- 1General Practice Ward/International Medical Center Ward, General Practice Medical Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2National Clinical Research Center for Geriatrics, Multimorbidity Laboratory, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Sarcopenia is a syndrome characterized by a decline in muscular mass, strength, and function with advancing age. The risk of falls, fragility, hospitalization, and death is considerably increased in the senior population due to sarcopenia. Although there is no conclusive evidence for drug treatment, resistance training has been unanimously recognized as a first-line treatment for managing sarcopenia, and numerous studies have also pointed to the combination of nutritional supplementation and resistance training as a more effective intervention to improve quality of life for people with sarcopenia. People with both malnutrition and sarcopenia have a higher mortality rate, so identifying people at risk of malnutrition and intervening early is extremely important to avoid sarcopenia and its associated problems. This article provides important information for dietary interventions in sarcopenia by summarizing the discoveries and developments of nutritional supplements such as protein, leucine, β-hydroxy-β-methylbutyric acid, vitamin D, vitamin C, vitamin E, omega-3 fatty acids, creatine, inorganic nitrate, probiotics, minerals, collagen peptides, and polyphenols in the management of sarcopenia.
1. Introduction
After the age of 50, muscle mass declines at a rate of 1%−2% per year in healthy adults, while muscle strength declines at a rate of 1.5% per year (1, 2). Sarcopenia was defined by the European Working Group on Sarcopenia in Older People (EWGSOP) in 2010 as “a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life, and death” (3). In 2018, the EWGSOP updated the definition, and muscle strength and function are now put in front because the two are more important than muscle mass (4). Sarcopenia is a major public health concern because it affects 20% of people over the age of 70 and 50% of people over the age of 80 (5). Currently, 6%−19% of the global population over the age of 60 suffers from sarcopenia (6).
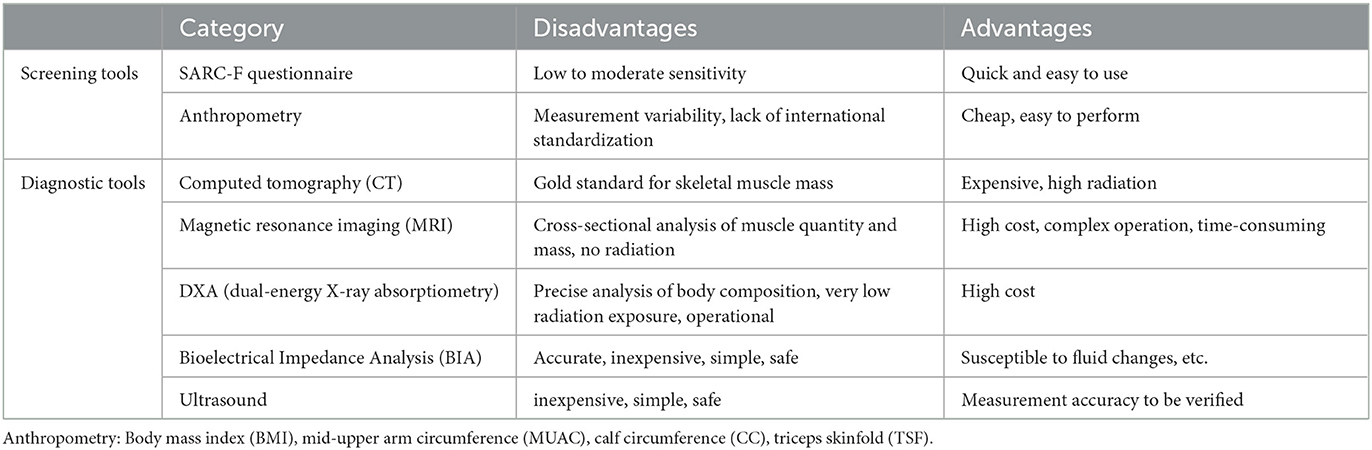
Sarcopenia should be suspected in patients who present with signs or symptoms such as falls, feeling weak, walking slowly, difficulty rising from a chair, weight loss, or muscle wasting, and EWGSOP2 recommends screening and evaluation starting with the Simple Five Item Scoring Questionnaire (SARC-F) (4). When low muscle strength is tested by grip strength or chair stand, it is considered very likely to have sarcopenia, because according to EWGSOP, the diagnosis of sarcopenia is “low muscle mass and low muscle function (strength or performance),” while muscle strength is the center of the diagnostic process (3). The Asian Working Group for Sarcopenia (AWGS) 2019 consensus defined “probable sarcopenia” as low muscle strength or physical fitness, specifically used in primary care or community-based health promotion (7). In addition, low muscle mass can be measured by instruments such as DXA, BIA, CT, and MRI. The severity of sarcopenia can be determined by measuring physical performance, such as gait speed, SPPB, TUG, and 400 m walk (4). Ideally, a primary care physician should begin screening older adults at risk for sarcopenia, then diagnose them through the use of appropriate diagnostic tools, and begin treatment as early as possible. This will prevent delays in the diagnosis and management of sarcopenia. Common tools for screening and diagnosing sarcopenia and their respective advantages and disadvantages are shown in Table 1.
It has been reported that older residents at risk of malnutrition in Asian communities range from 16 to 73% (9), and the combined prevalence of moderate to high malnutrition risk among elderly people in Europe is 48.4% (10). In Latin America, two out of every five hospitalized patients are at risk of malnutrition (11). Malnutrition increases the risk of sarcopenia by two to three times, and people with sarcopenia who are undernourished have a higher mortality rate (12, 13). According to the AWGS consensus, older adults who present with low body mass index (BMI), unintentional weight loss, and low muscle mass or exhibit poor muscle strength at any time should be assessed for malnutrition, and those at risk of malnutrition should be rescreened every 3 months (9). Some evidence has shown that adequate intake of protein, vitamin D, antioxidant nutrients, and long-chain polyunsaturated fatty acids is beneficial for improving sarcopenia (14). Healthcare professionals are advised to provide nutritional counseling on dietary habits for older adults with sarcopenia and to collaborate with a dietitian to develop a diet and protein optimization plan for the patient (15). Therefore, identifying individuals at risk for malnutrition to provide early intervention is an important public health strategy to prevent the development of sarcopenia and related complications.
2. Nutritional intervention for sarcopenia
2.1. Protein, leucine, β-hydroxy-β-methylbutyric acid
2.1.1. Mechanisms
On the metabolic front, temporal fluctuations in muscle protein synthesis (MPS) and muscle protein breakdown (MPB) rates determine the net increase or decrease in skeletal muscle protein, which continuously remodels skeletal muscle mass. Protein degradation exceeding protein synthesis results in a negative nitrogen balance and triggers sarcopenia. Dietary protein or amino acid intake is the primary physiological stimulus for MPS (16), stimulating muscle protein synthesis, increasing muscle mass, and reducing muscle loss during bed rest and aging (17).
First, the ubiquitin–proteasome system (UPS) is the major pathway for cellular protein degradation, and studies have demonstrated that activating the UPS leads to increased protein degradation and finally results in sarcopenia (18, 19), while protein or amino acid nutritional support can effectively downregulate the levels of MuRF-1 and Atrogin-1 and ameliorate UPP-mediated sarcopenia (20, 21). Second, the oxidative response is one of the inducers of sarcopenia (22). Protein or amino acid nutritional support contributes to promoting Sirt1 expression, activating FoxO family proteins, enhancing the expression of SOD, and reducing the oxidative response (23). Third, enhanced autophagy evokes sarcopenia (24). Protein or amino acid nutritional support can enhance the activity of the PI3K/Akt/mTOR signaling pathway to suppress cell autophagy (25). There are some potential new mechanisms, including altering miRNA profiles and gut microbiota (26).
2.1.2. Clinical studies
2.1.2.1. Protein
To measure the effect of protein supplementation on muscle health, Hanach et al. analyzed 14 RCTs (27), with milk protein or protein-based dairy products for not <12 weeks as the intervention. The results showed that milk protein significantly increased limb muscle mass, although there was no effect on grip strength or leg muscle strength, and there was no conclusive evidence of an effect on physical activity. Kirwan et al. (28) analyzed 28 RCTs and showed that among older adults who performed resistance training, those who consumed higher protein increased lean limb mass and grip strength compared to controls who were supplemented with lower protein; however, without resistance training, there was no additional benefit from protein supplementation alone. In healthy older adults from Asia and other countries, the combination of protein supplementation and exercise significantly increased lower extremity strength compared to exercise alone or placebo, although no significant differences were found in upper extremity strength, muscle mass, or gait speed (29). Therefore, it is recommended to supplement protein in combination with resistance training to increase muscle mass and strength (30). Regarding the relationship between dietary protein intake and skeletal muscle mass, a cross-sectional analysis of 3,213 middle-aged and elderly residents in the mainland Chinese community found that participants who consumed more than 0.96 g/kg of protein per day had higher muscle mass than those who consumed no more than 0.96 g/kg of protein per day (31). In elderly subjects aged 70–85 years, those who consumed 1.5 g/(kg/day) of protein continuously for 12 weeks had higher skeletal muscle mass and mass index and higher gait speed, while the other two groups (0.8 and 1.2 g/kg/day of protein, respectively) did not differ significantly in terms of muscle mass and physical performance (32). A dose-dependent increase in whole-body net protein balance during recovery from resistance exercise in older healthy men randomly assigned to consume 0 g, 15 g, 30 g, or 45 g of milk protein concentrate suggests that the dose of protein consumed after exercise is a key factor in the magnitude of the muscle protein synthesis response (33). The World Health Organization and the U.S. The National Academy of Sciences currently recommends a protein daily allowance (RDA) of 0.8 g/kg/day for adults, but this value applies to all ages, regardless of gender, physical activity, or health status. Evidence from RCTs in elderly populations, as well as the protein requirements of elderly individuals measured using the indicator amino acid oxidation (IAAO) technique, suggests that this dose does not meet the physiological protein requirements of elderly individuals. The estimated average protein requirement (EAR) and RDA measured using IAAO technology were 0.94 and 1.24 g/kg/day in older men and 0.96 and 1.29 g/kg/day in older women, respectively (34, 35). According to the European Society of Clinical Nutrition and Metabolism (ESPEN), the diet of the elderly should provide at least 1.0–1.5 g protein/kg body weight/day, with 25–30 g protein allocated to each meal (36). However, patients with severe chronic kidney disease should limit their protein intake. In summary, most studies confirm that protein intake is positively correlated with muscle mass and strength and that higher protein intake has a positive effect on skeletal muscle health during aging. However, protein supplementation is not recommended as an independent intervention to improve muscle mass and strength. Protein supplementation only during resistance training can significantly improve grip strength and physical function, and the combination of the two can improve sarcopenia significantly more than resistance training alone (37–39). There is bias and heterogeneity in the evidence for protein supplementation on measures of muscle mass and the effects of strength and physical performance, and differences in the type and dose of protein supplementation, as well as variations in exercise regimen and duration, need to be taken into account when interpreting the results. More carefully designed large-scale randomized controlled trials exploring the effects of protein supplementation on these measures are needed in the future.
