- 1Department of Orthopaedic Surgery, Third Xiangya Hospital of Central South University, Changsha, China
- 2Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, United States
Purpose: To conduct a solid evidence by synthesizing meta-analyses and updated RCTs about the effects of vitamin D on all-cause mortality in different health conditions.
Methods: Data sources: Pubmed, Embase, Web of Science, the Cochrane Library, Google Scholar from inception until 25th April, 2022. Study selection: English-language, meta-analyses and updated RCTs assessing the relationships between vitamin D and all-cause mortality. Data synthesis: Information of study characteristics, mortality, supplementation were extracted, estimating with fixed-effects model. A Measurement Tool to Assess Systematic Reviews, Grading of Recommendations Assessment, Development and Evaluation, and funnel plot was used to assess risk of bias. Main outcomes: All-cause mortality, cancer mortality, cardiovascular disease mortality.
Results: In total of 27 meta-analyses and 19 updated RCTs were selected, with a total of 116 RCTs and 149, 865 participants. Evidence confirms that vitamin D reduces respiratory cancer mortality (RR, 0.56 [95%CI, 0.33 to 0.96]). All-cause mortality is decreased in patients with COVID-19 (RR, 0.54[95%CI, 0.33 to 0.88]) and liver diseases (RR, 0.64 [95%CI, 0.50 to 0.81]), especially in liver cirrhosis (RR, 0.63 [95%CI, 0.50 to 0.81]). As for other health conditions, such as the general health, chronic kidney disease, critical illness, cardiovascular diseases, musculoskeletal diseases, sepsis, type 2 diabetes, no significant association was found between vitamin D and all-cause mortality.
Conclusions: Vitamin D may reduce respiratory cancer mortality in respiratory cancer patients and all-cause mortality in COVID-19 and liver disorders' patients. No benefits showed in all-cause mortality after vitamin D intervention among other health conditions. The hypothesis of reduced mortality with vitamin D still requires exploration.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=252921, identifier: CRD42021252921.
1. Introduction
The relationship between vitamin D and mortality has become a spotlight topic of increasing research interest, evidenced by the ever-expanding body of scientific literature on the topic at present (1). This growing interest is likely due to the high prevalence of vitamin D deficiency in patients with numerous life-threatening diseases, such as COVID-19 and cancer (2), which may greatly affect the mortality rate. As a result, vitamin D supplementation has emerged as a potential treatment to reduce mortality. However, blindly taking high-doses of vitamin D without any scientific guidance has become an universal phenomenon in popular health. For example, in the U.S., the average daily intake of vitamin D has reached 100 mcg (4, 000 IU) or more for an adult (3). This pattern of use is not only causing increased healthcare costs, but having an unknown impact on its clinical effectiveness or even all-cause mortality (4). Numerous researches have been carried out to uncover the exact relationship between vitamin D and all-cause mortality in order to guide the scientific use of vitamin D.
In 1999, Bostick et al. conducted a research about the relationship between vitamin D intake and ischemic heart disease mortality, and presented a non-significant result (5). More recently, Zittermann et al. pointed out that vitamin D deficiency is associated with excess mortality explicitly, and advocated for the urgent need to clarify the correlation between vitamin D and survival in specific patient populations in 2009 (6). Since then, several landmark randomized controlled trials (RCTs) and meta-analyses assessing the effect of vitamin D on mortality have been published. However at the same time, it was from this growing body of literature that much controversy over the utility of vitamin D began to emerge. The first notable research controversy arose in 2014, where among countless studies, a conclusion that vitamin D may reduce all-cause mortality was clearly proposed by Bjelakovic et al. (7) and Bolland et al. (8). This result was widely accepted at first, but was vehemently questioned by studies published in 2019. Zhang et al. definitively suggested that no statistically significant difference was observed between vitamin D and placebo groups in both all-cause mortality and cardiovascular diseases (CVD)-related mortality. However, there did exist a significant discrepancy between these two groups for cancer-related mortality (9). Surprisingly, these conclusions regarding the effect of vitamin D on cancer-related mortality were overturned again with a non-significant conclusion in 2022 (10). To date, these fierce debates are still ongoing and unsettled. Due to the jagged quality of the evidence, the controversy related to this topic has been difficult to clarify.
Within this context, an umbrella review of prior meta-analyses and systematic reviews of RCTs may fill this gap and obliterate some of the controversy in previously published studies. An umbrella review is a popular method for systematically assessing evidence from multiple sources and delivering the highest level of evidence, due to the minimizing bias and outstanding breadth and validity of such a study design (2, 11, 12). Whether vitamin D can reduce all-cause mortality is unclear. Thus, we sought to conduct a comprehensive umbrella review of existing meta-analyses of RCTs, in order to generate an evidence map for the effects of vitamin D on all-cause mortality in different populations. It was hypothesized that vitamin D intake may only reduce mortality in populations with specific health conditions.
2. Methods
2.1. Protocol, registration, and study design
An umbrella review was conducted to estimate the effects of vitamin D on all-cause mortality in populations with different health conditions, with a comprehensive evidence collection and critical evaluation performed on the existing body of literature of meta-analyses exploring this topic (13). This review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14). The pre-specified protocol was registered 3rd June 2021 to PROSPERO prior to conducting this review (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=252921; Registration number: CRD42021252921).
2.2. Data sources and search strategy
Pubmed, Embase, Web of Science, the Cochrane Library, Google Scholar were queried using the following search terms: “Vitamin D” + “Mortality” + Patient” + “Meta-analy*” OR “Metaanaly*” OR “Systematic review.” From these search results, we extracted meta-analyses published in English involving human patients exploring the relationship between vitamin D and mortality from inception through 25th April 2022. In addition, eligible RCTs were also screened. We excluded the RCTs already involved in the existed meta-analyses, rest of which were identified as additional updated RCTs from the search beginning until 25th April 2022. Additional sources included bibliographies of correlative references and studies.
2.3. Study selection and eligibility criteria
The predetermined eligibility criteria were systematic reviews, meta-analyses of RCTs and updated RCTs in recency assessing the efficacy of vitamin D on mortality or death outcomes of interest in patients with different diseases, which were all written in English. We included the above three types of studies regardless of the form, dosage, intaking methods of vitamin D, the baseline characteristics (clinical setting, age, sex, or race) of the examined population and the date of publication.
More specifically, systematic reviews and meta-analyses of observational cohort studies, narrative reviews or those reporting effects of vitamin D on other outcomes [e.g., vitamin D receptor (VDR), vitamin D metabolism gene polymorphisms or multiple interventions] were excluded. Letters, editorials, and articles published in languages other than English were also excluded. Composite systematic reviews or meta-analyses of RCTs and observational studies were reviewed only for data related to RCTs. For some systematic reviews and meta-analyses with overlapping or redundant interventions and outcomes; the most recent, largest, and updated study was included, unless there were concerns with quality of the study's design. Existing umbrella reviews were excluded, but were reviewed for any systematic reviews or meta-analyses not captured in the initial literature search.
Systematic reviews, meta-analyses and recent RCTs catering to pre-defined search strategies and inclusion criteria were conducted by two authors (MC, CH) under the supervision of another author (JH). Study selection was performed in a four-stage process. Firstly, duplications were removed by viewing titles, publication years, and authors' name. Secondly, titles and abstracts of these potentially eligible articles were examined according to predetermined eligibility criteria. Thirdly, full texts were screened and assessed for eligibility. Lastly, the data extraction and quality assessment was conducted. Disagreements and discrepancies were resolved by discussion between two authors (MC, JH). Study selection was conducted and recorded according to the PRISMA protocol (15).
2.4. Data extraction and outcomes
Our method for data extraction included recording data related to the first author's name, year of publication, number of studies included (by study design), participants information (such as the age, sex, disease), the intervention (such as dose, follow-up time), the comparison (such as placebo implement), the outcomes or effect sizes (such as mortality, morbidity, sample sizes, variables), and the quality (such as the reliability, bias assessment, heterogeneity).
Detailed information was extracted and presented in the order noted above. The pool of clinical trials was then reviewed by identifying trials contained in the eligible meta-analyses and trials published after which were screened. Finally, we organized the data collected above by removing duplicates and categorized them by different patient populations.
The main outcome of interest was all-cause mortality in different patient specific populations. The secondary outcomes were CVD mortality in patients with CVD, chronic kidney disease (CKD), type 2 diabetes (T2DM) and liver diseases, and cancer mortality in the cancer population.
2.5. Quality assessment
The methodological quality of each eligible meta-analysis and systematic review was assessed using A Measurement Tool to Assess Systematic Reviews (AMSTAR), a widely utilized instrument with high reliability, validity and practicality. On the basis of representative assessment tools with reference value, empirical evidence formed in the process of long-term use and expert consensus, AMSTAR has been the first priority of methodological quality assessment for systematic reviews and meta-analyses when conducting umbrella reviews (16). We applied 11 items to identify the methodological quality of each included meta-analysis, with each item ranked with “yes”, “no”, “unclear”, and “partially yes”. Under the assignment of “yes” = 1, “no” = 0, “unclear” = 0, and “partially yes” = 0, the meta-analyses can be divided into “high quality” (9~11), “moderate quality” (5~8), and “low quality” (0~4).
We assessed the evidence certainty of included meta-analyses and systematic reviews with Grading of Recommendations Assessment, Development and Evaluation (GRADE), a tool without the stereotyped restriction of study types, presenting the evidence certainty and assessment considerable objectivity and reliability. The influence factors can be categorized into “study limitation”, “indirectness”, “inconsistency”, “imprecision”, and “publication bias”. Eligible meta-analyses and systematic reviews were classified as “high”, “moderate”, “low”, and “very low” (17).
As for the included updated RCTs not contained within the available meta-analyses and systematic reviews, the Cochrane Risk of Bias (Cochrane ROB) was applied to evaluate evidence reliability. Updated RCTs were categorized as “low risk”, “unclear”, and “high risk” under 7 aspects of bias assessment.
Two authors (MC, JH) independently applied AMSTAR, GARDE, and Cochrane ROB for the quality assessment and made a consensus when studies with discordance were encountered.
2.6. Data synthesis and statistical analysis
We created an evidence map presenting the certainty of prior evidence for the effect of vitamin D on all-cause mortality in different diseases via Microsoft Excel 2016. We use the area representing the quality of the included studies in each section, which was evaluated using AMSTAR, GRADE, and The Cochrane ROB.
We implemented a fixed effect model for estimation, and heterogeneity was assessed by I2 statistics. If I2 is >50%, it suggested a high level of heterogeneity, in which we transitioned to implementing a random effect model for a more objective estimate; in order to draw an overall more precise conclusion. We also assessed possible sources of heterogeneity across studies by using subgroup analyses in order to reduce publication bias.
Statistical analyses were conducted using Review Manager (RevMan), version5.4, The Cochrane Collaboration, 2020. The effect measure was risk ratio for the outcome. Significant level was set at 0.05 for all analyses, while which was adjusted to 0.10 when ongoing the Egger regression test for its limited statistical power. Estimates of publication bias were considered using funnel plots if there were more than 10 included studies.
