
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 27 July 2023
Sec. Nutrition and Food Science Technology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1223638
This article is part of the Research TopicEmerging Active, Smart and Intelligent Packaging Solutions in the Fourth Phase of the Industrial Revolution (Industry 4.0)View all 5 articles
This literature review provides a focus on the potential of integrating the latest scientific and technological advances in the biological field to improve the status of the key steps of a food packaging life cycle: production, usage, post-usage, and long-term fate. A case study of such multi-biological food packaging is demonstrated based on the use of PHAs (polyhydroxyalkanoates) polymer, a microbiologically produced polymer from non-food renewable resources, activated by the use of bioactive components to enhance its usage benefits by reducing food loss and waste, displaying potential for reusability, compostability as post-usage, and finally, being ultimately biodegradable in most common natural conditions to considerably reduce the negative impact that persistent plastics have on the environment. We discuss how designing safe and efficient multi “bio” food packaging implies finding a compromise between sometimes contradictory functional properties. For example, active antimicrobials help preserve food but can hamper the ultimate biodegradation rate of the polymer. This review presents such antagonisms as well as techniques (e.g., coatings, nanoencapsulation) and tools (e.g., release kinetic) that can help design optimized, safe, and efficient active food packaging.
Although packaging plays a crucial role in the food supply chain by maintaining the quality and safety of food for a certain period, environmental problems due to resource depletion and persistent plastic accumulation from food packaging are of increasing concern. Indeed, the world’s plastic production is expected to increase three-fold by 2060, and ⅓ of this production will be used for packaging development (1, 2). In the meantime, 90% of this plastic ends up in the environment (through littering, landfilling, mismanagement, etc.): 3% directly in the ocean and 87% on the terrestrial continent (in soil), where they are fragmented through physical abrasion into micro and nanoparticles that diffuse into all environmental compartments (air, soil, water, and living organisms), resulting in considerable environmental and biological damage (1, 3). In this context, the emergence of new materials allowing the overall packaging sustainability to be increased is paramount: it clearly means (1) minimizing overall resource use and process step impacts, (2) ensuring product preservation to reduce food loss and waste, (3) reducing the burden of post-usage plastic waste management (e.g., by being compostable or recyclable) and, (4) paying special attention to the long-term outcome that can lead to the accumulation of plastic in the environment in the final stage of the materials life chain (3–5). One solution to address these multiple issues is to focus on materials that provide a biological solution at each stage: a biological resource, bioprocess transformation, bioactivity to better preserve food, and finally biological digestibility in the post-usage stages (Figure 1).
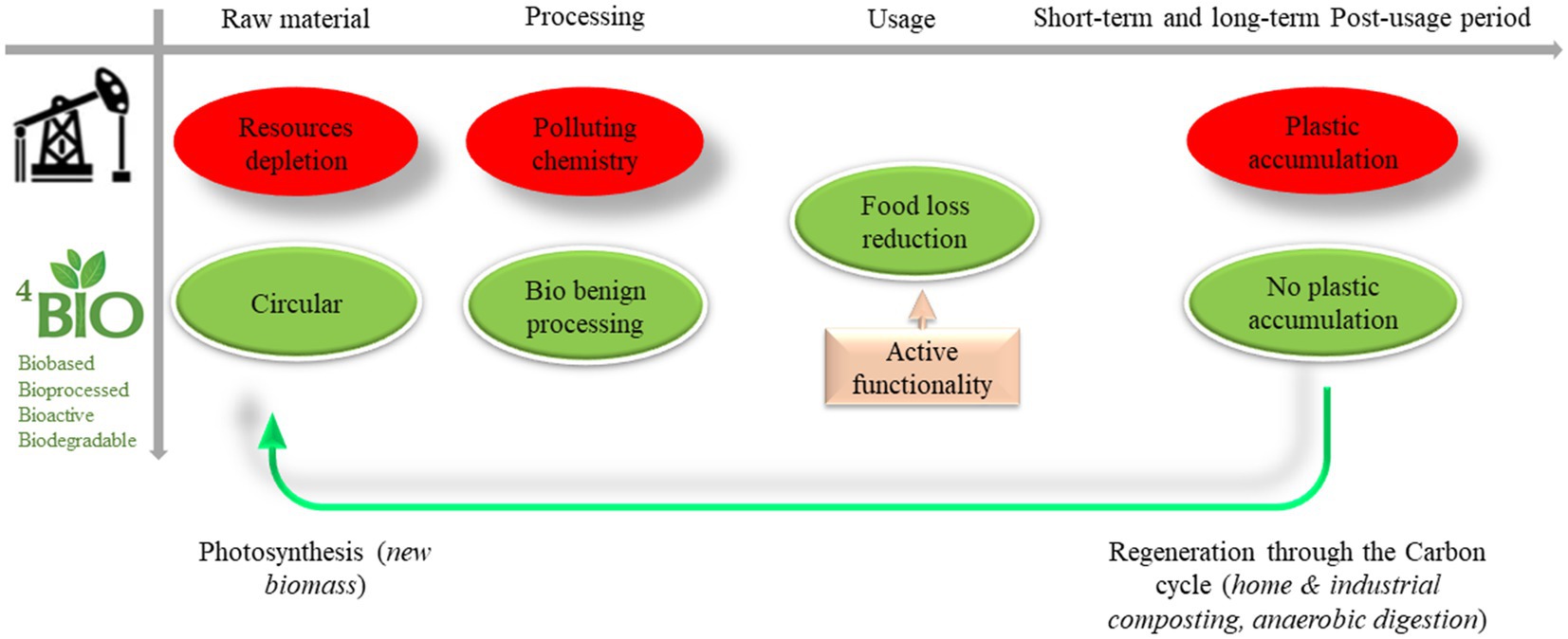
Figure 1. The expected effect of substituting fossil-based plastic materials with BIO4 ones – in the life cycle of food packaging materials (BIO4 = biobased, bioprocessed, bioactive, and biodegradable).
Therefore, to correctly use biobased resources for packaging development (e.g., plant or microbial polymers), it is necessary to use materials that are compatible with biomass temporal availability and the regeneration time and to avoid competition with food and feed uses. Moreover, bioprocess transformation refers to the use of biotechnologies to synthesize the material (e.g., microbial cells) with the net advantage that the range of resources to be used is considerably enlarged. For instance, with biotechnologies, it is possible to use organic wastes and other residues (6, 7). Bioprocesses also appear to be good alternatives to conventional chemical synthesis, because they require less time and additives, which can result in health problems in living marine and terrestrial organisms (endocrine disruptors, etc.) (8–10). Bioactive packaging refers to the integration of active bio components in the polymer, such as natural antimicrobials or antioxidants which can increase the packaging usage benefit by preserving food from deterioration, which leads to food loss and waste (FLW) mitigation, as demonstrated by several authors (11–13). Finally, biodegradable packaging means that the material could be disintegrated and then assimilated by microorganisms and digested into small unitary molecules (H2O, CO2, CH4) to produce a new microbial biomass. Ultimate biodegradation is currently the only solution to reduce conventional plastic accumulation in the environment (3).
Although several articles have already highlighted the importance of biodegradable packaging [see among others (14)], or the relevance of active, and smart biodegradable packaging for the food sector (15), or the applicability of biobased packaging for long-shelf-life foods (16), no publications to date have addressed all the four ‘bio’ aspects in a holistic approach to the packaging sustainability. It should be noted that in the web of sciences with the keywords “biodegradable, bioactive packaging” AND “biodegradable, bioactive, biobased packaging” AND “biodegradable, antimicrobial packaging” AND “biodegradable, biobased, antimicrobial packaging,” we obtained 90, 5, 215, and 6 review articles, respectively (carried out in February 2023).
In this context, the aim of this paper is to discuss the relevance of developing active packaging materials combining the four “bio” aspects (biobased, bioprocessed, bioactive, and biodegradable) by highlighting their challenges and limitations. Examples will be provided from commercial products or polymers that are still in development (e.g., PHAs, a family of polymers that, promisingly, covers the four “bio” aspects). This review highlights the importance of a reasoned-integrated approach to develop bio-benign, biodegradable, efficient active food packaging starting from the food needs in terms of preservation without compromising the ultimate biodegradability of the material. The importance of the use of biobased, bioprocessed, and biodegradable material will be discussed first, with examples of different polymers more or less in accordance with these aspects. The importance of the strategy of bioactive molecule incorporation is then developed, with the presentation of cutting-edge ways for incorporating active ingredients into biopolymers. Finally, the post-usage fate of the bioactive, biodegradable packaging materials is discussed.
The fear of a near future where oil-based resources are lacking and the societal demand for more ‘green’ materials have led to the development of polymers derived from renewable biological resources (‘biobased’).
For this purpose, research efforts have long focused on transforming plant or other non-fossil biogenic feedstocks into materials identical (e.g., bio-PET, bio-PE) or close to those derived from petroleum (e.g., PLA). At the same time, efforts have been made regarding the development of biodegradable solutions to solve the issue of plastic persistence in the environment, leading to the emergence, in the early 1990s, of the ‘bioplastics’ market, which includes both biodegradable and biobased plastics, with unfortunately a high degree of confusion regarding the distinction between them (17, 18). “Biobased” means materials partially or fully produced from biomass resources (such as sugar cane, hydrolyzed maize, rice or potato starch, etc.), irrespective of the end-of-life fate of the material (17). As such, a biobased polymer may be not biodegradable, such as bio-polyethylene (bio-PE), which is not more biodegradable than its oil-based counterpart. Several publications have sought to clarify this terminology and denounce the variations coming from the overall misinterpretation of the ‘bio’ prefix (17–19).
Two categories of biobased polymers can be considered (20): the first one consists of materials whereby the chemical structure of the biomass feedstock is not maintained. This is the case, for instance, when biomass is used to obtain soluble sugars, which are then fermented to produce monomers for the polymerization (e.g., lactic acid to produce poly(lactic acid) or ethylene to produce biobased polyethylene). This is the route followed to obtain polymers that are identical to those derived from petroleum (e.g., bio-PET, bio-PE). The second category corresponds to a group of polymers directly synthetized by living organisms (plants, algae, microorganisms, etc.) for which the initial chemical structure is preserved after extraction and purification and then used as such [e.g., polyhydroxyalkanoates (21)], polymalic acid (22) or only slightly modified [e.g., celluloses acetates (23)].
To manufacture compounds belonging to the first category, significant efforts must be placed on the plant transformation, with selective chemical processes required to remove chemical functionality afforded naturally by many biobased feedstocks (e.g., many natural molecules contain oxygen or nitrogen, while this is not the case for fossil-based hydrocarbons). Agricultural crops produce precursors: mostly starch and soluble sugars, that can be directly used to obtain biobased polymers, or building blocks derived from selective transformation used to extract, fractionate, and deconstruct plant cell-wall polymers (cellulose, hemicellulose, pectin, lignin, etc.) (24). Additional chemical transformations are often needed to obtain the necessary functionality for polymerization of the biobased building blocks into polymers.
