- 1Department of Food Science and Human Nutrition, College of Applied and Health Sciences, A’Sharqiyah University, Ibra, Oman
- 2Department of Clinical Nutrition, Al-Azhar University – Gaza, Gaza, Palestine
- 3Faculty of Pharmacy, Al-Azhar University – Gaza, Gaza, Palestine
Background: This study assessed serum, dietary zinc levels, and other risk factors during the third trimester among pregnant women with and without pregnancy-induced hypertension (PIH).
Methods: This case-control study was conducted in 2022, in the three main Obstetrics and Gynecology departments in Gaza Strip, Palestine. One hundred sixty pregnant women, during the third trimester, aged ≥20 years, were selected using a convenient sampling method. Data were obtained using an interview-based questionnaire, food frequency questionnaire, anthropometric measures, and biochemical tests. Statistical analysis was performed using SPSS version 24.
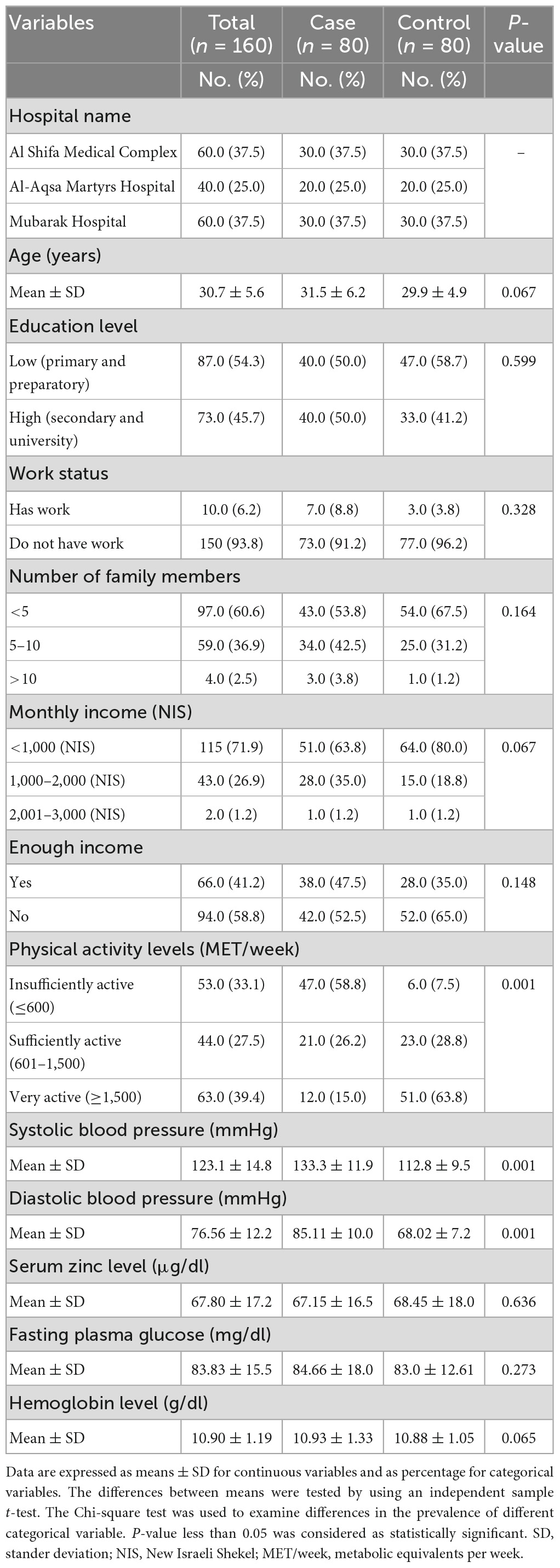
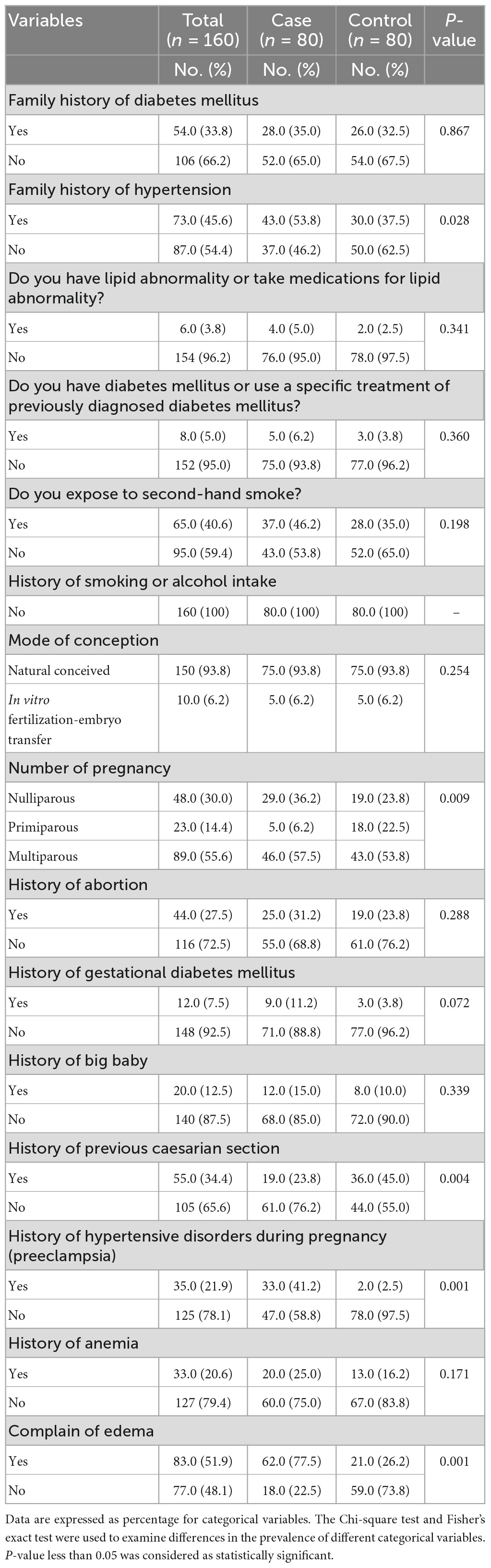
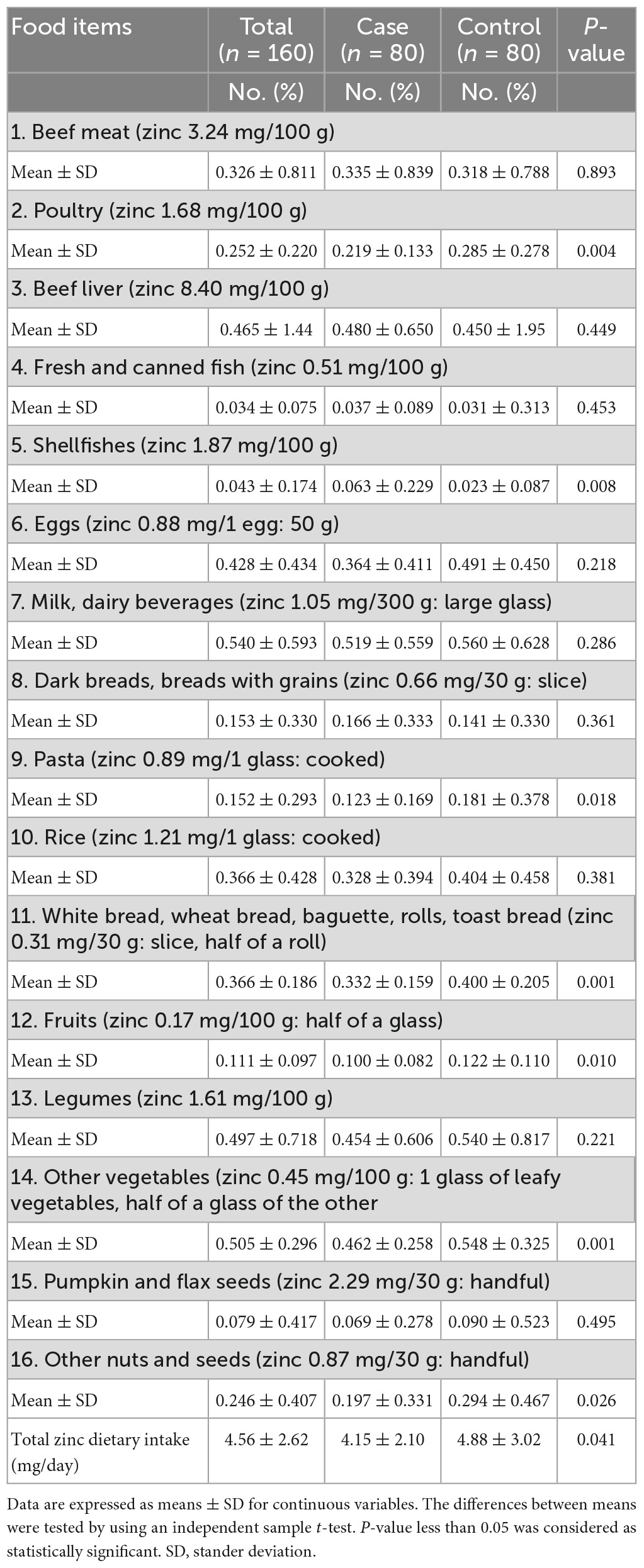
Results: The participants’ mean age was 30.7 ± 5.6 years. A total of 47 (58.8%) of cases and 6 (7.5%) of controls were insufficiently active; and the mean of blood pressure (mmHg) was 133.3 ± 11.9/85.11 ± 10.0 for cases and 112.8 ± 9.5/68.02 ± 7.2 for controls with significant differences between the two groups (P = <0.005). The mean serum zinc level (μg/dl) was 67.15 ± 16.5 for cases and 68.45 ± 18.0 for controls without significant differences between the two groups (P = 0.636). For newborns, the mean birth weight (g) was 2,904.6 ± 486 for cases, and 3,128.3 ± 501 for controls, and the mean Apgar score was 8.03 ± 0.62 for cases and 8.30 ± 1.17 for controls, with significant differences between the two groups (P = <0.005). Furthermore, 43 (53.8%) of cases have family history of hypertension; 5 (6.2%) were primiparous; 19 (23.8%) have previous caesarian section; 33 (41.2%) have history of preeclampsia; and 62 (77.5%) have edema, with significant differences between the two groups (P = <0.005). Additionally, the total zinc dietary daily intake (mg/day) was 4.15 ± 2.10 for cases and 4.88 ± 3.02 for controls, with significant differences between the two groups (P = 0.041). After adjustment for confounding variables, participants in the case group have higher odds of having low total zinc dietary intake compared to those in the control group [OR = 1.185, 95% CI = (1.016–1.382), P = 0.030].
