- 1College of Pharmacy, Jinan University, Guangzhou, China
- 2State Key Laboratory of Natural Medicines, Research Center of Biostatistics and Computational Pharmacy, China Pharmaceutical University, Nanjing, China
- 3Institute of Biomedicine, Anhui Medical University, Hefei, China
- 4National Clinical Research Center for Kidney Disease, State Key Laboratory for Organ Failure Research, Guangdong Provincial Key Laboratory of Renal Failure Research, Guangzhou Regenerative Medicine and Health Guangdong Laboratory, Division of Nephrology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 5Key Laboratory of Precision Nutrition and Food Quality, Department of Nutrition and Health, College of Food Sciences and Nutritional Engineering, China Agricultural University, Beijing, China
- 6Shenzhen Evergreen Medical Institute, Shenzhen, China
- 7Graduate School at Shenzhen, Tsinghua University, Shenzhen, China
- 8Department of Cardiology, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 9School of Health Administration, Anhui Medical University, Hefei, China
- 10Department of Clinical Laboratory, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 11Department of Clinical Laboratory, Fuwai Hospital Chinese Academy of Medical Sciences, Shenzhen, China
Background: There is growing concern regarding elevated levels of circulating unmetabolized folic acid (UMFA) due to excessive intake of folic acid (FA). However, no randomized clinical trial has been conducted to examine the FA-UMFA dose-response relationship.
Objective: This study aimed to investigate the FA-UMFA dose-response relationship in Chinese adults with hypertension and elevated homocysteine (H-type hypertension), a population with clear clinical indication for FA treatment.
Methods: The data for this study were derived from a randomized, double-blind, multicenter clinical trial of 8 FA dosages on efficacy of homocysteine (Hcy) lowering. The parent trial had three 3 stages: screening period (2–10 days), run-in period (0–2 weeks, baseline visit), and double-blind treatment period (8 weeks) with follow-up visits at the end of the 2nd, 4th, 6th, and 8th weeks of treatment. Participants were randomly assigned to 8 treatment groups corresponding to FA dosages of 0, 0.4, 0.6, 0.8, 1.2, 1.6, 2.0 mg to 2.4 mg.
Results: This study included 1,567 Chinese adults aged ≥45 years with H-type hypertension. There was a positive but non-linear association between FA supplementation and UMFA levels in the dosage range of 0 mg to 2.4 mg. In the regression analysis, the coefficients for the linear and quadratic terms of FA dosage were both statistically significant (P < 0.001). Notably, the slope for UMFA was greater for FA dosages >0.8 mg (ß = 11.21, 95% CI: 8.97, 13.45) compared to FA dosages ≤0.8 mg (ß = 2.94, 95% CI: 2.59, 3.29). Furthermore, FA dosages higher than 0.8 mg did not confer additional benefits in terms of increasing 5-methyl tetrahydrofolic acid (5-MTHF, active form of folate) or reducing homocysteine (Hcy).
Conclusion: In Chinese adults with H-type hypertension, this study showed a positive, non-linear, dosage-response relationship between FA supplementation ranging from 0 to 2.4 mg and circulating UMFA levels. It revealed that 0.8 mg FA is an optimal dosage in terms of balancing efficacy (increasing 5-MTHF and lowering Hcy) while minimizing undesirable elevation of UMFA.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT03472508?term=NCT03472508&draw=2&rank=1, identifier NCT03472508.
Introduction
H-type hypertension, defined as essential hypertension with an increased plasma Hcy level (≥10 μmol/L), accounts for about 75% of hypertension among Chinese patients (1, 2). Many studies have indicated that FA supplementation can effectively lower blood pressure (BP), Hcy levels, and coagulation factors, and remarkably improves prothrombotic status in patients with H-type hypertension (3).
After absorption, however, FA needs to be reduced to tetrahydrofolate (THF) to activate its metabolism, subsequently further metabolized to 5-methyl tetrahydrofolic acid (5-MTHF), which is considered the most biologically active and functional form of FA. This activation process consists of two steps, both catalyzed by dihydrofolate reductase (DHFR). In the first step, FA is transformed into dihydrofolate (DHF); in the second step, DHF is transformed into THF (4). Notably, DHFR activity in humans is inefficient and easily saturated (5). Once the catalytic ability of DHFR has been saturated, UMFA accumulates in biological fluids including plasma. Early studies in adults showed that 0.2 mg of oral FA supplementation can lead to detectable concentrations of UMFA in the bloodstream; a daily dosage of 0.4 mg brought out a continuous occurrence of circulatory FA. However, newer, more sensitive methodologies have detected very low concentrations (∼0.8 nmol/L) of UMFA, even in people not taking FA supplementation (6). The presence of UMFA in the circulation is nearly ubiquitous, as it has been detected even in the cord blood of newly delivered infants (7).
Mandatory FA grain fortification in the US (starting in 1998) and in over 50 other countries has raised the circulating levels of UMFA in the general population (8, 9). This practice has also led to a growing concern regarding potential unintended adverse consequences due to high circulating UMFA from FA intake (10). NHANES (the National Health and Nutrition Examination Survey, a US representative sample), a study of elderly participants from 1999 to 2002, showed that UMFA was related to increased odds of anemia among participants who used alcohol (11), alterations in cytokine mRNA expression, a decreased number and weakened cytotoxicity of natural killer (NK) cells (12, 13), and cognitive impairment among seniors (14), as well as showed an association with insulin resistance and metabolic syndrome (15). However, uncertainty remains regarding the exact association between FA supplementation and circulating UMFA levels and what factors may modify the association.
The best study design to address this knowledge gap, is a randomized FA trial. Therefore, we conducted a study using data derived from a randomized, double-blind control, multicenter clinical trial (RCT) to delineate the dosage-response relationship between 8 different FA dosages (ranging from 0 to 2.4 mg daily by mouth) with circulating levels of UMFA. The original trial was conducted in H-type hypertension patients who all had elevated Hcy. As demonstrated in publications from our group (16) and others (17), FA supplementation is a well-established and effective treatment for this patient population. The goal of the current study was to identify the optimal FA dosage that would find the balance between maximizing efficacy in lowering Hcy and minimizing UMFA levels, while considering pertinent individual characteristics. This study represents a significant step toward a future vision of precision nutrition (18).
