
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 20 June 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1211575
This article is part of the Research TopicPerioperative Nutrition and Surgical OutcomesView all 9 articles
 Tyler McKechnie1,2
Tyler McKechnie1,2 Yung Lee1,3
Yung Lee1,3 Joanna Dionne2
Joanna Dionne2 Aristithes Doumouras1,2,4
Aristithes Doumouras1,2,4 Sameer Parpia2
Sameer Parpia2 Mohit Bhandari2,5
Mohit Bhandari2,5 Cagla Eskicioglu1,4*
Cagla Eskicioglu1,4*Purpose: To optimize patients prior to bariatric surgery, very low energy diets (VLEDs) are often employed for 2–4 weeks preoperatively. They are known to result in preoperative weight loss, decrease liver volume, and decrease surgeon-perceived operative difficulty. Their impact on postoperative morbidity has been less extensively studied. We performed a focused systematic review and meta-analysis with the aim of comparing preoperative VLEDs prior to bariatric surgery with controls in terms of overall postoperative morbidity.
Methods: MEDLINE, Embase, and CENTRAL were searched from database inception to February 2023. Articles were eligible for inclusion if they were randomized controlled trials (RCTs) comparing postoperative morbidity in adult patients (i.e., over the age of 18) receiving a VLED with liquid formulation to those receiving a non-VLED control prior to elective bariatric surgery. Outcomes included overall 30-day postoperative morbidity and preoperative weight loss. An inverse variance meta-analysis was performed with GRADE assessment of the quality of evidence.
Results: After reviewing 2,525 citations, four RCTs with 294 patients receiving preoperative VLEDs with liquid formulation and 294 patients receiving a non-VLED control met inclusion. Patients receiving VLED experienced significantly more preoperative weight loss than patients receiving control (mean difference (MD) 3.38 kg, 95% confidence interval (CI) 1.06–5.70, p = 0.004, I2 = 95%). According to low certainty evidence, there was a non-significant reduction in 30-day postoperative morbidity in patients receiving VLED prior to bariatric surgery (risk ratio (RR) 0.67, 95%CI 0.39–1.17, p = 0.16, I2 = 0%).
Conclusion: The impact of preoperative VLEDs on postoperative outcomes following bariatric surgery remains unclear. It is possible that VLEDs may contribute to decreased postoperative morbidity, but further larger prospective trials are required to investigate the signal identified in this study.
Obesity is a worldwide epidemic. More than 10% of the world population qualifies as obese, and this proportion is only expected to increase (1). Along with the rising prevalence of obesity, we have witnessed an explosion of weight reduction interventions. Among the most popular and effective to date is bariatric surgery (2). Bariatric surgery is widely regarded as the most sustainable form of weight loss and can improve several obesity-related comorbidities, such as type II diabetes, obstructive sleep apnea, and non-alcoholic fatty liver disease (3–5).
To optimize patients prior to bariatric surgery, very low energy diets (VLEDs) are often employed for 2–4 weeks preoperatively. Current Canadian Adult Obesity Clinical Practice Guidelines recommend 2–3 weeks of preoperative VLED aiming for 650–900 kilocalories (kcal) consumed daily with the use of commercially available liquid supplements such as OptifastⓇ and ModifastⓇ (6). Similarly, enhanced recovery after surgery (ERAS) guidelines for bariatric surgery recommend a 2–4-week period of VLED consumption (7). Prior randomized controlled trials (RCTs) evaluating VLEDs in bariatric surgery have shown effective preoperative weight loss with these interventions, as well as significant reductions in liver volume and visceral fat volume, with corresponding decreases in surgeon-perceived intraoperative difficulty (8–11). However, their impact on postoperative morbidity has been less extensively studied.
One of the earliest RCTs performed by Van Nieuwenhove et al. demonstrated a significant reduction in overall 30-day postoperative morbidity in patients receiving preoperative VLEDs prior to bariatric surgery (8). These results have not been reliably reproduced by subsequent RCTs (12, 13). A systematic review and meta-analysis in 2011 by Cassie et al. reported a significant reduction in postoperative complications in bariatric surgery patients receiving preoperative weight loss interventions compared with controls, but they combined randomized and non-randomized data (14). Additionally, multiple RCTs have been published. Contemporary systematic reviews have focused on reductions in liver volume and preoperative weight loss, with no meta-analyzing of postoperative morbidity data (15–17). As such, we performed a focused systematic review and meta-analysis with the aim of comparing preoperative VLEDs prior to bariatric surgery with controls in terms of overall postoperative morbidity. We hypothesize that preoperative VLEDs will induce significant weight reduction and associated with reduced postoperative morbidity as compared with control interventions.
