- 1Clinical Public Health Center, Shenzhen Qianhai Shekou Free Trade Zone Hospital, Shenzhen, Guangdong, China
- 2Shenzhen Qianhai Shekou Free Trade Zone Hospital, Shenzhen, Guangdong, China
- 3Department of Oncology, Shenzhen Qianhai Shekou Free Trade Zone Hospital, Shenzhen, Guangdong, China
- 4School of Public Health, Sun Yat-sen University, Guangzhou, Guangdong, China
- 5School of Public Health, Shantou University, Shantou, Guangdong, China
- 6Department of Biostatistics and Epidemiology, Shenzhen University Health Science Center, School of Public Health, Shenzhen, Guangdong, China
- 7Department of Biostatistics and Epidemiology, School of Public Health, Xi'an Medical University, Xi'an, Shanxi, China
Objectives: To conduct a systematic review and meta-analysis of prospective cohort studies to investigate the association between total, vegetable, fruit, cereal, soluble and insoluble fiber intake and risk of all causes, cardiovascular disease (CVD), and cancer mortality and quantitatively assess the dose–response relation.
Methods: Eligible studies were identified by searching PubMed, Embase and Web of science before August 2023. Random effects models were used to calculate summary relative risk (RR) and 95% confidence intervals (CI) and restricted cubic splines to model the linear/non-linear association.
Results: The summary RR for all-cause, CVD and cancer mortality of dietary fiber was 0.90 (95% CI: 0.86,0.93), 0.87 (0.84,0.91), 0.91 (0.88,0.93), respectively. Significant association was observed for all-cause and CVD mortality with fruit, vegetable cereal and soluble fiber intake and cancer mortality with cereal fiber intake. No significant association was found for insoluble fiber, vegetable or fruit fiber intake and cancer mortality. Dose-response analysis showed a significant non-linear relation of dietary fiber intake with all-cause mortality, and linear relation for others.
Conclusions: Higher dietary fiber including different type and food sources of fiber intake were associated with lower risk of mortality. Our findings provide more comprehensive evidence on dietary fiber intake with mortality.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier: CRD42022338837.
1. Introduction
Cardiovascular disease (CVD) and cancer are the leading causes of death globally (1). It has been estimated that global deaths from coronary heart disease, stroke, and cancer will reach up to 18.6 million, 12.2 million, and 10.0 million, respectively in 2019–2020 (1–3). Poor diet contributed to one of the largest risk factors for death, accounting for 8.3% of all deaths (4). The WHO recommends a daily intake of dietary fiber >25 g/day for adults (5); however, the consumption of dietary fiber remains low in many high-income countries (18.3 g/day in the United States, 14.8 g/day in the United Kingdom, 16.9 g/day in France, and 15.0 g/day in Japan) (6). Major nutrition shifts occur in developing countries with an increase in fat intake and a decrease in whole grain and fiber intake. The dietary fiber consumption level was reported to be even lower in middle-income countries (9.7 g per capita/day in China) (7, 8). Accumulating evidence indicated that dietary fiber might decrease the risks of various chronic diseases (9, 10), including obesity, diabetes, hypertension, CVD (11–14), and cancer (15–17).
Inconsistent results were found in previous studies examining the effect of dietary fiber on mortality. Most of the previous studies detected an inverse association between dietary fiber and all-cause, CVD, or cancer mortality (18–20), but no association was found in other studies (21, 22). Although few systematic reviews and meta-analyses were conducted to analyze the relationship between fiber intake and mortality, some of those meta-analyses focused on specific populations such as patients and cancer survivors (23–25) and unstable findings have been reported with controversial results in many subgroups. A most recent meta-analysis conducted in 2019 analyzed the relationship between total fiber and a series of health outcomes, which included 68,183 deaths, but did not take into consideration the specific types of dietary fiber (26). More than 10 studies (18–20, 22, 27–33) have been reported since the last meta-analysis, with approximately 424,953 participants and 30,215 deaths that could be further added in this updated meta-analysis. Therefore, it is necessary to conduct an updated meta-analysis to explore the association between dietary fiber intake and all-cause, CVD, or cancer mortality and provide evidence on their dose–response relationship.
Dietary fiber can be classified into insoluble and soluble fibers based on solubility (34). Studies on associations between insoluble or soluble fiber intake and mortality have also been inconclusive. In a cohort study of 92,924 Japanese consumption of both insoluble and soluble fibers was associated with a lower risk of all-cause mortality (20). While some observational studies have not found a significant association between soluble or insoluble fiber intake and all-cause mortality mortality (28, 35). Only one previous systematic review and meta-analysis investigated the association between soluble and insoluble fiber intake and CVD mortality (36); however, the study did not assess the association between all-cause between all-cause mortality and cancer mortality.
The levels and sources of dietary fiber intake may be substantially different among countries. For example, grain products are the main source of dietary fiber for the US population (37), while dietary fiber mainly comes from vegetables for the Japanese population (38). Bean, fruit, and vegetable fibers but not cereal fibers are associated with reduced risk of all-cause mortality in a study conducted in Japan (39), whereas others reported no associations of individual food sources of dietary fiber (including fibers from cereals, fruits, or vegetables) with the risk of ischemic heart disease mortality mortality (40). Although a previous meta-analysis investigated the association between cereal fiber intake and all-cause, cardiovascular, and cancer mortality (41), the study included general participants and people with diseases, and several cohort studies with large sample sizes have been published in recent years (20, 28). Different from previous meta-analyses, this meta-analysis explored dietary fibers from different sources and cardiovascular or cancer mortality. To the best of our knowledge, most previous meta-analyses (24, 26, 36, 41–45) did not analyze the relationship between fibers from different sources and mortality. A meta-analysis (46) was conducted on the association between dietary fibers obtained from different sources including cereal, fruit, legume, and vegetable fibers and cardiovascular mortality.
Hence, our study aimed to conduct an updated systematic review and meta-analysis of prospective cohort studies to investigate the risk of all-cause, cardiovascular, and cancer mortality associated with dietary fiber intake and different food sources and different types (soluble and insoluble fiber) of dietary fiber intake in general populations and further explore the dose–response relationship.
2. Methods
The systematic review and meta-analysis were registered in the prospective register of systematic reviews database (PROSPERO) (https://www.crd.york.ac.uk/prospero/index.asp, identifier CRD42022338837) and conducted and reported according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (47).
2.1. Search strategy
We systematically searched the PubMed, Embase, and Web of Science electronic databases from their inception up to 25 August 2023. We used a combination of MeSH terms and free-text terms to identify relevant publications assessing dietary fiber intake and fibers from different food sources in relation to all-cause, CVD, and cancer mortality, with restriction to the English language and without date limitation. Moreover, the reference lists from the retrieved articles, systematic reviews, and meta-analyses were searched for further relevant studies. Study authors were contacted, but non-peer-reviewed sources were not considered. Details of the search terms used for querying literature are shown in Supplementary Table 1. The literature search was conducted by two independent investigators (F. Y. and P. Q.).
2.2. Inclusion and exclusion criteria
The PICOS (participants, interventions/exposures, comparators, outcomes, and study design) criteria were used to identify studies that were eligible for inclusion: (1) the study design was prospective cohort studies; (2) the exposure of interest was dietary fiber intake; (3) the outcome of interest included all-cause, CVD, or cancer mortality; and (4) the risk estimates, including adjusted hazard ratios (HR) or risk ratios (RR), with their corresponding 95% confidence intervals (CIs) were reported. When reports pertained to overlapping participants, we included only the study with a larger population to avoid duplication of data.
Reviews, abstracts, comments, or unpublished results were excluded. Studies on children, adolescents, or patients with chronic kidney disease, or who were undergoing hemodialysis, end-stage cancer, or critical illnesses were excluded.
2.3. Data extraction and quality assessment
The data extraction and quality assessment were conducted by F. Y. and P. Q., and any discrepancies were discussed with a third investigator (C. H.). The following characteristics from each study finally included in the meta-analysis were extracted using a standardized form: name of first author, publication year, country or region, the name of the study, sample size and number of deaths, follow-up period, types of outcomes, gender, age, types of fibers, amount of intake, measurement of fiber, assessment of interested outcomes, RRs/HRs and 95% CIs, and variables adjusted for in the analysis. When separate risk estimates for men and women were available in a study, their RRs were combined using a fixed-effects model to generate a pooled risk estimate. For dose–response meta-analysis, the risk estimates should be provided for at least three quantitative categories of fiber intake.
