- 1Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, United States
- 2Department of Medicine, Emory University School of Medicine, Atlanta, GA, United States
- 3Nutrition and Health Sciences Doctoral Program, Laney Graduate School, Emory University, Atlanta, GA, United States
- 4Department of Internal Medicine, Division of Cardiology, Virginia Commonwealth University School of Medicine, Richmond, VA, United States
- 5Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University School of Medicine, Atlanta, GA, United States
Objective: Poor diet quality contributes to metabolic dysfunction. This study aimed to gain a greater understanding of the relationship between dietary macronutrient quality and glucose homeostasis in adults with cystic fibrosis (CF).
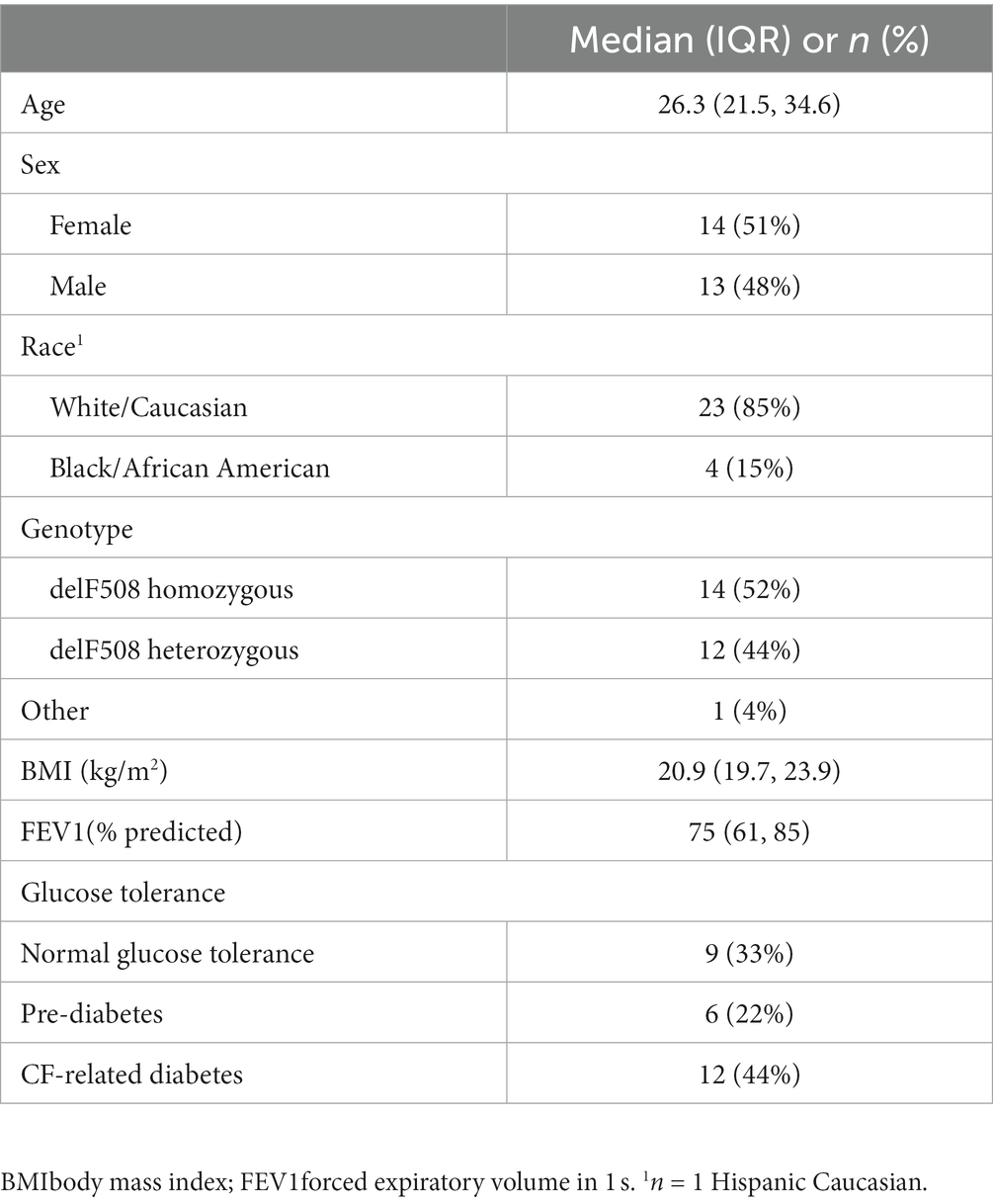
Design: This was a cross-sectional study of N = 27 adults with CF with glucose tolerance ranging from normal (n = 9) to prediabetes (n = 6) to being classified as having cystic fibrosis-related diabetes (CFRD, n = 12). Fasted blood was collected for analysis of glucose, insulin, and C-peptide. Insulin resistance was assessed by Homeostatic Model Assessment for Insulin Resistance (HOMA2-IR). Subjects without known CFRD also underwent a 2-h oral glucose tolerance test. Three-day food records were used to assess macronutrient sources. Dietary variables were adjusted for energy intake. Statistical analyses included ANOVA, Spearman correlations, and multiple linear regression.
Results: Individuals with CFRD consumed less total fat and monounsaturated fatty acids (MUFA) compared to those with normal glucose tolerance (p < 0.05). In Spearman correlation analyses, dietary glycemic load was inversely associated with C-peptide (rho = −0.28, p = 0.05). Total dietary fat, MUFA, and polyunsaturated fatty acids (PUFA) were positively associated with C-peptide (rho = 0.39–0.41, all p < 0.05). Plant protein intake was inversely related to HOMA2-IR (rho = −0.28, p = 0.048). Associations remained significant after adjustment for age and sex.
Discussion: Improvements in diet quality are needed in people with CF. This study suggests that higher unsaturated dietary fat, higher plant protein, and higher carbohydrate quality were associated with better glucose tolerance indicators in adults with CF. Larger, prospective studies in individuals with CF are needed to determine the impact of diet quality on the development of CFRD.
