- 1The First People’s Hospital of Yulin, Yulin, Guangxi, China
- 2Department of Cardiology, Affiliated Anzhen Hospital, Capital Medical University, Beijing, China
Background: Identifying risk factors associated with cardiac intensive care unit (CICU) patients’ prognosis can help clinicians intervene earlier and thus improve their prognosis. The correlation between the geriatric nutrition risk index (GNRI), which reflects nutritional status, and in-hospital mortality among CICU patients has yet to be established.
Method: The present study retrospectively enrolled 4,698 CICU patients. Based on the nutritional status, the participants were categorized into four groups. The primary endpoint was in-hospital mortality. The length of hospital stay and length of CICU stay were the secondary endpoints. To explore the correlation between nutritional status and in-hospital mortality, a logistic regression analysis was conducted. The nonlinear associations of GNRI with in-hospital mortality were evaluated using restricted cubic spline (RCS). Furthermore, subgroup analyses were conducted to evaluate the effect of the GNRI on in-hospital mortality across different subgroups, with calculation of the p for interaction.
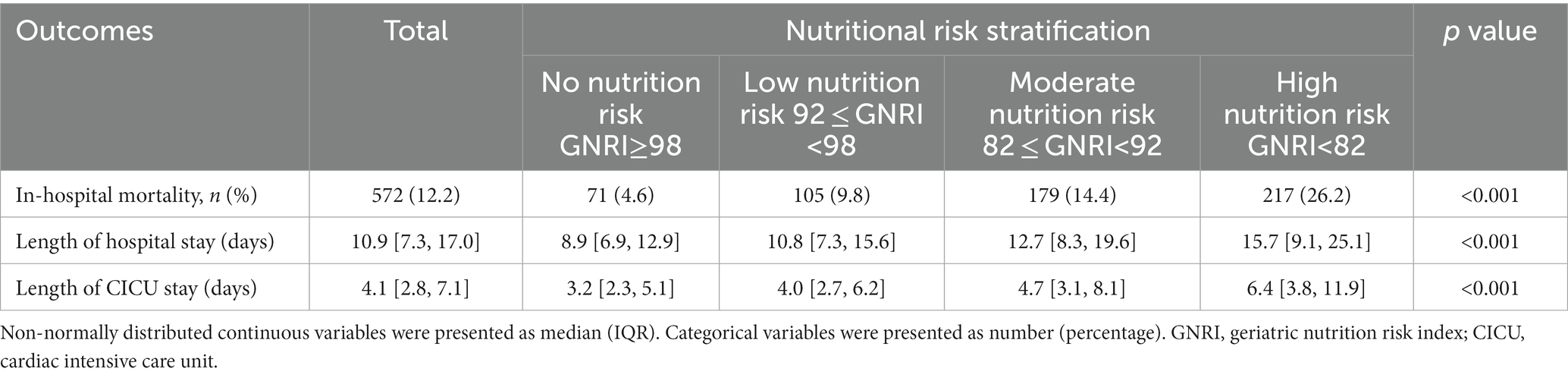
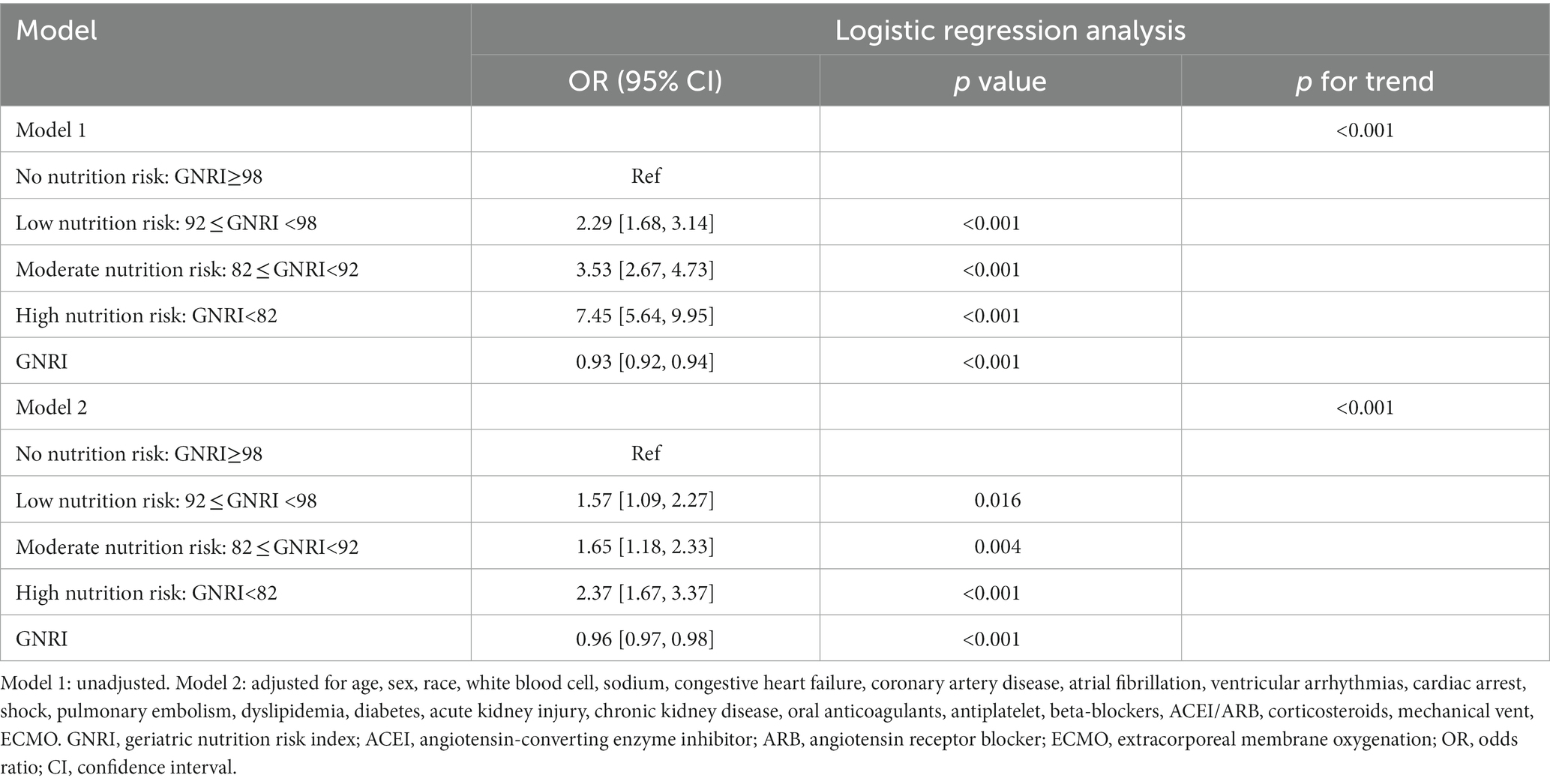
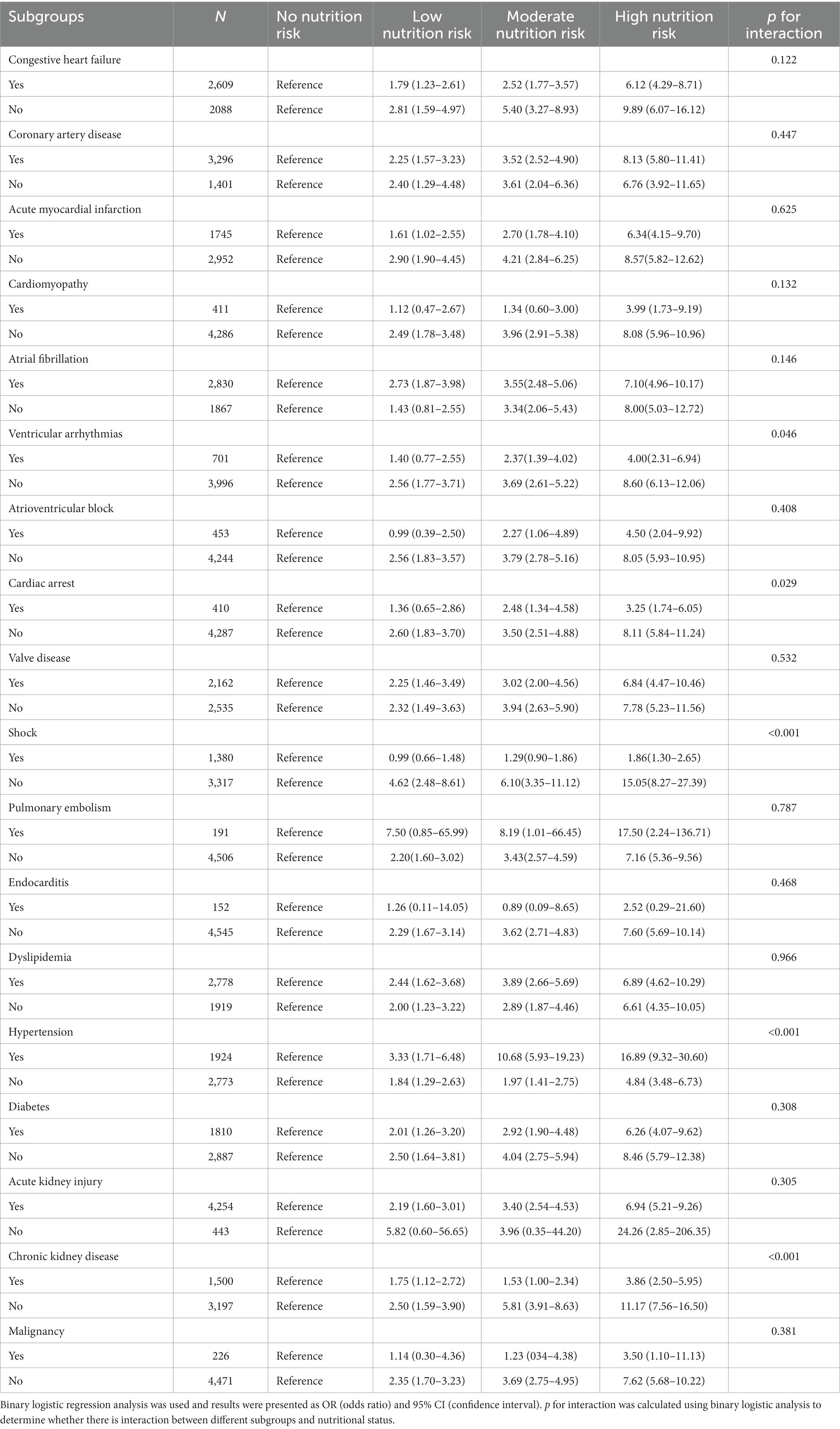
Result: A higher risk of malnutrition was significantly linked to an increased incidence of in-hospital mortality (High risk vs. No risk: 26.2% vs. 4.6%, p < 0.001), as well as a longer length of hospital stay (High risk vs. No risk: 15.7, 9.1–25.1 vs. 8.9, 6.9–12.9, p < 0.001) and CICU stay (High risk vs. No risk: 6.4, 3.8–11.9 vs. 3.2, 2.3–5.1, p < 0.001). An elevated GNRI was significantly associated with an increased risk of in-hospital mortality even after controlling for pertinent confounding