
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Nutr., 25 July 2023
Sec. Nutrition and Microbes
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1186927
This article is part of the Research TopicBeer - From Tradition to InnovationView all 5 articles
As a long-established fermented beverage, beer is rich in many essential amino acids, vitamins, trace elements, and bioactive substances that are involved in the regulation of many human physiological functions. The polyphenols in the malt and hops of beer are also important active compounds that interact in both directions with the gut microbiome. This review summarizes the mechanisms by which polyphenols, fiber, and other beneficial components of beer are fermentatively broken down by the intestinal microbiome to initiate the mucosal immune barrier and thus participate in immune regulation. Beer degradation products have anti-inflammatory, anticoagulant, antioxidant, and glucolipid metabolism-modulating potential. We have categorized and summarized reported data on changes in disease indicators and in vivo gut microbiota abundance following alcoholic and non-alcoholic beer consumption. The positive effects of bioactive substances in beer in cancer prevention, reduction of cardiovascular events, and modulation of metabolic syndrome make it one of the candidates for microecological modulators.
Beer, also known as “liquid bread,” is the oldest alcoholic beverage in human history, recorded by the Babylonians as early as 6,000 BC using clay tablets. At the same time, beer is the most widely produced and consumed beverage globally. It is second only to water and tea in terms of total consumption. Archeological research has found evidence of beer consumption in China dating back as far as 9,000 years (1). In response to consumer demand for new taste, smell, and visual stimuli, and for a variety of beer types, including non-alcoholic beers, beers produced by producers who add fruits, spices, vegetables, and natural foods to the fermentation process are becoming very popular around the world (2). At the same time, the health properties of beer that cannot be ignored are also gaining attention. It has been reported that beer consumption has a regulatory effect on various physiological functions of the human body. Moderate consumption of beer helps in preventing arteriosclerosis (3) and heart disease (4), inhibits cancer (5), and improves blood circulation and immune function (6). Beer has also been shown to have antioxidant and anti-aging effects (7), promote estrogen production (8), reduce radiation damage (9), and help prevent cardiovascular events (10).
Beer is brewed from malt, hops, yeast, and brewing water as well as starchy and sugary auxiliary ingredients through liquid pasting, saccharification, and liquid fermentation (11). Like yogurt, wine, cider, and many other fermented beverages, beer is rich in many nutrients. It contains many essential amino acids, vitamins, trace elements, and biologically active substances such as polyphenols and flavonoids. Beer also contains many minerals such as calcium, magnesium, zinc, copper, selenium, and iron. Beta-glucan and arabinose-oligosaccharides stored in malting barley also make beer a source of dietary fiber (12). In addition, the polyphenols in the malt and hops of beer are essential active compounds with antioxidant activity that act synergistically with dietary components. The amino acids, vitamins, inorganic salts, and low molecular sugars in beer are quickly digested and absorbed in the small intestine. Polyphenols that are not hydrolyzed by the small intestine reach the colon and are metabolized by the body’s microbiota (13).
Nutrients in the host’s diet can influence the growth of the gut microbiome. In the meantime, specific dietary components can stimulate the gut microbiome to secrete metabolites that affect the host’s physiological status. This ‘super-organ’ residing in the gut is an essential medium for the body’s intake of nutrients. It is involved in the absorption and metabolism of nutrients, strengthens the integrity of the gut, prevents the spread of pathogens, promotes immune tolerance to antigens, and regulates host immunity, thereby directly influencing human health and disease (14).
This review focuses on the potential mechanisms by which polyphenols and other beneficial components in beer exert prebiotic effects, interact with the gut microbiome, and participate in immune regulation, thus exploring beer’s immune utility and application prospects as a health food.
Drinking alcohol, which is a regular habit for many people, is controversial in terms of its effects on human health. It is well known that beer, as an alcoholic beverage, can induce serious tissue damage and organ lesions such as increased intestinal inflammation, endotoxemia, and alcoholic liver disease if consumed inappropriately or in excess (15). It can also amplify the damage caused by cardiac insufficiency and depression in patients, adolescents, and pregnant women (16).
