
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 22 May 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1155947
This article is part of the Research TopicWomen's Health in an Interdisciplinary Dimension – Determinants of Nutritional DisordersView all 16 articles
Introduction: Obesity and iron deficiency are prevalent health problems that affect billions of people all over the world. Obesity is postulated to relate to iron deficiency via reduced intestinal iron absorption due to increased serum hepcidin level, which is mediated by chronic inflammation. Weight loss in individuals with overweight or obesity and iron deficiency anemia is believed to be associated with an improvement in iron status however the evidence from clinical trials is scarce. This study was conducted to evaluate the effect of diet-induced weight loss on iron status and its markers among young women with overweight/obesity and iron deficiency anemia.
Methods: The study design was a single-blinded, randomized controlled trial with two parallel arms (weight loss intervention vs control). Study participants were recruited using the convenience sampling method through public advertisements posted and disseminated through social media. Interested and potential participants were asked to visit the Diet Clinic for eligibility screening. A total of 62 women were recruited and randomized into weight loss intervention and control group. The intervention duration was three months. The intervention group received individual consultation sessions with the dietitian and tailored energy-restricted diets. Physical activity levels, dietary intake, anthropometric measurements and clinical markers were measured at baseline and end of the trial.
Results: There was a significant decrease (p < 0.001) in body weight of the intervention group (-7.4 ± 2.7 kg) that was associated with significant improvements in iron status and its markers (p < 0.01). The intervention group experienced a significant increase in hemoglobin (0.5 ± 0.6 g/dL), serum ferritin (5.6 ± 5.8 ng/mL), and serum iron (13.0 ± 16.2 µg/dL), and a significant decrease in high-sensitivity C-reactive protein (-5.2 ± 5.6 mg/L), and serum hepcidin level (-1.9 ± 2.2 ng/mL) at the end of the trial.
Conclusion: Our findings indicate that diet-induced weight loss among participants was associated with an improvement in iron status and its related clinical markers.
Clinical Trial Registration: [https://www.thaiclinicaltrials.org/show/TCTR20221009001], identifier [TCTR20221009001].
Obesity and iron deficiency are serious public health issues affecting billions of people worldwide (1, 2). Obesity is widely prevalent among adults and associated with high morbidity and mortality rates due to adverse health effects caused by excessive fat accumulation in the body, including elevated serum lipids (3–5). Concurrently, iron deficiency is still one of the most prevalent nutritional deficiency problems at the global level (2). This deficiency will lead to iron deficiency anemia (IDA), a critical health problem, which adversely affects cognitive function, physical performance, and work productivity (6). Previously, iron deficiency has been linked to pediatric and adulthood obesity, in which obesity is considered an emerging risk factor for iron deficiency incidence (7, 8). The connection between obesity and iron deficiency could be explained by the state of low-grade chronic inflammation in obesity, which stimulates the expression of hepcidin, a key regulator of iron homeostasis (9, 10).
Young women are susceptible to weight gain and become overweight or obese due to many factors, such as unhealthy dietary patterns, sedentary lifestyles, and pregnancy (11). At the same time, young women are also at high risk of iron deficiency as their dietary iron requirements are higher than older women due to menstrual losses (12). Dieting is common among women with overweight or obesity, particularly at this stage of life (13). Energy-restricted diets have long-term beneficial health effects, but can negatively affect dietary iron intake (14–16).
Several studies have reported that individuals with overweight and obesity were associated with lower iron status and elevated systematic inflammation and serum hepcidin compared to those with normal body weight (17–26). However, very limited studies investigated the effect of weight loss on iron status, systematic inflammation, and serum hepcidin among individuals with overweight or obesity, especially in clinical trials (27). Therefore, this study was conducted to evaluate the effect of diet-induced weight loss on iron status, chronic inflammation, and serum hepcidin level among overweight or obese young women with IDA.