factors (High risk vs. No risk: OR, 95% CI: 2.37, 1.67–3.37, p < 0.001, p for trend <0.001). Additionally, the RCS model showed a linear relationship between GNRI and in-hospital mortality, with the risk of in-hospital mortality significantly decreasing as GNRI increased (non-linear p = 0.596). Furthermore, in the subgroups of hypertension, ventricular arrhythmias, cardiac arrest, shock, and chronic kidney disease, there was a significant interaction between nutritional status and in-hospital mortality.
Conclusion: Among CICU patients, a low GNRI was a significant predictor of in-hospital mortality. Furthermore, patients with a higher risk of malnutrition, as indicated by low GNRI values, experienced significantly longer hospital and CICU stays.
1. Introduction
Since its establishment in the 1960s with the objective of resuscitating patients with acute myocardial infarction (AMI), the coronary care unit (CCU) has undergone a transformation into a cardiac intensive care unit (CICU) (1–3). With the complexity of the clinical condition of patients, the current indications for CICU cover AMI, advanced heart failure (HF), cardiogenic shock (CS), organ failure, and multi-systemic critical illness (4). Patients admitted to the CICU often have many non-cardiac conditions in addition to cardiac disease, such as sepsis, acute renal failure, and acute respiratory failure (5, 6). These complications were associated not only with the severity of the underlying disease and the need for intensive care, but also with elevated morbidity and mortality rates, leading to greater resource utilization and medical costs (7–11). Therefore, identifying risk factors related to the prognosis of CICU patients is crucial for clinical physicians, which can help clinicians to intervene early in the treatment of patients and thus improve their prognosis.
