- 1Department of Food Processing, Technical Vocational School, Atatürk University, Erzurum, Türkiye
- 2Department of Plant and Animal Sciences, Technical Vocational School, Atatürk University, Erzurum, Türkiye
This study investigated the effects of loquat (Eriobotrya japonica L.) marmalade (LM) supplementation in probiotic yogurt during a 21 days storage period. In addition, the viability of Bifidobacterium and its effect on yogurt quality were investigated. Four types of yogurt, including plain yogurt (LM0) and yogurts with 5%, 10%, and 15% LM, were prepared. On days 1, 7, 14, and 21 of storage, physicochemical properties, microbial growth, and textural and sensory properties were investigated. The addition of LM to yogurt significantly affected the total dry matter, fat, pH, titratable acidity, syneresis, water holding capacity, and color parameters (L*, a*, b*). The addition of LM caused a decrease in L* (from 87.52 to 81.78) and an increase in a* values (from −35.42 to −30.14). Yogurts containing 10 and 15% LM demonstrated lower syneresis than control samples during storage. During storage, the pH of yogurts continuously decreased (P < 0.01). The viability of Bifidobacterium in yogurt was not affected by the LM addition. During storage, the viable count of Bifidobacterium ssp. decreased in all yogurt types. Lactobacillus delbrueckii ssp. bulgaricus decreased more than Streptococcus thermophilus did during storage. In all yogurt samples, coliform bacteria stayed below detectable concentrations. When a general evaluation was made by considering the physicochemical quality, sensory, and textural properties of all yogurt samples, it was revealed that LM-added yogurts can be produced and stored for 21 days.
1. Introduction
The majority of probiotic foods are milk-based, and their consumption has increased in recent years. Dairy foods containing probiotic bacteria play an important role as functional foods, and several studies have revealed their benefits to human health. Fermented milk and various yogurts are widely used and preferred for probiotic bacterial supplementation by customers worldwide, and their regular consumption has been shown to provide health benefits (1–3). Probiotics can be defined as live microbial mono- or mixed-culture dietary supplements that benefit human or animal hosts by improving the properties of native microbial flora (4, 5). Probiotics are live microorganisms that offer health benefits when consumed in food or supplements (6). Lactobacillus species are known for their antimicrobial and antiviral properties and play an important role in treating gastrointestinal disorders (7, 8). Probiotic species play a vital role in the improvement of lactose intolerance, inhibiting pathogens, lowering cholesterol, immune responses, prevention of intestinal and vaginal infections, against certain cancer varieties, food allergies, improving calcium absorption, immunostimulation and immunomodulation, constipation treatments, and SARS coronavirus (COVID-19) (5, 9, 10). It is now understood that intestinal microflora is closely related to human health (11, 12). However, adequate viable cells must be consumed regularly for the probiotic effect to occur in the consumer. Probiotic products must contain a minimum of 6–7 log CFUg–1 of viable cells of the probiotic microorganism (13).
Eriobotrya japonica L. is an evergreen tree in the Rosaceae family native to southeastern China that also grows in Korea, Japan, India, and other countries (14). Loquat has high medicinal value and has been used as a folk medicine for over 1000 years. Loquat extract has been used to counteract inflammation, cough, diabetes, chronic bronchitis, inflammation, cancer, and other health problems (15). Loquat can be eaten raw, but it is also used in processed foods such as jelly and jam (14). Consuming fruits and vegetables has been demonstrated in research to offer considerable protective health effects against chronic disease (16, 17). Consumption of fiber helps prevent obesity, atherosclerosis, colon cancer, and diabetes and also helps strengthen the natural gut flora (18). Loquat is rich in fiber and low in calories. The bioactive compounds in loquat have anti-cancer, anti-inflammatory, anti-diabetic, and anti-aging properties. The fiber content of loquat is 0.8–1.7 g/100 g per fruit (19).
This study aimed to investigate the potential benefits of incorporating loquat as a functional ingredient in probiotic fruit yogurts. Loquat is known for its high fiber content, which promotes the growth of probiotic bacteria and can contribute to better intestinal health. In addition, the use of loquat can enhance the nutritional value and taste of yogurt, potentially leading to increased consumption. We evaluated the physicochemical, sensory, and textural properties of the yogurts, along with their microbiological changes. A test panel assessment was conducted to evaluate the sensory properties of the different concentrations of loquat used in the yogurts (ranging from 5 to 15%). The goal of this study is to contribute to the existing literature on probiotic yogurt enriched with fiber-rich fruits, with the ultimate aim of promoting better consumer health.
2. Materials and methods
2.1. Material
The cow milk used for yogurt production was obtained from a small-scale dairy factory at Atatürk University (Erzurum, Turkey). The somatic cell count of the milk was 110.000 CFU/ml. Commercial probiotic yogurt cultures containing S. thermophilus, L. bulgaricus, and Bifidobacterium species (Bifidobacterium longum, B. bifidum, and B. infantis), labeled as Green Label ABY- 1, was supplied from Chr. Hansen (Istanbul, Turkey). The cultures were used in direct vat inoculation form and were inoculated following the manufacturer’s instructions. Fresh Eriobotrya japonica L. was purchased from local supermarkets in Erzurum. Orange-colored, plump, and fresh fruits were selected.
2.2. Preparation of loquat marmalade
Firstly, fresh, mature loquats were sorted and washed. The seeds and skin of the loquat were carefully removed, and the fruits were smashed with an Ultra Turrax homogenizer (Ika T25 Plus, Germany). An equal percentage of granulated sugar was incorporated into the fruit pulp. After the pulp was pasteurized at 90°C for 5 min, it was transferred to a sterile glass container and stored in the refrigerator until use.
2.3. Production of yogurts
Raw cow’s milk was concentrated by heating to 12% nonfat milk solids to increase total solids and cooled to 43 °C for final incubation. To prepare the primary culture, as recommended by the manufacturer, 50 units of DVS cultures were dissolved in 500 mL of milk, and 24 mL of this mixture was taken and added to 12 L of milk. The inoculated milk was divided equally into four batches. All batches were incubated for final fermentation at 43°C until pH reached 4.6. After incubation, the yogurt samples were cooled to 4°C and stored overnight. One group was reserved as control yogurt (LM 0), and the other three yogurt groups were mixed with LM at 5% (LM 5), 10% (LM 10), and 15% (LM 15). In the final step, the probiotic yogurt samples were filled into sterile 170 mL jars and stored at +4°C. The samples were subjected to physicochemical, microbiological, and sensory analyses on days 1, 7, 14, and 21.
2.4. Microbiological analysis
The yogurt samples (10 g) were aseptically weighed into a sterile Stomacher bag and homogenized in 90 mL of 1/4 Ringer’s solution (Merck, Germany) for 2 min to obtain a 10–1 dilution. Sequential dilutions were made with 1/4 Ringer’s solution up to 10–6 and then spread on the plates in duplicate. M17 agar (Merck, Germany) was used for counting S. thermophilus, and plates were incubated at 35–37°C for 48 h. Typical colonies (small, cream-colored colonies) were counted at the end of incubation. De Man, Rogosa, and Sharpe Agar (MRS) (Merck, Germany) and a CO2 atmosphere in anaerobic jars (Anaerocult C, Merck, Germany) at 37°C for 3 days were used to count L. bulgaricus. The bifidobacterial counts were determined using bifidobacterial-selective agar (BSM, Fluka). BSM plates were incubated under anaerobic conditions (Anaerocult C, Merck, Germany) at 37°C for 72 h (1). The BSM medium was prepared as follows: 0.116 g of BSM supplement (Fluka 83055) was dissolved in 10 mL of sterile water, added to sterilize warm BSM agar, and poured into petri dishes. BSM Agar was specifically selected for the enumeration of Bifidobacterium strains and inhibited Lactobacillus and Streptococcus strains. Plate Count Agar (PCA) (Merck, Germany) was used to enumerate the total viable aerobic mesophilic bacteria. The PCA plates were incubated at 30–32°C for 48 h. Violet Red Bile Agar (VRB; Merck, Germany) was used to count coliform bacteria, and the plates were incubated at 35–37°C for 24 h (20). Plates with 25–250 colonies were counted and expressed as colony-forming units (CFU) per gram of material.
