
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 07 March 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1132234
This article is part of the Research TopicImpact of Diet-Related Disorders on Musculoskeletal HealthView all 5 articles
Introduction: The mechanism by which socioeconomic status (SES) affects bone mineral density (BMD) remains unknown, and body mass index (BMI) may be a potential mediator. The purpose of this study was to investigate whether BMI mediates the relationship between SES [education level and poverty income ratio (PIR)] and lumbar BMD and the proportion it mediates.
Methods: This study included a total of 11,075 adults from the National Health and Nutrition Examination Survey (NHANES). Lumbar BMD was measured at the lumbar spine by dual-energy X-ray absorptiometry (DXA). Multivariate linear regression and smoothing curve fitting were used to investigate the relationship between SES and lumbar BMD. Mediator analysis was used to investigate the proportion of BMI mediating the association between SES and BMD.
Results: In the fully adjusted model, there was a positive correlation between SES and BMD (education level: β = 0.025, 95% CI: 0.005, 0.045; PIR: β = 0.007, 95% CI: 0.002, 0.011). Mediation analysis showed that BMI mediated the relationship between PIR, education level, and lumbar BMD with a range of mediation proportions from 13.33 to 18.20%.
Conclusion: BMI partially mediated the positive association between SES and BMD, and this association may be largely mediated by factors other than BMI.
Osteoporosis is a bone disease characterized by impaired bone strength that puts individuals at increased risk of fractures in the spine and joint areas (1, 2). As the global population ages, osteoporosis imposes a heavy socioeconomic and public health burden (3). The annual cost of osteoporosis fracture prevention and treatment in the United States is expected to exceed $50 billion 20 years from now (4, 5).
Investigation of risk factors for osteoporosis is an important tool for maintaining bone mass and reducing fracture risk (6). In addition to common laboratory and screening indicators (e.g., blood lipids, body composition, etc.) (7, 8), sociological factors are receiving increasing attention in bone metabolism (9). Wang and Dixon used multiple linear regression to investigate and find a significant positive association between education level, poverty income ratio (PIR), and BMD in menopausal women (10). A recent cross-sectional study in adult men again validated this association and highlighted the importance of socioeconomic status (SES) in the management of osteoporosis (11). However, the mechanisms behind the association between SES and BMD are complex and unclarified. Available evidence suggests that this association may arise primarily from the indirect effects of potential mediators, and exploring the main mediators is important for targeting groups with unequal SES for the prevention and management of osteoporosis (12, 13).
Individuals with low SES are often associated with problems such as inadequate energy intake (14) and lack of essential nutrients (15), which may lead to an unhealthy body mass index (BMI) or waist circumference. On the other hand, BMI has long been considered to be strongly associated with SES as a protective factor against bone loss (16, 17). Given these associations, BMI is considered to be a potentially important factor in mediating the relationship between SES and BMD.
Therefore, a cross-sectional study based on the four cycles of National Health and Nutrition Examination Survey (NHANES) 2011–2018 was carried out, to investigate the mediating role of BMI in the association between SES and lumbar BMD.
The NHANES is a comprehensive, national survey that collects health and nutrition information from non-institutionalized civilian residents in the United States (18, 19). The National Center for Health Statistics (NCHS) Research Ethics Review Board authorized the study protocol. At the time of recruiting, all subjects provided written consent. According to inclusion exclusion criteria, excluded 20,434 participants without SES data or BMD data, 7,228 participants age less than 20 years and 419 samples with cancer or malignancy. The study eventually included 11,075 participants (Figure 1).
