- 1Graduation Program in Health Sciences, Health Sciences Center, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil
- 2Center for Translational Medicine, Semmelweis University, Budapest, Hungary
Introduction: Body composition (BC) assessment can supply accurate information for in-hospital nutritional evaluation. The aim of this study was to explore in the literature how the studies assessed BC, for what purpose, and investigate the role of BC findings in COVID-19 hospitalized patients’ outcomes.
Methods: A scoping review was conducted according to the methodology available on the Joanna Briggs Institute website. We used the PCC acronym for the systematic search (population: adults with COVID-19, concept: assessment of BC, context: hospital setting) and performed it on PubMed, Scopus, and the Web of Science on 16 September 2022. Eligibility criteria consisted of the utilization of BC assessment tools in COVID-19 patients. Studies in which BC was solely measured with anthropometry (perimeters and skinfolds) were excluded. No language restriction was applied.
Results: Fifty-five studies were eligible for the review. Out of the 55 studies, 36 used computed tomography (CT), 13 used bioelectrical impedance (BIA), and 6 used ultrasound (US). No studies with D3-creatinine, 24 h urine excretion, dual-energy X-ray absorptiometry, or magnetic resonance were retrieved. BC was mainly assessed to test associations with adverse outcomes such as disease severity and mortality.
Discussion: Studies assessing BC in hospitalized patients with COVID-19 used mainly CT and BIA and associated the parameters with severity and mortality. There is little evidence of BC being assessed by other methods, as well as studies on BC changes during hospitalization.
1. Introduction
The coronavirus disease 2019 (COVID-19) has been, for over the past 3 years, the most serious public health emergency on several continents. On 11 March 2020, the World Health Organization (WHO) declared it a global pandemic (1). According to the WHO, by 23 April 2023, there were over 764 million confirmed cases and over 6.9 million deaths due to the disease worldwide (2). In this regard, some risk factors were found to be associated with COVID-19 severity and mortality. Obesity (body mass index >30 kg/m2) and/or high quantities of visceral adipose tissue (VAT) have been reported as predictors for hospitalization, severe state, and mortality in COVID-19 patients since they are linked with a high production of proinflammatory cytokines and an exacerbated inflammatory state (3–5).
Like obesity, reduced muscle mass (MM) or low skeletal muscle density (SMD) were found to be associated with worse prognosis in COVID-19 patients (6, 7). As COVID-19, like many other inflammatory diseases, has an impact on nutritional status due to the high consumption of protein and decreased protein synthesis (8, 9), changes in body composition (BC) might be exacerbated during the acute phase of the disease (10, 11). Therefore, BC assessment tools can be adopted to collect more accurate data on the presence of obesity as well as MM parameters.
Several BC assessment tools can be used to evaluate the adipose and muscle tissues in hospitalized patients, including at the bedside, and hence improve nutritional care and management. Image methods, such as computed tomography (CT), magnetic resonance imaging (MRI), dual-energy X-ray absorptiometry (DXA), ultrasound (US), and bioelectrical impedance (BIA), have been used for BC assessment in various clinical settings, due to their “opportunistic nature” during hospitalization (12). Abnormal BC as a predictor of negative outcomes is largely reported in some hospitalized populations, however, it has not yet been explored in COVID-19 patients (11). Thus, identifying how clinicians are currently assessing and monitoring BC in clinical settings is necessary for the implementation of adequate nutritional care.
Many observational studies investigated the predictive power of BC to assess the severity of COVID-19. The aim of this review was to identify the studies using parameters derived from BC assessment tools, report how the assessment was conducted, highlight the abnormalities in BC during hospitalization and summarize the main results. As the clinical question is broad and leads to other sub-questions, we chose to perform a scoping review. Thus, by identifying possible gaps in this topic, we can improve the research and the clinical practice regarding BC assessment in COVID-19 hospitalized patients as well as other hospitalized patients under acute inflammatory states.
2. Materials and methods
2.1. Study design
A scoping review was conducted, drawing inspiration from the Joanna Briggs Institute (JBI) (13). The PRISMA checklist for scoping reviews was filled out and is presented as Supplementary material (14). A review protocol was built for the scoping review, however, it was used only by our review team and not registered. This study analyzed qualitative and quantitative data presented in studies in which COVID-19 patients have undergone BC assessment.
2.2. Review question
From the available literature about BC assessment in COVID-19 hospitalized patients, what tools were utilized by the studies, and what are the gaps in the literature regarding BC assessment?
In this review, the acronym for population, concept, and context (PCC) for scoping reviews was as follows: Population (P) – adults older than 18 years hospitalized with COVID-19; Concept (C) – BC evaluated by non-anthropometric BC assessment tools; and Context (C) – hospital setting.
For this review, four research sub-questions were raised:
1. Regarding the tools, how did the studies with COVID-19 patients evaluate BC?
2. What were the objectives of the studies with COVID-19 patients submitted to BC assessment?
3. What were the main findings regarding BC parameters and COVID-19 prognosis?
4. What BC alterations occurred in patients with COVID-19 during hospitalization?
2.3. Eligibility criteria
All the studies evaluating hospitalized adults over 18 years of age with a diagnosis of COVID-19 and assessed by BC assessment tools were eligible for the scoping review. Our exclusion criteria consisted of non-targeted populations such as children, adolescents, pregnant women, and outpatients, studies that only assessed other body compartments such as epicardial fat thickness or the diaphragm muscle for cardiovascular and respiratory capacity assessment, and inappropriate study design, e.g., reviews, and case reports. No language restriction was applied, and only peer-reviewed, published data were eligible for inclusion. Although we focused on hospitalized patients, a few studies reported hospitalization as an outcome for outpatients (15, 16) and therefore they were also included.
2.4. Search strategy
A search strategy was constructed, and one reviewer (IPAV) systematically carried out the searches on electronic databases to find eligible articles published until 16 September 2022. The databases accessed were PubMed (accessed through the Medical Literature Analysis and Retrieval System Online MEDLINE), Web of Science, and Scopus. Subsequently, the titles and abstracts were exported to the citation manager EndNote software version 20.4.1 (Clarivate Analytics, Philadelphia, PA, United States) for manual duplicate removal. After the duplicate removal, the remaining references were shared among reviewers for the study selection. IPAV performed the title and abstract reading. The full-text assessment was performed by IPAV and IMS. In case of disagreements, ADLB decided whether the reference would be eligible for inclusion or not. Data extraction was performed by IPAV and checked by IMS. Again, in case of disagreements regarding the data extraction, a third reviewer was invited to resolve it (ADLB). We additionally carried out manual searches in reference lists of selected published studies to include eligible articles in case they were not available within the results yielded by the search strategy. No language nor time restriction was applied for our search. Additional contact with the authors was not necessary. The search key used on PubMed is presented in Box 1.