2.5. Physical and chemical analyses
The total solids content of the yogurt samples was determined by drying the samples at 103°C to a constant weight, and the fat content was determined by the Gerber method according to Association of Official Agricultural Chemists [AOAC] (21). The amount of synergism present in the yogurt samples was determined using the method described by Bulca et al. (22). 25 g of yogurt samples were weighed and filtered for 2 h at 4°C via a funnel using filter paper (Whatman No. 1, UK). The following formula was used to determine syneresis:
WHC was also determined using the method described by Bulca et al. (22). The yogurt samples (10 g) were centrifuged (4500 × g for 30 min at 4°C). The filtrates were weighed, and the WHC values were calculated according to the following formula:
The pH of yogurt samples was determined using a pH meter (Hanna, pH 211, Portugal) after performing a 2-point calibration. The titratable acidity (TA) of yogurt samples was determined by mixing a 10 g sample with the same volume of distilled water and titrating with 0.1 N NaOH using phenolphthalein as an indicator. TA was expressed as the volume (mL) of NaOH consumed in the titration, and was calculated according to the following formula:
Where m is the weight of the sample.
2.6. Colorimetric analyses
The color parameters were measured using a color meter (PCE XXM-20, PCE instrument), and the results were expressed using the CIELAB color system, with L*, a*, and b* values at illuminant D 65. The parameters were L*; 0–100 (black-white), a*; (−a*, +a*) (greenness, redness), and b*; (−b*, +b* (blueness, yellowness). Three replications were performed for each sample.
2.7. Sensory and texture analyses
The sensory assessment of the yogurt samples was performed on a sensory rating scale from one to nine (poor to very good) for all attributes described (odor, texture, flavor, syneresis, acidity, sweetness, and overall acceptability), as defined by Gürsel and Karacabey (23) and Roland et al. (24). All yogurt samples were served at 5°C to seven panelists selected from among non-smokers. The textural properties of the yogurts were assessed using a texture analyzer (TA-XT Plus; Stable Micro System Ltd, UK) fitted with a 500 g load cell. The yogurt samples were analyzed immediately after removal from the refrigerator (5°C). An aluminum extrusion cylinder probe (P25) with a diameter of 25 mm was used and adjusted to a penetration depth of 30 mm at a speed of 1.0 mm/sec. The P 25 probe speed was set to 1.0 mm/sec during the compression and release of the sample. Texture profile analysis (TPA) was used to calculate the basic textural parameters expressed as hardness, adhesiveness, springiness, cohesiveness, chewiness, and resilience values. The force-time curves were analyzed using Exponent Micro System software (v. 4.0.9.0). All measurements were performed in duplicate.