This study was a single-blinded, randomized controlled trial (RCT) with two parallel arms design (weight loss intervention vs. control). The study was conducted at a private diet clinic (Gharaibeh Diet Clinic, Ajloun, Jordan). The study protocol was approved by Human Research Ethics Committee at Universiti Sultan Zainal Abidin, Terengganu, Malaysia (UniSZA/UHREC/2020/172) and Ajloun Health Directorate, Ministry of Health, Ajloun, Jordan (No.: 22/8/140). The inclusion criteria were young adult Jordanian women (18–30 years)-with Arab ethnicity, being overweight [body mass index (BMI) = 25–29.9] or obese (BMI ≥ 30), and diagnosed with mild or moderate IDA [hemoglobin = 8.0–11.9 g/dL, mean corpuscular volume (MCV) < 80 fL and serum ferritin ≤30 ng/mL]. The exclusion criteria included symptomatic patients, pregnant or lactating within the past 12 months, presence of any chronic disease or significant medical condition, being vegetarian, tobacco smoker, alcohol drinker, or drug abuser, had unstable body weight within the past 6 months (weight change ≥3% of initial body weight), irregular menstrual cycle within the past 12 months, undergone bariatric surgery, or full or partial hysterectomy, donated blood or history of hemorrhage within the past 6 months, consumption of iron supplement within the past 6 months, or any other dietary supplement within the past 3 months, and use of medications that may influence weight, iron, or inflammatory status within the past 3 months such as contraceptive medication. Study participants were recruited using the convenience sampling method through public advertisements posted and disseminated through social media. Interested and potential participants were asked to visit the Diet Clinic for eligibility screening. Informed consent was obtained from all participants who met the inclusion criteria following the Helsinki Declaration prior to randomization. Block randomization was handled by an independent collaborator with an equal allocation using a computer-generated randomization schedule. The allocation was concealed until the intervention started. The allocation sequence was concealed from the researcher and participants in sequentially numbered, opaque, sealed, and stapled envelopes. The study randomization was blinded for measurers and data collectors. All methods were performed in accordance with the relevant guidelines and regulations. In this trial, exposure was diet-induced weight loss, while primary outcomes were changes in iron status markers, high-sensitivity C-reactive protein (hsCRP), and serum hepcidin. No important changes to methods or trial outcomes after trial commencement were applied.
The intervention duration was 3 months. The intervention group received individual consultation sessions with the dietitian on days 0, 15, 30, 45, 60, and 75 for 30 min. During the consultations, participants received tailored energy-restricted diets, i.e., energy requirement with a deficit of 500 kcal with 50, 30, and 20% of daily energy from carbohydrate, fat, and protein, respectively, education about iron-rich dietary sources, and method of recording food intake. The weight loss target was 1–2 kg for 2 weeks. The serving size of foods included in the diet was based on American food lists (28), and Jordanian food lists (29, 30). The control group was asked to continue on habitual dietary patterns throughout the participation period.
Data were collected at baseline (day 0) and the end of the trial (day 90). Sociodemographic and physical activity data were collected using a questionnaire during face-to-face interviews. Physical activity levels were assessed using the Global Physical Activity Questionnaire (GPAQ), which has acceptable reliability and validity for measuring adult physical activity levels (31, 32). Physical activity was categorized into three levels: low, moderate, and high (33, 34). Dietary intake was measured using 7-day food record method. Participants were required to record all foods and beverages consumed during the day in a specific form for 7 consecutive days preceding the diet clinic visit at baseline and during the study preceding each follow-up visit. Nutrition analysis software (Food processor, version 11.9, ESHA Research, Salem, OR, United States) was used to determine average daily nutrient intake.
Participants’ height, weight, waist circumference, hip circumference, and body fat percentage were measured by the dietitian in the morning after overnight fasting using standard procedures. Height was measured to the nearest 0.1 cm using a calibrated stadiometer (Seca 213, Germany) in the standing position without shoes. Weight was measured to the nearest 0.1 kg using a calibrated digital weight scale (Seca 876, Germany) while wearing minimal clothes without shoes. The BMI was determined by dividing the weight (kg) by squared height (m2) and classified into overweight (25–29.9 kg/m2) or obese (≥30 kg/m2) (35). The waist and hip circumferences were measured to the nearest 0.1 cm using anthropometric tape (Seca 203, Germany). The waist-hip ratio (WHR) was calculated by dividing the waist circumference by the hip circumference. The body fat percentage of participants was measured using a bioelectrical impedance analysis device (Tanita body composition monitor, BC-601F, Tokyo, Japan) according to the manufacturer’s instructions.