Malnutrition is widespread in critically ill patients and is related to a worse prognosis (12–14). Calculated from serum albumin, height, and weight, the GNRI is a convenient and accessible indicator to evaluate the nutritional status of patients (15, 16). Patients with lower GNRI scores were considered to have poorer nutritional status and had worse outcomes (17, 18). The GNRI score is now used as a risk index for a variety of diseases, such as uremia, sepsis, and cardiovascular diseases (CVD) (19–21). Previous studies have linked GNRI to a poor outcome in various CVDs, including acute HF, coronary artery disease (CAD), and acute ST-segment elevation myocardial infarction (22–25). Hence, in critically ill patients admitted to the CICU, employing GNRI as a tool to assess nutritional status might enhance risk stratification, and providing timely nutritional support could potentially enhance long-term prognosis. However, no studies have been undertaken to investigate the impact of nutritional status on the prognosis of CICU patients. The aim of this study was to explore an association between GNRI and in-hospital mortality in CICU patients.
2. Methods
2.1. Population selection criteria
This was an observational, retrospective study that included patients from the CICU and CCU, extracted from the Medical Information Mart for Intensive Care IV (MIMIC-IV version 2.0). The database provides comprehensive and high-quality data on patients admitted to intensive care units at Beth Israel Deaconess Medical Center between 2008 and 2019 (26). As shown in Figure 1, all patients who were initially admitted to the hospital for a duration of more than two days were included. Patients with the following conditions were excluded: (1) non-cardiac hospitalization; (2) weight, height and albumin data missing; (3) age < 18 years. A total of 4,698 patients were enrolled.
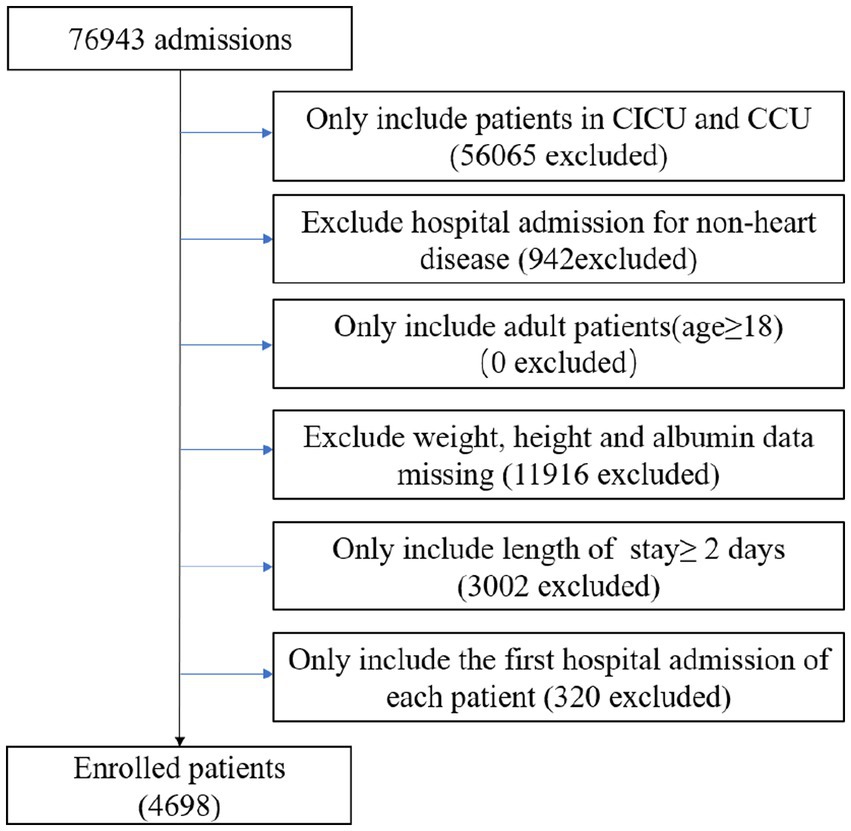
Figure 1. Flow chart of study population. CCU, coronary artery care unit; CICU, cardiac intensive care unit.
2.2. Data extraction
The data utilized in this study was extracted from the publicly available critical care database known as MIMIC-IV (26). The following information was collected: demographics, vital sign, comorbidities and medical history, laboratory parameter and treatment (Details can be found in Supplementary material).