2.8. Statistical analysis
A general linear model and statistical analysis were used to assess microbiological, physicochemical, and sensory characteristics on days 1, 7, 14, and 21. All analyses were performed in duplicate. Data from this study were compared with Duncan’s multiple range test (P < 0.05) using SPSS 20.0.
3. Results and discussion
3.1. Measurement of pH and TA
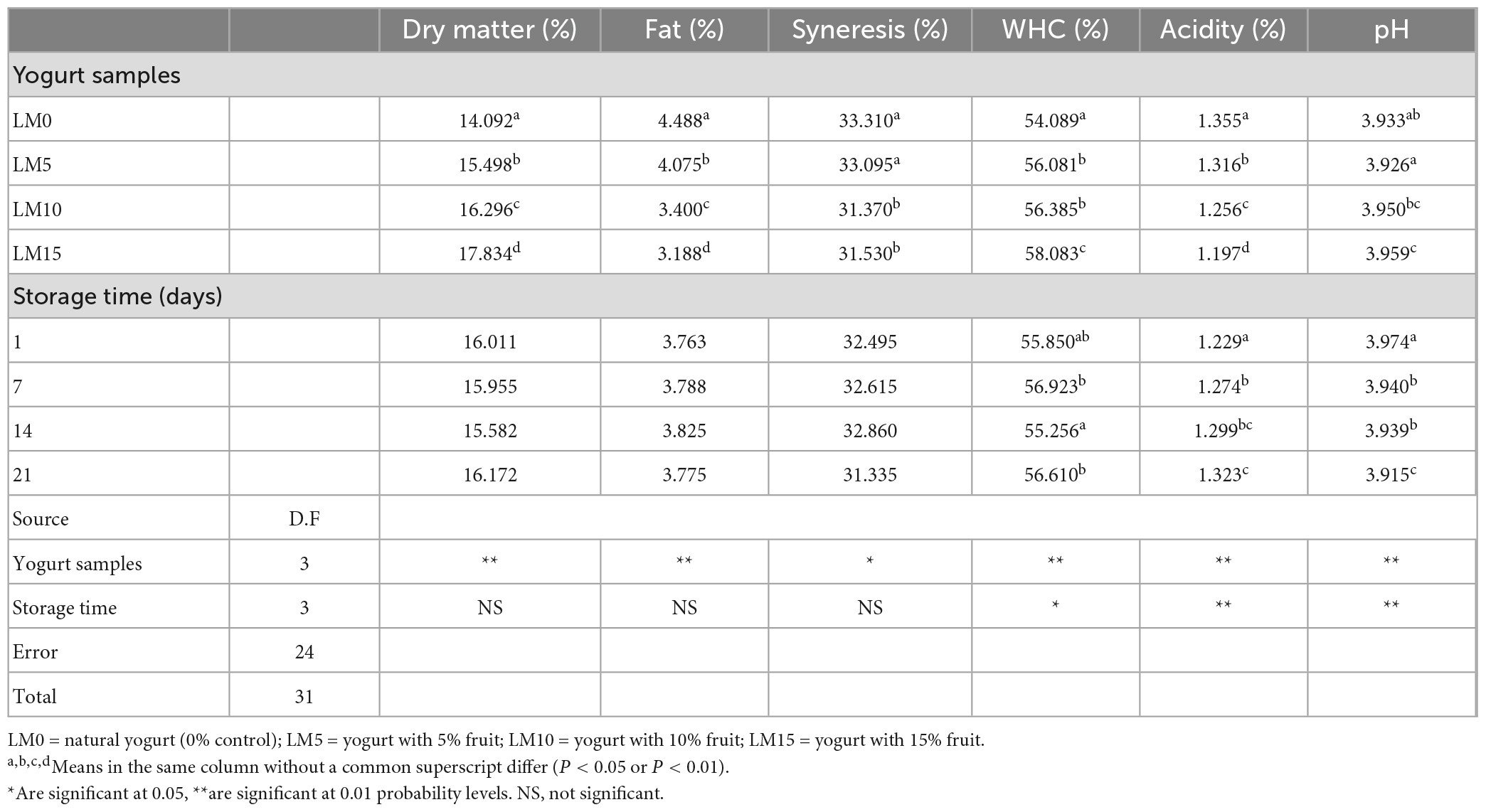
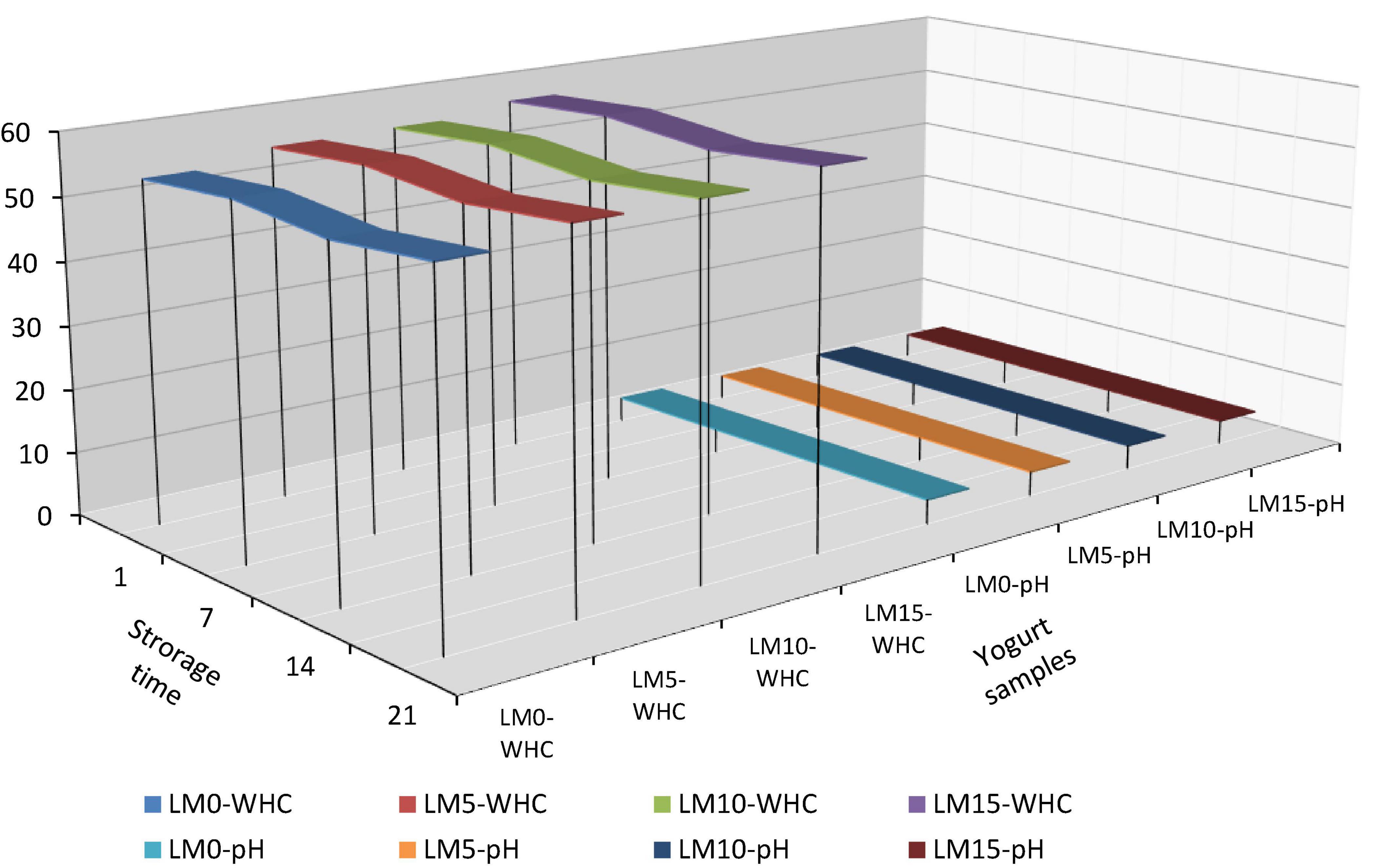
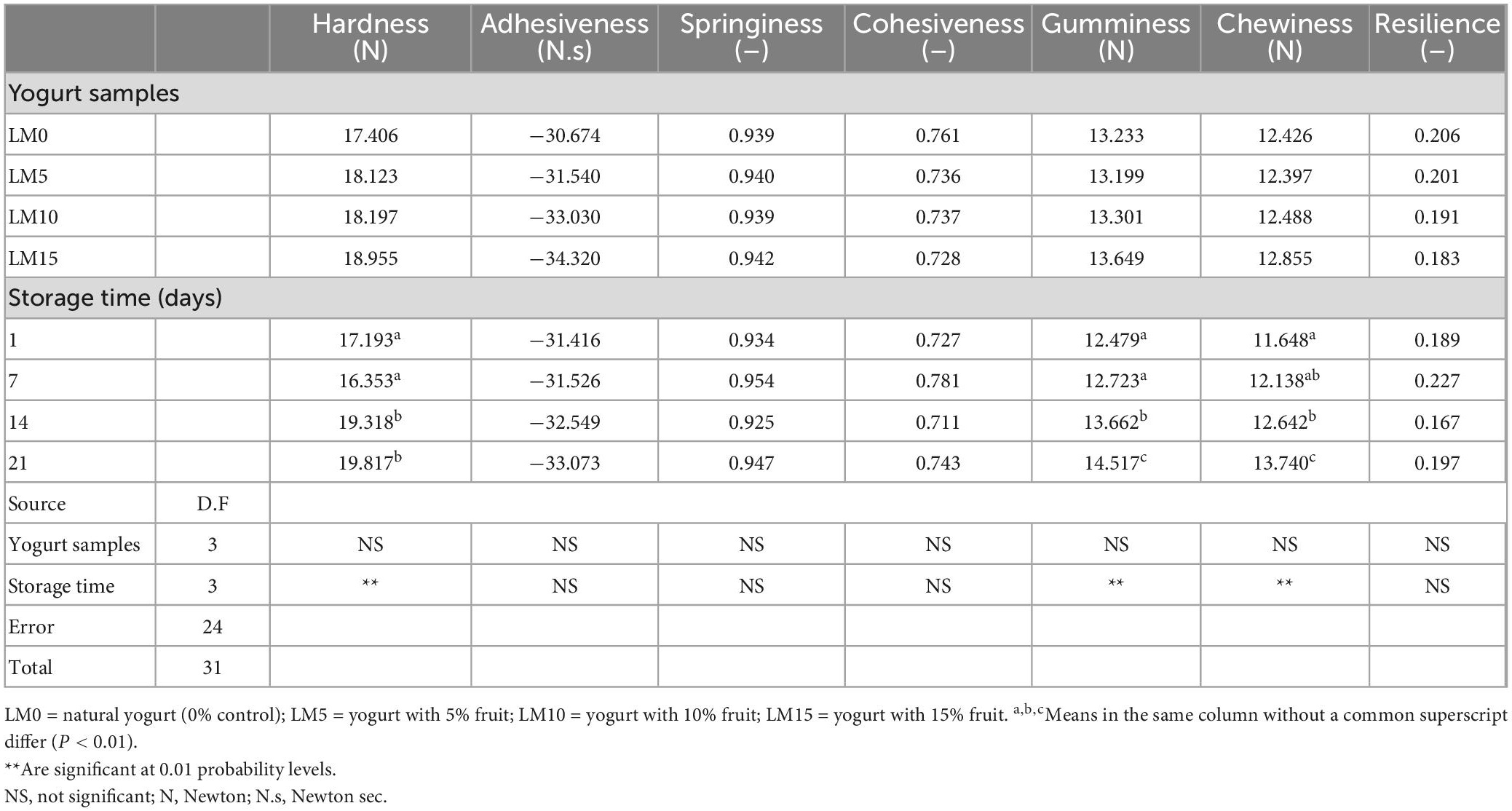
The results of the physicochemical and microbiological analyses are presented in Table 1. The pH and TA values of yogurt samples were significantly affected by LM addition and storage time (P < 0.01). LM-supplemented yogurts showed higher pH values and lower acidity than control yogurts (Figure 1). The initial pH of the yogurt samples varied between 3.95 and 4.01. The mean pH of the yogurt samples ranged from 3.93 (LM0 and LM5) to 3.96 (LM15). The initial TA values of the yogurt samples varied between 1.197 and 1.355%, and the mean TA values for yogurt samples ranged from 1.197% for LM15 yogurt to 1.355% for the control yogurts (Table 1). Çakmakçı et al. (25) stated that the pH value of probiotic yogurts prepared with banana fruit ranged from 4.07 to 4.60 during the storage period. However, the pH values in our study were lower than those previously stated. Hossain et al. (26) reported that the natural acidity of fruit causes an increase in the acidity of fruit yogurt. Donkor et al. (27) stated that yogurt cultures were responsible for the increase in yogurt acidity during storage. Arslan and Bayrakci (28) found that the viability of yogurt bacteria is adversely affected by the sugar concentration. This data is compatible with that of our study. This can be explained by the antagonistic effect of sugar content on yogurt culture. Kumar and Kumar (29) reported that the TA acidity of probiotic fruit yogurts ranged from 0.45 to 0.71%. These values were higher than these values. In this study, the pH of all yogurts decreased and TA values increased during the 21 days of storage. This process is called post-acidification and influences the viability of yogurt bacteria. The decline in pH in yogurts during storage is known as “post-acidification” (1). The lowest TA value (1.229%) was observed on the 1st day for the LM15 yogurt, and the highest value (1.323%) was observed on the 21st day for the control yogurts (Table 1). This increase can be attributed to the multiplication of lactic acid bacteria and the generation of lactic acid during storage. Bakırcı and Kavaz (30) found similar results.
