- 1Field Application Specialist, PerkinElmer, New Delhi, India
- 2National Institute of Food Technology Entrepreneurship and Management, Sonipat, Haryana, India
- 3Amity Institute of Biotechnology, Amity University Chhattisgarh, Raipur, India
- 4Department of Food Science, University of Massachusetts Amherst, Amherst, MA, United States
- 5Department of Food Science & Bioengineering, Zhejiang Gongshang University, Hangzhou, Zhejiang, China
- 6Centre for Ocean Research (DST-FIST Sponsored Centre), MoES-Earth Science and Technology Cell (Marine Biotechnological Studies), Sathyabama Research Park, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India
- 7Department of Food Technology, Jamia Hamdard University, New Delhi, India
- 8Department of Basic Medical Sciences, College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 9School of Chemical Engineering, Yeungnam University, Gyeongsan, Republic of Korea
The multifaceted role of vitamin C in human health intrudes several biochemical functions that are but not limited to antioxidant activity, homoeostasis, amino acid synthesis, collagen synthesis, osteogenesis, neurotransmitter production and several yet to be explored functions. In absence of an innate biosynthetic pathway, humans are obligated to attain vitamin C from dietary sources to maintain its optimal serum level (28 μmol/L). However, a significant amount of naturally occurring vitamin C may deteriorate due to food processing, storage and distribution before reaching to the human gastrointestinal tract, thus limiting or mitigating its disease combating activity. Literature acknowledges the growing prevalence of vitamin C deficiency across the globe irrespective of geographic, economic and population variations. Several tools have been tested to address vitamin C deficiency, which are primarily diet diversification, biofortification, supplementation and food fortification. These strategies inherit their own advantages and limitations. Opportunely, nanotechnology promises an array of delivery systems providing encapsulation, protection and delivery of susceptible compounds against environmental factors. Lack of clear understanding of the suitability of the delivery system for vitamin C encapsulation and fortification; growing prevalence of its deficiency, it is a need of the hour to develop and design vitamin C fortified food ensuring homogeneous distribution, improved stability and enhanced bioavailability. This article is intended to review the importance of vitamin C in human health, its recommended daily allowance, its dietary sources, factors donating to its stability and degradation. The emphasis also given to review the strategies adopted to address vitamin c deficiency, delivery systems adopted for vitamin C encapsulation and fortification.
1. Introduction
Vitamin C (ascorbic acid) has been well documented for its antioxidant activity and other biological activities (1). Humans cannot synthesize this essential nutrient and so must obtain it from their diet (2, 3). Vitamin C deficiency has been linked to several diseases in humans (4, 5), most notably scurvy, which are related to its key role in numerous biochemical functions, including collagen synthesis, amino acid synthesis, blood pressure control, atherogenesis, homoeostasis, neurotransmitter production, and osteogenesis (5–7). In addition to its role as a vitamin, ascorbic acid has also been shown to exhibit various nutraceutical functions, including anticancer effects. There have been an increasing number of publications on vitamin C over the past few decades, with many of them focusing on food fortification (Figure 1). The World Health Organization (WHO) states that food fortification is one of the most effective, safe, and economical ways of addressing nutrient deficiencies (8). However, the inclusion of vitamin C in functional foods and beverages is often challenging due to its chemical instability and low bioavailability (9–12).
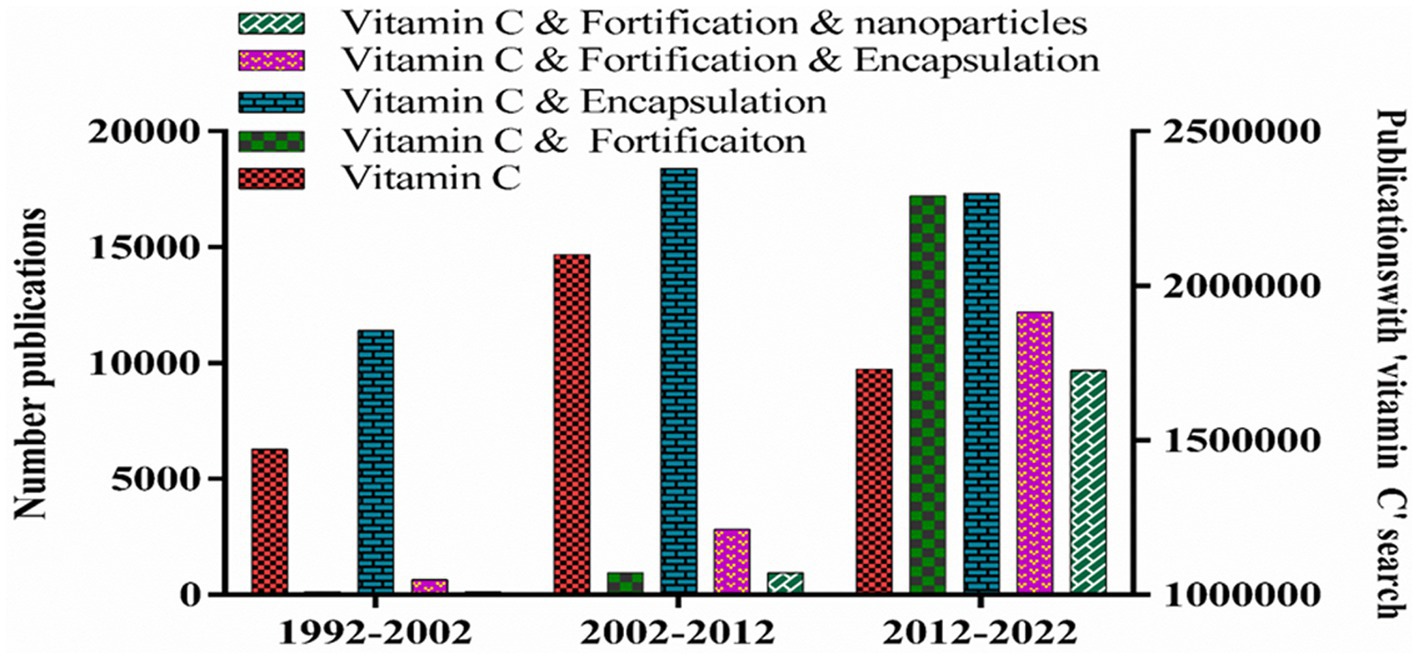
Figure 1. Number of publications with keywords “vitamin C”, “vitamin C and fortification” or “vitamin C and fortification and encapsulation” and “vitamin C and fortification and nanoparticles.”
Consequently, many researchers have focused on developing strategies to overcome these challenges (13–17). This review will begin by discussing the physicochemical attributes, biosynthesis, food sources, recommended dietary allowance, and available fortification strategies for vitamin C. Then the challenges associated with food fortification are discussed, and the important role of colloidal delivery systems in encapsulating, protecting, and delivering vitamin C is highlighted. The knowledge presented in this article may facilitate the development of more efficacious strategies for fortifying foods and beverages with this important micronutrient.
1.1. History
The connection between vitamin C deficiency and scurvy can be traced back to 1700 BC when the Ebers Papyrus defined the characteristic features of scurvy. There is also evidence of the role of food deficiencies in the writings of several ancient scholars, including Hippocrates in Greece (460 BC), Susrutrain India (400 BC), and Chang Chi in China (200AD). From the sixteenth to eighteenth centuries, there was a growing understanding that dietary deficiencies were causing diseases in sailors (18). Eventually, this led to the first scientific publication on the subject, “A Treatise of Scurvy”, by James Lind in 1753, where he emphasizes the importance of lemons, oranges, and fresh green vegetables in the prevention of scurvy (19). Almost, two centuries later, Albert Szent-Gyorgyi published his observations on the extraction of vitamin C (a “sugar-like crystal”) in the Biochemical Journal under the title “Observation on the function of peroxidase systems and the chemistry of the adrenal cortex: Description of a new carbohydrate derivate” (20). Later, W. M. Haworth elucidated the structure of these sugar-like crystals and named it hexuronic acid. Then, King and Waugh (21) extracted these sugar-like crystals from lemon juice and named it ascorbic acid, which was described in their article “The chemical nature of vitamin C” (21). Albert Szent-Gyorgyi was eventually granted the Nobel prize in Physiology or Medicine for his research on vitamin C (22).
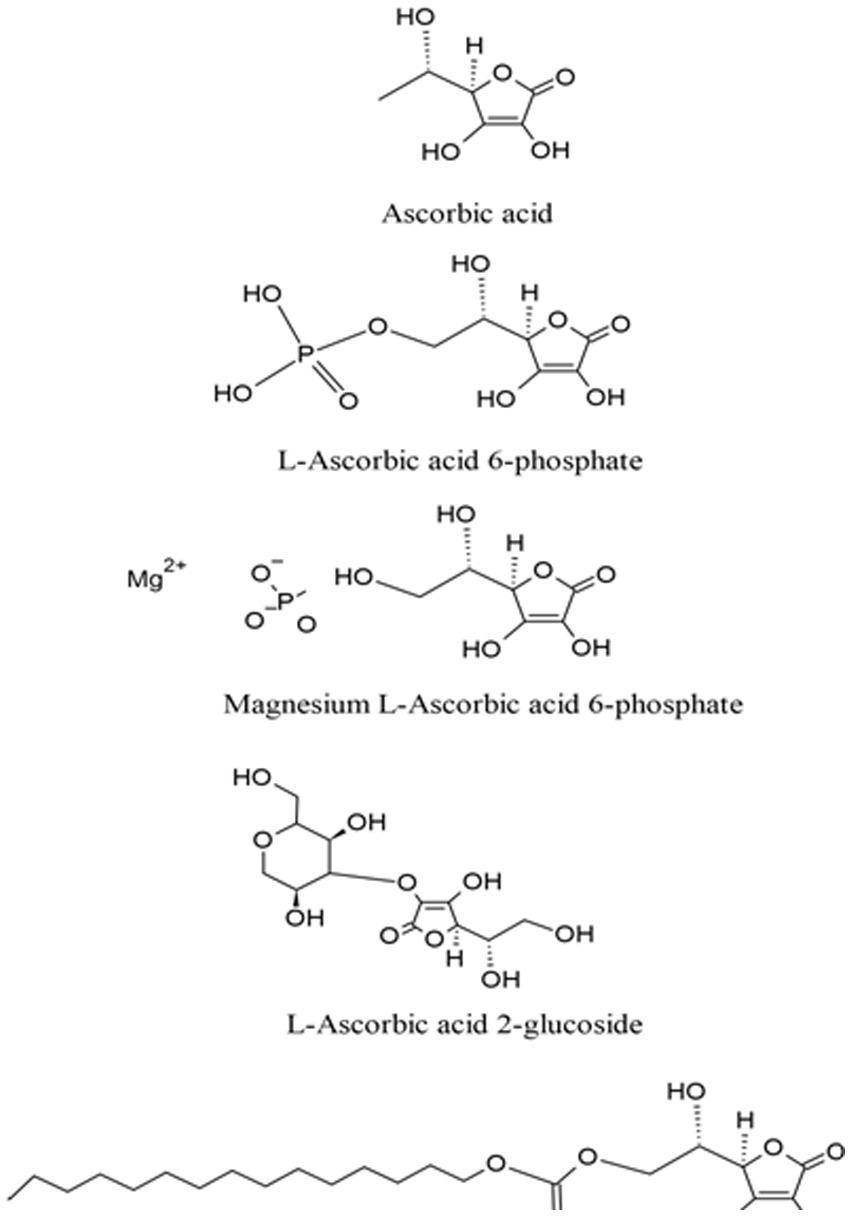
1.2. Molecular and physicochemical properties
Vitamin C is a low molecular weight carbohydrate with an enediol structure (Figure 2), which makes it a natural electron donor. The enediol structure also makes it susceptible to chemical degradation when exposed to changes in environmental conditions, such as pH, temperature, humidity, salt, and radiation (23). Several vitamin C analogs with differing physicochemical characteristics have also been synthesized (Figure 2). Researchers have classified these vitamin C analogs depending on their water-solubility and ability to raise vitamin C serum levels. Based on their physicochemical characteristics, these analogs can be categorized into:
i. Hydrophilic ascorbic acid: This group includes L-ascorbic acid 2-glucoside, magnesium L-ascorbic acid 6-phosphate, and L-Ascorbic acid 6-phosphate
ii. Hydrophobic ascorbic acid: This group includes tetra-isopalmitoyl ascorbic acid and L-ascorbyl 6-palmitate (10).
Based on their ability to increase blood serum levels of vitamin C they can also be categorized into two groups according to their potency (10):
i. Strong potency: This group includes L-ascorbate 2-phosphate, 6-bromo-6-deoxy-L-ascorbic acid, L-ascorbate 2-triphosphate, and ascorbic acid 2-O-α-glucoside;
ii. Weak potency: This group includes L-ascorbate-O-methyl ether, L-ascorbyl-2-sulfate, and L-ascorbyl palmitate.
1.3. Biosynthesis
Some animal species and green plants can naturally synthesize vitamin C through the innate glucuronic acid biochemical pathway (24). During evolution, however, humans appear to have lost a key enzyme (L-gulono-1,4 lactone oxidase) within the ascorbic acid biosynthesis pathway (25–27). Consequently, they must obtain this vitamin from their diet.
Dietary vitamin C is usually present in two different forms: ascorbic acid (reduced form) and dehydroascorbic acid (oxidized form). Initially, researchers speculated that vitamin C was only absorbed by the human body through passive diffusion due to its highly hydrophilic nature. Later, however, researchers identified a sodium-dependent vitamin C transporter responsible for the absorption of ascorbic acid (28). While other researchers found that the absorption of dehydroascorbic acid was mainly through glucose transporter isoforms GLUT1 and GLUT3 (29).
Researchers also observed a shift in the absorption mode depending on the vitamin C levels in dietary sources. For instance, at higher concentrations the uptake of vitamin C occurs mainly by passive diffusion while at lower concentrations it mainly follows carrier-mediated active transport (30). However, the precise threshold at which this transition occurs is still a matter of investigation. The absorption efficiency of vitamin C within the human gastrointestinal tract (GIT) is also dose-dependent, i.e., at lower vitamin C doses (<180 mg/day) it has been reported to be up to 80–90% but at higher doses it is reported to be considerably lower (30).
2. Dietary sources
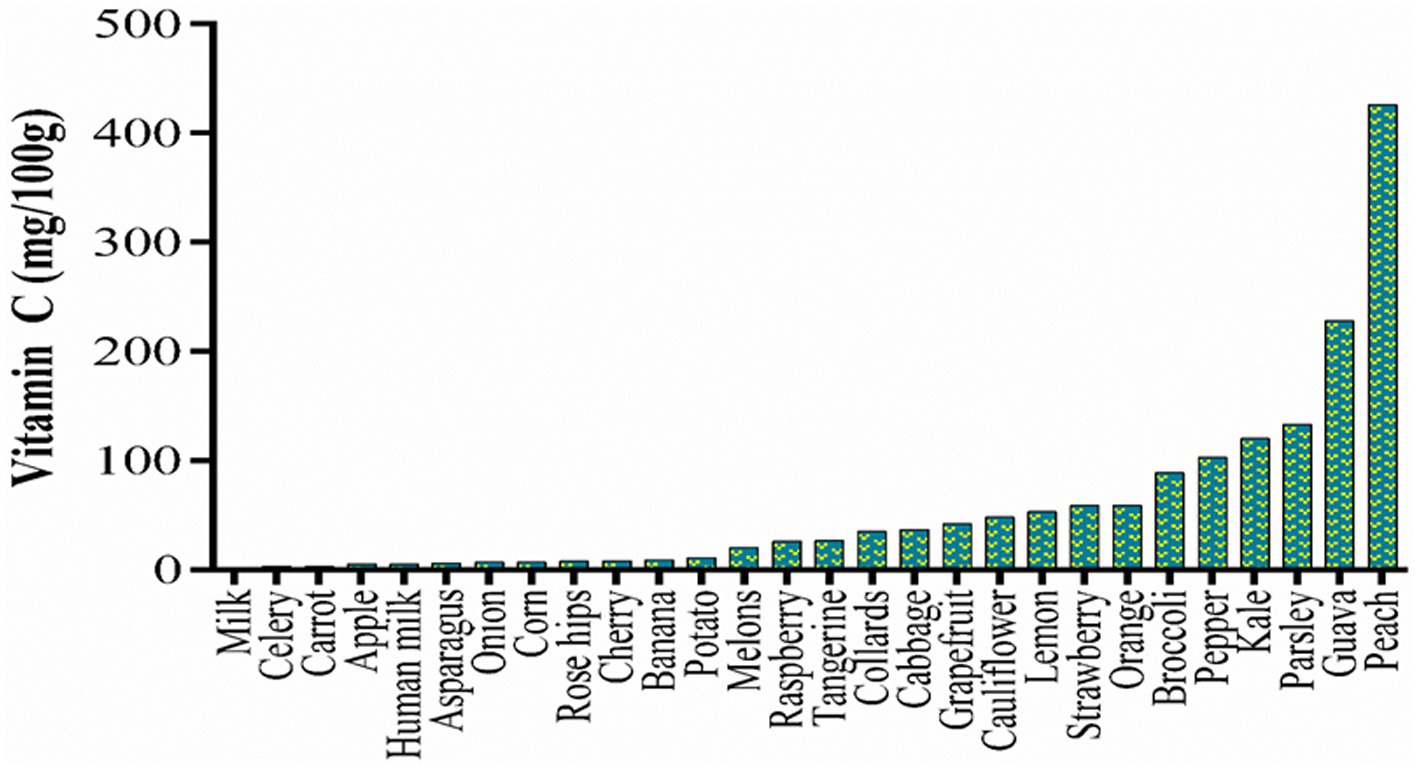
Due to the lack of a vitamin C biosynthetic pathway, humans rely on dietary sources to maintain an optimal vitamin C serum level. Fruits and vegetables are the major sources of vitamin C in the human diet (around 90%), with the remainder coming from animal sources (4). Several studies have reported the vitamin C content of different food types (Figure 3) including milk (31, 32), apple (33), banana (34), cherry (35), grapes (36), guava (37), lemons (38), melon (39), orange (35), peach (40), raspberry (41), rosehip (42), strawberry (43), tangerine (44), asparagus (45), broccoli (46), cabbage (47), carrot (48), celery (49), collards (50), kale (45), onions (51), and pepper (45). Vitamin C may also be consumed in the form of dietary supplements, such as capsules or tablets.