Beyond the economic and environmental cost of all this necessary chemistry, the use of dedicated agricultural crops for producing biobased polymers is highly controversial in a period of fears for worldwide food security (25). Most lifecycle analyses (LCA) show that biobased polymers are better than their oil-based equivalents in aspects such as GHG emissions and fossil fuel consumption but not for other indicators such as eutrophication, land occupation, etc., which leaves the question of environmental superiority of biobased polymers for further debate (26, 27).
In light of the questionable economic viability and environmental issues of biobased polymers chemically synthesized from biobased building blocks, a reasonable guideline would be to focus on the second category of polymers, those directly synthetized by living organisms and used after extraction/purification with minimal chemical transformations. To not compete with the food chain, they should be produced from agricultural or food by-products or residues. The use of residues and even organic wastes as feedstock to produce polymers is gaining more and more attention, especially in line with the development of circular economy schemes (7). They provide significant environmental benefit, provided that their collection, transport, and transformation are regionally-reasoned and do not necessitate expensive pre-treatments and transportation (28, 29).
Biodegradability is the intrinsic property of a material to be fully degraded by living microorganisms (e.g., soil microorganisms) into a new biomass and small non-toxic molecules such as water, carbon dioxide (CO2), and/or methane (CH4) in widespread natural environmental conditions and in a reasonable timeframe compatible with human life cycles (17). The term biodegradable is often and misleadingly applied to plastics that are biobased but not necessarily biodegradable, or that undergo degradation (e.g., lowering of the molar masses of macromolecules that form the material) but without complete, ultimate mineralization (30). The biodegradability of a polymer depends only on its chemical structure and not the carbon source. Polymers that are compostable are not necessarily biodegradable under ambient environmental conditions. For instance, PLA needs to reach a minimal temperature of approximately 60°C to be biodegraded, which can be achieved in industrial composting plants but not in soil for instance or even domestic compost (19). The risk of considering a polymer that turns out not to be biodegradable under ambient environmental conditions is the production of persistent micro and nano plastics with long-term adverse effects for the entire biosphere (3).
In light of the above, nonfood, nonfeed biobased, and minimally bioprocessed polymers appear to be the best compromise of biobased and bioprocessed polymers. In addition, care must be taken regarding the type of biodegradation that the material can achieve: ultimate biodegradation, e.g., complete degradation into mineral molecules, under common ambient environmental conditions, and in a reasonable timeframe are paramount.
Table 1 summarizes the various types of biopolymers already marketed and commercially available and also those that are still in development. Clearly, currently marketed biopolymers are not fully “bio” from their resources nor their end-of-life, or present limited usage benefit. They are either derived from food resources (e.g., PLA, PBSA, starch-based blends, or commercial PHBV) or not fully biodegradable under natural conditions (e.g., PLA), or not water resistant (e.g., starch-based blends).
Only cellulose-based materials and PHAs obtained from non-food resources (such as PHBV) appear to fulfill all the criteria.
PHAs comprise a family of polymers with very versatile properties (water-insoluble, hydrophobic, thermoplastic). They can be molded into rigid or semi-rigid items using thermomechanical processes, and in their final shape, they exhibit good oxygen barrier properties that make them promising for fresh and other processed food packaging applications (31).
Commercial PHAs are currently nearly exclusively the copolymer polyhydroxy(butyrate-co-valerate), P(HB-co-HV). P(HB-co-HV) is a copolymer, meaning that it is a combination of two monomers, namely hydroxy-butyrate and hydroxy-valerate. P(HB-co-HV) is currently produced using pure microbial cultures fed with high-purity substrates that require (1) sterility (2) high energy, and (3) pure glucose or corn steep liquor as feedstock (48% of the total production cost). This contributes to a prohibitive market price (5 €/kg) to be used as a commodity polymer.
To address this issue, and to create a virtuous cycle rather than depleting one by using organic residues instead of apure carbon source, researchers have developed bioconversion processes of agri-food residues using optimized eco-efficient mixed microbial cultures (MMC). These processes decrease the investments and operating costs of the P(HB-co-HV) conversion with respect to pure culture and make it easier to use cheaper by-products such as feedstock (6, 32, 33). However, these processes are not yet available at an industrial scale.
Once produced, PHA macromolecules must be extracted and purified to remove all impurities (e.g., proteins, inorganic compounds) that can hamper its further use as a thermo-mechanical processable polymer. To do this, after harvesting cells from the bioreactor, flocculation, centrifugation, or filtration is used to remove the aqueous phase from the sedimented biomass. After a thermal drying or lyophilization step, the PHA-rich biomass is ready for extraction and purification (34, 35). Most people utilize methods on a laboratory scale such as solvent-based extraction (using halogenated solvents or halogen-free solvents such as butanol, or acetone, etc.), enzymatic (Alcalase®, lysozyme) or mechanical techniques (ultrasonication, high-pressure homogenization, osmotic pressure), or combinations of these methods. These methods result in cell disruption, enabling polymer solubilization in the solvent used. The last steps are then washing and drying the polymer to obtain a powder that can be stored and subsequently used (36).
PHA granules are often damaged by random and chain-end scission during solvent-based extraction processes. The final PHA purity and quality (e.g., molecular weight and polydispersity index) are thus affected by the extraction method. The solvent extraction method (especially halogenated solvents) provides the highest recovery with the highest purity and molecular weight (up to 98% and 0.8 MDa, respectively) (33, 35–38). However, because of the cost and the environmental impact of solvents, their use remains possible only at the laboratory scale, for research purposes. Beyond extraction, purification is also very important and has been found to greatly impact the processability of the polymer and its final properties (39); it was observed that multiple purification steps lead to better P(HB-co-HV) mechanical properties after processing. Because considerable quantities of hazardous solvents and energy are necessary during the extraction and purification steps, PHA production is not fully bio-benign. Moreover, the halogenated solvents used for the extraction can cause severe health issues in living organisms as well as environmental problems (40).
Above all, PHAs are biodegradable under various composting conditions (including home-compositing) and other natural environments (e.g., soil), making them particularly suitable to fight pollution by persistent plastics. As a result of successful tests on the biodegradation of PHA in activated sludge, compost, aqueous environments, and soil, the biodegradation of PHA blends with natural fillers in composting conditions is faster than with pure PHAs (41–43). This high biodegradation rate is the result of the involvement of a wide range of microorganisms and fungi, similar to the ones used for PHA biosynthesis, that can biodegrade PHAs in anaerobic and aerobic conditions (37, 44–48). Environmental factors such as temperature, pH, moisture, oxygen concentration, sunlight, the number/amount of microorganisms, as well as the chemical structure of PHA such as the percentage of 3 HV, are known to affect the rate of biodegradation of these polymers (49).
In summary, PHAs can be synthesized from agri-food wastes and residues using bioprocessed (microbial synthesis) and they are biodegradable under natural conditions and ambient temperature. They are thus promising candidates to turn the current linear plastic chain into a more sustainable cycle. Moreover, PHAs exhibit good usage benefits, with high gas/water barrier properties as well as good crystallization and mechanical properties; making them good candidates to prolong the shelf-life of food products. PHAs can also be converted into bioactive materials to increase their packaging usage benefit of reducing food losses.
Commission Regulation [EC 450/2009 (50)] defined Active Packaging (AP) materials as follows “Materials and articles that are intended to extend the shelf-life, or to maintain or improve the condition, of packaged food; they are designed to deliberately incorporate components that would release or absorb substances into or from the packaged food or the environment surrounding the food.” As described in Table 2, various scavenging and releasing systems have been developed, aimed at decreasing the deterioration processes in food, such as microorganism growth, oxidation, etc. (61).
For example (62), showed that 0.5% of gallic acid in PHBV trays of meat, fish, or cheese packaging can induce the absorption of 7.7% of oxygen in 10 days (derived from food desorption or oxygen entrance through the packaging), thus limiting oxidation and microbial aerobic growth. Similarly, several authors have shown that the inclusion of different essential oils such as buriti oil (1.5 wt.%), orange leaf essential oil (2 wt.%), or thymol (6 or 8 wt.%) in chitosan, gelatin, or PLA films exhibited antimicrobial activity against E. coli, P. aeruginosa, S. aureus, and B. subtilis, as well as C. albicans and A. niger, making them suitable strategies to increase food shelf-lives in different sectors (63–66). Going beyond these studies demonstrating antimicrobial effects on only a single microbial species Alparslan et al. (67) tried to quantify the shelf-life gain obtained on fresh shrimp packed in gelatin film containing 2% orange leaf essential oil: the shelf-life increased from 8 days with gelatin film to 14 days with the active film. With the same objective of benefit quantification Suwanamornlert et al. (66) reported that the shelf-life of bread increased from 6 days (control with PLA packaging) to 9 days with PLA film containing 6 wt.% thymol.
It should be noted that essential oils extracted from natural aromatic plants (e.g., oregano, thyme, eucalyptus, rosemary, clove, cinnamon etc.) is often used as natural antioxidant, antimicrobial, and antifungal compounds for AP (68–70). Indeed, consumer demand for food products without synthetic chemical preservatives has promoted EO usage in the food packaging industry in recent years (68). As for all AP, the use of active components directly in the packaging material instead of in the food allow reduction of the use of preservatives, as the active component is slowly released toward the surface where it is needed (on the food surface).
To obtain safe and efficient active materials, the quantity of active compounds added in the formulation of active systems is defined by the expected efficiency but also regulatory needs and sensory aspects (e.g., active compounds that are also flavoring agents). Indeed, a specific admissible daily intake (ADI = the quantity of this specific compound that can be eaten without a negative impact on the health) is defined by regulations for all potential active substances (71). The use of the active molecules is possible only if the natural exposure of consumers to those substances from other (natural) sources (natural occurrence in food products due to their composition) does not exceed the ADI (Figure 2). The natural exposure depends on the country, food consumption habits, and the consumer’s age. This knowledge allows determination of the maximal quantity of the AC that can be used for active packaging (ADI minus the natural exposure) (Figure 2).
