
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 30 March 2023
Sec. Nutrition and Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1140019
This article is part of the Research Topic New Insights on the Management of Obesity with Nutrition and Physical Activity View all 10 articles
Background: Overweight and obesity are considered as one of the major risk factors for cardiovascular diseases (CVD). At present, many studies have proved that multiple nutritional supplements play an active role in metabolic diseases. However, the comparative efficacy of different nutritional supplements in improving indicators of cardiometabolic risk in obese and overweight patients is uncertain.
Methods: Cochrane Library, PubMed, Embase, and Web of Science were searched for the period from January 1990 to March 2022. A random-effect model was built in the Bayesian network meta-analysis. The surface under the cumulative ranking analysis (SUCRA) and clustering rank analysis was performed for ranking the effects.
Results: The study included 65 RCTs with 4,241 patients. In terms of glucose control, probiotic was more conductive to improve FBG (MD: −0.90; 95%CrI: −1.41 to −0.38), FINS (MD: −2.05; 95%CrI: −4.27 to −0.02), HOMA-IR (MD: −2.59; 95%CI −3.42 to −1.76). Probiotic (MD: −11.15, 95%CrI −22.16 to −1.26), omega-3 (MD: −9.45; 95%CrI: −20.69 to −0.93), VD (MD: −17.86; 95%CrI: −35.53 to −0.27), and probiotic +omega-3 (MD: 5.24; 95%CrI: 0.78 to 9.63) were beneficial to the improvement of TGs, TC and HDL-C, respectively. The SUCRA revealed that probiotic might be the best intervention to reduce FBG, FINS, HOMA-IR; Simultaneously, α-lipoic acid, VD, and probiotic + omega-3 might be the best intervention to improve TGs, TC, and HDL-C, respectively. Cluster-rank results revealed probiotic had the best comprehensive improvement effect on glucose metabolism, and probiotic + omega-3 may have a better comprehensive improvement effect on lipid metabolism (cluster-rank value for FBG and FINS: 3290.50 and for TGs and HDL-C: 2117.61).
Conclusion: Nutritional supplementation is effective on CVD risk factors in overweight and obese patients. Probiotic supplementation might be the best intervention for blood glucose control; VD, probiotic + omega-3 have a better impact on improving lipid metabolism. Further studies are required to verify the current findings.
The World Health Organization (WHO) defines overweight and obesity as abnormal or excessive fat accumulation that may damage health (1). Obesity is a threat to global population health in terms of prevalence and disease burden. In recent research, 2 billion people was diagnosed with overweight or obesity (2). In obese and overweight patients, the active metabolism of adipose tissue induces metabolic changes, such as increased production of reactive oxygen species, oxidative stress and inflammation, leading to type 2 diabetes mellitus (T2DM), arterial hypertension and dyslipidemia, which are the most important precursor risk factors for cardiovascular diseases (CVD) (3). Cardiometabolic biomarkers, such as blood glucose, insulin resistance and lipid profiles, are important risk indicators of subclinical disease and a valuable tool for monitoring CVD (4–6). Therefore, improving the metabolic status of overweight and obese patients is an important preventive strategy to prevent the development of more serious metabolic diseases.
Since most of the drugs used to treat obesity have been withdrawn from the market due to improper use or side effects, lifestyle change and diet control are the safest and most cost-effective interventions for obese and overweight people to control their weight (7–9). Some nutrients not only have antioxidant, anti-inflammatory and immune-enhancing biological activities, but also have greater safety compared with drugs. Currently available nutritional supplements such as vitamins, minerals, fatty acids, and plant compounds have been shown to improve obesity by improving carbohydrate metabolism, increasing lipolysis or energy expenditure, and reducing hunger (10). Therefore, they have attracted extensive attention in the treatment of metabolic diseases. According to previous meta-analysis, resveratrol, Vitamin D (VD)/VD + calcium (Ca), probiotics, α-lipoic acid, omega-3, curcumin, and magnesium (Mg) were used to improve multiple comorbidities of metabolic disorders (11–17). While most RCTs and meta-analysis to date have proved the beneficial effect of nutritional supplements on metabolism diseases patients, limited data are available regarding their effects on other indicators of CVD risk, i.e., metabolic syndrome (MetS) (18), elevated blood pressure (19), endothelial function (20), and in other at-risk populations. Obesity, particularly intra-abdominal obesity, predisposes people to several modifiable risk factors of CVD and T2DM, i.e., cardiometabolic risk (21). Furthermore, it is difficult to determine the comprehensive efficiency of different nutritional strategies using pair-wise meta-analysis.
The effect of different nutritional supplements for overweight and obesity patients on cardiovascular risk factors, as well as which intervention is most effective, remain to be verified. Therefore, in this study, we aimed to conduct systematic review and network meta-analysis (NMA) by comparing the adjuvant therapy of different nutritional supplements for overweight and obese adults, so as to provide reference for clinical practice.
This systematic review was prepared according to the preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) (22) as well as the PRISMA extension statement for network meta-analysis (23) (Supplementary File 1) and was registered at the international Prospective Register of Systematic Reviews (CRD42022371086).
Two independent researchers searched PubMed, EMBASE, Web of Science and Cochrane Library from the inception of each database to March 20, 2022, and the search strategy was based on the standards established by the Cochrane Collaboration. The search was limited to human subjects’ studies and English language publications. We use both medical subject heading (MeSH) and extensive free-text keywords, and search terms included: random*, adults, obesity, overweight, supplementation, nutrition, resveratrol, Vitamin D, probiotics, α-lipoic acid, omega-3, curcumin, magnesium. The search strategy is shown in Supplementary File 2.
In this network meta-analysis, randomized controlled trials (RCTs) which fulfilled the following criteria for participants, interventions, comparisons, outcomes, and study design (PICOS) were included: (1) Participants: We included studies of overweight or obese adults and excluded studies of other cardiovascular diseases (i.e., type 2 diabetes, insulin resistance, non-alcoholic fatty liver disease, hyperlipemia, hypertension), children, adolescents or pregnant women. Overweight and obesity are defined as body mass index (BMI) ≥ 25 and 30 kg/m2, respectively. (2) Intervention: The intervention group used at least one of the following seven nutrition supplements: resveratrol, VD, probiotics, α-lipoic acid, omega-3, curcumin, Mg. The duration was at least 4 weeks. (3) Comparisons: Control, including groups that received placebo or those who received any nutrition supplements on the basis of nutritional treatment or maintaining the usual diet. (4) Outcomes: The parameters in the research results include at least two of the following parameters: cardiovascular risk factors [including systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FBG), fasting insulin level (FINS), homeostatic model assessment of insulin resistance (HOMA-IR), hemoglobin A1c (HbA1c), triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C)] and body composition [including Weight, Waist circumference (WC), and BMI]. (5) Study design: Parallel or cross-over design.
Two researchers (D.Z. and Z.Y.) independently screened and assessed the titles and abstracts according to the prespecified criteria. The full texts of articles that potentially met the eligibility criteria were reviewed and data extracted using the same standardized data extraction methods. If more than one article from the same study was found, only the article with more detailed information was selected to avoid data duplication. The data was independently extracted and cross-checked by two researchers (D.Z. and Z.Y.), and any disagreement was resolved by the judgment of the third researcher (X.L.).
Information about study design was extracted, including study-level characteristics (i.e., first author name, year of publication, and geographic location), participant-level characteristics (i.e., age, proportion of male participants, and diet control or daily exercise), program-level characteristics (i.e., study design, sample size in each group, type and dose of nutritional supplementation, and outcome data). We extracted the preintervention/postintervention (pre/post) change data to conduct this NMA. Regarding the RCTs with multiple time points, only the last time point was considered and intermediary time points were omitted.
Assessment of risk of bias in randomized trials was performed using the Cochrane Risk of Bias Tool for RCTs (24) by two investigators independently (Z.Y. and D.Z.), and studies were assessed from the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each domain was classified as low risk of bias, high risk of bias, or unclear risk of bias. Any disagreement was resolved by discussions with the third author (X.L.).
For continuous data, mean and standard deviation (SD) were extracted. For studies presenting median and interquartile range, mean was estimated by (first quartile + third quartile)/2, and SD was estimated by (third quartile − first quartile)/1.35 (25). For studies presenting 95% confidence intervals (CIs), standard error (SE) was estimated by (upper limit − lower limit)/3.92 and SD was calculated as SE × √n (26). After data extraction, we unified the unit of the outcomes previously reported, and the FBG, FINS, and the lipid markers levels (i.e., TGs, TC, HDL-C, LDL-C) were encoded in mmol/L, μIU/mL, and mg/dL, respectively.
First, we performed a pairwise meta-analysis for every intervention comparison. Continuous data were analyzed using Weighted mean differences (WMDs) and 95% CIs to express the effect size and I2 statistic and Q test were used to assess the heterogeneity of the treatment effect which was deemed significant when P was <0.05 or I2 was more than 50%. In this analysis, heterogeneity was present, thus, all results were reported using the random-effect model.
Second, network meta-analysis was performed using a random effects model based on the Bayesian framework and this model using the Markov-chain Monte Carlo (MCMC) method to obtain the non-informative uniform and normal prior distributions (27). Four iteration chains, with 50,000 iterations per chain, were set to fit the model and calculate the posterior distributions of model parameters. The thinning interval was set at 10 and the burn-ins at 1,000 for each chain. In this NMA, mean differences (MDs) with 95% credible intervals (CrIs) were generated from the posterior distribution medians, which did not contain 0 indicating significant differences between interventions. Deviance information criterion (DIC) was obtained from consistency and inconsistency models for each endpoint and difference between each pair of DICs (dDIC) were calculated to assess global inconsistency. A value of dDIC < 10 was deemed to have no appreciable global inconsistency. A node-split model was used to check the consistency assumption of direct evidence and indirect evidence with p < 0.05 indicating significant local inconsistency (28). The consistency model was adopted only if global inconsistency tests and node-split tests both reported no significant inconsistency. We performed meta-regression analysis to evaluate the potential impact of confounding factors (e.g., age, life style, proportion of male, total number of participants and intervention duration) on the model based on non-negligible differences in participant baseline characteristics (29). Surface under the cumulative ranking curve analysis (SUCRA) derived from posterior probabilities was used to rank the relative efficacy of interventions with larger SUCRA value indicating better interventions (30). Clustered-ranking plots were used for the determination of the most comprehensive intervention choice.