2.3. Definition of nutritional status and endpoints
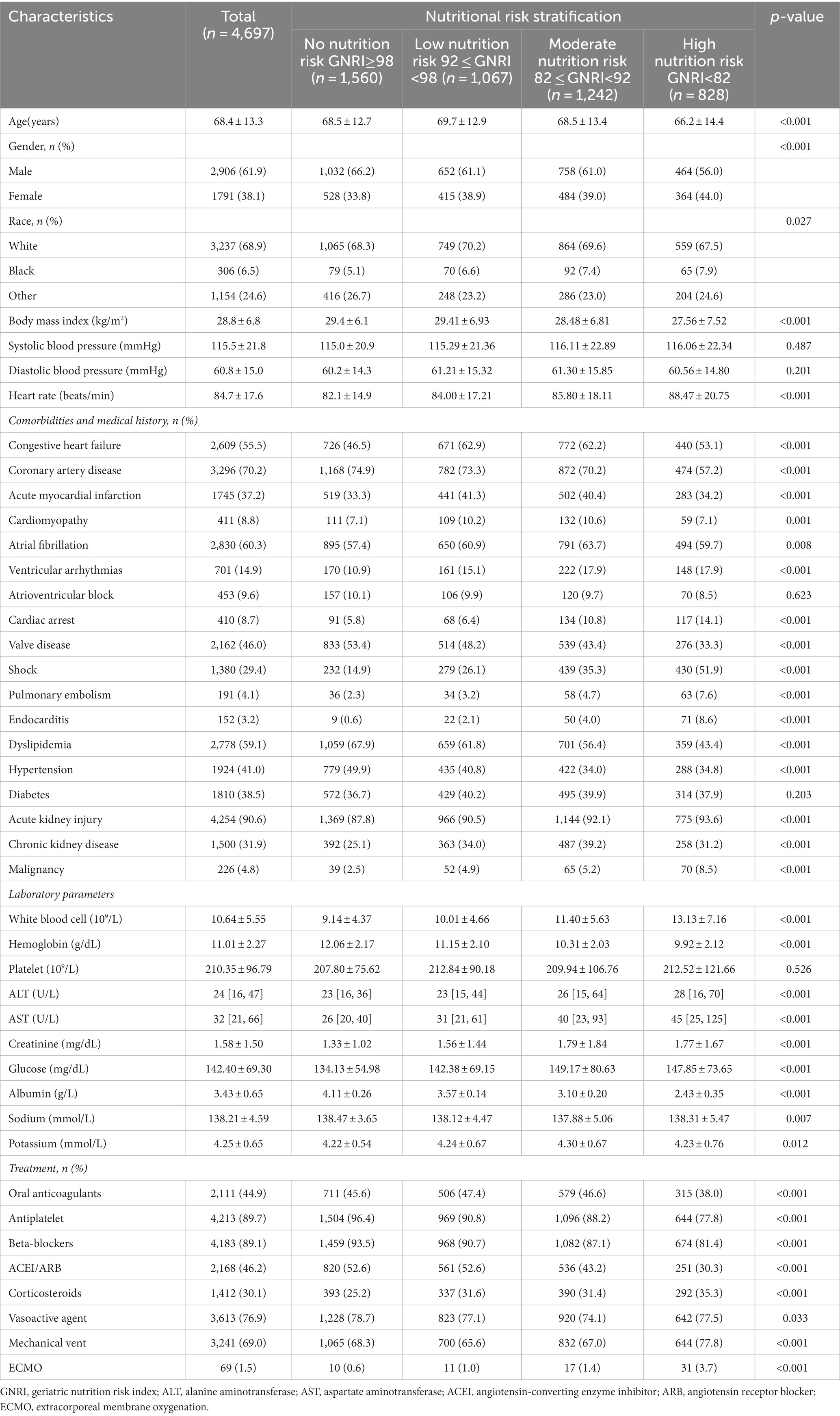
According to GNRI, all patients were classified into four groups (15): No nutrition risk: GNRI≥98 (n = 1,560), Low nutrition risk: 92 ≤ GNRI <98 (n = 1,067), Moderate nutrition risk: 82 ≤ GNRI <92 (n = 1,214), High nutrition risk: GNRI<82 (n = 828). The GNRI index was calculated as follows: GNRI = [14.89 × serum albumin (g/dL)] + [41.7 × actual BMI/ideal BMI] (27). Ideal BMI was set to 22 kg/m2 (28). If the patient’s BMI exceeded the ideal BMI, the “actual BMI/ideal BMI” ratio was set to 1. The primary endpoint was in-hospital mortality. The secondary endpoints were length of hospital stay and length of CICU stay.
2.4. Statistical analysis
The baseline characteristics were reported as mean ± standard deviation (SD) for normally distributed quantitative data, median [interquartile range (IQR)] for skewed data, and number (%) for categorical data. Analysis of variance, Kruskal-Wallis, and chi-square tests were conducted to compare patient characteristics according to nutritional status. Binary logistic regression analysis was used to determine the association between nutritional status and in-hospital mortality, and the results were presented as odds ratios (OR) with corresponding 95% confidence intervals (CI). To account for relative confounding variables, a multivariate logistic analysis using the stepwise method with removal at p > 0.05 was performed on all baseline covariates listed in Table 1 (Details can be found in Supplementary material). Furthermore, we created a restricted cubic spline curve (RCS) based on the multivariate logistic regression model to investigate the relationship between GNRI and in-hospital mortality. Three knots were chosen for examination. In subgroup analysis, univariate binary logistic regression was used to assess the correlation between nutritional status and in-hospital mortality in various comorbidity subgroups. The results were expressed as OR and 95% CI, with p for interaction computed.
All tests were two-sided, and statistical significance was defined as p < 0.05. R software was used to perform all data analysis.
3. Results
3.1. Patient characteristics
The patients were classified into four groups based on their nutritional status: No nutrition risk (n = 1,560), Low nutrition risk (n = 1,067), Moderate nutrition risk (n = 1,214), High nutrition risk (n = 828). Table 1 summarized the characteristics of the different nutritional states. Patients with high nutrition risk were younger, female sex, less often white, had a lower BMI but a higher heartrate, and were more likely to have a history of congestive HF, cardiomyopathy, atrial fibrillation, ventricular arrhythmias, acute myocardial infarction, cardiac arrest, pulmonary embolism, endocarditis, acute kidney injury, chronic kidney disease, shock and malignancy, but less often had coronary artery disease, valve disease, hypertension, and diabetes. Furthermore, patients with a high nutritional risk had higher levels of white blood cells, ALT, AST, creatinine, glucose, and potassium, while having lower levels of hemoglobin, sodium, and albumin. In addition, they received more corticosteroids, mechanical ventilation, and extracorporeal membrane oxygenation (ECMO), while receiving less oral anticoagulant, antiplatelet, beta-blocker, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), and vasoactive agent therapy.
3.2. Association between nutritional status and adverse outcomes
Overall, in-hospital mortality rate was 12.2%. As nutrition risk groups increased, in-hospital mortality increased significantly (High risk vs. No risk: 26.2% vs. 4.6%, p < 0.001) (Table 2). Higher nutrition risk was significantly associated with the increased length of hospital stay (High risk vs. No risk: 15.7, 9.1–25.1 vs. 8.9, 6.9–12.9, p < 0.001) and CICU stay (High risk vs. No risk: 6.4, 3.8–11.9 vs. 3.2, 2.3–5.1, p < 0.001) respectively (Table 2). As shown in Table 3, in model 1, higher nutrition risk was associated with the increased risk of in-hospital mortality (High risk vs. No risk: OR, 95% CI: 7.45, 5.64–9.95, p < 0.001, p for trend <0.001). In Model 2, we adjusted for relevant confounding variables and found that a higher nutrition risk was significantly associated with an increased risk of in-hospital mortality (High risk vs. No risk: OR, 95% CI: 2.37, 1.67–3.37, p < 0.001, p for trend <0.001). When analyzing GNRI as a continuous variable, we found that an increase of one unit in GNRI was associated with a reduction in the risk of in-hospital mortality by approximately 0.07-fold in Model 1 and 0.04-fold in Model 2, respectively.
