- 1Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran
- 2Department of Nutrition, Faculty of Public Health, Kerman University of Medical Sciences, Kerman, Iran
Introduction: Neurological disorders have been considered the major contributors to global long-term disability and lower quality of life. Lifestyle factors, such as dietary patterns, are increasingly recognized as important determinants of neurological function. Some dietary behaviors, such as Nordic diet (ND) were likely to have protective effects on brain function. However, an understanding of the effectiveness of the ND pattern to improve neurological function and brain health is not fully understood. We review the current evidence that supports the ND pattern in various aspects of neurological function and addresses both proven and less established mechanisms of action based on its food ingredients and biochemical compounds.
Methods: In this systematic review, PubMed, Web of Science, and Scopus databases were searched from inception to February 2023. Observational and intervention studies were included.
Results: Of the 627 screened studies, 5 observational studies (including three cohorts and two cross-sectional studies) and 3 intervention studies investigating the association between ND and neurological function. Observational studies investigated the association of ND with the following neurological functions: cognition, stroke, and neuropsychological function. Intervention studies investigated the effects of ND on cognition and depression.
Discussion: Despite the limited literature on ND and its association with neurological function, several aspects of ND may lead to some health benefits suggesting neuroprotective effects. The current state of knowledge attributes the possible effects of characteristic components of the ND to its antioxidant, anti-inflammatory, lipid-lowering, gut-brain-axis modulating, and ligand activities in cell signaling pathways. Based on existing evidence, the ND may be considered a recommended dietary approach for the improvement of neurological function and brain health.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD2023451117].
1. Introduction
Neurological disorders have been considered the major contributors to global long-term disability and lower quality of life (1). Recent reports have documented an alarming increase in the global population living with brain health disorders such as depression, anxiety, migraine, multiple sclerosis, Parkinson’s disease (PD), and Alzheimer’s disease (AD) (2–5). A neurological disorder is any illness of the body’s nervous system including biochemical, structural, or electrical abnormalities in the peripheral nerves, spinal cord, or brain which leads to a wide range of complications. Examples of complications include weakness, confusion, loss of sensation, chronic pain, seizures, and poor coordination (6). Considerable evidence has reported that oxidative and nitrosative stress, neuroinflammation, and mitochondrial dysfunction play a crucial role in the occurrence and progression of these disorders (7). Moreover, cardiometabolic conditions such as obesity, insulin resistance, metabolic syndrome, type 2 diabetes, cardiovascular diseases, and hypertension are involved in some brain health problems and cognitive performance (8).
Diet is increasingly recognized as an important determinant of neurological function. Animal studies demonstrated that nutrition can be involved in neurological function. Some dietary behaviors such as a Western diet, which contains high amounts of saturated fat, refined sugars, and processed foods, may have an interaction with impairment of learning and memory (9, 10). On the other hand, diverse aspects of diet and dietary patterns including the Mediterranean diet (MD), vegetarian diet, and Nordic diet (ND) could be important protective factors for cognitive health (11–14).
The ND, also understood as the Baltic Sea diet, originated in Nordic or Northern European countries including Denmark, Norway, Sweden, Iceland, and Finland (15). A high ND score indicates a high intake of different healthy food items including vegetables, legumes, whole grains (rye, oat, and barley), fruits (berries, apples, and pears), low-fat dairy, rapeseed oil, low-fat types of meat (game and poultry), shellfish, fatty fish (mackerel, herring, and salmon), seafood, salt restriction, and also low intake of sugar-sweetened products as well as moderate consumption of alcohol (16, 17). In the Danish population, previous research has demonstrated that a higher ND score is related to an 11% lower mortality rate (18). Further observational studies also indicated an inverse relationship between adherence to the ND and risk of abdominal obesity (19), markers of inflammation (20), and cardiometabolic risk indicators (17, 21, 22).
Considering that better cardiometabolic status is related to a lower risk of vascular dementia, the ND can decrease the rate of cognitive decline with aging (23). Moreover, the majority of ND features have previously been related to preserved cognition. For example, ND is characterized by the abundant use of berries, providing different types of antioxidants, flavonoids, and other bioactive compounds (24). A 2-year multi-domain lifestyle intervention has shown high berry consumption had a positive effect on cognitive performance compared with no berry consumption (25). Other main components of the ND are rye, oat, and barley; providing high fiber contents. A rapeseed oil-based diet high in α-linolenic acid has been postulated to protect against cognitive decline (24).
A comprehensive search of scientific literature in electronic databases did not find any ongoing or published systematic reviews on a particular type of diet, namely the Nordic diet, and its effects on neurological function. As a result, the primary goal of this research is to conduct a review of scientific literature to determine how ND affects neurological function. Also, the purpose of this review is to provide a summary of the primary molecular mechanisms that are proposed to explain the relationship between the ND, its components, neurological function, and brain health.
2. Methods
A comprehensive search was conducted up to February 2023 using Medline/PubMed, Scopus, and Web of Science to recognize observational and intervention studies reporting the effects of ND on neurological function. The search focused on the following terms: (Nordiet OR “Nordic dietary pattern” OR “Nordic diet” OR “Baltic Sea dietary pattern” OR “Baltic Sea diet”) AND (mental OR cognitive OR cognition OR memory OR “neurocognitive disorders” OR aging OR depression OR anxiety OR Alzheimer’s disease OR stroke OR dementia OR brain OR Parkinson’s disease OR migraine OR “multiple sclerosis” OR “neurological diseases”). The search was confined to English language publications, covering all years available in the database. There was no age limit. Moreover, reference lists of retrieved publications were manually searched for relevant articles. The abstract was reviewed to select relevant articles.
Studies that reported the effect of (intervention studies) or the association (observational studies) of the Nordic diet (as exposure) on neurological function (as outcome) were considered for review.
The inclusion criteria were as follows: (a) assessment of the ND as main exposure; (b) studies focusing on neurological function and disorders, cognitive spectrum (including cognition, memory, attention span, learning), and psychosocial and emotional aspects (including depression and anxiety) were included; (c) enrolled humans at all ages; (d) analytical epidemiological studies, i.e., observational studies (prospective cohort studies, case–control, or cross-sectional) and intervention studies (randomized control trials, and non-randomized trials, i.e., pre-post studies); and (e) studies written in English and published in reputable journals.
The exclusion criteria were: (a) no original studies (e.g., editorials, review articles, non-research letters); (b) case series or case reports; (c) lack of data on the ND (e.g., investigated only specific nutrients-rich in the ND); and (d) studies not conducted in humans. Moreover, intervention studies that utilized lifestyle interventions alongside the ND intervention (such as behavioral management or exercise) were excluded.
EndNote referencing software (version X8, Thomson Reuters) was used to import identified publications, and two authors (RJ and VB) independently examined the titles and abstracts of those publications. Full-text articles were retrieved if a suitable record was found. Any disagreements and discrepancies were addressed and resolved by consensus between the authors. The reference lists of selected records and review articles were checked for additional potentially relevant articles following the retrieval of full-text studies.
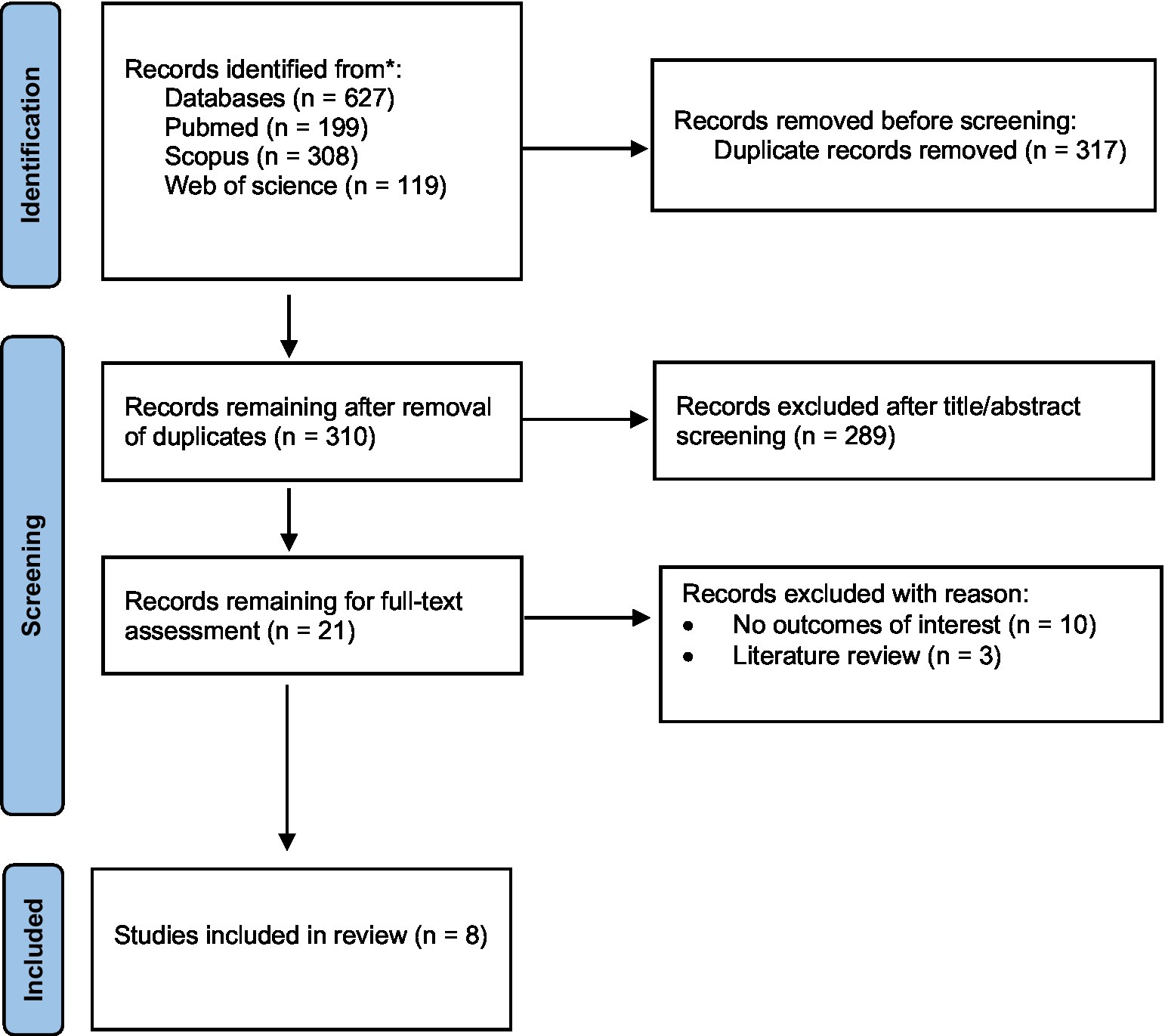
Data extraction was done by two authors (RJ and VB) on the included articles using a predefined data extraction form. The following data were extracted: first author, publication year, country, study design, duration, participants and sample size, dietary assessment method, details of control and intervention groups (intervention studies), outcome assessment, and main findings. Discrepancies in data extraction were addressed and resolved by consensus between the authors (Figure 1).