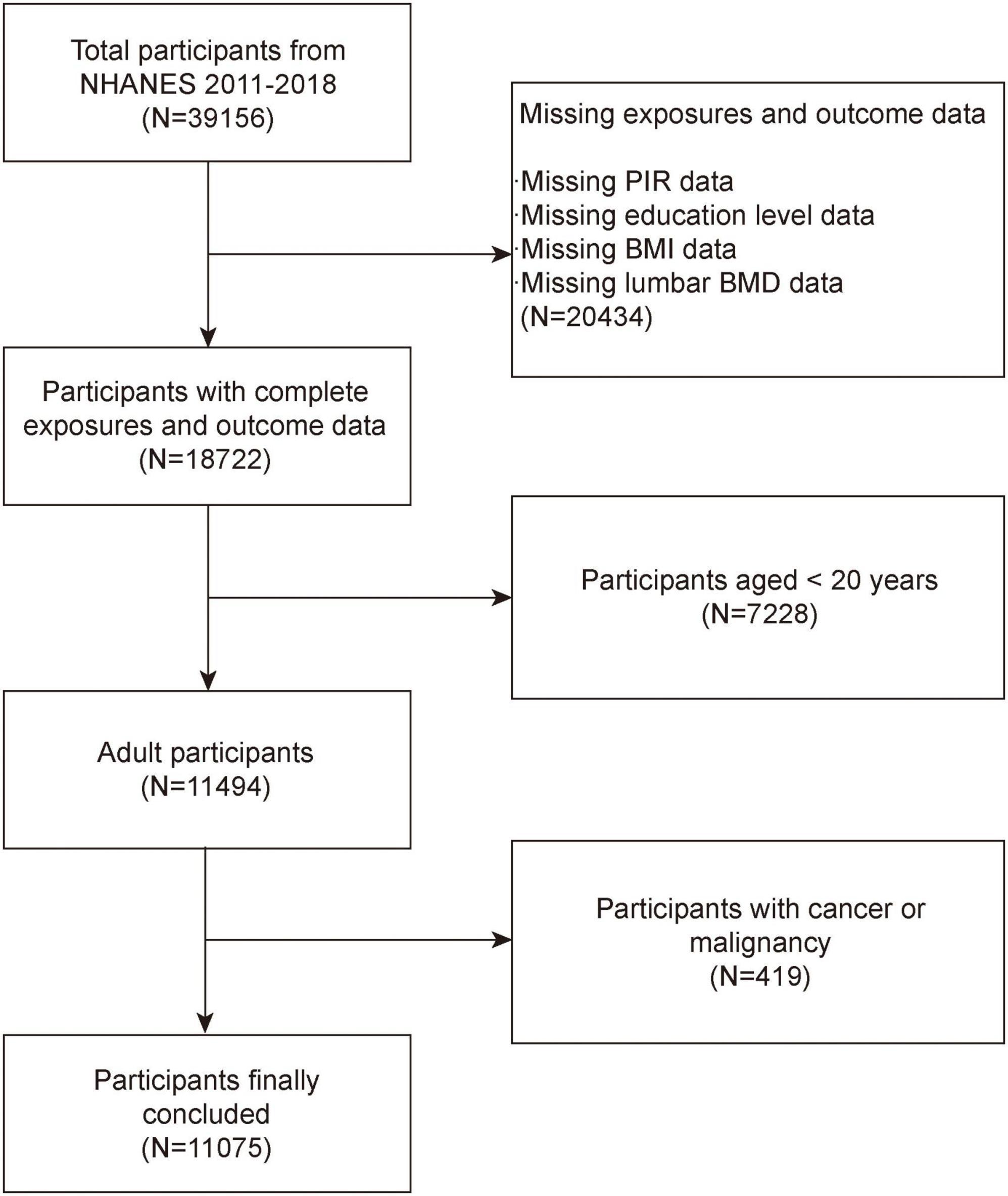
Figure 1. Flow chart of participants selection. NHANES, National Health and Nutrition Examination Survey; BMD, bone mineral density; BMI, body mass index; PIR, poverty income ratio.
The exposure variable is SES, which consists of PIR and educational attainment. PIR is a continuous variable, which is the rate of self-reported household income, based on household or family size, household age composition and year. Educational level is a categorical variable and is divided into three groups less than high school, high school, and more than high school. BMI was calculated according to international standards: weight divided by height squared. Outliers will receive reasonable verification to ensure the credibility of the data. For BMI classification according to WHO standards (underweight <18.5, normal 18.5–24.9, overweight 25–29.9, obese ≥30 kg/m2). Lumbar BMD was measured as the primary outcome of this study by dual-energy X-ray absorptiometry. Age, gender, race, diabetes status, smoke status, high blood pressure status, total calcium, serum phosphorus, blood urea nitrogen, activities status, direct HDL cholesterol, serum creatinine, and total cholesterol were all covariates in this study. The interpretation, measurement and calculation of all variables can be found on the official NHANES website.1
All analyses were performed with R (version 4.2) and Empowerstats (version 4.1). The Chi-square test and t-test were used to assess the demographic characteristics of the participants by BMI subgroups. Multivariate logistic regression analyses were used to investigate the association between SES, BMI and lumbar BMD (20–22). The potential mediated effect of BMI on the association between SES and lumbar BMD was estimated by parallel mediator analysis. The parallel mediation model uses individual indicators as mediators. The direct effect (DE) is the effect of SES on lumbar BMD without mediators. Indirect effects (IE) are the consequences of SES on lumbar BMD that are mediated by mediators. The fraction of mediators was estimated by dividing IE by TE (total effect).