Hematological and biochemical assays were conducted at a private certified medical laboratory (Ajloun Medical Labs, Ajloun, Jordan). Blood samples were collected from participants in the morning after overnight fasting on days 0 and 90. Complete blood count (CBC), including red cell (RBC) count, hemoglobin, hematocrit, MCV, mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), serum iron, total iron binding capacity (TIBC), serum ferritin, and hsCRP were measured according to standard medical laboratory procedures. Transferrin saturation (TS) was calculated by dividing serum iron on TIBC multiplied by 100%. Serum hsCRP was evaluated by sandwich immunodetection method using an AFIAS-6 automated immunoassay analyzer (Boditech Med Inc., Chuncheon-si, South Korea). Serum hepcidin level was determined using a human hepcidin immunoassay ELISA kit (Quantikine ELISA DHP250, R&D Systems, Minneapolis, MN, United States) according to the manufacturer’s instructions.
The sample size was calculated using G*Power software, Version 3.1.9.4, assuming that the test family is t-tests, the statistical test is the difference between two independent means (two groups), the tails are two, the effect size is equal to 0.80, the level of significance is equal to 0.05, and the power is equal to 0.80 (36). The total calculated sample size was 52 participants (26 participants in each group). After adding 20% to account for possible attrition, the required sample size was 62 participants (31 participants in each group).
IBM SPSS Statistics for Windows (Version 26, United States) was used for data analysis. Changes in dietary intake data were calculated by subtracting the during-intervention value from the baseline value (change value = during-intervention value−baseline value). Changes in anthropometric measures, iron status markers, hsCRP, and serum hepcidin were calculated by subtracting the post-intervention value from the baseline value (change value = post-intervention value−baseline value). The normality of data was assessed by visual inspection of histograms and the Shapiro–Wilk test. Categorical variables were presented as numbers and percentages. Continuous variables were presented as means and standard deviations. Chi-squared test was conducted to determine differences in categorical variables between the intervention group and the control group. Wilcoxon signed-rank test was conducted to determine differences in physical activity levels over time between baseline and post-intervention. Independent samples t-test was conducted to determine the mean differences for baseline, during-intervention and change values between the intervention group and the control group. Paired-samples t-test was conducted to determine the differences in means between baseline and during/post-intervention. ANCOVA was used to determine the differences in post-intervention means between the intervention group and control group after adjusting for baseline values. Pearson’s correlation test was run to assess the correlation between changes in anthropometric measures, iron status markers, hsCRP, and serum hepcidin. All reported p values were made based on two-tailed tests. Differences were considered statistically significant at values of p < 0.05.
The study was conducted from March to September 2021. Overall, 230 women were screened, 167 did not meet the inclusion criteria, and one withdrew from participating. Sixty-two participants were randomized (1:1) into intervention and control groups and enrolled in the study. However, four participants from the intervention and four from the control group dropped out due to pregnancy. The final sample size who completed the study was 54 (27 from each group; Figure 1).
The mean age of all participants was 26.5 ± 3.7. There were no significant differences in sociodemographic characteristics, anthropometric measurements (except waist circumference), and clinical data (except RBC count and hematocrit) between the intervention group and the control group at baseline (Table 1).
About half of the participants in each group (intervention group and control group) had high physical activity levels at baseline and post-intervention. There were no significant differences in physical activity levels between the intervention group and the control group at baseline and post-intervention. Furthermore, there were no significant differences in physical activity levels over time between baseline and post-intervention for participants in the intervention group and the control group (Table 2).
Iron intake was significantly reduced in the intervention group (−2.2 ± 3.0 mg/day, p = 0.001). The mean of change in iron intake for the intervention group (−2.2 ± 3.0 mg/day) was significantly different from that reported for the control group (0.1 ± 1.9 mg/day; p = 0.001). Similar results were reported for the intake of energy, protein, carbohydrate, and fat (Table 3).