BOX 1 Search strategy used on PubMed
((Diagnostic Imaging[MeSH Terms]) OR (Imaging, Diagnostic[Title/Abstract])) OR (Medical Imaging[Title/Abstract])) OR (Imaging, Medical[Title/Abstract])) OR (Ultrasonography[MeSH Terms])) OR (Diagnostic Ultraso*[Title/Abstract])) OR (Ultraso* Imaging[Title/Abstract])) OR (Medical Sonography[Title/Abstract])) OR (Echography[Title/Abstract])) OR (Computer Echotomography[Title/Abstract])) OR (Ultrasonic Tomography[Title/Abstract])) OR (Diagnostic Techniques and Procedures[MeSH Terms])) OR (Diagnostic Testing[Title/Abstract])) OR (Tomography, X-Ray Computed[MeSH Terms])) OR (Tomography, X-Ray Computerized[Title/Abstract])) OR (X-Ray Computer Assisted Tomography[Title/Abstract])) OR (Computerized Tomography, X Ray[Title/Abstract])) OR (CT X Ray*[Title/Abstract])) OR (Tomography, X Ray Computed[Title/Abstract])) OR (Tomography, X Ray Computed[Title/Abstract])) OR (CAT Scan, X Ray[Title/Abstract])) OR (Tomography, Transmission Computed[Title/Abstract])) OR (CT Scan, X-Ray[Title/Abstract])) OR (Computed Tomography, X-Ray[Title/Abstract])) OR (computed tomography[Title/Abstract])) OR (magnetic resonance imaging[MeSH Terms])) OR (magnetic resonance imaging[Title/Abstract])) OR (NMR Imaging[Title/Abstract])) OR (Tomography, NMR[Title/Abstract])) OR (MR Tomography[Title/Abstract])) OR (Magnetic Resonance Image*[Title/Abstract])) OR (MRI Scan*[Title/Abstract])) OR (Absorptiometry, Photon[MeSH Terms])) OR (Photon Absorptiometry[Title/Abstract])) OR (X-Ray Densitometry[Title/Abstract])) OR (X-Ray Photodensitometry[Title/Abstract])) OR (Dual-Energy X-Ray Absorptiometry Scan[Title/Abstract])) OR (DXA Scan*[Title/Abstract])) OR (DEXA Scan*[Title/Abstract])) OR (Dual-Photon Absorptiometry[Title/Abstract])) OR (Dual-Energy Radiographic Absorptiometry[Title/Abstract])) OR (X Ray Absorptiometry[Title/Abstract])) OR (Dual Energy X Ray Absorptiometry[Title/Abstract])) OR (DPX Absorptiometry[Title/Abstract])) OR (Dual X-Ray Absorptiometry[Title/Abstract])) OR (Tomography, Emission Computed [Title/Abstract])) OR (Densitometry [Title/Abstract])) OR (imaging techniques[Title/Abstract])) OR (bioelectrical impedance analysis[Title/Abstract])) OR (BIA[Title/Abstract])) OR (bioimpedance analysis[Title/Abstract])) OR (bioelectrical impedance analysis[Title/Abstract])) OR (neutron-activation analysis[Title/Abstract]) OR (Electric Impedance[MeSH Terms])) OR (ultrasound[Title/Abstract]) OR (sonography[Title/Abstract]) OR (CT scan[Title/Abstract]) AND (“COVID-19”[Mesh] OR COVID 19 OR COVID-19 Virus Disease OR COVID 19 Virus Disease OR COVID-19 Virus Diseases OR Disease, COVID-19 Virus OR Virus Disease, COVID-19 OR COVID-19 Virus Infection OR COVID 19 Virus Infection OR COVID-19 Virus Infections OR Infection, COVID-19 Virus OR Virus Infection, COVID-19 OR 2019-nCoV Infection OR 2019 nCoV Infection OR 2019-nCoV Infections OR Infection, 2019-nCoV OR Coronavirus Disease-19 OR Coronavirus Disease 19 OR 2019 Novel Coronavirus Disease OR 2019 Novel Coronavirus Infection OR 2019-nCoV Disease OR 2019 nCoV Disease OR 2019-nCoV Diseases OR Disease, 2019-nCoV OR COVID19 OR Coronavirus Disease 2019 OR Disease 2019, Coronavirus OR SARS Coronavirus 2 Infection OR SARS-CoV-2 Infection OR Infection, SARS-CoV-2 OR SARS CoV 2 Infection OR SARS-CoV-2 Infections OR COVID-19 Pandemic OR COVID 19 Pandemic OR COVID-19 Pandemics OR Pandemic, COVID-19 OR “SARS-CoV-2”[Mesh] OR Coronavirus Disease 2019 Virus OR 2019 Novel Coronavirus OR 2019 Novel Coronaviruses OR Coronavirus, 2019 Novel OR Novel Coronavirus, 2019 OR Wuhan Seafood Market Pneumonia Virus OR SARS-CoV-2 Virus OR SARS CoV 2 Virus OR SARS-CoV-2 Viruses OR Virus, SARS-CoV-2 OR 2019-nCoV OR COVID-19 Virus OR COVID 19 Virus OR COVID-19 Viruses OR Virus, COVID-19 OR Wuhan Coronavirus OR Coronavirus, Wuhan OR SARS Coronavirus 2 OR Coronavirus 2, SARS OR Severe Acute Respiratory Syndrome Coronavirus) AND ((sarcopenia[Title/Abstract])) OR (sarcopenic obesity[Title/Abstract])) OR (Skeletal Muscle*[Title/Abstract])) OR (Voluntary Muscle*[Title/Abstract])) OR (Soleus Muscle[Title/Abstract])) OR (Plantaris Muscle[Title/Abstract])) OR (Anterior Tibial Muscle[Title/Abstract])) OR (Gastrocnemius Muscle[Title/Abstract])) OR (Muscle*[Title/Abstract])) OR (Muscle Tissue*[Title/Abstract])) OR (Skeletal muscle cutoff values[Title/Abstract])) OR (appendicular lean soft tissue[Title/Abstract])) OR (skeletal muscle mass[Title/Abstract])) OR (skeletal muscle area[Title/Abstract])) OR (skeletal muscle mass index[Title/Abstract])) OR (appendicular skeletal muscle mass index[Title/Abstract])) OR (fat-free mass index[Title/Abstract])) OR (muscle mass[Title/Abstract])) OR (Quadriceps Muscle*[Title/Abstract])) OR (Quadriceps Femoris[Title/Abstract])) OR (Vastus Medialis[Title/Abstract])) OR (Vastus Intermedius[Title/Abstract])) OR (Rectus Femoris[Title/Abstract])) OR (Vastus Lateralis[Title/Abstract])) OR (appendicular lean mass[Title/Abstract])) OR (appendicular skeletal muscle mass[Title/Abstract])) OR (Appendicular lean tissue mass[Title/Abstract])) OR (body surface area[Title/Abstract])) OR (fat-free mass[Title/Abstract])) OR (third lumbar vertebra[Title/Abstract])) OR (total abdominal muscle area[Title/Abstract])) OR (thigh muscle area[Title/Abstract]))) OR (psoas muscle index[Title/Abstract])) OR (psoas muscle area[Title/Abstract])) OR (body skeletal muscle mass[Title/Abstract])) OR (muscle indices[Title/Abstract])) OR (lean mass measures[Title/Abstract])) OR (lean mass[Title/Abstract])) OR (muscle tissue[Title/Abstract])) OR (Muscle wasting[Title/Abstract])) OR (muscle size[Title/Abstract]) OR (Body Fat Distribution[Title/Abstract])) OR (Adiposity [Title/Abstract]) OR (Body Constitution [Title/Abstract])) OR (Body Composition[Title/Abstract]) OR (Fatty Tissue[Title/Abstract])) OR (Adipose Tissue[Title/Abstract]) OR (Abdominal Fat[Title/Abstract])) OR (Abdominal Adipose Tissue[Title/Abstract]) OR (Subcutaneous Fat [Title/Abstract])) OR (Subcutaneous Adipose Tissue[Title/Abstract]) OR (phase angle[Title/Abstract]) OR (intramuscular adipose[Title/Abstract]) OR (muscle quantity[Title/Abstract]) OR (Subcutaneous fat area[Title/Abstract]) OR (visceral Adipose Tissue[Title/Abstract]) OR (visceral fat[Title/Abstract]).
The search strategy performed on Scopus and Web of Science is available in Supplementary material 1.