Table 1 shows the weighted characteristics of the participants stratified by BMI. A total of 5,717 male and 5,358 female adults participated, of whom 190 were underweight (1.72%), 3,235 were normal (29.21%), 3,465 were overweight (31.29%), and 4,185 were obese (37.77%). All variables except smoking status differed significantly (P < 0.05) at baseline characteristics according to BMI category. Underweight and obese participants tended to have lower income and education and lower lumbar BMD compared to normal weight and overweight participants.
Table 2 shows the results of multivariate logistic regression analysis with a positive association between SES and lumbar BMD. There was a significant positive linear association between PIR and lumbar BMD, with an increase in lumbar BMD of 0.007 g/cm2 per unit increase in PIR (β = 0.007, 95% CI: 0.002, 0.011). This association also existed between education level and lumbar BMD, participants with more than high school education having a 0.025 g/cm2 higher lumbar BMD than those with less than high school education (β = 0.025, 95% CI: 0.005, 0.045). And participants with high school education having a 0.019 g/cm2 higher lumbar BMD than those with less than high school education (β = 0.019, 95% CI: 0.003, 0.035).
The results of the multiple logistic regression analysis showed a positive relationship between BMI and lumbar BMD, and this association remained significant and stable in all models (Table 2). For every 1 kg/m2 increase in BMI, lumbar BMD increased by 0.001 g/cm2 (β = 0.001, 95% CI: 0.001, 0.002). In contrast, when BMI was transformed into a categorical variable for analysis, this relationship became reversed and insignificant in overweight participants. When subgroup analysis was performed by gender, the relationship between BMI and lumbar BMD showed a positive association in both male and female participants.