The quality and digestibility of proteins are distinguishing features between animal and plant proteins, with differences in amino acid content and absorption kinetics. Animal proteins such as meat, fish, and dairy products are consistently high-quality proteins, while plant proteins vary in quality depending on the sources, with soy protein being recognized as a high-quality plant protein. Therefore, with respect to the quality of protein, animal-derived protein may be more effective in maintaining muscle health. Regarding potential differences between animal and plant proteins affecting muscle health, a meta-analysis of 16 RCTs (51) showed that protein sources did not affect changes in absolute lean body mass or muscle strength; however, animal protein was more beneficial for percent lean body mass. It was shown in a retrospective study that men and women with higher animal protein intake had higher percentages of skeletal muscle mass regardless of physical activity, while the beneficial effects of plant protein were only shown in physically active adults (52). In contradiction to these findings, a negative correlation between walking speed and relative animal protein intake and a positive correlation with relative plant protein intake have also been reported (53). Gazzani et al. (54) also supported the positive effect of plant proteins on physical performance and suggested that this could be related to other components of plant foods that affect muscle mass and strength, such as antioxidants. It is unclear how protein intake from different sources provides the best benefit for preventing sarcopenia, and further research is needed to refine protein dietary guidelines that promote muscle health.
2.1.2.2. Leucine
Amino acids are important raw materials for protein synthesis, and their homeostasis is essential for maintaining muscle health. Muscle protein synthesis is regulated at multiple physiological levels, including dietary protein digestion and amino acid absorption, visceral amino acid retention, postprandial insulin release, skeletal muscle tissue perfusion, muscle uptake of amino acids, and intracellular signaling in myocytes (55). Therefore, some scholars have proposed that the anabolic potential of proteins correlates with amino acid composition, which is supported by the finding that plasma concentrations of leucine, isoleucine, and tryptophan are reduced in patients with sarcopenia (56). Synthesis by activating rapamycin complex 1 (mTORC1), a target that acts as a “switch” for the MPS process, which initiates translation in the intracellular signaling cascade (57). Thus, the “leucine trigger” hypothesis has been proposed, which predicts that the magnitude and rate of postprandial blood leucine increase may modulate the magnitude of the postprandial MPS response to protein intake. Sixteen of the 29 eligible studies provided sufficient evidence to support the hypothesis (58). Thus, leucine content may be a key factor in promoting the muscle protein synthesis response. The effect of 25 g of whey protein on maintaining skeletal muscle protein synthesis and improving muscle loss is similar to that of 10 g of milk with leucine in older adults (59). Compared to isonitrogenous protein drinks, protein drinks with higher concentrations of leucine are more beneficial for myogenic fibronectin synthesis (60). Leucine supplementation has been reported to have beneficial effects on body weight, body mass index, and lean body mass in older adults with a tendency toward sarcopenia, although the effects on muscle strength are inconclusive (61). Besides, according to a systematic review, protein supplements rich in leucine can improve markers of sarcopenia, regardless of physical activity, however, leucine supplementation alone and no exercise did not improve sarcopenia (62). Current evidence tends to recommend a higher intake of leucine in older adults to increase muscle mass. Considering the importance of leucine in muscle protein synthesis, leucine requirements in elderly individuals, measured using the indicator amino acid oxidation method, are more than twice the current recommendations, averaging 77.8 mg/(kg/day) for men and 78.2 mg/(kg/day) for women (63).
2.1.2.3. β-hydroxy-β-methylbutyric acid
β-hydroxy-β-methylbutyric acid (HMB) is a metabolite of leucine. The International Society of Sports Nutrition believes that HMB can reduce exercise-induced skeletal muscle damage and is most effective when consumed for two consecutive weeks before exercise, so athletes are recommended to take 38 mg per kg of body weight per day to promote their skeletal muscle growth and improve strength (64). The manufacturer usually recommends taking 3 g of HMB per day (40), the dose being equivalent to the intake of 60 g of leucine (65). However, if 60 g of leucine is consumed directly, the activity of rate-limiting enzymes for catabolism increases, and the oxidation of branched-chain amino acids increases, which can lead to depletion of valine and isoleucine in body fluids and ultimately an imbalance in the concentration of branched-chain amino acids, thus possibly having a negative impact on protein metabolism (66); however, there is wide heterogeneity in the conclusions drawn from published articles regarding the effects of HMB supplementation on muscle health and physical performance. Supporting research findings indicate that HMB intake promotes both upper and lower-extremity muscle strength in older adults (67). Supplementation with 3 g of HMB is most beneficial for improving strength and body composition in people over 65 years of age, especially when bed-rested and untrained (68). In the opposing study, Phillips et al. (69) stated through systematic evaluation and meta-analysis that the current evidence is insufficient to assess the effects of HMB supplementation on muscle function, as the evidence supports little and is inconsistent. In a randomized controlled trial carried out among 40 young adult men (70), the intervention group was supplemented with the leucine metabolites alpha-hydroxyisocaproic acid (α-HICA) and β-hydroxy-β-methylbutyric acid (HMB); as a result, supplementation with leucine metabolites did not enhance resistance training-induced changes in muscle thickness compared to placebo (71). In conclusion, more high-quality primary studies are needed in the future to investigate the effects of HMB in patients with sarcopenia, and the current evidence does not yet provide unambiguous support for recommending HMB supplementation to alleviate sarcopenia.
2.2. Vitamins
2.2.1. Vitamin D
2.2.1.1. Mechanisms
The vitamin D/VDR axis plays a key role in regulating biological processes central to sarcopenic muscle atrophy, such as proteolysis, mitochondrial function, cellular senescence, and adiposity (72). First, vitamin D deficiency appears to lead to increased muscle protein breakdown via the ubiquitin-proteasomal pathway (UPP) and autophagy and upregulation of AMPK and members of the renin-angiotensin system (73, 74). Second, permanent exit from the cell cycle (senescence) is a critical aging phenomenon, and the vitamin D/VDR axis has been shown to have regulatory control (75). Third, low vitamin D states may lead to impaired mitochondrial function (76), and active 1,25(OH)2D3 can increase oxygen consumption rates and fission/fusion dynamics (77, 78). Fourth, low vitamin D states may lead to increased adiposity in muscle (79), and those who are overweight have an increased risk of deficits in muscle mass and function (80).
2.2.1.2. Clinical studies
Vitamin D is a fat-soluble vitamin synthesized in the skin, 90% of which comes from UV exposure and 10% from the diet. Vitamin D deficiency is now considered a global public health problem, and elderly individuals are at greater risk of vitamin D deficiency due to poor intestinal absorption, reduced sun exposure, and chronic renal insufficiency. Lower 25-(OH)-VD levels are thought to be associated with adverse changes in muscle mass and physical function (81). Yang et al. (82) fed mice a vitamin D-deficient diet for 24 weeks and immobilized them to determine the extent of skeletal muscle atrophy. As a result, vitamin D deficiency accelerated the decrease in gastrocnemius muscle mass, muscle fiber cross-sectional area, and grip strength; moreover, vitamin D supplementation inhibited the decrease in grip strength. The team also performed a cross-sectional analysis of 4,139 older adults, and linear regression analysis showed that serum 25 hydroxyvitamin D and physical activity were linearly associated and interacted with timed running time and grip strength. However, in another study in which the control group took a placebo daily and the intervention group took 800 IU of vitamin D orally daily, no differences were found between the two groups in leg push-up strength, function, or lean body mass after 1 year (83). According to the systematic reviews and meta-analyses, vitamin D supplementation alone did not improve muscle strength or SPPB scores, on the contrary, significantly decreased SPPB scores (84). When vitamin D was taken together with whey protein and leucine, the muscle mass of the limbs of patients with sarcopenia could be effectively increased even without physical exercise, and when combined with physical exercise, not only muscle mass increases but muscle strength and performance could also be significantly improved (85). However, we cannot be sure of the effectiveness of vitamin D supplementation alone, due to the presence of protein and amino acids. In summary, the exact role of vitamin D supplementation in the prevention and treatment of sarcopenia remains uncertain due to the high heterogeneity of studies and the conflicting results of RCTs.
2.2.2. Vitamin C and vitamin E
2.2.2.1. Mechanisms
With aging, the body's endogenous antioxidant defense system is impaired, and excessive accumulation of reactive oxygen species (ROS) in the body leads to oxidative muscle damage, which may be directly or indirectly involved in skeletal muscle atrophy (86). In addition, mitochondrial dysfunction occurs abnormally during muscle aging, which has been associated with aberrant ROS generation and oxidative damage (87). Antioxidant vitamins are thought to prevent oxidative stress and may be able to play a role in the treatment of sarcopenia. Therefore, whether antioxidant supplementation can improve age-related muscle mass and performance is becoming an issue of interest to researchers. Vitamins C and E are widely used antioxidant vitamins that have the ability to scavenge ROS and enhance cellular antioxidant capacity.
2.2.2.2. Related studies
Vitamin E is composed of two subgroups called tocopherols and tocotrienols. There are four isomers of tocopherols and tocotrienols (α, β, γ, and δ) depending on the number and location of the methyl groups, and their main dietary sources are vegetable oils, nuts, seeds, fish, shellfish, and vegetables (88). In vitro, studies have shown that alpha-tocopherol prevents myogenic cell atrophy and increases myotube survival (89), and the tocotrienol-rich fraction reverses the aging of myogenic cells by increasing the regenerative capacity of cells (90). Vitamin E contributes to the recovery of myogenic cell membranes and has a potential therapeutic effect on muscle cells (91), although further studies are needed to confirm the mechanisms involved. In a cross-sectional study, a significant positive association was found between increased dietary vitamin E intake and skeletal muscle mass index, bone mineral density status, and risk of total hip and hip fracture in middle-aged and older men and women, with effects ranging from 0.88 to 1.91% (92).
Vitamin C is the major water-soluble nonenzymatic antioxidant in plasma and tissues and must be obtained through dietary intake because it cannot be synthesized in vivo. A positive trend in quintiles of dietary vitamin C and lean body mass measurements suggests that dietary and circulating vitamin C is positively associated with skeletal muscle mass measurements in middle-aged and older men and women (93).
However, contrary studies have also been reported. In a systematic evaluation and meta-analysis, vitamins C and E did not promote muscle growth after strength training and may have diminished muscle hypertrophy over time (94). When young athletes were given vitamin C and E supplements, despite serum samples suggesting a reduction in oxidative stress in the body, participants' lower limb strength did not increase, and muscle damage could not be reduced (95). In summary, based on the existing evidence, there is not enough convincing evidence to support the use of vitamin E and vitamin C for the prevention and treatment of sarcopenia.
2.3. Omega-3 fatty acids
Omega-3 fatty acids (also known as n-3 fatty acids) are polyunsaturated fatty acids with many potential health benefits and are available in three main dietary forms: alpha-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3). ALA is considered an essential fatty acid because it cannot be synthesized in the human body and is found in nuts, seeds, canola oil, etc. EPA and DHA are mainly found in fish oil.
2.3.1. Mechanisms
Skeletal muscle atrophy involves an inflammatory phase that leads to cell death and tissue remodeling and activates endoplasmic reticulum stress (ERS) and autophagy (96, 97). Both EPA and DHA potentially attenuate ERS and autophagy in skeletal muscles undergoing atrophy by attenuating the increase in PERK and ATG14 expression (98). In addition, DHA promotes mitochondrial biogenesis and skeletal muscle fiber remodeling (99) and delays muscle wasting by stimulating intermediate oxidative stress and inhibiting proteasomal degradation of muscle proteins (100).
