
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 03 August 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1211194
This article is part of the Research Topic Intermittent Feeding in Critically Ill Patients View all 8 articles
 Huoyan Liang1,2†
Huoyan Liang1,2† Qingqing Mu3†
Qingqing Mu3† Wenju Sun1†
Wenju Sun1† Liming Liu1
Liming Liu1 Simin Qiu3
Simin Qiu3 Zili Xu3
Zili Xu3 Yuqing Cui1
Yuqing Cui1 Yan Yan1
Yan Yan1 Tongwen Sun1,2*
Tongwen Sun1,2*Background: An increasing number of studies indicate that vitamin C (VC) reduces the mortality of adult septic patients, while some articles suggest otherwise. We performed this systematic review and meta-analysis to resolve the discrepancies in reported results concerning the efficacy of VC in septic patients.
Methods: We comprehensively searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled trials for randomized controlled trials (RCTs) evaluating the efficacy of intravenous VC (IVVC) on adult septic patients published from inception to November 28, 2022. The quality of outcomes for eligible studies was assessed using the Recommendations Assessment, Development, and Evaluation methodology. The results were analyzed using the pooled mean difference (MD) or risk ratio (RR) and 95% confidence intervals (CIs).
Results: Twenty-two studies (3,570 adult septic patients) were included. IVVC treatment did not improve 28-day mortality compared to the control group (RR, 0.92; 95% CI, 0.81–1.04; I2 = 26%; evidence risk, moderate). IVVC monotherapy decreased mortality (RR, 0.69; 95% CI, 0.52–0.93; I2 = 57%), whereas combination therapy did not affect mortality (RR, 1.03; 95% CI, 0.90–1.17; I2 =0%). IVVC had a trend to decrease the mortality of septic patients (RR, 0.83; 95% CI, 0.69–1.00; I2 = 33%) but did not affect septic shock patients (RR, 1.01; 95% CI, 0.85–1.21; I2 = 18%). IVVC reduced the duration of vasopressor use (MD, −8.45; 95% CI, −15.43 to −1.47; evidence risk, very low) but did not influence the incidence of AKI, ICU length of stay, duration of mechanical ventilation.
Conclusions: IVVC treatment did not improve the 28-day mortality in septic patients. Subgroup analysis indicated that VC had a trend to decrease the 28-day mortality in patients with sepsis but not septic shock. IVVC monotherapy, rather than combination therapy, decreased the 28-day mortality in septic patients. The findings imply that Hydrocortisone, Ascorbic acid, Thiamine (HAT) combination therapy is not superior to IVVC monotherapy for septic patients. These findings warrant further confirmation in future studies, which should also investigate the mechanisms underlying the enhanced efficacy of IVVC monotherapy in septic patients.
Systematic review registration: https://inplasy.com/.
Sepsis is a dysregulated host response to infection (1), and it is the leading cause of intensive care unit (ICU) mortality worldwide (2). Sepsis can result in multiple organ failure, placing a significant economic burden on healthcare systems (3). The primary treatments for sepsis include antimicrobial therapy, source control, and organ support.
In sepsis, the oxidative stress response is substantially enhanced, causing mitochondrial damage, which is a driving factor for sequential organ failure (4–6). Vitamin C (VC) is an anti-inflammatory and antioxidant agent that protects against reactive oxygen species (ROS)-induced damage to the epithelial barrier (7). The previous indicated that VC could interact with tocopherol, glutathione, and thioredoxin, and stimulate the biosynthesis and activation of catalase and glutathione peroxidase (8). VC can also increase the bioavailability of NO, potentially improving microcirculatory perfusion. The mechanism behind this is that VC prevents the oxidation of tetrahydrobiopterin (BH4), which maintains the coupled activity of endothelial NOS, preventing the production of superoxide and the aggravation of oxidative damage (9). Humans cannot synthesize VC, and its levels are low in many critically ill patients (10), making supplementation potentially beneficial.
Previous studies (11–14) have demonstrated that intravenous VC (IVVC) therapy could reduce oxidative stress levels and improve clinical outcomes. Furthermore, some randomized controlled trials (RCTs) have further confirmed the therapeutic efficacy of IVVC for sepsis compared to placebo (15–17). Meta-analyses have evaluated the effect of VC treatment either as combination therapy with thiamine or other agents (18–23) or as mono-intravenous VC therapy (24), often including retrospective data to obtain pooled results, which limits the interpretability of their findings (19, 24–29). Therefore, as the effect of VC on sepsis remains unclear, numerous RCTs have been further conducted to evaluate the effect of VC on sepsis (30–32). This study aimed to assess the effect of VC on septic patients to address the discrepancies in the results concerning the benefit of VC administration in sepsis.
The protocol of this systematic review and meta-analysis was registered on INPLASY (INPLASY2022110147).
We conducted this meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria. The three databases MEDLINE, EMBASE, and the Cochrane Central Register of Controlled trials were searched to identify eligible studies published from the inception of the databases to November 28, 2022. The keyword search terms were VC, critically ill patients, and sepsis (detailed search strategy in Supplementary Table 1).
We included RCTs that met the following criteria: (1) design: RCTs; (2) population: adult patients (≥18 years) with a diagnosis of sepsis or septic shock. The sequential organ failure assessment (SOFA) score of two points or more is defined as organ dysfunction; (3) intervention: septic patients treated with VC; (4) control group: no VC administration; (5) reported outcomes: at least one of the following 28-day mortality, risk of incidence of acute kidney injury (AKI) after enrolment, intensive care unit length of stay (ICU-LOS), change in SOFA score at day 3, 4, or 5 from baseline, and duration of mechanical ventilation (MV); (6) language: published in English. The RCTs were excluded if only the abstract was published or the full text was unavailable.
The primary outcome was 28-day all-cause mortality. To investigate the effect of VC on septic patients, the data of 28-day hospital mortality were pooled. The ICU-LOS, vasopressor use, duration of MV, new-onset or worsening of AKI, and change in SOFA score after enrolment. Furthermore, we performed the subgroup analysis in this meta-analysis; importantly, if the included articles including the septic and septic shock patients, we considered these patients of studies as septic patients.
RevMan 5.3 (Cochrane IMS, Oxford, United Kingdom) and STATA 14.0 (College Station, Texas 77845 USA) were used for the random effects model analysis. The dichotomous and continuous data were assessed using the pooled risk ratio (RR) and mean difference (MD) with their 95% confidence interval (CI), respectively. The potential publication bias was assessed using the Egger's linear regression, and funnel plots were used for visually assessing asymmetry. P < 0.1 in Egger's test suggested low heterogeneity.
In the meta-analysis, we initially collected 4,050 references, and 3,100 remained after removing duplicates. Screening the title and abstract, and 44 RCTs were identified, of which 22 RCTs (3,570 patients) were included ultimately (Figure 1). The characteristics of the eligible studies are listed in Table 1. Ten RCTs (16, 30, 32–39) focused on 1,362 septic shock patients, while 12 RCTs (14, 15, 17, 31, 40–47) examined 2,208 septic patients. In nine RCTs (14, 16, 17, 30, 31, 36, 41, 42, 47), 1,498 patients received VC monotherapy, and in 13 RCTs (15, 32–35, 37–40, 43–46), and 2,072 patients received combination therapy.