The choice of the best molecule to develop an active antimicrobial packaging also depends on the capacity of the molecules to act on the targeting of specific microorganisms. Consequently, the appropriate AC can differ from one product to another, depending on the microorganisms prone to growth on the product. To correctly design the active packaging, it is necessary to confirm that the quantity that can be used for the active packaging, while remaining below the difference between the ADI and natural exposure, is enough to reach the minimal inhibitory concentration (MIC) of the targeted microorganisms [EC 450/2009 (50)] (Figure 2). The use of modeling tools, taking into account the release and migration (for volatile molecules) or diffusion characteristics (for non-volatile molecules) of the AC in defined storage conditions (temperature, duration) of the product, as well as MIC for the targeted microorganisms allows precise determination of the quantities of antimicrobial agents to use in packaging to preserve the food product from deterioration (13, 72). For some molecules with high odor or taste, such as essential oils, the quantity of AC to use should be below the odor threshold and avoid organoleptic modifications (Figure 2), as stipulated in the European regulations [1935/2004 EC (73)] and [EC 450/2009 (50)].
The impact of antimicrobial compounds, such as essential oil, on microorganism inhibition in food is dependent on the release and migration capacity of the molecules, and as a consequence on (1) the strategy of AC incorporation in the polymer material, (2) the volatility of the AC, and (3) the interactions and bonds between the component and the polymer material (74, 75). Indeed, different authors have shown that a film containing a homogenous concentration of AC throughout generated a slower and more sustained release than a film with an active coating concentrating the entire quantity of AC on the film surface (Figure 3) (76–78). This structure of AP and strategy of AC incorporation impact the preservative action of the AC on the food (Figure 4). For example, Wicochea-Rodríguez et al. (79) showed a four-fold lower release constant for carvacrol included in soy protein isolate film compared with the equivalent solution (carvacrol in soy protein isolate) coated on paper. Moreover, the authors also showed that the production of the same films with eugenol instead of carvacrol considerably increased the release of the molecule (release rate constant 100 times higher) due to the higher volatility of the molecule and lower retention by polymer, thereby inducing a different mechanism of release.
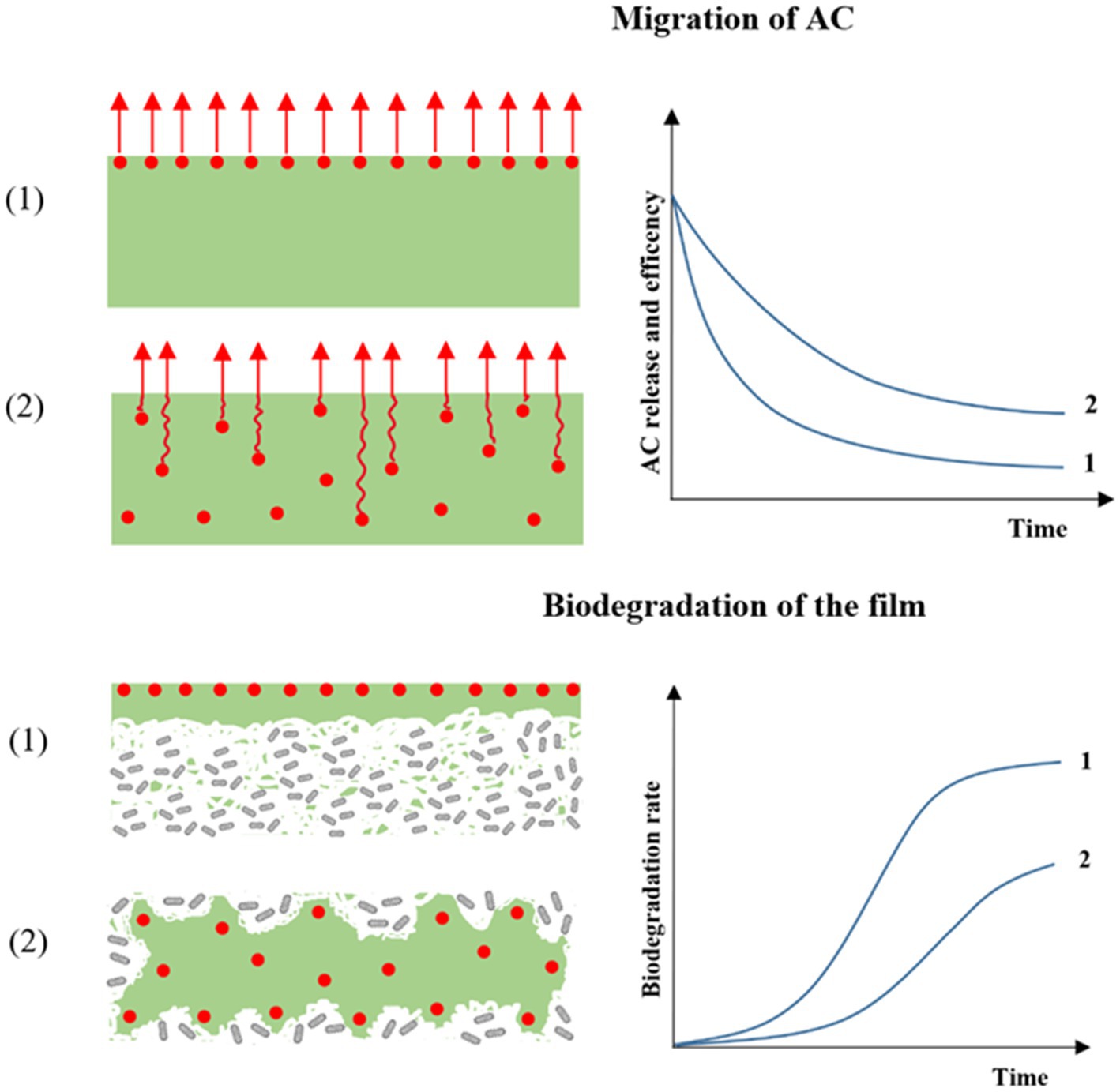
Figure 3. Hypothetical dependence of the active functionality incorporation methods, meaning (1) in coated and (2) “Throughout” material on the migration of AC and biodegradation of the film.
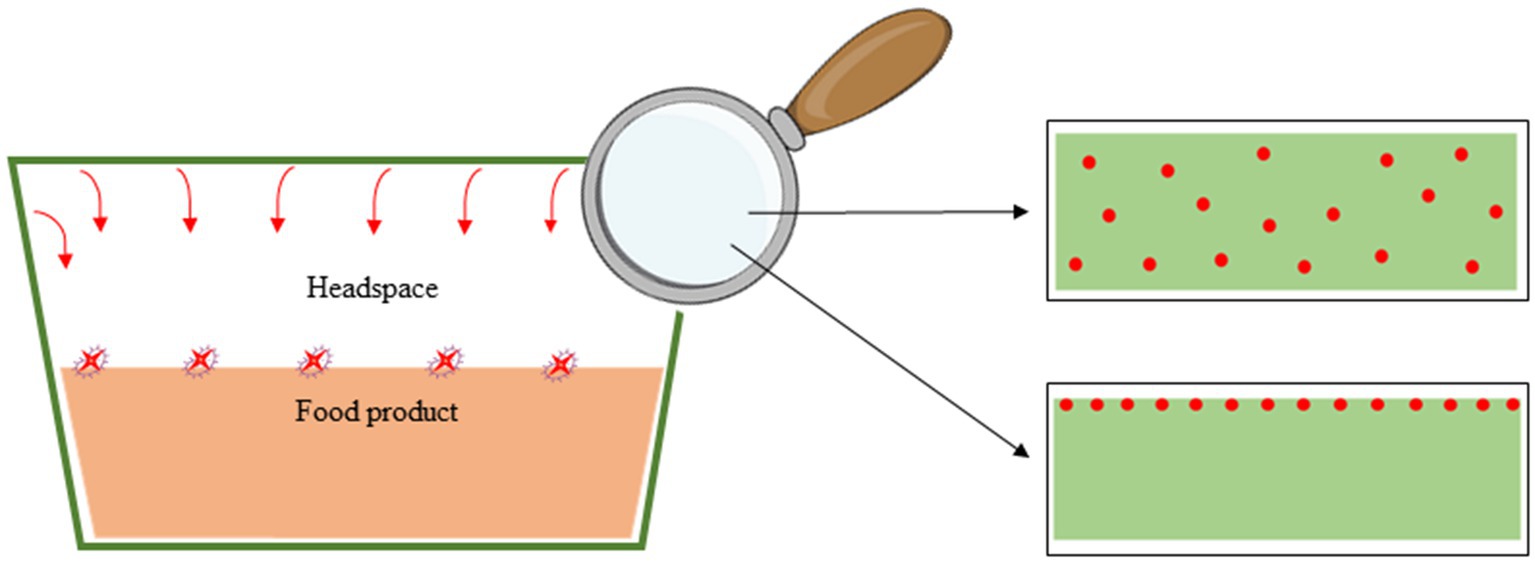
Figure 4. The various film structures and active volatile compound incorporation strategy (Active Compound Throughout and Active Compound in a surface coating) in packaging.
The choice to integrate AC throughout versus on the surface of polymers can also generate different degrees of loss of the molecules, depending on the production processing. Indeed, integration of AC directly in the polymer usually implies that the AC is subjected to thermal processes, such as extrusion or compression molding, meaning that highly sensitive molecules can be degraded, thus reducing their antimicrobial activity. By contrast, integration of AC on the surface of the polymer usually implies no thermal coating process, such as a bar, knife, spray coaters, or electrospinning, which preserves AC and its antimicrobial activity. The main principle of both of these physical incorporation methods is that the active components are physically contained or dispersed within the packaging material. These methods do not involve any chemical reactions or modifications of the active components, unlike sol–gel processes, surface immobilization, cross-linking, and covalent binding methods that involve chemical reactions (covalent and non-covalent bonds) between antimicrobial molecules and the film surface (77, 78, 80). The advantages and disadvantages of these incorporation methods are listed in Table 3.