Stata software (version 12.0, StataCorp, College Station, TX) were used to produce the network evidence relationship plot and comparison-adjusted funnel plots. R software (version 3.6.2, MathSoftCorp, AT&T Bell Laboratories) with GeMTC (version 0.8-8) and JAGS packages (version 4.1.0, https://sourceforge.net/projects/mcmc-jags/files/) was used to perform the pairwise and network meta-analysis.
Of the 3,863 publications retrieved via literature search, 2,233 records left after removing duplicates. After reviewing the title and abstract, 81 studies were selected for further review. Then 16 studies were excluded (8 included patients with other cardiovascular diseases, 4 were without control group, and 4 did not meet our inclusion criteria). Finally, a total of 65 studies (31–95) and 4,241 obesity or overweight patients were ultimately included in this NMA, with 2,395 in the experimental group and 1,846 in the control group. The detailed selection process is described in Figure 1. All 65 included studies consist of 55 two-arms, 3 three-arms and 8 four-arms and were published between 2005 and 2022. The intervention duration of all studies was more than 4 weeks. The average age of the participants was 43.1 years and the percentage of male patients was about 40.7%. the average BMI of subjects is more than 30 kg/m2. Table 1 details the study characteristics.
Figure 2 shows the network plots of the included studies. We included 13 kinds of nutritional supplementations in our NMA: resveratrol, VD, probiotics, probiotics + VD, VD + Ca, α-lipoic acid, omega-3, omega-3 + α-lipoic acid, probiotics + α-lipoic acid, curcumin, probiotics + omega-3, Mg, and placebo.
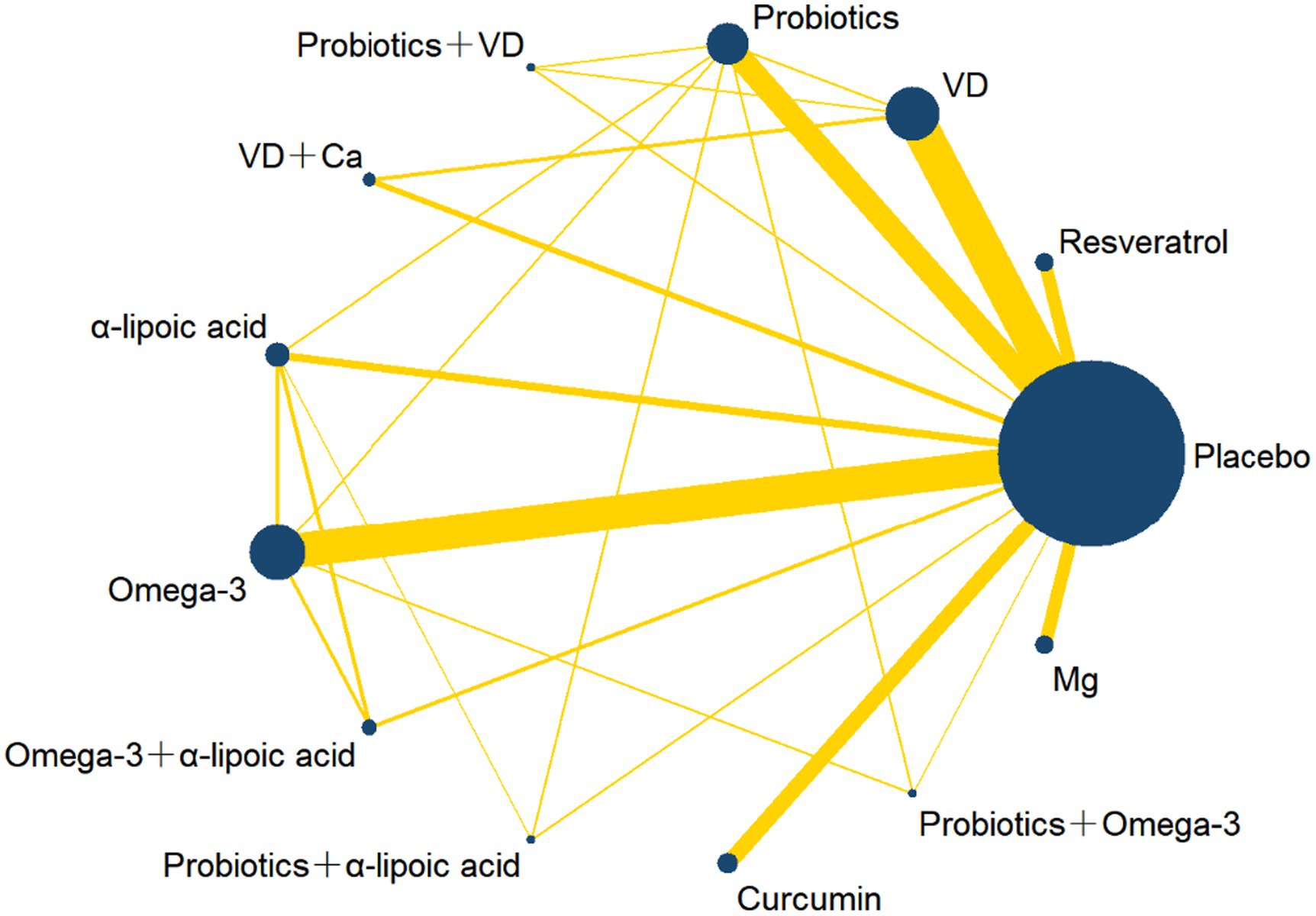
Figure 2. Network plot of different nutrition supplements for overweight and obese treatment. The width of the line is directly proportional to the number of treatments for each pair; the area of the circle represents the cumulative number of patients per intervention. VD, Vitamin D; Ca, Calcium; Mg, Magnesium.
The results of quality assessment were summaries in Supplementary Figure S1. The revised Cochrane Risk-of bias Tool for RCTs (RoB 2.0) was used to assess the quality of 65 included RCTs. All eligible RCTs mentioned randomization and were classified as “low risk.” 28 articles showed “unclear risk” and 1 article showed “high risk” in adequate allocation concealment. Six articles showed “unclear risk” and 4 articles showed “high risk” in blinding of participants and personnel. 28 articles showed “unclear risk” and 1 article showed “high risk” in terms of adequate allocation concealment. Six articles showed “unclear risk” and 6 articles showed “high risk” in the aspect of blinding of outcome assessment. For complete outcome assessment and selective reporting, 22 articles were deemed as “unclear risk” in complete data, whereas 65 articles showed no selecting outcomes to report. The 22 articles were considered as “unclear risk” in other bias.
Across all primary and secondary outcomes, model fit and iteration convergence were both good. All the dDIC value of the outcomes are less than 5 and the I2 value of all outcomes were less than 25%, indicating that there is no significant difference between the global consistency model and inconsistency model (Supplementary Table S2). There are some closed-loop network structures in the comparison of Weight, WC, BMI, FBG, FINS, TGs, TC, HDL-C, LDL-C, and no inconsistency between direct and indirect evidence was found by node-splitting method (all p > 0.05 in Supplementary Table S3). Network meta-regression showed no association among our all outcomes and life styles, proportion of male, total number of participants and intervention duration; however, we found some potential heterogeneity in the mean age of the patients with respect to weight and WC (Supplementary Figure S2).
The change in blood pressure was recorded in 7 studies with 1787 patients. Pairwise meta-analysis and NMA results both revealed that there was no significant difference in SDP and DBP change in all the 9 interventions and placebo (Supplementary Tables S1, S4). The rankings were shown in Table 2 and Supplementary Figures S3A,B.
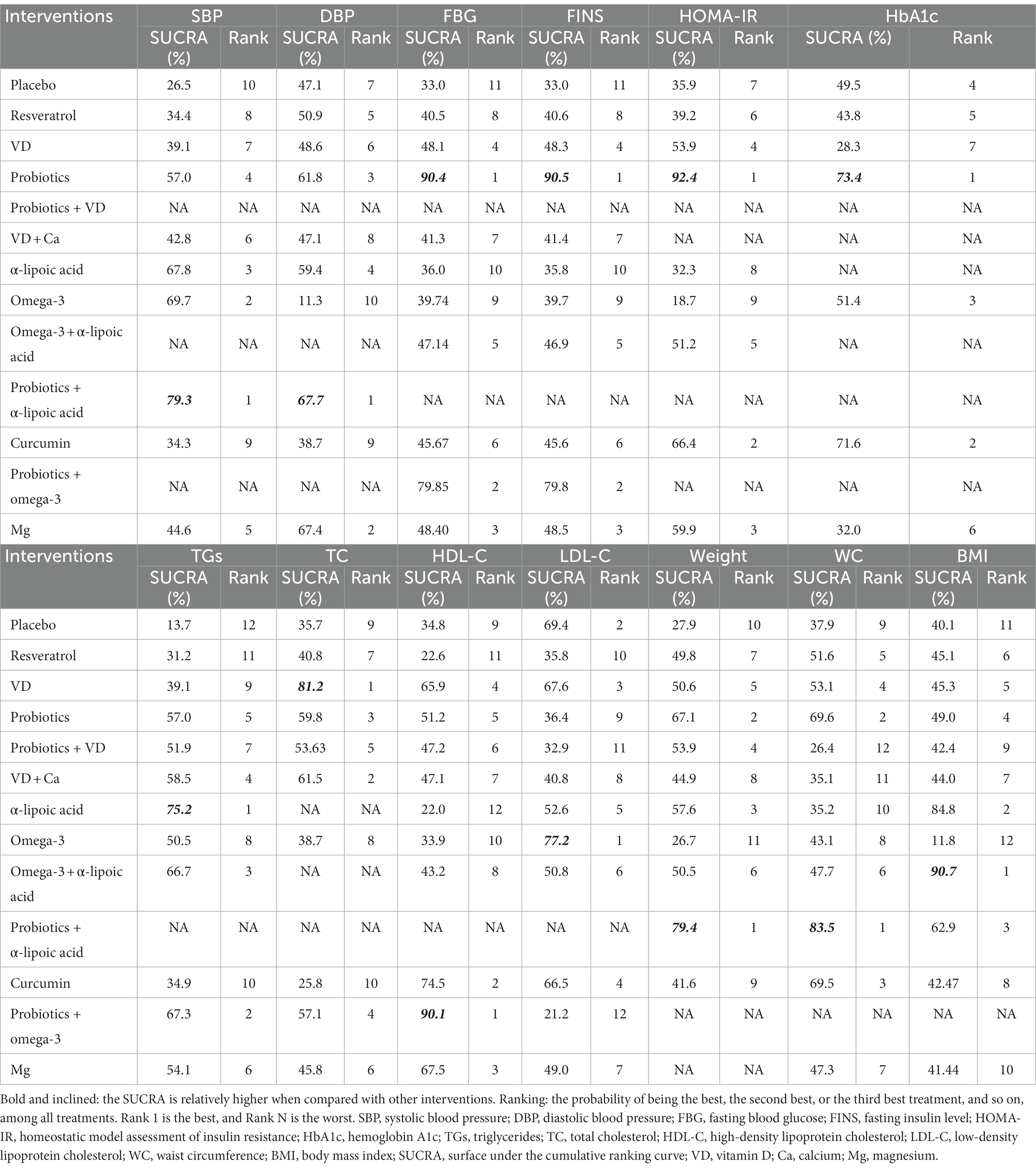
Table 2. Surface under the cumulative ranking curve and ranking probability of different nutrition supplements on each outcome.
