
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 19 July 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1225376
Objective: The causal relationship between early life adiposity and gestational diabetes mellitus (GDM) and the underlying mechanisms remains unclear. This study aimed to investigate the independent causal association between early life adiposity and GDM and identify potential metabolic mediators and their mediating effects on this relationship.
Methods: Using genome-wide association study (GWAS) summary statistics from the publicly available database of early life adiposity (5,530 cases and 8,318 controls) and GDM (11,279 cases and 179,600 controls), a two-step, two-sample Mendelian randomization (MR) was conducted to estimate the causal mediation effects of lipidomic biomarkers including low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride, apolipoprotein A-Ι, and apolipoprotein B on the relationship between early life adiposity and GDM.
Results: Genetically predicted childhood adiposity was positively associated with risk of GDM (OR: 1.21, 95%CI: 1.09–1.34, p = 4.58 × 10−4). This causal relationship remained after accounting for adult adiposity traits in the multivariable MR analyses. Two-step MR identified three candidate mediators that partially mediated the effect of early life adiposity on GDM, including HDL-C (5.81, 95%CI: 3.05–8.57%), apolipoprotein A-Ι (4.16, 95%CI: 1.64–6.69%), and triglyceride (2.20, 95%CI: 0.48–3.92%).
Conclusion: This MR study demonstrated that the causal effect of childhood obesity on future GDM risk was independent of adult adiposity. We identified three mediators, including HDL-C, apolipoprotein A-Ι, and triglyceride, in this association pathway. Our results provide insights into the pathogenesis of GDM and suggest additional prevention and treatment targets for GDM related to early life adiposity.
Gestational diabetes mellitus (GDM) is hyperglycemia that develops during pregnancy and usually resolves after birth (1). GDM affects up to 30% of pregnant women worldwide depending on the population, screening method, and diagnostic criteria (1). GDM has long been linked to adverse obstetric and neonatal outcomes and is mainly associated with higher infant birth weight (2). Moreover, GDM has been recognized as a risk factor for future cardiometabolic diseases in mothers and offspring (3). Observational and interventional studies have identified several modifiable and nonmodifiable risk factors for GDM, including advanced maternal age, family history of diabetes, previous GDM, previous macrosomia, overweight/obesity, and cigarette smoking, some of which are targeted in preventive and therapeutic strategies (4–7).
Childhood obesity poses a major threat to global public health, with an increasing prevalence in most parts of the world over the past decades (8). Irrespective of adiposity later in life, increasing childhood obesity may have significant consequences for population health, given evidence from observational studies and Mendelian randomization (MR) studies linking early life excess body weight to higher risks of chronic diseases, including hypertension and type 2 diabetes (9–11). Thus, childhood and adolescence may be critical periods during which adiposity affects the risk of developing metabolic disorders in adulthood. However, few studies have examined the impact of childhood obesity on the risk of developing GDM. Thus, whether childhood obesity contributes to the development of GDM later in life remains unclear.
MR uses genetic variation as an instrumental variable (IV) to estimate the causal association between exposure and outcome (12). Compared with traditional observational analyses, MR analyses are less prone to confounding and reverse causation, given the random allocation and fixed nature of genetic variants (13). Univariate MR (UVMR) analysis can estimate the total effect of early life adiposity on GDM risk (14). Moreover, multivariable MR (MVMR) allows the estimation of the independent effects of childhood obesity on GDM risk, independent of adult body size (15–17). It can also be used to examine the mediation between early life adiposity and GDM (18).
In this study, we conducted a two-sample MR analysis to examine the independent causal association between childhood obesity and GDM risk. Furthermore, we used two-step MR to investigate the potential mediators and quantify their mediating effects on this relationship.
MR was conducted in two stages. In stage 1, we performed a two-sample UVMR using summary-level data to assess the causal effect of childhood obesity on the risk of GDM. We then used MVMR to estimate the independent effect of childhood obesity on GDM after accounting for adult adiposity measures. In stage 2, we first screened for lipid traits, including low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride, apolipoprotein A-Ι, and apolipoprotein B, as potential mediators of the association between childhood obesity and GDM. We then performed a two-step MR to evaluate the mediation effect of each selected mediator on the causal relationship between childhood obesity and GDM.
