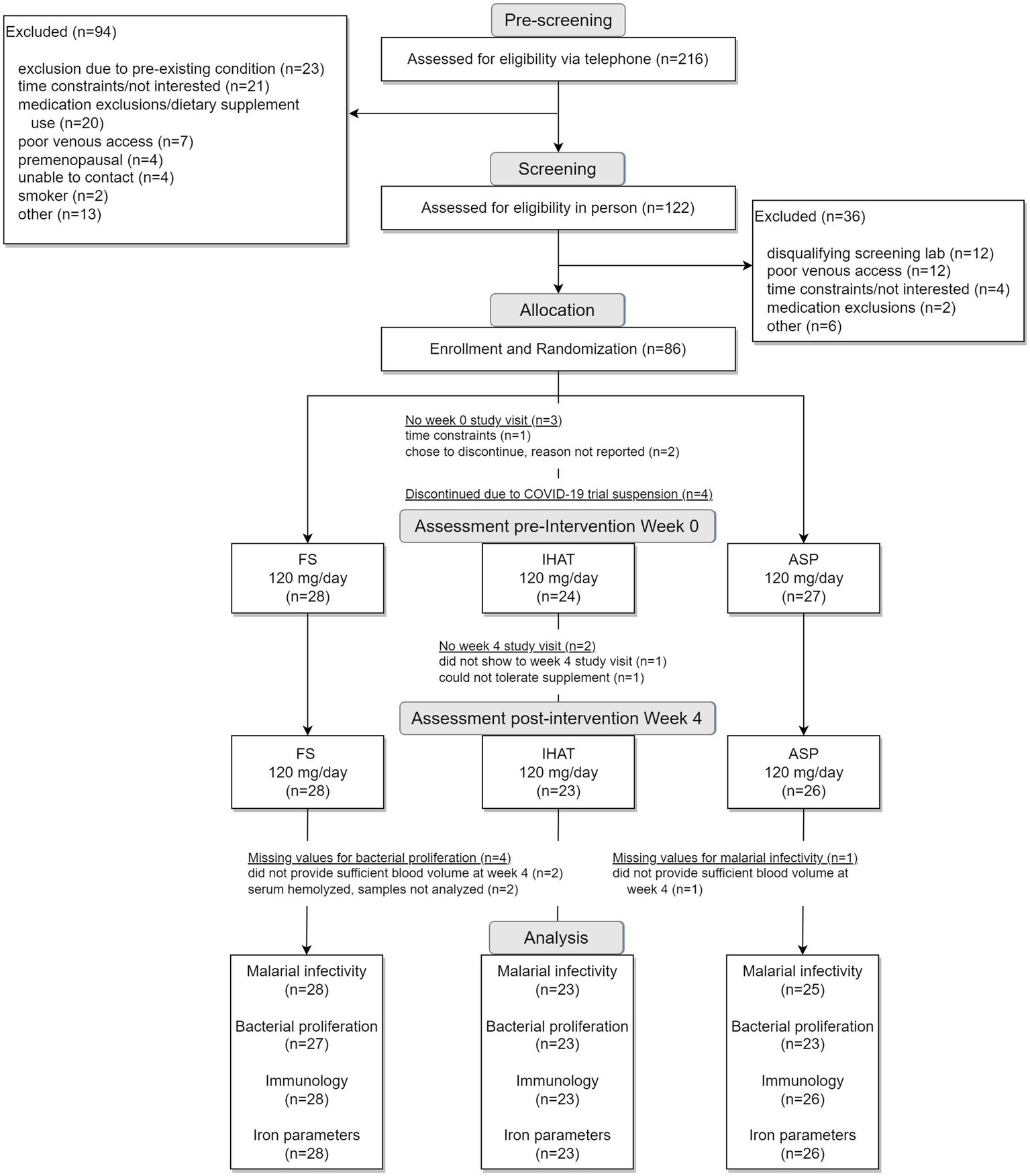
The two phases of this study were conducted sequentially, i.e., Phase I (Figure 1) was completed in its entirety prior to the initiation of Phase II (Figure 2). For each phase of the study, a randomization scheme for entering participants was generated using SIMD-oriented Fast Mersenne Twister algorithm (v. 1.5.1)-based randomization functions in the StatsBase.jl Julia package,1 stored, and known only to the data manager who held the unblinded key under lock and key data collection for the entire study. For Phase I, 160 participants were randomly assigned to one of the six treatment arms at the time they were enrolled in the study (Figure 1): placebo; FS daily (60 mg iron/d); FS weekly (420 mg iron/wk); FS + MNP (60 mg iron/d); IHAT (60 mg iron/d); and ASP (60 mg iron/d). The dose of 60 mg iron /d was based on the WHO recommendation for daily supplementation for non-anemic, pregnant females (30–60 mg iron/d) (37). A separate treatment consisting of weekly supplementation of 420 mg iron in four capsules taken once weekly; this dose regimen has been recommended as feasible, cost-effective in population-base programs (38–40), and may produce fewer gastrointestinal complaints than daily FS doses. In addition, a treatment involving the co-administration of FS and MNP was included to examine the safety of this common practice, as components of MNP, particularly ascorbic acid, have been shown to promote the enteric absorption of ferrous iron (41). The corn starch placebo provided a control for prospective changes over time, effects of daily ingesting capsules, and any time-related and/or and seasonal effects. For Phase II, 86 participants were randomly assigned to one of three treatment arms (Figure 2): IHAT (120 mg iron/d); ASP (120 mg iron/d); and FS (120 mg iron/d). All groups received four physically similar capsules to ensure blinding of the treatments. The dose of 120 mg Fe/day was based on the report of the Food and Nutrition Board (42), which suggest low adverse effects levels (constipation, diarrhea, black stools, gut irritation) of FS at that dose. Because this phase comprised direct comparisons between the three sources of iron, placebo control was not included.
At enrollment, informed consent was obtained and instructions for stool sample collection were reviewed. The participant was then randomly assigned to a treatment group and was scheduled for a baseline, pre-intervention visit (wk 0) at which a stool sample and a fasting blood sample (7 ml) were collected, and the participant was then given a standard continental breakfast (English muffin or bagel, spreads, coffee, juice, fruit excluding orange products, and teas). Immediately following the breakfast, the participant was given the assigned blinded intervention agent for the baseline (wk 0) dose. Instructions for the intervention were reviewed, and the participant was sent home with a pre-loaded calendar pack containing 90 capsules (three to be taken each day for 30 days). For the weekly FS group, treatment concealment was deemed impractical and therefore was not used; each participant received 16 capsules (four to be taken once weekly for 4 weeks). Each instructed to take all their scheduled capsules within a few minutes, was also given a compliance calendar to record capsule intake and was instructed to return the calendar with any unused capsules at the final study visit. Adherence to supplement protocol was assessed by counting the number of capsules remaining in the returned planner. Two hours after the breakfast, a second, post-prandial (PP) blood sample (7 ml) was drawn for use in bacterial proliferation assays.
2.3. Primary endpoints
The study was powered to detect treatment effects on the following endpoints:
2.3.1. Ex vivo assessment of malarial infectivity
Red blood cells (RBCs) were prepared from whole blood by three washes with RPMI-1640 (4°C), with recovery by low-speed centrifugation (500 × g for 10 min at 4°C). Packed cells were mixed with an equal volume of a freezing solution (28% glycerol, 3% mannitol, 0.65% sodium chloride); 1 ml aliquots were flash frozen and held at −80°C. For use in the invasion assay, samples were thawed with a stepwise sodium chloride gradient (43). Plasmodium falciparum 3D7 parasites were cultured in vitro in complete malaria media (CMM) containing RPMI-1640 supplemented with 0.5% Albumax II, 25 mM HEPES, 50 mg/L Hypoxanthine, 50 mg/L Gentamicin at 37°C and maintained with a gas mixture of 5% CO2, 3% O2, balanced by N2 as described previously (44). Malarial viability was confirmed by microscopic examination of blood smears.
For the invasion assay, P. falciparum schizonts were added in a 96-well plate, each well containing 5 μl of packed participant RBCs at a starting parasitemia of 0.5–1% per well. After incubation for 24 h at 37°C, when schizont concentration reached ca. 2.5% in a total volume of 200 μl CMM, parasites were fixed using a solution of 2% paraformaldehyde and 0.2% glutaraldehyde in PBS at 4°C for 45 min. Parasitemia was quantified by flow cytometry using an LSRII instrument (BD Biosciences, San Jose, CA, USA) with staining with Hoechst 33342 dye (Mafatlal Dyes and Chemicals Ltd., Mubai, Mahrashtra, India) and a 450/65 filter to measure the signal. A total of 100,000 events were captured for each measurement. Each assay was performed in duplicate and included two kinds of controls: an uninfected sample of participant RBCs, and a sample of non-frozen blood to normalize for any differences observed in parasite infectivity between invasion assays. For each participant, the 4-week change in malarial infectivity was quantified using the logarithm of the ratio of percent parasitized erythrocytes at baseline (wk 0) and post-intervention (wk 4).
2.3.2. Ex vivo assessment of bacterial proliferation potential
The potential for participant plasma to facilitate the proliferation of pathogenic bacteria was assessed ex vivo using five bacterial strains: (a) Staphylococcus aureus MW2 (45), an important cause of global morbidity and mortality (46); (b) Acinetobacter baumannii EGA50 [AB307-0294] (47), a nosocomial pathogen (48); (c) S. enterica, serovar Typhimurium (49), which can cause life-threatening bacteremia in young African children (50) and is closely related to S. enterica, serovar Typhi, an important cause of sepsis in low- and middle-income countries (51); (d) Klebsiella pneumoniae MGH 78578 (52), an opportunistic pathogen (53); and (e) extraintestinal pathogenic Escherichia coli ST131 [ExPEC] (54), an important cause of bacteremia (55).
Iron-depleted bacterial growth media was generated by adding 25 g Chelex 100 resin (BioRad #1421253) to 500 ml of LB media in a sterile container with stirring for 1 h at RT. The resin was then removed by filtration through a 0.22 μm filter. Aliquots of participant serum were heated at 55°C for 30 min to inactivate complement, and then held at −80°C until use. Each bacterial strain was grown on LB agar plates overnight at 37°C; they were harvested by scraping the plate and resuspended in 3 ml Chelex-treated LB. Bacterial suspensions were diluted in Chelex-treated LB to a final Optical density at 600 nm (OD600) of 0.5. Growth assays were performed in 96-well microtiter plates. All five bacteria were tested for growth on the same plate in sera from a single participant (1.25 ml aliquots of each pre- (wk 0) and post-supplementation (wk 4) serum were thawed at RT for 1 h prior to use). Chelex-treated LB (45 μl) was inoculated with 5 μl of each 0.5 OD600 bacterial suspension, and then mixed with 50 μl participant serum in triplicate wells each of which were then covered with 50 μl light mineral oil to minimize evaporation and cross contamination. Covered plates were a continuously slow shaking plate reader at 37°C. OD600 was determined then and subsequently at 20 min intervals for 18.5 h. Three controls were included on each plate: growth in iron-replete LB to confirm bacterial viability and growth consistency; participant serum alone with Chelex-treated LB to confirm serum sterility; and wells containing either LB or Chelex-treated LB to normalize OD readings and assess well-to-well cross contamination. Contamination was rare and never resulted in data elimination.