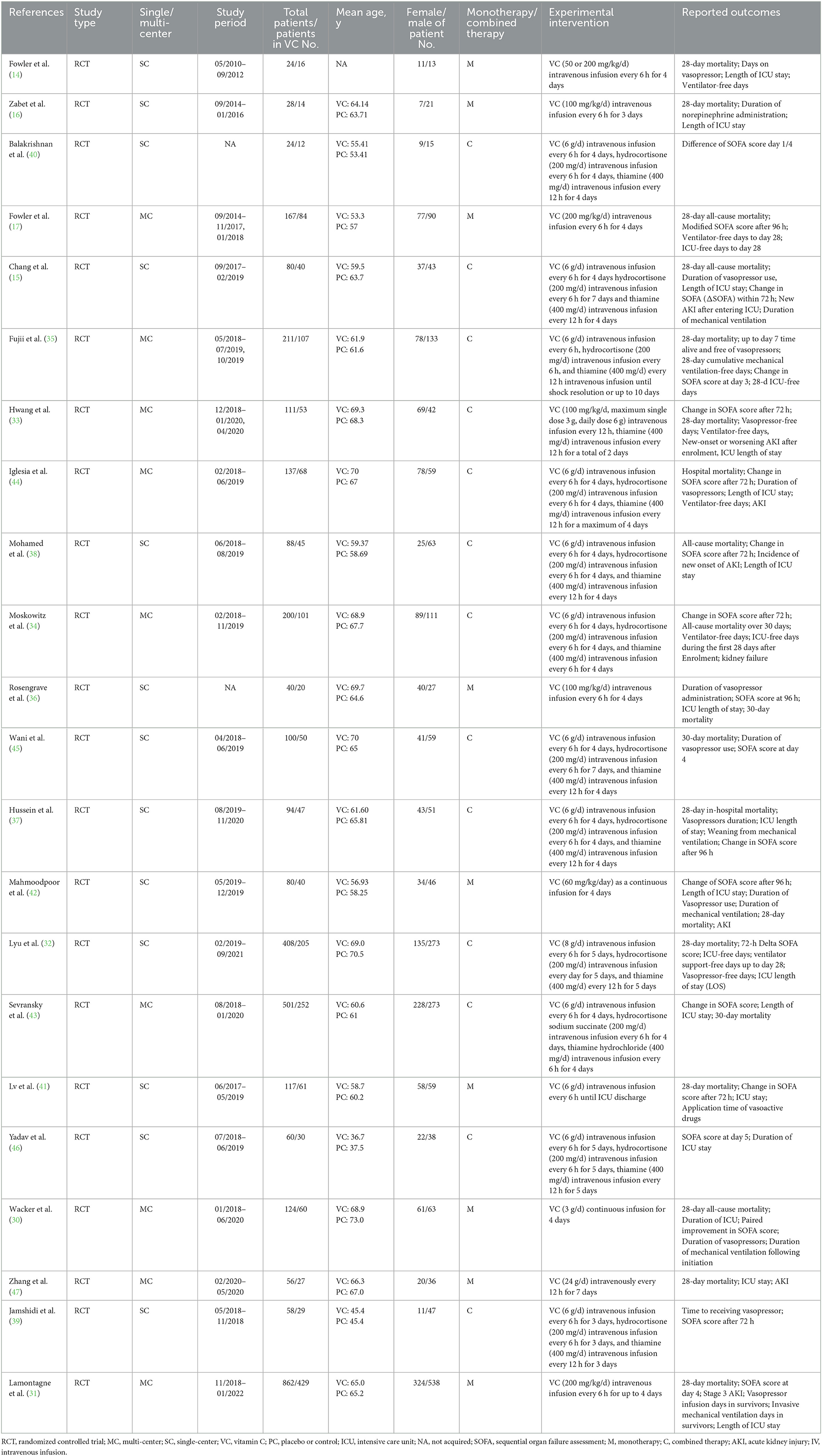
Table 1. Characteristic of the included studies in the meta-analysis of vitamin C vs. placebo or standard supportive care in adult septic patients.
Our meta-analysis indicated that VC could not improve 28-day overall mortality (RR, 0.92; 95% CI, 0.81–1.04; I2 = 26%; evidence risk, moderate; Figure 2). Nine studies (14, 16, 17, 30, 31, 36, 41, 42, 47) examined VC monotherapy for adult septic patients, while 10 (15, 32–35, 37, 38, 43–45) investigated the effect of combination therapy, which mostly involved IVVC combined with intravenous thiamine and intravenous hydrocortisone. No potential publication bias was found in the primary outcome of this study (P = 0.559; Supplementary Figure 1). Sensitivity analysis indicated that the models were credible (Supplementary Figure 2). IVVC exhibited no effect to mortality in patients treated with a high dose of VC (>100 mg/d or >6 g/d; RR, 0.82; 95% CI, 0.61–1.08; I2 = 29%; Figure 3). Similarly, a low dose of VC had no effect on mortality (RR, 0.95; 95% CI, 0.82–1.09; I2 = 21%; Figure 3). Additionally, in 12 trials (17, 30–36, 38, 43–45), therapy was administered <24 h from ICU admission, and mortality was not affected by VC (RR, 1.00; 95% CI, 0.90–1.11; I2 = 0%; Figure 4). Subgroup analysis demonstrated that IVVC administration (monotherapy) was associated with a decrease in 28-day mortality (RR, 0.69; 95% CI, 0.52–0.93; I2 = 57%; Figure 5). However, the combination of IVVC with other medicines had no effect on all-cause mortality in septic patients (RR, 1.03; 95% CI, 0.90–1.17; I2 = 0%; Figure 5). We also analyzed the difference in mortality concerning whether hydrocortisone was used in the control group. We observed that the mortality of patients in the intervention group was not improved compared to patients without hydrocortisone therapy in the control group (RR, 0.84; 95% CI, 0.68–1.03; I2 = 35%; Figure 6). In the subgroup analysis of patients treated with hydrocortisone, mortality in the control group showed no difference compared to that in the VC group (RR, 1.02; 95% CI, 0.88–1.19; I2 = 0%; Figure 6). Additionally, 10 RCTs (14, 15, 17, 31, 41–45, 47) included septic patients, and nine trials enrolled patients with septic shock (16, 30, 32–38). IVVC treatment was associated with a trend reduction in 28-day mortality in septic patients (RR, 0.83; 95% CI, 0.69–1.00; I2 = 33%; Figure 7), whereas it did not affect septic shock patients (RR, 1.01; 95% CI, 0.85–1.21; I2 = 18%; Figure 7). For ventilated patients (17, 30, 41, 47), mortality was reduced by parenteral IVVC treatment (RR, 0.61; 95% CI, 0.47–0.80; I2 = 0%; Figure 8).
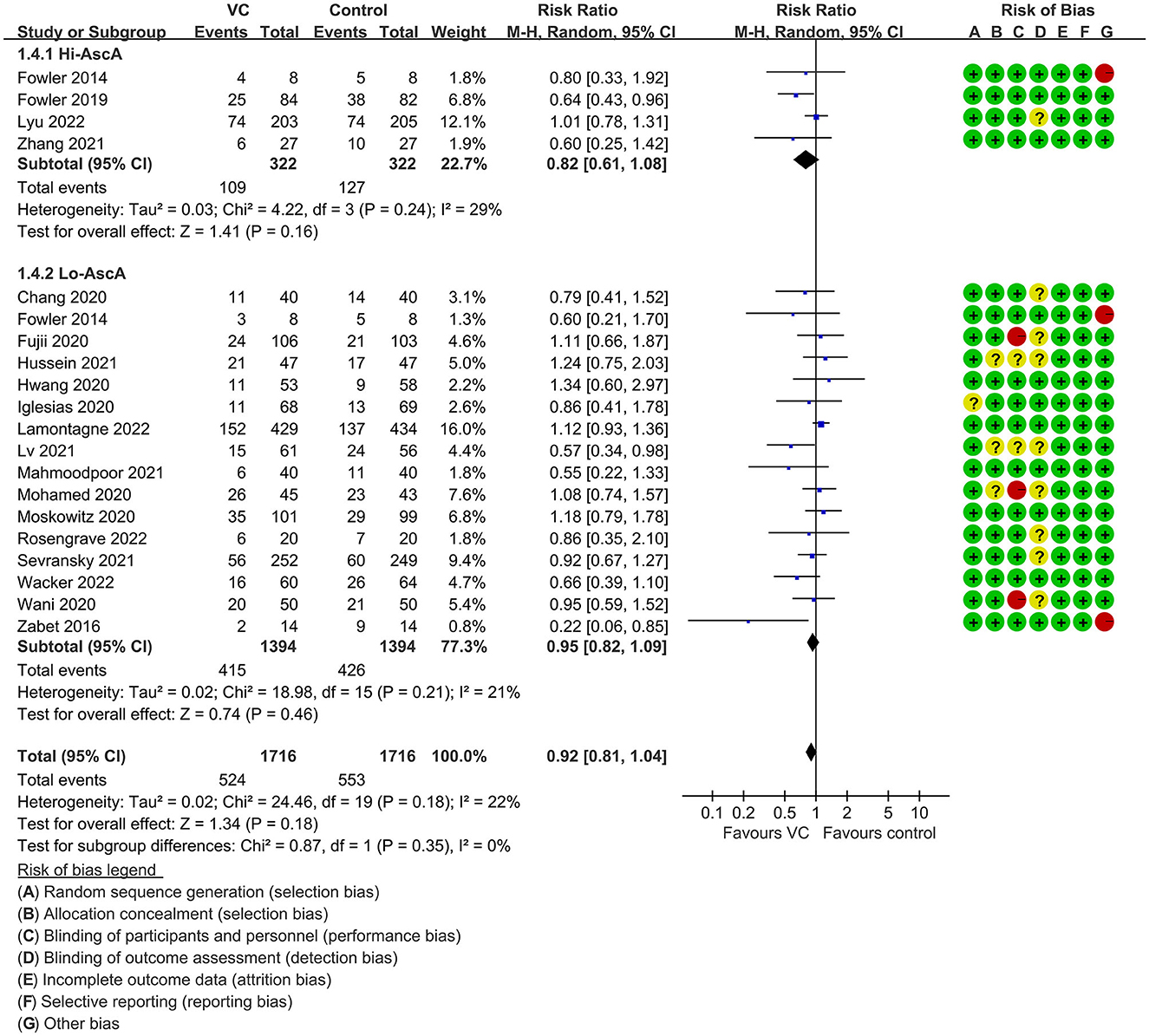
Figure 3. Subgroup analysis of primary outcomes in septic patients based on IVVC therapy for patients with high-dose VC (more than 100 mg/kg/d or 6 g/d) administration or low-dose administration (50–100 mg/kg/d or 6 g/d).