3. Results
3.1. Search results
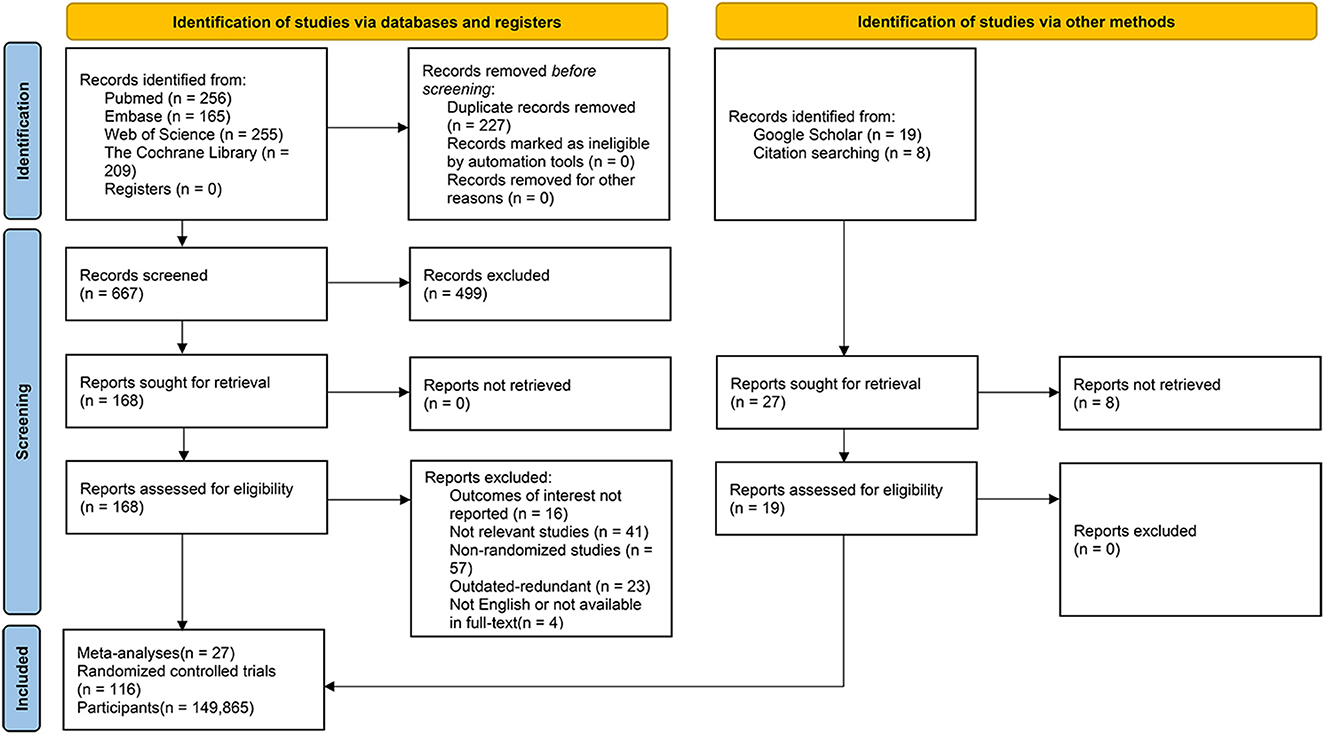
We identified 885 records from database searches. After removing overlapping studies and screening at the title and abstract level, a total of 168 articles were remained for full-text review. Of these 168 studies, 141 articles were excluded, including 16 studies which did not update their outcomes of interest, 41 reviews focused on non-relevant themes, 57 meta-analyses which were composed of non-randomized studies or irrelevant study types (e.g., umbrella review, narrative review, cross-sectional, observational cohort studies et al.), 23 studies which were outdated and redundant (we included the most recent iterations of such studies), as well as 4 articles with language issues in full-text reading or the full-text was not readily accessible. Overall, a total of 27 (7–9, 18–41) meta-analyses were pooled in this umbrella review and 19 updated RCTs newly published were added, with the inclusion of a total of 116 RCTs (10, 42–156) overall comprising 149, 865 participants altogether (Figure 1).
The baseline characteristics of the included RCTs and meta-analyses/systematic reviews in this umbrella review were presented in Supplementary Tables 1, 2.
3.2. Quality assessment
Methodological assessment noted that among the 27 eligible meta-analyses and systematic reviews included, 16 ranked “high” level, 9 ranked “moderate” level, 2 evaluated with “low” methodological level (Detailed information of AMSTAR assessment listed in Supplementary Table 3). According to the results of GRADE, the evidence certainty in this umbrella review was classified as 13 “high”, 7 “moderate”, 4 “low” and 3 “very low” (Detailed information of GRADE assessment was listed in Supplementary Table 4). The Cochrane ROB assessment and detailed information of updated RCTs is listed in Supplementary Table 5 and Supplementary Figures 1, 2. Results for the heterogeneity test performed are presented in Supplementary Figures 3–8.
3.3. General population
Forty-three citations (10, 42–82) were assessed for the risk of all-cause mortality in general population with vitamin D intervention. Publication dates ranged from 1983 to 2022. The general finding reports that there is no statistically significant difference between the effects of vitamin D and placebo on all-cause mortality in general population (RR, 0.99[95%CI, 0.96 to 1.03]). Subgroup analyses among female, male, and menopausal women populations indicate the same non-significant result. Results were quite consistent among the 43 RCTs, only two reported a harmful effect of vitamin D on all-cause mortality in general population compared with placebo (63, 64) (RR, 3.70[95%CI, 1.06 to 12.92], 1.20[95%CI, 1.04 to 1.37]), while others presented non-significant results (Supplementary Figure 9).
3.4. Cancer population
3.4.1. All-cause mortality in cancer population
Nine retrieved RCTs (61, 68, 79, 83–88) were assessed the risk for all-cause mortality in cancer population with vitamin D intervention. Publication dates ranged from 2003 to 2019. Results presents that no significant effects were seen for all-cause mortality in cancer patients treated with vitamin D (RR, 0.93[95%CI, 0.83 to 1.05]). Out of the 9 RCTs, one revealed that vitamin D may increase the all-cause mortality in prostate cancer patients (87) (RR, 1.25[95%CI, 1.04 to 1.50]), while the study lead by Avenell reported an opposite result (84) (RR, 0.78[95%CI, 0.62 to 0.97]) (Supplementary Figure 10).
3.4.2. Cancer mortality in cancer population
Nine retrieved articles (61, 68, 79, 83–88) were assessed for the risk of cancer mortality in the cancer patient population with vitamin D supplementation. Publication dates ranged from 2003 to 2019. Results shows non-significant difference in cancer mortality between cancer patients treated with vitamin D and placebo (RR, 0.91[95%CI, 0.80 to 1.03]). However, subgroup analyses imply that supplementation of vitamin D was associated with decreased risk of respiratory tract cancer mortality (RR, 0.56[95%CI, 0.33 to 0.96]), while subgroup analyses among prostate cancer and digestive tract cancer patients presented non-significant results (Figure 2).
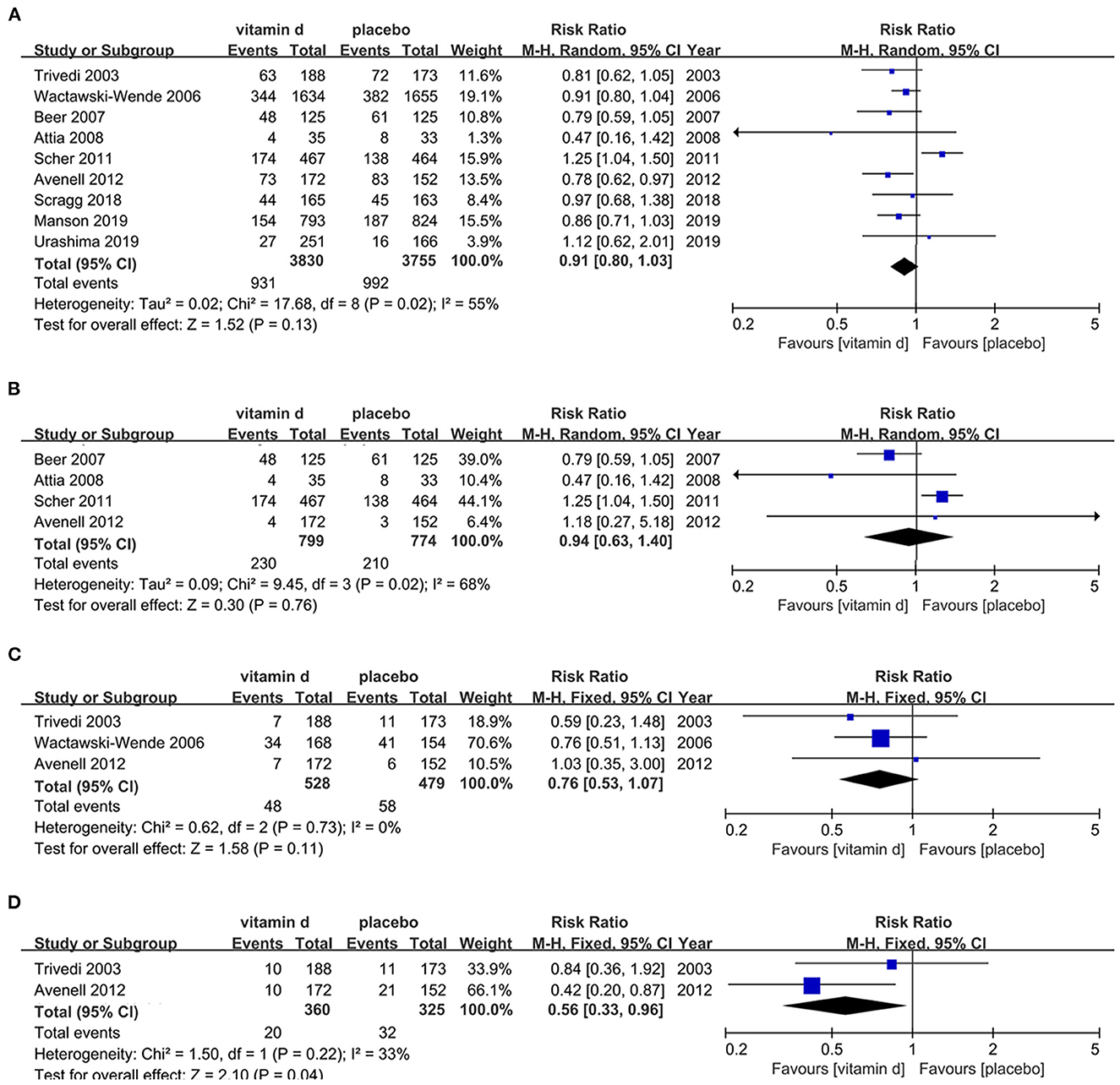
Figure 2. Effects of vitamin D on cancer mortality in cancer population. (A) Forest plot showing effects of vitamin D on cancer mortality in all kinds of cancer population. (B) Subgroup analysis on prostate cancer mortality in prostate cancer population. (C) Subgroup analysis on digestive cancer mortality in digestive cancer population. (D) Subgroup analysis on respiratory cancer mortality in respiratory cancer population.
3.5. CKD population
3.5.1. All-cause mortality in CKD population
Twenty-four RCTs (89–112) were cited to estimate the risk for all-cause mortality in CKD population with vitamin D supplement. Publication dates ranged from 1981 to 2021. No statistically significant discrepancy was found in all-cause mortality between vitamin D and placebo supplement, no matter in all stage CKD population (RR, 1.10[95%CI, 0.89 to 1.34]) nor late-stage patients (dialysis-dependent patients) (RR, 1.09[95%CI, 0.88 to 1.34]) (Supplementary Figures 11A, B).
3.5.2. CVD mortality in CKD population
Nine retrieved (92, 95–98, 101, 103, 107, 109) studies were evaluated for risk of CVD mortality in CKD population treated with vitamin D. Publication dates ranged from 1995 to 2015. All included citations noted common conclusions, that no differences were shown in this outcome regardless of the stage of CKD (all stage CKD population: RR, 1.20[95%CI, 0.52 to 2.75]; dialysis population: RR, 0.80[95%CI, 0.39 to 1.65]) (Supplementary Figures 11C, D).
3.6. Respiratory disease population
A total of 17 RCTs (113–129) were estimated for the risk of all-cause mortality in respiratory diseases patients with vitamin D supplementation. Publication dates ranged from 2009 to 2021. The analyze results shows that there's no statistically significant difference between vitamin D and placebo among respiratory diseases population (RR, 0.88[95%CI, 0.70 to 1.13]). However, subgroup analysis indicates that vitamin D may reduce the all-cause mortality among COVID-19 patients (RR, 0.54[95%CI, 0.33 to 0.88]). Other respiratory diseases including pulmonary tuberculosis (PTB), chronic obstructive pulmonary diseases (COPD) and pneumonia, subgroups analyses all present irrelevant results (Figure 3).
