
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 19 September 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1219976
Background: Hemodialysis (HD) patients often experience a significant reduction in quality of life (QOL). The source of dietary protein intake may influence the renal function and complications of HD patients. The present study assessed the relationship between plant and animal protein intake and QOL in HD patients.
Methods: 264 adult patients under dialysis for at least three months were included in this cross-sectional study. Dietary intakes were collected using a valid and reliable 168-item semi-quantitative food frequency questionnaire (FFQ) over the past year. Total, animal, and plant proteins were calculated for each patient. To evaluate QOL, Kidney Disease Quality of Life Short Form (KDQOL-SF 1/3) was used. Anthropometric measures were assessed according to standard protocols.
Results: In this study, the average age of participants was 58.62 ± 15.26 years old; most (73.5%) were men. The mean of total, plant, and animal proteins intake were 66.40 ± 34.29 g/d, 34.60 ± 18.24 g/d, and 31.80 ± 22.21 g/d. Furthermore, the mean score of QOL was 59.29 ± 18.68. After adjustment for potential confounders, a significant positive association was found between total dietary protein intake and QOL (β = 0.12; p = 0.03). Moreover, there was a significant association between plant-based protein intake and QOL (β = 0.26; p < 0.001). However, the association between animal protein intake and QOL was insignificant (β = 0.03; p = 0.60).
Conclusion: Higher total and plant proteins intake were associated with better QOL in HD patients. Further studies, particularly prospective ones, are needed to corroborate these associations.
End-stage renal disease (ESRD) is the final and irreversible stage of chronic kidney disease (CKD) (1). The prevalence of ESRD is estimated to range between 8 and 16% worldwide (2) and is increasing annually. Hemodialysis (HD) is the primary and most prevalent treatment for ESRD patients (1). According to the International Society of Nephrology, approximately 2.5 million people are undergoing dialysis treatment (2). Unfortunately, despite advancements in dialysis treatment and medications, the mortality rate for these patients is still high (3). HD patients may experience complications and symptoms such as constipation, nausea, fatigue, pruritus, sleep disturbances, and depression that can reduce their ability to perform daily activities independently and ultimately reduce their quality of life (QOL) (4). QOL refers to a person’s satisfaction with their life concerning their expectations, goals, relationships, and independence, consisting of mental and physical well-being (5). In addition, QOL is an essential clinical measure that demonstrates the efficacy of health care and can be used to predict patient mortality (6). Studies revealed that HD patients have a lower QOL than the general population (7).
Diet and nutritional status play critical roles in HD complications and QOL (8). Protein intake is one of the most important dietary factors for these patients (9). Inadequate protein intake can lead to malnutrition, especially in elderly patients (6). Furthermore, it can increase the risk of inflammation and worsen the HD patient’s complications, directly impacting the patients’ physical and mental well-being (7). However, on the other side, high protein consumption elevates levels of urea and creatinine, which increase uremic symptoms in HD patients (10). According to the Kidney Disease Outcomes Quality Initiative (KDOQI) guideline, the recommended dietary protein intake during HD is 1.0–1.2 g/kg-day to maintain a stable nutritional status (11).
Moreover, recent studies have investigated that protein sources (plant or animal) may potentially impact kidney function and complications in HD patients (12, 13). They are advised to consume limited plant-based protein to help control their serum phosphorus and potassium levels (14). Some guidelines recommend that at least half of their dietary protein should come from animal sources to ensure they get enough essential amino acids (15, 16). Animal-based protein have a higher biological value than plant-based protein (17). However, several studies have shown that plant-based diets and higher intakes of plant-based protein have positive effects on patients’ status (18–20). A cohort study revealed that higher intake of vegetables and fruits in HD patients was associated with lower mortality (21). Another longitudinal cohort study indicated adherence to a plant-based diet in these patients was not related to hyperkalemia and appeared to be associated with improved nutritional status. In this study, the mean of total, plant, and animal proteins intake were 59.13 g/d, 21/8 g/d, and 37.33 g/d (22). Furthermore, recent studies have found that a plant-based diet is not associated with malnutrition in HD patients, and higher intake of plant-based protein may provide adequate quantity and quality, especially when consumed from diverse sources (23, 24). Additionally, plant-based proteins decrease acidosis, whereas animal-based proteins increase the acid load and can cause hyperfiltration and proteinuria (25, 26). Moreover, plant-based proteins are rich in phytochemicals and antioxidants, while red meat and processed meat are high in saturated fatty acids (SFA) and sodium, which can increase inflammation and exacerbate patients’ complications (27).
Previous studies evaluated the association between dietary protein sources and CKD patients’ risk, progression, and mortality. Nevertheless, to our knowledge, no study has been conducted to examine the relationship between the origin of protein and QOL in HD patients. Therefore, we designed a cross-sectional study to investigate the association between plant and animal protein intake and QOL in patients undergoing dialysis.