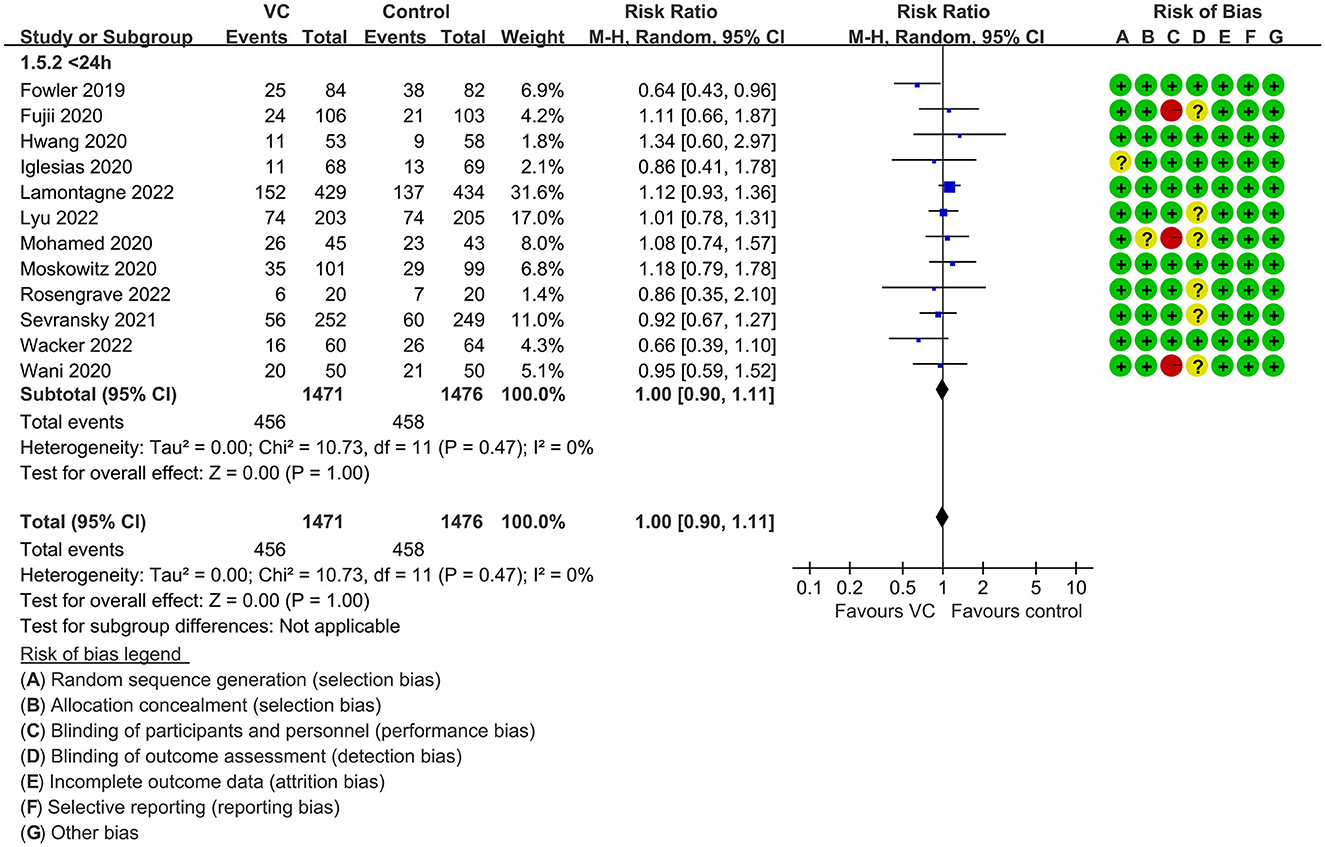
Figure 4. Subgroup analysis of primary outcomes in septic patients based on IVVC therapy for patients with therapy initiation within 24 h of ICU admission or sepsis diagnosis.
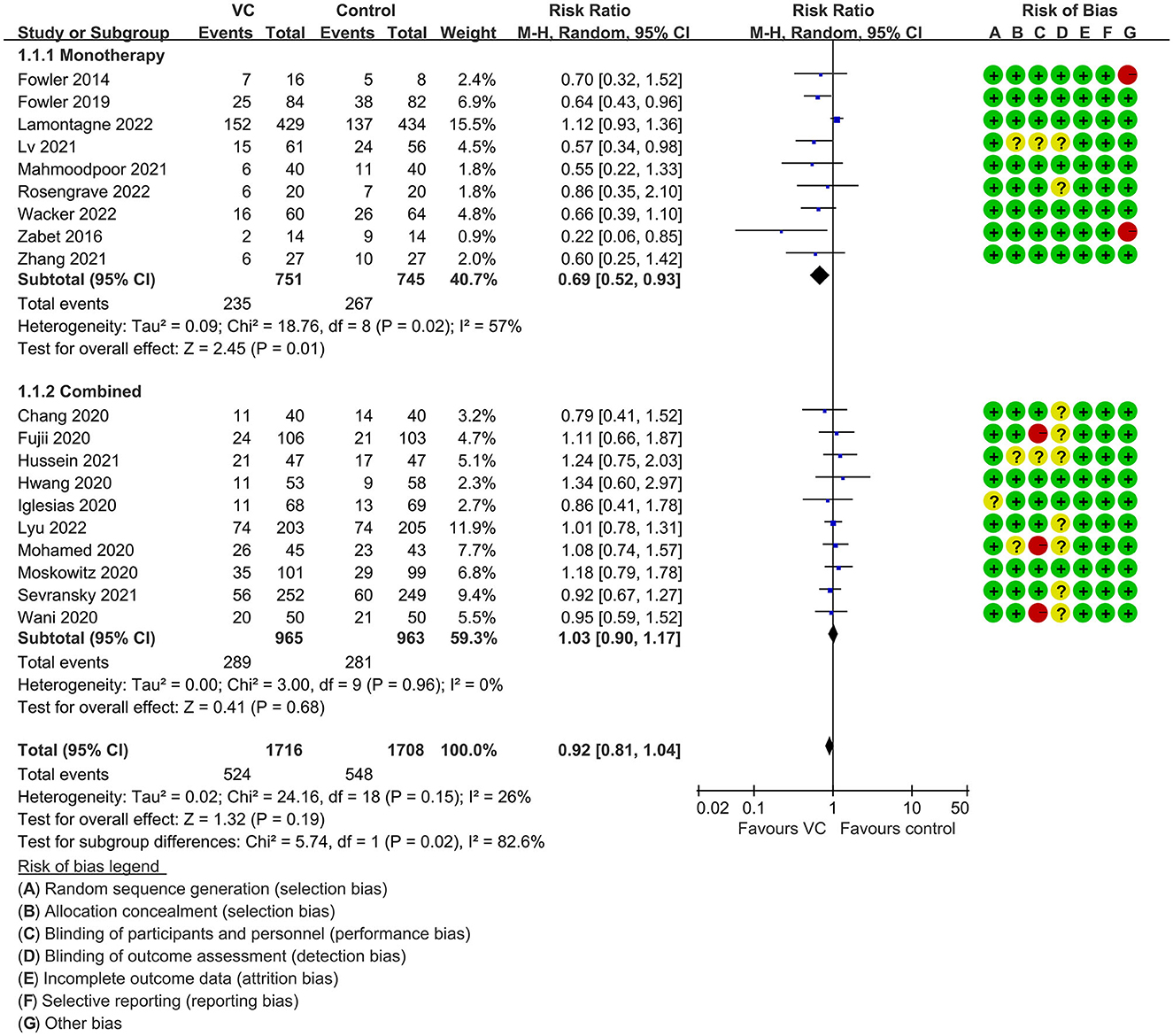
Figure 5. Subgroup analysis of primary outcomes in septic patients based on IVVC monotherapy or combination therapy.