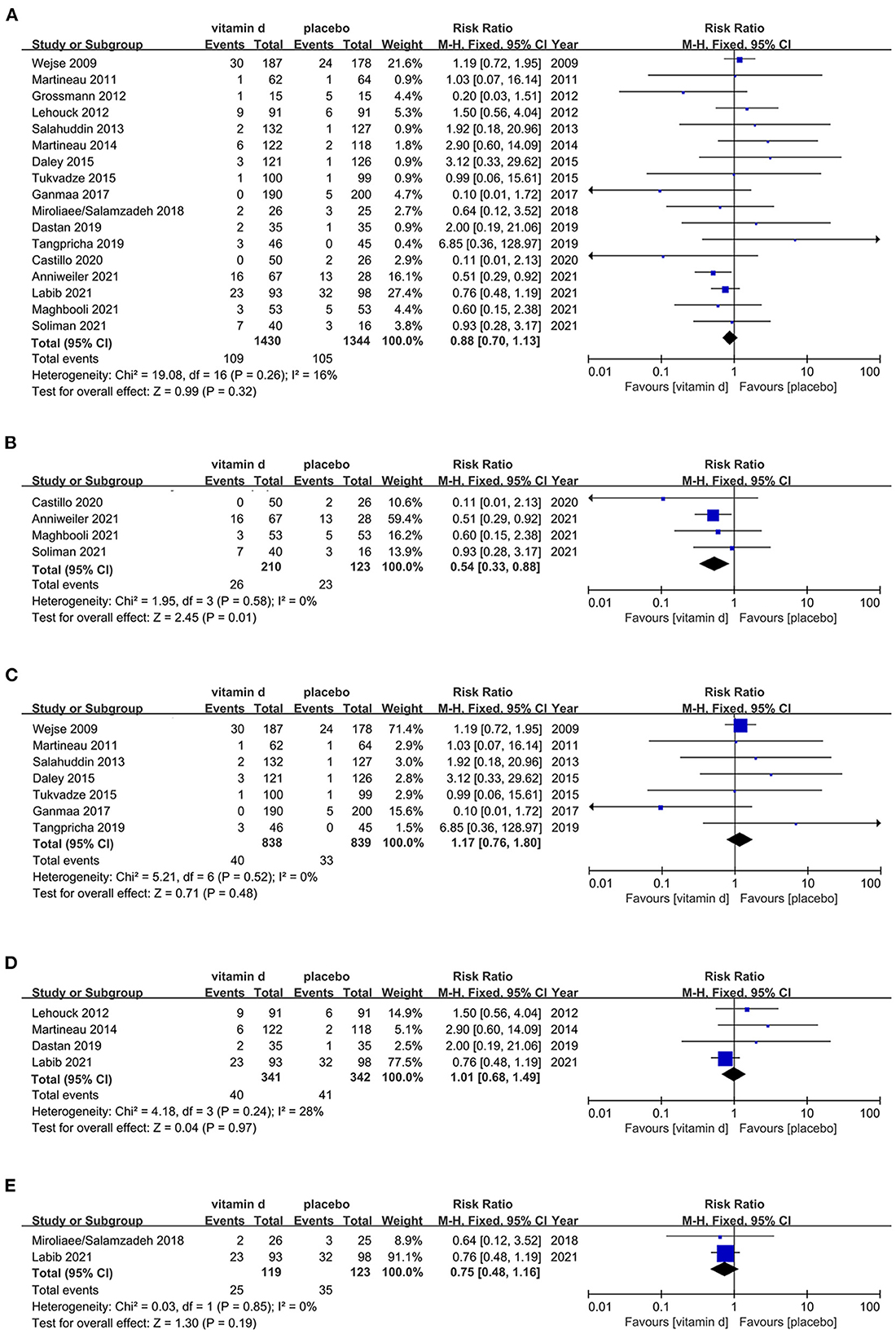
Figure 3. Effects of vitamin D on all-cause mortality in respiratory disease population. (A) Forest plot showing effects of vitamin D on all-cause mortality in all kinds of respiratory diseases population. (B) Subgroup analysis on all-cause mortality in COVID-19 population. (C) Subgroup analysis on all-cause mortality in PTB population. (D) Subgroup analysis on all-cause mortality in COPD population. (E) Subgroup analysis on all-cause mortality in pneumonia population.
3.7. Critically ill population
A total of 13 studies (114, 124, 130–140) were retrieved. Publication dates ranged from 2014 to 2020. No significant associations was observed about the risk of all-cause mortality in critically ill patients with vitamin D intervention (RR, 0.93[95%CI, 0.81 to 1.07]). Furthermore, according to the subgroup analyses, regardless of the follow-up time (7d, 30d, 90d, 180d) nor the severity of the illness (ICU stay, hospitalization), the supplement of vitamin D has nothing to do with affecting all-cause mortality in critically ill patients (Supplementary Figure 12).
3.8. CVD population
3.8.1. All-cause mortality in CVD population
In this part of the review, which was based on 8 RCTs (68, 78, 79, 108, 141–144), vitamin D did not demonstrate any clinically significant differences in all-cause mortality in CVD patients compared with placebo (RR, 1.03[95%CI, 0.91 to 1.18]). Publication dates ranged from 2003 to 2019 (Supplementary Figure 13A).
3.8.2. CVD mortality in CVD population
Five studies (68, 78, 79, 108, 144) were assessed in this section. Publication dates ranged from 2003 to 2019. No significant association between vitamin D supplementation and CVD mortality in CVD population was reported among the 5 included RCTs (RR, 1.03[95%CI, 0.90 to 1.18]) (Supplementary Figure 13B).
3.9. Musculoskeletal disease population
Six citations (84, 145–149) were evaluated the risk for all-cause mortality in musculoskeletal disease patient population with vitamin D supplementation. Publication dates ranged from 1973 to 2016. No clinically significant relevance was demonstrated in this outcome (RR, 0.97[95%CI, 0.87 to 1.08]). The subgroups of osteoarthritis (OA), rheumatoid arthritis (RA) and post-fracture operation also reported non-significant results (Supplementary Figure 14).
3.10. Sepsis population
Four identified RCTs (136, 139, 150, 151) has been tested for the effect of vitamin D on all-cause mortality in the sepsis patient population. Publication dates ranged from 2014 to 2021. On the basis of this analysis, there's no therapeutic nor harmful effect of vitamin D treatment on all-cause mortality in sepsis population (RR, 0.82[95%CI, 0.58 to 1.15]) (Supplementary Figure 15).
3.11. T2DM population
3.11.1. All-cause mortality in T2DM population
Three interventions (96, 152, 153) were evaluated the risk for all-cause mortality in T2DM population with vitamin D intervention. Publication dates ranged from 2010 to 2016. Result demonstrated that vitamin D intervention has no association with all-cause mortality in T2DM patients (RR, 0.80[95%CI, 0.21 to 2.97]) (Supplementary Figure 16A).
3.11.2. CVD mortality in T2DM population
Two studies (96, 153) evaluated the risk for CVD mortality in the T2DM population with vitamin D intervention. Publication dates ranged from 2010 to 2012. Both studies did not demonstrate any statistically significant findings for this outcome (RR, 1.00[95%CI, 0.14 to 7.09]) (Supplementary Figure 16B).
3.12. Liver disease population
3.12.1. All-cause mortality in liver disease population
Only 3 studies (154–156) matched our inclusion criteria and were assessed for the risk of all-cause mortality in liver diseases population with vitamin D supplementation. Publication dates ranged from 1984 to 2021. In this section, it implies that vitamin D supplement shows statistically significant reduction of all-cause mortality in liver disease patients (RR, 0.64[95%CI, 0.50 to 0.81]), which suggests that vitamin D might be beneficial in the liver disease patient population. Especially for cirrhotic patients, vitamin D may be therapeutic (RR, 0.63[95%CI, 0.50 to 0.81]), however this benefit may not be as applicable in hepatitis patients (Figures 4A, B).
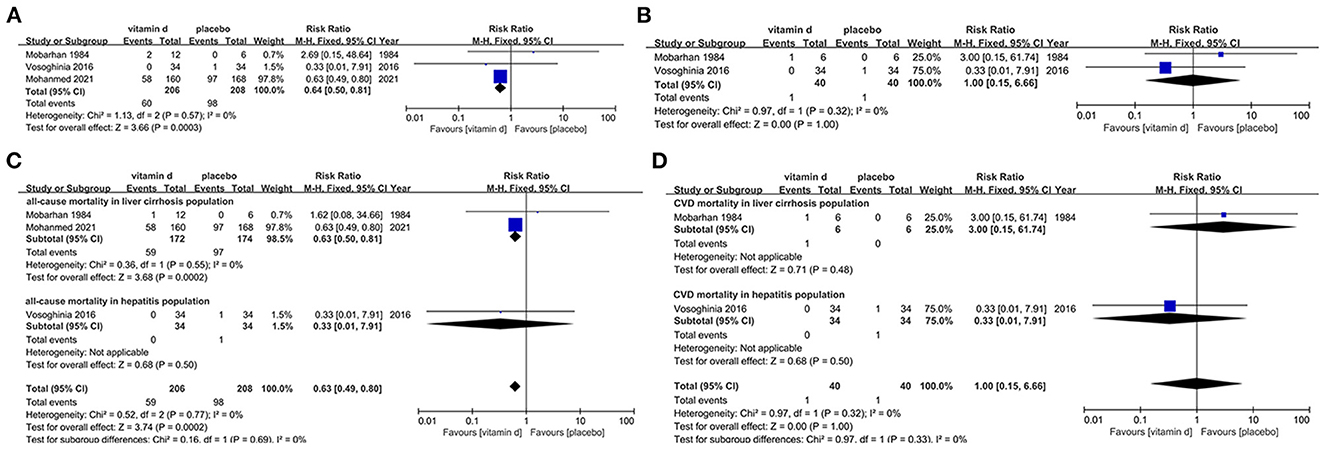
Figure 4. Effects of vitamin D on mortality in liver disease population. (A) Forest plot showing effects of vitamin D on all-cause mortality in all kinds of liver diseases population. (B) Forest plot showing effects of vitamin D on CVD mortality in all kinds of liver diseases population. (C) Subgroup analysis on all-cause mortality in liver cirrhosis and hepatitis population. (D) Subgroup analysis on CVD mortality in liver cirrhosis and hepatitis population.
3.12.2. CVD mortality in liver disease population
Two studies (154, 156) were evaluated for the risk of CVD mortality in the liver disease population with vitamin D intervention. According to the analysis result, vitamin D supplementation is not significantly associated with risk for this outcome regardless of the severity of the exacerbation of liver disease (RR, 1.00[95%CI, 0.15 to 6.66]) (Figures 4C, D).
3.13. Evidence map
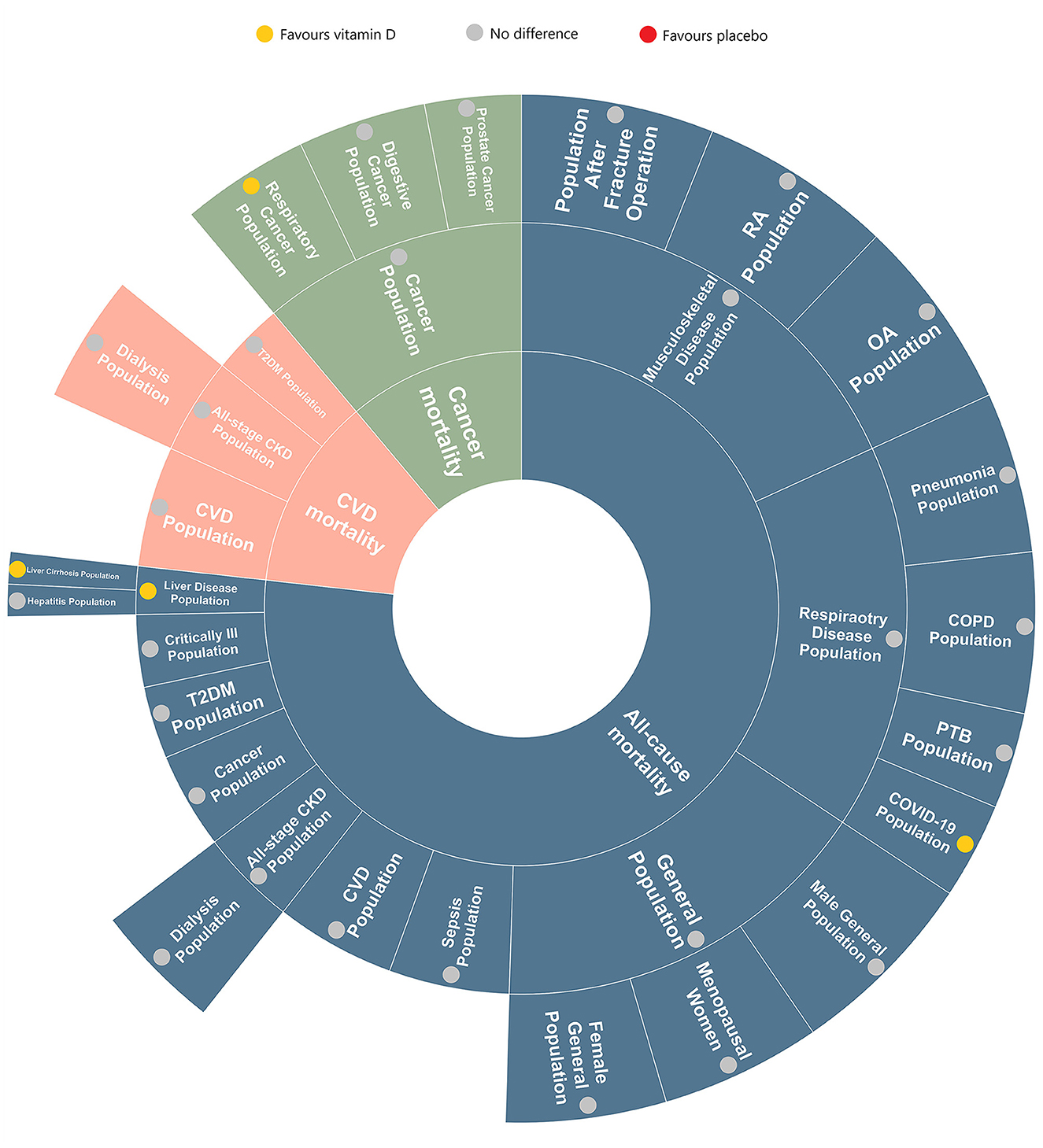
Figure 5 is an evidence map summarizing the effects of vitamin D on all-cause mortality in different diseases amongst the included RCTs in our review. The area of different section in this map represents the quality of the articles (based on the standardized mean scores of AMSTAR, GRADE, and The Cochrane ROB) related to the disease. For example, in the quality assessment, the standardized mean score of critically ill population is 3, while which in general population is 5, so the latter has a larger area than the former.