Conclusion: The current study showed the main risk factors of PIH among pregnant women in the Gaza Strip, Palestine. Furthermore, low maternal dietary zinc intake was associated with a high level of PIH. Moreover, having PIH could increase the risk of low birth weight and low Apgar scores. Therefore, reducing the main risk factors of PIH could reduce the adverse effect on both mother and birth outcomes.
Introduction
Zinc is an essential trace mineral element vital for many physiological functions and plays an important role in growth, reproduction, and the immune system (1). Adequate zinc stores in the body are extremely important during periods of pregnancy. However, zinc deficiency is common in developing countries, and low maternal serum zinc concentrations have previously been associated with pregnancy complications (2). Zinc deficiency during pregnancy adversely affects both the mother and fetus and subsequent birth outcomes. Major problems associated with zinc deficiency include growth retardation, delayed immune system development, cognitive impairment, impaired glucose tolerance, low birth weight, congenital malformations, pregnancy-induced hypertension (PIH), increased risk of abortion, miscarriage, stillbirths, preterm labor, postpartum hemorrhage, and prolonged labor (3).
Previous studies have provided evidence that zinc deficiency is a worldwide public health problem, especially in developing countries, including Palestine (4, 5). The World Health Organization (WHO) reported that the estimated prevalence of zinc deficiency ranges from 4 to 73% across various regions of the world’s population (6). In developing countries, zinc deficiency is 1 of the 10 significant factors contributing to the burden of disease (7). Zinc deficiency is predicted to be responsible for 1% of all deaths globally and 4.4% in children aged 6 months to 5 years (8). Additionally, the WHO prioritized minimizing zinc deficiency in developing countries to eradicate extreme poverty and hunger (9).
Diet is the main factor that determines zinc status (2). In the United States and Australia, an additional 2–4 mg of zinc per day is recommended for pregnant women compared to non-pregnant women (10, 11). It is widely acknowledged that many pregnant women do not meet this recommendation, particularly in developing countries with plant-based diets (12). Adequate maternal nutrition, particularly before and during pregnancy, is imperative to the mother’s and child’s health (13). Poor nutrition in pregnancy may lead to inappropriate nutrient partitioning between the mother and fetus, which can be deleterious to the health of both (14).