Introduction
Cystic fibrosis (CF) is an autosomal recessive genetic disease that leads to multi-organ impairment, including the lungs, pancreas, and gastrointestinal system (1). The disease results from cystic fibrosis transmembrane conductance regulator (CFTR) protein defects resulting in the inability to transport chloride and bicarbonate across epithelial cell membranes, subsequent mucosal abnormalities, and downstream chronic inflammation and tissue damage. Cystic fibrosis-related diabetes (CFRD) is one of the most common co-morbidities of CF and is associated with significantly increased morbidity and mortality (2). It is currently proposed that a decline in β-cell function begins in early childhood and results in diminished insulin secretion and delayed first phase insulin response (2, 3). In addition, individuals with CF have a diminished incretin response that contributes to impaired insulin secretion (4). Secondary factors (either intrinsic or extrinsic) may hasten the development of CFRD. Some of these factors are known, such as having first degree relatives with type 2 diabetes and having pancreatic exocrine insufficiency (5). Whether there are modifiable secondary factors contributing to CFRD development, such as dietary intake, is unknown.
The CF diet is typically high in energy-dense, nutrient-poor foods. Historically, individuals with CF were prescribed high-energy, high-fat diets to maintain their hypermetabolic state and offset malabsorption. Current CF dietary guidelines recommend an energy intake of 1.2 to 1.5 times that of the general population (6), but many in the CF community are revisiting the validity of this recommendation and there are growing calls to formulate evidence based dietary guidelines. Many people living with CF consume a diet abundant in high glycemic index (GI) foods, sugar sweetened beverages (SSBs), and refined sugars (6, 7). Further, the clinical recommendation for a high-fat diet in individuals with CF has historically resulted in an over-reliance on dietary saturated fats (7–9). Dietary intake analyses of individuals with CF reflect diets that are generally low in quality, as indicated by a disproportionately high intake of energy-dense, yet nutrient-poor, foods in children (7) and high added sugar and trans-fatty acid intake, as well as low Healthy Eating Index scores, in adults (10).
In non-CF populations, prolonged consumption of excess added sugars and saturated fat appear to promote a decline in β-cell function and increase insulin resistance (11–14). Although data are mixed, many studies suggest that fat quality is important in preventing the onset of type 2 diabetes (15). More specifically, diets high in saturated fat are associated with reduced insulin secretion, whereas diets high in monounsaturated fatty acids (MUFA) are associated with enhanced insulin secretion (15). Increased consumption of MUFA and polyunsaturated fatty acids (PUFA) can also lead to a reduction in HbA1C and insulin resistance (15–17). While dietary protein has known insulinotropic effects (18), observational studies in non-CF populations have indicated increased risk for diabetes with higher consumption of animal proteins and lower or no diabetes risk with higher plant protein intake (19, 20). The impact of dietary sources of macronutrients on glucose homeostasis for individuals with CF is unknown.
To date, there is a paucity of studies that investigate the metabolic sequelae of poor diet quality for individuals with CF. Thus, the purpose of this study was to quantify the relationships of diet quality indicators at the macronutrient level (e.g., carbohydrate, fat, protein) with measures of glucose homeostasis in adults with CF across the glucose tolerance spectrum. Such data may guide evidence-based updates to dietary recommendations for people living with CF.
Methods
Materials and methods
Subjects and study design
This was a prospective, cross-sectional study, which included 27 adults with clinically stable CF enrolled between 2014 and 2018. Details about the study were previously reported for a sub-set of participants and, in comparison, healthy controls (10). Briefly, inclusion criteria were, for those with CF, a confirmed CF diagnosis via chloride sweat test and/or CFTR genetic test with at least one Class I, II, or III CFTR mutation. All individuals with CF had pancreatic exocrine insufficiency and received pancreatic enzyme replacement therapy. For testing, participants were required to be on a stable medical regimen, including no oral or intravenous antibiotics or glucocorticoids, for at least 3 weeks. Exclusion criteria were current pregnancy, inability to fast overnight (including enteral tube feeds), and the most recent forced expiratory volume in 1 s (FEV1) expressed as a percentage of the predicted value (FEV1%) of <40%. All testing was performed following an overnight fast within the Georgia Clinical and Translational Science Institute Emory University Hospital Clinical Research Center (GCRC). Height and weight were assessed within the GCRC for determination of body mass index (BMI). Most recent lung function reported as percent predicted forced expiratory volume in one second (FEV1% predicted), was extracted from the electronic medical record based on spirometry performed at the Emory University Hospital Adult CF Clinic. The study was approved by the Emory Institutional Review Board, and written informed consent was conducted before any testing.
Assessment of glucose metabolism
For participants without previously diagnosed CFRD (n = 18), a standard 2-h oral glucose (75 g) tolerance test (OGTT) was performed and glucose tolerance status (normal glucose tolerance, pre-diabetes, or CFRD) was determined using cut-off glucose values recommended by the American Diabetes Association (21). For participants who already had a clinical diagnosis of CFRD within the medical chart (n = 9), short-acting insulin was not taken in the morning, as applicable, and only fasted blood was drawn. Blood was collected and processed on the day of the study. Serum was stored at −80°C until ready for analysis. Serum glucose concentrations (fasted and 2-h) were determined in real time in the Emory University Hospital Clinical Laboratory using a standard enzymatic assay for clinical care. Fasted serum insulin and C-peptide were assayed at the University of Alabama-Birmingham Metabolism Core in paired replicates using an immunofluorescence assay (TOSOH AIA 900, TOSOH Bioscience, South San Francisco, CA). The assay does not distinguish between types of insulin. The inter-assay (variation of controls between assays) and intra-assay (variation of replicates within the same assay) coefficients of variation within the Core are 3.95 and 1.49% for insulin and 6.81 and 1.67% for C-peptide, respectively. Insulin resistance was calculated with fasted glucose and insulin levels using the updated computer Homeostatic Model Assessment of Insulin Resistance (HOMA2-IR) (22).
Dietary intake
Participants were provided detailed instructions for completion of a three-day food record to include two consecutive weekdays and one weekend day. On receipt of the food record, a registered dietitian reviewed the record with the participant and asked probing questions for missed details. Records were analyzed for total kilocalories (kcal) and macronutrient composition using the Nutrition Data System for Research software (NDSR, Nutrition Coordinating Center, University of Minnesota, MN, USA; database version 2018). Dietary intake information was previously reported for a sub-set of participants (10). The current analysis focused on assessment of macronutrients from varying sources (e.g., saturated fats vs. monounsaturated fats, plant vs. animal protein intake). Dietary information was not available from one participant.