2.3.2. Related studies
It has been proposed that elevated plasma levels of proinflammatory cytokines affect muscle catabolic and anabolic signaling pathways and thus may play a key role in the development and progression of sarcopenia, with data showing significantly elevated levels of IL-6 and TNFα in elderly Chinese individuals with sarcopenia (101). Therefore, reducing chronic inflammation associated with aging is emerging as a potential therapeutic target for sarcopenia, and NSAIDs may not be recommended for the treatment of sarcopenia due to the high risk of adverse events that may occur with their use in elderly individuals. Increasing evidence indicates that omega-3 polyunsaturated fatty acids reduce the expression of inflammatory genes and have anti-inflammatory activity (102), particularly eicosapentaenoic acid, docosahexaenoic acid, and alpha-linolenic acid. It was concluded from a systematic evaluation and meta-analysis that omega-3 fatty acid supplementation promotes lean body mass, skeletal muscle mass, and isometric contraction maximal muscle strength in the quadriceps (103). Dietary omega-3 fatty acid levels are negatively associated with sarcopenia (104), and more than 2 g/day of omega-3 fatty acids may increase muscle mass and improve walking speed, especially for those with sarcopenia who have been receiving the intervention for more than 6 months (105). However, linear regression analysis concluded that there was no association between plasma omega-3 levels and grip strength in older adults (106). When cancer patients were supplemented with omega-3 fatty acids, their muscle maintenance, quality of life, and body weight were not improved (107). According to expert opinions (108), doses of 3,000 mg/day DHA plus EPA or more (with preferably more than 800 mg/day EPA) may be required for positive physical performance in older adults (109, 110), because lower doses have no significant effects on muscle strength (111). In conclusion, omega-3 fatty acids may improve sarcopenia, but well-designed, large prospective cohort studies and randomized controlled trials are needed to confirm these findings.
2.4. Creatine
Creatine is a natural nonprotein amino acid compound. Approximately half of the daily creatine requirement comes from the diet, mainly in red meat and seafood (112), and the other half is synthesized endogenously in the kidneys and liver (113). Creatine is mainly stored in muscle (95%), ~2/3 is in the form of PCr, and the rest is free creatine. Approximately 1%−2% of intramuscular creatine is degraded to creatinine and excreted in the urine each day (114, 115); therefore, the body needs to replenish ~1–3 g of creatine per day to maintain normal creatine stores and to obtain the free energy provided by catabolism, depending on muscle mass (116).
2.4.1. Mechanisms
The energy produced by phosphocreatine (PCr) degradation is used to resynthesize ADP and Pi back into ATP to maintain cell function. Increasing PCr and creatine in muscles provides energy reserves to meet anaerobic energy needs, providing a critical source of energy, especially during ischemia, injury, and/or response to impairment (117, 118). Creatine has been shown to activate signaling pathways in the muscle protein synthesis pathway (119), and creatine also protects against mitochondrial damage caused by oxidation, which may reduce inflammation and muscle damage (120, 121).
2.4.2. Clinical studies
After examining the effects of different creatine dosing strategies (lower: 5 g/day, higher: >5 g/day) and the presence or absence of a creatine loading phase (20 g/day for 5–7 days) on lean tissue mass and strength, overall, creatine increased lean tissue mass and strength, but when studies involving a creatine loading phase were excluded from the analysis, creatine had no greater benefit on muscle mass and strength compared to placebo and was effective only during the resistance training phase (122). In another study, creatine supplementation significantly increased upper extremity strength but had no effect on lower extremity strength or muscle mass. However, when resistance training was continued for at least 24 weeks, a significant increase was found in upper and lower extremity muscle strength among older females (123). Overall, creatine intake during resistance training in older adults may increase lean tissue mass, as well as muscle strength in the upper and lower extremities (124). Therefore, it is recommended that older adults supplement creatine concurrently with resistance training. Creatine supplementation appears to enhance the muscular adaptive response to training by increasing the ability to exercise at high intensities and enhancing postexercise recovery and adaptation (125). Differences in creatine dose and frequency of intake during resistance training need to be considered when interpreting the heterogeneity between these studies (126).
2.5. Inorganic nitrate
The health benefits of a diet rich in vegetables are partly explained by their high nitrate content, which is an important biologically active cardioprotective component of vegetables due to its effects on endogenous nitric oxide and vascular health (127). Approximately 80% of total dietary nitrate intake comes from vegetables, with leafy greens and beet being the most abundant and the rest from fruits and meat (128).
Skeletal muscle tissue is the largest reservoir of nitrate in the body and one of the main sites of nitrate and nitrite metabolism (129), which is sensitive to dietary nitrate intake, contributing to nitric oxide production during exercise, and it enhances human muscle contraction by increasing the free intracellular calcium concentration and the calcium sensitivity of myofilaments themselves (130, 131). A cross-sectional analysis revealed that higher nitrate intake (mean 31.2 mg/day) is associated with stronger grip strength and faster timed runs (132). Researchers evaluated participants' habitual dietary intake over 12 years in a cohort study, and individuals with the highest nitrate intake (mean 91 mg/day) had stronger knee extension and faster timed starts, and the results were unaffected by physical activity (133). In randomized controlled trials, nitrate is given almost in the form of concentrated beetroot as an acute dose ranging from 6.4 to 15.9 mmol, and the results show that intake significantly increases muscle strength, with an average increase of ~5% (134). Its potential benefits on muscle strength and endurance are not affected by dose, frequency of intake, level of training, muscle group, or type of contraction (135). It may improve grip strength in older adults by accelerating muscle oxygenation and muscle strength recovery after exercise (136). Most of the current research suggests that a nitrate-rich diet has potential benefits for muscle strength and physical function in older adults, but due to the lack of research, more evidence is needed to validate this claim.
2.6. Probiotics, prebiotics, synbiotics
Probiotics are beneficial bacteria that are mainly found in our digestive system. Prebiotics are mainly oligosaccharides that promote the growth and proliferation of beneficial bacteria in the body but are not digested and absorbed by the host (137). Preparations that mix probiotics and prebiotics are called synbiotics (138), and the benefits of both are unified.
2.6.1. Mechanisms
Changes in the structure of the intestinal flora are closely related to human health and disease. The major phyla of the healthy intestinal microbiota are the thick-walled phylum, the phylum Bacteroidetes, the phylum Actinomycetes, and to a lesser extent, the phylum Wolbachia and the phylum Aspergillus. In weak and cachectic humans, these beneficial bacteria are reduced, while an increase in opportunistic pathogens of Enterobacteriaceae occurs (139). Probiotics promote the production of metabolites such as short-chain fatty acids (SCFAs), secondary bile acids (BA), and some amino acids that regulate homeostasis in skeletal muscle by improving insulin sensitivity (140, 141). In addition, alterations in the ecosystem composition of the gut microbiota, such as reduced production of beneficial metabolites (e.g., SCFA) in the intestinal lumen, lead to intestinal leakage and bacterial endotoxins such as lipopolysaccharides (LPS) entering the peripheral blood (142), producing systemic inflammation associated with aging and muscle wasting (143, 144). Probiotics can limit inflammation and oxygen stress (145).
2.6.2. Related studies
The experimental model of germ-free mice provides valuable evidence for the potential role of the microbiota in controlling muscle mass and function. Compared to conventional mice, germ-free mice, and antibiotic-treated mice, their muscle mass and strength are decreased (146–148). Interestingly, this change in muscle mass and function can be restored by transplantation of microbiota or under natural conditions (146, 147). The elderly were divided into high-functioning (HF) and low-functioning (LF) groups based on physical function, stool samples from both groups were transferred to germ-free mice, and grip strength was significantly increased in mice with HF compared to mice with LF (149). In mouse and human models, reduced intestinal permeability usually coincides with improved muscle mass or strength (150). The use of probiotics, prebiotics, and synbiotics may thus reduce muscle mass loss by stimulating the growth of the bacterial flora and restoring the balance of the gut microbiome, ultimately resulting in a more beneficial metabolite profile and lower intestinal permeability. COPD patients with sarcopenia who were continuously supplemented with a multistrain probiotic for 16 weeks showed reduced markers of intestinal permeability and neuromuscular junction degeneration in plasma, along with improved grip strength, gait speed, and SPPB scores compared to the placebo group (151). However, the causal relationship between microbiota and muscle health remains uncertain due to the lack of targeted studies and the effects of a large number of covariates (including diet, exercise, polydipsia, and multiple drugs) on microbiota composition and function (152). In addition, specific strains that optimize muscle mass and function are not yet available due to the scarcity of human studies and the difficulty of accurate measurements. Future studies should be conducted in humans and should focus on the effects of different bacterial genera and strains on microbiome balance, metabolite profiles, gut function, and muscle mass in sarcopenia.
2.7. Magnesium, selenium, calcium, and other minerals
Growing evidence shows that low micronutrient intake is associated with an increased risk of sarcopenia (153). It has been shown from systematic evaluations that patients with sarcopenia have lower intakes of calcium, magnesium, sodium and selenium than older adults with healthy muscles (154), and magnesium, selenium and calcium appear to be the most promising minerals for the prevention or treatment of sarcopenia (155).
Magnesium is involved in numerous physiological processes as a cofactor in many enzymatic reactions, and it also plays an important role in maintaining muscle mass and protecting muscle tissue from oxidative damage (156, 157). Mg2+ supplementation in aged mice induces myogenic differentiation, promotes protein synthesis, provides protection against the loss of muscle regeneration potential and muscle mass during aging, significantly promotes muscle regeneration, and preserves muscle mass and strength (158). The study suggests that intramuscular ionized magnesium is negatively correlated with age and positively correlated with the strength of knee extension in females. This may be because females have chronic underlying magnesium deficiency and therefore have significantly lower intramuscular ionized magnesium than males (159). In a cross-sectional study involving 2,570 women aged 18–79 years (156), a positive association between dietary magnesium intake and skeletal muscle mass and explosive leg strength index was observed, and data from another prospective cohort study suggested that higher magnesium intake is associated with greater grip strength and higher skeletal muscle mass (157). In follow-up surveys over 5 years, increased magnesium intake was associated with increased SPPB scores in older women, but no such association was observed in men (160). Higher intake of magnesium has been shown to be positively correlated with appendicular muscle mass and change in appendicular muscle mass in a longitudinal study (161), and positive associations between magnesium intake and grip strength have been shown in a cross-sectional study (162). There is some consistency in the current studies of magnesium's ability to improve sarcopenia, suggesting that magnesium supplementation may slow age-related skeletal muscle mass loss, although the evidence is mainly observational and cross-sectional studies.
Selenium is one of the essential trace elements, and it has been reported that patients with selenium deficiency develop skeletal muscle disease, manifested by muscle pain, fatigue, proximal limb weakness, and elevated serum creatine kinase (163). Although dietary supplements of selenium alone or in combination with vitamins are being widely used, the effects of selenium on muscle performance have not been adequately studied. Experiments conducted in mice show that selenium supplementation increases calcium release from the sarcoplasmic reticulum, thereby improving skeletal muscle performance, and that increased expression of selenoprotein N in muscle enhances oxidative stress tolerance (164). Selenium concentrations were found to be negatively associated with restricted physical function in a cross-sectional study, with a reduced incidence of physical frailty when baseline selenium levels were doubled (165). In the only clinical randomized controlled study, combined vitamin E, vitamin C, zinc, and selenium supplementation for 17 weeks improved maximal voluntary contraction and endurance limit time in the quadriceps muscle by reducing oxidative stress and enhancing antioxidant defense (166). Although selenium intake is low in elderly individuals and correlated with poorer skeletal muscle function, prospective analysis indicates no significant effect of selenium intake on skeletal muscle function (167). Nevertheless, the daily dietary intake of selenium is 20–75 μg for adults according to the EU recommendations (45). Since most of the evidence is from observational studies, we are not yet able to conclusively determine the effect of selenium supplementation in patients with sarcopenia; thus, large randomized controlled trials are required in the future to demonstrate this.