2.5. Data collection and charting
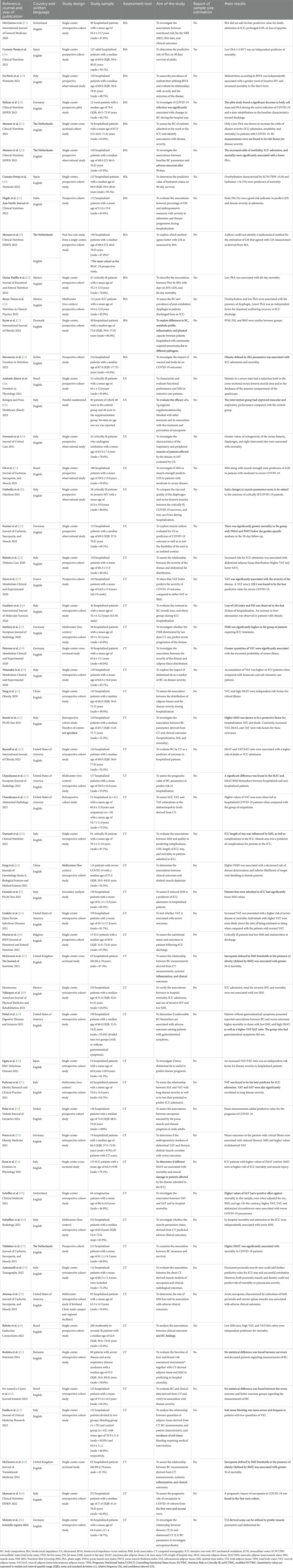
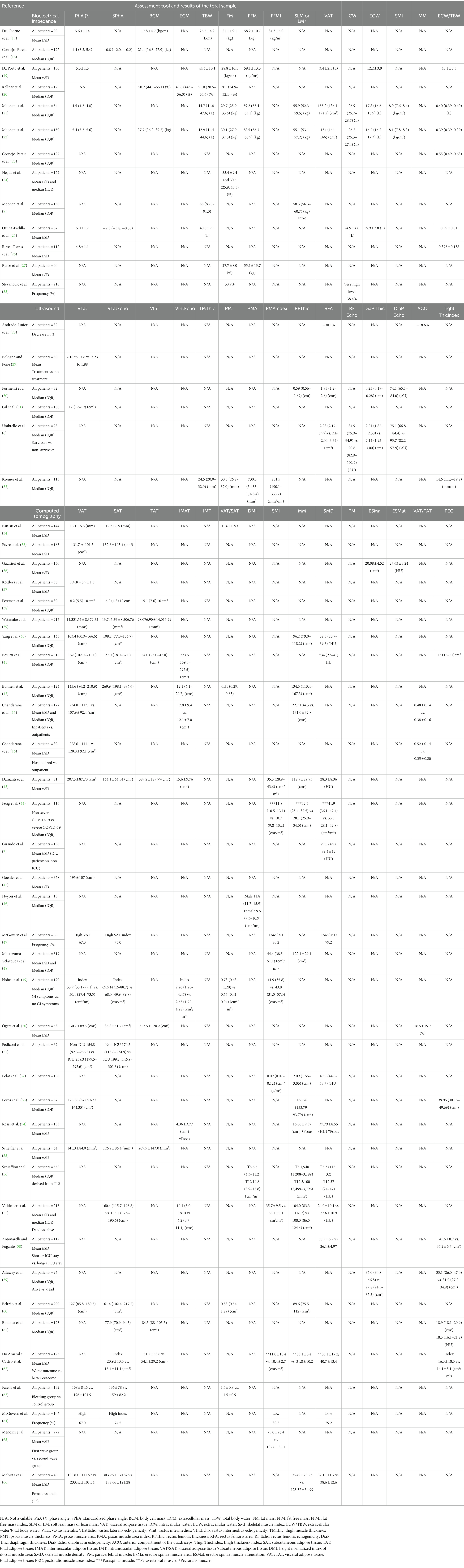
To address the research questions, the following data were extracted from each included study: (i) first author, year of publication, and journal; (ii) country and language; (iii) study design; (iv) population characteristics (sample size; sex; age and health status); (v) aim of the research paper; (vi) sample size estimation; (vii) main results of the study; (viii) type of BC assessment tool; (ix) moment of the assessment; (x) frequency of the assessment; (xi) report of the tool performer and report of the assessor of the BC tool; (xii) body markers/compartments measured; (xiii) report of the protocol; (xiv) exclusion criteria; (xv) criteria for the classification of the markers of BC; and (xvi) results of the BC assessment when available. After curating the information, the data were extracted to an Excel sheet and later exported and standardized into three tables (Tables 1–3). The first table summarizes the main characteristics of the studies, the second gives further information on the BC assessment, and the third provides the quantitative findings derived from the BC assessment.
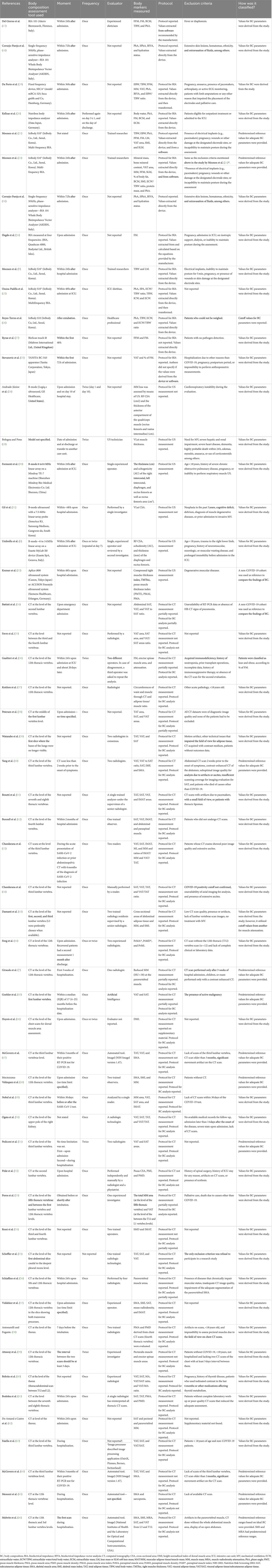
Table 2. General characteristics about the body composition tool, measurements, and parameters utilized.
2.6. Reporting items
The PRISMA checklist workflow for scoping reviews was used for reporting items in this scoping review (13) and it is available in the Supplementary material 2. No quality appraisal for the studies was performed since it is not recommended for scoping reviews according to JBI (14).
3. Results
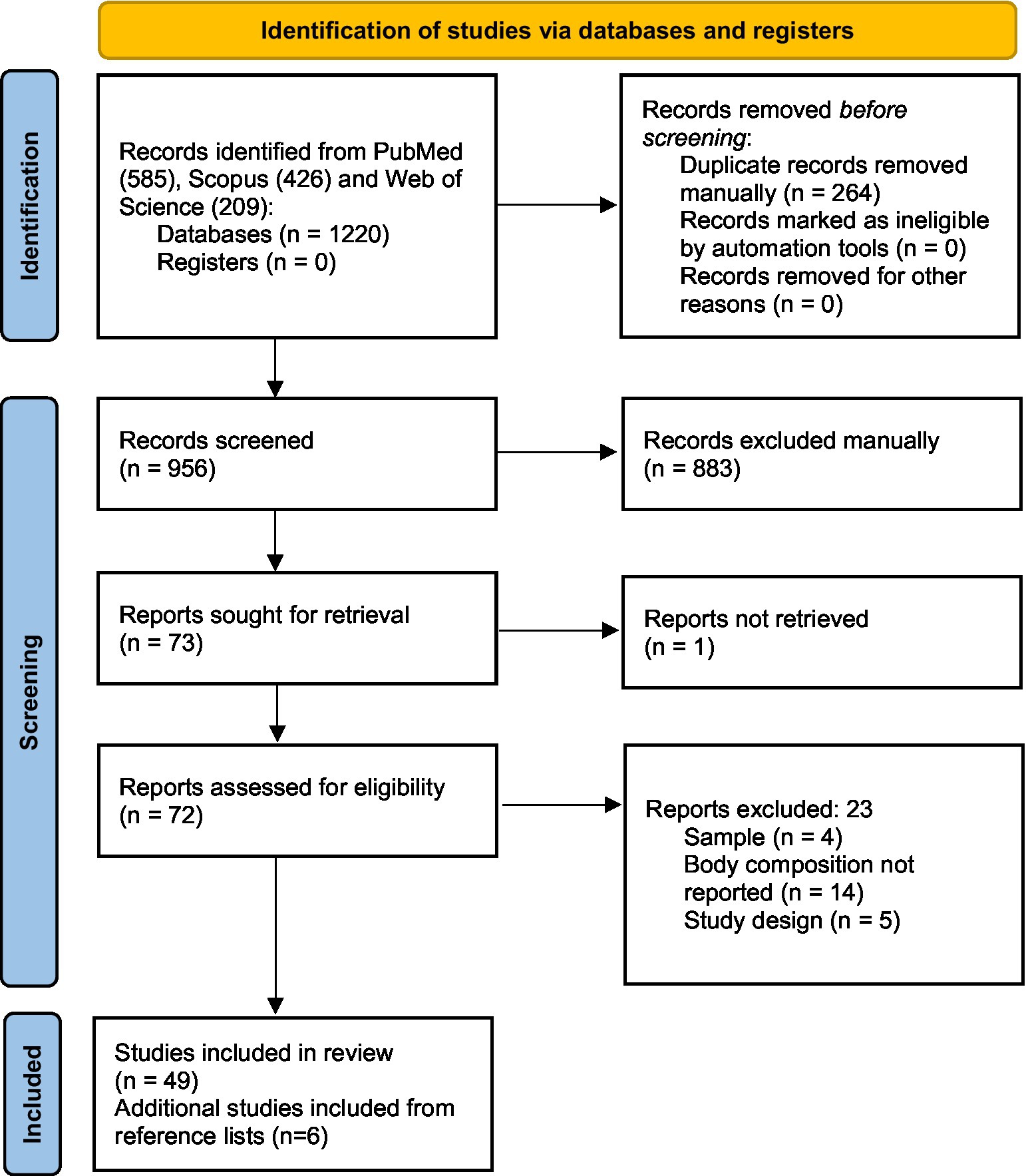
From the 1,220 potentially relevant citations yielded from the systematic searches, 264 records were excluded due to duplication. After the manual deletion of selected articles, 956 articles were eligible for the title and abstract readings of which 74 were eligible for the full-text assessment. After the full-text selection, 55 studies were eligible for data extraction and inclusion in our study. The flowchart of the study selection is shown in Figure 1.
Concerning the tools utilized, 36 used CT, 13 used BIA, and 6 used US. No studies with D3-creatinine, 24 h urine excretion, DXA, nor MRI for BC assessment were found.
Table 1 presents the studies performing BC assessments in patients hospitalized with COVID-19.