3. Stability in foods
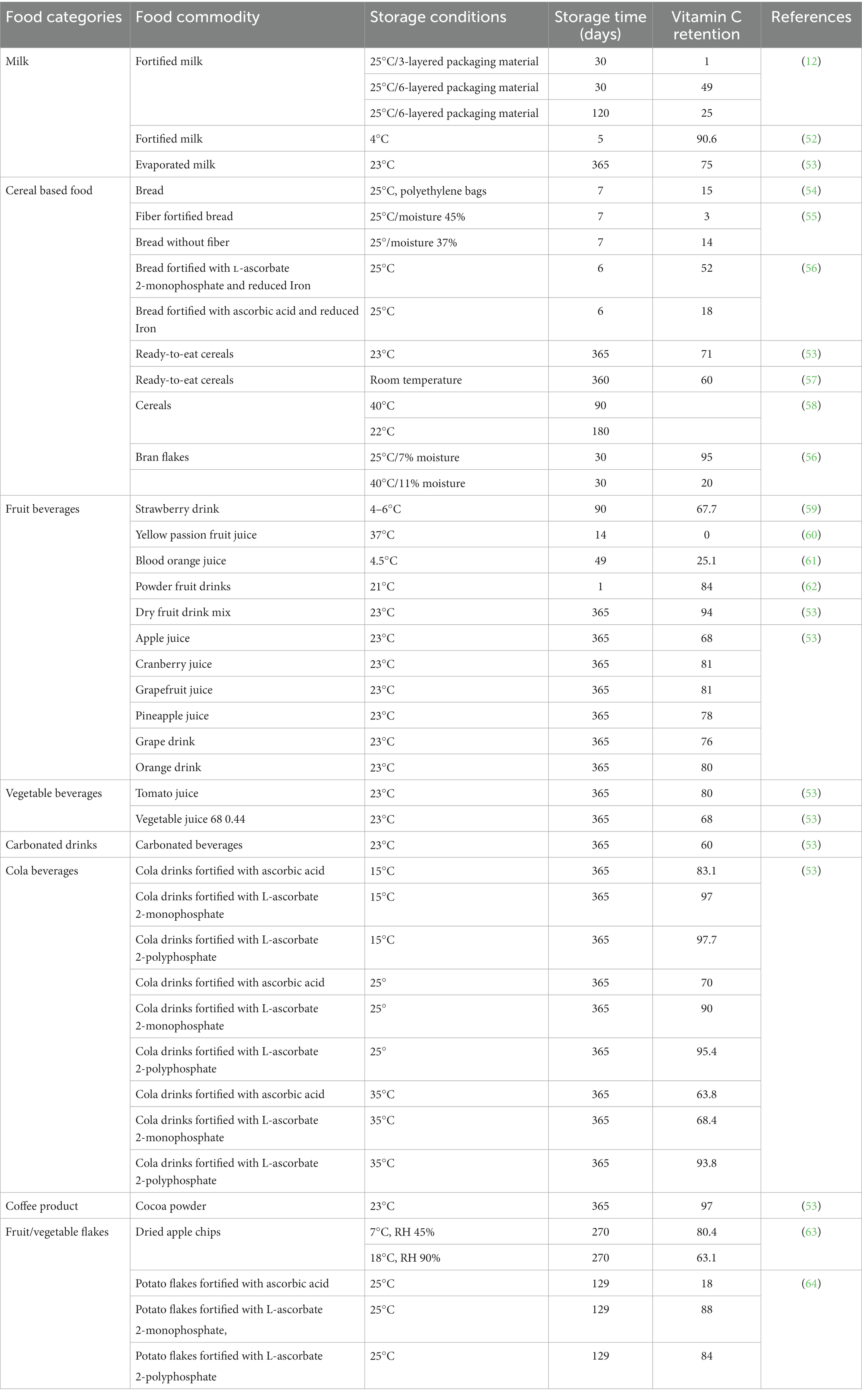
Vitamin C is a chemically active molecule that may undergo appreciable degradation in foods during processing, storage, and distribution (12). This degradation is mainly due to the hydrolytic opening of the lactone ring, thus resulting in the formation of a biologically inactive compound 2,3-deketogluconic acid (12). This degradation reaction is accelerated when the vitamin C is exposed to oxygen, transition metals, heat, and alkaline conditions (Table 1). For example, a significant proportion of vitamin C has been reported to be lost during the storage of potatoes, cabbages, and apples (64). The degree of loss depends on food matrix type and environmental conditions (stress). For example, boiling of potatoes was reported to cause a 40% loss in vitamin C content (2). Among the cooking methods steam seem to be most detrimental method for vitamin C. In contrast, some food processing operations stabilize vitamin C by inactivating enzymes (such as oxidases) that might promote its oxidation (2).
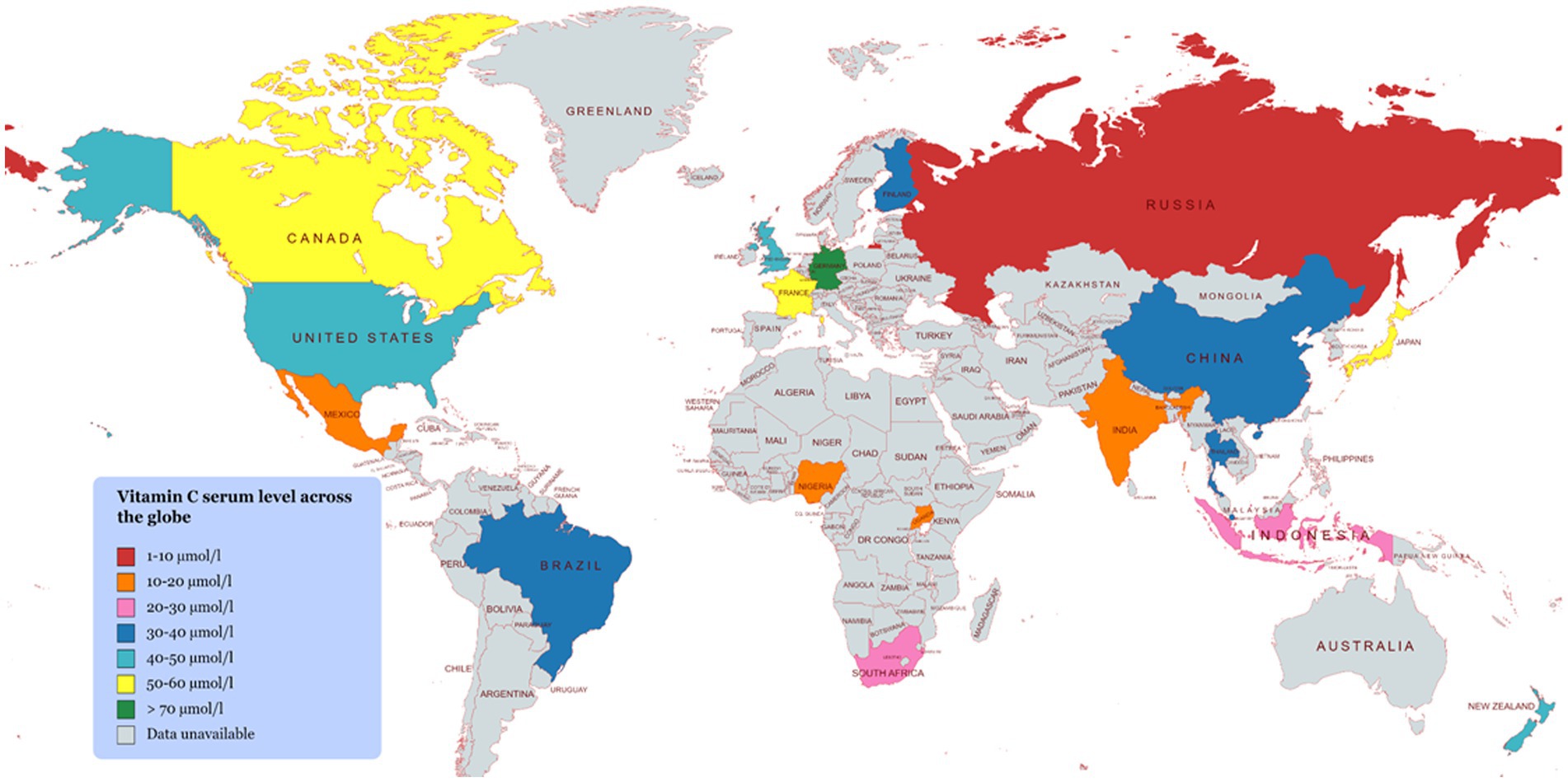
4. Vitamin C bioavailability
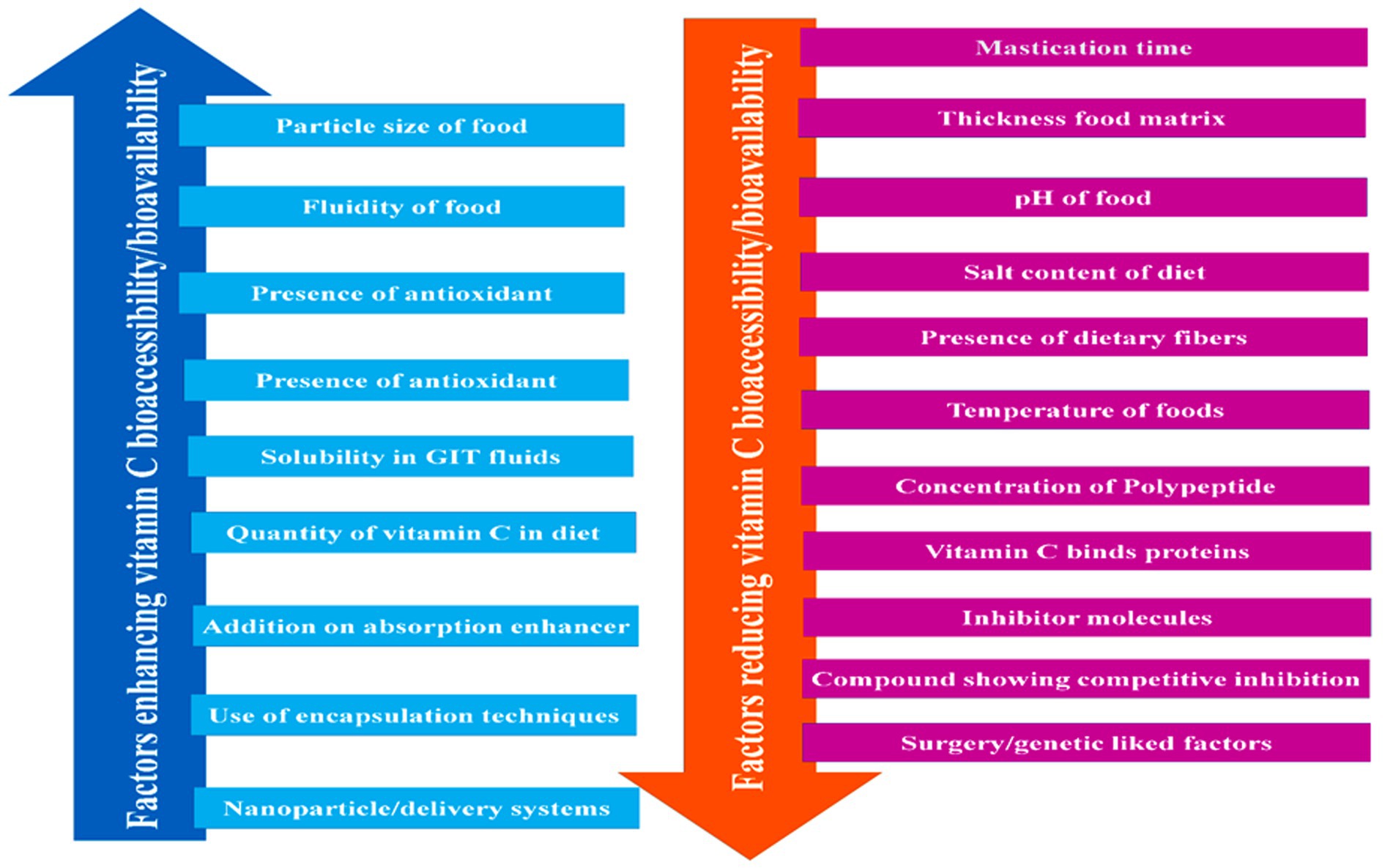
The biological efficacy of a vitamin depends on the quantity absorbed and utilized by the body rather than the amount consumed. The proportion of a vitamin absorbed in its active state is referred to as its bioavailability (2). The bioavailability of vitamin C depends on an array of factors, including the dose consumed, the composition and structure of the food matrix, the environmental conditions experienced during processing, storage, and distribution, and passage through the gastrointestinal tract (Figure 4 and Table 2). The bioavailability of vitamin C in foods is often considered to be equivalent to that of the purified form when the dose lies within the required nutritional range (15–200 mg) (2). However, it tends to fall by more than 50% when higher amounts (e.g., >1000 mg) are ingested (2). The conversion of ascorbic acid to dehydroascorbic acid in foods or the gastrointestinal tract can also reduce the bioactivity of vitamin C. A range of chemically synthesized ascorbic analogs have been developed to improve the chemical stability and bioavailability of vitamin C, including ascorbate 2-sulfate, ascorbate 2-monophosphate, and ascorbate 2-triphosphate (2).
5. Vitamin C deficiency
5.1. Indicators for vitamin C deficiency
The vitamin C status of a person or population may be established by diet-based assessments or analytical measurements. Diet-based assessments rely on analysis of food consumption patterns and frequencies. In this approach, subjects typically complete questionnaires related to their daily food consumption and then their vitamin C intake can be calculated from food databases. Analytical methods rely on measurement of the vitamin C levels in the serum of individuals, which can be achieved using various analytical methods including liquid chromatography and mass spectrometry. Plasma/serum vitamin C level is recognized as a biomarker for vitamin C status: <11 μmol/L (deficient), ≥11–28 μmol/L (suboptimal), and >28 μmol/L (sufficient) (4).
5.2. Vitamin C deficiency across the globe
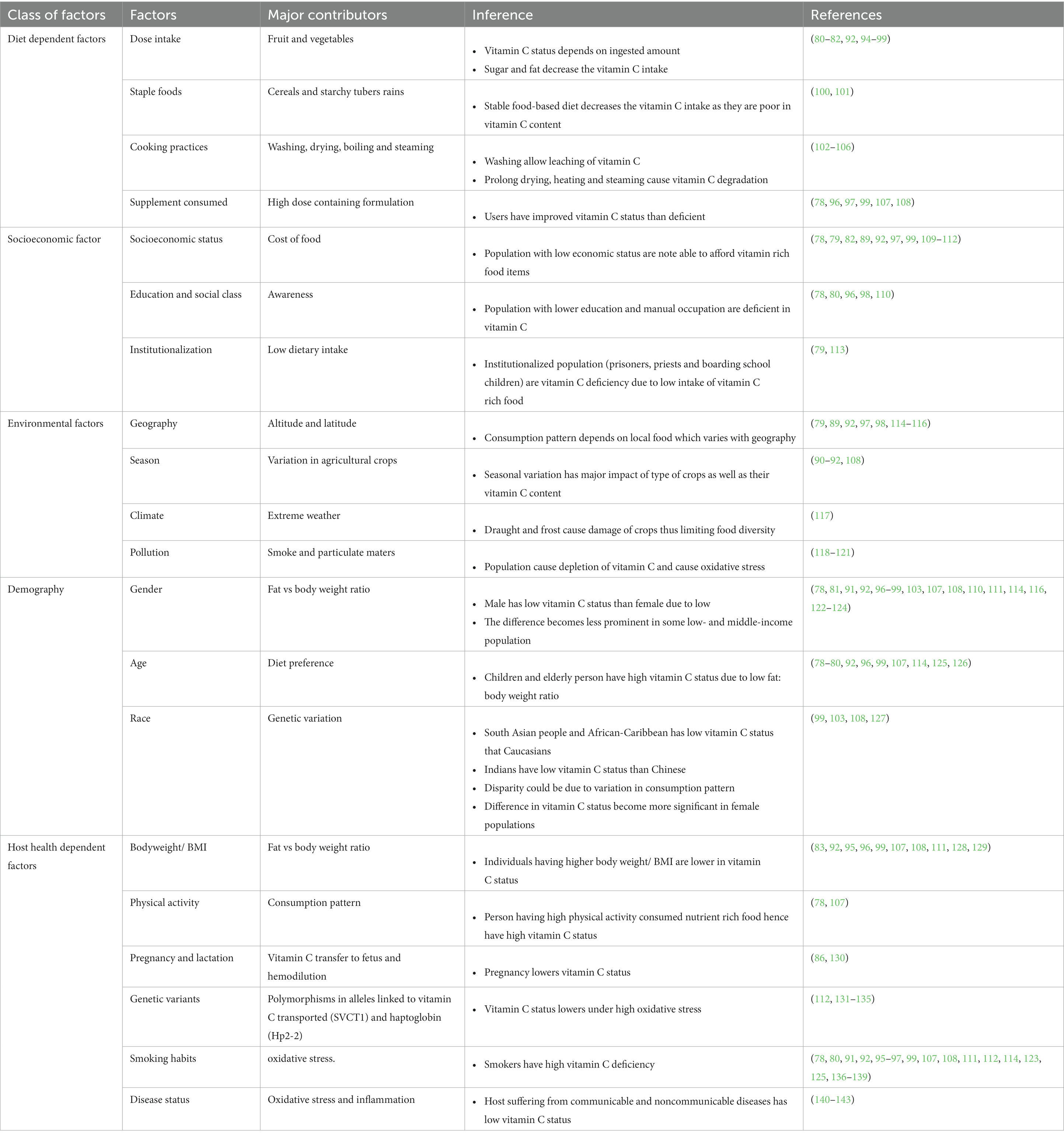
Despite improvements in the human diet over the past century, there are still high levels of vitamin C deficiency in some populations around the world (Figure 5) (73–76). For example, the EPIC-Norfolk survey conducted a vitamin C assessment of a relatively large sample size (22,400 participants) and observed a higher vitamin C deficiency in males than females (77, 78). This difference in vitamin deficiency status between male and female population is mainly governed by life style (smoking), low consumption of vitamin C supplements. Researchers have also reported significant differences in vitamin C status for different populations e.g., a lower level of vitamin C deficiency in the overall British population (14%) than in the Scottish one (20%) (79, 80). European and American populations also significantly vary in their vitamin C status (80). The prevalence of vitamin C deficiency is more pronounced in some other countries. For instance, its prevalence was widespread (up to 60%) in the female population in Quinto, Ecuador and similar observations have been made in other South American and African populations (81–89). In Asia, vitamin C status also significantly varied between and within countries. For example, low vitamin C status was recorded in the Indian population as compared to the Chinese population, with this difference being more prominent in the female population (90–92). This discrepancy in vitamin C status among different populations is mainly attributed to differences in the types of foods that are available and commonly consumed (4).
5.3. Health concerns related to vitamin C deficiency
Various factors contribute to the vitamin C requirements and status of individuals, including geographical, demographic, diet, socioeconomic environmental, and health factors (4, 93) (Table 3). Due to its many roles in human health; suboptimal vitamin C levels lead to a variety of undesirable health effects, including oxidative stress, malfunction of key biochemical pathways, and inhibition of the synthesis of key biological components, which can lead to diseases, such as scurvy. Moreover, studies have highlighted the important role of vitamin C in the prevention of cardiometabolic disorders, diabetes, and cancer (144, 145). Vitamin C also plays role in hormone regulation, neurotransmitter production, immunological functions, connective tissue development, and several other important biological functions (2, 6, 146, 147).
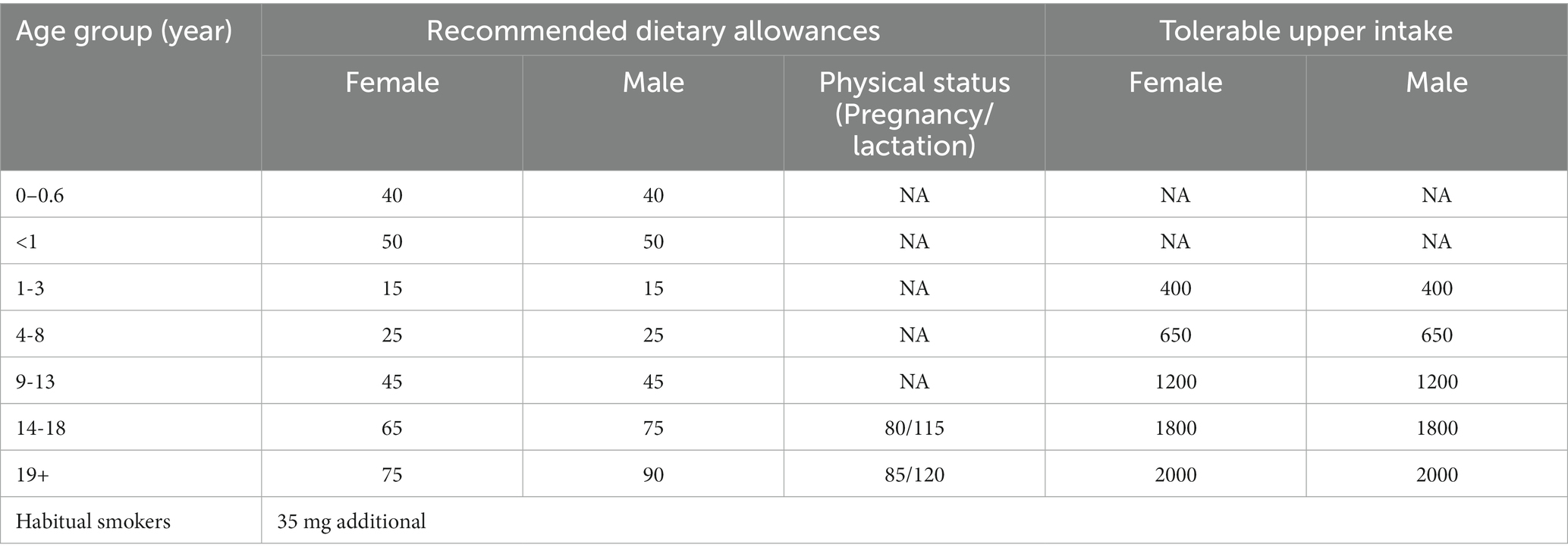
5.4. Recommended dietary allowance
The daily requirement of vitamin C depends somewhat on the gender, age, health status, and lifestyle of individuals (Table 4). However, the Institute of Medicine advises 75 mg/day (female) and 90 mg/day (adult men) as the recommended dietary allowance (RDA) (146, 148–152). The RDA is the amount of vitamin C that should be consumed daily to maintain good health [Dietary Reference Intakes: Thiamin R and Choline (153); IOM and FNB (128, 154)]. The Institute of Medicine also recommends the intake of35 mg of additional vitamin C for habitual smokers over the general population (2, 155).