We obtained genome-wide association study (GWAS) summary statistics of childhood obesity from the Early Growth Genetics (EGG) Consortium (19). The GWAS meta-analysis consisted of 14 relevant studies, with 5,530 cases (≥95% body mass index [BMI] reached before the age of 18 years) and 8,318 controls (<50% BMI consistent throughout all measures during childhood) (Table 1). The childhood BMI was calculated from the height and weight measurements obtained at ages 2–18 years, except for Avon Longitudinal Study of Parents and Children (ALSPAC), which leveraged BMI data available from the first four clinical examinations prior to 2 years old. The summary-level data for adult BMI were accessed from a GWAS meta-analysis, which included association results for up to from 125 studies, 82 with GWAS results (n = 236,231) and 43 with results from Metabochip (n = 103,047) (20). The GWAS summary statistics for adult waist circumference (WC) and waist-to-hip ratio (WHR) were obtained from the previously described meta-analysis, which included 142,762 individuals of European ancestry from 57 cohorts genotyped with GWAS and 67,326 individuals from 44 cohorts genotyped with the Metabochip (21) (Table 1).
For lipid traits, we obtained summary statistics for LDL-C, HDL-C, triglyceride, apolipoprotein A-Ι, and apolipoprotein B from the GWAS data provided by the UK Biobank (22) (Table 1). The GWAS of lipids and apolipoproteins in the UK biobank included a sample size ranging between 393,193 and 441,016 individuals, with a mean age of 56.9 years and a female representation of 54.2%.
Summary statistics for GDM were obtained from Release 8 results of GWAS data from the FinnGen consortium. This GWAS data included 11,279 GDM cases (identified using registry data on the International Classification of Diseases [ICD] 9 and 10 codes O24.4) with a mean age of 31.2 years (23) (Table 1).
Details of the recruitment, information on genetic data, and measurements of baseline characteristics of each cohort are obtained from the original study.
To obtain reliable IVs, three key assumptions of MR must be satisfied (24). First, IVs are strongly associated with early life adiposity/lipid traits. Second, IVs are independent of confounders of the exposure-outcome relationship (excluding mediators). Third, IVs affect the outcome only through exposure and mediators, and not through any other paths.
We screened for genetic variants with genome-wide significance (p < 5 × 10−8) and linkage disequilibrium (r2 < 0.001) within a 10,000 kb window (25). Palindromic single-nucleotide polymorphisms (SNPs) with incompatible alleles were also removed. Proxy SNPs were used when the SNPs were unavailable in the GWAS outcome data. The final IVs for subsequent MR studies consisted of rigorously selected SNPs. To avoid a weak IVs bias, the F-statistic was calculated to evaluate the strength of the selected IVs (26).
For UVMR analyses, the inverse variance-weighted (IVW) method was used as the main analysis (13). The MR-Egger (27), weighted median (28), and weighted mode methods (29) were used as sensitivity analyses to assess the robustness of the IVW estimate. We performed MR pleiotropy residual sum and outliers (MR-PRESSO) to detect outliers with horizontal pleiotropy among the chosen SNPs (30). We evaluated heterogeneity using Cochran’s Q statistic and identified horizontal pleiotropy based on the MR-Egger regression model intercept (27, 31).
For MVMR analyses, the multivariate inverse variance-weighted (MV-IVW) method was used as the main analysis. The multivariate MR Egger (MVMR-Egger) method was used for sensitivity analysis.
To control for false positive rates, we used a Bonferroni-corrected threshold of p < 0.01 (α = 0.05/5) when analyzing the causal effects of childhood obesity on lipid traits, lipid traits on GDM, and lipid traits on childhood obesity.
We then conducted two-step MR analyses to assess and quantify the mediating effect of the selected mediators on the causal relationship between childhood obesity and GDM. The first step was to estimate the causal effect (β1) of childhood obesity on each chosen mediator using a UVMR analysis. The second step estimated the causal effect (β2) of each mediator on GDM risk, adjusted for childhood obesity, using an MVMR analysis. We calculated each mediator’s proportion of the total effect of childhood obesity on GDM by dividing the mediation effect (β1 × β2) by the total effect (32). We derived the standard errors for the mediation effects using the delta method (33).