In fact, data on the prevalence and determinants of zinc deficiency among pregnant women are scanty and inconclusive (15). In addition, to the best of our knowledge, no baseline information exists about zinc levels and PIH among pregnant women in Gaza Strip, Palestine. Therefore, the aim of the current study was to assess the serum, dietary zinc levels, and other risk factors during the third trimester among pregnant women with and without PIH in Gaza Strip, Palestine, to determine whether maternal serum zinc or dietary zinc intake are important factors associated with PIH. Understanding this association may help improve maternal and fetal outcomes and reduce possible complications. This is the first study, which examined this association among pregnant women in Gaza Strip, Palestine, and the results of this study will provide baseline information about zinc levels and the main risk factors of PIH during the third trimester among pregnant women in Gaza Strip, Palestine.
Materials and methods
Study design
This case-control study was conducted among pregnant women attended the main governmental hospitals in Gaza Strip, Palestine.
Study setting and period
This study was conducted in the year 2022 in the three main governmental hospitals (Al Shifa Medical Complex, Al-Aqsa Martyrs Hospital, and Mubarak Hospital) of the Palestinian Ministry of Health (MOH), which are considered the main governmental hospitals providing the Obstetrics and Gynecology services to the Palestinians pregnant woman.
Study population
All pregnant women with and without PIH during the third trimester who attended the main governmental hospitals in Gaza Strip, Palestine, during the study period. The study protocol was approved by the Palestinian Health Research Council (PHRC/HC/116/22). The study was conducted according to the tenets of the Helsinki Declaration. Written informed consent was obtained from each participant. The inclusion criteria include pregnant women aged ≥20 years, women during the third trimester, women who were attending the Obstetrics and Gynecology Departments of Palestinian MOH during the study period and lived in the Gaza Strip for at least 3 years. The inclusion criteria for the cases included pregnant women diagnosed with PIH, and for the controls, we included pregnant women without PIH. On the contrary, pregnant women who were previously diagnosed with chronic hypertension (HTN) or other types of serious illness such as cancer, thyroid diseases, acute myocardial infarction, or end-stage kidney disease; pregnant women taking drugs affecting zinc balance; and pregnant women with a previous history of (antiphospholipid syndrome, ulcerative colitis, Crohn’s disease, short bowel syndrome, chronic diarrhea, chronic liver or kidney diseases, and sickle cell anemia) were also excluded.
Sample size and sampling technique
The number of pregnant women who attended the governmental hospitals in the Gaza Strip in 2017 was 17.226. In addition, the number of high-risk pregnancies was 5,278 cases, according to a recent report from the Palestinian MOH in 2017 (16). The representative sample size in the current study was 160 pregnant women, which was calculated using the Charan and Biswas formula (17). Pregnant women during the third trimester, aged ≥20 years (80 cases and 80 controls) were selected by a convenient sampling method according to the availability of subjects who fit eligibility criteria during the data collocation period. Additionally, the study sample was distributed based on the population density on the three leading governmental hospitals in Gaza Strip, Palestine.
Data collection
Interview-based questionnaire
Data regarding the characteristics of the study participants, demographic and socioeconomic, medical and gestational history, and diet compliance variables were obtained with an interview-based questionnaire. Additionally, reports and all relevant documentation, including the participant’s medical records, were checked.
Anthropometric measurements
For pregnant women, height (cm) and weight (kg) were measured on admission using standard methods (18). Furthermore, for the newborns, length (cm), weight (g), and head and chest circumference (cm) were measured after delivery using standard methods (19).
Blood pressure measurements (mmHg) for pregnant women on admission
Blood pressure was measured from the left arm (mmHg) by mercury sphygmomanometer. Three readings on different days, while the participant seated, after relaxing for at least 15 min in a quiet environment, empty bladder, and the average of three measurements was recorded (20).
Biochemical measurements
After 12 h fasting, venous blood samples were collected from all participants (case and control) on admission and at the beginning of the third trimester by well-trained and experienced nurses. Venous blood (5.0 ml) was drawn and was used for serum zinc level (μg/dl), hemoglobin level (g/dl), and fasting blood sugar (mg/dl). Mindray BS-300 and Mindray BA-88A chemistry analyzers were used for blood chemistry and serum zinc level analysis (21). In addition, serum zinc was analyzed by colorimetric method of Mindray BA-88A analyzer, using commercially available colorimetric determination kits of serum zinc. This is a direct colorimetric assay based on the 5-Br-PAPS method. The laboratory tests were analyzed in a private licensed laboratory.
Clinical examination
All participants were examined by the physicians for signs and symptoms of any diseases, including PIH, and for signs and symptoms of zinc deficiency. In addition, the WHO criteria were used by the physicians for the diagnosis of PIH (22). In the current study, PIH is defined as systolic blood pressure >140 mmHg and diastolic blood pressure >90 mmHg. It is refers to one of four conditions: (a) pre-existing hypertension, (b) gestational hypertension and preeclampsia, (c) pre-existing hypertension plus superimposed gestational hypertension with proteinuria, and (d) unclassifiable hypertension (22–24).
Apgar score
The Apgar score was used to assess the health status of the newborn. The Apgar score is a quick way for various members of the healthcare team, including midwives, nurses, or physicians, to evaluate the health of all newborns at 1 and 5 min after birth and in response to resuscitation (25).
Dietary assessment
In the current study, a validated Palestinian Arabic version semi-quantitative food frequency questionnaire (FFQ) was used to assess the dietary zinc intake of the pregnant women (26). The FFQ is relatively easy and inexpensive to administer and can be used to measure dietary intake over a prolonged time period, in addition the semi-quantitative FFQ is adequate to identify deficiency of zinc and other trace elements (20). The FFQ in our study contains a list of 98 food items; it was developed and validated among the Palestinian population in 2014 (26). All participants were asked to estimate the number of times per day, week, or month they consumed these particular food products and the amount usually eaten per food item by making comparisons with the specified reference portion. Common household measures, including measuring cups, spoons, and a ruler were shown to assist the participants in the estimation process. Additionally, dietary intakes were converted into grams per day. The net amount of dietary zinc intake (mg/day) consumed by the study participants was calculated based on the USDA Food Composition Database (27).
Assessment of physical activity levels
Data on physical activity was obtained using the international physical activity questionnaire (IPAQ) short version. According to the IPAQ scoring protocol, the participants were classified based on their weekly energy expenditure as follows: insufficiently active: ≤600 MET/week; sufficiently active: 601–1,500 MET/week; and very active: ≥1,500 MET/week (28).
Pilot study
Pilot study was carried out on 20 participants; the questionnaire and data collection process were modified according to the result of the pilot study. The data was collected by five qualified data collectors (two nurses and three nutritionists).
