- 1Ketogenic Metabolic Therapy Laboratory, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
- 2Faculty of Medicine, Department of Pediatrics, University of São Paulo, São Paulo, Brazil
- 3Laboratory of Food Education and Sport Nutrition, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
- 4Department of Child Neurology and Psychiatry, Istituto di Ricerca e Cura a Carattere Scientifico Mondino Foundation, Pavia, Italy
- 5Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
Introduction: Headaches are a prevalent disorder worldwide, and there is compelling evidence that certain dietary interventions could provide relief from attacks. One promising approach is ketogenic therapy, which replaces the brain's glucose fuel source with ketone bodies, potentially reducing the frequency or severity of headaches.
Aim: This study aims to conduct a systematic review of the scientific literature on the impact of ketosis on migraine, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method.
Results: After a careful selection process and bias evaluation, 10 articles were included in the review, primarily from Italy. The bias assessment indicated that 50% of the selected articles had a low risk of bias in all domains, with the randomization process being the most problematic domain. Unfortunately, the evaluation of ketosis was inconsistent between articles, with some assessing ketonuria, some assessing ketonemia, and some not assessing ketosis levels at all. Therefore, no association could be made between the level of ketosis and the prevention or reduction of migraine attacks. The ketogenic therapies tested in migraine treatments included the very low-calorie ketogenic diet (VLCKD, n = 4), modified Atkins diet (MAD, n = 3), classic ketogenic diet (cKDT, n = 2), and the administration of an exogenous source of beta-hydroxybutyrate (BHB). The meta-analysis, despite reporting high heterogeneity, found that all interventions had an overall significant effect (Z = 9.07, p < 0.00001; subgroup differences, Chi2 = 9.19, dif = 3, p = 0.03; I2, 67.4%), regardless of the type of endogenous or exogenous induction of ketosis.
Conclusion: The initial findings of this study suggest that metabolic ketogenic therapy may provide some benefit in treating migraines and encourage further studies, especially randomized clinical trials with appropriate and standardized methodologies. The review strongly recommends the use of the adequate measurement of ketone levels during ketogenic therapy to monitor adherence to the treatment and improve knowledge of the relationship between ketone bodies and efficacy.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022330626.
1. Introduction
Headache is one of the most common disorders in the general population, and it is frequently diagnosed in childhood (1), with a prevalence of 54.4–58.4% in children and adolescents (2). It is the third biggest cause of disability across the world (3), and in 2019 it was ranked 14th overall for global causes of disability-adjusted life years, rising to 10th place for females and ranked 2nd and 5th among individuals aged 10–24 and 25–49, respectively (4). According to the International Classification of Headache Disorders 3rd edition (ICHD-3) (5), headache is classified into primary headache, with no underlying organic causes, and secondary headache (6). Primary headache onset often occurs in childhood or adolescence and its prevalence grows with age, heavily impacting a child's quality of life, in particular on school performance, sports, and social activities (3). Tension-type headache (prevalence of 20–25%) is the most common cause of primary headache, followed by migraine (7). Migraine prevalence is ~15% in the general population, with a peak between 35 and 39 years (8). Moreover, migraine affects 7.7–9.1% of children, and girls are the most impacted (2). According to the ICHD-3, the three main categories of migraine are migraine without aura, migraine with aura, and chronic migraine. Aura consists of reversible focal neurologic symptoms (visual scintillations, scotoma, and, less often, spreading hemisensory symptoms or speech dysfunction) that develop gradually over a period of 5–60 min (or less) followed by a subsequent headache, typically unilateral, pulsatile, and aggravated by physical activity. Common accompanying symptoms are nausea, vomiting, photophobia, and phonophobia (5).
Migraine is a neurovascular disease that tends to run in families and likely has a genetic basis (9). The most widely accepted etiopathogenetic theory is Moskowitz's trigeminovascular hypothesis (10, 11). According to this theory, patients are predisposed to migraine attacks due to an over-sensitization of the trigeminal and trigemino-cervical neurons, which is associated with a lowered threshold of activation of nociceptive terminals by vasoactive peptides, the most important of which is CGRP (12). In addition, there is strong evidence to suggest that migraine is a brain energy deficit syndrome: several magnetic resonance spectroscopy studies have highlighted that the brains of migraine sufferers experience an energy deficit during attacks, likely in response to hypometabolism and increased oxidative stress in the brain (13). Current prophylactic therapies for migraine often suffer from a lack of specificity, poor tolerability, potential side effects, and limited efficacy, leading to unsatisfactory results in a large proportion of patients (14). Although several promising monoclonal antibodies have been developed for the adult population and are now being implemented in the pediatric population, most of them are not currently available in clinical practice. In parallel with the development of novel therapeutics for migraine, there is a growing body of literature on the use of neuromodulatory non-pharmacologic approaches, such as nutraceuticals (e.g., riboflavin, coenzyme Q10, magnesium, vitamin D, and melatonin) or behavioral therapies (15).
Nutrition is a widely discussed environmental factor that may affect the course of migraine (16). While there is a debate over how certain foods can act as favorable or protective factors in relation to migraine attacks and the pro-inflammatory state, it is accepted that migraine is sensitive to diet, including food amount and meal timing, and that some dietary ingredients can trigger migraine attacks (16). A long list of foods involved in the mechanism of migraine has been identified, such as chocolate, citrus fruits, nuts, ice cream, tomatoes, onions, dairy products, alcoholic beverages, coffee, caffeine, monosodium glutamate, histamine, tyramine, phenylethylamine, nitrites, aspartame, sucralose, and gluten. Some foods or ingredients can trigger headaches only in subgroups of patients (e.g., celiac groups), while others can cause migraines in case of abstinence (such as caffeine) (17). Even when these elements are correctly identified, the use of diets with certain food restrictions remains controversial (17). Low-fat or weight-loss dietary interventions for migraine have so far been inconclusive. The mechanisms through which nutrition could impact migraine could be related to decreasing inflammation, such as with high omega-3/low omega-6 diets, which can bring beneficial effects. Nonetheless, more studies are needed (18).
