
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 30 June 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1218453
This article is part of the Research Topic Role of Nutrition in Skeletal Muscle Atrophy and Sarcopenia View all 6 articles
 Qiaoqiao Du1
Qiaoqiao Du1 Yanhui Lu2
Yanhui Lu2 Fan Hu2
Fan Hu2 Xinglin Feng3
Xinglin Feng3 Yunquan Zhang4
Yunquan Zhang4 Shaojie Li5
Shaojie Li5 Chi Zhang6
Chi Zhang6 Hua Zhang7
Hua Zhang7 Yi Zeng8
Yi Zeng8 Yao Yao5
Yao Yao5 Zhaohui Lu1
Zhaohui Lu1 Wenya Zhang1*
Wenya Zhang1* Xiangyang Gao9*
Xiangyang Gao9*Background: Sarcopenia is a common geriatric disease. Many dietary factors may contribute to the development of sarcopenia. Few studies have been conducted on dietary diversity and sarcopenia in Chinese older adults. Among a nationwide sample, the objective of this study is to assess the association between the dietary diversity score (DDS) and the prevalence of possible sarcopenia. We considered the different patterns of dietary diversity in relation to possible sarcopenia.
Methods: We conducted this analysis utilizing the cross-sectional data from the 2012, 2014, and 2018 waves of the Chinese longitudinal healthy longevity survey (CLHLS). A standard developed by the Asian Working Group for Sarcopenia 2019 (AWGS2019) was used to assess the possibility of sarcopenia. On the basis of the DDS generated by previous studies, we have constructed four new indicators as follows: total diet, animal-based diet, plant-based diet, and plant-based diet without the consumption of legume products and nuts. We used the generalized estimation equation (GEE) model to evaluate the associations between the DDS of the total diet, animal-based diet, plant-based diet, and plant-based diet without the intake of legume products and nuts and possible sarcopenia. These associations were statistically adjusted for a variety of potential confounders. Sensitivity analysis was performed by excluding some participants who were long-term bedridden, had Alzheimer's disease, or were terminally ill.
Results: The analysis included 6,624 participants (mean age 83.4 years at baseline). In our study, we found that participants with a higher DDS of the total diet (OR = 0.62; 95% CI: 0.51–0.77), animal-based diet (OR = 0.62; 95% CI: 0.49–0.79), and plant-based diet (OR = 0.64;95% CI: 0.51–0.80) were at a lower risk of developing sarcopenia. In sensitivity analyses, the associations remained unchanged.
Conclusion: Taking a diversified diet, including animal foods, may reduce the risk of developing sarcopenia. According to the findings of this study, adopting a diversified diet might reduce the risk of sarcopenia for older adults.
Sarcopenia is a common geriatric disease characterized by loss of skeletal muscle mass, low muscle strength, and/or low physical performance and is a serious public health problem faced by aging societies in the current times (1–4). The prevalence of this disease in the elderly population is largely influenced by factors, such as gender, age, pathological conditions, and diagnostic criteria (4). The results of a systematic review indicate that the prevalence ranges from 0.2 to 86.5% (0.3–91.2% in women and 0.4–87.7% in men), using all classifications for sarcopenia (5). The most commonly used classifications were the European Working Group on Sarcopenia in Older People (EWGSOP; prevalence range: 0.4–57.4%) and the Asian Working Group for Sarcopenia (AWGS; prevalence range: 0.3–53.0%) (2, 5, 6). In a meta-analysis, the overall prevalence of sarcopenia among individuals older than 60 years was estimated at 10–27% (5). While it is generally associated with advanced aging, muscle loss begins as early as 40 years of age and affects the elderly as they experience changes in body composition, including a reduction in muscle mass, deterioration of muscle quality, and an increase in fat mass (5, 7). It is necessary to screen out people who may suffer from sarcopenia at an early stage (8).
There is growing evidence that sarcopenia is closely related to a variety of adverse outcomes, including falls, functional disability, frailty, and mortality (9, 10). In clinical practice, case-finding is warranted when a patient exhibits signs or symptoms of sarcopenia (i.e., feeling faint, walking slowly, falling, or having difficulty getting up from a chair) (9). In accordance with the recommendations of the International Conference on Sarcopenia and Frailty Research (ICFSR), subjects who screen positive for sarcopenia should be referred for further evaluation to confirm their diagnosis (10). However, the diagnosis of sarcopenia must be based comprehensively on three aspects as follows: muscle quality, muscle strength, and muscle function (2). Most of these methods are time-consuming, laborious, need to be carried out by professionally trained staff, and are unsuitable for large-scale population surveys (8). AWGS 2019 has defined a new concept of “possible sarcopenia” with the aim of facilitating timely lifestyle intervention in the primary healthcare setting and in preventive services and preventing further development of sarcopenia, which will contribute to greater awareness of sarcopenia prevention and interventions across diverse healthcare settings (2, 11). Based on the AWGS criteria, 29.6% of older adults (≥60 years) were diagnosed with possible sarcopenia from China Health and Retirement Longitudinal Study (CHARLS) (12). A cross-sectional study showed that 61% of Iranian elderly (≥60 years) living in the community were considered to have possible sarcopenia judging by handgrip strength according to the EWGSOP2 (13). Early screening for sarcopenia is not only helpful in identifying individuals who may suffer from sarcopenia at an early stage but can also provide references for the development and prognosis of other diseases (8).