The following databases covering the period from database inception through February 2023 were searched: Medline, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). The search was designed and conducted by a medical research librarian with input from study investigators. Search terms included “bariatric surgery”, “gastric bypass”, “very low energy diet”, and “very low calorie diet” (complete search strategy available in Supplementary Tables 1, 2). The references of studies meeting inclusion criteria were searched manually to ensure that all relevant articles were included. This systematic review and meta-analysis are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) a priori (CRD 42023403021; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=403021).
Articles were eligible for inclusion if they were randomized controlled trials (RCTs) comparing postoperative morbidity in adult patients (i.e., over the age of 18) receiving a VLED with liquid formulation to those receiving a non-VLED control prior to elective bariatric surgery. Only studies evaluating VLEDs with liquid formulation (e.g., OptifastⓇ, ModifastⓇ) were considered for inclusion to assess the impact of liquid formulation-based protocols on preoperative weight loss and postoperative morbidity. Studies evaluating lifestyle-based interventions (e.g., dieting, exercise) were not considered for inclusion. Studies in which VLEDs with liquid formulation were used in both the intervention and control groups were excluded. Any reported postoperative morbidity (i.e., overall postoperative morbidity, infectious morbidity, and wound complications) was considered adequate for inclusion. Studies that did not report postoperative morbidity were excluded. Single-armed studies evaluating VLEDs or comparative studies comparing two different types of VLEDs were not considered for inclusion. Finally, non-randomized studies, systematic reviews, meta-analyses, and editorials were excluded.
The outcomes were overall 30-day postoperative morbidity and preoperative weight loss in kilograms (kg). The majority of studies evaluating preoperative VLEDs with liquid formulation prior to bariatric surgery do not clearly define postoperative morbidity as it is not a commonly reported outcome and has yet to be analyzed as a primary outcome (8, 10, 12, 13, 18, 19). For the purposes of this review, postoperative morbidity was defined as any deviation from the expected postoperative course within 30 days of the index operation as reported by each included study. If studies reported overall morbidity as a pooled outcome, this was extracted preferentially, followed by overall infectious morbidity, gastrointestinal morbidity, and wound complications.
Two reviewers independently evaluated the systematically searched titles and abstracts using a standardized, pilot-tested form. Discrepancies that occurred at the title and abstract screening phases were resolved by the inclusion of the study. At the full-text screening stage, discrepancies were resolved by consensus between the reviewers. If disagreement persisted, an additional reviewer was consulted. Two reviewers independently conducted data extraction into a data collection form designed a priori. The extracted data included study characteristics (e.g., author, year of publication, and study design), patient demographics (e.g., age, gender, body mass index [BMI], and comorbidities), treatment characteristics (e.g., VLED details, type of bariatric surgery), and postoperative morbidity (e.g., overall, infectious, wound).
The risk of bias for RCTs was assessed using the Cochrane Risk of Bias Tool for Randomized Controlled Trials 2.0 (20). Quality of evidence for estimates derived from meta-analyses was assessed by Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) (21). Two reviewers assessed the risk of bias and certainty of evidence independently. Discrepancies were discussed among the reviewers until a consensus was reached.
All statistical analyses were performed on STATA version 15 (StataCorp, College, TX) and Cochrane Review Manager 5.3 (London, United Kingdom). A meta-analysis was performed using an inverse variance random effects model for all comparative data. Pooled effect estimates for binary outcomes were estimated with risk ratios (RR) along with their respective 95% confidence intervals (CI). Pooled effect estimates for continuous outcomes were estimated with mean differences (MD) along with their respective 95% CI. Mean and standard deviation (SD) were estimated for studies that only reported median and interquartile range (IQR) using the method described by Wan et al. (22). Missing SD data were, then, calculated according to the prognostic method (23). Assessment of the between-study heterogeneity was carried out using the I2 statistic. An I2 greater than 40% was considered to represent considerable heterogeneity (24). Bias in meta-analyzed outcomes was assessed with funnel plots when data from more than 10 studies were included in the analysis (25). A leave-one-out sensitivity analysis was performed by iteratively removing one study at a time from the inverse variance random effects model to ensure that pooled effect estimates were not driven by a single study. A systematic narrative summary was provided for each outcome.
From 2,525 unique citations, four RCTs with 294 patients receiving preoperative VLEDs with liquid formulation (mean age: 40.8 years, female: 74.6%, and mean BMI: 44.1 kg/m2) and 294 patients receiving a non-VLED control (mean age: 41.4 years, female: 73.0%, and mean BMI: 44.1 kg/m2) were included (8, 12, 13, 19). A total of 21 studies were excluded at the full-text review stage (Appendix A1). A PRISMA flow diagram of the study screening process is presented in Figure 1. Included studies were conducted between 2011 and 2019. Postoperative morbidity was a secondary or tertiary outcome in all included studies. Study characteristics and demographic details for the included studies are presented in Table 1.