Figure 2 displayed the use of restricted cubic splines (RCS) to visually represent the relationship between MACE and GNRI, as well as fit the model. After potential confounders were considered, a linear association between GNRI and in-hospital mortality was confirmed (non-linear p = 0.596). As GNRI increased, the risk of in-hospital mortality decreased significantly.
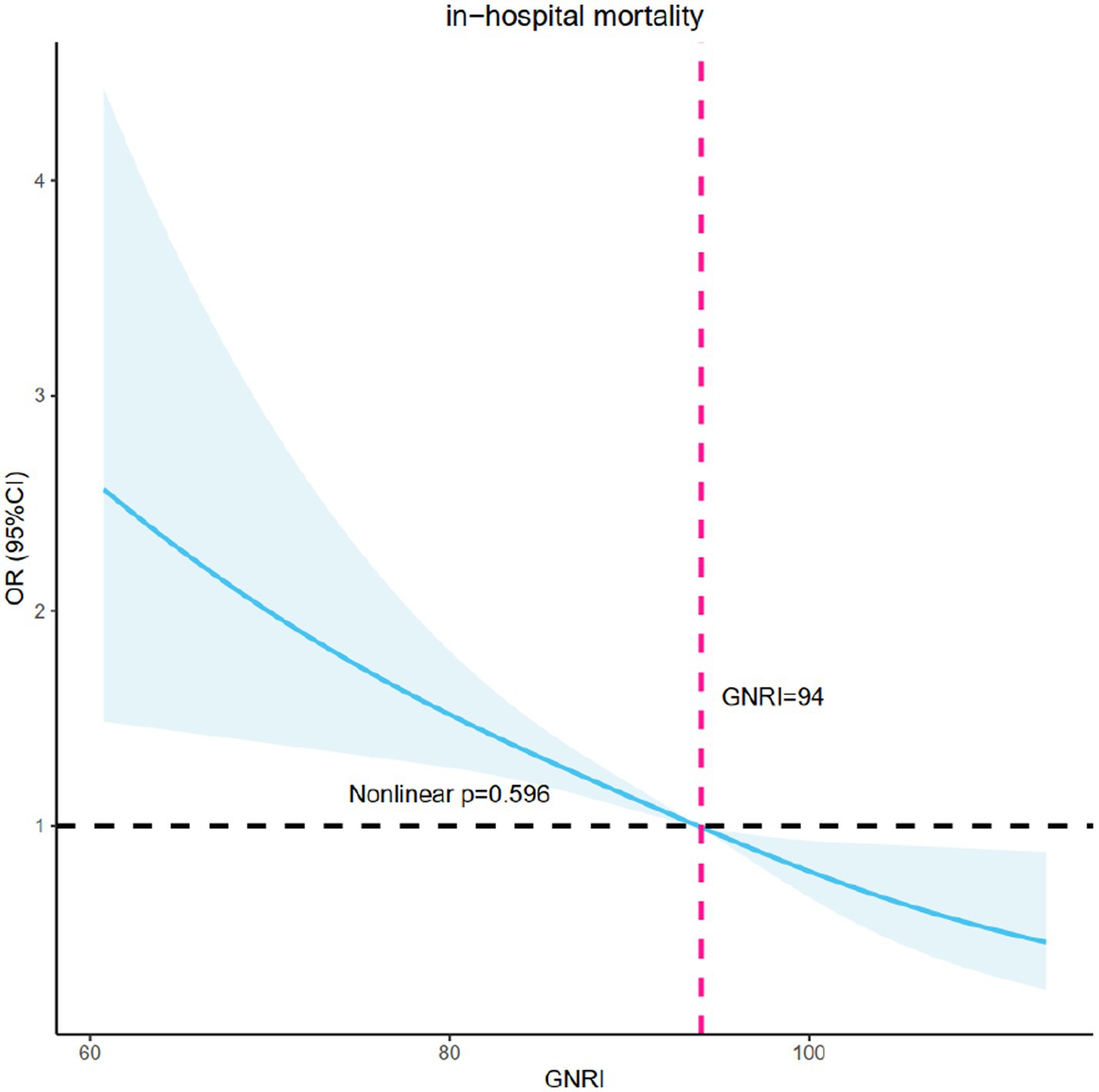
Figure 2. RCS model showing the association between the nutritional status and in-hospital mortality. RCS, restricted cubic spline curve.
3.3. Subgroup analysis
In all subgroup analyses (Table 4), we found that patients with hypertension (p for interaction<0.001) had increased risks of in-hospital mortality for higher nutrition risk. But patients with ventricular arrhythmias (p for interaction = 0.046), cardiac arrest (p for interaction = 0.029), shock (p for interaction<0.001), and chronic kidney disease (p for interaction<0.001) had lower risks of in-hospital mortality. In the remaining subgroups, no significant interactions were found.
4. Discussion
Our findings revealed that GNRI was an independent predictor of in-hospital mortality among CICU patients. The RCS analysis further confirmed a linear relationship between GNRI and in-hospital mortality. Furthermore, we found that higher nutrition risk was significantly related to the increased length of hospital stay and CICU stay. Significant interactions were observed in the relationship between GNRI and in-hospital mortality in hypertension, ventricular arrhythmias, cardiac arrest, shock, and chronic kidney disease subgroups.
Malnutrition, a condition characterized by an imbalance between the body’s energy intake and demands, has been unequivocally linked to cardiovascular disease (29). However, the underlying mechanism responsible for this association was multifaceted, with inflammation, metabolism, and aging all implicated in this pathological relationship (30, 31). Indeed, previous investigations have demonstrated that malnutrition was intricately linked to inflammation (30, 32). The inflammatory reaction, in turn, could antagonize albumin synthesis, a key protein involved in maintaining optimal nutritional status, and further aggravate malnutrition, engendering a self-perpetuating cycle of deleterious consequences (33). Furthermore, emerging evidence has suggested that malnutrition could precipitate the onset of various pathologies, such as free radical damage, impaired insulin secretion, lipolysis, and lipid oxidation. These adverse events, in turn, could incite tissue damage, diabetes, and fatty liver disease, thus perpetuating the vicious cycle of malnutrition (34–36). Importantly, previous research has also highlighted the unfavorable prognostic implications of malnutrition, manifesting as an adverse prognosis in various diseases, such as HF, CAD, and peripheral arterial disease (37–40).