In recent years, the use of high-fat low-carbohydrate diets has gained popularity as a possible treatment for migraine. The International Ketogenic Diet Study Group cites migraine as one of the neurological diseases that can potentially benefit from ketogenic dietary treatment (KDT) (19). Interestingly, the first report of using KDT for migraine appeared in 1928 (20), only a few years after the diet's first use for epilepsy. This study obtained poor results (some relief in nine of the 23 adult patients), but the author reported being encouraged enough to continue with the high-fat method. In the last few years, several studies described in a review by Caminha et al. (21) have investigated the potentially protective effects of ketosis-inducing diets in migraine. Ketogenic diet therapies may affect migraine in several ways: (i) by replacing brain fuel from glucose to ketone bodies (KBs); (ii) through the positive influence of systemic ketosis on pathways of migraine pathophysiology; (iii) as signaling molecules, KBs could increase mitochondrial functioning, reduce oxidative stress, alter cerebral excitability, change cortical spreading depression, reduce systemic inflammation, and change the gut microbiome (22). The concept of a gut-brain axis can stimulate the use of probiotics for several neurological diseases, but studies investigating probiotics for migraine are limited and often mix the application of probiotics with other components (17).
Based on the protective effects of ketone bodies, an interesting question that has yet to be answered is whether the levels of ketosis, measured by circulating levels of beta-hydroxybutyrate (BHB), are related to the efficacy of migraine attack prevention. To achieve high systemic ketosis, it is necessary to follow a diet that is high in fat, moderate in protein, and very low in carbohydrates, such as the classical ketogenic diet with 3:1 or 4:1 ketogenic ratios. However, there are physiological differences between children and adults, and even when treated with diets at the same ketogenic ratio, adults have much lower blood ketone levels than children (22, 23). Furthermore, the classic ketogenic diet is more burdensome for patients, requiring a strict dietary plan and weighing of foods to the gram. Alternative dietary approaches, such as the medium-chain triglycerides diet, modified Atkins diet, and low glycemic index therapy, have been developed to allow for greater flexibility for patients in epilepsy treatment. From a clinical perspective, it is of paramount importance to establish target levels of ketosis that are sufficient to maintain the beneficial effect on migraine while limiting dietary restrictions.
This systematic review and meta-analysis are an initial effort to systematize information on the efficacy and tolerability of different ketogenic diets and levels of ketosis in the prevention of migraine in children, adolescents, and adults.
2. Materials and methods
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24). A comprehensive search was performed in electronic databases, including PubMed/Medline, Scopus, Web of Science, LILACS, Science Direct, and the Cochrane Library. The search was limited to articles published in the last 10 years, and only studies published in English, Italian, Portuguese, or Spanish were included.
To investigate the effect of ketosis on migraine, both clinical trials and observational studies were included regardless of whether they were controlled or randomized. The study protocol was registered on the PROSPERO platform (registration number: CRD42022330626).
2.1. Literature search
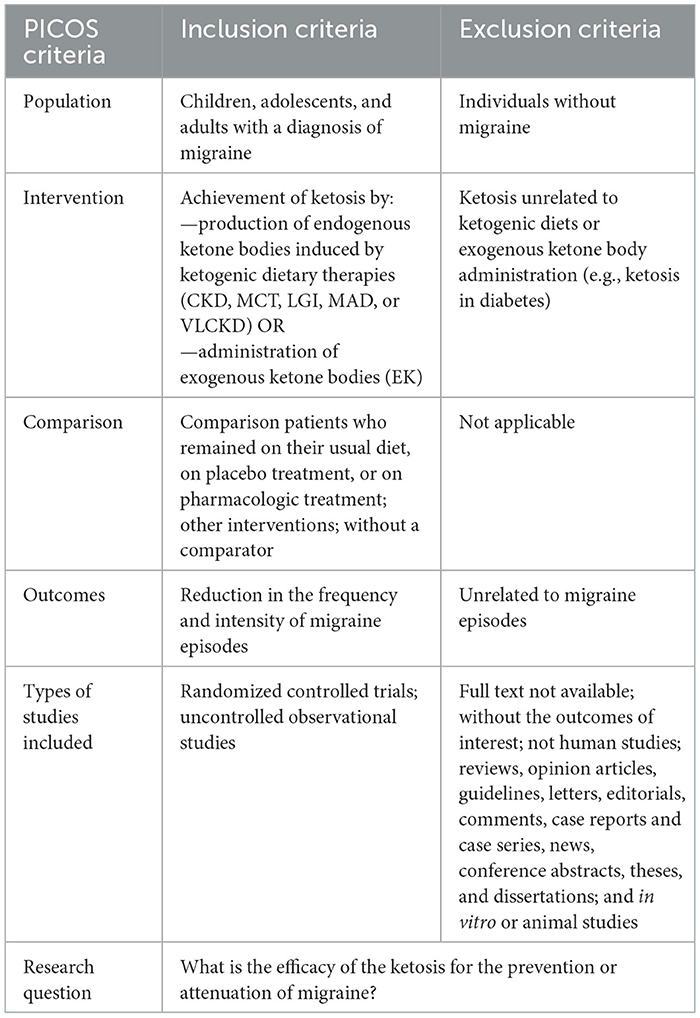
An electronic search was conducted using subject index terms such as “migraine,” “migraine disorders,” “headache,” “headache disorders,” “cephalalgia,” and “cephalalgia” in combination with keywords such as “ketones,” “3-hydroxybutyric acid,” “ketone bodies,” “acetoacetates,” “ketosis,” “ketoacidos*,” “metabolic keto*,” “acetonemi*,” “ketonemi*,” “ketoacidemi*,” “ketonuri*,” “ketoaciduri*,“ or “acetonuri*”. The final search strategy is described in detail in the Supplementary material. In addition, gray literature was searched using Google Scholar. Some newer references from more up-to-date studies or references found in previous review articles were included manually. The populations of interest were adults, adolescents, and children, and the comparison group was any control diet or placebo group. Detailed inclusion and exclusion criteria are described in Table 1.
2.2. Study selection
The research and study selection were carried out independently by two authors (LCLN and GC) using Rayyan software (25) in two steps: (1) reading the titles and abstracts, and (2) evaluating the complete articles selected in the previous stage and including other studies present in the references of the selected articles. The decision to include the articles was based on the PICOS strategy [Population (P), children and adults; Intervention (I), ketosis/ketone bodies; Control (C), placebo; Outcome (O), relief in migraine symptoms; Study type (S), clinical trials]. In cases of disagreement, a third author (MG) reviewed the full-text articles to decide. The articles found in the electronic databases were organized using the Mendeley reference manager. Google Scholar was used to search for gray literature.
2.3. Study quality
The risk of bias was assessed using the RoB 2.0 Cochrane tool (26), which checked five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. After selecting articles, the quality of evidence was checked using the Mixed Methods Appraisal Tool system (MMAT system) (27). Data tables were constructed based on article details, and data for generating the meta-analysis were exported to Review Manager (RevMan) 5.4 software (28).