5.5. Vitamin C intake and the current supply
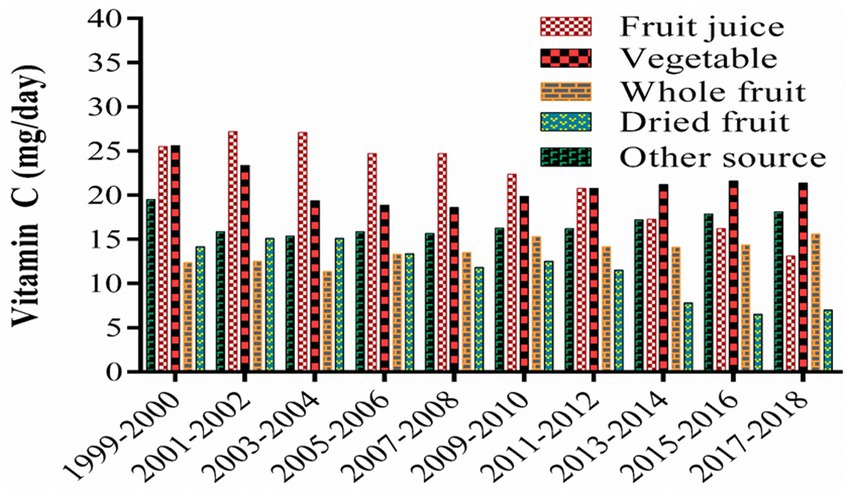
Food consumption patterns vary according to geography, demography, socioeconomic status, and dietary preferences. Researchers have reported that a significant proportion of vitamin C in the diet comes from fruit juices, vegetables, whole fruits, and dried fruits (Figure 6) and that vitamin C consumption has declined between 1999 and 2018 (148). In many populations, the current supply of vitamin C is sufficient to meet the RDA but, in some populations, this is not the case. As a result, strategies need to be developed to address potential vitamin C deficiencies in these populations.
6. Strategies adopted to address vitamin C deficiency
In general, there are several approaches to addressing vitamin deficiency: (i) diet; (ii) biofortification; (iii) food fortification; and (iv) dietary supplements (15–17). In this section, several of these approaches are discussed in the context of vitamin C.
6.1. Diet
Diet-based approaches involve the addition of vitamin C-rich food items to the diet, such as lemons, oranges, kiwi, and other fruits and vegetables (156–158). However, this strategy depends on the affordability and availability of these items, as well as typical eating patterns in the target populations (10, 15, 17). The inclusion of vitamin C-rich food items that have a high bioavailability is crucial for the success of this approach (10). Educating the target population about health concerns linked with vitamin C deficiency, as well as the sources of affordable vitamin C-rich foods are also important.
6.2. Supplementation
Dietary supplements are also a successful means of ensuring that people have sufficient levels of vitamins and minerals in their diet to prevent health problems (159, 160). Supplements are typically available as capsules, tablets, or powders containing vitamin C alone or in combination with other nutrients (161). These formulations are designed to contain levels of vitamin C or its analogs that help ensure people meet the RDA. They must be carefully formulated to ensure the vitamins remain stable during storage and after ingestion. The major limitation of supplementation is that they are not affordable or desirable for many people.
6.3. Biofortification
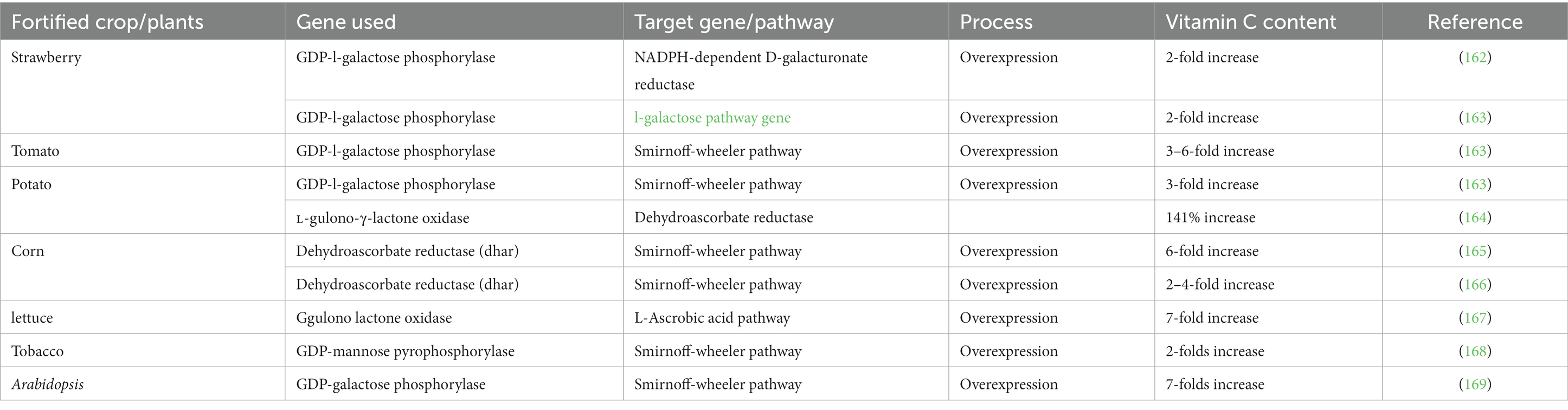
Biofortification approaches rely on increasing the micronutrient levels in agricultural staple crops using selective breeding, crop management, and/or genetic engineering approaches. Researchers have already used these approaches to increase the vitamin C levels in various kinds of fruits and vegetables, including strawberries, tomatoes, and potatoes (Table 5). For instance, researchers overexpressed the GDP-l-galactose phosphorylase (GGPor VTC2) gene in transgenic tomatoes to enhance their vitamin C content by 3- to 6-fold (163). Similarly, genes have been inserted into corn to increase its vitamin C content appreciably (165). Another study reported a 2-fold increase in the overexpression of the GDP-l-galactose phosphorylase gene in strawberries, leading to an increase in their vitamin C level (163).
6.4. Food fortification
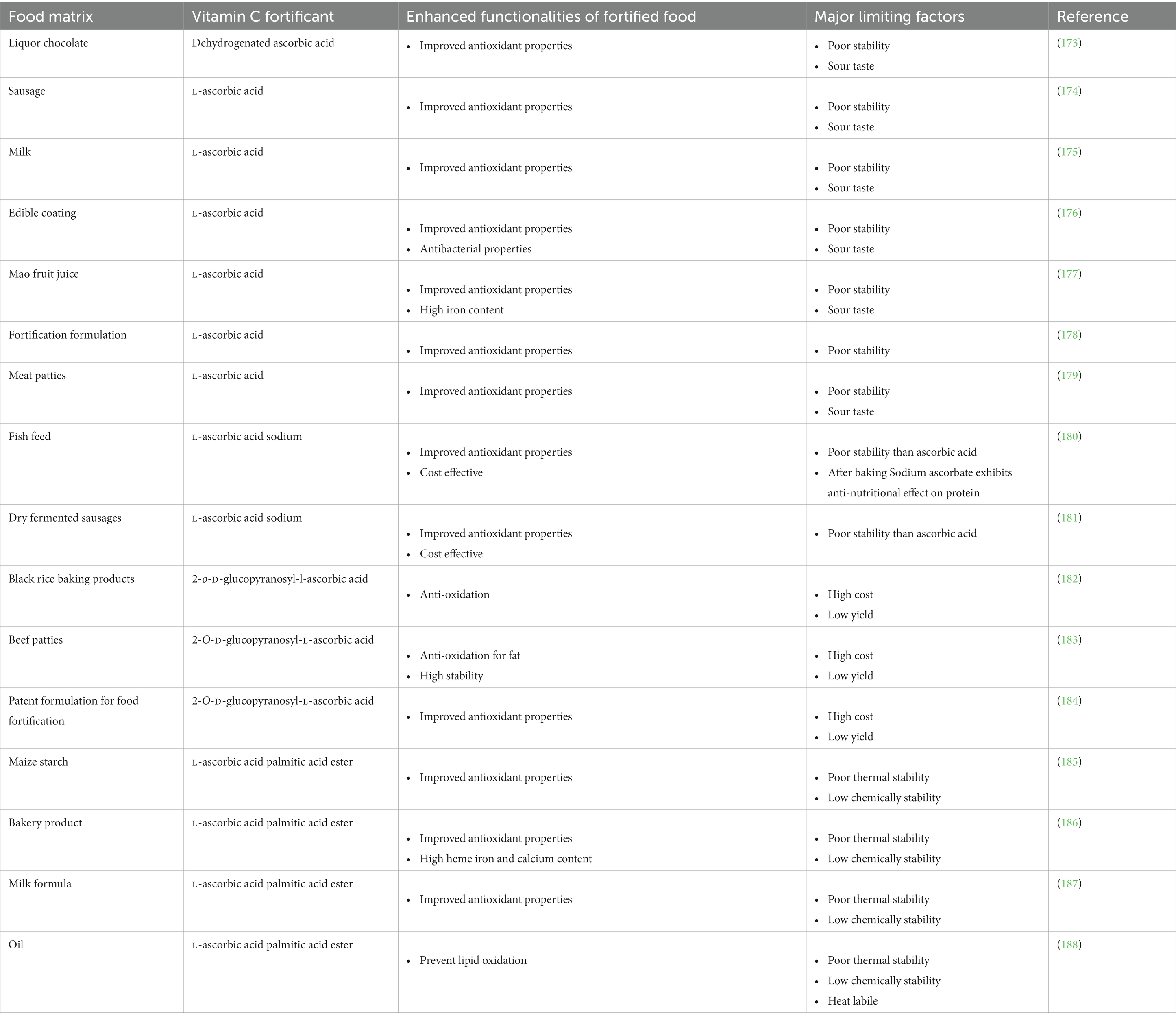
Food fortification is an effective, safe, and affordable approach to meeting the nutritional requirements of certain populations (170). The efficacy of vitamin fortification is enhanced when it can be integrated into an existing food supply network (15, 17). However, it is important to select appropriate food types for fortification with vitamin C. Knowledge of the vitamin C requirements and status of the target population is required. Information about the dietary patterns of this population is also required to establish the most common types and amounts of foods and beverages consumed. An understanding of the physicochemical properties of vitamin C and its analogs is also required, such as its stability, solubility, and interaction characteristics. Then, effective methods of incorporating vitamin C into these products in a stable and bioavailable form, without adversely impacting their organoleptic attributes or affordability, are required (171, 172). Vitamin C is a water-soluble molecule that can often be simply dissolved into aqueous solutions and food matrices. However, it may physically interact with other components or chemically degrade, which can reduce its efficacy or decrease food quality attributes. Consequently, fortification must be carried out carefully.
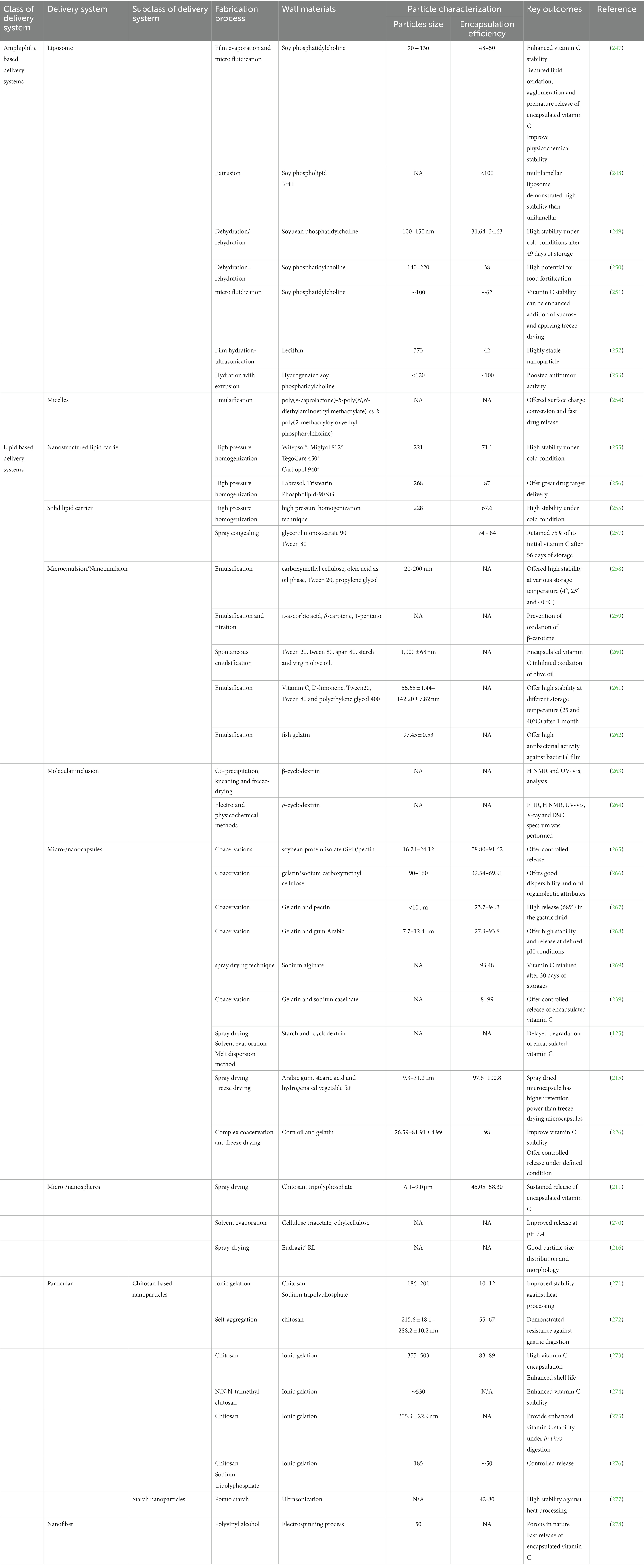
Various food matrices have already been fortified with vitamin C, however, significant losses can occur during storage, processing, and distribution (Tables 1, 6). The extent of these losses depends on food matrix effects and the environmental conditions the foods are exposed to (189–207). For instance, thermal processing and trace metals can promote rapid degradation of vitamin C (40, 46, 104, 106), thereby reducing its potentially beneficial health effects. These challenges can often be overcome using suitable encapsulation techniques (Figure 7 and Supplementary Table S1).
7. Encapsulation technologies and delivery systems
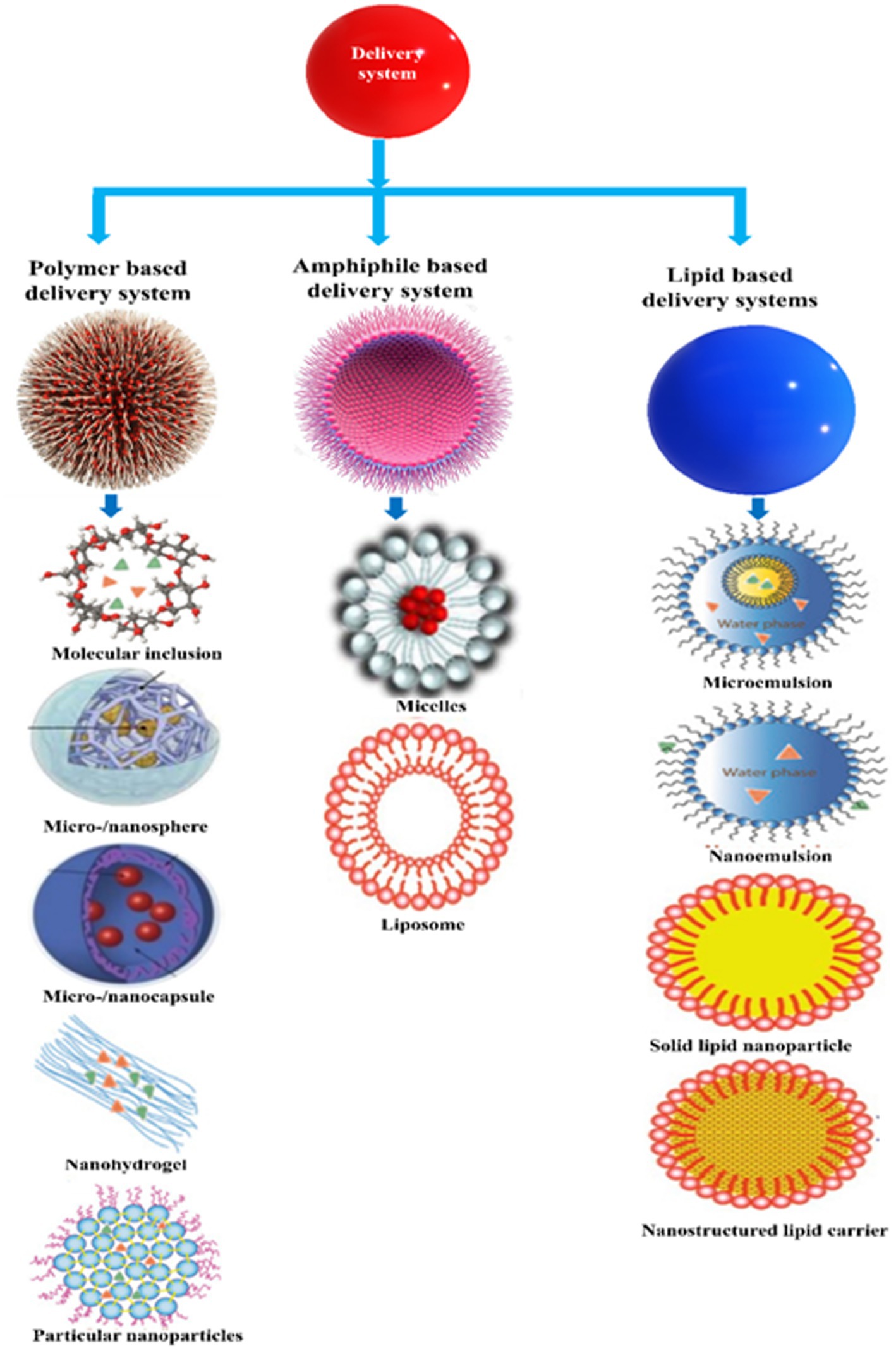
Encapsulation technologies are being used to improve the matrix compatibility, stability, and bioavailability of vitamins in fortified foods (15, 17, 171). A wide range of these technologies are available [Table 7 and Figure 8; (9, 11, 245, 246). At present, however, a universal encapsulation technology has not been developed that is applicable to all food products. Instead, they usually must be designed for the specific application, taking into account food matrix effects, and the nature of the processing, storage, and distribution conditions the food product will experience throughout its lifetime. Additionally, the knowledge of the performance of different vitamin C encapsulation technologies is still limited. For the sake of convenience, some of the most common encapsulation technologies developed for this purpose are classified into three groups based on the major ingredients used to fabricate them: polymer-, lipid-, and amphiphile-based systems (Supplementary Table S1). Nevertheless, some of these encapsulation technologies do combine two or more of these ingredients together.
7.1. Polymer-based delivery systems
Researchers have exploited the scaffolding ability of natural or synthetic polymers to fabricate polymer-based delivery systems compatible with vitamin C encapsulation. This class of delivery system includes nanofibers, inclusion complexes, capsules, and particles (Table 8). These delivery systems can be assembled using a range of ingredients and fabrication methods, including simple mixing, electrostatic complexation, antisolvent precipitation, injection-gelation, spray drying, freeze drying, cold setting, ionic gelation, and electrospinning, which are discussed in detail elsewhere (279–282).