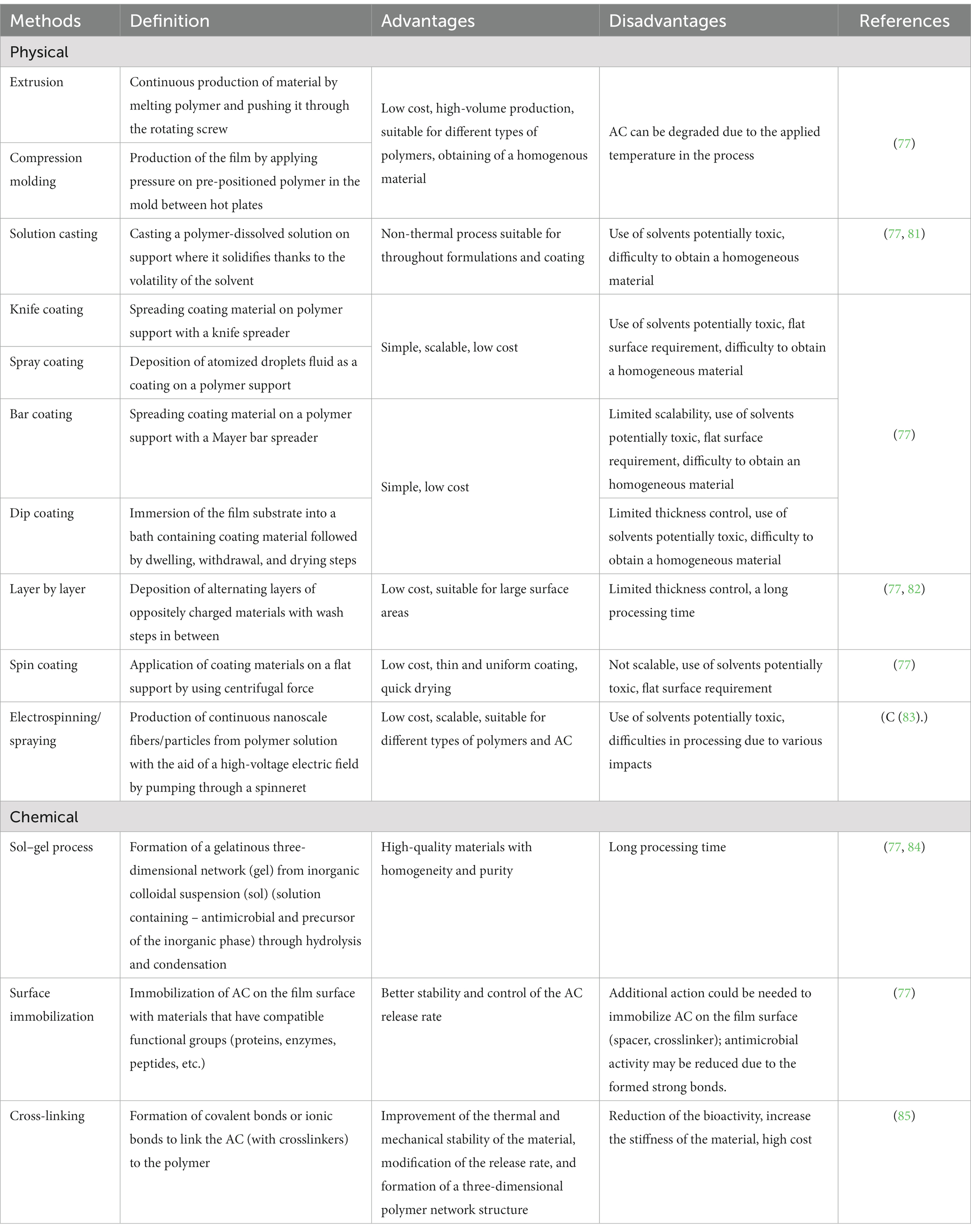
Table 3. Advantages and disadvantages of physical and chemical active functionality incorporation methods.
To overcome the issue of thermal degradation during thermomechanical shaping processes, attention has been given in the past two decades to different encapsulation strategies. Beyond mitigation and protection of AC from thermal degradation during packaging production, encapsulation may also help to control the release of the active molecules, especially if they are volatiles, and it is also used for that purpose (86). The term “encapsulation” was defined by Becerril et al. (87) and Stoleru and Brebu (88) as the technique that permits a substance (the active agent) to be encapsulated within another, resulting in tiny particles, the contents of which are gradually released under certain conditions. Hence, encapsulation strategies permit savings in regard to some active components as it is no longer necessary to compensate for the AC thermal degradation by spiking the initial load of AC in the material. In the same way, it allows minimization of the negative impact of the EOs on odors and organoleptic characteristics of the food products (89–91). Finally, encapsulation allows AC losses during storage (e.g., AC trapped into a polymer capsule) to be avoided and to trigger the release only when it is necessary, e.g., in food contact by action of humidity for instance as is the case for cyclodextrin-based capsules (92). Various carriers have been tested to develop encapsulation strategies, such as cyclodextrins, (nano)clays, nanofibers, and nanoparticles, all of which exhibit some advantages and disadvantages, as listed in Table 4 (87, 94).
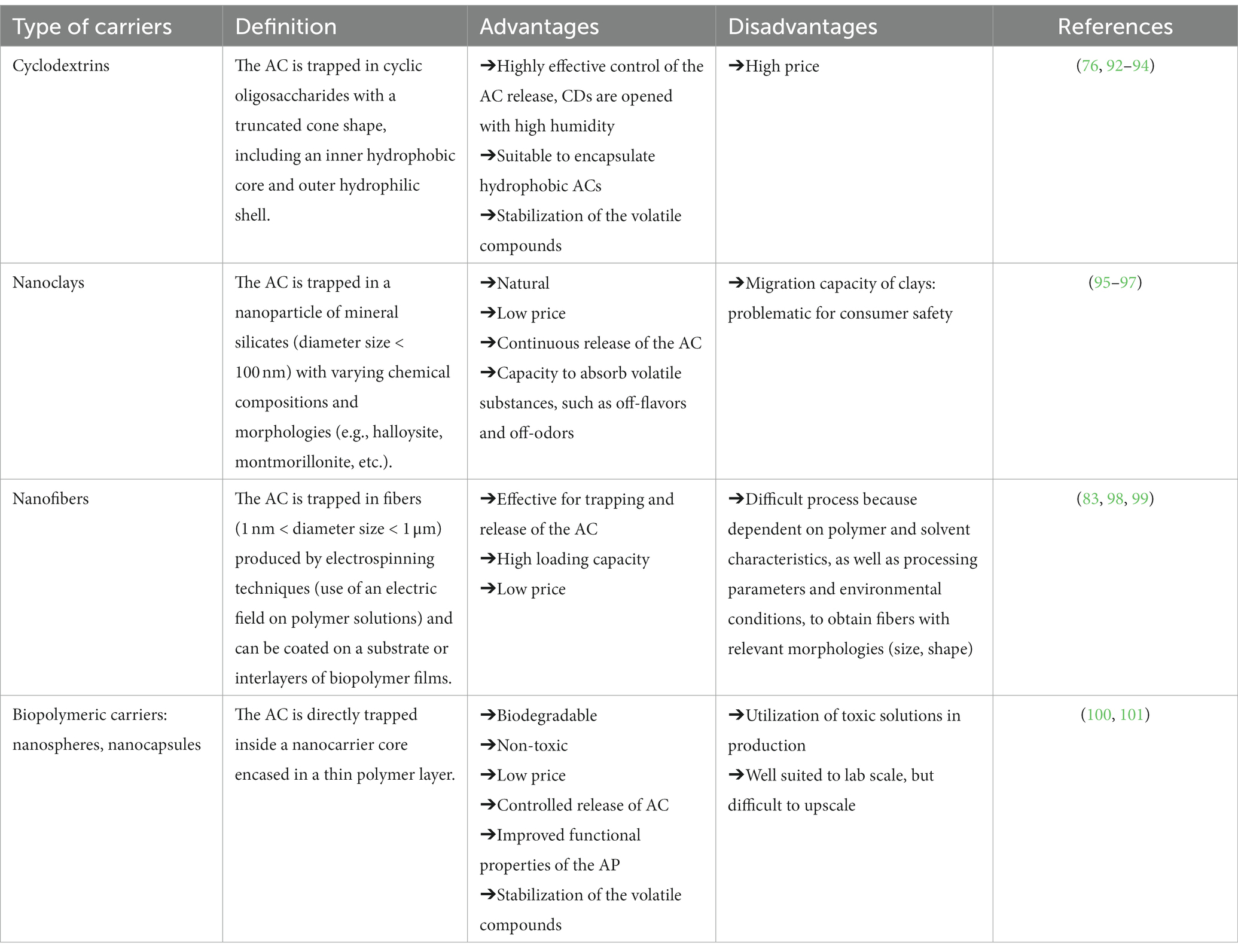
Table 4. Advantages and disadvantages of the various encapsulation strategies used for active packaging.
To design bioactive and biodegradable packaging, it is crucial to consider the impact of natural antimicrobial components in polymers on the degradation capabilities of the entire packaging. Thus, it is necessary to achieve the right balance between the antimicrobial characteristics of packaging materials to increase the food shelf-life during the usage phase and the biodegradation of packaging during the post-usage phase. The influences of the incorporated bioactive components on the biodegradation properties of active biobased materials are reviewed in Table 5.
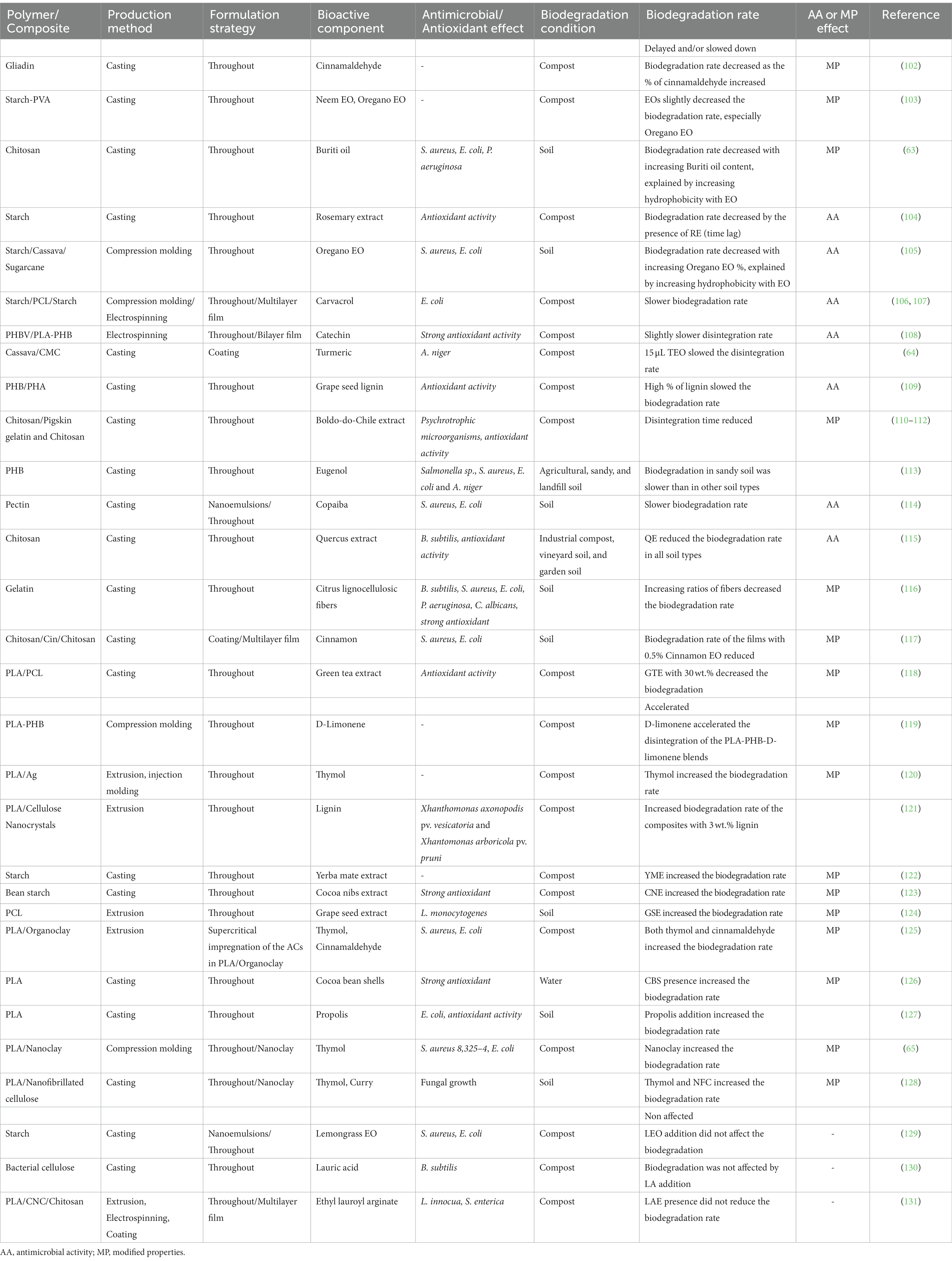
Table 5. Effects of bioactive components on the antimicrobial/antioxidant activity and biodegradation of biopolymers.
