
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 21 September 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1218166
This article is part of the Research Topic Value-Based Nutritional Intervention to Reduce the Progression of Chronic Human Diseases View all 14 articles
Background: Hyperuricemia is a common condition that can lead to gout and other related diseases. It has been suggested that Inflammatory factors play important role in the development and progression of hyperuricemia. The dietary inflammatory index (DII) enables the assessment of the inflammatory potential of an individual’s diet. This study aimed to investigate the association between DII and hyperuricemia.
Methods: This study was performed based on a cross-sectional dataset from the National Health and Nutrition Examination Survey (NHANES) 2005–2018. Participants aged 18 years and above with dietary intake and serum uric acid level information were included. DII scores were calculated using dietary intake data, based on which participants were categorized into tertiles. Multivariable logistic regression analysis was adopted to investigate the association between DII and hyperuricemia.
Results: Among a total of 31,781 participants in the analysis, 5,491 had hyperuricemia. After adjusting confounding factors, the odds of hyperuricemia are significantly higher in the second (OR 1.17, 95% CI 1.07–1.29) and third tertiles (OR 1.31, 95% CI 1.19–1.44) relative to the first one.
Conclusion: This study suggested that diet with higher inflammatory potential, as measured by DII, is associated with increased hyperuricemia risk. These findings indicated that dietary modification may be a potential approach for hyperuricemia’s prevention and control.
Hyperuricemia is defined as the overproduction or under-excretion of uric acid, and it tops the list of global disease burdens associated with gout and a wide spectrum of other diseases, affecting patients of all ages and sexes (1, 2). It is an independent risk factor for various systemic diseases, including gout, cardiovascular diseases, hypertension, chronic kidney disease, and many others (3). Up to 2016, the global prevalence of hyperuricemia has hit 21% (4). Its prevalence in U.S. ranged from 14.6 to 20% (5) and showed a tendency to affect younger people (38.6 ± 11.8 years), even non-obese adults (6, 7).
Unhealthy eating habit is closely related to hyperuricemia since its influence on inflammation (8). For instance, Western dietary patterns characterized by high caloric content and significant fat levels have been linked to an increased presence of inflammatory markers in the body (9). Correspondingly, anti-inflammatory diets could reduce this level, and dietary management targeting at asymptomatic hyperuricemia may be of great benefit (10).
Since inflammation is an essential factor for hyperuricemia, measuring inflammation level might be useful for the prediction of and protection against hyperuricemia. The Dietary Inflammatory Index (DII) was firstly proposed by Shivappa et al. in 2009 based on published literatures (11) and was updated in 2014 (12). It specifically aimed to measure dietary inflammation potential. DII, as is closely related to the expression of blood inflammatory markers, has been widely used in the investigation of the association between inflammation caused by diet and the onset and progression of diverse ailments (13).
Up to now, there are only two studies on the association between DII and hyperuricemia. One was a cross-sectional study in China, whose results showed that higher DII scores were associated with higher hyperuricemia risk after covariates adjustment (14). The other was a case–control study in Korea, which reported that higher pro-inflammatory dietary intake was significantly associated with the risk of hyperuricemia only in males (6). However, both studies focused on Asian population, and can only provide limited reference for U.S. population due to the differences between Eastern and Western dietary habits (15). This study aimed to explore such a relationship in U.S. population.
The National Health and Nutrition Examination Survey (NHANES) is a sweeping cross-sectional study administered by the National Center for Health Statistics. Its objective is to compile crucial data about individuals’ health conditions by conducting a range of interviews, physical assessments, and laboratory examinations. To ensure an accurate representation of the overall U.S. population, a sophisticated multistage sampling technique was employed for this particular survey. In order to evaluate the dietary patterns of NHANES participants, two comprehensive interviews were conducted to obtain dietary-recall information. Specifically, the first interview was implemented face-to-face by highly trained dietary interviewers in NHANES Mobile Examination Center (MEC), while the second interview was conducted over phone three to ten days after the MEC interview (the next week of MEC interview). During the dietary-recall interviews, U.S. Department of Agriculture Automated Multiple-Pass Method dietary interview approach was used to collect information on food intake. Participants utilized measuring guides during in-person interviews while a food model booklet was used during the telephone interviews to quantify food. The methodology and materials used for the survey underwent ethical review and were approved by the Ethics Review Board of the National Center for Health Statistics. Further, prior to participating in the survey, written informed consent was obtained from all individuals involved. Data from NHANES 2005–2018 cycle were selected in this study, including a total of 70,190 participants initially. Exclusion criteria were as follows: (a) participants without dietary data for DII calculation (n = 9,549), (b) participants with missing uric acid data (n = 18,995), (c) individuals under the age of 18 years old (n = 6,267), (d) pregnant individuals (n = 642), and (e) individuals with missing estimated glomerular filtration rate (eGFR) or eGFR values less than 60 mL/min/1.73 m (2) (n = 2,956). Ultimately, 31,781 participants were included in the analysis, as depicted in Figure 1.