Statistical analyses
Descriptive statistics were performed on all variables. To account for differences of total caloric intake, dietary variables (except total kcal and glycemic index) were adjusted per 1,000 kcal for statistical analyses. Kruskal-Wallis tests (for continuous variables) or Fischer’s exact test (for categorical variables) were used to compare variables between CF participants across the glucose tolerance groups. Post-hoc Steel-Dwass nonparametric multiple comparison tests were performed for variables as indicated (23). Associations between dietary intake variables and glucose tolerance outcomes were analyzed using Spearman rank correlations. Relationships were subsequently assessed using multiple linear regression, with adjustment for age and sex, and with log-transformed outcomes variables as needed based on visual inspection of a normal distribution. All analyses were conducted in JMP® Pro software version 15.0.0 (SAS Institute, Cary, NC), using two-sided tests with an alpha significance value of 0.05.
Results
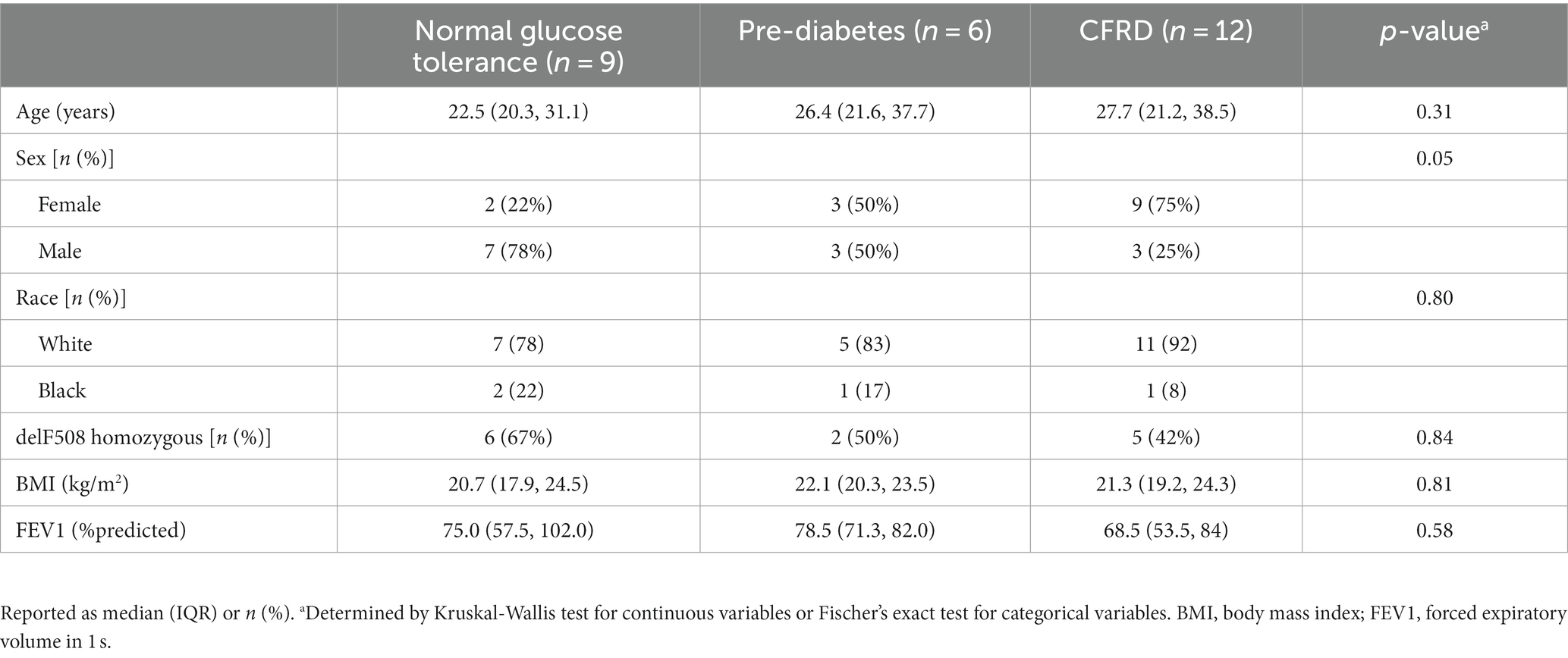
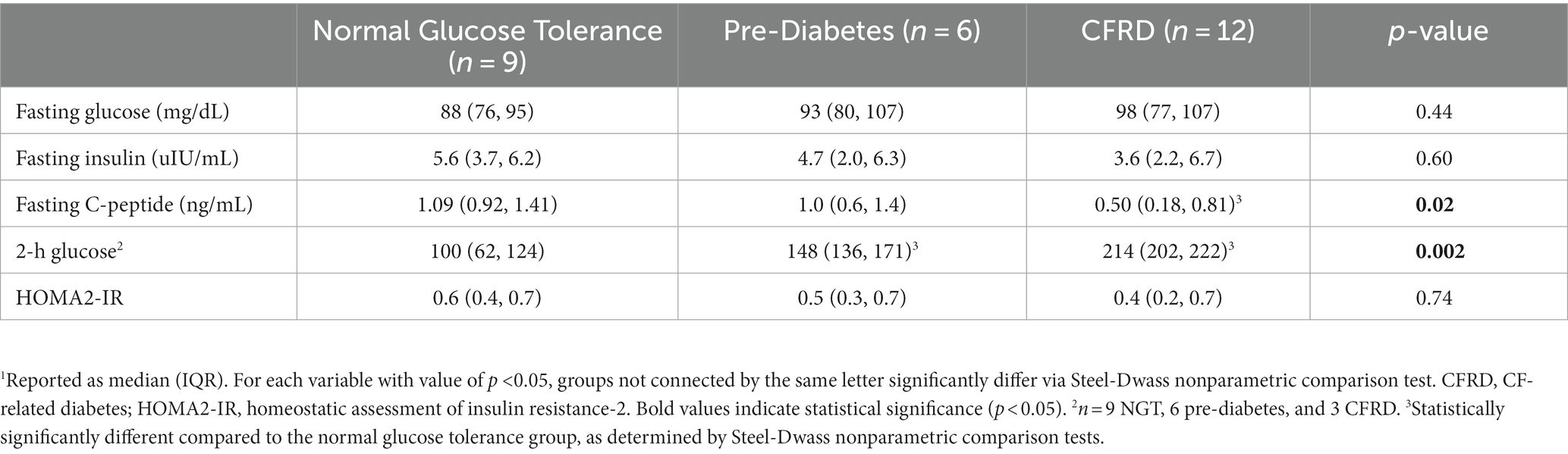
Demographic information of the N = 27 study participants with CF is provided in Table 1. Briefly, the mean age was 26 years. Approximately half the cohort was female (51%), and approximately half (52%) were homozygous for the delF508 mutation. The mean BMI (21.4 kg/m2) was below target recommendations (10, 24). Participants had moderate lung disease based on mean FEV1 (75% predicted). A total of 44% of participants had CFRD, 22% had pre-diabetes, and 33% had normal glucose tolerance. Table 2 provides demographic information based on glucose tolerance status. CFRD was more prevalent in females compared to males (75% vs. 25%, p = 0.05). Fasting glucose, insulin, or HOMA2-IR did not significantly differ between glucose tolerance groups (all p > 0.05, Table 3). Nine participants with CFRD were being treated with exogenous insulin for glucose control, three of whom were on a basal insulin regimen. Post-hoc exclusion of three participants on a basal insulin regimen did not alter the results. As expected, individuals with CFRD had significantly lower fasting C-peptide compared to those with normal glucose tolerance (p < 0.05), and those with pre-diabetes and CFRD had significantly higher 2-h glucose values following the OGTT compared to those with normal glucose tolerance (p < 0.05).