7.1.1. Microfibers/nanofibers
Microfibers or nanofibers can be prepared using one or more natural or synthetic polymers (283, 284). Typically, a microfiber has a diameter above a few hundred nanometers whereas a nanofiber had a diameter below this value, but there is no clear cut off. A range of fabrication techniques have been used to create polymer fibers including centrifugal spinning and electrospinning, with the latter being most explored for encapsulation applications (285–291). The functional properties of electrospun fibers can be controlled by varying their composition, dimensions, and surface properties, which is useful for controlling the dispersibility, stability, and release behaviors of encapsulated vitamins (292–296). The use of polymer fibers for vitamin C encapsulation is still in its infancy, with few published studies in this area. One group developed vitamin C-loaded polyvinyl alcohol/β–cyclodextrin nanofibers suitable for applications in cosmetics, personal-care products, and topical drug delivery (297). Fish oil/gelatin nanofibers produced by electrospinning have also been used to encapsulate vitamin C (298).
7.1.2. Molecular inclusion complexes
Generally, polymeric molecular inclusion complexes are produced from polymers and other host molecules capable of binding guest molecules. Cyclodextrins are the most widely used substances to encapsulate bioactive compounds. They have a cavity that can accommodate guest molecules due to the formation of a helix by α(1,4)-linked glucose chains (299). Vitamin C can be incorporated into this cavity (300). Molecule inclusion complexes can be prepared using various methods, including solvent evaporation, isoelectric precipitation, mixing, and freeze-drying (301–306). The structural and physicochemical properties of vitamin C-loaded β-cyclodextrin molecular inclusion complexes formulated using different approaches (co-precipitation, kneading, and freeze-drying) have been characterized (263). Other researchers have also reported that β-cyclodextrin can be used to encapsulate vitamin C (264).
7.1.3. Polymer capsules and particles
Polymer capsules consist of polymeric shells surrounding fluid cores, whereas the whole of polymer particles consist of a polymer network. For the sake of clarity, these will both be referred to as polymer particles, unless otherwise stated. Polymer particles may be assembled from synthetic and/or natural polymers. In the food industry, proteins and polysaccharides are typically used for this purpose. Typically, microcapsules/microparticles have diameters more than a few hundred nanometers, whereas nanocapsules/nanoparticles have smaller dimensions. Polymer particles can be formed by various methods, including injection-gelation, coacervation, spray-drying, freeze-drying, solvent displacement, templating, and molding (307–312). A few studies have demonstrated the potential of polymer particles for vitamin C encapsulation. For instance, vitamin C was encapsulated in gelatin-based microparticles prepared using the coacervation method to improve its stability and control its release (226). Similarly, casein hydrolysate/soy protein/pectin particles have been used to improve vitamin C stability (265). Furthermore, polymer microparticles were fabricated using coacervation for co-encapsulation of vitamin C and quercetin (262). Some researchers have investigated the effects of different fabrication methods on the release of vitamin C from polymer microparticles (236). The retention and release behavior of vitamin C in gelatin-caseinate microparticles has also been studied (239). Studies have shown that vitamin C is released from gelatin-pectin microparticles under simulated gastrointestinal conditions (267). Alginate-based microparticles have been shown to retain vitamin C throughout 30 days of storage (269). Some researchers have examined the impact of introducing encapsulated vitamin C into food products. For instance vitamin C-loaded microparticles have been incorporated into bakery products (215). In this case, encapsulation was shown to increase the stability of the vitamin.
Several different kinds of food-grade polymer particles are summarized in Table 8. Chitosan is often used to assemble these systems because it is a cationic polysaccharide that can bind anionic vitamin C. For instance, vitamin C-chitosan nanoparticles have been shown to improve the bioavailability of the vitamin (276). In another study, researchers developed vitamin C-loaded nanoparticles using the ionic gelation method to improve vitamin C stability against heat processing (271). Encapsulation of vitamin C in chitosan-based nanoparticles has also been shown to improve it stability during storage (273) and to prolong its release under gastric fluid conditions (275). Vitamin C was also encapsulated in starch-derived nanoparticles to improve its stability and bioavailability (277). Typically, the composition, dimensions, structure, surface properties, and pore size of polymer particles must be manipulated to control the retention, release, and stability of vitamin C.
7.2. Amphiphile-based delivery systems
Amphiphilic-based delivery systems are typically assembled from amphiphilic ingredients, such as phospholipids and surfactants. These amphiphilic substances tend to assemble into a colloidal structure like micelles, microemulsions, or liposomes due to the hydrophobic effect.
7.2.1. Liposomes
Liposomes typically consist of phospholipid bilayers organized into one or more concentric shells. As a result, they have both hydrophilic domains (polar head groups and inner core) and lipophilic domains (non-polar tail groups). A variety of preparation methods have been developed to fabricate liposomes (313–317). Liposomes with diameters below a few hundred nanometers are often referred to as nanoliposomes. Several studies have shown that vitamin C can be encapsulated in liposomes (231, 233). For instance, researchers have shown that encapsulation of vitamin C in liposomes assembled from phosphatidylcholine, tocopherol, and cholesterol could improve its stability (231). Similarly, loading vitamin C into liposomes assembled from soybean phosphatidylcholine was shown to improve its resistance to oxidation and to premature release during digestion (247). Vitamin C has been co-encapsulated with vitamin A and methionine in liposomes (248). Vitamin C has been loaded into liposomes fabricated using a film hydration-sonication technique, which improved its stability (252). Some researchers have reported that vitamin C-loaded liposomes can be incorporated into milk products (233).
7.2.2. Micelles and microemulsions
Conventional micelles and oil-in-water microemulsions consists of small colloidal particles assembled from surfactants, where the non-polar tails are the interior, and the polar heads are exposed to the surrounding water (318–320). Conversely, in reverse micelles and water-in-oil (W/O) microemulsions the polar heads are in the interior and the non-polar tails are exposed to the surrounding oil. These kinds of association colloids are thermodynamically stable systems under specific compositional and environmental conditions (321). Consequently, they can often be formed by simply mixing the different components together. The particle size of these systems is typically very low (<50 nm), which means that are optically transparent and highly resistant to gravitational separation (322). A variety of fabrication methods are available for encapsulating bioactive substances within association colloids, including solvent evaporation and spontaneous emulsification (319, 322–327). It is often difficult to encapsulate and retain vitamin C into micelles and oil in water (O/W) microemulsions because of its hydrophilic nature. However, it can be trapped within the internal water domain of reverse micelles or W/O microemulsions when the continuous phase is oil. Only a few studies have so far been conducted on the use of association colloids for vitamin C encapsulation and delivery. For instance, vitamin C has been loaded into micelles assembled from modified-phosphorylcholine and used as an antitumor drug delivery system (254). Researchers have fabricated microemulsions from carboxymethyl cellulose, oleic acid, Tween 20, and propylene glycol and observed them to be highly stable at different storage temperatures (4°, 25°, and 40°C) (258). The researchers also investigated the influence of surfactant/co-surfactant and hydrophilic-lipophilic balance on vitamin C-loaded microemulsions (261). In general, this kind of delivery system is likely to be most useful for applications where the vitamin C needs to be trapped within an oil phase, then reverse micelles or W/O microemulsions can be used.
7.3. Lipid-based delivery systems
This group of delivery systems includes colloidal dispersions primarily assembled from edible fats and oils, including emulsions, solid lipid nanoparticles, and nanostructured lipid carriers (Table 8).
7.3.1. Emulsions
Emulsions are thermodynamically unstable colloidal dispersions because of the positive free energy associated with the oil-water interface. Emulsions with droplets below a few hundred nanometers are often referred to as nanoemulsions. A range of fabrication methods has been developed to form emulsions, including mechanical approaches (like microfluidization, homogenization, and sonication methods) and physicochemical approaches (like phase inversion and spontaneous emulsification methods) (328–330). Emulsions can be classified as oil-in-water or water-in-oil types depending on whether the oil phase makes up the droplets or the surrounding medium, respectively. O/W emulsions are rarely used to encapsulate vitamin C because it is hydrophilic and therefore tends to be soluble in the external aqueous phase, rather than inside the oil droplets. Researchers have encapsulated vitamin C within the internal aqueous phase of W/O/W multiple emulsions, but they did not measure its retention or stability over time (331). In another study, the same authors showed that the vitamin C was rapidly released from these emulsions, which can be attributed to the fact that it has some solubility in oil and can therefore diffuse out of the W/O droplets into the surrounding water (239). Emulsions therefore appear to have limited application for the encapsulation of vitamin C.
7.3.2. Solid lipid nanoparticles and nanostructured lipid carriers
This type of colloidal delivery system is like an emulsion, but the lipid droplets are fully or partially crystalline. Typically, an oil-in-water emulsion is formed at a temperature above the melting point of the fat phase, and then the system is cooled to promote crystallization and form solid lipid nanoparticles (SLNs) or nanostructured lipid carriers (NLCs) (332–335). In SLNs, the lipid phase is completely crystalline, whereas in NLCs it is only partly crystalline. The advantages of using these kinds of delivery systems are that the solid nature of the lipid phase can slow down molecular diffusion processes, which can improve the retention and stability of encapsulated substances. SLNs and NLCs are typically used to encapsulate lipophilic bioactive substances but some researchers have examined their application to vitamin C. For instance, vitamin C-loaded SLNs have been prepared using a hot homogenization method (255). Another study reported that vitamin C was retained in SLNs at a relatively high level (>75%) after 56 days of storage (257). High-pressure homogenization has been used to produce vitamin C-loaded NLCs, which was shown to prolong the release of the vitamin (256). Nevertheless, further research is required in this area. Like emulsions, it may be difficult to trap and retain the hydrophilic vitamin C molecules within the hydrophobic interior of the particles in SLNs and NLCs.
8. Fate of vitamin C loaded delivery systems in gastrointestinal tract
It is often important to design food-grade delivery systems that can increase the bioavailability of nutrients and/or control the region they are released and absorbed in the gastrointestinal tract (9, 13–17, 67). This can often be achieved by controlling the compositions, sizes, structures, physical states, aggregation states, and interfacial properties of the colloidal particles they contain. Most research studies on vitamin C fortification using delivery systems have focused on the following aspects: morphological characterization, degree of stability enhancement, release kinetics, compatibility with food matrices, stability in food matrices, and impact on food properties (9). Some studies have also examined the bioaccessibility (in vitro models) or bioavailability (in vivo models) of encapsulated vitamin C.
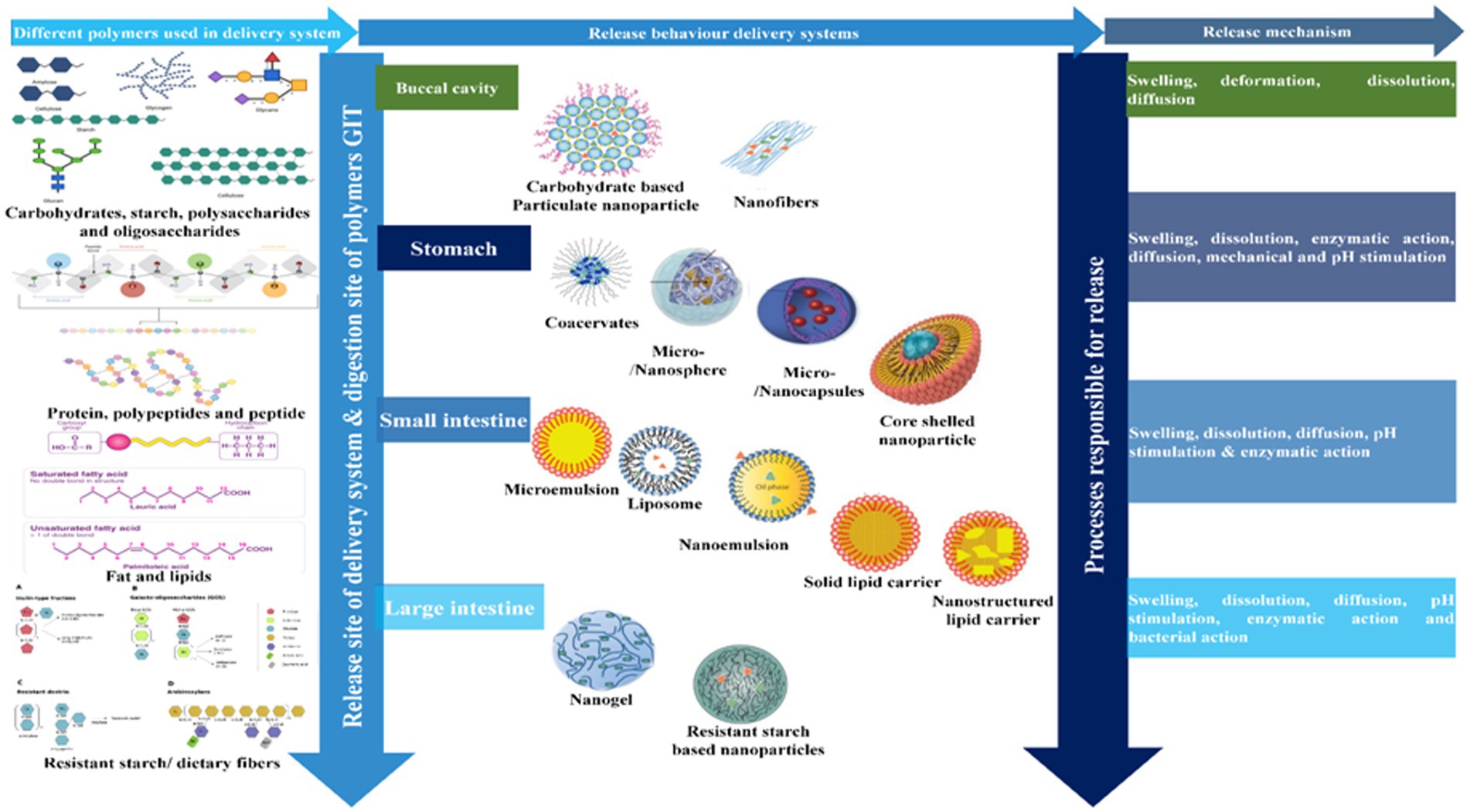
It is important that any encapsulated vitamin C is released within the gastrointestinal tract in an active form that can be absorbed by the enterocytes. The hydrophilic nature of vitamin C means that it is usually highly soluble in gastrointestinal fluids, which ensures it has a high bioaccessibility (67). However, it may chemically degrade within the gastrointestinal environment, which can be inhibited using well-designed delivery systems. Nevertheless, there is still a need for a systematic comparison of the efficacy of different kinds of delivery systems for improving the bioavailability of vitamin C in different food matrices. A schematic diagram of the fate of vitamin C-loaded delivery systems in the human gastrointestinal tract is shown in Figure 8: (i) the delivery system should initially contain a sufficiently high concentration of the vitamin to have a biological effect; (ii) the delivery system should retain and protect the vitamin in the mouth and stomach; (iii) the delivery system should release the vitamin in the small intestine where absorption normally occurs; (iv) the delivery system might be designed to protect the vitamin and promote its absorption in the small intestine; (v) the delivery system itself should be safe for application within foods. Clearly, further studies are needed in this area.
9. Safety compliance and risks of vitamin C delivery systems
It is important that any vitamin C delivery systems are safe for human consumption and do not have any unforeseen adverse health effects (336, 337). Synthetic polymers or surfactants may have some undesirable health impacts and therefore natural alternatives may be better (338). Similarly, the use of organic solvents, alcohols, or synthetic chemicals during the production of the delivery systems should be avoided, or they should be completely removed prior to sale, to reduce health risks (338). In general, the impact of their short- and long-term effects on human health should be assessed (338). The Food and Drug Administration (FDA) in the United States has released guidelines regarding the incorporation of nanoparticles in foods (339). The European Food Safety Authority (EFSA) in the European Union has developed regulations on the utilization of nanomaterials as delivery systems in foods (340). Methods to perform risk assessments of nanomaterials applied in foods have been given (341).
10. Conclusion
In many countries, the general population consumes enough fruits and vegetables to have sufficient levels of vitamin C in their diets. However, there are some populations that do suffer from vitamin C deficiencies, which lead to debilitating diseases like scurvy. Moreover, vitamin C may act as a nutraceutical ingredient that can exhibit a range of other beneficial health effects, especially due to its antioxidant activity. The biological activity of vitamin C in many foods and beverages is limited because of its tendency to chemical degrade. Consequently, there is interest in improving the chemical stability and bioavailability of this bioactive substance using encapsulation technologies. There have been many studies on the use of colloidal delivery systems to encapsulate, protect, and release hydrophobic vitamins (like vitamins A, D, and E) but to far fewer on their application to hydrophilic vitamins (like vitamin C). There appears to be a range of colloidal delivery systems available that can be used for this purpose, especially those that have hydrophilic domains inside the particles (like polymer particles, W/O/W emulsions, and liposomes) but further work is needed to establish their relative merits and limitations. Moreover, research is required to establish whether they can be affordably produced at sufficiently high quantities for commercial applications, and whether they are robust enough and effective under real life situations.
Author contributions
VM: conceptualization, methodology, writing – original draft, writing – review and editing, and data curation. AS: methodology, writing – review and editing, writing – original draft, and conceptualization. DM: project administration, supervision, visualization, writing – review and editing. RS: data curation, formal analysis, and visualization. KB: investigation, data curation, methodology, and formal analysis. TR: methodology, formal analysis, and resources. JL: funding acquisition, resources, validation, and review. ES: funding acquisition, resources, and validation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Priority Research Centres Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2014R1A6A1031189) and by an NRF grant funded by the Korean government (MSIT) (Grant No. 2021R1A2C1008368).
Conflict of interest
VM was employed by the company PerkinElmer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1229243/full#supplementary-material
References
2. Combs, GF Jr, and McClung, JP. The vitamins: fundamental aspects in nutrition and health. Cambridge, Massachusetts, United States: Academic press (2016).
3. Davies, M. B., Austin, J., and Partridge, D. A. (1991). Vitamin C: its chemistry and biochemistry: Royal society of chemistry.
4. Carr, AC, and Rowe, S. Factors affecting vitamin C status and prevalence of deficiency: A global health perspective. Nutrients. (2020) 12:1963. doi: 10.3390/nu12071963
6. Granger, M, and Eck, P. Dietary vitamin C in human health. Adv Food Nutr Res. (2018) 83:281–310. doi: 10.1016/bs.afnr.2017.11.006
7. Iqbal, K, Khan, A, and Khattak, M. Biological significance of ascorbic acid (vitamin C) in human health-a review. Pakistan J Nutr. (2004) 3:5–13. doi: 10.3923/pjn.2004.5.13
8. Orriss, GD . Food fortification: Safety and legislation. Food Nutr Bull. (1998) 19:109–16. doi: 10.1177/156482659801900204
9. Abbas, S, Da Wei, C, Hayat, K, and Xiaoming, Z. Ascorbic acid: microencapsulation techniques and trends—a review. Food Rev. Int. (2012) 28:343–74. doi: 10.1080/87559129.2011.635390
10. Caritá, AC, Fonseca-Santos, B, Shultz, JD, Michniak-Kohn, B, Chorilli, M, and Leonardi, GR. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed Nanotechnol Biol Med. (2020) 24:102117. doi: 10.1016/j.nano.2019.102117
11. Comunian, T, Babazadeh, A, Rehman, A, Shaddel, R, Akbari-Alavijeh, S, Boostani, S, et al. Protection and controlled release of vitamin C by different micro/nanocarriers. Crit Rev Food Sci Nutr. (2020) 62:3301–22. doi: 10.1080/10408398.2020.1865258
12. Lešková, AS-MM-E . Vitamin C degradation during storage of fortified foods. J Food Nutr Res. (2006) 45:55–61. Available at: https://iifiir.org/en/fridoc/vitamin-c-degradation-during-storage-of-fortified-foods-127136
13. Maurya, VK, and Aggarwal, M. Enhancing bio-availability of vitamin D by Nano-engineered based delivery systems-an overview. Int J Curr Microbiol App Sci. (2017) 6:340–53. doi: 10.20546/ijcmas.2017.607.040
14. Maurya, VK, Aggarwal, M, Ranjan, V, and Gothandam, KM. Improving bioavailability of vitamin A in food by encapsulation: an update. Nanosci Med. (2020) 1:117–45. doi: 10.1007/978-3-030-29207-2_4
15. Maurya, VK, Bashir, K, and Aggarwal, M. Vitamin D microencapsulation and fortification: trends and technologies. J Steroid Biochem Molecul Biol. (2020) 196:105489. doi: 10.1016/j.jsbmb.2019.105489
16. Maurya, VK, Shakya, A, Aggarwal, M, Gothandam, KM, Bohn, T, and Pareek, S. Fate of β-carotene within loaded delivery systems in food: state of knowledge. Antioxidants. (2021) 10:426. doi: 10.3390/antiox10030426
17. Maurya, VK, Shakya, A, Bashir, K, Kushwaha, SC, and McClements, DJ. Vitamin A fortification: recent advances in encapsulation technologies. Comprehens Rev Food Sci Food Safety. (2022) 21:2772–819. doi: 10.1111/1541-4337.12941
18. McCord, CP . SCURVY as an occupational disease: the Sappington memorial lecture. J Occup Med. (1959) 1:315–8. doi: 10.1097/00043764-197110000-00007
20. Szent-Györgyi, A . Observations on the function of peroxidase systems and the chemistry of the adrenal cortex: Description of a new carbohydrate derivative. Biochem J. (1928) 22:1387–409. doi: 10.1042/bj0221387
21. King, C. G., and Waugh, W. A. (1932). The chemical nature of vitamin C. Science, 75, 357–358, doi: 10.1126/science.75.1944.357.b
22. Carpenter, KJ . The history of scurvy and vitamin C. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim: Cambridge University Press (1988).