3.2. Syneresis and WHC
The release of serum, known as syneresis, is an important quality criterion for yogurt. A higher syneresis level indicates lower-quality yogurt (31). The highest syneresis value was found in the control (33.31%), and the lowest was in LM15 yogurt (31.53%) (Table 1). The decrease in pH stimulated syneresis in yogurt samples (Figure 1). The addition of LM decreased the syneresis values of the yogurt. However, the differences were not statistically significant (P > 0.05). In general, the syneresis values for all yogurt samples decreased during storage. The highest syneresis values (32.50%) were found on day one and the lowest values (31.335%) on day 21 (Table 2). However, the differences were not statistically significant (P > 0.05). The results showed that increasing the percentage of LM in yogurt resulted in lover syneresis. LM 15 yogurt had the lowest syneresis value compared with the other yogurts. Korkmaz et al. (32) reported that syneresis decreased in maca (Lepidium meyenii) powder and propolis-enriched yogurts. This result is consistent with the results of our study. WHC is generally referred to as the ability of food to retain natural or added water, and is important for the creation of food structure (33). There was a significant difference (P < 0.01) in WHC values between yogurt varieties. The WHC values of yogurts ranged from 54.09 to 58.083% (Table 2). The control yogurt had the lowest WHC values, followed by the LM-supplemented yogurts. WHC values increased proportionally with increasing LM percentages in yogurt samples. During the storage period, the WHC levels of the yogurts increased slightly but significantly (P < 0.05). These WHC values were lower than those reported by Karaca et al. (34) and similar to the results obtained by Bakırcı et al. (35).
3.3. Color analysis
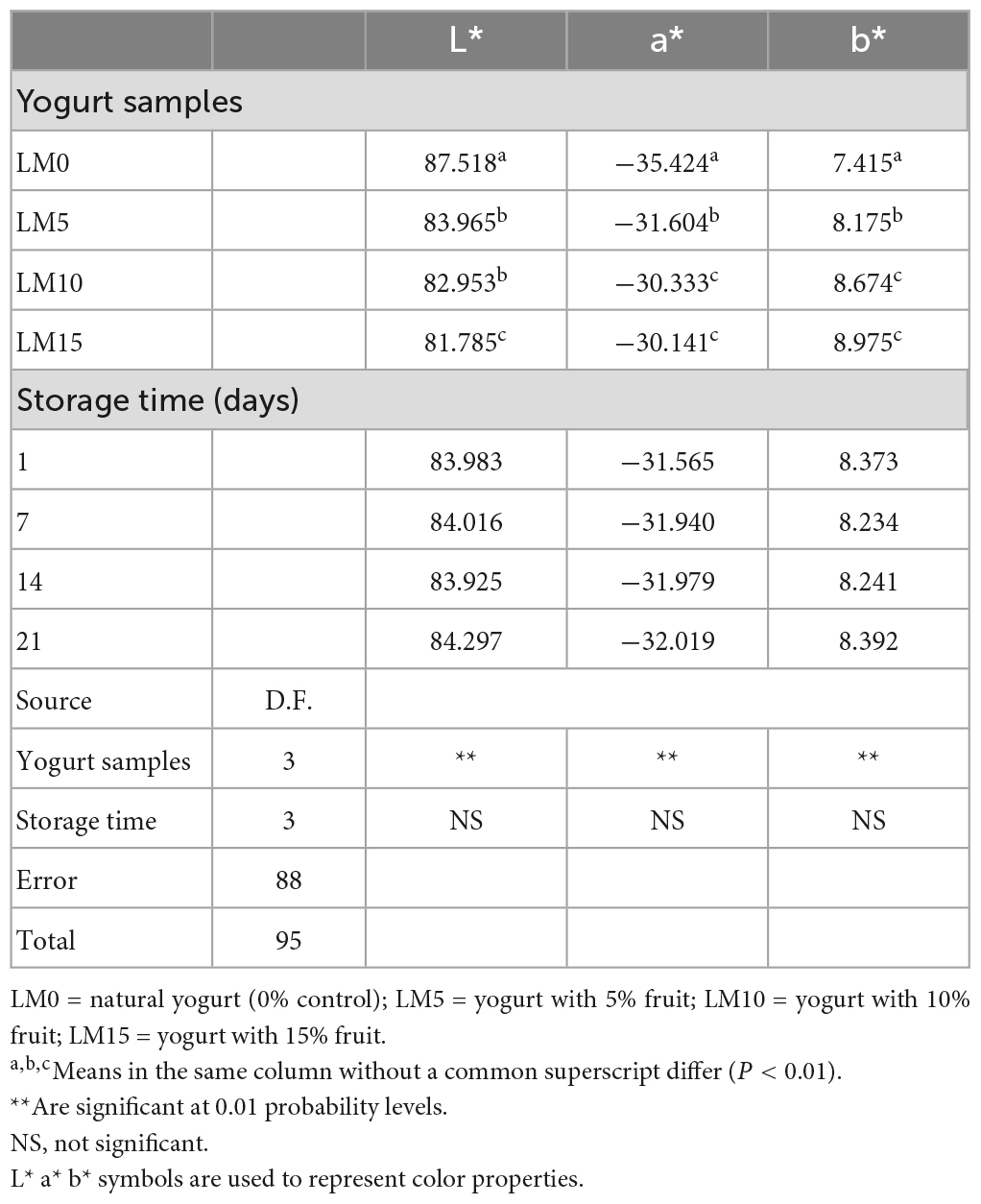
The color of fruit yogurts is a notable factor in consumer preferences and is often used to determine the sensory quality of yogurt. Table 2 shows the changes in instrumental color parameters for the control and LM-added yogurt samples. The addition of LM significantly affected (P < 0.01) the L*, a*, and b* values of the samples. The amount of LM decreased the L* value of yogurt but increased the a* and b* values. The highest L* value (87.52) was found in the control yogurt, and the highest a* and b* values (−30.14 and 8.97, respectively) were found in the LM15 yogurt. The L*, a*, and b* values of the yogurt samples were not affected by the storage time. In terms of color parameters, there was no significant difference between yogurt stored for one and 21 days (P > 0.05). Although a slight reduction in a* values was found during the storage period, these increases were not statistically significant. This can be explained by the fact that the natural pigments in the loquat were stable during the storage period and were not affected by the acidity of the yogurt. Ścibisz et al. (36) reported that the loss of color properties of the yogurt they produced with blueberries during storage depended on the fruit type and storage time.