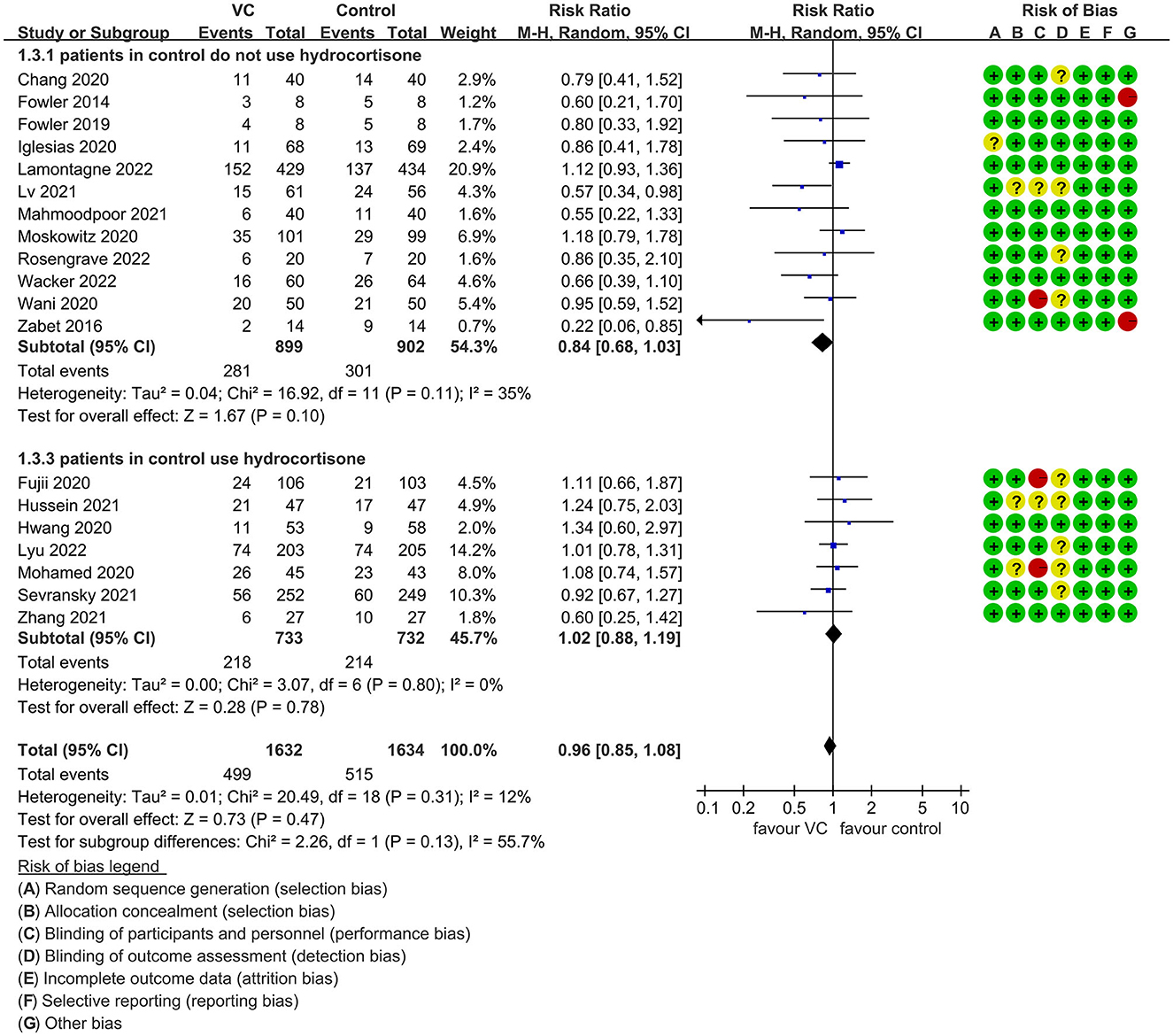
Figure 6. Subgroup analysis of primary outcomes in septic patients based on IVVC therapy for patients with or without hydrocortisone use in the control group.
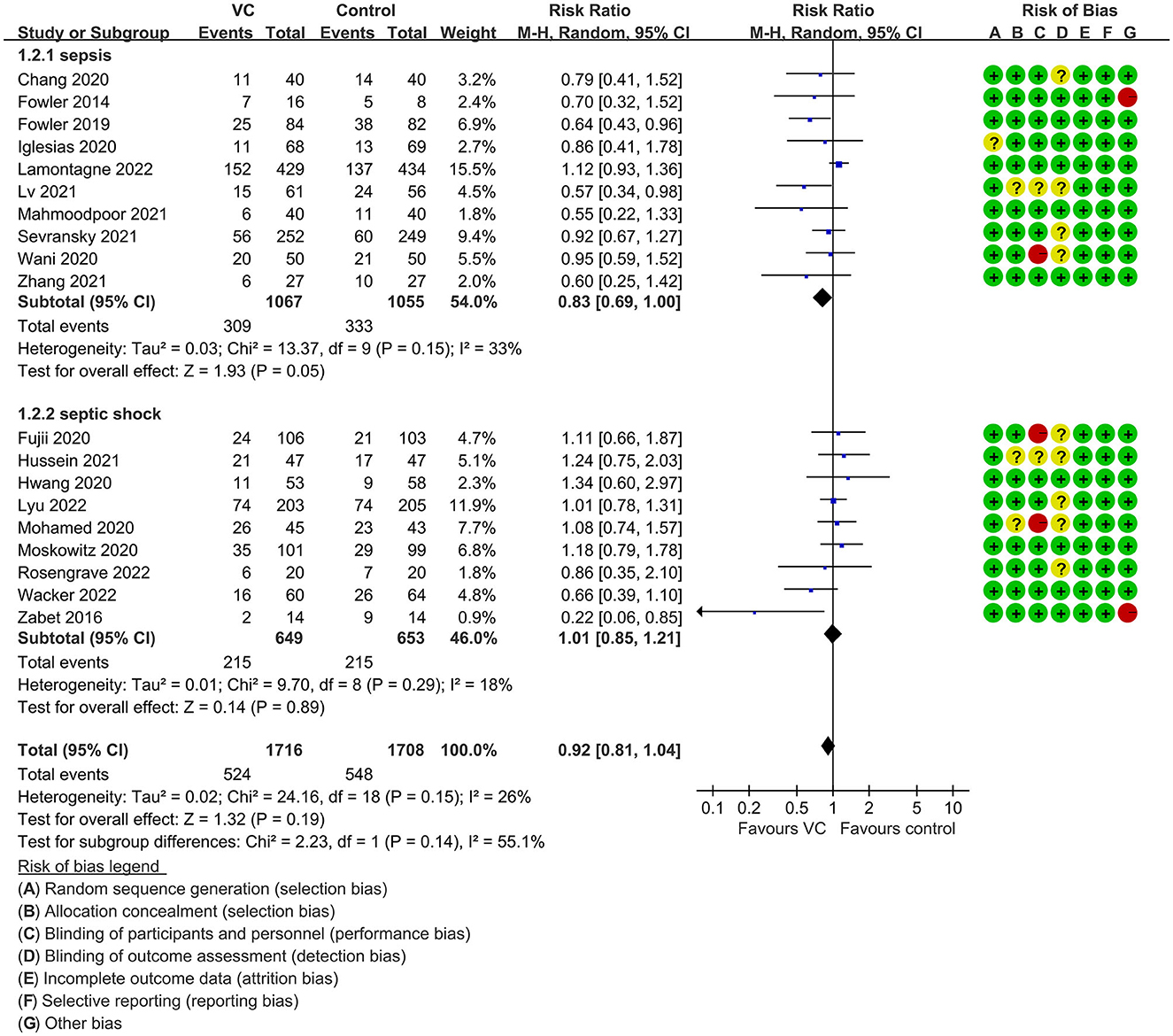
Figure 7. Subgroup analysis of primary outcomes in septic patients based on IVVC therapy for patients with sepsis or septic shock.