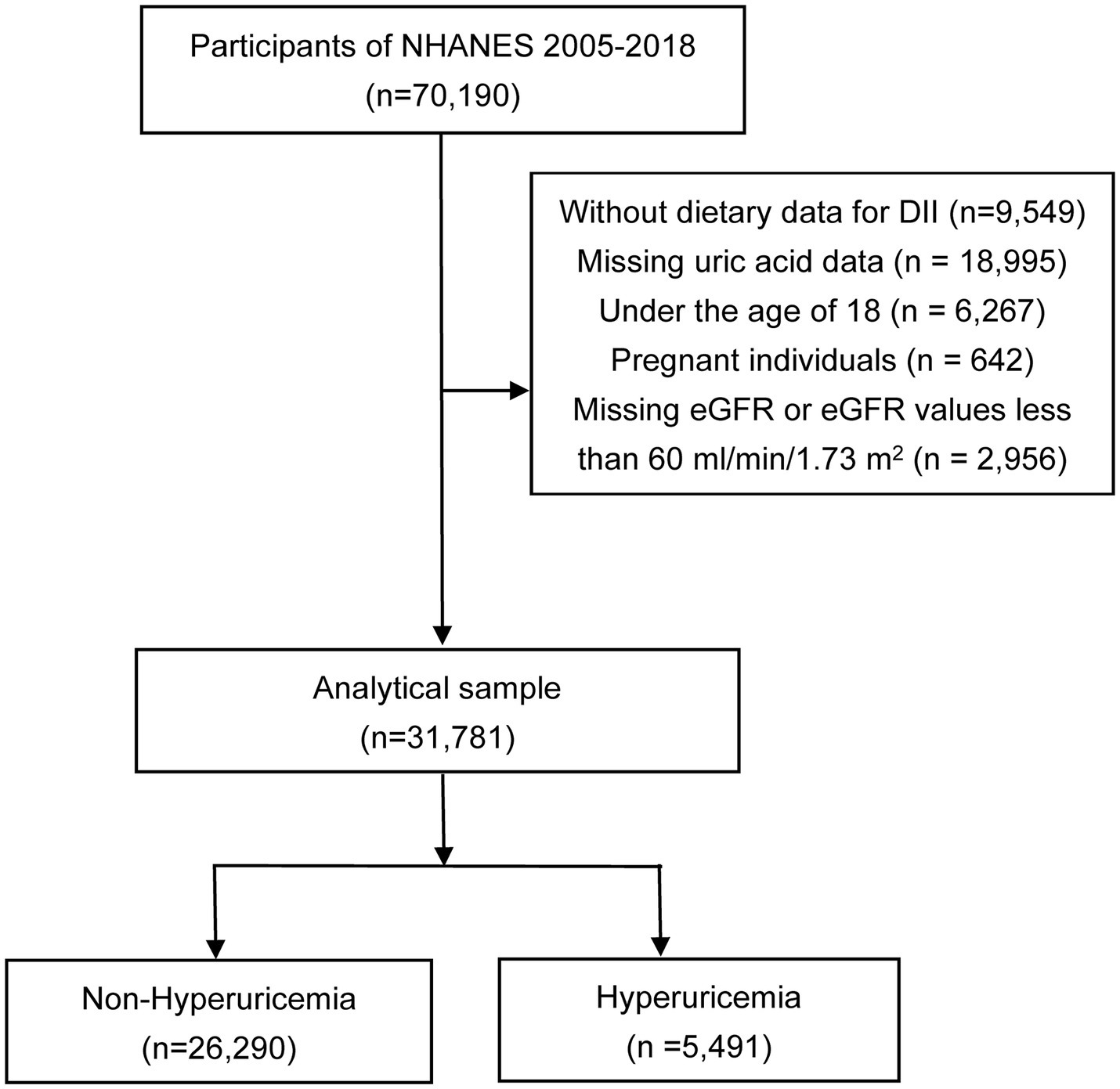
Figure 1. Flowchart of the study. NHANES, the National Health and Nutrition Examination Survey; DII, dietary inflammatory index; eGFR, estimated glomerular filtration rate.
DII developed by Shivappa et al. was computed to measure the inflammatory potential of different dietary patterns (12). DII calculation was conducted by a standardized global database that contains daily dietary intake information of 11 regionally representative populations. Both the standard mean and standard deviation were provided for all DII food parameters from the world database. To ensure that the investigation is thorough and accurate, a scoring system was created. A score of “+1” is given to dietary components that increase the levels of CRP, TNF-a, IL-1b, and IL-6, or decrease the levels of IL-4 and IL-10. Conversely, a score of “−1” is assigned to dietary components that reduce the levels of CRP, TNF-a, IL-1b, and IL-6, or increase the levels of IL-4 and IL-10. This comprehensive approach allows for a more detailed examination of how dietary components affect these specific indicators. These values were weighted according to the study design. The z-score for each food parameter was calculated by subtracting the standard mean from the value of consumption reported by each individual and then dividing that result by the standard deviation. These z-scores were transformed into proportions (ranging from 0 to 1) to minimize the effect of positive skewing. To obtain a symmetrical distribution centered around zero with bounds between −1 and + 1, each proportion was doubled, and then 1 was subtracted. This value was then multiplied by the corresponding inflammatory effect score for each food parameter. In this study, there were 28 parameters available in NHANES data that could be utilized to calculate DII, including energy, protein, carbohydrate, dietary fiber, total fatty acid, total saturated fatty acid, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), cholesterol, β-carotene, niacin, folate, magnesium, iron, zinc, selenium, caffeine, alcohol, n3 polyunsaturated fatty acid, n6 polyunsaturated fatty acid, and vitamins A, B1, B2, B6, B12, C, D, and E. An elevated DII score signifies the consumption of a diet that triggers inflammation, whereas a reduced score indicates the adoption of an anti-inflammatory diet.
This investigation was primarily focused on hyperuricemia condition, which is characterized by elevated uric acid level in bloodstream. The measurement of serum uric acid level was carried out using the Beckman UniCel® DxC800 Synchron or Beckman Synchron LX20 (Beckman Coulter, Inc., Brea, CA, United States), which employs an oxidizing process to convert uric acid to allantoin and H2O2. Following established diagnostic standards, hyperuricemia was delineated as a serum uric acid threshold of 7.0 mg/dL or more in males and 6.0 mg/dL or more in females (16).
In this study, demographic, lifestyle variables, physical measurements, laboratory tests, and self-reported health status were assessed in a computer-assisted personal interview. Demographic information includes age, sex, race/ethnicity, and educational level; health status contains smoking, drinking, physical activity, and disease history (hypertension, diabetes and hyperlipidemia); physical health examination involves height, body mass, and blood pressure measurements; and laboratory tests covers uric acid, serum glucose level.
The smoking status was divided into three distinct groups for this study. The first group consisted of individuals who had never smoked, meaning that they had smoked less than 100 cigarettes in their entire lifetime. The second group comprised individuals who were former smokers, having smoked more than 100 cigarettes in their lifetime and subsequently quit smoking at the time of the survey. Finally, the third group consisted of current smokers, who had smoked more than 100 cigarettes in their lifetime and continued to smoke at least every few days. Current drinking was classified into three categories: heavy drinking (≥3 drinks per day for females; ≥4 drinks per day for males; binge drinking on 5 or more days per month), moderate drinking (≥2 drinks per day for females; ≥3 drinks per day for males; binge drinking ≥2 days per month), and mild drinking (others). To determine the individual’s metabolic equivalent of task (MET)/week, a calculation was performed by multiplying the total number of minutes spent on various activities during the week by the metabolic equivalents estimated by the Compendium of Physical Activities. This approach allowed for an accurate assessment of the intensity and frequency of the individual’s physical activity over the course of the week. By utilizing the Compendium of Physical Activities, which provides standardized estimates of metabolic equivalents for various activities, the calculation was based on scientifically informed data and avoided potential inaccuracies and bias. The level of physical activity was determined in terms of hours of activity per week (MET/week), and results were divided into three groups: low (<600 METs/week), moderate (600–1,199 METs/week), and vigorous (≥1,200 METs/week). The eGFR was determined through the application of the Chronic Kidney Disease Epidemiology Collaboration creatinine equation. This formula calculates eGFR values based on specific parameters for both male and female. For male, the equation is as follows: eGFR = (140-age) × body weight (kg) × 1.23/creatinine (mmol/L). Similarly, for female, the equation is: eGFR = (140-age) × body weight (kg) × 1.03/creatinine (mmol/L). Hypertension was defined as blood pressure ≥ 140/90 mmHg, a diagnosis of hypertension, or a prescription of antihypertensive drugs in health questionnaire. Diabetes was diagnosed when patients met one or more of the following criteria: (1) a medical diagnosis of diabetes as recorded by the patient’s healthcare provider (“doctor told you have diabetes”), (2) glycohemoglobin A1c (HbA1c) level of greater than 6.5%, (3) a fasting blood glucose level of equal to or greater than 7.0 mmol/L, (4) a random blood glucose level of equal to or greater than 11.1 mmol/L, or (5) a two-hour blood glucose level of equal to or greater than 11.1 mmol/L following an oral glucose tolerance test (OGTT). Hyperlipidemia was defined by TG levels equal to or greater than 150 mg/dL, hypercholesterolemia, or medication that lowers lipid levels. Individuals who met any of the following criteria were considered to have hypercholesterolemia: (1) TC levels equal to or greater than 200 mg/dL, (2) LDL-C levels equal to or greater than 130 mg/dL, or (3) HDL-C levels less than 40 mg/dL for males and less than 50 mg/dL for females.