3.1. General characteristics of the studies
3.1.1. Year and country of publication
Regarding the year of publication, eight studies were published in 2020 (17, 34–40), 29 in 2021 (6, 7, 15, 16, 18–22, 28, 30, 31, 41–57), and 18 in 2022 (9, 23–27, 29, 32, 33, 58–66). Interestingly, the highest frequency of studies evaluating BC were from the European continent, with 37 studies (67.3%), followed by America with 13 publications (23.6%), Asia with four studies (7.3%), and Eurasia with one study (Table 1).
3.1.2. Study design and objectives
Out of the 55 studies, 40 were single-center (72.7%) (6, 9, 17–23, 25, 27, 28, 30–32, 34, 36, 38, 42, 43, 45–50, 52, 53–55, 58, 60–66) and seven were multicenter cohorts (12.7%) (15, 26, 37, 44, 51, 56, 59); the remaining eight studies did not specify the number of centers (14.6%) (7, 16, 24, 29, 33, 35, 41, 57). Most of the studies (n = 46; 83.6%) investigated the associations between abnormal BC markers and changes with COVID-19 outcomes such as ICU admission, disease severity, length of hospital stay (LOS), mechanical ventilation (MV), and death (6, 7, 15–25, 28, 30–34, 36–38, 40–43, 45–49, 50, 52–54, 56–62, 66).
From the studies that did not evaluate BC and COVID-19 outcomes through BIA, one evaluated post-extubation dysphagia (26), one analyzed the agreement of lean mass (LM) between BIA and other measurement tools (9), and one analyzed the BC characteristics between different groups infected by viral and bacterial pathogens (27). Out of the studies with US, one evaluated the treatment and prevention of sarcopenia by arginine supplementation (29). CT on the other hand was used in five studies with different objectives, e.g., assessing nutritional status and outcomes in patients after ICU discharge (46), examining the relationship between patients admitted with COVID-19 and frailty and other prognostic factors (64), investigating obesity or sarcopenia through the T12 and L3 scans (66), and assessing tissue bleeding and BC parameters (63). Finally, Gualtieri, evaluated the differences in BC of aldults with obesity and without obesity during ICU stay (36).
3.2. General characteristics of the participants
3.2.1. Sample size
Sample size varied greatly between studies, from 15 participants (46) to 519 (48) in single-center studies, and from 58 (37) to 552 (56) in multicenter studies evaluating BC through CT. When evaluating through BIA, the number of patients enrolled varied from 12 (20) to 216 (33), and for US, it varied from 28 (6) to 186 (31). Out of the included studies, 11 reported the sample size estimation either on the manuscript or in supplementary material (6, 18, 23, 25, 30–32, 36, 39, 43, 60).
3.2.2. Clinical characteristics
Participants evaluated by BIA did not vary greatly between studies. One paper included critically ill patients only (25), Moonen et al. (21) also included ICU patients, and another included post-ICU patients (26). The studies with US focused on the muscular changes of the critically ill (6, 28, 30), and measured the predictive value of one measurement on the prognosis of the moderately to severely ill (31) and non-ICU patients (32). Meanwhile, of the studies that used CT scans, three only evaluated ICU patients (36, 46, 54). In some studies, a cohort of non-COVID-19 patients was enrolled for comparison to the group affected by the disease (32, 34). Regarding the sex of the participants, the most prevalent was male in most of the studies, with frequencies varying from 53.3% (41) to 93.3% (28). A few studies had smaller percentages of males in the sample, the studies of Kremer et al. that had 50% of males (32), McGovern et al. with 47.3% (47), Yang et al. with 46.9% (40), and Faiella et al. (63) with 50% and 46% in the bleeding group and control group, respectively.
The information on the parameters and moment and frequency of evaluation are presented in Table 2.
3.3. Body composition methods used in the studies
3.3.1. Body composition assessment tools and parameters assessed
BIA was used in 13 studies (9, 17–27, 33) and the measurements performed differed significantly among them. Some studies only evaluated the parameters of the hydration status, while others evaluated the parameters of fat mass (FM) and LM. In the studies that evaluated BC through BIA, various parameters of hydration and BC were reported. Two studies that utilized the same sample of the ward (n = 141) and ICU patients (n = 49) relied on predictive equations to assess other BC parameters (9, 22) as well as the study enrolling 54 patients (21). The remaining studies in which ward patients were included explored other BC parameters such as FM and fat-free mass (FFM) Ryrsø et al. (27), Da Porto et al. (19), Del Giorno et al. (17), Stevanovic et al. (33), and Hegde et al. (24) reported data of FM or VAT and not FFM.
Regarding the use of US to evaluate BC, all the studies that assessed either the parameters for MM quantity or quality were reported A total of five studies (5/6, 83.3%) reported the associations between muscle quality and quantity parameters and worse outcomes (6, 28, 30–32). No studies reported data on FM. Additionally, three of the six studies repeated the measurements before discharge or a few days after the first assessment to evaluate MM during hospitalization. In two studies, critically ill patients were included and analyzed through measurements of the diaphragm and rectus femoris echogenicity and thickness (6, 30). The echogenicity, or echodensity, in arbitrary units (AU) was measured in two studies (6, 30).
CT stood out as the most frequently used method to describe BC, with 36 studies (7, 15, 16, 34–37, 39–66). VAT was evaluated in 21 studies (15, 16, 34–36, 39–42, 45, 47, 49–51, 53, 55, 60, 63, 64, 66). MM was evaluated in another 24 studies through muscle quantity (7, 16, 36, 37, 40, 42–44, 48, 49, 52, 53, 56–62, 65, 66), SMD (36, 41, 44, 46, 47, 52, 54, 57, 58, 61, 66), or index (47, 49, 43).
3.3.2. Moment and frequency of evaluation
BIA was mostly evaluated once; Kellnar et al. on the other hand evaluated BC upon admission and on the day of discharge. US was evaluated twice during hospitalization (6, 28, 29) since the objectives were to compare the changes in the muscular tissue during hospitalization. CT, however, was mostly evaluated only once in 30 studies (7, 15, 16, 34, 35, 37–43, 45, 47–50, 52–54, 56–58, 60–66), once or twice in the studies by Hoyois et al. and Feng et al., and twice in the studies by Faiella et al., Pediconi et al., and Gualtieri et al.
Regarding the time of the evaluation, BIA was evaluated mostly within 24 h of admission (9, 17, 20–22) but other authors evaluated it upon 48 h (25, 27) of admission or even 72 h of admission (18, 23, 33). Measurements with US had a narrower interval for the first measurement of a maximum of 24 to 48 h upon admission in the cohorts in Kremer et al., Gi et al., Umbrello et al., and Formenti et al. However, the remaining studies did not provide further information on the moment of evaluation.
The numerical values of the BC parameters of the three tools are presented in Table 3.
3.4. Main findings
Some of the studies with BIA found significant results between the parameters derived from the tools and worse prognoses, for instance, phase angle (PhA) (18, 20–22, 25) and percentage of FM (24, 33). Moonen et al. also estimated BC parameters but found only PhA increased the odds of morbidity and mortality in COVID-19 patients (22). The studies utilizing BIA showed mostly PhA to be a strong indicator of severe illness (22), morbidity (21, 22), and mortality according to the studies’ findings (18, 21, 22, 25). Nevertheless, the indicator was not associated with LOS in the studies by Osuna-Padilla et al. (25) and Del Giorno et al. (17). Furthermore, Del Giorno et al. evaluated the associations between BIA parameters and mortality with ICU admission; however, a significant association using the measurements was not found (17).
Regarding the use of US, in three studies, there was an expressive reduction in muscular tissue (6, 28, 29). Furthermore, the reduction of the thickness in the rectus femoris muscle area (28) and vastus lateralis area (31) were predictors of a severe state and LOS, respectively. Higher values of echogenicity of the rectus femoris, diaphragm, and right intercostal sites also showed an association with worse outcomes in the study by Formenti et al. (30) as well as muscle area and thickness (6, 28, 31, 32).
In the studies that aimed to analyze the associations between the VAT and adverse outcomes, many of them found an association between higher values and hospitalization (16, 41), disease severity (35, 45, 55), critical illness (40, 53), MV (41), ICU admission (34, 39, 51), or mortality (41, 45, 60). Additionally, the ratio of visceral adipose tissue/subcutaneous adipose tissue (VAT/SAT) was a predictor of mortality (42, 49), such as visceral adipose tissue/muscle area (VAT/MA) (60) and the ratio of visceral adipose tissue/total adipose tissue (VAT/TAT) being predictors of disease severity (50). Furthermore, some studies found significant associations between MM and negative outcomes. Regarding SMD, MV (41), disease severity (44), and death (41, 54) were frequent among the patients with lower values. On the other hand, higher quantities of MM were indirectly related to frequencies of mortality (60, 62), and ICU admission (7, 56, 58). Nevertheless, some authors did not find significant associations between the parameters derived from CT and worse outcomes (61, 62). Similarly, Antonarelli et al. did not find associations between pectoralis muscle quantity and density with mortality nor disease severity, and Moctezuma-Velázquez et al. did not find significant associations between skeletal muscle index (SMI) and ICU admission, MV, or mortality (48).