Methods
Study design
This study used data from a randomized, double-blind control, multicenter clinical study. The primary aim of the trial was to evaluate Hcy reduction efficacy by different dosages of FA among hypertension patients, stratified by MTHFR C677T genotypes (19).
The parent clinical trial consisted of 3 stages: (1) a screening period (2–10 days), (2) a run-in period (0–2 weeks), and (3) a double-blind treatment period (8 weeks). There were 6 study visits: the first at the beginning of the run-in period; the second at the beginning of the double-blind treatment period; and the third, fourth, fifth, and sixth at the end of the 2nd, 4th, 6th, and 8th weeks of treatment, respectively. Hypertension patients enrolled in the study who showed good tolerance for and compliance with angiotensin converting enzyme inhibitor (ACEI) drugs, and who had already been genotyped for the MTHFR C677T polymorphism during the run-in period, could directly enter the double-blind randomized treatment period. Drugs that could affect the efficacy evaluation were not permitted to be taken at any stage of the trial.
Participants
Eligible participants were identified from patients presenting at hospitals located in the cities of Wuyuan, Anqing, and Lianyungang between March 2018 and June 2019. The inclusion criteria for the run-in period included patients aged 45 years or older who had been diagnosed with primary hypertension or were currently taking blood pressure medications; or for those who had not taken blood pressure medications within the past 2 weeks, had been newly diagnosed with hypertension, defined as diastolic blood pressure (DBP) ≥90 mmHg or systolic blood pressure (SBP) ≥140 mmHg; and Hcy ≥ 10 μmol/L. For these participants, two BP readings were taken, at least 1 day apart with the patient seated. Three measurements were taken at each visit, and the mean value of the three was used to determine hypertensive status at both visits. The 2nd BP was measured at visit 1. To qualify for the treatment period, patients had to have complete information on the detection of the MTHFR C677T gene polymorphism, and exhibit good tolerance to enalapril and good medication adherence (>80%).
Patients were excluded if they had secondary hypertension, cardiovascular diseases, digestive diseases (viral hepatitis, abnormal liver function, gastrointestinal dysfunction, etc.), urinary diseases, diabetes, corpulmonale, chronic obstructive pulmonary disease (COPD), stroke, malignancy, malnutrition, hematopoietic disorders and other serious diseases. Patients who were taking folate, B12 or B6, as well as those with frequent use of FA supplements or compounds containing FA within the previous 3 months were also excluded. Further exclusion criteria included anyone pregnant and/or lactating, and/or with an allergy or intolerance to enalapril and/or FA. Patients whose mental or nervous system dysfunction, inability to express desire, were excluded.
Ethics approval
The clinical trial was carried out conforming to the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of The Second Affiliated Hospital of Nanchang University Local Ethical Review process (February 4th, 2018) in China. Trial registration was completed before the beginning of recruitment (NCT03472508). All participants provided written, informed consent prior to any data collection. Supporting data will be provided by the corresponding author upon request, after consent from the Ethics Committee of The Second Affiliated Hospital of Nanchang University has been obtained.
Allocation
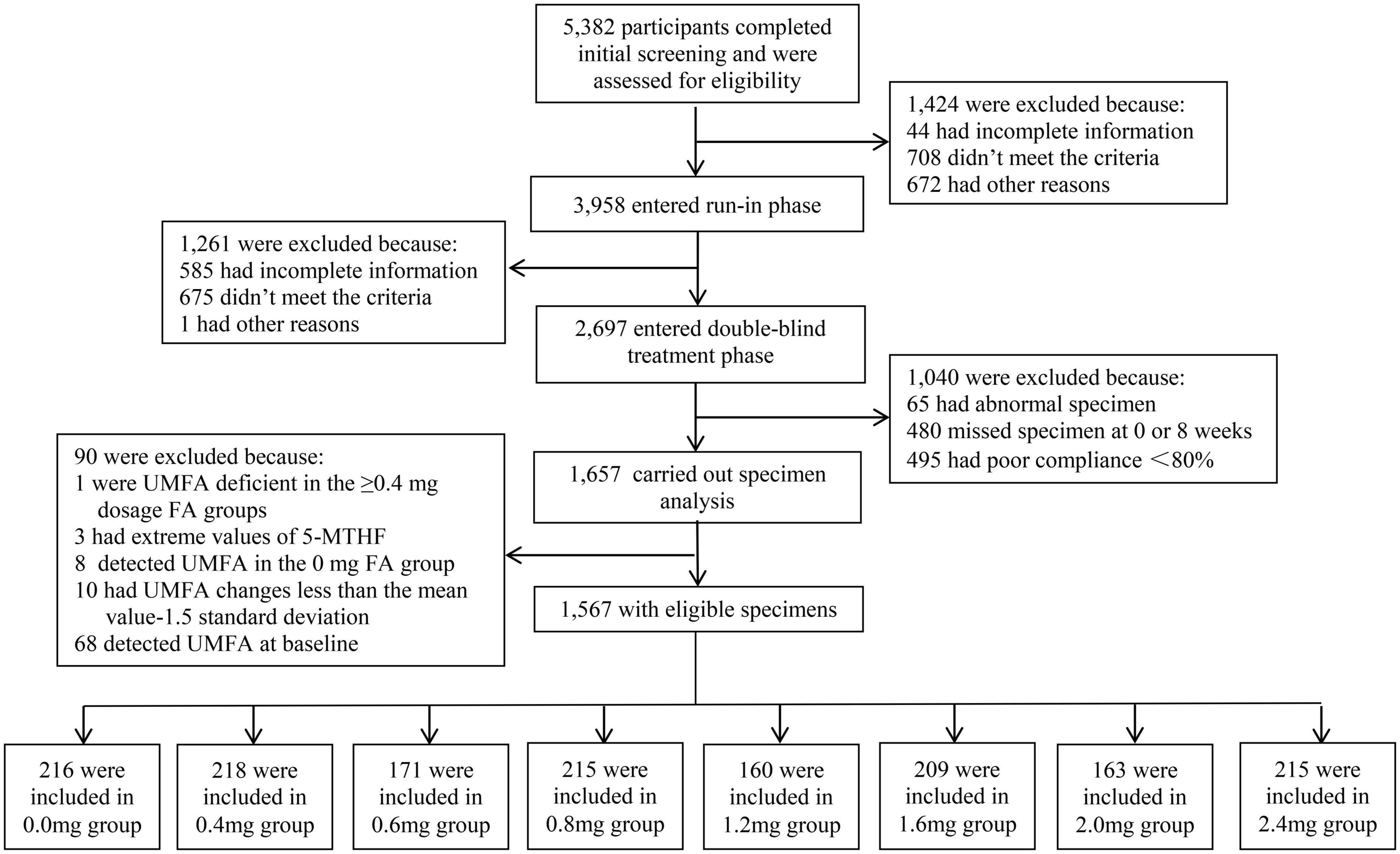
This study utilized data from a randomized, double blind, clinical trial in order to objectively evaluate the efficacy of different FA dosages on circulating UMFA levels, in addition to circulating folate and Hcy. A total of 5,382 patients were screened for the trial, and ultimately 2,697 patients entered into randomization and the double-blind treatment phase. All patients were first stratified by sex and the MTHFR C677T genotype (CC, CT, TT) for a total of six strata. Each of the six strata was then randomized into eight treatment groups according to a random list generated by SAS software, using quadratic block randomization as the randomization grouping method, consisting of either enalapril only (10 mg), or one of the other 7 treatment combinations with various dosages of FA (see Figure 1). All drugs were supplied by AUSA Pharmaceutical Limited; all test drugs were packed into safety capsules that appeared identical. Neither the clinicians/investigators nor the patients knew the contents of the capsules.