Considering that the results were not significant in the sensitivity analysis, smoothed curve fitting was further utilized to confirm the non-linear relationship between BMI and lumbar spine BMD. The results showed a non-linear positive relationship between BMI and lumbar BMD with saturated values (Figure 2).
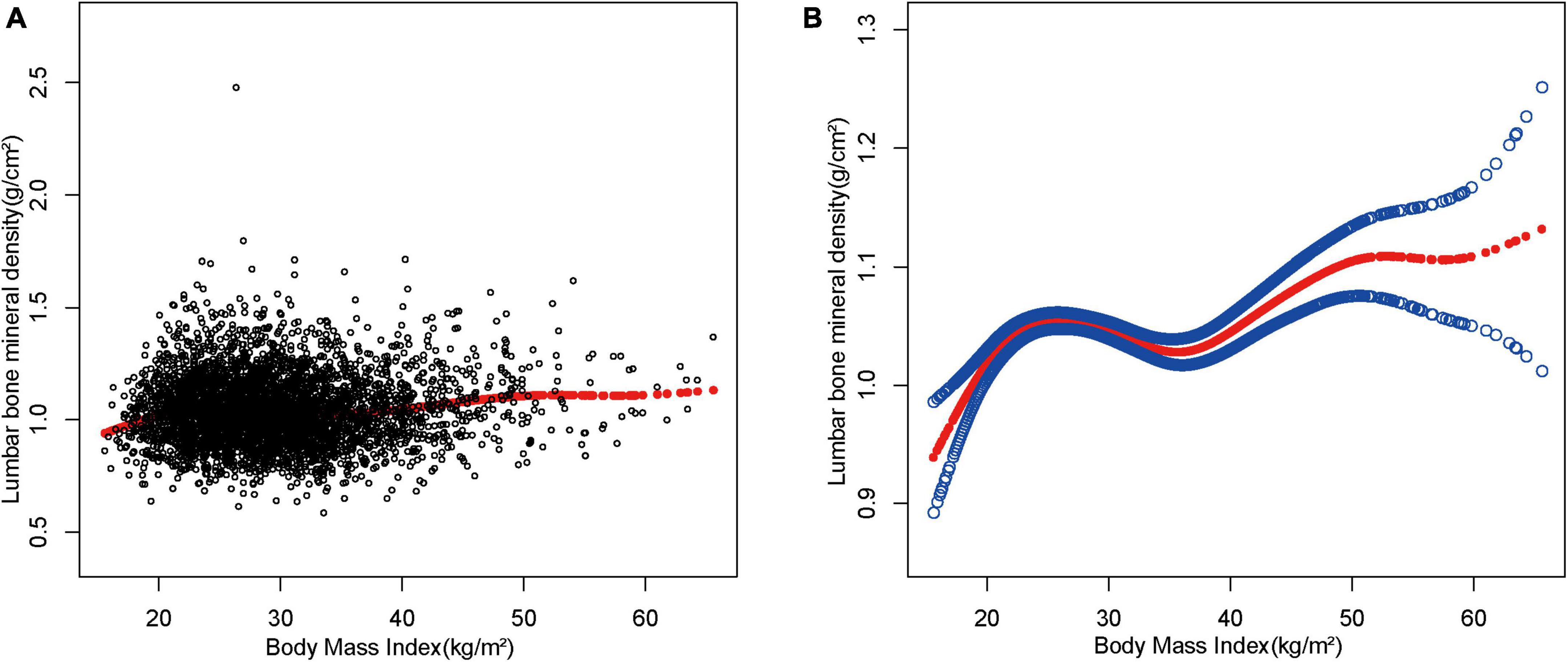
Figure 2. The association between body mass index and lumbar bone mineral density. (A) Each black point represents a sample. (B) The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit.
The mediation analysis investigated whether and to what extent BMI mediated the association between SES and lumbar BMD. Table 3 shows the total effect, which is the effect of SES on lumbar BMD; the direct effect, which is the effect of PIR, education level on lumbar BMD, not mediated by BMI; and the indirect effect, which is the effect of PIR, education level on lumbar BMD, mediated by BMI. In general, the direct effect greatly exceeded the indirect effect, although the statistical significance of the latter was significant. The proportion of BMI mediating the effect of PIR and education level on lumbar BMD was 18.20 and 13.33%, respectively (Figure 3).
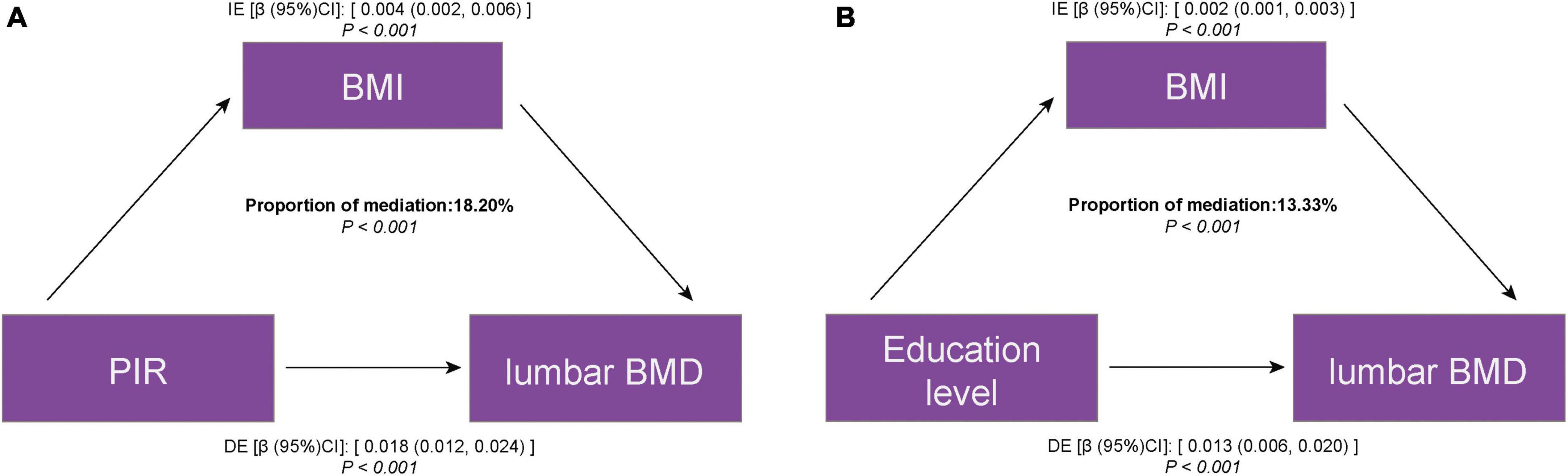
Figure 3. Estimated proportion of the association between SES and lumbar BMD mediated by BMI. (A) PIR and lumbar BMD; (B) education level and lumbar BMD. IE, indirect effect; DE, direct effect; mediation proportion = IE/DE + IE.