FBG was measured in 39 studies and involved 2,731 patients. The pairwise meta-analysis revealed that compared with placebo, probiotics + omega-3 (WMD: −5.55 mmol/l; 95%CI: −6.69 to −4.40) resulted in a significant reduction in FBG (Supplementary Table S1). However, in NMA (Table 3), compared with placebo (MD: −0.90 mmol/L; 95%CrI: −1.41 to −0.38), VD (MD: −0.75 mmol/L; 95%CrI: −1.47 to −0.01), and omega-3 (MD: −0.84 mmol/L; 95%CrI: −0.20 to −1.47), probiotics resulted in a greater reduction in FBG. According to the SCURA values, probiotics (SUCRA 90.4%) may be the best intervention for decreasing FBG (Table 2; Supplementary Figure S3C).
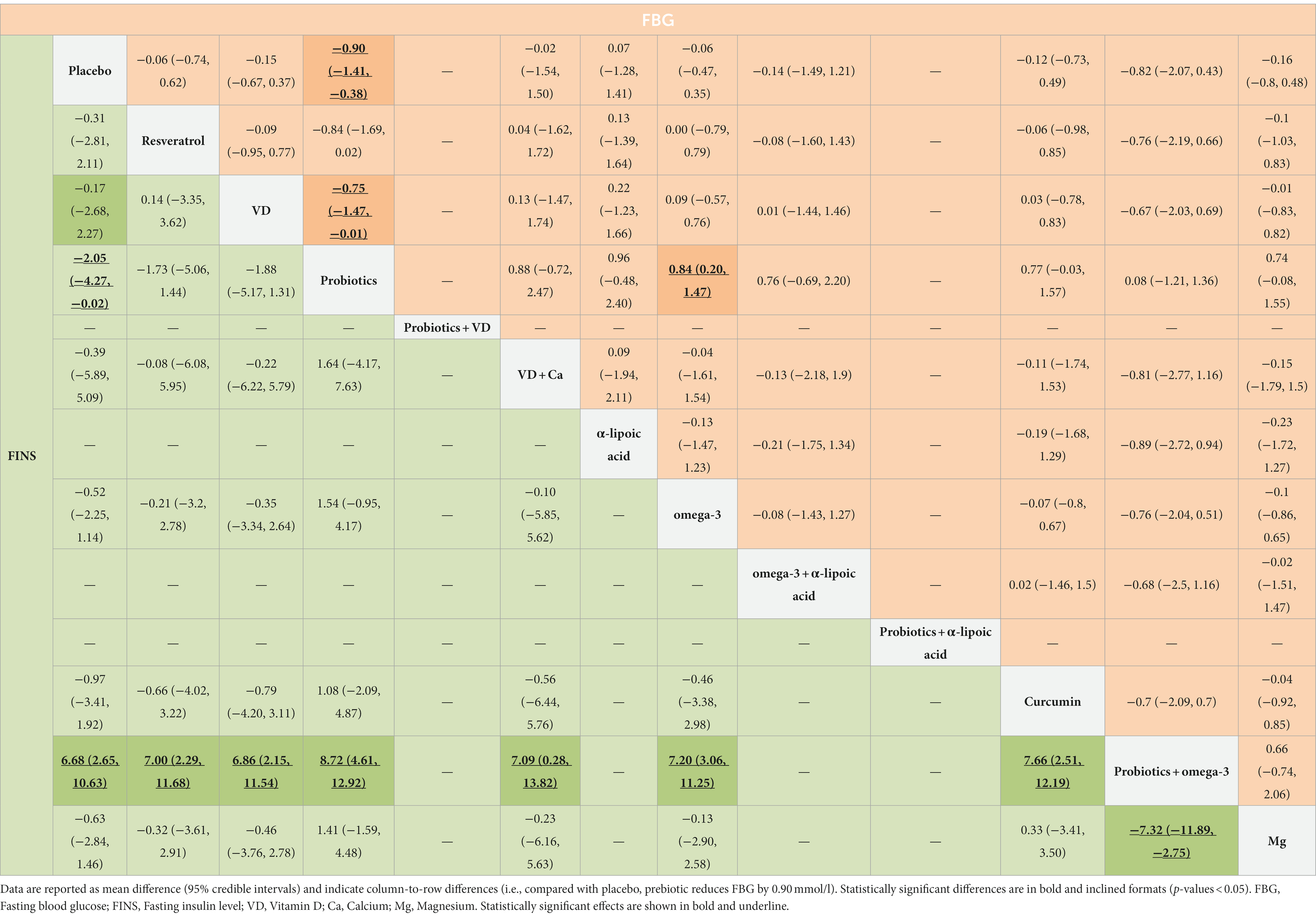
Table 3. Results of the network meta-analysis of FINS (lower-left quadrant) and FBG (upper-right quadrant).
FINS was measured in 37 studies involving 1936 patients. The pairwise meta-analysis revealed that compared with placebo, α-lipoic acid + probiotics (WMD: −2.51 μIU/mL; 95%CI: −3.33 to −1.69) and probiotics + omega-3 (WMD: −4.04 μIU/mL; 95%CI: −4.94 to −3.14) resulted in a significant reduction in FINS (Supplementary Table S1). However, NMA revealed that probiotics might be more effective than placebo (MD: −2.05 μIU/mL; 95%CrI: −4.27 to −0.02) and probiotics + omega-3 (MD: −8.72 μIU/mL; 95% CrI: −4.61 to −12.92; Table 3). According to the SCURA values, probiotics (SUCRA 90.5%) may be the best intervention to reduce FINS (Table 2; Supplementary Figure S3D).
HOMA-IR was measured in 25 studies involving 1,436 patients. The pairwise meta-analysis revealed that compared with placebo, α-lipoic acid + probiotics (WMD: −2.59; 95%CI: −3.42 to −1.76) and curcumin (WMD: −0.41; 95%CI: −0.74 to −0.08, I2 = 0%; p = 0.60) resulted in a greater benefit in improving HOMA-IR (Supplementary Table S1). However, NMA showed that probiotics might be more effective than placebo (MD: −1.43; 95%CrI: −2.46 to −0.31) and omega-3 (MD: −1.92; 95%CrI: −0.20 to −3.55; Supplementary Table S5). According to the SCURA values, probiotics (SUCRA 93.4%) may be the best intervention to improve HOMA-IR (Table 4; Supplementary Figure S3E).
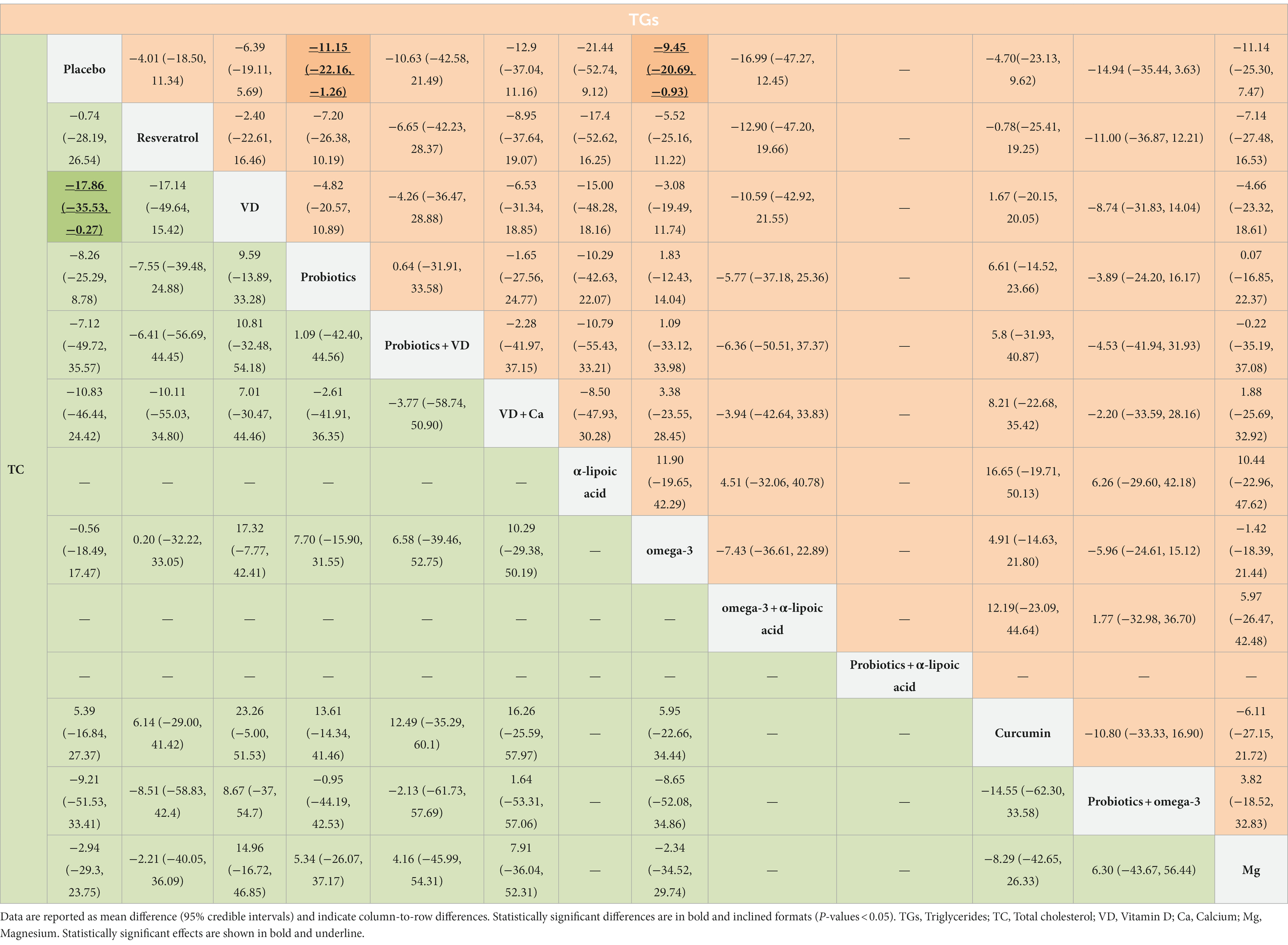
Table 4. Results of the network meta-analysis of TC (lower-left quadrant) and TGs (upper-right quadrant).