Most of the analyzed results implies that vitamin D has no beneficial nor harmful effect on all-cause mortality in certain populations. However, a portion of data shows that vitamin D plays a therapeutic role in reducing respiratory cancer mortality in respiratory cancer patients and all-cause mortality in COVID-19 and liver diseases population (especially for cirrhotic patients). Due to the limitation of available RCTs, there was a paucity of data evaluating the impact of vitamin D on all-cause mortality in the T2DM population, sepsis population and liver disease population, which remains to be discovered and corrected.
4. Discussion
Based on this evidence map and umbrella review of 27 meta-analyses and 116 RCTs with 149, 865 participants, we found that the effect of vitamin D on mortality varies depending on different health condition. Our analysis suggests that vitamin D may reduce respiratory cancer mortality in patients with respiratory cancer, as well as all-cause mortality in patients with COVID-19 or liver diseases (especially cirrhosis). However, vitamin D can't reduce cancer mortality in cancer patients or all-cause mortality in the populations with health conditions such as cancer, CKD, respiratory diseases (COVID-19, PTB, COPD, and pneumonia), critical illness, CVD, musculoskeletal diseases (OA, RA, and post-fracture surgery), sepsis, T2DM, and hepatitis.
Comparing our evidence with previous studies, some inconsistency did exist. On the one hand, our results shows that vitamin D has a therapeutic effect on mortality in cirrhotic, respiratory cancer and COVID-19 patients. However, there is still disagreement in the literature. In hepatic patients, Bjelakovic et al. (41) reported that vitamin D has non-significant effects on mortality in cirrhosis, which was questioned by Mohamed (155) with a therapeutic result, consistent with our finding. In cancer patients, divergent opinions are even greater. A reduced cancer mortality after vitamin D supplementation was reported by a meta-analysis basing on 74,655 participants in 2019 (9). However, a subsequent large-scale RCT study pointed out that higher cancer mortality may present after excluding the first 2 years of follow-up (10). What's more, since COVID-19 outbreak, the effectiveness of vitamin D as a treatment has been widely discussed. Four RCTs evaluated mortality with vitamin D supplementation, and only one flaw-designed RCT showed a weak benefit of vitamin D treatment on all-cause mortality in COVID-19 patients (114), which is consistent with our findings. The other RCTs reported similar results between vitamin D and placebo groups (114, 121, 126). On the other hand, we found no significant differences between vitamin D and placebo in the other population groups we studied. Most available studies support our perspective, but a 2014 meta-analysis on vitamin D supplementation's effect on all-cause mortality in the general population showed inconsistencies upon further review (7).
Reasons behind these discrepancies in vitamin D supplementation efficacy require further consideration. The top of which regards to follow-up time, which indicates the onset and duration of drug effectiveness. Some studies excluded the first 2 years of follow-up analysis, assuming that vitamin D might not have reached an adequate onset time in first 2 years (10, 68). On the contrary, other studies suggested that the effects of vitamin D may disappear within one or two years after the cessation of supplementation (84). These findings raise concerns about the accuracy and reliability of included trials in our review. Due to the chronic nature of many diseases in this review, a significant induction period may be required to capture outcomes (1). Insufficient follow-up time may only reveal changes in progression rather than final outcomes, leading to discrepancies in mortality that are difficult to analyze. Based on this situation, the null hypothesis is usually not rejected due to the high possibility of type 2 error Thus, different follow-up times may be one of the reasons of the inconsistency.
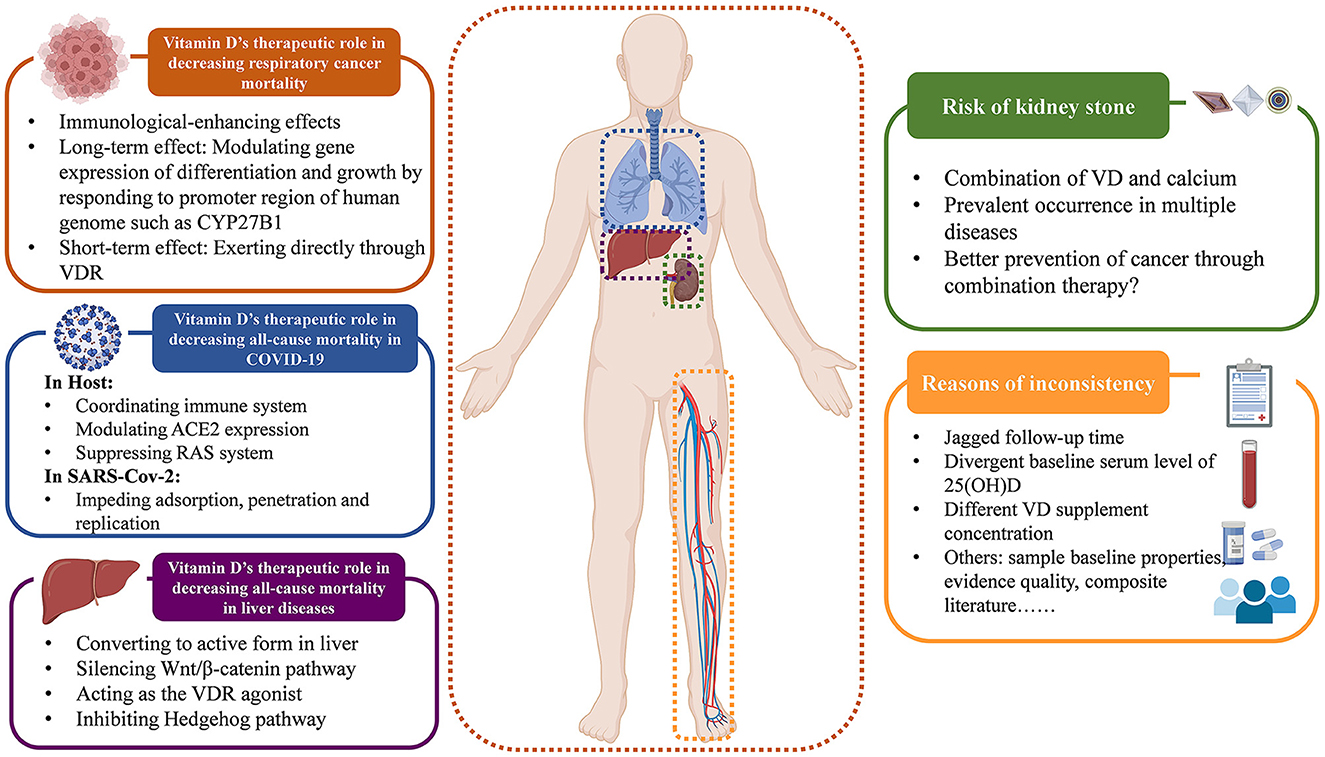
Moreover, the variation in baseline serum vitamin D level may be another reason of the inconsistency, which could affect appropriate supplementation doses and any potential impact on mortality. The baseline vitamin D level in the RCTs included in our review range from 128.7 ± 47.3 nmol/L (76) to 16.4 ± 5.2 nmol/L (140), with daily supplement doses varying from 600,000 IU (60) to 800IU (65). It's acknowledged that sun exposure promotes the cutaneous vitamin D3 formation (157), therefore different levels of sun exposure and different health conditions lead to varying vitamin D metabolic status, resulting in different serum 25(OH)D levels. Thus, different doses of vitamin D supplementation are required to achieve protective effects (158). Some investigators suggested that the protective vitamin D concentration is 75–110 nmol/L, to achieve such a level, about 1,800–4,000 IU per day is needed orally. That is to say, a low dose (< 800 IU/d) may not be sufficient for protection (77, 159). Nevertheless, high-dose vitamin D supplementation also requires prudent consideration. Some argued that high vitamin D concentrations may be harmful (160), while others suggested that only high doses have positive effects. Based on this, there is an urgent need to find the best dosage for optimal treatment outcomes. However, no study has identified the ideal vitamin D supplementation dose that caters to all health conditions. The pathological heterogeneity results in different recommended doses for different diseases. For respiratory infections, a daily supplementation of 400–1,000 IU is most effective (161), and osteoporosis patients are advised to take 800 IU/day (162), while primary hyperparathyroidism requires a higher dose of 2,800 IU/day due to the low serum calcium trait of PTH (163). Therefore, a universal agreement has been reached that the key to supplementing vitamin D is achieving optimal serum 25(OH)D levels, rather than chasing the best supplementary dose. The goal of vitamin D supplementation should be to raise abnormal serum levels back to normal, instead of simply providing a fixed dose. A serum vitamin D level of at least 20 ng/ml is generally considered ideal for most health conditions as recommended (164). However, the common intervention of RCTs is giving a fixed dose, instead of achieving a fixed level, which likely to result in a situation of sufficient vitamin D baseline supplemented with low dose of vitamin D (165), leading to inconsistent results. Additionally, the administration route of vitamin D is also a concern for its effects. The common ways of supplementing vitamin D are oral intake and intramuscular injection. Studies suggest that for general population, oral supplementation is better for loading, while the two routes seem equally effective for maintenance therapy (166). Thus oral administration may be a safer way to take supplementation. What's more, the relationship between serum vitamin D level and disease occurrence is complex. Some suggest that low levels of serum vitamin D may be the cause of various diseases, while others argue it as the consequence of disease. Amiel et al. (167) pointed out that vitamin D deficiency is tightly linked with the susceptibility of SARS-CoV-2, while Smolders et al. (168) alludes that decreased vitamin D status is just the consequence of systematic inflammation in COVID-19. Besides, Bolland et al. (8) presented the idea of “reverse causality”, that low serum vitamin D level is not a consequence of health problems, but rather a cause of various diseases occurring due to reduced sun exposure. However, this viewpoint has been opposed by others based on the theory of statistical type 2 error (169). In this case, vitamin D supplementation might be futile if the vitamin D deficiency is just a consequence of diseases instead of the cause. Based on our umbrella review and evidence map, it's clear that vitamin D supplementation remarkably reduces respiratory cancer mortality. Numerous large-scale observational studies also support the therapeutic superiority of vitamin D for cancer (170, 171). The mechanisms behind that require deep consideration. Many studies have shown that vitamin D has immune-enhancing effects, boosting the immune system by activating specific immune cells for resisting viruses, bacterium, and tumor cells. This theory supports our findings in cancer mortality. Current explanation mostly supports that vitamin D affects gene expression in two ways: long-term by binding to specific gene promoters and modulating protein expression related to cell differentiation and growth, such as CYP27B1 (172, 173), and short-term by directly affecting VDR without involving protein production through The Central Law (174, 175). Both mechanisms can modify the inflammatory response and alter the tumor microenvironment, resulting in an immunological effect (176). Research published in 2020 suggested that the gene regulation modulated by vitamin D is not only evident in patients, but also in tumor cells. Vitamin D may be involved in the re-programming and adhesion-modifying of tumor cells, and result in better evasion of immune surveillance (177, 178). This paradox needs more comprehensive studies to explain.
These mechanisms were also explored of the reduced all-cause mortality in liver diseases. Scientific f research has revealed a high prevalence of vitamin D deficiency in liver diseases, particularly cirrhosis, which significantly impacts the mortality of patients with hepatic disorders (179–181). The first hydroxylation of vitamin D occurs in the liver, which is crucial for its absorption and activation in the body. Therefore, cirrhosis-related complications like portal hypertension can hinder the conversion of vitamin D to its active form (179). What's more, patients with hepatic disorders often lack bile salts, which are necessary for absorbing fat-soluble vitamins in the gastrointestinal tract (181, 182). With the function of infection preventing, angiogenesis influencing, apoptosis modulating, and differentiation and proliferation affecting (179), vitamin D supplementation has therefore become a therapy to improve liver cirrhosis. It was widely acknowledged that β-catenin plays a crucial role in fibrogenesis. In 2018, it was confirmed that vitamin D can silence the Wnt1/β-catenin pathway, which suppresses the activation of hepatic stellate cells and reduces collagen fiber secretion, leading to the inhibition of type I/III collagen formation, thereby ameliorating the deterioration of liver cirrhosis (183). In addition, the polymorphism of VDR in immune cells and hepatocytes also presents a high correlation with liver cirrhosis, owing to the co-mediation of VDR and bile salts for vitamin D uptake (181, 184, 185). More importantly, supraphysiological concentrations of 25(OH)D3 resulting from artificial vitamin D supplementation can act as a VDR agonist (186), inhibiting Hedgehog (Hh) pathway, reducing the production of hepatitis C virus (HCV) largely, blocking an important predisposing factor for liver cirrhosis (187). Vitamin D is able to directly combine with smoothened released by the combination of Hh and patched, thus suppressing the transcription factor glioma-associated, impeding the replication of HCV effectively and improving sustained virologic responses in patients (188). These findings corroborate and cater to the result of our subgroup analysis on liver cirrhosis.