However, when alcohol consumption is controlled within safe limits, the nutrients in beer and the combined effects on the gut microbiome have a positive effect on the regulation of human immune function (15) (Figure 1). Beer reduces leukocyte adhesion molecules and biological risk markers of inflammation and increases plasma antioxidant capacity, whereas ethanol alone does not (17, 18). We will describe the effect of beer intake on organismal immunity through the aspect of conventional, and non-alcoholic beer intake in humans, respectively. Studies on the relationship between beer and immunity can be found in the supplementary data (Table 1).
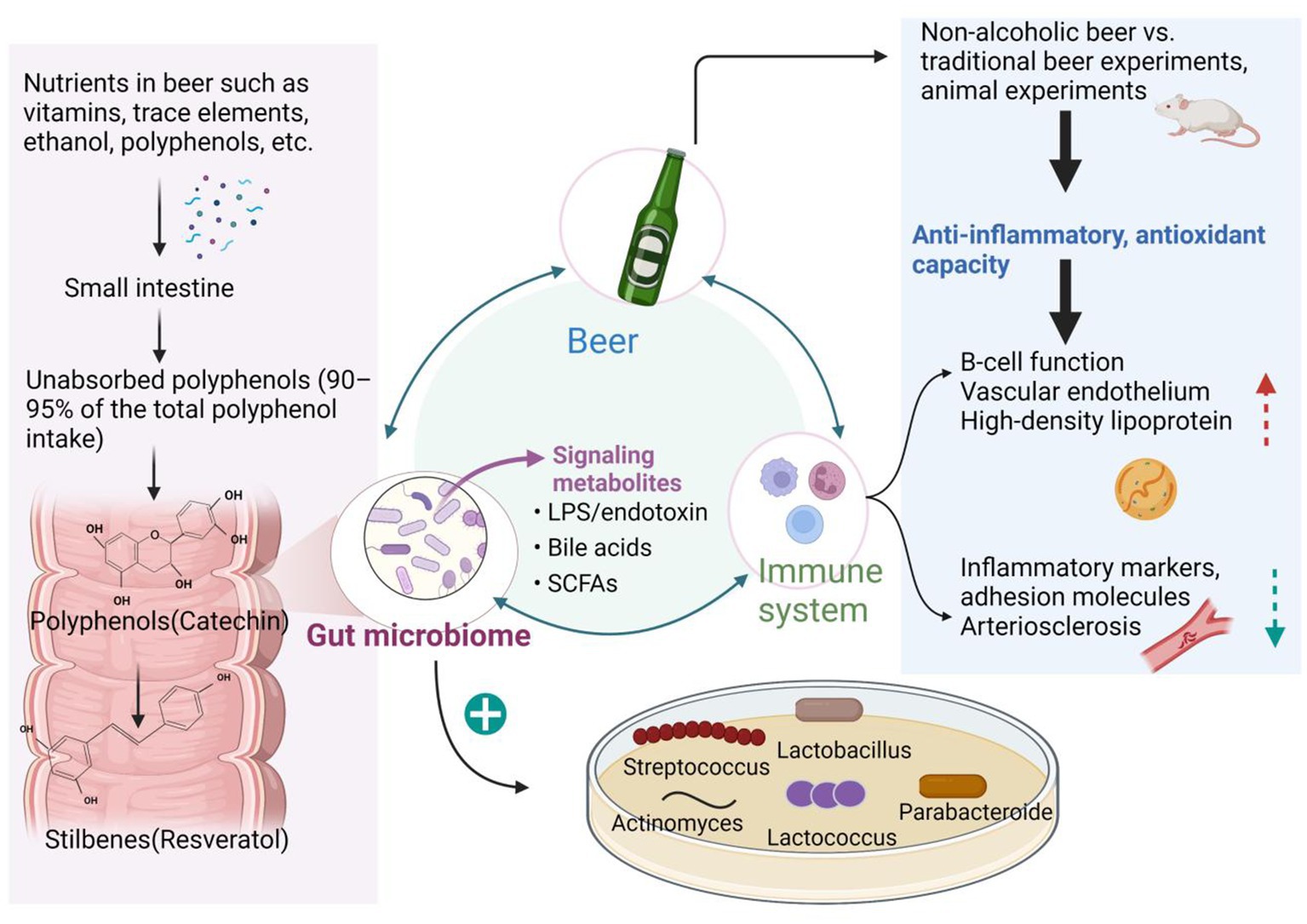
Figure 1. Relationship between beer, immunity, and the gut microbiome. When beer is consumed in moderation, the phenols and other nutrients it contains are fermented and broken down by the microbial community that resides in the outer mucosal layer of the gut. This miraculous digestive process produces a wealth of metabolites that, through the interaction of multiple microbes in the inner mucus, in turn, promote the growth of beneficial flora that exert a range of anti-inflammatory, antioxidant, and immunomodulatory effects, creating a virtuous cycle. This gives beer, a fermented beverage, a place in the improvement of cardiovascular disease, obesity, diabetes, neurodegenerative diseases, cancer, non-alcoholic fatty liver disease, and the prevention of infections.
Epidemiological studies have shown an association between moderate intake of fermented beverages and lower levels of inflammatory biomarkers, which is mainly attributed to the polyphenols, antioxidants, vitamins, and alcohol that are present in the beverages (33, 34). The polyphenolic component of beer interferes with pro-inflammatory pathways and induces inhibition of transcription factors or macromolecular complexes, thus reducing the synthesis and release of pro-inflammatory cytokines such as interleukins (IL) IL-1β, IL-6, IL-8, and tumor necrosis factor (TNF) (35). Studies have shown that moderate beer consumption increased IgG, IgM, and IgA concentrations as well as IL-2, IL-4, IL-10, and interferon (IFN)-γ production, resulting in decreased IFN-γ/IL-10 ratio in men and women, suggesting that moderate beer intake can produce the immunomodulatory effect (36).
The stimulation effect of low concentrations of ethanol on cellular immune responses was noted in the delayed cutaneous hypersensitivity-like responses evaluation (37). It has been noted that the intake of moderate amounts of alcohol is associated with a reduced risk of developing rhinovirus and the common cold (38). A complete immune response cannot be achieved without phagocytosis and the oxidative burst function of leukocytes. It has been noted that after 30 days of moderate beer consumption, there was a significant increase in the oxidative capacity of both neutrophils and monocytes (39). It can be speculated that moderate beer consumption may be associated with an increase in first-line immunity. In addition to those results, an in vitro study suggested that the beneficial effects on health may be related to the ability of beer to interfere with the pro-inflammatory cytokine cascade (40). It has even been suggested that this beverage has a dual anti-inflammatory effect of increasing IL-10 and attenuating the monocyte inflammatory response (41).
The abundant polyphenols in beer can exhibit a wide range of anti-inflammatory, antioxidant, and anti-aggregation activities mediated by the gut microbiome (36, 42). In mouse models of stress and depression, polyphenols can reduce the neuroinflammatory state by inhibiting the firing of neuronal cells (43).
Xanthohumol (XN) in beer inhibits the activity of inducible nitric oxide synthase and thus exerts anti-inflammatory effects. In addition, XN and humulone can produce anti-inflammatory effects by inhibiting TNF-α-induced endogenous synthesis of prostaglandin E2 by cyclooxygenase 2 (20, 44). It has been observed that the ingestion of XN via a natural beer matrix improves the wound-healing process in rats. This suggests that XN plays a role in reducing inflammation and oxidative stress, and in promoting angiogenesis (45).
Animal model studies have shown that isohumulone, a bitter substance in hops, can inhibit atherosclerosis. When ApoE-deficient mice (suffering from significant hyperlipidemia and progressive atherosclerotic lesions) were treated with isohumulone for 10 weeks, the area of atherosclerotic lesions in the aortic arch and aortic valve was significantly smaller than in controls. A significant decrease in serum IL-6 levels was also observed, suggesting that isohumulone slows the progression of atherosclerosis by inhibiting the inflammatory response during the atherosclerotic process (46). In addition, studies in hamsters noted that consumption of light or dark beer significantly inhibited atherosclerosis and reduced cholesterol and triglyceride levels (20). This suggests that moderate consumption of beer induces positive biochemical changes in blood composition that contribute to improving and preventing atherosclerosis.
Combining these results of studies in humans and animals, there is a consensus that moderate beer consumption has a beneficial effect on the immune system compared to states of alcohol abuse or abstinence (47, 48) (Figure 2).