3.4. Viable counts
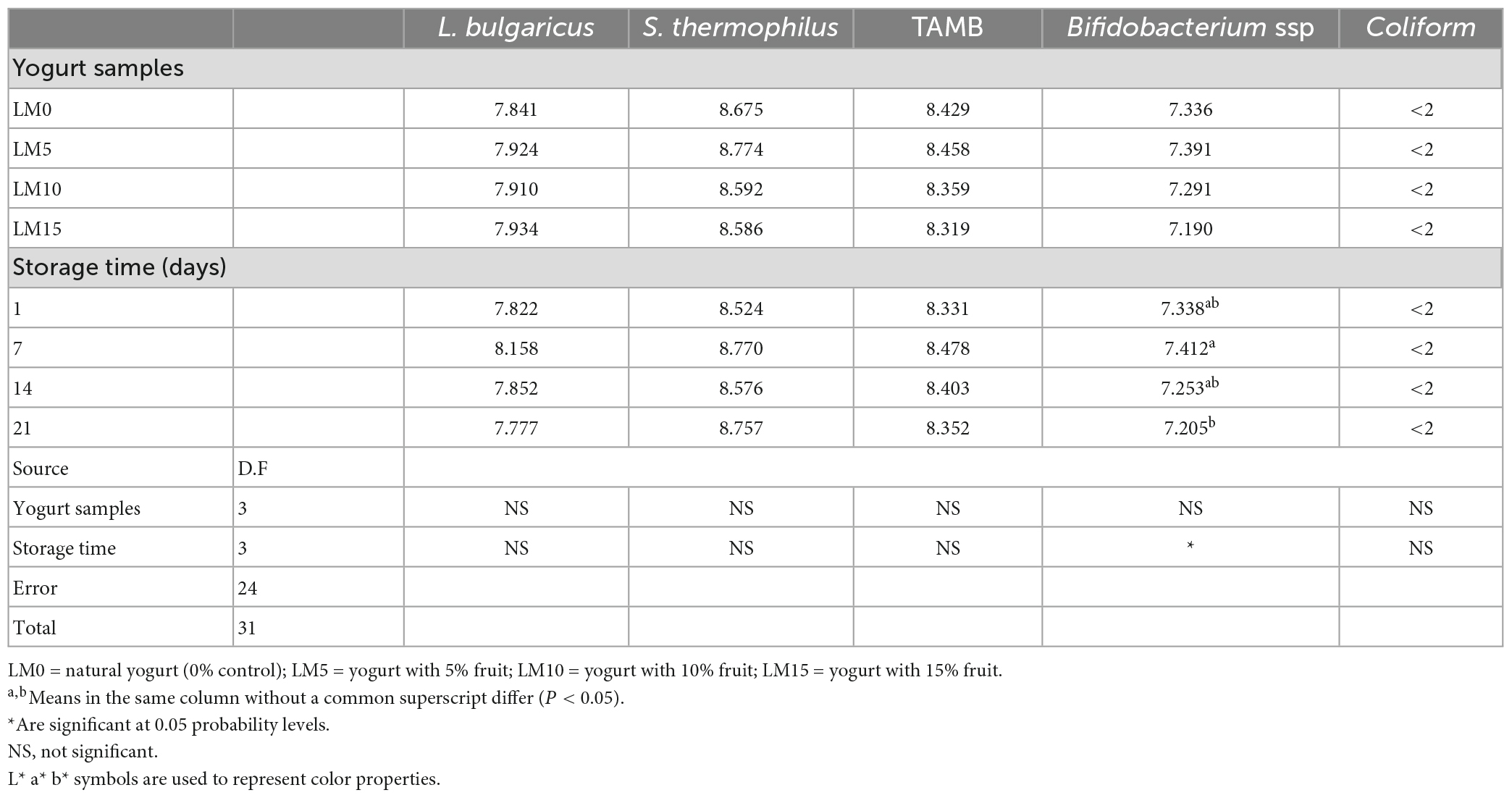
Probiotic dairy products can provide a reasonably high number of bacteria while also providing additional advantages (10). Yogurt cultures that can survive in the small intestine are thought to be beneficial for human health (37). Table 3 shows the changes in the number of S. thermophilus, L. bulgaricus, Bifidobacterium ssp., and TAMB in the yogurts. The coliforms group remained below the detectable level in all analyzed yogurt samples (<2 log CFU/g). A small increase in S. thermophilus was observed during the storage period for all yogurt varieties. The decrease in pH during storage may be due to the increase in S. thermophilus, while the number of L. bulgaricus remained almost constant throughout the storage period. LM addition and storage time did not significantly affect (P > 0.05) the numbers of viable S. thermophilus and L. bulgaricus. Akın and Akın (38) reported a decrease in S. thermophilus and L. bulgaricus by about one log unit. Birollo et al. (39) reported a slight increase in S. thermophilus and L. bulgaricus in yogurts during a 60 days storage period, followed by a decrease. However, in our study, we found that the numbers of S. thermophilus and L. bulgaricus increased by only 0.2 and 0.1 log units, respectively. This result shows that a storage time longer than 20 days is a critical factor affecting the viability of S. thermophilus and L. bulgaricus. The difference between the TAMB counts of the control and LM yogurts was not significant (P > 0.05). The highest number of TAMB was found in the control yogurt group, and the lowest number was found in LM15 yogurts (Table 3). Çon et al. (40) stated that adding fruit to yogurt had no significant effect on his TAMB counts. This result is consistent with that of the present study. The number of TAMB in all yogurt samples increased slightly during the storage period, but these increases were not statistically significant (P > 0.05). The control yogurt and LM 5 yogurt had the highest number of Bifidobacterium and the LM 15 yogurt had the lowest. However, differences in bifidobacterial counts by yogurt type were not significant (P > 0.05) (Table 3). This result may be attributed to differences in the amount of sugar used in the preparation of the LM yogurts. Arslan and Bayrakçı (28) stated that sugar concentration negatively affects the viability of yogurt bacteria. The Bifidobacterial counts decreased in all yogurt types during storage, however, these decreases were not statistically significant (P > 0.05). It can be said that the number of Bifidobacterium in this study was well preserved. This can be explained by the fact that the applied heat treatment increased the dry matter and reduced the dissolved oxygen and redox potential. Dave and Shah (41) stated that the reduction in redox potential and dissolved oxygen promoted the growth of Bifidobacterium. The mean Bifidobacterium ssp. the number was found to be lower than both L. bulgaricus and S. thermophilus numbers of about 0.8 and 1.5 log units, respectively. Ranadheera et al. (42) reported similar results. Çakmakçı et al. (25) stated that food containing probiotic bacteria should have a minimum recommended content above 6 log CFU/g for optimal therapeutic effect. In this study, although the number of Bifidobacterium decreased during storage, all yogurt samples retained their probiotic characteristics throughout the storage period.