In the cross-sectional analysis, daily calcium intake was negatively correlated with overall fat percentage and positively correlated with extremity bone mass. After adjusting for age, sex, BMI, total energy intake, and lifestyle factors, daily calcium intake was significantly lower in patients with sarcopenia than in those without sarcopenia (168). However, 6 months of calcium supplementation does not have a significant effect on skeletal muscle strength and serum testosterone in young adult men, as found in one randomized controlled trial (169). There is a lack of studies on the effect of calcium on patients with sarcopenia.
2.8. Collagen and collagen peptides
Collagen accounts for one-third of the total protein in the human body, is the most abundant form of structural protein in the body, and contributes about 65%−80% of tendon dry weight (170). Extramyocellular connective tissue transmits contractility to tendons and bone, and collagen is a core structural component of extracellular connective tissue and is therefore essential for the strength, regulation, and regeneration of this tissue (171). Dietary collagens, such as collagen peptides or gelatin, are most commonly extracted from the skin, bones, or scales of pigs, cattle, and other poultry (172), and because they contain large amounts of glycine and proline and hydroxyproline, similar to the amino acid distribution of muscle connective tissue, it has been proposed that increasing their intake may help to stimulate muscle connective tissue synthesis to the greatest extent (173), thereby increasing muscle mass and strength and improving sarcopenia possibly.
2.8.1. Mechanisms
Consumption of proline-rich and glycine-rich collagen may be more suitable than high-quality protein sources such as casein or whey protein (providing only 6% proline and 2% glycine) to provide specific amino acid precursors required to support de novo synthesis of connective tissue proteins since the amount of glycine and proline provided in the usual diet is insufficient to provide metabolism and promote increased rates of tissue collagen synthesis (174, 175). In an in vitro model, tendons in growth mediums containing proline and ascorbic acid showed increased collagen content and improved mechanical properties (176). In rats, a glycine-rich diet made the Achilles tendinitis model more resistant to maximum tolerated loads (177). In addition, peptides produced by collagen hydrolysis, which are easily absorbed in the digestive tract before entering the circulation (178), can enhance fibroblast elastin synthesis, while inhibiting elastin degradation and promoting fibroblast proliferation (179), and thus may enhance connective tissue remodeling in muscle.
2.8.2. Clinical studies
Regarding the effect of collagen supplementation on body composition, Zdzieblik et al. (180) showed that elderly men with sarcopenia exercised three times a week and ingested 15 g of collagen peptide per day for 12 weeks, and their changes in body composition were very significant, with a mean increase in fat-free mass of 4.2 kg compared to only 2.9 kg in the placebo group. The same test in young, healthy men resulted in a mean increase in fat-free mass of 2.6 kg in the collagen peptide group and only 0.7 kg in the placebo-supplemented group (181). In premenopausal women, it was also found that the combination of resistance training with collagen supplementation significantly increased fat-free mass and increased hand grip strength. The above studies showed that collagen peptide supplementation was effective in improving muscle mass and strength while resisting resistance exercise. Regarding the effect of collagen on muscle protein synthesis, Oikawa et al. (182) supplemented 30 g of whey protein or collagen peptide twice a day in older adults who lacked physical activity and low-energy status, and only the whey protein group enhanced fat-free mass and muscle protein synthesis in the lower extremities during return to activity. Two other studies have similarly observed increased muscle protein synthesis with whey protein compared to collagen supplementation (183, 184). This suggests that collagen has little anabolic potential compared to isonitrogenous higher-quality protein sources. According to systematic reviews and meta-analyses, collagen supplements are most beneficial in reducing joint pain and improving joint function, with some improvement in body composition, strength, and muscle recovery (170). In conclusion, collagen supplementation with resistance exercise can increase muscle mass and strength, but there is insufficient evidence that collagen is more effective in improving sarcopenia than traditional high-quality protein sources such as casein or whey protein.
2.9. Polyphenols
Polyphenols are a range of plant compounds with antioxidant and anti-inflammatory properties containing one or more phenolic rings attached to hydroxyl groups (185). They are divided into four classes: phenolic acids, flavonoids, stilbenes, and lignans (186) and are particularly abundant in fruits, vegetables, coffee, tea, cocoa, vanilla, and spices (187). Because there are a wide variety of polyphenols available and there are many factors that can alter their concentration in food, it is difficult to establish reference composition tables (188).
2.9.1. Mechanisms
The effects of polyphenolic compounds in dystrophia are mainly through the inhibition of E3 ubiquitin ligases and upstream regulators in inflammation, oxidative stress, and mitochondrial damage (189, 190). It also increases protein synthesis by effectively activating the Akt/mTOR pathway (191). Moreover, PPs modulated the expression of miRNAs, IGF-1 signaling pathway, follistatin, mitochondrial biogenesis, and myogenic differentiation factors involved in myogenesis (192).
2.9.2. Related studies
Resveratrol (RSV) is a natural polyphenol. In animal experiments, high doses of RSV (400 mg/kg/day) have been reported to attenuate muscle fiber atrophy following hindlimb suspension in rodents (193). Lower doses of RSV (5 mg/kg/day) still promoted skeletal muscle hypertrophy and reduced exercise-induced muscle necrosis in wild-type mice (194). In clinical studies, elderly subjects were supplemented with 500 mg/day resveratrol during exercise, and muscle mitochondrial density and muscle fatigue resistance were higher in elderly subjects compared with placebo-supplemented groups (195). Resveratrol at 1,000 mg/day increased the 6-min walk distance by 33.1 m in older adults, which was higher than the mean walking distance in the 500 mg/day group (196). Patients with chronic kidney disease received 500 mg resveratrol and 500 mg curcumin orally daily, and muscle mass and bone mass increased significantly after 12 weeks. However, no improvement in walking ability with resveratrol was observed in elderly subjects with peripheral arterial disease (197), mitochondrial function in skeletal muscle was not improved and lean body mass was decreased in COPD patients receiving 150 mg/day resveratrol (198). To determine the effects of polyphenols on muscle, multiple systematic reviews and meta-analyses have assessed the effectiveness of polyphenols on muscle pain and muscle recovery after exercise in healthy adults, and the results have shown that consumption of polyphenol-rich foods, juices, and concentrates accelerates the recovery of muscle function and reduces muscle soreness at doses ranging from 150 to 1,500 mg/day (199–201). A meta-analysis suggests that polyphenol supplementation is unlikely to enhance exercise-induced changes in body composition or performance, and that only isoflavones may increase lean body mass in postmenopausal women (202), and another meta-analysis suggests that short-term polyphenols intake, although attenuating the inflammatory response after exercise, does not affect the anabolic response to protein and exercise in healthy elderly men (203). In summary, polyphenol supplementation is believed to reduce muscle pain and accelerate the recovery of muscle function after exercise, but the effect on body composition and physical performance in patients with sarcopenia is inconclusive and remains to be explored.
3. Conclusions
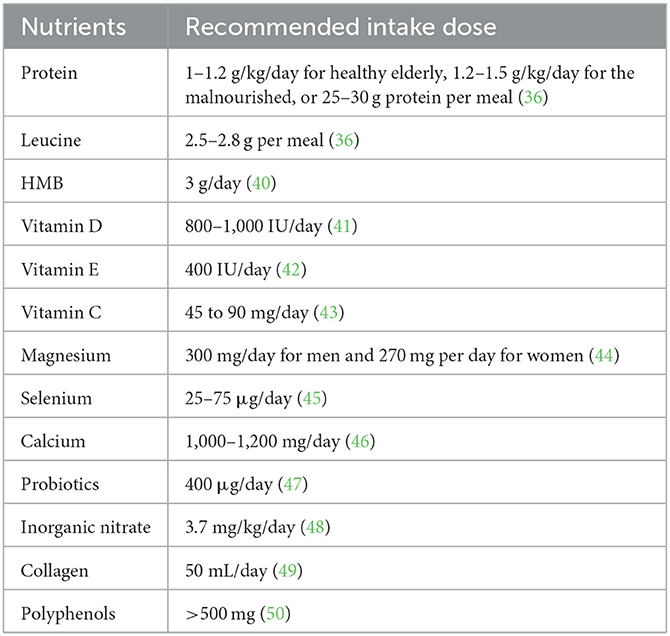
Clinicians or health care providers need to screen older adults at risk for sarcopenia, especially those with comorbid malnutrition, and use appropriate diagnostic tools to make the diagnosis. Related professionals should then provide resistance training and diet and protein optimization programs to patients with diagnosed sarcopenia (15). This article summarizes the research progress of nutritional supplements in the improvement of sarcopenia, including the possible cellular and molecular mechanisms involved, so as to provide a reference for medical staff and researchers. The currently acceptable recommended intakes for each nutrient are shown in Table 2.
In the future, patients may benefit from complex hybrid nutritional supplements, as well as the development of nutrigenomics and metabolomics (204), so that nutritional interventions provided are tailored to an individual's nutritional and metabolic status. In addition, when the molecular mechanisms of muscle targets are well studied, they may play a key role in developing targeted treatment and prevention strategies.