We conducted a multi-center cross-sectional study between September 2021 and March 2022 on 264 HD patients in 5 hemodialysis centers in Isfahan, Iran. Patients were included in the study if they were ≥ 18 years old, alert, and receiving HD for at least three months. We excluded patients with incomplete questionnaires, mental disabilities, pregnant women, or if their daily energy intake was less than 800 kcal/d or above 4,200 kcal/d (28). Before being recruited for the study, each patient provided written informed consent. The Isfahan University of Medical Sciences ethical committee accepted this study’s protocol (IR.MUI.RESEARCH.REC.1399.605). This study is based on the M.S. thesis of MD.
Dietary intake was evaluated using a validated semi-quantitative food frequency questionnaire (FFQ) that contained 168 food items, designed specifically for use in Iran (29). Previous studies showed that this FFQ could accurately reflect the dietary patterns of the Iranian population (29–32). Expert dietitians questioned patients face-to-face to complete questionnaires and describe the frequency of each food item ingested daily, weekly, monthly, or yearly in the previous year. The reported frequency for each food item was converted to a daily intake. For example, a response of “two serving/week” was converted to 0.28 servings/day. Each food serving size was converted from household measurements to grams. Nutritionist IV software was used to calculate total energy, macronutrients, and micronutrients. Protein consumption was divided into two groups: plant protein and animal protein. Legumes, nuts, seeds, grains, vegetables, and fruits were referred to as plant proteins. The animal protein group consisted of red and processed meat, poultry, eggs, fish, and dairy products.
Kidney Disease Quality of Life Short Form (KDQOL-SF 1/3) was used to measure QOL in HD patients. The questionnaire was administered orally by trained interviewers during the first hour of dialysis treatment. This questionnaire has 36 items and two main sections: 12 generic items that evaluate the general mental and physical status and 24 specific CKD-disease items that assess symptoms, effects, and the burden of kidney disease. The average scores for the five subscales ranged from 0–100, and higher scores represented better QOL. The validity and reliability of this questionnaire have been previously confirmed in Iranian patients with HD (33).
The patient’s height was measured, without shoes, using non-elastic tape with an accuracy of 0.1 cm. After a dialysis session, dry weight was measured with the fewest clothes and without shoes with an accuracy of 0.1 kg using a calibrated digital floor scale when no signs or symptoms of hypovolemia or hypervolemia were detected (35). The body mass index (BMI) was determined by dividing dry weight by squared height.
Age, sex, marital and employment status, dialysis vintage, the frequency and duration of the dialysis, urea reduction ratio (URR), urea kinetics (Kt/V), and the major cause of renal failure were collected from medical records at baseline.
The Statistical Package for Social Sciences (SPSS, version 22, Chicago, IL, United States) was used for all analyses in this study. p value ≤0.05 was considered statistically significant. The Kolmogorov–Smirnov test was used to determine the normality of distribution for all variables. Continuous variables are represented by means ± standard deviations, while categorical variables are represented by percentages (%). To compare categorical variables, the Chi-square test was performed, and the one-way analysis of variance (ANOVA) was utilized to evaluate continuous variables among tertiles of total, plant, and animal protein intake. Analysis of covariance (ANCOVA) was conducted to compare dietary intake after controlling for energy intake. Linear regression analysis was used to examine the association between total and type of dietary protein and QOL, with adjustment for gender, job, height, weight, URR, and total energy consumption.
The mean ± SD age of 264 HD patients who contributed to the current study was 58.62 ± 15.26 years. Most patients were men (73.5%), married (76.20%), either retired (34.5%) or unemployed (28.4%). Most patients’ primary cause of ESRD was diabetic nephropathy (37.5%). The mean ± SD dialysis vintage was 47.11 ± 45.64 months. Moreover, the mean ± SD BMI was 24.53 ± 4.54 kg/m2. According to nutritional status, 7.7% of patients were undernourished, 49.8% had normal weight, 31.8% were overweight, and 10.7% were obese. Furthermore, the means of anthropometric characteristics, such as height and dry weight, were 164.93 ± 9.22 cm and 66.98 ± 14.64 kg, respectively. The mean of total, plant, and animal proteins intake were 66.40 ± 34.29 g/d, 34.60 ± 18.24 g/d, and 31.80 ± 22.21 g/d. Moreover, this study’s mean Q.O.L. score of H.D. patients was 59.29 ± 18.68.
The general characteristics of HD patients among tertiles of the total plant and animal protein intake are presented in Table 1. As shown in Table 1, the percentage of men was significantly higher in the last tertile of total, plant, and animal proteins compared with the lower tertiles (p < 0.001 for all tertiles of protein intake). Furthermore, there were mostly retired subjects in the top tertile of all types of protein intake whereas most unemployed individuals were in the lowest tertile (p < 0.001 for all tertiles of protein intake). Across different tertiles of total protein intake, patients in the top tertiles had significantly higher dry weight (p = 0.01) and height (p < 0.001) compared to the patients in the first tertiles. Additionally, patients in the top tertiles of total protein intake, had significantly lower dialysis sessions (p = 0.02) and URR (p < 0.001) than the lowest tertile. Within the plant-based protein, patients in higher tertiles had significantly higher height (p < 0.001) and URR (p = 0.03) compared to the patients in the lower tertiles and those in lower tertiles had significantly higher dialysis sessions (p = 0.02) than patients in the higher tertiles. Patients in higher tertiles of animal protein had significantly higher height (p < 0.001) compared to the patients in the bottom tertile. Moreover, the major cause of ESRD in most patients in the top tertile of animal protein intake was diabetes mellitus, whereas in the lowest tertile was acute kidney injury (p = 0.02). No significant differences were observed regarding other characteristics throughout the tertiles of all types of protein intake.