We assessed study quality with the Newcastle–Ottawa Scale (NOS) for cohort studies (48). A maximum score of 1 for each question in the checklist can be awarded. Scores were calculated according to three major aspects: selection of participants, adjustment for confounders, and ascertainment of outcomes and nine questions. Scores of 0–2, 3–5, 5–7, and 7–9 were considered poor, fair, good, and high quality, respectively.
2.4. Statistical methods
For studies reporting HRs for fiber consumption, we assumed that the HR was approximately equal to the RR (49). The missing number of cases in each category was calculated by using the reported RRs/HRs and the number of total cases (50). The average or midpoint of each defined quartile was used for the dose amount. If the category dose range was open-ended, we assumed the length of the open-ended interval to be the same as that of the adjacent interval. For studies reporting risk estimates compared to medium or highest dietary fiber intake, the RR was recalculated by setting the lowest category of dietary fiber intake as the reference.
We computed the highest vs. lowest estimates by using a random-effects model (51), which considered variations (heterogeneities) both within and between studies. We calculated summary RRs (95% CIs) of all-cause, CVD, and cancer mortality per 10 g/day increment. We used the generalized least squares regression to estimate study-specific dose–response associations (52) and the random-effects model to pool the study-specific dose–response RR estimates (51). To examine possible linear or non-linear associations, we used restricted cubic splines for each study with more than three categories of exposure, with three fixed knots at 25%, 50%, and 75% of the total distribution of the reported intake, and combined them using multivariable meta-analysis (53). The significance of non-linearity was calculated using null hypothesis testing (53). We combined the study-specific slopes using random-effects models.
Heterogeneity was assessed using Cochran's Q test and I2 statistic (54), with a value of I2 > 50% considered to represent potentially important heterogeneity, and P < 0.1 was considered statistically significant for the Q statistic (55). Publication bias was assessed using Egger's test and funnel plots. When Egger's test indicated bias, a trim and fill method was used to detect the effect of probable missing studies on the overall effect. We further carried out subgroup analyses stratified by study characteristics, including duration of follow-up (>10 vs. ≤10), number of cases (≤1,000 vs. >1,000), geographical location, study quality (>7 vs. ≤7), adjustment for confounding factors (physical activity (PA), comorbidity at baseline, carbohydrate, protein), and dietary assessment methods, and meta-regression to investigate potential sources of heterogeneity. We also conducted sensitivity analyses excluding each study at a time from each analysis to clarify if the results are robust. A two-tailed P < 0.05 was considered significant. The Stata version 15.0 software (Stata Corp., TX) was used for the analyses.
3. Results
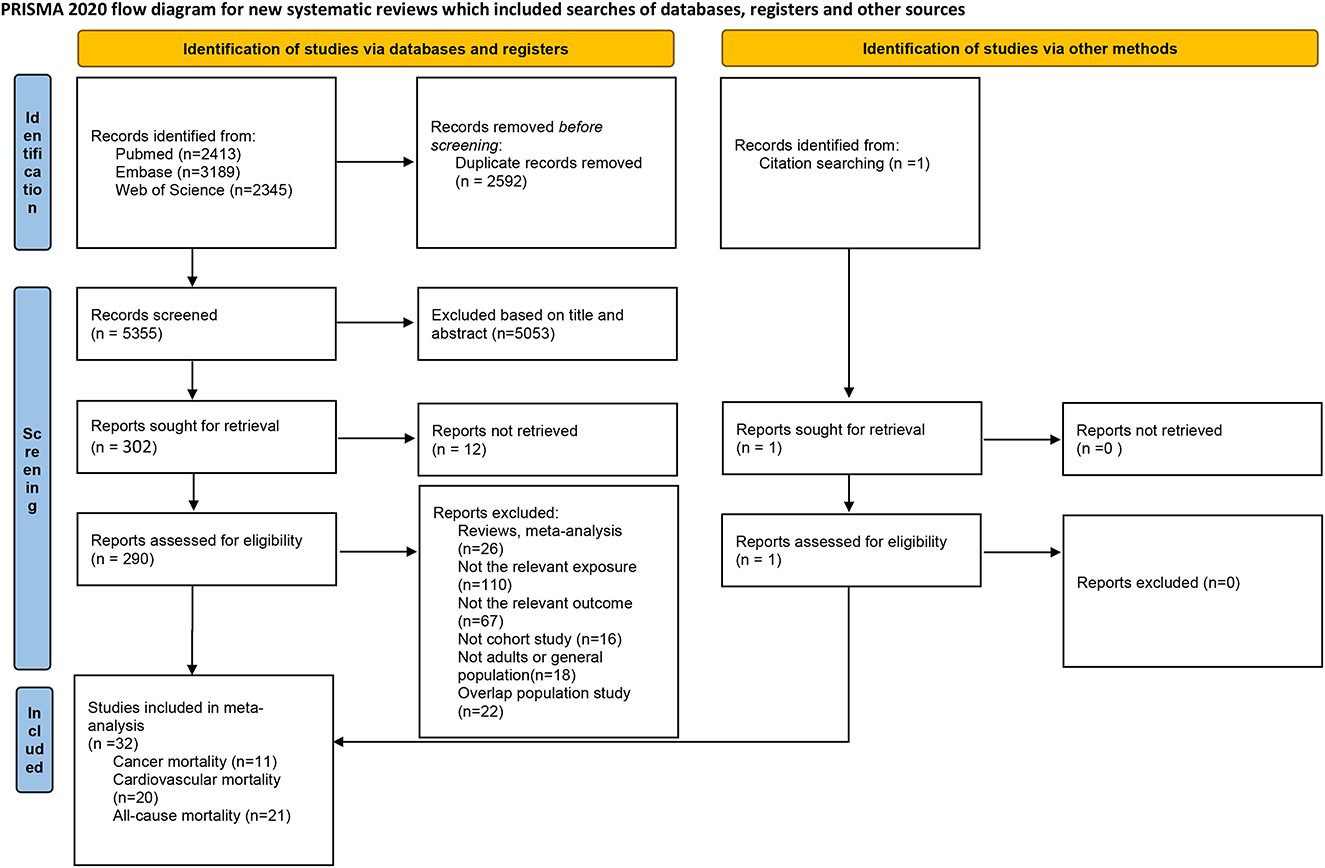
The flowchart for the selection is presented in Figure 1. We found 7,947 studies through the database search and reference lists. After removing duplicates, 5,355 records remained. After reviewing the title and abstract of these studies, 5,053 studies were subsequently excluded, and 302 full-text studies were then assessed. After full-text screening, a total of 290 publications were excluded because of duplicated data from the same cohort studies (n = 22), reviews (n = 11), or meta-analyses (n = 15), not relevant exposure (n = 110), not relevant outcome (n = 67), not cohort study (n = 16), or not adults or general population (n = 18). Finally, 32 publications were included in the systematic review and meta-analysis.
3.1. Study characteristics
A total of 32 articles (18–22, 28, 29, 32, 35, 40, 56–77) were included in the systematic review and the present meta-analysis. The characteristics of the studies included in the meta-analysis are listed in Table 1. The number of participants in these studies ranged from 314 to 452,717, with a mean or median age ranging from 16 to 99 years. Ten studies were from the United States (18, 21, 35, 56, 62, 64, 65, 67, 72, 77), four from the United Kingdom (19, 70, 71, 74), three cohorts conducted in Australia (58, 61, 63), two conducted among multiple nations (40, 59), two from Spain (28, 57), one from Dutch (69), one from Finland (68), one from France (22), one from Israeli (66), three from Japan (20, 29, 60), one from Korea (32), one from China (75), one from Malaysia (76), and one from Sweden (73). The follow-up period ranged from 2 to 40 years. Notably, 22 studies assessed dietary fiber intake using the food frequency questionnaire (FFQ) (20, 28, 32, 40, 56–63, 65–67, 70–74), and 10 using 24-h dietary records (18, 19, 21, 22, 35, 64, 69, 75–77). A total of 21 studies adjusted for physical activities (18, 20–22, 28, 32, 35, 40, 56, 57, 59, 60, 62, 65, 67, 68, 71, 72, 75), and others did not adjust for physical activities, and only one study did not adjust for age (69).