In the present study, the results of the multiple regression analysis suggest that US adults with higher SES are associated with higher lumbar BMD. More importantly, this study found that BMI mediated the positive association between SES and lumbar BMD, although the proportion of mediation was less than 20%. This suggests that the association between SES and BMD may be primarily due to factors other than BMI, such as genetics, dietary intake, and levels of systemic inflammation.
For most causes of morbidity and mortality, SES is of great importance and impact (23–25). Therefore, the study of SES as a risk factor for bone health is essential to reduce the public health burden. The association between SES and BMD has been of interest to researchers for 30 years, but results have been inconsistent due to differences in populations and study methods (26). Fehily et al. in 1992 investigated factors that may have influenced BMD during the development of over 500 14 years-olds and showed that males in manual occupations may have higher BMD (27). Results from the Louisiana Osteoporosis Study suggest a positive association between SES and BMD in the total population, with males with lower education and females with lower income being the most susceptible to relatively lower BMD (28). A meta-analysis including eight epidemiological studies showed that most population-based studies support the idea that participants with higher income levels and education are more likely to have higher BMD (29). This finding was also validated in this cross-sectional study, which included 11,075 representative US participants. However, the reasons behind the positive association between SES and BMD are complex and unexplained. Based on the available evidence, the negative effects of SES on BMD are thought to possibly stem from unhealthy lifestyles, including factors such as food insecurity (30), lack of essential nutrients (31, 32), and exposure to harmful substances (33). Health outcomes of an unhealthy lifestyle, such as underweight (34) and visceral fat accumulation (35, 36), may further negatively affect bone metabolism.
In the past, obesity and being overweight have been considered a protective factor. A positive association between BMI and BMD was found in several studies as early as 20 years ago (37, 38). Researchers concluded that BMI reduced the risk of bone loss and fracture in gender-specific populations and groups of menopausal women (39, 40). The results of multivariate logistic regression and subgroup analyses also indicated a positive association between BMI and BMD, which was maintained significantly in both men and women. The mechanisms underlying the positive association between obesity and BMD have long been described. The main include: (1) the mechanical overload generated in the presence of obesity leads to bone deformation, which triggers a series of transduction signals that stimulate increased bone mass through increased osteoblast activity; (2) increased osteogenic differentiation and osteoblast maturation of mesenchymal stem cells through adipocyte production of sex steroids; and (3) adipose tissue is a substrate for sex hormone synthesis and secretes adipokines and cytokines, which play a role in bone metabolism. Given these mechanisms, obesity is thought to be a potentially important mediator of the association between SES and BMD, and the results of the mediation analysis support this hypothesis.