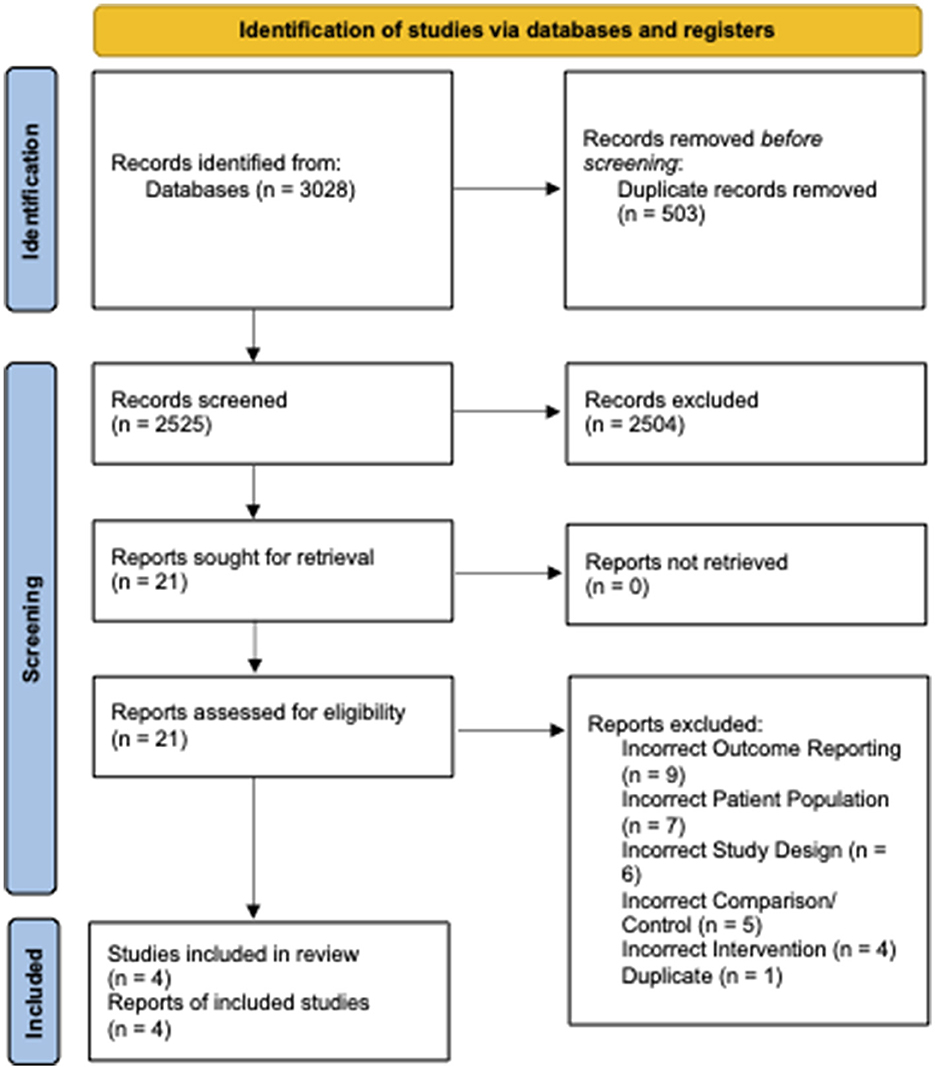
Figure 1. PRISMA diagram—transparent reporting of systematic reviews and meta-analysis flow diagram outlining the search strategy results from the initial search to included studies.
A detailed description of the preoperative VLEDs with liquid supplementation utilized for each of the included studies is shown in Table 2. Two of the studies utilized OptifastⓇ, one utilized ProdimedⓇ, and the other utilized a skim-milk liquid supplementation. All diets targeted 650–800 kcal per day. Duration of the preoperative VLED ranged from 2 to 4 weeks. Adherence was only reported by Contreras et al. they reported that 94% of patients included in the intervention arm consumed 80% or more of the prescribed doses of liquid supplementation (i.e., “high adherence”) (19).
Specific diets were assigned to the control group in two of the RCTs. Gils-Contreras et al. randomly assigned patients 1:1 to VLED or low energy diet (LED). The LED had the same macronutrient composition (%) as the VLED; however, patients consumed approximately 1,200 kcal per day (vs. 800 kcal in the VLED group). Schouten et al. prescribed a strict “standard diet” to their control group that consisted of 657 kcal per day, 86 g of protein, 20 g of carbohydrate, and 25 g of fat. The control group in the RCT performed by Van Nieuwenhove et al. (8) did not alter the control group's diet. Patients were instructed to “have their regular diet” up to the evening prior to surgery. The most recent RCT by Chakravartty et al. (12) did not describe the control intervention.
Anthropometric data for each included study are presented in Table 3. The mean pre-intervention weight loss for patients receiving VLEDs was 6.2 kg and for the patients receiving control was 3.4 kg. The mean reported weight change for all intervention groups across all studies was negative (i.e., on average, all patients, regardless of study arm lost weight). Upon pooling data from all four included studies, patients receiving VLED experienced significantly more weight loss than patients receiving control (MD 3.38 kg, 95%CI 1.06–5.70, p = 0.004, I2 = 95%) (Figure 2). The results were similar to the leave-one-out sensitivity analysis.