All analyses were performed using R (Version 4.1.3) with the R package “TwosampleMR,” “Mendelian Randomization,” and “MR-PRESSO” (34, 35).
In UVMR, genetically predicted childhood obesity was positively associated with GDM risk (OR: 1.21, 95%CI: 1.09–1.34, p = 4.58 × 10−4) (Figure 1). Sensitivity analyses using weighted median (OR: 1.41, 95%CI: 1.11–1.36, p = 9.81 × 10−4), weighted mode (OR: 1.24, 95%CI: 1.10–1.41, p = 0.03), and MR-PRESSO methods (OR: 1.21, 95%CI: 1.09–1.34, p = 0.02) supported the robustness of the IVW method (Supplementary Table S1). MR-PRESSO did not detect any outlier SNPs. The MR-Egger intercept test showed no evidence of pleiotropy (p intercept = 0.76). Cochran’s Q statistic indicated no potential heterogeneity among the selected SNPs (p heterogeneity = 0.07). The mean F-statistic for the selected IVs was 45, indicating that the estimates did not suffer from weak instrumental bias.

Figure 1. UVMR and MVMR estimates for the causal, independent effect of childhood obesity on GDM risk.
In MVMR analyses, the causal effect of childhood obesity on GDM remained significant after accounting for adult BMI (OR: 1.17, 95%CI: 1.01–1.36, p = 0.03), WC (OR: 1.10, 95%CI: 1.01–1.20, p = 0.03), or WHR (OR: 1.21, 95%CI: 1.07–1.36, p = 1.94 × 10−3) (Figure 1). Sensitivity analyses using the MVMR-Egger method further confirmed the robustness of the MV-IVW method (Supplementary Table S2).
The IVW results suggested that childhood obesity was negatively associated with the genetically determined level of LDL-C (β: -0.02, 95CI%: −0.03 – −0.004, p = 7.49 × 10−3), HDL-C (β: −0.04, 95CI%: −0.05 – −0.03, p = 1.35 × 10−7), and apolipoprotein A-Ι (β: −0.04, 95CI%: −0.05 – −0.02, p = 2.28 × 10−5) and was positively associated with the genetically determined level of triglyceride (β: 0.02, 95CI%: 0.005–0.03, p = 5.29 × 10−3) (Table 2). The mean F-statistics for the genetic instruments were greater than 10, indicating limited weak instrument bias (Table 2). The MR-Egger intercept test indicated no evidence of horizontal pleiotropy between childhood obesity and the selected mediators (Supplementary Table S3). Moreover, Cochran’s Q statistics showed no significant evidence of heterogeneity among the IVs (Supplementary Table S3).
MVMR analyses indicated that the causal effect of HDL-C (OR: 0.76, 95%CI: 0.65–0.89, p = 7.36 × 10−4), triglyceride (OR: 1.30, 95%CI: 1.11–1.53, p = 1.49 × 10−3), and apolipoprotein A-Ι (OR: 0.75, 95%CI: 0.63–0.89, p = 8.66 × 10−4) on GDM remained significant after accounting for childhood obesity (Table 3). The MVMR-Egger sensitivity analysis confirmed the robustness of the MV-IVW method (Supplementary Table S4).
Two-step MR analyses indicated three potential mediators, including HDL-C, apolipoprotein, and triglyceride, which might be responsible for the causal effect of childhood obesity on GDM risk. HDL-C mediated the total effect of childhood obesity on GDM risk (5.81, 95%CI: 3.05–8.57%), followed by apolipoprotein A-Ι (4.16, 95%CI: 1.64–6.69%), and triglyceride (2.20, 95%CI: 0.48–3.92%) (Figure 2).