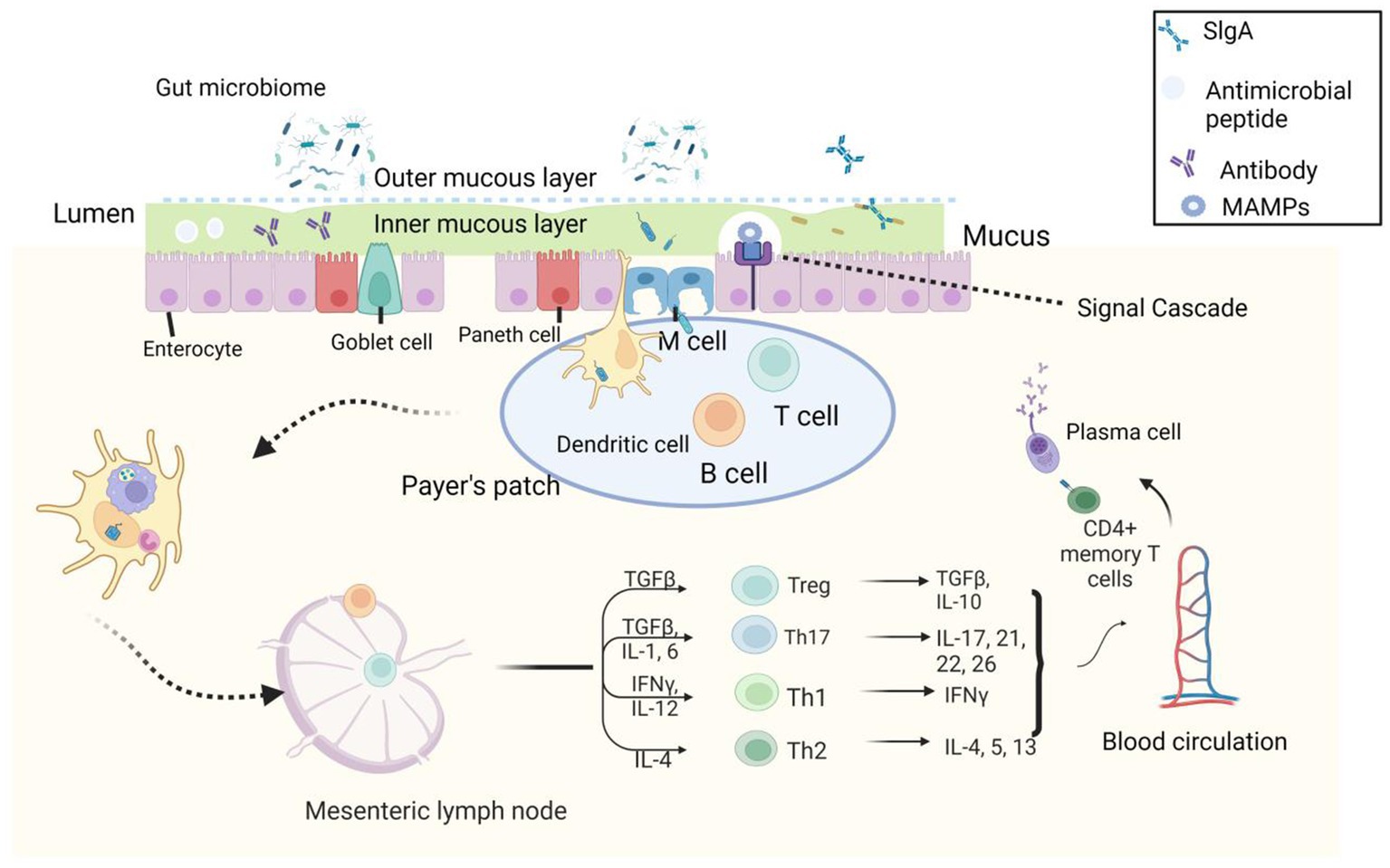
Figure 2. Microbial-associated molecular patterns (MAMPs) produced by commensal microorganisms stimulate Pattern recognition receptors (PRRs) in epithelial cells and induce the production of antimicrobial peptides. A few microorganisms are endocytosed into Peyer’s patches by M and DC cells, affecting the production of pro-inflammatory cytokines by intestinal epithelial cells (IECs) and dendritic cells (DCs) and macrophages present in the lamina propria (GALT) and Peyer’s patches. These cytokines such as thymic stromal lymphopoietin (TSLP), transforming growth factor (TGF), and interleukin-10 (IL-10) further recruit T and B cells, drive the production of mature, isolated lymphoid follicles, and release plasma cells that produce lgA. lgA binds to plgR to form a dimer that reaches the inner mucosa from the lamina propria after cleavage of plgR protein to release lgA, allowing it to exert its antimicrobial effect. Antigenic components obtained by a transmembrane sampling of DC cells can drain through the lymphatics to the mesenteric lymph nodes, inducing expansion of T cells and regulation of Th-1, Th-2, and Th-3 cell numbers (49–56).
Several studies have shown that moderate beer intake can reduce the expression of inflammasome signaling pathways in human macrophages. In such studies, the normal beer groups had significantly reduced intracellular protein levels of pro-IL-1β in primed macrophages, and the release of cleaved IL-1β protein was blocked. Transcription of pro-inflammatory interleukins such as IL-1β and TNF-α was also significantly reduced in the non-alcoholic beer group (57). That is, non-alcoholic beer may also enhance the immune response, leading to a more effective defense.
A 45-day study of postmenopausal women consuming 500 mL of non-alcoholic beer per day found that intake of non-alcoholic beer lowered cholesterol levels in subjects with blood cholesterol greater than 240 mg/dL (58), which supports the role of long-term non-alcoholic beer consumption in combating mild chronic inflammation and preventing metabolic disorders. Similarly, supplementing mothers’ diets with non-alcoholic beer reduced oxidative damage in lactating mothers, thereby increasing the antioxidant capacity of breast milk (32).
In several studies in animals and humans, physiological stress resulting from prolonged high-intensity exercise was found to be associated with transient inflammation, immune dysfunction, and increased incidence of upper respiratory tract disease compared to moderate physical activity (59–63). In a study on healthy male runners, subjects consumed non-alcoholic beer daily for 3 weeks before and 2 weeks after the marathon. There was a reduction in IL-6 and total white blood cell count, and upper respiratory tract disease 24 h post-race. This positive result may be attributed to the polyphenolic compounds in non-alcoholic beer, which have antioxidant, anti-pathogenic, and anti-inflammatory properties (33).
The consumption of alcohol, as part of most people’s habits, has been controversial in terms of its effects on human health. If consumed inappropriately or in excess, it can trigger toxic reactions and social health burdens.
Alcohol causes dysbiosis of the gut microbiota in rodents and humans, as well as decreased production of beneficial metabolites such as short-chain fatty acids (SCFAs) (64, 65). Alcohol contributes to increased intestinal permeability and the transfer of bacterial products from Gram-negative microorganisms by enhancing oxidative stress in mitochondria (66) and disrupting the tight junctions of intestinal epithelial cells (67). The reflux of these toxic substances into the liver through the portal vein induces hepatic pathological damage such as alcoholic liver disease (ALD) (68). In addition, ethanol and its toxic metabolite acetaldehyde trigger intestinal barrier dysfunction, which can induce endotoxemia and systemic inflammation in the brain, intestine, and pancreas (69).