3.5. Textural and sensory analysis
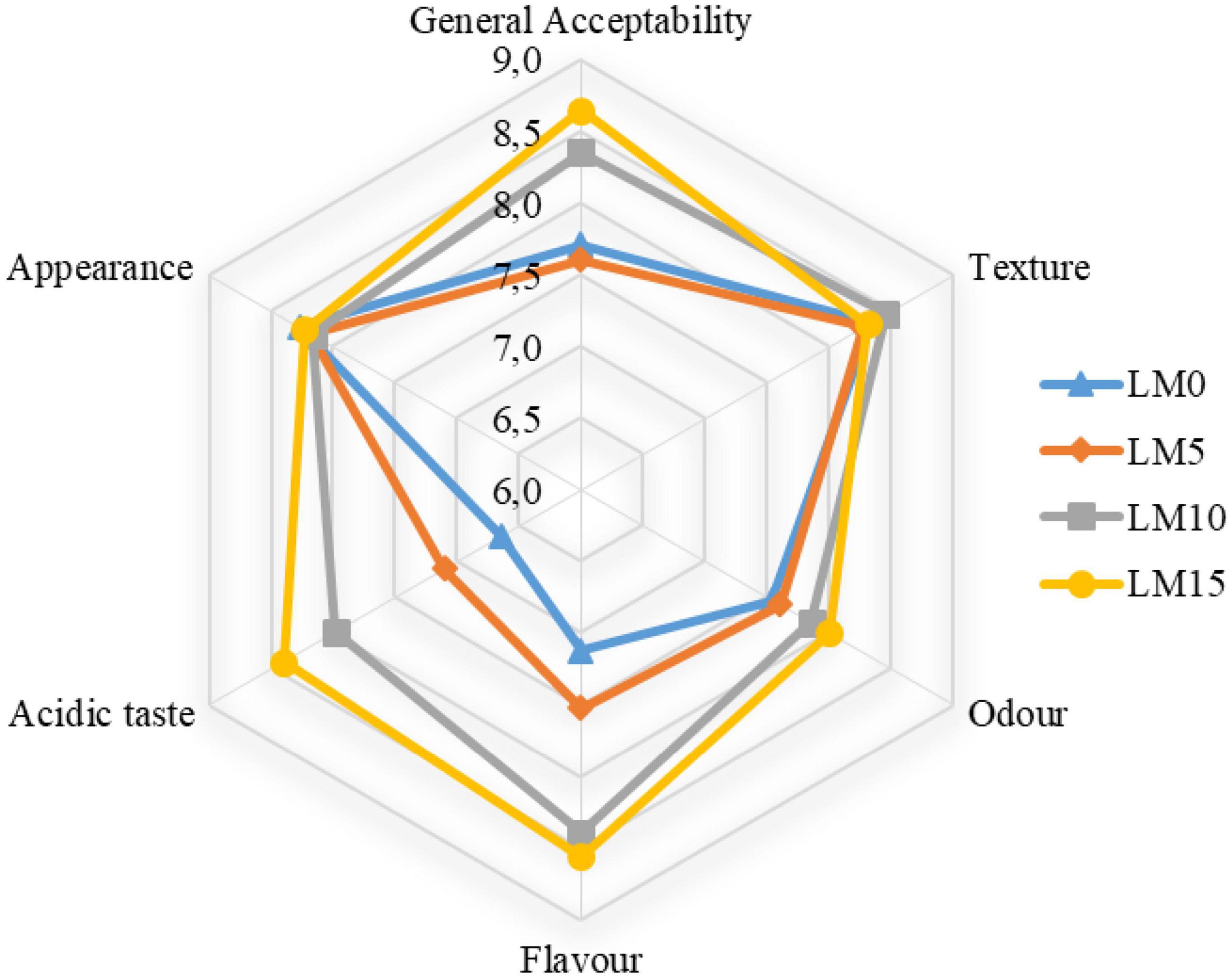
The textural properties of fermented milk products, such as yogurt, are one of the main criteria that determine their acceptance by consumers (43). Table 4 shows the changes in the texture parameters of the yogurts during storage. The addition of LM to yogurt samples increased textural properties, such as hardness, gumminess, and chewiness, and decreased adhesiveness, cohesiveness, and resilience. However, these differences were not statistically significant (P > 0.05). All texture scores, except for the adhesiveness of LM 10 and LM15 yogurt samples, were higher than those of the control yogurt. There was a significant difference (P < 0.01) between textural properties and storage time. As expected, the scores of the hardness, gumminess, and chewiness of the yogurts increased significantly during the storage, and the differences were significant (P < 0.01) from day 14 (Figure 2). Some researchers have reported that long storage periods affect textural properties (such as hardness, firmness, and gumminess (18, 43). Najgebauer-Lejko et al. (44) stated that the textural properties of vegetables added to yogurts, such as hardness, gumminess, and cohesiveness, were insignificant. These results were consistent with our findings. Sensory analysis, especially taste and flavor evaluation, is a key attribute that plays an important role in general acceptance Falah et al. (45). Figure 3 shows the sensory evaluation results of the yogurt samples. The addition of LM increased the flavor, odor, acidic taste, and overall acceptability of yogurt samples. The appearance scores were slightly lower in the LM-supplemented yogurt samples than in the control group, but the difference was not significant (P > 0.05). The acidic taste and aroma scores of the LM-supplemented yogurts differed from each other and also from the control sample depending on the LM ratio. The highest acidic taste scores were obtained for LM15, LM10, and LM5 yogurts. Similarly, the highest general acceptability scores were obtained in the LM15 and LM10 yogurts (Figure 3). Sensory evaluation scores, including appearance, flavor, acidic taste, and overall acceptability scores, tended to decrease in the yogurt samples during storage. As predicted, acidic taste scores steadily decreased with storage. As yogurt is a living product, the bacterial content in yogurt causes an increase in the amount of lactic acid (Table 1). However, after the 14th day of storage, there was not much change in the acidic taste scores. Yogurts containing 15% LM were generally preferred by the panelists over all other yogurt samples.