We initiated our analysis by comparing the baseline data. Based on sex, participants were divided into two groups according to the presence of hyperuricemia for baseline characteristic analysis. Given that different components of the DII might exhibit varying impacts on inflammation and could demonstrate exponential growth or decline, we presented the distribution of different DII components among different groups in the form of medians and interquartile ranges. Mean ± standard deviation was used to report continuous variables, while percentages were used to represent categorical variables. For variables with a normal distribution, analysis was conducted using Student’s t-test or chi-squared test. When variables exhibited skewed distribution, non-parametric tests or Fisher’s exact probability test were employed for analysis. Furthermore, a multivariable logistic regression model was utilized in both the overall population and sex-stratified subgroups to estimate odds ratios and 95% confidence intervals. The DII score was included in the model as an independent variable, both in continuous and tertile forms (T1 (−5.28 to 0.79, n = 10,594), T2 (0.79 to 2.60, n = 10,593), T3 (2.60 to 5.79, n = 10,594)), to explore potential correlations with hyperuricemia. The transformation of DII into tertile variables aims to assess whether DII exhibits correlations with hyperuricemia across different variable type states. Additionally, linear trend tests were conducted to assess linear relationships, and a Generalized Additive Model (GAM) along with smoothed curve fitting and a two-part logistic regression model were employed to explore nonlinear correlations. Subgroup and interaction analyses were performed for covariates such as sex, age, race/ethnicity, hypertension, diabetes, and BMI, while controlling for potential confounding factors. All statistical analyses were conducted using R (version 3.5.3) and EmpowerStats,1 with statistical significance defined as p < 0.05.
Table 1 displays the baseline characteristics based on sex for the hyperuricemia and non-hyperuricemia groups. The male group comprises 15,828 individuals, while the female group consists of 15,953 individuals. Among males, age (p = 0.067), METs/week (p = 0.355), and diabetes (p = 0.837) showed no statistically significant differences between the hyperuricemia and non-hyperuricemia groups. However, there were statistically significant differences between the groups in terms of race/ethnicity, education level, smoking, alcohol consumption, BMI, hypertension, hyperlipidemia, and eGFR. Among males, those with lower education levels, higher BMI, higher blood pressure, lower eGFR, history of smoking, moderate-to-heavy alcohol consumption, and higher lipid levels were more prone to hyperuricemia. Furthermore, among the female population, statistically significant differences were observed among all variables.
Additionally, Table 2 presents the scores for each component of the DII based on sex-specific grouping for the hyperuricemia and non-hyperuricemia groups. From Table 2, it can be observed that, for both males and females, there are no statistically significant differences in cholesterol intake and caffeine intake between the groups. Among males, there are no statistically significant differences in vitamin B6 and niacin intake between the hyperuricemia and non-hyperuricemia groups. Among females, there are no statistically significant differences in β-Carotene intake between the groups. However, for the other components of the DII, statistically significant differences are observed between the hyperuricemia and non-hyperuricemia groups for both males and females.
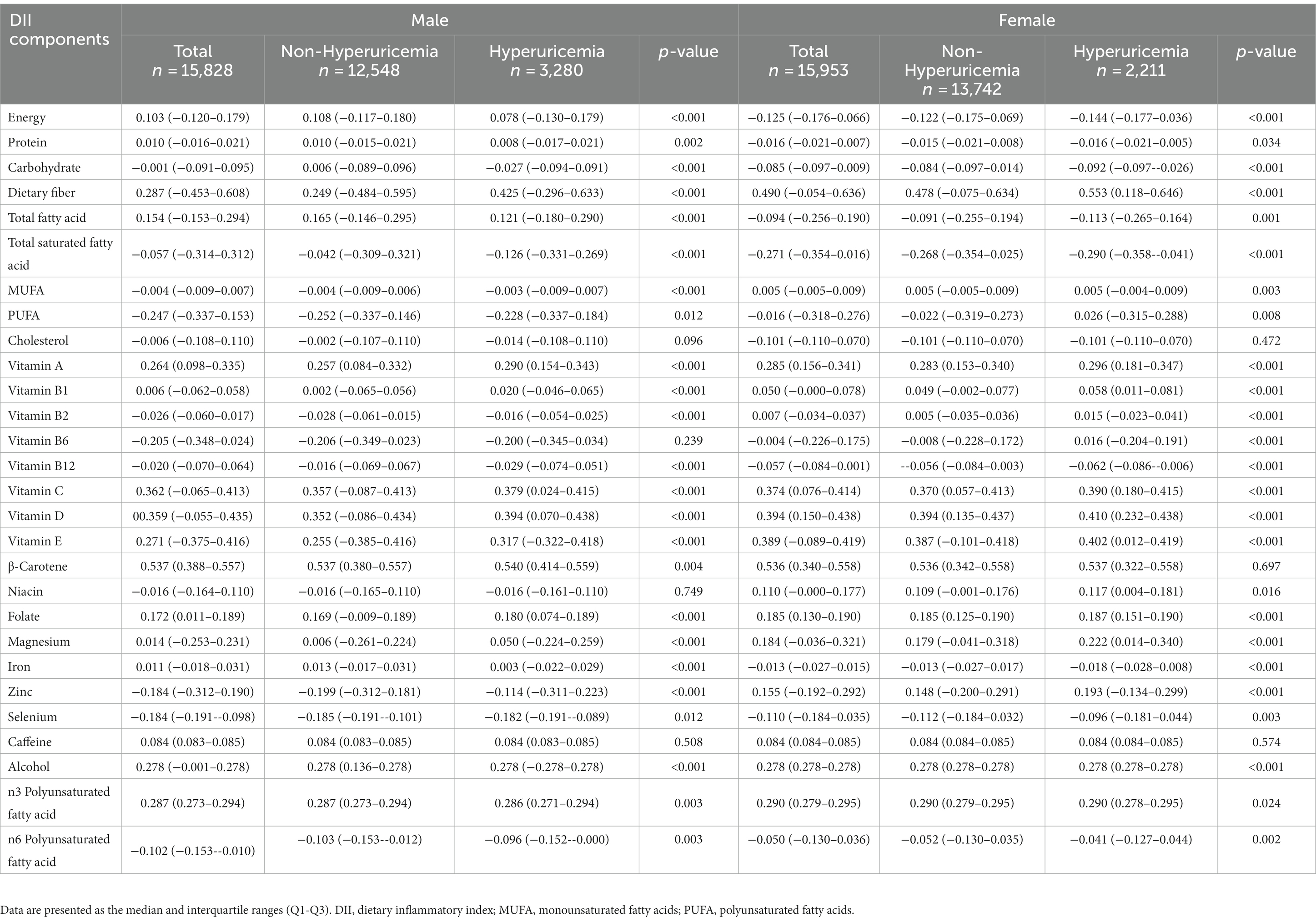
Table 2. Comparison of each component of DII scores between individuals with hyperuricemia and individuals without hyperuricemia in different sexes.