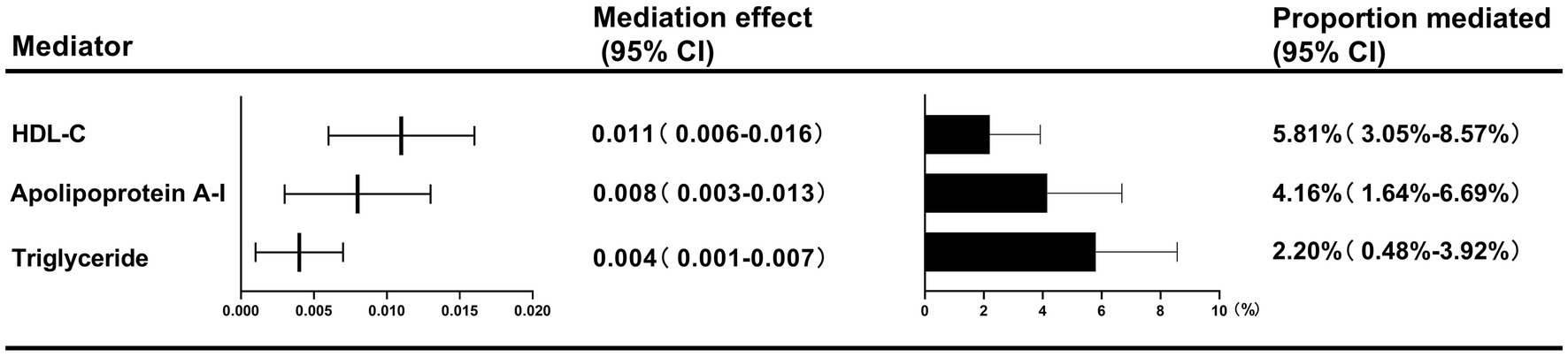
Figure 2. Two-step MR estimates for the causal influence of childhood obesity on GDM risk by each potential mediator.
In this MR study, we utilized two-sample and two-step MR approaches, for the first time, to investigate the causal associations between childhood obesity and GDM. Notably, we comprehensively explored the roles of lipid profiles in mediating the relationships of childhood obesity with GDM risk. Our main findings were threefold. First, we demonstrated that genetically predicted childhood obesity increased the risk of GDM, and this deleterious effect persisted after adjusting for adult adiposity traits, including BMI, WC, and WHR. Second, we found that childhood obesity was causally related to decreased levels of LDL-C, HDL-C, and apolipoprotein A-Ι and increased levels of triglyceride, while HDL-C, apolipoprotein A-Ι, and triglyceride were further causally associated with risk of GDM. Third, we identified HDL-C, apolipoprotein A-Ι, and triglyceride as potential mediators of the causal effect of childhood obesity on GDM.
Previous studies have identified a causal role of childhood obesity in the development of multiple chronic diseases, including hypertension and type 2 diabetes. However, few studies have focused on the association between childhood obesity and GDM. A retrospective study of 13,031 women with anthropometric information measured during childhood showed that a higher childhood BMI was associated with an increased risk of GDM (36). A cohort of 1,386 GDM patients found no association between heavy body shape at 10 years of age and the risk of self-reported GDM (37). A longitudinal study also showed that mean childhood BMI was not associated with the risk of GDM (38). The influence of potential confounders such as adult adiposity may partly explain the conflicting observations in existing studies. Using MR approaches to minimize potential confounding factors, our study built on previous evidence by demonstrating that childhood obesity causally and adversely affects GDM independently of adult adiposity. These findings highlight the significance of childhood obesity as a key indicator in GDM risk prediction and prevention.
To identify the biochemical mechanisms through which childhood obesity influences the risk of GDM, we further explored whether there are causal mediators of subsequent life trajectories that modulate the relationship between childhood obesity and GDM. We identified HDL-C, apolipoprotein A-Ι, and triglyceride levels as causal mediators of the impact of childhood obesity on GDM. Our findings are in line with the results of previous studies using metabolomic and lipidomic approaches, which showed that altered lipid traits associated with insulin resistance, inflammation regulation, and oxidative stress were involved in the pathophysiology of GDM (39, 40). However, further studies are required to confirm the mechanisms underlying childhood obesity-related GDM.
This study had several strengths. First, we designed a rigorous MR framework to establish causality between childhood obesity and GDM as well as mediation mechanisms. Second, we employed multiple complementary sensitivity analyses to verify the reliability of the MR findings. Third, we used large-scale GWAS summary statistics, which increased the statistical power and accuracy of the causal effect estimates.
This study also had several limitations. First, we assumed that the associations between childhood obesity and GDM were linear in both UVMR and MVMR analyses. Further research utilizing individual-level data is warranted to examine the potential nonlinear causal connections between childhood obesity and GDM. Second, we concentrated on the lipid traits that were theoretically linked to childhood obesity or GDM as candidate mediators. However, the mechanisms connecting childhood obesity and GDM were not fully elucidated.