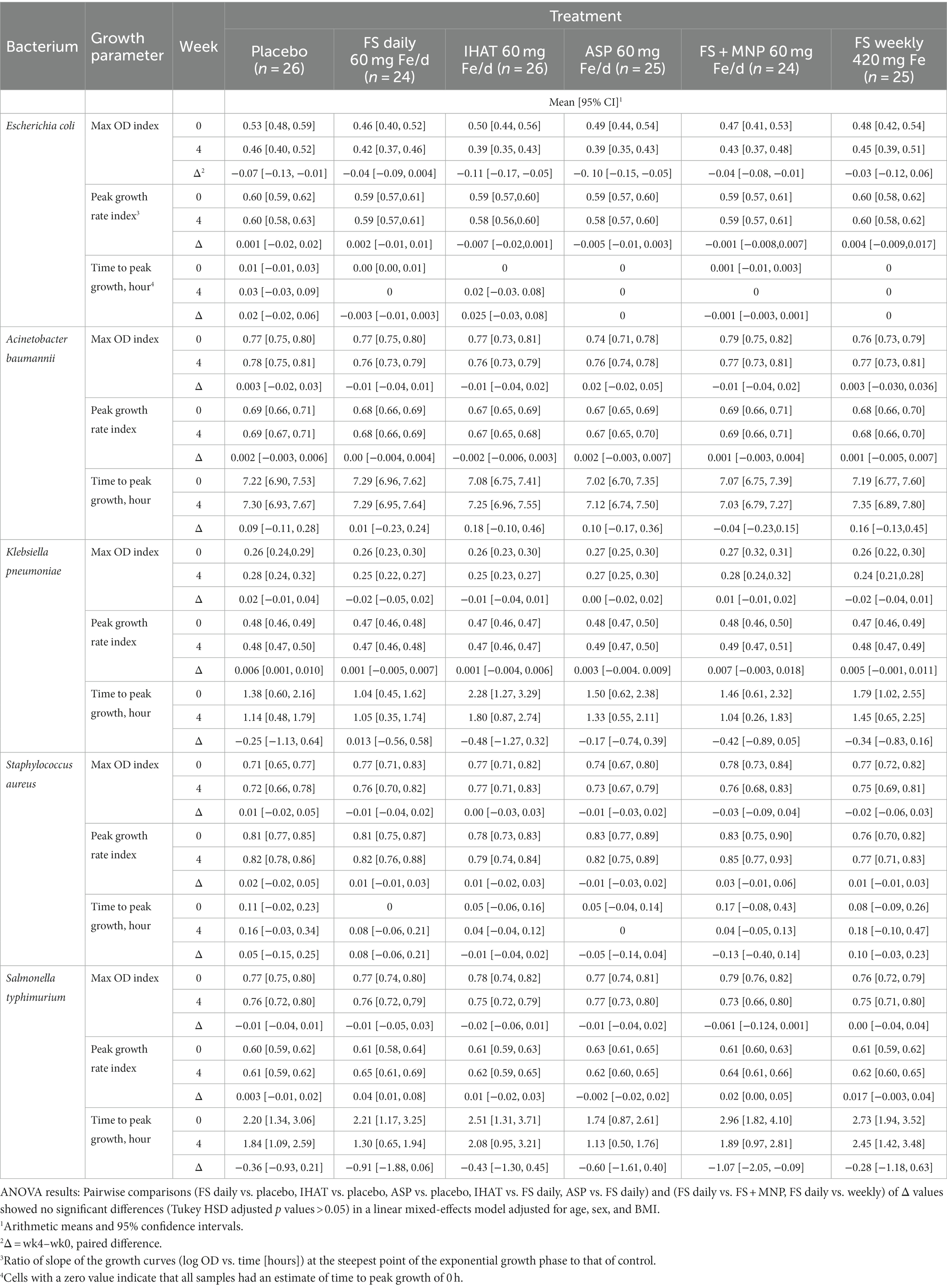
Bacterial proliferation data were modeled using polynomial growth curves similar to those previously reported (56). Eighteen-hour growth curves were fit from the mean OD of three replicates measured every 20 min, with OD expressed in natural logarithm. Fourth degree polynomials were fit using ordinary least squares. Three growth parameters were calculated from the estimated curves: maximum OD (max OD): the normalized maximum optical density reached after the exponential growth period, peak growth rate index (the normalized rate during the exponential growth period, i.e., the steepest slope of the growth curve in the log OD vs. time plot), and time to peak growth (in hours). Max OD and peak growth rate are expressed as indices to indicate the values were normalized. Control values on each plate were used to normalize growth parameters to account for day-to-day variation and utilization of different plate readers, except for time to peak growth due to a high proportion of zero values.
2.3.3. Markers of gut inflammation
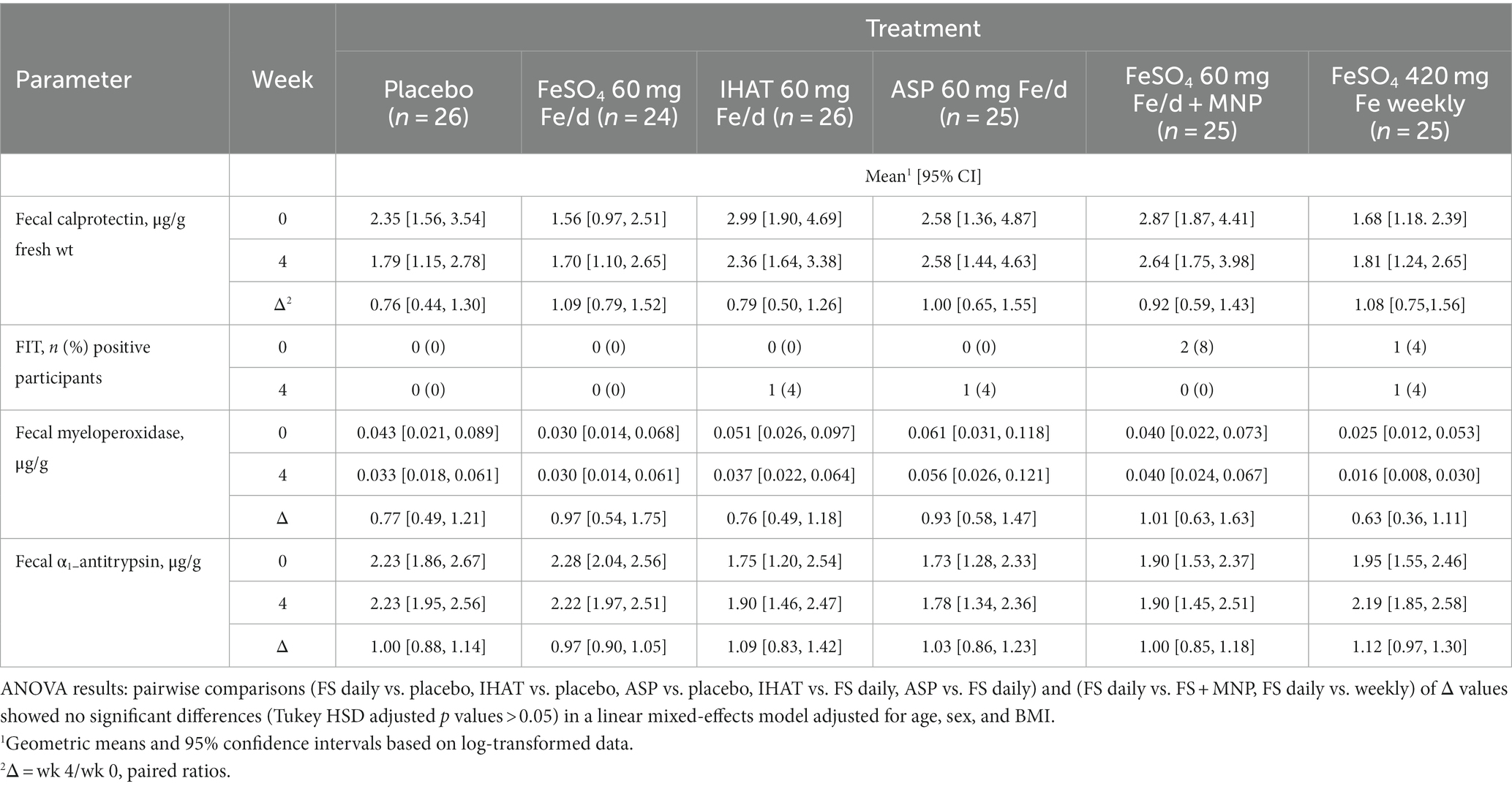
Gut inflammation was assessed using multiple fecal biomarkers of intestinal inflammation. Participant stool samples were collected in plastic bags, cooled with frozen gel packs, and homogenized and extracted within 24 h using phosphate-buffered saline containing 1 mM phenylmethylsulfonyl fluoride, 1% bovine serum albumin, and 0.05% Tween-20. Extracts were held at −80°C until analysis. Sandwich ELISA kits were used to determined fecal concentrations of calprotectin (R&D Systems, Minneapolis, MN), myeloperoxidase (R&D Systems, Minneapolis, MN) and α-1-antitrypsin (Cat. #: DY1268, R&D Systems, Minneapolis, MN). The fecal immunochemical test (FIT) for occult blood in stool samples (57) was performed by the Hematology Laboratory, Tufts Medical Center.
2.4. Secondary endpoints
Also addressed were the following secondary endpoints for which the study was not powered:
2.4.1. Assessment of general health status and dietary patterns
In each phase, the health of each participant was monitored weekly. The study physician (JBM), a practicing gastroenterologist, was available for support. Each participant was also given a 24 h dietary recall questionnaire administered weekly by telephone (Figure 3). Recalls were analyzed by the multiple-pass 24-h recall method, using the Nutrition Data System for Research software v. 2016 and v. 2020 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN).
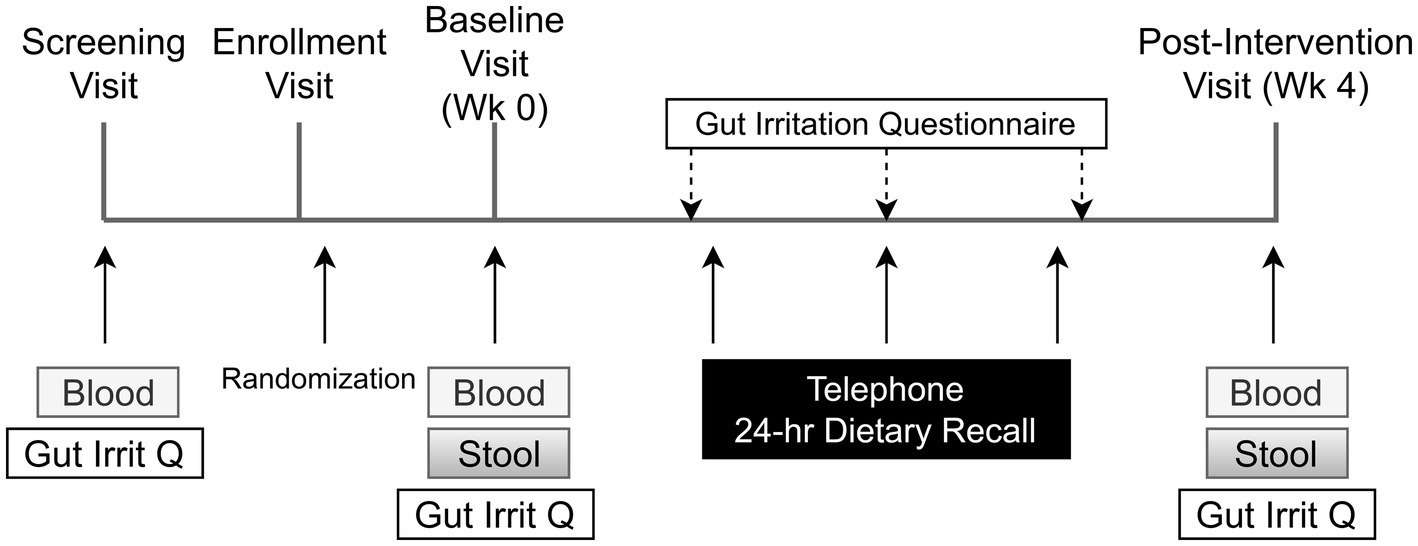
Figure 3. Schedule of subject activities in both phases of the study. The title of each figure is shown above. Figure legends are not applicable.