Sarcopenia is a condition common in older people who are not physically active, do not exercise, and do not obtain sufficient nutrition. Numerous dietary factors may contribute to the development of sarcopenia, according to a wide range of evidence, such as inadequate protein intake, deficiency of vitamin D, and lack of long-chain polyunsaturated fatty acids and antioxidant nutrients (4, 9). Sarcopenia is characterized by malnutrition, particularly protein-energy malnutrition. Several previous studies have demonstrated that inadequate protein intake (both vegetables and animal proteins) negatively affects muscle protein synthesis in older people (14–17). When coupled with a monotonous dietary pattern, food intake declines by ~25% between the ages of 40 and 70 years, which may result in inadequate nutrient intake (4). Previous studies have focused on the association between food components or types and sarcopenia, making it difficult to identify complex interactions and synergistic effects (4, 18).
To address the limitations of previous studies, it is necessary to study the overall diet (18). Dietary diversity is defined as “the number of different foods or food groups consumed during a given reference period” and assessed by the dietary diversity score (DDS) (19). DDS usually entails assigning scores to different food and evaluating dietary diversity based on the sum of those scores, with higher scores indicating dietary variety. An increase in individual DDS is associated with increased dietary intake of macronutrients and micronutrients (20, 21). DDS is a well-recognized indicator for evaluating diet quality and nutrition of food security status (21). The DDS can be widely used in large-scale studies and surveys (such as the Demographic and Health Survey) as a convenient and inexpensive index for assessing nutrient adequacy and overall dietary quality (19). The National Health and Nutrition Examination Survey (NHANES) concluded that DDS could be viewed as a convenient and fast indicator for assessing dietary diversity and observed that improved diet quality was associated with increased dietary diversity (19, 22). Previous studies have additionally demonstrated an association between DDS and chronic diseases, such as cardiovascular diseases, cancer, diabetes, and metabolic syndrome, as well as its correlation with improved health status and prolonged longevity (21–23). According to data from several investigations, higher DDS was also associated with better cognitive function, fewer physical limitations, and lower psychological stress in the elderly (24, 25).
Several studies have reported an association between diet and sarcopenia (7, 13, 18, 26–29). There is, however, a lack of evidence from developing countries, as most of these studies were based on data from developed countries (23). While older people are at a higher risk of sarcopenia, few studies have been conducted still on dietary diversity and sarcopenia in Chinese setting, which has the world's largest older people population. Based on nationwide data, the objective of this study is to assess the association between DDS and the prevalence of possible sarcopenia in Chinese older adults and to provide fundamental information for establishing dietary guidelines for preventing sarcopenia in the population.
Data from the CLHLS were utilized in this study. CLHLS was conducted in 23 provinces out of the 31 provinces in China using a multistage, stratified cluster sampling design. Details of the sampling strategy have been discussed in our previous studies (30, 31). The association between DDS and possible sarcopenia was examined using data from the waves in 2012, 2014, and 2018. A total of 32,831 participants participated in the survey in 2012 (n = 9,765), 2014 (n = 7,192), and 2018 (n = 15,874). A total of 274 individuals aged below 65 years and 25,933 individuals with missing or incomplete information regarding calf circumference, muscle strength, or physical performance were excluded. In this study, 6,624 adults were enrolled in the final analytical sample (Figure 1). Informed consent was obtained from all participants and/or their families, and the study was approved by the Ethics Committee of Peking University (IRB00001052-13074).
The DDS was developed according to the dietary guidelines for Chinese populations which give suggestions regarding the consumption of 10 food groups (23). At the baseline, DDS was measured using a food frequency questionnaire relating to the 10 primary food groups, which included cereals, vegetables, fruits, legume products, nuts, meat, eggs, fish, dairy products, and funguses (23, 32). Those who consume cereals, vegetables, and fruits “every day/virtually every day or frequently” will receive one point for that food group. Funguses, legume products, nuts, meat, eggs, fish, and dairy products consumed “virtually every day or at least once a week” would be assigned one point. We calculated DDS for each participant with a total score of 10 and further assessed different dietary diversity by using the animal food group and the plant food group according to the previous research (33, 34). There are four main sources of animal-enriched diet food as follows: meat, fish, eggs, and dairy products. Using the frequency of intake of animal food, we calculated the DDS of the animal-based diet, which ranged from 0 to 4. There are six sources of plant-based food as follows: cereals, vegetables, fruits, legume products, nuts, and funguses. Our plant-based DDS ranged from 0 to 6, based on the frequency of plant-based food consumption. We scored low on DDS of a protein plant-based diet by using cereals, vegetables, fruits, and funguses on a scale of 0–4, excluding legume products and nuts.