According to each of the included studies, postoperative morbidity is presented in Table 4. Three of the included studies reported 30-day postoperative morbidity as a composite of all system/organ-specific complications within 30 days of the index surgery, while Chakravartty et al. (12) reported 30-day postoperative morbidity without defining the types of complications (12). Upon pooling data from all four included studies, there was no significant difference in 30-day postoperative morbidity between patients receiving and not receiving VLED prior to bariatric surgery (RR 0.67, 95%CI 0.39–1.17, p = 0.16, I2 = 0%) (Figure 3). The results were similar to the leave-one-out sensitivity analysis.
According to the Cochrane Tool, the risk of bias for RCTs 2.0 for each of the included studies is presented in Figure 4. The study by Schouten et al. was at low risk of bias across all domains (13). The studies by Van Nieuwenhove et al. and Contreras et al. were at unclear risk of bias due to deviations from the intervention without blinding of participants or the research team (8, 19). The study by Chakravartty et al. was at unclear risk of bias due to an imbalance of baseline covariates between the groups (12).
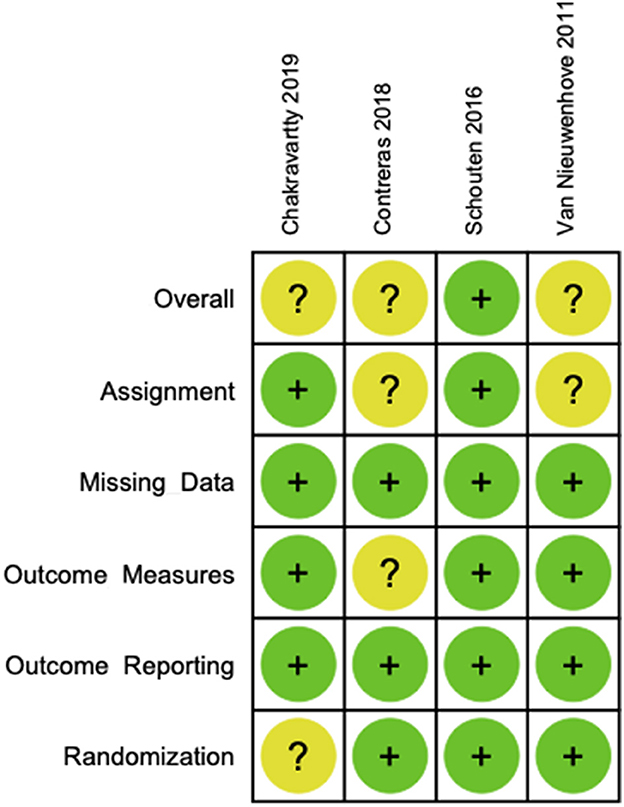
Figure 4. Cochrane risk of bias tool for randomized controlled trials 2.0—individual study analyses.
The GRADE certainty of evidence summary table is presented in Figure 5. Overall, certainty of evidence for 30-day postoperative morbidity was low. The certainty of evidence supporting this outcome was downgraded due to heterogeneity in VLEDs, a small overall pooled sample size (n = 588), and a low outcome event rate (n = 50). Overall, certainty of evidence for preoperative weight loss was very low. The certainty of evidence supporting this outcome was downgraded due to heterogeneity in VLEDs, heterogeneity in the pooled estimate (i.e., I2 > 40%), wide 95% CIs for the pooled effect estimate, and high risk of bias due to lack of blinding.
While preoperative VLEDs for bariatric surgery are well established and are associated with benefits such as decreased visceral fat, surgeon-perceived difficulty, and operative time, data pertaining to their impact on postoperative morbidity are less robust (14, 15). This review identified four RCTs comparing VLEDs with liquid formulation to controls prior to bariatric surgery in terms of postoperative morbidity. Preoperative VLEDs with liquid formulation resulted in significantly more preoperative weight loss than control interventions. Overall, there was a point estimate suggesting a 33% reduction in the risk of overall 30-day postoperative morbidity with the use of VLEDs with liquid formulation—a point estimate suggesting an important benefit; however, the wide 95% CIs and resultant type II error risk create uncertainty (RR 0.67, 95% CI 0.39–1.17, p = 0.16). The certainty of evidence according to GRADE was low.