Various systems are commonly employed in clinical practice to assess nutritional status, including subjective global assessment (SGA) (41) and mini-nutritional assessment (MNA) (42, 43). Nonetheless, many of these indicators have been discarded due to their complexity and vulnerability to subjective influences (41–43). Meanwhile, laboratory indices such as albumin (44) and hemoglobin (45) have been utilized to assess nutritional status and their association with patient prognosis has been established. However, these indicators are limited in that they only reflect a singular aspect and their predictive ability can be influenced by external factors. In recent years, GNRI has gained popularity as a commonly used tool in clinical nutrition assessment, primarily due to its convenience and accessibility (46). Moreover, it has been clinically established that a correlation between GNRI and the development and prognosis of several cardiovascular diseases, including HF, CAD, and stroke (47–49). A study that enrolled 2,299 patients with non-ST-segment elevation acute coronary syndrome found that a lower GNRI was significantly related to poor prognosis (50). An observational study showed that patients undergoing coronary artery bypass grafting with decreased GNRI had an increased incidence of MACE and a lower survival rate during long-term follow-up (51). According to a meta-analysis, low baseline GNRI was identified as a reliable predictor of cardiovascular events in CAD patients. In addition, another study conducted on elderly patients with HF demonstrated that a lower GNRI could independently predict MACE, thereby affirming the risk stratification ability of GNRI (22).
In the realm of scoring systems, GNRI exerts its preeminence by virtue of its remarkable faculty for risk stratification. The singularity of GNRI lies not only in its robustness, but also in its simplicity, which sets it apart from more intricate scoring mechanisms (52). As far as we knew, this study was the first to examine the correlation between GNRI and in-hospital mortality among CICU patients. As with prior research, the GNRI has been shown to be a reliable predictor of in-hospital mortality among CICU patients. This discovery reinforced the use of GNRI as a prognostic indicator in clinical settings and enhanced risk assessment and stratification based on traditional risk factors. Notably, among patients without ventricular arrhythmias, shock, chronic kidney disease or cardiac arrest, the effect of nutritional status on in-hospital mortality was enhanced, implying that clinicians should not ignore CICU patients without diseases that had a high case fatality rate, as paying attention to nutritional status and intervening accordingly could benefit patients more.
The RCS curve revealed a linear negative relationship between GNRI and in-hospital mortality: as nutritional status improved as measured by GNRI, the in-hospital mortality risk decreased, suggesting that clinicians might be able to improve poor outcomes by increasing GNRI with more aggressive treatment and better care. Furthermore, as the level of nutrition risk increased, the length of hospitalization and CICU stay rose significantly, compounding the emotional, physical, and financial stress experienced by patients. The potential explanation for this phenomenon was that patients with optimal nutritional status exhibited a more rapid convalescence from the ailment, thereby resulting in expedited hospital discharge and diminished expenses associated with hospitalization. As a result, indicators like the GNRI, which is more cost-effective and accessible, should receive more attention. When a full assessment of a patient’s health status is not possible in an emergency, the use of GNRI could quickly identify high-risk patients and provide clinicians with new treatment suggestions. This is especially true in medical settings that are deprived of adequate resources and infrastructure, such as those in geographically isolated regions or areas with poor healthcare facilities. Taken together, we believe that for patients with comorbid malnutrition in the CICU, the earlier their nutritional status is improved, the better their prognosis is likely to be.
While this study had some limitations. (1) This study only assessed the initial GNRI of CICU patients and did not record and analyze the dynamic changes in GNRI. (2) The use of public databases limited the collection of relevant information that could have influenced the model, such as detailed causes of death, left ventricular ejection fraction, specific coronary artery lesions, revascularization, types of myocardial infarction, and precise clinical symptoms. (3) Due to the retrospective nature of our study, we were unable to determine a specific cause for hospitalization. (4) Since it was a single-center retrospective study, it was susceptible to certain biases that might compromise the accuracy of the findings, thereby reducing their strength and rendering them incapable of establishing causality. Multi-central research is needed to further verify the current discovery among a wider range of people.
5. Conclusion
GNRI, being a simple and easily measurable tool in clinical practice, contributed significantly to the prognosis of in-hospital mortality among patients admitted to the CICU. Moreover, we found that higher nutrition risk, as indicated by low GNRI values, was significantly associated with prolonged hospital and CICU stays. Prospective, randomized studies are needed to establish whether interventions aimed at improving nutritional status could improve clinical outcomes. Moreover, we observed that higher nutrition risk, as indicated by low GNRI values, was significantly associated with prolonged hospital and CICU stays.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://doi.org/10.13026/6mm1-ek67.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL and ZW: conceptualization. TS and BZ: methodology. YL and ZW: writing – original draft. XL: writing – review and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1218738/full#supplementary-material