As shown in the study flow chart (Figure 1), there were 5,382 patients who completed the initial screening, and those who were ineligible for enrollment (n = 1,424) were disqualified. Although 2,697 patients entered into the randomization, only 1,657 patients had biospecimens for the lab analyses. The final sample for this study included 1,567 patients, of whom 218 patients received supplementation with 0.4 mg FA daily (Group 2), 171 with 0.6 mg FA daily (Group 3), 215 with 0.8 mg FA daily Group 4), 160 with 1.2 mg FA daily (Group 5), 209 with 1.6 mg FA daily (Group 6), 163 with 2 mg FA daily (Group 7), 215 with 2.4 mg FA daily (Group 8), and finally, 216 patients who received no FA supplementation who served as a control group (Group 1).
Assessments
Sex, age, and BMI measures
Information on participant sex, age and body mass index (BMI) were collected and recorded at the baseline visit. Males and females were randomized to each group, in which all participants were aged over 45 years. BMI was calculated as the formula BMI = weight in kg/height in meters2.
Physical examination and lifestyle survey
Each participant completed a physical examination and questionnaire survey, covering lifestyle and disease history and medication use. All participants kept a diary throughout the study in which they reported their daily intake of capsules, any illnesses they experienced, and their use of medication. Habits of smoking and drinking were also recorded.
Biochemical measurements
To determine MTHFR C677T (rs1801133) polymorphisms, an ABI Prism 7900HT sequence detection system (Life Technologies) was used with the TaqMan assay. Detection of serum B12 levels collected at both the baseline visit during the run-in period and the double-blind treatment period was completed by chemiluminescent immunoassay in a commercial laboratory (New Industrial). Serum Hcy, creatinine, fasting lipids, estimated glomerular filtration rate (eGFR), and glucose levels at baseline were measured by automatic clinical analyzers (BeckmanCoulter). Quantification of UMFA and 5-MTHF in plasma was determined by stable-isotope dilution ultra-high performance liquid chromatography-tandem mass spectrometry. All the above measurements were conducted at the central laboratory of the Shenzhen Tailored Medical Laboratory, which obtained certificates of ISO9001:2015, ISO14001:2015, and ISO45001:2018.
Statistical analysis
Continuous variables were presented as means ± SD or medians (IQR); and IQRs were expressed as 25th and 75th percentiles. Categorical variables were presented as numbers (%). The absolute changes in UMFA levels from values at baseline to values at the 8th week of the intervention were calculated. The differences in baseline characteristics between those receiving different dosages of FA in the intervention group were compared by ANOVA tests or Chi square tests. After adjusting for covariables, changes in UMFA for each of the different dosages were determined by graphical smoothing curves using a generalized linear model. The smoothing and regression models were adjusted for center, sex, age, BMI, smoking and drinking status, C677T, baseline eGFR, baseline fasting glucose (GLU), baseline high density lipoprotein cholesterol (HDL-C), baseline total cholesterol (TC), baseline triglycerides (TG), SBP, and DBP. The additive effects of the linear and quadratic terms of the model were tested using chi-square tests (degrees of freedom: 2). The interaction between subgroups of each parameter and FA supplement dosage was assessed by the Wald test, which was used for measuring interactions on a multiplicative scale. Threshold analysis in the correlation of serum folate with UMFA levels was done with a 2-piecewise Cox regression model by a smoothing function, with cutoffs at ≤0.8 and >0.8 mg/day. A likelihood-ratio test and bootstrap resampling methods were employed to confirm the threshold level (i.e., inflection point). All analysis of data were conducted using the statistical package R1 and Stata software, version 14.0 (Stata Corp). A two-tailed P < 0.05 was supposed to have statistical significance.