HbA1c was reported in 12 studies involving 724 patients. The pairwise meta-analysis revealed that only curcumin (WMD: −0.36; 95%CI: −0.70 to −0.01; I2 = 16%; p = 0.30) resulted in a significant reduction in HbA1c compared to placebo (Supplementary Table S1). However, there was no significant difference between all interventions and placebo in NMA (Supplementary Table S5).
TGs was measured in 43 studies involving 2,795 patients. The pairwise meta-analysis revealed that compared with placebo, probiotics (WMD: −0.21 mg/dL; 95%CI −0.39 to −0.04; I2 = 49%; p = 0.10), omega-3 (WMD: −0.29 mg/dL; 95%CI −0.46 to −0.13; I2 = 0%; p = 0.89) and probiotics + omega-3 (WMD: −0.60 mg/dL; 95%CI: −1.12 to −0.09) all resulted in a significant reduction in TGs (Supplementary Table S1). Similarly, NMA revealed that probiotics (MD: −11.15 mg/dL; 95%CrI: −22.16 to −1.26) and omega-3 (MD: −9.45 mg/dL; 95%CrI: −20.69 to −0.93) might be more effective than placebo (Table 4). According to the SCURA values, α-lipoic acid (SUCRA 75.2%) may be the best intervention to reduce TGs (Table 4; Supplementary Figure S3G).
TC was measured in 37 studies involving 2,379 patients. The pairwise meta-analysis showed that compared with placebo, probiotics (WMD: −0.36 mg/dL; 95%CI: −0.57 to −0.15; I2 = 23%; p = 0.24), α-lipoic acid + probiotics (WMD: −2.51 mg/dL; 95%CI: −3.33 to −1.69), and probiotics + omega-3 (WMD: −0.94 mg/dL; 95%CI: −1.48 to −0.41) all resulted in a significant reduction in TC (Supplementary Table S1). However, NMA revealed that only VD (MD: −17.86 mg/dL; 95%CrI: −35.53 to −0.27) might be more effective than placebo (Table 4). According to the SCURA values, VD (SUCRA 81.2%) may be the best intervention to reduce TC (Table 2; Supplementary Figure S3H).
HDL-C was reported in 43 studies involving 2,804 patients. The pairwise meta-analysis demonstrated that curcumin (WMD: 0.35 mg/dL; 95%CI: 0.12 to 0.57; I2 = 0%; p = 0.70) and probiotics + omega-3 (WMD: 3.07 mg/dL; 95%CI: 2.31 to 3.83) resulted in a significant increase in HDL-C compared to placebo (Supplementary Table S1). Likewise, NMA revealed that probiotics + omega-3 might be more effective than placebo (MD: 5.09 mg/dL; 95%CrI: 0.77 to 9.38), resveratrol (MD: 6.36 mg/dl; 95%CrI: 0.92 to 11.58) and omega-3 (MD: 5.24 mg/dL; 95%CrI 0.78 to 9.63; Supplementary Table S6). According to the SCURA values, probiotics + omega-3 (SUCRA 90.1%) may be the best intervention to increase HDL-C (Table 2; Supplementary Figure S3I).
LDL-C was reported in 42 studies involving 2,737 patients. The pairwise meta-analysis described that compared with placebo, probiotics (WMD: −0.32 mg/dL; 95%CI: −0.52 to −0.12; I2 = 25%; p = 0.22) and probiotics + omega-3 (WMD: −1.37 mg/dL; 95%CI: −1.94 to −0.80) resulted in a significant reduction in LDL-C (Supplementary Table S1). However, there was no significant difference between all interventions and placebo in NMA (Supplementary Table S6).
According to the cluster-rank results in Figure 3, probiotics ranked highest in decreasing the FBG and FINS and was the optimum strategy (cluster-rank value = 3290.50); probiotics + omega-3 ranked highest in decreasing the TGs meanwhile increasing the HDL-C and has the greatest potential to be the optimum strategy (cluster-rank value = 2117.61).
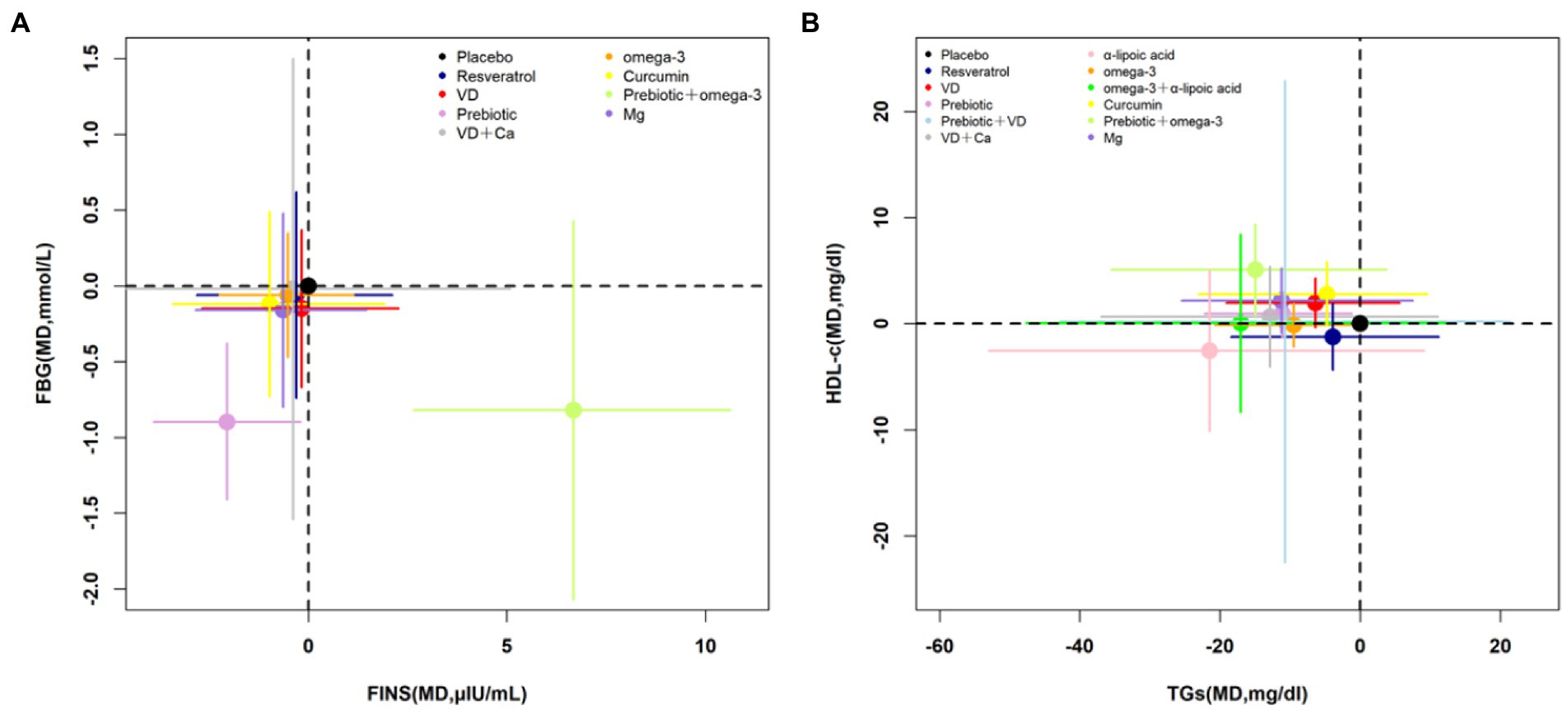
Figure 3. Cluster-rank plots. (A) The cluster-rank plot of FBG and FINS. (B) The cluster-rank plot of HDL-C and TGs. (The cluster-rank value is the product of the abscissa and ordinate of each treatment.) FBG, fasting blood glucose; FINS, fasting insulin level; TGs, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; VD, vitamin D; Ca, calcium; Mg, magnesium.
In this NMA, body weight, WC and BMI were reported in 38, 33, and 45 studies, respectively. The results of this NMA showed that there was no significant difference in weight loss and WC reduction among all interventions. The effect of omega-3 + α-lipoic acid (MD: −6.70 Kg/m2; 95%CI: −13.13 to −0.24) on reducing BMI was significantly better than that of placebo (Supplementary Table S8). According to the SCURA values, α-lipoic acid + probiotics might be the best intervention to reduce body weight (SUCRA 79.4%) and WC (SUCRA 83.5%), and omega-3 + α-lipoic acid (SUCRA 90.7%) might be the best intervention to reduce BMI (Table 2; Supplementary Figures S3K–M).