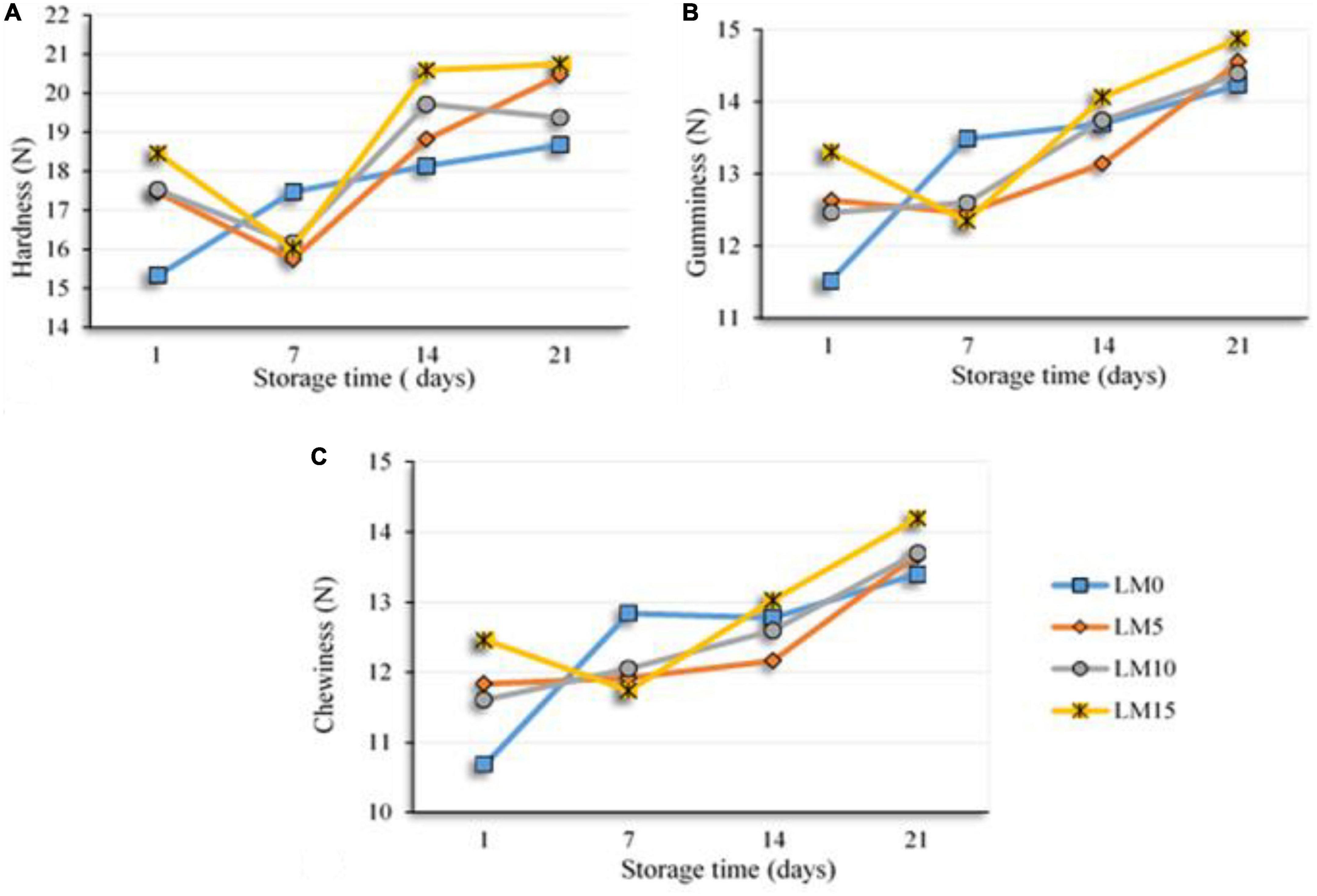
Figure 2. Changes in textural parameters of the yogurt samples during storage hardness (A), gumminess (B), and chewiness (C).
4. Conclusion
A novel probiotic fruit yogurt has been developed to deliver health benefits through probiotic bacteria to the human host. The present study reveals that the incorporation of 15% LM had a remarkably positive impact on the sensory and textural properties of the yogurt. However, the effect was uncertain with the use of 5% LM. Therefore, it can be concluded that a 10% LM ratio can serve as the acceptable threshold limit for optimal sensory and textural properties of loquat fruit yogurt.
The results of this study demonstrate that neither the addition of LM nor 21 days storage has any effect on the vitality of probiotic bacteria in yogurt; this is only affected after 21 days of storage. All yogurt types preserved their probiotic properties until the expiration of the storage period. Changes in pH values were compatible with the WHC and syneresis values. The addition of LM caused an increase in a* and b* values, but the values changed harmoniously according to the color of the fruit. Determining the number of probiotic bacteria in fermented foods like yogurt is difficult because of the presence of other lactic acid bacteria, however, in this study, the BSA medium was found to be effective for the enumeration of Bifidobacteria. The addition of LM improved the flavor, odor, acidic taste, and overall acceptability scores. This concludes that loquat is a suitable choice for probiotic yogurt production in terms of its sensory properties.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TT prepared and revised the manuscript, methodology, and project management. AD provided the feedback during writing, mentoring, and project management. Both authors contributed to the manuscript and approved the submitted version.
Funding
This study was supported by the Atatürk University Research Projects Unit (BAP) (BAP, FBA 6318). The funding agency declares that it does not bear the costs of publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Turgut T, Cakmakci S. Probiotic strawberry yogurts: microbiological, chemical and sensory properties. Probiotics Antimicrob Proteins. (2018) 10:64–70. doi: 10.1007/s12602-017-9278-6
2. Cruz AG, Castro WF, Faria JAF, Bolini HMA, Celeghini RMS, Raices RS, et al. Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage. Food Res Int. (2013) 51:723–8. doi: 10.1016/j.foodres.2013.01.028
3. Batista ALD, Silva R, Cappato LP, Almada CN, Garcia RKA, Silva MC, et al. Quality parameters of probiotic yogurt added to glucose oxidase compared to commercial products through microbiological, physical-chemical and metabolic activity analyses. Food Res Int. (2015) 77:627–35. doi: 10.1016/j.foodres.2015.08.017
4. Turgut T, Cakmakci S. Investigation of the possible use of probiotics in ice cream manufacture. Int J Dairy Technol. (2009) 62:444–51. doi: 10.1111/j.1471-0307.2009.00494.x
5. Olson DW, Aryana KJ. Probiotic Incorporation into yogurt and various novel yogurt-based products. Appl Sci. (2022) 12:12607. doi: 10.3390/app122412607
6. Novik G, Savich V. Beneficial microbiota. Probiotics and pharmaceutical products in functional nutrition and medicine. Microbes Infect. (2020) 22:8–18. doi: 10.1016/j.micinf.2019.06.004
7. Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T. Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol. (2016) 69:187–203. doi: 10.1136/jclinpath-2015-202976
8. Sunmola AA, Ogbole OO, Faleye TO, Adetoye A, Adeniji JA, Ayeni FA. Antiviral potentials of Lactobacillus plantarum, Lactobacillus amylovorus, and Enterococcus hirae against selected Enterovirus. Folia Microbiol. (2019) 64:257–64. doi: 10.1007/s12223-018-0648-6
9. Sarvari F, Mortazavian AM, Fazeli MR. Biochemical characteristics and viability of probiotic and yogurt bacteria in yogurt during the fermentation and refrigerated storage. Appl Food Biotechnol. (2014) 1:55–61.
10. Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. (2017) 61:1600240. doi: 10.1002/mnfr.201600240
11. Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genom Proteom Bioinform. (2018) 16:33–49. doi: 10.1016/j.gpb.2017.06.002
12. Xiong J, Wang K, Wu J, Qiuqian L, Yang K, Qian Y, et al. Changes in intestinal bacterial communities are closely associated with shrimp disease severity. Appl Microbiol Biotechnol. (2015) 99:6911–9. doi: 10.1007/s00253-015-6632-z
13. Mohan A, Hadi J, Gutierrez-Maddox N, Li Y, Leung IK, Gao Y, et al. Sensory, microbiological and physicochemical characterisation of functional Manuka honey yogurts containing probiotic Lactobacillus reuteri DPC16. Foods. (2020) 9:106. doi: 10.3390/foods9010106
14. Henmi A, Shoji M, Nomura M, Inoue T. Fatty acid composition and applications of Eriobotrya japonica seed oil. J Oleo Sci. (2019) 68:599–606. doi: 10.5650/jos.ess18178
15. Liu Y, Zhang W, Xu C, Li X. Biological activities of extracts from loquat (Eriobotrya japonica Lindl.): a review. Int J Mol Sci. (2016) 17:1983. doi: 10.3390/ijms17121983
16. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. (2003) 78:517–20S. doi: 10.1093/ajcn/78.3.517S
17. Aguilera Y, Martin- Cabrejas MA, González de Mejia E. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: their role in prevention of chronic diseases. Phytochem Rev. (2016) 15:405–23. doi: 10.1007/s11101-015-9443-z
18. Espírito-Santo AP, Perego P, Converti A, Oliveira MD. Influence of milk type and addition of passion fruit peel powder on fermentation kinetics, texture profile and bacterial viability in probiotic yoghurts. LWT. (2012) 47:393–9. doi: 10.1016/j.lwt.2012.01.038
19. Dhiman A, Suhag R, Thakur D, Gupta V, Prabhakar PK. Current status of loquat (Eriobotrya Japonica lindl.): bioactive functions, preservation approaches, and processed products. Food Rev Int. (2021) 38:1–31. doi: 10.1080/87559129.2020.1866007
21. Association of Official Agricultural Chemists [AOAC]. Official methods of analysis of AOAC international. 16th ed. Rockville, MA: AOAC International (1995).