Results
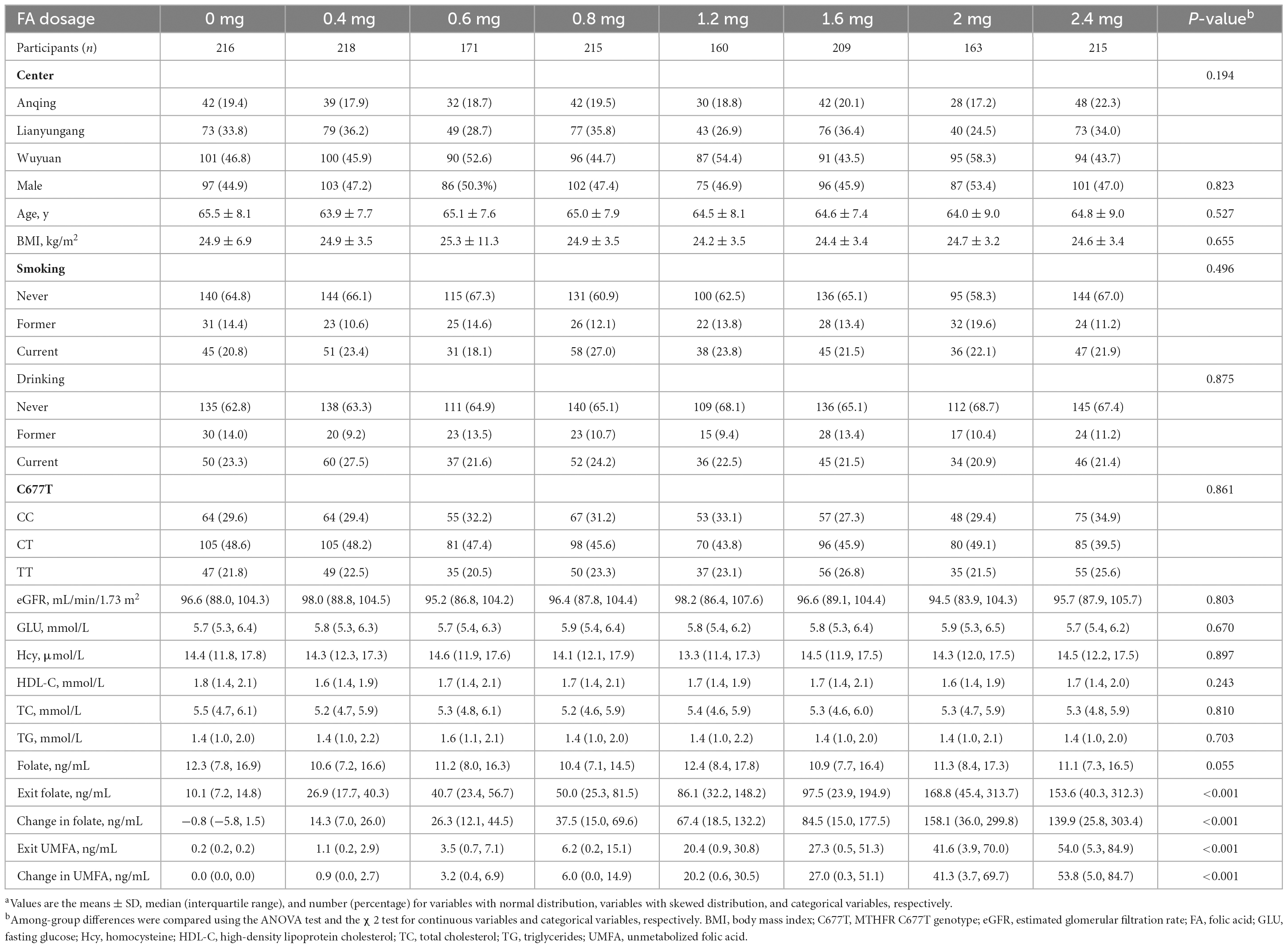
Many of the baseline characteristics between the eight dosage treatment groups (Table 1) were quite similar, including the MTHFR C677T genotype, baseline folate levels, and Hcy levels. Compared with the control group, the exit folate and exit UMFA levels in the FA groups were significantly higher, and these increased with increasing FA dosage. In comparing eligible patients with ineligible patients, high-density lipoprotein cholesterol (HDL-C) and exit Hcy (p < 0.001) differed significantly (Supplementary Table 1).
Figure 2 shows the smoothing curves illustrating the association between different dosages of FA and changes in UMFA (a); folate (b); 5-MTHF (c); and Hcy (d), indicating 0.8 mg/day as the critical FA dosage for stimulating UMFA change. As shown in Figure 2A, as FA dosage increased from 0 to 2.4 mg/day, change in UMFA increased continuously: for FA dosages higher than 0.8 mg/day, UMFA jumped dramatically, while for FA dosages lower than 0.8 mg/day, change in UMFA tended to increase at a much slower rate.
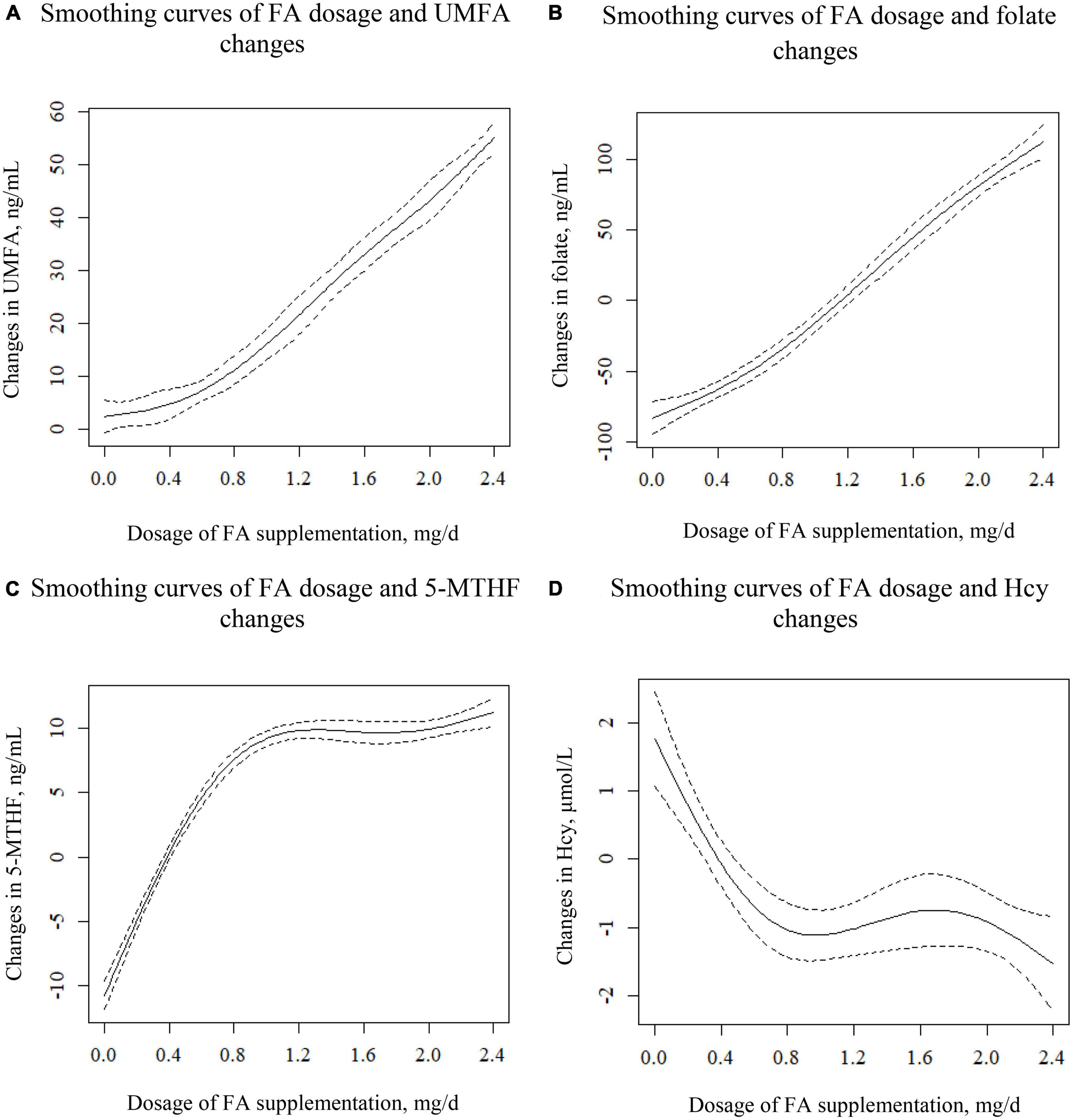
Figure 2. Smoothing curves illustrating the association between different dosages of FA and the changes in UMFA (A), folate (B), 5-MTHF (C), and Hcy (D). Adjusted for center, sex, age, BMI, smoking and drinking status, C677T, baseline eGFR, baseline GLU, baseline HDL-C, baseline TC, baseline TG, SBP, and DBP.
As shown in Figure 2B, changes in folate displayed a positively curved relationship with different dosages of FA. For FA dosages higher than 0.8 mg/day, the slope of the curve was slightly greater than for FA dosages lower than 0.8 mg/day.
As shown in Figure 2C, as the FA dosage increased from 0 to 0.8 mg, changes in 5-MTHF levels rose sharply to 10 ng/mL, after which it remained nearly unchanged. For FA dosages higher than 0.8 mg, the 5-MTHF level appeared to level off at 10 ng/mL.
As shown in Figure 2D, after a sharp initial plunge, the curve flattened out at FA dosage 0.8 mg, and then fell again at FA dosage 1.6 mg. Specifically, as the FA dosage increased from 0 to 0.8 mg, change in Hcy decreased from 2 to −1 μmol/L; and with an FA dosage between 0.8 and 1.6 mg, change in Hcy remained constant at −1 μmol/L, while an FA dosage above 1.6 mg led to a Hcy reduction of −1 to −2 μmol/L.