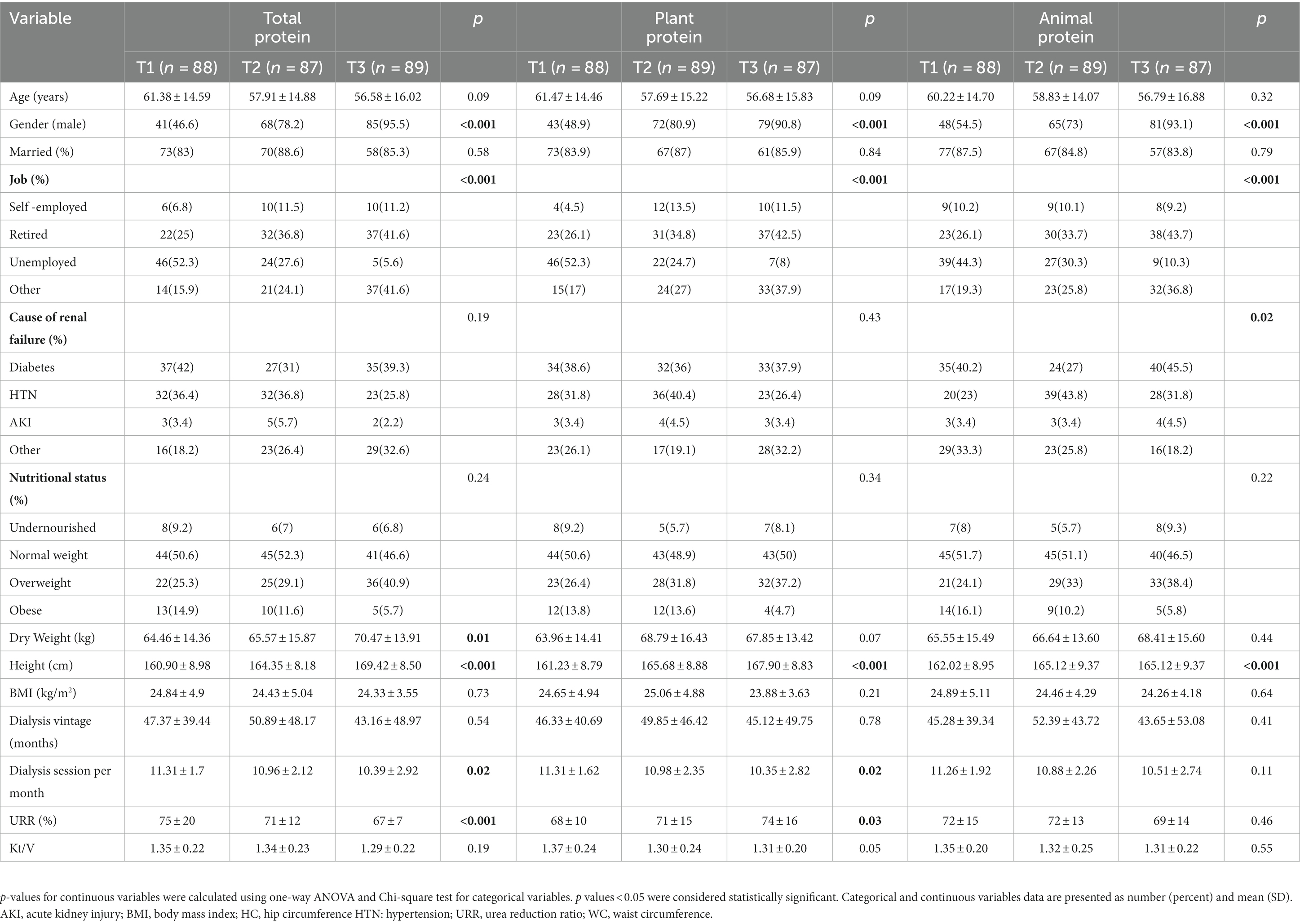
Table 1. The baseline characteristics of study population across tertiles of total, plant and animal protein intake (n = 264).