Statistical analysis
Statistical analysis was performed using SPSS version 24. Dietary intakes were converted into grams per day. The net amount of zinc dietary intake (mg/day) consumed by the study participants were calculated based on the USDA Food Composition Database (27). In addition, data are expressed as means ± standard deviation (SD) for continuous variables and as percentage for categorical variables. The Chi-square test and Fisher’s exact test were used to examine the significant differences in the prevalence of different categorical variables. The differences between mean were tested by independent samples t-test. P-value less than 0.05 was considered as statistically significant.
Results
The current study was conducted in the three main governmental hospitals of the Palestinian MOH. The results demonstrated that the majority of the study participants were from Al Shifa Medical Complex and Mubarak Hospital. The mean of age for the study participants was 30.7 ± 5.6 years without significant differences between the cases and control groups (P = 0.067). Concerning physical activity levels, a large percentage of the cases were insufficiently active, while the majority of controls were very active, with significant differences found between the case and control group (P = 0.001). In addition, the mean blood pressure for women in the cases group was slightly high compared to women in controls group, but still within the normal levels, with significant differences between the two groups (P = < 0.005). Moreover, the mean serum zinc and hemoglobin levels was slightly low for women in the cases group compared with women in the control group; while the mean of fasting plasma glucose was slightly high compared to women in controls group, without significant differences between the two groups (P > 0.005 for all), as shown in Table 1.
Table 2 presented the anthropometric measures for the mothers and for the newborns. For mothers, the results showed that the mean BMI (kg/m2) was slightly high for women in cases group compared with women in the controls group, without significant differences between the two groups (P > 0.005). For newborns, the results revealed that the mean birth weight (g) and the mean of Apgar score were low in the cases group compared with the controls group, with significant differences between the two groups (P = 0.005 and 0.001, respectively).
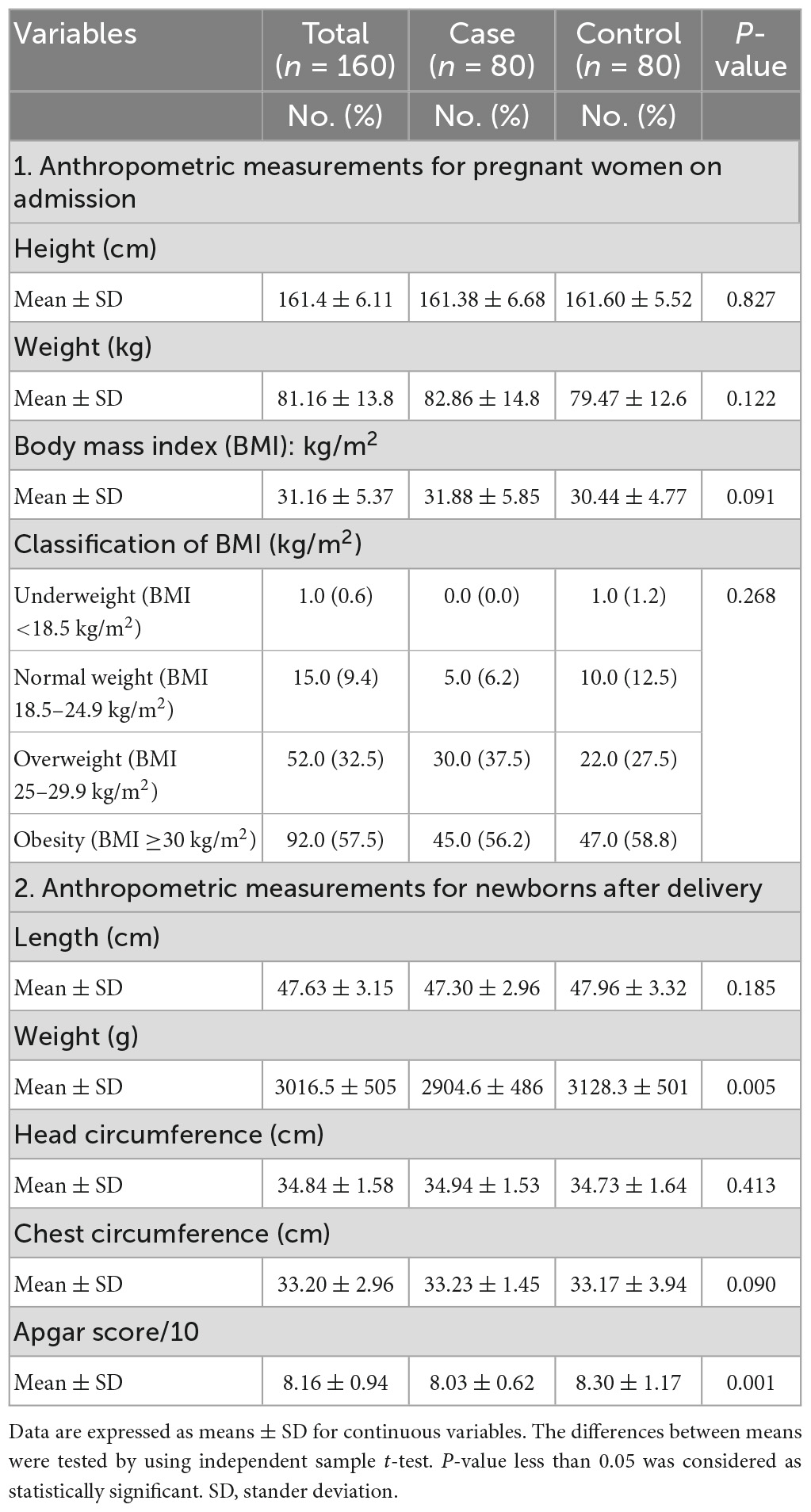
Table 2. Anthropometric measurements for the study participants, newborns, and the results of Apgar score.
Table 3 presents selected factors that may contribute to the development of PIH. The results showed that a large percentage of the cases have a family history of HTN compared with the controls, with a statistically significant difference between the two groups (P = 0.028). In addition, women in the cases group have more family history of DM compared with the controls, without significant differences between the two groups (P = 0.867). The results also revealed that women in the cases group have more history of preeclampsia and complained of edema more than women in the controls group, with significant differences between the two groups (P < 0.005 for all).