23. Rucker, RB, Zempleni, J, Suttie, JW, and McCormick, DB. Handbook of vitamins. England & Wales: Crc Press (2007).
24. Chatterjee, I . The history of vitamin C research in India. J Biosci. (2009) 34:185. doi: 10.1007/s12038-009-0021-7
25. Chatterjee, I . Evolution and the biosynthesis of ascorbic acid. Science. (1973) 182:1271–2. doi: 10.1126/science.182.4118.1271
26. Drouin, G, Godin, J-R, and Pagé, B. The genetics of vitamin C loss in vertebrates. Curr Genom. (2011) 12:371–8. doi: 10.2174/138920211796429736
27. Jukes, TH, and King, JL. Evolutionary loss of ascorbic acid synthesizing ability. J Hum Evol. (1975) 4:85–8. doi: 10.1016/0047-2484(75)90002-0
28. Sotiriou, S, Gispert, S, Cheng, J, Wang, Y, Chen, A, Hoogstraten-Miller, S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. (2002) 8:514–7. doi: 10.1038/0502-514
29. Rumsey, SC, Kwon, O, Xu, GW, Burant, CF, Simpson, I, and Levine, M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. (1997) 272:18982–9. doi: 10.1074/jbc.272.30.18982
30. Lykkesfeldt, J, and Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients. (2019) 11:2412. doi: 10.3390/nu11102412
31. Kon, SK, and Watson, MB. The vitamin C content of cow's milk. Biochem J. (1937) 31:223. doi: 10.1042/bj0310223
32. Romeu-Nadal, M, Morera-Pons, S, Castellote, A, and Lopez-Sabater, M. Rapid high-performance liquid chromatographic method for Vitamin C determination in human milk versus an enzymatic method. J Chromatography B. (2006) 830:41–6. doi: 10.1016/j.jchromb.2005.10.018
33. Klimczak, I, and Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. (2015) 175:100–5. doi: 10.1016/j.foodchem.2014.11.104
34. Hernández, Y, Lobo, MG, and González, M. Determination of vitamin C in tropical fruits: a comparative evaluation of methods. Food Chem. (2006) 96:654–64. doi: 10.1016/j.foodchem.2005.04.012
35. Pfendt, LB, Vukašinović, VL, Blagojević, NZ, and Radojević, MP. Second order derivative spectrophotometric method for determination of vitamin C content in fruits, vegetables and fruit juices. Eur Food Res Technol. (2003) 217:269–72. doi: 10.1007/s00217-003-0746-8
36. Chebrolu, KK, Jayaprakasha, GK, Jifon, J, and Patil, BS. Production system and storage temperature influence grapefruit vitamin C, limonoids, and carotenoids. J Agric Food Chem. (2012) 60:7096–103. doi: 10.1021/jf301681p
37. Bashir, HA, and Abu-Goukh, A-BA. Compositional changes during guava fruit ripening. Food Chem. (2003) 80:557–63. doi: 10.1016/S0308-8146(02)00345-X
38. Kumar, GV, Kumar, A, Raghu, K, Patel, GR, and Manjappa, S. Determination of vitamin C in some fruits and vegetables in Davanagere city,(Karanataka)–India. Int J Pharm Life Sci. (2013) 4:2489–91.
39. Laur, LM, and Tian, L. Provitamin A and vitamin C contents in selected California-grown cantaloupe and honeydew melons and imported melons. J Food Composit Anal. (2011) 24:194–201. doi: 10.1016/j.jfca.2010.07.009
40. Liu, H, Cao, J, and Jiang, W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT Food Sci Technol. (2015) 63:1042–8. doi: 10.1016/j.lwt.2015.04.052
41. Klesk, K, Qian, M, and Martin, RR. Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. J Agric Food Chem. (2004) 52:5155–61. doi: 10.1021/jf0498721
42. Leahu, A, Damian, C, Oroian, M, Ropciuc, S, and Rotaru, R. Influence of processing on vitamin C content of rosehip fruits. Sci Papers Anim Sci Biotechnol. (2014) 47:116–20.
43. Koyuncu, MA, and Dilmaçünal, T. Determination of vitamin C and organic acid changes in strawberry by HPLC during cold storage. Not Botanic Horti Agrob Cluj-Napoca. (2010) 38:95–8. doi: 10.15835/nbha3834819
44. Izuagie, A, and Izuagie, F. Iodimetric determination of ascorbic acid (vitamin C) in citrus fruits. Res J Agric Biol Sci. (2007) 3:367–9.
45. Padayatty, SJ, Katz, A, Wang, Y, Eck, P, Kwon, O, Lee, J-H, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am College Nutr. (2003) 22:18–35. doi: 10.1080/07315724.2003.10719272
46. Somsub, W, Kongkachuichai, R, Sungpuag, P, and Charoensiri, R. Effects of three conventional cooking methods on vitamin C, tannin, myo-inositol phosphates contents in selected Thai vegetables. J Food Composit Anal. (2008) 21:187–97. doi: 10.1016/j.jfca.2007.08.002
47. Ko, Y, Ashok, S, Ainala, SK, Sankaranarayanan, M, Chun, AY, Jung, GY, et al. Coenzyme B12 can be produced by engineered Escherichia coli under both anaerobic and aerobic conditions. Biotechnol J. (2014) 9:1526–35. doi: 10.1002/biot.201400221
48. Matějková, J, and Petříková, K. Variation in content of carotenoids and vitamin C in carrots. Notul Sci Biol. (2010) 2:88–91. doi: 10.15835/nsb245108
49. Sowbhagya, H . Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): an overview. Critic Rev Food Sci Nutr. (2014) 54:389–98. doi: 10.1080/10408398.2011.586740
50. Phillips, KM, Tarrago-Trani, MT, Gebhardt, SE, Exler, J, Patterson, KY, Haytowitz, DB, et al. Stability of vitamin C in frozen raw fruit and vegetable homogenates. J Food Composit Analysis. (2010) 23:253–9. doi: 10.1016/j.jfca.2009.08.018
51. Colina-Coca, C, de Ancos, B, and Sánchez-Moreno, C. Nutritional composition of processed onion: S-Alk (en) yl-L-cysteine sulfoxides, organic acids, sugars, minerals, and vitamin C. Food Bioprocess Technol. (2014) 7:289–98. doi: 10.1007/s11947-013-1150-4
52. Biringen Löker, G, Ugˇur, M, and Yıldız, M. A parrtial supplementation of pasteurized milk with vitamin C, iron and zinc. Food/Nahrung. (2003) 47:17–20. doi: 10.1002/food.200390001
53. De Ritter, E . Stability characteristics of vitamins in processed foods. Food Technol. (1976) 48-54. Available at: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL7638010100
54. Park, H, Seib, PA, Chung, OK, and Seitz, LM. Fortifying bread with each of three antioxidants. Cereal Chem. (1997) 74:202–6. doi: 10.1094/CCHEM.1997.74.3.202
55. Park, H, Seib, PA, and Chung, OK. Fortifying bread with a mixture of wheat fiber and psyllium husk fiber plus three antioxidants. Cereal Chem. (1997) 74:207–11. doi: 10.1094/CCHEM.1997.74.3.207
56. Wang, X, Seib, P, and Ra, K. L-Ascorbic acid and its 2-phosphorylated derivatives in selected foods: vitamin C fortification and antioxidant properties. J Food Sci. (1995) 60:1295–300. doi: 10.1111/j.1365-2621.1995.tb04578.x
58. Anderson, RH, Maxwell, DL, Mulley, AE, and Fritsch, CW. Effects of processing and storage on micronutrients in breakfast cereals. Food Technol. (1976)
59. Murtaza, MA, Huma, N, Javaid, J, Shabbir, MA, Din, GMU, and Mahmood, S. Studies on stability of strawberry drink stored at different temperatures. Int. J. Agri. Biol. (2004) 6:58–60. doi: 10.1046/j.1537-2995.1979.19680104104.x
60. Talcott, ST, Percival, SS, Pittet-Moore, J, and Celoria, C. Phytochemical composition and antioxidant stability of fortified yellow passion fruit (Passiflora edulis). J Agric Food Chem. (2003) 51:935–41. doi: 10.1021/jf020769q
61. Choi, M, Kim, G, and Lee, H. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res Int. (2002) 35:753–9. doi: 10.1016/S0963-9969(02)00071-6
62. Mehansho, H, Mellican, RI, Hughes, DL, Compton, DB, and Walter, T. Multiple-micronutrient fortification technology development and evaluation: from lab to market. Food Nutr Bull. (2003) 24:S111–9. doi: 10.1177/15648265030244S108
63. Konopacka, D, and Markowski, J. Retention of ascorbic acid during apple chips production and storage. Polish J Food Nutr Sci. (2004) 13:237–42.
64. Wang, XY, Kozempel, MG, Hicks, KB, and Seib, PA. Vitamin C stability during preparation and storage of potato flakes and reconstituted mashed potatoes. J Food Sci. (1992) 57:1136–9. doi: 10.1111/j.1365-2621.1992.tb11282.x
65. Brandon, EFA, Bakker, MI, Kramer, E, Bouwmeester, H, Zuidema, T, and Alewijn, M. Bioaccessibility of vitamin A, vitamin C and folic acid from dietary supplements, fortified food and infant formula. Int J Food Sci Nutr. (2014) 65:426–35. doi: 10.3109/09637486.2013.869795
66. Rodríguez-Roque, MJ, Rojas-Graü, MAA, Elez-Martínez, P, and Martín-Belloso, O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blended fruit juice. J Agric Food Chem. (2013) 61:1859–67. doi: 10.1021/jf3044204
67. Yaman, M, Çatak, J, Uğur, H, Gürbüz, M, Belli, İ, Tanyıldız, SN, et al. The bioaccessibility of water-soluble vitamins: A review. Trends Food Sci Technol. (2021) 109:552–63. doi: 10.1016/j.tifs.2021.01.056
68. Pérez-Vicente, A, Gil-Izquierdo, A, and García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J Agric Food Chem. (2002) 50:2308–12. doi: 10.1021/jf0113833
69. Rodríguez-Roque, MJ, de Ancos, B, Sánchez-Moreno, C, Cano, MP, Elez-Martínez, P, and Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J Funct Foods. (2015) 14:33–43. doi: 10.1016/j.jff.2015.01.020
70. Cilla, A, Perales, S, Lagarda, MJ, Barberá, R, Clemente, G, and Farré, R. Influence of storage and in vitro gastrointestinal digestion on total antioxidant capacity of fruit beverages. J Food Composit Analysis. (2011) 24:87–94. doi: 10.1016/j.jfca.2010.03.029
71. Cilla, A, Alegría, A, de Ancos, B, Sánchez-Moreno, C, Cano, MP, Plaza, L, et al. Bioaccessibility of tocopherols, carotenoids, and ascorbic acid from milk-and soy-based fruit beverages: Influence of food matrix and processing. J Agric Food Chem. (2012) 60:7282–90. doi: 10.1021/jf301165r
72. Aschoff, JK, Kaufmann, S, Kalkan, O, Neidhart, S, Carle, R, and Schweiggert, RM. In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. J Agric Food Chem. (2015) 63:578–87. doi: 10.1021/jf505297t
73. Fain, O . Vitamin C deficiency. La Revue Med Inter. (2004) 25:872–80. doi: 10.1016/j.revmed.2004.03.009
74. Nakade, M, Jungari, ML, Ambad, R, and Dhingra, G. Status of vitamins and minerals in pregnancy: still a point of concern in Central India. Int J Cur Res Rev|. (2020) 12:45–9. doi: 10.31782/ijcrr.2020.4549
75. Rowe, S, and Carr, AC. Global vitamin C status and prevalence of deficiency: a cause for concern? Nutrients. (2020) 12:2008. doi: 10.3390/nu12072008
76. van den Berg, H, van der Gaag, M, and Hendriks, H. Influence of lifestyle on vitamin bioavailability. Int J Vitamin Nutr Res. (2002) 72:53–9. doi: 10.1024/0300-9831.72.1.53
77. Canoy, D, Wareham, N, Welch, A, Bingham, S, Luben, R, Day, N, et al. Plasma ascorbic acid concentrations and fat distribution in 19 068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am J Clin Nutr. (2005) 82:1203–9. doi: 10.1093/ajcn/82.6.1203
78. McCall, SJ, Clark, AB, Luben, RN, Wareham, NJ, Khaw, K-T, and Myint, PK. Plasma Vitamin C levels: Risk factors for deficiency and association with self-reported functional health in the European Prospective Investigation into Cancer-Norfolk. Nutrients. (2019) 11:1552. doi: 10.3390/nu11071552
79. Bates, CJ, Prentice, A, Cole, T, Van der Pols, J, Doyle, W, Finch, S, et al. Micronutrients: highlights and research challenges from the 1994–5 National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr. (1999) 82:7–15. doi: 10.1017/S0007114599001063
80. Wrieden, WL, Hannah, MK, Bolton-Smith, C, Tavendale, R, Morrison, C, and Tunstall-Pedoe, H. Plasma vitamin C and food choice in the third Glasgow MONICA population survey. J Epidemiol Commun Health. (2000) 54:355–60. doi: 10.1136/jech.54.5.355
81. Charlton, KE, Kolbe-Alexander, TL, and Nel, JH. Micronutrient dilution associated with added sugar intake in elderly black South African women. Eur J Clin Nutr. (2005) 59:1030–42. doi: 10.1038/sj.ejcn.1602208
82. de Oliveira, AM, Rondó, PHC, Mastroeni, SS, and Oliveira, JM. Plasma concentrations of ascorbic acid in parturients from a hospital in Southeast Brazil. Clin Nutr. (2008) 27:228–32. doi: 10.1016/j.clnu.2007.11.006
83. García, OP, Ronquillo, D, Caamaño, MDC, Camacho, M, Long, KZ, and Rosado, JL. Zinc, vitamin A, and vitamin C status are associated with leptin concentrations and obesity in Mexican women: results from a cross-sectional study. Nutr Metab. (2012) 9:59. doi: 10.1186/1743-7075-9-59
84. Halestrap, P, and Scheenstra, S. Outbreak of scurvy in Tana River County, Kenya: A case report. Afr J Primary Health Care Family Med. (2018) 10:1–3. doi: 10.4102/phcfm.v10i1.1811
85. Hamer, DH, Sempértegui, F, Estrella, B, Tucker, KL, Rodríguez, A, Egas, J, et al. Micronutrient deficiencies are associated with impaired immune response and higher burden of respiratory infections in elderly Ecuadorians. J Nutr. (2008) 139:113–9. doi: 10.3945/jn.108.095091
86. Kiondo, P, Tumwesigye, NM, Wandabwa, J, Wamuyu-Maina, G, Bimenya, GS, and Okong, P. Plasma vitamin C assay in women of reproductive age in Kampala, Uganda, using a colorimetric method. Tropical Med Inter Health. (2012) 17:191–6. doi: 10.1111/j.1365-3156.2011.02907.x
87. Nwagha, UI, Iyare, EE, Ejezie, FE, Ogbodo, SO, Dim, CC, and Anyaehie, BU. Parity related changes in obesity and some antioxidant vitamins in non-pregnant women of South-Eastern Nigeria. Nigerian J Clin Pract. (2012) 15:380–4. doi: 10.4103/1119-3077.104506
88. Ugwa, EA, Iwasam, EA, and Nwali, MI. Low serum vitamin C status among pregnant women attending antenatal care at general hospital Dawakin kudu, Northwest Nigeria. Int J Prevent Med. (2016) 7:40. doi: 10.4103/2008-7802.176166
89. Villalpando, S, Montalvo-Velarde, I, Zambrano, N, García-Guerra, A, Ramírez-Silva, CI, Shamah-Levy, T, et al. Vitamins A, and C and folate status in Mexican children under 12 years and women 12-49 years: a probabilistic national survey. Salud Public Mexico. (2003) 45:508–19. doi: 10.1590/S0036-36342003001000007
90. Frankenfeld, CL, Lampe, JW, Shannon, J, Gao, DL, Li, W, Ray, RM, et al. Fruit and vegetable intakes in relation to plasma nutrient concentrations in women in Shanghai, China. Public Health Nutr. (2012) 15:167–75. doi: 10.1017/S1368980011001029
91. Lam, TK, Freedman, ND, Fan, J-H, Qiao, Y-L, Dawsey, SM, Taylor, PR, et al. Prediagnostic plasma vitamin C and risk of gastric adenocarcinoma and esophageal squamous cell carcinoma in a Chinese population. Am J Clin Nutr. (2013) 98:1289–97. doi: 10.3945/ajcn.113.061267
92. Ravindran, RD, Vashist, P, Gupta, SK, Young, IS, Maraini, G, Camparini, M, et al. Prevalence and risk factors for vitamin C deficiency in north and south India: a two centre population based study in people aged 60 years and over. PLoS One. (2011) 6:e28588. doi: 10.1371/journal.pone.0028588
94. Carter, B, Monsivais, P, and Drewnowski, A. Absorption of folic acid and ascorbic acid from nutrient comparable beverages. Journal of food science. (2010) 75:H289–93. doi: 10.1111/j.1750-3841.2010.01844.x
95. Drewnowski, A, Rock, CL, Henderson, SA, Shore, AB, Fischler, C, Galan, P, et al. Serum beta-carotene and vitamin C as biomarkers of vegetable and fruit intakes in a community-based sample of French adults. Am J Clin Nutr. (1997) 65:1796–802. doi: 10.1093/ajcn/65.6.1796
96. Langlois, K, Cooper, M, and Colapinto, CK. Vitamin C status of Canadian adults: findings from the 2012/2013 Canadian Health Measures Survey Statistics Canada (2016).