Comparison-adjusted funnel plot was showed in Supplementary Figure S4. All studies on the funnel plot were symmetrically distributed on either side of the vertical line of X = 0, indicating that there was no significant small sample effects or publication bias.
At present, nutritional supplements have been shown to be effective as a complementary therapy to improve glucose and lipid metabolism in patients with metabolic diseases (97, 98). However, the effects of common nutritional supplements on improving cardiometabolic risk factors in overweight and obese patients are inconsistent. Network meta-analysis can directly and indirectly compare the efficacy of various nutritional supplements to determine the best nutritional strategy (99).
Overall, among the interventions we included in the comparison, probiotic showed significantly higher efficacy in reducing FBG, FINS, and HOMA-IR than placebo and other interventions; probiotic and omega-3 seemed to be more effective than placebo and other nutrients in reducing TGs; probiotic + omega-3 seemed to be more effective than placebo and other nutrients in increasing HDL-C; however, none of the interventions had a significant impact on body weight, WC, and BMI.
In terms of blood glucose metabolism, SUCRA shows that probiotic was the best way for improving the FBG, FINS, and HOMA-IR. The result is inconsistent with Zarezadeh, who believes that probiotics supplementation does not reduce glucose metabolism in patients with metabolic syndrome and obesity (97). We believe that this is mainly due to the difference in intervention dose and duration. The study intervention included in our meta lasted 8 weeks, and were all medium dose probiotics (more than 108 or 109 CFU). Our NMA also provides compelling evidence for the benefits of probiotics in improving blood glucose metabolism. Different studies have explained the potential hypoglycemic mechanisms of probiotics, and most studies believe that it may be related to gut bacteria, increasing satiety and reducing appetite (13, 100–102). A variety of probiotics, such as Bifidobacterium and Lactobacillus, were used in the included studies. These composite probiotics can decrease the number of harmful bacteria such as Acinobacteria, Escherichina, and Gram-negative bacteria, and promote the growth of short chain fatty acids (SCFAs) (103). SCFAs play an important role in regulating glucose storage in the muscle, adipose tissue, and liver (104). Moreover, probiotics can improve insulin signaling pathway (105).
In terms of lipid metabolism, NMA shows that probiotic and omega-3 seemed to be more effective than placebo in reducing TGs, however, the SUCRA shows that α-lipoic acid might be the most successful intervention among these treatments. This research showed that α-lipoic acid reduced triglycerides to a greatest extent. α-lipoic acid is a potent antioxidant and free radical scavenger, but the mechanism of its regulation of blood lipids is still unclear (106). Butler et al. found that α-lipoic acid offset the rise of TGs in blood and liver by inhibiting liver lipogenic gene expressions, and stimulate clearance of TG-rich lipoproteins by lowering the secretion of hepatic TGs (107). Lee WJ ‘s experimental study showed that α-lipoic acid decreased lipid accumulation in skeletal muscle and hepatic steatosis by activating AMP-activated kinase (AMPK) (108). The SCURA shows that VD was the best for decreasing TC. Makariou (51) and Jiang XJ (109) found a significant negative correlation between VD supplementation and TC. Jorde et al. found that vitamin D 40,000 IU/wk. did not significantly improve serum lipids and other cardiovascular risk factors compared with placebo (110). In contrast, Ramiro-Lozano and Calvo-Romero presented that oral vitamin D 16,000 IU/wk. showed a reduction in TC but not LDL-C and TGs in type 2 diabetes participants (111). In this NMA, the doses of vitamin D in most studies exceeded 2,000 IU/d, which may prove that high VD levels were associated with a favorable serum lipid profile. With regard to cholesterol level, Major’s experiment study showed that vitamin D may increase calcium intestinal absorption and insoluble calcium-fatty soap formation in the gut, resulting in reduced fatty acid absorption and increased fecal fatty acids (47). The effect of probiotics +omega-3 on HDL-C was significant, and SCURA shows that probiotics +omega-3 might be the most successful intervention. The results on HDL-C increasing are compatible with those of Jone’s (112) and Venturini’s (113) experiment studies, but discordant with those of Chang’s (114, 115), possibly owing to the different proportions and dose of omega-3. The effect of omega-3 on HDL cholesterol has been well established (116), but the mechanism of synergistic effect in combination with probiotics remains unclear.
This study comprehensively analyzed the effect of intervention on blood glucose control and lipid metabolism, and the results of cluster rank analysis were consistent with those of SUCRA. Probiotic was found to have statistically significant advantages in decreasing FBG and FINS simultaneously. For the effect of lowering TGs and improving HDL-C, the cluster rank analysis showed that omega-3 + probiotics might be the most effective intervention. Previous meta-analysis results showed different nutrients have different effects on body composition in obese and overweight patients (117, 118). In this NMA, no effective change was found in body weight, WC, and BMI, however, meta-regression shows that age may be a source of heterogeneity in the results of body weight and WC. A low-calorie diet and regular physical exercise were also important ways to improve cardiometabolic indicators in the early prevention of overweight and obesity patients, but meta-regression indicated that the results were consistent, and no matter the daily exercise or a low-calorie diet alone or a combination of the two life styles had no significant effect on the outcomes.
In this review, chronic cardiovascular diseases with complex pathogenesis are excluded, only overweight and obese patients are included, which reduces clinical heterogeneity to some extent and improves the comparability of results. A comprehensive search of treatment strategies for common nutritional supplements in adjuvant therapy was conducted, including a sufficient number of RCT experiments and nutrients were compared as much as possible. The statistical stability and reliability of network meta-analysis depends on the uniform standards of similarity, homogeneity and consistency. No inconsistency was observed in this NMA through consistency test and node splitting method, and the NMA results are robust. Based on SCURA and cluster rank analysis, the results of this NMA will be useful for clinical decision making.
There are also some limitations shown in the study. First, we did not perform further subgroup analysis. On the one hand, subgroups could not include all supplements in this study. On the other hand, this meta-regression showed the reported results have good consistency, which was not affected by the imputation models. Second, although all the included studies are RCTs, the most common; used is a placebo as a control. Due to the variety of interventions, a small number of direct comparisons of some treatments impairs the robustness of the final results. Third, although SCURA ranking has been widely used to give clinically significant results, due to the minimum absolute difference between the highest rank and others rankings, cautious interpretation is required.
Nutrition supplements might be positive efficacious intervention from which patients with overweight or obesity will derive benefit in improving some CVD risk factors. Probiotics supplementation might be potentially the preferred the intervention for glycemic control. VD, α-lipoic acid, probiotic + omega-3 have a better impact on lipid metabolism. Further studies are required to verify the current findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
XL, ZY, and DZ conceived and designed the research, analyzed the data, interpreted the data, and wrote the first draft. ZY and DZ retrieved the literature and identified eligible studies. XL and DZ extracted the data and checked the statistical methods. XL and ZY reviewed the manuscript and revised the important content. All authors contributed to the article and approved the submitted version.
This work was supported by a research grant from Science and Technology Project of Henan Province (no. 222102310176).
We would like to thank Xinxin Liu for her assistance in developing the search strategy and Zhengzhou University for providing facilities to search in electronic databases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1140019/full#supplementary-material
CVD, Cardiovascular disease; VD, Vitamin D; Ca, Calcium; Mg, Magnesium; WC, Waist circumference; BMI, Body mass index; FBG, Fasting blood glucose; FINS, Fasting insulin level; HOMA-IR, Homeostatic model assessment of insulin resistance; HbA1c, Hemoglobin A1c; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; TGs, Triglycerides; TC, Total cholesterol; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol.
1. World Health Organization . obesity, (2018) Obesity. Available at https://www.who.int/topics/obesity/en/ [Accessed February 5, 2023].