References
1. Leong, DP, Joseph, PG, McKee, M, Anand, SS, Teo, KK, Schwalm, JD, et al. Reducing the global burden of cardiovascular disease, Part 2: prevention and treatment of cardiovascular disease. Circ Res. (2017) 121:695–10. doi: 10.1161/CIRCRESAHA.117.311849
2. Morrow, DA, Fang, JC, Fintel, DJ, Granger, CB, Katz, JN, Kushner, FG, et al. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation. (2012) 126:1408–28. doi: 10.1161/CIR.0b013e31826890b0
3. Katz, JN, Shah, BR, Volz, EM, Horton, JR, Shaw, LK, Newby, LK, et al. Evolution of the coronary care unit: clinical characteristics and temporal trends in healthcare delivery and outcomes. Crit Care Med. (2010) 38:375–1. doi: 10.1097/CCM.0b013e3181cb0a63
4. Jentzer, JC, van Diepen, S, Barsness, GW, Katz, JN, Wiley, BM, Bennett, CE, et al. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J. (2019) 215:12–9. doi: 10.1016/j.ahj.2019.05.012
5. Morrow, DA. Trends in cardiac critical care: reshaping the cardiac intensive care unit. Circ Cardiovasc Qual Outcomes. (2017) 10:e004010. doi: 10.1161/CIRCOUTCOMES.117.004010
6. Sinha, SS, Sjoding, MW, Sukul, D, Prescott, HC, Iwashyna, TJ, Gurm, HS, et al. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ Cardiovasc Qual Outcomes. (2017) 10:e003616. doi: 10.1161/CIRCOUTCOMES.117.003616
7. Stevens, V, Geiger, K, Concannon, C, Nelson, RE, Brown, J, and Dumyati, G. Inpatient costs, mortality and 30-day re-admission in patients with central-line-associated bloodstream infections. Clin Microbiol Infect. (2014) 20:O318–24. doi: 10.1111/1469-0691.12407
8. Ouimet, S, Kavanagh, BP, Gottfried, SB, and Skrobik, Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. (2007) 33:66–73. doi: 10.1007/s00134-006-0399-8
9. Hein, OV, Birnbaum, J, Wernecke, K, England, M, Konertz, W, and Spies, C. Prolonged intensive care unit stay in cardiac surgery: risk factors and long-term-survival. Ann Thorac Surg. (2006) 81:880–5. doi: 10.1016/j.athoracsur.2005.09.077
10. Cook, DJ, Griffith, LE, Walter, SD, Guyatt, GH, Meade, MO, Heyland, DK, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care. (2001) 5:368–5. doi: 10.1186/cc1071
11. Garrouste-Orgeas, M, Timsit, JF, Vesin, A, Schwebel, C, Arnodo, P, Lefrant, JY, et al. Selected medical errors in the intensive care unit: results of the IATROREF study: parts I and II. Am J Respir Crit Care Med. (2010) 181:134–2. doi: 10.1164/rccm.200812-1820OC
12. van Zanten, ARH, De Waele, E, and Wischmeyer, PE. Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care. (2019) 23:368. doi: 10.1186/s13054-019-2657-5
13. Singer, P. Preserving the quality of life: nutrition in the ICU. Crit Care. (2019) 23:139. doi: 10.1186/s13054-019-2415-8
14. Wischmeyer, PE. Nutrition therapy in sepsis. Crit Care Clin. (2018) 34:107–5. doi: 10.1016/j.ccc.2017.08.008
15. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–3. doi: 10.1093/ajcn/82.4.777
16. Cereda, E, and Pedrolli, C. The geriatric nutritional risk index. Curr Opin Clin Nutr Metab Care. (2009) 12:1–7. doi: 10.1097/MCO.0b013e3283186f59
17. Gärtner, S, Kraft, M, Krüger, J, Vogt, LJ, Fiene, M, Mayerle, J, et al. Geriatric nutritional risk index correlates with length of hospital stay and inflammatory markers in older inpatients. Clin Nutr. (2017) 36:1048–53. doi: 10.1016/j.clnu.2016.06.019
18. Rus, VA, Chitu, M, Cernea, S, Benedek, I, Hodas, R, Zavate, R, et al. Altered nutritional status, inflammation and systemic vulnerability in patients with acute myocardial infarction undergoing percutaneous coronary revascularisation: A prospective study in a level 3 cardiac critical care unit. Nutr Diet. (2020) 77:212–2. doi: 10.1111/1747-0080.12536
19. Matsukuma, Y, Tanaka, S, Taniguchi, M, Nakano, T, Masutani, K, Hirakata, H, et al. Association of geriatric nutritional risk index with infection-related mortality in patients undergoing hemodialysis: the Q-Cohort Study. Clin Nutr. (2019) 38:279–7. doi: 10.1016/j.clnu.2018.01.019
20. Takahashi, H, Ito, Y, Ishii, H, Aoyama, T, Kamoi, D, Kasuga, H, et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol. (2014) 64:32–6. doi: 10.1016/j.jjcc.2013.10.018
21. Lee, JS, Choi, HS, Ko, YG, and Yun, DH. Performance of the geriatric nutritional risk index in predicting 28-day hospital mortality in older adult patients with sepsis. Clin Nutr. (2013) 32:843–8. doi: 10.1016/j.clnu.2013.01.007
22. Li, H, Cen, K, Sun, W, and Feng, B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. (2021) 33:1477–86. doi: 10.1007/s40520-020-01656-3
23. Wada, H, Dohi, T, Miyauchi, K, Doi, S, Naito, R, Konishi, H, et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. (2017) 119:1740–5. doi: 10.1016/j.amjcard.2017.02.051
24. Honda, Y, Nagai, T, Iwakami, N, Sugano, Y, Honda, S, Okada, A, et al. Usefulness of geriatric nutritional risk index for assessing nutritional status and its prognostic impact in patients aged ≥65 years with acute heart failure. Am J Cardiol. (2016) 118:550–5. doi: 10.1016/j.amjcard.2016.05.045
25. Jia, Y, Gao, Y, Li, D, Cao, Y, Cheng, Y, Li, F, et al. Geriatric nutritional risk index score predicts clinical outcome in patients with acute st-segment elevation myocardial infarction. J Cardiovasc Nurs. (2020) 35:E44–e52. doi: 10.1097/JCN.0000000000000674
26. Johnson, A, Bulgarelli, L., Pollard, T., Horng, S., Celi, L. A., and Mark, R MIMIC-IV (version 2.0). PhysioNet (2022).