3. Results
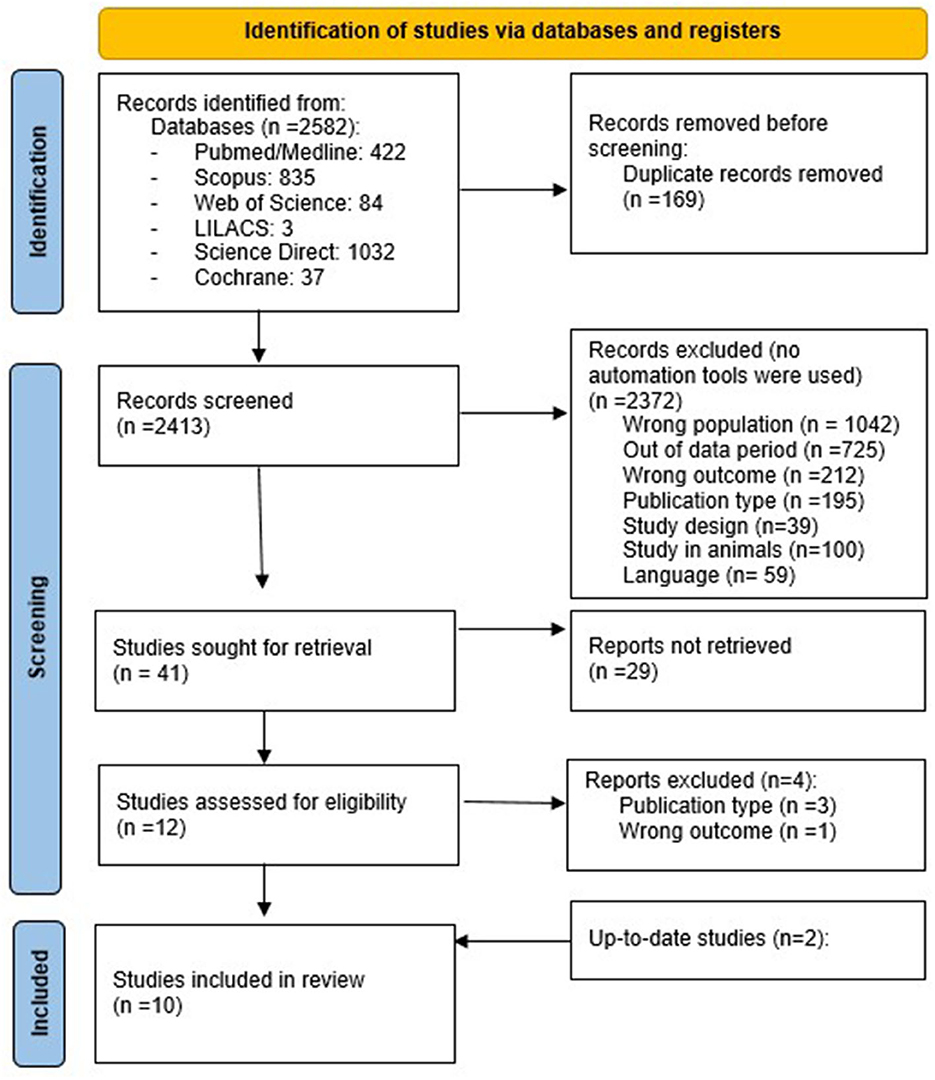
A total of 2,582 articles were identified from all databases. After a systematic selection of articles that excluded duplicates (carried out by two authors), only 10 articles remained (eight conducted in Italy, one in Switzerland, and one in Australia). The article selection process is illustrated in the flow chart (Figure 1), following the PRISMA method (24).
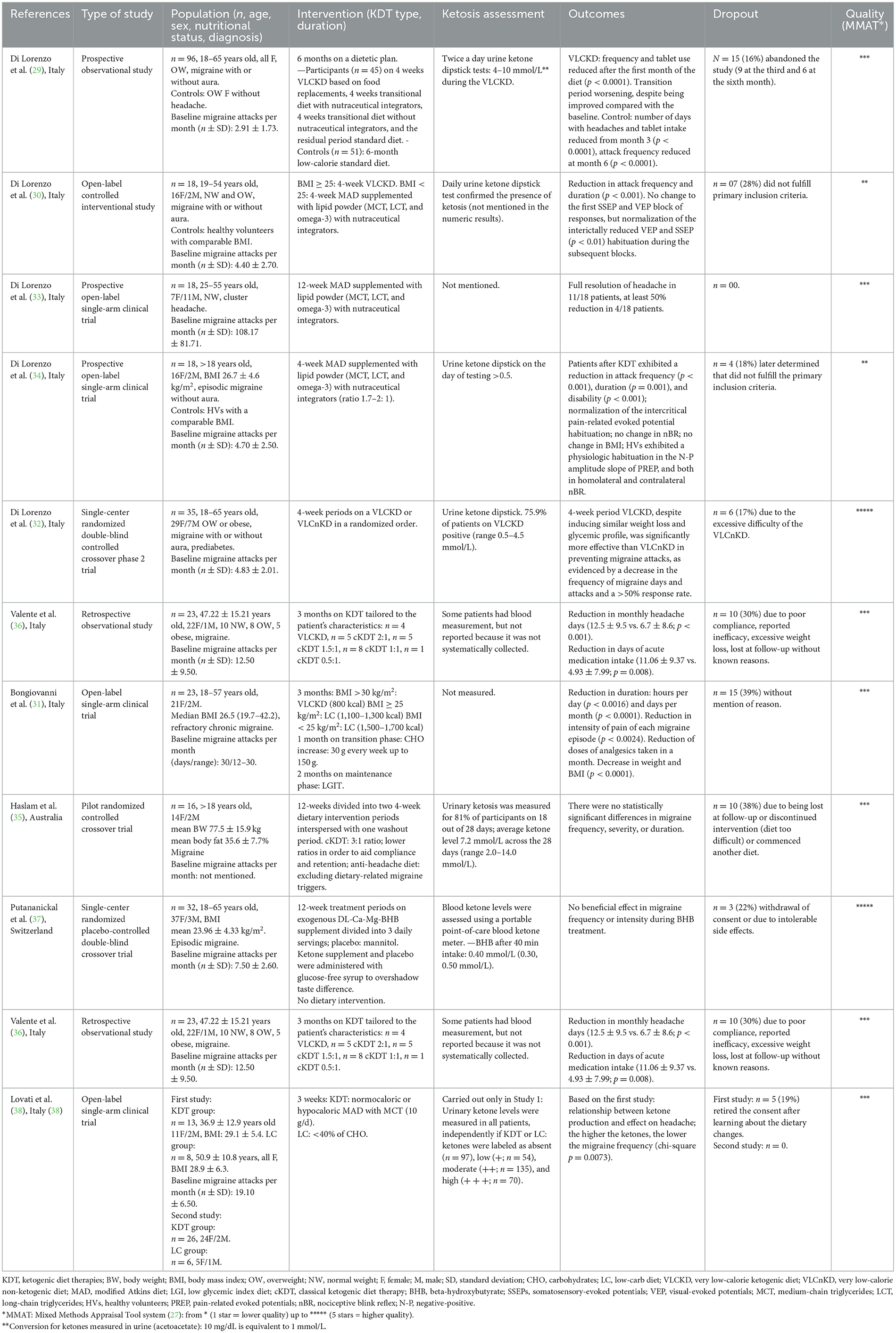
The study population, type and duration of ketogenic therapy intervention, levels of urine or blood ketosis (if reported), outcomes, and dropout rates in the selected articles are synthesized in Table 2. All studies were conducted on adults affected by migraine, except for one that concerned the treatment of cluster headaches. Half of the selected articles used an interventional open-label trial design, and only one article used retrospective observational data. The different ketogenic dietary treatments used in the selected studies are described in the following section.