3. Nordic diet
The ND is designed to provide better health-related quality of life. As its name implies, the ND is a dietary pattern focusing on the consumption of traditional, organic, plant-based, and local foods originating from the Scandinavian countries including Iceland, Finland, Norway, Sweden, and Denmark (26). It is also known as the Baltic Sea diet which is recommended for healthy subjects to introduce healthier options for eating according to foods that are commonly available in the Scandinavian countries (27, 28).
The new ND, a gastronomic interpretation of the ND, was designed in 2004 by a group of nutritionists, scientists, and leading Nordic chefs to increase the focus on local cuisine and promote the excellence of Nordic food internationally (26, 29). It is based on four crucial principles: sustainability, health, Nordic identity, and gastronomic potential (30). The new ND Manifesto emphasizes wild, foraged, local, fresh, and very tasty foods. Generally, this dietary pattern includes less fat, less sugar, less alcohol, high fiber, and high in fish and seafood. There are some foods and ways to prepare them that are the same in Scandinavian countries, including berries, apples, cabbage, peas, root vegetables, barley, oats, rye, fermented milk, fat-free or low-fat dairy products, fish (salmon, Baltic herring, and mackerel), and rapeseed oil consumption. It also suggests an increase in legume consumption, which increases total protein intake, but processed meat products and red meat are consumed less (31, 32). Moreover, a critical part of the diet in the Scandinavian nations is fish and other seafood, due to their rich marine archipelago (30, 33). Considering the geographical location of the Nordic nations and the growing conditions of rapeseed plant in the winter, rapeseed oil (also recognized as canola oil) is the main source of fats in the ND (34). Water is generally recommended as a drink in the ND, and salt intake is limited to 5–6 gr/day. Moreover, in some Scandinavian nations, taking a vitamin D supplement is advised as a dietary supplement (35–37). More information on the components of the ND is given in Figure 2.
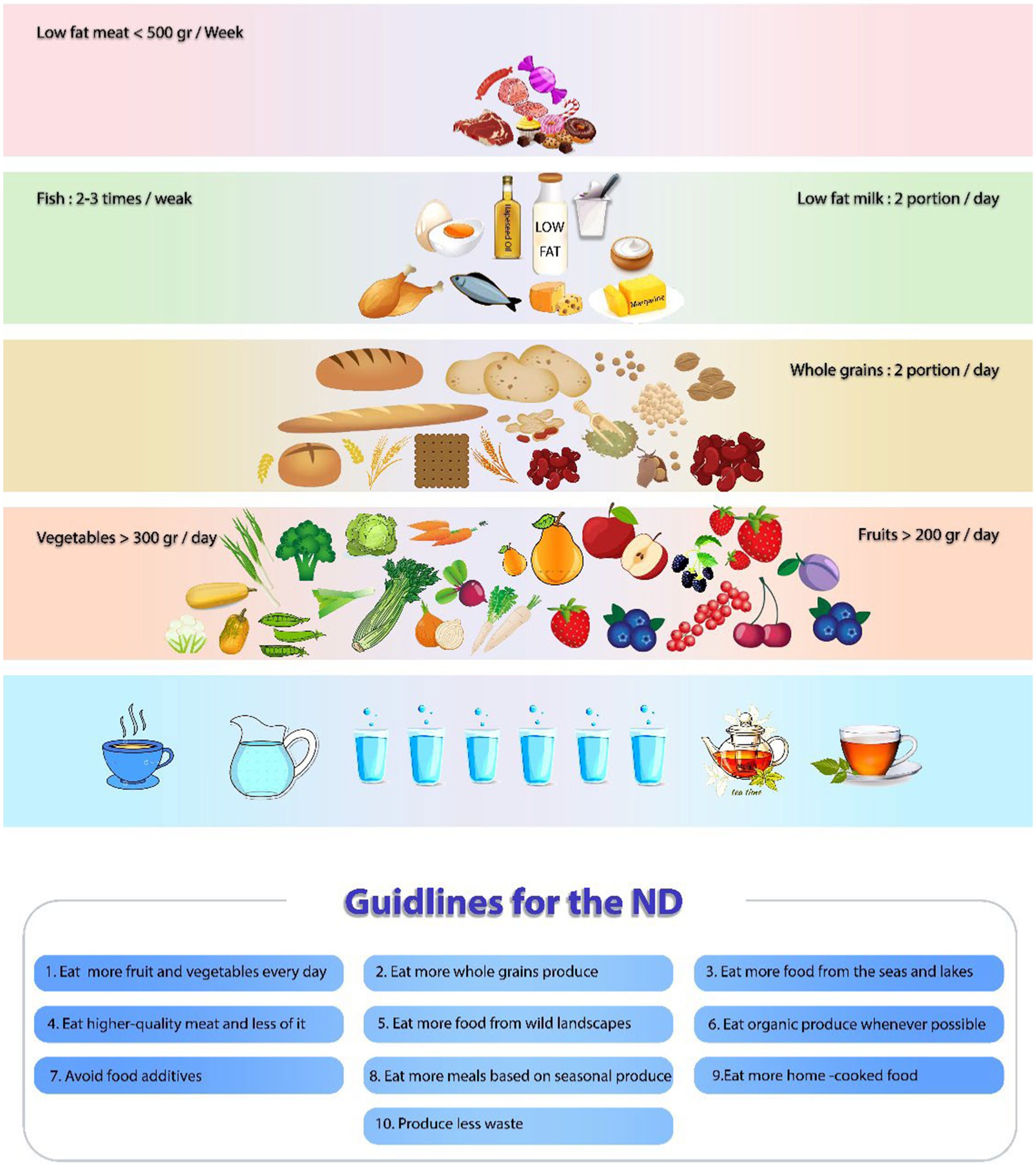
Figure 2. The pyramid illustrates the overall characteristics of the Nordic diet. The main components of the ND including Nordic vegetables, roots, cabbages, peas, Nordic fruits, apples, pears, and berries, are placed at the bottom of the pyramid. Whole-grain cereals (rye, oats, and barley), potatoes, legumes, nuts, and seeds are placed in the middle of the pyramid. Above them are located fatty fish, low-fat or fat-free dairy products, low-fat choices of meat (poultry and game), egg, margarine, and rapeseed oil. Foods that should be consumed carefully, such as animal fats (butter), red and processed meat (beef, pork, processed meat products, and sausage), sweets, and chocolate, are at the top of the pyramid.
After decades of collaboration among the Scandinavian countries, guidelines for dietary composition and reference values for nutrient intake were published through the joint publication of the Nordic Nutrition Recommendations (NNRs). Based on NNRs, the amount of macronutrient distribution range (as calorie percent) is 10–20% of total energy intake should be derived from proteins, 45–60% from carbohydrates, and 25–40% from fats (34, 38). In terms of dietary recommendations, the ND has similarities with the MD, both recommend healthy foods for the general population, that are rich in vegetables, fruits, legumes, nuts, seeds, whole-grain cereals, and seafood, and low in red meat and processed foods (39). The notable point difference between these dietary patterns is the type of oil used in each dietary pattern. The ND is based on rapeseed oil (more polyunsaturated fat), while the MD mainly recommends olive oil (34).
The ND is also similar to the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study which investigated whether diet, vascular management, exercise, and cognitive training could improve cognitive function in at-risk older adults (40). It emphasizes fruits, vegetables, whole grains, and fish while limiting red meat, sucrose, and saturated fats. The FINGER trial showed that this comprehensive program could increase white-matter integrity and improve cognition, executive function, and processing speed in older adults with elevated dementia risk (41, 42).
A growing body of evidence suggests that the components of ND are related to beneficial health effects, but little is recognized about the overall health-related impacts of ND (43, 44). Several studies reported that the ND is related to improving cardiovascular risk indicators including hypertension and dyslipidemia (45, 46). Several observational and intervention studies have indicated an inverse association between adherence to the ND and risk of type 2 diabetes mellitus, colorectal cancer, stroke, inflammation, coronary heart disease, and all-cause mortality (22, 47–51). So, the health benefits of the ND have increasingly become an area of interest.