4. Discussion
The objectives of this scoping review were to determine how BC was evaluated in the studies assessing hospitalized COVID-19 patients. Our findings suggest that CT followed by BIA and US were the main assessment tools utilized in COVID-19 adult populations. Several reasons may explain this preference. Regarding the studies with CT, the radiologic tool was routinely applied in all COVID-19 patients included in the studies to check pulmonary states. Chest scans often contain the 12th thoracic vertebrae, widely reported in the included studies as the reference scan to assess BC (7, 36, 37, 48, 53, 56, 57, 59, 60, 65, 66) and the third lumbar vertebrae as well (15, 16, 35, 40, 43, 47, 49, 51, 54, 56, 63, 64, 66). The remaining studies also utilized scans but from alternative levels (34, 38, 39, 41, 42, 45, 46, 52, 55, 58, 61, 62).
4.1. Regarding the tools, how did the studies with COVID-19 patients evaluate body composition?
Regarding the studies included in our review, there was a discrepancy in how the protocols were reported. Although a great deal of the studies described in detail how the CT scan was performed and how they proceeded with the analysis of the images, a few articles did not report the protocols for CT scanning (16, 54, 59, 64) or the image analysis (35, 52, 65, 66), neither in the manuscript nor the supplementary material. Another finding was the incomplete exploration of the results. Some studies did not report more than one parameter of BC derived from the assessment tool in their studies (7, 46, 65). An outstanding finding of the studies included was the utilization of artificial intelligence tools to determine body compartments through CT (45). This strategy can bring a faster and more accurate data report, facilitating the work of clinicians and researchers.
Not surprisingly, BIA was not reported to be used as much as CT in the hospital setting, but its characteristics (portability, non-invasiveness, convenience, and inexpensiveness) facilitate its use in routine care and research. Some requirements are needed for the evaluation, and CT outstands as a BC assessment tool for not needing them. For BIA, there are prerequisites on body size, temperature, and fluid and electrolyte balance that must be observed before the evaluation. Failing to fulfill such requirements may compromise the results (67). COVID-19 patients, especially in the intensive care unit (ICU), do not fit most of these demands, hence impairing the assessment with BIA. It is crucial to emphasize that most studies evaluating the critically-ill did not assess FM nor FFM, but the parameters which are feasible for ICU patients, like PhA and other crude values of BIA (25, 26). Comparably to BIA, which can be used at the bedside, US stood out as the third most used BC tool.
Recently, the interest in evaluating BC through US has been increasing due to its good suitability in critically ill patients (68, 69). Since some of the hospitalized patients with COVID-19 are prone to critical illness and require MV, US can be a useful method to assess MM changes due to prolonged hospitalization, allowing clinicians to make early nutritional interventions. Thus, not surprisingly, US was used in studies with critically ill patients (6, 28, 30) as well as in studies that aimed to evaluate changes in MM during hospitalization (6, 28, 29).
4.2. What were the objectives of the studies with COVID-19 patients submitted to body composition assessment?
Most studies aimed to investigate the associations between the prognosis of COVID-19 and the parameters derived from the tools. However, the studies using BIA by Moonen et al. (9), Reyes-Torres et al. (26), and Ryrsø et al. (27), the studies using US by Andrade-Júnior et al. (28) and Bologna and Pone (29), and the studies using CT by Hoyois et al. (46), Faiella et al. (63), McGovern et al. (64), and Molwitz et al. (66) all had other objectives but reported data on at least one BC parameter.
It is a fact that COVID-19 manifests itself more severely, with easier infection, and with higher morbidity and mortality in those who suffer from obesity (3–5, 70, 71). This is because obesity affects most physiological processes and presents an exacerbated inflammatory state (72), worsening the immune response. Also, the degrees of obesity according to body mass index (BMI) were directly proportional to the risk for hospitalization, ICU admission, invasive MV, and in-hospital mortality (73). However, BMI alone is not the best indicator of obesity, as it does not reflect adipose tissue content nor its distribution (74), and most previous studies did not evaluate adiposity itself but an estimation that may not have provided reliable clinical data.
Besides obesity, reduced MM or low SMD were associated with a worse prognosis in patients with COVID-19 (6, 7). Some studies have shown associations between muscle quality and quantity parameters and worse results (6, 28, 30–32), using US as an evaluation tool. This shows us that it is essential to know the muscle quantity and quality of individuals. Others using the BIA tool verified the relationship of PhA (18, 20–22, 25) and percentage of FM (24, 33) with a worse prognosis. Furthermore, in more current studies, authors showed controversial results in which the amount of MM is not associated with negative results, such as frequency of mortality (56, 60) and ICU admission (56, 58).
These controversial results might have occurred due to the limitations of the evaluation tool used, since the studies that showed no relationship between the amount of MM and negative results used CT, which can be influenced by the size of the patient and tissues such as subcutaneous adipose tissue; for example, even muscles may not appear in the cross-sectional image (75).
4.3. What were the main findings regarding body composition parameters and COVID-19 patients?
Our results show that a great number of studies aimed to analyze the associations between BC and COVID-19 prognosis. The study by Moonen et al. was the most comprehensive, including data not only on hydration status but on VAT, MM, and FFM among others (21, 22).
BIA can estimate BC based on prediction equations, but unfortunately, the equations are used for specific populations, increasing the possibility of misestimation (76). Although 10 studies evaluated BIA and outcomes in COVID-19, most of these studies did not find an association between the BC estimation and risk of severe disease (17, 18, 21, 22). Nevertheless, Hegde et al. found the percentage of FM to be an indicator of LOS and disease severity upon admission (24). Many reasons may have contributed to the lack of evidence, e.g., small samples, utilization of inadequate equations, and non-attendance to the prerequisites of BIA evaluation among others.
In the study by Moonen et al. (22) that aimed to assess the differences in BC between ward patients (n = 30), and ICU patients (n = 24), several parameters (soft lean mass, percentage of FM, FFM, FM, dry weight, VAT area, and SMI) were assessed to find possible associations between BC and prognoses, but no significant results were found. Reliable results may be affected by the hydration state of the ICU patients.
Another study regarding BC assessment in COVID-19 patients through US (28) found that patients in a severe state had a reduction in both the cross-sectional rectus femoris muscle area and in the thickness of the anterior compartment of the quadriceps (28). MM was also a predictor of LOS in patients with moderate to severe disease in the study by Gil et al. (62), and changes in muscle parameters (echogenicity) were a predictor of mortality in the critically ill (30). In inflammatory diseases like COVID-19, impaired protein synthesis and catabolism leading to sarcopenia are associated with high CRP concentration; however, this relationship is not yet clear (11).
However, there is no data to support the validity of US to assess BC in specific populations for predicting COVID-19 prognosis (77). This could be due to the lack of standardization of the measurements and the absence of cutoff values for US parameters, e.g., the thickness of the vastus intermedius muscles and the rectus femoris, and the thickness of the quadriceps muscle layer, to evaluate the loss of MM and quality (78). Nevertheless, CT cutoff values for visceral obesity, low muscularity, muscle attenuation, and SMI were determined from many populations in the included studies. The associations between a worse prognosis and CT parameters were reported not only in original articles but also in secondary analyses.
In a meta-analysis with four studies evaluating BC and outcomes in COVID-19 patients, a higher VAT area was significantly associated with ICU admission and MV (79). Furthermore, in another meta-analysis with 539 patients utilizing CT cross-sectional images (slices), increased TAT and higher VAT areas had a significant association with COVID-19 disease severity (80).
4.4. What body composition alterations occurred in patients with COVID-19 during hospitalization?
A few studies evaluated the status of MM during hospitalization, and the most used tool for this assessment was the US. It was evident in three studies that the thickness of MM decreased (6, 28, 29). The loss of the tissue can be explained by a few reasons. COVID-19 patients have a combination of symptoms that may reduce nutritional intake as well as a systemic inflammation state that accelerates the MM loss during hospitalization (11, 81). Additionally, the immobilization and poor nutrition throughout the hospital stay also impair the maintenance of MM (82). Therefore, COVID-19 patients may suffer from decreased functional capacity and low physical function, as well as a hindered conduct of daily-life activities after hospital discharge (83). In the retrospective study by Bologna and Pone (29) which used US to verify the preservation of MM during hospitalization after arginine supplementation, the treated group had a significant maintenance of the MM when compared to the control group. It is important that, in clinical practice, not only must the identification of the patient’s risk for nutritional deterioration be addressed, but also the implementation of an adequate nutritional strategy. Hence, individualized, multi-modal nutritional care must be implemented from the beginning of admission (82).