22. Bulca S, Umut F, Koç A. The influence of microbial transglutaminase on camel milk yogurt. LWT. (2022) 160:113339. doi: 10.1016/j.lwt.2022.113339
23. Gürsel A, Karacabey A. Dondurma Teknolojisine Ýlişkin Hesaplamalar, Reçeteler ve Kalite Kontrol Testleri. Ankara: Ankara Üniv. (1998).
24. Roland AM, Phillips LG, Boor KJ. Effects of fat content on the sensory properties, melting, color and hardness of ice cream. J Dairy Sci. (1999) 82:32–8. doi: 10.3168/jds.S0022-0302(99)75205-7
25. Çakmakçı S, Çetin B, Turgut T, Gürses M, Erdoðan A. Probiotic properties, sensory qualities, and storage stability of probiotic banana yogurts. Turk J Vet Anim Sci. (2012) 36:231–7. doi: 10.3906/vet-1007-2
26. Hossain MN, Fakruddin M, Islam MN. Quality comparison and acceptability of yoghurt with different fruit juices. J Food Process Technol. (2012) 3:1–5. doi: 10.4172/2157-7110.1000171
27. Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int Dairy J. (2006) 16:1181–9. doi: 10.1016/j.idairyj.2005.10.008
28. Arslan S, Bayrakci S. Physicochemical, functional, and sensory properties of yogurts containing persimmon. Turk J Agric For. (2016) 40:68–74. doi: 10.3906/tar-1406-150
29. Kumar A, Kumar D. Development of antioxidant rich fruit supplemented probiotic yogurts using free and microencapsulated Lactobacillus rhamnosus culture. J Food Sci Technol. (2016) 53:667–75. doi: 10.1007/s13197-015-1997-7
30. Bakırcı I, Kavaz A. An investigation of some properties of banana yogurts made with commercial ABT-2 starter culture during storage. Int J Dairy Technol. (2008) 61:270–6. doi: 10.1111/j.1471-0307.2008.00409.x
31. Wu H, Hulbert GJ, Mount JR. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov Food Sci Emerg Technol. (2000) 1:211–8. doi: 10.1016/S1466-8564(00)00020-5
32. Korkmaz S, Parmaksiz A, Ahmet S, Korkmaz B. The effects of solutions of maca (Lepidium meyenii) powder as a food/feed supplement on the viability of murine macrophage cells by digital image analysis. J Advn Vet Bio Sci Tech. (2021) 6:116–20. doi: 10.31797/vetbio.934630
33. Gyavali R, Ibrahim SA. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends Food Sci Technol. (2016) 56:61–76. doi: 10.1016/j.tifs.2016.07.013
34. Karaca OB, Saydam IB, Güven M. Physicochemical, mineral and sensory properties of set-type yoghurts produced by addition of grape, mulberry and carob molasses (Pekmez) at different ratios. Int J Dairy Technol. (2012) 65:111–7. doi: 10.1111/j.1471-0307.2011.00731.x
35. Bakırcı S, Dagdemir E, Boran OS, Hayaloglu AA. The effect of pumpkin fibre on quality and storage stability of reduced-fat set-type yogurt. Int J Food Sci Technol. (2017) 52:180–7. doi: 10.1111/ijfs.13264
36. Ścibisz I, Ziarno M, Mitek M. Color stability of fruit yogurt during storage. J Food Sci Technol. (2019) 56:1997–2009. doi: 10.1007/s13197-019-03668-y
37. Yadav A, Jaiswal P, Jaiswal M, Kumar N, Sharma R, Raghuwanshi S, et al. Concise review: importance of probiotics yogurt for human health improvement. J Environ Sci Toxicol Food Technol. (2015) 9:25–30.
38. Akın GMB, Akın MS. Effects of cysteine and different incubation temperatures on the microflora, chemical composition and sensory characteristics of bio-yogurt made from goat’s milk. Food Chem. (2007) 100:788–93. doi: 10.1016/j.foodchem.2005.10.038
39. Birollo GA, Reinheimer JA, Vinderola CG. Enterococci vs non-lactic acid microflora as hygiene indicators for sweetened yoghurt. Food Microbiol. (2001) 18:597–604. doi: 10.1006/fmic.2001.0435
40. Çon AH, Çakmakçı S, Çaðlar A, Gökalp HY. Effects of different fruits and storage periods on microbiological qualities of fruit-flavored yogurt produced in Turkey. J Food Prot. (1996) 59:402–6. doi: 10.4315/0362-028X-59.4.402
41. Dave RI, Shah NP. Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int Dairy J. (1997) 7:31–41. doi: 10.1016/S0958-6946(96)00046-5
42. Ranadheera CS, Evans CA, Adams MC, Baines SK. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. (2012) 135:1411–8. doi: 10.1016/j.foodchem.2012.06.025
43. Delikanlı B, Ozcan T. Improving the textural properties of yogurt fortified with milk proteins. J Food Process Preserv. (2017) 41:e13101. doi: 10.1111/jfpp.13101
44. Najgebauer-Lejko D, Tabaszewska M, Grega T. The effect of addition of selected vegetables on the microbiological, textural and flavour profile properties of yoghurts. Acta Sci Pol Technol Aliment. (2015) 14:45–53. doi: 10.17306/J.AFS.2015.1.5
45. Falah F, Zareie Z, Vasiee A, Tabatabaee Yazdi F, Mortazavi SA, Alizadeh Behbahani B. Production of synbiotic ice-creams with Lactobacillus brevis PML1 and inulin: functional characteristics, probiotic viability, and sensory properties. J Food Meas Charact. (2021) 15:5537–46. doi: 10.1007/s11694-021-01119-x
Keywords: Bifidobacterium, Eriobotrya japonica L., probiotics, probiotic yogurt, yogurt texture
Citation: Turgut T and Diler A (2023) The effect of addition Eriobotrya japonica L. marmalade on physicochemical, microbiological, and sensory properties of probiotic yogurts. Front. Nutr. 10:1151037. doi: 10.3389/fnut.2023.1151037
Received: 30 January 2023; Accepted: 10 March 2023;
Published: 30 March 2023.
Edited by:
Hayriye Sebnem Harsa, İzmir Institute of Technology İYTE, TürkiyeReviewed by:
Malgorzata Ziarno, Warsaw University of Life Sciences, PolandNasim Khorshidian, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2023 Turgut and Diler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamer Turgut, dHR1cmd1dEBhdGF1bmkuZWR1LnRy
 Tamer Turgut
Tamer Turgut Abdulkerim Diler
Abdulkerim Diler