The logistic regression modeling results shown in Table 3 indicates the association between DII score and hyperuricemia. After adjusting for all covariates (age, sex, BMI, race/ethnicity, educational level, smoking, drinking, MET, eGFR, diabetes, hypertension, and hyperlipidemia), a positive association was established between these two (OR 1.06, 95% CI 1.04–1.09). The second and third tertile groups showed higher odds than the first tertile (T2: OR 1.17, 95% CI 1.07–1.29; T3: OR 1.31, 95% CI 1.19–1.44), which was also confirmed by the trend test (p<0.05). At the same time, we also conducted sex based stratified analysis and found that the relationship between DII and hyperuricemia remained unchanged among different sexes. Additionally, we used a GAM and a smooth curve fit to evaluate the correlation between them and evaluated their nonlinearity using a two part logistic regression model. When DII was treated as a continuous variable, a positive correlation was observed between DII and uric acid (Figure 2A). When DII was treated as a categorical variable with three tertiles, the relationship between DII and uric acid remained unchanged (Figure 2B). The results of a two part logistic regression model also show that there is no nonlinear relationship between DII and uric acid (Supplementary Table S1).
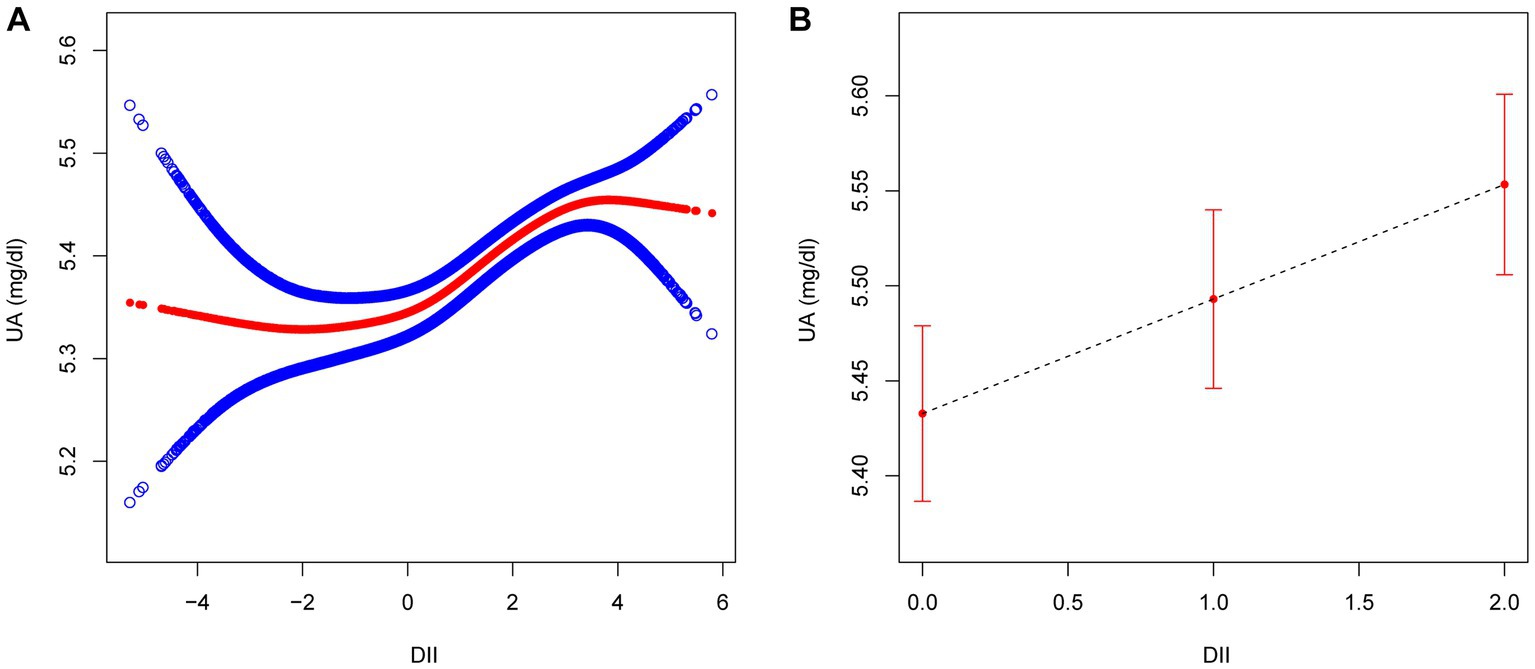
Figure 2. The association between DII ((A) as continuous variable; (B) as categorical variable) and UA. Age, sex, BMI, race/ethnicity, educational level, smoking, drinking, MET, eGFR, diabetes, hypertension, and hyperlipidemia were adjusted. DII, dietary inflammatory index; UA, uric acid; BMI, body mass index; MET, metabolic equivalent of task; eGFR, estimated glomerular filtration rate.
The forest plot illustrates interactions between DII and hyperuricemia concerning age and diabetes (p < 0.05). Borderline significance levels for interactions in hypertension and BMI are observed at a p value of 0.05. However, interactions between DII and hyperuricemia are not significant for sex and race/ethnicity (p > 0.05) (Figure 3).