This scoping review has several limitations. The first is the non-inclusion of potential scientific productions. Our searches were conducted in three different scientific literature databases and resulted in 1,220 citations and another six citations were added from the bibliography lists available in the selected articles (18, 43, 53, 54, 57, 61). These three databases cover most of the medical literature regarding BC and COVID-19. However, studies published in journals not indexed in these databases were probably not included. Furthermore, a great number of the included journal papers that evaluated the associations between BC and prognosis in COVID-19 patients had very low levels of scientific evidence due to, e.g., their small sample sizes and observational designs. Additionally, the variability between studies was high in terms of sample size, statistical analysis, methodologies applied at the moment of the evaluation, clinical conditions of the patients, and the parameters retrieved from the assessments. Notably, this could be due to the number of centers enrolled in the studies, which is also a determinant of external validity as well as the sample size estimation for each study.
Although our study presents several limitations, its strengths must be addressed. This was the first scoping review evaluating BC assessment in COVID-19 patients. Our main findings suggest that BC tools were used specially to provide predictive value to COVID-19 prognosis. Henceforth, the interrelations between BC and COVID-19 must be further investigated through original articles and secondary studies, preferably for each kind of assessment tool. Our perspectives are addressed to clinicians and researchers that may have a better overview regarding the state of the art of BC and COVID-19. Thus, health practitioners and researchers may conduct BC assessments in clinical practice or elucidate through systematic reviews better thresholds for BC in COVID-19 patients for the early detection of severity risk.
5. Conclusion
Our findings suggest that CT was the most common BC assessment tool, followed by BIA and US. This finding may be due to the opportunistic nature of CT, as patients had the scans to assess lung impairment during the disease. Most studies evaluated BC to find associations with adverse events, such as LOS and mortality. There is little evidence about BC changes during hospitalization. As the COVID-19 pandemic continues worldwide, new studies to be published may fill this gap in the literature.
Author contributions
IV: methodology, data curation, writing-original draft preparation, and writing-reviewing, and editing. IS and AB: writing-reviewing and editing. AF: conceptualization, methodology, data curation, writing-original draft preparation, and writing-reviewing, and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001. AF received a productivity scholarship from the Brazilian National Council for Scientific and Technological Development (CNPq).
Acknowledgments
The authors would like to thank Flávia Moraes Silva for the methodological contributions to this scoping review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1176441/full#supplementary-material
References
1. Guo, G , Ye, L , Pan, K , Chen, Y , Xing, D , Yan, K, et al. New insights of emerging SARS-CoV-2: epidemiology, etiology, clinical features, clinical treatment, and prevention. Front Cell Dev Biol. (2020) 8:1–22. doi: 10.3389/fcell.2020.00410
2. WHO . COVID-19 weekly epidemiological update—140. World Health Organization (2023). 1–13. Available at: https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update
3. Silverio, R , Gonçalves, DC , Andrade, MF , and Seelaender, M . Coronavirus disease 2019 (COVID-19) and nutritional status: the missing link. Adv Nutr. (2020) 2019:1–11. doi: 10.1093/advances/nmaa125
4. Banerjee, M , Gupta, S , Sharma, P , Shekhawat, J , and Gauba, K . Obesity and COVID-19: a fatal Alliance. Indian J Clin Biochem. (2020) 35:410–7. doi: 10.1007/s12291-020-00909-2
5. Calder, PC , Carr, AC , Gombart, AF , and Eggersdorfer, M . Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. (2020) 12:1–3. doi: 10.3390/nu12082326
6. Umbrello, M , Guglielmetti, L , Formenti, P , Antonucci, E , Cereghini, S , Filardo, C, et al. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19–related ARDS. Nutrition. (2021) 91–92:111449. doi: 10.1016/j.nut.2021.111449
7. Giraudo, C , Librizzi, G , Fichera, G , Motta, R , Balestro, E , Calabrese, F, et al. Reduced muscle mass as predictor of intensive care unit hospitalization in COVID-19 patients. PLoS One. (2021) 16:e0253433. doi: 10.1371/journal.pone.0253433
8. Wang, P , Li, Y , and Wang, Q . Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition. (2021) 84:111104. doi: 10.1016/j.nut.2020.111104
9. Pfx, H , Jh, A , Jans, I , Rh, A , and Zanten, V . Protein requirements and provision in hospitalised COVID-19 ward and ICU patients: agreement between calculations based on body weight and height, and measured bioimpedance lean body mass. Clin Nutr ESPEN. (2022) 49:474–82. doi: 10.1016/j.clnesp.2022.03.001
10. Bano, G , Trevisan, C , Carraro, S , Solmi, M , Luchini, C , Stubbs, B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. (2017) 96:10–5. doi: 10.1016/j.maturitas.2016.11.006
11. Virgens, IPA , Santana, NM , Lima, SCVC , and Fayh, APT . Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br J Nutr. (2020) 126:1–25. doi: 10.1017/S0007114520004420
12. Smith, LO , Olieman, JF , Berk, KA , Ligthart‐Melis, GC , and Earthman, CP . Clinical applications of body composition and functional status tools for nutritional assessment of hospitalized adults: a systematic review. J Parenter Enter Nutr. (2022) 47:11–29. doi: 10.1002/jpen.2444
13. Peters, MDJ , Godfrey, C , McInerney, P , Munn, Z , Tricco, AC , and Khalil, H . “Chapter 11: Scoping Reviews (2020 version),” in JBI Manual for Evidence Synthesis, JBI, 2020. eds. E. Aromataris and Z. Munn. (2020). Available from https://synthesismanual.jbi.global
14. Tricco, AC , Lillie, E , Zarin, W , O’Brien, KK , Colquhoun, H , Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
15. Chandarana, H , Pisuchpen, N , Krieger, R , Dane, B , and Mikheev, A . Association of body composition parameters measured on CT with risk of hospitalization in patients with Covid-19. Eur J Radiol. (2021) 145:110031–7. doi: 10.1016/j.ejrad.2021.110031
16. Chandarana, H , Dane, B , Mikheev, A , Taffel, MT , Feng, Y , and Rusinek, H . Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom Radiol. (2021) 46:818–25. doi: 10.1007/s00261-020-02693-2
17. Del Giorno, R , Quarenghi, M , Stefanelli, K , Capelli, S , Giagulli, A , Quarleri, L, et al. Nutritional risk screening and body composition in covid-19 patients hospitalized in an internal medicine ward. Int J Gen Med. (2020) 13:1643–51. doi: 10.2147/IJGM.S286484
18. Cornejo-Pareja, I , Vegas-Aguilar, IM , García-Almeida, JM , Bellido-Guerrero, D , Talluri, A , Lukaski, H, et al. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: a longitudinal cohort study. Clin Nutr. (2021) 41:3106–14. doi: 10.1016/j.clnu.2021.02.017
19. Da Porto, A , Tascini, C , Peghin, M , Sozio, E , Colussi, G , Casarsa, V, et al. Prognostic role of malnutrition diagnosed by bioelectrical impedance vector analysis in older adults hospitalized with covid-19 pneumonia: a prospective study. Nutrients. (2021) 13:4085. doi: 10.3390/nu13114085
20. Kellnar, A , Hoppe, JM , Brunner, S , and Stremmel, C . Hospitalization for COVID-19 is associated with significant changes in body composition. Clin Nutr ESPEN. (2021) 45:499–502. doi: 10.1016/j.clnesp.2021.07.033
21. Moonen, HP , van Zanten, FJL , Driessen, L , de Smet, V , Slingerland-Boot, R , Mensink, M, et al. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: the BIAC-19 study. Clin Nutr. (2021) 40:2328–36. doi: 10.1016/j.clnu.2020.10.023