Body weight was significantly reduced in both groups. However, the weight loss was higher in the intervention compared to the control group (−7.4 ± 2.7 kg vs. −0.9 ± 1.5 kg). After adjusting for baseline values, the post-intervention weight mean for the intervention group (79.2 ± 12.1 kg) was significantly lower than the control group (83.5 ± 11.4 kg; p < 0.001). Similar results were reported for other anthropometric variables (BMI, body fat percentage, waist circumference, hip circumference, and WHR), except that changes in hip circumference and WHR were not statistically significant for the control group (Table 4).
The intervention group experienced a significant increase in hemoglobin (0.5 ± 0.6 g/dL), serum ferritin (5.6 ± 5.8 ng/mL), and serum iron (13.0 ± 16.2 µg/dL), and a significant decrease in hsCRP (−5.2 ± 5.6 mg/L), and serum hepcidin level (−1.9 ± 2.2 ng/mL) at the end of the trial. Conversely, changes in these variables were not statistically significant in the control group. After adjusting for baseline values, post-intervention means for hemoglobin (11.7 ± 1.1 g/dL), serum ferritin (15.5 ± 10.4 ng/mL), and serum iron (50.2 ± 22.4 μg/dL) for the intervention group were significantly higher than the control group (11.4 ± 1.0 g/dL, 10.3 ± 7.7 ng/mL, and 46.5 ± 24.2 μg/dL, respectively; p < 0.05). However, post-intervention means for hsCRP (4.9 ± 6.0 mg/L), and serum hepcidin level (3.8 ± 3.0 ng/mL) for the intervention group were significantly lower than the control group (8.4 ± 7.7 mg/L and 4.9 ± 2.8 ng/mL, respectively) after adjusting for baseline values (p < 0.05; Table 4).
A significant negative correlation was observed between changes in weight and changes in RBC count (r = −0.529), hematocrit (r = −0.415), MCV (r = −0.293), MCH (r = −0.294), serum ferritin (r = −0.305), serum iron (r = −0.278), and TS (r = −0.308). Conversely, a significant positive correlation was observed between changes in weight and changes in TIBC (r = 0.290), hsCRP (r = 0.359), and serum hepcidin (r = 0.393; Table 5).
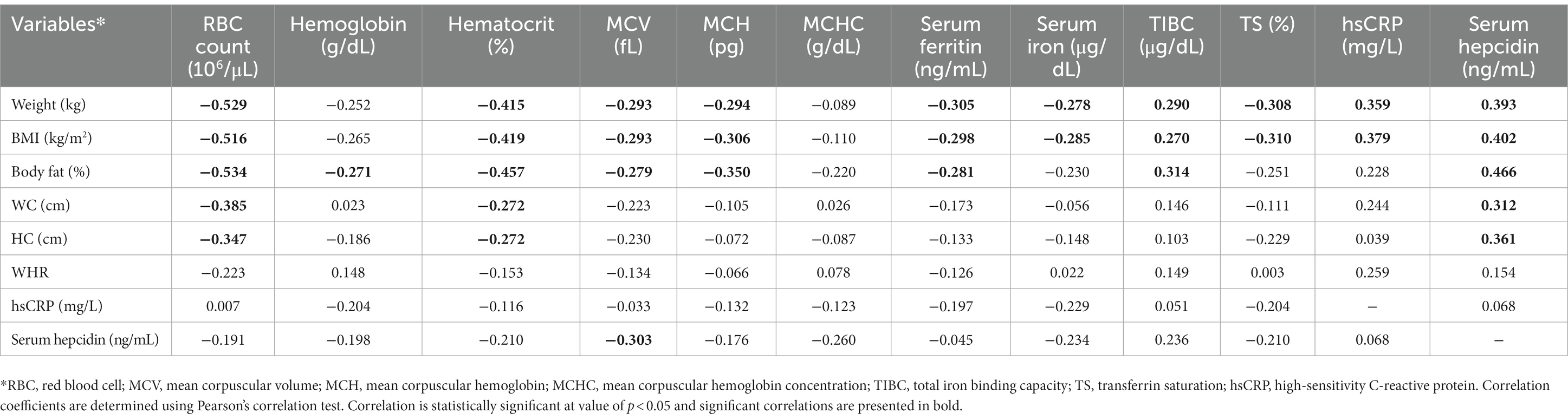
Table 5. Pearson’s correlation between changes in anthropometric measures, iron status markers, hsCRP, and serum hepcidin for all study participants (n = 54).