All studies (Table 5) showed that the impact of active components on polymer biodegradation was due to either (1) the antimicrobial activities of the AC or (2) modification of polymer properties owing to the presence of AC (132, 133).
In the first case, essential oils and natural bioactive components have a high probability to hamper the biodegradation of biopolymers, as they are per se antimicrobial compounds and poorly biodegradable due to the presence of quaternary carbon atoms and fused or bridged ring systems (134). Most of these AC are extracted from thyme, mint, sage, parsley seed, and spearmint oil, and they are toxic to microorganisms, including those involved in biodegradation (135, 136). Various effects can be expected: a delay in the biodegradation kinetics (time lag) without further impact on the biodegradation rate, a slowing down of the biodegradation rate, or a decrease of the maximal biodegradation level, as shown in Figure 5. A combination of these three different impacts may also occur. An impact on the disintegration (the first step of the biodegradation process before assimilation by the microorganisms) has also been reported (108).
For instance, Tampau et al. (107), showed that biodegradation of a starch/PCL/starch multilayer containing carvacrol (15 wt.% PCL) in compost remained incomplete, with a maximum of 85%, after 45 days compared to the starch/PCL/starch film without carvacrol that is 100% biodegraded in the same conditions and duration, while Piñeros-Hernandez et al. (104) showed that biodegradation of starch film containing between 5 and 20% rosemary extract (polyphenol) was retarded in compost conditions (Table 5) but that the final biodegradation level was not affected.
However, although a decrease in biodegradation because of antimicrobial activity of AC was often observed, the majority of studies cover only active packaging with a non-encapsulation AC throughout the entity (Table 5). Consequently, the impact of the incorporation strategies, i.e., encapsulation or not, coating vs. incorporation throughout, etc. is very absent from the literature. Indeed, the presence of the AC on the surface of the packaging instead of throughout the entity increases the release of the molecules during the usage stage (see Section 3.2). Therefore, biodegradation should be impacted less, as the concentration of AC is lower and the polymer is more available for microorganisms at the post-usage stage (Figure 3). In this vein, Mustapha et al. (64), showed that a decrease in the coating thickness of starch with turmeric oil induced an increase in biodegradation. Consequently, a high level of importance should be placed on the design of active packaging to find the best formulation allowing the shelf-life to be increased without delaying the biodegradation of the material. For this, mathematical modeling could be developed, coupling the alteration of the microorganism’s growth in food and their impact on the product shelf-life, as well as the growth of the biodegradation microorganism and the impact on the end-of-life of the material.
In the second case, the addition of AC in polymers can either reduce or increase the polymer biodegradation due to modification of their properties. A decrease in biodegradation can be due to an increase in the molecular weight or hydrophobicity, or a decrease in flexibility, as has been observed with the presence of cinnamaldehyde (1.5 wt.% to 5 wt.%) in gliadin film during compost (102) or cinnamon (0.5 wt.%) in chitosan/starch film during biodegradation in soil (117). Indeed, EOs formed bonds with the polymer chains, and this cross-linking increased the glass transition temperature. It also restricted the entrance of water into the polymer matrix, which led to less swelling and lower water vapor diffusion coefficients. By contrast, the increase in biodegradation can be due to an increase in hydrophilicity, thermal stability, and chain mobility and a decrease in the molecular weight and crystallinity, as has been observed with the presence of green tea extract (from 10 to 30%) in PCL/PLA film in compost (118), or olive pomace (15%) in PHBV film during biodegradation in soil (137). It should be noted that AC encapsulated in nano clays, such as thymol in PLA-based nano-composites, cinnamaldehyde in PLA/organoclay, or curry/thymol in nanofibrillated cellulose/PLA, led to an increase in the film’s biodegradation owing to higher sensitivity to hydrolysis of the polymeric chains, which highlights the relevance of encapsulation of the AC (65, 125, 128). However, the impact of AC on the biodegradation of the polymer can differ due to the amount of the AC and reach a breaking point with an increase/or decrease of the AC quantity, whereby polymer biodegradation can decrease because of significant deterioration of the polymer properties. For example, the presence of 20 wt.% green tea extract in PCL/PLA accelerated the biodegradation of the entire material owing to increased hydrophilicity, but 30 wt.% green tea extract in PCL/PLA decreased the biodegradation of the entire material owing to an increase in crystallinity (118). By contrast, the integration of 3 wt.% lignin into a cellulose nanocrystals/PLA system resulted in acceleration of the disintegration of the film, but the presence of only 1 wt.% lignin in the system resulted in slower biodegradation due to a higher degree of crystallization of the material (121).
Finally, in some rare cases, the presence of AC in the polymer did not impact the biodegradation of the entire material, as observed with lemongrass EO and lauric acid in starch (129, 130), and ethyl lauroyl arginate (LAE) in a PLA/PLA-LAE/PLA-CNC/Chi structure (131).
Beyond AC incorporation that results in modification of the polymer properties, conditions met during biodegradation also affect the biodegradation rate (humidity, nature of the soil, etc.). Therefore, the type of active molecules, their incorporation strategy, their interaction with polymer, and on polymer properties, are among the many parameters that can affect (positively or negatively) the biodegradation of the bioactive material and should hence be taken into account. Beyond these observations, the comprehension of the interactions of these different parameters still needs to be deepened to better design active packaging, allowing for an increase in food shelf-life by maintaining or even enhancing the biodegradation of the packaging.
The continuous increase in plastic accumulation and production worldwide, and the very substantial environmental impact of food loss and waste, are the main reasons for improving the overall sustainability of food packaging materials. Therefore, an appropriate choice of material is paramount to tailoring sustainable food packaging. The use of materials derived from biobased sources, which do not compete with food and feed resources, has resulted in bioprocessing for environmentally friendly production. They are fully biodegradable by ensuring their digestibility under natural conditions, as is the case for PHAs, and they are showing great promise to enhance sustainability. Furthermore, bioactive materials help preserve food by minimizing deterioration, such as microbial development or oxidation, consequently reducing food loss and waste and its negative environmental impact. However, to design efficient antimicrobial packaging, the AC has to be present in sufficient quantity to reach the minimal inhibitory concentration of target microorganisms without surpassing the admissible daily intake. To achieve this goal, various strategies for the incorporation of AC in film (coating or throughout the polymer) have been developed with different encapsulation options, thereby enabling control of the release of the AC. However, it is crucial to achieve a proper equilibrium between antimicrobial functions during usage and biodegradability in the post-usage phase. Yet, in the case of natural active components (EOs, plant extracts, etc.), it has been shown that the amount and type of added AC, its incorporation strategy into the polymers, and its impact on the polymer’s properties can either positively or negatively impact the biodegradation properties of the biopolymer. Consequently, in the future, better comprehension and prediction of the impact of AC on the shelf-life of products and biodegradation of the material should be considered as a whole, through the use of modeling tools, to better design sustainable packaging.
AR and FC wrote the first draft of the manuscript. All authors contributed to the conceptualization, design of the present review, manuscript revision, read, and approved the submitted version.