Combined with Figure 2A, the threshold effect analysis on the effect of FA dosage on UMFA is shown in Table 2. The results of the analysis indicated a positive correlation between the two. All patients were divided into two groups according to FA supplementation with 0.8 mg/day as a new grouping point: low-dosage (≤0.8 mg) and high-dosage FA group (>0.8 mg). For each 0.2, or 0.4 mg/day FA dosage increase, the slope for UMFA was greater for the high-dosage FA group (ß = 11.21, 95% CI (8.97, 13.45) compared to the low-dosage FA group (ß = 2.94, 95% CI: 2.59, 3.29) (P for interaction, <0.001). The coefficients for the linear and quadratic terms for FA dosages were both statistically significant (P < 0.001) in the total sample regression analysis (Supplementary Table 2).
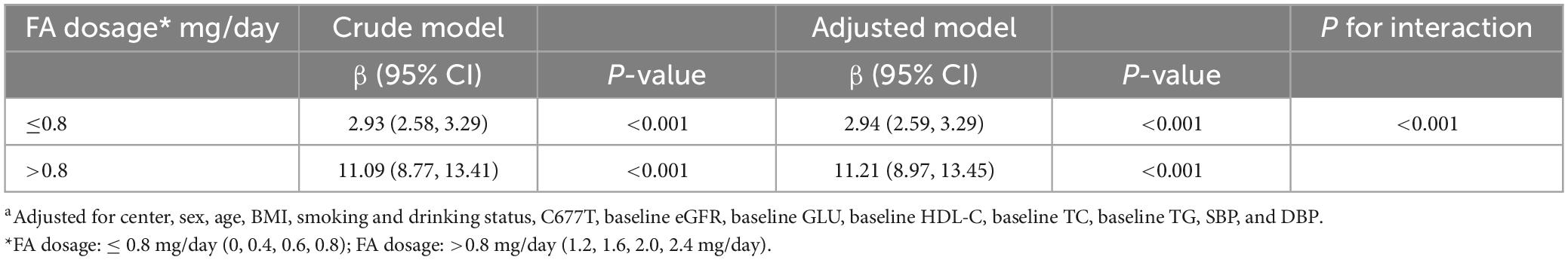
Table 2. Threshold effect analysis: the dose-response relationship of folic acid supplementation with circulating unmetabolized folic acid (UMFA), stratified by FA dosage subgroups (≤ 0.8 vs. >0.8 mg/day)a.
Table 3 shows the results of a stratified analysis of the effect of FA dosage on UMFA. For those in the low-dosage FA group (≤0.8 mg/day), those with a lower BMI showed a stronger association between FA dosage and UMFA (< 24.5 vs. ≥ 24.5 kg/m2; P for interaction, <0.001). In addition, hospital center (p for interaction, <0.001) and smoking status (p for interaction, 0.019) positively modified the association between FA dosage and UMFA levels in the low-dosage group, with a stronger correlation found among current smokers and patients from Anqing or Wuyuan. In the high-dosage FA group (1.2–2.4 mg/day) none of the other variables, including age, sex, hospital center, BMI, SBP, DBP, TC, triglycerides, HDL-C, GLU, eGFR, MTHFR C677T, rs70991108 (a polymorphism consisting of a 19-bp deletion in the first intron of the DHFR gene), smoking status, or drinking status, significantly modified the relationship between FA dosage and UMFA levels.