97. Mosdøl, A, Erens, B, and Brunner, EJ. Estimated prevalence and predictors of vitamin C deficiency within UK's low-income population. J Public Health. (2008) 30:456–60. doi: 10.1093/pubmed/fdn076
98. Paalanen, L, Prättälä, R, Alfthan, G, Salminen, I, and Laatikainen, T. Vegetable and fruit consumption, education and plasma vitamin C concentration in Russian and Finnish Karelia, 1992–2002. Public Health Nutr. (2014) 17:2278–86. doi: 10.1017/S1368980013002243
99. Schleicher, RL, Carroll, MD, Ford, ES, and Lacher, DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. (2009) 90:1252–63. doi: 10.3945/ajcn.2008.27016
101. World health in 1970 The Medical journal of Australia. (1971) 2:453–5. doi: 10.5694/j.1326-5377.1971.tb50666.x
102. Gittelsohn, J, Thapa, M, and Landman, LT. Cultural factors, caloric intake and micronutrient sufficiency in rural Nepali households. Soc Sci Med. (1997) 44:1739–49. doi: 10.1016/S0277-9536(96)00375-9
103. Hughes, K, and Ong, C-N. Vitamins, selenium, iron, and coronary heart disease risk in Indians, Malays, and Chinese in Singapore. J Epidemiol Commun Health. (1998) 52:181–5. doi: 10.1136/jech.52.3.181
104. Navarre, DA, Shakya, R, Holden, J, and Kumar, S. The effect of different cooking methods on phenolics and vitamin C in developmentally young potato tubers. Am J Potato Res. (2010) 87:350–9. doi: 10.1007/s12230-010-9141-8
105. Santos, PHS, and Silva, MA. Retention of vitamin C in drying processes of fruits and vegetables—a review. Dry Technol. (2008) 26:1421–37. doi: 10.1080/07373930802458911
106. Soares, A, Carrascosa, C, and Raposo, A. Influence of different cooking methods on the concentration of glucosinolates and vitamin C in broccoli. Food Bioprocess Technol. (2017) 10:1387–411. doi: 10.1007/s11947-017-1930-3
107. Jungert, A, and Neuhäuser-Berthold, M. The lower vitamin C plasma concentrations in elderly men compared with elderly women can partly be attributed to a volumetric dilution effect due to differences in fat-free mass. Br J Nutr. (2015) 113:859–64. doi: 10.1017/S0007114515000240
108. Ness, AR, Cappuccio, FP, Atkinson, RW, Khaw, K-T, and Cook, DG. Plasma vitamin C levels in men and women from different ethnic backgrounds living in England. Int J Epidemiol. (1999) 28:450–5. doi: 10.1093/ije/28.3.450
109. Bates, B., Collins, D., Cox, L., Nicholson, S., Page, P., Roberts, C., et al. (2019). National Diet and Nutrition Survey Years 1 to 9 of the Rolling Programme (2008/2009–2016/2017): Time trend and income analyses. Public Health England: London, UK, 56–60.
110. Chiplonkar, SA, Agte, VV, Mengale, SS, and Tarwadi, KV. Are lifestyle factors good predictors of retinol and vitamin C deficiency in apparently healthy adults? Eur J Clin Nutr. (2002) 56:96–104. doi: 10.1038/sj.ejcn.1601291
111. Pearson, JF, Pullar, JM, Wilson, R, Spittlehouse, JK, Vissers, M, Skidmore, PML, et al. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: findings of the CHALICE cohort study. Nutrients. (2017) 9:831. doi: 10.3390/nu9080831
112. Timpson, NJ, Forouhi, NG, Brion, M-J, Harbord, RM, Cook, DG, Johnson, P, et al. Genetic variation at the SLC23A1 locus is associated with circulating concentrations of L-ascorbic acid (vitamin C): evidence from 5 independent studies with> 15,000 participants. Am J Clin Nutr. (2010) 92:375–82. doi: 10.3945/ajcn.2010.29438
113. Viroonudomphol, D, Mahaisiriyodom, A, Mingkhawn, R, Sadomthian, P, Korchasri, N, Jittngamkhum, S, et al. Relationship between serum antioxidant vitamins A, E, and C and lipid profiles in priest subjects at the Priest Hospital. Southeast Asian J Trop Med Public Health. (2005) 36:246–53. Available at: https://pubmed.ncbi.nlm.nih.gov/16438218/#:~:text=All%20serum%20vitamins%2C%20A%2C%20E,subjects%20compared%20with%20the%20controls
114. Faure, H, Preziosi, P, Roussel, AM, Bertrais, S, Galan, P, Hercberg, S, et al. Factors influencing blood concentration of retinol, α-tocopherol, vitamin C, and β-carotene in the French participants of the SU. VI. MAX trial. Eur J Clin Nutr. (2006) 60:706–17. doi: 10.1038/sj.ejcn.1602372
115. Matilainen, T, Vartiainen, E, Puska, P, Alfthan, G, Pokusajeva, S, Moisejeva, N, et al. Plasma ascorbic acid concentrations in the Republic of Karelia, Russia and in North Karelia, Finland. Eur J Clin Nutr. (1996) 50:115–20.
116. Olmedilla, B, Granado, F, Southon, S, Wright, AJA, Blanco, I, Gil-Martinez, E, et al. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br J Nutr. (2001) 85:227–38. doi: 10.1079/BJN2000248
117. Cheung, E, Mutahar, R, Assefa, F, Ververs, M-T, Nasiri, SM, Borrel, A, et al. An epidemic of scurvy in Afghanistan: assessment and response. Food Nutr Bull. (2003) 24:247–55. doi: 10.1177/156482650302400303
118. Preston, AM, Rodríguez, C, and Rivera, CE. Plasma ascorbate in a population of children: influence of age, gender, vitamin C intake, BMI and smoke exposure. Puerto Rico Health Sci J. (2006) 25:1–10.
119. Preston, AM, Rodriguez, C, Rivera, CE, and Sahai, H. Influence of environmental tobacco smoke on vitamin C status in children. Am J Clin Nutr. (2003) 77:167–72. doi: 10.1093/ajcn/77.1.167
120. Strauss, RS . Environmental tobacco smoke and serum vitamin C levels in children. Pediatrics. (2001) 107:540–2. doi: 10.1542/peds.107.3.540
121. Wilson, KM, Finkelstein, JN, Blumkin, AK, Best, D, and Klein, JD. Micronutrient levels in children exposed to secondhand tobacco smoke. Nicotine Tobacco Res. (2011) 13:800–8. doi: 10.1093/ntr/ntr076
122. Dherani, M, Murthy, GVS, Gupta, SK, Young, IS, Maraini, G, Camparini, M, et al. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Investig Ophthalmol Visual Sci. (2008) 49:3328–35. doi: 10.1167/iovs.07-1202
123. Galan, P, Viteri, FE, Bertrais, S, Czernichow, S, Faure, H, Arnaud, J, et al. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. (2005) 59:1181–90. doi: 10.1038/sj.ejcn.1602230
124. Itoh, R, Yamada, K, Oka, J, Echizen, H, and Murakami, K. Sex as a factor in levels of serum ascorbic acid in a healthy elderly population. Int J Vitamin Nutr Res. (1989) 59:365–72.
125. Birlouez-Aragon, I, Delcourt, C, Tessier, F, Papoz, L, and Group, PS. Associations of age, smoking habits and diabetes with plasma vitamin C of elderly of the POLA study. Int J Vitamin Nutr Res. (2001) 71:53–9. doi: 10.1024/0300-9831.71.1.53
126. NyyssÖnen, K, Parviainen, MT, Salonen, R, Tuomilehto, J, and Salonen, JT. Vitamin C deficiency and risk of myocardial infarction: prospective population study of men from eastern Finland. BMJ. (1997) 314:634. doi: 10.1136/bmj.314.7081.634
127. Khan, RM, and Iqbal, MP. Deficiency of vitamin C in South Asia. Pakistan J Med Sci. (2006) 22:347.
128. Iom, IOM, and FNB, FANB. Dietary reference intakes: energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington: The National Academy (2005).
129. Saito, K, Yokoyama, T, Yoshida, H, Kim, H, Shimada, H, Yoshida, Y, et al. A significant relationship between plasma vitamin C concentration and physical performance among Japanese elderly women. J Gerontol. (2012) 67A:295–301. doi: 10.1093/gerona/glr174
130. Juhl, B, Lauszus, FF, and Lykkesfeldt, J. Poor vitamin C status late in pregnancy is associated with increased risk of complications in type 1 diabetic women: a cross-sectional study. Nutrients. (2017) 9. doi: 10.3390/nu9030186
131. Bendich, A, and Langseth, L. The health effects of vitamin C supplementation: a review. J Am College Nutr. (1995) 14:124–36. doi: 10.1080/07315724.1995.10718484
132. Cahill, LE, and El-Sohemy, A. Vitamin C transporter gene polymorphisms, dietary vitamin c and serum ascorbic acid. Lifestyle Genom. (2009) 2:292–301. doi: 10.1159/000314597
133. Cahill, LE, and El-Sohemy, A. Haptoglobin genotype modifies the association between dietary vitamin C and serum ascorbic acid deficiency. Am J Clin Nutr. (2010) 92:1494–500. doi: 10.3945/ajcn.2010.29306
134. Corpe, CP, Tu, H, Eck, P, Wang, J, Faulhaber-Walter, R, Schnermann, J, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Investig. (2010) 120:1069–83. doi: 10.1172/JCI39191
135. Na, N, Delanghe, JR, Taes, YEC, Torck, M, Baeyens, WRG, and Ouyang, J. Serum vitamin C concentration is influenced by haptoglobin polymorphism and iron status in Chinese. Clin Chim Acta. (2006) 365:319–24. doi: 10.1016/j.cca.2005.09.015
136. Boonpangrak, S, Tantimongcolwat, T, Treeratanapiboon, L, Leelahakul, P, and Prachayasittikul, V. Lifestyle behaviors and serum vitamin C in the Thai population in Bangkok Metropolitan. EXCLI J. (2018) 17:452–66. doi: 10.17179/excli2018-1203
137. Marangon, K, Herbeth, B, Lecomte, E, Paul-Dauphin, A, Grolier, P, Chancerelle, Y, et al. Diet, antioxidant status, and smoking habits in French men. Am J Clin Nutr. (1998) 67:231–9. doi: 10.1093/ajcn/67.2.231
138. Schectman, G, Byrd, JC, and Gruchow, HW. The influence of smoking on vitamin C status in adults. Am J Public Health. (1989) 79:158–62. doi: 10.2105/AJPH.79.2.158
139. Tribble, DL, Giuliano, LJ, and Fortmann, SP. Reduced plasma ascorbic acid concentrations in nonsmokers regularly exposed to environmental tobacco smoke. Am J Clin Nutr. (1993) 58:886–90. doi: 10.1093/ajcn/58.6.886
141. Carr, AC, and Cook, J. Intravenous vitamin C for cancer therapy – identifying the current gaps in our knowledge. Front Physiol. (2018) 9. doi: 10.3389/fphys.2018.01182
142. Carr, AC, and Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. (1999) 69:1086–107. doi: 10.1093/ajcn/69.6.1086
143. McGregor, GP, and Biesalski, HK. Rationale and impact of vitamin C in clinical nutrition. Curr Opin Clin Nutr Metab Care. (2006) 9:697–703. doi: 10.1097/01.mco.0000247478.79779.8f
144. Gordon, DS, Rudinsky, AJ, Guillaumin, J, Parker, VJ, and Creighton, KJ. Vitamin C in health and disease: A companion animal focus. Topics Compan Anim Med. (2020) 39:100432. doi: 10.1016/j.tcam.2020.100432
145. Nowak, D . Vitamin C in Human Health and Disease, vol. 13 Multidisciplinary Digital Publishing Institute (2021). 1595 p.
146. Carr, AC, and Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: a review of RDA criteria and underlying health perspectives. Crit Rev Food Sci Nutr. (2021) 61:742–55. doi: 10.1080/10408398.2020.1744513
147. Chisnall, M, and Macknight, R. Importance of vitamin C in human health and disease. In: Ascorbic acid in plant growth, development and stress tolerance : Springer (2017). 491–501.
148. Brauchla, M, Dekker, MJ, and Rehm, CD. Trends in Vitamin C Consumption in the United States: 1999–2018. Nutrients. (2021) 13:420. doi: 10.3390/nu13020420
149. Frei, B, and Traber, MG. The new US Dietary Reference Intakes for vitamins C and E. Redox Rep. (2001) 6:5–9. doi: 10.1179/135100001101535978
150. Levine, M, Rumsey, SC, Daruwala, R, Park, JB, and Wang, Y. Criteria and recommendations for vitamin C intake. JAMA. (1999) 281:1415–23. doi: 10.1001/jama.281.15.1415
151. Levine, M, Wang, Y, Padayatty, SJ, and Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proceed Natl Acad Sci. (2001) 98:9842–6. doi: 10.1073/pnas.171318198
152. Requirements of ascorbic acid, vitamin D, vitamin B12, folate, and iron. Report of a Joint FAO/WHO Expert Group, (1970).
153. Dietary Reference Intakes . Institute of Medicine, Food and Nutrition Board. Washington, DC: National Academy Press (1998).
154. Young, VR . Evidence for a recommended dietary allowance for vitamin C from pharmacokinetics: a comment and analysis. Proceed Natl Acad Sci U S A. (1996) 93:14344–8. doi: 10.1073/pnas.93.25.14344
155. Smith, JL, and Hodges, RE. Serum levels of vitamin C in relation to dietary and supplemental intake of vitamin C in smokers and nonsmokers. Ann New York Acad Sci. (1987) 498:144–52. doi: 10.1111/j.1749-6632.1987.tb23758.x
156. Marik, PE, and Liggett, A. Adding an orange to the banana bag: vitamin C deficiency is common in alcohol use disorders. Critical Care. (2019) 23:1–6. doi: 10.1186/s13054-019-2435-4
157. Nair, MK, Augustine, LF, and Konapur, A. Food-based interventions to modify diet quality and diversity to address multiple micronutrient deficiency. Front Public Health. (2016) 3:277. doi: 10.3389/fpubh.2015.00277
158. Ojiewo, C., Tenkouano, A., Hughes, J. D. A., and Keatinge, J. D. H. (2013). Diversifying diets: using indigenous vegetables to improve profitability, nutrition and health in Africa. London: Routledge, 291–302
159. Lykkesfeldt, J, and Poulsen, HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr. (2010) 103:1251–9. doi: 10.1017/S0007114509993229
160. Spoelstra-de Man, AME, Elbers, PWG, and Oudemans-Van Straaten, HM. Vitamin C: should we supplement? Curr Opin Critic Care. (2018) 24:248–55. doi: 10.1097/MCC.0000000000000510
161. Chugh, PK, and Lhamo, Y. An assessment of vitamin supplements in the Indian market. Indian J Pharmac Sci. (2012) 74:469. doi: 10.4103/0250-474X.108431
162. Agius, F, González-Lamothe, R, Caballero, JL, Muñoz-Blanco, J, Botella, MA, and Valpuesta, V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. (2003) 21:177–81. doi: 10.1038/nbt777
163. Bulley, S, Wright, M, Rommens, C, Yan, H, Rassam, M, Lin-Wang, K, et al. Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol J. (2012) 10:390–7. doi: 10.1111/j.1467-7652.2011.00668.x
164. Hemavathi, UCP, Akula, N, Young, KE, Chun, SC, Kim, DH, and Park, SW. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett. (2010) 32:321–30. doi: 10.1007/s10529-009-0140-0
165. Naqvi, S, Zhu, C, Farre, G, Ramessar, K, Bassie, L, Breitenbach, J, et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proceed Natl Acad Sci. (2009) 106:7762–7. doi: 10.1073/pnas.0901412106
166. Chen, Z, Young, TE, Ling, J, Chang, S-C, and Gallie, DR. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proceed Natl Acad Sci. (2003) 100:3525–30. doi: 10.1073/pnas.0635176100
167. Jain, AK, and Nessler, CL. Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Molec Breed. (2000) 6:73–8. doi: 10.1023/A:1009680818138
168. Badejo, AA, Tanaka, N, and Esaka, M. Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene. Plant Cell Physiol. (2008) 49:126–32. doi: 10.1093/pcp/pcm164
169. Bulley, SM, Rassam, M, Hoser, D, Otto, W, Schünemann, N, Wright, M, et al. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot. (2009) 60:765–78. doi: 10.1093/jxb/ern327
170. Dary, O., and Hurrell, R. (2006). Guidelines on food fortification with micronutrients. World Health Organization, Food and Agricultural Organization of the United Nations: Geneva, Switzerland, 1–376.
171. Dwyer, JT, Wiemer, KL, Dary, O, Keen, CL, King, JC, Miller, KB, et al. Fortification and health: challenges and opportunities. Adv Nutr. (2015) 6:124–31. doi: 10.3945/an.114.007443
172. Korzeniowska, M, Wojdylo, A, and Barrachina, AAC. Advances in food fortification with vitamins and co-vitamins. In: Food Biofortification Technologies : CRC Press (2017). 61–96.
173. Yu, H, He, Y, Wang, M, Yang, F, Xie, Y, Guo, Y, et al. Regenerative efficacy of tert-butyl hydroquinone (TBHQ) on dehydrogenated ascorbic acid and its corresponding application to liqueur chocolate. Food Biosci. (2021) 42:101129. doi: 10.1016/j.fbio.2021.101129
174. Fredriksen, J, Løken, EB, Borgejordet, Å, Gjerdevik, K, and Nordbotten, A. Unexpected sources of vitamin C. Food Chem. (2009) 113:832–4. doi: 10.1016/j.foodchem.2008.05.019
175. Kurzer, AB, Dunn, ML, Pike, OA, Eggett, DL, and Jefferies, LK. Antioxidant effects on retinyl palmitate stability and isomerization in nonfat dry milk during thermally accelerated storage. Int Dairy J. (2014) 35:111–5. doi: 10.1016/j.idairyj.2013.11.001
176. Przekwas, J, Wiktorczyk, N, Budzyńska, A, Wałecka-Zacharska, E, and Gospodarek-Komkowska, E. Ascorbic acid changes growth of food-borne pathogens in the early stage of biofilm formation. Microorganisms. (2020) 8:553. doi: 10.3390/microorganisms8040553
177. Sripakdee, T, Mahachai, R, and Chanthai, S. Phenolics and ascorbic acid related to antioxidant activity of MaoFruit juice and their thermal stability study. Oriental J Chem. (2017) 33:74–86. doi: 10.13005/ojc/330108
178. Khalid, N, Kobayashi, I, Neves, MA, Uemura, K, Nakajima, M, and Nabetani, H. Monodisperse W/O/W emulsions encapsulating l-ascorbic acid: Insights on their formulation using microchannel emulsification and stability studies. Colloids Surfaces A. (2014) 458:69–77. doi: 10.1016/j.colsurfa.2014.04.019
179. Hwang, KE, Kim, HW, Song, DH, Kim, YJ, Ham, YK, Choi, YS, et al. Effect of Mugwort and Rosemary either singly, or combination with ascorbic acid on shelf stability of pork patties. J Food Process Preserv. (2017) 41:e12994. doi: 10.1111/jfpp.12994
180. Yoshitomi, B . Depletion of ascorbic acid derivatives in fish feed by the production process. Fish Sci. (2004) 70:1153–6. doi: 10.1111/j.1444-2906.2004.00917.x
181. Berardo, A, De Maere, H, Stavropoulou, DA, Rysman, T, Leroy, F, and De Smet, S. Effect of sodium ascorbate and sodium nitrite on protein and lipid oxidation in dry fermented sausages. Meat Sci. (2016) 121:359–64. doi: 10.1016/j.meatsci.2016.07.003
182. Zhang, W, Huang, Q, Yang, R, Zhao, W, and Hua, X. 2-OD-glucopyranosyl-L-ascorbic acid: Properties, production, and potential application as a substitute for L-ascorbic acid. J Funct Foods. (2021) 82:104481. doi: 10.1016/j.jff.2021.104481
183. Lee, BJ, Hendricks, DG, and Cornforth, DP. A comparison of carnosine and ascorbic acid on color and lipid stability in a ground beef Pattie model system. Meat Sci. (1999) 51:245–53. doi: 10.1016/s0309-1740(98)00121-1
184. Yamamoto, I, Muto, N, and Miyake, T. α-Glycosyl-L-ascorbic acid, and its preparation and uses Google Patents (1992).
185. Bamidele, OP, Duodu, KG, and Emmambux, MN. Encapsulation and antioxidant activity of ascorbyl palmitate with maize starch during pasting. Carbohydrate Polymers. (2017) 166:202–8. doi: 10.1016/j.carbpol.2017.02.095
186. Alemán, M, Bou, R, Tres, A, Polo, J, Codony, R, and Guardiola, F. Oxidative stability of a heme iron-fortified bakery product: effectiveness of ascorbyl palmitate and co-spray-drying of heme iron with calcium caseinate. Food Chem. (2016) 196:567–76. doi: 10.1016/j.foodchem.2015.09.031
187. Lin, F, Chen, Y, and He, S. Application of L-ascorbyl palmitate in formula milk. Modern Food Sci Technol. (2010) 26:1114–6. Available at: https://www.cabdirect.org/cabdirect/abstract/20113222459
188. He, S, Lin, F, and Chen, Y. Effect of L-ascorbyl palmitate on the stability of frying oil. Modern Food Sci Technol. (2010) 26:972–4. doi: 10.1007/bf02541662
189. Abe-Matsumoto, LT, de Araújo, YA, and Medeiros, ML. Stability of vitamin C in enriched jelly. Braz. J. Anal. Chem. (2020) 7:14–9. doi: 10.30744/brjac.2179-3425.ar-38-2019
190. Burch, R . The stability and shelf life of vitamin-fortified foods In: D Kilcast and P Subramaniam, editors. Food and Beverage Stability and Shelf Life : Woodhead Publishing (2011). 743–54.