2.4.2. Assessment of iron status
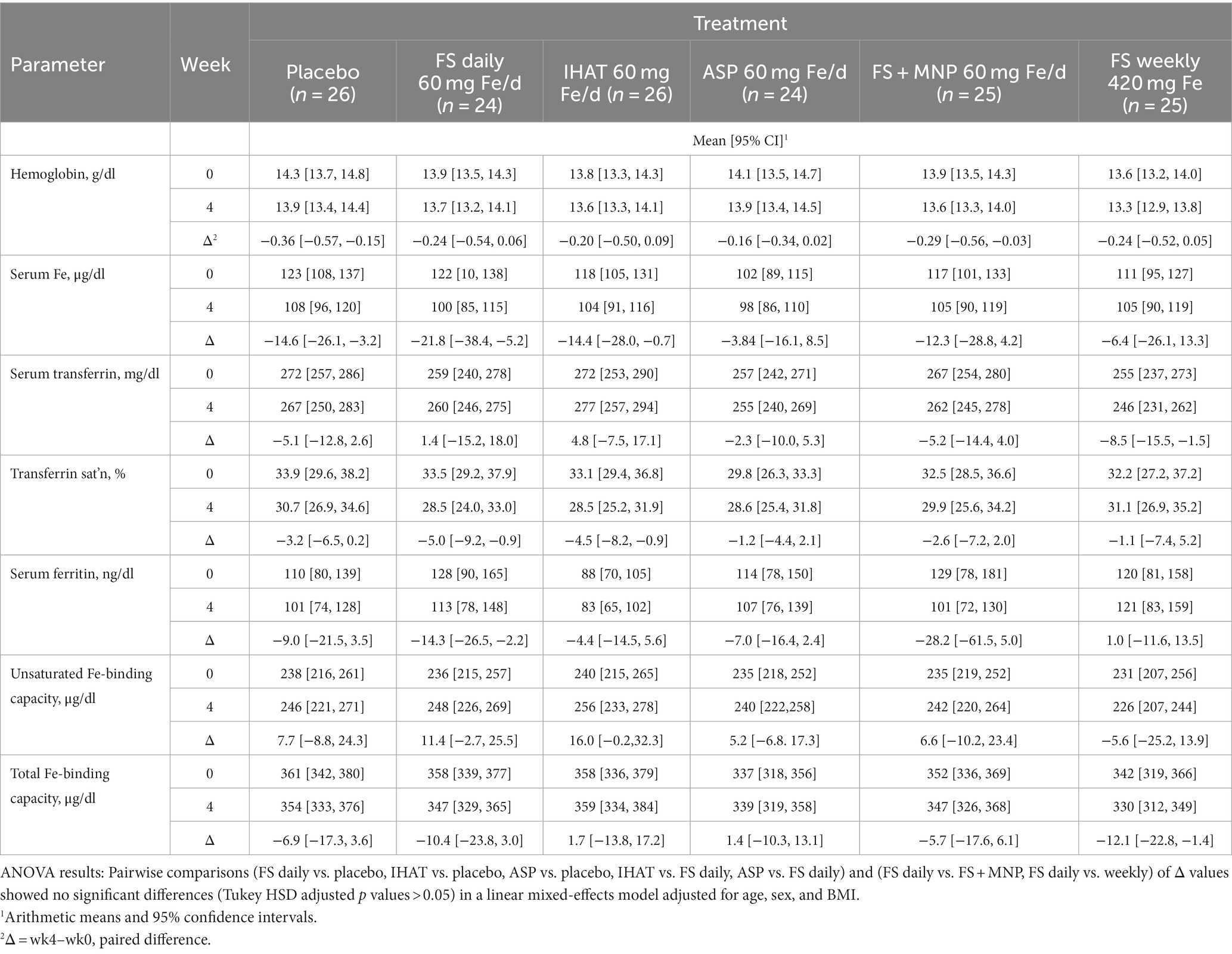
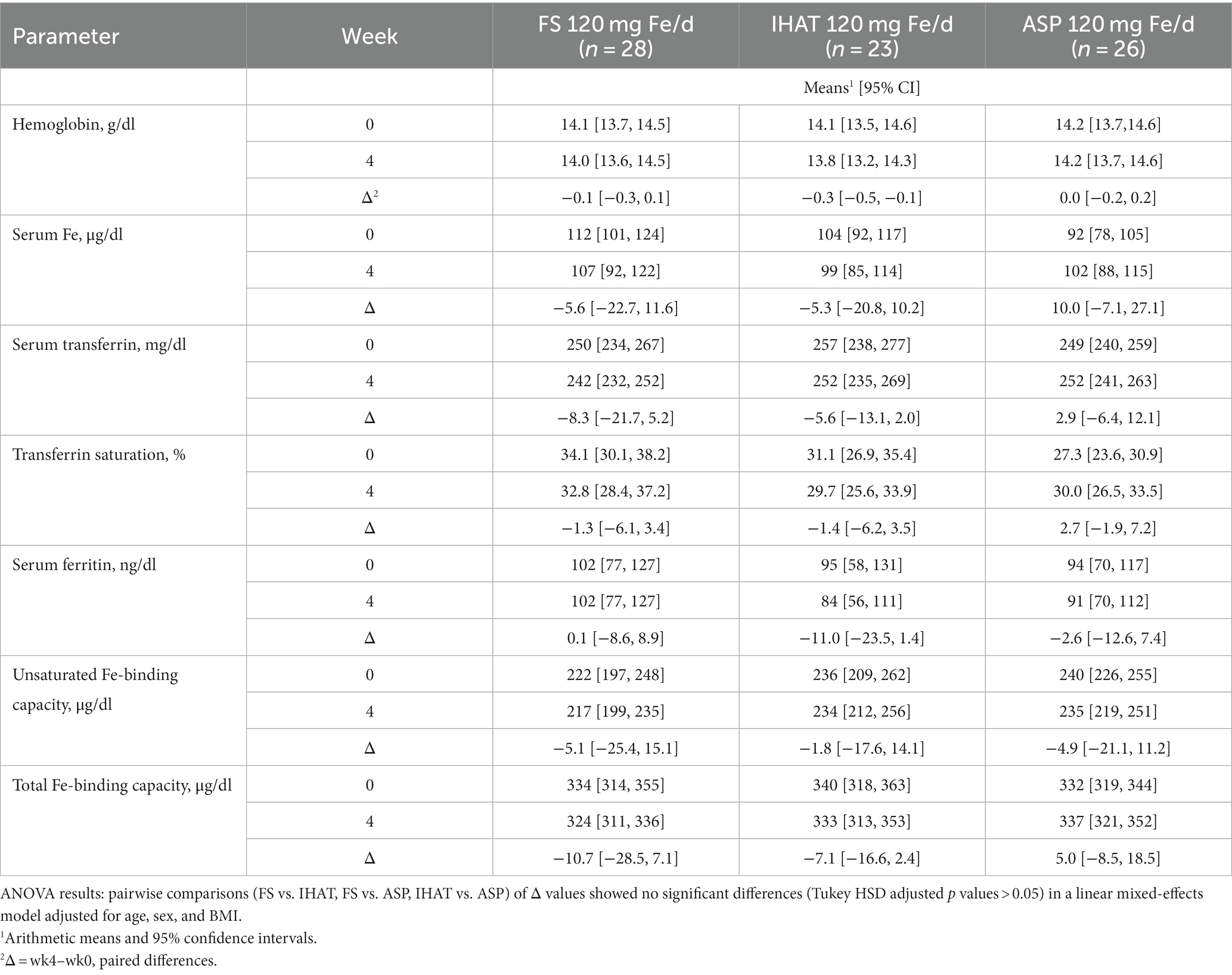
Hemoglobin and hematocrit were determined in whole blood as part of a 20-parameter hematology profile determined by cytochemistry, impedance, and analysis of cellular structure by light absorbance using a hematology analyzer (Pentra 60c + Hematology Analyzer, HORIBA ABX SAS, HORIBA Instruments Incorporated, Albany, NY). Serum iron was measured by an endpoint colorimetric procedure (58) using a clinical chemistry analyzer (AU480 Clinical Chemistry Analyzer, Beckman Coulter, Inc., Brea, CA). Unsaturated iron binding capacity in serum was measured by a colorimetric procedure using a clinical chemistry analyzer (AU480 Clinical Chemistry Analyzer, Beckman Coulter, Inc., Brea, CA). Average intra- and inter-assay CVs were less than 4.0 and 6.0%, respectively. Total iron binding capacity (TIBC) was determined from the total serum iron and unsaturated iron binding capacity measured using a clinical chemistry analyzer (AU480 Clinical Chemistry Analyzer, Beckman Coulter, Inc., Brea, CA). Transferrin was determined in serum by an immunoturbidimetric assay using a clinical chemistry analyzer (AU480 Clinical Chemistry Analyzer, Beckman Coulter, Inc., Brea, CA); transferrin saturation was calculated from the relationship of serum iron to total iron-binding capacity. Average intra- and inter- assay CVs were <2.5 and <3.0%, respectively. Ferritin was measured in serum by solid-phase, two-site chemiluminescent immunometric assays using the IMMULITE 2000 (Siemens Healthcare Diagnostics, Los Angeles, CA) according to Babson (59). The average intra- and inter-assay CVs were less than 5 and 6%, respectively.
2.4.3. Assessment of gastrointestinal symptoms
A questionnaire designed and validated to address symptoms of gastrointestinal irritation after FS supplementation (60) was administered after the initial fasting blood draw and again weekly by telephone.
2.5. Data management
Data were managed using a REDCap (61) database and handled in compliance with HIPAA and 21CFR11. Participant identifiers were recorded in separate electronic case reports and were maintained separately from research data. Each participant’s identity and records of telephone pre-screening, final screening, and study data were kept in locked files or eCRF, with only the PI (GFC), Study Physician (JBM), and Study Coordinators having access to participant identifying information if needed. Otherwise, only de-identified data were made available to study co-investigators. Data entered into the database underwent external validation checks; meta-data files and data dictionaries were used to provide information necessary for proper use and understanding of the data files. Laboratory specimens were identified by unique study codes; the master list linking the identities of participants and specimens was kept in a locked file and on a separate, password-protected database on a secure server. REDCap was used for edit resolution; trouble shooting data entry problems; an audit trail of database editing; performing range checks for cleaning, daily backup, cleaning of transitional databases; and, ultimately, transferring master databases for statistical analysis. At key points in data acquisition, clean-up and analysis, an additional off-site copy of the data was stored using the LabArchives electronic laboratory notebook software (LabArchives LLC, San Marcos, CA, USA). Access to the study database was restricted until the study was completed and unblinded. Protected participant health information was automatically de-identified for all viewing and data exports.