Figure 8. Subgroup analysis of primary outcomes in septic patients based on IVVC therapy for ventilated patients.
The IVVC treatment showed no difference in AKI incidence (RR, 1.05; 95% CI, 0.94–1.18; I2 = 0%; Supplementary Figure 3), ICU-LOS (MD, 0.07; 95% CI, −0.54–0.68; I2 = 42%; Supplementary Figure 4), change in SOFA score (MD, 0.04; 95% CI, −0.55–0.63; I2 = 96%; Supplementary Figure 5), and duration of MV (MD, 0.96; 95% CI, −0.27–2.18; I2 = 84%; evidence risk, moderate; Supplementary Figure 6). IVVC treatment for septic patients could reduce the vasopressor duration (MD, −8.45; 95% CI, −15.43 to −1.47; I2 = 80%; evidence risk, very low; Supplementary Figure 7). The funnel plot and Egger's test showed no publication bias in AKI incidence (P = 0.128), ICU-LOS (P = 0.784), change in SOFA score (P = 0.92), duration of MV (P = 0.324), and duration of vasopressor use (P = 0.145; Supplementary Figures 8–12) for funnel plot. This meta-analysis indicated that the models of AKI incidence, ICU-LOS, change in SOFA score, duration of MV, and duration of vasopressor use were credible (Supplementary Figures 13–17). Subgroup analysis showed that IVVC monotherapy used in seven trials (14, 16, 30, 31, 36, 41, 42) was association with lower risk of the duration of vasopressor use (MD, −12.11; 95% CI, −21.46 to −2.75, I2 = 74%; Supplementary Figure 18), but combination therapy used in eight trials (15, 32, 33, 35, 37, 39, 44, 45) exhibited no effect (MD, −4.82; 95% CI, −16.00–6.36; I2 = 83%; Supplementary Figure 18). Furthermore, IVVC administration may be related with a reduction in both duration of vasopressor use < 7 days (MD, −15.06; 95% CI, −23.43 to −6.70; I2 = 0%; Supplementary Figure 19) and duration of vasopressor use ≥ 7 days (MD, −7.06; 96% CI, −15.05–0.93; I2 = 82%; Supplementary Figure 19). Furthermore, the risk of bias and the evidence rank of each outcome in this meta-analysis are tabulated in Table 2.
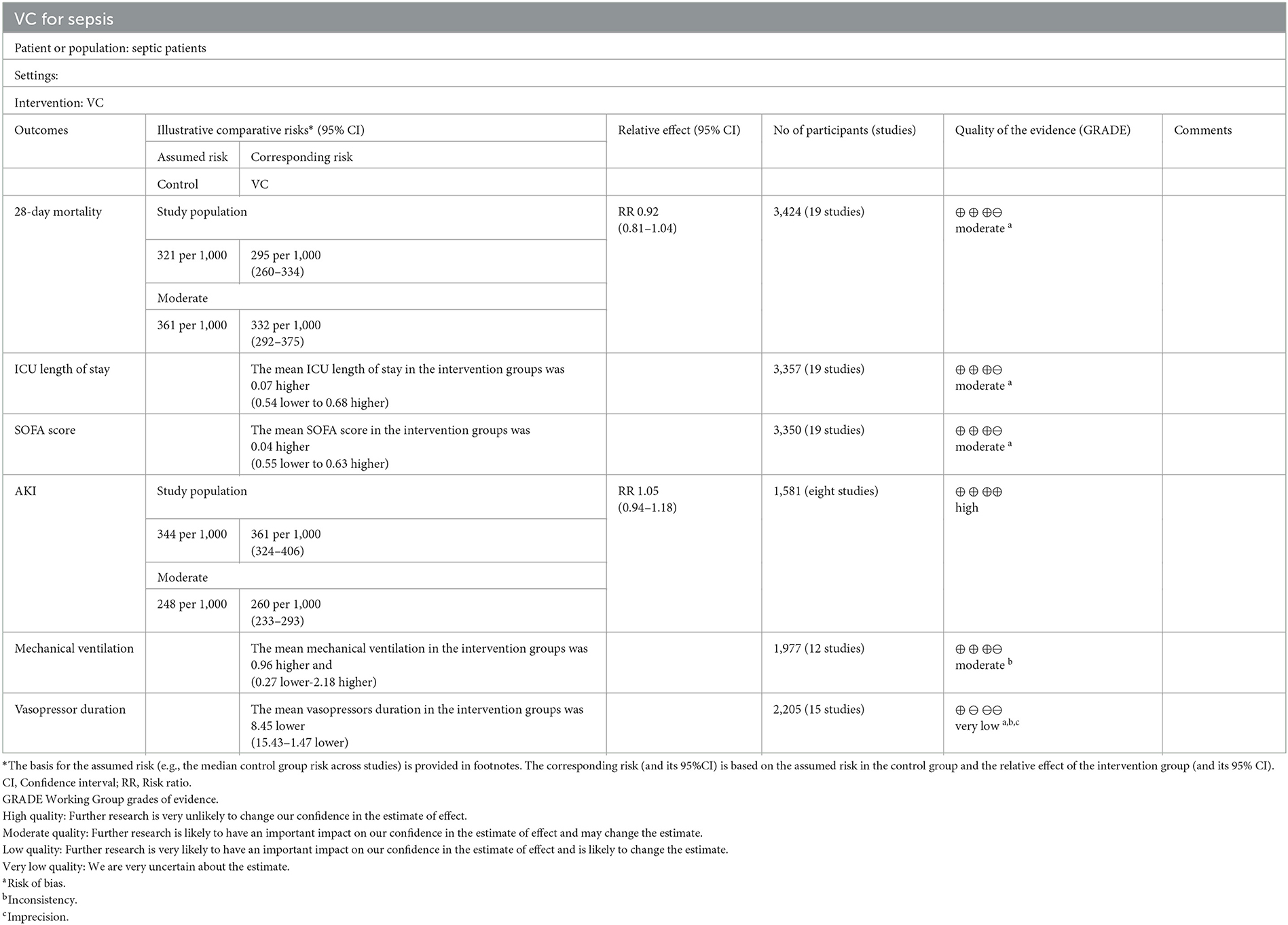
Table 2. Findings and evidence rank of the included studies with vitamin C vs. placebo treatment in septic patients.
The results of this meta-analysis suggest that IVVC may not improve the 28-day overall mortality in septic patients. However, the effect of the drug is influenced by various factors such as dosage, timing of therapy, drug combination, frequency of hydrocortisone use in the control group, and severity of illness (septic shock and ventilation). There is no reduction mortality with high-dose VC and low-dose VC. Similarly, the VC therapy initiation of therapy within 24h of ICU admission or sepsis diagnosis did not impact the mortality of septic patients. Moreover, the use of IVVC monotherapy, but not combination therapy, was found to decrease the 28-day overall mortality in septic patients. In subgroup analysis, the mortality was not reduced in the control group when hydrocortisone was used. IVVC treatment was associated with a trend of decreasing the mortality of septic patients compared to septic shock patients, and ventilated patients benefited more from IVVC.
Previous met-analysis study (26) only included 10 studies showed that VC does not affect in-hospital or ICU mortality of septic patients, which differs from our study findings. However, the study (26) assessed pooled data from only three retrospective studies, making the methodology quality low. Including a small number of retrospective studies decreases the precision of the conclusion. One study (48) conducted that VC treatment or combination of glucocorticoids and vitamin B1 could not reduce the long-term mortality compare with control group, whereas it not reported the short-term mortality. A recent meta-analysis (49) compared the effect of IVVC on critical ill patients with that of a placebo and found that IVVC could reduce 28-day mortality, which was similar to our study findings. However, this study (49) is limited because of not indicating that the IVVC monotherapy reduce in the duration of vasopressor use and lack of discussion about VC do not affect AKI incidence. An additional meta-analysis (50) showed VC could reduce the 28-day mortality, whereas the author did not conduct subgroup analysis on patients with different disease severity levels, such as those with sepsis and septic shock.