However, when alcohol consumption is controlled within safe limits, the combined effects of alcohol and other component metabolism on the intestinal flora deserve a more comprehensive analysis (70).
It is well known that the gut microbiome uses diet as a bridge to form a complex and dynamic mutually beneficial symbiosis with the host. At the same time, the intestinal mucosa and its vast micro-ecosystem together form a complex and dynamic immune barrier that participates in disease prevention and regulation of immune function through the interaction of microorganisms with the immune system (71).
Most of the water, vitamins, and other low molecular weight substances in beer are absorbed in the stomach and small intestine. Polyphenols and other important substances reach the colon, where they meet the intestinal microbiota and are eventually transformed (72, 73). Their metabolites inhibit pathogenic bacteria, stimulate the proliferation and activity of healthy flora such as Lactobacilli and Bifidobacteria, and regulate the intestinal microbiota (74). In other words, the polyphenols in beer have prebiotic properties (75).
Studies have shown that phenolic substrates and the resulting aromatic metabolites supplied to intestinal bacteria through dietary intake may in turn cause fluctuations in microbial community composition through selective prebiotic effects and modulation of antimicrobial activity against enteropathogenic bacteria (76–78).
It has been noted that aromatic metabolites produced by the metabolism of epicatechin, catechin, 3-O-methylmalonic acid, and caffeic acid in beer significantly inhibit the growth of pathogenic bacteria such as Clostridium perfringens and Clostridium difficile without affecting the growth of Bifidobacterium (75). Flavonols in beer induce the growth of Lactobacillus and Bifidobacterium and may decrease plasma C-reactive protein concentrations, suggesting that beer may provide immunological benefits (79). In the human intestine, six species of Streptococcus are associated with immunomodulatory functions (80). An increase in the abundance of flora with immunomodulatory functions has been documented with the consumption of non-alcoholic and alcoholic beers: including Streptococcus, Actinomyces, Veillonella, Bacillus, Lactococcus, and Weissella. Among them, Actinomyces has important functions in the regulation of intestinal permeability, the immune system, metabolism, and the gut-brain axis. In an article on the effects of moderate beer consumption on human gut health (71), it was found that 30 days of non-alcoholic beer daily consumption resulted in increased numbers of Streptococcus spp., Bacillus spp., and Paramecium spp. as well as changes in glucose metabolism, and lipopolysaccharide and phenylalanine synthesis in the intestine. Of interest is the fact that the phenylalanine synthesis pathway is associated with resveratrol production (80) which may be beneficial in the treatment of cancer, neurological diseases, cardiovascular diseases, and nonalcoholic fatty liver disease (81).
It is known that uropathogens can form biofilms that protect bacteria from the host immune responses and antimicrobial treatments, making the treatment of urinary tract infections a challenge. A study isolated Lactobacillus fermentum TIU19 from beer and showed that it may act as a potential probiotic against biofilm formation and inhibit the activity of the multi-drug resistant uropathogenic E. coli and E. faecalis (82). A similar study was conducted on Vibrio vulnificus DU14 isolated from traditional fermented rice beer from the Sivsagar district of Assam, which also has probiotic potential (83).
Intestinal microbiota act in coordination with the intestinal barrier, protecting the body from disease and stimulating the immune system (84). The production of short-chain fatty acids after polyphenol ingestion improves intestinal permeability, thus reducing intestinal inflammation and endotoxemia (85). In addition, short-chain fatty acids are key substrates of the cross-feeding system, which is a component of the intestinal flora. For example, cross-feeding occurs between Bifidobacterium longum and Eubacterium rectale, and both strains consume arabinoxylan, which is the main source of dietary fiber in beer. However, Bifidobacterium longum was additionally stimulated by consuming monosaccharides released by extracellular degradation of arabinoxylan by Eubacterium rectale, resulting in a reciprocal cross-feeding effect. In addition, Faecalibacterium prausnitzii can produce butyric acid from lactic acid produced by Bifidobacteria. In the presence of arabinoxylan, Bifidobacteria and butyric acid-producing colonic bacteria (Clostridium praecalibacterium, Eubacterium rectum, and Roseburia spp.) were stimulated simultaneously, leading to a significant increase in butyric acid production (13).
Ferulic acid is the most abundant polyphenol in beer (86). A study in mice showed that ferulic acid altered the composition of the intestinal microbiota by modulating the ratio of thick-walled to bacterial flora, making it closely associated with specific intestinal microbiota and the genetic regulation of triglyceride and total cholesterol metabolism in nonalcoholic fatty liver disease (87). Recent studies have reported that the gut microbiome can convert ellagic acid to urolithin (88). Urolithins can cross the blood–brain barrier (89) and may affect mice with Alzheimer’s disease by reducing neuronal inflammation (81). In addition, we found different conclusions in experiments using barley beer waste pellets fed to rats (90) and in experiments analyzing fecal samples from abstainers and moderate drinkers (13). Both studies showed that the intake of beer modulates the levels of butyric acid, which then interacts directly with the host’s immune system and reduces the levels of inflammatory markers (14).
We have reason to believe that beer, mediated by the gut microbiome (20), exerts beneficial immunomodulatory effects in humans. Studies on the relationship between beer, the gut microbiome, and immunity can be found in the supplementary data (Table 2) (96, 97).
Beer is a long-established alcoholic beverage that is rich in a variety of nutrients and micronutrients. This review focuses specifically on the interactions and mechanisms between beer and the gut microbiome in the regulation of body immunity.
The risk of death is lower in light and moderate drinkers and increased in heavy drinkers. Previous evidence from the literature on animal experiments (87–90, 96, 97), and human experiments (33–39) suggests that low or moderate beer consumption, with or without alcohol, shows positive health effects by stimulating the development of a healthy microbiota. In particular, it has been shown to be effective in reducing the incidence of coronary heart disease, Alzheimer’s disease, metabolic syndrome, cancer, and Type-2 diabetes. Disease-related indicators have also been noted including reductions in fibrinogen (anticoagulation) and C-reactive protein (anti-inflammatory); and increases in HDL (anti-atherosclerotic) and adiponectin (improves glucose homeostasis) levels (5).