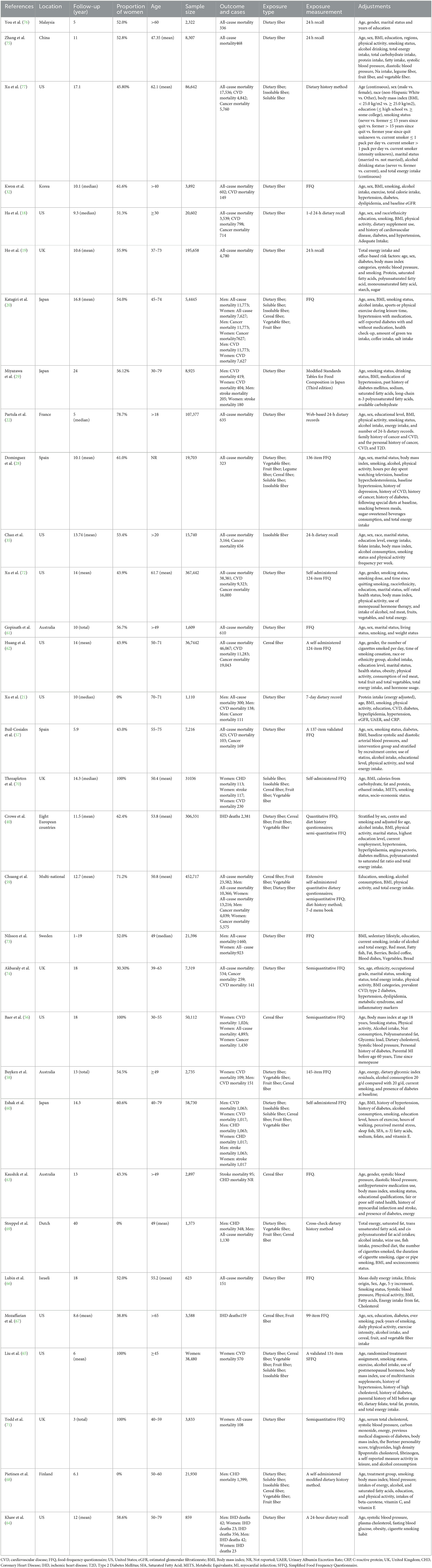
Table 1. Main characteristics of prospective studies examined the association of dietary fiber intake with all-cause, cardiovascular, cancer mortality.
In all, 22 prospective cohort studies were summarized for meta-analysis to evaluate the possible relationships between dietary fiber consumption and mortality risk, totaling 171,751 deaths (164,183 for all-cause, 95,879 for CVD, and 107,114 for cancer mortality) among 2,567,890 participants. A total of 21 articles reported RRs of all-cause mortality (18–22, 28, 32, 35, 57, 59, 61, 62, 66, 69, 71–77), 11 reported RRs of cancer mortality (18–22, 28, 32, 35, 57, 59, 61, 62, 66, 69, 71–74, 77), 5 reported RRs of mortality from coronary heart disease (60, 63, 68–70), 14 reported RRs of mortality from CVD (18, 20, 21, 29, 32, 56–58, 60, 62, 65, 70, 72, 77), three reported RRs of mortality from ischemic heart disease (40, 64, 67), and four reported RRs of mortality from stroke (29, 60, 63, 70). Assessment of quality of the included studies for the association between dietary fiber and mortality is shown in Supplementary Table 2. By applying the NOS, the mean quality assessment score of included studies was 7.39 (range 5–8), with 28 studies assessed as high quality (more than 7 points) (18–22, 28, 29, 32, 35, 40, 56–60, 62–68, 70–73, 75, 77) and the other four (61, 69, 74, 76) as good quality.
The results of the highest vs. lowest meta-analyses on the associations between intake of dietary fiber and all-cause, CVD, and cancer mortality are shown in Table 1, Supplementary Figures 1–12.
3.2. Dose–response meta-analysis
3.2.1. Dietary fiber
A total of 14 studies (18–22, 28, 32, 57, 59, 69, 72, 75–77) with a total of 1,367,285 participants and 97,469 deaths were included in the dose–response meta-analysis of dietary fiber intake and all-cause mortality. The summary RR for a 10-g/day increment of dietary fiber intake was 0.90 (95% CI: 0.86–0.93; I2 = 86.1%, Pheterogeneity < 0.001; Table 2, Supplementary Figure 19). Evidence of heterogeneity between subgroups in stratified analyses was not found (Supplementary Figure 7). A non-linear dose–response association was found between dietary fiber intake and all-cause mortality (Pnon−linearity = 0.0096, Figure 2). The shape of the non-linear curve was steeper with a dietary fiber intake of < 15 g/day, but the increase was more gradual after 15 g/day.
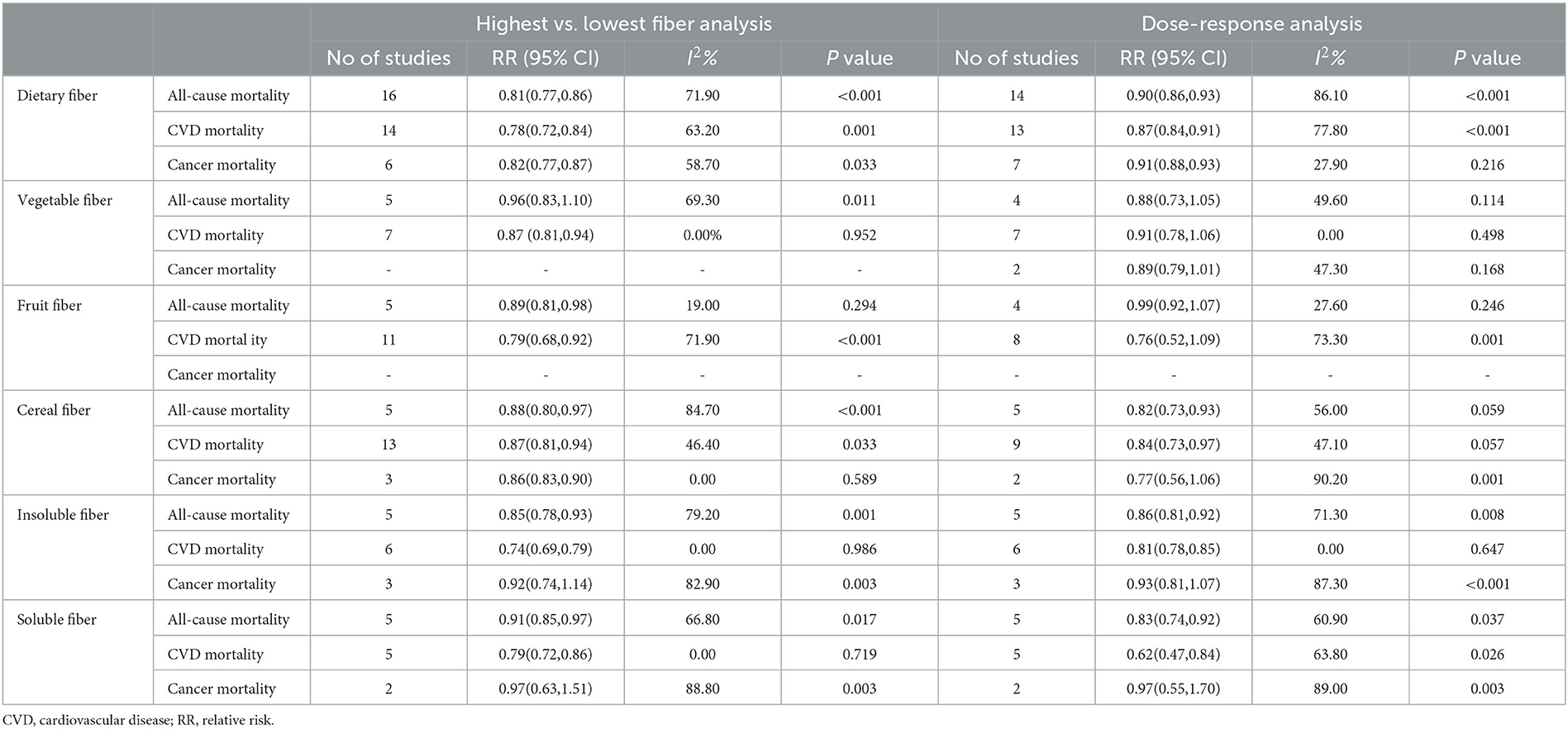
Table 2. Dietary fiber intake and risk of all-cause, cardiovascular, and cancer mortality for the highest versus lowest and dose-response meta-analysis.