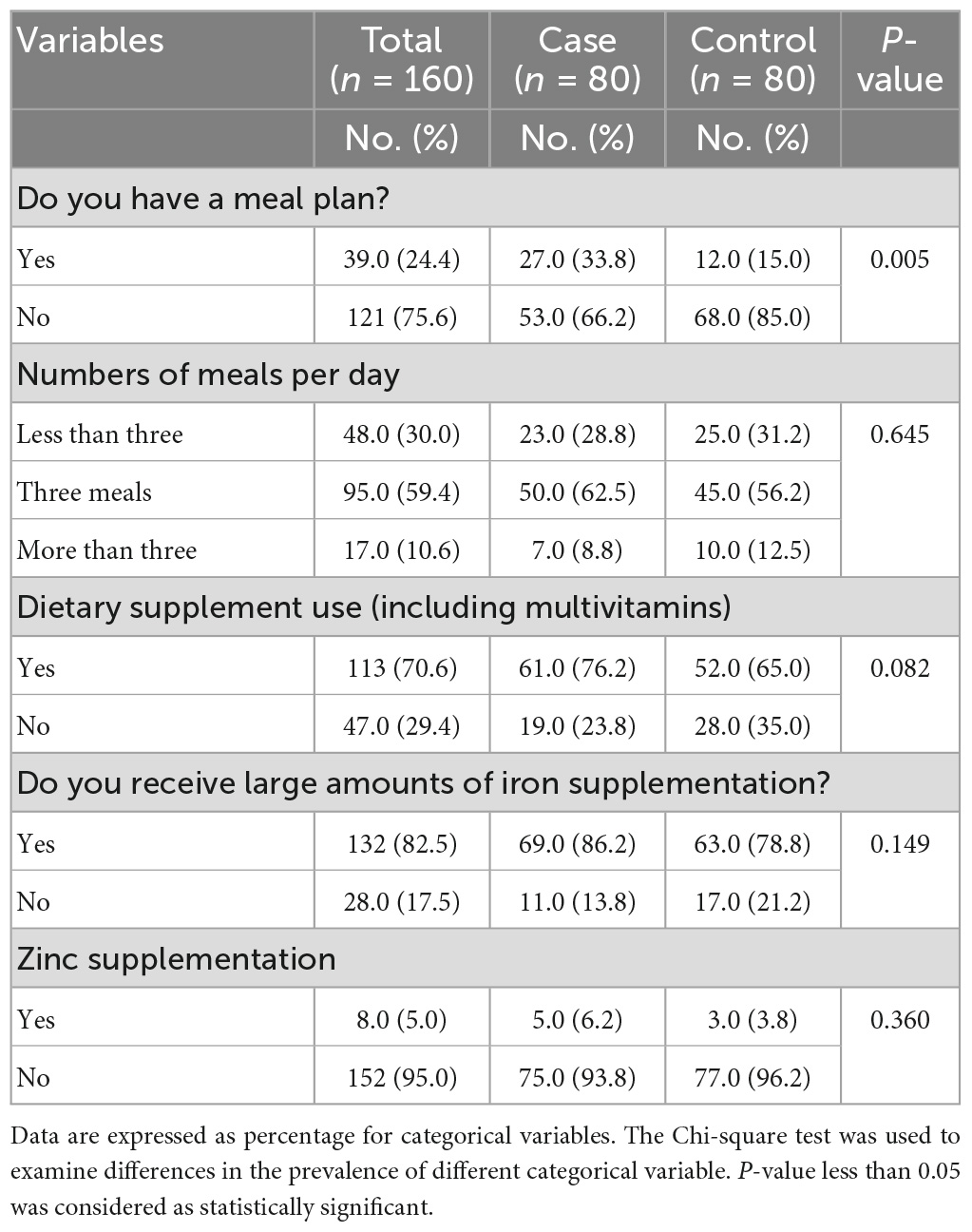
Table 4 showed that a large percentage of cases have a meal plan and have three meals per day, without significant differences between the two groups (P < 0.005). In addition, women in the cases group using dietary supplements, including multivitamins, received large amounts of iron supplementation, and used zinc supplementation more than women in the controls group, without significant differences between the two groups (P = >0.05 for all).
Table 5 presents the consumption patterns of dietary zinc intake (mg/day). The total dietary zinc daily intake (mg/day) were slightly low for women in the cases group compared with women in the control group, with significant differences between the two groups (P = 0.041).
Finally, Table 6 presented the odd ratio (OR) and confidence interval (CI) for serum zinc level (μg/dl) and total dietary zinc intake (mg/day) of the case and control group. After adjustment for confounding variables, participants in the case group have higher odds of having low total zinc dietary intake, compared to those in the control group [OR = 1.185, 95% CI = (1.016–1.382), P = 0.030].
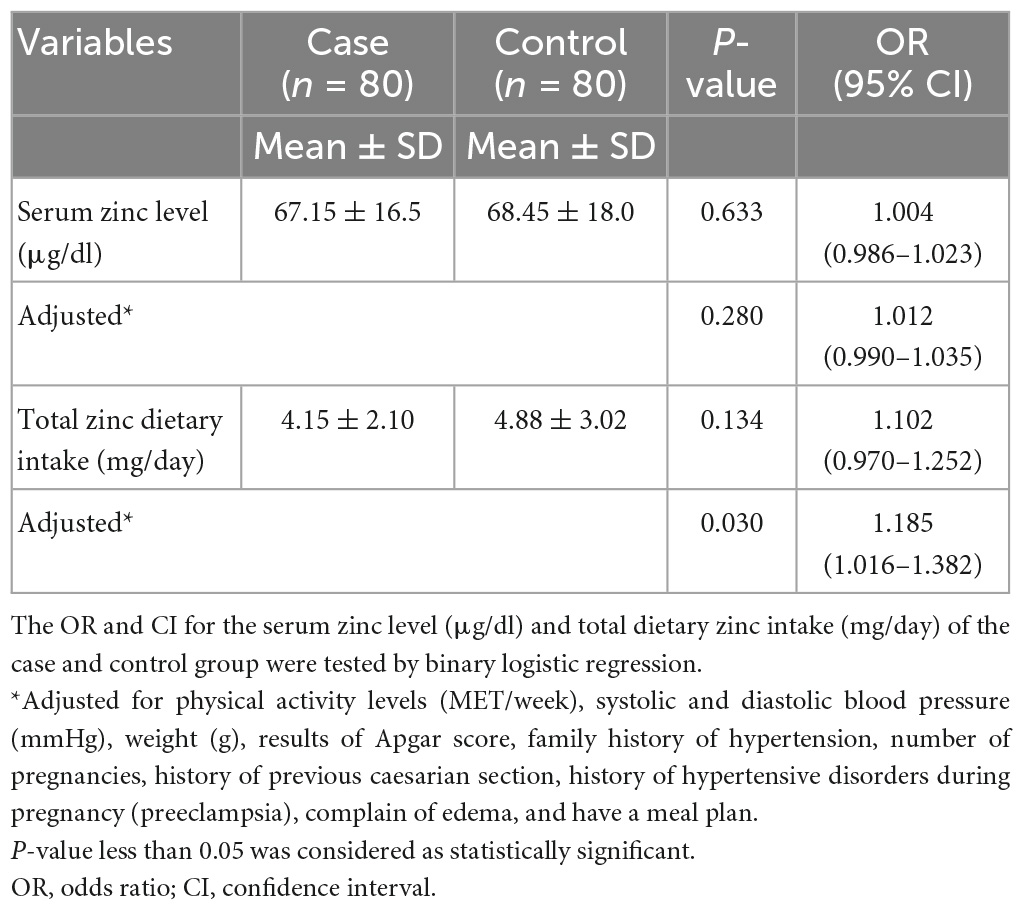
Table 6. Odd ratio and confidence interval for the serum zinc level (μg/dl) and total dietary zinc intake (mg/day) of the case and control group.