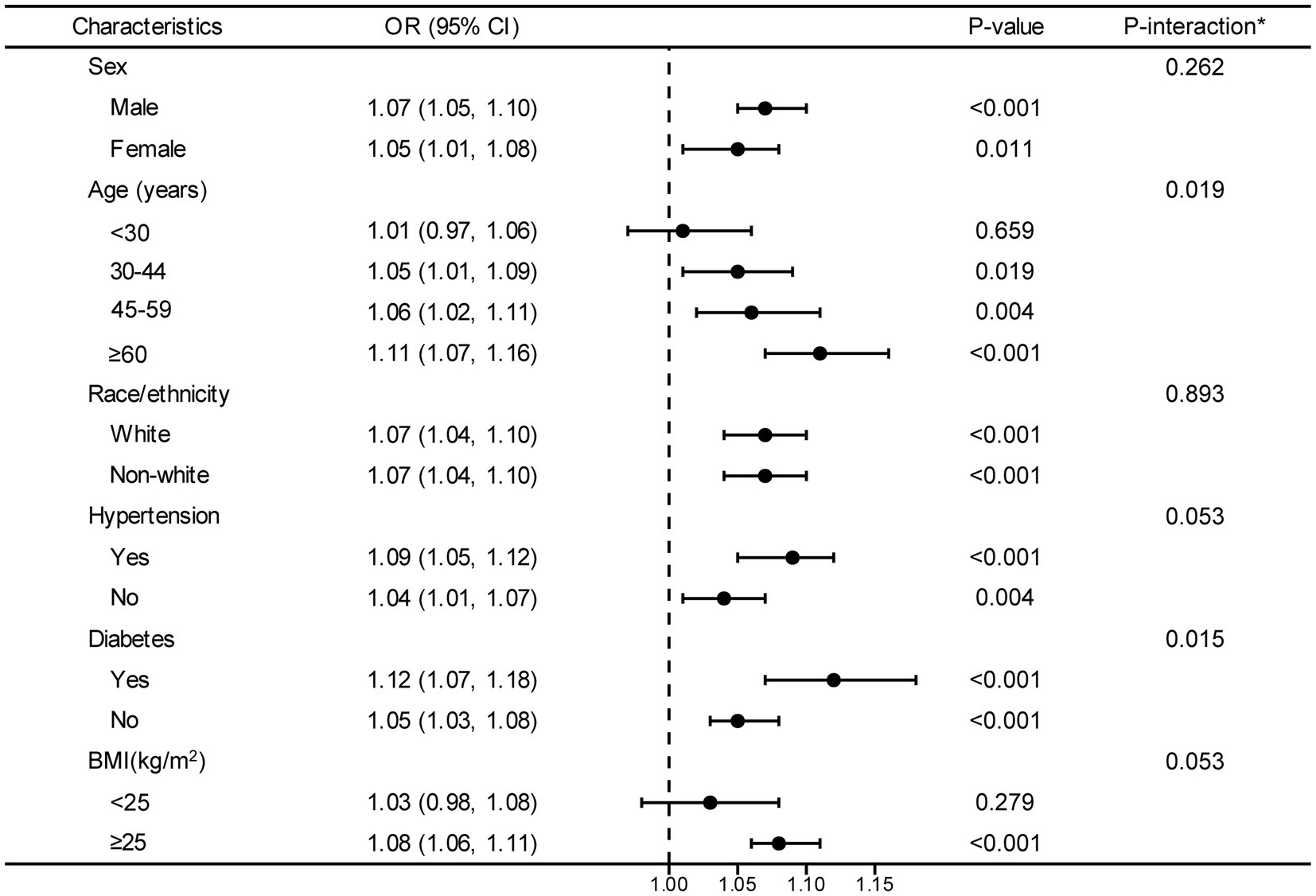
Figure 3. Stratified analyses between DII and hyperuricemia. OR values are based on different population stratifications, estimated using a multivariable logistic regression model to assess the odds ratios and 95% confidence intervals between DII and hyperuricemia. The first column of p-values represents the significance of OR values after stratification by different population groups. The second column of P-values pertains to the significance of models considering interaction terms between DII and various variables (sex, age, race/ethnicity, hypertension, diabetes and BMI). *Each stratification adjusted for all the factors (age, sex, BMI, race/ethnicity, educational level, smoking, drinking, MET, eGFR, diabetes, hypertension, and hyperlipidemia) except the stratification factor itself. OR, odd ratio; CI, confidence interval; DII, dietary inflammatory index; BMI, body mass index; MET, metabolic equivalent of task; eGFR, estimated glomerular filtration rate.
In this cross-sectional study using NHANES data, we found that DII, which is an indicative index for pro-inflammatory diet, was in significant positive association with hyperuricemia after adjusting for multiple covariates. Compared with the lowest tertile subgroup of the DII, the highest tertile subgroup of the DII increased the risk of hyperuricemia by 31% for all participants, including 37 and 23% for male and female, respectively. Likewise, this positive correlation has also been validated in the GAM and through smooth curve fitting.
The results between the DII components and hyperuricemia showed that both male and female, the hyperuricemia group had higher intakes of dietary fiber, PUFA, vitamin A, vitamin B1, vitamin B2, vitamin B12, vitamin C, vitamin D, vitamin E, folate, magnesium, zinc, selenium, and n6 polyunsaturated fatty acids, as compared with the non-hyperuricemia group. In addition, female participants had higher levels of vitamin B6 and niacin intake in the hyperuricemia group than in the non-hyperuricemia group; and the male hyperuricemia group had higher levels of beta-carotene intake than the non-hyperuricemia group. Moreover, the differences between the cholesterol and caffein were not significant in either male or female. Hyperuricemia group had a slightly higher intake of dietary fiber than the other. The possible mechanism behind might be that the viscosity and bulk of dietary fiber interfere with the absorption of purines or adenine in the digestive system (17), or enhance intestinal motility and potentially binding uric acid in the intestine to facilitate its excretion (18). Additionally, In addition, our study found significant differences in certain vitamins in DII between the hyperuricemia and non-hyperuricemia groups, regardless of sex differences, which these vitamins are associated with hyperuricemia in various ways. For example, vitamin C mediates serum uric acid level by effectively preventing the impairment of renal epithelial function caused by uric acid crystals (19). Vitamin B12, as summarized by Zhang et al., its combination with folic acid could inactivate xanthine oxidoreductase, interfere with the conversion of purines to uric acid, and also reduce homocysteinemia level that may induce significant DNA damage and release purine nucleotides, ultimately reducing uric acid (20). Vitamin D affects the secretion and transport of uric acid by influencing serum parathyroid hormone level. Vitamin E acts by inhibiting xanthine oxidase activity and reducing uric acid formation (21). In addition, an experimental study reported that vitamin E also promotes uric acid excretion in deoxycorticosterone-salt-treated rats (22). In the end, a significant difference was also identified in alcohol intake between two groups. In addition, in this study we observed that cholesterol and caffeine intake did not differ significantly between the hyperuricemia and non-hyperuricemia groups of male and female, which may be related to the prevalence of caffeine consumption as well as to the Western dietary pattern in the US, which is characterized by a high-calorie and fat diet and is usually associated with higher levels of inflammatory markers in the body (9, 23, 24). Most Americans follow this pattern of dietary consumption, eliminating or diminishing its role between the sexes.