4. Nordic diet and neurological function: current evidence
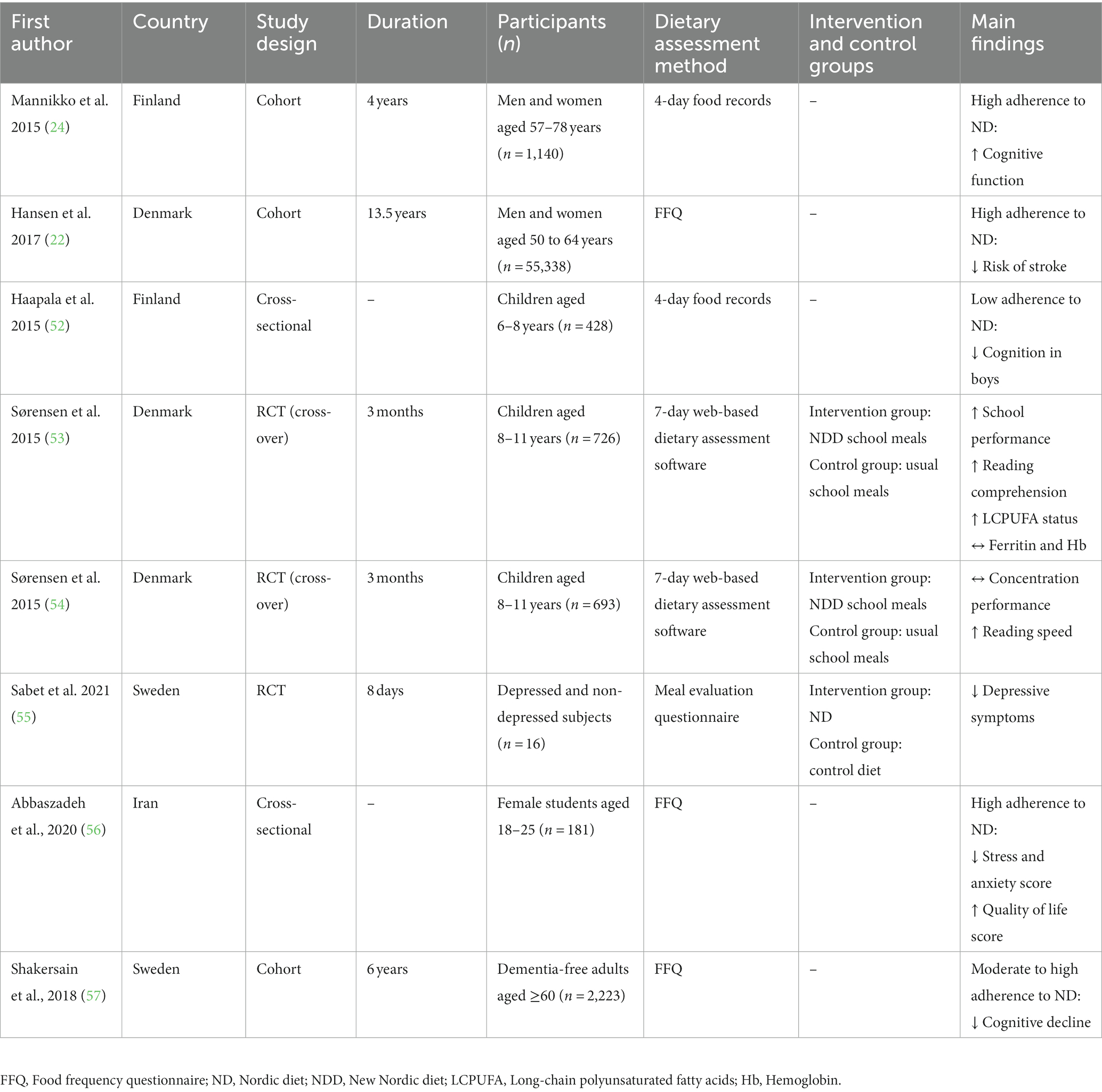
To date, a few studies have examined the role of the ND in neurological diseases. There are five observational studies (including three cohorts and two cross-sectional studies) and three intervention studies investigating the association between the ND and neurological function (Table 1).
4.1. Observational studies
4.1.1. Cognition
In 2015, a 4-year study involving 1,140 women and men aged 57–78 years with normal cognition was done to estimate the cross-sectional and longitudinal associations of the ND with cognitive performance at baseline and 4-year follow-up in a Finnish population-based random sample. Assessment of cognition by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery and the Mini-mental State Examination (MMSE) indicated that subjects with better adherence to the ND experienced higher scores in global cognitive functioning over a four-year study period compared to baseline after adjustment for lifestyle and demographic confounders (24). A population cohort study on 2,223 dementia-free adults aged ≥60 followed up for 6 years reported that moderate and high adherence to the ND was more firmly connected with a better-preserved cognitive performance, assessed by MMSE, among Swedish older adults compared to low adherence to the ND (57). Another cross-sectional study including 428 children aged 6–8 years demonstrated that lower scores of the Baltic Sea diet, based on 4-day food records, were related to lower cognitive performance (assessed by Raven’s Colored Progressive Matrixes) among children in Finland (52).
4.1.2. Stroke
A Danish cohort study among 55,338 men and women aged 50 to 64 years with a median follow-up of 13.5 years revealed that adhering to a healthy ND, evaluated with a healthy Nordic food index score, was related to a lower risk of ischemic stroke in the Danish Diet, Cancer, and Health cohort (22).
4.1.3. Neuropsychological function
A cross-sectional study containing 181 female students aged between 18 and 25 years was conducted to examine the association between adherence to the health-promoting ND with neuropsychological activity. A collection of standardized questionnaires such as insomnia severity index, quality of life, depression anxiety stress scale, cognitive ability questionnaire, and Epworth sleep scale was used to evaluate the neurological function determinants such as anxiety, stress, memory, and sleep disorders. Analyses of data reported that adherence to the ND was inversely associated with anxiety and stress scores and directly linked to health-related quality of life (56).
4.2. Intervention studies
4.2.1. Depression
A 8-day randomized controlled pilot trial conducted in Sweden involving 16 women and men aged between 18 and 65 years with major depressive disorder in a controlled setting with provided foods, demonstrated that a healthy ND compared to a control diet was related to a greater reduction in depressive symptoms (55). Studies with a stronger methodology, that consider a longer period and larger sample size, may help to confirm the findings of this study.
4.2.2. Cognitive
A school meal, randomized, controlled, cross-over trial involving 693 Danish children aged 8–11 years was conducted to investigate the effects of optimal well-being, development, and health for Danish children (OPUS) through a healthy new Nordic school meal on the concentration and school performance for 3 months. A healthy Nordic school meal program did not affect concentration function compared to the usual packed lunch from home but increased reading speed (54). Secondary analyses of the OPUS school meal study on 726 Danish children aged 8 to 11 years old indicated that increased fish intake through a healthy Nordic school meal program during 3 months was associated with greater improvements in school performance, reading comprehension, as well as whole blood long-chain polyunsaturated fatty acids status (53).
5. Mechanisms linking the ND to neurological function
5.1. Vegetables and fruits
The ND is rich in vegetables and fruits which are the main sources of nutritious components such as minerals, natural antioxidants, vitamins, and dietary fibers (58, 59). These components have multiple beneficial effects in preventing and improving several chronic disorders. Their benefits for health are mediated by polyphenolic compounds to provide antioxidant, anticarcinogenic, antimicrobial, antiviral, and anti-inflammatory effects (58, 60–62). Thus, daily consumption of foods rich in plant-derived polyphenols has been encouraged, especially in the Western world (61, 63).
Berries are important and abundant wild fruits in the Nordic countries with significant health-promoting outcomes, attributed mainly to a special class of polyphenols known as anthocyanins (64). Anthocyanins are organic water-soluble glycosidic pigments widely found in dark-colored vegetables, fruits, and grains with considerable inherent antioxidant properties (65). It has been reported that anthocyanins have a wide and various range of beneficial impacts such as anti-inflammatory, immunomodulatory, cardioprotective, hepatoprotective, neuroprotective, anticancer, antidiabetic, anti-obesity, and antioxidant effects, which make them promising factors for the prevention and treatment of pathological circumstances including neurological disorders (66, 67). Multiple lines of evidence suggest that anthocyanins may have neuroprotective and anti-neuroinflammatory activities that result in improving neural function (68). Neuroinflammation defines specific processes related to the trafficking of immune cells in the central nervous system, which leads to a pathological condition by affecting certain pathways. During the neuroinflammatory process, activation of microglia and astrocytes can cause proinflammatory cytokines to be released and, in turn, activate a second level of inflammatory signaling pathways like nuclear factor-kappa B (NF-κB) (69, 70). Thus, oxidative stress and neuroinflammation can disrupt normal brain function (71). It is suggested that increased generation of reactive oxygen species (ROS) may trigger oxidative injury, cellular damage, mitochondrial dysfunction, and impaired deoxyribonucleic acid repair system which are detrimental factors for neurological function (72, 73). Polyphenols, including anthocyanins, can prevent neuroinflammation, protect neurons against neurotoxin-induced damage, and promote cognitive performance, learning, memory, and neuron survival through their anti-inflammatory and antioxidant activities (74–76). In this regard, Poulose et al. demonstrated that anthocyanins protect the microglial cells subjected to lipopolysaccharide (LPS), a bacterial endotoxin, against neuroinflammation by preventing the activation of p38 and NF-κB and reducing proinflammatory factors such as cyclooxygenase-2 (COX-2) and tumor necrosis factor-α (TNF-α) (77). These findings are consistent with another study in which anthocyanins prevented the up-regulation of inflammatory pathways including Phosphoinositide 3-kinase (PI3K)/AKT, NF-κB, and mitogen-activated protein kinases (MAPKs) in microglial cells, decreasing the generation of pro-inflammatory factors, such as TNF-α, nitric oxide (NO), interleukin (IL)-1β, and prostaglandin E2 (PGE2) (78).
It is noteworthy that the relatively low bioavailability of anthocyanins in the brain may limit their efficacy. Indeed, low concentrations of circulating anthocyanins are considered to be due to the metabolism by gut microbiota to produce different phenolic acid and aldehyde metabolites (79). Some studies indicated that protocatechuic acid (PA), a key anthocyanin metabolite, is a neuroprotective factor through anti-inflammatory and antioxidant features (79, 80).
Cruciferous vegetables including kale, cabbage, Brussels sprouts, horseradish, cauliflower, radish, turnips, and broccoli sprouts are also identified as one of the main components in the ND (81). These vegetables contain a wide variety of nutrients such as protein, carbohydrate, vitamins, minerals, phytochemicals (Tannins, flavonoids, anthocyanins, and carotenoids), phytosterols, and non-nutritive metabolites such as nitrogen-containing compounds and sulfur compounds (glucosinolates and S-methyl cysteine sulfoxide) (82). Several studies have examined the health-promoting effects of cruciferous vegetables in humans and showed that higher consumption of these vegetables is related to a reduced risk of several chronic disorders (83). Besides phytochemicals, the beneficial health characteristics of these vegetables are mainly related to the functions of glucosinolates. In case of plant tissue damage, glucosinolates are hydrolyzed by enzymatic activity, and metabolites such as isothiocyanate, nitriles, and thiocyanates are formed. Also, glucosinolates hydrolysis can occur by intestinal microbiota (84, 85). Cruciferous vegetables, due to their glucosinolates and other metabolites, exhibit several biological properties related to neurological and psychiatric conditions (86, 87). Several studies have provided mechanistic insight into the advantageous effects of glucosinolates on brain health through modulating the hypothalamic–pituitary–adrenal axis, reducing neuroinflammation, oxidative stress, beta-amyloid, and tau production, inhibiting DNA methyltransferases, and increasing brain-derived neurotrophic factor and cellular lifespan (88–90). In a clinical study, daily oral administration of isothiocyanate sulforaphane increased peripheral and brain glutathione, an antioxidant, in healthy human subjects (91). In addition, Ghazizadeh-Hashemi et al. showed that a 6-week sulforaphane administration safely decreased depressive symptoms in patients with a history of cardiac interventions and presence of mild to moderate depression (92). Although, a variety of animal model studies has reported a good effectiveness of sulforaphane interventions for psychiatric and neurodegenerative disorders such as depression, anxiety, schizophrenia, cognitive function, learning and memory, multiple sclerosis, and AD (86, 93–96). Therefore, considering that the positive impacts of cruciferous vegetables and their metabolites are mainly associated with antioxidant and anti-inflammatory mechanisms, the ND may also have beneficial outcomes on neurological function.