To identify and diagnose older adults with or at risk of sarcopenia, AWGS2019 has developed a set of criteria, including case-finding, assessment, and diagnostic protocols suitable for use in hospitals, research settings, and in community healthcare and screening (2). A possible sarcopenia assessment was conducted using the AWGS2019 standards, which include three components as follows: calf circumference, muscle strength, or physical performance (2). The grip strength of the dominant hand and the non-dominant hand was measured as hard as possible in each hand. Muscle strength was determined by measuring the grip strength of both hands twice, utilizing a digital grip strength dynamometer. A maximum reading was obtained from at least two trials using either both hands or the dominant hand. In accordance with the recommendations of AWGS 2019, men and women were deemed to have low grip strength at a weight of 28 and 18 kg, respectively (2). AWGS 2019 recommends calf circumference < 34 cm for men and < 33 cm for women for screening or case-finding (2, 35). Physical performance was assessed with the question “how do you stand up after sitting on a chair?” Respondents were asked to select from three responses: “no problem,” “with problem,” and “not able to do it.” Responses of “with problem” or “not able to do it” indicated low physical performance (35).
To obtain more reliable findings, we controlled for a broad set of potential confounders, which included gender (male or female), age groups (65–79, 80–99, and ≥100 years), education (0, 1–6, and ≥7 years), marital status (married/unmarried, divorced, or widowed), pre-retirement occupation (peasant or no-peasant), household income (< 100,000/≥100,000 yuan), smoking, drinking, and physical activity status (never/previous/current). Weight, height, and waist circumference were measured using standard anthropometric methods. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters. Biochemical indices, including vitamin D3 and albumin, were obtained from the analysis of blood samples.
The data were presented as mean ± SD or percentages. Group comparisons used the chi-squared tests for qualitative variables and the independent t-test for quantitative variables. To fill in the missing covariates, multiple imputation methods were used. The odds ratios (ORs) of possible sarcopenia with the DDS quartile were calculated using generalized estimation equation (GEE) models (working correlation matrix structure: AR). Model 1 was an unadjusted model. Model 2 further controlled for gender, age, household income, level of education, marital status, and occupation before retirement. In Model 3, physical activities, smoking, drinking, BMI, and waist circumference were additionally controlled, and in Model 4, vitamin D3 and albumin were additionally controlled. To examine the robustness of the estimation, we performed a sensitivity analysis by excluding older adults who were long-term bedridden, had Alzheimer's disease, or were terminally ill. IBM SPSS statistics version 21.0 for Windows was used to analyze the data. Forest plots were performed using MedCalc version 20. Statistical significance was determined by a two-tailed p-value of 0.05.
According to the sarcopenia status, the baseline characteristics of participants (n = 4,378) are shown in Table 1. The prevalence of possible sarcopenia was 66.9% (2,931/4,378). A total of 2,006 (41.8%) participants were men, 2,372 (58.2%) were women, and 1,835 (41.9%) were married. The participants were aged 83.4 ± 11.0 years, including 1,524 participants aged 65–79 years, 2,065 participants aged 80–99 years, and 789 participants aged ≥100 years. The overall education level of the participants was low, with only 9.9% of participants having received more than 7 years of education. A total of 2,767 (63.2%) participants were residing in rural areas and 4,094 (93.5%) had a household income of < 100,000 yuan. Of them, 18.8% smoked, 17.5% drank alcohol, and 22.0% did physical activities. Participants with possible sarcopenia had lower BMI, waist circumference, calf circumference, grip strength, vitamin D3, and albumin (all P < 0.001). The mean of DDS was 5.14 ± 1.76. DDS in the possible sarcopenia group was significantly lower than that in the non-sarcopenia group (P < 0.001; Table 1).
Based on the total DDS and classification of dietary sources of protein, GEE models were carried out to calculate the odds of possible sarcopenia. In the crude model, the participants who had the higher DDS (fourth quartile vs. the first quartile) showed lower odds of having possible sarcopenia, total diet (OR = 0.61; 95% CI: 0.52–0.72), animal-based diet (OR = 0.76; 95%CI: 0.67–0.87), plant-based diet (OR = 0.59; 95%CI: 0.49–0.71), and plant-based diet without legume products and nuts (OR = 0.49; 95% CI: 0.37–0.65) were significantly associated with decreased odds of possible sarcopenia. After adjustment for sex, age, household income, education level, marital status, and pre-retirement occupation in model 2, all associations were still significant. A further adjustment for physical activities, smoking, drinking, BMI, and waist circumference in model 3 revealed an inverse relationship between the odds of possible sarcopenia and total diet (OR = 0.66; 95% CI: 0.54–0.80), animal-based diet (OR = 0.63; 95% CI: 0.50–0.79), and plant-based diet (OR = 0.69; 95% CI: 0.55–0.86). No significant association was observed between a plant-based diet without legume products and nuts (OR = 0.81; 95% CI: 0.58–1.14) and possible sarcopenia. In the final model, which controlled all the confounding risk variables, total diet (OR = 0.62; 95%CI: 0.51–0.77), animal-based diet (OR = 0.62; 95% CI: 0.49–0.79), and plant-based diet (OR = 0.64; 95% CI: 0.51–0.80) were still associated with decreased odds of possible sarcopenia. A plant-based diet without legume products and nuts was not seemingly associated with possible sarcopenia (OR = 0.80; 95% CI: 0.57–1.13; Table 2 and Figure 2).