In addition, most people tend to consume food in several dietary patterns or their combinations. Current assessment only focused on the effect of single nutrient, which may lead to one-sided outcomes due to its neglect on synergistic effects of nutritional patterns (25). Further regression analysis showed that there is a positive association between DII and hyperuricemia risk after adjusting for potential confounders, with similar associations for male and female participants. A study among a Chinese population indicated a highly positive correlation between DII score and serum uric acid levels, regardless of sex (14). While, a Korean study found that only female with higher DII scores had a higher risk of HUA (26). The discrepancy could be partially due to the differences in the eating habits of different populations. The Korean diet is characterized by containing large amounts of fermented foods and seafood (27). In contrast to the dietary habits in Korea, Western diets are characterized by high fat intake, which contain processed meats and red meats (24). According to the DII concept (12), a pro-inflammatory diet contains mainly of red and processed meats, fried foods, high-sugar foods and refined grains, which are associated with high uric acid levels (28). An anti-inflammatory diet includes more vegetables, fruits, soy products, whole grains and nuts. Therefore, a healthy diet might be able to reduce the risk of hyperuricemia.
Previous studies have confirmed that serum uric acid level is determined by the production-excretion balance of uric acid, in which excretion via kidney plays a major role (29). In addition, it has been shown that intestine, other than kidney, is an important potential organ for the excretion of uric acid, and it works primarily through the action of intestinal flora and uric acid transporters (30). Thus the potential mechanism by which DII influence hyperuricemia are as follows. DII is closely associated with the development of hyperuricemia through direct effects of host purine metabolism or indirect effect of gut microbiota. On one hand, diets that promote inflammation can have adverse effects on an individual’s health by inducing oxidative stress and disrupting the immune system. Such diets can significantly increase the levels of inflammatory cytokines, exacerbating the problem. Additionally, they could alter the gastrointestinal microbial ecosystem, leading to chronic inflammation and other related health issues (31). Chronic inflammation is one of the main mechanism causing renal injury, which may ultimately impede uric acid excretion (32). On the other hand, anti-inflammatory diets in DII downregulate adenosine deaminase and xanthine oxidase activities, and improve intestinal barrier function and restores intestinal microbiota metabolism (33). Therefore, it might be a new therapeutic strategy targeting at metabolic balance of purines along with the regulation of intestinal excretion of uric acid, to prevent and alleviate hyperuricemia in the future.
In the final forest plot, our study indicates that there is an interaction between age and diabetes concerning the relationship between DII and hyperuricemia (p value for interaction <0.05). Additionally, blood pressure and BMI might also have a certain influence on the association between DII and hyperuricemia (borderline significant p value for interaction). Previous study pointed out that the population of participants who were ≥ 60 years old had the highest hyperuricemia risk (34). Possible explanations for this discrepancy could be attributed to different leptins levels in different age groups. It has been shown that leptin plays a critical role in inflammatory and immune responses (35), and is an important independent variable of uric acid values across all age groups (36). Also, a study by Francesco et al. showed that fasting leptin was higher in elderly subjects than in younger subjects, even after adjusting for the covariate of fat mass (37). Based on these studies, it is reasonable to deduce that the age-based DII-hyperuricemia relationship might be influenced by leptin levels. Additionally, we found that DII and hyperuricemia had a stronger association in patients with diabetes, which was quite expectable and was consistent with other studies which have confirmed a linear and positive correlation between serum uric acid levels in diabetes mellitus and serum insulin levels (38). A longitudinal study had reported that patients with diabetes at baseline were related to an increased hyperuricemia risk (39). Diabetes may regulate the association between DII and hyperuricemia by insulin resistance. Insulin resistance is a prevalent medical condition that could potentially cause an upsurge in the activity of hexose monophosphate shunt. This could lead to an augment in purine biosynthesis and turnover, ultimately resulting in an increase in the level of uric acid (40). Moreover, we found that as DII scores increase, the risk of hyperuricemia in individuals with a BMI > 25 also increases. This is consistent with a previous analysis of a representative sample of U.S. adults, which indicated that obesity plays a mediating role in the relationship between diet and hyperuricemia, with an indirect effect proportion of BMI as high as 53.34% (41). In the meanwhile, some studies have reached conclusions that are inconsistent with ours. A Korean case–control study evaluating a pro-inflammatory diet and the risk of hyperuricemia found that participants with a BMI <25 had a higher risk of developing hyperuricemia (26, 42). Additionally, research suggests that not only overweight but also being underweight is associated with higher levels of inflammation. This could explain why low BMI is also correlated with an increased risk of hyperuricemia (43, 44). It is worth emphasizing that these studies are currently based on studies conducted in single-country populations, leading to limitations in the extrapolation of findings. Meanwhile, similar to our findings, a survey from the U.S. indicated participants with hypertension had an elevated DII compared with those without hypertension (45). Although the underlying mechanisms are not clear, it is possible that the underlying hypertension risk or improved dietary pattern of these participants played a partial role in the correlation between DII and HUA risk (46). Hence, further investigation is needed to elucidate the question of whether hypertension might play a role in the relationship between DII and hyperuricemia. In contrast, a study showed a positive association between DII score and hyperuricemia that did not vary by hypertension status. The discrepancy could be partially due to the criteria for inclusion and exclusion, and sample size of the study participants. In our study, to minimize the potential adverse effects of renal impairment on nutritional intake, we only included individuals with an eGFR of ≥60 mL/min/1.73 m (2). In terms of sample size, we incorporated a larger number of samples (n = 31,781 VS n = 19,004), which might provide a more representative reflection of the characteristics of a larger population.
There are some advantages and limitations of this study. This is the first study to confirm the association between DII and hyperuricemia using data from a large-scale U.S. adults based on NHANES. However, present results should be interpreted with caution as cross-sectional observational studies cannot demonstrate causation and directionality. Secondly, although confounding factors have been extensively adjusted, other elements cannot be completely ruled out. Besides this, a possible limitation is the self-reporting of some conditions by the study participants. Information about self-reported may be recall biased or interview subjects diagnosed with hyperuricemia might change their diet pattern. Hyperuricemia represents a major global public health burden, thus further longitudinal studies should be conducted to provide stronger evidence for the relationship between DII and hyperuricemia.