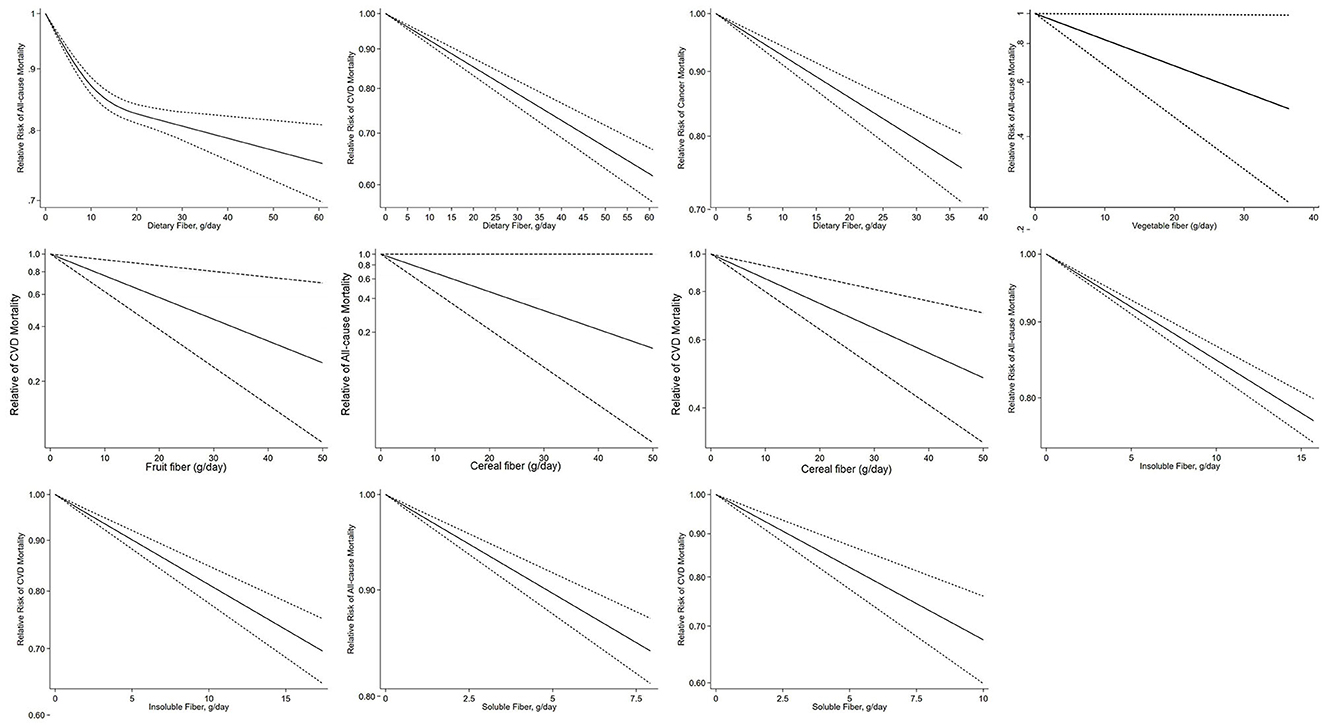
Figure 2. Dose–response association of per 10 g/day increase in total fiber, vegetable, fruit, cereal, soluble and insoluble fiber intake with all-cause, cardiovascular, and cancer mortality by restricted cubic splines.
Thirteen studies (18, 20, 21, 29, 32, 40, 57, 58, 60, 64, 65, 72, 77) on the association between dietary fiber intake and CVD mortality were included in the dose–response analysis, which included 945,653 participants and 78,735 deaths. The summary RR for a 10-g/day increment of dietary fiber intake was 0.87 (95% CI: 0.84–0.91; I2 = 79.2%, Pheterogeneity < 0.001; Table 2, Supplementary Figure 19). Evidence of heterogeneity between subgroups was observed in the analysis stratified by adjustment for comorbidity at baseline (P = 0.032) (Supplementary Figure 7). No evidence of a non-linear dose–response association was found between dietary fiber intake and risk of CVD mortality (Pnon−linearity = 0.247, Figure 2). Dose–response analysis of six studies (18, 20, 21, 57, 59, 72) showed an inverse association between dietary fiber and cancer mortality (summary RR 0.90, 95% CI: 0.87–0.94; I2 = 35.4%, Pheterogeneity = 0.17; Table 2, Supplementary Figure 19). Evidence of heterogeneity between subgroups was observed in the analysis stratified by adjustment for region (P = 0.032) (Supplementary Figure 7). There was no indication of non-linearity between dietary fiber intake and risk of cancer mortality (Pnon−linearity = 0.995, Figure 2).
Sensitivity analysis showed that the exclusion of any single study from the analysis did not appreciably alter the summary effect sizes (Supplementary Table 9).
3.2.2. Vegetable fiber
Four studies (22, 28, 59, 69) were included in the dose–response meta-analysis of vegetable fiber intake and all-cause mortality. The summary RR for a 10-g/day increment of vegetable fiber intake was 0.88 (95% CI: 0.73–1.05; I2 = 49.6%, Pheterogeneity = 0.11; Table 2, Supplementary Figure 20). No evidence of heterogeneity between subgroups was observed (Supplementary Figure 8). The non-linearity between dietary fiber intake and risk of cancer mortality approached significance (P = 0.07, Figure 2).
No significant association was seen between vegetable fiber intake and CVD mortality based on six studies (40, 58, 65, 68–70). The summary RR for a 10-g/day increment of vegetable fiber was 0.91 (95% CI: 0.78–1.06; I2 = 0%, Pheterogeneity = 0.50; Table 2, Supplementary Figure 20). No evidence of heterogeneity between subgroups was observed (Supplementary Figure 8). A non-linear dose–response association was found between vegetable fiber intake and risk of cancer mortality (Pnon−linearity = 0.01, Figure 2). The association between vegetable fiber and CVD mortality has a J-shape, with the lowest estimates at 5 g/day.
In the sensitivity analysis, exclusion of the study by Partula et al. (22) and Streppel et al. (69) resulted in a change in the significant inverse association between vegetable fiber intake and all-cause mortality to a marginally significant inverse association, but the summary estimate of vegetable fiber intake and CVD mortality remained robust (Supplementary Table 10).
3.2.3. Fruit fiber
No significant association was seen between fruit fiber intake and all-cause mortality based on four studies (22, 28, 59, 69). The summary RR for a 10-g/day increment of fruit fiber intake was 0.99 (95% CI: 0.92–1.07; I2 = 27.6%, Pheterogeneity = 0.25; Table 2, Supplementary Figure 21). No significant association with fruit fiber intake was found in subgroup analyses, and no evidence of heterogeneity between subgroups was observed (Supplementary Figure 9). There was no indication of non-linearity between fruit fiber intake and all-cause mortality (Pnon−linearity = 0.25, Figure 2).
No significant association was found between fruit fiber intake and CVD mortality based on seven studies studies (40, 58, 60, 65, 68–70). The summary RR for a 10-g/day increment of fruit fiber intake was 0.76 0.52–1.09; I2 = 73.3%, Pheterogeneity = 0.001; Table 2, Supplementary Figure 21). No evidence of heterogeneity between subgroups was observed (Supplementary Figure 9). There was no evidence of non-linearity between fruit fiber intake and CVD mortality (Pnon−linearity = 0.13, Supplementary Figure 14).
A sensitivity analysis showed that exclusion of the studies by Eshak et al. (60) or Pietinen et al. (68) resulted in a change from the non-significant association between fruit fiber intake and CVD mortality to a significant inverse association (Supplementary Table 11).
3.2.4. Cereal fiber
In the dose–response analysis of cereal fiber intake and all-cause mortality, based on five studies studies (22, 28, 56, 59, 69), a significant inverse association was found. The summary RR for a 10-g/day increment cereal fiber intake was 0.82 (95% CI: 0.73–0.93; I2 = 56.0%, Pheterogeneity = 0.06; Table 2, Supplementary Figure 22). No evidence of heterogeneity between subgroups was observed (Supplementary Figure 10). There was no indication of non-linearity between soluble fiber intake and CVD disease mortality (P = 0.24, Figure 2).
In the dose–response analysis of cereal fiber intake and CVD mortality, based on nine studies (40, 56, 58, 60, 63, 65, 68–70), a significant inverse association was found. The summary RR for a 10-g/day increment of cereal fiber intake was 0.84 (95% CI: 0.73–0.97; I2 = 47.1%, Pheterogeneity = 0.06; Table 2, Supplementary Figure 22). No evidence of heterogeneity between subgroups was observed (Supplementary Figure 10). There was no indication of non-linearity between cereal fiber intake and CVD mortality (Pnon−linearity = 0.45, Figure 2), with nine studies included (40, 56, 58, 60, 63, 65, 68–70).