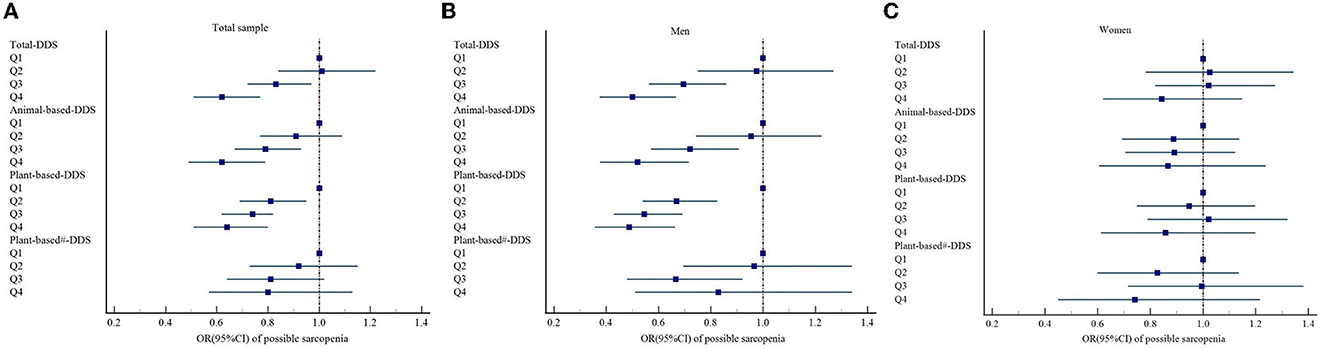
Figure 2. Forest plots of the association between DDS and possible sarcopenia based on Model 4 in (A) total sample, (B) men, and (C) women, respectively; Model 4: Adjusted for age, household income, education level, marital status, pre-retirement occupation, physical activities, smoking, drinking, BMI, waist circumference, vitamin D3, and albumin. DDS, dietary diversity score. #A plant-based diet without legume products and nuts.
According to the subgroup analyses using the full model (model 4) by sex, associations were statistically significant for men but not for women. As shown in Table 3, the subgroup analyses by sex revealed that higher DDS of the total diet (OR = 0.50; 95% CI: 0.38–0.67), animal-based diet (OR = 0.52; 95% CI: 0.38–0.72), and plant-based diet (OR = 0.49; 95% CI: 0.38–0.66) was still associated with a decreased risk of possible sarcopenia in male participants (Figure 2).
In a sensitivity analysis of the different DDSs, we obtained similar results using model 4. All the relationships between DDS (total diet, animal-based diet, plant-based diet, and plant-based diet without legumes products and nuts) and possible sarcopenia were consistent with model 4 in Table 2, after excluding participants who were long-term bedridden, had Alzheimer's disease, or were terminally ill (n = 118; Table 4).
Based on the findings of this study, it was found that the total DDS, the animal-based DDS, and the plant-based DDS were significantly lower in Chinese older people with possible sarcopenia than in those people without sarcopenia. In sensitivity analyses, these associations between DDS and possible sarcopenia remained unchanged among older adults. According to subgroup analyses, the association was only found in men but not in women with significance. However, the association between a plant-based diet without legume products and nuts and possible sarcopenia was not marked.
Our findings support evidence from several previous studies among older adults that diet has a great influence on sarcopenia, including dietary variety, dietary quality, and dietary patterns. These different approaches for the study of the overall diet are complementary; thus, it is difficult to determine the best approach. Dietary patterns commonly strike a balance between the health benefits of food and individuals' dietary habits. The evidence from nutritional epidemiology suggests that the Mediterranean diet was positively associated with muscle function, such as slowing the decrease in walking speed and the risk of developing a new mobility disability (28). People with a high diet quality usually have more diversified eating habits. In a systematic review of diet quality and sarcopenia in developing economies, diet quality defined by diversity and nutrient adequacy was found to be positively associated with the components of sarcopenia (muscle mass, muscle strength, and physical performance) (27). Although dietary variety analyses do not necessarily take into account the “healthfulness” of foods within food groups as diet quality or patterns (such as Mediterranean diet), most dietary guidelines recommend adding a variety of foods. Several prospective studies provided strong support that a higher DDS leads to an increase in handgrip strength and general gait speed and that adherence to a diverse diet is associated with a healthier aging process in elderly people (23, 36).