In conclusion, our study based on an existing database highlighted the association between a diet with higher inflammatory potential measured by DII and increased risk of developing hyperuricemia. These findings suggest that adjusting dietary habits to reduce inflammation may be an effective prevention and control strategy for hyperuricemia. This study provides valuable evidence for healthcare professionals and individuals to consider when making dietary choices to maintain optimal health. Future research aiming to perform interventions based on dietary modifications may yield promising results for combating hyperuricemia and other related health conditions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All data came from NHANES, which was approved by National Centre for Health Statistics Institutional Ethics Review Board, and all the subjects agreed on the survey and signed written consent. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HW: writing-most of manuscript, data curation and processing. SQ: writing-part of the manuscript and data curation. FL: writing-part of the manuscript. HZ: software, writing—review and editing. LZ: methodology, writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
We acknowledge the National Center for Health Statistics at the CDC, which was responsible for designing, collecting, and administering the NHANES data and making the data available for public use. Thanks to Zhang Jing (Second Department of Infectious Disease, Shanghai Fifth People’s Hospital, Fudan University) for his work on the NHANES database. His outstanding work, nhanesR package and web page, makes it easier for us to explore NHANES database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1218166/full#supplementary-material
1. George, C, and Minter, DA. Hyperuricemia In: Salah Aboubakr MD, editor. StatPearls, vol. 2022. Treasure Island (FL): StatPearls publishing LLC (2022)
2. Smith, E, Hoy, D, Cross, M, Merriman, TR, Vos, T, Buchbinder, R, et al. The global burden of gout: estimates from the global burden of disease 2010 study. Ann Rheum Dis. (2014) 73:1470–6. doi: 10.1136/annrheumdis-2013-204647
3. Borghi, C, Agabiti-Rosei, E, Johnson, RJ, Kielstein, JT, Lurbe, E, Mancia, G, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. (2020) 80:1–11. doi: 10.1016/j.ejim.2020.07.006
4. Fang, XY, Qi, LW, Chen, HF, Gao, P, Zhang, Q, Leng, RX, et al. The interaction between dietary fructose and gut microbiota in hyperuricemia and gout. Front Nutr. (2022) 9:890730. doi: 10.3389/fnut.2022.890730
5. Chen-Xu, M, Yokose, C, Rai, SK, Pillinger, MH, and Choi, HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and nutrition examination survey, 2007-2016. Arthritis Rheumatol. (2019) 71:991–9. doi: 10.1002/art.40807
6. Li, Z, Gao, L, Zhong, X, Feng, G, Huang, F, and Xia, S. Association of Visceral fat Area and Hyperuricemia in non-obese US adults: a Cross-sectional study. Nutrients. (2022) 14:3992. doi: 10.3390/nu14193992
7. Gu, T, Cao, G, Luo, M, Zhang, N, Xue, T, Hou, R, et al. A systematic review and meta-analysis of the hyperuricemia risk from certain metals. Clin Rheumatol. (2022) 41:3641–60. doi: 10.1007/s10067-022-06362-1
8. Bolte, LA, Vich Vila, A, Imhann, F, Collij, V, Gacesa, R, Peters, V, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. (2021) 70:1287–98. doi: 10.1136/gutjnl-2020-322670
9. Tayyem, RF, Qalqili, TR, Ajeen, R, and Rayyan, YM. Dietary patterns and the risk of inflammatory bowel disease: findings from a case-control study. Nutrients. (2021) 13:1889. doi: 10.3390/nu13061889
10. Gong, M, Wen, S, Nguyen, T, Wang, C, Jin, J, and Zhou, L. Converging relationships of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab. Syndr. Obes. (2020) 13:943–62. doi: 10.2147/DMSO.S232377
11. Cavicchia, PP, Steck, SE, Hurley, TG, Hussey, JR, Ma, Y, Ockene, IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
12. Shivappa, N, Steck, SE, Hurley, TG, Hussey, JR, and Hébert, JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
13. Marx, W, Veronese, N, Kelly, JT, Smith, L, Hockey, M, Collins, S, et al. The dietary inflammatory index and human health: an umbrella review of Meta-analyses of observational studies. Adv. Nutr. (2021) 12:1681–90. doi: 10.1093/advances/nmab037
14. Ye, C, Huang, X, Wang, R, Halimulati, M, Aihemaitijiang, S, and Zhang, Z. Dietary inflammatory index and the risk of hyperuricemia: a Cross-sectional study in Chinese adult residents. Nutrients. (2021) 13:4504. doi: 10.3390/nu13124504
15. Tseng, M, Wright, DJ, and Fang, CY. Acculturation and dietary change among Chinese immigrant women in the United States. J Immigr Minor Health. (2015) 17:400–7. doi: 10.1007/s10903-014-0118-4
16. Feig, DI, Kang, DH, and Johnson, RJ. Uric acid and cardiovascular risk. N Engl J Med. (2008) 359:1811–21. doi: 10.1056/NEJMra0800885
17. Koguchi, T, Koguchi, H, Nakajima, H, Takano, S, Yamamoto, Y, Innami, S, et al. Dietary fiber suppresses elevation of uric acid and urea nitrogen concentrations in serum of rats with renal dysfunction induced by dietary adenine. Int J Vitam Nutr Res. (2004) 74:253–63. doi: 10.1024/0300-9831.74.4.253
18. Zhang, Y, and Qiu, H. Dietary magnesium intake and hyperuricemia among US adults. Nutrients. (2018) 10:296. doi: 10.3390/nu10030296
19. Li, H, Liu, X, Lee, MH, and Li, H. Vitamin C alleviates hyperuricemia nephropathy by reducing inflammation and fibrosis. J Food Sci. (2021) 86:3265–76. doi: 10.1111/1750-3841.15803
20. Zhang, Y, and Qiu, H. Folate, Vitamin B6 and vitamin B12 intake in relation to hyperuricemia. J Clin Med. (2018) 7:210. doi: 10.3390/jcm7080210
21. Bursać-Mitrović, M, Milovanović, DR, Mitić, R, Jovanović, D, Sovrlić, M, Vasiljević, P, et al. Effects of l-ascorbic acid and ALPHA-tocopherol on biochemical parameters of swimming-induced oxidative stress in serum of GUINEA pigs. Afr J Tradit Complement Altern Med. (2016) 13:29–33. doi: 10.21010/ajtcam.v13i4.5