3.1. Ketogenic therapies tested in migraine treatment
3.1.1. Very low-calorie ketogenic diet
Most articles (four out of 10) used a Very Low-Calorie Ketogenic Diet (VLCKD) (29–32). In these studies, the diet consisted of a semi-starvation regimen ( ≤ 800 kcal) composed of low-carbohydrate (about 30 g/day carbohydrates), low-fat (15–20 g lipids), and a normal-protein (1.0–1.5 g/kg of desired weight proteins) amount. Patients were instructed to avoid rice, grains, cereals and derivatives (bread, pasta, crackers, cookies, etc.), legumes, starchy vegetables (potatoes, corn, and green peas), fruits, and dairy products other than cheese, cream, or butter. The diet plans of the studies included Italian dietary products developed by industries. Owing to dietary restrictions, nutraceutical integrators were prescribed. Salads were allowed ad libitum, dressed with a spoonful of olive oil. After the VLCKD period, patients progressively reintroduced carbohydrates from breakfast to dinner to wean themselves from ketogenesis. At the end of the transition period or for the control group, patients received a standard diet (SD) or low glycemic index diet (LGID). In these cases, the SD was considered a low-calorie diet (1,200–1,500 kcal) subdivided into five daily meals with the following profile: 46% of total energy from carbohydrates, 24% as protein, and 30% as total fat (< 8% saturated fat). LGID consisted of exchanging the provision of carbohydrates into a low glycemic index (< 50) carbohydrate profile.
3.1.2. Modified atkins diet
Another ketogenic diet therapy approach used in three articles (30, 33, 34) was the modified Atkins diet (MAD). In these studies, the approach consisted of a low-carbohydrate (~15 g/day), normal/low-protein (about 0.7–1.2 g/kg/day), and high-fat (approximately little more than the weight of carbohydrates and proteins together) diet prepared from common foods. Most articles allowed no more than 10 g of carbohydrates per day in the first month (after that, up to 20–30 g/day) subdivided into three regular-size meals a day or four to five smaller meals. Each meal consisted of a liberal combination of fat and protein in the form of fish, shellfish, poultry, red meat, eggs, or low-carbohydrate and high-fat cheese, dressed with butter, heavy whipping cream, mayonnaise, olive oil, and other vegetable oils. Almonds, nuts, and oilseeds were suitable as snacks. Salad vegetables were suggested twice per day, dressed with oil, mayonnaise or sour cream, vinegar (without added sugars, no balsamic), and salt. Leafy vegetables, up to 200 g per portion, were permitted, while other vegetables were limited. All spices (without added sugar) were allowed.
3.1.3. Classic ketogenic diet
Only two articles (35, 36) used classical ketogenic diet therapy (cKDT), which is commonly used for refractory epilepsy. This ketogenic therapy was presented in individualized meal plans, calculated by a dietitian according to ratios of grams of total fat to carbohydrate plus protein (3:1, 2:1, and 1:1). Haslam et al. (35) provided meal plans for a rotating 7-day 1,600 (females) or 1,800 (males) kcal/day, presented as three meals per day. Participants had the option to snack on additional items from an approved list with a 3:1 ratio of total fat to combined carbohydrates plus protein (e.g., 30 g of nuts) or choose extra meals ad libitum from the meal plan to manage hunger and/or fatigue as needed. Participants were advised that they could flavor the meals with herbs, spices, salt, and pepper, as desired, but should avoid using pre-packaged sauces to moderate total carbohydrate intake. They were instructed to abstain from alcohol and not deviate from the meal plans. Black tea, coffee, and artificially sweetened beverages, such as diet soft/soda drinks, were recommended ad libitum. All participants started on a 3:1 diet plan and were monitored by a dietitian, with weekly follow-ups via email or telephone. If a participant indicated he/she was struggling to adhere to the initial 3:1 diet due to food portion sizes or was not tolerating the high-fat content or carbohydrate restriction, he/she was downgraded onto the 2:1 and then 1:1 to aid compliance and retention in the study, while achieving ketosis.
3.1.4. Other approaches
One study used a low-carb (LC) approach with a low-calorie but high-protein diet (31), while another study (37) did not use a specific dietary approach and instead relied solely on an exogenous source of beta-hydroxybutyrate (BHB).
All the aforementioned articles can be found in detail in Table 2.
3.2. Efficacy on migraine
The studies by Di Lorenzo et al. (29, 30, 33, 34) and Bongiovanni et al. (31) demonstrated a positive effect on the frequency and intensity of migraine attacks. Some studies reported that the percentage of patients achieving a decrease in the number of migraine attacks (generally of at least 50%) ranged from 58 to 83% of patients (31, 32, 36, 38), and full resolution of attacks was reported in 63% of patients in the study by Di Lorenzo et al. (33) (data not shown). There were five single-arm intervention studies (29–31, 33, 34) and one randomized trial (32).
On the other hand, two other clinical trials (35, 37) did not reach statistically significant results on the efficacy of ketogenic therapy. Unfortunately, owing to a shortage of included studies and the lack of clinical data availability, a correlation of KD efficacy on migraine type was not feasible.
3.3. Ketosis assessment
The assessment of ketosis varied greatly among the articles, with ketonemia measured in only two articles (36, 37) and ketonuria measured in six articles (29, 30, 32, 34, 35, 38).