In this study, we found that diet-induced weight loss among young women with overweight or obesity was associated with an improvement in iron indicators including a decrease in chronic inflammation and serum hepcidin level. There are few studies that previously investigated the effect of weight loss on iron status (37–40). One study was conducted among 15 obese children from Italy who participated in a 6-month weight loss program. By the end of that study, the children had a significant weight loss, a significant increase in iron absorption, and a significant decrease in serum hepcidin (37). In another study, children with overweight and obesity who participated in a school-based physical exercise study for 8 months showed a significant decrease in BMI z-score, C-reactive protein (CRP), and serum hepcidin, and a significant increase in serum iron (38). A study among young women with overweight and obesity showed that participants who achieved at least 10% weight loss had significantly higher mean serum iron and mean TS compared to those who lost less than 5% of baseline weight (39). Additionally, a recent study concluded that weight loss improved serum iron markers via a positive effect on low-grade chronic inflammation based on significant changes in body weight, CRP level, and iron markers among premenopausal Turkish women with overweight and obesity who participated in a weight loss trial (40). The findings of all the above-mentioned studies were in harmony with the current study results, which reported significant improvements in iron markers and a significant decrease in hsCRP and serum hepcidin.
Interestingly, our results revealed that diet-induced weight loss in the intervention group improved iron status despite a lower mean intake of dietary iron. A study reported that the iron supplement was less effective in improving iron status in children with high BMI-for-age z-scores (41). These findings indicate that iron status in individuals with overweight and obesity may be affected by chronic inflammation and hepcidin levels above and beyond dietary intake of iron although this requires further investigation.
Although a significant positive correlation was observed between changes in weight and changes in both hsCRP and serum hepcidin. The levels of hsCRP and serum hepcidin were not correlated. Current evidence proposed that the elevation in serum hepcidin associated with obesity is affecting iron absorption through inflammatory pathways (7). The regulation of hepcidin by inflammation occurs in response to certain pro-inflammatory cytokines such as interleukin-6. Interleukin-6 triggers hepcidin synthesis via signal transducer and activator of transcription 3-dependent pathways (42). In this study, only hsCRP is used as a measure of pro-inflammatory activity. Interleukin-6 could have a higher correlation with serum hepcidin.
To our knowledge, this is the first study conducted among participants with overweight/obesity and IDA using a RCT design. RCT is a rigorous study design used to examine cause-effect relationships between an intervention and outcome. In addition, we used the gold standard 7-day food record to measure dietary intake, which helped to reduce measurement bias. Nonetheless, this study was conducted at a single site, which may limit the population source; however, appropriate randomization techniques were applied to avoid bias. While metabolic measures such as serum glucose level and lipid profile were not included in this study, the use of only hsCRP to indicate chronic inflammation limited the outcomes. The intervention duration also involved festive seasons, which may affect the intervention and outcomes. Long-term studies of more than 3 months, using multi-centers, with participants from multi-ethnic backgrounds, and including interleukin-6 level as another indicator of pro-inflammatory activity are highly recommended for future studies. Nevertheless, the results have proven the importance of diet-induced weight loss to correct iron deficiency in individuals with overweight or obesity. This evidence could be used as the basis for the development of low-cost early treatment for IDA, as opposed to supplementation, which eventually will help reduce the overall treatment cost for health sectors.
Our findings indicate that diet-induced weight loss among young women with overweight/obesity and IDA was associated with an improvement in iron status and its related clinical markers. This effect was suggested to link with reduced chronic inflammation and serum hepcidin levels due to reduced intestinal iron absorption mechanism. This finding proves the positive effects of diet-induced weight loss and can be used as one of the bases for treatment guidelines in women at risk of IDA, particularly those overweight or obese.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee at Universiti Sultan Zainal Abidin, Terengganu, Malaysia (UniSZA/UHREC/2020/172) and Ajloun Health Directorate, Ministry of Health, Ajloun, Jordan (No.: 22/8/140). The patients/participants provided their written informed consent to participate in this study.