AR acknowledges one-year Ph.D. salary funding from the Azerbaijan National Academy of Sciences (ANAS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Europe, Plastics. Plastics - the Facts 2021. (2021) Available at: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/
3. Gontard, N, David, G, Guilbert, A, and Sohn, J. Recognizing the long-term impacts of plastic particles for preventing distortion in decision-making. Nat Sustain. (2022) 5:472–8. doi: 10.1038/s41893-022-00863-2
4. Andrade, M. S., Ishikawa, O. H., Costa, R. S., Seixas, M. V., Rodrigues, R. C., and Moura, E. A. Development of sustainable food packaging material based on biodegradable polymer reinforced with cellulose nanocrystals. Food Packag Shelf Life. (2022) 31:100807. doi: 10.1016/j.fpsl.2021.100807
5. Guillard, V, Gaucel, S., Fornaciari, C., Angellier-Coussy, H, Buche, P, and Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front Nutr. (2018) 5:121. doi: 10.3389/fnut.2018.00121
6. Albuquerque, M. G., Adiutori, R, Trindade, N. M., Carmo, I. T. D., Oliveira, C. S. S., Pardelha, F, et al. Biobased feedstock valorisation through polyhydroxyalkanoate production: from excess cheese whey to eco-efficient bioplastics. Environ Eng Manag. J. (2012) 11. Available at: http://omicron.ch.tuiasi.ro/EEMJ/
7. Gontard, N., Sonesson, U., Birkved, M., Majone, M., Bolzonella, D., Celli, A., and Sebok, A. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit Rev Environ Sci Technol Adv. (2018) doi: 10.1080/10643389.2018.1471957
8. Hermabessiere, L., Dehaut, A., Paul-Pont, I, Lacroix, C., Jezequel, R, Soudant, P, et al. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere. (2017) 182:781–93. doi: 10.1016/j.chemosphere.2017.05.096
9. Pires, A., Cuccaro, A, Sole, M, and Freitas, R. Micro(nano)plastics and plastic additives effects in marine annelids: A literature review. Environ. Res. (2022) 214:113642. doi: 10.1016/j.envres.2022.113642
11. Catarino, M. D., Alves-Silva, J. M., Fernandes, R. P., Gonçalves, M. J., Salgueiro, L. R., Henriques, M. F., et al. Development and performance of whey protein active coatings with Origanum virens essential oils in the quality and shelf life improvement of processed meat products. Food Control. (2017) 80:273–80. doi: 10.1016/j.foodcont.2017.03.054
12. Correa, J. P., Molina, V, Sanchez, M., Kainz, C., Eisenberg, P, and Massani, MB. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag Shelf Life. (2017) 11:31–9. doi: 10.1016/j.fpsl.2016.11.004
13. Menezes, N. M. C., Martins, W. F., Longhi, D. A., and Aragão, G. M. F.de. Modeling the effect of oregano essential oil on shelf-life extension of vacuum-packed cooked sliced ham. Meat Science. (2018) 139, 113–119. doi: 10.1016/j.meatsci.2018.01.017
14. Siracusa, V, Rocculi, P., Romani, S., and Rosa, MD. Biodegradable polymers for food packaging: a review. Trends Food Sci Technol. (2008) 19:634–43. doi: 10.1016/j.tifs.2008.07.003
15. Amin, U., Khan, M. K. I., Maan, A. A., Nazir, A., Riaz, S., Khan, M. U., and Lorenzo, J. M. (2022). Biodegradable active, intelligent, and smart packaging materials for food applications. Food Packag Shelf Life. 33:100903. doi: 10.1016/j.fpsl.2022.100903
16. Peelman, N., Ragaert, P., Verguldt, E., Devlieghere, F., and Meulenaer, B.de (2016). Applicability of biobased packaging materials for long shelf-life food products. Packag Res. 1. doi: 10.1515/pacres-2016-0002
17. Aubin, S., Beaugrand, J, Berteloot, M., Boutrou, R, Buche, P, Gontard, N., et al. Plastics in a circular economy: Mitigating the ambiguity of widely-used terms from stakeholders consultation. Environ Sci Pol. (2022) 134:119–26. doi: 10.1016/j.envsci.2022.04.011
18. Kawashima, N., Yagi, T, and Kojima, K. How Do Bioplastics and Fossil-Based Plastics Play in a Circular Economy? Macromol Mater Eng. (2019) 304:1900383. doi: 10.1002/mame.201900383
19. Lambert, S., and Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chemical Society Reviews. (2017) 46:6855–71. doi: 10.1039/c7cs00149e
20. EN 17228. European Standard EN 17228:2019 Plastics - Bio-based polymers, plastics, and plastics products - Terminology, characteristics and communication. (2019). Available at: https://www.en-standard.eu/search/?q=EN+17228%3A2019
21. Chen, G-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. (2009) 38:2434–46. doi: 10.1039/b812677c
22. Zou, X., Cheng, C, Feng, J, Song, X., Lin, M, and Yang, S-T. Biosynthesis of polymalic acid in fermentation: Advances and prospects for industrial application. Crit Rev Biotechnol. (2019) 39:408–21. doi: 10.1080/07388551.2019.1571008
23. Candido, R. G., Godoy, G. G., and Gonçalves, A. R. Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydr Polym. (2017) 167:280–9. doi: 10.1016/j.carbpol.2017.03.057
24. Cywar, R. M., Rorrer, N. A., Hoyt, C. B., Beckham, G. T., and Chen, EY-X. Bio-based polymers with performance-advantaged properties. Nat Rev Materials. (2022) 7:83–103. doi: 10.1038/s41578-021-00363-3
25. Kopittke, P. M., Menzies, N. W., Wang, P, McKenna, B. A., and Lombi, E. Soil and the intensification of agriculture for global food security. Environ Int. (2019) 132:105078. doi: 10.1016/j.envint.2019.105078
26. Pellis, A., Malinconico, M, Guarneri, A., and Gardossi, L. Renewable polymers and plastics: Performance beyond the green. Biotechnol. (2021) 60:146–58. doi: 10.1016/j.nbt.2020.10.003
27. Walker, S., and Rothman, R. Life cycle assessment of bio-based and fossil-based plastic: A review. J Clean Prod. (2020) 261:121158. doi: 10.1016/j.jclepro.2020.121158
28. Donner, M., Gohier, R., and Vries, H.de (2020). A new circular business model typology for creating value from agro-waste. Sci Total Environ. 716,:137065. doi: 10.1016/j.scitotenv.2020.137065
29. Donner, M., Verniquet, A., Broeze, J., Kayser, K., and Vries, H.de. Critical success and risk factors for circular business models valorising agricultural waste and by-products. Resour Conserv Recycl. (2021) 165:105236. doi: 10.1016/j.resconrec.2020.105236
30. Vert, M., Doi, Y., Hellwich, K.-H., Hess, M., Hodge, P., Kubisa, P., et al. (2012). Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl Chem. 84, 377–410. doi: 10.1351/PAC-REC-10-12-04
31. Angellier-Coussy, H., Guillard, V., Buche, P, and Gontard, N. Barquettes agro-sourcées à base de sous-produits des industries agro-alimentaires: Projet FP7 EcoBioCAP. Innovat Agronom. (2017) 58:61–7.
32. Duque, A. F., Oliveira, C. S. S., Carmo, I. T. D., Gouveia, A. R., Pardelha, F, Ramos, A. M., et al. Response of a three-stage process for PHA production by mixed microbial cultures to feedstock shift: Impact on polymer composition. New Biotechnol. (2014) 31:276–88. doi: 10.1016/j.nbt.2013.10.010
33. Kourmentza, C, Plácido, J., Venetsaneas, N., Burniol-Figols, A., Varrone, C., Gavala, H. N., et al. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering. (2017)4: doi: 10.3390/bioengineering4020055
34. Koller, M. Established and advanced approaches for recovery of microbial polyhydroxyalkanoate (PHA) biopolyesters from surrounding microbial biomass. The EuroBiotech J. (2020a) 4:113–26. doi: 10.2478/ebtj-2020-0013
35. Koller, M., Niebelschütz, H., and Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng Life Sci. (2013) 13:549–62. doi: 10.1002/elsc.201300021
36. Pagliano, G., Galletti, P, Samorì, C, Zaghini, A, and Torri, C. Recovery of Polyhydroxyalkanoates From Single and Mixed Microbial Cultures: A Review. Front Bioeng Biotechnol. (2021) 9:624021. doi: 10.3389/fbioe.2021.624021
37. Koller, M. (Ed.) (2020b). The Handbook of Polyhydroxyalkanoates : Volume 3. Postsynthetic Treatment, Processing and Application. Taylor&Francis.
38. Pérez-Rivero, C., López-Gómez, J. P., and Roy, I. A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: State-of-the-art and latest developments. Biochem Eng J. (2019) 150:107283. doi: 10.1016/j.bej.2019.107283
39. Bossu, J, Angellier-Coussy, H., Totee, C., Matos, M., Reis, M., and Guillard, V. Effect of the Molecular Structure of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-3HV)) Produced from Mixed Bacterial Cultures on Its Crystallization and Mechanical Properties. Biomacromolecules. (2020) 21:4709–23. doi: 10.1021/acs.biomac.0c00826
40. Haque, M. A., Priya, A., Hathi, Z. J., Qin, Z-H, Mettu, S, and Lin, C. S. K. Advancements and current challenges in the sustainable downstream processing of bacterial polyhydroxyalkanoates. Curr Opin Green Sustain Chem. (2022) 36:100631. doi: 10.1016/j.cogsc.2022.100631
41. Arcos-Hernandez, M. V., Laycock, B., Pratt, S., Donose, B. C., Nikolić, M. A., Luckman, P., et al. (2012). Biodegradation in a soil environment of activated sludge derived polyhydroxyalkanoate (PHBV). Polym Degrad Stab. 97, 2301–2312. doi: 10.1016/j.polymdegradstab.2012.07.035
42. David, G, Michel, J, Gastaldi, E., Gontard, N., and Angellier-Coussy, H. How Vine Shoots as Fillers Impact the Biodegradation of PHBV-Based Composites. Int J Mol Sci. (2019) 21. doi: 10.3390/ijms21010228
43. Sashiwa, H, Fukuda, R., Okura, T, Sato, S, and Nakayama, A. Microbial Degradation Behavior in Seawater of Polyester Blends Containing Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx). Mar Drugs. (2018) 16. doi: 10.3390/md16010034
44. Emadian, S. M., Onay, T. T., and Demirel, B. Biodegradation of bioplastics in natural environments In: Waste Management, vol. 59. (New York, N.Y.): (2017). 526–36. doi: 10.1016/j.wasman.2016.10.006
45. Kumaravel, S, Hema, R., and Lakshmi, R. Production of Polyhydroxybutyrate (Bioplastic) and its Biodegradation by Pseudomonas Lemoignei and Aspergillus Niger. E-J Chem. (2010) 7:S536–42. doi: 10.1155/2010/148547
46. Lee, K-M, Gimore, D. F., and Huss, M. J. Fungal Degradation of the Bioplastic PHB (Poly-3-hydroxy-butyric acid). J Polym Environ. (2006) 14:213. doi: 10.1007/s10924-006-0013-8
47. Meereboer, K. W., Misra, M, and Mohanty, A. K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. (2020) 22:5519–58. doi: 10.1039/D0GC01647K
48. Roohi, Z. M. R., and Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym Adv Technol. (2018) 29:30–40. doi: 10.1002/pat.4126
49. Weng, Y-X, Wang, X-L, and Wang, Y-Z. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polym Testing. (2011) 30:372–80. doi: 10.1016/j.polymertesting.2011.02.001
50. EC 450/2009. Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Official Journal of European Union. (2009) Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R0450