Based on our results, vitamin D has been shown to reduce all-cause mortality in SARS-CoV-2 infection. The available beneficial mechanisms of vitamin D supplementation can be broadly divided into the effects on hosts and effects on viruses. On the one hand, vitamin D coordinates hosts' immune system, the angiotensin converting enzyme 2 (ACE2) expression and the renin-angiotensin (RAS) system to resist SARS-CoV-2 (189, 190). Vitamin D can activate VDR on cell membranes to awake immune response (189). High levels of 25(OH)D3 combine with vitamin D-binding proteins in monocytes, facilitating its spread via the bloodstream and increasing the likelihood of binding to VDR. This induces autophagy, where autophagosomes act as antigen presenting stimulants and induce the adaptive immunity (191, 192). What's more, vitamin D also decreases ACE2 expression, effectively blocking COVID-19 entry (189). Moreover, vitamin D supplementation reduces damage to the host by decreasing RAS activity through inducing ACE2/Ang1-7 pathway (189, 193, 194). On the other hand, vitamin D can also hinder the adsorption, penetration and replication of SARS-CoV-2 by inducing the release of cathelicidin, defensins, and soluble ACE2 (195–199). Researchers also noted that the timing of the first vitamin D supplement is crucial for its effectiveness against COVID-19. If taken too late after symptoms appear, it may be ineffective in reducing virus viability and preventing organ damage caused by cytokine storms (200). Generally, observational studies and meta-analyses suggest that vitamin D may be beneficial for COVID-19 (201–203) and deficiency is commonly observed. However, with only a few trials conducted, our knowledge of vitamin D therapy for SARS-CoV-2 may just be the tip of the iceberg. More and better experimental investigations are urgently needed to guide the clinical treatment in the future.
Although our umbrella review found no negative effects of vitamin D on mortality, we did observe some adverse events during the intervention. One common adverse event was kidney stone occurrence, especially when vitamin D treatment was combined with calcium, which was not only occurred in CKD patients, but also reported in patients with cancer (28, 53, 61, 62, 152, 204–207), CVD (20, 208–210), and even respiratory diseases (211). However, some studies suggested that combining vitamin D and Ca2+ may offer greater protection against cancer than using vitamin D alone (212), but the potential benefits of this combination for preventing cancer and the risks of kidney stones are still uncertain. Rigorous trials are needed to provide solid evidence of its effectiveness in future clinical treatments.
We summarize the relevant opinions discussed above in a figure for easy reading in Figure 6.
Nevertheless, our review also has a certain number of limitations. The inherent secondary limitations of the included articles is one of the shortcomings that cannot be avoided. Some meta-analyses with a small range of trials leave the quality uncertain and the result skeptical. Although umbrella review is the top evidence to draw integrated conclusions nowadays, we still need to consider the inherent disadvantages of baseline studies. Secondly, the available RCTs of some focus in this review are still lacking (e.g., only three RCTs were retrieved for liver disease), the insufficiency of baseline studies is bound to impede the analysis and exploration in some populations, resulting in poor data capacity and overall results quality. Moreover, the wide range of vitamin D dosing amount is also one of the limitations that cannot be neglected. We aim to find out the effect of vitamin D on all-cause mortality, thus we prefer not to set boundaries on vitamin D type (vitamin D2, vitamin D3 et al.), intake method (oral intake, muscle injection et al.), combination use (calcium et al.), supplementation concentration, and dosing frequency, which inevitably introduce bias into our research. Besides we didn't focus on the variations in serum vitamin D level (213–215) or the differences in VDR expression among different races, which may affect the effects of vitamin D supplements and introduce bias into our results (216). Last, the comprehensiveness of included diseases' types is faulty. We manually excluded the data of Parkinson (217) and AIDS (218), because these data have not meet the statistical criteria, leaving the results a tiny flaw.
5. Conclusions
In summary, this evidence map and umbrella review suggests that vitamin D may reduce respiratory cancer-related mortality in respiratory tract cancer patients, and decrease all-cause mortality in COVID-19 or liver disease population (particularly in liver cirrhosis patients). However, there is no evidence to support the beneficial or harmful effects of vitamin D on all-cause mortality and other specific-cancer mortality in other health conditions. Simultaneously, this study may provide clinicians a statistical foundation to adjust their vitamin D supplementation regimen for different health conditions. However, due to the discrepancy in follow-up time and inadequate RCTs, there is a clear need for better designed trials and further studies to draw a more convincing conclusion on the role of vitamin D in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MC and CH performed the data collection. MC conducted the statistical analysis of all data. MC and JH drafted and edited the original manuscript and participated in the conception and design of the original study. MG and SW revised the manuscript and participated the original study design. All authors read and approved the final manuscript.
Funding
The Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University (YX202209) fund this study.
Acknowledgments
The authors express their gratitude to the Third Xiangya Hospital of Central South University, the University of Pittsburgh, and the Central South University. The authors also gratefully acknowledge Biorender for granting permission to use the vector images (Agreement number: HC25HADD6T).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1132528/full#supplementary-material
Abbreviations
ACE2, angiotensin converting enzyme 2; AIDS, Acquired Immune Deficiency Syndrome; AMSTAR, a measurement tool to assess systematic reviews; CI, critically ill; CKD, chronic kidney disease; Cochrane ROB, cochrane risk of bias; COPD, chronic obstructive pulmonary diseases; COVID-19, corona virus disease-2019; CVD, cardiovascular diseases; GRADE, grading of recommendations assessment, development and evaluation; HCV, hepatitis C virus; Hh, Hedgehog; OA, osteoarthritis; PRISMA, preferred reporting items for systematic reviews and meta-Analyses; PTB, pulmonary tuberculosis; RA, rheumatoid arthritis; RAS, renin-angiotensin; RCT, randomized controlled trial; Revman, review manager; SARS-CoV-2, the severe acute respiratory syndrome coronavirus 2; T2DM, type 2 diabetes; VDR, vitamin D receptor.
References
1. Michaëlsson K. The puzzling world of vitamin D insufficiency. Lancet Diabetes Endocrinol. (2014) 2:269–329. doi: 10.1016/S2213-8587(14)70008-7
2. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. (2014) 348:g2035. doi: 10.1136/bmj.g2035
3. Rooney MR, Harnack L, Michos ED, Ogilvie RP, Sempos CT, Lutsey PL. Trends in Use of High-Dose Vitamin D Supplements Exceeding 1000 or 4000 International Units Daily, 1999-2014. JAMA. (2017) 317:2448–50. doi: 10.1001/jama.2017.4392
4. Liu D, Meng X, Tian Q, Cao W, Fan X, Wu L, et al. Vitamin D and multiple health outcomes: an umbrella review of observational studies, randomized controlled trials, and mendelian randomization studies. Adv Nutr. (2021) 13:1044–62. doi: 10.1093/advances/nmab142
5. Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. (1999) 149:151–61. doi: 10.1093/oxfordjournals.aje.a009781
6. Zittermann A, Gummert JF, Börgermann J. Vitamin D deficiency and mortality. Curr Opin Clin Nutr Metab Care. (2009) 12:634–9. doi: 10.1097/MCO.0b013e3283310767
7. Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. (2014) 10:CD007470. doi: 10.1002/14651858.CD007470.pub3
8. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. (2014) 2:307–20. doi: 10.1016/S2213-8587(13)70212-2
9. Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. (2019) 366:l4673. doi: 10.1136/bmj.l4673
10. Neale RE, Baxter C, Romero BD, McLeod DSA, English DR, Armstrong BK, et al. The D-Health Trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. (2022) 10:120–8. doi: 10.1016/S2213-8587(21)00345-4
11. Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. (2017) 357:j2376. doi: 10.1136/bmj.j2376
12. Köhler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, et al. Mapping risk factors for depression across the lifespan: An umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res. (2018) 103:189–207. doi: 10.1016/j.jpsychires.2018.05.020
13. Ioannidis JPA. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Can Med Assoc J. (2009) 181:488–93. doi: 10.1503/cmaj.081086
14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
16. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. (2007) 7:10. doi: 10.1186/1471-2288-7-10
17. Khan SU, Khan MU, Riaz H, Valavoor S, Zhao D, Vaughan L, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. (2019) 171:190–8. doi: 10.7326/M19-0341
18. Nudy M, Krakowski G, Ghahramani M, Ruzieh M, Foy AJ. Vitamin D supplementation, cardiac events and stroke: A systematic review and meta-regression analysis. IJC Heart Vascul. (2020) 28:100537. doi: 10.1016/j.ijcha.2020.100537
19. Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. (2019) 4:765. doi: 10.1001/jamacardio.2019.1870
20. Zittermann A, Prokop S. The Role of Vitamin D for Cardiovascular Disease and Overall Mortality. In: Sunlight, Vitamin D and Skin Cancer. New York, NY: Springer New York. (2014). doi: 10.1007/978-1-4939-0437-2_6
21. Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2011) 96:1931–42. doi: 10.1210/jc.2011-0398
22. Autier P, Gandini S. Vitamin D Supplementation and Total MortalityA Meta-analysis of Randomized Controlled Trials. Arch Intern Med. (2007) 167:1730. doi: 10.1001/archinte.167.16.1730
23. Haykal T, Samji V, Zayed Y, Gakhal I, Dhillon H, Kheiri B, et al. The role of vitamin D supplementation for primary prevention of cancer: meta-analysis of randomized controlled trials. J Commun Hospital Internal Med Perspect. (2019) 9:480–8. doi: 10.1080/20009666.2019.1701839
24. Vaughan-Shaw PG, Buijs LF, Blackmur JP, Theodoratou E, Zgaga L, Din FVN, et al. The effect of vitamin D supplementation on survival in patients with colorectal cancer: systematic review and meta-analysis of randomised controlled trials. Br J Cancer. (2020) 123:1705–12. doi: 10.1038/s41416-020-01060-8
25. Zhang X, Niu W. Meta-analysis of randomized controlled trials on vitamin D supplement and cancer incidence and mortality. Biosci Rep. (2019) 39:BSR20190369. doi: 10.1042/BSR20190369
26. Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol. (2019) 30:733–43. doi: 10.1093/annonc/mdz059
27. Shahvazi S, Soltani S, Ahmadi S, de Souza R, Salehi-Abargouei A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. (2019) 51:11–21. doi: 10.1055/a-0774-8809
28. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, Madrid E, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochr Datab System Rev. (2020) 2003:CD002141. doi: 10.1002/14651858.CD002141.pub3
29. Junarta J, Jha V, Banerjee D. Insight into the impact of vitamin D on cardiovascular outcomes in chronic kidney disease. Nephrology. (2019) 24:781–90. doi: 10.1111/nep.13569
30. Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GFM. Meta-analysis: Vitamin D Compounds in Chronic Kidney Disease. Ann Intern Med. (2007) 147:840. doi: 10.7326/0003-4819-147-12-200712180-00004
31. Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochr Datab System Rev. (2009) 2009:CD005633. doi: 10.1002/14651858.CD005633.pub2
32. Lu RJ, Zhu SM, Tang FL, Zhu XS, Fan ZD, Wang GL, et al. Effects of vitamin D or its analogues on the mortality of patients with chronic kidney disease: an updated systematic review and meta-analysis. Eur J Clin Nutr. (2017) 71:683–93. doi: 10.1038/ejcn.2017.59
33. Peng L, Li L, Wang P, Chong W, Li Y, Zha X, et al. Association between Vitamin D supplementation and mortality in critically ill patients: A systematic review and meta-analysis of randomized clinical trials. PLoS ONE. (2020) 15:e0243768. doi: 10.1371/journal.pone.0243768
34. Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, et al. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J Crit Care. (2017) 38:109–14. doi: 10.1016/j.jcrc.2016.10.029
35. Juhász MF, Varannai O, Németh D, Szakács Z, Kiss S, Izsák VD, et al. Vitamin D supplementation in patients with cystic fibrosis: A systematic review and meta-analysis. J Cystic Fibrosis. (2021) 20:729–36. doi: 10.1016/j.jcf.2020.12.008
36. Wu H, Xiong X, Zhu M, Wei J, Zhuo K, Cheng D. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. (2018) 18:108. doi: 10.1186/s12890-018-0677-6
37. Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism. (2021) 119:154753. doi: 10.1016/j.metabol.2021.154753
38. Pal R, Banerjee M, Bhadada SK, Shetty AJ, Singh B, Vyas A. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Invest. (2022) 45:53–68. doi: 10.1007/s40618-021-01614-4
39. Lan S-H, Lai C-C, Chang S-P, Lu L-C, Hung S-H, Lin W-T. Vitamin D supplementation and the outcomes of critically ill adult patients: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. (2020) 10:14261. doi: 10.1038/s41598-020-71271-9
40. Wang T, Liu Z, Fu J, Min Z. Meta-analysis of vitamin D supplementation in the treatment of chronic heart failure. Scandinavian Cardiov J. (2019) 53:110–6. doi: 10.1080/14017431.2019.1612084
41. Bjelakovic G, Nikolova D, Bjelakovic M, Gluud C. Vitamin D supplementation for chronic liver diseases in adults. Cochr Datab System Rev. (2017) 2017:CD011564. doi: 10.1002/14651858.CD011564.pub2
42. Albert CM, Cook NR, Pester J, Moorthy MV, Ridge C, Danik JS, et al. Effect of marine omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA. (2021) 325:1061. doi: 10.1001/jama.2021.1489
43. Aloia JF, Talwar SA, Pollack S, Yeh J, A. randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. (2005) 165:1618–23. doi: 10.1001/archinte.165.14.1618
44. Bæksgaard L, Andersen KP, Hyldstrup L. Calcium and Vitamin D Supplementation Increases Spinal BMD in Healthy, Postmenopausal Women. Osteoporosis Int. (1998) 8:255–60. doi: 10.1007/s001980050062
45. Björkman M, Sorva A, Risteli J, Tilvis R. Vitamin D supplementation has minor effects on parathyroid hormone and bone turnover markers in vitamin D-deficient bedridden older patients. Age Ageing. (2008) 37:25–31. doi: 10.1093/ageing/afm141
46. Brazier M, Grados F, Kamel S, Mathieu M, Morel A, Maamer M, et al. Clinical and laboratory safety of one year's use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. (2005) 27:1885–93. doi: 10.1016/j.clinthera.2005.12.010
47. Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP, et al. higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. (2007) 55:234–9. doi: 10.1111/j.1532-5415.2007.01048.x
48. Burleigh E, McColl J, Potter J. Does vitamin D stop inpatients falling? A randomised controlled trial Age and Ageing. (2007) 36:507–13. doi: 10.1093/ageing/afm087
49. Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. (2002) 13:257–64. doi: 10.1007/s001980200023
50. Corless D, Dawson E, Fraser F, Ellis M, Evans SJ, Perry JD, et al. Do vitamin D supplements improve the physical capabilities of elderly hospital patients? Age Ageing. (1985) 14:76–84. doi: 10.1093/ageing/14.2.76
51. Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner-Binder D, Damm TN, et al. Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily. J Am Geriatr Soc. (2004) 52:230–6. doi: 10.1111/j.1532-5415.2004.52060.x
52. Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial: vitamin D treatment to prevent falls. J Am Geriatr Soc. (2005) 53:1881–8. doi: 10.1111/j.1532-5415.2005.00468.x
53. Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. (2001) 86:3618–28. doi: 10.1210/jcem.86.8.7703
54. Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150, 000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res. (2012) 27:170–6. doi: 10.1002/jbmr.524
55. Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, et al. 1, 25-Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. J Clin Endocrinol Metab. (1991) 73:1111–7. doi: 10.1210/jcem-73-5-1111
56. Hin H, Tomson J, Newman C, Kurien R, Lay M, Cox J, et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int. (2017) 28:841–51. doi: 10.1007/s00198-016-3833-y
57. Inkovaara J, Gothoni G, Halttula R, Heikinheimo R, Tokola O. Calcium, vitamin D and anabolic steroid in treatment of aged bones: double-blind placebo-controlled long-term clinical trial. Age Ageing. (1983) 12:124–30. doi: 10.1093/ageing/12.2.124
58. Kärkkäinen M, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Effect of calcium and vitamin D supplementation on bone mineral density in women aged 65-71 years: a 3-year randomized population-based trial (OSTPRE-FPS). Osteoporos Int. (2010) 21:2047–55. doi: 10.1007/s00198-009-1167-8
59. Komulainen M, Kröger H, Tuppurainen MT, Heikkinen A-M, Alhava E, Honkanen R, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D 3 in early postmenopausal women: a population-based 5-year randomized trial 1. J Clin Endocrinol Metab. (1999) 84:546–52. doi: 10.1210/jcem.84.2.5496
60. Krieg MA, Jacquet AF, Bremgartner M, Cuttelod S, Thiébaud D, Burckhardt P. Effect of supplementation with vitamin D 3 and calcium on quantitative ultrasound of bone in elderly institutionalized women: a longitudinal study. Osteopor Int. (1999) 9:483–8. doi: 10.1007/s001980050265
61. Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. (2006) 354:684–96. doi: 10.1056/NEJMoa055222
62. Lappe J, Watson P, Travers-Gustafson D, Recker R, Garland C, Gorham E, et al. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA. (2017) 317:1234–43. doi: 10.1001/jama.2017.2115
63. Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc. (2003) 51:291–9. doi: 10.1046/j.1532-5415.2003.51101.x
64. Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age Ageing. (2006) 35:482–6. doi: 10.1093/ageing/afj080
65. Levis S, Gómez-Marín O. Vitamin D and Physical Function in Sedentary Older Men. J Am Geriatr Soc. (2017) 65:323–31. doi: 10.1111/jgs.14510
66. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D Supplementation and Fracture Incidence in Elderly Persons: A Randomized, Placebo-Controlled Clinical Trial. Ann Intern Med. (1996) 124:400. doi: 10.7326/0003-4819-124-4-199602150-00003
67. Lyons RA, Johansen A, Brophy S, Newcombe RG, Phillips CJ, Lervy B, et al. Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporos Int. (2007) 18:811–8. doi: 10.1007/s00198-006-0309-5
68. Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med. (2019) 380:33–44. doi: 10.1056/NEJMoa1809944
69. Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation With Oral Vitamin D 3 and Calcium During Winter Prevents Seasonal Bone Loss: A Randomized Controlled Open-Label Prospective Trial. J Bone Miner Res. (2004) 19:1221–30. doi: 10.1359/JBMR.040511
70. Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can Vitamin D Supplementation Reduce the Risk of Fracture in the Elderly? A Randomized Controlled Trial. J Bone Miner Res. (2002) 17:709–15. doi: 10.1359/jbmr.2002.17.4.709
71. Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab. (1995) 80:1052–8. doi: 10.1210/jcem.80.4.7714065
72. Owusu JE, Islam S, Katumuluwa SS, Stolberg AR, Usera GL, Anwarullah AA, et al. Cognition and Vitamin D in Older African-American Women- Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J Am Geriatr Soc. (2019) 67:81–6. doi: 10.1111/jgs.15607
73. Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D 3) for prevention of fractures in primary care. BMJ. (2005) 330:1003. doi: 10.1136/bmj.330.7498.1003
74. Prince RL. Effects of Ergocalciferol Added to Calcium on the Risk of Falls in Elderly High-Risk Women. Arch Intern Med. (2008) 168:103. doi: 10.1001/archinternmed.2007.31
75. Reid IR, Horne AM, Mihov B, Gamble GD, Al-Abuwsi F, Singh M, et al. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J Intern Med. (2017) 282:452–60. doi: 10.1111/joim.12651
76. Salovaara K, Tuppurainen M, Kärkkäinen M, Rikkonen T, Sandini L, Sirola J, et al. Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial–the OSTPRE-FPS. J Bone Miner Res. (2010) 25:1487–95. doi: 10.1002/jbmr.48
77. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual High-Dose Oral Vitamin D and Falls and Fractures in Older Women: A Randomized Controlled Trial. JAMA. (2010) 303:1815. doi: 10.1001/jama.2010.594
78. Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, et al. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. (2017) 2:608. doi: 10.1001/jamacardio.2017.0175
79. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. (2003) 326:469–469. doi: 10.1136/bmj.326.7387.469
80. Uusi-Rasi K, Patil R, Karinkanta S, Kannus P, Tokola K, Lamberg-Allardt C, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med. (2015) 175:703–11. doi: 10.1001/jamainternmed.2015.0225
81. Witham MD, Price RJG, Struthers AD, Donnan PT, Messow C-M, Ford I, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. (2013) 173:1672–9. doi: 10.1001/jamainternmed.2013.9043
82. Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab. (2008) 93:743–9. doi: 10.1210/jc.2007-1466
83. Attia S, Eickhoff J, Wilding G, McNeel D, Blank J, Ahuja H, et al. Randomized, Double-Blinded Phase II Evaluation of Docetaxel with or without Doxercalciferol in Patients with Metastatic, Androgen-Independent Prostate Cancer. Clin Cancer Res. (2008) 14:2437–43. doi: 10.1158/1078-0432.CCR-07-4274
84. Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-Term Follow-Up for Mortality and Cancer in a Randomized Placebo-Controlled Trial of Vitamin D 3 and/or Calcium (RECORD Trial). J Clin Endocrinol Metab. (2012) 97:614–22. doi: 10.1210/jc.2011-1309
85. Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT investigators. JCO. (2007) 25:669–74. doi: 10.1200/JCO.2006.06.8197
86. Scher HI, Jia X, Chi K, de Wit R, Berry WR, Albers P, et al. Randomized, Open-Label Phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. JCO. (2011) 29:2191–8. doi: 10.1200/JCO.2010.32.8815
87. Scragg R, Khaw K-T, Toop L, Sluyter J, Lawes CMM, Waayer D, et al. Monthly high-dose vitamin D supplementation and cancer risk: a post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol. (2018) 4:e182178. doi: 10.1001/jamaoncol.2018.2178
88. Urashima M, Ohdaira H, Akutsu T, Okada S, Yoshida M, Kitajima M, et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA. (2019) 321:1361. doi: 10.1001/jama.2019.2210
89. Alshahawey M, El Borolossy R, El Wakeel L, Elsaid T, Sabri NA. The impact of cholecalciferol on markers of vascular calcification in hemodialysis patients: A randomized placebo controlled study. Nutr Metab Cardiovasc Dis. (2021) 31:626–33. doi: 10.1016/j.numecd.2020.09.014
90. Alvarez JA, Law J, Coakley KE, Zughaier SM, Hao L, Shahid Salles K, et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2012) 96:672–9. doi: 10.3945/ajcn.112.040642
91. Bhan I, Dobens D, Tamez H, Deferio JJ Li YC, Warren HS, et al. Nutritional vitamin D supplementation in dialysis: a randomized trial. CJASN. (2015) 10:611–9. doi: 10.2215/CJN.06910714
92. Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. (2004) 43:877–90. doi: 10.1053/j.ajkd.2004.01.012
93. Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, et al. Paricalcitol Capsule for the Treatment of Secondary Hyperparathyroidism in Stages 3 and 4 CKD. Am J Kidney Diseases. (2006) 47:263–76. doi: 10.1053/j.ajkd.2005.10.007
94. Delanaye P, Weekers L, Warling X, Moonen M, Smelten N, Médart L, et al. Cholecalciferol in haemodialysis patients: a randomized, double-blind, proof-of-concept and safety study. Nephrol Dialysis Transpl. (2013) 28:1779–86. doi: 10.1093/ndt/gft001
95. de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. (2010) 376:1543–51. doi: 10.1016/S0140-6736(10)61032-X
96. Frazão JM, Elangovan L, Maung HM, Chesney RW, Acchiardo SR, Bower JD, et al. Intermittent doxercalciferol (1α-Hydroxyvitamin D2) therapy for secondary hyperparathyroidism. Am J Kidney Dis. (2000) 36:550–61. doi: 10.1053/ajkd.2000.16193
97. Hamdy NAT, Kanis JA, Beneton MNC, Brown CB, Juttmann JR, Jordans JGM, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. (1995) 310:358–63. doi: 10.1136/bmj.310.6976.358
98. Hewitt NA, O'Connor AA, O'Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. CJASN. (2013) 8:1143–9. doi: 10.2215/CJN.02840312
99. Li L, Lin M, Krassilnikova M, Ostrow K, Bader A, Radbill B, et al. Effect of cholecalciferol supplementation on inflammation and cellular alloimmunity in hemodialysis patients: data from a randomized controlled pilot trial. PLoS ONE. (2014) 9:e109998. doi: 10.1371/journal.pone.0109998