Author contributions
SLiu contributed to drafting the paper. LZ had primary responsibility for final content. LZ and SLi revised the final draft of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Sichuan Science and Technology Program (Grant number 2023YFS0247) and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Grant number Z2021JC005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. (2002) 76:473–81. doi: 10.1093/ajcn/76.2.473
2. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. (2000) 88:1321–6. doi: 10.1152/jappl.2000.88.4.1321
3. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
4. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
6. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) 16:21. doi: 10.1186/s40200-017-0302-x
7. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7. doi: 10.1016/j.jamda.2019.12.012
8. Ackermans L, Rabou J, Basrai M, Schweinlin A, Bischoff SC, Cussenot O, et al. Screening, diagnosis and monitoring of sarcopenia: when to use which tool? Clin Nutr ESPEN. (2022) 48:36–44. doi: 10.1016/j.clnesp.2022.01.027
9. Chen LK, Arai H, Assantachai P, Akishita M, Chew STH, Dumlao LC, et al. Roles of nutrition in muscle health of community-dwelling older adults: evidence-based expert consensus from Asian Working Group for Sarcopenia. J Cachexia Sarcopenia Muscle. (2022) 13:1653–72. doi: 10.1002/jcsm.12981
10. Leij-Halfwerk S, Verwijs MH, van Houdt S, Borkent JW, Guaitoli PR, Pelgrim T, et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis. Maturitas. (2019) 126:80–9. doi: 10.1016/j.maturitas.2019.05.006
11. Correia M, Sulo S, Brunton C, Sulz I, Rodriguez D, Gomez G, et al. Prevalence of malnutrition risk and its association with mortality: nutritionDay Latin America survey results. Clin Nutr. (2021) 40:5114–21. doi: 10.1016/j.clnu.2021.07.023
12. Tan VMH, Pang BWJ, Lau LK, Jabbar KA, Seah WT, Chen KK, et al. Malnutrition and sarcopenia in community-dwelling adults in Singapore: Yishun Health Study. J Nutr Health Aging. (2021) 25:374–81. doi: 10.1007/s12603-020-1542-x
13. Gümüşsoy M, Atmiş V, Yalçin A, Bahşi R, Yigit S, Ari S, et al. Malnutrition-sarcopenia syndrome and all-cause mortality in hospitalized older people. Clin Nutr. (2021) 40:5475–81. doi: 10.1016/j.clnu.2021.09.036
14. Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. (2018) 37:1121–32. doi: 10.1016/j.clnu.2017.08.016
15. Zanker J, Sim M, Anderson K, Balogun S, Brennan-Olsen SL, Dent E, et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J Cachexia Sarcopenia Muscle. (2023) 14:142–56. doi: 10.1002/jcsm.13115
16. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. (2013) 41:169–73. doi: 10.1097/JES.0b013e318292f3d5
17. Cholewa JM, Dardevet D, Lima-Soares F, de Araújo Pessôa K, Oliveira PH, Dos Santos Pinho JR, et al. Dietary proteins and amino acids in the control of the muscle mass during immobilization and aging: role of the MPS response. Amino Acids. (2017) 49:811–20. doi: 10.1007/s00726-017-2390-9
18. Bilodeau PA, Coyne ES, Wing SS. The ubiquitin proteasome system in atrophying skeletal muscle: roles and regulation. Am J Physiol Cell Physiol. (2016) 311:C392–403. doi: 10.1152/ajpcell.00125.2016
19. Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. (2015) 6:6670. doi: 10.1038/ncomms7670
20. Zhao F, Yu Y, Liu W, Zhang J, Liu X, Liu L, et al. Small molecular weight soybean protein-derived peptides nutriment attenuates rat burn injury-induced muscle atrophy by modulation of ubiquitin-proteasome system and autophagy signaling pathway. J Agric Food Chem. (2018) 66:2724–34. doi: 10.1021/acs.jafc.7b05387
21. Suer MK, Crane JD, Trappe TA, Jemiolo B, Trappe SW, Harber MP. Amino acid infusion alters the expression of growth-related genes in multiple skeletal muscles. Aviat Space Environ Med. (2013) 84:669–74. doi: 10.3357/ASEM.3379.2013
22. Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. (2007) 102:2389–97. doi: 10.1152/japplphysiol.01202.2006
23. El Assar M, Angulo J, Walter S, Carnicero JA, García-García FJ, Sánchez-Puelles JM, et al. Better nutritional status is positively associated with mRNA expression of SIRT1 in community-dwelling older adults in the Toledo study for healthy aging. J Nutr. (2018) 148:1408–14. doi: 10.1093/jn/nxy149
24. Pan YJ, Zhou SJ, Feng J, Bai Q, A LT, Zhang AH. Urotensin II induces mice skeletal muscle atrophy associated with enhanced autophagy and inhibited Irisin precursor (fibronectin type III domain containing 5) expression in chronic renal failure. Kidney Blood Press Res. (2019) 44:479–95. doi: 10.1159/000499880
25. Zheng R, Huang S, Zhu J, Lin W, Xu H, Zheng X. Leucine attenuates muscle atrophy and autophagosome formation by activating PI3K/AKT/mTOR signaling pathway in rotator cuff tears. Cell Tissue Res. (2019) 378:113–25. doi: 10.1007/s00441-019-03021-x
26. Zhang J, Yu Y, Wang J. Protein nutritional support: the classical and potential new mechanisms in the prevention and therapy of sarcopenia. J Agric Food Chem. (2020) 68:4098–108. doi: 10.1021/acs.jafc.0c00688
27. Hanach NI, McCullough F, Avery A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv Nutr. (2019) 10:59–69. doi: 10.1093/advances/nmy065
28. Kirwan RP, Mazidi M, Rodríguez García C, Lane KE, Jafari A, Butler T, et al. Protein interventions augment the effect of resistance exercise on appendicular lean mass and handgrip strength in older adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. (2022) 115:897–913. doi: 10.1093/ajcn/nqab355
29. Li L, He Y, Jin N, Li H, Liu X. Effects of protein supplementation and exercise on delaying sarcopenia in healthy older individuals in Asian and non-Asian countries: a systematic review and meta-analysis. Food Chem X. (2022) 13:100210. doi: 10.1016/j.fochx.2022.100210
30. Gielen E, Beckwée D, Delaere A, De Breucker S, Vandewoude M, Bautmans I. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev. (2021) 79:121–47. doi: 10.1093/nutrit/nuaa011
31. Li CY, Fang AP, Ma WJ, Wu SL, Li CL, Chen YM, et al. Amount rather than animal vs plant protein intake is associated with skeletal muscle mass in community-dwelling middle-aged and older Chinese adults: results from the Guangzhou Nutrition and Health Study. J Acad Nutr Diet. (2019) 119:1501–10. doi: 10.1016/j.jand.2019.03.010
32. Park Y, Choi JE, Hwang HS. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2018) 108:1026–33. doi: 10.1093/ajcn/nqy214
33. Holwerda AM, Paulussen KJM, Overkamp M, Goessens JPB, Kramer IF, Wodzig W, et al. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J Nutr. (2019) 149:221–30. doi: 10.1093/jn/nxy263
34. Rafii M, Chapman K, Owens J, Elango R, Campbell WW, Ball RO, et al. Dietary protein requirement of female adults >65 years determined by the indicator amino acid oxidation technique is higher than current recommendations. J Nutr. (2015) 145:18–24. doi: 10.3945/jn.114.197517
35. Rafii M, Chapman K, Elango R, Campbell WW, Ball RO, Pencharz PB, et al. Dietary protein requirement of men >65 years old determined by the indicator amino acid oxidation technique is higher than the current estimated average requirement. J Nutr. (2015) 146:681–7. doi: 10.3945/jn.115.225631
36. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
37. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. (2018) 52:376–84. doi: 10.1136/bjsports-2017-097608
38. Mertz KH, Reitelseder S, Bechshoeft R, Bulow J, Højfeldt G, Jensen M, et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: a randomized controlled trial. Am J Clin Nutr. (2021) 113:790–800. doi: 10.1093/ajcn/nqaa372
39. Wu PY, Huang KS, Chen KM, Chou CP, Tu YK. Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: a systematic review and network meta-analysis. Maturitas. (2021) 145:38–48. doi: 10.1016/j.maturitas.2020.12.009
40. Kim D, Kim J. Effects of β-hydroxy-β-methylbutyrate supplementation on recovery from exercise-induced muscle damage: a mini-review. Phys Act Nutr. (2022) 26:41–5. doi: 10.20463/pan.2022.0023
41. Dawson-Hughes B. Vitamin D and muscle function. J Steroid Biochem Mol Biol. (2017) 173:313–6. doi: 10.1016/j.jsbmb.2017.03.018
42. Higgins MR, Izadi A, Kaviani M. Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int J Environ Res Public Health. (2020) 17:8452. doi: 10.3390/ijerph17228452
43. Institute Institute of Medicine Panel on Dietary A, Related C. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academies Press (US). Copyright 2000 by the National Academy of Sciences. All rights reserved (2000).
44. Dietary reference values for food energy and nutrients for the United Kingdom. Report of the panel on dietary reference values of the committee on medical aspects of food policy. Rep Health Soc Subj. (1991) 41:1–210.
45. Robertson A, Tirado C, Lobstein T, Jermini M, Knai C, Jensen JH, et al. Food and health in Europe: a new basis for action. WHO Reg Publ Eur Ser. (2004) i–xvi, 1–385, back cover.
46. van der Velde RY, Brouwers JR, Geusens PP, Lems WF, van den Bergh JP. Calcium and vitamin D supplementation: state of the art for daily practice. Food Nutr Res. (2014) 58:9. doi: 10.3402/fnr.v58.21796
47. Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. (2011) 3:118–34. doi: 10.3390/nu3010118
48. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. (2009) 90:1–10. doi: 10.3945/ajcn.2008.27131
49. Borumand M, Sibilla S. Daily consumption of the collagen supplement Pure Gold Collagen® reduces visible signs of aging. Clin Interv Aging. (2014) 9:1747–58. doi: 10.2147/CIA.S65939
50. Williamson G, Holst B. Dietary reference intake (DRI) value for dietary polyphenols: are we heading in the right direction? Br J Nutr. (2008) 99(Suppl 3):S55–8. doi: 10.1017/S0007114508006867
51. Lim MT, Pan BJ, Toh DWK, Sutanto CN, Kim JE. Animal protein versus plant protein in supporting lean mass and muscle strength: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2021) 13:661. doi: 10.3390/nu13020661
52. Bradlee ML, Mustafa J, Singer MR, Moore LL. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J Gerontol A Biol Sci Med Sci. (2017) 73:88–94. doi: 10.1093/gerona/glx070
53. Coelho-Junior HJ, Calvani R, Gonçalves IO, Rodrigues B, Picca A, Landi F, et al. High relative consumption of vegetable protein is associated with faster walking speed in well-functioning older adults. Aging Clin Exp Res. (2019) 31:837–44. doi: 10.1007/s40520-019-01216-4
54. Gazzani D, Zamboni F, Spelta F, Ferrari P, Mattioli V, Cazzoletti L, et al. Vegetable but not animal protein intake is associated to a better physical performance: a study on a general population sample of adults. Food Nutr Res. (2019) 63:3–4. doi: 10.29219/fnr.v63.3422
55. Gorissen SH, Rémond D, van Loon LJ. The muscle protein synthetic response to food ingestion. Meat Sci. (2015) 109:96–100. doi: 10.1016/j.meatsci.2015.05.009