We also observed some variations in the association between different DDSs and possible sarcopenia, while the associations between DDS (total diet, animal-based diet, and plant-based diet) and possible sarcopenia were more pronounced. When constructing a DDS, the food groups included were different across studies and may contribute to the inconsistent findings, partly due to the study population and purpose (21, 32, 33, 37). In the literature, the classification of DDS was based on the US Department of Agriculture (USDA) food guide pyramid (five food groups), the Chinese Dietary Guideline (10 food groups), and the Food and Agriculture Organization (FAO) guidelines (9 food groups) (32, 34, 38). In our study, we calculated the DDS based on the dietary guidelines for Chinese populations, which additionally includes some food groups which play important roles in the Chinese diet. We also referred to previous research on the food classification of an animal/plant-based diet. Research on dietary diversity can consider the intake of a variety of foods and nutrients at the same time, effectively avoided the limitations imposed by specific nutrients and food (39), and has more practical significance and popularization value.
In sarcopenia, several areas were identified as essential in terms of diet as follows: protein, vitamin D, calcium, folate, and antioxidants (4, 40). Dietary protein intake has been consistently reported to be positively associated with muscle mass, independent of study designs and populations (41, 42). Our results of the protein-based diet were consistent with previous studies that observed a significant association between high protein intake and a reduced prevalence of possible sarcopenia after classifying participants by protein dietary diversity. Even though animal protein was generally considered to be more effective at stimulating muscle protein synthesis than plant protein, meta-analyses demonstrated that changes in absolute lean mass and muscle strength were not affected by the protein source (16). Low intake of dietary protein including plant-based protein was associated with a higher risk of low muscle mass in older adults (43).
Plant protein quality is dependent on the food source and can have an equivalent nutritional value to that of animal protein. A recent meta-analysis concluded that soybean protein resulted in similar muscle mass and strength gains, similar to those of animal protein, on the basis of moderation and dietary variety (44). Protein quality depends on the amino acid composition and its capacity to be digested, absorbed, and utilized to meet the body's needs (16). Animal protein was considered to be of “high quality” as it provides adequate amounts of all the essential amino acids and tends to be well-digested (16). There was evidence to suggest that leucine may activate the signaling pathways leading to protein synthesis (4). Leucine and its metabolites, such as β-methyl butyrate and β-hydroxy, have been demonstrated as important constituents in the fight against sarcopenia (43). A high proportion of leucine was considered to be necessary to reverse suboptimal muscle protein synthesis and increase lean body mass in the elderly (4). Therefore, it is possible that the intentional consumption of proteinaceous food may mitigate the risk of factors associated with sarcopenia.
We also observed some variations in the associations by sex. The association between DDS (total diet, animal-based diet, and plant-based diet) and possible sarcopenia was only found to be statistically significant in older men. Hormonal decline associated with aging was also probable to impact the loss of muscle mass, with a reduction in testosterone and estrogen levels in women (4). Testosterone has been demonstrated to function in the development, maintenance, or rejuvenation of muscle tissues (45). Age-related declines of such hormones were observed in sarcopenia patients (46). Since the women in our research were older, it is likely that they may have lost more muscle because they had been postmenopausal for years and had lower sex hormones. These results reinforced the preventive role of diet intervention in the premenopausal period (47). Other studies also showed that the proper intake of meat in men and the sufficient intake of milk in women significantly reduced the risk ratio of sarcopenia (7).
In our study, a plant-based diet consisting only of grain-combined fruits, vegetables, and funguses was not significantly associated with possible sarcopenia. Previous research showed that fruits and vegetables also play a role in preventing sarcopenia, but no such associations were observed in women (29, 48). Fruits and vegetables, the primary source of antioxidants, may reduce the risk of sarcopenia due to the catabolic effects of oxidative stress on the skeletal muscle (48). It is uncertain whether the antioxidant effects of fruits and vegetables are limited. In addition, longitudinal analyses from different regions or countries did not always yield similar results due to differences in food availability and food preparation methods between regions (18). Previous studies have shown that diet and its diverse components may play an important role in regulating inflammation. The inflammatory potential of a diet have direct and indirect effects on low muscle mass, grip strength, and muscle mass, through one and the other, in aging (49). The consumption of ultra-processed foods is strongly associated with frailty risk in older adults (50). An anti-inflammatory diet may indirectly reduce the risk of physical disabilities or frailty, by slowing age-related sarcopenia and muscle wasting (50). Promoting an anti-inflammatory diet has the potential to prevent sarcopenia early in life. Further studies are required to clarify the relationship between different dietary diversity and sarcopenia.
Therefore, improvement of diet quality through a varied and adequate intake of protein may be essential for the prevention of sarcopenia. The interaction and mutual supplement of nutrients in a balanced diet play an important role in protecting body functions. For older adults, it is necessary to distinguish between men's and women's food. While some evidence suggested the benefits of healthier dietary patterns, the role of a non-exercise nutritional intervention in treating sarcopenia was much less clear. Questions about the appropriate intake of key nutrients (such as protein) and how these nutrients should be taken in terms of timing and distribution throughout the day remain controversial (1). The nutritional guidelines for treating and/or preventing sarcopenia, formulated by the Society for Sarcopenia, Cachexia, and Wasting Disease (SCWD), recommend a protein consumption of at least 1.0–1.5 g/kg BW/day in combination with appropriate exercise to prevent the loss of muscle mass and function with age (51). They point to the potential for sex effects that require further exploration.