Discussion
Zinc deficiency is common in developing countries, and low maternal serum zinc concentrations have previously been associated with pregnancy complications (2). Zinc deficiency during pregnancy has an adverse effect on both the mother and fetus and on subsequent birth outcomes. Previous studies have provided evidence that zinc deficiency is a worldwide public health problem, especially in developing countries (4, 5). In the current study, no statistically significant associations were found concerning socioeconomic variables with the risk of PIH between the case and control group. Owiredu et al. (29) also showed that socioeconomic status was not directly associated with PIH. However, low socioeconomic factors may act as risk factors for PIH (30). Low socioeconomic factors could be associated with nutritional issues, reduced antenatal care and unsanitary hygienic conditions (31). A study in Australia found that working women compared to non-working ones, had a higher risk of developing pre-eclampsia and eclampsia; this may be related to the stress that women get during work (32). Several previous studies showed significant associations between maternal education and income level with PIH (30, 33).
Two of previous studies showed that aerobic exercise for about 30–60 min for at least twice per week during pregnancy is associated with a reduced risk of gestational hypertensive disorders, PIH, and cesarean delivery (34, 35). Our results support these findings of these studies.
The International Zinc Nutrition Consultative Group defined zinc deficiency as blood zinc levels below 56 μg/dl in the first trimester or 50 μg/dl in the second or third trimesters (36). Tesfa et al. (37) showed that the mean serum zinc level was significantly reduced in women with PIH as compared to normotensive pregnant women. On the contrary, Lewandowska et al. (38) showed no association between serum zinc concentrations and PIH. The same results were obtained by Mistry et al. (39) and Ugwuja et al. (40). The results of the current study, indicated that all of the study participants have normal serum zinc level, fasting plasma glucose, and hemoglobin level during the study period, therefore, it is not yet clear whether zinc levels play a role in PIH.
van Middendorp et al. (41) showed that having family history of HTN had about five time’s greater odds of developing PIH than those who did not have family history of HTN. This is consistent with the results of our study. Umegbolu and Ogamba (42) reported that first pregnancy is a risk factor for PIH and its occurrence is more common in nulliparous than multiparous women. Howell (43) showed that in women with prior cesarean delivery, PIH was significantly higher. The results of our study support these findings. The etiology of PIH is not fully understood yet, among many factors, genetic, metabolic, immunological, and environmental factors are taken into consideration (44, 45). Further future studies with large sample size on the main risk factors for PIH are recommended.
Hromadnikova et al. (45) demonstrated that there was a significant association of PIH with low birth weight, and women who delivered low birth weight babies were five times more likely to have had PIH. Furthermore, high blood pressure during pregnancy can affect the development of the placenta, causing the nutrient and oxygen supply to the baby to be limited, which associated with a lower mean Apgar score (46). In agreement with these studies, our results demonstrated that, for newborns, the mean birth weight (g) and the mean Apgar score were lower in the cases compared to women in the control group.
Pregnant women need up to 2.6 mg of zinc per day during the second half of their pregnancy (47). The WHO recommends a zinc intake between 1.1 and 2.0 mg/day for pregnant mothers (48). However, studies have reported that people in low and middle-income nations tend to eat plant-based foods that are high in phytate, and starchy roots and tubers, which have a low zinc concentration, which could lead to zinc insufficiency (49, 50). We found a lower intake of zinc during pregnancy in our cohort, although this does not correlate with low serum zinc levels suggesting that the study participants have adequate total zinc intake during pregnancy. Hennigar et al. (51) showed that dietary zinc intake is not substantially correlated with serum zinc concentration, because many factors have been identified to affect serum zinc independent of an individual’s zinc status, such as meal consumption, time of day, inflammation, and infection. However, as zinc intake appears to correlate with the frequency of PIH a changing diet might be warranted. Increasing the intake of animal-source foods, cutting out or cutting back on coffee, and taking zinc supplements could improve (52).
Previous studies showed that inadequate nutrition at home could be attributed to a number of factors, including lack of variety in the diet, low consumption of animal products, socioeconomic status, maternal education, pregnancy, hemoglobin concentration, maternal workload, chronic illness, vegetarianism, and a lack of antenatal care (53, 54) can contribute to inefficient zinc intake. Further research on the relationship between serum and dietary zinc levels during the third trimester among pregnant women with and without PIH and pregnancy outcomes needs more studies in the future.
Strengths and limitations
The main strength of this study is that the results will provide baseline information about zinc levels and PIH during the third trimester among pregnant women in Gaza Strip, Palestine. The main limitations of this study are its non-probability sampling techniques, which limit the generalizability of the results. Furthermore, the possibility of recall bias and misreporting by using FFQ for maternal dietary zinc intake. Unfortunately, we did not record the gestational age at the time of blood collection nor at the time of delivery.