Oxidant molecules play a critical role in host defense by impacting cellular signaling, regulating vascular tone, and modulating production of prostaglandins. A massive early production of oxidants followed by a quick decline may result in mitochondrial and endothelial dysfunction, which can lead to the condition deterioration in sepsis (9). Based on the problematic role of oxidants, IVVC is suggested as an aggressive early therapy, followed by quick cessation after the imbalance of ROS has been corrected. The huge burst of ROS is generated within minutes after the start of reperfusion. Therefore, a late start of vitamin infusion may lead to negative results (51). However, the subgroup analysis of early drug administration (time of therapy from ICU admission or diagnosis of sepsis or septic shock within 24 h) did not significantly influence mortality, which is contrary to our expectations. Further trials are needed to explore this finding.
Our meta-analysis suggests that IVVC monotherapy could significantly decrease the 28-day mortality, while combined therapy has no effect. However, the mechanisms underlying the beneficial effects of VC on sepsis mortality are not yet fully understood. An in vitro study has shown that hydrocortisone may reduce the inflammatory response by increasing the level of sodium-coupled VC transporter 2, which facilitates the uptake of VC into the cell (52). However, the recent meta-analysis showed that combining VC with other therapies did not lead to a reduction in mortality in septic patients (23). In the early stage of sepsis, the release of many cytokines and the dysregulation of the inflammatory response caused by damaged tissues can damage the vascular endothelial cells, leading to acute organ dysfunction. Therefore, the restoration of vascular endothelial integrity and capillary function, as well as the early reduction of inflammation in sepsis are important goals for treating sepsis. Based on the pharmacological mechanisms of hydrocortisone, VC, and thiamine, and the results of Chang et al.'s study, it is speculated that early combination therapy may be beneficial, but the efficacy may vary among patients with different stages of sepsis (15). VC may be beneficial during initial anabatic inflammatory responses, however at reactive immunosuppression VC is harmful to the body (53, 54). The hydrocortisone may aggravate the disadvantage of VC due to its promoting to immunosuppression. Antagonism between hydrocortisone and VC may weigher than synergism. The use of hydrocortisone for treating septic shock has been controversial, with studies yielding mixed results. Two recent RCTs, ADRENAL and APROCCHSS, suggest that glucocorticoids are effective in treating critically ill patients with septic shock (55, 56). The therapeutic effect of hydrocortisone on sepsis may be related to disease severity and medication dosage. One study demonstrated that thiamine supplementation could lower blood lactate levels in some sepsis patients with thiamine deficiency at enrollment, indicating that baseline thiamine levels may be associated with the efficacy of the Hydrocortisone, Ascorbic acid, Thiamine (HAT) regimen (57). VC may not have a synergistic effect with hormones and thiamine, and its therapeutic effect may be maximized when administered as monotherapy. Therefore, monitoring plasma levels of VC and thiamine upon admission is necessary to evaluate efficacy and guide drug administration.
The finding suggests that adding hydrocortisone and thiamine to VC therapy does not reduce 28-day mortality. VC combination therapy reportedly does not affect sepsis patients, indicating that future studies should focus on confirming the impact of VC monotherapy. Furthermore, this study showed no difference in secondary outcomes such as AKI incidence, change in SOFA score, ICU-LOS, and duration of MV between monotherapy and combination therapy. VC monotherapy is associated with a significant reduction in the duration of vasopressor use. The incidence of adverse events was extremely low, and we acknowledge that there are accidental factors and individual differences. Only one trial reported increased rates of hypernatremia following VC treatment (34). Although a patient with hypoglycemia who received VC experienced a severe episode (31), this was an iatrogenic injury unrelated to VC therapy. Moreover, three patients exhibited hypotension and tachycardia (42). Furthermore, we compared mortality between arms in subgroups where hydrocortisone monotherapy was mandated or could be used in the control group. When steroids were only used as part of the co-intervention in patients requiring high-dose vasopressors, the outcomes suggest no difference in mortality between VC and control groups.
Patients with septic shock represent the worsening subtype of sepsis. Lyu et al.'s study showed that baseline SOFA scores (mean 10) were higher in patients with septic shock. Higher SOFA scores are associated with an increased risk of death, which may be one reason why no beneficial effect of VC on mortality was observed in the study (32). VC prevents the accumulation of activated neutrophils in alveolar spaces, increasing alveolar fluid clearance in ventilated patients. This also depends on the effect of VC promoting alveolar epithelial water-channel expression and preventing damage (11). Additionally, VC reduces vascular injury by preventing neutrophil extracellular trap formation (58), suggesting a heightened mortality benefit in patients requiring ventilation.
Oxidative stress resulting from sepsis contributes to multiple organ failure. Inadequate antioxidants to counter elevated levels of ROS and nitrogen lead to cellular injury and endothelial barrier dysfunction (59, 60). VC can reduce nitric oxide production via the iNOS pathway and decrease vasoconstriction and vascular permeability (61). Additionally, plasma VC levels decrease rapidly under acute inflammation, accompanied by significant human tissue alterations, including dysregulated inflammation, increased endothelial permeability, and edema (10, 62, 63). VC is associated with increased superoxide dismutase activity and decreased malondialdehyde levels (64). With short longevity and water-solution, VC displays saturable enteral absorption kinetics, indicating that VC supplementation may improve clinical outcomes in septic patients. However, (62) it showed that suggesting the wide heterogeneity an antioxidant decreases in response to ICU patients when planning to use antioxidant therapy. Similarly, the previous article showed that (65) the antioxidant needs for personalized approaches: Right species, Right place, Right time, Right level, and Right target, suggesting precision redox is the key for antioxidant pharmacology. Furthermore, one report (66) suggested that Gut microbiota participates in the pathogenesis of sepsis by influencing the inflammatory state and immune response of the host. However, Whether VC will also affect the prognosis of sepsis patients by changing the host's gut microbiota, and it will be further to research in the future studies.
Our meta-analysis has several strengths. We searched for the latest and most comprehensive studies to estimate the effect of VC on septic patients. Importantly, we set explicit inclusion criteria to limit bias. Thirdly, all included studies were RCTs, which enhances the quality of our findings. The risk of bias was low among the eligible studies. We explored the influence of dosage, timing of therapy, drug combination, hydrocortisone frequency in the control group, and severity (septic shock and ventilation) on intravenous VC's therapeutic effect.
Some limitations of this study are noted, including that most included studies did not report baseline plasma VC levels (11, 12, 14, 54), leading to a missed signal in patients with baseline deficiency. The endpoint of secondary outcomes is various effecting the reliability. Besides, some of the sample size of subgroup is too small to get precise results and most tests of subgroup differences are not statistically significant.
IVVC administration could not improve the 28-day all-cause mortality of septic patients. IVVC monotherapy, rather than combination therapy, could decrease all-cause mortality in septic patients. The findings suggest that the effect of HAT combination therapy on septic patients is not superior to IVVC monotherapy. However, subgroup analysis suggested that IVVC had a trend to reduce the 28-day mortality in patients with sepsis but not septic shock. This effect should be further confirmed in future studies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
TS and HL provided the idea for the meta-analysis. QM, SQ, LL, and ZX contributed to the data extraction. QM and LL computed the pooled outcomes. HL and QM wrote the article. TS revised the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1211194/full#supplementary-material
Supplementary Figure 1. Funnel plot assessing the potential publication bias for primary outcomes in septic patients based on IVVC administration.
Supplementary Figure 2. Sensitivity analysis for assessing the robustness of primary outcomes in septic patients based on IVVC administration.
Supplementary Figure 3. Forest plot assessing the risk of AKI incidence in septic patients based on IVVC administration.
Supplementary Figure 4. Forest plot assessing the effect of ICU-LOS in septic patients based on IVVC administration.
Supplementary Figure 5. Forest plot assessing the effect of SOFA score in septic patients based on IVVC administration.
Supplementary Figure 6. Forest plot assessing the effect of duration of MV in septic patients based on IVVC administration.
Supplementary Figure 7. Forest plot assessing the effect of duration of vasopressor use in septic patients based on IVVC administration.
Supplementary Figure 8. Funnel plot assessing the potential publication bias for the effect of AKI incidence in septic patients.
Supplementary Figure 9. Funnel plot assessing the potential publication bias for the effect of ICU-LOS in septic patients.