Our study has some strengths. First, we used a large nationally representative sample of older adults in China for our study. We included a variety of covariates which allowed us to adjust for major potential confounders that were measured in the study population. Second, we utilized DDS which provides an overview of the diet and does not impose any restrictions based on specific nutrients or foods. Moreover, we performed a sensitivity analysis by excluding participants who were long-term bedridden, had Alzheimer's disease, or were terminally ill, and the results remained stable. Regarding the study limitations, we could not completely exclude memory bias since dietary data were self-reported and might not accurately reflect actual food intake. Furthermore, the dietary diversity in this study was based on frequency, which is a semi-quantitative measure, and it did not demonstrate a relationship between quantitative dietary diversity and outcomes. Additionally, the outcome indicators were part of epidemiological investigations, which may be subjected to a measurement bias. Due to the limitations of measurement conditions, equipment, and time, the measurement of calf circumference and grip strength (physical function) was completed in only a few eligible areas. Moreover, we excluded older adults with poorer health status and cognitive function since they may refuse or be unable to complete the survey and physical examination. Finally, only 6,624 cases were included. Although the fact that our study adjusted for a wide set of confounding factors, it was not possible to completely exclude residual and unmeasured confounding. Because of our cross-sectional study design, the study cannot conclude causality.
In summary, higher DDS was associated with a reduced risk of sarcopenia in the elderly. Adoption of a diverse diet may provide a cost-effective intervention for the prevention of sarcopenia. Prospective and/or intervention studies are necessary to analyze the causal relationship between dietary variety and the risk of sarcopenia.
Publicly available datasets were analyzed in this study. This data can be found here: https://doi.org/10.18170/DVN/WBO7LK.
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University, Peking University, Beijing, China. The patients/participants provided their written informed consent to participate in this study.
YY, WZ, and XG contributed to the conception and design of the research. YL, FH, YZh, SL, CZ, and YZe contributed to data collection and acquisition. QD, XF, and HZ contributed to statistical analyses and interpretation. QD drafted the manuscript. YY, ZL, and XG revised the submitted version. All authors have read and agreed to approve the final manuscript.
We received funding from the National Key R&D Program of China (2020YFC2008905), the National High Level Hospital Clinical Research Funding (BJ-2022-133), Suzhou Kejiaoxingwei Youth Science and Technology Project (KJXW2021014), the National Natural Sciences Foundation of China (72061137004), and the U.S. National Institute of Aging/National Institute of Health (P01AG031719).
The authors are grateful to the CLHLS research team, the field team, and every respondent for the time and efforts that they have devoted to the CLHLS project, which provided the data for this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
2. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7 e2. doi: 10.1016/j.jamda.2019.12.012
3. Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. (2022) 44:101264. doi: 10.1016/j.eclinm.2021.101264
4. Papadopoulou SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. (2020) 12:1293. doi: 10.3390/nu12051293
5. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
6. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
7. Lim HS. Association of dietary variety status and sarcopenia in Korean elderly. J Bone Metab. (2020) 27:143–9. doi: 10.11005/jbm.2020.27.2.143
8. Xie WQ, Xiao GL, Hu PW, He YQ, Lv S, Xiao WF. Possible sarcopenia: early screening and intervention-narrative review. Ann Palliat Med. (2020) 9:4283–93. doi: 10.21037/apm-20-967
9. Cho MR, Lee S, Song SK. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J Korean Med Sci. (2022) 37:e146. doi: 10.3346/jkms.2022.37.e146
10. Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. (2018) 22:1148–61. doi: 10.1007/s12603-018-1139-9
11. Marcos-Pardo PJ, Gonzalez-Galvez N, Lopez-Vivancos A, Espeso-Garcia A, Martinez-Aranda LM, Gea-Garcia GM, et al. Sarcopenia, diet, physical activity and obesity in european middle-aged and older adults: the lifeage study. Nutrients. (2020) 13:8. doi: 10.3390/nu13010008