Conclusion
The current study showed the main risk factors of PIH among pregnant women in the Gaza Strip, Palestine are family history of hypertension, primiparous, previous caesarian section, history of preeclampsia, complaints of edema, high maternal BMI, physical inactivity, and low maternal dietary zinc intake. Furthermore, low maternal dietary zinc intake was associated with high levels of PIH. Moreover, having PIH could increase the risk of low birth weight and low Apgar scores. Therefore, reducing the main risk factors of PIH could reduce the adverse effect on both mother and birth outcomes. Further future studies with large sample size are recommended.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Palestinian Health Research Council (Helsinki Ethical Committee of Research PHRC/HC/116/22). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SA and AME collected, analyzed, and interpreted the data and wrote the first draft of the manuscript. SA, AME, MT, and AHE contributed in the study design and the critical review of the manuscript. AHE contributed to the analysis and interpretation of data and the critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the Palestinian Ministry of Health, for their support and funding. We also thank the study participants for their time.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. (2017) 25:11–24. doi: 10.1007/s10787-017-0309-4
2. Wilson R, Grieger J, Bianco-Miotto T, Roberts C. Association between maternal zinc status, dietary zinc intake and pregnancy complications: a systematic review. Nutrients. (2016) 8:641. doi: 10.3390/nu8100641
3. Kumari D, Garg S, Bhawrani P. Zinc homeostasis in immunity and its association with preterm births. Scand J Immunol. (2022) 95:e13142. doi: 10.1111/sji.13142
4. Akhtar S. Zinc status in south asian populations an update. J Health Populat Nutr. (2013) 31:139–49. doi: 10.3329/jhpn.v31i2.16378
5. Hambidge K, Krebs N. Zinc deficiency: a special challenge. J Nutr. (2007) 137:1101–5. doi: 10.1093/jn/137.4.1101
6. Kumera G, Awoke T, Melese T, Eshetie S, Mekuria G, Mekonnen F, et al. Prevalence of zinc deficiency and its association with dietary, serum albumin and intestinal parasitic infection among pregnant women attending antenatal care at the university of gondar hospital. Gondar Northwest Ethiopia. BMC Nutr. (2015) 1:31. doi: 10.1186/s40795-015-0026-6
7. Khalid N, Ahmed A, Bhatti M, Randhawa M, Ahmad A, Rafaqat R. A question mark on zinc deficiency in 185 million people in pakistan—possible way out. Crit Rev Food Sci Nutr. (2014) 54:1222–40. doi: 10.1080/10408398.2011.630541
8. Bzikowska-Jura A, Sobieraj P, Michalska-Kacymirow M, Wesołowska A. Investigation of iron and zinc concentrations in human milk in correlation to maternal factors: an observational pilot study in poland. Nutrients. (2021) 13:303. doi: 10.3390/nu13020303
9. Oruamabo R. Child malnutrition and the millennium development goals: much haste but less speed? Arch Dis Childhood. (2015) 100:S19–22. doi: 10.1136/archdischild-2013-305384
10. Slater K, Rollo M, Szewczyk Z, Ashton L, Schumacher T, Collins C. Do the dietary intakes of pregnant women attending public hospital antenatal clinics align with Australian guide to healthy eating recommendations? Nutrients. (2020) 12:2438. doi: 10.3390/nu12082438
11. Hyde N, Brennan-Olsen S, Bennett K, Moloney D, Pasco J. Maternal nutrition during pregnancy: intake of nutrients important for bone health. Maternal Child Health J. (2017) 21:845–51. doi: 10.1007/s10995-016-2178-7
12. Hunt J. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. (2003) 78:633S–9S. doi: 10.1093/ajcn/78.3.633S
13. Sebastiani G, Borrás-Novell C, Alsina Casanova M, Pascual Tutusaus M, Ferrero Martínez S, Gómez Roig M, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. (2018) 10:1008. doi: 10.3390/nu10081008
14. Redmer D, Milne J, Aitken R, Johnson M, Borowicz P, Reynolds L, et al. Decreasing maternal nutrient intake during the final third of pregnancy in previously overnourished adolescent sheep: effects on maternal nutrient partitioning and feto-placental development. Placenta. (2012) 33:114–21. doi: 10.1016/j.placenta.2011.11.023
15. Mekonnen A, Terefe W, Belachew A, Adhanu A, Gezae K. Prevalence and associated factors of zinc deficiency among pregnant women attending antenatal care at Gambella Hospital, Gambella, Ethiopia, 2018. Am J Life Sci. (2019) 7:91.
16. Palestinian Ministry of Health. (2020). Available online at: https://www.moh.gov.ps/portal/ (accessed January 7, 2023)
17. Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. (2013) 35:121–6.
18. Farag H, Hosseinzadeh-Attar M, Muhammad B, Esmaillzadeh A, El Bilbeisi A. Effects of vitamin D supplementation along with endurance physical activity on lipid profile in metabolic syndrome patients: a randomized controlled trial. Diab Metab Syndr Clin Res Rev. (2019) 13:1093–8. doi: 10.1016/j.dsx.2019.01.029
19. Tiruneh C, Teshome D. Prediction of birth weight by using neonatal anthropometric parameters at birth in finote selam hospital, Ethiopia. Pediatric Health Med Therap. (2021) 2021:259–67. doi: 10.2147/PHMT.S309573
20. El Bilbeisi A, Hosseini S, Djafarian K. Association of dietary patterns with diabetes complications among type 2 diabetes patients in gaza strip. Palestine: a cross sectional study. J Health Populat Nutr. (2017) 36:1–1.
21. Al-Maweri S, Halboub E, Al-Sharani H, Shamala A, Al-Kamel A, Al- Wesabi M, et al. Association between serumzinc levels and recurrent aphthous stomatitis: a meta-analysis with trial sequential analysis. Clin Oral Invest. (2021) 25:407–15. doi: 10.1007/s00784-020-03704-8
22. DeCherney A. Current diagnosis & treatment: obstetrics & gynecology. In: Nathan L, Laufer N, Roman A, Education M editors. McGraw-Hill Education. (2019).
23. Kintiraki E, Papakatsika S, Kotronis G, Goulis D, Kotsis V. Pregnancy- induced hypertension. Hormones. (2015) 14:211–23. doi: 10.14310/horm.2002.1582
24. Aghajanian P, Ainbinder S, Akhter M, Andrew D, Anti D, Archie C. Current diagnosis and treatment obstetrics & gynecology. Mc Graw-Hill Companies. (2007) 10:925–1281.
26. Hamdan M, Monteagudo C, Lorenzo-Tovar M, Tur J, Olea-Serrano F, Mariscal-Arcas M. Development and validation of a nutritional questionnaire for the Palestine population. Public Health Nutr. (2014) 17:2512–8. doi: 10.1017/S1368980013002711
27. USDA. The USDA food composition database. (2023). Available online at: https://fdc.nal.usda.gov/. (accessed January 10, 2023).
28. El Bilbeisi A, Hosseini S, Djafarian K. The association between physical activity and the metabolic syndrome among type 2 diabetes patients in Gaza strip, Palestine. Ethiopian J Health Sci. (2017) 27:273–82. doi: 10.4314/ejhs.v27i3.9
29. Owiredu W, Ahenkorah L, Turpin C, Amidu N, Laing E. Putative risk factors of pregnancy-induced hypertension among ghanaian pregnant women. J Med Biomed Sci. (2012) 1:62–76.
30. Ramesh K, Gandhi S, Rao V. Socio-demographic and other risk factors of pre eclampsia at a tertiary care hospital, karnataka: case control study. J Clin Diagnost Res. (2014) 8:JC01. doi: 10.7860/JCDR/2014/10255.4802
31. Ghimire P, Agho K, Akombi B, Wali N, Dibley M, Raynes-Greenow C, et al. Perinatal mortality in South Asia: systematic review of observational studies. Int J Environ Res Public Health. (2018) 15:1428. doi: 10.3390/ijerph15071428
32. Cerón-Mireles P, Harlow S, Sánchez-Carrillo C, Nunez R. Risk factors for pre-eclampsia/eclampsia among working women in Mexico City. Paediatric Perinatal Epidemiol. (2001) 15:40–6. doi: 10.1046/j.1365-3016.2001.00315.x
33. Silva L, Coolman M, Steegers E, Jaddoe V, Moll H, Hofman A, et al. Low socioeconomic status is a risk factor for preeclampsia: the generation r study. J Hypert. (2008) 26:1200–8.