Dietary intake by glucose tolerance status
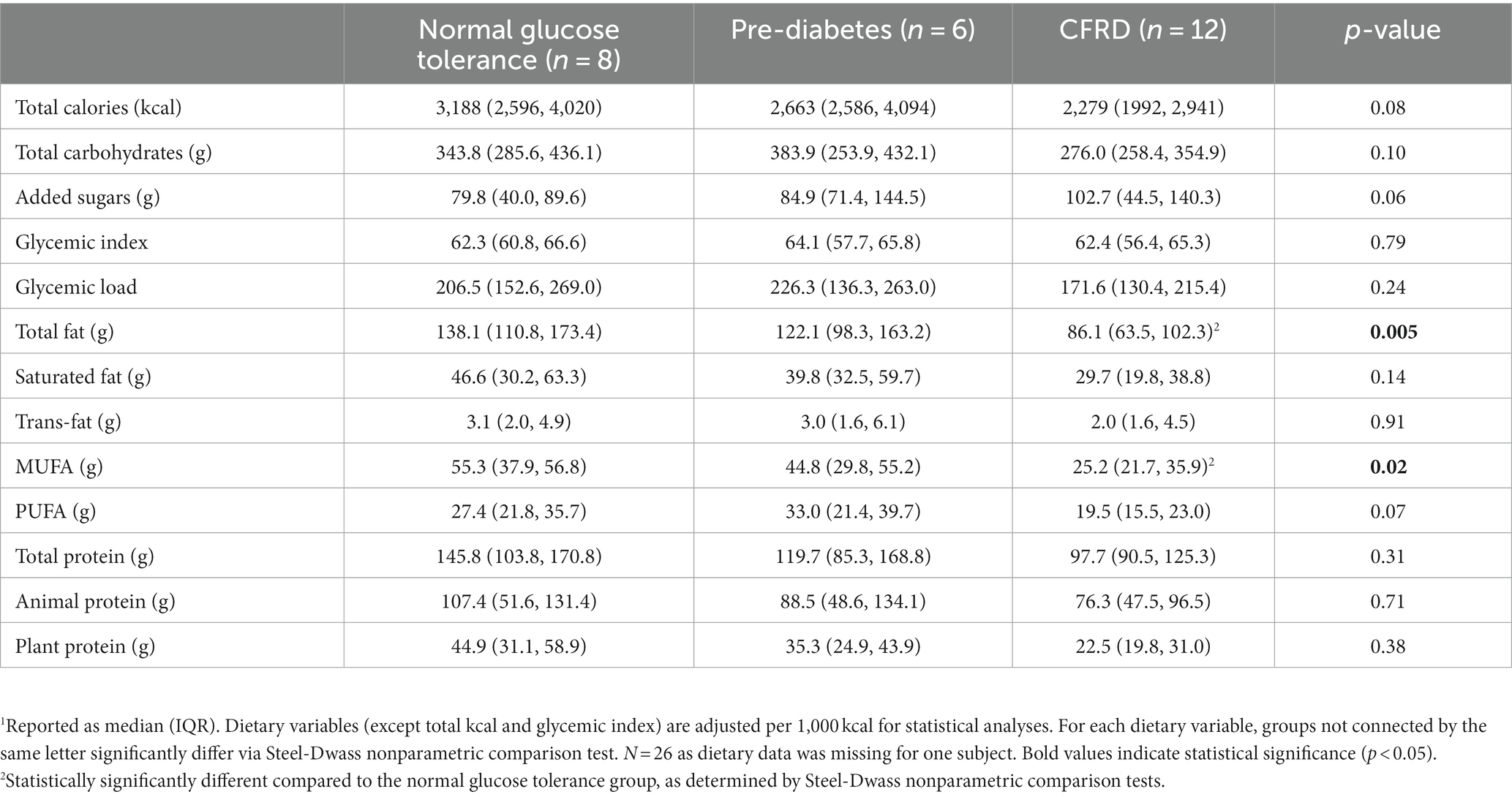
Dietary intake information by glucose tolerance status is provided in Table 4. Both total fat intake and MUFA intake were significantly lower in those with CFRD compared to those with normal glucose tolerance (p = 0.005 and 0.02, respectively). Total kcal, total dietary carbohydrates, added sugars, or dietary protein intake did not differ significantly by glucose tolerance group (all p > 0.05).