Dietary intake among tertiles of the total, plant, and animal proteins intake are shown in Table 2. All dietary protein sources significantly increased across the tertile of total protein intake (p < 0.05). Furthermore, total energy intake, carbohydrate, fat, SFA, cholesterol, sodium, potassium, phosphorus, calcium, vitamin B1, B2, B3, B6, B12, folic acid and vitamin C significantly increased across the tertile of total protein intake (p < 0.05). Moreover, patients in the top tertile of plant protein consumed higher grains, vegetables, fruits, legumes, nuts and seeds, energy, carbohydrate, sodium, potassium, phosphorus, calcium, vitamin B1, B2, B3, B6, folic acid and vitamin C compared with the lower tertiles (p < 0.001). In contrast, they significantly had lower intakes of red and processed meats, poultries, fishes, eggs, dairy products, fat, SFA, cholesterol and vitamin B12 (p < 0.05). Across different tertiles of animal protein intake, patients in the top tertiles had significantly higher consumption of red and processed meats, poultries, fishes, eggs, dairy products, energy, fat, SFA, cholesterol, sodium, potassium, phosphorus, calcium, vitamin B1, B2, B3, B6, B12, and folic acid (p < 0.05), and they significantly had lower intakes of grains, vegetables, fruits, legumes, nuts and seeds, carbohydrate, and vitamin C compared with the lower tertiles (p < 0.001).
The results of linear regression between the QOL and different types of dietary protein intake among HD patients are presented in Table 3. In the unadjusted model, significant positive associations were seen between total (β = 0.16; p < 0.001), plant (β = 0.32; p < 0.001), and animal (β = 0.16; p < 0.001) protein intake and QOL. Similarly, in model 2 after adjusting for sex, job, height, weight and URR, significant positive associations were seen between total (β = 0.14; p < 0.001), plant (β = 0.29; p < 0.001), and animal (β = 0.12; p = 0.01) protein intake and QOL. Furthermore, in model 3, after adjusting for model 2 confounding variables in addition to total energy intake significant positive associations were seen just between total (β = 0.12; p = 0.03) and plant (β = 0.26; p < 0.001) protein intake and QOL. However, in model 3, the association between animal protein intake and QOL was not significant (β = 0.03; p = 0.60).
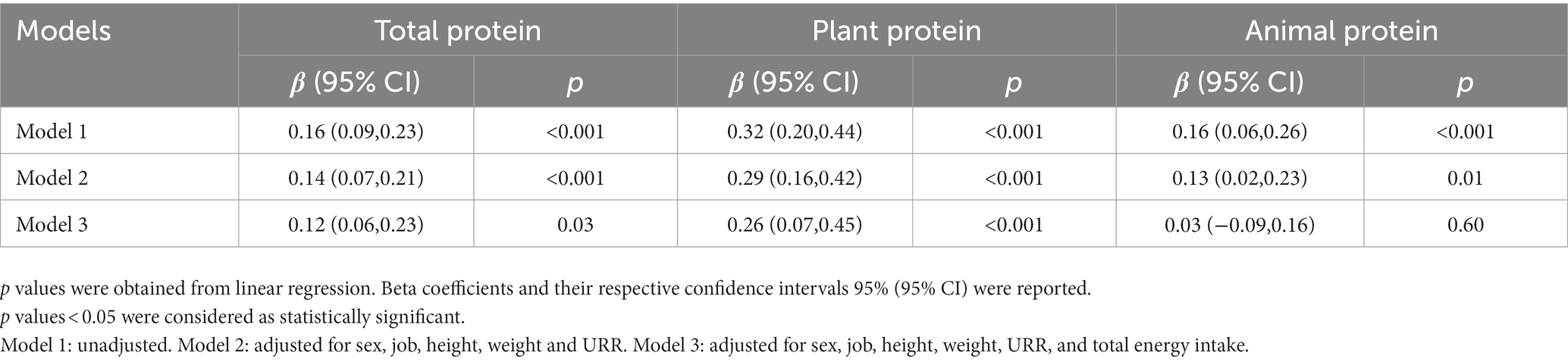
Table 3. The association between quality of life and different types of dietary protein intake in hemodialysis patients (n = 264).
Nutritional status, especially dietary protein intake, is an essential factor that influences the QOL of HD patients. However, few studies are available on the association of dietary protein with QOL in these patients (34–36). In addition to the quantity of protein, the source of protein may be an influential factor in the complications and QOL of HD patients (21, 37). This study is the first to evaluate the association between the source of protein and QOL in HD patients. We observed that higher consumption of total and plant proteins were associated with better QOL in HD patients, while there was no significant association between animal protein intake and QOL.
There are limited studies on the association between total dietary protein and QOL in HD patients (6, 36, 38). In line with our study, Shahrin et al. showed a significant positive correlation between protein intake and QOL in HD patients (6). However, Sharin’s study was conducted in Malaysia and their patients were older than our study. Moreover, the mean of total dietary protein intake in their study was lower than our study. Two other cross-sectional studies revealed that low dietary protein intake, as indicated by low serum albumin levels, is independently associated with poor QOL among HD patients (36, 39). Possible mechanisms may include the important role of dietary protein intake in the catabolic process in HD patients, which helps prevent muscle wasting and decrease the risk of infection (40). Furthermore, low protein consumption may cause anemia, weakness, and fatigue, directly affecting QOL’s physical and mental components (8, 41). In contrast, Yusop et al. found that lower protein intake was associated with better QOL in HD patients. This study also revealed that patients who did not achieve the protein intake recommendation still had good QOL scores and better BMI status (42). The discrepancy between the results of our study and those reported by Yusop et al. could be due to the different methodologies used to assess dietary intakes. Yusop et al. used 24-h diet recall, whereas we used the FFQ, which evaluated dietary intakes over the past year and may be more accurate.