100. Marckmann P, Agerskov H, Thineshkumar S, Bladbjerg E-M, Sidelmann JJ, Jespersen J, et al. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dialy Transpl. (2012) 27:3523–31. doi: 10.1093/ndt/gfs138
101. Massart A, Debelle FD, Racapé J, Gervy C, Husson C, Dhaene M, et al. Biochemical parameters after cholecalciferol repletion in hemodialysis: results from the vita dial randomized trial. Am J Kidney Dis. (2014) 64:696–705. doi: 10.1053/j.ajkd.2014.04.020
102. Memmos DE, Eastwood JB, Talner LB, Gower PE, Curtis JR, Phillips ME, et al. Double-blind trial of oral 1, 25-dihydroxy vitamin D3 versus placebo in asymptomatic hyperparathyroidism in patients receiving maintenance haemodialysis. BMJ. (1981) 282:1919–24. doi: 10.1136/bmj.282.6280.1919
103. Merino JL, Teruel JL, Fernández-Lucas M, Villafruela JJ, Bueno B, Gomis A, et al. Effects of a Single, High Oral Dose of 25-Hydroxycholecalciferol on the Mineral Metabolism Markers in Hemodialysis Patients: Dialysis and High Dose of 25(OH)D. Ther Apher Dial. (2015) 19:212–9. doi: 10.1111/1744-9987.12279
104. Moe SM, Zekonis M, Harezlak J, Ambrosius WT, Gassensmith CM, Murphy CL, et al. A placebo-controlled trial to evaluate immunomodulatory effects of paricalcitol. Am J Kidney Dis. (2001) 38:792–802. doi: 10.1053/ajkd.2001.27697
105. Morrone L, Palmer SC, Saglimbene VM, Perna A, Cianciolo G, Russo D, et al. Calcifediol supplementation in adults on hemodialysis: a randomized controlled trial. J Nephrol. (2021) 35:517–525. doi: 10.1007/s40620-021-01104-z
106. Obi Y, Ichimaru N, Sakaguchi Y, Iwadoh K, Ishii D, Sakai K, et al. Correcting anemia and native vitamin D supplementation in kidney transplant recipients: a multicenter, 2 × 2 factorial, open-label, randomized clinical trial. Transpl Int. (2021) 34:1212–25. doi: 10.1111/tri.13885
107. Przedlacki J, Manelius J, Huttunen K. Bone Mineral Density Evaluated by Dual-Energy X-Ray Absorptiometry after One-Year Treatment with Calcitriol Started in the Predialysis Phase of Chronic Renal Failure. Nephron. (1995) 69:433–7. doi: 10.1159/000188515
108. The J-DAVID. Investigators, Shoji T, Inaba M, Fukagawa M, Ando R, Emoto M, et al. Effect of Oral alfacalcidol on clinical outcomes in patients without secondary hyperparathyroidism receiving maintenance hemodialysis: The J-DAVID randomized clinical trial. JAMA. (2018) 320:2325. doi: 10.1001/jama.2018.17749
109. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. JAMA. (2012) 307:674. doi: 10.1001/jama.2012.120
110. Wang AY-M, Fang F, Chan J, Wen Y-Y, Qing S, Chan IH-S, et al. Effect of paricalcitol on left ventricular mass and function in CKD—The OPERA trial. JASN. (2014) 25:175–86. doi: 10.1681/ASN.2013010103
111. Wasse H, Huang R, Long Q, Singapuri S, Raggi P, Tangpricha V. Efficacy and safety of a short course of very-high-dose cholecalciferol in hemodialysis. Am J Clin Nutr. (2012) 95:522–8. doi: 10.3945/ajcn.111.025502
112. Wasse H, Huang R, Long Q, Zhao Y, Singapuri S, McKinnon W, et al. Very High-dose Cholecalciferol and Arteriovenous Fistula Maturation in ESRD: A Randomized, Double-blind, Placebo-Controlled Pilot Study. J Vasc Access. (2014) 15:88–94. doi: 10.5301/jva.5000187
113. Annweiler C, Beaudenon M, Simon R, Guenet M, Otekpo M, Célarier T, et al. Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: Extension phase of the GERIA-COVID study. J Steroid Biochem Mol Biol. (2021) 213:105958. doi: 10.1016/j.jsbmb.2021.105958
114. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. (2020) 203:105751. doi: 10.1016/j.jsbmb.2020.105751
115. Daley P, Jagannathan V, John KR, Sarojini J, Latha A, Vieth R, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. (2015) 15:528–34. doi: 10.1016/S1473-3099(15)70053-8
116. Dastan F, Salamzadeh J, Pourrashid MH, Edalatifard M, Eslaminejad A. Effects of high-dose vitamin D replacement on the serum levels of systemic inflammatory biomarkers in patients with acute exacerbation of chronic obstructive pulmonary disease. COPD. (2019) 16:278–83. doi: 10.1080/15412555.2019.1666812
117. Ganmaa D, Munkhzul B, Fawzi W, Spiegelman D, Willett WC, Bayasgalan P, et al. High-Dose Vitamin D 3 during tuberculosis treatment in mongolia. A Randomized Controlled Trial. Am J Respir Crit Care Med. (2017) 196:628–37. doi: 10.1164/rccm.201705-0936OC
118. Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. (2012) 4:191–7. doi: 10.4161/derm.20332
119. Labib JR, Ibrahem SK, Ismail MM, Fatah SAMAE, Sedrak AS, Attia MAS, et al. Vitamin D supplementation and improvement of pneumonic children at a tertiary pediatric hospital in Egypt: A randomized controlled trial. Medicine. (2021) 100:25011. doi: 10.1097/MD.0000000000025011
120. Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. (2012) 156:105. doi: 10.7326/0003-4819-156-2-201201170-00004
121. Maghbooli Z, Sahraian MA, Jamalimoghadamsiahkali S, Asadi A, Zarei A, Zendehdel A, et al. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: a pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocrine Practice. (2021) 27:1242–51. doi: 10.1016/j.eprac.2021.09.016
122. Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, et al. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. (2011) 377:242–50. doi: 10.1016/S0140-6736(10)61889-2
123. Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Vitamin D 3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. (2015) 3:120–30. doi: 10.1016/S2213-2600(14)70255-3
124. Miroliaee AE, Salamzadeh J, Shokouhi S, Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J Critical Care. (2018) 44:300–5. doi: 10.1016/j.jcrc.2017.08.040
125. Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study Supplementary Cholecalciferol in recovery from tuberculosis: A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect Dis. (2013) 13:22. doi: 10.1186/1471-2334-13-22
126. Soliman AR, Abdelaziz TS, Fathy A. Impact of Vitamin D Therapy on the Progress COVID-19: Six weeks follow-up study of Vitamin D deficient elderly diabetes patients. In: Proceedings of Singapore Healthcare. (2021). doi: 10.1177/20101058211041405
127. Tangpricha V, Lukemire J, Chen Y, Binongo JNG, Judd SE, Michalski ES, et al. Vitamin D for the Immune System in Cystic Fibrosis (DISC): a double-blind, multicenter, randomized, placebo-controlled clinical trial. Am J Clin Nutr. (2019) 109:544–53. doi: 10.1093/ajcn/nqy291
128. Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, Shenvi N, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. (2015) 102:1059–69. doi: 10.3945/ajcn.115.113886
129. Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as Supplementary Treatment for Tuberculosis: A Double-blind, Randomized, Placebo-controlled Trial. Am J Respir Crit Care Med. (2009) 179:843–50. doi: 10.1164/rccm.200804-567OC
130. Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of High-Dose Vitamin D 3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA. (2014) 312:1520. doi: 10.1001/jama.2014.13204
131. The The National Heart Lung and Blood Institute PETAL Clinical Trials Network. Early high-dose vitamin D 3 for critically ill, vitamin D-deficient patients. N Engl J Med. (2019) 381:2529–2540. doi: 10.1056/NEJMoa1911124
132. Han JE, Jones JL, Tangpricha V, Brown MA, Hao L, Hebbar G, et al. High dose vitamin D administration in ventilated intensive care unit patients: A pilot double blind randomized controlled trial. J Clin Transl Endocrinol. (2016) 4:59–65. doi: 10.1016/j.jcte.2016.04.004
133. Hasanloei MAV, Rahimlou M, Eivazloo A, Sane S, Ayremlou P, Hashemi R. Effect of oral versus intramuscular vitamin d replacement on oxidative stress and outcomes in traumatic mechanical ventilated patients admitted to intensive care unit. Nutr Clin Pract. (2020) 35:548–58. doi: 10.1002/ncp.10404
134. Ingels C, Vanhorebeek I, Van Cromphaut S, Wouters PJ, Derese I, Dehouwer A, et al. Effect of intravenous 25OHD supplementation on bone turnover and inflammation in prolonged critically ill patients. Horm Metab Res. (2020) 52:168–78. doi: 10.1055/a-1114-6072
135. Karsy M, Guan J, Eli I, Brock AA, Menacho ST, Park MS. The effect of supplementation of vitamin D in neurocritical care patients: RandomizEd Clinical TrIal oF hYpovitaminosis D (RECTIFY). J Neurosurg. (2020) 133:1103–12. doi: 10.3171/2018.11.JNS182713
136. Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. (2014) 190:533–41. doi: 10.1164/rccm.201405-0988OC
137. Miri M, Kouchek M, Rahat Dahmardeh A, Sistanizad M. Effect of high-dose vitamin D on duration of mechanical ventilation in ICU patients. Iran J Pharm Res. (2019) 18:1067–72. doi: 10.22037/ijpr.2019.1100647
138. Naguib SN, Sabry NA, Farid SF, Alansary AM. Short-term effects of alfacalcidol on hospital length of stay in patients undergoing valve replacement surgery: a randomized clinical trial. Clin Ther. (2021) 43:e1–18. doi: 10.1016/j.clinthera.2020.11.008
139. Quraishi SA, De Pascale G, Needleman JS, Nakazawa H, Kaneki M, Bajwa EK, et al. Effect of cholecalciferol supplementation on Vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med. (2015) 43:1928–37. doi: 10.1097/CCM.0000000000001148
140. Sistanizad M, Kouchek M, Miri M, Salarian S, Shojaei S, Moeini Vasegh F, et al. High dose vitamin D improves total serum antioxidant capacity and ICU outcome in critically ill patients - A randomized, double-blind clinical trial. Eur J Integr Med. (2021) 42:101271. doi: 10.1016/j.eujim.2020.101271
141. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. (2006) 83:754–9. doi: 10.1093/ajcn/83.4.754
142. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo MET. The Effects of Vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ: Heart Failure. (2010) 3:195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899
143. Witte KK, Byrom R, Gierula J, Paton MF, Jamil HA, Lowry JE, et al. Effects of Vitamin D on cardiac function in patients with chronic HF. J Am College Cardiol. (2016) 67:2593–603. doi: 10.1016/j.jacc.2016.03.508
144. Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. (2017) 38:2279–86. doi: 10.1093/eurheartj/ehx235
145. Arden NK, Cro S, Sheard S, Doré CJ, Bara A, Tebbs SA, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthr Cartilage. (2016) 24:1858–66. doi: 10.1016/j.joca.2016.05.020
146. Berggren M, Stenvall M, Olofsson B, Gustafson Y. Evaluation of a fall-prevention program in older people after femoral neck fracture: a one-year follow-up. Osteoporos Int. (2008) 19:801–9. doi: 10.1007/s00198-007-0507-9
147. Brohult J, Jonson B. Effects of large doses of calciferol on patients with rheumatoid arthritis: a double-blind clinical trial. Scand J Rheumatol. (1973) 2:173–6. doi: 10.3109/03009747309097085
148. Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ, A. randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age Ageing. (2004) 33:45–51. doi: 10.1093/ageing/afh002
149. Jin X, Jones G, Cicuttini F, Wluka A, Zhu Z, Han W, et al. Effect of Vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. (2016) 315:1005. doi: 10.1001/jama.2016.1961
150. Aggarwal B, Subramaniam R, Wig N, Baidya DK, Bhattacharyya A. Effect of early administration of vitamin D on clinical outcome in critically ill sepsis patients: a randomized placebo-controlled trial. Indian J Crit Care Med. (2021) 25:1147–54. doi: 10.5005/jp-journals-10071-23993
151. Wang Y, Yang Z, Gao L, Cao Z, Wang Q. Effects of a single dose of vitamin D in septic children: a randomized, double-blinded, controlled trial. J Int Med Res. (2020) 48:030006052092689. doi: 10.1177/0300060520926890
152. Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, et al. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab. (2016) 101:1647–55. doi: 10.1210/jc.2015-4013
153. The TIDE Trial Investigators. Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE) randomised controlled trial. Diabetologia. (2012) 55:36–45. doi: 10.1007/s00125-011-2357-4
154. Mobarhan SA, Russell RM, Recker RR, Posner DB, Iber FL, Miller P. Metabolic bone disease in alcoholic cirrhosis: a comparison of the effect of vitamin D 2 25-Hydroxyvitamin D, or supportive treatment. Hepatology. (1984) 4:266–73. doi: 10.1002/hep.1840040216
155. Mohamed AA, Al-Karmalawy AA, El-Kholy AA, El-Damas DA, Abostate HM, Mostafa SM, et al. Effect of Vitamin D supplementation in patients with liver cirrhosis having spontaneous bacterial peritonitis: a randomized controlled study. Eur Rev Med Pharmacol Sci. (2021) 25:6908–19. doi: 10.26355/eurrev_202111_27239