2.6. Statistical methods
The sample sizes used in both phases were based on power calculations for each primary outcome as previously described (26). For malarial infectivity, a standard deviation (SD) of 0.202 was estimated from published studies with P. falciparum infectivity (62). Based on an independent two-sample t-test with 0.05 type I error, it was determined that 25 participants per group would yield 90% power to detect a 20% reduction in parasite infectivity. For bacterial proliferation, between-participant estimates of SD for doubling time during the exponential phase of growth were estimated for several species from published data (59). This indicated that, with 25 participants per treatment group and 0.01 type I error (adjusted for planned analyses with five species), power was >0.99 for a minimal detectable difference in doubling time of 1 h. Mean fecal calprotectin was reported previously to be 1.9 μg/g in participants with minimal inflammation (irritable bowel syndrome) and 27.6 μg/g in participants with mild inflammation (collagenous colitis) with a SD of 1.3 in log concentration (63). A sample size of 25 participants per group was estimated to allow the detection of a 3.5-fold change in fecal calprotectin values with 90% power at a type I error level of 0.05.
An intention-to-treat analysis was performed with all participants according to their randomization assignment. Analysis excluding participants with low supplement adherence was also performed, however did not substantially affect results; thus, the results from the intention-to-treat approach are presented. Participant characteristics and dietary intake were summarized for each intervention group using mean, median, or frequency, along with SD, range, or interquartile range, as appropriate. Statistical testing was structured to test the study hypotheses and objectives in a series of planned pair-wise comparisons. The formal hypotheses of the study (26) were tested by evaluating the following outcomes: metabolic responses as assessed by biomarkers of iron status; the susceptibility of participant RBCs to infection by P. falciparum as assessed ex vivo; the ability of participant plasma to facilitate proliferation of selected species of pathogenic bacteria as assessed ex vivo; and inflammatory responses as assessed by fecal biomarkers of inflammation. Treatment effects were evaluated by the following sets of pairwise comparisons:
• Phase I (six treatments each providing 60 mg Fe/day):
New iron forms: IHAT vs. FS; ASP vs. FS; FS vs. placebo; IHAT vs. placebo; and ASP vs. placebo
Modes of administration: FS daily vs. FS + MNP daily; and FS daily vs. FS weekly
• Phase II (three treatments each providing 120 mg Fe/day):
New iron forms: IHAT vs. FS; ASP vs. FS; IHAT vs. ASP
An alpha level was designated for each set of comparisons to achieve a familywise error rate of 0.05 for each outcome. Models were fit using the lmer4 package in R v. 4.2 and p values from pairwise comparisons were adjusted based on Tukey’s HSD method applied in the emmeans package v. 1.7.4-1 (64). Four-week changes were assessed using linear models adjusted for age, sex, and BMI. A participant-specific random effect was added to the models for outcomes that were assessed both at fasting and 2-h PP. Additionally, noninferiority tests were performed for the primary outcomes in Phase I. These tests were not prespecified but were added to the analysis to characterize comparisons of the supplemental iron forms to FS daily, given the interpretation of these comparisons as one-sided equivalence hypotheses. A conservative noninferiority margin was set at 50% of the 4 wk. change between placebo and FS daily. Data were evaluated graphically to assess modeling assumptions including outlier detection, normality, and linearity with covariates. Log transformations were used when appropriate to satisfy normality assumptions.
3. Results
3.1. Results of phase I
3.1.1. Participants
The Phase I recruitment scheme is shown in Figure 1. This produced a cohort of 160 participants comprised of mostly college-educated females and males aged 50–80 yrs. (Table 1), recruited between June 2016 and June 2019. During the study, participants consumed ca. 1940 kcal/day, including ca. 78 g/day of protein more than half of which was from foods of animal origin. The estimated dietary iron intakes of participants were 12–15 mg/day (Table 1). Of the 151 participants that completed the study, 142 reported the number of returned capsules. Adherence is reported as the number of participants with an intake of at least 80% of the total provided supplements based on returned capsules. All participants on daily supplementation regimens reported taking >80% of the allocated capsules during the 4-week study, whereas 23 of the 25 participants in the weekly supplementation group reported >80% adherence.
3.1.2. Primary endpoints
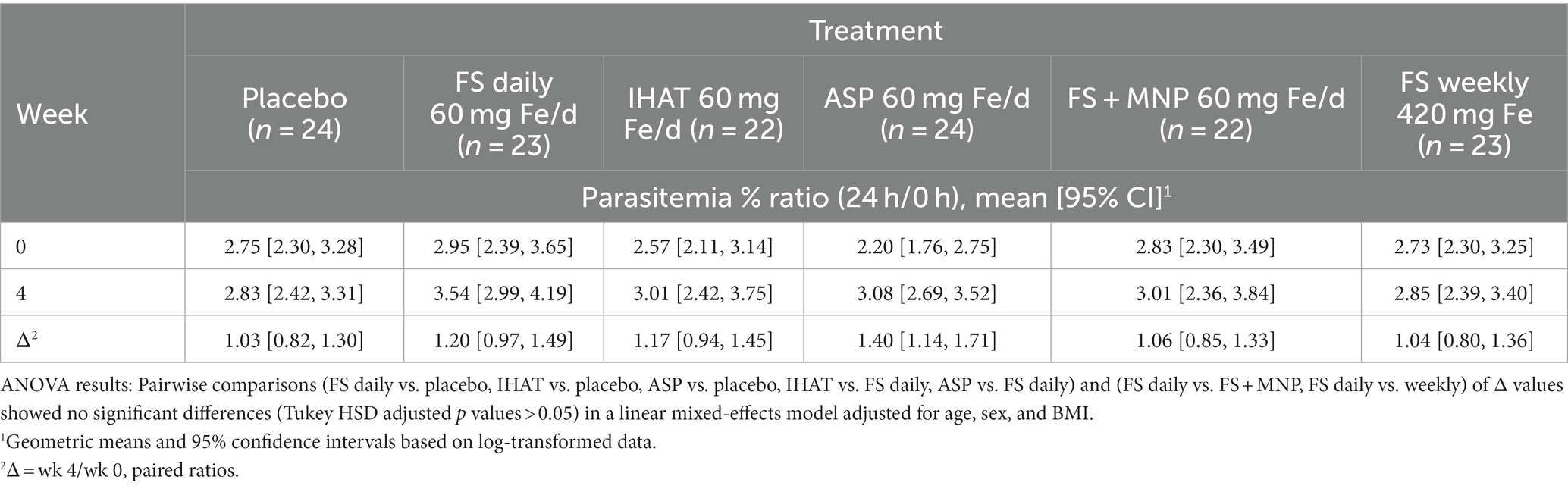
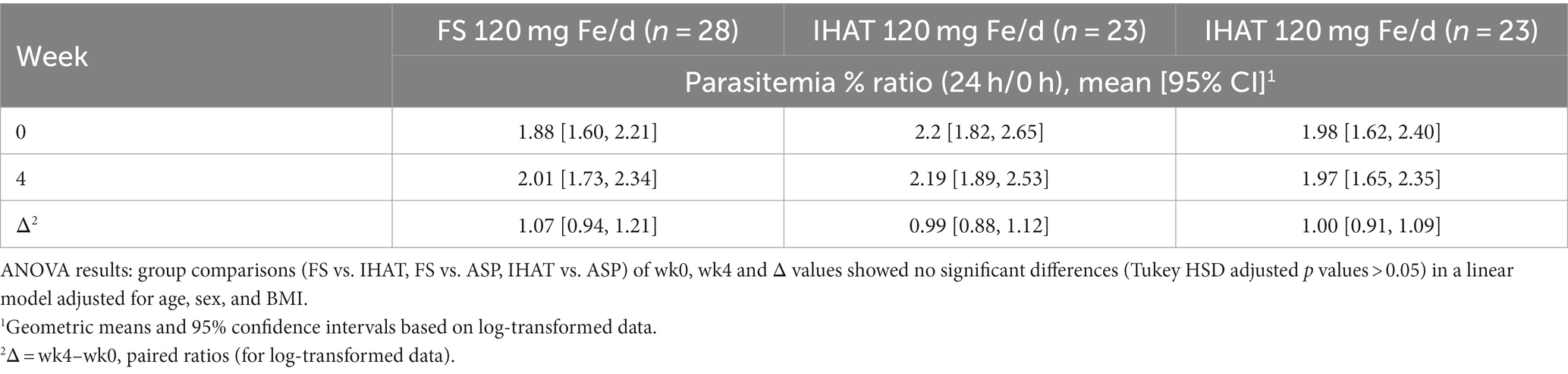
a. Ex vivo malarial invasion. No form of Fe affected the 28-day change in ex vivo susceptibility of participant erythrocytes to P. falciparum parasitemia, and that the mode of administering FS (daily v. weekly) had no effect on that endpoint (Table 2). None of the supplemental iron groups showed evidence of noninferiority to FS daily (Supplementary Table 2).