In addition to the total protein intake, the source of protein can also affect kidney function and complications in HD patients (23, 24, 43, 44). In this study, we found that a higher intake of plant protein is associated with better QOL. Considering that there has been no study on the association of protein sources with QOL in these patients, the mechanism underlying this relationship has not been adequately addressed. However, various studies have been conducted on the source of protein intake and the complications of HD patients, which can affect the mental and physical dimensions of QOL in these patients. Cardiovascular disease (CVD) is one of the most common complications that crucially affect QOL and is the first cause of death in HD patients (45). Plant-based diets and increased protein intake from plant sources can reduce the risk of CVD in these patients (46). Plant-based proteins are rich in polyphenols and fiber, which significantly lower inflammatory factors, total and low density lipoprotein (LDL) cholesterol (47). Furthermore, patients who consume higher levels of plant protein have higher serum levels of threonine and histidine amino acids. The high level of these amino acids is associated with better blood pressure control. In contrast, red and processed meat are rich in SFA and sodium, increasing inflammatory markers, cholesterol, and serum concentrations of alanine and methionine. These factors are associated with hypertension and increased CVD complications in patients (48, 49). Moreover, higher plant protein intake can reduce the dietary acid load (DAL), while higher consumption of animal protein yields a higher DAL, which increases acidosis and decreases kidney function (50). A cross-sectional study revealed that higher DAL was associated with depression and sleep disorders. Therefore, plant-based proteins may have favorable effects on the mental health and QOL of patients (51). Further, uremic toxins accumulate in HD patients and cause various adverse effects, such as increased inflammation, oxidative stress, insulin resistance, increased CVD risk, and suppression of appetite (52). Plant-based proteins reduce the production of uremic toxins by modulating gut microbiota, improving patients’ conditions (53). Also, increasing the intake of plant protein may be associated with better appetite and calorie intake, which decrease the risk of malnutrition and ultimately improve QOL (54). Regardless of the benefits of plant-based proteins, one of the biggest concerns of using these sources in HD patients is the risk for hyperkalemia. However, the studies showed that high intake of these sources, despite their higher potassium contents, has not been shown to cause hyperkalemia in these patients (55). This may be because they are high in fiber, facilitating potassium excretion, whereas animal-based proteins worsen constipation and increase the risk of hyperkalemia (23). Another concern about plant-based protein is the risk for hyperphosphatemia. Although some plant-based protein like legumes, seeds and nuts have a high phosphate content, phosphates in these sources are stored in phytate form, which are difficult to digest, and only 10–30% of them are absorbed. In contrast, phosphates in animal-based protein have much higher bioavailability, and 40–60% of them are absorbed in the human gut (56, 57). A cross-sectional study showed that HD patients on vegetarian diets had significantly lower serum phosphate levels than non-vegetarians (58). Despite these considerations, hyperkalemia and hyperphosphatemia can have severe health consequences. Therefore, plant-based protein should still be consumed with caution in HD patients. Because of the benefits of plant-based proteins on the different complications of HD patients and the effects of these complications on the QOL, there may be a positive association between the plant protein and the QOL of these patients.
While this is the first study that has examined the association between the source of protein and QOL among HD patients, there are several limitations. The main limitation of this study was the absence of laboratory outcomes. Including biochemical assessments would have allowed for a more precise investigation of this association. Moreover, since this was a cross-sectional study, causality cannot be established. Furthermore, although a validated FFQ was used in this study, there may be some measurement errors and recall biases for a dietary intake assessment. Finally, many factors may have influenced the QOL that the researcher could not control.
In conclusion, our findings indicate that higher consumption of plant protein than animal protein is associated with better QOL in HD patients. However, clinical trials and cohort studies are required to make a clear conclusion.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Isfahan University of Medical Sciences (Code: IR.MUI.RESEARCH.REC.1399.605). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MR designed the study. MD collected data and entered data into the software. MR analyzed data. MD wrote the initial manuscript. S-AK revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Isfahan University of Medical sciences (grant number: 199466). The funders had no role in study design; collection, analysis, and interpretation of data; writing of the report; the decision to submit the report for publication.