56. Dai M, Lin T, Yue J, Dai L. Signatures and clinical significance of amino acid flux in sarcopenia: a systematic review and meta-analysis. Front Endocrinol. (2021) 12:725518. doi: 10.3389/fendo.2021.725518
57. Mai K, Cando P, Trasino SE. mTOR1c activation with the leucine “trigger” for prevention of sarcopenia in older adults during lockdown. J Med Food. (2022) 25:117–20. doi: 10.1089/jmf.2021.0094
58. Zaromskyte G, Prokopidis K, Ioannidis T, Tipton KD, Witard OC. Evaluating the leucine trigger hypothesis to explain the post-prandial regulation of muscle protein synthesis in young and older adults: a systematic review. Front Nutr. (2021) 8:685165. doi: 10.3389/fnut.2021.685165
59. Lynch HM, Buman MP, Dickinson JM, Ransdell LB, Johnston CS, Wharton CM. No significant differences in muscle growth and strength development when consuming soy and whey protein supplements matched for leucine following a 12 week resistance training program in men and women: a randomized trial. Int J Environ Res Public Health. (2020) 17:3871. doi: 10.3390/ijerph17113871
60. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, et al. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: a randomized, controlled trial. Am J Clin Nutr. (2018) 107:217–26. doi: 10.1093/ajcn/nqx028
61. Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. (2015) 19:437–46. doi: 10.1007/s12603-014-0559-4
62. Conde Maldonado E, Marqués-Jiménez D, Casas-Agustench P, Bach-Faig A. Effect of supplementation with leucine alone, with other nutrients or with physical exercise in older people with sarcopenia: a systematic review. Endocrinol Diabetes Nutr. (2022) 69:601–13. doi: 10.1016/j.endien.2022.11.012
63. Szwiega S, Pencharz PB, Rafii M, Lebarron M, Chang J, Ball RO, et al. Dietary leucine requirement of older men and women is higher than current recommendations. Am J Clin Nutr. (2021) 113:410–9. doi: 10.1093/ajcn/nqaa323
64. Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, et al. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB). J Int Soc Sports Nutr. (2013) 10:6. doi: 10.1186/1550-2783-10-6
65. Holeček M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. (2017) 8:529–41. doi: 10.1002/jcsm.12208
66. Holecek M, Siman P, Vodenicarovova M, Kandar R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr Metab. (2016) 13:12. doi: 10.1186/s12986-016-0072-3
67. Lin Z, Zhao A, He J. Effect of β-hydroxy-β-methylbutyrate (HMB) on the muscle strength in the elderly population: a meta-analysis. Front Nutr. (2022) 9:914866. doi: 10.3389/fnut.2022.914866
68. Costa Riela NA, Alvim Guimarães MM, Oliveira de. Almeida D, Araujo EMQ. Effects of beta-hydroxy-beta-methylbutyrate supplementation on elderly body composition and muscle strength: a review of clinical trials. Ann Nutr Metab. (2021) 77:16–22. doi: 10.1159/000514236
69. Phillips SM, Lau KJ, D'Souza AC, Nunes EA. An umbrella review of systematic reviews of β-hydroxy-β-methyl butyrate supplementation in ageing and clinical practice. J Cachexia Sarcopenia Muscle. (2022) 13:2265–75. doi: 10.1002/jcsm.13030
70. Teixeira FJ, Matias CN, Monteiro CP, Valamatos MJ, Reis JF, Tavares F, et al. Leucine metabolites do not enhance training-induced performance or muscle thickness. Med Sci Sports Exerc. (2019) 51:56–64. doi: 10.1249/MSS.0000000000001754
71. Osuka Y, Kojima N, Nishihara K, Sasai H, Wakaba K, Tanaka K, et al. β-Hydroxy-β-methylbutyrate supplementation may not enhance additional effects of exercise on muscle quality in older women. Med Sci Sports Exerc. (2022) 54:543–50. doi: 10.1249/MSS.0000000000002836
72. Bollen SE, Bass JJ, Fujita S, Wilkinson D, Hewison M, Atherton PJ. The vitamin D/vitamin D receptor (VDR) axis in muscle atrophy and sarcopenia. Cell Signal. (2022) 96:110355. doi: 10.1016/j.cellsig.2022.110355
73. Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology. (2013) 154:4018–29. doi: 10.1210/en.2013-1369
74. Gogulothu R, Nagar D, Gopalakrishnan S, Garlapati VR, Kallamadi PR, Ismail A. Disrupted expression of genes essential for skeletal muscle fibre integrity and energy metabolism in vitamin D deficient rats. J Steroid Biochem Mol Biol. (2020) 197:105525. doi: 10.1016/j.jsbmb.2019.105525
75. Bass JJ, Kazi AA, Deane CS, Nakhuda A, Ashcroft SP, Brook MS, et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J Physiol. (2021) 599:963–79. doi: 10.1113/JP280652
76. Dzik KP, Skrobot W, Kaczor KB, Flis DJ, Karnia MJ, Libionka W, et al. Vitamin D deficiency is associated with muscle atrophy and reduced mitochondrial function in patients with chronic low back pain. Oxid Med Cell Longev. (2019) 2019:6835341. doi: 10.1155/2019/6835341
77. Romeu Montenegro K, Carlessi R, Cruzat V, Newsholme P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J Steroid Biochem Mol Biol. (2019) 193:105423. doi: 10.1016/j.jsbmb.2019.105423
78. Schnell DM, Walton RG, Vekaria HJ, Sullivan PG, Bollinger LM, Peterson CA, et al. Vitamin D produces a perilipin 2-dependent increase in mitochondrial function in C2C12 myotubes. J Nutr Biochem. (2019) 65:83–92. doi: 10.1016/j.jnutbio.2018.11.002
79. Chang E, Kim Y. Vitamin D ameliorates fat accumulation with AMPK/SIRT1 activity in C2C12 skeletal muscle cells. Nutrients. (2019) 11:2806. doi: 10.3390/nu11112806
80. Gimigliano F, Moretti A, de Sire A, Calafiore D, Iolascon G. The combination of vitamin D deficiency and overweight affects muscle mass and function in older post-menopausal women. Aging Clin Exp Res. (2018) 30:625–31. doi: 10.1007/s40520-018-0921-1
81. Conzade R, Grill E, Bischoff-Ferrari HA, Ferrari U, Horsch A, Koenig W, et al. Vitamin D in relation to incident sarcopenia and changes in muscle parameters among older adults: the KORA-age study. Calcif Tissue Int. (2019) 105:173–82. doi: 10.1007/s00223-019-00558-5
82. Yang A, Lv Q, Chen F, Wang Y, Liu Y, Shi W, et al. The effect of vitamin D on sarcopenia depends on the level of physical activity in older adults. J Cachexia Sarcopenia Muscle. (2020) 11:678–89. doi: 10.1002/jcsm.12545
83. Shea MK, Fielding RA, Dawson-Hughes B. The effect of vitamin D supplementation on lower-extremity power and function in older adults: a randomized controlled trial. Am J Clin Nutr. (2019) 109:369–79. doi: 10.1093/ajcn/nqy290
84. Prokopidis K, Giannos P, Katsikas Triantafyllidis K, Kechagias KS, Mesinovic J, Witard OC, et al. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:1642–52. doi: 10.1002/jcsm.12976
85. Chang MC, Choo YJ. Effects of whey protein, leucine, and vitamin D supplementation in patients with sarcopenia: a systematic review and meta-analysis. Nutrients. (2023) 15:521. doi: 10.3390/nu15030521
86. Damiano S, Muscariello E, La Rosa G, Di Maro M, Mondola P, Santillo M. Dual role of reactive oxygen species in muscle function: can antioxidant dietary supplements counteract age-related sarcopenia? Int J Mol Sci. (2019) 20:3815. doi: 10.3390/ijms20153815
87. Kolesar JE, Safdar A, Abadi A, MacNeil LG, Crane JD, Tarnopolsky MA, et al. Defects in mitochondrial DNA replication and oxidative damage in muscle of mtDNA mutator mice. Free Radic Biol Med. (2014) 75:241–51. doi: 10.1016/j.freeradbiomed.2014.07.038
88. Otsuka Y, Iidaka T, Horii C, Muraki S, Oka H, Nakamura K, et al. Dietary intake of vitamin E and fats associated with sarcopenia in community-dwelling older Japanese people: a cross-sectional study from the Fifth survey of the ROAD Study. Nutrients. (2021) 13:1730. doi: 10.3390/nu13051730
89. von Grabowiecki Y, Licona C, Palamiuc L, Abreu P, Vidimar V, Coowar D, et al. Regulation of a Notch3-Hes1 pathway and protective effect by a tocopherol-omega alkanol chain derivative in muscle atrophy. J Pharmacol Exp Ther. (2015) 352:23–32. doi: 10.1124/jpet.114.216879
90. Lim JJ, Ngah WZ, Mouly V, Abdul Karim N. Reversal of myoblast aging by tocotrienol rich fraction posttreatment. Oxid Med Cell Longev. (2013) 2013:978101. doi: 10.1155/2013/978101
91. Howard AC, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. Nat Commun. (2011) 2:597. doi: 10.1038/ncomms1594
92. Mulligan AA, Hayhoe RPG, Luben RN, Welch AA. Positive associations of dietary intake and plasma concentrations of vitamin E with skeletal muscle mass, heel bone ultrasound attenuation and fracture risk in the EPIC-Norfolk Cohort. Antioxidants. (2021) 10:159. doi: 10.3390/antiox10020159
93. Lewis LN, Hayhoe RPG, Mulligan AA, Luben RN, Khaw KT, Welch AA. Lower dietary and circulating vitamin C in middle- and older-aged men and women are associated with lower estimated skeletal muscle mass. J Nutr. (2020) 150:2789–98. doi: 10.1093/jn/nxaa221
94. Dutra MT, Martins WR, Ribeiro ALA, Bottaro M. The effects of strength training combined with vitamin C and E supplementation on skeletal muscle mass and strength: a systematic review and meta-analysis. J Sports Med. (2020) 2020:3505209. doi: 10.1155/2020/3505209
95. de Oliveira DCX, Rosa FT, Simões-Ambrósio L, Jordao AA, Deminice R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition. (2019) 63–64:29–35. doi: 10.1016/j.nut.2019.01.007
96. Song S, Tan J, Miao Y, Zhang Q. Crosstalk of ER stress-mediated autophagy and ER-phagy: involvement of UPR and the core autophagy machinery. J Cell Physiol. (2018) 233:3867–74. doi: 10.1002/jcp.26137
97. Londhe P, Guttridge DC. Inflammation induced loss of skeletal muscle. Bone. (2015) 80:131–42. doi: 10.1016/j.bone.2015.03.015
98. Marzuca-Nassr GN, Kuwabara WMT, Vitzel KF, Murata GM, Torres RP, Mancini-Filho J, et al. Endoplasmic reticulum stress and autophagy markers in soleus muscle disuse-induced atrophy of rats treated with fish oil. Nutrients. (2021) 13:2298. doi: 10.3390/nu13072298
99. Chen W, Chen Y, Wu R, Guo G, Liu Y, Zeng B, et al. DHA alleviates diet-induced skeletal muscle fiber remodeling via FTO/m(6)A/DDIT4/PGC1α signaling. BMC Biol. (2022) 20:39. doi: 10.1186/s12915-022-01239-w
100. Lee JH, Jeon JH, Lee MJ. Docosahexaenoic acid, a potential treatment for sarcopenia, modulates the ubiquitin-proteasome and the autophagy-lysosome systems. Nutrients. (2020) 12:2597. doi: 10.3390/nu12092597
101. Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. (2017) 22:25. doi: 10.1186/s40001-017-0266-9
102. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. (2017) 45:1105–15. doi: 10.1042/BST20160474
103. Bird JK, Troesch B, Warnke I, Calder PC. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: a scoping systematic review and meta-analysis. Clin Nutr ESPEN. (2021) 46:73–86. doi: 10.1016/j.clnesp.2021.10.011
104. Zhang Y, Guo H, Liang J, Xiao W, Li Y. Relationship between dietary omega-3 and omega-6 polyunsaturated fatty acids level and sarcopenia. A meta-analysis of observational studies. Front Nutr. (2021) 8:738083. doi: 10.3389/fnut.2021.738083