34. Arvizu M, Minguez-Alarcon L, Stuart J, Mitsunami M, Rosner B, Rich- Edwards J, et al. Physical activity before pregnancy and the risk of hypertensive disorders of pregnancy. Am J Obstetr Gynecol MFM. (2022) 4:100556. doi: 10.1016/j.ajogmf.2021.100556
35. Longo-Mbenza B, Tshimanga B, Buassa-bu-Tsumbu B, Kabangu J. Diets rich in vegetables and physical activity are associated with a decreased risk of pregnancy induced hypertension among rural women from Kimpese. DR Congo Nigerian J Med. (2008) 17:265–9.
36. Gebremedhin S, Enquselassie F, Umeta M. Prevalence of prenatal zinc deficiency and its association with socio-demographic, dietary and health care related factors in Rural Sidama. Southern Ethiopia: a cross-sectional study. BMC Public Health. (2011) 11:898. doi: 10.1186/1471-2458-11-898
37. Tesfa E, Nibret E, Munshea A. Maternal serum zinc level and pre-eclampsia risk in african women: a systematic review and meta-analysis. Biol Trace Element Res. (2021) 2021:1–8. doi: 10.1007/s12011-021-02611-7
38. Lewandowska M, Sajdak S, Marciniak W, Lubiński J. First trimester serum copper or zinc levels, and risk of pregnancy-induced hypertension. Nutrients. (2019) 11:2479. doi: 10.3390/nu11102479
39. Mistry H, Gill C, Kurlak L, Seed P, Hesketh J, Méplan C, et al. SCOPE consortium. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks’ gestation in nulliparous women who subsequently develop preeclampsia. Free Rad Biol Med. (2015) 78:147–55. doi: 10.1016/j.freeradbiomed.2014.10.580
40. Ugwuja E, Ejikeme B, Ugwu N, Obeka N, Akubugwo E, Obidoa O. Comparison of plasma copper, iron and zinc levels in hypertensive and non- hypertensive pregnant women in Abakaliki, South Eastern Nigeria. Pak J Nutr. (2010) 9:1136–40.
41. van Middendorp D, Asbroek A, Bio F, Edusei A, Meijjer L, Newton S, et al. Rural and urban differences in blood pressure and pregnancy- induced hypertension among pregnant women in Ghana. Globalization Health. (2013) 9:59. doi: 10.1186/1744-8603-9-59
42. Umegbolu E, Ogamba J. Incidence of gestational hypertension among pregnant women (2006–2015) in enugu state, Southeast Nigeria: a retrospective study. Int J Commun Med Public Health. (2017) 4:357. doi: 10.18203/2394-6040.ijcmph20170255
43. Howell E. Reducing disparities in severe maternal morbidity and mortality. Clin Obstetr Gynecol. (2018) 61:387. doi: 10.1097/GRF.0000000000000349
44. Phipps E, Thadhani R, Benzing T, Karumanchi S. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. (2019) 15:275–89. doi: 10.1038/s41581-019-0119-6
45. Hromadnikova I, Dvorakova L, Kotlabova K, Krofta L. The prediction of gestational hypertension, preeclampsia and fetal growth restriction via the first trimester screening of plasma exosomal C19MC microRNAs. Int J Mol Sci. (2019) 20:2972. doi: 10.3390/ijms20122972
46. Eskild A, Haavaldsen C, Vatten L. Placental weight and placental weight to birthweight ratio in relation to Apgar score at birth: a population study of 522 360 singleton pregnancies. Acta Obstetr Gynecol Scand. (2014) 93:1302–8. doi: 10.1111/aogs.12509
47. Tamura T, Goldenberg R, Ramey S, Nelson K, Chapman V. Effect of zinc supplementation of pregnant women on the mental and psychomotor development of their children at 5 y of age. Am J Clin Nutr. (2003) 77:1512–6. doi: 10.1093/ajcn/77.6.1512
48. Blumfield M, Hure A, Macdonald-Wicks L, Smith R, Collins C. A systematic review and meta- analysis of micronutrient intakes during pregnancy in developed countries. Nutr Rev. (2013) 71:118–32.
49. Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant- based diets compared to meat-eaters: a systematic review. Nutrients. (2022) 14:29. doi: 10.3390/nu14010029
50. Foster M, Samman S. Implications of a plant-based diet on zinc requirements and nutritional status. In: Mariotti F editor. Vegetarian and plant-based diets in health and disease prevention. (2017). p. 683–713. doi: 10.1016/B978-0-12-803968-7.00038-1
51. Hennigar S, Lieberman H, Fulgoni V, McClung J. Serum zinc concentrations in the US population are related to sex, age, and time of blood draw but not dietary or supplemental zinc. J Nutr. (2018) 148:1341–51. doi: 10.1093/jn/nxy105
52. Mousa A, Naqash A, Lim S. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients. (2019) 11:443. doi: 10.3390/nu11020443
53. Kumssa D, Joy E, Ander E, Watts M, Young S, Walker S, et al. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci Rep. (2015) 5:10974. doi: 10.1038/srep10974
Keywords: assessment, Palestine, pregnancy-induced hypertension, pregnant women, serum zinc level
Citation: El Bilbeisi AH, Abo Khosa SM, Taleb MH and El Afifi AM (2023) Assessment of serum, dietary zinc levels, and other risk factors during the third trimester among pregnant women with and without pregnancy-induced hypertension: a case-control study. Front. Nutr. 10:1155529. doi: 10.3389/fnut.2023.1155529
Received: 31 January 2023; Accepted: 22 May 2023;
Published: 05 June 2023.
Edited by:
Imre Lengyel, Queen’s University Belfast, United KingdomReviewed by:
Lana McClements, University of Technology Sydney, AustraliaAli Tarighat-Esfanjani, Tabriz University of Medical Sciences, Iran
Copyright © 2023 El Bilbeisi, Abo Khosa, Taleb and El Afifi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdel Hamid El Bilbeisi, YWJlZF9hekBob3RtYWlsLmNvbQ==
 Abdel Hamid El Bilbeisi
Abdel Hamid El Bilbeisi Sahar M. Abo Khosa2
Sahar M. Abo Khosa2