Supplementary Figure 10. Funnel plot assessing the potential publication bias for the effect in SOFA score in septic patients.
Supplementary Figure 11. Funnel plot assessing the potential publication bias for the duration of MV in septic patients.
Supplementary Figure 12. Funnel plot assessing the potential publication bias for the duration of vasopressor use in septic patients.
Supplementary Figure 13. Sensitivity analysis assessing the robustness of AKI incidence in septic patients based on IVVC administration.
Supplementary Figure 14. Sensitivity analysis assessing the robustness of ICU-LOS in septic patients based on IVVC administration.
Supplementary Figure 15. Sensitivity analysis assessing the robustness of SOFA score of septic patients based on IVVC administration.
Supplementary Figure 16. Sensitivity analysis for assessing the robustness of the duration of MV in septic patients based on IVVC administration.
Supplementary Figure 17. Sensitivity analysis assessing the robustness of the duration of vasopressor use in septic patients based on IVVC administration.
Supplementary Figure 18. Subgroup analysis of the duration of vasopressor use in septic patients based on IVVC monotherapy or combination therapy.
Supplementary Figure 19. Subgroup analysis of the duration of vasopressor use in septic patients based on IVVC therapy.
Supplementary Table 1. Detailed search strategy.
RR, risk ratio; RCTs, randomized controlled trials; MD, mean difference; CI, confidence interval; ICU, intensive care unit; AKI, acute kidney injury; LOS, length of stay; MV, mechanical ventilation; SOFA, sequential organ failure assessment; HAT, Hydrocortisone, Ascorbic acid, Thiamine; ROS, reactive oxygen species; VC, vitamin C.
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). J Am Med Assoc. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Marshall JC, Vincent JL, Guyatt G, Angus DC, Abraham E, Bernard G, et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. (2005) 33:1708–16. doi: 10.1097/01.CCM.0000174478.70338.03
3. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. (2001) 29:1303–10. doi: 10.1097/00003246-200107000-00002
4. Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. (2004) 4:729–41. doi: 10.1016/j.mito.2004.07.023
5. Mantzarlis K, Tsolaki V, Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev. (2017) 2017:5985209. doi: 10.1155/2017/5985209
6. May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. (2013) 19:2068–83. doi: 10.1089/ars.2013.5205
7. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. (2017) 9:111211. doi: 10.3390/nu9111211
8. Gegotek A, Skrzydlewska E. Ascorbic acid as antioxidant. Vitam Horm. (2023) 121:247–70. doi: 10.1016/bs.vh.2022.10.008
9. Spoelstra-de Man AME, Elbers PWG, Oudemans-van Straaten HM. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit Care. (2018) 22:70. doi: 10.1186/s13054-018-1996-y
10. Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. (2017) 21:300. doi: 10.1186/s13054-017-1891-y
11. Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA 3rd, et al. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med. (2011) 39:1454–60. doi: 10.1097/CCM.0b013e3182120cb8
12. Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Puri P, Massey HD, et al. Attenuation of sepsis-induced organ injury in mice by vitamin C. J Parenter Enteral Nutr. (2014) 38:825–39. doi: 10.1177/0148607113497760
13. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. (2017) 151:1229–38. doi: 10.1016/j.chest.2016.11.036
14. Fowler AA 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. (2014) 12:32. doi: 10.1186/1479-5876-12-32
15. Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, et al. Combined treatment with hydrocortisone, vitamin C, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest. (2020) 158:174–82. doi: 10.1016/j.chest.2020.02.065
16. Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose Ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract. (2016) 5:94–100. doi: 10.4103/2279-042X.179569
17. Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. J Am Med Assoc. (2019) 322:1261–70. doi: 10.1001/jama.2019.11825
18. Li T, Zeng J, Li DH, Yang GY, Wang K, Deng HF, et al. Efficacy of intravenous vitamin C intervention for septic patients: a systematic review and meta-analysis based on randomized controlled trials. Am J Emerg Med. (2021) 50:242–50. doi: 10.1016/j.ajem.2021.08.012
19. Somagutta MKR, Pormento MKL, Khan MA, Hamdan A, Hange N, Kc M, et al. The efficacy of vitamin C, thiamine, and corticosteroid therapy in adult sepsis patients: a systematic review and meta-analysis. Acute Crit Care. (2021) 36:185–200. doi: 10.4266/acc.2021.00108
20. Fong KM, Au SY, Ng GWY. Steroid, ascorbic acid, and thiamine in adults with sepsis and septic shock: a systematic review and component network meta-analysis. Sci Rep. (2021) 11:15777. doi: 10.1038/s41598-021-95386-9
21. Ge Z, Huang J, Liu Y, Xiang J, Gao Y, Walline JH, et al. Thiamine combined with vitamin C in sepsis or septic shock: a systematic review and meta-analysis. Eur J Emerg Med. (2021) 28:189–95. doi: 10.1097/MEJ.0000000000000812
22. Assouline B, Faivre A, Verissimo T, Sangla F, Berchtold L, Giraud R, et al. Thiamine, ascorbic acid, and hydrocortisone as a metabolic resuscitation cocktail in sepsis: a meta-analysis of randomized controlled trials with trial sequential analysis. Crit Care Med. (2021) 49:2112–20. doi: 10.1097/CCM.0000000000005262
23. Zayed Y, Alzghoul BN, Banifadel M, Venigandla H, Hyde R, Sutchu S, et al. Vitamin C, thiamine, and hydrocortisone in the treatment of sepsis: a meta-analysis and trial sequential analysis of randomized controlled trials. J Intensive Care Med. (2022) 37:327–36. doi: 10.1177/0885066620987809
24. Cai B, Lv X, Lin M, Feng C, Chen C. Clinical efficacy and safety of vitamin C in the treatment of septic shock patients: systematic review and meta-analysis. Ann Palliat Med. (2022) 11:1369–80. doi: 10.21037/apm-22-225
25. Wu T, Hu C, Huang W, Xu Q, Hu B, Li J. Effect of combined hydrocortisone, ascorbic acid and thiamine for patients with sepsis and septic shock: a systematic review and meta-analysis. Shock. (2021) 56:880–9. doi: 10.1097/SHK.0000000000001781
26. Wei XB, Wang ZH, Liao XL, Guo WX, Wen JY, Qin TH, et al. Efficacy of vitamin C in patients with sepsis: An updated meta-analysis. Eur J Pharmacol. (2020) 868:172889. doi: 10.1016/j.ejphar.2019.172889
27. Scholz SS, Borgstedt R, Ebeling N, Menzel LC, Jansen G, Rehberg S. Mortality in septic patients treated with vitamin C: a systematic meta-analysis. Crit Care. (2021) 25:17. doi: 10.1186/s13054-020-03438-9
28. Zhang M, Jativa DF. Vitamin C supplementation in the critically ill: a systematic review and meta-analysis. SAGE Open Med. (2018) 6:2050312118807615. doi: 10.1177/2050312118807615
29. Brown J, Robertson C, Sevilla L, Garza J, Rashid H, Benitez AC, et al. A systematic review and meta-analysis on possible role of vitamin C in sepsis. Cureus. (2022) 14:e32886. doi: 10.7759/cureus.32886
30. Wacker DA, Burton SL, Berger JP, Hegg AJ, Heisdorffer J, Wang Q, et al. Evaluating vitamin C in septic shock: a randomized controlled trial of vitamin C monotherapy. Crit Care Med. (2022) 50:e458–67. doi: 10.1097/CCM.0000000000005427
31. Lamontagne F, Masse MH, Menard J, Sprague S, Pinto R, Heyland DK, et al. Intravenous vitamin C in adults with sepsis in the intensive care unit. N Engl J Med. (2022) 386:2387–98. doi: 10.1056/NEJMoa2200644
32. Lyu QQ, Zheng RQ, Chen QH, Yu JQ, Shao J, Gu XH. Early administration of hydrocortisone, vitamin C, and thiamine in adult patients with septic shock: a randomized controlled clinical trial. Crit Care. (2022) 26:295. doi: 10.1186/s13054-022-04175-x