22. Moonen, HP , Bos, AE , and Jh, A . Bioelectric impedance body composition and phase angle in relation to 90-day adverse outcome in hospitalized COVID-19 ward and ICU patients: the prospective BIAC-19 study. Clin Nutr. (2021) 46:185–92. doi: 10.1016/j.clnesp.2021.10.010
23. Cornejo-Pareja, I , Vegas-Aguilar, IM , Lukaski, H , Talluri, A , Bellido-Guerrero, D , Tinahones, FJ, et al. Overhydration assessed using bioelectrical impedance vector analysis adversely affects 90-day clinical outcome among SARS-CoV2 patients: a new approach. Nutrients. (2022) 14:2726. doi: 10.3390/nu14132726
24. Hegde, SG , Dhareshwar, S , Bandyopadhyay, S , Kuriyan, RR , Idiculla, J , Ghosh, S, et al. Central obesity in low BMI as a risk factor for COVID-19 severity in south Indians. Asia Pac J Clin Nutr. (2022) 31:142–6. doi: 10.6133/apjcn.202203_31(1).0015
25. Osuna-Padilla, IA , Rodríguez-Moguel, NC , Rodríguez-Llamazares, S , Aguilar-Vargas, A , Casas-Aparicio, GA , Ríos-Ayala, MA, et al. Low phase angle is associated with 60-day mortality in critically ill patients with COVID-19. J Parenter Enter Nutr. (2022) 46:828–35. doi: 10.1002/jpen.2236
26. Reyes-Torres, CA , Flores-López, A , Osuna-Padilla, IA , Hernández-Cárdenas, CM , and Serralde-Zúñiga, AE . Phase angle and overhydration are associated with post-extubating dysphagia in patients with COVID-19 discharged from the ICU. Nutr Clin Pract. (2022) 37:110–6. doi: 10.1002/ncp.10781
27. Ryrsø, CK , Dungu, AM , Hegelund, MH , Jensen, AV , Sejdic, A , Faurholt-Jepsen, D, et al. Body composition, physical capacity, and immuno-metabolic profile in community-acquired pneumonia caused by COVID-19, influenza, and bacteria: a prospective cohort study. Int J Obes. (2022) 46:817–24. doi: 10.1038/s41366-021-01057-0
28. de Andrade-Junior, MC , Salles, ICD d , de Brito, CMM , Pastore-Junior, L , Righetti, RF , and Yamaguti, WP . Skeletal muscle wasting and function impairment in intensive care patients with severe COVID-19. Front Physiol. (2021) 12:1–13. doi: 10.3389/fphys.2021.640973
29. Bologna, C , and Pone, E . Clinical study on the efficacy and safety of arginine administered orally in association with other active ingredients or the prevention and treatment of sarcopenia in patients with COVID-19-related pneumonia, hospitalized in a sub-intensive care unit. Healthcare. (2022) 10:162. doi: 10.3390/healthcare10010162
30. Formenti, P , Umbrello, M , Castagna, V , Cenci, S , Bichi, F , Pozzi, T, et al. Respiratory and peripheral muscular ultrasound characteristics in ICU COVID 19 ARDS patients. J Crit Care. (2021) 67:14–20. doi: 10.1016/j.jcrc.2021.09.007
31. Gil, S , Jacob Filho, W , Shinjo, SK , Ferriolli, E , Busse, AL , Avelino-Silva, TJ, et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: a prospective observational study. J Cachexia Sarcopenia Muscle. (2021) 12:1871–8. doi: 10.1002/jcsm.12789
32. Kremer, WM , Labenz, C , Kuchen, R , Sagoschen, I , Bodenstein, M , Schreiner, O, et al. Sonographic assessment of low muscle quantity identifies mortality risk during COVID-19: a prospective single-Centre study. J Cachexia Sarcopenia Muscle. (2022) 13:169–79. doi: 10.1002/jcsm.12862
33. Stevanovic, D , Zdravkovic, V , Poskurica, M , Petrovic, M , Cekerevac, I , Zdravkovic, N, et al. The role of bioelectrical impedance analysis in predicting COVID-19 outcome. Front Nutr. (2022) 9:1–8. doi: 10.3389/fnut.2022.906659
34. Battisti, S , Pedone, C , Napoli, N , Russo, E , Agnoletti, V , Nigra, SG, et al. Computed tomography highlights increased visceral adiposity associated with critical illness in covid-19. Diabetes Care. (2020) 43:e129–30. doi: 10.2337/dc20-1333
35. Favre, G , Legueult, K , Pradier, C , Raffaelli, C , Ichai, C , Iannelli, A, et al. Visceral fat is associated to the severity of COVID-19. Metabolism. (2020) 115:154440. doi: 10.1016/j.metabol.2020.154440
36. Gualtieri, P , Falcone, C , Romano, L , Macheda, S , Correale, P , Arciello, P, et al. Body composition findings by computed tomography in sars-cov-2 patients: increased risk of muscle wasting in obesity. Int J Mol Sci. (2020) 21:1–13. doi: 10.3390/ijms21134670
37. Kottlors, J , Zopfs, D , Fervers, P , Bremm, J , Abdullayev, N , Maintz, D, et al. Body composition on low dose chest CT is a significant predictor of poor clinical outcome in COVID-19 disease—a multicenter feasibility study. Eur J Radiol. (2020) 132:109274. doi: 10.1016/j.ejrad.2020.109274
38. Petersen, A , Bressem, K , Albrecht, J , Thieß, HM , Vahldiek, J , Hamm, B, et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. (2020) 110:154317. doi: 10.1016/j.metabol.2020.154317
39. Watanabe, M , Caruso, D , Tuccinardi, D , Risi, R , Zerunian, M , Polici, M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. (2020) 111:154319. doi: 10.1016/j.metabol.2020.154319
40. Yang, Y , Ding, L , Zou, X , Shen, Y , Hu, D , Hu, X, et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with Sars‐COV‐2. Obesity. (2020) 28:2040–8. doi: 10.1002/oby.22971
41. Besutti, G , Pellegrini, M , Ottone, M , Cantini, M , Milic, J , Bonelli, E, et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLoS One. (2021) 16:1–16. doi: 10.1371/journal.pone.0251768
42. Bunnell, KM , Thaweethai, T , Buckless, C , Shinnick, DJ , Torriani, M , Foulkes, AS, et al. Body composition predictors of outcome in patients with COVID-19. Int J Obes. (2021) 45:1–6. doi: 10.1038/s41366-021-00907-1
43. Damanti, S , Cristel, G , Alvise, G , Paola, E , Da, V , Gobbi, A, et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin Nutr. (2021) 41:2965–72. doi: 10.1016/j.clnu.2021.08.004
44. Feng, Z , Zhao, H , Kang, W , Liu, Q , Wu, J , Bragazzi, NL, et al. Association of Paraspinal Muscle Measurements on chest computed tomography with clinical outcomes in patients with severe coronavirus disease 2019. J Gerontol Ser A Biol Sci Med Sci. (2021) 76:E78–84. doi: 10.1093/gerona/glaa317
45. Goehler, A , Hsu, TMH , Seiglie, JA , Siedner, MJ , Lo, J , Triant, V, et al. Visceral adiposity and severe COVID-19 disease: application of an artificial intelligence algorithm to improve clinical risk prediction. Open Forum Infect Dis. (2021) 8:ofab275. doi: 10.1093/ofid/ofab275
46. Hoyois, A , Ballarin, A , Thomas, J , Lheureux, O , Preiser, JC , Coppens, E, et al. Nutrition evaluation and management of critically ill patients with COVID-19 during post–intensive care rehabilitation. J Parenter Enter Nutr. (2021) 45:1–11. doi: 10.1002/jpen.2101
47. McGovern, J , Dolan, R , Richards, C , Laird, BJ , McMillan, DC , and Maguire, D . Relation between body composition, systemic inflammatory response, and clinical outcomes in patients admitted to an urban teaching hospital with COVID-19. J Nutr. (2021) 151:1–9. doi: 10.1093/jn/nxab142
48. Moctezuma-Velazquez, P , Miranda-Zazueta, G , Ortiz-Brizuela, E , Gonzalez-Lara, MF , Tamez-Torres, KM , Roman-Montes, CM, et al. Low thoracic skeletal muscle area is not associated with negative outcomes in patients with COVID-19. Am J Phys Med Rehabil. (2021) 100:413–8. doi: 10.1097/PHM.0000000000001716
49. Nobel, YR , Su, SH , Anderson, MR , Luk, L , Small-Saunders, JL , Reyes-Soffer, G, et al. Relationship between body composition and death in patients with COVID-19 differs based on the presence of gastrointestinal symptoms. Dig Dis Sci. (2021) 67:4484–91. doi: 10.1007/s10620-021-07324-4
50. Ogata, H , Mori, M , Jingushi, Y , Matsuzaki, H , Katahira, K , Ishimatsu, A, et al. Impact of visceral fat on the prognosis of coronavirus disease 2019: an observational cohort study. BMC Infect Dis. (2021) 21:1–8. doi: 10.1186/s12879-021-06958-z
51. Pediconi, F , Rizzo, V , Schiaffino, S , Cozzi, A , Della Pepa, G , Galati, F, et al. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes Res Clin Pract. (2021) 15:89–92. doi: 10.1016/j.orcp.2020.12.002