The gut microbiome has long played an important role in human health. This review demonstrates the environment-diet-microbial-host interactions through Figure 1. It is clear from the figure that when beer is consumed in moderation, the phenols and other nutrients it contains are fermented and broken down by the microbial community that resides in the outer mucosal layer of the gut. This miraculous digestive process produces a large number of metabolites that, through the interaction of multiple microorganisms in the inner mucosa, in turn, promote changes in the abundance of beneficial flora, exerting a range of anti-inflammatory, antioxidant, and immunomodulatory effects. In this virtuous cycle, the gut microbiota provides a platform for information exchange. We demonstrate the mechanism by which these fermentation breakdown products function on this platform in Figure 2 (49–56): fermentation products promote the growth of beneficial flora, and microbial-associated molecular patterns (MAMPs) produced by these microbes stimulate pattern recognition receptors (PRRs) in epithelial cells and induce the production of antimicrobial peptides. A few are endocytosed into Peyer’s patches by M and dendritic cells (DCs) cells, which produce pro-inflammatory cytokines or chemokines (thymic stromal lymphopoietin (TSLP), transforming growth factor (TGF) and interleukin-10 (IL-10)) that act on effector cells and induce maturation of lymphoid follicles and release of lgA. antigenic components acquired by DC cells can drain through the lymphatic vessels to the mesenteric lymph nodes, induce the expansion of T cells, regulate the number of Th-1, Th-2, and Th-3 cells, and initiate the mucosal immune barrier.
Evidence from the literature (16, 19–32) suggests that the immunomodulatory effects of beer are attributed to the three components it contains: polyphenols, fiber, and ethanol. Due to the conversion of beer substrates, the formation of bioactive end products, and the presence of microorganisms, some of its components exert “similar” or even greater effects than probiotics. Polyphenols and fiber show the potential to enhance the development of a healthy gut microbiome through probiotic mechanisms, dominated by sugar-catabolizing, short-chain fatty acid-producing bacteria (Bifidobacterium spp. and Saccharomyces spp.). Polyphenols and picric acid constitute the antioxidants of beer, exerting a counteracting effect against intestinal flora dysbiosis and local anti-inflammatory effects. Beer contains dietary fibers such as non-starchy, non-digestible carbohydrates (β-glucan, arabinoxylan, mannose, fructose polymers, etc.) that, through fermentation, establish contact with the intestinal microbiota and act as nutritional substrates to stimulate the development of the gut microbiome. Most of all these reported studies assessed the effect of the above substances on microbiota by monitoring changes in SCFAs. In addition, the acetate produced by alcohol may stimulate energy metabolism and prevent cardiovascular disease.
To date, the mechanisms of interaction between beer and the gut microbiome in immunomodulation have not been adequately studied, and the available results are based on inference rather than quantification of specific effects. Low or non-alcoholic beers are good candidates for functional foods. Health beers made by fortifying them with bioactive substances such as fiber, antioxidants, and probiotics would provide health benefits to consumers. Whether beer can be used in the future as a micro-ecological regulator or even as an alternative therapy for chronic diseases such as hypertension, diabetes, and obesity is a question that deserves further research.
HL: conceptualization and modification. SZ: manuscript writing, figure drawing, and modification. SJ: manuscript writing. CZ and SH: modification. All authors contributed to the article and approved the submitted version.
This study was supported by the Open Research Fund of State Key Laboratory of Biological Fermentation Engineering of Beer, under grant no. K202101.
We also thank Wendy Hempstock, Ph.D. from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
CZ and SH were employed by Tsingtao Brewery Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang, J, Jiang, L, and Sun, H. Early evidence for beer drinking in a 9000-year-old platform mound in southern China. PLoS One. (2021) 16:e0255833. doi: 10.1371/journal.pone.0255833
2. Nardini, M. An overview of bioactive phenolic molecules and antioxidant properties of beer: emerging trends. Molecules. (2023) 28:3221. doi: 10.3390/molecules28073221
3. Nishiwaki, M, Yamaguchi, T, Nishida, R, and Matsumoto, N. Dose of alcohol from beer required for acute reduction in arterial stiffness. Front Physiol. (2020) 11:1033. doi: 10.3389/fphys.2020.01033
4. Sancen, M, Leniz, A, Macarulla, MT, Gonzalez, M, Milton-Laskibar, I, and Portillo, MP. Features of non-alcoholic beer on cardiovascular biomarkers. Can it be a substitute for conventional beer? Nutrients. (2023) 15:173.
5. Zugravu, CA, Medar, C, Manolescu, LSC, and Constantin, C. Beer and microbiota: pathways for a positive and healthy interaction. Nutrients. (2023) 15
6. Romeo, J, Wärnberg, J, Díaz, L, and Marcos, GG. Effects of moderate beer consumption on first-line immunity of healthy adults. J Physiol Biochem. (2007) 63:153–9. doi: 10.1007/BF03168226
7. Wang, L, Hong, K, Agbaka, JI, Song, Y, Lv, C, and Ma, C. Characterization of bitter-tasting and antioxidant activity of dry-hopped beers. J Sci Food Agric. (2022) 102:4843–53. doi: 10.1002/jsfa.11847
8. Aichinger, G, Bliem, G, and Marko, D. Systemically achievable doses of beer flavonoids induce estrogenicity in human endometrial cells and cause synergistic effects with selected pesticides. Front Nutr. (2021) 8:691872. doi: 10.3389/fnut.2021.691872
9. Sohn, JG, Ha, TY, Hwang, CH, and Lee, YH. Studies on radiation protection effect of the beer. Korea: Republic of Korean Journal of Radiotherapy (2007) 19:83–90.
10. Hendriks, HFJ. Alcohol and human health: what is the evidence? Annu Rev Food Sci Technol. (2020) 11:1–21.
11. Preedy, VR. Beer in health and disease prevention: beer in health and disease prevention. Cambridge, MA: Academic Press (2009).
12. Goi, I, Díaz-Rubio, M, and Saura-Calixto, F. “Dietary fiber in beer: Content, composition, colonic fermentability, and contribution to the diet,” in Beer in Health and Disease Prevention. ed. V. R. Preedy, (2009) 299–307.
13. González-Zancada, N, Redondo-Useros, N, Díaz, LE, Gómez-Martínez, S, and Nova, E. Association of moderate beer consumption with the gut microbiota and SCFA of healthy adults. Molecules. (2020) 25:4772. doi: 10.3390/molecules25204772
14. Vacca, M, Celano, G, Calabrese, FM, Portincasa, P, and Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms. (2020) 8:573. doi: 10.3390/microorganisms8040573
15. Engen, PA, Green, SJ, Voigt, RM, Forsyth, CB, and Keshavarzian, A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. (2015) 37:223–36.