Association between dietary intake and glucose homoeostasis measures
Fasting glucose or fasting insulin did not significantly correlate with the dietary variables (Table 5). Fasting C-peptide concentrations were inversely associated with total carbohydrate intake and dietary glycemic load (rho = −0.28, p = 0.05 for both). Fasting C-peptide was significantly, positively associated with total dietary fat (rho = 0.41, p = 0.003), MUFA (rho = 0.40, p = 0.004) and PUFA (rho = 0.39, p = 0.006). There was a possible association between HOMA2-IR and added sugar intake (rho = 0.25, p = 0.08) although it did not reach statistical significance, and it was inversely associated with plant protein intake (rho = −0.28, p = 0.048). Relationships were similar after adjusting for age and sex using multiple linear regression (MLR) modeling (Supplementary Table S1). Of note, the inverse relationship between fasting C-peptide concentrations and total carbohydrate intake and dietary glycemic load became statistically significant after adjusting for age and sex (β = −0.01 ± 0.005, p = 0.01 and β = −0.02 ± 0.007, p = 0.04, respectively). The relationship between HOMA2-IR and added sugar intake likewise became statistically significant (β = 0.008 ± 0.003, p = 0.03). After adjustment for age and sex, statistically significant inverse relationships of 2-h glucose with total fat and MUFA emerged (β = −5.6 ± 1.86, p = 0.01 and β = −9.51 ± 4.32, p = 0.046, respectively). Regression plots for relationships that were similar in both Spearman and MLR analyses are shown in Figure 1. Results were similar after post-hoc exclusion of three participants on a basal insulin regimen.
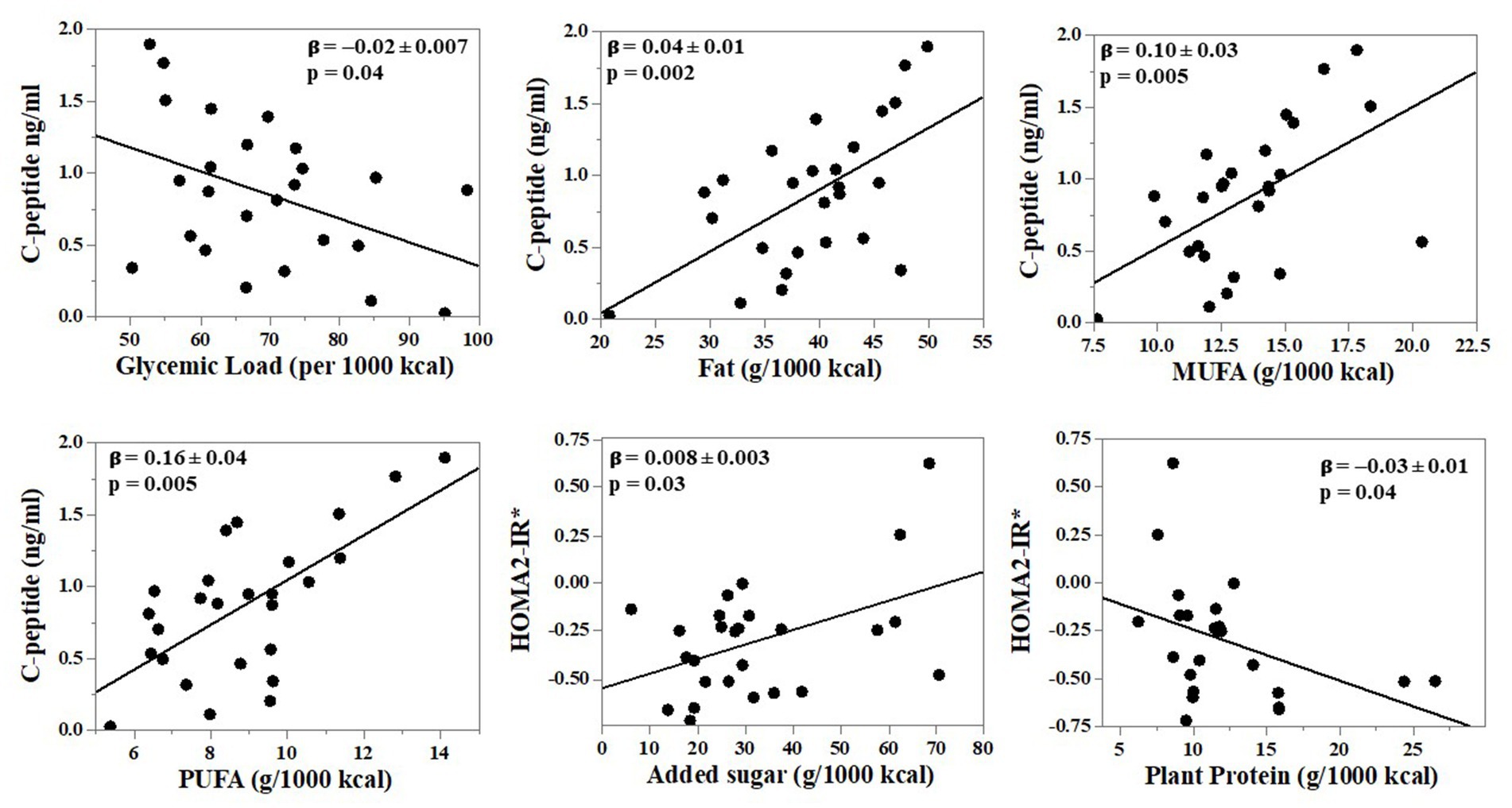
Figure 1. Independent relationships between nutrients reflecting diet quality and glucose tolerance outcomes in adults with cystic fibrosis (CF). N = 26 adults with CF. Dietary intake was collected via 3-day food records and analyzed using Nutrition Data Systems for Research. Multiple linear regression (MLR) analyses were performed with adjustment for age and sex. Shown are relationships that were statistically significant in both Spearman correlation and MLR analyses. Results of additional MLR analysis are provided in Supplementary Table S1. The asterisk in figure with HOMA2-IR =*Log10-transformed for analyses.
Discussion
The present study provides an analysis of the typical diet of adults with CF across glucose spectrum to determine whether dietary intake, with special attention paid to the nutrient source and diet quality of macronutrients, is associated with unfavorable outcomes for glucose homeostasis. We found that the source of macronutrients, including added sugars and glycemic load, MUFA, and plant protein intake, were significant correlates of glucose tolerance indicators, drawing attention to the importance of diet quality.
Studies investigating diet quality in CF, have focused on carbohydrate quality in the form of sugar sweetened beverages, glycemic index, or glycemic load (7, 10). Sutherland et al. found that children with CF consumed twice as many calories from sweetened drinks, confectionary sugars and packaged snacks when compared to control children (7), resulting in a diet high in added sugars and saturated fats with very sub-optimal micronutrients in the diet (7). We previously reported that adults with CF consumed large amounts of added sugar compared to controls, and this was associated with increased visceral adipose tissue (10), a risk factor for insulin resistance. In this updated analysis, the median total added sugar intake (83.2 g, 12.6% of total kcal daily) was above the recommended limits suggested by the American Heart Association and the 2020 Dietary Guidelines for Americans (<6 and < 10% of total kcal intake, respectively) (10, 25).