Two studies (56, 59) reported data on cereal fiber intake and cancer mortality. The summary RR for a 10-g/day increment of cereal fiber intake was 0.77 (95% CI: 0.56–1.06; I2 = 90.2%, Pheterogeneity = 0.001; Table 2, Supplementary Figure 22).
The sensitivity analysis showed that the summary estimate is robust (Supplementary Table 12).
3.2.5. Insoluble fiber
Five studies (20, 22, 28, 35, 77) assessed the dose–response meta-analysis of insoluble fiber intake and all-cause mortality. The summary RR for a 10-g/day increment of insoluble fiber intake was 0.86 (95% CI: 0.81–0.92, I2 = 71.3%, Pheterogeneity = 0.008; Table 2, Supplementary Figure 23). Evidence of heterogeneity between subgroups was observed in the analysis stratified by the number of cases included in the study (0.034) and whether adjusted for region (P = 0.040) (Supplementary Figure 11). There was no indication of non-linearity between insoluble fiber intake and all-cause mortality (Pnon−linearity = 0.909, Figure 2), with five studies included (20, 22, 28, 35).
Six studies (20, 60, 65, 68, 70, 77) on the association between insoluble fiber intake and CVD mortality were included in the dose–response analysis. The summary RR for a 10-g/day increment of insoluble fiber intake was 0.81 (95% CI: 0.78–0.85; I2 = 0.00%, Pheterogeneity = 0.65; Table 2, Supplementary Figure 23). No evidence of heterogeneity between subgroups was observed (Supplementary Figure 11). There was no evidence of non-linear dose–response association between insoluble fiber intake and CVD mortality (Pnon−linearity = 0.52, Figure 2), with six studies included (20, 60, 65, 68, 70).
The dose–response analysis of three studies (20, 35, 77) showed no significant association between insoluble fiber and cancer mortality (summary RR: 0.93, 95% CI: 0.81–1.07), with no significant heterogeneity among the studies (I2 = 87.3%, Pheterogeneity < 0.001; Table 2, Supplementary Figure 23). There was no evidence of non-linear dose–response association between insoluble fiber intake and CVD mortality (Pnon−linearity = 0.699, Figure 2), with two studies included (20, 35).
In the sensitivity analysis, the summary estimate is robust for all-cause and CVD mortality. Exclusion of the study by Katagiri et al. (20) resulted in a change from the non-significant association between insoluble fiber intake and cancer mortality to a significant inverse association (Supplementary Table 13).
3.2.6. Soluble fiber
Five prospective studies (20, 22, 28, 35, 77) were included in the dose–response meta-analysis of soluble fiber intake and all-cause mortality. The summary RR for a 10-g/day increment of soluble fiber intake was 0.83 (95% CI: 0.74–0.92; I2 = 60.9%, Pheterogeneity = 0.037; Table 2, Supplementary Figure 24). Evidence of heterogeneity between subgroups was observed in the analysis stratified by dietary fiber measurement (P = 0.032) (Supplementary Figure 12). There was no indication of non-linearity between soluble fiber intake and all-cause mortality (Pnon−linearity = 0.785, Figure 2), with five studies included (20, 22, 28, 35).
Five studies (60, 65, 68, 70, 77) provided RRs of soluble fiber intake and CVD mortality. The summary RR for a 10-g/day increment of soluble fiber intake was 0.62 (95% CI: 0.47–0.84; I2 = 63.8%, Pheterogeneity = 0.026; Table 2, Supplementary Figure 24). Evidence of heterogeneity between subgroups in stratified analyses was not observed (Supplementary Figure 12). There was no indication of non-linearity between soluble fiber intake and CVD mortality (Pnon−linearity = 0.587, Figure 2).
In the sensitivity analysis, the summary estimate is robust, except that exclusion of the study by Katagiri et al. and Xu et al. (20, 77) lead to a non-significant association between soluble fiber intake and all-cause mortality (Supplemental Table 14).
3.3. Publication bias
In the highest vs. lowest meta-analysis, Egger's linear regression test and visual inspection of the funnel plots (Supplementary Figure 25) indicated possible publication bias for the association between dietary fiber intake and CVD mortality (P = 0.001), and vegetable fiber intake and all-cause mortality (P = 0.038), but no evidence of publication bias for other outcomes. In the dose–response analyses, Egger's linear regression test and visual inspection of the funnel plots indicated possible publication bias for the association between dietary fiber intake and cancer mortality (P = 0.043) (Supplementary Figures 31, 36). No evidence of significant publication bias was found in other analyses (Supplementary Figures 26–30, 32–35). Application of the trim and fill method did not result in a change in the average effect size, further suggesting that the results were not affected by publication bias.
4. Discussion
The present systematic review and meta-analysis investigated the association between dietary fiber intake and different sources and types of fiber intake and all-cause, CVD, and cancer mortality by applying highest vs. lowest, linear, and non-linear dose–response analyses. We found that dietary fiber intake was inversely associated with all-cause, CVD, and cancer mortality. The inverse association was also found for cereal fiber intake. All categories of fibers were inversely associated with CVD mortality. The inverse association of cancer mortality was only detected for cereal fiber and dietary fiber. Significant associations were also found for other fiber intake and all-cause mortality, except for fruit and vegetable fiber intake. Besides, a non-linear relationship was found for all-cause mortality.
A large number of longitudinal cohort studies have reported the health benefits of dietary fiber intake (78–80). Several systematic reviews and meta-analyses suggested that high dietary fiber intake was associated with a reduced risk of all-cause, CVD, and cancer mortality (42, 46), which was consistent with the findings from our systematic review and meta-analysis. The subgroup analysis also showed the stability of the findings, which was different from previous meta-analyses meta-analyses (24). This may account for the fact that our study has additionally included more than 14 related studies studies (18–22, 28, 29, 32, 35, 61, 62, 75–77) published in recent years, with than 2,614,294 participants included, compared to the previous meta-analysis. This study found a non-linear relationship between dietary fiber and all-cause mortality, showing that the protective effect of dietary fiber is relatively constant when the daily intake is >15 g. A meta-analysis including five papers concluded that risk reduction associated with all-cause mortality was greatest when the daily intake of dietary fiber was between 25 and 29 g, while the dose–response data suggested that amounts >30 g/day confer additional benefits (26). The inconsistent findings might be because of the relatively large number of studies included: publications since 2016 were not included in their dose–response analyses of dietary fiber intake and all-cause mortality (26), and ~14 more articles updated to 2023 were included in our dose–response meta-analysis. The non-linear relationship of dietary fiber was not found among all-cause and cancer mortality because the effect of dietary fiber on different health outcomes may have different mechanisms.
Dietary fibers from different food sources have a distinctive mix of different types of compounds and may have a different effect on all-cause and CVD mortality (81, 82). The present systematic review and meta-analysis found the inverse association between vegetable and fruit fiber intake and CVD mortality as well as the significantly inverse association between cereal fiber intake and all-cause, CVD, and cancer mortality, but no association of vegetable fiber with cancer or all-cause mortality. A meta-analysis also found that cereal fiber intake was protectively associated with all-cause, CVD, and cancer mortality, although it included general participants and people with diseases (41). Our study also showed that cereal fiber but not fruit fiber or vegetable fiber was significantly associated with lower total mortality in the dose–response analysis, which was in line with an earlier meta-analysis (45). The recommended level of dietary fiber intake is 25 g for adult women and 38 g for adult men, and the public should consume adequate amounts of dietary fiber from a variety of plant foods (83). Plant foods contain more than just dietary fiber, so any protective properties of plant-based diets may be linked to other dietary components, such as vitamins, minerals, or phytochemicals, and not just isolated dietary fiber (84). The unstable findings in the subgroup analysis suggest that more studies are further needed on the association between fruit fiber and CVD mortality.