16. De Gaetano, G, Costanzo, S, Di Castelnuovo, A, Badimon, L, Bejko, D, Aa, A, et al. Effects of moderate beer consumption on health and disease: a consensus document. Nutr Metab Cardiovasc Dis. (2016) 26:443–67. doi: 10.1016/j.numecd.2016.03.007
17. Mathur, N, and Pedersen, BK. Exercise as a mean to control low-grade systemic inflammation. Mediat Inflamm. (2008) 2008:109502:1–6. doi: 10.1155/2008/109502
18. Murphy, EA, Davis, JM, Carmichael, MD, Gangemi, JD, Ghaffar, A, and Mayer, EP. Exercise stress increases susceptibility to influenza infection. Brain Behavior Immunity. (2008) 22:1152–5. doi: 10.1016/j.bbi.2008.06.004
19. Amandine, E, Lucie, G, Marie, VR, Delzenne, NM, Cani, PD, and Daniel, T. Tetrahydro iso-alpha acids from hops improve glucose homeostasis and reduce body weight gain and metabolic endotoxemia in high-fat diet-fed mice. PLoS One. (2012) 7:e33858. doi: 10.1371/journal.pone.0033858
20. Gerhuser, C. Beer constituents as potential cancer chemopreventive agents. Eur J Cancer. (2005) 41:1941–54. doi: 10.1016/j.ejca.2005.04.012
21. Kok, BP, Andrea, G, Littlejohn, NK, Verena, A, Cristina, G, Woojoo, K, et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol Metab. (2018) 16:76–87. doi: 10.1016/j.molmet.2018.07.013
22. Jastrzębski, Z, Gorinstein, S, Czyżewska-Szafran, H, Leontowicz, H, Leontowicz, M, Trakhtenberg, S, et al. The effect of short-term lyophilized beer consumption on established hypertension in rats. Food Chem Toxicol. (2007) 45:296–302. doi: 10.1016/j.fct.2006.08.007
23. Rita, N, Raquel, C, Delfim, D, et al. Xanthohumol-supplemented beer modulates angiogenesis and inflammation in a skin wound healing model. Involvement of local adipocytes. J Cell Biochem. (2011) 113:100–9. doi: 10.1002/jcb.23332
24. Costa, R, Negrão, R, Valente, I, Castela, Â, Duarte, D, Guardão, L, et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J Nat Prod. (2013) 76:2047–53. doi: 10.1021/np4002898
25. Shela, CA, Libman, I, Leontowicz, H, Leontowicz, M, Tashma, Z, et al. Bioactivity of beer and its influence on human metabolism. Int J Food Sci Nutr. (2007) 58:94–107. doi: 10.1080/09637480601108661
26. Nurmi, K, Virkanen, J, Rajamaki, K, Niemi, K, Kovanen, PT, and Eklund, KK. Ethanol inhibits activation of NLRP3 and AIM2 inflammasomes in human macrophages–a novel anti-inflammatory action of alcohol. PLoS One. (2013) 8:e78537. doi: 10.1371/journal.pone.0078537
27. Karatzi, K, Rontoyanni, VG, Protogerou, AD, Georgoulia, A, and Sidossis, LS. Acute effects of beer on endothelial function and hemodynamics: a single-blind, crossover study in healthy volunteers. Nutrition. (2013) 29:1122–6. doi: 10.1016/j.nut.2013.02.016
28. Chiva-Blanch, G, Magraner, E, Condines, X, Valderas-Martínez, P, Roth, I, Arranz, S, et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: a randomized feeding trial. Nutr Metab Cardiovasc Dis. (2015) 25:36–45. doi: 10.1016/j.numecd.2014.07.008
29. Gorinstein, S, Zemser, M, Weisz, M, Halevy, S, and Trakhtenberg, S. The influence of alcohol-containing and alcohol-free beverages on lipid levels and lipid peroxides in serum of rats. J Nutr Biochem. (1998) 9:682–6. doi: 10.1016/S0955-2863(98)00069-2
30. Teresa, P, Mo-G, N, Gemma, V, Patricia, C, Alba, D, Rosa, A, et al. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients. (2018) 10:1237.
31. Chiva-Blanch, G, Condines, X, Magraner, E, Roth, I, Valderas-Martínez, P, Arranz, S, et al. The non-alcoholic fraction of beer increases stromal cell derived factor 1 and the number of circulating endothelial progenitor cells in high cardiovascular risk subjects: a randomized clinical trial. Atherosclerosis. (2014) 233:518–24. doi: 10.1016/j.atherosclerosis.2013.12.048
32. Codoñer-Franch, P, Hernández-Aguilar, MT, Navarro-Ruiz, A, López-Jaén, AB, Borja-Herrero, C, and Valls-Bellés, V. Diet supplementation during early lactation with non-alcoholic beer increases the antioxidant properties of breastmilk and decreases the oxidative damage in breastfeeding mothers. Breastfeed Med. (2013) 8:164–9. doi: 10.1089/bfm.2012.0059
33. Karvaj, M. Overall alcohol intake, beer, wine and systemic markers of inflamation in western europe: results from three MONICA samples (Augsburg, Glasgow, Lille). Neuro Endocrinol Lett (2007);28 Suppl 4:10
34. Romeo, J, Wrnberg, J, Nova, E, Díaz, L, Gómez-Martinez, S, and Marcos, A. Moderate alcohol consumption and the immune system: a review. Br J Nutr. (2007) 98:S111–5. doi: 10.1017/S0007114507838049
35. Oliveira, I, Sousa, A, Morais, JS, Ferreira, I, Bento, A, Estevinho, L, et al. Chemical composition, and antioxidant and antimicrobial activities of three hazelnut (Corylus avellana L.) cultivars. Food Chem Toxicol. (2008) 46:1801–7. doi: 10.1016/j.fct.2008.01.026
36. Gemma, CB, Sara, A, Lamuela-Raventos, RM, and Ramon, E. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: evidences from human studies. Alcohol Alcoholism. (2013) 48:270–7.