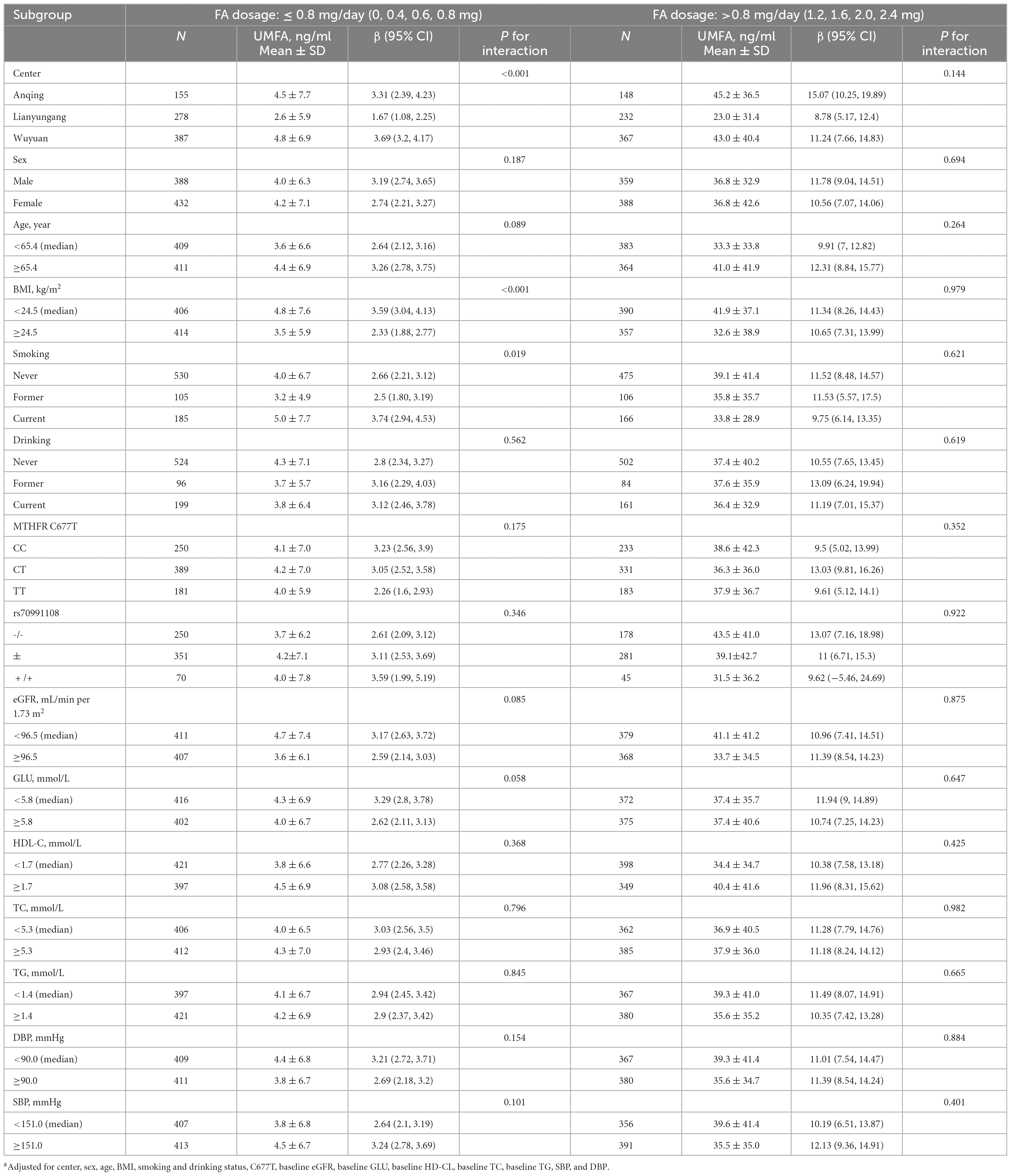
Table 3. Stratified analyses by participant characteristics on the effect of folic acid dosage on unmetabolized folic acid (UMFA)a.
Discussion
To our knowledge, this is the largest study utilizing data from a RCT to assess the dosage-response relationship between FA supplementation (eight different FA dosages) and circulating levels of UMFA, and is the first such study in a Chinese population. The results of this research provide critical insights into the optimal dosage of FA supplementation while balancing efficacy and adverse effects. These findings offer practical implications for public health professionals and clinicians.
Our research results show that high intake of FA can lead to the occurrence of UMFA in plasma, which is consistent with that of several previous studies. A study in an elderly Irish cohort of 137 participants with a mean age of 67.4 years demonstrated that an oral FA dosage above 200 μg (threshold dosage) resulted in UMFA in plasma (20). Stamm et al. reported on a study of 117 women who received a daily multivitamin containing 1000 μg FA throughout pregnancy and lactation until 8 weeks postpartum, at which point, UMFA was detected in nearly all non-fasted blood samples (21). Sweeney et al. tested the postprandial serum FA response to multiple dosages of FA in fortified bread and found the appearance of UMFA in the serum of all participants at all test dosages, showing apparent accumulation effects (22). However, these results only proved that higher intake of FA leads to higher occurrence of UMFA in the serum, without identifying which dosage of FA is the optimum, and offered no practical guiding significance for clinical application.