We would like to thank all the participants for contributing to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ifudu, O. Care of patients undergoing hemodialysis. N Engl J Med. (1998) 339:1054–62. doi: 10.1056/NEJM199810083391507
2. Bello, AK, Okpechi, IG, Osman, MA, Cho, Y, Htay, H, Jha, V, et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. (2022) 18:378–95. doi: 10.1038/s41581-022-00542-7
3. Goodkin, DA, Young, EW, Kurokawa, K, Prütz, K-G, and Levin, NW. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis. (2004) 44:16–21. doi: 10.1016/S0272-6386(04)01100-X
4. Al-Mansouri, A, Al-Ali, FS, Hamad, AI, Ibrahim, MIM, Kheir, N, Ibrahim, RA, et al. Assessment of treatment burden and its impact on quality of life in dialysis-dependent and pre-dialysis chronic kidney disease patients. Res Soc Adm Pharm. (2021) 17:1937–44. doi: 10.1016/j.sapharm.2021.02.010
5. Broers, NJ, Usvyat, LA, Kooman, JP, Van Der Sande, FM, Lacson, E Jr, Kotanko, P, et al. Quality of life in dialysis patients: a retrospective cohort study. Nephron. (2015) 130:105–12. doi: 10.1159/000430814
6. Shahrin, FIM, Yu, LZ, Omar, N, Zakaria, NF, and Daud, ZAM. Association of socio-demographic characteristics, nutritional status, risk of malnutrition and depression with quality of life among elderly haemodialysis patients. Malays J Nutr. (2019) 25, 1–11. doi: 10.31246/mjn-2018-0101
7. Günalay, S, Öztürk, YK, Akar, H, and Mergen, H. The relationship between malnutrition and quality of life in haemodialysis and peritoneal dialysis patients. Rev Assoc Med Bras. (2018) 64:845–52. doi: 10.1590/1806-9282.64.09.845
8. Raimundo, P, Ravasco, P, Proença, V, and Camilo, M. Does nutrition play a role in the quality of life of patients under chronic haemodialysis? Nutr Hosp. (2006) 21:139–144.
9. Malhotra, R, Lipworth, L, Cavanaugh, KL, Young, BA, Tucker, KL, Carithers, TC, et al. Protein intake and long-term change in glomerular filtration rate in the Jackson heart study. J Ren Nutr. (2018) 28:245–50. doi: 10.1053/j.jrn.2017.11.008
10. Metzger, M, Yuan, WL, Haymann, J-P, Flamant, M, Houillier, P, Thervet, E, et al. Association of a low-protein diet with slower progression of CKD. Kidney Int Rep. (2018) 3:105–14. doi: 10.1016/j.ekir.2017.08.010
11. Ikizler, TA, Burrowes, JD, Byham-Gray, LD, Campbell, KL, Carrero, JJ, Chan, W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. (2020) 76:S1–s107. doi: 10.1053/j.ajkd.2020.05.006
12. Bernier-Jean, A, Prince, RL, Lewis, JR, Craig, JC, Hodgson, JM, Lim, WH, et al. Dietary plant and animal protein intake and decline in estimated glomerular filtration rate among elderly women: a 10-year longitudinal cohort study. Nephrol Dial Transplant. (2021) 36:1640–7. doi: 10.1093/ndt/gfaa081
13. Mirmiran, P, Yuzbashian, E, Aghayan, M, Mahdavi, M, Asghari, G, and Azizi, F. A prospective study of dietary meat intake and risk of incident chronic kidney disease. J Ren Nutr. (2020) 30:111–8
14. Campbell, KL, and Carrero, JJ. Diet for the Management of Patients with Chronic Kidney Disease; it is not the quantity, but the quality that matters. J Ren Nutr. (2016) 26:279–81. doi: 10.1053/j.jrn.2016.07.004
15. Kopple, JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. (2001) 37:S66–70. doi: 10.1053/ajkd.2001.20748
16. Cupisti, A, Brunori, G, Di Iorio, BR, D'Alessandro, C, Pasticci, F, Cosola, C, et al. Nutritional treatment of advanced CKD: twenty consensus statements. J Nephrol. (2018) 31:457–73. doi: 10.1007/s40620-018-0497-z
17. Ferrari, L, Panaite, S-A, Bertazzo, A, and Visioli, F. Animal– and plant-based protein sources: a scoping review of human health outcomes and environmental impact. Nutrients [Internet]. (2022) 14:nu14235115. doi: 10.3390/nu14235115
18. Carrero, JJ, Gonzalez-Ortiz, A, Avesani, CM, Bakker, SJ, Bellizzi, V, Chauveau, P, et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat Rev Nephrol. (2020) 16:525–42. doi: 10.1038/s41581-020-0297-2
19. Kelly, JT, and Carrero, JJ. Dietary sources of protein and chronic kidney disease progression: the proof may be in the pattern. J Ren Nutr. (2017) 27:221–4. doi: 10.1053/j.jrn.2017.04.001
20. Haring, B, Selvin, E, Liang, M, Coresh, J, Grams, ME, Petruski-Ivleva, N, et al. Dietary protein sources and risk for incident chronic kidney disease: results from the atherosclerosis risk in communities (ARIC) study. J Ren Nutr. (2017) 27:233–42. doi: 10.1053/j.jrn.2016.11.004