2. Armstrong, A , Jungbluth Rodriguez, K , Sabag, A , Mavros, Y , Parker, HM , Keating, SE, et al. Effect of aerobic exercise on waist circumference in adults with overweight or obesity: a systematic review and meta-analysis. Obes Rev. (2022) 23:e13446. doi: 10.1111/obr.13446
3. Lau, ES , Paniagua, SM , Zarbafian, S , Hoffman, U , Long, MT , Hwang, SJ, et al. Cardiovascular biomarkers of obesity and overlap with Cardiometabolic dysfunction. J Am Heart Assoc. (2021) 10:e020215. doi: 10.1161/jaha.120.020215
4. Permatasari, HK , Nurkolis, F , Gunawan, WB , Yusuf, VM , Yusuf, M , Kusuma, RJ, et al. Modulation of gut microbiota and markers of metabolic syndrome in mice on cholesterol and fat enriched diet by butterfly pea flower kombucha. Curr Res Food Sci. (2022) 5:1251–65. doi: 10.1016/j.crfs.2022.08.005
5. Santos, HO , Price, JC , and Bueno, AA . Beyond fish oil supplementation: the effects of alternative plant sources of Omega-3 polyunsaturated fatty acids upon lipid indexes and Cardiometabolic biomarkers-an overview. Nutrients. (2020) 12:3159. doi: 10.3390/nu12103159
6. Neeland, IJ , Poirier, P , and Després, JP . Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. (2018) 137:1391–406. doi: 10.1161/circulationaha.117.029617
7. Halford, JC , Boyland, EJ , Blundell, JE , Kirkham, TC , and Harrold, JA . Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol. (2010) 6:255–69. doi: 10.1038/nrendo.2010.19
8. Harrold, JA , Dovey, TM , Blundell, JE , and Halford, JC . CNS regulation of appetite. Neuropharmacology. (2012) 63:3–17. doi: 10.1016/j.neuropharm.2012.01.007
9. Panidis, D , Tziomalos, K , Papadakis, E , Vosnakis, C , Chatzis, P , and Katsikis, I . Lifestyle intervention and anti-obesity therapies in the polycystic ovary syndrome: impact on metabolism and fertility. Endocrine. (2013) 44:583–90. doi: 10.1007/s12020-013-9971-5
10. Watanabe, M , Risi, R , Masi, D , Caputi, A , Balena, A , Rossini, G, et al. Current evidence to propose different food supplements for weight loss: a comprehensive review. Nutrients. (2020) 12:2873. doi: 10.3390/nu12092873
11. Akbari, M , Ostadmohammadi, V , Lankarani, KB , Tabrizi, R , Kolahdooz, F , Khatibi, SR, et al. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Metabolism. (2018) 87:56–69. doi: 10.1016/j.metabol.2018.07.002
12. Tabrizi, R , Vakili, S , Lankarani, KB , Akbari, M , Mirhosseini, N , Ghayour-Mobarhan, M, et al. The effects of Curcumin on glycemic control and lipid profiles among patients with metabolic syndrome and related disorders: a systematic review and Metaanalysis of randomized controlled trials. Curr Pharm Des. (2018) 24:3184–99. doi: 10.2174/1381612824666180828162053
13. Ghorbani, Z , Kazemi, A , Bartolomaeus, TUP , Martami, F , Noormohammadi, M , Salari, A, et al. The effect of probiotic and synbiotic supplementation on lipid parameters among patients with cardiometabolic risk factors: a systematic review and meta-analysis of clinical trials. Cardiovasc Res. (2022) 1–13. doi: 10.1093/cvr/cvac128
14. Akbari, M , Tamtaji, OR , Lankarani, KB , Tabrizi, R , Dadgostar, E , Haghighat, N, et al. The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. (2020) 19:25. doi: 10.1186/s12944-020-1198-x
15. AlAnouti, F , Abboud, M , Papandreou, D , Mahboub, N , Haidar, S , and Rizk, R . Effects of vitamin D supplementation on lipid profile in adults with the metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2020) 12:3352. doi: 10.3390/nu12113352
16. Toprak, O , Kurt, H , Sarı, Y , Şarkış, C , Us, H , and Kırık, A . Magnesium replacement improves the metabolic profile in obese and pre-diabetic patients with mild-to-moderate chronic kidney disease: a 3-month, randomised, double-blind, Placebo-Controlled Study. Kidney Blood Press Res. (2017) 42:33–42. doi: 10.1159/000468530
17. Fatahi, S , Sohouli, MH , da Silva Magalhães, EI , da Cruz Silveira, VN , Zanghelini, F , Rahmani, P, et al. Comparing the effects of docosahexaenoic and eicosapentaenoic acids on cardiovascular risk factors: pairwise and network meta-analyses of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2022) 33:11–21. doi: 10.1016/j.numecd.2022.09.013
18. Jang, H , and Park, K . Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: a systematic review and meta-analysis. Clin Nutr. (2020) 39:765–73. doi: 10.1016/j.clnu.2019.03.032
19. Fogacci, F , Tocci, G , Presta, V , Fratter, A , Borghi, C , and Cicero, AFG . Effect of resveratrol on blood pressure: a systematic review and meta-analysis of randomized, controlled, clinical trials. Crit Rev Food Sci Nutr. (2019) 59:1605–18. doi: 10.1080/10408398.2017.1422480
20. Hallajzadeh, J , Milajerdi, A , Kolahdooz, F , Amirani, E , Mirzaei, H , and Asemi, Z . The effects of curcumin supplementation on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2019) 33:2989–95. doi: 10.1002/ptr.6477
21. Zhang, X , Zhang, M , Zhao, Z , Huang, Z , Deng, Q , Li, Y, et al. Geographic variation in prevalence of adult obesity in China: results from the 2013-2014 National Chronic Disease and risk factor surveillance. Ann Intern Med. (2020) 172:291–3. doi: 10.7326/m19-0477
22. Page, MJ , McKenzie, JE , Bossuyt, PM , Boutron, I , Hoffmann, TC , Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
23. Hutton, B , Salanti, G , Caldwell, DM , Chaimani, A , Schmid, CH , Cameron, C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
24. Higgins, J , Sterne, J , and Savovic, J . A revised tool for assessing risk of bias in randomized trails. Cochrane Database Syst Rev. (2016) 10:29–31. doi: 10.1136/bmj.l4898
25. Wan, X , Wang, W , Liu, J , and Tong, T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
26. Higgins, JP , and Deeks, JJ . Choosing effect measures and computing estimates of effect. Cochrane Handbook Syst Rev Intervent. (2019) 6:143–76. doi: 10.1002/9781119536604.ch6
27. Brooks, SP . A.: general methods for monitoring convergence of iterative simulations. J ComputGraph Stat. (1998) 7:434–55.
28. Veroniki, AA , Vasiliadis, HS , Higgins, JP , and Salanti, G . Evaluation of inconsistency in networks of interventions. Int J Epidemiol. (2013) 42:332–45. doi: 10.1093/ije/dys222
29. Dias, S , Sutton, AJ , Welton, NJ , and Ades, AE . Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med Decis Mak. (2013) 33:618–40. doi: 10.1177/0272989X13485157
30. Salanti, G , Ades, AE , and Ioannidis, JP . Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
31. Arzola-Paniagua, MA , García-Salgado López, ER , Calvo-Vargas, CG , and Guevara-Cruz, M . Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: a randomized controlled trial. Obesity. (2016) 24:1454–63. doi: 10.1002/oby.21523
32. Batista-Jorge, GC , Barcala-Jorge, AS , Silveira, MF , Lelis, DF , Andrade, JMO , de Paula, AMB, et al. Oral resveratrol supplementation improves metabolic syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. (2020) 256:117962. doi: 10.1016/j.lfs.2020.117962
33. Kantartzis, K , Fritsche, L , Bombrich, M , Machann, J , Schick, F , Staiger, H, et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes Metab. (2018) 20:1793–7. doi: 10.1111/dom.13268
34. Poulsen, MM , Vestergaard, PF , Clasen, BF , Radko, Y , Christensen, LP , Stødkilde-Jørgensen, H, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. (2013) 62:1186–95. doi: 10.2337/db12-0975
35. Timmers, S , Konings, E , Bilet, L , Houtkooper, RH , van de Weijer, T , Goossens, GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. (2011) 14:612–22. doi: 10.1016/j.cmet.2011.10.002
36. van der Made, SM , Plat, J , and Mensink, RP . Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: a randomized, placebo-controlled crossover trial. PLoS One. (2015) 10:e0118393. doi: 10.1371/journal.pone.0118393
37. Wong, RH , Berry, NM , Coates, AM , Buckley, JD , Bryan, J , Kunz, I, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. (2013) 31:1819–27. doi: 10.1097/HJH.0b013e328362b9d6
38. Al-Bayyari, N , Al-Zeidaneen, S , Hailat, R , and Hamadneh, J . Vitamin D3 prevents cardiovascular diseases by lowering serum total homocysteine concentrations in overweight reproductive women: a randomized, placebo-controlled clinical trial. Nutr Res. (2018) 59:65–71. doi: 10.1016/j.nutres.2018.07.012
39. Carrillo, AE , Flynn, MG , Pinkston, C , Markofski, MM , Jiang, Y , Donkin, SS, et al. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr. (2013) 32:375–81. doi: 10.1016/j.clnu.2012.08.014
40. Chandler, PD , Scott, JB , Drake, BF , Ng, K , Chan, AT , Hollis, BW, et al. Bennett: impact of vitamin D supplementation on adiposity in African-Americans. Nutr Diabetes. (2015) 5:e147. doi: 10.1038/nutd.2014.44
41. Cheshmazar, E , Hosseini, AF , Yazdani, B , Razmpoosh, E , and Zarrati, M . Effects of vitamin D supplementation on Omentin-1 and Spexin levels, inflammatory parameters, lipid profile, and anthropometric indices in obese and overweight adults with vitamin D deficiency under low-calorie diet: a randomized placebo controlled trial. Evid Based Complement Alternat Med. (2020) 2020:1–10. doi: 10.1155/2020/3826237
42. Ebadi, SA , Sharifi, L , Rashidi, E , Ebadi, SS , Khalili, S , Sadeghi, S, et al. Supplementation with vitamin D and insulin homeostasis in healthy overweight and obese adults: a randomized clinical trial. Obes Res Clin Pract. (2021) 15:256–61. doi: 10.1016/j.orcp.2021.03.004
43. Farag, HAM , Hosseinzadeh-Attar, MJ , Muhammad, BA , Esmaillzadeh, A , and El Bilbeisi, AH . Comparative effects of vitamin D and vitamin C supplementations with and without endurance physical activity on metabolic syndrome patients: a randomized controlled trial. Diabetol Metab Syndr. (2018) 10:80. doi: 10.1186/s13098-018-0384-8