NA, AA, and HA-J: conceptualization, methodology, validation, investigation, resources, supervision, project administration, and visualization. NA: software, formal analysis, data curation, writing—original draft preparation, and funding acquisition. AA and HA-J: writing—review and editing. All authors contributed to the article and approved the submitted version.
This study was self-funded by NA (PhD Candidate).
The authors would like to thank Hedayh Gharaibeh and Sana’a Smadi from Gharaibeh Diet Clinic, Ajloun, Jordan, Fakher Gharaibeh from Gharaibeh Medical Clinic, Ajloun, Jordan, Khaled Alshlool and his professional team from Ajloun Medical Labs, Ajloun, Jordan, and Yasmeen Jaber from Masoud Est. for Medical and Scientific Supplies, Amman, Jordan for their contribution in study conducting, data collection, and blood analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, Body mass index; IDA, Iron deficiency anemia; GPAQ, Global physical activity questionnaire; WC, Waist circumference; HC, Hip circumference; WHR, Waist-hip ratio; RBC, Red blood cell; MCV, Mean corpuscular volume; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; TIBC, Total iron binding capacity; TS, Transferrin saturation; hsCRP, High-sensitivity C-reactive protein; CRP, C-reactive protein; RCT, randomized controlled trial.
1. Inoue, Y, Qin, B, Poti, J, Sokol, R, and Gordon-Larsen, P. Epidemiology of obesity in adults: latest trends. Curr. Obes. Rep. (2018) 7:276–88. doi: 10.1007/s13679-018-0317-8
2. Lopez, A, Cacoub, P, Macdougall, IC, and Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet. (2016) 387:907–16. doi: 10.1016/S0140-6736(15)60865-0
3. Alagal, RI, AlFaris, NA, AlTamimi, JZ, Alshwaiyat, NM, Ahmad, A, Alzaheb, RA, et al. Differences in overweight and obesity prevalence among young men from twelve middle eastern and Asian countries living in Saudi Arabia. Healthcare. (2022) 10:690. doi: 10.3390/healthcare10040690
4. AlTamimi, JZ, Alshwaiyat, NM, AlFaris, NA, AlKehayez, NM, Ahmad, A, and Alagal, RI. Differences in overweight and obesity prevalence in middle-aged men from twelve middle eastern and Asian countries living in Saudi Arabia. Int J Gen Med. (2022) 15:3333–43. doi: 10.2147/IJGM.S359639
5. Abdelaal, M, le Roux, CW, and Docherty, NG. Morbidity and mortality associated with obesity. Ann Transl Med. (2017) 5:161. doi: 10.21037/atm.2017.03.107
6. Camaschella, C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. (2017) 31:225–33. doi: 10.1016/j.blre.2017.02.004
7. Alshwaiyat, NM, Ahmad, A, Wan Hassan, WMR, and Al-Jamal, HAN. Association between obesity and iron deficiency (review). Exp Ther Med. (2021) 22:1268. doi: 10.3892/etm.2021.10703
8. Aigner, E, Feldman, A, and Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients. (2014) 6:3587–600. doi: 10.3390/nu6093587
9. Coimbra, S, Catarino, C, and Santos-Silva, A. The role of adipocytes in the modulation of iron metabolism in obesity. Obes. Rev. (2013) 14:771–9. doi: 10.1111/obr.12057
10. Verga Falzacappa, MV, Vujic Spasic, M, Kessler, R, Stolte, J, Hentze, MW, and Muckenthaler, MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. (2007) 109:353–8. doi: 10.1182/blood-2006-07-033969
11. Hendryx, M, Chojenta, C, and Byles, JE. Obesity risk among young Australian women: a prospective latent class analysis. Obesity. (2020) 28:154–60. doi: 10.1002/oby.22646
12. Percy, L, and Mansour, D. Iron deficiency and iron-deficiency anaemia in women’s health. Obstetric Gynaecol. (2017) 19:155–61. doi: 10.1111/tog.12368
13. Fayet, F, Petocz, P, and Samman, S. Prevalence and correlates of dieting in college women: a cross sectional study. Int. J. Women’s Health. (2012) 4:405–11. doi: 10.2147/IJWH.S33920
14. Manaf, NUA, Ahmad, A, and Yusoff, NAM. A systematic review on long term effects of weight loss diet on body weight and lipid profile: findings from randomized controlled trials. Univ J Public Health. (2018) 6:40–8. doi: 10.13189/ujph.2018.060202
15. Kretsch, MJ, Fong, AKH, Green, MW, and Johnson, HL. Cognitive function, iron status, and hemoglobin concentration in obese dieting women. Eur. J. Clin. Nutr. (1998) 52:512–8. doi: 10.1038/sj.ejcn.1600598
16. O’Connor, H, Munas, Z, Griffin, H, Rooney, K, Cheng, HL, and Steinbeck, K. Nutritional adequacy of energy restricted diets for young obese women. Asia Pac. J. Clin. Nutr. (2011) 20:206–11.