Further, we found a significant positive relationship between added sugar intake and insulin resistance and a significant inverse relationship between dietary glycemic load and fasting C-peptide, independent of age and sex. The role of chronic high glycemic load intake on insulin secretion in people with CF is unknown, but it is possible that chronic glucose stimulation by high carbohydrate, high glycemic load diets induces glucose toxicity to pancreatic β-cells (26). In another adult CF study, added sugar intake and glycemic load significantly correlated with higher glucose variability and less time in the euglycemic range during continuous glucose monitoring (27). Whether interventions to modify carbohydrate quality influence glucose tolerance in adults with CF is unknown, although a low glycemic index behavioral intervention in a pediatric CF cohort decreased, albeit not statistically significantly, fasting glucose levels (28). We did not find significant relationships between dietary variables and fasting glucose; however, fasting glucose is not a hallmark feature of CFRD (29), and it has been hypothesized that people with CF have enhanced glucose utilization (30).
Aside from achieving a goal of 35–40% of calories consumed from fats, there have historically been no recommended restrictions on the type of fats to consume in the CF diet. Like added sugars, the clinical recommendation for a high-fat diet in individuals with CF has resulted in an over-reliance on dietary saturated fats (7–9), in agreement with results of this study showing a median saturated fat intake of 12% of total kcal. Less emphasis in adults with CF has been placed on intake of unsaturated fats (MUFA and PUFA). Current study findings revealed positive correlations between fasted C-peptide concentrations and MUFA and PUFA. In non-CF populations, increased consumption of unsaturated fatty acids may improve glucose homeostasis (17, 31), with human acute meal challenges showing insulin secretory effects of MUFA through the action of the incretin glucagon-like peptide-1 (GLP-1) (32–34). The links between unsaturated fat consumption and glucose homeostasis generated evidence-based dietary guidelines for the general population that suggest diets high in vegetables, vegetable oils, nuts, and fish can decrease the risk of developing type 2 diabetes (17). In models adjusted for age and sex, the two-hour serum glucose level was also inversely correlated with MUFA intake. While further dietary interventions and meal challenge testing is required in adults with CF, these data suggest general population recommendations for increased intake of unsaturated fatty acids with concomitant decrease in saturated fatty acids (35), should apply to adults with CF.
We found a novel inverse relationship between HOMA-IR and plant protein intake among participants with CF. Plant proteins, compared to animal proteins, have previously been shown to correlate with a reduced prevalence or risk of developing type 2 diabetes (36, 37) and lower fasting insulin and glucose in populations with type 2 diabetes (38). Likewise, some, but not all (39), plant-based intervention studies in overweight adults have shown improvements in β-cell function and insulin sensitivity (40). Whether plant protein has a direct effect on insulin action, is not clear, as several nutrients commonly found in plant foods may play a role in mitigating the effects of insulin resistance, including polyphenols, such as genistein (41). Plant-based dietary interventions for glycemic control have not been studied in CF populations, likely owing to the historical emphasis on consumption of energy-dense foods.
Our study suggests that modifications to the typical CF diet, with decreased consumption of added sugars and increased consumption of MUFA, PUFA, and plant proteins, may improve glucose tolerance in adults with CF. As the lifespan of individuals with CF continues to increase, understanding the long-term sequelae of an unrestricted diet is of upmost importance for individuals living with CF. Evidence-based research in diet quality is gaining momentum but far from robust. This is particularly important, because in non-CF populations, energy dense, nutrient poor diets can lead to chronic diseases, like diabetes, which place a significant health burden on the population (7). There is an increasing prevalence of overweight and obesity among individuals with CF, even among individuals with CF who are pancreatic insufficient and who have severe CFTR mutations (2). The shift toward over-nutrition in CF is likely not only rooted in improved medical therapies, but also high-calorie, nutrient poor dietary intake. Thus, the need for changes in diet quality and recommendations that decrease the risk of chronic disease in the aging CF population are becoming a more urgent need.
This study adds to the limited body of literature that highlights diet quality and the role sources of dietary carbohydrates, fats, and proteins may have on improved glucose homeostasis in adults with CF. Limitations of the study include the small, single center, cross-sectional study design, limiting our ability to infer causality in our findings. Reverse causality is a possibility, where a diagnosis of diabetes or glucose intolerance leads to changes in dietary intake. As an exploratory study, correction for multiple correlations was not performed, thus spurious significant relationships may also have arisen. Planning data was not available to address sample size and power considerations; however, the reported data will inform future prospective studies. It is possible that outcomes were influenced by basal insulin regimens in three participants; however, results involving insulin resistance were similar if these participants were excluded. Further, the current study primarily focused on fasted measures of glucose homeostasis, which may be more reflective of hepatic glucose metabolism and do not represent dynamic changes in glucose tolerance (42). Larger, prospective studies are needed to determine the impact of dietary fats and other macronutrients on glucose tolerance and risk for CFRD using robust, dynamic measures of insulin secretion and sensitivity. If proven effective in the CF population, interventions for diet modification represent a non-invasive and inexpensive measure that could prevent or delay metabolic abnormalities for individuals with CF.
Conclusion
In conclusion, we showed novel relationships linking the quality of dietary fat, carbohydrates, and protein to glucose homeostasis in adults with CF across the glucose spectrum. While added sugars and dietary glycemic load were associated with metabolic impairment, unsaturated fatty acids and plant proteins were associated with better markers of glucose metabolism. Rigorous clinical trials are warranted to determine if modifying the macronutrient quality of the CF diet will influence glycemic outcomes, such as insulin secretion or insulin sensitivity, and ultimately mitigate decline in glucose tolerance among individuals with CF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Emory University Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
TD and JA: initial drafting and finalization of manuscript. BC, P-DN, EI, MB, WH, TZ, and JA: data collection. KE and JA: data analysis. TD, BC, P-DN, PV, MV, AS, TZ, and JA: interpretation of results. All authors contributed to this work, including study conception and design, and reviewed and approved the final manuscript.