44. Hajipoor, S , Hekmatdoost, A , Rezaei, M , Nachvak, SM , Alipour, M , Eskandari, S, et al. The effect of yogurt co-fortified with probiotic and vitamin D on lipid profile, anthropometric indices and serum 25-hydroxi vitamin D in obese adult: a double-blind randomized-controlled trial. Food Sci Nutr. (2021) 9:303–12. doi: 10.1002/fsn3.1996
45. Lithgow, HM , Florida-James, G , and Leggate, M . The combined effect of high-intensity intermittent training and vitamin D supplementation on glycemic control in overweight and obese adults. Physiol Rep. (2018) 6:e13684. doi: 10.14814/phy2.13684
46. Mai, S , Walker, GE , Vietti, R , Cattaldo, S , Mele, C , Priano, L, et al. Acute vitamin D₃ supplementation in severe obesity: evaluation of Multimeric Adiponectin. Nutrients. (2017) 9:459. doi: 10.3390/nu9050459
47. Major, GC , Alarie, F , Doré, J , Phouttama, S , and Tremblay, A . Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. (2007) 85:54–9. doi: 10.1093/ajcn/85.1.54
48. Mason, C , Liren, X , Ikuyo, I , Duggan, C , Ching-Yun, W , Korde, L, et al. Vitamin D3 supplementation during weight loss: a double-blind randomized controlled trial. Am J Clin Nutr. (2014) 99:1015–25. doi: 10.3945/ajcn.113.073734
49. Rajaie, H , Bellissimo, N , Keshavarzi, S , and Faghih, S . The effect of calcium and vitamin D supplementation on body composition and weight reduction: a randomized, triple-blind, controlled trial. Prog Nutr. (2018) 20:153–62. doi: 10.23751/pn.v20i2-S.6116
50. Rajaie, H , Rabiee, MR , Bellissimo, N , and Faghih, S . Independent and combined effects of calcium and vitamin D supplementation on blood lipids in overweight or obese premenopausal women: a triple-blind randomized controlled clinical trial. Int J Prev Med. (2021) 12:52. doi: 10.4103/ijpvm.IJPVM_294_19
51. Makariou, SE , Elisaf, M , Challa, A , Tellis, C , Tselepis, AD , and Liberopoulos, EN . Effect of combined vitamin D administration plus dietary intervention on oxidative stress markers in patients with metabolic syndrome: a pilot randomized study. Clin Nutr ESPEN. (2019) 29:198–202. doi: 10.1016/j.clnesp.2018.10.004
52. Makariou, SE , Elisaf, M , Challa, A , Tentolouris, N , and Liberopoulos, EN . No effect of vitamin D supplementation on cardiovascular risk factors in subjects with metabolic syndrome: a pilot randomised study. Arch Med Sci Atheroscler Dis. (2017) 2:52–e60. doi: 10.5114/amsad.2017.70504
53. Salehpour, A , Shidfar, F , Hosseinpanah, F , Vafa, M , Razaghi, M , Hoshiarrad, A, et al. Vitamin D3 and the risk of CVD in overweight and obese women: a randomised controlled trial. Br J Nutr. (2012) 108:1866–73. doi: 10.1017/s0007114512000098
54. Salekzamani, S , Mehralizadeh, H , Ghezel, A , Salekzamani, Y , Jafarabadi, MA , Bavil, AS, et al. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Investig. (2016) 39:1303–13. doi: 10.1007/s40618-016-0507-8
55. Zittermann, A , Frisch, S , Berthold, HK , Goetting, C , Kuhn, J , Kleesiek, K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. (2009) 89:1321–7. doi: 10.3945/ajcn.2008.27004
56. Huerta, AE , Navas-Carretero, S , Prieto-Hontoria, PL , Martínez, JA , and Moreno-Aliaga, MJ . Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity. (2015) 23:313–21. doi: 10.1002/oby.20966
57. Nasiri, G , Bastani, A , Haji-Aghamohammadi, AA , Nooshabadi, MR , Shahmirzalou, P , and Haghighian, HK . Effects of probiotic and alpha-lipoic acid supplements, separately or in combination on the anthropometric indicators and maintenance of weight in overweight individuals. Clin Nutr ESPEN. (2021) 41:242–8. doi: 10.1016/j.clnesp.2020.12.007
58. Romo-Hualde, A , Huerta, AE , González-Navarro, CJ , Ramos-López, O , Moreno-Aliaga, MJ , and Martínez, JA . Untargeted metabolomic on urine samples after α-lipoic acid and/or eicosapentaenoic acid supplementation in healthy overweight/obese women. Lipids Health Dis. (2018) 17:103. doi: 10.1186/s12944-018-0750-4
59. Bateni, Z , Rahimi, HR , Hedayati, M , Afsharian, S , Goudarzi, R , and Sohrab, G . The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: a randomized, double-blind clinical trial. Phytother Res. (2021) 35:3945–53. doi: 10.1002/ptr.7109
60. Campbell, MS , and Ouyang, A, M. K. I, R. J. Charnigo, P. M. Westgate and B. S. Fleenor . Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: a double-blinded, randomized, controlled trial. Nutrition. (2019) 62:135–9. doi: 10.1016/j.nut.2019.01.002
61. Cicero, AFG , Sahebkar, A , Fogacci, F , Bove, M , Giovannini, M , and Borghi, C . Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. Eur J Nutr. (2020) 59:477–83. doi: 10.1007/s00394-019-01916-7
62. Dolati, S , Namiranian, K , Amerian, R , Mansouri, S , Arshadi, S , and Azarbayjani, MA . The effect of curcumin supplementation and aerobic training on anthropometric indices, serum lipid profiles, C-reactive protein and insulin resistance in overweight women: a randomized, double-blind, placebo-controlled trial. J Obes Metab Syndr. (2020) 29:47–57. doi: 10.7570/jomes19055
63. Javandoost, A , Afshari, A , Saberi-Karimian, M , Sahebkar, A , Safarian, H , Moammeri, M, et al. The effects of curcumin and a modified curcumin formulation on serum Cholesteryl Ester transfer protein concentrations in patients with metabolic syndrome: a randomized, placebo-controlled clinical trial. Avicenna J Phytomed. (2018) 8:330–7.
64. Karandish, M , Mozaffari-khosravi, H , Mohammadi, SM , Cheraghian, B , and Azhdari, M . The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: a phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother Res. (2021) 35:4377–87. doi: 10.1002/ptr.7136
65. Mohammadi, A , Sahebkar, A , Iranshahi, M , Amini, M , Khojasteh, R , Ghayour-Mobarhan, M, et al. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: a randomized crossover trial. Phytother Res. (2013) 27:374–9. doi: 10.1002/ptr.4715
66. Yang, YS , Su, YF , Yang, HW , Lee, YH , Chou, JI , and Ueng, KC . Lipid-lowering effects of Curcumin in patients with metabolic syndrome: a randomized, double-blind, Placebo-Controlled Trial. Phytother Res. (2014) 28:1770–7. doi: 10.1002/ptr.5197
67. Abbott, KA , Burrows, TL , Acharya, S , Thota, RN , and Garg, ML . DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostaglandins Leukot Essent Fatty Acids. (2020) 159:102154. doi: 10.1016/j.plefa.2020.102154
68. Baxheinrich, A , Stratmann, B , Lee-Barkey, YH , Tschoepe, D , and Wahrburg, U . Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of α-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br J Nutr. (2012) 108:682–91. doi: 10.1017/s0007114512002875
69. Browning, LM , Krebs, JD , Moore, CS , Mishra, GD , O'Connell, MA , and Jebb, SA . The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. (2007) 9:70–80. doi: 10.1111/j.1463-1326.2006.00576.x
70. de Luis, D , Domingo, JC , Izaola, O , Casanueva, FF , Bellido, D , and Sajoux, I . Effect of DHA supplementation in a very low-calorie ketogenic diet in the treatment of obesity: a randomized clinical trial. Endocrine. (2016) 54:111–22. doi: 10.1007/s12020-016-0964-z
71. DeFina, LF , Marcoux, LG , Devers, SM , Cleaver, JP , and Willis, BL . Effects of omega-3 supplementation in combination with diet and exercise on weight loss and body composition. Am J Clin Nutr. (2011) 93:455–62. doi: 10.3945/ajcn.110.002741
72. Gammelmark, A , Madsen, T , Varming, K , Lundbye-Christensen, S , and Schmidt, EB . Low-dose fish oil supplementation increases serum adiponectin without affecting inflammatory markers in overweight subjects. Nutr Res. (2012) 32:15–23. doi: 10.1016/j.nutres.2011.12.007
73. Jaacks, LM , Sher, S , Staercke, C , Porkert, M , Alexander, WR , Jones, DP, et al. Pilot randomized controlled trial of a Mediterranean diet or diet supplemented with fish oil, walnuts, and grape juice in overweight or obese US adults. BMC Nutr. (2018) 4:26. doi: 10.1186/s40795-018-0234-y
74. Kratz, M , Swarbrick, MM , Callahan, HS , Matthys, CC , Havel, PJ , and Weigle, DS . Effect of dietary n-3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr. (2008) 87:347–53. doi: 10.1093/ajcn/87.2.347
75. Munro, IA , and Garg, ML . Dietary supplementation with n-3 PUFA does not promote weight loss when combined with a very-low-energy diet. Br J Nutr. (2012) 108:1466–74. doi: 10.1017/s0007114511006817
76. Munro, IA , and Garg, ML . Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: a double-blinded randomised controlled trial. Food Funct. (2013) 4:650–8. doi: 10.1039/c3fo60038f
77. Neale, EP , Muhlhausler, B , Probst, YC , Batterham, MJ , Fernandez, F , and Tapsell, LC . Short-term effects of fish and fish oil consumption on total and high molecular weight adiponectin levels in overweight and obese adults. Metabolism. (2013) 62:651–60. doi: 10.1016/j.metabol.2012.10.014
78. Rajkumar, H , Mahmood, N , Kumar, M , Varikuti, SR , Challa, HR , and Myakala, SP . Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediat Inflamm. (2014) 2014:348959. doi: 10.1155/2014/348959
79. Rivas, E , Wooten, JS , Newmire, DE , and Ben-Ezra, V . Omega-3 fatty acid supplementation combined with acute aerobic exercise does not alter the improved post-exercise insulin response in normoglycemic, inactive and overweight men. Eur J Appl Physiol. (2016) 116:1255–65. doi: 10.1007/s00421-016-3387-x
80. Sjoberg, NJ , Milte, CM , Buckley, JD , Howe, PR , Coates, AM , and Saint, DA . Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br J Nutr. (2010) 103:243–8. doi: 10.1017/s000711450999153x
81. Anggeraini, AS , Massi, MN , Hamid, F , Ahmad, A , As'ad, S , and Bukhari, A . Effects of synbiotic supplement on body weight and fasting blood glucose levels in obesity: a randomized placebo-controlled trial. Ann Med Surg. (2021) 68:102548. doi: 10.1016/j.amsu.2021.102548