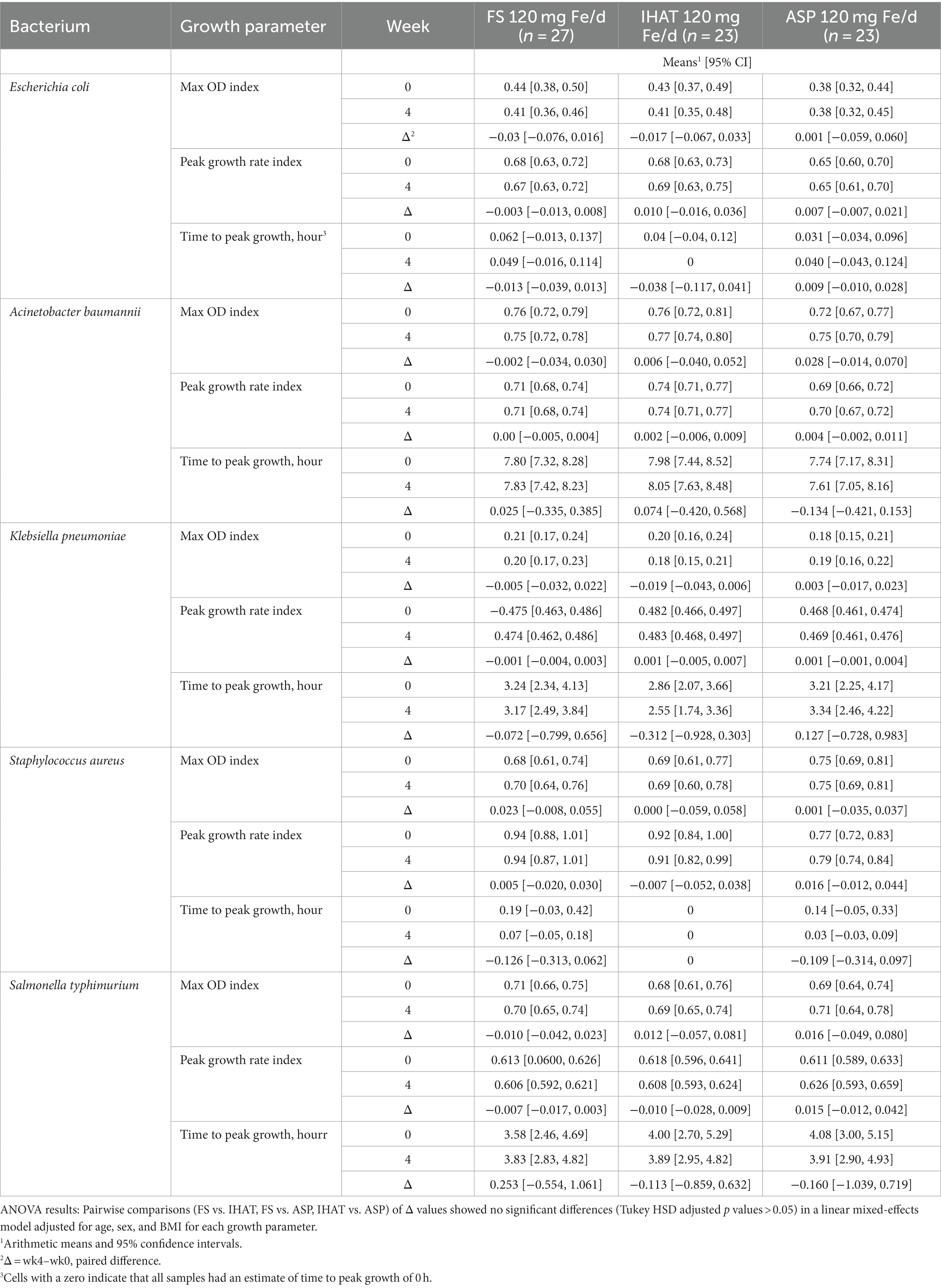
b. Ex vivo bacterial proliferation. No form of iron affected the ex vivo proliferation of E. coli, K. pneumoniae, S. aureus, or S. typhimurium when assessed in participant fasting plasma (Table 3). However, when assessed in 2-h PP sera, the 4-week changes in max OD and peak growth rate index for Acinobacter baumannii were significantly different (p = 0.041 and p = 0.015, respectively) for participants given ASP as compared to those given FS; these data are included in the Supplementary Data (Supplementary Table 1). For peak growth rate in E.coli and S. typhimurium, a noninferiority margin was established using data from the placebo group. IHAT was shown to be noninferior to FS daily for both E.coli (p = 0.049 for noninferiority) and S. typhimurium (p = 0.005 for noninferiority). Additionally, noninferiority of ASP, FS + MNP and FS weekly were statistically significant for S. typhimurium (Supplementary Table 2).
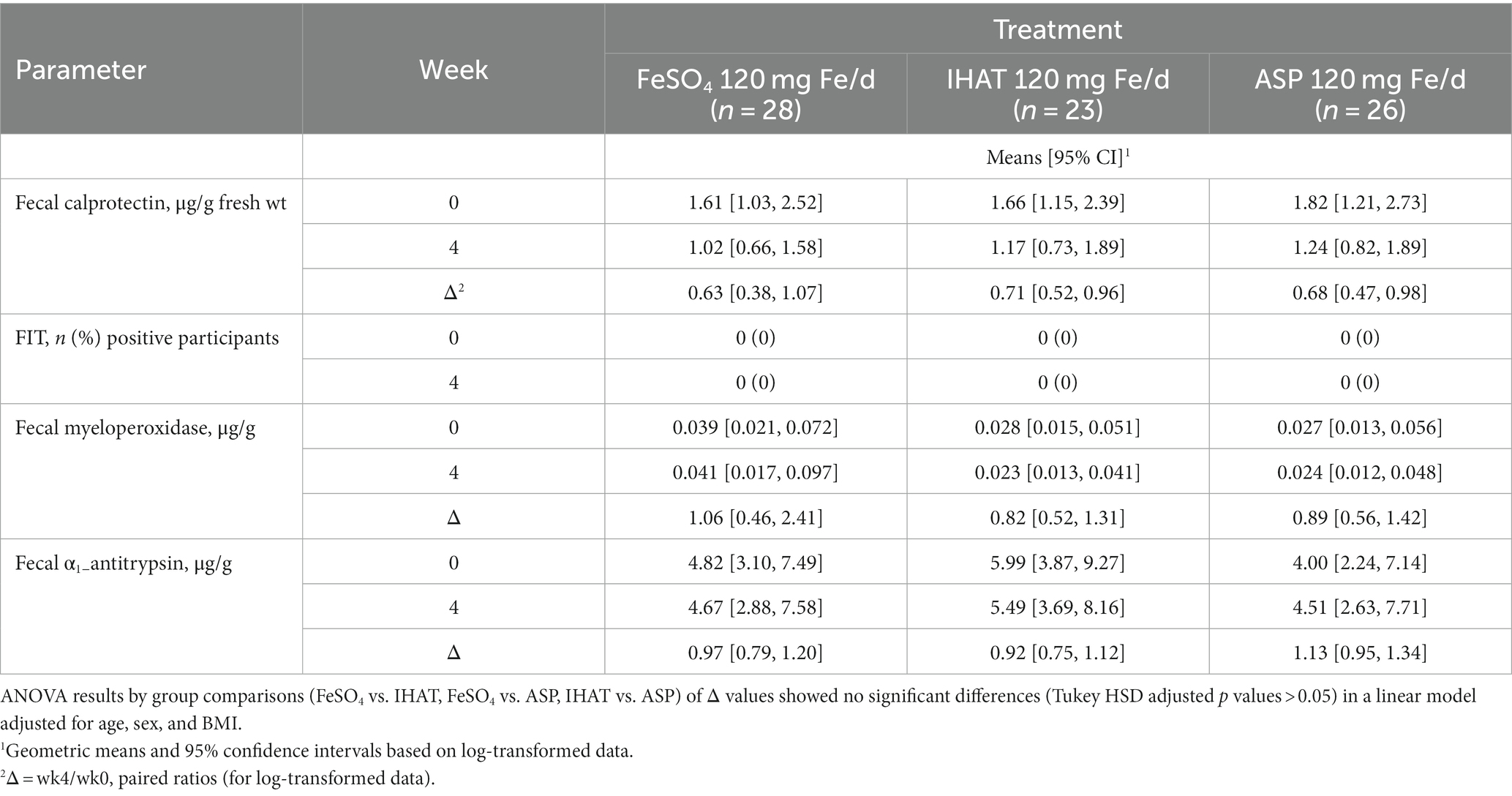
c. Gut inflammation. No form of Fe affected the 4-week changes in any markers of gut inflammation (Table 4). Moreover, none of the markers at week four in any intervention groups were significantly different than that observed in the placebo group. IHAT was shown to be noninferior to FS daily for fecal calprotectin (p = 0.04 for noninferiority).
3.1.3. Secondary endpoints
a. Iron Status. No form of supplemental Fe significantly affected any parameter of iron status assessed in fasting blood samples over the 4-week intervention period (Table 5). The adjusted mean difference between ASP and placebo was 0.17 g/dl [95% CI: −0.28, 0.61]. The four-week change in fasting serum iron was not significantly different for FS weekly compared to FS daily, with an adjusted mean difference of 23.2 μg/dl [95% CI: −15.4, 61.8].
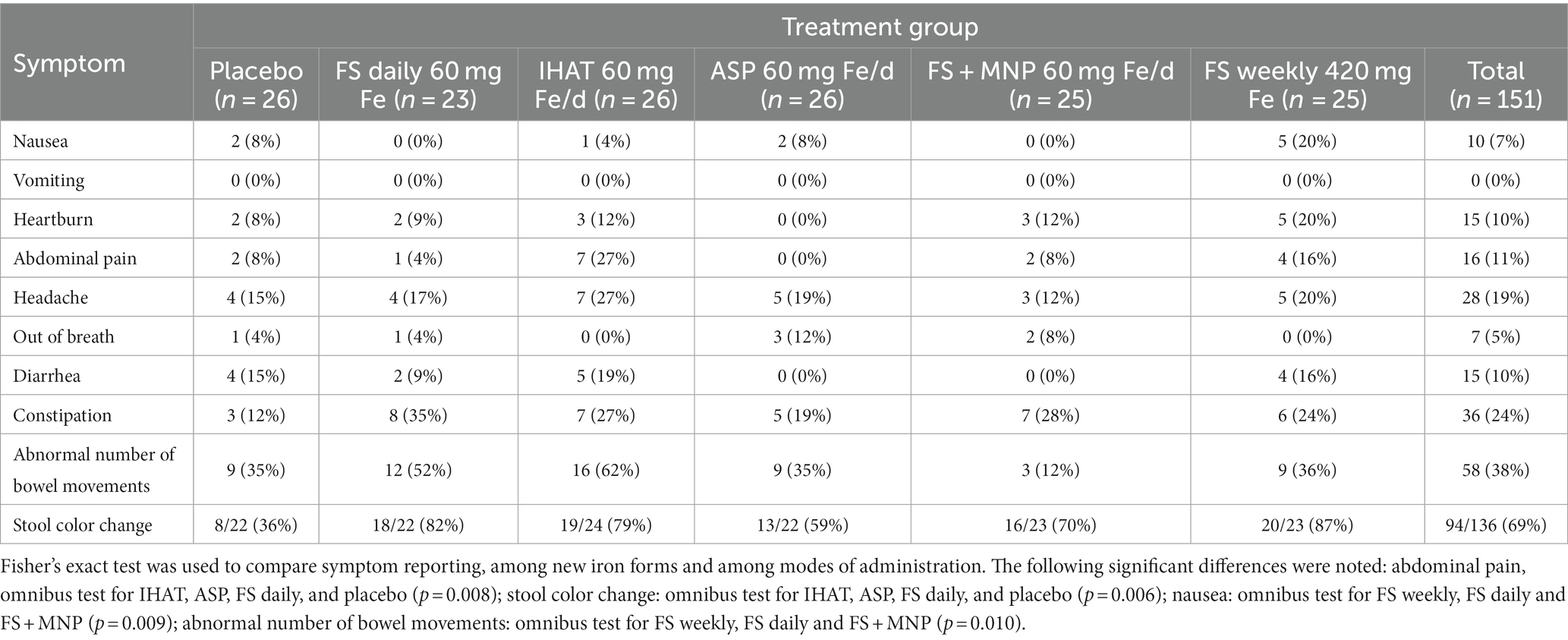
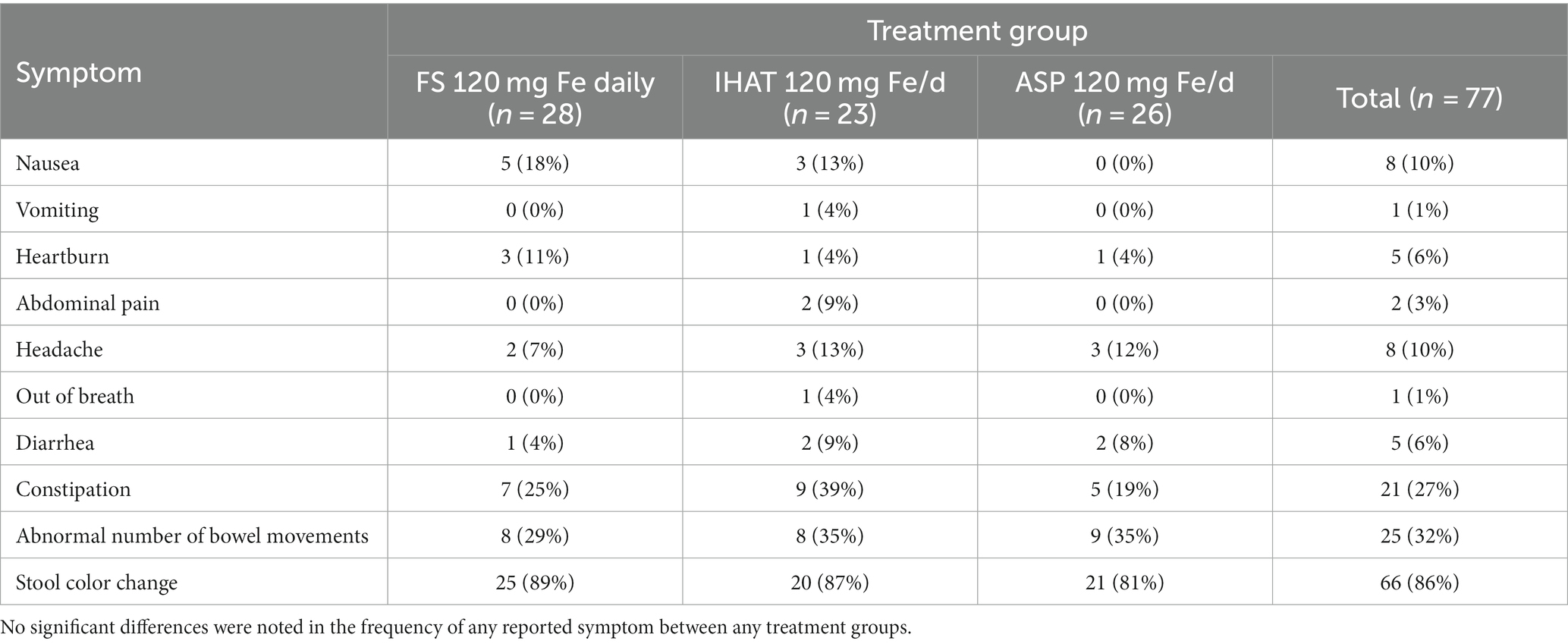
b. Gastrointestinal Symptoms. Most (81%) participants reported at least one symptom, which were mostly of mild intensity and inconvenience. The leading symptom was change in stool color, reported by 69% of participants, followed by abnormal number of bowel movements (38%) and constipation (24%) (Table 6). The frequencies of these symptoms did not differ between iron treatments except for abdominal pain (omnibus test for IHAT, ASP, FS daily, and placebo, p = 0.008); stool color darkening (omnibus test for IHAT, ASP, FS daily, and placebo, p = 0.006); nausea (omnibus test for FS weekly, FS daily and FS + MNP, p = 0.009); and abnormal number of bowel movements (omnibus test for FS weekly, FS daily and FS + MNP, p = 0.010). Upon review by the study physician (JBM), the few instances of FIT positivity were not found to have corresponding anemia or abdominal pain.