Apples and pears are widely consumed in the Nordic regions and are generally recognized as healthy food sources (97). Some studies demonstrated that apples are a good source of antioxidants with various phytochemical compounds. The main phytochemicals in apples are flavonoids (including quercetins, catechin, epicatechin, and proanthocyanidins) and phenolic acids (including p-coumaric acid, chlorogenic acid, and caffeic acid) (97). Quercetin, (3,5,7,3′,4′-pentahydroxyflavone), plays a principal neuroprotective role by inhibiting the proinflammatory mediators that are released by glial cells. Indeed, it is a strong scavenger of free radicals such as ROS and reactive nitrogen species (98). It has been proposed that quercetin prevents neuroinflammation by inhibiting the production of NO in glial cells, which further inhibits the NF-κB signaling pathway and prevents inflammation-related neuronal damage (99). Similarly, it ameliorated activated astrocytes which mediate the generation of ROS and cytokines and further influence neuronal cells and degeneration (100). Most importantly, quercetin may protect the neuronal cells against amyloid beta peptide toxicity by activating the nuclear factor (erythroid-derived 2)-like 2 (Nrf2)- antioxidant response elements (ARE) pathway (101). Activation of the Nrf2-ARE signaling pathway promotes neuroprotection against oxidative injury and cell death (102). Another area of interest regarding the neuroprotective mechanisms provided by quercetin is sirtuins. Quercetin has been shown to activate sirtuin-1, which leads to the protection of cells from apoptosis (101).
5.2. Whole grains
The ND is characterized by a high content in whole grain cereals versus refined grains. Whole wheat, oat, rye, and barley are among the main food sources of carbohydrates and other nutrients such as dietary fibers, minerals, and vitamins in the Scandinavian nations. It has been reported that whole-grain cereals are high in phytochemicals such as phytosterols, phenolic acid, and β-glucans which are responsible for the high antioxidant properties (103). A Finnish study revealed that whole-grain consumption was very high among the subjects in Finland with the lowest quartile median consumption (79 g/day), which was higher than the highest quintile mean consumption (45.6 g/day) found in the US population (32). There is ample evidence to support that whole grain cereals have health-benefits effects (30). Mounting evidence demonstrated that whole-grain cereals are good sources of fermentable carbohydrates, including resistant starch, dietary fibers, and oligosaccharides, that cannot usually be broken down by the digestive enzymes of humans. However, the gut microflora selectively breaks down and ferments them into gases and short-chain fatty acids (SCFAs) (104). SCFAs are absorbed and utilized by the gastrointestinal epithelial cells, as well as beneficial bacteria such as lactobacilli and bifidobacterial; therefore improving the gut microbial community, decreasing the gut pH, and enhancing the immunity of the body (105). Extensive research efforts have indicated that an appropriate increase in whole-grain intake (barley and oats) can enhance the growth of probiotics and enhance intestinal and distal organ functions (106, 107). On the other hand, evidence is emerging supporting the role of intestinal microbiota in the brain function by the gut-brain axis, thereby regulating neurological activities such as behavior, cognitive function, and mood (108, 109). Gut-brain axis involves a complex structure of immunological, neural, and endocrinological factors, that can be an important target for manipulating brain health (109). A cross-sectional study demonstrated an inverse relationship between fiber supplementation and depressive symptoms (110). However, there was no significant association in Gopinath et al. study (111). Overall, there are complex mechanistic processes involved in the impacts of dietary fiber-derived whole grains on brain health. Metabolites from bacterial fermentation, such as SCFAs, may play a variety of regulatory roles including histone acetylation, signaling through G protein-coupled receptors, and affect the host’s immune system by altering the circulating levels of inflammatory mediators and reducing neuroinflammation (112). In animal models of endotoxin-induced sickness behavior, pectin as the only source of fiber decreased brain TNF-α and IL-1β (113).
Moreover, gut microbiota modulates serotonin concentration, a neurotransmitter, and the metabolism of tryptophan, which is often targeted by anti-depressant medications in the central nervous system (114). Butyrate, one of the SCFAs, can increase the transcripts of brain-derived neurotrophic factor (BDNF), an important molecule for maintaining the integrity of neurons and cognitive function, in the brain (115). It is worth noting that the gut microbiome may directly or indirectly affect the generation of neurotransmitters, such as dopamine, γ-aminobutyric acid (GABA), and acetylcholine which modulate cognition and mood status (109). The potential action mechanisms of the ND on the gut-brain axis are shown in Figure 3.
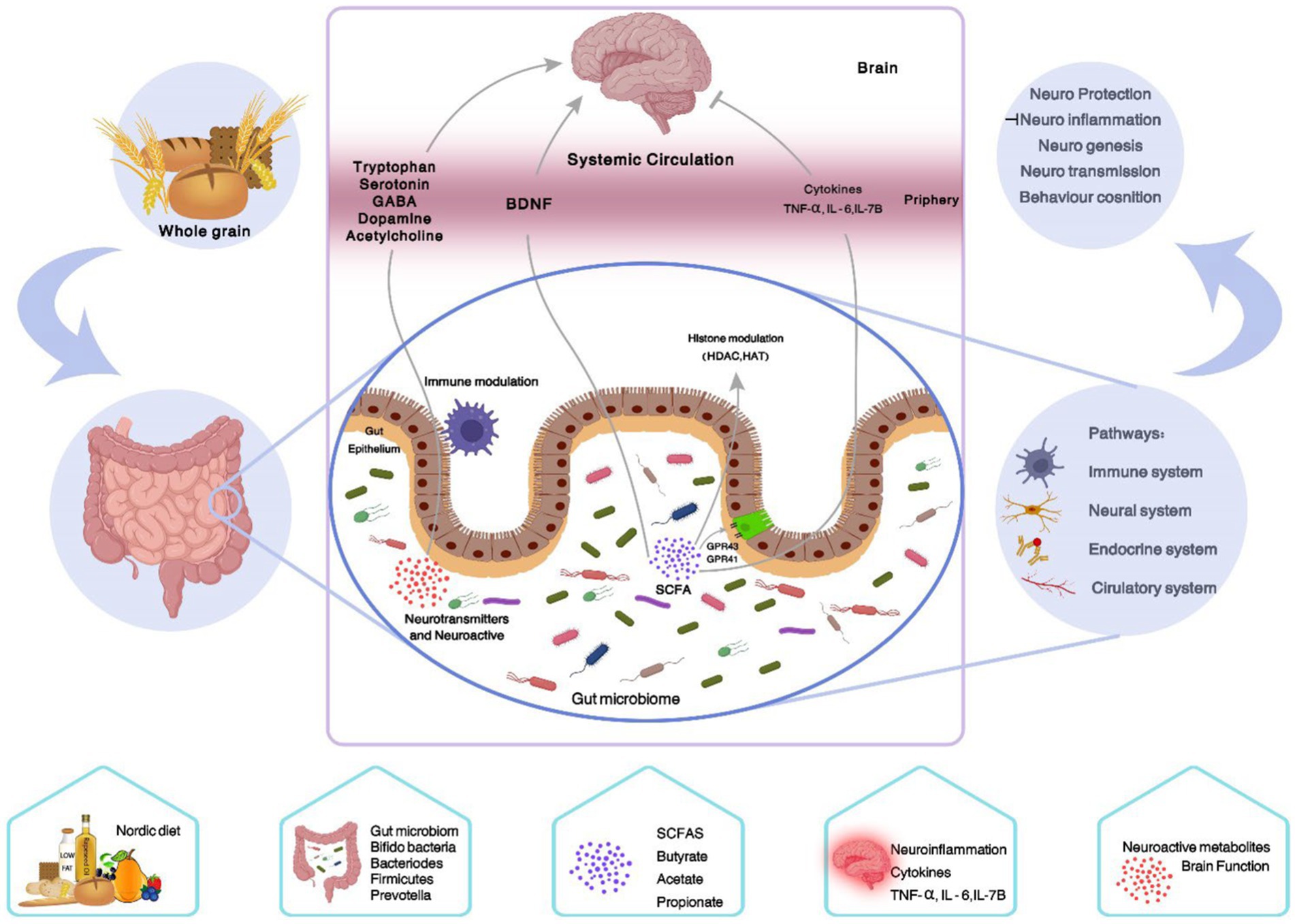
Figure 3. Summary of the effects of the ND on the gut-brain axis. One of the components of the Nordic diet is whole grains, which are a good source of fermentable carbohydrates. The intestinal microbial flora selectively ferments them into gases and SCFAs, which in several ways lead to the improvement of the gut barrier function and effect on the central nervous system (gut-brain-axis). (1) Modulating histone through GPR, (2) reducing cytokines such as TNF-α, IL-6, and IL-1β, (3) modulating neurotransmitters and neuroactive (tryptophan, serotonin, GABA, dopamine, and acetylcholine), (4) increasing BDNF. GABA, Gamma-Aminobutyric Acid; BDNF, Brain-derived neurotrophic factor; TNF-α, Tumor necrosis factor-alpha; IL-6, Interleukin-6; IL-1β, Interleukin-1beta; SCFAs, Short chain fatty acids; GPR, G protein-coupled receptor.
Besides fibers, a growing body of evidence has revealed that whole-grain cereals are rich in a variety of phytochemicals, including phytic acid, flavonoids, terpenes, coumarins, and phenolic compounds, which have various physiological activities, including hypotensive, antibacterial, anti-hyperglycemia, immunomodulation, antioxidant, anti-tumor, anti-inflammation, and anti-aging (103). These compounds are documented to exert antioxidant and anti-inflammatory activities, resulting in a neuroprotective effect (103). It is worth noting that studies on animals have shown that diets high in carbohydrates and low in protein could lead to the longest lifespan and improved cognition. It may be concluded that the quality of dietary carbohydrates is more important than its quantity on nerve function and brain health (116, 117).
5.3. Legumes
Legumes are nutritionally valuable, providing several nutritious components including proteins with essential amino acids, minerals, vitamins, dietary fibers, complex carbohydrates, unsaturated fats, and non-nutritional compounds such as alkaloids, isoflavone, phytic acid, phenolic compounds (tannins), and saponins (113). Dietary isoflavones derived from legumes may have beneficial effects on brain health, receiving new attention regarding neuroprotective properties (118). These compounds are classified as phytoestrogens which are most extensively investigated. Isoflavones can mimic the transcriptional effects of estrogen via binding to estrogen-receptor beta (ERβ), mediating estrogen action, and thereby inhibiting apoptotic cell death (119). Estrogen and its mimics modulate synaptic plasticity, neuronal survival and growth, and brain function through an estrogen receptor-mediated pathway, and subsequently regulation of gene transcription and second messenger systems (120).