191. Crotti, L, Dresch, G, and Ramallo, LA. Stability of vitamin C-fortified yerba mate and sensory evaluation of the drink. J Food Process Preserv. (2008) 32:306–18. doi: 10.1111/j.1745-4549.2008.00180.x
192. Cvetković, BR, and Jokanović, MR. Effect of preservation method and storage condition on ascorbic acid loss in beverages. Acta Period Technol. (2009) 40:1–7. doi: 10.2298/apt0940001c
193. Dennison, DB, and Kirk, JR. Oxygen effect on the degradation of ascorbic acid in a dehydrated food system. J Food Sci. (1978) 43:609–18. doi: 10.1111/j.1365-2621.1978.tb02365.x
194. Dennison, DB, and Kirk, JR. Effect of trace mineral fortification on the storage stability of ascorbic acid in a dehydrated model food system. J Food Sci. (1982) 47:1198–200. doi: 10.1111/j.1365-2621.1982.tb07647.x
195. Godoy, HT, Amaya-Farfan, J, and Rodriguez-Amaya, DB. Degradation of vitamins. In: Chemical changes during processing and storage of foods : Elsevier (2021). 329–83.
196. Ilic, DB, and Ashoor, SH. Stability of vitamins A and C in fortified yogurt. J Dairy Sci. (1988) 71:1492–8. doi: 10.3168/jds.S0022-0302(88)79712-X
197. Kirk, J, Dennison, D, Kokoczka, P, and Heldman, D. Degradation of ascorbic acid in a dehydrated food system. J Food Sci. (1977) 42:1274–9. doi: 10.1111/j.1365-2621.1977.tb14477.x
198. Lipasek, RA, Taylor, LS, and Mauer, LJ. Effects of anticaking agents and relative humidity on the physical and chemical stability of powdered vitamin C. J Food Sci. (2011) 76:C1062–74. doi: 10.1111/j.1750-3841.2011.02333.x
199. Majid, I, Hussain, S, and Nanda, V. Impact of sprouting on the degradation kinetics of color and vitamin C of onion powder packaged in different packaging materials. J Food Process Preserv. (2019) 43:e13849. doi: 10.1111/jfpp.13849
200. Masamba, KG, and Mndalira, K. Vitamin C stability in pineapple, guava and baobab juices under different storage conditions using different levels of sodium benzoate and metabisulphite. Afr J Biotechnol. (2013) 12:186–91. doi: 10.5897/ajb10.501
201. Ottaway, PB . Stability of vitamins in food. In: The technology of vitamins in food : Springer (1993). 90–113.
202. Peleg, M . Theoretical study of aerobic vitamin C loss kinetics during commercial heat preservation and storage. Food Res Int. (2017) 102:246–55. doi: 10.1016/j.foodres.2017.10.008
203. Polydera, AC, Stoforos, NG, and Taoukis, PS. Comparative shelf life study and vitamin C loss kinetics in pasteurised and high pressure processed reconstituted orange juice. J Food Engin. (2003) 60:21–9. doi: 10.1016/S0260-8774(03)00006-2
204. Rahman, MS, Al-Rizeiqi, MH, Guizani, N, Al-Ruzaiqi, MS, Al-Aamri, AH, and Zainab, S. Stability of vitamin C in fresh and freeze-dried capsicum stored at different temperatures. J Food Sci Technol. (2015) 52:1691–7. doi: 10.1007/s13197-013-1173-x
205. Remini, H, Mertz, C, Belbahi, A, Achir, N, Dornier, M, and Madani, K. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurised blood orange juice during storage. Food Chem. (2015) 173:665–73. doi: 10.1016/j.foodchem.2014.10.069
206. Tikekar, RV, Anantheswaran, RC, and LaBorde, LF. Ascorbic acid degradation in a model apple juice system and in apple juice during ultraviolet processing and storage. J Food Sci. (2011) 76:H62–71. doi: 10.1111/j.1750-3841.2010.02015.x
207. Zhang, Z-H, Zeng, X-A, Brennan, CS, Brennan, M, Han, Z, and Xiong, X-Y. Effects of pulsed electric fields (PEF) on vitamin C and its antioxidant properties. Int J Molec Sci. (2015) 16:24159–73. doi: 10.3390/ijms161024159
208. Yan, B, Davachi, SM, Ravanfar, R, Dadmohammadi, Y, Deisenroth, TW, Pho, TV, et al. Improvement of vitamin C stability in vitamin gummies by encapsulation in casein gel. Food Hydrocolloids. (2021) 113:106414. doi: 10.1016/j.foodhyd.2020.106414
209. Estevinho, BN, Carlan, I, Blaga, A, and Rocha, F. Soluble vitamins (vitamin B12 and vitamin C) microencapsulated with different biopolymers by a spray drying process. Powder Technol. (2016) 289:71–8. doi: 10.1016/j.powtec.2015.11.019
210. Desai, KG, and Park, HJ. Effect of manufacturing parameters on the characteristics of vitamin C encapsulated tripolyphosphate-chitosan microspheres prepared by spray-drying. J Microencaps. (2006) 23:91–103. doi: 10.1080/02652040500435436
211. Desai, KGH, and Park, HJ. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J Microencaps. (2005) 22:179–92. doi: 10.1080/02652040400026533
212. Pereira, HVR, Saraiva, KP, Carvalho, LMJ, Andrade, LR, Pedrosa, C, and Pierucci, APTR. Legumes seeds protein isolates in the production of ascorbic acid microparticles. Food Res Int. (2009) 42:115–21. doi: 10.1016/j.foodres.2008.10.008
213. Leyva-López, R, Palma-Rodríguez, HM, López-Torres, A, Capataz-Tafur, J, Bello-Pérez, LA, and Vargas-Torres, A. Use of enzymatically modified starch in the microencapsulation of ascorbic acid: Microcapsule characterization, release behavior and in vitro digestion. Food Hydrocolloids. (2019) 96:259–66. doi: 10.1016/j.foodhyd.2019.04.056
214. Hoyos-Leyva, JD, Chavez-Salazar, A, Castellanos-Galeano, F, Bello-Perez, LA, and Alvarez-Ramirez, J. Physical and chemical stability of l-ascorbic acid microencapsulated into taro starch spherical aggregates by spray drying. Food Hydrocolloids. (2018) 83:143–52. doi: 10.1016/j.foodhyd.2018.05.002
215. Alvim, ID, Stein, MA, Koury, IP, Dantas, FBH, and Cruz, CL. Comparison between the spray drying and spray chilling microparticles contain ascorbic acid in a baked product application. LWT Food Sci Technol. (2016) 65:689–94. doi: 10.1016/j.lwt.2015.08.049
216. Esposito, E, Cervellati, F, Menegatti, E, Nastruzzi, C, and Cortesi, R. Spray dried Eudragit microparticles as encapsulation devices for vitamin C. Int J Pharmac. (2002) 242:329–34. doi: 10.1016/S0378-5173(02)00176-X
217. Finotelli, P. V., and Rocha-Leão, M. H. (2005). Microencapsulation of ascorbic acid in maltodextrin and capsul using spray-drying. Paper presented at the Anais do 4° Mercosur Congress on Process Systems Engineering.
218. Righetto, AM, and Netto, FM. Vitamin C stability in encapsulated green West Indian cherry juice and in encapsulated synthetic ascorbic acid. J Sci Food Agric. (2006) 86:1202–8. doi: 10.1002/jsfa.2469
219. Pierucci, APTR, Andrade, LR, Baptista, EB, Volpato, NM, and Rocha-Leão, MHM. New microencapsulation system for ascorbic acid using pea protein concentrate as coat protector. J Microencaps. (2006) 23:654–62. doi: 10.1016/j.jfca.2009.08.018
220. Trindade, MA, and Grosso, CRF. The stability of ascorbic acid microencapsulated in granules of rice starch and in gum Arabic. J Microencaps. (2000) 17:169–76. doi: 10.1080/026520400288409
221. Barra, PA, Márquez, K, Gil-Castell, O, Mujica, J, Ribes-Greus, A, and Faccini, M. Spray-drying performance and thermal stability of L-ascorbic acid microencapsulated with sodium alginate and gum Arabic. Molecules. (2019) 24:2872. doi: 10.3390/molecules24162872
222. Osman, A, Goda, HA, Abdel-Hamid, M, Badran, SM, and Otte, J. Antibacterial peptides generated by alcalase hydrolysis of goat whey. LWT Food Sci Technol. (2016) 65:480–6. doi: 10.1016/j.lwt.2015.08.043
223. Sartori, T, Consoli, L, Hubinger, MD, and Menegalli, FC. Ascorbic acid microencapsulation by spray chilling: production and characterization. LWT Food Sci Technol. (2015) 63:353–60. doi: 10.1016/j.lwt.2015.03.112
224. Carvalho, JDDS, Oriani, VB, De Oliveira, GM, and Hubinger, MD. Characterization of ascorbic acid microencapsulated by the spray chilling technique using palm oil and fully hydrogenated palm oil. LWT. (2019) 101:306–14. doi: 10.1016/j.lwt.2018.11.043
225. Carvalho, JDDS, Oriani, VB, De Oliveira, GM, and Hubinger, MD. Solid lipid microparticles loaded with ascorbic acid: Release kinetic profile during thermal stability. J Food Process Preserv. (2021) 45:e15557. doi: 10.1111/jfpp.15557
226. Comunian, TA, Thomazini, M, Alves, AJG, de Matos Junior, FE, de Carvalho Balieiro, JC, and Favaro-Trindade, CS. Microencapsulation of ascorbic acid by complex coacervation: protection and controlled release. Food Res Int. (2013) 52:373–9. doi: 10.1016/j.foodres.2013.03.028
227. Tsai, W-C, and Rizvi, SSH. Simultaneous microencapsulation of hydrophilic and lipophilic bioactives in liposomes produced by an ecofriendly supercritical fluid process. Food Res Int. (2017) 99:256–62. doi: 10.1016/j.foodres.2017.05.029
228. Khalid, N, Kobayashi, I, Neves, MA, Uemura, K, Nakajima, M, and Nabetani, H. Formulation of monodisperse water-in-oil emulsions encapsulating calcium ascorbate and ascorbic acid 2-glucoside by microchannel emulsification. Colloids Surfaces. (2014) 459:247–53. doi: 10.1016/j.colsurfa.2014.07.014
229. Comunian, TA, Abbaspourrad, A, Favaro-Trindade, CS, and Weitz, DA. Fabrication of solid lipid microcapsules containing ascorbic acid using a microfluidic technique. Food Chem. (2014) 152:271–5. doi: 10.1016/j.foodchem.2013.11.149
230. Knezevic, Z, Gosak, D, Hraste, M, and Jalsenjako, I. Fluid-bed microencapsulation of ascorbic acid. J Microencaps. (1998) 15:237–52. doi: 10.3109/02652049809006853
231. Kirby, C, Whittle, C, Rigby, N, Coxon, D, and Law, B. Stabilization of ascorbic acid by microencapsulation in liposomes. Int J Food Sci Technol. (1991) 26:437–49. doi: 10.1111/j.1365-2621.1991.tb01988.x
232. Farhang, B, Kakuda, Y, and Corredig, M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Sci Technol. (2012) 92:353–66. doi: 10.1007/s13594-012-0072-7
233. Sharma, R., and Lal, D. (2005). Fortification of milk with microencapsulated vitamin C and its thermal stability. Available at: http://krishi.icar.gov.in/PDF/ICAR_Data_Use_Licence.pdf).
235. Chang, D, Abbas, S, Hayat, K, Xia, S, Zhang, X, Xie, M, et al. Encapsulation of ascorbic acid in amorphous maltodextrin employing extrusion as affected by matrix/core ratio and water content. Int J Food Sci Technol. (2010) 45:1895–901. doi: 10.1111/j.1365-2621.2010.02348.x
236. Uddin, MS, Hawlader, MN, and Zhu, HJ. Microencapsulation of ascorbic acid: effect of process variables on product characteristics. J Microencaps. (2001) 18:199–209. doi: 10.1080/02652040010000352
237. Özdemir, KS, and Gökmen, V. Effect of microencapsulation on the reactivity of ascorbic acid, sodium chloride and vanillin during heating. J Food Engin. (2015) 167:204–9. doi: 10.1016/j.jfoodeng.2015.03.029
238. Baek, J, Ramasamy, M, Willis, NC, Kim, DS, Anderson, WA, and Tam, KC. Encapsulation and controlled release of vitamin C in modified cellulose nanocrystal/chitosan nanocapsules. Curr Res Food Sci. (2021) 4:215–23. doi: 10.1016/j.crfs.2021.03.010
239. Fraj, J, Petrovic, L, Dekic, L, Budincic, JM, Bucko, S, and Katona, J. Encapsulation and release of vitamin C in double W/O/W emulsions followed by complex coacervation in gelatin-sodium caseinate system. J Food Engin. (2021) 292. doi: 10.1016/j.jfoodeng.2020.110353
240. Lee, J-B, Ahn, J, Lee, J, and Kwak, H-S. L-ascorbic acid microencapsulated with polyacylglycerol monostearate for milk fortification. Biosci Biotechnol Biochem. (2004) 68:495–500. doi: 10.1271/bbb.68.495
241. Lee, JB, Ahn, J, and Kwak, HS. Microencapsulated ascorbic acid for milk fortification. Archives Pharmacal Res. (2003) 26:575–80. doi: 10.1007/BF02976884
242. Kim, S-S, Han, Y-J, Hwang, T-J, Roh, H-J, Hahm, T-S, Chung, M-S, et al. Microencapsulation of ascorbic acid in sucrose and lactose by cocrystallization. Food Sci Biotechnol. (2001) 10:101–7.
243. Desai, KGH, Liu, C, and Park, HJ. Characteristics of vitamin C immobilized particles and sodium alginate beads containing immobilized particles. J Microencaps. (2005) 22:363–76. doi: 10.1080/02652040500098861
244. Han, J, Guenier, A-S, Salmieri, S, and Lacroix, M. Alginate and chitosan functionalization for micronutrient encapsulation. J Agric Food Chem. (2008) 56:2528–35. doi: 10.1021/jf703739k
245. Bedhiafi, T, Idoudi, S, Fernandes, Q, Al-Zaidan, L, Uddin, S, Dermime, S, et al. Nano-vitamin C: A promising candidate for therapeutic applications. Biomed. Pharmacother. (2023) 158:114093.
246. Bleiel, D, Nitschke, I, and Noack, MJ, Barbe, AG . Impact of care level, setting and accommodation costs on a newly developed oral care nursing plan format for elderly patients with care needs–Results from a cross‐sectional study. Int. J. Dent. Hyg. (2022) 20: 543–52.
247. Zhou, W, Liu, W, Zou, L, Liu, W, Liu, C, Liang, R, et al. Storage stability and skin permeation of vitamin C liposomes improved by pectin coating. Colloids Surfaces B Biointer. (2014) 117:330–7. doi: 10.1016/j.colsurfb.2014.02.036
248. Monroig, Ó, Navarro, JC, Amat, F, and Hontoria, F. Enrichment of Artemia nauplii in vitamin A, vitamin C and methionine using liposomes. Aquaculture. (2007) 269:504–13. doi: 10.1016/j.aquaculture.2007.02.056
249. Jiao, Z, Wang, X, Yin, Y, and Xia, J. Preparation and evaluation of vitamin C and folic acid-coloaded antioxidant liposomes. Particulate Sci Technol. (2019) 37:453–9. doi: 10.1080/02726351.2017.1391907
250. Marsanasco, M, Márquez, AL, Wagner, JR, del Alonso, S, and Chiaramoni, NS. Liposomes as vehicles for vitamins E and C: An alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res Int. (2011) 44:3039–46. doi: 10.1016/j.foodres.2011.07.025
251. Yang, S, Liu, C, Liu, W, Yu, H, Zheng, H, Zhou, W, et al. Preparation and Characterization of Nanoliposomes Entrapping Medium-Chain Fatty Acids and Vitamin C by Lyophilization. Int J Molec Sci. (2013) 14:19763–73. doi: 10.3390/ijms141019763
252. Yang, S, Liu, C-M, Liu, W, Liu, W-L, Tong, G-H, Zhang, Y, et al. Preparation and stability of vitamin C liposomes. Food Sci. (2010) 20:5–12.
253. Lipka, D, Gubernator, J, Filipczak, N, Barnert, S, Süss, R, Legut, M, et al. Vitamin C-driven epirubicin loading into liposomes. Int J Nanomed. (2013) 8:3573–85. doi: 10.2147/IJN.S47745
254. Wu, Z, Chen, B, Gan, Z, Chen, F, and Luo, X. Exogenous vitamin C-triggered surface charge conversion of pH/reduction-responsive micelles for the enhanced tumor-specific activity of loaded doxorubicin. Molec Pharmac. (2020) 17:954–64. doi: 10.1021/acs.molpharmaceut.9b01183
255. Üner, M, Wissing, SA, Yener, G, and Müller, RH. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for application of ascorbyl palmitate. Die Pharmazie. (2005) 60:577–82. Available at: https://pubmed.ncbi.nlm.nih.gov/16124399/
256. Jain, A, Garg, NK, Jain, A, Kesharwani, P, Jain, AK, Nirbhavane, P, et al. A synergistic approach of adapalene-loaded nanostructured lipid carriers, and vitamin C co-administration for treating acne. Drug Develop. Industr. Pharm. (2016) 42:897–905. doi: 10.3109/03639045.2015.1104343
257. Matos-Jr, FE, Di Sabatino, M, Passerini, N, Favaro-Trindade, CS, and Albertini, B. Development and characterization of solid lipid microparticles loaded with ascorbic acid and produced by spray congealing. Food Res Inter. (2015) 67:52–9. doi: 10.1016/j.foodres.2014.11.002
258. Vitamin, P . Formulation and physical characterization of microemulsions based carboxymethyl cellulose as vitamin c carrier. Malay J Anal Sci. (2015) 19:275–83.
259. Szymula, M . Atmospheric oxidation of β-carotene in aqueous, pentanol, SDS microemulsion systems in the presence and absence of vitamin C. J Dispersion Sci Technol. (2004) 25:129–37. doi: 10.1081/DIS-120030659
260. Osanloo, M, Jamali, N, and Nematollahi, A. Improving the oxidative stability of virgin olive oil using microformulated vitamin-C. Food Sci Nutr. (2021) 9:3712–21. doi: 10.1002/fsn3.2332
261. Ramli, S, Chyi, KT, Zainuddin, N, Mokhtar, W, and Abdul Rahman, I. The influence of surfactant/co-surfactant hydrophilic-lipophilic balance on the formation of limonene-based microemulsion as Vitamin C carrier. Sains Malaysiana. (2019) 48:1035–42. http://dx.doi.org/10.17576/jsm-2019-4805-12.