51. Malhotra, B., Keshwani, A., and Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front Microbiol. (2015) 6:611. doi: 10.3389/fmicb.2015.00611
52. Yildirim, S., Rocker, B., and Pettersen, M. K., Nilsen-Nygaard, J., Ayhan, Z., Rutkaite, R., and Coma, V. Active Packaging Applications for Food. Comprehen Rev Food Sci Food Safety Adv. (2017) doi: 10.1111/1541-4337.12322
53. Rüegg, N., Röcker, B., and Yildirim, S. Application of palladium-based oxygen scavenger to extend the mould free shelf life of bakery products. Food Packag Shelf Life. (2022) 31:100771. doi: 10.1016/j.fpsl.2021.100771
54. Aday, M. S., Caner, C., and Rahvalı, F. Effect of oxygen and carbon dioxide absorbers on strawberry quality. Postharvest Biol Technol. (2011) 62:179–87. doi: 10.1016/j.postharvbio.2011.05.002
55. Pantaleao, I, Pintado, M, and Pocas, M. Evaluation of two packaging systems for regional cheese. Food Chem. (2007) 102:481–7. doi: 10.1016/j.foodchem.2006.05.058
56. Wei, H., Li, L., Zhang, T., Seidi, F, and Xiao, H. Platinum-loaded dendritic mesoporous silica as novel ethylene scavenger to extend shelf life of banana (Musa nana). Food Chem. (2023) 424:136415. doi: 10.1016/j.foodchem.2023.136415
57. Hansen, A. Å., Moen, B., Rødbotten, M., Berget, I., and Pettersen, M. K. Effect of vacuum or modified atmosphere packaging (MAP) in combination with a CO2 emitter on quality parameters of cod loins (Gadus morhua). Food Packag Shelf Life.(2016) 9, 29–37. doi: 10.1016/j.fpsl.2016.05.005
58. Hempel, A. W., O’Sullivan, M. G., Papkovsky, D. B., and Kerry, J. P. Use of smart packaging technologies for monitoring and extending the shelf-life quality of modified atmosphere packaged (MAP) bread: application of intelligent oxygen sensors and active ethanol emitters. Eur Food Res Technol. (2013) 237:117–24. doi: 10.1007/s00217-013-1968-z
59. Sirocchi, V, Devlieghere, F., Peelman, N, Sagratini, G., Maggi, F, Vittori, S, et al. Effect of Rosmarinus officinalis L. Essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. (2017) 221:1069–76. doi: 10.1016/j.foodchem.2016.11.054
60. Graciano-Verdugo, A. Z., Soto-Valdez, H., Peralta, E., Cruz-Zárate, P., Islas-Rubio, A. R., Sánchez-Valdes, S., et al. Migration of α-tocopherol from LDPE films to corn oil and its effect on the oxidative stability. Food Res Int. (2010) 43, 1073–1078. doi: 10.1016/j.foodres.2010.01.019
61. Van Boekel, M. A. Kinetic Modeling of Food Quality: A Critical Review. Comp Rev Food Sci Food Safety. (2008) 7:144–58. doi: 10.1111/j.1541-4337.2007.00036.x
62. Di Giuseppe, F, Coffigniez, F, Aouf, C., Guillard, V., and Torrieri, E. Activated gallic acid as radical and oxygen scavenger in biodegradable packaging film. Food Packag Shelf. (2022) 31:100811. doi: 10.1016/j.fpsl.2022.100811
63. de, F. S. M., Lopes, P. S., Da Silva, C. F., and Yoshida, C. M. P. Active packaging material based on buriti oil - Mauritia flexuosa L.f. (Arecaceae) incorporated into chitosan films. J Appl Polym Sci. (2016) 133:n/a-n/a. doi: 10.1002/app.43210
64. Mustapha, F. A., Jai, J, Nik Raikhan, N. H., Sharif, Z., and Yusof, N. M. Response surface methodology analysis towards biodegradability and antimicrobial activity of biopolymer film containing turmeric oil against Aspergillus niger. Food Control. (2019) 99:106–13. doi: 10.1016/j.foodcont.2018.12.042
65. Ramos, M., and Fortunati, E., Beltrán, A., Peltzer, M., Cristofaro, F., Visai, L., et al. Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers. (2020) 12. doi: 10.3390/polym12091878
66. Suwanamornlert, P, Kerddonfag, N., Sane, A, Chinsirikul, W, Zhou, W, and Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Packag Shelf. (2020) 25:100515. doi: 10.1016/j.fpsl.2020.100515
67. Alparslan, Y., Yapıcı, H. H., Metin, C., Baygar, T, Günlü, A., and Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT. (2016) 72:457–66. doi: 10.1016/j.lwt.2016.04.066
68. Falleh, H., Ben Jemaa, M., Saada, M., and Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. (2020) 330:127268. doi: 10.1016/j.foodchem.2020.127268
69. Guo, Q., Du, G., Jia, H., Fan, Q., Wang, Z., Gao, Z., et al. (2021). Essential oils encapsulated by biopolymers as antimicrobials in fruits and vegetables: A review. Food Biosci. 44:101367. doi: 10.1016/j.fbio.2021.101367
70. Seow, Y. X., Yeo, C. R., Chung, H. L., and Yuk, H-G. Plant Essential Oils as Active Antimicrobial Agents. Crit Rev Food Sci Nutr. (2014) 54. doi: 10.1080/10408398.2011.599504
71. Corrales, M, Fernández, A., and Han, J. H. Chapter 7 - Antimicrobial Packaging Systems In: JH Han, editor. Food Science and Technology. Innovations in Food Packaging. (Second Edition) ed. San Diego: Academic Press (2014). 133–70. doi: 10.1016/B978-0-12-394601-0.00007-2
72. Gavara, R., Lopez-Carballo, G., Hernandez-Munoz, P., Muriel-Galet, V, Ramon Catala, P., Cerisuelo, J, et al. Practical Guide to Antimicrobial Active Packaging. Retrieved from: Smithers Rapra Publishing (2015) Available at: https://books.google.fr/books?id=wKl5jgEACAAJ
73. 1935/2004 EC. REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Official Journal of European Union. (2004) Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R1935
75. Nerin, C., and Silva, F., Manso, S., and Becerril, R. (2016). The Downside of Antimicrobial Packaging. In Antimicrobial Food Packaging (pp. 81–93). Elsevier. doi: 10.1016/B978-0-12-800723-5.00006-1
76. Almasi, H., Jahanbakhsh Oskouie, M, and Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit Rev Food Sci Nutr. (2021) 61:2601–21. doi: 10.1080/10408398.2020.1783199
77. Fu, Y, and Dudley, E. G. Antimicrobial-coated films as food packaging: A review. Compr Rev Food Sci Food Saf. (2021) 20:3404–37. doi: 10.1111/1541-4337.12769
78. Vasile, C, and Baican, M. Progresses in Food Packaging, Food Quality, and Safety-Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules. (Basel, Switzerland), (2021) 26. doi: 10.3390/molecules26051263
79. Wicochea-Rodríguez, J. D., Chalier, P, Ruiz, T, and Gastaldi, E. Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release. Front Chem. (2019) 7:398. doi: 10.3389/fchem.2019.00398
80. Vilela, C., Kurek, M., Hayouka, Z., Röcker, B., Yildirim, S., Antunes, M. D. C., et al. (2018). A concise guide to active agents for active food packaging. Trends Food Sci Technol. 80, 212–222. doi: 10.1016/j.tifs.2018.08.006
81. Beltrán Sanahuja, A., and Valdés García, A. New Trends in the Use of Volatile Compounds in Food Packaging. Polymers. (2021) 13. doi: 10.3390/polym13071053
82. Qie, R., Zajforoushan Moghaddam, S, and Thormann, E. Dopamine-Assisted Layer-by-Layer Deposition Providing Coatings with Controlled Thickness, Roughness, and Functional Properties. ACS Omega. (2023) 8:2965–72. doi: 10.1021/acsomega.2c05620
83. Zhang, C., Li, Y., Wang, P., and Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr Rev Food Sci Food Saf. (2020) 19:479–502. doi: 10.1111/1541-4337.12536
84. Modan, E. M., and Plaiasu, A. G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. The Annals of “Dunarea De Jos” University of Galati. Fascicle IX, Metallurgy and Materials. Science. (2020) 43:53–60. doi: 10.35219/mms.2020.1.08
85. Hussein, K. H., Park, K.-M., Lee, Y.-S., Woo, J.-S., Kang, B.-J., Choi, K.-Y., et al. (2017). New insights into the pros and cons of cross-linking decellularized bioartificial organs. Inter J Art Organs. 40, 136–141. doi: 10.5301/ijao.5000541
86. Westlake, J. R., Tran, M. W., Jiang, Y., Zhang, X., Burrows, A. D., and Xie, M. Biodegradable Active Packaging with Controlled Release: Principles, Progress, and Prospects. ACS Food Sci Technol. (2022) 2:1166–83. doi: 10.1021/acsfoodscitech.2c00070
87. Becerril, R., Nerín, C., and Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules. (2020) 25. doi: 10.3390/molecules25051134
88. Stoleru, E., and Brebu, M. Stabilization Techniques of Essential Oils by Incorporation into Biodegradable Polymeric Materials for Food Packaging. Molecules.(2021) 26. doi: 10.3390/molecules26206307
89. Pedrós-Garrido, S., Clemente, I., Calanche, J. B., Condón-Abanto, S., Beltrán, J. A., Lyng, J. G., and Whyte, P. Antimicrobial activity of natural compounds against listeria spp. and their effects on sensory attributes in salmon (Salmo salar) and cod (Gadus morhua). Food Control. (2020) 107, 106768. doi: 10.1016/j.foodcont.2019.106768
90. Reis, D. R., Ambrosi, A., and Di Luccio, M. Encapsulated essential oils: A perspective in food preservation. Future Foods. (2022) 5:100126. doi: 10.1016/j.fufo.2022.100126
91. Zanetti, M., Carniel, T. K., Dalcanton, F., dos Anjos, R. S., Gracher Riella, H., Araújo, P. H.de, et al. (2018). Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci Technol, 81, 51–60. doi: 10.1016/j.tifs.2018.09.003
92. Kurek, M., Laridon, Y., Torrieri, E., Guillard, V., Pant, A., Stramm, C., et al. (2017). A mathematical model for tailoring antimicrobial packaging material containing encapsulated volatile compounds. Innov Food Sci Emerg Technol. 42, 64–72. doi: 10.1016/j.ifset.2017.05.014
93. Nedovic, V., Kalusevic, A., Manojlovic, V., Levic, S., and Bugarski, B. An overview of encapsulation technologies for food applications. Proc Food Sci. (2011) 1:1806–15. doi: 10.1016/j.profoo.2011.09.265
94. Rezaei, A., Fathi, M., and Jafari, S. M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Hydrocoll. (2019) 88:146–62. doi: 10.1016/j.foodhyd.2018.10.003
95. Bumbudsanpharoke, N., and Ko, S. Nano-food packaging: An overview of market, migration research, and safety regulations. J Food Sci. (2015) 80:R910–23. doi: 10.1111/1750-3841.12861
96. Saad, H., Ayed, A., Srasra, M., Attia, S., Srasra, E., Bouhtoury, FC-E., et al. New Trends in Clay-Based Nanohybrid Applications: Essential Oil Encapsulation Strategies to Improve Their Biological Activity: 2 In: W Oueslati, editor. Nanoclay. Rijeka: IntechOpen (2022) doi: 10.5772/intechopen.106855
97. Ribeiro-Santos, R., Andrade, M., Melo, N. R.de, and Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci Techno. (2017) 61, 132–140. doi: 10.1016/j.tifs.2016.11.021
98. Topuz, F, and Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res Int. (2020) 130:108927. doi: 10.1016/j.foodres.2019.108927
99. Zhang, W., Jiang, H., Rhim, J-W., Cao, J., and Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. (2022) 367:130671. doi: 10.1016/j.foodchem.2021.130671
100. Abdul Rahman, N. Applications of Polymeric Nanoparticles in Food Sector In: S Siddiquee, GJH Melvin, and MM Rahman, eds. Nanotechnology: Applications in Energy, Drug and Food. Cham: Springer International Publishing (2019). 345–59.