Obesity is associated with an increased risk of a number of postoperative complications across an array of surgical specialties (26). It induces systemic dysregulation across a number of biochemical pathways that, when combined with the physiologic stress of surgery, increase the vulnerability of the host to adverse events (27). In particular, obesity, which is often associated with insulin resistance, can have a major impact on surgical wound healing (8, 28). Not only there is heightened mechanical stress but also impaired cellular immune function due to insulin resistance and other obesity-associated comorbidities can increase the risk of surgical site infection, wound hematomas, wound seromas, and dehiscence (29, 30). Moreover, impaired immune function also places these patients at higher risk of postoperative infectious complications such as urinary tract infection and pneumonia (31). Obesity impacts the ability to mobilize in general, and this is exacerbated postoperatively, which could, in part, explain the increased rates of atelectasis and venous thromboembolism in these patients (32, 33). Obese patients are even at significantly greater risk of postoperative mortality compared with non-obese counterparts (31). Altogether, interventions targeted at reducing weight preoperatively for obese surgical patients should thus have the potential to impact postoperative outcomes. Moreover, preoperative VLEDs can improve glycemic control in patients with type II diabetes and thus may contribute to a reduction in postoperative complications by mitigating the adverse impacts of insulin resistance (34). Preoperative VLEDs in the present meta-analysis demonstrated an ability to reduce weight in a short period of time (mean weight loss of 6.2 kg). The meta-analysis of postoperative morbidity resulted in a point estimate suggesting an important benefit; however, the wide 95% CIs and resultant type II error risk create significant uncertainty as to whether the preoperative weight loss induced by the VLEDs significantly influenced postoperative morbidity (RR 0.67, 95%CI 0.39–1.17, p = 0.16, I2 = 0%).
Due to the systemic complications associated with obesity, as well as increased visceral and subcutaneous fat volume, obese patients across all surgical specialties, not just bariatric surgery, are at heightened postoperative risk (26, 35, 36). Thus, all obese surgical patients may also stand to benefit from preoperative VLEDs. Preoperative optimization via VLEDs for obese patients undergoing non-bariatric surgery has been studied with small RCTs and retrospective data. A systematic review by our research group published in 2022 identified 13 studies, evaluating their use prior to non-bariatric surgery (37). Postoperative morbidity was only reported in three RCTs, varying across orthopedic surgery and hepatobiliary surgery (35, 38, 39). However, similar to the present study, preoperative VLEDs were effective at inducing preoperative weight loss, with studies reporting preoperative weight loss ranging from 3.2 kg to 19.2 kg (37). The systematic review also noted consistent decreases in operative time and estimated blood loss, which can be associated with decreased postoperative morbidity (37). Along with the data from the present systematic review, we believe that preoperative VLEDs are safe for obese patients undergoing both bariatric and non-bariatric surgery that has the potential to decrease overall postoperative morbidity, but further study is required by way of large, high-quality RCTs.
The meta-analysis for overall 30-day postoperative morbidity in the present study suggested minimal between-study heterogeneity (I2 = 0%). However, there was significant heterogeneity in observed preoperative weight loss between studies (I2 = 95%). While there were insufficient data to explore heterogeneity through subgroup analyses, there were significant differences between the dietary interventions among the included studies that may explain the heterogeneity. Van Nieuwenhove et al. and Contreras et al. utilized Optifast™, Schouten et al. utilized Prodimed™, and Chakravartty et al. utilized a skim-milk liquid supplement (8, 12, 13, 19). Moreover, the duration of the intervention period varied across studies, with Van Nieuwenhove et al. and Schouten et al. intervening for 2 weeks, Contreras et al. intervening for 3 weeks, and Chakravartty et al. intervening for 4 weeks (8, 12, 13, 19). Both Optifast programs induced significant preoperative weight loss. The weight loss experienced by the patients in the intervention group compared with the control group in the Van Nieuwenhove et al. cohort was greater than the Contreras et al. cohort, despite the intervention period being a week shorter (4.5 kg vs. 2.3 kg) (8, 19). The only cohort that did not experience significant preoperative weight loss compared with control was that reported by Schouten et al., which utilized the Prodimed program (13). This program is much less commonly relied upon as a VLED than other programs such as Optifast, Modifast, and Formulite (15, 17, 40). The study that reported the largest MD in preoperative weight loss between intervention and control was Chakravartty et al., which also employed the longest intervention period (4 weeks). This may indicate that there is increased weight loss with longer intervention periods (12). Altogether, there remains significant heterogeneity in research and clinical practice in terms of VLED liquid formulation products and duration. Further study is required to determine the optimal intervention formulation and duration.
The strengths of this systematic review and meta-analysis include the thorough methodology, quality of the included studies, comprehensive risk of bias analysis, evaluation of the certainty of evidence with GRADE, and novelty. The study limitations include a small number of included studies (n = 4), small number of pooled participants (n = 588), small number of pooled outcome events (n = 50), lack of adherence data and heterogeneity of the included dietary interventions, and resultant weight loss. The small number of included studies and participants limited statistical power, such that the meta-analysis was underpowered to detect a difference in overall 30-day postoperative morbidity. Heterogeneity in VLED interventions, specifically the type of liquid supplementation and duration of the intervention, may impact compliance, weight loss outcomes, and postoperative outcomes (19, 40). Similarly, significance between study heterogeneity existed in terms of control interventions, with some studies including active controls and others not, thus potentially attenuating the relative risk reduction observed with the intervention. These effects could not be explored via subgroups due to the deficit in the quantity of included data. The data from the included studies were limited due to a high risk of bias, mostly due to a lack of blinding and a paucity of compliance data.