27. Yamada, K, Furuya, R, Takita, T, Maruyama, Y, Yamaguchi, Y, Ohkawa, S, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. (2008) 87:106–3. doi: 10.1093/ajcn/87.1.106
28. Shah, B, Sucher, K, and Hollenbeck, CB. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract. (2006) 21:312–9. doi: 10.1177/0115426506021003312
29. Bellanti, F, Lo Buglio, A, Quiete, S, and Vendemiale, G. Malnutrition in hospitalized old patients: screening and diagnosis. Clin Outcomes Manag Nutr. (2022) 14:910. doi: 10.3390/nu14040910
30. Nakagomi, A, Kohashi, K, Morisawa, T, Kosugi, M, Endoh, I, Kusama, Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. (2016) 23:713–7. doi: 10.5551/jat.31526
31. Wells, JL, and Dumbrell, AC. Nutrition and aging: assessment and treatment of compromised nutritional status in frail elderly patients. Clin Interv Aging. (2006) 1:67–79. doi: 10.2147/ciia.2006.1.1.67
32. Hasper, D, Hummel, M, Kleber, FX, Reindl, I, and Volk, HD. Systemic inflammation in patients with heart failure. Eur Heart J. (1998) 19:761–5. doi: 10.1053/euhj.1997.0858
33. Purser, JL, Kuchibhatla, MN, Fillenbaum, GG, Harding, T, Peterson, ED, and Alexander, KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. (2006) 54:1674–81. doi: 10.1111/j.1532-5415.2006
34. Thompson, DS, Bourdon, C, Massara, P, Boyne, MS, Forrester, TE, Gonzales, GB, et al. Childhood severe acute malnutrition is associated with metabolic changes in adulthood. JCI Insight. (2020) 5:e141316. doi: 10.1172/jci.insight.141316
35. Spoelstra, MN, Mari, A, Mendel, M, Senga, E, van Rheenen, P, van Dijk, TH, et al. Kwashiorkor and marasmus are both associated with impaired glucose clearance related to pancreatic β-cell dysfunction. Metabolism. (2012) 61:1224–30. doi: 10.1016/j.metabol.2012.01.019
36. Badaloo, AV, Forrester, T, Reid, M, and Jahoor, F. Lipid kinetic differences between children with kwashiorkor and those with marasmus. Am J Clin Nutr. (2006) 83:1283–8. doi: 10.1093/ajcn/83.6.1283
37. Nakamura, T, Matsumoto, M, Haraguchi, Y, Ishida, T, and Momomura, SI. Prognostic impact of malnutrition assessed using geriatric nutritional risk index in patients aged ⩾80 years with heart failure. Eur J Cardiovasc Nurs. (2020) 19:172–7. doi: 10.1177/1474515119864970
38. Matsuo, Y, Kumakura, H, Kanai, H, Iwasaki, T, and Ichikawa, S. The geriatric nutritional risk index predicts long-term survival and cardiovascular or limb events in peripheral arterial disease. J Atheroscler Thromb. (2020) 27:134–3. doi: 10.5551/jat.49767
39. Kunimura, A, Ishii, H, Uetani, T, Aoki, T, Harada, K, Hirayama, K, et al. Impact of geriatric nutritional risk index on cardiovascular outcomes in patients with stable coronary artery disease. J Cardiol. (2017) 69:383–8. doi: 10.1016/j.jjcc.2016.09.004
40. Arikawa, R, Kanda, D, Ikeda, Y, Tokushige, A, Sonoda, T, Anzaki, K, et al. Prognostic impact of malnutrition on cardiovascular events in coronary artery disease patients with myocardial damage. BMC Cardiovasc Disord. (2021) 21:479. doi: 10.1186/s12872-021-02296-9
41. Detsky, AS, McLaughlin, JR, Baker, JP, Johnston, N, Whittaker, S, Mendelson, RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. (1987) 11:8–13.
42. Guigoz, Y, Vellas, B, and Garry, PJ. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. (1996) 54:S59–65. doi: 10.1111/j.1753-4887.1996.tb03793.x
43. Vellas, B, Guigoz, Y, Garry, PJ, Nourhashemi, F, Bennahum, D, Lauque, S, et al. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. (1999) 15:116–2. doi: 10.1016/S0899-9007(98)00171-3
44. Nelson, JJ, Liao, D, Sharrett, AR, Folsom, AR, Chambless, LE, Shahar, E, et al. Serum albumin level as a predictor of incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. (2000) 151:468–7. doi: 10.1093/oxfordjournals.aje.a010232
45. Pei, J, Wang, X, Chen, P, Zheng, K, and Hu, X. Hb Levels and sex differences in relation to short-term outcomes in patients with acute myocardial infarction. Front Cardiovasc Med (2021). 16:653351. doi:, et al.10.3389/fcvm.2021.653351
46. Narumi, T, Arimoto, T, Funayama, A, Kadowaki, S, Otaki, Y, Nishiyama, S, et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol. (2013) 62:307–3. doi: 10.1016/j.jjcc.2013.05.007
47. Cheng, YL, Sung, SH, Cheng, HM, Hsu, PF, Guo, CY, Yu, WC, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. (2017) 6:e004876. doi: 10.1161/JAHA.116.004876
48. Lee, M, Lim, JS, Kim, Y, Lee, JH, Kim, CH, Lee, SH, et al. Association between geriatric nutritional risk index and post-stroke cognitive outcomes. Nutrients. (2021) 13:1776. doi: 10.3390/nu13061776
49. Fan, Y, He, L, Zhou, Y, and Man, C. Predictive value of geriatric nutritional risk index in patients with coronary artery disease: a meta-analysis. Front Nutr. (2021) 8:736884. doi: 10.3389/fnut.2021.736884
50. Zhao, Q, Zhang, TY, Cheng, YJ, Ma, Y, Xu, YK, Yang, JQ, et al. Impacts of geriatric nutritional risk index on prognosis of patients with non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Nutr Metab Cardiovasc Dis. (2020) 30:1685–96. doi: 10.1016/j.numecd.2020.05.016
51. Tasbulak, O, Guler, A, Duran, M, Sahin, A, Bulut, U, Avci, Y, et al. Association between nutritional indices and long-term outcomes in patients undergoing isolated coronary artery bypass grafting. Cureus. (2021) 13:e16567. doi: 10.7759/cureus.16567
Keywords: MIMIC-IV database, cardiac intensive care unit, geriatric nutritional risk index, nutritional status, in-hospital mortality
Citation: Li Y, Wang Z, Sun T, Zhang B and Liang X (2023) Geriatric nutritional risk index was associated with in-hospital mortality among cardiac intensive care unit patients. Front. Nutr. 10:1218738. doi: 10.3389/fnut.2023.1218738
Edited by:
Silvia Lai, Sapienza University of Rome, ItalyReviewed by:
Hongli Gao, Capital Medical University, ChinaLuca Salomone, Sapienza University of Rome, Italy
Cheng-fu Cao, Peking University People's Hospital, China
Copyright © 2023 Li, Wang, Sun, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangwen Liang, bGlhbmd4aWFuZ3dlbjMxNUAxNjMuY29t
†These authors have contributed equally to this work
 Yuefeng Li
Yuefeng Li Zhengdong Wang1†
Zhengdong Wang1† Tienan Sun
Tienan Sun Biyang Zhang
Biyang Zhang