Funding
This work was supported by Cystic Fibrosis Foundation (ALVARE19A0, COUSIN21H0, DALEY16E0) and National Institutes of Health grant [P30 DK125013 (Georgia Cystic Fibrosis Research and Translation Core Center), UL1 TR002378 (Georgia Clinical and Translational Science Alliance), K01 DK102851 and R01 DK133523 (JA), K24 DK096574 (TZ), T32 CA093423 (MB), K23 DK11324-01A (PV)].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1158452/full#supplementary-material
References
1. Ratjen, F, Bell, SC, Rowe, SM, Goss, CH, Quittner, AL, and Bush, A. Cystic fibrosis. Nat Rev Dis Primers. (2015) 1:15010. doi: 10.1038/nrdp.2015.10
2. Granados, A, Chan, CL, Ode, KL, Moheet, A, Moran, A, and Holl, R. Cystic fibrosis related diabetes: pathophysiology, screening and diagnosis. J Cyst Fibros. (2019) 18:S3–s9. doi: 10.1016/j.jcf.2019.08.016
3. Prentice, BJ, Ooi, CY, Strachan, RE, Hameed, S, Ebrahimkhani, S, Waters, SA, et al. Early glucose abnormalities are associated with pulmonary inflammation in young children with cystic fibrosis. J Cyst Fibros. (2019) 18:869–73. doi: 10.1016/j.jcf.2019.03.010
4. Frost, F, Jones, GH, Dyce, P, Jackson, V, Nazareth, D, and Walshaw, MJ. Loss of incretin effect contributes to postprandial hyperglycaemia in cystic fibrosis-related diabetes. Diabet Med. (2019) 36:1367–74. doi: 10.1111/dme.14121
5. Blackman, SM, and Tangpricha, V. Endocrine disorders in cystic fibrosis. Pediatr Clin N Am. (2016) 63:699–708. doi: 10.1016/j.pcl.2016.04.009
6. Balzer, BW, Graham, CL, Craig, ME, Selvadurai, H, Donaghue, KC, Brand-Miller, JC, et al. Low glycaemic index dietary interventions in youth with cystic fibrosis: a systematic review and discussion of the clinical implications. Nutrients. (2012) 4:286–96. doi: 10.3390/nu4040286
7. Sutherland, R, Katz, T, Liu, V, Quintano, J, Brunner, R, Tong, CW, et al. Dietary intake of energy-dense, nutrient-poor and nutrient-dense food sources in children with cystic fibrosis. J Cyst Fibros. (2018) 17:804–10. doi: 10.1016/j.jcf.2018.03.011
8. Smith, C, Winn, A, Seddon, P, and Ranganathan, S. A fat lot of good: balance and trends in fat intake in children with cystic fibrosis. J Cyst Fibros. (2012) 11:154–7. doi: 10.1016/j.jcf.2011.10.007
9. Calvo-Lerma, J, Hulst, J, Boon, M, Martins, T, Ruperto, M, Colombo, C, et al. The relative contribution of food groups to macronutrient intake in children with cystic fibrosis: a European Multicenter assessment. J Acad Nutr Diet. (2019) 119:1305–19. doi: 10.1016/j.jand.2019.01.003
10. Bellissimo, MP, Zhang, I, Ivie, EA, Tran, PH, Tangpricha, V, Hunt, WR, et al. Visceral adipose tissue is associated with poor diet quality and higher fasting glucose in adults with cystic fibrosis. J Cyst Fibros. (2019) 18:430–5. doi: 10.1016/j.jcf.2019.01.002
11. Davis, JN, Ventura, EE, Shaibi, GQ, Weigensberg, MJ, Spruijt-Metz, D, Watanabe, RM, et al. Reduction in added sugar intake and improvement in insulin secretion in overweight Latina adolescents. Metab Syndr Relat Disord. (2007) 5:183–93. doi: 10.1089/met.2006.0038
12. Paschen, M, Moede, T, Valladolid-Acebes, I, Leibiger, B, Moruzzi, N, Jacob, S, et al. Diet-induced β-cell insulin resistance results in reversible loss of functional β-cell mass. FASEB J. (2019) 33:204–18. doi: 10.1096/fj.201800826R
13. Acosta-Montaño, P, and García-González, V. Effects of dietary fatty acids in pancreatic Beta cell metabolism, Implications in Homeostasis. Nutrients. (2018) 10:393. doi: 10.3390/nu10040393
14. von Frankenberg, AD, Marina, A, Song, X, Callahan, HS, Kratz, M, and Utzschneider, KM. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur J Nutr. (2017) 56:431–43. doi: 10.1007/s00394-015-1108-6
15. Qian, F, Korat, AA, Malik, V, and Hu, FB. Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. (2016) 39:1448–57. doi: 10.2337/dc16-0513
16. Gulseth, HL, Gjelstad, IMF, Tiereny, AC, McCarthy, D, Lovegrove, JA, Defoort, C, et al. Effects of dietary fat on insulin secretion in subjects with the metabolic syndrome. Eur J Endocrinol. (2019) 180:321–8. doi: 10.1530/eje-19-0022
17. Imamura, F, Micha, R, Wu, JHY, de Oliveira Otto, MC, Otite, FO, Abioye, AI, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. (2016) 13:e1002087. doi: 10.1371/journal.pmed.1002087
18. Rietman, A, Schwarz, J, Tomé, D, Kok, FJ, and Mensink, M. High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr. (2014) 68:973–9. doi: 10.1038/ejcn.2014.123
19. Chen, Z, Zuurmond, MG, van der Schaft, N, Nano, J, Wijnhoven, HAH, Ikram, MA, et al. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: the Rotterdam study. Eur J Epidemiol. (2018) 33:883–93. doi: 10.1007/s10654-018-0414-8
20. van Nielen, M, Feskens, EJ, Mensink, M, Sluijs, I, Molina, E, Amiano, P, et al. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. Diabetes Care. (2014) 37:1854–62. doi: 10.2337/dc13-2627
21. American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S002
22. Wallace, TM, Levy, JC, and Matthews, DR. Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. doi: 10.2337/diacare.27.6.1487
23. Douglas, CE, and Michael, FA. On distribution-free multiple comparisons in the one-way analysis of variance. Commun Stat Theory Methods. (1991) 20:127–39. doi: 10.1080/03610929108830487
24. Stallings, VA, Stark, LJ, Robinson, KA, Feranchak, AP, Quinton, H, Clinical Practice Guidelines on Growth and Nutrition Subcommittee, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. (2008) 108:832–9. doi: 10.1016/j.jada.2008.02.020
25. Dietary Guidelines Advisory Committee. Scientific Report of the Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC: US Department of Agriculture, Agricultural Research Service (2020).