In conclusion, this MR study supports the causal effect of childhood obesity on GDM risk. Furthermore, our findings suggest that this association is partially mediated by the lipid traits, HDL-C, apolipoprotein, and triglyceride. Understanding the causal relationships between childhood obesity, dyslipidemia, and GDM is crucial for elucidating the pathogenesis of GDM and identifying potential targets for early intervention.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics committee of Shengjing Hospital of China Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. The animal study was reviewed and approved by Ethics committee of Shengjing Hospital of China Medical University.
CXL and HL conceptualized and designed the study. CL and NL performed the analyses and interpretation of data. CL wrote the first draft of the manuscript. CL, CXL, and HL contributed to manuscript review and editing, and read. All authors contributed to the article and approved the submitted version.
The authors thank the participants of the UK Biobank study and the genome-wide association study consortiums (EGG, GIANT, and FinnGen) who made their summary statistics publicly available for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1225376/full#supplementary-material
1. White, SL, Ayman, G, Bakhai, C, Hillier, TA, and Magee, LA. Screening and diagnosis of gestational diabetes. BMJ. (2023) 381:e071920. doi: 10.1136/bmj-2022-071920
2. Zhang, C, Rawal, S, and Chong, YS. Risk factors for gestational diabetes: is prevention possible? Diabetologia. (2016) 59:1385–90. doi: 10.1007/s00125-016-3979-3
3. Johns, EC, Denison, FC, Norman, JE, and Reynolds, RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. (2018) 29:743–54. doi: 10.1016/j.tem.2018.09.004
4. Solomon, CG, Willett, WC, Carey, VJ, Rich-Edwards, J, Hunter, DJ, Colditz, GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. (1997) 278:1078–83. doi: 10.1001/jama.1997.03550130052036
5. Cypryk, K, Szymczak, W, Czupryniak, L, Sobczak, M, and Lewinski, A. Gestational diabetes mellitus – an analysis of risk factors. Endokrynol Pol. (2008) 59:393–7.
6. Yang, H, Wei, Y, Gao, X, Xu, X, Fan, L, He, J, et al. Risk factors for gestational diabetes mellitus in Chinese women: a prospective study of 16,286 pregnant women in China. Diabet Med. (2009) 26:1099–104. doi: 10.1111/j.1464-5491.2009.02845.x
7. Bar-Zeev, Y, Haile, ZT, and Chertok, IA. Association between prenatal smoking and gestational diabetes mellitus. Obstet Gynecol. (2020) 135:91–9. doi: 10.1097/AOG.0000000000003602
8. Collaborators, GBDO, Afshin, A, Forouzanfar, MH, Reitsma, MB, Sur, P, Estep, K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
9. Bibbins-Domingo, K, Coxson, P, Pletcher, MJ, Lightwood, J, and Goldman, L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. (2007) 357:2371–9. doi: 10.1056/NEJMsa073166
10. Hannon, TS, Rao, G, and Arslanian, SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. (2005) 116:473–80. doi: 10.1542/peds.2004-2536
11. Hu, B, He, X, Li, F, Sun, Y, Sun, J, and Feng, L. Childhood obesity and hypertension in pregnancy: a two-sample Mendelian randomization analysis. J Hypertens. (2023) 41:1152–8. doi: 10.1097/HJH.0000000000003442
12. Smith, GD, and Ebrahim, S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
13. Lawlor, DA, Harbord, RM, Sterne, JA, Timpson, N, and Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
14. Papadimitriou, N, Bull, CJ, Jenab, M, Hughes, DJ, Bell, JA, Sanderson, E, et al. Separating the effects of early and later life adiposity on colorectal cancer risk: a Mendelian randomization study. BMC Med. (2023) 21:5. doi: 10.1186/s12916-022-02702-9
15. Burgess, S, and Thompson, SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251–60. doi: 10.1093/aje/kwu283
16. Sanderson, E, Davey Smith, G, Windmeijer, F, and Bowden, J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. (2019) 48:713–27. doi: 10.1093/ije/dyy262
17. Sanderson, E, Richardson, TG, Morris, TT, Tilling, K, and Davey Smith, G. Estimation of causal effects of a time-varying exposure at multiple time points through multivariable mendelian randomization. PLoS Genet. (2022) 18:e1010290. doi: 10.1371/journal.pgen.1010290