12. Hu Y, Peng W, Ren R, Wang Y, Wang G. Sarcopenia and mild cognitive impairment among elderly adults: the first longitudinal evidence from CHARLS. J Cachexia Sarcopenia Muscle. (2022) 13:2944–52. doi: 10.1002/jcsm.13081
13. Esmaeily Z, Tajary Z, Daei S, Rezaei M, Eyvazkhani A, Dorosty Motlagh AR, et al. Association between Healthy Eating Index-2015 scores and probable sarcopenia in community-dwelling Iranian older adults: a cross-sectional study. J Nutr Sci. (2021) 10:e20. doi: 10.1017/jns.2021.12
14. Putra C, Konow N, Gage M, York CG, Mangano KM. Protein source and muscle health in older adults: a literature review. Nutrients. (2021) 13:743. doi: 10.3390/nu13030743
15. Berrazaga I, Micard V, Gueugneau M, Walrand S. The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: a critical review. Nutrients. (2019) 11:1825. doi: 10.3390/nu11081825
16. Lim MT, Pan BJ, Toh DWK, Sutanto CN, Kim JE. Animal protein versus plant protein in supporting lean mass and muscle strength: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2021) 13:661. doi: 10.3390/nu13020661
17. Hevia-Larrain V, Gualano B, Longobardi I, Gil S, Fernandes AL, Costa LAR, et al. High-protein plant-based diet versus a protein-matched omnivorous diet to support resistance training adaptations: a comparison between habitual vegans and omnivores. Sports Med. (2021) 51:1317–30. doi: 10.1007/s40279-021-01434-9
18. Jang EH, Han YJ, Jang SE, Lee S. Association between diet quality and sarcopenia in older adults: systematic review of prospective cohort studies. Life. (2021) 11:811. doi: 10.3390/life11080811
19. Nachvak SM, Abdollahzad H, Mostafai R, Moradi S, Pasdar Y, Rezaei M, et al. Dietary diversity score and its related factors among employees of Kermanshah University of medical sciences. Clin Nutr Res. (2017) 6:247–55. doi: 10.7762/cnr.2017.6.4.247
20. Isabirye N, Bukenya JN, Nakafeero M, Ssekamatte T, Guwatudde D, Fawzi W. Dietary diversity and associated factors among adolescents in eastern Uganda: a cross-sectional study. BMC Public Health. (2020) 20:534. doi: 10.1186/s12889-020-08669-7
21. Moustakim R, Mziwira M, El Ayachi M, Belahsen R. Dietary diversity score and the incidence of chronic kidney disease in an agricultural Moroccan adults population. Rocz Panstw Zakl Hig. (2022) 73:293–301. doi: 10.32394/rpzh.2022.0221
22. Vadiveloo M, Dixon LBM, T., Elbel B, Parekh N. Dietary variety is inversely associated with body adiposity among US adults using a novel food diversity index. J Nutr. (2015) 145:555–63. doi: 10.3945/jn.114.199067
23. Zhang J, Zhao A. Dietary diversity and healthy aging: a prospective study. Nutrients. (2021) 13:1787. doi: 10.3390/nu13061787
24. Zhang J, Zhao A, Wu W, Yang C, Ren Z, Wang M, et al. dietary diversity is associated with memory status in chinese adults: a prospective study. Front Aging Neurosci. (2020) 12:580760. doi: 10.3389/fnagi.2020.580760
25. Y Yokoyama MN, Murayama H, Amano H, Taniguchi Y, Nofuji Y, Narita M, et al. Association of dietary variety with body composition and physical function in community-dwelling elderly Japanese. J Nutr Health Aging. (2016) 20:691–6. doi: 10.1007/s12603-015-0632-7
26. Bagheri A, Soltani S, Hashemi R, Heshmat R, Motlagh AD, Esmaillzadeh A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr J. (2020) 19:129. doi: 10.1186/s12937-020-00649-2
27. Ramadas A, Law HH, Krishnamoorthy R, Ku JWS, Mohanty P, Lim MZC, et al. Diet quality and measures of sarcopenia in developing economies: a systematic review. Nutrients. (2022) 14:868. doi: 10.3390/nu14040868
28. Granic A, Sayer AA, Robinson SM. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients. (2019) 11:745. doi: 10.3390/nu11040745
29. Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet quality and sarcopenia in older adults: a systematic review. Nutrients. (2018) 10:308. doi: 10.3390/nu10030308
30. Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. (2017) 389:1619–29. doi: 10.1016/S0140-6736(17)30548-2
31. Yao Y, Chen H, Chen L, Ju SY, Yang H, Zeng Y, et al. Type of tea consumption and depressive symptoms in Chinese older adults. BMC Geriatr. (2021) 21:331. doi: 10.1186/s12877-021-02203-z
32. Meng L, Wang Y, Li T, Loo-Bouwman CAV, Zhang Y, Man-Yau Szeto I. Dietary diversity and food variety in Chinese children aged 3(-)17 years: are they negatively associated with dietary micronutrient inadequacy? Nutrients. (2018) 10:1674. doi: 10.3390/nu10111674
33. Doustmohammadian A, Amirkalali B, Gholizadeh E, Khoonsari M, Faraji AH, Nikkhah M, et al. Mediators of dietary diversity score (DDS) on NAFLD in Iranian adults: a structural equation modeling study. Eur J Clin Nutr. (2023) 77:370–9. doi: 10.1038/s41430-022-01240-0