Soluble fiber is found in oat bran, barley, beans, lentils, peas, and some fruits and vegetables, while insoluble fiber is rich in foods such as wheat bran, whole grains, nuts, and seeds (77). Although mounting evidence has suggested the protective role of dietary fiber against various chronic diseases (13, 22), the health effect may depend on the dietary fiber type (85, 86), and the findings on soluble and insoluble fiber intake and mortality are contradictory (20, 22). Our study found the inverse association between both soluble and insoluble fiber intake and all-cause and CVD mortality. The finding on CVD mortality was in line with one previous systematic review and meta-analysis (87), and our study included eight additional studies (18, 20, 21, 29, 32, 57, 72, 77) after 2012 and found a linear relationship. To the best of our knowledge, this is the first study to explore soluble and insoluble fiber intake and all-cause and cancer mortality. No significant association was found between insoluble or soluble fiber intake and cancer mortality in the present study, which may be explained by the limited number of studies included. Insoluble fiber is characterized by a fecal-bulking ability, which may reduce the risk of cancer mortality (77); however, evidence regarding soluble or insoluble fiber on cancer mortality remains limited and inconsistent, and only three studies (20, 35, 77) conducted in Japan and the United States were included in our systematic review and meta-analysis. Further prospective studies on soluble and insoluble fiber intake and cancer mortality are therefore needed.
The mechanism underlying the inverse relationship between dietary fiber and mortality is unclear, but there are several plausible explanations. The protective effect of dietary fiber on cholesterol (88, 89), blood pressure (90), insulin sensitivity (85), and blood glucose (91) as well as the anti-inflammatory effects (92) may partly explain the protection from mortality. A study demonstrated that the inclusion of a practical dose of dietary fiber (11.6 g) in a bakery product significantly reduced postprandial glucose and insulin responses in healthy adults (93). Insulin is known to promote the action of the hepatic enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (94). Inhibition of HMG-CoA reductase may result in the prevention of excess cholesterol being synthesized and released into circulation by the liver and may thereby reduce the risk of CVD (95). Moreover, alteration of intestinal microbiota composition and function may be an important reason for the potential benefits of dietary fiber (96). Experimental studies also suggested that the reduction of soluble fiber may influence the synthesis of microbial metabolites that are important for regulating metabolic, immune, behavioral, and neurobiological outcomes (97).
This review has some strengths. First, the present study was a comprehensive systematic review and meta-analysis of prospective cohort studies to investigate the association between dietary fiber intake and mortality, using high vs. low analysis and dose–response analysis. Second, the different types and food sources of dietary fiber were also considered, which can provide valuable insight into the mechanisms and evidence for strategies to derive the greatest benefit from balanced consumption of dietary fiber. Furthermore, a large number of participants and deaths have been included and allowed us to quantitatively assess the association between dietary fiber intake and risk of mortality.
In terms of study limitations, first of all, most studies did not consider other nutrients as confounding factors, such as protein, carbohydrate, or fiber from other food sources, which may affect the magnitude of the association between dietary fiber intake and mortality. Besides, comorbidity at baseline was not controlled in a few studies, which could affect the association between dietary fiber and mortality. Second, different dietary fiber assessment tools were used, which might lead to variation in the study results. Third, only three studies (20, 35, 77) reported risk estimates on soluble or insoluble fiber intake and cancer mortality, which limit us to conduct the subgroup and sensitivity analyses and suggest the necessity of further studies. Fourth, different diet assessment tools were used (FFQ, 24-h dietary recall, semiquantitative FFQ), and therefore measurement error was unavoidable. Fifth, sensitivity analyses demonstrated a profound lack of robustness among summary estimates for vegetable fiber and fruit fiber intake on mortality in the dose–response meta-analysis. Sixth, high heterogeneity exists in our meta-analysis of fruit fiber–CVD mortality and dietary fiber–all-cause mortality associations, although sensitive and subgroup analyses were conducted to show stable findings. The meta-regression analysis was also conducted, and we found that the heterogeneity may come from different levels of study quality for studies included in the meta-analysis of dietary fiber and all-cause mortality and different durations of follow-up for the studies on the association of fruit fiber and CVD mortality.
In conclusion, the present systematic review and meta-analysis found that higher dietary fiber intake was associated with a lower risk of all-cause, CVD, and cancer mortality. For different food sources of dietary fibers, fruit, vegetable, and cereal fiber intake were related to reduced risk of mortality, but there was no association of vegetable or fruit fiber with cancer mortality, showing a significant non-linear relationship between dietary fiber intake and all-cause mortality and a linear relation for other fibers. Our study incorporates different types and food sources of dietary fibers, which provide valuable insight into the mechanisms and may provide evidence for strategies to derive the greatest benefit from a balanced consumption of dietary fiber. The association between insoluble or soluble fiber intake and mortality and the difference between sources of dietary fiber and cancer mortality warrants further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FY and PQ conceived, designed, performed the study, and drafted the manuscript. FY, JM, CH, XZ, YC, RL, and PQ extracted, analyzed, or interpreted the data. FY, JM, YC, RL, CH, XZ, FH, and PQ revised the manuscript. All authors approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number: 82103940) and the Natural Science Foundation of Guangdong Province (Grant Number: 2022A1515010503).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1153165/full#supplementary-material
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–249. doi: 10.3322/caac.21660
4. Li S, Liu Z, Joseph P, Hu B, Yin L, Tse LA, et al. Modifiable risk factors associated with cardiovascular disease and mortality in China: a PURE substudy. Eur Heart J. (2022) 43:2852–63. doi: 10.1093/eurheartj/ehac268
5. WHO. Diet, nutrition and the prevention of chronic diseases. World Health Organization technical report series. World Health Organ Tech Rep Ser. (2003) 916:1–149.
6. Stephen AM, Champ MM, Cloran SJ, Fleith M, van Lieshout L, Mejborn H, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. (2017) 30:149–90. doi: 10.1017/S095442241700004X
7. Teo KK, Rafiq T. Cardiovascular risk factors and prevention: a perspective from developing countries. Can J Cardiol. (2021) 37:733–43. doi: 10.1016/j.cjca.2021.02.009
8. Yu D, Zhao L, Zhao W. Status and trends in consumption of grains and dietary fiber among Chinese adults (1982-2015). Nutr Rev. (2020) 78:43–53. doi: 10.1093/nutrit/nuz075
9. Shinozaki K, Okuda M, Sasaki S, Kunitsugu I, Shigeta M. Dietary fiber consumption decreases the risks of overweight and hypercholesterolemia in Japanese children. Ann Nutr Metab. (2015) 67:58–64. doi: 10.1159/000434634