3.1.4. Adverse events
Two participants reported adverse events. One participant, assigned to the placebo, was withdrawn from the study due to discomfort in taking the placebo. Another, receiving ASP, experienced diarrhea on intervention days 8 and 9, although that participant’s report on the Gut Irritation Questionnaire on day 8 was normal. Out of an abundance of caution, that participant was dropped from the study.
3.2. Results of phase II
3.2.1. Volunteers
The Phase II recruitment scheme is shown in Figure 2. This produced a cohort of 86 volunteers comprised of mostly college-educated females and males aged 50–80 years (Table 7), recruited between June 2019 and October 2021. A total of 61 of these had also participated in Phase I but had completed that phase at least 1-year prior to enrolling in Phase II. During the study, participants consumed ca. 2,169 kcal/day, including ca. 85 g/day of protein more than half of which was from foods of animal origin. Their estimated dietary iron intakes were 14–16 mg/day (Table 7). Of the 79 participants that completed the study, 77 reported the number of returned capsules. All participants reported taking >80% of the allocated capsules during the study.
3.2.2. Primary endpoints
a. Ex vivo malarial invasion. No form of iron administered at the level of 120 mg Fe/day affected the susceptibility of participant erythrocytes to P. falciparum infection (Table 8).
b. Ex vivo bacterial proliferation potential. No form of iron administered at the level of 120 mg Fe/day affected the proliferation of any bacterium in participant plasma (Table 9).
c. Gut inflammation. No form of Fe affected the 4-week changes in markers of gut inflammation (Table 10).
3.2.3. Secondary endpoints
a. Iron Status. No form of supplemental Fe significantly affected 4-week changes in any parameter of iron status assessed in fasting blood samples, including transferrin saturation, which remained 30–40% (Table 11). The four-week changes in transferrin saturation for between ASP and FS (adjusted mean difference of 4.32% [95% CI: −3.82, 12.46]), and between ASP and IHAT (adjusted mean difference of 4.24% IHAT [95% CI: −4.23, 12.72]) were not significantly different.
b. Gastrointestinal Symptoms. Most (87%) participants reported at least one symptom, which were mostly of mild intensity and inconvenience. The most frequently reported symptom was stool color change (86% of participants), abnormal number of bowel movements (32%), and constipation (27%) (Table 12). The presence of all symptoms declined in the last (4th) week. There was no significant difference in the frequency of reporting of any symptom between the intervention groups.
3.2.4. Adverse event
After 2 weeks of supplementation, one participant experienced moderate nausea 30–40 min of taking ASP. This was accompanied by constipation and irregular bowel movements. The supplement was discontinued, the participant was dropped from the study and was advised to contact the study physician. This participant did not participate in the Phase I study.
4. Discussion
The goal of the Safe Iron Study was to examine the safety of two novel forms of iron, IHAT and ASP, in comparison to that of FS at two levels of supplementation, 60 and 120 mg/day. In addition, the effects of daily vs. weekly FS dosing were compared, as were the effects of co-administration of MNP with FS.
4.1. Primary endpoints
a. Susceptibility to malarial infection. The magnetic isolation of mature parasites yielded nearly homogeneous populations of schizonts without complications of uninfected erythrocytes. Schizont morphology, as examined microscopically, showed no effects of participant iron supplementation; merozoites released from purified schizonts were able to invade erythrocytes directly. That merozoites were also able to re-invade participant erythrocytes (data not shown), demonstrated normal development of parasites under these conditions. That no form or level of iron affected malarial invasion of participant erythrocytes (Tables 2, 8) suggests that iron supplementation of these iron-replete adults did not affect their apparent risk to clinical malaria infection. This finding is consistent with minimal effects on plasma NTBI, which has been proposed to increase the intensity of malarial infections (19, 20). Still, the possibility that the ex vivo assay may not fully reflect susceptibility to infection in clinical settings cannot be excluded. This study did not address the role in the malarial life cycle of hepatocytes in which schizonts are produced from sporozoites delivered by Anopheles injection into the bloodstream. Therefore, these results cannot be taken as dispositive of the hypothesis that iron supplementation of young iron-replete individuals can enhance clinical malarial infection as proposed by Clark et al. (62).
b. Bacterial proliferation potential. The ex vivo bacterial growth assays performed in this study showed no significant effects of iron supplementation (Tables 3, 9). These findings differ from those of Cross et al. (59), who found oral supplementation of participants of marginal iron status with FS (2 mg/kg) to increase, within 4 h, the ex vivo growth of pathogenic bacteria (E. coli, Yersinia enterocolitica, S. enterica serovar Typhimurium, and Staphylococcus epidermidis) in participant sera. Those ex vivo responses were strongly correlated with increase in transferrin saturation in all cases except Staphylococcus aureus, which is known to scavenge heme iron and did not respond to participant iron supplementation. In the present study, no treatment effects were observed on transferrin saturation or any other parameter of iron status. Nevertheless, significant increases in max OD and peak growth rates of E. coli, K. pneumoniae, S. aureus, S. typhimurium, and A. baumannii were observed within 2 h of the first weekly dose of 420 mg Fe as FS. IHAT was shown to be noninferior to FS daily in both E. coli and S. typhimurium, and the other iron supplementation groups were shown to be noninferior to FS daily in S. typhimurium. Short-term responses were not observed for any treatment over the 4-week course of supplementation. Such null results are consistent with iron-replete participants having low enteric absorption of iron, as indicated by their null transferrin saturation responses in both phases of the study (Tables 5, 11). We did, however, note a significant difference in the 4-week changes in proliferation potential of A. baumannii for participants given ASP as compared to those given FS; however, that difference was noted only when assessed in PP samples (see Supplementary Data, Supplementary Table 1).
c. Gut inflammation. Markers of gut inflammation revealed no significant impacts of iron supplementation on the gut inflammatory tone (Tables 4, 10), and noninferiority of IHAT was shown for fecal calprotectin. This suggest that unabsorbed iron from moderate to high levels of supplementation do not promote intestinal inflammation in healthy, iron-replete adults, and that markers of intestinal inflammation are not useful indicators clinical symptoms of abdominal pain and constipation that can be experienced with iron supplements.
4.2. Secondary endpoints
a. Iron status. The participants in both phases of this study were iron-replete, as indicated by their normal hemoglobin levels, their estimated dietary iron intakes, which exceeded the recommended daily allowance (8 mg) (42), and their baseline values of biomarkers of iron status (Tables 5, 11). In no case did any form of supplemental iron, at either the 60 mg/day or 120 mg/day dose level, increase iron status (e.g., transferrin saturation remained ca. 30%). That no increase in serum iron or transferrin saturation was detected 2 h after iron dosing (data not shown), is as expected for iron-replete individuals within the timeframe of these studies, suggesting that absorption was low for all iron supplements and implying that iron treatment did not increase NTBI in the plasma (19) and that lower gut exposure to non-absorbed iron was significant.
b. Gastrointestinal symptoms. Most participants reported changes in stool color (darkening); some reported abnormal number of bowel movements and/or constipation neither of which they considered more than mildly inconvenient (Tables 6, 12). In Phase I, participants receiving FS, IHAT or ASP more frequently reported stool color changes than the placebo group. Those receiving IHAT more frequently reported abdominal pain than the FS or ASP groups. Reports of abnormal bowel movements associated with daily supplementation with FS were fewer for participants also receiving MNP and those taking FS weekly in phase I; no treatment differences were observed in phase II. In Phase I abdominal pain was reported by 7 participants (27%) taking IHAT; however, in phase II, in which iron doses were doubled, abdominal pain was reported by only 2 participants (9%) both of whom were taking IHAT. The profile of reported gastrointestinal symptoms is consistent with that expected for oral iron supplements, while those associated with FS were lower than expected from other studies (65). Three participants were withdrawn from the study due to adverse events including abdominal pain, nausea, and constipation; as most of these cases involved non-specific symptoms, these low numbers may not be related to treatment.
While the Food and Nutrition Board (42) suggested 120 mg Fe/day as the LOAEL for FS, a more recent meta-analysis of more than 40 clinical trials (65) found that the prevalence of gastrointestinal complaints associated with FS administration appears to be idiosyncratic and not related to dose. Accordingly, the observation that, in Phase II of this study, neither IHAT nor ASP providing 120 mg iron/day produced a pattern of reported gastrointestinal symptoms different from FS is not surprising, as the insolubility of each in the post-duodenal gut would suggest that neither form has substantial interactions with those epithelial surfaces.