Moreover, the antioxidant action of isoflavones has been suggested to be involved in the presumed effect of legumes on brain health. These compounds afford protection against neuroinflammation by ameliorating oxidative damage, upregulating endogenous antioxidant signaling pathways, and protecting the integrity of the mitochondria (119, 121). These antioxidant activities may be attributed to the inhibition of the NF-κB signaling pathway (121). In this regard, some studies demonstrated that isoflavones prevent microglial activation and inflammation (122, 123). In vitro studies have shown that isoflavone treatment exhibited protection against apoptosis via regulating the antiapoptotic bcl-2 protein suppression and modulating cell survival signaling (120, 121). Other protective effects of dietary isoflavones on brain health can mediate by reductions in proapoptotic proteins such as Bax and Bad, and modulation of kinase signaling (119).
5.4. Fish/seafood
Fish, especially fatty fish, and seafood are one of the natural components in the ND, with high amounts of polyunsaturated fatty acids (PUFAs) containing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). In addition, these food sources contain many other valuable nutrients such as vitamins and minerals (30). It was commonly suggested that the major dietary components of fish are DHA and EPA, which are the main building blocks for nerve cell membranes and play a crucial role in proper brain development and function, neurotransmission, and ion channel modulation, and thus are effective in neuroprotection. Some studies reported that there is a relationship between the reduction of omega-3 PUFA concentration in the blood serum and erythrocyte membranes with several neuropsychiatric disorders such as depression, schizophrenia and also dyspraxia, dyslexia, autism, and attention-deficit hyperactivity disorder (ADHD) (124–126). Omega-3 PUFAs have several mechanisms of action in the cerebral vascular system and brain that could improve brain function (127). First, they seem to attenuate inflammation by reducing the production of pro-inflammatory cytokines from arachidonic acid. Omega-3 PUFAs compete with arachidonic acid for incorporation into phospholipid membranes in the cyclooxygenase pathway by preventing the generation of proinflammatory eicosanoids, including prostaglandin E2 and thromboxane B2. EPA and DHA can also prevent the secretion of other pro-inflammatory mediators including interleukins (IL-1, IL-1β, IL-2, IL-6), TNF-α, and interferon-gamma (IFN-γ). Other anti-inflammatory mechanisms are the reduction of T-cell proliferation and inhibition of leukocyte migration. Considering that inflammation is involved in the pathophysiology of neurological disorders, these fatty acids might exert neuroprotective properties through their anti-inflammatory effects (127–129). Second, long-chain omega-3 fatty acids decrease cardiovascular risk factors, including hypertriglyceridemia, and enhance the blood flow in the brain, thus reducing cerebrovascular disease by modulating risk factors (127).
Moreover, a growing body of evidence suggests that omega-3 fatty acids affect the receptor function, the neurotransmitter level, and its metabolism. In this regard, some studies indicated that these fatty acids are related to the activity of the serotoninergic system. There is a relationship between low DHA levels and low concentrations of 5-hydroxyindolacetic acid (5-HIAA), the main metabolite of serotonin, in the cerebrospinal fluid, observed in people suffering from depression and schizophrenia. This can imply that low levels of omega-3 fatty acids are associated with impaired serotonergic neurotransmission (130, 131). DHA induces BDNF synthesis, thus sustaining the life of neurons. In addition, PUFAs deficiency may impair the release and uptake of neurotransmitters, enzymatic processes, and function of ion and receptor channels such as dopaminergic, GABAergic, and cholinergic in nervous system cells, contributing to the development of psychiatric disorders including depression, anxiety, and aggression (126, 132). Some studies suggest that low consumption of fish is associated with increased prevalence of depression (129).
It is also known that the consumption of omega-3 PUFAs is recommended for the prevention of AD. Consuming fish once a week decreases the risk of AD by 60%. EPA and DHA can reduce amyloid-β production and its accumulation in plaques, and improve its clearance (127). In the Cardiovascular Health and Cognition study, consumption of fatty fish (≥ 2 servings per week) reduced the incidence of dementia by 28% and reduced the risk of AD by 41% (133).
5.5. Rapeseed oil
Rapeseed oil is regarded to be a key oilseed product in Nordic countries. It is characterized by a low level of saturated fatty acids (7%), and substantial amounts of mono- and polyunsaturated fatty acids such as alpha-linolenic acid, linoleic acid, and oleic acid (134). These fatty acids are known as neuroactive molecules (135). Oleic acid (18:1ω9 monounsaturated fatty acid) is critical for the survival of neural stem cells, which are the source of newborn neurons in the brain and are involved in mood, memory, learning, and stress response. In a rodent model study, oleic acid bound to the orphan nuclear receptor TLX/NR2E1 (nuclear receptor subfamily 2, group E, member 1), converted it from a transcriptional repressor to a transcriptional activator of cell-cycle and neurogenesis genes and induced neurogenesis and brain development (136). Similar beneficial effects were also shown in another animal study, in which oleic acid administration demonstrated its neuroprotective effects in transient and permanent focal cerebral ischemia (137). It is believed that oleic acid is associated with the onset of neurodegeneration disease through anti-inflammatory and vascular activities (138).
Moreover, multiple regression analysis of cohort study data revealed that daily consumption of oleic acid has a significantly positive effect on cognitive decline in community-dwelling Japanese elderly subjects (139). Certain dietary patterns, such as MD which contains high amounts of oleic acid, have been strongly associated with protection against age-related cognitive decline (140–142). Therefore, it is highly likely that rapeseed oil, as a source of oleic acid and PUFAs, may be a beneficial food that is closely related to neurological disorders.
6. Strengths and limitations
To the best of our knowledge, this is the first review study demonstrating the potential differential effects of ND on brain function in Nordic versus non-Nordic countries. This is important when considering promoting the adoption of the ND in non-Nordic countries to improve neurological function. Our review also discussed the association of several aspects of the ND with brain health and neurological function that may be less well-regarded. Moreover, what differentiates this review is that it was not limited to a specific neurological function and included research that investigated different neurological domains. This review had some limitations. The methods of dietary assessment and neurological function tests varied from one study to another, which is considered confounding factors.
7. Conclusion
Knowing the ingredients and nutritional content of the food we eat, provides important insight into how diet affects our brain health. Several aspects of the ND may lead to some health benefits suggesting neuroprotective effects. High consumption of fruits, vegetables, whole grains, legumes, rapeseed oil, fish, and seafood leads to the high ingestion of dietary phytochemicals, antioxidants, fibers, and mono- and polyunsaturated fatty acids in the ND pattern. The current state of knowledge attributes the possible effects of characteristic components of the ND to its antioxidant, anti-inflammatory, lipid-lowering, gut-brain-axis modulating, and ligand activities in cell signaling pathways. Based on existing evidence, the ND may be considered a recommended dietary approach for the improvement of neurological function and brain health.
7.1. Future directions
Future directions should focus on precise dietary patterns and their applications in brain health. The neurological benefits of ND are not very well investigated. On the other hand, considering the different designs of studies and limitations in the number of studies, we chose the narrative approach for the present review. There is a need to investigate the randomized trial in placebo versus Nordic diet group in different populations. Overall, more and long-term human studies are needed to confirm the neuroprotective effects of the ND.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RJ and VB researched the latest knowledge and publications in the field and wrote the article. VB designed, supervised, and corrected the article. All authors approved the final version of this article for publication.
Acknowledgments
This article is the result of the research project approved by the student research committee of Kerman University of Medical Sciences under the number 401000952 which was carried out with the financial support of the research and technology vice-chancellor of this university.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Godos, J, Currenti, W, Angelino, D, Mena, P, Castellano, S, Caraci, F, et al. Diet and mental health: review of the recent updates on molecular mechanisms. Antioxidants. (2020) 9:346. doi: 10.3390/antiox9040346
2. Friedrich, MJ. Depression is the leading cause of disability around the world. JAMA. (2017) 317:1517. doi: 10.1001/jama.2017.3828
3. James, SL, Abate, D, Abate, KH, Abay, SM, Abbafati, C, Abbasi, N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. (2018) 392:1789–858.
4. Riccò, M, Vezzosi, L, Balzarini, F, Gualerzi, G, Ranzieri, S, Signorelli, C, et al. Prevalence of Parkinson disease in Italy: a systematic review and meta-analysis. Acta Bio Medica. (2020) 91:e2020088. doi: 10.23750/abm.v91i3.9443
5. Jack, CR, Therneau, TM, Weigand, SD, Wiste, HJ, Knopman, DS, Vemuri, P, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging–Alzheimer’s association research framework. JAMA Neurol. (2019) 76:1174–83. doi: 10.1001/jamaneurol.2019.1971
6. Austen, W. An approach on the biological disease of psychiatric and neurological disorders in young patients. J Mult Scler. (2021) 8:1–2.