In studies using VLCKD, Bongiovanni et al. (31) did not measure ketone levels, while Di Lorenzo et al. used ketonuria as the preferred method of ketosis monitoring. The authors described that a daily urine dipstick confirmed the presence of ketosis without reporting values in one paper (30) and values in the range of 0.5–10 mmol/L were reported in the other two studies (29, 32). Ketonuria was the preferred method of ketosis monitoring in all studies using MAD (30, 33, 34), but values were not detailed.
In the study using cKDT, Valente et al. (36) reported that many patients measured blood ketones, but the authors did not systematically collect or report any data. On the contrary, in the study by Haslam et al. (35), urinary ketosis was measured by 81% of participants in 18 out of 28 days, with an average level of 7.2 mmol/L (range 2.0–14.0 mmol/L). Putananickal et al. (37) measured blood ketones and described average values of 0.4 mmol/L 40 min after the intake of ketone supplements.
Therefore, some authors did not report the results from this assessment or did not measure it, impairing the analysis of adherence to the treatment. Surprisingly, studies that reported similar values of ketonuria (29, 35) found contrasting results on outcomes with the intervention. On the other hand, Lovati et al. (38) found an inverse correlation between ketonuria and the frequency of migraine episodes in 13 patients.
3.4. Tolerability
In general, all studies showed good tolerability of ketosis with some common related side effects, including constipation (32, 35, 36), fatigue, nausea (35, 36), bloating (33, 35), difficulty sleeping, dizziness, irritability, flatulence (35), muscle cramps (32), abdominal pain, excessive weight loss (36), and hair loss (33). Some studies reported no side effects of the intervention (38). An average of a 21% (0–39%) dropout rate could be a reason for the small final sample sizes in all studies. Some studies justified the high dropout rate due to difficulties with diet compliance, which is often reported as incompatible with a normal social life (31).
3.5. Meta-analysis
A meta-analysis was performed only with studies that had available data regarding the average number of migraine attacks per month before and after intervention, along with their standard deviation.
The meta-analysis was performed on subgroups based on the type of intervention. The VLCKD intervention had four articles (29–32), but one of them (31) did not have complete data so it was excluded. Four studies were considered for the MAD intervention (30, 33, 34, 38), and one of them (33) had more expressive results. Only one study was included for the interventions with exogenous BHB (37) and cKDT (36).
Despite high reported heterogeneity, all interventions had an overall significant positive effect, as seen in Figure 2, regardless of the type of endogenous or exogenous induction of ketosis. For the EK and cKDT approaches, as they are alone in the category, heterogeneity was not applicable.
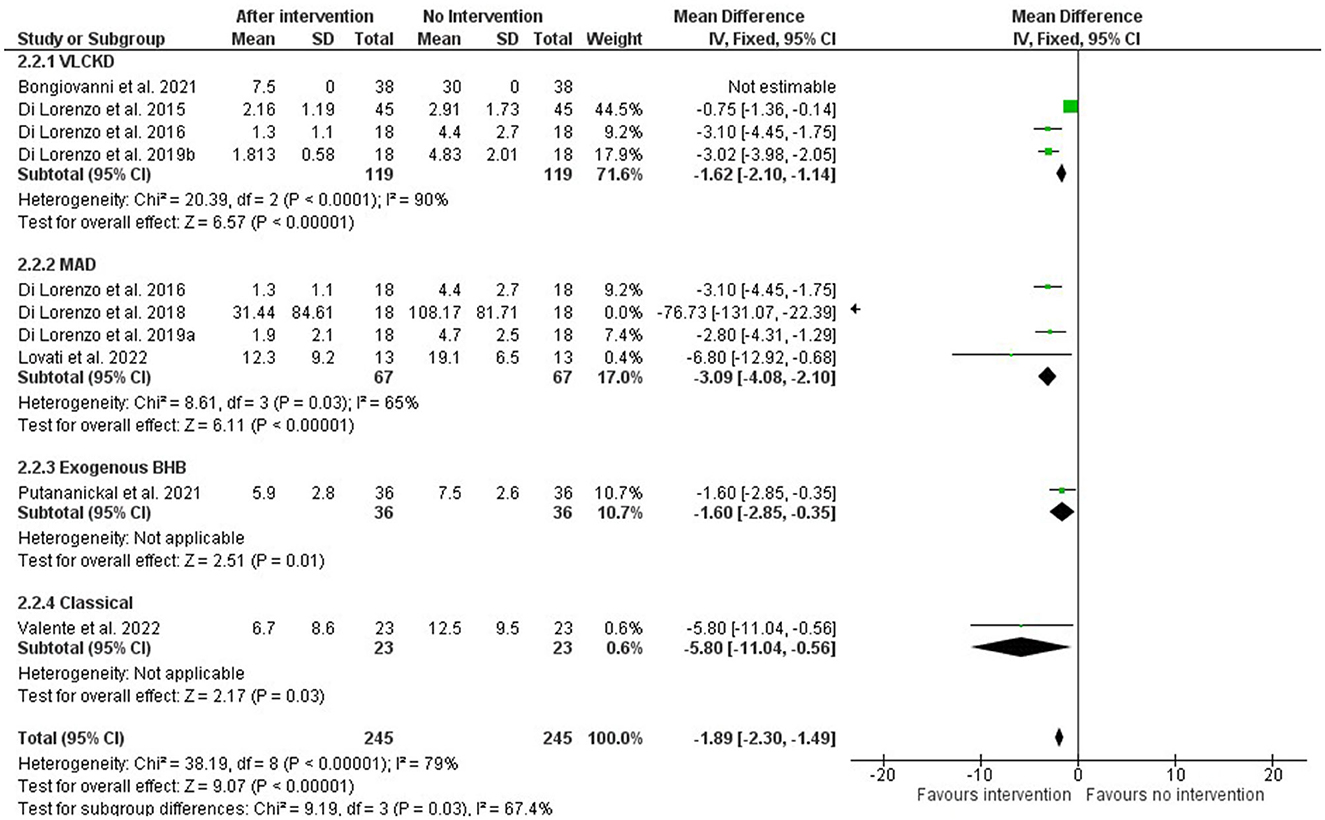
Figure 2. Forest plot of the meta-analysis of different types of ketosis-inducing interventions on migraine frequency attacks (number per month).