156. Vosoghinia H, Esmaeilzadeh A, Ganji A, Hosseini SM-R, Jamehdar SA, Salehi M, et al. Vitamin D in Standard HCV Regimen (PEG-Interferon Plus Ribavirin), its effect on the early virologic response rate: a clinical trial. Razavi Int J Med. (2016) 4:27–34. doi: 10.17795/rijm36632
157. Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. (1989) 68:882–7. doi: 10.1210/jcem-68-5-882
158. Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. (2012) 108:1557–61. doi: 10.1017/S0007114511007161
159. Anagnostis P, Karras SN, Athyros VG, Annweiler C, Karagiannis A. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes. Lancet Diab Endocrinol. (2014) 2:362–3. doi: 10.1016/S2213-8587(14)70095-6
160. Manson JE, Bassuk SS, Lee I-M, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. (2012) 33:159–71. doi: 10.1016/j.cct.2011.09.009
161. Jolliffe DA. Camargo CA, Sluyter JD, Mary A, Aloia JF, Ganmaa D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. (2021) 9:276–92. doi: 10.1136/thorax-2020-BTSabstracts.105
162. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. (2019) 104:1595–622. doi: 10.1210/jc.2019-00221
163. Dos Santos LM, Ohe MN, Pallone SG, Nacaguma IO, Kunii IS, da Silva REC, et al. Levels of bioavailable, and free forms of 25(OH)D after supplementation with vitamin D3 in primary hyperparathyroidism. Endocrine. (2023) 80:183–90. doi: 10.1007/s12020-022-03265-8
164. Erdmann J, Wiciński M, Szyperski P, Gajewska S, Ohla J, Słupski M. Vitamin D supplementation and its impact on different types of bone fractures. Nutrients. (2022) 15:103. doi: 10.3390/nu15010103
165. Pilz S, Trummer C, Theiler-Schwetz V, Grübler MR, Verheyen ND, Odler B, et al. Critical appraisal of large Vitamin D randomized controlled trials. Nutrients. (2022) 14:303. doi: 10.3390/nu14020303
166. Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. (2018) 175:125–35. doi: 10.1016/j.jsbmb.2017.01.021
167. Dror AA, Morozov N, Daoud A, Namir Y, Yakir O, Shachar Y, et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS ONE. (2022) 17:e0263069. doi: 10.1371/journal.pone.0263069
168. Smolders J, van den Ouweland J, Geven C, Pickkers P, Kox M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism. (2021) 115:154434. doi: 10.1016/j.metabol.2020.154434
169. Gillie O. Controlled trials of vitamin D, causality and type 2 statistical error. Public Health Nutr. (2016) 19:409–14. doi: 10.1017/S1368980014002304
170. Madden JM, Murphy L, Zgaga L, Bennett K, De. novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res Treat. (2018) 172:179–90. doi: 10.1007/s10549-018-4896-6
171. Gnagnarella P, Muzio V, Caini S, Raimondi S, Martinoli C, Chiocca S, et al. Vitamin D supplementation and cancer mortality: narrative review of observational studies and clinical trials. Nutrients. (2021) 13:3285. doi: 10.3390/nu13093285
172. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin d receptor null mice. Endocr Rev. (2008) 29:726–76. doi: 10.1210/er.2008-0004
173. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev. (2012) 33:456–92. doi: 10.1210/er.2012-1000
174. Baran DT, Sorensen AM. Rapid actions of 1−25-dihydroxyvitamin D3 physiologic role. Exp Biol Med. (1994) 207:175–9. doi: 10.3181/00379727-207-43803
175. Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1α,25-Dihydroxyvitamin D 3 modulates human adipocyte metabolism via nongenomic action. FASEB J. (2001) 15:1–15. doi: 10.1096/fj.01-0584fje
176. Ahmed F. A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection. Front Immunol. (2020) 11:590459. doi: 10.3389/fimmu.2020.590459
177. Fernández-Barral A, Bustamante-Madrid P, Ferrer-Mayorga G, Barbáchano A, Larriba MJ, Muñoz A. Vitamin D effects on cell differentiation and stemness in cancer. Cancers. (2020) 12:2413. doi: 10.3390/cancers12092413
178. Migliaccio S, Di Nisio A, Magno S, Romano F, Barrea L, Colao AM, et al. Vitamin D deficiency: a potential risk factor for cancer in obesity? Int J Obes (Lond). (2022) 46:707–17. doi: 10.1038/s41366-021-01045-4
179. Buonomo AR, Scotto R, Zappulo E, Nerilli M, Pinchera B, Perruolo G, et al. Severe Vitamin D deficiency increases mortality among patients with liver cirrhosis regardless of the presence of HCC. In Vivo. (2019) 33:177–82. doi: 10.21873/invivo.11456
180. Kim H-S, Rotundo L, Kothari N, Kim S-H, Pyrsopoulos N. Vitamin D is associated with severity and mortality of non-alcoholic fatty liver disease: a US population-based study. J Clin Transl Hepatol. (2017) 5:185–92. doi: 10.14218/JCTH.2017.00025
181. Skaaby T, Husemoen LLN, Borglykke A, Jørgensen T, Thuesen BH, Pisinger C, et al. Vitamin D status, liver enzymes, and incident liver disease and mortality: a general population study. Endocrine. (2014) 47:213–20. doi: 10.1007/s12020-013-0107-8
182. Pappa HM, Bern E, Kamin D, Grand RJ. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. (2008) 24:176–83. doi: 10.1097/MOG.0b013e3282f4d2f3
183. Huang GR, Wei SJ, Huang YQ, Xing W, Wang LY, Liang LL. Mechanism of combined use of vitamin D and puerarin in anti-hepatic fibrosis by regulating the Wnt/β-catenin signalling pathway. WJG. (2018) 24:4178–85. doi: 10.3748/wjg.v24.i36.4178
184. Zúñiga S, Firrincieli D, Housset C, Chignard N. Vitamin D and the vitamin D receptor in liver pathophysiology. Clin Res Hepatol Gastroenterol. (2011) 35:295–302. doi: 10.1016/j.clinre.2011.02.003
185. Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, et al. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. (2012) 32:845–51. doi: 10.1111/j.1478-3231.2011.02735.x
186. Lou Y-R, Molnár F, Peräkylä M, Qiao S, Kalueff AV, St-Arnaud R, et al. 25-Hydroxyvitamin D3 is an agonistic vitamin D receptor ligand. J Steroid Biochem Molec Biol. (2010) 118:162–70. doi: 10.1016/j.jsbmb.2009.11.011
187. Gal-Tanamy M, Bachmetov L, Ravid A, Koren R, Erman A, Tur-Kaspa R, et al. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology. (2011) 54:1570–9. doi: 10.1002/hep.24575
188. Ravid A, Rapaport N, Issachar A, Erman A, Bachmetov L, Tur-Kaspa R, et al. 25-Hydroxyvitamin D Inhibits Hepatitis C Virus Production in Hepatocellular Carcinoma Cell Line by a Vitamin D Receptor-Independent Mechanism. IJMS. (2019) 20:2367. doi: 10.3390/ijms20092367
189. Peng MY, Liu WC, Zheng JQ, Lu CL, Hou YC, Zheng CM, et al. Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D. IJMS. (2021) 22:5251. doi: 10.3390/ijms22105251
190. Chiu SK, Tsai KW, Wu CC, Zheng CM, Yang CH, Hu WC, et al. Putative Role of Vitamin D for COVID-19 Vaccination. IJMS. (2021) 22:8988. doi: 10.3390/ijms22168988
191. Matsumura T, Sugiyama N, Murayama A, Yamada N, Shiina M, Asabe S, et al. Antimicrobial peptide LL-37 attenuates infection of hepatitis C virus: Anti-HCV effect of LL-37. Hepatol Res. (2016) 46:924–32. doi: 10.1111/hepr.12627
192. Tian Y, Wang ML, Zhao J. Crosstalk between autophagy and type I interferon responses in innate antiviral immunity. Viruses. (2019) 11:132. doi: 10.3390/v11020132
193. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. (2017) 16:7432–8. doi: 10.3892/mmr.2017.7546
194. Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, et al. 1, 25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic amp response element in the renin gene promoter. J Biol Chem. (2007) 282:29821–30. doi: 10.1074/jbc.M705495200
195. Mangoni ML, McDermott AM, Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol. (2016) 25:167–73. doi: 10.1111/exd.12929
196. Weidmann J, Craik DJ. Discovery, structure, function, and applications of cyclotides: circular proteins from plants. EXBOTJ. (2016) 67:4801–12. doi: 10.1093/jxb/erw210
197. Jia HP, Look DC, Tan P, Shi L, Hickey M, Gakhar L, et al. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Molec Physiol. (2009) 297:L84–96. doi: 10.1152/ajplung.00071.2009
198. Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: Role of renin-angiotensin system. Redox Biol. (2019) 26:101295. doi: 10.1016/j.redox.2019.101295
199. Cherrie M, Clemens T, Colandrea C, Feng Z, Webb DJ, Weller RB, et al. Ultraviolet A radiation and COVID-19 deaths in the USA with replication studies in England and Italy. Br J Dermatol. (2021) 185:363–70. doi: 10.1111/bjd.20093
200. Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a Single High Dose of Vitamin D 3 on hospital length of stay in patients moderate to severe COVID-19: a randomized clinical trial. JAMA. (2021) 325:1053. doi: 10.1001/jama.2020.26848
201. Oristrell J, Oliva JC, Casado E, Subirana I, Domínguez D, Toloba A, et al. Vitamin D supplementation and COVID-19 risk: a population-based, cohort study. J Endocrinol Invest. (2022) 45:167–79. doi: 10.1007/s40618-021-01639-9
202. Nogues X, Ovejero D, Pineda-Moncusí M, Bouillon R, Arenas D, Pascual J, et al. Calcifediol Treatment and COVID-19-Related Outcomes. J Clin Endocrinol Metab. (2021) 106:e4017–27. doi: 10.1210/clinem/dgab405
203. Alcala-Diaz JF, Limia-Perez L, Gomez-Huelgas R, Martin-Escalante MD, Cortes-Rodriguez B, Zambrana-Garcia JL, et al. Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study. Nutrients. (2021) 13:1760. doi: 10.3390/nu13061760
204. Helde Frankling M, Klasson C, Sandberg C, Nordström M, Warnqvist A, Bergqvist J, et al. ‘Palliative-D'—Vitamin D supplementation to palliative cancer patients: a double blind, randomized placebo-controlled multicenter trial. Cancers. (2021) 13:3707. doi: 10.3390/cancers13153707
205. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. (2007) 85:1586–91. doi: 10.1093/ajcn/85.6.1586
206. Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology. (2016) 150:114–22. doi: 10.1053/j.gastro.2015.09.010
207. Akiba T, Morikawa T, Odaka M, Nakada T, Kamiya N, Yamashita M, et al. Vitamin D supplementation and survival of patients with non-small cell lung cancer: a randomized, double-blind, placebo-controlled trial. Clin Cancer Res. (2018) 24:4089–97. doi: 10.1158/1078-0432.CCR-18-0483
208. Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. (2005) 365:1621–8. doi: 10.1016/S0140-6736(05)63013-9
209. Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus Vitamin D Supplementation and the Risk of Fractures. N Engl J Med. (2006) 354:669–83.
210. Cooper L, Clifton-Bligh PB, Nery ML, Figtree G, Twigg S, Hibbert E, et al. Vitamin D supplementation and bone mineral density in early postmenopausal women. Am J Clin Nutr. (2003) 77:1324–9. doi: 10.1093/ajcn/77.5.1324
211. Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. (2009) 137:1396–404. doi: 10.1017/S0950268809002404
212. Guo H, Guo J, Xie W, Yuan L, Sheng X. The role of vitamin D in ovarian cancer: epidemiology, molecular mechanism and prevention. J Ovarian Res. (2018) 11:71. doi: 10.1186/s13048-018-0443-7
213. Wang W, Li G, He X, Gao J, Wang R, Wang Y, et al. Serum 25-Hydroxyvitamin D Levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem. (2015) 35:1999–2005. doi: 10.1159/000374007
214. Akbar MR, Wibowo A, Pranata R, Setiabudiawan B. Low Serum 25-hydroxyvitamin D (Vitamin D) level is associated with susceptibility to COVID-19, severity, and mortality: a systematic review and meta-analysis. Front Nutr. (2021) 8:660420. doi: 10.3389/fnut.2021.660420
215. Li Y, Ding S. Serum 25-Hydroxyvitamin D and the risk of mortality in adult patients with Sepsis: a meta-analysis. BMC Infect Dis. (2020) 20:189. doi: 10.1186/s12879-020-4879-1
216. Xu H, Liu Z, Shi H, Wang C. Prognostic role of vitamin D receptor in breast cancer: a systematic review and meta-analysis. BMC Cancer. (2020) 20:1051. doi: 10.1186/s12885-020-07559-w
217. Sato Y, Manabe S, Kuno H, Oizumi K. Amelioration of osteopenia and hypovitaminosis D by 1alpha-hydroxyvitamin D3 in elderly patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. (1999) 66:64–8. doi: 10.1136/jnnp.66.1.64
Keywords: vitamin D, all-cause mortality, umbrella review, patients, COVID-19
Citation: Cao M, He C, Gong M, Wu S and He J (2023) The effects of vitamin D on all-cause mortality in different diseases: an evidence-map and umbrella review of 116 randomized controlled trials. Front. Nutr. 10:1132528. doi: 10.3389/fnut.2023.1132528
Received: 02 February 2023; Accepted: 08 June 2023;
Published: 22 June 2023.
Edited by:
Owen Kelly, Sam Houston State University, United StatesReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaCaiqi Xu, Shanghai Jiao Tong University, China
Copyright © 2023 Cao, He, Gong, Wu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinshen He, Smluc2hlbi5oZUBob3RtYWlsLmNvbQ==
 Mingyu Cao
Mingyu Cao Chunrong He2
Chunrong He2 Matthew Gong
Matthew Gong Jinshen He
Jinshen He