7. Pavón, S, Lázaro, E, Martínez, O, Amayra, I, López-Paz, J, Caballero, P, et al. Ketogenic diet and cognition in neurological diseases: a systematic review. Nutr Rev. (2021) 79:802–13. doi: 10.1093/nutrit/nuaa113
8. Mayeux, R, and Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. (2012) 2:a006239. doi: 10.1101/cshperspect.a006239
9. Francis, HM, Mirzaei, M, Pardey, MC, Haynes, PA, and Cornish, JL. Proteomic analysis of the dorsal and ventral hippocampus of rats maintained on a high fat and refined sugar diet. Proteomics. (2013) 13:3076–91. doi: 10.1002/pmic.201300124
10. Pistell, PJ, Morrison, CD, Gupta, S, Knight, AG, Keller, JN, Ingram, DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. (2010) 219:25–32. doi: 10.1016/j.jneuroim.2009.11.010
11. Yoon, J-H, and Baek, SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. (2005) 46:585–96. doi: 10.3349/ymj.2005.46.5.585
12. Estruch, R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. (2010) 69:333–40. doi: 10.1017/S0029665110001539
13. Dominguez, LJ, Veronese, N, Vernuccio, L, Catanese, G, Inzerillo, F, Salemi, G, et al. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients. (2021) 13:4080. doi: 10.3390/nu13114080
14. Pagliai, G, Sofi, F, Vannetti, F, Caiani, S, Pasquini, G, Molino Lova, R, et al. Mediterranean diet, food consumption and risk of late-life depression: the Mugello study. J Nutr Health Aging. (2018) 22:569–74. doi: 10.1007/s12603-018-1019-3
15. Nouripour, F, and Hejazi, N. Nordic diet and cardio-metabolic diseases: a review. Int J Nutr Sci. (2019) 4:105–8. doi: 10.30476/IJNS.2019.82686.1025
16. Ramezani-Jolfaie, N, Mohammadi, M, and Salehi-Abargouei, A. The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr. (2019) 58:2159–74. doi: 10.1007/s00394-018-1804-0
17. Adamsson, V, Reumark, A, Fredriksson, IB, Hammarström, E, Vessby, B, Johansson, G, et al. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: a randomized controlled trial (NORDIET). J Intern Med. (2011) 269:150–9. doi: 10.1111/j.1365-2796.2010.02290.x
18. Olsen, A, Egeberg, R, Halkjær, J, Christensen, J, Overvad, K, and Tjønneland, A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr. (2011) 141:639–44. doi: 10.3945/jn.110.131375
19. Kanerva, N, Kaartinen, NE, Schwab, U, Lahti-Koski, M, and Männistö, S. Adherence to the Baltic Sea diet consumed in the Nordic countries is associated with lower abdominal obesity. Br J Nutr. (2013) 109:520–8. doi: 10.1017/S0007114512001262
20. Kanerva, N, Loo, B-M, Eriksson, JG, Leiviskä, J, Kaartinen, NE, Jula, A, et al. Associations of the Baltic Sea diet with obesity-related markers of inflammation. Ann Med. (2014) 46:90–6. doi: 10.3109/07853890.2013.870020
21. Gunge, V, Andersen, I, Kyrø, C, Hansen, C, Dahm, C, Christensen, J, et al. Adherence to a healthy Nordic food index and risk of myocardial infarction in middle-aged Danes: the diet, cancer and health cohort study. Eur J Clin Nutr. (2017) 71:652–8. doi: 10.1038/ejcn.2017.1
22. Hansen, CP, Overvad, K, Kyrø, C, Olsen, A, Tjønneland, A, Johnsen, SP, et al. Adherence to a healthy Nordic diet and risk of stroke: a Danish cohort study. Stroke. (2017) 48:259–64. doi: 10.1161/STROKEAHA.116.015019
23. Gorelick, PB, Scuteri, A, Black, SE, DeCarli, C, Greenberg, SM, Iadecola, C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2011) 42:2672–713. doi: 10.1161/STR.0b013e3182299496
24. Männikkö, R, Komulainen, P, Schwab, U, Heikkilä, HM, Savonen, K, Hassinen, M, et al. The Nordic diet and cognition–the DR's EXTRA study. Br J Nutr. (2015) 114:231–9. doi: 10.1017/S0007114515001890
25. Eskelinen, MH, Solomon, A, Ngandu, T, Antikainen, R, Hänninen, T, Laatikainen, T, et al. Effect of berries and fruits on cognitive change during a 2-year multi-domain lifestyle intervention: the Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER) prevention (nonpharmacological)/nutrition. Alzheimers Dement. (2020) 16:e042431. doi: 10.1002/alz.042431
26. Mithril, C, Dragsted, LO, Meyer, C, Blauert, E, Holt, MK, and Astrup, A. Guidelines for the new Nordic diet. Public Health Nutr. (2012) 15:1941–7. doi: 10.1017/S136898001100351X
27. Flight, I, and Clifton, P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: a review of the literature. Eur J Clin Nutr. (2006) 60:1145–59. doi: 10.1038/sj.ejcn.1602435
28. Kanerva, N, Kaartinen, NE, Schwab, U, Lahti-Koski, M, and Männistö, S. The Baltic Sea diet score: a tool for assessing healthy eating in Nordic countries. Public Health Nutr. (2014) 17:1697–705. doi: 10.1017/S1368980013002395
29. Neuman, N, and Leer, J. Nordic Cuisine but National Identities. “New Nordic Cuisine” and the gastronationalist projects of Denmark and Sweden. Anthropol Food. (2018) 13 doi: 10.4000/aof.8723
30. Mithril, C, Dragsted, LO, Meyer, C, Tetens, I, Biltoft-Jensen, A, and Astrup, A. Dietary composition and nutrient content of the new Nordic diet. Public Health Nutr. (2013) 16:777–85. doi: 10.1017/S1368980012004521
31. Perälä, M-M, von Bonsdorff, M, Männistö, S, Salonen, MK, Simonen, M, Kanerva, N, et al. A healthy Nordic diet and physical performance in old age: findings from the longitudinal Helsinki birth cohort study. Br J Nutr. (2016) 115:878–86. doi: 10.1017/S0007114515005309
32. Åkesson, A, Andersen, LF, Kristjánsdottir, AG, Roos, E, Trolle, E, Voutilainen, E, et al. Health effects associated with foods characteristic of the Nordic diet: a systematic literature review. Food Nutr Res. (2013) 57:22790. doi: 10.3402/fnr.v57i0.22790
33. Bere, E, and Brug, J. Towards health-promoting and environmentally friendly regional diets–a Nordic example. Public Health Nutr. (2009) 12:91–6. doi: 10.1017/S1368980008001985
34. Krznarić, Ž, Karas, I, Ljubas Kelečić, D, and Vranešić, BD. The Mediterranean and Nordic diet: a review of differences and similarities of two sustainable, health-promoting dietary patterns. Front Nutr. (2021):339. doi: 10.3389/fnut.2021.683678
35. Bechthold, A, Boeing, H, Tetens, I, Schwingshackl, L, and Nöthlings, U. Perspective: food-based dietary guidelines in Europe—scientific concepts, current status, and perspectives. Advances in nutrition. (2018) 9:544–60.
36. Magnusdottir, OK, Gunnarsdottir, I, and Birgisdóttir, BE. Dietary guidelines in type 2 diabetes: the Nordic diet or the ketogenic diet? Curr Opin Endocrinol Diabetes Obes. (2017) 24:315–9. doi: 10.1097/MED.0000000000000361
37. Recommendations NN. Nordic Nutrition Recommendations 2012: Integrating nutrition and physical activity. Copenhagen, Denmark: Nordic Council of Ministers (2014). 627 p.
38. Fogelholm, M. New Nordic nutrition recommendations are here. New York: Taylor & Francis (2013). 22903 p.
39. Hemler, EC, and Hu, FB. Plant-based diets for personal, population, and planetary health. Adv Nutr. (2019) 10:S275–83. doi: 10.1093/advances/nmy117
40. Ngandu, T, Lehtisalo, J, Solomon, A, Levälahti, E, Ahtiluoto, S, Antikainen, R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. (2015) 385:2255–63. doi: 10.1016/S0140-6736(15)60461-5
41. Lehtisalo, J, Levälahti, E, Lindström, J, Hänninen, T, Paajanen, T, Peltonen, M, et al. Dietary changes and cognition over 2 years within a multidomain intervention trial—the Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER). Alzheimers Dement. (2019) 15:410–7. doi: 10.1016/j.jalz.2018.10.001
42. Stephen, R, Solomon, A, Ngandu, T, Levälahti, E, Rinne, JO, Kemppainen, N, et al. White matter changes on diffusion tensor imaging in the FINGER randomized controlled trial. J Alzheimers Dis. (2020) 78:75–86. doi: 10.3233/JAD-200423
43. Lankinen, M, Schwab, U, Kolehmainen, M, Paananen, J, Poutanen, K, Mykkänen, H, et al. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the Sysdimet study. PLoS One. (2011) 6:e22646. doi: 10.1371/journal.pone.0022646
44. Brader, L, Uusitupa, M, Dragsted, LO, and Hermansen, K. Effects of an isocaloric healthy Nordic diet on ambulatory blood pressure in metabolic syndrome: a randomized SYSDIET sub-study. Eur J Clin Nutr. (2014) 68:57–63. doi: 10.1038/ejcn.2013.192
45. Adamsson, V, Reumark, A, Cederholm, T, Vessby, B, Risérus, U, and Johansson, G. What is a healthy Nordic diet? Foods and nutrients in the NORDIET study. Food Nutr Res. (2012) 56:18189. doi: 10.3402/fnr.v56i0.18189
46. Uusitupa, M, Hermansen, K, Savolainen, MJ, Schwab, U, Kolehmainen, M, Brader, L, et al. Effects of an isocaloric healthy N ordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome–a randomized study (SYSDIET). J Intern Med. (2013) 274:52–66. doi: 10.1111/joim.12044
47. Lacoppidan, SA, Kyrø, C, Loft, S, Helnæs, A, Christensen, J, Hansen, CP, et al. Adherence to a healthy Nordic food index is associated with a lower risk of type-2 diabetes—the Danish diet, cancer and health cohort study. Nutrients. (2015) 7:8633–44. doi: 10.3390/nu7105418
48. Roswall, N, Sandin, S, Löf, M, Skeie, G, Olsen, A, Adami, H-O, et al. Adherence to the healthy Nordic food index and total and cause-specific mortality among Swedish women. Eur J Epidemiol. (2015) 30:509–17. doi: 10.1007/s10654-015-0021-x
49. Kyrø, C, Skeie, G, Loft, S, Overvad, K, Christensen, J, Tjønneland, A, et al. Adherence to a healthy Nordic food index is associated with a lower incidence of colorectal cancer in women: the diet, Cancer and health cohort study. Br J Nutr. (2013) 109:920–7. doi: 10.1017/S0007114512002085
50. Kanerva, N, Rissanen, H, Knekt, P, Havulinna, A, Eriksson, J, and Männistö, S. The healthy Nordic diet and incidence of type 2 diabetes—10-year follow-up. Diabetes Res Clin Pract. (2014) 106:e34–7. doi: 10.1016/j.diabres.2014.08.016
51. Kolehmainen, M, Ulven, SM, Paananen, J, de Mello, V, Schwab, U, Carlberg, C, et al. Healthy Nordic diet downregulates the expression of genes involved in inflammation in subcutaneous adipose tissue in individuals with features of the metabolic syndrome. Am J Clin Nutr. (2015) 101:228–39. doi: 10.3945/ajcn.114.092783
52. Haapala, EA, Eloranta, A-M, Venäläinen, T, Schwab, U, Lindi, V, and Lakka, TA. Associations of diet quality with cognition in children–the physical activity and nutrition in children study. Br J Nutr. (2015) 114:1080–7. doi: 10.1017/S0007114515001634
53. Sørensen, LB, Damsgaard, CT, Dalskov, S-M, Petersen, RA, Egelund, N, Dyssegaard, CB, et al. Diet-induced changes in iron and n-3 fatty acid status and associations with cognitive performance in 8–11-year-old Danish children: secondary analyses of the optimal well-being, development and health for Danish children through a healthy new Nordic diet school meal study. Br J Nutr. (2015) 114:1623–37. doi: 10.1017/S0007114515003323
54. Sørensen, LB, Dyssegaard, CB, Damsgaard, CT, Petersen, RA, Dalskov, S-M, Hjorth, MF, et al. The effects of Nordic school meals on concentration and school performance in 8-to 11-year-old children in the OPUS school meal study: a cluster-randomised, controlled, cross-over trial. Br J Nutr. (2015) 113:1280–91. doi: 10.1017/S0007114515000033
55. Sabet, JA, Ekman, MS, Lundvall, AS, Risérus, U, Johansson, U, Öström, Å, et al. Feasibility and acceptability of a healthy Nordic diet intervention for the treatment of depression: a randomized controlled pilot trial. Nutrients. (2021) 13:902. doi: 10.3390/nu13030902
56. Abbaszadeh, A, Saharkhiz, M, Khorasanchi, Z, Karbasi, S, Askari, M, Hoseini, ZS, et al. Impact of a Nordic diet on psychological function in young students. Nutr Health. (2021) 27:97–104. doi: 10.1177/0260106020964981
57. Shakersain, B, Rizzuto, D, Larsson, SC, Faxén-Irving, G, Fratiglioni, L, and Xu, W-L. The Nordic prudent diet reduces risk of cognitive decline in the Swedish older adults: a population-based cohort study. Nutrients. (2018) 10:229. doi: 10.3390/nu10020229
58. Serafini, M, and Peluso, I. Functional foods for health: the interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr Pharm Des. (2016) 22:6701–15. doi: 10.2174/1381612823666161123094235