105. Huang YH, Chiu WC, Hsu YP, Lo YL, Wang YH. Effects of omega-3 fatty acids on muscle mass, muscle strength and muscle performance among the elderly: a meta-analysis. Nutrients. (2020) 12:3739. doi: 10.3390/nu12123739
106. Batista RAB, de Branco FMS, Nehme R, de Oliveira EP, Pena GDG. Association between plasma omega-3 and handgrip strength according to glycohemoglobin levels in older adults: results from NHANES 2011-2012. Nutrients. (2022) 14:4060. doi: 10.3390/nu14194060
107. Lam CN, Watt AE, Isenring EA, de van der Schueren MAE, van der Meij BS. The effect of oral omega-3 polyunsaturated fatty acid supplementation on muscle maintenance and quality of life in patients with cancer: a systematic review and meta-analysis. Clin Nutr. (2021) 40:3815–26. doi: 10.1016/j.clnu.2021.04.031
108. Troesch B, Eggersdorfer M, Laviano A, Rolland Y, Smith AD, Warnke I, et al. Expert opinion on benefits of long-chain omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients. (2020) 12:2555. doi: 10.3390/nu12092555
109. Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. (2015) 102:115–22. doi: 10.3945/ajcn.114.105833
110. Logan SL, Spriet LL. Omega-3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community-dwelling older females. PLoS ONE. (2015) 10:e0144828. doi: 10.1371/journal.pone.0144828
111. Rolland Y, Barreto PS, Maltais M, Guyonnet S, Cantet C, Andrieu S, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain lifestyle intervention on muscle strength in older adults: secondary analysis of the multidomain Alzheimer preventive trial (MAPT). Nutrients. (2019) 11:1931. doi: 10.3390/nu11081931
112. Brosnan ME, Brosnan JT. The role of dietary creatine. Amino Acids. (2016) 48:1785–91. doi: 10.1007/s00726-016-2188-1
113. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. (2000) 80:1107–213. doi: 10.1152/physrev.2000.80.3.1107
114. Harris R. Creatine in health, medicine and sport: an introduction to a meeting held at Downing College, University of Cambridge, July 2010. Amino Acids. (2011) 40:1267–70. doi: 10.1007/s00726-011-0913-3
115. Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol. (1996) 81:232–7. doi: 10.1152/jappl.1996.81.1.232
116. Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. (2017) 14:18. doi: 10.1186/s12970-017-0173-z
117. Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. (2011) 40:1271–96. doi: 10.1007/s00726-011-0877-3
118. Marshall RP, Droste JN, Giessing J, Kreider RB. Role of creatine supplementation in conditions involving mitochondrial dysfunction: a narrative review. Nutrients. (2022) 14:529. doi: 10.3390/nu14030529
119. Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics. (2008) 32:219–28. doi: 10.1152/physiolgenomics.00157.2007
120. Barbieri E, Guescini M, Calcabrini C, Vallorani L, Diaz AR, Fimognari C, et al. Creatine prevents the structural and functional damage to mitochondria in myogenic, oxidatively stressed C2C12 cells and restores their differentiation capacity. Oxid Med Cell Longev. (2016) 2016:5152029. doi: 10.1155/2016/5152029
121. Giallauria F, Cittadini A, Smart NA, Vigorito C. Resistance training and sarcopenia. Monaldi Arch Chest Dis. (2016) 84:738. doi: 10.4081/monaldi.2015.738
122. Forbes SC, Candow DG, Ostojic SM, Roberts MD, Chilibeck PD. Meta-analysis examining the importance of creatine ingestion strategies on lean tissue mass and strength in older adults. Nutrients. (2021) 13:1912. doi: 10.3390/nu13061912
123. Dos Santos EEP, de Araújo RC, Candow DG, Forbes SC, Guijo JA, de Almeida Santana CC, et al. Efficacy of creatine supplementation combined with resistance training on muscle strength and muscle mass in older females: a systematic review and meta-analysis. Nutrients. (2021) 13:3757. doi: 10.3390/nu13113757
124. Chilibeck PD, Kaviani M, Candow DG, Zello GA. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med. (2017) 8:213–26. doi: 10.2147/OAJSM.S123529
125. Dolan E, Artioli GG, Pereira RMR, Gualano B. Muscular atrophy and sarcopenia in the elderly: is there a role for creatine supplementation? Biomolecules. (2019) 9:642. doi: 10.3390/biom9110642
126. Candow DG, Forbes SC, Chilibeck PD, Cornish SM, Antonio J, Kreider RB. Variables influencing the effectiveness of creatine supplementation as a therapeutic intervention for sarcopenia. Front Nutr. (2019) 6:124. doi: 10.3389/fnut.2019.00124
127. Bondonno CP, Blekkenhorst LC, Liu AH, Bondonno NP, Ward NC, Croft KD, et al. Vegetable-derived bioactive nitrate and cardiovascular health. Mol Aspects Med. (2018) 61:83–91. doi: 10.1016/j.mam.2017.08.001
128. Bondonno CP, Blekkenhorst LC, Prince RL, Ivey KL, Lewis JR, Devine A, et al. Association of vegetable nitrate intake with carotid atherosclerosis and ischemic cerebrovascular disease in older women. Stroke. (2017) 48:1724–9. doi: 10.1161/STROKEAHA.117.016844
129. Gilliard CN, Lam JK, Cassel KS, Park JW, Schechter AN, Piknova B. Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide. (2018) 75:1–7. doi: 10.1016/j.niox.2018.01.010
130. Wylie LJ, Park JW, Vanhatalo A, Kadach S, Black MI, Stoyanov Z, et al. Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J Physiol. (2019) 597:5565–76. doi: 10.1113/JP278076
131. Coggan AR, Peterson LR. Dietary nitrate enhances the contractile properties of human skeletal muscle. Exerc Sport Sci Rev. (2018) 46:254–61. doi: 10.1249/JES.0000000000000167
132. Sim M, Lewis JR, Blekkenhorst LC, Bondonno CP, Devine A, Zhu K, et al. Dietary nitrate intake is associated with muscle function in older women. J Cachexia Sarcopenia Muscle. (2019) 10:601–10. doi: 10.1002/jcsm.12413
133. Sim M, Blekkenhorst LC, Bondonno NP, Radavelli-Bagatini S, Peeling P, Bondonno CP, et al. Dietary nitrate intake is positively associated with muscle function in men and women independent of physical activity levels. J Nutr. (2021) 151:1222–30. doi: 10.1093/jn/nxaa415
134. Coggan AR, Baranauskas MN, Hinrichs RJ, Liu Z, Carter SJ. Effect of dietary nitrate on human muscle power: a systematic review and individual participant data meta-analysis. J Int Soc Sports Nutr. (2021) 18:66. doi: 10.1186/s12970-021-00463-z
135. Alvares TS, Oliveira GV, Volino-Souza M, Conte-Junior CA, Murias JM. Effect of dietary nitrate ingestion on muscular performance: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2022) 62:5284–306. doi: 10.1080/10408398.2021.1884040
136. de Oliveira GV, Morgado M, Conte-Junior CA, Alvares TS. Acute effect of dietary nitrate on forearm muscle oxygenation, blood volume and strength in older adults: a randomized clinical trial. PLoS ONE. (2017) 12:e0188893. doi: 10.1371/journal.pone.0188893
137. Jäger R, Zaragoza J, Purpura M, Iametti S, Marengo M, Tinsley GM, et al. Probiotic administration increases amino acid absorption from plant protein: a placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob Proteins. (2020) 12:1330–9. doi: 10.1007/s12602-020-09656-5
138. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. (2018) 48:1044–60. doi: 10.1111/apt.15001
139. Ticinesi A, Mancabelli L, Tagliaferri S, Nouvenne A, Milani C, Del Rio D, et al. The gut-muscle axis in older subjects with low muscle mass and performance: a proof of concept study exploring fecal microbiota composition and function with shotgun metagenomics sequencing. Int J Mol Sci. (2020) 21:8946. doi: 10.3390/ijms21238946
140. Saint-Criq V, Lugo-Villarino G, Thomas M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res Rev. (2021) 66:101235. doi: 10.1016/j.arr.2020.101235
141. Kreznar JH, Keller MP, Traeger LL, Rabaglia ME, Schueler KL, Stapleton DS, et al. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Rep. (2017) 18:1739–50. doi: 10.1016/j.celrep.2017.01.062
142. Giron M, Thomas M, Dardevet D, Chassard C, Savary-Auzeloux I. Gut microbes and muscle function: can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle. (2022) 13:1460–76. doi: 10.1002/jcsm.12964
143. Picca A, Fanelli F, Calvani R, Mulè G, Pesce V, Sisto A, et al. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators Inflamm. (2018) 2018:7026198. doi: 10.1155/2018/7026198
144. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. (2001) 79:243–53. doi: 10.1007/s001090100226
145. Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. (2021) 12:578386. doi: 10.3389/fimmu.2021.578386
146. Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. (2019) 11:eaan5662. doi: 10.1126/scitranslmed.aan5662
147. Nay K, Jollet M, Goustard B, Baati N, Vernus B, Pontones M, et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab. (2019) 317:E158–e71. doi: 10.1152/ajpendo.00521.2018
148. Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, et al. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol Endocrinol Metab. (2019) 316:E956–e66. doi: 10.1152/ajpendo.00510.2018
149. Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol. (2019) 127:110722. doi: 10.1016/j.exger.2019.110722
150. van Krimpen SJ, Jansen FAC, Ottenheim VL, Belzer C, van der Ende M, van Norren K. The effects of pro-, pre-, and synbiotics on muscle wasting, a systematic review-gut permeability as potential treatment target. Nutrients. (2021) 13:1115. doi: 10.3390/nu13041115
151. Karim A, Muhammad T, Shahid Iqbal M, Qaisar R. A multistrain probiotic improves handgrip strength and functional capacity in patients with COPD: a randomized controlled trial. Arch Gerontol Geriatr. (2022) 102:104721. doi: 10.1016/j.archger.2022.104721
152. Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. (2019) 11:1633. doi: 10.3390/nu11071633
153. Robinson S, Granic A, Sayer AA. Micronutrients and sarcopenia: current perspectives. Proc Nutr Soc. (2021) 80:311–8. doi: 10.1017/S0029665121001956
154. Santiago ECS, Roriz AKC, Ramos LB, Ferreira AJF, Oliveira CC, Gomes-Neto M. Comparison of calorie and nutrient intake among elderly with and without sarcopenia: a systematic review and meta-analysis. Nutr Rev. (2021) 79:1338–52. doi: 10.1093/nutrit/nuaa145
155. van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. (2018) 19:6–11. doi: 10.1016/j.jamda.2017.05.026
156. Welch AA, Kelaiditi E, Jennings A, Steves CJ, Spector TD, MacGregor A. Dietary magnesium is positively associated with skeletal muscle power and indices of muscle mass and may attenuate the association between circulating C-reactive protein and muscle mass in women. J Bone Miner Res. (2016) 31:317–25. doi: 10.1002/jbmr.2692
157. Welch AA, Skinner J, Hickson M. Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: cross-sectional findings from the UK BIOBANK COHORT. Nutrients. (2017) 9:1189. doi: 10.3390/nu9111189
158. Liu Y, Wang Q, Zhang Z, Fu R, Zhou T, Long C, et al. Magnesium supplementation enhances mTOR signalling to facilitate myogenic differentiation and improve aged muscle performance. Bone. (2021) 146:115886. doi: 10.1016/j.bone.2021.115886