82. Eslamparast, T , Zamani, F , Hekmatdoost, A , Sharafkhah, M , Eghtesad, S , Malekzadeh, R, et al. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: a randomised, double-blind, placebo-controlled pilot study. Br J Nutr. (2014) 112:438–45. doi: 10.1017/s0007114514000919
83. Hadi, A , Sepandi, M , Marx, W , Moradi, S , and Parastouei, K . Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: a randomized clinical trial. Complement Ther Med. (2019) 47:102216. doi: 10.1016/j.ctim.2019.102216
84. Hess, AL , Benítez-Páez, A , Blædel, T , Larsen, LH , Iglesias, JR , Madera, C, et al. The effect of inulin and resistant maltodextrin on weight loss during energy restriction: a randomised, placebo-controlled, double-blinded intervention. Eur J Nutr. (2020) 59:2507–24. doi: 10.1007/s00394-019-02099-x
85. Rabiei, S , Hedayati, M , Rashidkhani, B , Saadat, N , and Shakerhossini, R . The effects of Synbiotic supplementation on body mass index, metabolic and inflammatory biomarkers, and appetite in patients with metabolic syndrome: a triple-blind randomized controlled trial. J Diet Suppl. (2019) 16:294–306. doi: 10.1080/19390211.2018.1455788
86. Rahayu, ES , Mariyatun, M , Putri Manurung, NE , Hasan, PN , Therdtatha, P , Mishima, R, et al. Effect of probiotic lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J Gastroenterol. (2021) 27:107–28. doi: 10.3748/wjg.v27.i1.107
87. Szulińska, M , Łoniewski, I , van Hemert, S , Sobieska, M , and Bogdański, P . Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and Cardiometabolic profile in obese postmenopausal women: a 12-week randomized clinical trial. Nutrients. (2018) 10:773. doi: 10.3390/nu10060773
88. Tripolt, NJ , Leber, B , Blattl, D , Eder, M , Wonisch, W , Scharnagl, H, et al. Short communication: effect of supplementation with lactobacillus casei Shirota on insulin sensitivity, β-cell function, and markers of endothelial function and inflammation in subjects with metabolic syndrome--a pilot study. J Dairy Sci. (2013) 96:89–95. doi: 10.3168/jds.2012-5863
89. Chacko, SA , Sul, J , Song, Y , Li, X , LeBlanc, J , You, Y, et al. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: a randomized, double-blind, controlled, crossover trial in overweight individuals. Am J Clin Nutr. (2011) 93:463–73. doi: 10.3945/ajcn.110.002949
90. Joris, PJ , Plat, J , Bakker, SJ , and Mensink, RP . Long-term magnesium supplementation improves arterial stiffness in overweight and obese adults: results of a randomized, double-blind, placebo-controlled intervention trial. Am J Clin Nutr. (2016) 103:1260–6. doi: 10.3945/ajcn.116.131466
91. Joris, PJ , Plat, J , Bakker, SJ , and Mensink, RP . Effects of long-term magnesium supplementation on endothelial function and cardiometabolic risk markers: a randomized controlled trial in overweight/obese adults. Sci Rep. (2017) 7:106. doi: 10.1038/s41598-017-00205-9
92. Lee, S , Park, HK , Son, SP , Lee, CW , Kim, IJ , and Kim, HJ . Effects of oral magnesium supplementation on insulin sensitivity and blood pressure in normo-magnesemic nondiabetic overweight Korean adults. Nutr Metab Cardiovasc Dis. (2009) 19:781–8. doi: 10.1016/j.numecd.2009.01.002
93. Mooren, FC , Krüger, K , Völker, K , Golf, SW , Wadepuhl, M , and Kraus, A . Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects—a double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab. (2011) 13:281–4. doi: 10.1111/j.1463-1326.2010.01332.x
94. Rodríguez-Moran, M , and Guerrero-Romero, F . Oral magnesium supplementation improves the metabolic profile of metabolically obese, normal-weight individuals: a randomized double-blind placebo-controlled trial. Arch Med Res. (2014) 45:388–93. doi: 10.1016/j.arcmed.2014.05.003
95. Solati, M , Kazemi, L , Shahabi Majd, N , Keshavarz, M , Pouladian, N , and Soltani, N . Oral herbal supplement containing magnesium sulfate improve metabolic control and insulin resistance in non-diabetic overweight patients: a randomized double blind clinical trial. Med J Islam Repub. Iran. (2019) 33:463–73. doi: 10.34171/mjiri.33.2
96. Huerta, AE , Prieto-Hontoria, PL , Fernández-Galilea, M , Sáinz, N , Cuervo, M , Martínez, JA, et al. Circulating irisin and glucose metabolism in overweight/obese women: effects of α-lipoic acid and eicosapentaenoic acid. J Physiol Biochem. (2015) 71:547–58. doi: 10.1007/s13105-015-0400-5
97. Zarezadeh, M , Musazadeh, V , Faghfouri, AH , Sarmadi, B , Jamilian, P , Jamilian, P, et al. Probiotic therapy, a novel and efficient adjuvant approach to improve glycemic status: an umbrella meta-analysis. Pharmacol Res. (2022) 183:106397. doi: 10.1016/j.phrs.2022.106397
98. Najafi, N , Mehri, S , Ghasemzadeh Rahbardar, M , and Hosseinzadeh, H . Effects of alpha lipoic acid on metabolic syndrome: a comprehensive review. Phytother Res. (2022) 36:2300–23. doi: 10.1002/ptr.7406
99. Ahn, E , and Kang, H . Concepts and emerging issues of network meta-analysis. Korean J Anesthesiol. (2021) 74:371–82. doi: 10.4097/kja.21358
100. Suzumura, C, EA , Bersch-Ferreira, A , Torreglosa, CR , da Silva, JT , Coqueiro, AY , Kuntz, MGF, et al. Cavalcanti: effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: a systematic review and meta-analyses of randomized trials. Nutr Rev. (2019) 77:430–50. doi: 10.1093/nutrit/nuz001
101. Million, M , Angelakis, E , Paul, M , Armougom, F , Leibovici, L , and Raoult, D . Comparative meta-analysis of the effect of lactobacillus species on weight gain in humans and animals. Microb Pathog. (2012) 53:100–8. doi: 10.1016/j.micpath.2012.05.007
102. Gibbons, SM , Gurry, T , Lampe, JW , Chakrabarti, A , Dam, V , Everard, A, et al. Perspective: leveraging the gut microbiota to predict personalized responses to dietary, prebiotic, and probiotic interventions. Adv Nutr. (2022) 13:1450–61. doi: 10.1093/advances/nmac075
103. Wang, C , Zhang, C , Li, S , Yu, L , Tian, F , Zhao, J, et al. Effects of probiotic supplementation on dyslipidemia in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Foods. (2020) 9:1540. doi: 10.3390/foods9111540
104. Kim, CH . Microbiota or short-chain fatty acids: which regulates diabetes? Cell Mol Immunol. (2018) 15:88–91. doi: 10.1038/cmi.2017.57
105. Zhang, J , Wang, S , Zeng, Z , Qin, Y , Shen, Q , and Li, P . Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J Funct Foods. (2020) 67:103843. doi: 10.1016/j.jff.2020.103843
106. Salehi, B , Berkay Yılmaz, Y , Antika, G , Boyunegmez Tumer, T , Fawzi Mahomoodally, M , Lobine, D, et al. Insights on the use of α-Lipoic acid for therapeutic purposes. Biomol Ther. (2019) 9:356. doi: 10.3390/biom9080356
107. Butler, JA , Hagen, TM , and Moreau, R . Lipoic acid improves hypertriglyceridemia by stimulating triacylglycerol clearance and downregulating liver triacylglycerol secretion. Arch Biochem Biophys. (2009) 485:63–71. doi: 10.1016/j.abb.2009.01.024
108. Lee, WJ , Song, KH , Koh, EH , Won, JC , Kim, HS , Park, HS, et al. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. (2005) 332:885–91. doi: 10.1016/j.bbrc.2005.05.035
109. Jiang, X , Peng, M , Chen, S , Wu, S , and Zhang, W . Vitamin D deficiency is associated with dyslipidemia: a cross-sectional study in 3788 subjects. Curr Med Res Opin. (2019) 35:1059–63. doi: 10.1080/03007995.2018.1552849
110. Jorde, R , Sneve, M , Torjesen, P , and Figenschau, Y . No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. (2010) 267:462–72. doi: 10.1111/j.1365-2796.2009.02181.x
111. Ramiro-Lozano, JM , and Calvo-Romero, JM . Effects on lipid profile of supplementation with vitamin D in type 2 diabetic patients with vitamin D deficiency. Ther Adv Endocrinol Metab. (2015) 6:245–8. doi: 10.1177/2042018815599874
112. Jones, PJ , Senanayake, VK , Pu, S , Jenkins, DJ , Connelly, PW , Lamarche, B, et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. (2014) 100:88–97. doi: 10.3945/ajcn.113.081133
113. Venturini, D , Simão, AN , Urbano, MR , and Dichi, I . Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition. (2015) 31:834–40. doi: 10.1016/j.nut.2014.12.016
114. Chang, WL , Azlan, A , Noor, SM , Ismail, IZ , and Loh, SP . Short-term intake of Yellowstripe Scad versus Salmon did not induce similar effects on lipid profile and inflammatory markers among healthy overweight adults despite their comparable EPA plus DHA content. Nutrients. (2021) 13:3524. doi: 10.3390/nu13103524
115. Badimon, L . New trials in the scene of cardiovascular disease: innovation, controversy, and reassurance. Cardiovasc Res. (2021) 117:e52–4. doi: 10.1093/cvr/cvab048
116. Fleming, JA , and Kris-Etherton, PM . The evidence for α-linolenic acid and cardiovascular disease benefits: comparisons with eicosapentaenoic acid and docosahexaenoic acid. Adv Nutr. (2014) 5:863s–76s. doi: 10.3945/an.114.005850
117. Askari, M , Mozaffari, H , Jafari, A , Ghanbari, M , and Darooghegi Mofrad, M . The effects of magnesium supplementation on obesity measures in adults: a systematic review and dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2021) 61:2921–37. doi: 10.1080/10408398.2020.1790498
Keywords: obesity, nutritional supplement, cardiovascular risk factors, body composition, probiotics, Bayesian network meta-analysis
Citation: Yu Z, Zhao D and Liu X (2023) Nutritional supplements improve cardiovascular risk factors in overweight and obese patients: A Bayesian network meta-analysis. Front. Nutr. 10:1140019. doi: 10.3389/fnut.2023.1140019
Received: 12 January 2023; Accepted: 09 March 2023;
Published: 30 March 2023.
Edited by:
Claire Tourny, Université de Rouen, FranceReviewed by:
William Ben Gunawan, Diponegoro University, IndonesiaCopyright © 2023 Yu, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxin Liu, bGl1eHhAenp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.