59. Bourre, J-M. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. (2006) 10:377.
60. Tardy, A-L, Pouteau, E, Marquez, D, Yilmaz, C, and Scholey, A. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients. (2020) 12:228. doi: 10.3390/nu12010228
61. Heber, D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. (2004) 50:145.
62. Liu, RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. (2013) 4:384S–92S. doi: 10.3945/an.112.003517
63. Rodriguez-Casado, A. The health potential of fruits and vegetables phytochemicals: notable examples. Crit Rev Food Sci Nutr. (2016) 56:1097–107. doi: 10.1080/10408398.2012.755149
65. Li, D, Wang, P, Luo, Y, Zhao, M, and Chen, F. Health benefits of anthocyanins and molecular mechanisms: update from recent decade. Crit Rev Food Sci Nutr. (2017) 57:1729–41. doi: 10.1080/10408398.2015.1030064
66. Wang, Y, Zhao, L, Lu, F, Yang, X, Deng, Q, Ji, B, et al. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules. (2015) 20:22395–410. doi: 10.3390/molecules201219785
67. Tsakiroglou, P, VandenAkker, NE, Del Bo’, C, Riso, P, and Klimis-Zacas, D. Role of berry anthocyanins and phenolic acids on cell migration and angiogenesis: an updated overview. Nutrients. (2019) 11:1075. doi: 10.3390/nu11051075
68. Henriques, JF, Serra, D, Dinis, TC, and Almeida, LM. The anti-neuroinflammatory role of anthocyanins and their metabolites for the prevention and treatment of brain disorders. Int J Mol Sci. (2020) 21:8653. doi: 10.3390/ijms21228653
69. Kim, WI, Ryu, HJ, Kim, J-E, Seo, CH, Lee, BC, Choi, I-G, et al. Differential nuclear factor-kappa B phosphorylation induced by lipopolysaccharide in the hippocampus of P2X7 receptor knockout mouse. Neurol Res. (2013) 35:369–81. doi: 10.1179/1743132812Y.0000000137
70. Sahin, C, Dursun, S, Cetin, M, and Aricioglu, F. The neuroinflammation perspective of depression: reuniting the outstanding mechanisms of the pathophysiology. Klinik Psikofarmakoloji Bülteni. (2016) 26:196–206. doi: 10.5455/bcp.20160520092044
71. Uddin, M, Kabir, M, Jakaria, M, Mamun, AA, Niaz, K, Amran, M, et al. Endothelial PPARγ is crucial for averting age-related vascular dysfunction by stalling oxidative stress and ROCK. Neurotox Res. (2019) 36:583–601. doi: 10.1007/s12640-019-00047-5
72. Van Houten, B, Santa-Gonzalez, GA, and Camargo, M. DNA repair after oxidative stress: current challenges. Curr Opin Toxicol. (2018) 7:9–16. doi: 10.1016/j.cotox.2017.10.009
73. Liguori, I, Russo, G, Curcio, F, Bulli, G, Aran, L, Della-Morte, D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. (2018) 13:757. doi: 10.2147/CIA.S158513
74. Vauzour, D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med Cell Longev. (2012) 2012:914273. doi: 10.1155/2012/914273
75. Choi, D-Y, Lee, Y-J, Hong, JT, and Lee, H-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res Bull. (2012) 87:144–53. doi: 10.1016/j.brainresbull.2011.11.014
76. Uttara, B, Singh, AV, Zamboni, P, and Mahajan, R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. (2009) 7:65–74. doi: 10.2174/157015909787602823
77. Poulose, SM, Fisher, DR, Larson, J, Bielinski, DF, Rimando, AM, Carey, AN, et al. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J Agric Food Chem. (2012) 60:1084–93. doi: 10.1021/jf203989k
78. Jeong, J-W, Lee, WS, Shin, SC, Kim, G-Y, Choi, BT, and Choi, YH. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways. Int J Mol Sci. (2013) 14:1502–15. doi: 10.3390/ijms14011502
79. Winter, AN, Brenner, MC, Punessen, N, Snodgrass, M, Byars, C, Arora, Y, et al. Comparison of the neuroprotective and anti-inflammatory effects of the anthocyanin metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxidative Med Cell Longev. (2017) 2017:6297080. doi: 10.1155/2017/6297080
80. Wang, H-y, Wang, H, Wang, J-h, Wang, Q, Ma, Q-f, and Chen, Y-y. Protocatechuic acid inhibits inflammatory responses in LPS-stimulated BV2 microglia via NF-κB and MAPKs signaling pathways. Neurochem Res. (2015) 40:1655–60. doi: 10.1007/s11064-015-1646-6
81. Galbete, C, Kröger, J, Jannasch, F, Iqbal, K, Schwingshackl, L, Schwedhelm, C, et al. Nordic diet, Mediterranean diet, and the risk of chronic diseases: the EPIC-Potsdam study. BMC Med. (2018) 16:1–13. doi: 10.1186/s12916-018-1082-y
82. Manchali, S, Murthy, KNC, and Patil, BS. Crucial facts about health benefits of popular cruciferous vegetables. J Funct Foods. (2012) 4:94–106. doi: 10.1016/j.jff.2011.08.004
83. Connolly, EL, Sim, M, Travica, N, Marx, W, Beasy, G, Lynch, GS, et al. Glucosinolates from cruciferous vegetables and their potential role in chronic disease: investigating the preclinical and clinical evidence. Front Pharmacol. (2021) 12:2964. doi: 10.3389/fphar.2021.767975
84. Kissen, R, Rossiter, JT, and Bones, AM. The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem Rev. (2009) 8:69–86. doi: 10.1007/s11101-008-9109-1
85. Luang-In, V, Narbad, A, Nueno-Palop, C, Mithen, R, Bennett, M, and Rossiter, JT. The metabolism of methylsulfinylalkyl-and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol Nutr Food Res. (2014) 58:875–83. doi: 10.1002/mnfr.201300377
86. Wu, S, Gao, Q, Zhao, P, Gao, Y, Xi, Y, Wang, X, et al. Sulforaphane produces antidepressant-and anxiolytic-like effects in adult mice. Behav Brain Res. (2016) 301:55–62. doi: 10.1016/j.bbr.2015.12.030
87. Panjwani, AA, Liu, H, and Fahey, JW. Crucifers and related vegetables and supplements for neurologic disorders: what is the evidence? Curr Opin Clin Nutr Metabolic Care. (2018) 21:451–7. doi: 10.1097/MCO.0000000000000511
88. Folkard, DL, Melchini, A, Traka, MH, Aa, A-B, Saha, S, Mulholland, F, et al. Suppression of LPS-induced transcription and cytokine secretion by the dietary isothiocyanate sulforaphane. Mol Nutr Food Res. (2014) 58:2286–96. doi: 10.1002/mnfr.201400550
89. Kim, J, Lee, S, Choi, BR, Yang, H, Hwang, Y, Park, JHY, et al. Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways. Mol Nutr Food Res. (2017) 61:1600194. doi: 10.1002/mnfr.201600194
90. Kim, J. Pre-clinical neuroprotective evidences and plausible mechanisms of sulforaphane in Alzheimer’s disease. Int J Mol Sci. (2021) 22:2929. doi: 10.3390/ijms22062929
91. Sedlak, TW, Nucifora, LG, Koga, M, Shaffer, LS, Higgs, C, Tanaka, T, et al. Sulforaphane augments glutathione and influences brain metabolites in human subjects: a clinical pilot study. Complex Psychiatry. (2017) 3:214–22. doi: 10.1159/000487639
92. Ghazizadeh-Hashemi, F, Bagheri, S, Ashraf-Ganjouei, A, Moradi, K, Shahmansouri, N, Mehrpooya, M, et al. Efficacy and safety of sulforaphane for treatment of mild to moderate depression in patients with history of cardiac interventions: a randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin Neurosci. (2021) 75:250–5. doi: 10.1111/pcn.13276
93. Zhang, J-c, Yao, W, Dong, C, Yang, C, Ren, Q, Ma, M, et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J Nutr Biochem. (2017) 39:134–44. doi: 10.1016/j.jnutbio.2016.10.004
94. Kim, HV, Kim, HY, Ehrlich, HY, Choi, SY, Kim, DJ, and Kim, Y. Amelioration of Alzheimer’s disease by neuroprotective effect of sulforaphane in animal model. Amyloid. (2013) 20:7–12. doi: 10.3109/13506129.2012.751367
95. Sunkaria, A, Bhardwaj, S, Yadav, A, Halder, A, and Sandhir, R. Sulforaphane attenuates postnatal proteasome inhibition and improves spatial learning in adult mice. J Nutr Biochem. (2018) 51:69–79. doi: 10.1016/j.jnutbio.2017.09.016
96. Lim, JL, van der Pol, SM, Baron, W, McCord, JM, de Vries, HE, and van Horssen, J. Protandim protects oligodendrocytes against an oxidative insult. Antioxidants. (2016) 5:30. doi: 10.3390/antiox5030030
97. Soler, C, Soriano, JM, and Mañes, J. Apple-products phytochemicals and processing: a review. Nat Prod Commun. (2009) 4:1934578X0900400504.