22. Seifi, B, Kadkhodaee, M, and Zahmatkesh, M. Effect of vitamin E therapy on serum uric acid in DOCA-salt-treated rats. Acta Physiol Hung. (2011) 98:214–20. doi: 10.1556/APhysiol.98.2011.2.13
23. Loftfield, E, Freedman, ND, Dodd, KW, Vogtmann, E, Xiao, Q, Sinha, R, et al. Coffee drinking is widespread in the United States, but usual intake varies by key demographic and lifestyle factors. J Nutr. (2016) 146:1762–8. doi: 10.3945/jn.116.233940
24. Malesza, IJ, Malesza, M, Walkowiak, J, Mussin, N, Walkowiak, D, Aringazina, R, et al. High-fat, Western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. (2021) 10:3164. doi: 10.3390/cells10113164
25. Hu, FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. (2002) 13:3–9. doi: 10.1097/00041433-200202000-00002
26. Kim, HS, Kwon, M, Lee, HY, Shivappa, N, R Hébert, J, Sohn, C, et al. Higher pro-inflammatory dietary score is associated with higher hyperuricemia risk: results from the case-controlled Korean genome and epidemiology study_Cardiovascular disease association Study. Nutrients. (2019) 11:1803. doi: 10.3390/nu11081803
27. Das, G, Heredia, JB, de Lourdes Pereira, M, Coy-Barrera, E, Oliveira, SMR, Gutiérrez-Grijalva, EP, et al. Korean traditional foods as antiviral and respiratory disease prevention and treatments: a detailed review. Trends Food Sci Technol. (2021) 116:415–33. doi: 10.1016/j.tifs.2021.07.037
28. Gao, X, Qi, L, Qiao, N, Choi, HK, Curhan, G, Tucker, KL, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. (2007) 50:306–12. doi: 10.1161/HYPERTENSIONAHA.107.091041
29. Lipkowitz, M. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. (2012) 14:179–88. doi: 10.1007/s11926-012-0240-z
30. Yin, H, and Liu, NCJ. The role of the intestine in the development of hyperuricemia. Front Immunol. (2022) 13:845684. doi: 10.3389/fimmu.2022.845684
31. Serino, M. Molecular paths linking metabolic diseases, gut microbiota Dysbiosis and Enterobacteria infections. J Mol Biol. (2018) 430:581–90. doi: 10.1016/j.jmb.2018.01.010
32. Méndez, LC. Renal effects of hyperuricemia. Contrib Nephrol. (2018) 192:8–16. doi: 10.1159/000484273
33. Wang, J, Chen, Y, Zhong, H, Chen, F, Regenstein, J, Hu, X, et al. The gut microbiota as a target to control hyperuricemia pathogenesis: potential mechanisms and therapeutic strategies. Crit Rev Food Sci Nutr. (2022) 62:3979–89. doi: 10.1080/10408398.2021.1874287
34. Wang, L, Liu, H, Wang, D, Huang, X, Hong, X, Wang, Y, et al. The correlation between dietary inflammatory index and risk of hyperuricemia in the U.S. population. Medicine. (2023) 102:e33374. doi: 10.1097/MD.0000000000033374
35. Lago, R, Gómez, R, Lago, F, Gómez-Reino, J, and Gualillo, O. Leptin beyond body weight regulation—current concepts concerning its role in immune function and inflammation. Cell Immunol. (2008) 252:139–45. doi: 10.1016/j.cellimm.2007.09.004
36. Leptin plasma levels in the general population influence of age, gender, body weight and medical history.
37. Di Francesco, V, Fantin, F, Omizzolo, F, Residori, L, Bissoli, L, Bosello, O, et al. The anorexia of aging. Digest Dis. (2007) 25:129–37. doi: 10.1159/000099477
38. Gill, A, Kukreja, S, Malhotra, N, and Chhabra, N. Correlation of the serum insulin and the serum uric acid levels with the glycated haemoglobin levels in the patients of type 2 diabetes mellitus. J Clin Diagn Res. (2013) 7:1295–7. doi: 10.7860/JCDR/2013/6017.3121
39. McAdams-DeMarco, MA, Law, A, Maynard, JW, Coresh, J, and Baer, AN. Risk factors for incident hyperuricemia during mid-adulthood in African American and white men and women enrolled in the ARIC cohort study. BMC Musculoskelet Disord. (2013) 14:347. doi: 10.1186/1471-2474-14-347
40. Modan, M, Halkin, H, Karasik, A, and Lusky, A. Elevated serum uric acid? A facet of hyperinsulinaemia. Diabetologia. (1987) 30:713–8. doi: 10.1007/BF00296994
41. Wang, J, Chen, S, Zhao, J, Liang, J, Gao, X, Gao, Q, et al. Association between nutrient patterns and hyperuricemia: mediation analysis involving obesity indicators in the NHANES. BMC Public Health. (2022) 22:1981. doi: 10.1186/s12889-022-14357-5
42. Ishizaka, N, Ishizaka, Y, Toda, A, Tani, M, Koike, K, Yamakado, M, et al. Changes in waist circumference and body mass index in relation to changes in serum uric acid in Japanese individuals. J Rheumatol. (2010) 37:410–6. doi: 10.3899/jrheum.090736
43. Remedios, C, Shah, M, Bhasker, AG, and Lakdawala, M. Hyperuricemia: a reality in the Indian obese. Obes Surg. (2012) 22:945–8. doi: 10.1007/s11695-012-0655-7
44. Kahraman, S, Yilmaz, R, Akinci, D, Arici, M, Altun, B, Erdem, Y, et al. U-shaped association of body mass index with inflammation and atherosclerosis in hemodialysis patients. J Ren Nutr. (2005) 15:377–86. doi: 10.1053/j.jrn.2005.07.004
45. Zhou, N, Xie, Z, Liu, Q, Xu, Y, Dai, SC, Lu, J, et al. The dietary inflammatory index and its association with the prevalence of hypertension: a cross-sectional study. Front Immunol. (2023) 13:1097228. doi: 10.3389/fimmu.2022.1097228
Keywords: dietary inflammatory index, hyperuricemia, adults, the United States, NHANES
Citation: Wang H, Qin S, Li F, Zhang H and Zeng L (2023) A cross-sectional study on the association between dietary inflammatory index and hyperuricemia based on NHANES 2005–2018. Front. Nutr. 10:1218166. doi: 10.3389/fnut.2023.1218166
Received: 06 May 2023; Accepted: 28 August 2023;
Published: 21 September 2023.
Edited by:
Mohammed S. Razzaque, Lake Erie College of Osteopathic Medicine, United StatesReviewed by:
Miguel Murguía-Romero, National Autonomous University of Mexico, MexicoCopyright © 2023 Wang, Qin, Li, Zhang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Zeng, emVuZ2xpbmc1MTBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.