33. Hwang SY, Ryoo SM, Park JE, Jo YH, Jang DH, Suh GJ, et al. Combination therapy of vitamin C and thiamine for septic shock: a multi-centre, double-blinded randomized, controlled study. Intensive Care Med. (2020) 46:2015–25. doi: 10.1007/s00134-020-06191-3
34. Moskowitz A, Huang DT, Hou PC, Gong J, Doshi PB, Grossestreuer AV, et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. J Am Med Assoc. (2020) 324:642–50. doi: 10.1001/jama.2020.11946
35. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. J Am Med Assoc. (2020) 323:423–31. doi: 10.1001/jama.2019.22176
36. Rosengrave P, Spencer E, Williman J, Mehrtens J, Morgan S, Doyle T, et al. Intravenous vitamin C administration to patients with septic shock: a pilot randomised controlled trial. Crit Care. (2022) 26:26. doi: 10.1186/s13054-022-03900-w
37. Hussein AA, Sabry NA, Abdalla MS, Farid SF. A prospective, randomised clinical study comparing triple therapy regimen to hydrocortisone monotherapy in reducing mortality in septic shock patients. Int J Clin Pract. (2021) 75:e14376. doi: 10.1111/ijcp.14376
38. Mohamed ZU, Prasannan P, Moni M, Edathadathil F, Prasanna P, Menon A, et al. Vitamin C therapy for routine care in septic shock (ViCTOR) trial: effect of intravenous vitamin C, thiamine, and hydrocortisone administration on inpatient mortality among patients with septic shock. Indian J Crit Care Med. (2020) 24:653–61. doi: 10.5005/jp-journals-10071-23517
39. Jamshidi MR, Zeraati MR, Forouzanfar B, Tahrekhani M, Motamed N. Effects of triple combination of hydrocortisone, thiamine, and Vitamin C on clinical outcome in patients with septic shock: a single-center randomized controlled trial. J Res Med Sci. (2021) 26:47. doi: 10.4103/jrms.JRMS_593_19
40. Balakrishnan M, Gandhi H, Shah K, Pandya H, Patel R, Keshwani S, et al. Hydrocortisone, vitamin C and thiamine for the treatment of sepsis and septic shock following cardiac surgery. Indian J Anaesth. (2018) 62:934–9. doi: 10.4103/ija.IJA_361_18
41. Lv SJ, Zhang GH, Xia JM Yu H, Zhao F. Early use of high-dose vitamin C is beneficial in treatment of sepsis. Ir J Med Sci. (2021) 190:1183–8. doi: 10.1007/s11845-020-02394-1
42. Mahmoodpoor A, Shadvar K, Sanaie S, Hadipoor MR, Pourmoghaddam MA, Saghaleini SH. Effect of vitamin C on mortality of critically ill patients with severe pneumonia in intensive care unit: a preliminary study. BMC Infect Dis. (2021) 21:616. doi: 10.1186/s12879-021-06288-0
43. Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, Buchman TG, et al. Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. J Am Med Assoc. (2021) 325:742–50. doi: 10.1001/jama.2020.24505
44. Iglesias J, Vassallo AV, Patel VV, Sullivan JB, Cavanaugh J, Elbaga Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial. Chest. (2020) 158:164–73. doi: 10.1016/j.chest.2020.02.049
45. Wani SJ, Mufti SA, Jan RA, Shah SU, Qadri SM, Khan UH, et al. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: results from an open label randomised controlled clinical trial and a review of the literature. Infect Dis. (2020) 52:271–8. doi: 10.1080/23744235.2020.1718200
46. Yadav AK, Singh VK, Psingh G, Vinitasingh A. Outcome of ulinastatin vs metabolic resuscitation using ascorbic acid, thiamine and glucocorticoid in early treatment of sepsis-a randomised controlled trial. J Clin Diagnost Res. (2021) 15:UC36–9. doi: 10.7860/JCDR/2021/47233.14946
47. Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. (2021) 11:5. doi: 10.1186/s13613-020-00792-3
48. Fujii T, Salanti G, Belletti A, Bellomo R, Carr A, Furukawa TA, et al. Effect of adjunctive vitamin C, glucocorticoids, and vitamin B1 on longer-term mortality in adults with sepsis or septic shock: a systematic review and a component network meta-analysis. Intensive Care Med. (2022) 48:16–24. doi: 10.1007/s00134-021-06558-0
49. Patel JJ, Ortiz-Reyes A, Dhaliwal R, Clarke J, Hill A, Stoppe C, et al. Vitamin C in critically ill patients: a systematic review and meta-analysis. Crit Care Med. (2022) 50:e304–12. doi: 10.1097/CCM.0000000000005320
50. Martimbianco ALC, Pacheco RL, Bagattini ÂM, de Fátima Carreira Moreira Padovez R, Azevedo LCP, Riera R. Vitamin C-based regimens for sepsis and septic shock: systematic review and meta-analysis of randomized clinical trials. J Crit Care. (2022) 71:154099. doi: 10.1016/j.jcrc.2022.154099
51. Lagowska-Lenard M, Stelmasiak Z, Bartosik-Psujek H. Influence of vitamin C on markers of oxidative stress in the earliest period of ischemic stroke. Pharmacol Rep. (2010) 62:751–6. doi: 10.1016/S1734-1140(10)70334-0
52. Fujii T, Deane AM, Nair P. Metabolic support in sepsis: corticosteroids and vitamins: the why, the when, the how. Curr Opin Crit Care. (2020) 26:363–8. doi: 10.1097/MCC.0000000000000736
53. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. (2013) 13:260–8. doi: 10.1016/S1473-3099(13)70001-X
54. Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. (2018) 24:248–55. doi: 10.1097/MCC.0000000000000510
55. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. (2018) 378:797–808. doi: 10.1056/NEJMoa1705835
56. Annane D, Brun-Buisson C, Cariou A, Martin C, Misset B, Renault A, et al. Erratum to: design and conduct of the activated protein C and corticosteroids for human septic shock (APROCCHSS) trial. Ann Intensive Care. (2016) 6:79. doi: 10.1186/s13613-016-0165-1
57. Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. (2016) 44:360–7. doi: 10.1097/CCM.0000000000001572
58. Mohammed BM, Fisher BJ, Kraskauskas D, Farkas D, Brophy DF, Fowler AA 3rd, et al. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. (2013) 5:3131–51. doi: 10.3390/nu5083131
59. Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res. (2003) 109:144–8. doi: 10.1016/S0022-4804(02)00083-5
60. Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care. (2014) 18:460. doi: 10.1186/s13054-014-0460-x
61. Berger MM, Oudemans-van Straaten HM. Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care. (2015) 18:193–201. doi: 10.1097/MCO.0000000000000148
62. Margaritelis NV, Paschalis V, Theodorou AA, Vassiliou V, Kyparos A, Nikolaidis MG. Rapid decreases of key antioxidant molecules in critically ill patients: a personalized approach. Clin Nutr. (2020) 39:1146–54. doi: 10.1016/j.clnu.2019.04.029
63. McNamara R, Deane AM, Anstey J, Bellomo R. Understanding the rationale for parenteral ascorbate (vitamin C) during an acute inflammatory reaction: a biochemical perspective. Crit Care Resusc. (2018) 20:174–9.
64. Hartmann SE, Waltz X, Kissel CK, Szabo L, Walker BL, Leigh R, et al. Cerebrovascular and ventilatory responses to acute isocapnic hypoxia in healthy aging and lung disease: effect of vitamin C. J Appl Physiol. (2015) 119:363–73. doi: 10.1152/japplphysiol.00389.2015
65. Meng J, Lv Z, Zhang Y, Wang Y, Qiao X, Sun C, et al. Precision redox: the key for antioxidant pharmacology. Antioxid Redox Signal. (2021) 34:1069–82. doi: 10.1089/ars.2020.8212
Keywords: vitamin C, sepsis, septic shock, hydrocortisone, meta-analysis
Citation: Liang H, Mu Q, Sun W, Liu L, Qiu S, Xu Z, Cui Y, Yan Y and Sun T (2023) Effect of intravenous vitamin C on adult septic patients: a systematic review and meta-analysis. Front. Nutr. 10:1211194. doi: 10.3389/fnut.2023.1211194
Received: 24 April 2023; Accepted: 06 July 2023;
Published: 03 August 2023.
Edited by:
Cristian Deana, Azienda Sanitaria Universitaria Integrata di Udine, ItalyReviewed by:
Zahra Vahdat Shariatpanahi, Shahid Beheshti University of Medical Sciences, IranCopyright © 2023 Liang, Mu, Sun, Liu, Qiu, Xu, Cui, Yan and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tongwen Sun, c3VudG9uZ3dlbkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.