4.3. Strengths and limitations
This was a rigorously designed study that addressed the effects of multiple modalities of iron supplementation of iron-replete individuals on key aspects of malarial infectivity, bacterial infections, and gut inflammation. Both phases of the study were powered on these primary outcomes which, thus, had type I error control. Limitations include that we did not perform power calculations for other outcomes, i.e., iron status parameters, markers of gut inflammation besides fecal calprotectin, and GI side effects, or noninferiority tests. That iron supplementation did not affect malarial infection of erythrocytes does not imply that such treatment may not affect susceptibility to malarial infection in clinical settings, as we did not access effects on hepatocytes, which are also important in the cycle of malarial infection.
5. Conclusion
This study demonstrated that two novel forms of bioavailable iron, IHAT and ASP (at either dose level of 60 mg/day in Phase I or 120 mg/day in Phase II) for 28 days did not produce significant different responses in the primary outcomes: malaria infectivity, bacterial proliferation, and gut inflammation. Similarly, there were no significant differences in parameters of iron status between these forms, with or without micronutrient supplementation. The majority (69–87%) of participants experienced changes in stool color, with over 25% of participants reporting changes in stool number and constipation consistent with iron supplementation. At the 60 mg/day dose used in phase I, IHAT produced abdominal pain in 27% of participants; unexpectedly, no treatment-related difference in symptoms were observed at the therapeutic dose in phase II. Taken overall, these results indicate that IHAT and ASP may be safe and as well tolerated as FS in these healthy, iron-replete adults.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Tufts Health Sciences Institutional Review Board. The participants provided their written informed consent to participate in this study.
Author contributions
GC, SM, EL, JM, JL, AC, DW, and KB designed the research. EL, EO, and MD coordinated the project. JM provided medical oversight. JL, MO, LM, and FN provided essential materials (bacterial cultures) and conducted bacterial proliferation studies. AC, CS, and AQ provided essential materials (malarial parasites) and conducted malarial infectivity studiers. CG conducted participant recruitment, dietary assessment, treatment administration, and sample collection. EO, GP, and WG conducted clinical analyses. KB, GM, and JW managed and analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is supported by the Bill & Melinda Gates Foundation (OPP1139998). Additional support for Dr. Chishti’s laboratory came from the NIH grant RO1-HL060961. EL was funded by a Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship.
Acknowledgments
The authors gratefully acknowledge the contributions of the applicants and enrolled participants in this study, as well as the following individuals: Kenneth Brown for his collaboration in the design of this project; Dora Pereira and Manju Reddy for help in obtaining IHAT and ASP, respectively. Kimberly Dupiton, Michelle Hallam, Andrew Howland, Janice Klian, Jean McShea, Marlaine Parker, Ryan Piccirillo, Helen Rasmussen, Meagan Reardon, Carmelle St. Victor, Kim Trinh, and Margaret Vilme for recruiting and managing participants; Shahin Sarkarati Smith and Stephanie Thea Leon Valliere for conducting clinical chemical analyses; Quynh Anh Phan and Bryan Makowski for contributing to the development of malaria infectivity assays; Jifan Wang for assistance with graphs and tables; and David Hamer and James McClung for providing participant safety oversight. We appreciate the contributions of the Medical Research Council, Cambridge, UK, and Nemysis, Ltd., Dublin, IE, for donating IHAT; Cura Global Health, Ames, IA, USA, for donating ASP (Aspiron™, now called Ultimine™); D. G. Keusch, Tufts University Medical Center, Boston, MA, for donating the culture of Salmonella typhimurium; DSM Nutritional Products, Geneva, SW, for donating MixMe™ micronutrient powder; Ingredion, Westchester, IL, USA, for donating Melojel® corn starch; and the Richardson Centre for Functional Foods and Nutraceutical at the University of Manitoba, Winnipeg, Manitoba, Canada, for performing the encapsulation of intervention agents.
Conflict of interest
DP is an inventor of the IHAT iron supplementation technology, for which she could receive future awards to inventors through the MRC Awards to Inventor scheme. She is also scientific advisor to Nemysis Ltd, who now hold the license for IHAT. She has received honoraria or consultancy fees from Vifor Pharma UK, Shield Therapeutics, Entia Ltd, Danone Nutricia, UN Food and Agriculture Organization (FAO) and Nemysis Ltd, for work concerning iron in health. DP has since moved to full employment in the iron and health industry with Vifor Pharma UK.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1230061/full#supplementary-material
Footnotes
References
1. Hurrell, R, and Egli, I. Iron bioavailability and dietary reference values. Am J Clin Nutr. (2010) 91:1461S–7S. doi: 10.3945/ajcn.2010.28674F
2. Jaeggi, T, Kortman, GA, Moretti, D, Chassard, C, Holding, P, Dostal, A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. (2015) 64:731–42. doi: 10.1136/gutjnl-2014-307720
3. Paganini, D, Uyoga, MA, and Zimmermann, MB. Iron fortification of foods for infants and children in low-income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients. (2016) 8:494–505. doi: 10.3390/nu8080494
4. Tang, M, Frank, DN, Sherlock, L, Ir, D, Robertson, CE, and Krebs, NF. Effect of vitamin E with therapeutic iron supplementation on iron repletion and gut microbiome in US Iron deficient infants and toddlers. J Pediatr Gastroenterol Nutr. (2016) 63:379–85. doi: 10.1097/MPG.0000000000001154
5. Zimmermann, MB, Chassard, C, Rohner, F, N'Goran, EK, Nindjin, C, Dostal, A, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in cote d'Ivoire. Am J Clin Nutr. (2010) 92:1406–15. doi: 10.3945/ajcn.110.004564
6. Mayo-Wilson, E, Imdad, A, Junior, J, Dean, S, and Bhutta, ZA. Preventive zinc supplementation for children, and the effect of additional iron: a systematic review and meta-analysis. BMJ Open. (2014) 4:e004647. doi: 10.1136/bmjopen-2013-004647
7. Soofi, S, Cousens, S, Iqbal, SP, Akhund, T, Khan, J, Ahmed, I, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. (2013) 382:29–40. doi: 10.1016/S0140-6736(13)60437-7
8. Barffour, MA, Schulze, KJ, Coles, CL, Chileshe, J, Kalungwana, N, Arguello, M, et al. High Iron Stores in the low Malaria Season Increase Malaria Risk in the high transmission season in a prospective cohort of rural Zambian children. J Nutr. (2017) 147:1531–6. doi: 10.3945/jn.117.250381
9. Gwamaka, M, Kurtis, JD, Sorensen, BE, Holte, S, Morrison, R, Mutabingwa, TK, et al. Iron deficiency protects against severe plasmodium falciparum malaria and death in young children. Clin Infect Dis. (2012) 54:1137–44. doi: 10.1093/cid/cis010
10. Jonker, FA, Calis, JC, van Hensbroek, MB, Phiri, K, Geskus, RB, Brabin, BJ, et al. Iron status predicts malaria risk in Malawian preschool children. PLoS One. (2012) 7:e42670. doi: 10.1371/journal.pone.0042670
11. Lynch, S, Stoltzfus, R, and Rawat, R. Critical review of strategies to prevent and control iron deficiency in children. Food Nutr Bull. (2007) 28:S610–20. doi: 10.1177/15648265070284S413
12. Moya-Alvarez, V, Cottrell, G, Ouedraogo, S, Accrombessi, M, Massougbodgi, A, and Cot, M. High Iron levels are associated with increased malaria risk in infants during the first year of life in Benin. Am J Trop Med Hyg. (2017) 97:497–503. doi: 10.4269/ajtmh.16-0001
13. Nyakeriga, AM, Troye-Blomberg, M, Dorfman, JR, Alexander, ND, Back, R, Kortok, M, et al. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. (2004) 190:439–47. doi: 10.1086/422331
14. Portugal, S, Carret, C, Recker, M, Armitage, AE, Goncalves, LA, Epiphanio, S, et al. Host-mediated regulation of superinfection in malaria. Nat Med. (2011) 17:732–7. doi: 10.1038/nm.2368
15. Sazawal, S, Black, RE, Ramsan, M, Chwaya, HM, Stoltzfus, RJ, Dutta, A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. (2006) 367:133–43. doi: 10.1016/S0140-6736(06)67962-2
16. Dogan, B, Suzuki, H, Herlekar, D, Sartor, RB, Campbell, BJ, Roberts, CL, et al. Inflammation-associated adherent-invasive Escherichia coli are enriched in pathways for use of propanediol and iron and M-cell translocation. Inflamm Bowel Dis. (2014) 20:1919–32. doi: 10.1097/MIB.0000000000000183
17. Werner, T, Wagner, SJ, Martinez, I, Walter, J, Chang, JS, Clavel, T, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut. (2011) 60:325–33. doi: 10.1136/gut.2010.216929
18. Prentice, AM, Doherty, CP, Abrams, SA, Cox, SE, Atkinson, SH, Verhoef, H, et al. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. (2012) 119:1922–8. doi: 10.1182/blood-2011-11-391219
19. Hurrell, R. Iron and malaria: absorption, efficacy and safety. Int J Vitam Nutr Res. (2010) 80:279–92. doi: 10.1024/0300-9831/a000035
20. Hurrell, RF. Safety and efficacy of iron supplements in malaria-endemic areas. Ann Nutr Metab. (2011) 59:64–6. doi: 10.1159/000332140
21. Kartikasari, AE, Georgiou, NA, Visseren, FL, van Kats-Renaud, H, van Asbeck, BS, and Marx, JJ. Endothelial activation and induction of monocyte adhesion by nontransferrin-bound iron present in human sera. FASEB J. (2006) 20:353–5. doi: 10.1096/fj.05-4700fje
22. Pereira, DI, Aslam, MF, Frazer, DM, Schmidt, A, Walton, GE, McCartney, AL, et al. Dietary iron depletion at weaning imprints low microbiome diversity and this is not recovered with oral Nano Fe(III). Microbiology. (2015) 4:12–27. doi: 10.1002/mbo3.213
23. US Food & drug GRAS Notices (2016). Available at: www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=82.
24. Codus Alimentarus International Food Standards (2019). Available at: www.fao.org › fao-who-codexalimentarius › sh-proxy.
25. Reddy, MB, and Armah, SM. Impact of Iron-enriched aspergillus oryzae on Iron bioavailability, safety, and gut microbiota in rats. J Agric Food Chem. (2018) 66:6213–8. doi: 10.1021/acs.jafc.8b01758
26. Lewis, ED, Wu, D, Mason, JB, Chishti, AH, Leong, JM, Barger, K, et al. Safe and effective delivery of supplemental iron to healthy older adults: the double-blind, randomized, placebo-controlled trial protocol of the safe Iron study. Gates Open Res. (2019) 3:1510. doi: 10.12688/gatesopenres.13039.2
27. Bries, AE, Wang, C, Agbemafle, I, Wels, B, and Reddy, MB. Assessment of acute serum Iron, non-transferrin-bound Iron, and gastrointestinal symptoms with 3-week consumption of Iron-enriched aspergillus oryzae compared with ferrous sulfate. Curr Dev Nutr. (2019) 3:nzz127. doi: 10.1093/cdn/nzz127
28. Matuszek, G., and Lewis, E. Safe and effective delivery of supplemental iron to healthy volunteers.
29. Multiple Micronutrient Powder Supply and Outlook (2016).UNICEF Supply Div. Available at: https://unicef.org/media/4926/file/MNP-market-note-September-2016.pdf.