18. Sanderson, E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. (2021) 11. doi: 10.1101/cshperspect.a038984
19. Bradfield, JP, Taal, HR, Timpson, NJ, Scherag, A, Lecoeur, C, Warrington, NM, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. (2012) 44:526–31. doi: 10.1038/ng.2247
20. Locke, AE, Kahali, B, Berndt, SI, Justice, AE, Pers, TH, Day, FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. (2015) 518:197–206. doi: 10.1038/nature14177
21. Shungin, D, Winkler, TW, Croteau-Chonka, DC, Ferreira, T, Locke, AE, Magi, R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. (2015) 518:187–96. doi: 10.1038/nature14132
22. Richardson, TG, Sanderson, E, Palmer, TM, Ala-Korpela, M, Ference, BA, Davey Smith, G, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med. (2020) 17:e1003062. doi: 10.1371/journal.pmed.1003062
23. Kurki, MI, Karjalainen, J, Palta, P, Sipilä, TP, Kristiansson, K, Donner, KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
24. Davies, NM, Holmes, MV, and Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
25. Hemani, G, Tilling, K, and Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
26. Burgess, S, and Thompson, SG, Collaboration CCG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
27. Burgess, S, Bowden, J, Fall, T, Ingelsson, E, and Thompson, SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. doi: 10.1097/EDE.0000000000000559
28. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
29. Hartwig, FP, Davey Smith, G, and Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
30. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
31. Bowden, J, Del Greco, MF, Minelli, C, Davey Smith, G, Sheehan, N, and Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
32. VanderWeele, TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. (2016) 37:17–32. doi: 10.1146/annurev-publhealth-032315-021402
33. Kong, L, Ye, C, Wang, Y, Zheng, J, Zhao, Z, Li, M, et al. Causal effect of lower birthweight on non-alcoholic fatty liver disease and mediating roles of insulin resistance and metabolites. Liver Int. (2023) 43:829–39. doi: 10.1111/liv.15532
34. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-base platform supports systematic causal inference across the human phenome. elife. (2018) 7:7. doi: 10.7554/eLife.34408
35. Yavorska, OO, and Burgess, S. Mendelian Randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46:1734–9. doi: 10.1093/ije/dyx034
36. Pedersen, DC, Bjerregaard, LG, Rasmussen, KM, Nohr, EA, and Baker, JL. Risk of gestational diabetes mellitus in nulliparous women – associations with early life body size and change in body mass index from childhood to adulthood. Diabetes Res Clin Pract. (2021) 171:108564. doi: 10.1016/j.diabres.2020.108564
37. Yeung, EH, Hu, FB, Solomon, CG, Chen, L, Louis, GM, Schisterman, E, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. (2010) 53:668–78. doi: 10.1007/s00125-009-1634-y
38. Wallace, M, Bazzano, L, Chen, W, and Harville, E. Maternal childhood cardiometabolic risk factors and pregnancy complications. Ann Epidemiol. (2017) 27:429–34. doi: 10.1016/j.annepidem.2017.06.002
39. Wang, Y, Huang, Y, Wu, P, Ye, Y, Sun, F, Yang, X, et al. Plasma lipidomics in early pregnancy and risk of gestational diabetes mellitus: a prospective nested case-control study in Chinese women. Am J Clin Nutr. (2021) 114:1763–73. doi: 10.1093/ajcn/nqab242
Keywords: early life, obesity, gestational diabetes mellitus, lipidomic biomarkers, Mendelian randomization
Citation: Li C, Li N, Liu C and Li H (2023) Causal effect of early life adiposity on gestational diabetes mellitus and mediating roles of lipidomic biomarkers. Front. Nutr. 10:1225376. doi: 10.3389/fnut.2023.1225376
Received: 22 May 2023; Accepted: 05 July 2023;
Published: 19 July 2023.
Edited by:
Diego Augusto Santos Silva, Federal University of Santa Catarina, BrazilReviewed by:
Ronny Westerman, Bundesinstitut für Bevölkerungsforschung, GermanyCopyright © 2023 Li, Li, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Li, bGlodWFuNzkzNHNqQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.