3.6. Bias assessment
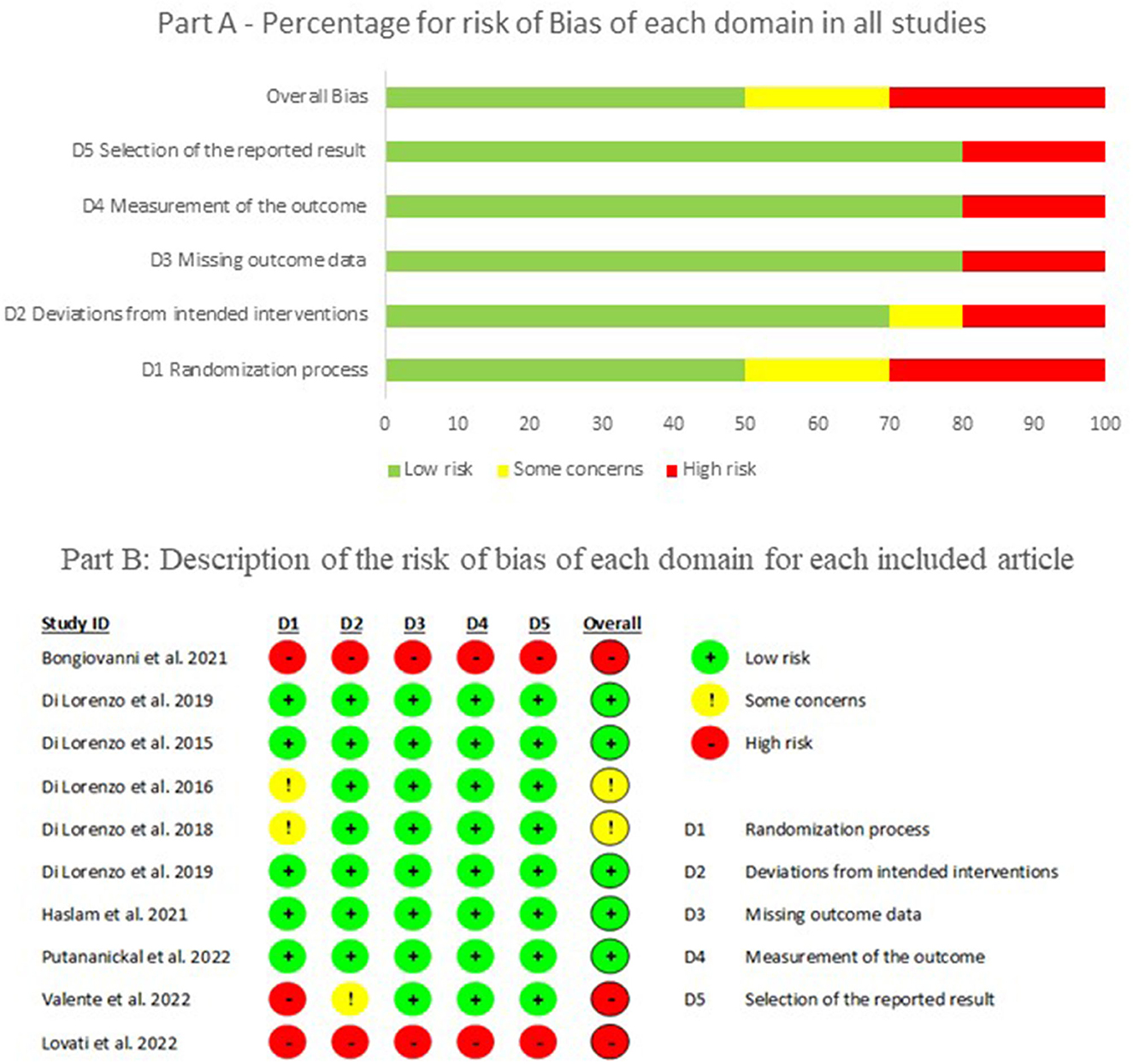
The assessment of bias showed that 50% of the selected articles had a low risk of bias in all domains. The main domain with problems was the randomization process due to the methodological design of the studies, and few randomized controlled trials were included. Figure 3 shows the overall percentage of bias and for each domain according to the Rob 2.0 Cochrane tool (part A), along with detailed information on the risk of bias from each article (part B). This is reflected in the quality of the studies (MMAT), in which only two articles reached a high level of quality (five stars), and the majority received a three-star quality rating (60%), as can be seen in Table 2.
4. Discussion
This is the first attempt to systematically relate ketosis with migraine improvement through a meta-analysis of the effect of different ketogenic interventions (dietary or not) on migraine attacks. Even though the main result of the article is ketosis based, for the authors, it was challenging to standardize the pattern of results between articles, considering that most of them provide results with no mention of ketosis.
The results from this paper could support some beneficial effect of ketone bodies on the prevention or relief of migraine attacks, regardless of the strategy to increase ketosis. Unfortunately, the paucity and heterogeneity of available studies, which included only adult populations, does not allow the identification of demographic, clinical, or dietetic parameters that might correlate with a better outcome.
In stating the role of ketosis levels in migraine amelioration (mainly using BHB as a measure for KDT implementation), many red flags must be considered. First, some studies correlate the improvement of migraine attacks to weight loss in overweight subjects, probably related to a reduction in pro-inflammatory adipokines (39). Second, the correlation between blood and urine ketones is not well-defined and might vary depending on hydration/urine volume, acid-base balance, renal hemodynamics, and excretion (40). Third, KDT mechanisms of action might rely on both direct and indirect actions, such as modeling mitochondrial function, increasing glutathione, reducing ROS, and inhibiting the NLRP3 inflammasome (41).
Most of the articles included in this systematic review mainly addressed headache pain related to migraine (frequency, duration, and severity); however, some studies also measured aspects related to general disability (34, 37), medication use (29, 31, 37), or changes in BMI (29–31, 34).
Valente et al. (36) aimed to clarify whether the observed effect of KDT on migraine was due only to weight and fat mass reduction or not. Interestingly, patients benefitted from KDT independently from the reduced weight and fat mass, implying that the beneficial effects of KDT might be related to mechanisms other than just weight change (36). According to Lovati et al. (38), these possible mechanisms could be likely related to ketone production. In fact, Lovati et al. (38) found a correlation between urine ketone levels and migraine attack frequency (p = 0.0073), although in a limited sample (n = 13). By contrast, patients on a diet with a similar carbohydrate content but not ketogenic had no beneficial outcomes. Thus, these encouraging results should be viewed with caution because of the limited data available in the selected articles. In fact, the study by Haslam et al. (35), the only one that reported a ketosis level (only urinary), found no significant benefits from a KDT intervention nor found correlations between urinary ketosis and migraine severity.