37. Mendenhall, CL, Theus, SA, Roselle, GA, Grossman, CJ, and Rouster, SD. Biphasic in vivo immune function after low- versus high-dose alcohol consumption. Alcohol. (1997) 14:255–60. doi: 10.1016/S0741-8329(96)00150-4
38. Cohen, S, Tyrrell, D, Russell, H, and Jarvis, MJ. Smoking, alcohol consumption, and susceptibility to the common cold. (2022)
39. Romeo, J, Wärnberg, J, Díaz, LE, González-Gross, M, and Marcos, A. Effects of moderate beer consumption on first-line immunity of healthy adults. J Physiol Biochem. (2007) 63:153–9. doi: 10.1007/BF03168226
40. Winkler, C, Wirleitner, B, Schroecksnadel, K, Schennach, H, and Fuchs, D. Beer down-regulates activated peripheral blood mononuclear cells in vitro. Int Immunopharmacol. (2006) 6:390–5. doi: 10.1016/j.intimp.2005.09.002
41. Mandrekar, P, Catalano, D, and White, B. Szabo G, Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. (2006) 30:135–9. doi: 10.1111/j.1530-0277.2006.00012.x
42. Kawabata, K, Yoshioka, Y, and Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. (2019) 24:370. doi: 10.3390/molecules24020370
43. Tal, F, and Giulio, P. Polyphenolic compounds ameliorate stress-induced depression by preventing NLRP3 inflammasome priming (P19-011-19). Curr. Dev. Nutr. (2019) 3:nzz049.P19-011-19. doi: 10.1093/cdn/nzz049.P19-011-19
44. Milligan, SR, Kalita, JC, Pocock, V, and Van, DK, V., Stevens, JF, Deinzer, ML, et al. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus) flavonoids. J Clin Endocrinol Metab (2000);85:4912–4915, doi: 10.1210/jcem.85.12.7168
45. Rita NegrãoCosta, R, Duarte, D, Gomes, TT, Coelho, P, Guimarães, JT, et al. Xanthohumol-supplemented beer modulates angiogenesis and inflammation in a skin wound healing model. Involvement of local adipocytes. J Cell Biochem. (2012) 113:100–9. doi: 10.1002/jcb.23332,
46. Kondo, K. Beer and health: preventive effects of beer components on lifestyle-related diseases. Biofactors. (2010) 22:303–10. doi: 10.1002/biof.5520220160
47. Romeo, J, Wärnberg, J, Nova, E, Díaz, LE, González-Gross, M, and Marcos, A. Changes in the immune system after moderate beer consumption. Ann Nutr Metab. (2007) 51:359–66. doi: 10.1159/000107679
48. Díaz, LE, Montero, A, González-Gross, M, Vallejo, AI, Romeo, J, and Marcos, A. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr. (2002) 56:S50–3. doi: 10.1038/sj.ejcn.1601486
49. Johansson, MEV, Larsson, H, Jessica, M, and Hansson, GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host--microbial interactions. Proc Natl Acad Sci U S A. (2011)
50. Petersson, J, Schreiber, O, Hansson, GC, Gendler, SJ, Velcich, A, Lundberg, JO, et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. (2011) 300:G327–33. doi: 10.1152/ajpgi.00422.2010
51. Shizuo, A. Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci. (2009)
52. Figdor, CG, Kooyk, YV, and Adema, GJ. Erratum: C-type lectin receptors on dendritic cells and langerhans cells. Nat Rev Immunol. (2002) 2:77. doi: 10.1038/nri723
53. Hooper, LV. Molecular analysis of commensal host-microbial relationships in the intestine. Science. (2001) 291:881–4. doi: 10.1126/science.291.5505.881
54. Sharma, R, Young, C, and Neu, J. Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol. (2009) 2010:305879. doi: 10.1155/2010/305879
55. Le, Y, Jiang, S, Hu, J, Gong, W, Su, S, Dunlop, NM, et al. N36, a synthetic N-terminal heptad repeat domain of the HIV-1 envelope protein gp41, is an activator of human phagocytes. Clin Immunol. (2000) 96:236–42. doi: 10.1006/clim.2000.4896
56. Maynard, CL, Elson, CO, Hatton, RD, and Weaver, CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. (2012) 489:231–41. doi: 10.1038/nature11551
57. Braga, AF, Ferreira, P, Oliveira, J, Rocha, I, and Faria, N. Heterologous production of resveratrol in bacterial hosts: current status and perspectives. World J Microbiol Biotechnol. (2018) 34:122. doi: 10.1007/s11274-018-2506-8
58. Alvarez, JRM, Bellés, VV, López-Jaén, AB, Marín, AV, and Codoñer-Franch, P. Effects of alcohol-free beer on lipid profile and parameters of oxidative stress and inflammation in elderly women. Nutrition. (2009) 25:182–7. doi: 10.1016/j.nut.2008.08.005
59. Chubak, J, Mctiernan, A, Sorensen, B, Wener, MH, Yasui, Y, Velasquez, M, et al. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. (2006) 119:937–942.e5. doi: 10.1016/j.amjmed.2006.06.033
60. Ekblom, B, Ö.Ekblom, Malm, C. Infectious episodes before and after a marathon race. Scand J Med Sci Sports (2006);16:287–293, doi: 10.1111/j.1600-0838.2005.00490.x
61. Petersen, AMW, and Pedersen, BK. The anti-inflammatory effect of exercise. J Appl Physiol. (2005) 98:1154–62. doi: 10.1152/japplphysiol.00164.2004
62. Nieman, DC, Henson, DA, Fagoaga, OR, Utter, AC, Vinci, DM, Davis, JM, et al. Change in salivary IgA following a competitive marathon race. Int J Sports Med. (2002) 23:69–75. doi: 10.1055/s-2002-19375
63. Scherr, J, Braun, S, Schuster, T, Hartmann, C, Moehlenkamp, S, Wolfarth, B, et al. 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med Sci Sports Exerc. (2011) 43:1819–27. doi: 10.1249/MSS.0b013e31821b12eb
64. Smirnova, E, Puri, P, Muthiah, MD, Daitya, K, Brown, R, Chalasani, N, et al. Fecal microbiome distinguishes alcohol consumption from alcoholic hepatitis but does not discriminate disease severity. Hepatology. (2020) 72:271–86. doi: 10.1002/hep.31178
65. Mutlu, E, Keshavarzian, A, Engen, P, Forsyth, CB, Sikaroodi, M, and Gillevet, P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. (2009) 33:1836–46. doi: 10.1111/j.1530-0277.2009.01022.x
66. Zhong, W, Mcclain, CJ, Cave, M, Kang, YJ, and Zhou, Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. (2010) 298:G625–33. doi: 10.1152/ajpgi.00350.2009
67. Frazier, TH, Dibaise, JK, and Mcclain, CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. J Parenter Enter Nutr. (2011) 35:14S–20S. doi: 10.1177/0148607111413772
68. Schnabl, B, and Brenner, DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. (2014) 146:1513–24. doi: 10.1053/j.gastro.2014.01.020
69. Meena, AS, Shukla, PK, Rao, R, Canelas, C, Pierre, JF, and Rao, R. TRPV6 deficiency attenuates stress and corticosterone-mediated exacerbation of alcohol-induced gut barrier dysfunction and systemic inflammation. Front Immunol. (2023) 14:1093584. doi: 10.3389/fimmu.2023.1093584
70. Engen, PA, Green, SJ, Voigt, RM, Forsyth, CB, and Keshavarzian, A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. (2015) 37:223–36.