17. Abdel Hamed, ER, Sallam, SF, Hamdy, HA, El Shafie, AI, El Kassas, GM, Khairy, SA, et al. Serum hepcidin level and iron status in a sample of obese Egyptian children. Med Res J. (2015) 14:7–11. doi: 10.1097/01.MJX.0000464332.84361.87
18. Alam, F, Memon, AS, and Fatima, SS. Increased body mass index may lead to hyperferritinemia irrespective of body iron stores. Pak J Med Sci. (2015) 31:1521–6. doi: 10.12669/pjms.316.7724
19. Kaner, G, Pekcan, G, Pamuk, G, Pamuk, BO, and Amoutzopoulos, B. Is iron deficiency related with increased body weight? A cross-sectional study. Prog Nutr. (2016) 18:102–10.
20. Lecube, A, Carrera, A, Losada, E, Hernández, C, Simó, R, and Mesa, J. Iron deficiency in obese postmenopausal women. Obesity. (2006) 14:1724–30. doi: 10.1038/oby.2006.198
21. Menzie, CM, Yanoff, LB, Denkinger, BI, McHugh, T, Sebring, NG, Calis, KA, et al. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J. Am. Diet. Assoc. (2008) 108:145–8. doi: 10.1016/j.jada.2007.10.034
22. Nead, KG, Halterman, JS, Kaczorowski, JM, Auinger, P, and Weitzman, M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. (2004) 114:104–8. doi: 10.1542/peds.114.1.104
23. Tussing-Humphreys, LM, Nemeth, E, Fantuzzi, G, Freels, S, Guzman, G, Holterman, AXL, et al. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity. (2010) 18:1449–56. doi: 10.1038/oby.2009.319
24. Yanoff, LB, Menzie, CM, Denkinger, B, Sebring, NG, McHugh, T, Remaley, AT, et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int. J. Obes. (2007) 31:1412–9. doi: 10.1038/sj.ijo.0803625
25. Zimmermann, MB, Zeder, C, Muthayya, S, Winichagoon, P, Chaouki, N, Aeberli, I, et al. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int. J. Obes. (2008) 32:1098–104. doi: 10.1038/ijo.2008.43
26. Shekarriz, R, and Vaziri, MM. Iron profile and inflammatory status of overweight and obese women in sari, north of Iran. Int J Hematol Oncol Stem Cell Res. (2017) 11:108–13.