101. Rehman, A., Jafari, S. M., Aadil, R M., Assadpour, E., Randhawa, M. A., and Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci Technol. (2020) 101:106–21. doi: 10.1016/j.tifs.2020.05.001
102. Balaguer, M. P., Villanova, J., Cesar, G., Gavara, R., and Hernandez-Munoz, P. Compostable properties of antimicrobial bioplastics based on cinnamaldehyde cross-linked gliadins. Chem Eng J. (2015) 262:447–55. doi: 10.1016/j.cej.2014.09.099
103. Cano, A. I., Cháfer, M., Chiralt, A., and González-Martínez, C. Biodegradation behavior of starch-PVA films as affected by the incorporation of different antimicrobials. Polym Degrad Stab. (2016) 132:11–20. doi: 10.1016/j.polymdegradstab.2016.04.014
104. Piñeros-Hernandez, D., Medina-Jaramillo, C., López-Córdoba, A., and Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocollo. (2017) 63:488–95. doi: 10.1016/j.foodhyd.2016.09.034
105. Ketkaew, S., Kasemsiri, P., Hiziroglu, S., Mongkolthanaruk, W., Wannasutta, R., Pongsa, U., et al. Effect of Oregano Essential Oil Content on Properties of Green Biocomposites Based on Cassava Starch and Sugarcane Bagasse for Bioactive Packaging. J Polym Environ. (2018) 26:311–8. doi: 10.1007/s10924-017-0957-x
106. Tampau, A., González-Martínez, C., and Chiralt, A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocollo. (2018) 79:158–69. doi: 10.1016/j.foodhyd.2017.12.021
107. Tampau, A., González-Martínez, C., and Chiralt, A. Biodegradability and disintegration of multilayer starch films with electrospun PCL fibres encapsulating carvacrol. Polym Degrad Stab. (2020) 173:109100. doi: 10.1016/j.polymdegradstab.2020.109100
108. Arrieta, M. P., Díez García, A., López, D., Fiori, S., and Peponi, L. (2019). Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials. 9. doi: 10.3390/nano9030346
109. Vostrejs, P., Adamcová, D., Vaverková, M. D., Enev, V., Kalina, M., Machovsky, M., et al. (2020). Active biodegradable packaging films modified with grape seeds lignin. RSC Adv. 10, 29202–29213. doi: 10.1039/d0ra04074f
110. Bonilla, J., Poloni, T., and Sobral, P. J. A. Active edible coatings with Boldo extract added and their application on nut products: reducing the oxidative rancidity rate. Int J Food Sci Technol. (2017) 53. doi: 10.1111/ijfs.13645
111. Bonilla, J., and Sobral, P. J. A. Gelatin-chitosan edible film activated with Boldo extract for improving microbiological and antioxidant stability of sliced Prato cheese. Int J Food Sci Technol. (2018) 53. doi: 10.1111/ijfs.14032
112. Bonilla, J., and Sobral, P. J. A. Disintegrability under composting conditions of films based on gelatin, chitosan and/or sodium caseinate containing boldo-of-Chile leafs extract. Int J Biol Macromol. (2020) 151:178–85. doi: 10.1016/j.ijbiomac.2020.02.051
113. Rech, C. R., Da Silva Brabes, K. C., Bagnara e Silva, B. E., Bittencourt, P. R. S., Koschevic, M. T., Da Silveira, T. F. S., et al. (2020). Biodegradation of eugenol-loaded polyhydroxybutyrate films in different soil types. Case Stud Chem Environ Eng, 2, 100014. doi: 10.1016/j.cscee.2020.100014
114. Norcino, L. B., Mendes, J. F., Natarelli, C., Manrich, A., Oliveira, J. E., and Mattoso, L. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. (2020) 106:105862. doi: 10.1016/j.foodhyd.2020.105862
115. Oberlintner, A., Bajić, M., Kalčíková, G., Likozar, B., and Novak, U. Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ Technol Innov. (2021) 21:101318. doi: 10.1016/j.eti.2020.101318
116. Ibrahim, S., Elsayed, H., and Hasanin, M. Biodegradable, Antimicrobial and Antioxidant Biofilm for Active Packaging Based on Extracted Gelatin and Lignocelluloses Biowastes. J Polym Environ. (2021) 29:472–82. doi: 10.1007/s10924-020-01893-7
117. He, X., Li, M., Gong, X., Niu, B., and Li, W. Biodegradable and antimicrobial CSC films containing cinnamon essential oil for preservation applications. Food Packag Shelf Life. (2021) 29:100697. doi: 10.1016/j.fpsl.2021.100697
118. Sadeghi, A., Razavi, S. M. A., and Shahrampour, D. Fabrication and characterization of biodegradable active films with modified morphology based on polycaprolactone-polylactic acid-green tea extract. Int J Biol Macromol. (2022) 205:341–56. doi: 10.1016/j.ijbiomac.2022.02.070
119. Arrieta, M. P., López, J., Hernández, A., and Rayón, E. (2014). Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur Polym J. 50, 255–270. doi: 10.1016/j.eurpolymj.2013.11.009
120. Ramos, M., and Fortunati, E., Peltzer, M., and Dominici, F., Jiménez, A., Del Garrigós, M. C., and Kenny, J. M. (2014). Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym Degrad Stab. 108, 158–165. doi: 10.1016/j.polymdegradstab.2014.02.011
121. Yang, W., and Fortunati, E., Dominici, F., Giovanale, G., Mazzaglia, A., Balestra, G. M., et al. (2016). Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int J Biol Macromol. 89, 360–368. doi: 10.1016/j.ijbiomac.2016.04.068
122. Medina Jaramillo, C., Gutiérrez, T. J., Goyanes, S., Bernal, C., and Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr Polym. (2016) 151:150–9. doi: 10.1016/j.carbpol.2016.05.025
123. Kim, S., Baek, S-K, Go, E., and Song, K. B. Application of Adzuki Bean Starch in Antioxidant Films Containing Cocoa Nibs Extract. Polymers. (2018) 10. doi: 10.3390/polym10111210
124. Lyu, J. S., Lee, J-S, and Han, J. Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process. Sci Rep. (2019) 9:20236. doi: 10.1038/s41598-019-56757-5
125. Villegas, C., and Arrieta, M. P., Rojas, A., Torres, A., Faba, S., Toledo, M. J., et al. (2019). PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos Part B. 176,:107336. doi: 10.1016/j.compositesb.2019.107336
126. Papadopoulou, E. L., Paul, U. C., Tran, T. N., Suarato, G., Ceseracciu, L., Marras, S., et al. (2019). Sustainable Active Food Packaging from Poly(lactic acid) and Cocoa Bean Shells. ACS Appl Mater Interfaces. 11, 31317–31327. doi: 10.1021/acsami.9b09755
127. Ulloa, P. A., Vidal, J., Dicastillo, C., Rodriguez, F., Guarda, A., Cruz, R. M. S., et al. [M. J.] (2019). Development of poly(lactic acid) films with propolis as a source of active compounds: Biodegradability, physical, and functional properties. J Appl Polym Sci, 136,:47090. doi: 10.1002/app.47090
128. Zabidi, N., Nazri, F., Tawakkal, I. S. M. A., Basri, M. S. M., Basha, R. K., and Othman, S. H. (2022). Characterization of active and pH-sensitive poly(lactic acid) (PLA)/nanofibrillated cellulose (NFC) films containing essential oils and anthocyanin for food packaging application. Int J Biol Macromol. 212, 220–231. doi: 10.1016/j.ijbiomac.2022.05.116
129. Mendes, J. F., Norcino, L. B., Martins, H., Manrich, A., Otoni, C. G., Carvalho, E., et al. (2020). Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocollo. 100,:105428. doi: 10.1016/j.foodhyd.2019.105428
130. Zahan, K. A., Azizul, N. M., Mustapha, M., Tong, W. Y., Abdul Rahman, M. S., and Sahuri, I. S. Application of bacterial cellulose film as a biodegradable and antimicrobial packaging material. Materials Today: Proceedings. (2020) 31:83–8. doi: 10.1016/j.matpr.2020.01.201
131. Patiño Vidal, C., Luzi, F., and Puglia, D., López-Carballo, G., and Rojas, A., Galotto, M. J., et al. (2023). Development of a sustainable and antibacterial food packaging material based in a biopolymeric multilayer system composed by polylactic acid, chitosan, cellulose nanocrystals and ethyl lauroyl arginate. Food Packag Shelf Life. 36,:101050. doi: 10.1016/j.fpsl.2023.101050
132. Atarés, L., and Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci Technol. (2016) 48:51–62. doi: 10.1016/j.tifs.2015.12.001
133. Lagaron, J. M., and Lopez-Rubio, A. Nanotechnology for bioplastics: opportunities, challenges and strategies. Trends Food Sci Technol. (2011) 22:611–7. doi: 10.1016/j.tifs.2011.01.007
134. Jenner, K. J., Kreutzer, G., and Racine, P. Persistency assessment and aerobic biodegradation of selected cyclic sesquiterpenes present in essential oils. Environ Toxicol Chem. (2011) 30:1096–108. doi: 10.1002/etc.492
135. Harder, J., Heyen, U., Probian, C., and Foß, S. Anaerobic utilization of essential oils bydenitrifying bacteria. Biodegradation. (2000) 11:55–63.
136. Suttinun, O., Müller, R., and Luepromchai, E. Cometabolic degradation of trichloroethene by Rhodococcus sp. Strain L4 immobilized on plant materials rich in essential oils. Appl Environ Microbiol. (2010) 76:4684–90. doi: 10.1128/AEM.03036-09
Keywords: sustainability, biodegradable, active food packaging, natural active components, usage benefit, post-usage fate
Citation: Rzayeva A, Coffigniez F, Zeynalov N, Gontard N and Guillard V (2023) Integrating the latest biological advances in the key steps of a food packaging life cycle. Front. Nutr. 10:1223638. doi: 10.3389/fnut.2023.1223638
Received: 16 May 2023; Accepted: 05 July 2023;
Published: 27 July 2023.
Edited by:
Sneh Punia Bangar, Clemson University, United StatesReviewed by:
Tian Ren, Shaanxi Normal University, ChinaCopyright © 2023 Rzayeva, Coffigniez, Zeynalov, Gontard and Guillard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanny Coffigniez, ZmFubnkuY29mZmlnbmllekB1bW9udHBlbGxpZXIuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.