Interestingly, there is no verified role for ketone levels in seizure control, even for epilepsy. Sharma et al. (42) recalled that, although some studies have demonstrated a positive effect between ketone levels in the blood and urine and seizure control, this finding has not been universal. In addition, it is possible that the age of the subject (42) and other variables may affect the correlation between seizure control and blood/urine ketone levels.
A dropout rate varying from 0 to 39% (average 21%) was reported in the included studies and was higher in patients undergoing cKDT (average 34%) and lower for the MAD (average 13%). As with epileptic patients, a lack of compliance can lie in many factors, with KDT inefficacy on disease symptoms as one of the most prominent. The meta-analysis of Ye et al. (43) on the efficacy and compliance to the cKDT and MAD in adult epileptic patients revealed an overall combined rate of compliance of 45%, with 38 and 56% compliance for cKDT and MAD, respectively. These findings are consistent with a previous study (44), in which compliance was significantly higher in patients undergoing the MAD than in patients following a cKDT. The dropout rate of 21% in the present study is not alarming when compared with the median of 30% of patients who report inconsistently adhering to recommended or prescribed acute migraine medication, or with the rate of only 24% of patients adhering to preventive medication (45). Future studies on migraine patients, both in the pediatric and adult age groups, should be designed to evaluate compliance rates according to migraine type and diverse ketogenic dietary interventions.
Many limitations of the present study must be considered. None of the selected studies included the pediatric population, making it impossible to assess KDT efficacy and tolerability in children/adolescents suffering from migraine. Only a few articles were considered eligible according to the inclusion criteria, the majority of which were from the same research group, potentially biasing the results. Moreover, the presence of bias was evidenced by the Rob2 instrument, especially due to the lack of randomization in most studies. Another limitation is the absence of regular ketosis measurement and the difficulty of assessing adherence to interventions.
Prospective randomized controlled trials are needed to confirm the efficacy and tolerability of KDT in migraine patients, both in the pediatric and adult age groups, and to study its optimal duration, repeatability, feasibility in normal weight subjects, and association with conventional migraine prophylaxis. Additionally, it is essential to underline that the phenotypic expressions of migraine vary greatly in the pediatric population compared with the adult population, thus inclusion criteria and population characteristics must be carefully considered when comparing outcomes.
5. Conclusion
The initial findings of the present review support some benefit of metabolic ketogenic therapy in migraine and encourage further studies, especially randomized clinical trials with appropriate and standardized methodology. Our Systematic Review strongly suggests the inclusion of adequate measurement of ketone levels during ketogenic therapy to check adherence to the treatment and improve knowledge on the relationship between ketone bodies and efficacy. Thus, we can assert with greater certainty that the strategy of increasing ketone bodies in the body can bring benefits in the relief and prevention of migraine.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LN, CF, and AT: conceptualization. LN, CF, LP, and AT: methodology. LN, GC, LP, MG, CF, EP, AC, and AT: investigation. LN, CF, MG, GC, LP, AC, and AT: data curation. LN, AT, GC, and LP: writing—original draft preparation. LN, CF, MG, GC, AT, EP, AC, and LP: writing—review and editing. AT: supervision. All authors have read and agreed to the present version of the manuscript.
Acknowledgments
We acknowledge Rebecca L. Haslam, Clare E. Collins, Erin Clarke, Maurizio Fadda, and Daria Bongiovanni for answering our email with additional data for meta-analysis. We also acknowledge Simona Fiorini, Claudia Trentani, and Leticia Pereira de Brito Sampaio for all their support during this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1204700/full#supplementary-material
References
1. Raucci U, Della Vecchia N, Ossella C, Paolino MC, Villa MP, Reale A, et al. Management of childhood headache in the emergency department. Review of the literature. Front Neurol. (2019) 10:886. doi: 10.3389/fneur.2019.00886
2. Tozzi E, Maiorani D, Ciancarelli I, Sech E. Cefalea in età evolutiva: epidemiologia. Gior Neuropsich Età Evol. (2012) 32:11–6.
3. Lundqvist C, Clench-Aas J, Hofoss D, Bartonova A. Self-reported headache in schoolchildren: parents underestimate their children's headaches. Acta Paediatr. (2006) 95:940–6. doi: 10.1080/08035250600678810
4. Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, Lifting The Burden: the Global Campaign against Headache. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. (2020) 21:137. doi: 10.1186/s10194-020-01208-0
5. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. (2013) 33:629–808. doi: 10.1177/0333102413485658
6. Blume HK. Childhood headache: a brief review. Pediatr Ann. (2017) 46:e155–65. doi: 10.3928/19382359-20170321-02
7. Jong MC, Boers I, van Wietmarschen HA, Tromp E, Busari JO, Wennekes R, et al. Hypnotherapy or transcendental meditation versus progressive muscle relaxation exercises in the treatment of children with primary headaches: a multi-centre, pragmatic, randomised clinical study. Eur J Pediatr. (2019) 178:147–54. doi: 10.1007/s00431-018-3270-3
8. Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. (2022) 23:34. doi: 10.1186/s10194-022-01402-2
10. Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. (2019) 18:795–804. doi: 10.1016/S1474-4422(19)30185-1
11. Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. (2009) 8:679–90. doi: 10.1016/S1474-4422(09)70090-0
12. Ashina M, Hansen JM, Á Dunga BO, Olesen J. Human models of migraine - short-term pain for long-term gain. Nat Rev Neurol. (2017) 13:713–24. doi: 10.1038/nrneurol.2017.137
13. Di Lorenzo C, Ballerini G, Barbanti P, Bernardini A, D'Arrigo G, Egeo G, et al. Applications of ketogenic diets in patients with headache: clinical recommendations. Nutrients. (2021) 13:1–26. doi: 10.3390/nu13072307
14. Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden. Headache. (2007) 47:475–9. doi: 10.1111/j.1526-4610.2007.00752.x
15. Barmherzig R, Rajapakse T. Nutraceuticals and behavioral therapy for headache. Curr Neurol Neurosci Rep. (2021) 21:33. doi: 10.1007/s11910-021-01120-3
16. Papetti L, Moavero R, Ferilli MAN, Sforza G, Tarantino S, Ursitti F, et al. Truths and myths in pediatric migraine and nutrition. Nutrients. (2021) 13:1–14. doi: 10.3390/nu13082714
18. Razeghi Jahromi S, Ghorbani Z, Martelletti P, Lampl C, Togha M. School of advanced studies of the European Headache Federation (EHF-SAS). J Headache Pain. (2019) 20:106. doi: 10.1186/s10194-019-1057-1
19. Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. (2018) 3:175–92. doi: 10.1002/epi4.12225
20. Schnabel TG. An Experience with a ketogenic dietary in migraine. Ann Intern Med. (1928) 2:341–7. doi: 10.7326/0003-4819-2-4-341
21. Caminha MC, Moreira AB, Matheus FC, Rieger DK, Moreira JD, Dalmarco EM, et al. Efficacy and tolerability of the ketogenic diet and its variations for preventing migraine in adolescents and adults: a systematic review. Nutr Rev. (2022) 80:1634–47. doi: 10.1093/nutrit/nuab080
22. Gross EC, Klement RJ, Schoenen J, D'Agostino DP, Fischer D. Potential protective mechanisms of ketone bodies in migraine prevention. Nutrients. (2019) 11:1–20. doi: 10.3390/nu11040811
23. Porper K, Zach L, Shpatz Y, Ben-Zeev B, Tzadok M, Jan E, et al. Dietary-induced ketogenesis: adults are not children. Nutrients. (2021) 13:1–8. doi: 10.3390/nu13093093
24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:1–34. doi: 10.1016/j.jclinepi.2009.06.006
25. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
26. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
27. Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. EFI. (2018) 34:285–91. doi: 10.3233/EFI-180221