71. Hernández-Quiroz, F, Nirmalkar, K, Villalobos-Flores, LE, Murugesan, S, and García-Mena, J. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol. (2019) 85:77–94. doi: 10.1016/j.alcohol.2019.05.006
72. Scalbert, A, Morand, C, Manach, C, and Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. (2002) 56:276–82. doi: 10.1016/S0753-3322(02)00205-6
73. Cardona, F, Andrés-Lacueva, C, Tulipani, S, Tinahones, FJ, and Queipo-Ortuño, M. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. (2013) 24:1415–22. doi: 10.1016/j.jnutbio.2013.05.001
74. Lee, HC, Jenner, AM, Low, CS, and Lee, YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol. (2006) 157:876–84. doi: 10.1016/j.resmic.2006.07.004
75. Gibson, GR, Hutkins, R, Sanders, ME, Prescott, SL, Reimer, RA, Salminen, SJ, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 14:491–502.
76. Tzounis, X, Rodriguez-Mateos, A, Vulevic, J, Gibson, GR, Kwik-Uribe, C, and Spencer, JPE. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr. (2011) 93:62–72. doi: 10.3945/ajcn.110.000075
77. Rastmanesh, R. High polyphenol, low probiotic diet for weight loss because of intestinal microbiota interaction. Chem Biol Interact. (2011) 189:1–8. doi: 10.1016/j.cbi.2010.10.002
78. Selma, MAV, Espín, JC, and Tomás-Barberán, FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. (2009) 57:6485–501. doi: 10.1021/jf902107d
79. Queipo-Ortuno, MI, Boto-Ordonez, M, Gomez-Zumaquero, JM, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. (2012) 95:1323–34.
80. Bartholomeus, VDB, Marjolein, M, Zoetendal, EG, Wells, JM, Michiel, K, and Benoit, F. Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS One. (2014) 9:e114277. doi: 10.1371/journal.pone.0114277
81. Tao, Y, Hang, M, Weixi, L, et al. Pomegranate's neuroprotective effects against Alzheimer's disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem Neurosci. (2015) 7:26–33. doi: 10.1021/acschemneuro.5b00260
82. Das, S, Vishakha, K, Banerjee, S, Bera, T, Mondal, S, and Ganguli, A. A novel probiotic strain of Lactobacillus fermentum TIU19 isolated from Haria beer showing both in vitro antibacterial and antibiofilm properties upon two multi resistant uro-pathogen strains. Curr Res Microb Sci. (2022) 3:100150. doi: 10.1016/j.crmicr.2022.100150
83. Borah, T, Gogoi, B, Khataniar, A, Gogoi, M, Das, A, and Borah, D. Probiotic characterization of indigenous Bacillus velezensis strain DU14 isolated from Apong, a traditionally fermented rice beer of Assam. Biocatal Agric Biotechnol. (2019) 18:101008.
84. Hernandez-Quiroz, F, Nirmalkar, K, Villalobos-Flores, LE, Murugesan, S, Cruz-Narvaez, Y, Rico-Arzate, E, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and beta-cell function. Alcohol. (2020) 85:77–94. doi: 10.1016/j.alcohol.2019.05.006
85. Redondo, N, Nova, E, Díaz-Prieto, LE, and Marcos, A. Effects of moderate beer consumption on health. Nutr Hosp. (2018) 35:41–4. doi: 10.20960/nh.2286
86. Sara, A, Gemma, C-B, Palmira, V-M, Medina-Remón, A, Lamuela-Raventós, RM, and Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. (2012) 4:759–81. doi: 10.3390/nu4070759
87. Ma, S. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE(−/−) mice. Biomed Pharmacother. (2019) 113:108753. doi: 10.1016/j.biopha.2019.108753
88. Romo-Vaquero, M, García-Villalba, R, González-Sarrías, A, Beltrán, D, Tomás-Barberán, F, Espín, J, et al. Interindividual variability in the human metabolism of ellagic acid: contribution of Gordonibacter to urolithin production. J Funct Foods. (2015) 17:785–91. doi: 10.1016/j.jff.2015.06.040
89. Gasperotti, M, Passamonti, S, Tramer, F, Masuero, D, Guella, G, Mattivi, F, et al. Fate of microbial metabolites of dietary polyphenols in rats: is the brain their target destination? ACS Chem Neurosci. (2015) 6:1341–52. doi: 10.1021/acschemneuro.5b00051
90. Anhê, F, Roy, D, Pilon, G, Dudonné, S, Matamoros, S, Varin, TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. (2015) 64:872–83. doi: 10.1136/gutjnl-2014-307142
91. Blatchford, PA, Parkar, SG, Hopkins, W, Ingram, JR, and Sutton, KH. Dose-dependent alterations to in vitro human microbiota composition and butyrate inhibition by a supercritical carbon dioxide hops extract. Biomol Ther. (2019) 9:390.
92. Nuñez-Sánchez, MA, Dávalos, A, González-Sarrías, A, Casas-Agustench, P, Visioli, F, Monedero-Saiz, T, et al. MicroRNAs expression in normal and malignant colon tissues as biomarkers of colorectal cancer and in response to pomegranate extracts consumption: critical issues to discern between modulatory effects and potential artefacts. Mol Nutr Food Res. (2015) 59:1973–86. doi: 10.1002/mnfr.201500357
93. Sierksma, A, Van, D, Kluft, C, and Hendriks, H. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr. (2002) 56:1130–6. doi: 10.1038/sj.ejcn.1601459
94. Russell, WR, Duncan, SH, Scobbie, L, Duncan, G, Cantlay, L, Calder, AG, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. (2013) 57:523–35. doi: 10.1002/mnfr.201200594
95. Diana, R, Rachel, C, Peter, K, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes. (2015) 64:2847–58. doi: 10.2337/db14-1916
96. Song, H, Chu, Q, Yan, F, Yang, Y, Han, W, and Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J Gastroenterol Hepatol. (2016) 31:1462–9. doi: 10.1111/jgh.13278
Keywords: beer, nutrition, gut microbiome, food immunomodulatory, mucosal barrier, antioxidant
Citation: Zhang S, Jin S, Zhang C, Hu S and Li H (2023) Beer-gut microbiome alliance: a discussion of beer-mediated immunomodulation via the gut microbiome. Front. Nutr. 10:1186927. doi: 10.3389/fnut.2023.1186927
Received: 24 March 2023; Accepted: 11 July 2023;
Published: 25 July 2023.
Edited by:
Carmen Hernandez-Brenes, Tecnologico de Monterrey, MexicoReviewed by:
Arlette Santacruz, Monterrey Institute of Technology and Higher Education (ITESM), MexicoCopyright © 2023 Zhang, Jin, Zhang, Hu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huajun Li, bGhqY211QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.