27. Teng, IC, Tseng, SH, Aulia, B, Shih, CK, Bai, CH, and Chang, JS. Can diet-induced weight loss improve iron homoeostasis in patients with obesity: a systematic review and meta-analysis. Obes. Rev. (2020) 21:e13080. doi: 10.1111/obr.13080
28. Wheeler, ML, Daly, A, Evert, A, Franz, MJ, Geil, P, Holzmeister, LA, et al. Choose your foods: exchange lists for diabetes, sixth edition, 2008: description and guidelines for use. J. Am. Diet. Assoc. (2008) 108:883–8. doi: 10.1016/j.jada.2008.02.002
29. Bawadi, HA, and Al-Sahawneh, SA. Developing a meal-planning exchange list for traditional dishes in Jordan. J. Am. Diet. Assoc. (2008) 108:840–6. doi: 10.1016/j.jada.2008.02.016
30. Bawadi, HA, Al-shwaiyat, NM, Tayyem, RF, Mekary, R, and Tuuri, G. Developing a food exchange list for middle eastern appetisers and desserts commonly consumed in Jordan. Nutr. Diet. (2009) 66:20–6. doi: 10.1111/j.1747-0080.2008.01313.x
31. Armstrong, T, and Bull, F. Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health. (2006) 14:66–70. doi: 10.1007/s10389-006-0024-x
32. Bull, FC, Maslin, TS, and Armstrong, T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J. Phys. Act. Health. (2009) 6:790–804. doi: 10.1123/jpah.6.6.790
33. AlFaris, NA, Alshwaiyat, NM, AlTamimi, JZ, Alagal, RI, Al-Jamal, HA, and AlKehayez, NM. Physical activity levels of a multi-ethnic population of middle-aged men living in Saudi Arabia and factors associated with physical inactivity. Int J Public Health. (2022) 66:1604328. doi: 10.3389/ijph.2021.1604328
34. AlTamimi, JZ, Alagal, RI, AlKehayez, NM, Alshwaiyat, NM, Al-Jamal, HA, and AlFaris, NA. Physical activity levels of a multi-ethnic population of young men living in Saudi Arabia and factors associated with physical inactivity. Front. Public Health. (2022) 9:734968. doi: 10.3389/fpubh.2021.734968
36. Faul, F, Erdfelder, E, Lang, A-G, and Buchner, A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
37. Amato, A, Santoro, N, Calabro, P, Grandone, A, Swinkels, DW, Perrone, L, et al. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int. J. Obes. (2010) 34:1772–4. doi: 10.1038/ijo.2010.204
38. Coimbra, S, Catarino, C, Nascimento, H, Inês Alves, A, Filipa Medeiros, A, Bronze-da-Rocha, E, et al. Physical exercise intervention at school improved hepcidin, inflammation, and iron metabolism in overweight and obese children and adolescents. Pediatr. Res. (2017) 82:781–8. doi: 10.1038/pr.2017.139
39. Cheng, HL, Griffin, HJ, Bryant, CE, Rooney, KB, Steinbeck, KS, and O’Connor, HT. Impact of diet and weight loss on iron and zinc status in overweight and obese young women. Asia Pac. J. Clin. Nutr. (2013) 22:574–82. doi: 10.6133/apjcn.2013.22.4.08
40. Kaner, G, Pekcan, AG, and Yürekli, BPŞ. Effect of a weight loss intervention on iron parameters in overweight and obese Turkish women. Prog. Nutr. (2019) 21:50–6. doi: 10.23751/pn.v21i1-S.5503
41. Baumgartner, J, Smuts, CM, Aeberli, I, Malan, L, Tjalsma, H, and Zimmermann, MB. Overweight impairs efficacy of iron supplementation in iron-deficient south African children: a randomized controlled intervention. Int. J. Obes. (2013) 37:24–30. doi: 10.1038/ijo.2012.145
Keywords: weight loss, obesity, iron status, chronic inflammation, hepcidin, iron deficiency anemia
Citation: Alshwaiyat NM, Ahmad A and Al-Jamal HAN (2023) Effect of diet-induced weight loss on iron status and its markers among young women with overweight/obesity and iron deficiency anemia: a randomized controlled trial. Front. Nutr. 10:1155947. doi: 10.3389/fnut.2023.1155947
Received: 01 February 2023; Accepted: 27 April 2023;
Published: 22 May 2023.
Edited by:
Karolina Krupa-Kotara, Medical University of Silesia in Katowice, PolandReviewed by:
Yaoshan Dun, Central South University, ChinaCopyright © 2023 Alshwaiyat, Ahmad and Al-Jamal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naseem Mohammad Alshwaiyat, c2hfbmFzZWVtQHlhaG9vLmNvbQ==; Aryati Ahmad, YXJ5YXRpYWhtYWRAdW5pc3phLmVkdS5teQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.