21. Saglimbene, VM, Wong, G, Ruospo, M, Palmer, SC, Garcia-Larsen, V, Natale, P, et al. Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. (2019) 14:250–60. doi: 10.2215/CJN.08580718
22. González-Ortiz, A, Xu, H, Ramos-Acevedo, S, Avesani, CM, Lindholm, B, Correa-Rotter, R, et al. Nutritional status, hyperkalaemia and attainment of energy/protein intake targets in haemodialysis patients following plant-based diets: a longitudinal cohort study. Nephrol Dial Transplant. (2021) 36:681–8. doi: 10.1093/ndt/gfaa194
23. Dupuis, L, Brown-Tortorici, A, Kalantar-Zadeh, K, and Joshi, S. A Mini review of plant-based diets in hemodialysis. Blood Purif. (2021) 50:672–7. doi: 10.1159/000516249
24. St-Jules, DE, Goldfarb, DS, Popp, CJ, Pompeii, ML, and Liebman, SE. Managing protein-energy wasting in hemodialysis patients: a comparison of animal-and plant-based protein foods. Semin Dial. (2019) 32:41–46. doi: 10.1111/sdi.12737
25. Adeva, MM, and Souto, G. Diet-induced metabolic acidosis. Clinical nutrition (Edinburgh, Scotland). (2011) 30:416–21. doi: 10.1016/j.clnu.2011.03.008
26. Kontessis, P, Jones, S, Dodds, R, Trevisan, R, Nosadini, R, Fioretto, P, et al. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. (1990) 38:136–44. doi: 10.1038/ki.1990.178
27. Shu, X, Calvert, JK, Cai, H, Xiang, Y-B, Li, H, Zheng, W, et al. Plant and animal protein intake and risk of incident kidney stones: results from the Shanghai Men's and Women's health studies. J Urol. (2019) 202:1217–23. doi: 10.1097/JU.0000000000000493
28. Alvirdizadeh, S, Yuzbashian, E, Mirmiran, P, Eghtesadi, S, and Azizi, F. A prospective study on total protein, plant protein and animal protein in relation to the risk of incident chronic kidney disease. BMC Nephrol. (2020) 21:1–7. doi: 10.1186/s12882-020-02079-y
29. Mirmiran, P, Hosseini-Esfahani, F, Mehrabi, Y, Hedayati, M, and Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2009) 13:654–62. doi: 10.1017/S1368980009991698
30. Mohammadifard, N, Sajjadi, F, Maghroun, M, Alikhasi, H, Nilforoushzadeh, F, and Sarrafzadegan, N. Validation of a simplified food frequency questionnaire for the assessment of dietary habits in Iranian adults: Isfahan healthy heart program, Iran. ARYA atherosclerosis. (2015) 11:139–46.
31. Esfahani, FH, Asghari, G, Mirmiran, P, and Azizi, F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol. (2010) 20:150–8. doi: 10.2188/jea.JE20090083
32. Ebrahimi-Mameghani, M, Behroozi-Fared-Mogaddam, A, and Asghari-Jafarabadi, M. Assessing the reliability and reproducibility of food frequency questionnaire and identify major dietary patterns in overweight and obese adults in Tabriz, Iran. J Mazandaran Univ Med Sci. (2014) 23:45–57.
33. Moro, T, Tinsley, G, Bianco, A, Marcolin, G, Pacelli, QF, and Battaglia, G. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. Journal of translational medicine. (2016) 14:1. doi: 10.1186/s12967-016-1044-0
34. Ayala, M, Marchant, M, Hertz, C, and Castillo, G. Intradialytic nutrition and quality of life in Chilean older patients in hemodialysis with protein-energy wasting. Int Urol Nephrol. (2022) 54:1947–55. doi: 10.1007/s11255-021-03077-1
35. Mazairac, AH, de Wit, GA, Penne, EL, van der Weerd, NC, Grooteman, MP, van den Dorpel, MA, et al. Protein-energy nutritional status and kidney disease-specific quality of life in hemodialysis patients. J Ren Nutr. (2011) 21:376–86.e1. doi: 10.1053/j.jrn.2010.08.004
36. Ohri-Vachaspati, P, and Sehgal, AR. Quality of life implications of inadequate protein nutrition among hemodialysis patients. J Ren Nutr. (1999) 9:9–13. doi: 10.1016/S1051-2276(99)90016-X
37. Kramer, H. Kidney disease and the westernization and industrialization of food. Am J Kidney Dis. (2017) 70:111–21. doi: 10.1053/j.ajkd.2016.11.012
38. Megawati, SW, Abdurachman, R, and Muliani, R. Relationship between nutritional status and quality of life of chronic kidney failure undergoing hemodialysis. Life Sci. (2019) 15:705–15. doi: 10.18502/kls.v4i13.5328
39. Kalantar-Zadeh, K, Kopple, JD, Block, G, and Humphreys, MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. (2001) 12:2797–806. doi: 10.1681/ASN.V12122797
40. Johansen, KL, Shubert, T, Doyle, J, Soher, B, Sakkas, GK, and Kent-Braun, JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. (2003) 63:291–7. doi: 10.1046/j.1523-1755.2003.00704.x
41. Heidari, B, Taheri, H, Hajian-Tilaki, K, Yolmeh, M, and Akbari, R. Low baseline serum albumin as a predictor of anemia in chronic hemodialysis patients. Caspian J Intern Med. (2015) 6:161–4.