10. Nie Y, Luo F. Dietary fiber: an opportunity for a global control of hyperlipidemia. Oxid Med Cell Longev. (2021) 2021:5542342. doi: 10.1155/2021/5542342
11. Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, heart failure. Nat Rev Cardiol. (2019) 16:137–54. doi: 10.1038/s41569-018-0108-7
12. Soliman GA. Dietary fiber, atherosclerosis, cardiovascular disease. Nutrients. (2019) 11:55. doi: 10.3390/nu11051155
13. Aleixandre A, Miguel M. Dietary fiber and blood pressure control. Food Funct. (2016) 7:1864–71. doi: 10.1039/C5FO00950B
14. Du P, Luo K, Wang Y, Xiao Q, Xiao J, Li Y, et al. Intake of dietary fiber from grains and the risk of hypertension in late midlife women: results from the SWAN study. Front Nutrit. (2021) 8:730205. doi: 10.3389/fnut.2021.730205
15. Ma Y, Hu M, Zhou L, Ling S, Li Y, Kong B, et al. Dietary fiber intake and risks of proximal and distal colon cancers: a meta-analysis. Medicine (Baltimore). (2018) 97:e11678. doi: 10.1097/MD.0000000000011678
16. Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, et al. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. (2018) 4:71–9. doi: 10.1001/jamaoncol.2017.3684
17. Kim K, Chang Y. Association of dietary fiber intake with metabolic syndrome among adult cancer survivors: a population-based cross-sectional study. Sci Rep. (2021) 11:11794. doi: 10.1038/s41598-021-91312-1
18. Ha K, Sakaki JR, Chun OK. Nutrient adequacy is associated with reduced mortality in US adults. J Nutr. (2021) 151:3214–22. doi: 10.1093/jn/nxab240
19. Ho FK, Gray SR, Welsh P, Petermann-Rocha F, Foster H, Waddell H, et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: prospective cohort study of UK Biobank participants. BMJ. (2020) 368:m688. doi: 10.1136/bmj.m688
20. Katagiri R, Goto A, Sawada N, Yamaji T, Iwasaki M, Noda M, et al. Dietary fiber intake and total and cause-specific mortality: the Japan Public Health Center-based prospective study. Am J Clini Nutr. (2020) 111:1027–35. doi: 10.1093/ajcn/nqaa002
21. Xu H, Huang XY, Riserus U, Krishnamurthy VM, Cederholm T, Arnlov J, et al. Dietary fiber, kidney function, inflammation, mortality risk. Clini J Am Soc Nephrol. (2014) 9:2104–10. doi: 10.2215/CJN.02260314
22. Partula V, Deschasaux M, Druesne-Pecollo N, Latino-Martel P, Desmetz E, Chazelas E, et al. Milieu Interieur, Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Sante cohort. Am J Clini Nutr. (2020) 112:195–207. doi: 10.1093/ajcn/nqaa063
23. Jayedi A, Emadi A, Khan TA, Abdolshahi A, Shab-Bidar S. Dietary fiber and survival in women with breast cancer: a dose-response meta-analysis of prospective cohort studies. Nutr Cancer. (2021) 73:1570–80. doi: 10.1080/01635581.2020.1803928
24. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. (2020) 17:1003053. doi: 10.1371/journal.pmed.1003053
25. Park SH, Hoang T, Kim J. Dietary factors and breast cancer prognosis among breast cancer survivors: a systematic review and meta-analysis of cohort studies. Cancers. (2021) 13. doi: 10.3390/cancers13215329
26. Reynolds A, Mann J, Cummings J, Winter N, Mete T, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet (London, England). (2019) 393:434–45. doi: 10.1016/S0140-6736(18)31809-9
27. Beydoun HA, Huang S, Beydoun MA, Hossain S, Zonderman AB. Mediating-moderating effect of allostatic load on the association between dietary approaches to stop hypertension diet and all-cause and cause-specific mortality: 2001-2010 national health and nutrition examination surveys. Nutrients. (2019) 11:2311. doi: 10.3390/nu11102311
28. Dominguez LJ, Bes-Rastrollo M, Toledo E, Gea A, Fresán U, Barbagallo M, et al. Dietary fiber intake and mortality in a Mediterranean population: the “Seguimiento Universidad de Navarra” (SUN) project. Eur J Nutr. (2019) 58:3009–22. doi: 10.1007/s00394-018-1846-3
29. Miyazawa I, Miura K, Miyagawa N, Kondo K, Kadota A, Okuda N, et al. Relationship between carbohydrate and dietary fibre intake and the risk of cardiovascular disease mortality in Japanese: 24-year follow-up of NIPPON DATA80. Eur J Clin Nutr. (2020) 74:67–76. doi: 10.1038/s41430-019-0424-y
30. Ricci C, Leitzmann MF, Freisling H, Schutte AE, Schutte R, Kruger SH, et al. Diet and sedentary behaviour in relation to mortality in US adults with a cardiovascular condition: results from the National Health and Nutrition Examination Survey linked to the US mortality registry. Br J Nutr. (2020) 124:1329–37. doi: 10.1017/S0007114520002391
31. King DE, Xiang J. A relationship between mortality and eating breakfast and fiber. J Am Board Family Med. (2021) 34:678–87. doi: 10.3122/jabfm.2021.04.210044
32. Kwon YJ, Lee HS, Park GE, Lee JW. Association Between Dietary Fiber Intake and All-Cause and Cardiovascular Mortality in Middle Aged and Elderly Adults With Chronic Kidney Disease. Front Nutr. (2022) 9:863391. doi: 10.3389/fnut.2022.863391
33. Zhang HR, Yang Y, Tian W, Sun YJ. Dietary fiber and all-cause and cardiovascular mortality in older adults with hypertension: a cohort study Of NHANES. J Nutr Health Aging. (2022) 26:407–14. doi: 10.1007/s12603-022-1770-3
34. Gorman MA, Bowman C. Position of The American Dietetic Association: health implications of dietary fiber. J am diet assoc. (1993) 93:1446–7. doi: 10.1016/0002-8223(93)92252-S
35. Chan CW, Lee PH. Association between dietary fibre intake with cancer and all-cause mortality among 15 740 adults: the National Health and Nutrition Examination Survey III. J Hum Nutr Dietet. (2016) 29:633–42. doi: 10.1111/jhn.12389
36. Liu L, Wang S, Liu J. Fiber consumption and all-cause, cardiovascular, and cancer mortalities: a systematic review and meta-analysis of cohort studies. Mol Nutr Food Res. (2015) 59:139–46. doi: 10.1002/mnfr.201400449
37. McGill CR, Fulgoni VL, Devareddy L. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: National Health and Nutrition Examination Survey 2001-2010. Nutrients. (2015) 7:1119–30. doi: 10.3390/nu7021119
38. Nakaji S, Sugawara K, Saito D, Yoshioka Y, MacAuley D, Bradley T, et al. Trends in dietary fiber intake in Japan over the last century. Eur J Nutr. (2002) 41:222–7. doi: 10.1007/s00394-002-0379-x
39. Jenkins DJA, Srichaikul KK, Kendall CWC, Sievenpiper JL. Bean, fruit, vegetable fiber but not cereal fiber are associated with reduced mortality in Japan. Am J Clin Nutr. (2020) 111:941–3. doi: 10.1093/ajcn/nqaa045
40. Crowe FL, Key TJ, Appleby PN, Overvad K, Schmidt EB, Egeberg R, et al. Dietary fibre intake and ischaemic heart disease mortality: the European Prospective Investigation into Cancer and Nutrition-Heart study. Eur J Clin Nutr. (2012) 66:950–6. doi: 10.1038/ejcn.2012.51
41. Hajishafiee M, Saneei P, Benisi-Kohansal S, Esmaillzadeh A. Cereal fibre intake and risk of mortality from all causes, CVD, cancer and inflammatory diseases: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. (2016) 116:343–52. doi: 10.1017/S0007114516001938
42. Yang Y, Zhao LG, Wu QJ, Ma X, Xiang YB. Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol. (2015) 181:83–91. doi: 10.1093/aje/kwu257
43. Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. (2018) 107:436–44. doi: 10.1093/ajcn/nqx082
44. Liu X, Yang W, Petrick JL, Liao LM, Wang W, He N, et al. Higher intake of whole grains and dietary fiber are associated with lower risk of liver cancer and chronic liver disease mortality. Nat Commun. (2021) 12:6388. doi: 10.1038/s41467-021-26448-9
45. Kim Y, Je Y. Dietary fiber intake and total mortality: a meta-analysis of prospective cohort studies. Am J Epidemiol. (2014) 180:565–73. doi: 10.1093/aje/kwu174
46. Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: a meta-analysis of prospective cohort studies. Arch Cardiovasc Dis. (2016) 109:39–54. doi: 10.1016/j.acvd.2015.09.005
47. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed). (2021) 372:n71. doi: 10.1136/bmj.n71
48. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) For Assessing The Quality of Nonrandomised Studies in Meta-Analyses. Available online at: www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed April 2022).
49. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. (2007) 298:2654–64. doi: 10.1001/jama.298.22.2654
50. Bekkering GE, Harris RJ, Thomas S, Mayer AM, Beynon R, Ness AR, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? Am J Epidemiol. (2008) 167:1017–26. doi: 10.1093/aje/kwn005
51. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
52. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
53. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. doi: 10.1093/aje/kwr265
54. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
55. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
56. Baer HJ, Glynn RJ, Hu FB, Hankinson SE, Willett WC, Colditz GA, et al. Risk factors for mortality in the nurses' health study: a competing risks analysis. Am J Epidemiol. (2010) 173:319–29. doi: 10.1093/aje/kwq368
57. Buil-Cosiales P, Zazpe I, Toledo E, Corella D, Salas-Salvadó J, Diez-Espino J, et al. Fiber intake and all-cause mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. (2014) 100:1498–507. doi: 10.3945/ajcn.114.093757
58. Buyken AE, Flood V, Empson M, Rochtchina E, Barclay AW, Brand-Miller J, et al. Carbohydrate nutrition and inflammatory disease mortality in older adults. Am J Clini Nutr. (2010) 92:634–43. doi: 10.3945/ajcn.2010.29390
59. Chuang SC, Norat T, Murphy N, Olsen A, Tjønneland A, Overvad K, et al. Fiber intake and total and cause-specific mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. (2012) 96:164–74. doi: 10.3945/ajcn.111.028415
60. Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, et al. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. Journal of Nutrition. (2010) 140:1445–53. doi: 10.3945/jn.110.122358
61. Gopinath B, Flood VM, Kifley A, Louie JC, Mitchell P. Association between carbohydrate nutrition and successful aging over 10 years. J Gerontol Biol Sci Med Sci. (2016) 71:1335–40. doi: 10.1093/gerona/glw091
62. Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals BMC Med. (2015) 13:59. doi: 10.1186/s12916-015-0294-7
63. Kaushik S, Wang JJ, Wong TY, Flood V, Barclay A, Brand-Miller J, et al. Glycemic Index, Retinal Vascular Caliber, Stroke Mortality. Stroke. (2009) 40:206–12. doi: 10.1161/STROKEAHA.108.513812
64. Khaw KT, Barrett-Connor E. Dietary fiber and reduced ischemic heart disease mortality rates in men and women: a 12-year prospective study. Am J Epidemiol. (1987) 126:1093–102. doi: 10.1093/oxfordjournals.aje.a114748
65. Liu SM, Buring JE, Sesso HD, Rimm EB, Willett WC, Manson JE, et al. prospective study of dietary fiber intake and risk of cardiovascular disease among women. J Am Coll Cardiol. (2002) 39:49–56. doi: 10.1016/S0735-1097(01)01695-3
66. Lubin F, Lusky A, Chetrit A, Dankner R. Lifestyle and ethnicity play a role in all-cause mortality. J Nutr. (2003) 133:1180–5. doi: 10.1093/jn/133.4.1180
67. Mozaffarian D, Kumanyika SK, Lemaitre RN, Olson JL, Burke GL, Siscovick DS. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA. (2003) 289:1659–66. doi: 10.1001/jama.289.13.1659
68. Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The alpha-tocopherol, beta-carotene cancer prevention study. Am J Epidemiol. (1996) 145:876–87. doi: 10.1093/oxfordjournals.aje.a009047
69. Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: the Zutphen Study. Am J Clin Nutr. (2008) 88:1119–25. doi: 10.1093/ajcn/88.4.1119
70. Threapleton DE, Greenwood DC, Burley VJ, Aldwairji M, Cade JE. Dietary fibre and cardiovascular disease mortality in the UK Women's Cohort Study. Eur J Epidemiol. (2012) 28:335–46. doi: 10.1007/s10654-013-9799-6
71. Todd S, Woodward M, Tunstall-Pedoe H, Bolton-Smith C. Dietary antioxidant vitamins and fiber in the etiology of cardiovascular disease and all-causes mortality: Results from the Scottish Heart Health Study. Am J Epidemiol. (1999) 150:1073–80. doi: 10.1093/oxfordjournals.aje.a009931
72. Xu M. Ready to eat cereal consumption with total and cause-specific mortality: Prospective analysis of 367,442 individuals. J Am Coll Nutr. (2016) 35:217–23. doi: 10.1080/07315724.2014.971193
73. Nilsson LM, Winkvist A, Brustad M, Jansson JH, Johansson I, Lenner P, et al. A traditional Sami diet score as a determinant of mortality in a general northern Swedish population. Int J Circumpolar Health. (2012) 71:1–12. doi: 10.3402/ijch.v71i0.18537
74. Akbaraly TN, Ferrie JE, Berr C, Brunner EJ, Head J, Marmot MG, et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clini Nutr. (2011) 94:247–53. doi: 10.3945/ajcn.111.013128
75. Zhang Z, Chen B, Zeng J, Fan M, Xu W, Li X, et al. Associations between consumption of dietary fibers and the risk of type 2 diabetes, hypertension, obesity, cardiovascular diseases, and mortality in Chinese adults: longitudinal analyses from the china health and nutrition survey. Nutrients. (2022) 14:32650. doi: 10.3390/nu14132650
76. You YX, Rivan NFM, Singh DKA, Rajab NF, Ludin AFM, Din NC, et al. Incidence and Predictors of Mortality among Community-Dwelling Older Adults in Malaysia: A 5 Years Longitudinal Study. Int J Environ Res Public Health. (2022) 19:8943. doi: 10.3390/ijerph19158943
77. Xu X, Zhang J, Zhang Y, Qi H, Wang P. Associations between dietary fiber intake and mortality from all causes, cardiovascular disease and cancer: a prospective study. J Transl Med. (2022) 20:344. doi: 10.1186/s12967-022-03558-6
78. Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. (2020) 112:603–12. doi: 10.1093/ajcn/nqaa161
79. Luo J, Xu X. Dietary fiber intake and the risk of bladder cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) cohort. Carcinogenesis. (2020) 41:478–82. doi: 10.1093/carcin/bgz187
80. Szmidt MK, Kaluza J, Harris HR, Linden A, Wolk A. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: a prospective cohort study of women. Eur J Nutr. (2020) 59:1869–79. doi: 10.1007/s00394-019-02038-w
81. Dhingra D, Michael M, Rajput H. Dietary fibre in foods: a review. (2012) 49:255–66. doi: 10.1007/s13197-011-0365-5
82. Song S, Song Y. Dietary fiber and its source are associated with cardiovascular risk factors in korean adults. Nutrients. (2021) 13:160. doi: 10.3390/nu13010160
83. Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. (2008) 108:1716–31. doi: 10.1016/j.jada.2008.08.007
84. Korczak R, Slavin JL. Definitions, regulations, and new frontiers for dietary fiber and whole grains. Nutr Rev. (2020) 78:6–12. doi: 10.1093/nutrit/nuz061
85. Dong Y, Chen L, Gutin B, Zhu H. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur J Clin Nutr. (2019) 73:1172–8. doi: 10.1038/s41430-018-0372-y
86. Deschasaux M, Pouchieu C, His M, Hercberg S, Latino-Martel P, Touvier M. Dietary total and insoluble fiber intakes are inversely associated with prostate cancer risk. J Nutr. (2014) 144:504–10. doi: 10.3945/jn.113.189670
87. Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ (Clinical research ed). (2013) 347:f6879. doi: 10.1136/bmj.f6879
88. Yang X, Dai J, Zhong Y, Wei X, Wu M, Zhang Y, et al. Characterization of insoluble dietary fiber from three food sources and their potential hypoglycemic and hypolipidemic effects. Food Funct. (2021) 12:6576–87. doi: 10.1039/D1FO00521A
89. Surampudi P, Enkhmaa B. Anuurad E. Lipid lowering with soluble dietary fiber. Curr Atheroscler Rep. (2016) 18:75. doi: 10.1007/s11883-016-0624-z
90. Xue Y, Cui L, Qi J, Ojo O, Du X, Liu Y. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: a randomized controlled trial. Nutr Metab Cardiovasc Dis. (2021) 31:2458–70. doi: 10.1016/j.numecd.2021.04.013
91. Jung C-H, Nutrients MCJ. Impact of high-carbohydrate diet on metabolic parameters in patients with type 2 diabetes. Nutrients. (2017) 9:322. doi: 10.3390/nu9040322
92. Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, et al. Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity. (2018) 48:992–1005. doi: 10.1016/j.immuni.2018.04.022
93. Stewart ML, Zimmer JP. Postprandial glucose and insulin response to a high-fiber muffin top containing resistant starch type 4 in healthy adults: a double-blind, randomized, controlled trial. Nutrition. (2018) 53:59–63. doi: 10.1016/j.nut.2018.01.002
94. Gunness P, Gidley MJ. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. (2010) 1:149–55. doi: 10.1039/c0fo00080a
95. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. (2014) 5:927–46.
96. Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. (2018) 23:705–715. doi: 10.1016/j.chom.2018.05.012
Keywords: dietary fiber intake, mortality, cancer, cardiovascular disease, meta-analysis
Citation: Yao F, Ma J, Cui Y, Huang C, Lu R, Hu F, Zhu X and Qin P (2023) Dietary intake of total vegetable, fruit, cereal, soluble and insoluble fiber and risk of all-cause, cardiovascular, and cancer mortality: systematic review and dose–response meta-analysis of prospective cohort studies. Front. Nutr. 10:1153165. doi: 10.3389/fnut.2023.1153165
Received: 29 January 2023; Accepted: 08 September 2023;
Published: 03 October 2023.
Edited by:
Raul Zamora-Ros, Institut d'Investigacio Biomedica de Bellvitge (IDIBELL), SpainReviewed by:
Nasser Laouali, Institut Gustave Roussy, FranceRonny Westerman, Bundesinstitut für Bevölkerungsforschung, Germany
Copyright © 2023 Yao, Ma, Cui, Huang, Lu, Hu, Zhu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei Qin, cWlucGVpMjI1QDE2My5jb20=
 Feifei Yao1
Feifei Yao1 Fulan Hu
Fulan Hu Pei Qin
Pei Qin