262. Ji, R, Cui, H, Duhoranimana, E, Hayat, K, Yu, J, Hussain, S, et al. Co-encapsulation of L-ascorbic acid and quercetin by gelatin/sodium carboxymethyl cellulose coacervates using different interlayer oils. Food Res Int. (2021) 145:110411. doi: 10.1016/j.foodres.2021.110411
263. Bratu, I, Muresan-Pop, M, Kacso, I, and Fărcaş, SI. Spectroscopic investigation of the interaction between β-cyclodextrin and ascorbic acid. J Physics. (2009) 182:012004. doi: 10.1088/1742-6596/182/1/012004
264. Saha, S, Roy, A, Roy, K, and Roy, MN. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci Rep. (2016) 6:35764. doi: 10.1038/srep35764
265. Mendanha, DV, Molina Ortiz, SE, Favaro-Trindade, CS, Mauri, A, Monterrey-Quintero, ES, and Thomazini, M. Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res Int. (2009) 42:1099–104. doi: 10.1016/j.foodres.2009.05.007
266. Ji, M, Wu, J, Sun, X, Guo, X, Zhu, W, Li, Q, et al. Physical properties and bioactivities of fish gelatin films incorporated with cinnamaldehyde-loaded nanoemulsions and vitamin C. LWT. (2021) 135:110103. doi: 10.1016/j.lwt.2020.110103
267. da Cruz, MCR, Perussello, CA, and Masson, ML. Microencapsulated ascorbic acid: development, characterization, and release profile in simulated gastrointestinal fluids. J Food Process Engin. (2018) 41:e12922. doi: 10.1111/jfpe.12922
268. Rodrigues da Cruz, MC, Andreotti Dagostin, JL, Perussello, CA, and Masson, ML. Assessment of physicochemical characteristics, thermal stability and release profile of ascorbic acid microcapsules obtained by complex coacervation. Food Hydrocolloids. (2019) 87:71–82. doi: 10.1016/j.foodhyd.2018.07.043
269. Marcela, F, Lucía, C, Esther, F, and Elena, M. Microencapsulation of L-ascorbic acid by spray drying using sodium alginate as wall material. J Encapsul Adsorp Sci. (2016) 6:1–8. doi: 10.4236/jeas.2016.61001
270. Khaldia, S, Lamia, B, Yasmina, K, and Lahcene, B. Preparation, characterization and antioxidant activity of microspheres made of cellulose triacetate (CTA) to control the release of vitamin C. J Chem Technol Biotechnol. (2020) 95:1800–7. doi: 10.1002/jctb.6378
271. Jang, K-I, and Lee, HG. Stability of chitosan nanoparticles for l-ascorbic acid during heat treatment in aqueous solution. J Agric Food Chem. (2008) 56:1936–41. doi: 10.1021/jf073385e
272. Cho, Y, Kim, JT, and Park, HJ. Size-controlled self-aggregated N-acyl chitosan nanoparticles as a vitamin C carrier. Carbohydrate Polymers. (2012) 88:1087–92. doi: 10.1016/j.carbpol.2012.01.074
273. Aresta, A, Calvano, CD, Trapani, A, Cellamare, S, Zambonin, CG, and De Giglio, E. Development and analytical characterization of vitamin(s)-loaded chitosan nanoparticles for potential food packaging applications. J Nanoparticle Res. (2013) 15:1592. doi: 10.1007/s11051-013-1592-7
274. de Britto, D, de Moura, MR, Aouada, FA, Mattoso, LHC, and Assis, OBG. N,N,N-trimethyl chitosan nanoparticles as a vitamin carrier system. Food Hydrocolloids. (2012) 27:487–93. doi: 10.1016/j.foodhyd.2011.09.002
275. Dudhani, AR, and Kosaraju, SL. Bioadhesive chitosan nanoparticles: Preparation and characterization. Carbohydrate Polymers. (2010) 81:243–51. doi: 10.1016/j.carbpol.2010.02.026
276. Alishahi, A, Mirvaghefi, A, Tehrani, MR, Farahmand, H, Koshio, S, Dorkoosh, FA, et al. Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss). Carbohydrate Polym. (2011) 86:142–6. doi: 10.1016/j.carbpol.2011.04.028
277. Shabana, S, Prasansha, R, Kalinina, I, Potoroko, I, Bagale, U, and Shirish, SH. Ultrasound assisted acid hydrolyzed structure modification and loading of antioxidants on potato starch nanoparticles. Ultrasonics Sonochem. (2019) 51:444–50. doi: 10.1016/j.ultsonch.2018.07.023
278. Tehrani, E, and Amiri, S. Synthesis and characterization PVA electro-spun nanofibers containing encapsulated vitamin C in chitosan microspheres. J Textile Institute. (2022) 113:212–23. doi: 10.1080/00405000.2020.1869405
279. Aditya, NP, Espinosa, YG, and Norton, IT. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol Advanc. (2017) 35:450–7. doi: 10.1016/j.biotechadv.2017.03.012
280. Joye, IJ, Davidov-Pardo, G, and McClements, DJ. Nanotechnology for increased micronutrient bioavailability. Trends Food Sci Technol. (2014) 40:168–82. doi: 10.1016/j.tifs.2014.08.006
281. Joye, IJ, and McClements, DJ. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr Opin Colloid Interface Sci. (2014) 19:417–27. doi: 10.1016/j.cocis.2014.07.002
282. Suganya, V, and Anuradha, V. Microencapsulation and nanoencapsulation: a review. Int J Pharm Clin Res. (2017) 9:233–9. doi: 10.25258/ijpcr.v9i3.8324
283. Rezaei, A, Nasirpour, A, and Fathi, M. Application of cellulosic nanofibers in food science using electrospinning and its potential risk. Comprehens Rev Food Sci Food Safety. (2015) 14:269–84. doi: 10.1111/1541-4337.12128
284. Sameen, DE, Ahmed, S, Lu, R, Li, R, Dai, J, Qin, W, et al. Electrospun nanofibers food packaging: trends and applications in food systems. Crit Rev Food Sci Nutr. (2021):1–14.
285. Coelho, SC, Estevinho, BN, and Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: electrospinning and electrospraying–a review. Food Chem. (2021) 339:127850. doi: 10.1016/j.foodchem.2020.127850
286. Lee, Y.-S., and Im, J. S. (2010). Preparation of functionalized nanofibers and their applications. Nanofibers: Rijeka, Croatia, 121–140.
287. Liu, Z, Chen, R, and He, J. Active generation of multiple jets for producing nanofibres with high quality and high throughput. Mat Design. (2016) 94:496–501. doi: 10.1016/j.matdes.2016.01.075
288. Wsoo, MA, Abd Razak, SI, Bohari, SPM, Shahir, S, Salihu, R, Kadir, MRA, et al. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: characterization, in-vitro drug release and cytotoxicity studies. Int J Biol Macromole. (2021) 181:82–98. doi: 10.1016/j.ijbiomac.2021.03.108
289. Yalcinkaya, F . Preparation of various nanofiber layers using wire electrospinning system. Arab J Chem. (2019) 12:5162–72. doi: 10.1016/j.arabjc.2016.12.012
290. Zare, M, Dziemidowicz, K, Williams, GR, and Ramakrishna, S. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials. (2021) 11:1968. doi: 10.3390/nano11081968
291. Zhao, Y, Qiu, Y, Wang, H, Chen, Y, Jin, S, and Chen, S. Preparation of nanofibers with renewable polymers and their application in wound dressing. Int J Polymer Sci. (2016) 2016:1–17. doi: 10.1155/2016/4672839
292. Fahami, A, and Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin A delivery. Food Hydrocolloids. (2018) 81:31–8. doi: 10.1016/j.foodhyd.2018.02.008
293. Fathi, M, Nasrabadi, MN, and Varshosaz, J. Characteristics of vitamin E-loaded nanofibres from dextran. Int J Food Proper. (2017) 20:2665–74. doi: 10.1080/10942912.2016.1247365
294. Jacobsen, C, García-Moreno, PJ, Mendes, AC, Mateiu, RV, and Chronakis, IS. Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Ann Rev Food Sci Technol. (2018) 9:525–49. doi: 10.1146/annurev-food-030117-012348
295. Lemma, SM, Scampicchio, M, Mahon, PJ, Sbarski, I, Wang, J, and Kingshott, P. Controlled release of retinyl acetate from β-cyclodextrin functionalized poly (vinyl alcohol) electrospun nanofibers. J Agric Food Chem. (2015) 63:3481–8. doi: 10.1021/acs.jafc.5b00103
296. Tehrani, E, and Amiri, S. Synthesis and characterization PVA electro-spun nanofibers containing encapsulated vitamin C in chitosan microspheres. J Textile Institute. (2020):1–12.
297. Okur, N, Saricam, C, Gocek, I, Kalav, B, and Sahin, UK. Functionalized polyvinyl alcohol nanofiber webs containing β–cyclodextrin/Vitamin C inclusion complex. J Indust Textiles. (2019) 50:1559–71. doi: 10.1177/1528083719866933
298. Liu, LJ, Tao, LN, Chen, JH, Zhang, T, Xu, JM, Ding, MZ, et al. Fish oil-gelatin core-shell electrospun nanofibrous membranes as promising edible films for the encapsulation of hydrophobic and hydrophilic nutrients. LWT Food Sci Technol. (2021) 146. doi: 10.1016/j.lwt.2021.111500
299. Matencio, A, Navarro-Orcajada, S, García-Carmona, F, and López-Nicolás, JM. Applications of cyclodextrins in food science. A review. Trends Food Sci Technol. (2020) 104:132–43. doi: 10.1016/j.tifs.2020.08.009
300. Fourmentin, S, Crini, G, and Lichtfouse, E. Cyclodextrin fundamentals, reactivity and analysis Springer (2018).
301. Adeoye, O, and Cabral-Marques, H. Cyclodextrin nanosystems in oral drug delivery: a mini review. Int. J. Pharmac. (2017) 531:521–31. doi: 10.1016/j.ijpharm.2017.04.050
302. Arima, H, Motoyama, K, and Higashi, T. Potential use of cyclodextrins as drug carriers and active pharmaceutical ingredients. Chem Pharmac Bull. (2017) 65:341–8. doi: 10.1248/cpb.c16-00779
303. Chanphai, P, Vesper, AR, Bariyanga, J, Bérubé, G, and Tajmir-Riahi, HA. Review on the delivery of steroids by carrier proteins. J Photochem Photobiol B Biol. (2016) 161:184–91. doi: 10.1016/j.jphotobiol.2016.05.015
304. Chilajwar, SV, Pednekar, PP, Jadhav, KR, Gupta, GJC, and Kadam, VJ. Cyclodextrin-based nanosponges: a propitious platform for enhancing drug delivery. Expert Opin Drug Delivery. (2014) 11:111–20. doi: 10.1517/17425247.2014.865013
305. Liu, F, Ma, C, Gao, Y, and McClements, DJ. Food-grade covalent complexes and their application as nutraceutical delivery systems: A review. Comprehens Rev Food Sci Food Safety. (2017) 16:76–95. doi: 10.1111/1541-4337.12229
306. Shafaei, Z, Ghalandari, B, Vaseghi, A, Divsalar, A, Haertlé, T, Saboury, AA, et al. β-Lactoglobulin: An efficient nanocarrier for advanced delivery systems. Nanomed Nanotechnol Biol Med. (2017) 13:1685–92. doi: 10.1016/j.nano.2017.03.007
307. Arayachukeat, S, Wanichwecharungruang, SP, and Tree-Udom, T. Retinyl acetate-loaded nanoparticles: dermal penetration and release of the retinyl acetate. Int J Pharmac. (2011) 404:281–8. doi: 10.1016/j.ijpharm.2010.11.019
308. Cho, E-C . Effect of polymer characteristics on the thermal stability of retinol encapsulated in aliphatic polyester nanoparticles. Bull Korean Chem Soc. (2012) 33:2560–6. doi: 10.5012/bkcs.2012.33.8.2560
309. Della Porta, G, Campardelli, R, Falco, N, and Reverchon, E. PLGA microdevices for retinoids sustained release produced by supercritical emulsion extraction: continuous versus batch operation layouts. J Pharmac Sci. (2011) 100:4357–67. doi: 10.1002/jps.22647
310. Jain, A, Sharma, G, Kushwah, V, Garg, NK, Kesharwani, P, Ghoshal, G, et al. Methotrexate and beta-carotene loaded-lipid polymer hybrid nanoparticles: a preclinical study for breast cancer. Nanomedicine. (2017) 12:1851–72. doi: 10.2217/nnm-2017-0011
311. Kim, D-G, Jeong, Y-I, Choi, C, Roh, S-H, Kang, S-K, Jang, M-K, et al. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int J Pharmac. (2006) 319:130–8. doi: 10.1016/j.ijpharm.2006.03.040
312. Lee, JS, Nam, YS, Kang, BY, Han, SH, and Chang, IS. Vitamin A microencapsulation within poly (methyl methacrylate)-g-polyethylenimine microspheres: Localized proton buffering effect on vitamin A stability. J Appl Polymer Sci. (2004) 92:517–22. doi: 10.1002/app.20028
313. Ajeeshkumar, KK, Aneesh, PA, Raju, N, Suseela, M, Ravishankar, CN, and Benjakul, S. Advancements in liposome technology: preparation techniques and applications in food, functional foods, and bioactive delivery: a review. Comprehen Rev Food Sci Food Safety. (2021) 20:1280–306. doi: 10.1111/1541-4337.12725
314. Bozzuto, G, and Molinari, A. Liposomes as nanomedical devices. Int J Nanomed. (2015) 10:975–99. doi: 10.2147/IJN.S68861
315. Daeihamed, M, Dadashzadeh, S, Haeri, A, and Faghih Akhlaghi, M. Potential of liposomes for enhancement of oral drug absorption. Curr Drug Deliv. (2017) 14:289–303. doi: 10.2174/1567201813666160115125756
316. Esposto, BS, Jauregi, P, Tapia-Blácido, DR, and Martelli-Tosi, M. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci Technol. (2021) 108:40–8. doi: 10.1016/j.tifs.2020.12.003
317. Maherani, B, Arab-Tehrany, E, Mozafari, R, Gaiani C, M, and Linder, M. Liposomes: a review of manufacturing techniques and targeting strategies. Curr Nanosci. (2011) 7:436–52. doi: 10.2174/157341311795542453
318. Chen, H, Wooten, H, Thompson, L, and Pan, K. Nanoparticles of casein micelles for encapsulation of food ingredients. In: Biopolymer nanostructures for food encapsulation purposes (2019). 39–68. doi: 10.1016/B978-0-12-815663-6.00002-1
319. Ranadheera, CS, Liyanaarachchi, WS, Chandrapala, J, Dissanayake, M, and Vasiljevic, T. Utilizing unique properties of caseins and the casein micelle for delivery of sensitive food ingredients and bioactives. Trends Food Sci Technol. (2016) 57:178–87. doi: 10.1016/j.tifs.2016.10.005
320. Sun, X, and Bandara, N. Applications of reverse micelles technique in food science: a comprehensive review. Trends Food Sci Technol. (2019) 91:106–15. doi: 10.1016/j.tifs.2019.07.001
321. Sadiq, U, Gill, H, and Chandrapala, J. Casein micelles as an emerging delivery system for bioactive food components. Foods. (2021) 10:1965. doi: 10.3390/foods10081965
322. Tang, C-H . Assembly of food proteins for nano- encapsulation and delivery of nutraceuticals (a mini-review). Food Hydrocolloids. (2021) 117:106710. doi: 10.1016/j.foodhyd.2021.106710
323. Gothwal, A, Khan, I, and Gupta, U. Polymeric micelles: recent advancements in the delivery of anticancer drugs. Pharmac Res. (2016) 33:18–39. doi: 10.1007/s11095-015-1784-1
324. Keskin, D, and Tezcaner, A. Micelles as delivery system for cancer treatment. Curr Pharmac Design. (2017) 23:5230–41. doi: 10.2174/1381612823666170526102757
325. Kim, S, Shi, Y, Kim, JY, Park, K, and Cheng, J-X. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle–cell interaction. Expert Opin Drug Delivery. (2010) 7:49–62. doi: 10.1517/17425240903380446
326. Tanbour, R, M Martins, A, G Pitt, W, and A Husseini, G. Drug delivery systems based on polymeric micelles and ultrasound: a review. Curr Pharmaceut Design. (2016) 22:2796–807. doi: 10.2174/1381612822666160217125215
327. Wakaskar, RR . General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J Drug Target. (2018) 26:311–8. doi: 10.1080/1061186X.2017.1367006
328. Dasgupta, N, Ranjan, S, and Gandhi, M. Nanoemulsion ingredients and components. Environ Chem Lett. (2019) 17:917–28. doi: 10.1007/s10311-018-00849-7
329. Harwansh, RK, Deshmukh, R, and Rahman, MA. Nanoemulsion: promising nanocarrier system for delivery of herbal bioactives. J Drug Deliver Sci Technol. (2019) 51:224–33. doi: 10.1016/j.jddst.2019.03.006
330. Kumar, DHL, and Sarkar, P. Encapsulation of bioactive compounds using nanoemulsions. Environ Chem Lett. (2018) 16:59–70. doi: 10.1007/s10311-017-0663-x
331. Fraj, JL, Petrovic, LB, Budincic, JRM, Katona, JM, Bucko, SD, and Spasojevic, LM. Properties of double W/O/W emulsions containing Vitamin C and E stabilized with a gelatin/sodium caseinate complex. J Serbian Chem Soc. (2019) 84:1427–38. doi: 10.2298/JSC190515075F
332. Basha, SK, Dhandayuthabani, R, Muzammil, MS, and Kumari, VS. Solid lipid nanoparticles for oral drug delivery. Materials Today. (2021) 36:313–24. doi: 10.1016/j.matpr.2020.04.109
333. Cruz, Z, García-Estrada, C, Olabarrieta, I, and Rainieri, S. Lipid nanoparticles: delivery system for bioactive food compounds In: LMC Sagis , editor. Microencapsulation and microspheres for food applications. San Diego: Academic Press (2015). 313–31.
334. Hou, D, Xie, C, Huang, K, and Zhu, C. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials. (2003) 24:1781–5. doi: 10.1016/S0142-9612(02)00578-1
335. Mehnert, W, and Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Delivery Rev. (2012) 64:83–101. doi: 10.1016/j.addr.2012.09.021
336. de Souza Simões, L, Madalena, DA, Pinheiro, AC, Teixeira, JA, Vicente, AA, and Ramos, OL. Micro-and nano bio-based delivery systems for food applications: In vitro behavior. Adv Colloid Interface Sci. (2017) 243:23–45. doi: 10.1016/j.cis.2017.02.010
337. Dima, C, Assadpour, E, Dima, S, and Jafari, SM. Bioavailability of nutraceuticals: role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Comprehens Rev Food Sci Food Safety. (2020) 19:954–94. doi: 10.1111/1541-4337.12547
338. Chau, C-F, Wu, S-H, and Yen, G-C. The development of regulations for food nanotechnology. Trends Food Sci Technol. (2007) 18:269–80. doi: 10.1016/j.tifs.2007.01.007
339. Amenta, V, Aschberger, K, Arena, M, Bouwmeester, H, Moniz, FB, Brandhoff, P, et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul Toxicol Pharmacol. (2015) 73:463–76. doi: 10.1016/j.yrtph.2015.06.016
340. Livney, YD . Nanostructured delivery systems in food: Latest developments and potential future directions. Curr Opin Food Sci. (2015) 3:125–35. doi: 10.1016/j.cofs.2015.06.010
Keywords: vitamin C, antioxidant, stability, fortification, encapsulation technique, delivery system
Citation: Maurya VK, Shakya A, McClements DJ, Srinivasan R, Bashir K, Ramesh T, Lee J and Sathiyamoorthi E (2023) Vitamin C fortification: need and recent trends in encapsulation technologies. Front. Nutr. 10:1229243. doi: 10.3389/fnut.2023.1229243
Edited by:
Antonio Morata, Polytechnic University of Madrid, SpainReviewed by:
Periasamy Vaiyapuri Subbarayan, King Saud University, Saudi ArabiaNagendraPrabhu Ponnuraj, University of Illinois at Urbana-Champaign, United States
Karthik Selvaraj, Linköping University, Sweden
Copyright © 2023 Maurya, Shakya, McClements, Srinivasan, Bashir, Ramesh, Lee and Sathiyamoorthi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vaibhav Kumar Maurya, VmFpYmhhdm1hdXJ5YS5uaWZ0ZW1AZ21haWwuY29t; Amita Shakya, YW1pdGEubmlmdGVtQGdtYWlsLmNvbQ==; Ezhaveni Sathiyamoorthi, ZXpoYXZlbmk4NkBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Vaibhav Kumar Maurya
Vaibhav Kumar Maurya Amita Shakya
Amita Shakya David Julian McClements4,5
David Julian McClements4,5 Ramachandran Srinivasan
Ramachandran Srinivasan Khalid Bashir
Khalid Bashir Jintae Lee
Jintae Lee Ezhaveni Sathiyamoorthi
Ezhaveni Sathiyamoorthi