42. Md Yusop, NB, Yoke Mun, C, Shariff, ZM, and Beng, HC. Factors associated with quality of life among hemodialysis patients in Malaysia. PLoS One. (2013) 8:e84152. doi: 10.1371/journal.pone.0084152
43. He, Y, Lu, Y, Yang, S, Li, Y, Yang, Y, Chen, J, et al. Dietary plant protein and mortality among patients receiving maintenance hemodialysis: a cohort study. Am J Kidney Dis. (2021) 78:649–57. e1. doi: 10.1053/j.ajkd.2021.03.023
44. Garcia-Torres, R, Young, L, Murray, DP, Kheda, M, and Nahman, NS Jr. Dietary protein source and phosphate levels in patients on hemodialysis. J Ren Nutr. (2020) 30:423–9. doi: 10.1053/j.jrn.2019.11.006
45. Cozzolino, M, Mangano, M, Stucchi, A, Ciceri, P, Conte, F, and Galassi, A. Cardiovascular disease in dialysis patients. Nephrol Dialysis Transplant. (2018) 33:iii28–34. doi: 10.1093/ndt/gfy174
46. Marx, W, Kelly, J, Marshall, S, Nakos, S, Campbell, K, and Itsiopoulos, C. The effect of polyphenol-rich interventions on cardiovascular risk factors in Haemodialysis: a systematic review and Meta-analysis. Nutrients. (2017) 9:9121345. doi: 10.3390/nu9121345
47. Xie, L-M, Ge, Y-Y, Huang, X, Zhang, Y-Q, and Li, J-X. Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int J Clin Exp Med. (2015) 8:1363.
48. Tuttle, KR, Milton, JE, Packard, DP, Shuler, LA, and Short, RA. Dietary amino acids and blood pressure: a cohort study of patients with cardiovascular disease. Am J Kidney Dis. (2012) 59:803–9. doi: 10.1053/j.ajkd.2011.12.026
49. Mirmiran, P, Yuzbashian, E, Aghayan, M, Mahdavi, M, Asghari, G, and Azizi, F. A prospective study of dietary meat intake and risk of incident chronic kidney disease. J Am Soc Nephrol. (2020) 30:111–8. doi: 10.1053/j.jrn.2019.06.008
50. Mirmiran, P, Yuzbashian, E, Bahadoran, Z, Asghari, G, and Azizi, F. Dietary Acid-Base load and risk of chronic kidney disease in adults: Tehran lipid and glucose study. Iran J Kidney Dis. (2016) 10:119–25.
51. Daneshzad, E, Keshavarz, S-A, Qorbani, M, Larijani, B, Bellissimo, N, and Azadbakht, L. Association of dietary acid load and plant-based diet index with sleep, stress, anxiety and depression in diabetic women. Br J Nutr. (2020) 123:901–12. doi: 10.1017/S0007114519003179
52. Vanholder, R, Schepers, E, Pletinck, A, Nagler, EV, and Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. (2014) 25:1897–907. doi: 10.1681/ASN.2013101062
53. Montemurno, E, Cosola, C, Dalfino, G, Daidone, G, De Angelis, M, Gobbetti, M, et al. What would you like to eat, Mr CKD microbiota? A Mediterranean diet, please! Kidney Blood Press Res. (2014) 39:114–23. doi: 10.1159/000355785
54. Joshi, S, Shah, S, and Kalantar-Zadeh, K. Adequacy of plant-based proteins in chronic kidney disease. J Ren Nutr. (2019) 29:112–7. doi: 10.1053/j.jrn.2018.06.006
55. St-Jules, DE, Goldfarb, DS, and Sevick, MA. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. (2016) 26:282–7. doi: 10.1053/j.jrn.2016.02.005
56. Noori, N, Sims, JJ, Kopple, JD, Shah, A, Colman, S, Shinaberger, CS, et al. Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran J Kidney Dis. (2010) 4:89–100.
57. Kalantar-Zadeh, K, Gutekunst, L, Mehrotra, R, Kovesdy, CP, Bross, R, Shinaberger, CS, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. (2010) 5:519–30. doi: 10.2215/CJN.06080809
Keywords: dietary protein, animal protein, plant protein, quality of life, hemodialysis
Citation: Darzi M, Rouhani MH and Keshavarz S-A (2023) The association between plant and animal protein intake and quality of life in patients undergoing hemodialysis. Front. Nutr. 10:1219976. doi: 10.3389/fnut.2023.1219976
Received: 09 May 2023; Accepted: 01 September 2023;
Published: 19 September 2023.
Edited by:
Fabiana Baggio Nerbass, Fundacão Pró-Rim, BrazilReviewed by:
Kulnipa Kittisakmontri, Chiang Mai University, ThailandCopyright © 2023 Darzi, Rouhani and Keshavarz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed-Ali Keshavarz, c2EuYWtlc2hhdmFyekB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.