34. Bahrami A, Shirani P, Sohouli M, Nasab SJ, Rafiee P, Naja F, et al. Dietary diversity score (DDS) and odds of colorectal cancer and adenoma: a case-control study. J Nutr Sci. (2022) 11:e34. doi: 10.1017/jns.2022.30
35. Tan JY, Zeng QL, Ni M, Zhang YX, Qiu T. Association among calf circumference, physical performance, and depression in the elderly Chinese population: a cross-sectional study. BMC Psychiatry. (2022) 22:278. doi: 10.1186/s12888-022-03925-z
36. Yokoyama Y, Murayama H, Amano H, Taniguchi Y, Nofuji Y, Narita M, et al. Dietary variety and decline in lean mass and physical performance in community J Nutr Health Aging. (2017) 21:11–6. doi: 10.1007/s12603-016-0726-x
37. Alenko A, Agenagnew L, Beressa G, Tesfaye Y, Woldesenbet YM, Girma S. COVID-19-related anxiety and its association with dietary diversity score among health care professionals in ethiopia: a web-based survey. J Multidiscip Healthc. (2021) 14:987–96. doi: 10.2147/JMDH.S305164
38. Rezazadegan M, Mirjalili F, Jalilpiran Y, Aziz M, Jayedi A, Setayesh L, et al. The association between dietary diversity score and odds of diabetic nephropathy: a case-control study. Front Nutr. (2022) 9:767415. doi: 10.3389/fnut.2022.767415
39. Lv X, Sun S, Wang J, Chen H, Li S, Hu Y, et al. Anti-inflammatory dietary diversity and depressive symptoms among older adults: a nationwide cross-sectional analysis. Nutrients. (2022) 14:5062. doi: 10.3390/nu14235062
40. Zupo R, Moroni A, Castellana F, Gasparri C, Catino F, Lampignano L, et al. A machine-learning approach to target clinical and biological features associated with sarcopenia: findings from northern and southern Italian aging populations. Metabolites. (2023) 13:565. doi: 10.3390/metabo13040565
41. Yoo JI, Lee KH, Choi Y, Lee J, Park YG. Poor dietary protein intake in elderly population with sarcopenia and osteosarcopenia: a nationwide population-based study. J Bone Metab. (2020) 27:301–10. doi: 10.11005/jbm.2020.27.4.301
42. Ganapathy A, Nieves JW. Nutrition and sarcopenia-what do we know? Nutrients. (2020) 12:1755. doi: 10.3390/nu12061755
43. Huang RY, Yang KC, Chang HH, Lee LT, Lu CW, Huang KC. The association between total protein and vegetable protein intake and low muscle mass among the community-dwelling elderly population in Northern Taiwan. Nutrients. (2016) 8:373. doi: 10.3390/nu8060373
44. Messina M, Lynch H, Dickinson JM, Reed KE. No difference between the effects of supplementing with soy protein versus animal protein on gains in muscle mass and strength in response to resistance exercise. Int J Sport Nutr Exerc Metab. (2018) 28:674–85. doi: 10.1123/ijsnem.2018-0071
45. Ardeljan A. D., Hurezeanu R. (2023). “Sarcopenia,” in StatPearls (Treasure Island, FL: StatPearls Publishing). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK560813/
46. Shin MJ, Jeon YK, Kim IJ. Testosterone and sarcopenia. World J Mens Health. (2018) 36:192–8. doi: 10.5534/wjmh.180001
47. Cuesta-Triana F, Verdejo-Bravo C, Fernandez-Perez C, Martin-Sanchez FJ. Effect of milk and other dairy products on the risk of frailty, sarcopenia, and cognitive performance decline in the elderly: a systematic review. Adv Nutr. (2019) 10(Suppl. 2):S105–9. doi: 10.1093/advances/nmy105
48. Kim J, Lee Y, Kye S, Chung YS, Kim KM. Association of vegetables and fruits consumption with sarcopenia in older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing. (2015) 44:96–102. doi: 10.1093/ageing/afu028
49. Zupo R, Watanabe M, De Pergola G, Castellana F. Editorial: nutrition and diet practices: impact on body components and functioning. Front Endocrinol. (2023) 14:1179751. doi: 10.3389/fendo.2023.1179751
50. De Nucci S, Zupo R, Donghia R, Castellana F, Lofu D, Aresta S, et al. Dietary profiling of physical frailty in older age phenotypes using a machine learning approach: the Salus in Apulia Study. Eur J Nutr. (2023) 62:1217–29. doi: 10.1007/s00394-022-03066-9
Keywords: dietary diversity, possible sarcopenia, plant-based diet, older adults, China
Citation: Du Q, Lu Y, Hu F, Feng X, Zhang Y, Li S, Zhang C, Zhang H, Zeng Y, Yao Y, Lu Z, Zhang W and Gao X (2023) Dietary diversity and possible sarcopenia among older people in China: a nationwide population-based study. Front. Nutr. 10:1218453. doi: 10.3389/fnut.2023.1218453
Received: 07 May 2023; Accepted: 05 June 2023;
Published: 30 June 2023.
Edited by:
Diego Augusto Santos Silva, Federal University of Santa Catarina, BrazilReviewed by:
Carlo Pedrolli, Azienda Provinciale per i Servizi Sanitari (APSS), ItalyCopyright © 2023 Du, Lu, Hu, Feng, Zhang, Li, Zhang, Zhang, Zeng, Yao, Lu, Zhang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangyang Gao, MTM4MTExMzA4MDhAMTI2LmNvbQ==; Wenya Zhang, end5MTkwM0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.