30. Powell, JJ, Bruggraber, SF, Faria, N, Poots, LK, Hondow, N, Pennycook, TJ, et al. A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomedicine. (2014) 10:1529–38. doi: 10.1016/j.nano.2013.12.011
31. Aslam, MF, Frazer, DM, Faria, N, Bruggraber, SF, Wilkins, SJ, Mirciov, C, et al. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice. FASEB J. (2014) 28:3671–8. doi: 10.1096/fj.14-251520
32. Latunde-Dada, GO, Pereira, DI, Tempest, B, Ilyas, H, Flynn, AC, Aslam, MF, et al. A nanoparticulate ferritin-core mimetic is well taken up by HuTu 80 duodenal cells and its absorption in mice is regulated by body iron. J Nutr. (2014) 144:1896–902. doi: 10.3945/jn.114.201715
33. Pereira, DI, Bruggraber, SF, Faria, N, Poots, LK, Tagmount, MA, Aslam, MF, et al. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine. (2014) 10:1877–86. doi: 10.1016/j.nano.2014.06.012
34. EFSAa Panel on Nutrition NFFood, A, Turck, D, Bohn, T, Castenmiller, J, De Henauw, S, et al. Safety of iron hydroxide adipate tartrate as a novel food pursuant to regulation (EU) 2015/2283 and as a source of iron in the context of directive 2002/46/EC. EFSA J. (2021) 19:e06935. doi: 10.2903/j.efsa.2021.6935
35. Reddy, MB, Armah, SM, Stewart, JW, and O'Brien, KO. Iron absorption from Iron-enriched aspergillus oryzae is similar to ferrous sulfate in healthy female subjects. Curr Dev Nutr. (2018) 2:nzy004. doi: 10.1093/cdn/nzy004
36. Bries, AE, Hurrell, RF, and Reddy, MB. Iron absorption from bouillon fortified with Iron-enriched aspergillus oryzae is higher than that fortified with ferric pyrophosphate in young women. J Nutr. (2020) 150:1109–15. doi: 10.1093/jn/nxaa035
37. World Health Organization. WHO recommendations on antenatal Care for a Positive Pregnancy Experience. Geneva: EVIMalaR, Glasgow, UK (2016). 196 p.
38. Bhatla, N, Kaul, N, Lal, N, Kriplani, A, Agarwal, N, Saxena, R, et al. Comparison of effect of daily versus weekly iron supplementation during pregnancy on lipid peroxidation. J Obstet Gynaecol Res. (2009) 35:438–45. doi: 10.1111/j.1447-0756.2008.00972.x
39. Haidar, J, Omwega, AM, Muroki, NM, and Ayana, G. Daily versus weekly iron supplementation 9nd prevention of iron deficiency anaemia in lactating women. East Afr Med J. (2003) 80:11–6. doi: 10.4314/eamj.v80i1.8661
40. Siddiqui, IA, Rahman, MA, and Jaleel, A. Efficacy of daily vs. weekly supplementation of iron in schoolchildren with low iron status. J Trop Pediatr. (2004) 50:276–8. doi: 10.1093/tropej/50.5.276
41. Troesch, B, Egli, I, Zeder, C, Hurrell, RF, and Zimmermann, MB. Fortification iron as ferrous sulphate plus ascorbic acid is more rapidly absorbed than as sodium iron EDTA but neither increases serum nontransferrin-bound iron in women. J Nutr. (2011) 141:822–7. doi: 10.3945/jn.110.136127
42. Food and Nutrition Board (2001). Dietary reference intakes: For vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, Iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. National Academy Press, Washington, DC. p 290–393.
43. Moll, K, Ljungstrom, I, Perlmann, H, Scherf, A, and Wahlgren, H. Methods in malaria research. 6th ed EVIMalaR Glasgow, UK, and MR4/ATCC, Manassas, VA, USA. (2013). 474 p.
44. Trager, W, and Jensen, JB. Human malaria parasites in continuous culture. Science. (1976) 193:673–5. doi: 10.1126/science.781840
45. Ding, Y, Onodera, Y, Lee, JC, and Hooper, DC. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J Bacteriol. (2008) 190:7123–9. doi: 10.1128/JB.00655-08
46. Taylor, TA, and Unakal, CG. Staphylococcus Aureus. Edtion ed. Treasure Island (FL): StatPearls (2022).
47. Geisinger, E, Mortman, NJ, Vargas-Cuebas, G, Tai, AK, and Isberg, RR. A global regulatory system links virulence and antibiotic resistance to envelope homeostasis in Acinetobacter baumannii. PLoS Pathog. (2018) 14:e1007030. doi: 10.1371/journal.ppat.1007030
48. Wong, D, Nielsen, TB, Bonomo, RA, Pantapalangkoor, P, Luna, B, and Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev. (2017) 30:409–47. doi: 10.1128/CMR.00058-16
49. Jorgensen, MG, van Raaphorst, R, and Veening, JW. Noise and stochasticity in gene expression: a pathogenic fate determinant. Methods Microbiol. (2013) 40:157. doi: 10.1016/B978-0-12-417029-2.00006-6
50. Graham, SM. Nontyphoidal salmonellosis in Africa. Curr Opin Infect Dis. (2010) 23:409–14. doi: 10.1097/QCO.0b013e32833dd25d
51. Pulford, CV, Perez-Sepulveda, BM, Canals, R, Bevington, JA, Bengtsson, RJ, Wenner, N, et al. Stepwise evolution of Salmonella Typhimurium ST313 causing bloodstream infection in Africa. Nat Microbiol. (2021) 6:327–38. doi: 10.1038/s41564-020-00836-1
52. Paczosa, MK, Silver, RJ, McCabe, AL, Tai, AK, McLeish, CH, Lazinski, DW, et al. Transposon mutagenesis screen of Klebsiella pneumoniae identifies multiple genes important for resisting antimicrobial activities of neutrophils in mice. Infect Immun. (2020) 88: 19. doi: 10.1128/IAI.00034-20
53. Zander, DS, and Farver, CF. Pulmonary pathology. 2nd ed. Philadelphia, PA: Elsevier (2018). 974 p.
54. Ciesielczuk, H, Doumith, M, Hope, R, Woodford, N, and Wareham, DW. Characterization of the extra-intestinal pathogenic Escherichia coli ST131 clone among isolates recovered from urinary and bloodstream infections in the United Kingdom. J Med Microbiol. (2015) 64:1496–503. doi: 10.1099/jmm.0.000179
55. Poolman, JT, and Wacker, M. Extraintestinal pathogenic Escherichia coli, a common human pathogen: challenges for vaccine development and Progress in the field. J Infect Dis. (2016) 213:6–13. doi: 10.1093/infdis/jiv429
56. Cross, JH, Bradbury, RS, Fulford, AJ, Jallow, AT, Wegmuller, R, Prentice, AM, et al. Oral iron acutely elevates bacterial growth in human serum. Sci Rep. (2015) 5:16670. doi: 10.1038/srep16670
57. Garber, JJ, and Chung, DC. Colonic polyps and polyposis syndromes In: M Feldman, LS Friedman, and LJ Brandt, editors. Sleisenger and Fordtran's gastrointestinal and liver disease. 11th ed. Philadelphia, PA: Elsevier (2021)
58. Diehl, H. The iron reagents: Bathophenanthroline, bathophenanthroline-disulfonic acid, 2,4,6-tripyidyl-s-triazine [sic, and] phenyl-2-pyridyl ketoxime. 2nd ed. Columbus, Ohio: G.F. Smith Chemical Co. (1965).
59. Babson, AL, Olson, DR, Palmieri, T, Ross, AF, Becker, DM, and Mulqueen, PJ. The Immulit assay tube: a new approach to heterogeneous ligand assay. Clin Chem. (1991) 37:1521–2. doi: 10.1093/clinchem/37.9.1521
60. Pereira, DI, Couto Irving, SS, Lomer, MC, and Powell, JJ. A rapid, simple questionnaire to assess gastrointestinal symptoms after oral ferrous sulphate supplementation. BMC Gastroenterol. (2014) 14:103. doi: 10.1186/1471-230X-14-103
61. REDCap Software (2020). Available at: https://projectredcap.org/software/
62. Clark, MA, Goheen, MM, Fulford, A, Prentice, AM, Elnagheeb, MA, Patel, J, et al. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat Commun. (2014) 5:4446. doi: 10.1038/ncomms5446
63. von Arnim, U, Wex, T, Ganzert, C, Schulz, C, and Malfertheiner, P. Fecal calprotectin: a marker for clinical differentiation of microscopic colitis and irritable bowel syndrome. Clin Exp Gastroenterol. (2016) 9:97–103. doi: 10.2147/CEG.S97701
64. CRAN Package emmeans (1980). Available at: https://CRAN.R-project.org/package=emmeans
Keywords: iron, ferrous sulfate, malarial infectivity, bacterial proliferation, gut inflammation, IHAT, fungal iron
Citation: Lewis ED, Ortega EF, Dao MC, Barger K, Mason JB, Leong JM, Osburne MS, Magoun L, Nepveux V FJ, Chishti AH, Schwake C, Quynh A, Gilhooly CH, Petty G, Guo W, Matuszek G, Pereira D, Reddy M, Wang J, Wu D, Meydani SN and Combs GF (2023) Safe and effective delivery of supplemental iron to healthy adults: a two-phase, randomized, double-blind trial – the safe iron study. Front. Nutr. 10:1230061. doi: 10.3389/fnut.2023.1230061
Edited by:
Elnaz Daneshzad, Tehran University of Medical Sciences, IranReviewed by:
Zeinab Ghorbani, Guilan University of Medical Sciences, IranRamesh Allipour Birgani, Tehran University of Medical Sciences, Iran
Copyright © 2023 Lewis, Ortega, Dao, Barger, Mason, Leong, Osburne, Magoun, Nepveux V, Chishti, Schwake, Quynh, Gilhooly, Petty, Guo, Matuszek, Pereira, Reddy, Wang, Wu, Meydani and Combs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simin N. Meydani, c2ltaW4ubWV5ZGFuaUB0dWZ0cy5lZHU=
†Present Address: Erin D. Lewis, KGK Science, Inc., London, Ontario, ON, Canada Weimin Guo, Department of Pathology and Laboratory Medicine, Boston University Chobanian & Avedisian School of Medicine, Boston, MA, United States Dora Pereira, Vifor Pharma UK Ltd., Cambridge, England, United Kingdom
 Erin D. Lewis1†
Erin D. Lewis1† Edwin F. Ortega
Edwin F. Ortega Maria Carlota Dao
Maria Carlota Dao Kathryn Barger
Kathryn Barger Joel B. Mason
Joel B. Mason John M. Leong
John M. Leong Marcia S. Osburne
Marcia S. Osburne Felix J. Nepveux V
Felix J. Nepveux V Gayle Petty
Gayle Petty Weimin Guo
Weimin Guo Manju Reddy
Manju Reddy Jifan Wang
Jifan Wang Dayong Wu
Dayong Wu Simin N. Meydani
Simin N. Meydani Gerald F. Combs Jr.
Gerald F. Combs Jr.