In summary, the impact of preoperative VLEDs on postoperative outcomes following bariatric surgery remains unclear. It is possible that VLEDs may contribute to decreased postoperative morbidity, but further larger prospective trials are required to investigate the signal identified in this study. Furthermore, large trials investigating the use of preoperative VLEDs in obese patients undergoing non-bariatric surgery are required to determine the generalizability of these interventions aimed toward optimizing a continuously growing patient population.
All authors involved in the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revision of the manuscript, approval of the final version of the manuscript, and agreement to be accountable for all aspects of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1211575/full#supplementary-material
1. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. (2019) 92:6–10. doi: 10.1016/j.metabol.2018.09.005
2. Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Jr WSY, Weidenbacher HJ, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. (2016) 151:1046–55. doi: 10.1001/jamasurg.2016.2317
3. Dixon JB le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. (2012) 379:2300–11. doi: 10.1016/S0140-6736(12)60401-2
4. Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2019) 17:1040–1060.e11. doi: 10.1016/j.cgh.2018.10.017
5. O'Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. (2004) 292:1724. doi: 10.1007/s11695-018-3525-0
6. Garneau P, Glazer S, Jackson T, Sampath S, Reed K, Christou N, et al. Guidelines for Canadian bariatric surgical and medical centres: a statement from the Canadian Association of Bariatric Physicians and Surgeons. Canadian J Surg. (2022) 65:E170–7. doi: 10.1503/cjs.020719
7. Stenberg E, Dos Reis Falcão LF, O'Kane M, Liem R, Pournaras DJ, Salminen P, et al. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J Surg. (2022) 46:729–51. doi: 10.1007/s00268-021-06394-9
8. Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, van Dielen F, Wiezer R, Janssen I, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass a randomized multicenter study. Arch Surg. (2011) 146:1300–5. doi: 10.1001/archsurg.2011.273
9. Heinberg LJ, Schauer PR. Pilot Testing of a Portion-Controlled, Commercially Available Diet on Presurgical Weight Loss and Metabolic Outcomes in Patients Undergoing Bariatric Surgery. Obes Surg. (2014) 24:1817–20. doi: 10.1007/s11695-014-1371-2
10. Carbajo MA, Castro MJ, Kleinfinger S, Gómez-Arenas S, Ortiz-Solórzano J, Wellman R, et al. Effects of a balanced energy and high protein formula diet (Vegestart complet®) vs. low-calorie regular diet in morbid obese patients prior to bariatric surgery (laparoscopic single anastomosis gastric bypass): A prospective, double-blind randomized study. Nutr Hosp. (2010) 25:939–48.
11. Bakker N, van den Helder RS, Geenen RWF, Hunfeld MA, Cense HA, Demirkiran A, et al. Four weeks of preoperative omega-3 fatty acids reduce liver volume: a randomised controlled trial. Obes Surg. (2019) 29:2037–2044. doi: 10.1007/s11695-019-03814-7
12. Chakravartty S, Vivian G, Mullholland N, Shaikh H, McGrath J, Sidhu PS, et al. Preoperative liver shrinking diet for bariatric surgery may impact wound healing: a randomized controlled trial. Surg Obes Relat Dis. (2019) 15:117–25. doi: 10.1016/j.soard.2018.10.001
13. Schouten R, van der Kaaden I, van't Hof G, Feskens PGBM. Comparison of Preoperative Diets Before Bariatric Surgery: a Randomized, Single-Blinded, Non-inferiority Trial. Obes Surg. (2016) 26:1743–9. doi: 10.1007/s11695-015-1989-8
14. Cassie S, Menezes C, Birch DW, Shi X, Karmali S. Effect of preoperative weight loss in bariatric surgical patients: A systematic review. Surg Obes Relat Dis. (2011) 7:760–7. doi: 10.1016/j.soard.2011.08.011
15. Naseer F, Shabbir A, Livingstone B, Price R, Syn NL, Flannery O. The efficacy of energy-restricted diets in achieving preoperative weight loss for bariatric patients: a systematic review. Obes Surg. (2018) 28:3678–90. doi: 10.1007/s11695-018-3451-1
16. Romeijn MM, Kolen AM, Holthuijsen DDB, Janssen L, Schep G, Leclercq WKG, et al. Effectiveness of a Low-Calorie Diet for Liver Volume Reduction Prior to Bariatric Surgery: a Systematic Review. Obes Surg. (2021) 31:350–356. doi: 10.1007/s11695-020-05070-6
17. Holderbaum M, Casagrande DS, Sussenbach S, Buss C. Effects of very low calorie diets on liver size and weight loss in the preoperative period of bariatric surgery: a systematic review. Surg Obes Relat Dis. (2018) 14:237–44. doi: 10.1016/j.soard.2017.09.531
18. Faria SL, Faria OP, Cardeal MDA, Ito MK. Effects of a very low calorie diet in the preoperative stage of bariatric surgery: A randomized trial. Surg Obes Relat Dis. (2015) 11:230–7. doi: 10.1016/j.soard.2014.06.007
19. Contreras AG, Sanjaume AB, Jaime MM, Soler AR, Pereferrer FS, López AM, et al. Effects of two preoperatory weight loss diets on hepatic volume, metabolic parameters, and surgical complications in morbid obese bariatric surgery candidates: a randomized clinical trial. Obes Surg. (2018) 28:3756–68. doi: 10.1007/s11695-018-3413-7
20. Higgins JP, Savovic J, Page MJ, Sterne J. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). Cochrane Handbook for Systematic Reviews of Interventions. (2019). Available online at: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool?authuser=0 (accessed April 29, 2023).
21. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
23. Weir CJ, Butcher I, Assi V, Lewis SC, Murray GD, Langhorne P, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: A systematic review. BMC Med Res Methodol. (2018) 18:25. doi: 10.1186/s12874-018-0483-0
24. Higgins J, Green S. Identifying and measuring heterogeneity. In: Cochrane Handbook for Systematic Reviews of Interventions 51 (John Wiley & Sons, Inc). (2011).
25. Lan J. The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
26. Pasulka P, Bistrian B, Benotti P, Blackburn G. The Risks of Surgery in Obese Patients. Ann Intern Med. (1986) 104:540–6. doi: 10.7326/0003-4819-104-4-540
27. O'Rourke RW. Inflammation in obesity-related diseases. Surgery. (2009) 145:255–9. doi: 10.1016/j.surg.2008.08.038
28. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive Insulin Therapy in Critically Ill Patients. New England J Med. (2001) 345:1359–67. doi: 10.1056/NEJMoa011300
29. Tjeertes EEKM, Hoeks SSE, Beks SSBJC, Valentijn TTM, Hoofwijk AAGM, Stolker RJRJ. Obesity - a risk factor for postoperative complications in general surgery? BMC Anesthesiol. (2015) 15:1–7. doi: 10.1186/s12871-015-0096-7
30. Wahl TS, Patel FC, Goss LE, Chu DI, Grams J, Morris MS. The obese colorectal surgery patient: Surgical site infection and outcomes. Dis Colon Rectum. (2018) 61:938–45. doi: 10.1097/DCR.0000000000001085
31. Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. (2007) 31:556–60. doi: 10.1007/s00268-006-0305-0
32. Eichenberger A, Proietti S, Wicky S, Frascarolo P, Suter M, Spahn DR, et al. Morbid Obesity and Postoperative Pulmonary Atelectasis: An Underestimated Problem. Anesth Analg. (2002) 95:1788–92. doi: 10.1097/00000539-200212000-00060
33. Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. (2005) 118:978–80. doi: 10.1016/j.amjmed.2005.03.012
34. Gumbiner B, Wendel J, McDermott M. Effects of diet composition and ketosis on glycemia during very-low-energy-diet therapy in obese patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. (1996) 63:110–5. doi: 10.1093/ajcn/63.1.110
35. Liljensøe A, Laursen JO, Bliddal H, Søballe K, Mechlenburg I. Weight Loss Intervention Before Total Knee Replacement: A 12-Month Randomized Controlled Trial. Scandinavian J Surg. (2021) 110:3–12. doi: 10.1177/1457496919883812
36. Geiger TM, Muldoon R. Complications following colon rectal surgery in the obese patient. Clin Colon Rectal Surg. (2011) 24:274–82. doi: 10.1055/s-0031-1295692
37. McKechnie T, Povolo CA, Lee J, Lee Y, Park L, Doumouras AG, et al. Very Low Energy Diets Prior to Non-Bariatric Surgery: A Systematic Review and Meta-Analysis. Surgery. (2022) 172:1733–1743. doi: 10.1016/j.surg.2022.09.006
38. Barth RJ Jr, Mills JB, Suriawinata AA, Putra J, Tosteson TD, Axelrod D, et al. Short-term Preoperative Diet Decreases Bleeding After Partial Hepatectomy. Ann Surg. (2019) 269:48–52. doi: 10.1097/SLA.0000000000002709
39. Burnand KM, Lahiri RP, Burr N, Jansen van Rensburg L, Lewis MPN. A randomised, single blinded trial, assessing the effect of a two week preoperative very low calorie diet on laparoscopic cholecystectomy in obese patients. HPB. (2016) 18:456–61. doi: 10.1016/j.hpb.2016.01.545
40. Davenport L, Johari Y, Klejn A, Laurie C, Smith A, Ooi GJ, et al. Improving compliance with very low energy diets (VLEDs) prior to bariatric surgery—a randomised controlled trial of two formulations. Obes Surg. (2019) 29:2750–7. doi: 10.1007/s11695-019-03916-2
41. Alami RS, Morton JM, Schuster R, Lie J, Sanchez BR, Peters A, et al. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Relat Dis. (2007) 3:141–5. doi: 10.1016/j.soard.2006.11.006