28. RevMan 5. Review Manager 5 (RevMan 5), Version 5.4. Copenhagen: The Cochrane Collaboration (2020).
29. Di Lorenzo C, Coppola G, Sirianni G, Di Lorenzo G, Bracaglia M, Di Lenola D, et al. Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur J Neurol. (2015) 22:170–7. doi: 10.1111/ene.12550
30. Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Evangelista M, Sirianni G, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain. (2016) 17:58. doi: 10.1186/s10194-016-0650-9
31. Bongiovanni D, Benedetto C, Corvisieri S, Del Favero C, Orlandi F, Allais G, et al. Effectiveness of ketogenic diet in treatment of patients with refractory chronic migraine. Neurol Sci. (2021) 42:3865–70. doi: 10.1007/s10072-021-05078-5
32. Di Lorenzo C, Pinto A, Ienca R, Coppola G, Sirianni G, Di Lorenzo G, et al. A randomized double-blind, cross-over trial of very low-calorie diet in overweight migraine patients: a possible role for ketones? Nutrients. (2019) 11:1–13. doi: 10.3390/nu11081742
33. Di Lorenzo C, Coppola G, Di Lenola D, Evangelista M, Sirianni G, Rossi P, et al. Efficacy of modified atkins ketogenic diet in chronic cluster headache: an open-label, single-arm, clinical trial. Front Neurol. (2018) 9:64. doi: 10.3389/fneur.2018.00064
34. Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Sirianni G, Rossi P, et al. A ketogenic diet normalizes interictal cortical but not subcortical responsivity in migraineurs. BMC Neurol. (2019) 19:136. doi: 10.1186/s12883-019-1351-1
35. Haslam RL, Bezzina A, Herbert J, Spratt N, Rollo ME, Collins CE. Can ketogenic diet therapy improve migraine frequency, severity and duration? Healthcare. (2021) 9:1–14. doi: 10.3390/healthcare9091105
36. Valente M, Garbo R, Filippi F, Antonutti A, Ceccarini V, Tereshko Y, et al. Migraine prevention through ketogenic diet: more than body mass composition changes. J Clin Med. (2022) 11:1–9. doi: 10.3390/jcm11174946
37. Putananickal N, Gross EC, Orsini A-L, Schmidt S, Hafner P, Gocheva V, et al. Efficacy and safety of exogenous beta-hydroxybutyrate for preventive treatment in episodic migraine: a single-centred, randomised, placebo-controlled, double-blind crossover trial. Cephalalgia. (2022) 42:302–11. doi: 10.1177/03331024211043792
38. Lovati C. d'Alessandro CM, Ventura SD, Muzio F, Pantoni L. Ketogenic diet in refractory migraine: possible efficacy and role of ketone bodies-a pilot experience. Neurol Sci. (2022) 43:6479–85. doi: 10.1007/s10072-022-06311-5
39. Peterlin BL, Sacco S, Bernecker C, Scher AI. Adipokines and migraine: a systematic review. Headache. (2016) 56:622–44. doi: 10.1111/head.12788
40. Carmant L. Assessing ketosis: approaches and pitfalls. Epilepsia. (2008) 49 Suppl 8:20–2. doi: 10.1111/j.1528-1167.2008.01826.x
41. Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy-a review. Nutrients. (2020) 12:1–19. doi: 10.3390/nu12061809
42. Sharma S, Whitney R, Kossoff EH, RamachandranNair R. Does the ketogenic ratio matter when using ketogenic diet therapy in pediatric epilepsy? Epilepsia. (2023) 64:284–91. doi: 10.1111/epi.17476
43. Ye F, Li X-J, Jiang W-L, Sun H-B, Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol. (2015) 11:26–31. doi: 10.3988/jcn.2015.11.1.26
44. Payne NE, Cross JH, Sander JW, Sisodiya SM. The ketogenic and related diets in adolescents and adults–a review. Epilepsia. (2011) 52:1941–8. doi: 10.1111/j.1528-1167.2011.03287.x
Keywords: migraine disorders, ketogenic diet, ketosis, systematic review, meta-analysis
Citation: Neri LdCL, Ferraris C, Catalano G, Guglielmetti M, Pasca L, Pezzotti E, Carpani A and Tagliabue A (2023) Ketosis and migraine: a systematic review of the literature and meta-analysis. Front. Nutr. 10:1204700. doi: 10.3389/fnut.2023.1204700
Received: 12 April 2023; Accepted: 23 May 2023;
Published: 12 June 2023.
Edited by:
Lais Bhering Martins, Swiss Food and Nutrition Valley, SwitzerlandReviewed by:
Shayne Mason, North-West University, South AfricaParisa Gazerani, Oslo Metropolitan University, Norway
Copyright © 2023 Neri, Ferraris, Catalano, Guglielmetti, Pasca, Pezzotti, Carpani and Tagliabue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cinzia Ferraris, Y2luemlhLmZlcnJhcmlzQHVuaXB2Lml0
 Lenycia de Cassya Lopes Neri
Lenycia de Cassya Lopes Neri Cinzia Ferraris
Cinzia Ferraris Guido Catalano
Guido Catalano Monica Guglielmetti
Monica Guglielmetti Ludovica Pasca
Ludovica Pasca Elena Pezzotti
Elena Pezzotti Adriana Carpani4
Adriana Carpani4 Anna Tagliabue
Anna Tagliabue