An understanding of the absorption and metabolism of FA in humans can reveal the reasons for the occurrence of UMFA in the serum. Previous studies have shown that FA is mainly absorbed in the proximal jejunum in a prototype form after oral administration, and oral doses of FA in excess of about 260–280 μg (589–634 nmol) leads to the direct appearance of UMFA in the systemic circulation (6). Absorbed folate, which may undergo biotransformation in the absorptive mucosa, is subsequently transferred via the mesenteric veins to the hepatic portal vein and carried to the liver where an extensive amount (liver “first-pass”) is removed (23). While the liver has a high affinity for the removal of FA, it has a low affinity for the removal of 5-MTHF (24). This may have important implications for FA use as a supplement or fortification since the human liver has a low capacity for reduction and may eventually give rise to saturation, resulting in significant (and potentially deleterious) UMFA entering systemic circulation (25). Our study investigated the saturation point of oral FA transformation through two indicators, namely, folate and 5-MTHF, as well as the saturation point of FA on lowering Hcy levels. Our study also identified that when the FA dosage is equal to 0.8 mg/day, the biotransformation capacity of FA into 5-MTHF in humans is saturated, and the effect of FA on reducing Hcy is also basically saturated.
This study also revealed individual characteristics that may modify the FA supplementation and UMFA association. There was a significant interaction between FA supplementation dosage and age on UMFA levels (Supplementary Table 3). Compared with participants aged ≥65.4 years, significantly lower UMFA levels (mean: 17.9 vs. 21.6, p < 0.001) were found among those aged <65.4 years. It is likely that aging may slow down the absorption and metabolism of FA: as we age the older the age, the weaker the FA absorption declines, and metabolism weakens and the higher the UMFA levels increase. M Wolters et al. considered that atrophic gastritis could result in declining gastric acid and pepsinogen secretion, and hence decreasing the intestinal digestion and absorption of both B vitamins (i.e., vitamin B12 and FA), wherein atrophic gastritis occurred with a frequency of approximately 20–50% in the elderly subjects (26). There was also a significant interaction effect between FA supplementation and BMI on UMFA levels. Compared with participants with BMI ≥ 24.4, significantly higher UMFA levels were found among those with BMI < 24.4 (Mean: 22.8 vs. 16.7, p < 0.001). This indicates that people of different BMI have different FA requirements, which is consistent with the results reported in the previous literature (27). One explanation for this observation is that, as body size increases, the distribution of folate changes, resulting in changes of freely available plasma/serum folate and folate in the cells (28, 29).
Overall, our study provides a significant contribution to the literature on FA supplementation and UMFA levels in hypertensive adults. The research findings have several practical implications for clinicians, public health professionals, and policymakers for developing more effective interventions and strategies to reduce the risk of UMFA accumulation and its potential adverse health outcomes.
The research highlights the importance of regularly monitoring FA supplementation in patients with hypertension. However, attention should be paid to the limitations of the present study. We did not compare the correlation between FA intake and circulating UMFA among participants from different racial groups or ethnicities or for individuals under 45 years of age. It is important for future research to explore the optimal dosage of FA supplementation in other populations with different characteristics to investigate the effects of long-term, high-dosage FA intake on UMFA levels and related health outcomes.
Conclusion
This study, utilizing data from a large, randomized nutrition trial, showed a positive, non-linear, dosage-response relationship between FA supplementation ranging from 0 mg to 2.4 mg and circulating UMFA levels in Chinese adults with H-type hypertension. Our findings indicate that, on average, supplementation with 0.8 mg/day FA appears to be an optimal dosage in balancing efficacy vs. UMFA levels.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of The Second Affiliated Hospital of Nanchang University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PC, JJ, SL, XQ, and XX designed the research. PC, YS, NZ, YW, ZZ, QH, BW, LL, XH, XC, GT, YD, and HZ conducted the research. PC, XQ, SS, and ZZ collected and analyzed the data. PC, LT, ZZ, and QH drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study funding came from the Jiangxi Science and Technology Innovation Platform Project (20165BCD41005); Key R&D Projects, Jiangxi (20203BBGL73173); the Project of Jiangxi Provincial Health Commission (202130440); the Department of Science and Technology of Guangdong Province (2020B121202010); the Science, Technology and Innovation Committee of Shenzhen (JSGG20180703155802047 and JSGG20201103153807021); and the National Natural Science Foundation of China (81960074). None of the funding agencies participated in the study design; collection, management, analysis, and interpretation of the data; nor in the preparation, review, or approval of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1191610/full#supplementary-material
Footnotes
References
1. Luo J. H-type hypertension-hypertension with elevated homocysteine. Adv Cardiovasc Dis. (2012) 33:250–2. doi: 10.3969/j.issn.1004-3934.2012.02.032
2. Li J, Jiang S, Zhang Y, Tang G, Wang Y, Mao G, et al. H-type hypertension and risk of stroke in Chinese adults: a prospective, nested case–control study. J Transl Int Med. (2015) 3:171–8. doi: 10.1515/jtim-2015-0027
3. Zhang S, Wang T, Wang H, Tang J, Hou A, Yan X, et al. Effects of individualized administration of folic acid on prothrombotic state and vascular endothelial function with H-type hypertension. Medicine. (2022) 101:e28628. doi: 10.1097/MD.0000000000028628