42. Baldry EL, Aithal GP, Kaye P, Idris IR, Bennett A, Leeder PC, et al. Effects of short-term energy restriction on liver lipid content and inflammatory status in severely obese adults: Results of a randomized controlled trial using 2 dietary approaches. Diab Obes Metab. (2017) 19:1179–83. doi: 10.1111/dom.12918
43. Nielsen LV, Nielsen MS, Schmidt JB, Pedersen SD, Sjödin A. Efficacy of a liquid low-energy formula diet in achieving preoperative target weight loss before bariatric surgery. J Nutr Sci. (2016) 5:e22. doi: 10.1017/jns.2016.13
44. Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD. Preoperative lifestyle intervention in bariatric surgery: initial results from a randomized, controlled trial. Obesity (Silver Spring). (2013) 21:254–60. doi: 10.1002/oby.20069
45. Lorenzo PM, Sajoux I, Izquierdo AG, Gomez-Arbelaez D, Zulet MA, Abete I, et al. Immunomodulatory effect of a very-low-calorie ketogenic diet compared with bariatric surgery and a low-calorie diet in patients with excessive body weight. Clin Nutr. (2022) 41:1566–77. doi: 10.1016/j.clnu.2022.05.007
46. Kandel D, Bojsen-Mϕller KN, Svane MS, Samkani A, Astrup A, Holst JJ, et al. Mechanisms of action of a carbohydrate-reduced, high-protein diet in reducing the risk of postprandial hypoglycemia after Roux-en-Y gastric bypass surgery. Am J Clin Nutr. (2019) 110:296–304. doi: 10.1093/ajcn/nqy310
47. Albanese A, Prevedello L, Markovich M, Busetto L, Vettor R, Foletto M. Pre-operative Very Low Calorie Ketogenic Diet (VLCKD) vs. Very Low Calorie Diet (VLCD): Surgical impact. Obes Surg. (2019) 29:292–6. doi: 10.1007/s11695-018-3523-2
48. Tauser M, Fumeli D, Busni D, Borroni F, Sebastianelli S, Nicolai G, et al. A very low calorie ketogenic diet improves weight loss and quality of life in patients with adjustable gastric banding. Ann Ital Chir. (2017) 88:143–8.
49. Hutcheon DA, Byham-Gray LD, Marcus AF, Scott JD, Miller M. Predictors of preoperative weight loss achievement in adult bariatric surgery candidates while following a low-calorie diet for 4 weeks. Surg Obes Relat Dis. (2017) 13:1041–51. doi: 10.1016/j.soard.2016.12.026
50. Parikh M, Dasari M, McMacken M, Ren C, Fielding G, Ogedegbe G. Does a preoperative medically supervised weight loss program improve bariatric surgery outcomes? A pilot randomized study. Surg Endosc. (2012) 26:853–61. doi: 10.1007/s00464-011-1966-9
51. Crujeiras AB, Gomez-Arbelaez D, Zulet MA, Carreira MC, Sajoux I, de Luis D, et al. Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: a marker of metabolic stress? Int J Obes (Lond). (2017) 41:1570–8. doi: 10.1038/ijo.2017.138
52. Jones B, Sands C, Alexiadou K, Minnion J, Tharakan G, Behary P, et al. The metabolomic effects of tripeptide gut hormone infusion compared to Roux-en-Y gastric bypass and caloric restriction. J Clin Endocrinol Metab. (2022) 107:e767–e82. doi: 10.1210/clinem/dgab608
Keywords: bariatric surgery, weight loss, very low energy diet, very low calorie diet, postoperative complications, randomized controlled trials, systematic review
Citation: McKechnie T, Lee Y, Dionne J, Doumouras A, Parpia S, Bhandari M and Eskicioglu C (2023) Very low energy diets prior to bariatric surgery may reduce postoperative morbidity: a systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 10:1211575. doi: 10.3389/fnut.2023.1211575
Received: 24 April 2023; Accepted: 23 May 2023;
Published: 20 June 2023.
Edited by:
Lidia Santarpia, University of Naples Federico II, ItalyReviewed by:
Luigi Schiavo, University of Salerno, ItalyCopyright © 2023 McKechnie, Lee, Dionne, Doumouras, Parpia, Bhandari and Eskicioglu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cagla Eskicioglu, ZXNraWNpb0BtY21hc3Rlci5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.