52. Polat, M , Salbaş, ÇS , Sari, S , Doğan, M , Çam, S , and Karadağ, A . The association between prognosis and sarcopenia assessed by psoas muscle measurements in elderly male patients with covid-19. Turk Geriatr Derg. (2021) 24:557–66. doi: 10.31086/tjgeri.2021.253
53. Poros, B , Sabine, B-PA , Sabel, B , Stemmler, HJ , Wassilowsky, D , Weig, T, et al. Anthropometric analysis of body habitus and outcomes in critically ill COVID-19 patients. Obes Med. (2021) 25:100358–6. doi: 10.1016/j.obmed.2021.100358
54. Rossi, AP , Gottin, L , Donadello, K , Schweiger, V , Brandimarte, P , Zamboni, GA, et al. Intermuscular adipose tissue as a risk factor for mortality and muscle injury in critically ill patients affected by COVID-19. Front Physiol. (2021) 12:1–6. doi: 10.3389/fphys.2021.651167
55. Scheffler, M , Genton, L , Graf, CE , Remuinan, J , Gold, G , Zekry, D, et al. Prognostic role of subcutaneous and visceral adiposity in hospitalized octogenarians with covid-19. J Clin Med. (2021) 10:5500. doi: 10.3390/jcm10235500
56. Schiaffino, S , Albano, D , Cozzi, A , Messina, C , Arioli, R , Bnà, C, et al. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology. (2021) 300:E328–36. doi: 10.1148/radiol.2021204141
57. Viddeleer, AR , Raaphorst, J , Min, M , Beenen, LFM , Scheerder, MJ , Vlaar, APJ, et al. Intramuscular adipose tissue at level Th12 is associated with survival in COVID-19. J Cachexia Sarcopenia Muscle. (2021) 12:823–7. doi: 10.1002/jcsm.12696
58. Antonarelli, M , and Fogante, M . Chest CT-derived muscle analysis in COVID-19 patients. Tomography. (2022) 8:414–22. doi: 10.3390/tomography8010034
59. Attaway, A , Welch, N , Dasarathy, D , Amaya-Hughley, J , Bellar, A , Biehl, M, et al. Acute skeletal muscle loss in SARS-CoV-2 infection contributes to poor clinical outcomes in COVID-19 patients. J Cachexia Sarcopenia Muscle. (2022) 13:2435–46. doi: 10.1002/jcsm.13052
60. Beltrão, FEL , Beltrão, DCA , Carvalhal, G , Beltrão, FNL , de Aquino, IM , Brito, TDS, et al. Low muscle mass and high visceral fat mass predict mortality in patients hospitalized with moderate-to-severe COVID-19: a prospective study. Endocr Connect. (2022) 11:e220290. doi: 10.1530/EC-22-0290
61. Bodolea, C , Nemes, A , Avram, L , Craciun, R , Coman, M , Ene-Cocis, M, et al. Nutritional risk assessment scores effectively predict mortality in critically ill patients with severe COVID-19. Nutrients. (2022) 14:2105. doi: 10.3390/nu14102105
62. do Amaral e Castro, A , Yokoo, P , Fonseca, EKUN , Otoni, JC , Haiek, SL , Shoji, H, et al. Prognostic factors of worse outcome for hospitalized COVID-19 patients, with emphasis on chest computed tomography data: a retrospective study. Einstein. (2022) 20:eAO6953–7. doi: 10.31744/einstein_journal/2022ao6953
63. Faiella, E , Castiello, G , Santucci, D , Pacella, G , Bernetti, C , Villamu, MM, et al. Analysis of risk factors of soft tissue bleeding in COVID-19 patients: a point of view after two years of pandemic. J Clin Med Res. (2022) 14:188–95. doi: 10.14740/jocmr4708
64. McGovern, J , Al-Azzawi, Y , Kemp, O , Moffitt, P , Richards, C , Dolan, RD, et al. The relationship between frailty, nutritional status, co-morbidity, CT-body composition and systemic inflammation in patients with COVID-19. J Transl Med. (2022) 20:1–8. doi: 10.1186/s12967-022-03300-2
65. Menozzi, R , Valoriani, F , Prampolini, F , Banchelli, F , Boldrini, E , Martelli, F, et al. Impact of sarcopenia in SARS-CoV-2 patients during two different epidemic waves. Clin Nutr ESPEN. (2022) 47:252–9. doi: 10.1016/j.clnesp.2021.12.001
66. Molwitz, I , Ozga, AK , Gerdes, L , Ungerer, A , Köhler, D , Ristow, I, et al. Prediction of abdominal CT body composition parameters by thoracic measurements as a new approach to detect sarcopenia in a COVID-19 cohort. Sci Rep. (2022) 12:1–10. doi: 10.1038/s41598-022-10266-0
67. Mazocco, L , Gonzalez, MC , Barbosa-Silva, TG , and Chagas, P . Sarcopenia in Brazilian rural and urban elderly women: is there any difference? Nutrition. (2019) 58:120–4. doi: 10.1016/j.nut.2018.06.017
68. Looijaard, WGPM , Molinger, J , and Weijs, PJM . Measuring and monitoring lean body mass in critical illness. Curr Opin Crit Care. (2018) 24:241–7. doi: 10.1097/MCC.0000000000000511
69. Mundi, MS , Patel, JJ , and Martindale, R . Body composition technology: implications for the ICU. Nutr Clin Pract. (2019) 34:48–58. doi: 10.1002/ncp.10230
70. Muscogiuri, G , Pugliese, G , Barrea, L , Savastano, S , and Colao, A . Obesity: the “Achilles heel” for COVID-19? Metabolism. (2020) 108:154251. doi: 10.1016/j.metabol.2020.154251
71. Sanchis-Gomar, F , Lavie, CJ , Mehra, MR , Henry, BM , and Lippi, G . Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. (2020) 95:1445–53. doi: 10.1016/j.mayocp.2020.05.006
72. Fischer-Posovszky, P , and Möller, P . The immune system of adipose tissue: obesity-associated inflammation. Pathologe. (2020) 41:224–9. doi: 10.1007/s00292-020-00782-z
73. Yang, J , Tian, C , Chen, Y , Zhu, C , Chi, H , and Li, J . Obesity aggravates COVID-19: an updated systematic review and meta-analysis. J Med Virol. (2021) 93:2662–74. doi: 10.1002/jmv.26677
74. Gonzalez, MC , Correia, MITD , and Heymsfield, SB . A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care. (2017) 20:314–21. doi: 10.1097/MCO.0000000000000395
75. Prado, CMM , and Heymsfield, SB . Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enter Nutr. (2014) 38:940–53. doi: 10.1177/0148607114550189
76. Gonzalez, MC , Barbosa-Silva, TG , and Heymsfield, SB . Bioelectrical impedance analysis in the assessment of sarcopenia. Curr Opin Clin Nutr Metab Care. (2018) 21:366–74. doi: 10.1097/MCO.0000000000000496
77. Sheean, P , Gonzalez, MC , Prado, CM , Mckeever, L , Hall, AM , and Braunschweig, CA . American Society for Parenteral and Enteral Nutrition clinical guidelines: The validity of body composition assessment in clinical populations preliminary remarks (intent of guidelines). (2019)
78. Albano, D , Messina, C , Vitale, J , and Sconfienza, LM . Imaging of sarcopenia: old evidence and new insights. Eur Radiol. (2020) 30:2199–208. doi: 10.1007/s00330-019-06573-2
79. Földi, M , Farkas, N , Kiss, S , Dembrovszky, F , Szakács, Z , Balaskó, M, et al. Visceral adiposity elevates the risk of critical condition in COVID-19: a systematic review and meta-analysis. Obesity. (2021) 29:521–8. doi: 10.1002/oby.23096
80. Pranata, R , Anthonius, M , Huang, I , and Yonas, E . Visceral adiposity, subcutaneous adiposity, and severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Clin Nutr ESPEN J. (2021) 43:163–8. doi: 10.1016/j.clnesp.2021.04.001
81. Anker, MS , Landmesser, U , von Haehling, S , Butler, J , Coats, AJS , and Anker, SD . Weight loss, malnutrition, and cachexia in COVID-19: facts and numbers. J Cachexia Sarcopenia Muscle. (2020) 12:1–5. doi: 10.1002/jcsm.12674
82. Cereda, E , Clavé, P , Collins, PF , Holdoway, A , and Wischmeyer, PE . Recovery focused nutritional therapy across the continuum of care: learning from covid-19. Nutrients. (2021) 13:3293. doi: 10.3390/nu13093293
Keywords: nutritional status, skeletal muscle mass, body fat, coronavirus disease 2019, hospitalized patient
Citation: das Virgens IPA, Sousa IM, Bezerra ADL and Fayh APT (2023) Assessment of body composition in adults hospitalized with acute COVID-19: a scoping review. Front. Nutr. 10:1176441. doi: 10.3389/fnut.2023.1176441
Edited by:
Klara Komici, University of Molise, ItalyCopyright © 2023 das Virgens, Sousa, Bezerra and Fayh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Paula Trussardi Fayh, YW5hLmZheWhAdWZybi5icg==
 Isabel Pinto Amorim das Virgens1,2
Isabel Pinto Amorim das Virgens1,2 Ana Paula Trussardi Fayh
Ana Paula Trussardi Fayh