4. Chen P, Tang Y, He Q, Liu L, Zhou Z, Song Y, et al. A sensitive UPLC-MS/MS method for simultaneous quantification of one-carbon metabolites & co-factors in human plasma. J Pharm Biomed Anal. (2022) 219:114944. doi: 10.1016/j.jpba.2022.114944
5. Bailey S, Ayling J. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci U S A. (2009) 106:15424–9. doi: 10.1073/pnas.0902072106
6. Kelly P, McPartlin J, Goggins M, Weir D, Scott J. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. (1997) 65:1790–5. doi: 10.1093/ajcn/65.6.1790
7. Patanwala I, King M, Barrett D, Rose J, Jackson R, Hudson M, et al. Folic acid handling by the human gut: implications for food fortification and supplementation. Am J Clin Nutr. (2014) 100:593–9. doi: 10.3945/ajcn.113.080507
8. Quinlivan E, Gregory JFG III Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. (2003) 77:221–5. doi: 10.1093/ajcn/77.1.221
9. Pfeiffer C, Sternberg M, Fazili Z, Yetley E, Lacher D, Bailey R, et al. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. (2015) 145:520–31. doi: 10.3945/jn.114.201210
10. Crider K, Bailey L, Berry R. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. (2011) 3:370–84. doi: 10.3390/nu3030370
11. Selhub J, Rosenberg I. Excessive folic acid intake and relation to adverse health outcome. Biochimie. (2016) 126:71–8. doi: 10.1016/j.biochi.2016.04.010
12. Paniz C, Bertinato J, Lucena M, Carli ED, Amorim P, Gomes G, et al. A daily dose of 5 mg folic acid for 90 days is associated with increased serum unmetabolized folic acid and reduced natural killer cell cytotoxicity in healthy brazilian adults. J Nutr. (2017) 147:1677–85. doi: 10.3945/jn.117.247445
13. Troen A, Mitchell B, Sorensen B, Wener M, Johnston A, Wood B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. (2006) 136:189–94. doi: 10.1093/jn/136.1.189
14. Morris M, Jacques P, Rosenberg I, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. (2010) 91:1733–44. doi: 10.3945/ajcn.2009.28671
15. Li Z, Gueant-Rodriguez R, Quilliot D, Sirveaux M, Meyre D, Gueant J, et al. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin Nutr. (2018) 37:1700–6. doi: 10.1016/j.clnu.2017.07.008
16. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman R, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in china: the CSPPT randomized clinical trial. JAMA. (2015) 313:1325–35. doi: 10.1001/jama.2015.2274
17. Zeng R, Xu C, Xu Y, Wang Y, Wang M. The effect of folate fortification on folic acid-based homocysteine-lowering intervention and stroke risk: a meta-analysis. Public Health Nutr. (2015) 18:1514–21. doi: 10.1017/S1368980014002134
18. Xu B, Shi H. Precision nutrition: concept, evolution, and future vision. Precis Nutr. (2022) 1:e00002. doi: 10.1097/PN9.0000000000000002
19. Bao H, Huang X, Liu L, Li J, Ding C, Xiong Y, et al. A randomized, double-blind, controlled trial on the homocysteine-lowering effects of different doses of folic acid among patients with hypertension and elevated homocysteine according to methylenetetrahydrofolate reductase C677T genotypes: rationale and methods. Precis Nutr. (2022) 1:e000007. doi: 10.1097/PN9.0000000000000004
20. Boilson A, Staines A, Kelleher C, Daly L, Shirley I, Shrivastava A, et al. Unmetabolized folic acid prevalence is widespread in the older Irish population despite the lack of a mandatory fortification program. Am J Clin Nutr. (2012) 96:613–21. doi: 10.3945/ajcn.111.026633
21. Stamm R, March K, Karakochuk C, Gray A, Brown R, Green T, et al. Lactating canadian women consuming 1000 μg folic acid daily have high circulating serum folic acid above a threshold concentration of serum total folate. J Nutr. (2018) 148:1103–8. doi: 10.1093/jn/nxy070
22. Sweeney M, McPartlin J, Weir D, Daly L, Scott J. Postprandial serum folic acid response to multiple doses of folic acid in fortified bread. Br J Nutr. (2006) 95:145–51. doi: 10.1079/bjn20051618
23. Rogers L, Pfeiffer C, Bailey L, Gregory JF. A dual-label stable-isotopic protocol is suitable for determination of folate bioavailability in humans: evaluation of urinary excretion and plasma folate kinetics of intravenous and oral doses of [13C5] and [2H2]folic acid. J Nutr. (1997) 127:2321–7. doi: 10.1093/jn/127.12.2321
24. Steinberg S, Campbell C, Hillman R. Kinetics of the normal folate enterohepatic cycle. J Clin Invest. (1979) 64:83–8. doi: 10.1172/JCI109467
25. Wright A, Dainty J, Finglas P. Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br J Nutr. (2007) 98:667–75. doi: 10.1017/S0007114507777140
26. Wolters M, Ströhle A, Hahn A. Age-associated changes in the metabolism of vitamin B(12) and folic acid: prevalence, aetiopathogenesis and pathophysiological consequences. Z Gerontol Geriatr. (2004) 37:109–35. doi: 10.1007/s00391-004-0169-6
27. Stern S, Matok I, Kapur B, Koren G. Dosage requirements for periconceptional folic acid supplementation: accounting for BMI and lean body weight. J Obstet Gynaecol Can. (2012) 34:374–8. doi: 10.1016/s1701-2163(16)35220-3
28. da Silva V, Hausman D, Kauwell G, Sokolow A, Tackett R, Rathbun S, et al. Obesity affects short-term folate pharmacokinetics in women of childbearing age. Int J Obes. (2013) 37:1608–10. doi: 10.1038/ijo.2013.41
Keywords: folic acid, dosage, unmetabolized folic acid, H-type hypertension, safety
Citation: Chen P, Tang L, Song Y, Wang B, Qin X, Zhang N, Wei Y, Xu X, Zhou Z, He Q, Liu L, Siddiqi SM, Huang X, Cheng X, Tang G, Duan Y, Zhou H, Jiang J and Li S (2023) Association of folic acid dosage with circulating unmetabolized folic acid in Chinese adults with H-type hypertension: a multicenter, double-blind, randomized controlled trial. Front. Nutr. 10:1191610. doi: 10.3389/fnut.2023.1191610
Received: 22 March 2023; Accepted: 29 August 2023;
Published: 14 September 2023.
Edited by:
Iain Brownlee, Northumbria University, United KingdomReviewed by:
Keyhan Lotfi, Tehran University of Medical Sciences, IranGuangyun Mao, Wenzhou Medical University, China
Copyright © 2023 Chen, Tang, Song, Wang, Qin, Zhang, Wei, Xu, Zhou, He, Liu, Siddiqi, Huang, Cheng, Tang, Duan, Zhou, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Jiang, amlhbmdqaWVAam51LmVkdS5jbg==; Sha Li, dGxpc2hhQGpudS5lZHUuY24=
 Ping Chen
Ping Chen Linlin Tang2
Linlin Tang2 Xianhui Qin
Xianhui Qin Nan Zhang
Nan Zhang Yaping Wei
Yaping Wei Xiping Xu
Xiping Xu Qiangqiang He
Qiangqiang He Sultan Mehmood Siddiqi
Sultan Mehmood Siddiqi Xiao Huang
Xiao Huang Sha Li
Sha Li