159. Cameron D, Welch AA, Adelnia F, Bergeron CM, Reiter DA, Dominguez LJ, et al. Age and muscle function are more closely associated with intracellular magnesium, as assessed by (31)P magnetic resonance spectroscopy, than with serum magnesium. Front Physiol. (2019) 10:1454. doi: 10.3389/fphys.2019.01454
160. Arias-Fernández L, Struijk EA, Caballero FF, Ortolá R, García-Esquinas E, Rodríguez-Artalejo F, et al. Prospective association between dietary magnesium intake and physical performance in older women and men. Eur J Nutr. (2022) 61:2365–73. doi: 10.1007/s00394-022-02808-z
161. Hayhoe RPG, Lentjes MAH, Mulligan AA, Luben RN, Khaw KT, Welch AA. Cross-sectional associations of dietary and circulating magnesium with skeletal muscle mass in the EPIC-Norfolk cohort. Clin Nutr. (2019) 38:317–23. doi: 10.1016/j.clnu.2018.01.014
162. Gedmantaite A, Celis-Morales CA, Ho F, Pell JP, Ratkevicius A, Gray SR. Associations between diet and handgrip strength: a cross-sectional study from UK Biobank. Mech Ageing Dev. (2020) 189:111269. doi: 10.1016/j.mad.2020.111269
163. Chariot P, Bignani O. Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve. (2003) 27:662–8. doi: 10.1002/mus.10304
164. Bodnár D, Ruzsnavszky O, Oláh T, Dienes B, Balatoni I, Ungvári É, et al. Dietary selenium augments sarcoplasmic calcium release and mechanical performance in mice. Nutr Metab. (2016) 13:76. doi: 10.1186/s12986-016-0134-6
165. García-Esquinas E, Carrasco-Rios M, Ortolá R, Sotos Prieto M, Pérez-Gómez B, Gutiérrez-González E, et al. Selenium and impaired physical function in US and Spanish older adults. Redox Biol. (2021) 38:101819. doi: 10.1016/j.redox.2020.101819
166. Passerieux E, Hayot M, Jaussent A, Carnac G, Gouzi F, Pillard F, et al. Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: a double-blind randomized controlled clinical trial. Free Radic Biol Med. (2015) 81:158–69. doi: 10.1016/j.freeradbiomed.2014.09.014
167. Perri G, Mendonça N, Jagger C, Walsh J, Eastell R, Mathers JC, et al. Dietary selenium intakes and musculoskeletal function in very old adults: analysis of the Newcastle 85+ study. Nutrients. (2020) 12:2068. doi: 10.3390/nu12072068
168. Seo MH, Kim MK, Park SE, Rhee EJ, Park CY, Lee WY, et al. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2009. Endocr J. (2013) 60:679–86. doi: 10.1507/endocrj.EJ12-0395
169. Saha S, Goswami R, Ramakrishnan L, Vishnubhatla S, Mahtab S, Kar P, et al. Vitamin D and calcium supplementation, skeletal muscle strength and serum testosterone in young healthy adult males: randomized control trial. Clin Endocrinol. (2018) 88:217–26. doi: 10.1111/cen.13507
170. Khatri M, Naughton RJ, Clifford T, Harper LD, Corr L. The effects of collagen peptide supplementation on body composition, collagen synthesis, and recovery from joint injury and exercise: a systematic review. Amino Acids. (2021) 53:1493–506. doi: 10.1007/s00726-021-03072-x
171. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. (2010) 123(Pt 24):4195–200. doi: 10.1242/jcs.023820
172. León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Álvarez G. Hydrolyzed collagen-sources and applications. Molecules. (2019) 24:4031. doi: 10.3390/molecules24224031
173. Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr. (2017) 105:136–43. doi: 10.3945/ajcn.116.138594
174. Holwerda AM, van Loon LJC. The impact of collagen protein ingestion on musculoskeletal connective tissue remodeling: a narrative review. Nutr Rev. (2022) 80:1497–514. doi: 10.1093/nutrit/nuab083
175. Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH, Bierau J, Verdijk LB, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. (2018) 50:1685–95. doi: 10.1007/s00726-018-2640-5
176. Paxton JZ, Grover LM, Baar K. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A. (2010) 16:3515–25. doi: 10.1089/ten.tea.2010.0039
177. Vieira CP, De Oliveira LP, Da Ré Guerra F, Dos Santos De Almeida M, Marcondes MC, Pimentel ER. Glycine improves biochemical and biomechanical properties following inflammation of the Achilles tendon. Anat Rec. (2015) 298:538–45. doi: 10.1002/ar.23041
178. Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. (2005) 53:6531–6. doi: 10.1021/jf050206p
179. Edgar S, Hopley B, Genovese L, Sibilla S, Laight D, Shute J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci Rep. (2018) 8:10474. doi: 10.1038/s41598-018-28492-w
180. Zdzieblik D, Oesser S, Baumstark MW, Gollhofer A, König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr. (2015) 114:1237–45. doi: 10.1017/S0007114515002810
181. Oertzen-Hagemann V, Kirmse M, Eggers B, Pfeiffer K, Marcus K, de Marées M, et al. Effects of 12 weeks of hypertrophy resistance exercise training combined with collagen peptide supplementation on the skeletal muscle proteome in recreationally active men. Nutrients. (2019) 11:1072. doi: 10.3390/nu11051072
182. Oikawa SY, McGlory C, D'Souza LK, Morgan AK, Saddler NI, Baker SK, et al. A randomized controlled trial of the impact of protein supplementation on leg lean mass and integrated muscle protein synthesis during inactivity and energy restriction in older persons. Am J Clin Nutr. (2018) 108:1060–8. doi: 10.1093/ajcn/nqy193
183. Oikawa SY, Kamal MJ, Webb EK, McGlory C, Baker SK, Phillips SM. Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: a randomized controlled trial. Am J Clin Nutr. (2020) 111:708–18. doi: 10.1093/ajcn/nqz332
184. Oikawa SY, Macinnis MJ, Tripp TR, McGlory C, Baker SK, Phillips SM. Lactalbumin, not collagen, augments muscle protein synthesis with aerobic exercise. Med Sci Sports Exerc. (2020) 52:1394–403. doi: 10.1249/MSS.0000000000002253
185. Frank J, Fukagawa NK, Bilia AR, Johnson EJ, Kwon O, Prakash V, et al. Terms and nomenclature used for plant-derived components in nutrition and related research: efforts toward harmonization. Nutr Rev. (2020) 78:451–8. doi: 10.1093/nutrit/nuz081
186. Colomer R, Sarrats A, Lupu R, Puig T. Natural polyphenols and their synthetic analogs as emerging anticancer agents. Curr Drug Targets. (2017) 18:147–59. doi: 10.2174/1389450117666160112113930
187. Scalbert A, Morand C, Manach C, Rémésy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. (2002) 56:276–82. doi: 10.1016/S0753-3322(02)00205-6
188. Francaux M, Deldicque L. Using polyphenol derivatives to prevent muscle wasting. Curr Opin Clin Nutr Metab Care. (2018) 21:159–63. doi: 10.1097/MCO.0000000000000455
189. Aslan A, Beyaz S, Gok O, Erman O. The effect of ellagic acid on caspase-3/bcl-2/Nrf-2/NF-kB/TNF-α /COX-2 gene expression product apoptosis pathway: a new approach for muscle damage therapy. Mol Biol Rep. (2020) 47:2573–82. doi: 10.1007/s11033-020-05340-7
190. Zhang M, Tang J, Li Y, Xie Y, Shan H, Chen M, et al. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1α/SIRT3 pathway involved. Chem Biol Interact. (2017) 277:168–75. doi: 10.1016/j.cbi.2017.09.018
191. Mukai R, Horikawa H, Lin PY, Tsukumo N, Nikawa T, Kawamura T, et al. 8-Prenylnaringenin promotes recovery from immobilization-induced disuse muscle atrophy through activation of the Akt phosphorylation pathway in mice. Am J Physiol Regul Integr Comp Physiol. (2016) 311:R1022–r31. doi: 10.1152/ajpregu.00521.2015
192. Nikawa T, Ulla A, Sakakibara I. Polyphenols and their effects on muscle atrophy and muscle health. Molecules. (2021) 26:4887. doi: 10.3390/molecules26164887
193. Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. (2011) 25:3646–60. doi: 10.1096/fj.10-177295
194. Woodman KG, Coles CA, Lamandé SR, White JD. Resveratrol promotes hypertrophy in wildtype skeletal muscle and reduces muscle necrosis and gene expression of inflammatory markers in Mdx mice. Molecules. (2021) 26:853. doi: 10.3390/molecules26040853
195. Alway SE, McCrory JL, Kearcher K, Vickers A, Frear B, Gilleland DL, et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J Gerontol A Biol Sci Med Sci. (2017) 72:1595–606. doi: 10.1093/gerona/glx089
196. Harper SA, Bassler JR, Peramsetty S, Yang Y, Roberts LM, Drummer D, et al. Resveratrol and exercise combined to treat functional limitations in late life: a pilot randomized controlled trial. Exp Gerontol. (2021) 143:111111. doi: 10.1016/j.exger.2020.111111
197. McDermott MM, Leeuwenburgh C, Guralnik JM, Tian L, Sufit R, Zhao L, et al. Effect of resveratrol on walking performance in older people with peripheral artery disease: the RESTORE randomized clinical trial. JAMA Cardiol. (2017) 2:902–7. doi: 10.1001/jamacardio.2017.0538
198. Beijers RJ, Gosker HR, Sanders KJ, de Theije C, Kelders M, Clarke G, et al. Resveratrol and metabolic health in COPD: a proof-of-concept randomized controlled trial. Clin Nutr. (2020) 39:2989–97. doi: 10.1016/j.clnu.2020.01.002
199. Rickards L, Lynn A, Harrop D, Barker ME, Russell M, Ranchordas MK. Effect of polyphenol-rich foods, juices, and concentrates on recovery from exercise induced muscle damage: a systematic review and meta-analysis. Nutrients. (2021) 13:2988. doi: 10.3390/nu13092988
200. Carey CC, Lucey A, Doyle L. Flavonoid containing polyphenol consumption and recovery from exercise-induced muscle damage: a systematic review and meta-analysis. Sports Med. (2021) 51:1293–316. doi: 10.1007/s40279-021-01440-x
201. Fernández-Lázaro D, Mielgo-Ayuso J, Seco Calvo J, Córdova Martínez A, Caballero García A, Fernandez-Lazaro CI. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: a systematic review. Nutrients. (2020) 12:501. doi: 10.3390/nu12020501
202. Martinez-Negrin G, Acton JP, Cocksedge SP, Bailey SJ, Clifford T. The effect of dietary (poly)phenols on exercise-induced physiological adaptations: a systematic review and meta-analysis of human intervention trials. Crit Rev Food Sci Nutr. (2022) 62:2872–87. doi: 10.1080/10408398.2020.1860898
203. Jackman SR, Brook MS, Pulsford RM, Cockcroft EJ, Campbell MI, Rankin D, et al. Tart cherry concentrate does not enhance muscle protein synthesis response to exercise and protein in healthy older men. Exp Gerontol. (2018) 110:202–8. doi: 10.1016/j.exger.2018.06.007
Keywords: sarcopenia, nutritional supplements, protein, leucine, β-hydroxy-β-methylbutyric acid, antioxidant, omega-3 fatty acids, creatine
Citation: Liu S, Zhang L and Li S (2023) Advances in nutritional supplementation for sarcopenia management. Front. Nutr. 10:1189522. doi: 10.3389/fnut.2023.1189522
Received: 19 March 2023; Accepted: 20 June 2023;
Published: 10 July 2023.
Edited by:
Nathan A. Berger, Case Western Reserve University, United StatesReviewed by:
Sousana Konstantinos Papadopoulou, International Hellenic University, GreeceRiccardo Caccialanza, San Matteo Hospital Foundation (IRCCS), Italy
Copyright © 2023 Liu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangqing Li, bHNxaHhqa0AxMjYuY29t
†These authors have contributed equally to this work
 Simin Liu1†
Simin Liu1† Lin Zhang
Lin Zhang Shuangqing Li
Shuangqing Li