26. Robertson, RP, Harmon, J, Tran, PO, Tanaka, Y, and Takahashi, H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. (2003) 52:581–7. doi: 10.2337/diabetes.52.3.581
27. Armaghanian, N, Atkinson, F, Taylor, N, Kench, A, Brand-Miller, J, Markovic, T, et al. Dietary intake in cystic fibrosis and its role in glucose metabolism. Clin Nutr. (2019) 39:2495–500. doi: 10.1016/j.clnu.2019.11.004
28. Gorji, Z, Modaresi, M, Yekanni-Nejad, S, and Mahmoudi, M. Effects of low glycemic index/high-fat, high-calorie diet on glycemic control and lipid profiles of children and adolescence with cystic fibrosis: a randomized double-blind controlled clinical trial. Diabetes Metab Syndr. (2020) 14:87–92. doi: 10.1016/j.dsx.2019.12.010
29. Potter, KJ, Bonhoure, A, Boudreau, V, Tremblay, F, Lavoie, A, Carricart, M, et al. Marginal association of fasting blood glucose with the risk of cystic fibrosis-related diabetes. Ann Endocrinol (Paris). (2022) 84:265–71. doi: 10.1016/j.ando.2022.09.025
30. Frohnert, BI, Ode, KL, Moran, A, Nathan, BM, Laguna, T, Holme, B, et al. Impaired fasting glucose in cystic fibrosis. Diabetes Care. (2010) 33:2660–4. doi: 10.2337/dc10-0613
31. Garg, A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr. (1998) 67:577S–82S. doi: 10.1093/ajcn/67.3.577S
32. Lopez, S, Bermudez, B, Ortega, A, Varela, LM, Pacheco, YM, Villar, J, et al. Effects of meals rich in either monounsaturated or saturated fat on lipid concentrations and on insulin secretion and action in subjects with high fasting triglyceride concentrations. Am J Clin Nutr. (2011) 93:494–9. doi: 10.3945/ajcn.110.003251
33. Beysen, C, Karpe, F, Fielding, BA, Clark, A, Levy, J, Frayn, KN, et al. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia. (2002) 45:1533–41. doi: 10.1007/s00125-002-0964-9
34. Rocca, AS, LaGreca, J, Kalitsky, J, and Brubaker, PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. (2001) 142:1148–55. doi: 10.1210/endo.142.3.8034
35. Snetselaar, LG, de Jesus, JM, DeSilva, DM, and Stoody, EE. Dietary guidelines for Americans, 2020-2025: understanding the scientific process, guidelines, and Key recommendations. Nutr Today. (2021) 56:287–95. doi: 10.1097/NT.0000000000000512
36. Chen, Z, Franco, OH, Lamballais, S, Ikram, MA, Schoufour, JD, Muka, T, et al. Associations of specific dietary protein with longitudinal insulin resistance, prediabetes and type 2 diabetes: the Rotterdam study. Clin Nutr. (2020) 39:242–9. doi: 10.1016/j.clnu.2019.01.021
37. Malik, VS, Li, Y, Tobias, DK, Pan, A, and Hu, FB. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol. (2016) 183:715–28. doi: 10.1093/aje/kwv268
38. Viguiliouk, E, Stewart, S, Jayalath, V, Ng, A, Mirrahimi, A, de Souza, R, et al. Effect of replacing animal protein with plant protein on Glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2015) 7:9804–24. doi: 10.3390/nu7125509
39. Chalvon-Demersay, T, Azzout-Marniche, D, Arfsten, J, Egli, L, Gaudichon, C, Karagounis, LG, et al. A systematic review of the effects of plant compared with animal protein sources on features of metabolic syndrome. J Nutr. (2017) 147:jn239574–292. doi: 10.3945/jn.116.239574
40. Kahleova, H, Tura, A, Hill, M, Holubkov, R, and Barnard, ND. A plant-based dietary intervention improves Beta-cell function and insulin resistance in overweight adults: a 16-week randomized clinical trial. Nutrients. (2018) 10:189. doi: 10.3390/nu10020189
41. Papuc, C, Goran, GV, Predescu, CN, Tudoreanu, L, and Stefan, G. Plant polyphenols mechanisms of action on insulin resistance and against the loss of pancreatic beta cells. Crit Rev Food Sci Nutr. (2022) 62:325–52. doi: 10.1080/10408398.2020.1815644
Keywords: cystic fibrosis, diabetes, diet quality, insulin secretion, insulin resistance, glucose tolerance, nutrition
Citation: Daley TC, Cousineau BA, Nesbeth P-DC, Ivie EA, Bellissimo MP, Easley KA, Vellanki P, Vos MB, Hunt WR, Stecenko AA, Ziegler TR and Alvarez JA (2023) Quality of dietary macronutrients is associated with glycemic outcomes in adults with cystic fibrosis. Front. Nutr. 10:1158452. doi: 10.3389/fnut.2023.1158452
Edited by:
James Shaw, Newcastle University, United KingdomReviewed by:
Katherine Kutney, University Hospitals of Cleveland, United StatesJurij Dolensek, University of Maribor, Slovenia
Copyright © 2023 Daley, Cousineau, Nesbeth, Ivie, Bellissimo, Easley, Vellanki, Vos, Hunt, Stecenko, Ziegler and Alvarez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tanicia C. Daley, dGFuaWNpYS5kYWxleUBlbW9yeS5lZHU=
 Tanicia C. Daley
Tanicia C. Daley Benjamin A. Cousineau
Benjamin A. Cousineau Paula-Dene C. Nesbeth
Paula-Dene C. Nesbeth Elizabeth A. Ivie2
Elizabeth A. Ivie2 Moriah P. Bellissimo
Moriah P. Bellissimo Arlene A. Stecenko
Arlene A. Stecenko Thomas R. Ziegler
Thomas R. Ziegler Jessica A. Alvarez
Jessica A. Alvarez