98. Boots, AW, Haenen, GR, and Bast, A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. (2008) 585:325–37. doi: 10.1016/j.ejphar.2008.03.008
99. Khan, A, Ali, T, Rehman, SU, Khan, MS, Alam, SI, Ikram, M, et al. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol. (2018) 9:1383. doi: 10.3389/fphar.2018.01383
100. Yang, Y, Liu, X, Wu, T, Zhang, W, Shu, J, He, Y, et al. Quercetin attenuates AZT-induced neuroinflammation in the CNS. Sci Rep. (2018) 8:1–8. doi: 10.1038/s41598-018-24618-2
101. Costa, LG, Garrick, JM, Roquè, PJ, and Pellacani, C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxidative Med Cell Longev. (2016) 2016:2986796. doi: 10.1155/2016/2986796
102. Gan, L, and Johnson, JA. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochimica et Biophysica Acta. (2014) 1842:1208–18. doi: 10.1016/j.bbadis.2013.12.011
103. Guo, H, Wu, H, Sajid, A, and Li, Z. Whole grain cereals: the potential roles of functional components in human health. Crit Rev Food Sci Nutr. (2022) 62:8388–402. doi: 10.1080/10408398.2021.1928596
104. Slavin, J. Whole grains and human health. Nutr Res Rev. (2004) 17:99–110. doi: 10.1079/NRR200374
105. Gill, SK, Rossi, M, Bajka, B, and Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. (2021) 18:101–16. doi: 10.1038/s41575-020-00375-4
106. Dvoncova, D, Havrlentova, M, Hlinkova, A, and Hozlar, P. Effect of fertilization and variety on the beta-glucan content in the grain of oats. Żywność Nauka Technologia Jakość. (2010) 17:108–16.
107. Peterson, DM, Wesenberg, DM, and Burrup, DE. β-Glucan content and its relationship to agronomic characteristics in elite oat germplasm. Crop Sci. (1995) 35:965–70. doi: 10.2135/cropsci1995.0011183X003500040005x
108. Mayer, EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. (2011) 12:453–66. doi: 10.1038/nrn3071
109. Kim, C-S, Cha, L, Sim, M, Jung, S, Chun, WY, Baik, HW, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol. (2021) 76:32–40. doi: 10.1093/gerona/glaa090
110. Xu, H, Li, S, Song, X, Li, Z, and Zhang, D. Exploration of the association between dietary fiber intake and depressive symptoms in adults. Nutrition. (2018) 54:48–53. doi: 10.1016/j.nut.2018.03.009
111. Gopinath, B, Flood, VM, Burlutksy, G, Louie, JC, and Mitchell, P. Association between carbohydrate nutrition and prevalence of depressive symptoms in older adults. Br J Nutr. (2016) 116:2109–14. doi: 10.1017/S0007114516004311
112. Kim, C-S, Byeon, S, and Shin, D-M. Sources of dietary fiber are differently associated with prevalence of depression. Nutrients. (2020) 12:2813. doi: 10.3390/nu12092813
113. Sherry, CL, Kim, SS, Dilger, RN, Bauer, LL, Moon, ML, Tapping, RI, et al. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav Immun. (2010) 24:631–40. doi: 10.1016/j.bbi.2010.01.015
114. O’Mahony, SM, Clarke, G, Borre, Y, Dinan, TG, and Cryan, J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. (2015) 277:32–48. doi: 10.1016/j.bbr.2014.07.027
115. Schroeder, FA, Lin, CL, Crusio, WE, and Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. (2007) 62:55–64. doi: 10.1016/j.biopsych.2006.06.036
116. Le Couteur, DG, Solon-Biet, S, Cogger, VC, Mitchell, SJ, Senior, A, de Cabo, R, et al. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci. (2016) 73:1237–52. doi: 10.1007/s00018-015-2120-y
117. Wahl, D, Solon-Biet, SM, Wang, Q-P, Wali, JA, Pulpitel, T, Clark, X, et al. Comparing the effects of low-protein and high-carbohydrate diets and caloric restriction on brain aging in mice. Cell Rep. (2018) 25:2234–43. e6. doi: 10.1016/j.celrep.2018.10.070
118. Gorzkiewicz, J, Bartosz, G, and Sadowska-Bartosz, I. The potential effects of phytoestrogens: the role in neuroprotection. Molecules. (2021) 26:2954. doi: 10.3390/molecules26102954
119. Schreihofer, DA. Neuroprotection by dietary isoflavones and their role in cerebral ischemia In: Bioactive nutraceuticals and dietary supplements in neurological and brain disease. Amsterdam: Elsevier (2015). 385–94.
120. Lee, Y-B, Lee, HJ, and Sohn, HS. Soy isoflavones and cognitive function. J Nutr Biochem. (2005) 16:641–9. doi: 10.1016/j.jnutbio.2005.06.010
121. Qian, Y, Guan, T, Huang, M, Cao, L, Li, Y, Cheng, H, et al. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem Int. (2012) 60:759–67. doi: 10.1016/j.neuint.2012.03.011
122. Jantaratnotai, N, Utaisincharoen, P, Sanvarinda, P, Thampithak, A, and Sanvarinda, Y. Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. Int Immunopharmacol. (2013) 17:483–8. doi: 10.1016/j.intimp.2013.07.013
123. Wang, X, Chen, S, Ma, G, Ye, M, and Lu, G. Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport. (2005) 16:267–70. doi: 10.1097/00001756-200502280-00013
124. Astorg, P, Couthouis, A, Bertrais, S, Arnault, N, Meneton, P, Guesnet, P, et al. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukot Essent Fat Acids. (2008) 78:171–82. doi: 10.1016/j.plefa.2008.01.003
125. Murakami, K, Miyake, Y, Sasaki, S, Tanaka, K, and Arakawa, M. Fish and n-3 polyunsaturated fatty acid intake and depressive symptoms: Ryukyus child health study. Pediatrics. (2010) 126:e623–30. doi: 10.1542/peds.2009-3277
126. Pawełczyk, T. The role of omega-3 polyunsaturated fatty acids in the etiopathogenesis and treatment of psychiatric disorders. Farmakoterapia w Psychiatrii i Neurologii. (2010) 26:71–7.
127. Fotuhi, M, Mohassel, P, and Yaffe, K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Rev Neurol. (2009) 5:140–52. doi: 10.1038/ncpneuro1044
128. Cardoso, C, Afonso, C, and Bandarra, NM. Dietary DHA and health: cognitive function ageing. Nutr Res Rev. (2016) 29:281–94. doi: 10.1017/S0954422416000184
129. Wendolowicz, A, Stefanska, E, and Ostrowska, L. Influence of selected dietary components on the functioning of the human nervous system. Rocz Panstw Zakl Hig. (2018) 69:15–21.
130. Morris, G, Berk, M, Carvalho, A, Caso, JR, Sanz, Y, Walder, K, et al. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol. (2017) 54:4432–51. doi: 10.1007/s12035-016-0004-2
131. Kennedy, DO. B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients. (2016) 8:68. doi: 10.3390/nu8020068
132. Rao, TS, Asha, M, Ramesh, B, and Rao, KJ. Understanding nutrition, depression and mental illnesses. Indian J Psychiatry. (2008) 50:77. doi: 10.4103/0019-5545.42391
133. Huang, TL, Zandi, P, Tucker, K, Fitzpatrick, A, Kuller, L, Fried, L, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE ε4. Neurology. (2005) 65:1409–14. doi: 10.1212/01.wnl.0000183148.34197.2e
134. Lin, L, Allemekinders, H, Dansby, A, Campbell, L, Durance-Tod, S, Berger, A, et al. Evidence of health benefits of canola oil. Nutr Rev. (2013) 71:370–85. doi: 10.1111/nure.12033
135. Galán-Arriero, I, Serrano-Muñoz, D, Gómez-Soriano, J, Goicoechea, C, Taylor, J, Velasco, A, et al. The role of Omega-3 and Omega-9 fatty acids for the treatment of neuropathic pain after neurotrauma. Biochimica et Biophysica Acta. (2017) 1859:1629–35. doi: 10.1016/j.bbamem.2017.05.003
136. Kandel, P, Semerci, F, Mishra, R, Choi, W, Bajic, A, Baluya, D, et al. Oleic acid is an endogenous ligand of TLX/NR2E1 that triggers hippocampal neurogenesis. Proc Natl Acad Sci. (2022) 119:e2023784119. doi: 10.1073/pnas.2023784119
137. Song, J, Kim, Y-S, Lee, DH, Lee, SH, Park, HJ, Lee, D, et al. Neuroprotective effects of oleic acid in rodent models of cerebral ischaemia. Sci Rep. (2019) 9:10732. doi: 10.1038/s41598-019-47057-z
138. López-Miranda, J, Pérez-Jiménez, F, Ros, E, De Caterina, R, Badimón, L, Covas, MI, et al. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis. (2010) 20:284–94. doi: 10.1016/j.numecd.2009.12.007
139. Sakurai, K, Shen, C, Shiraishi, I, Inamura, N, and Hisatsune, T. Consumption of oleic acid on the preservation of cognitive functions in Japanese elderly individuals. Nutrients. (2021) 13:284. doi: 10.3390/nu13020284
140. Willcox, DC, Scapagnini, G, and Willcox, BJ. Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev. (2014) 136:148–62. doi: 10.1016/j.mad.2014.01.002
141. Omar, SH. Mediterranean and MIND diets containing olive biophenols reduces the prevalence of Alzheimer’s disease. Int J Mol Sci. (2019) 20:2797. doi: 10.3390/ijms20112797
142. Panza, F, Solfrizzi, V, Colacicco, A, Dintrono, A, Capurso, C, Torres, F, et al. Mediterranean diet and cognitive decline. Public Health Nutr. (2004) 7:959–63. doi: 10.1079/PHN2004561
Glossary
Keywords: diet, Nordic, brain, neuroprotection, cognition
Citation: Jafari RS and Behrouz V (2023) Nordic diet and its benefits in neurological function: a systematic review of observational and intervention studies. Front. Nutr. 10:1215358. doi: 10.3389/fnut.2023.1215358
Edited by:
Barbara Shukitt-Hale, Tufts University, United StatesReviewed by:
Zahra Yari, National Nutrition and Food Technology Research Institute, IranDevin Wahl, Colorado State University, United States
Copyright © 2023 Jafari and Behrouz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vahideh Behrouz, dmFoaWRlaC5iZWhyb3V6QGdtYWlsLmNvbQ==
 Reyhaneh Sadat Jafari
Reyhaneh Sadat Jafari Vahideh Behrouz
Vahideh Behrouz