Exploring the main mediators of the relationship between SES and BMD is important for the prevention and management of osteoporosis (11). The data suggest that the effects of SES on BMD are broad and complex and may affect bone metabolism in a variety of ways, including through diet, inflammation, and physical activity patterns (32, 41–43). However, a significant proportion of these can have a large effect on body size, with changes in both diet and physical activity patterns leading to corresponding changes in BMI, which can further influence bone metabolic processes (44). The results of mediating effects analysis suggest that BMI is indeed a mediator of the relationship between SES and BMD, but the proportion of mediators for both PIR and education level is below 20%, implying that there may be other major mediators. Dietary intake factors may be worth investigating. Lim et al. investigated calcium intake among adults in six regions of Korea, and the authors found significant regional differences in calcium intake. Furthermore, participants with lower SES had inadequate calcium intake and low diet quality, and inadequate calcium and energy intake may have a negative impact on bone metabolism (45). In addition, inflammation levels may also be an important factor in the association between SES and BMD (46). It has been shown that lower SES is associated with increased psychosocial stress and elevated blood inflammation levels, and higher levels of systemic inflammation have been shown to be negatively associated with BMD in menopausal women (47). In addition, higher dietary inflammatory potential has also been suggested as a risk factor for bone health, and a meta-analysis that included more than 100,000 participants suggested that a diet high in pro-inflammatory components may increase the risk of osteoporosis and fracture (48).
Our study has some limitations. First, due to the design of the cross-sectional study, the current study were unable to determine the causal relationship between SES and lumbar BMD. In addition, self-reported SES may lead to data bias and affect the accuracy of conclusions (49, 50). Despite these shortcomings, our study has several advantages. This study includes data from a large and representative cross-sectional survey. More importantly, this study confirms the association between SES and lumbar BMD and extends these studies for the first time to the potential mediation effects of BMI.
According to the findings of this study, BMI partially mediates the positive relationship between SES and BMD. Further investigation is needed to determine whether there are higher mediating variables than BMI in this association, such as dietary intake and inflammation levels.
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes.
The studies involving human participants were reviewed and approved by the NCHS Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
YZ and WT designed the research and revised the manuscript. YZ and CT collected and analyzed the data and drafted the manuscript. All authors contributed to the manuscript and approved the submitted version.
The authors appreciate the time and effort given by participants during the data collection phase of the NHANES project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMD, bone mineral density; BMI, body mass index; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; MEC, mobile examination center; SES, socioeconomic status; PIR, poverty income ratio; DE, direct effect; IE, indirect effect; TE, total effect.
1. Cummings S, Melton L. Epidemiology and outcomes of osteoporotic fractures. Lancet. (2002) 359:1761–7.
3. Khosla S, Hofbauer L. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. (2017) 5:898–907.
4. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. (2005) 16(Suppl 2):S3–7.
5. Kahwati L, Weber R, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US preventive services task force. Jama. (2018) 319:1600–12.
6. Kushchayeva Y, Pestun I, Kushchayev S, Radzikhovska N, Lewiecki E. Advancement in the treatment of osteoporosis and the effects on bone healing. J Clin Med. (2022) 11:7477. doi: 10.3390/jcm11247477
7. Xie R, Huang X, Liu Q, Liu M. Positive association between high-density lipoprotein cholesterol and bone mineral density in U.S. adults: the NHANES 2011-2018. J Orthop Surg Res. (2022) 17:92. doi: 10.1186/s13018-022-02986-w
8. Ma M, Liu X, Jia G, Geng B, Xia Y. The association between body fat distribution and bone mineral density: evidence from the US population. BMC Endocr Disord. (2022) 22:170. doi: 10.1186/s12902-022-01087-3
9. Montgomery B, Joseph G, Segovia N, Koltsov J, Thomas T, Vorhies J, et al. The influence of race, income, and sex on treatment and complications of common pediatric orthopedic fractures. Orthopedics. (2023) [Online ahead of print]. doi: 10.3928/01477447-20230104-06
10. Wang M, Dixon L. Socioeconomic influences on bone health in postmenopausal women: findings from NHANES III, 1988–1994. Osteoporosis Int. (2006) 17:91–8. doi: 10.1007/s00198-005-1917-1
11. Xiao P, Fuerwa C, Hsu C, Peng R, Cui A, Jiang N, et al. Socioeconomic status influences on bone mineral density in American men: findings from NHANES 2011-2020. Osteoporos Int. (2022) 33:2347–55. doi: 10.1007/s00198-022-06498-5
12. Brennan S, Pasco J, Urquhart D, Oldenburg B, Hanna F, Wluka A. The association between socioeconomic status and osteoporotic fracture in population-based adults: a systematic review. Osteoporos Int. (2009) 20:1487–97. doi: 10.1007/s00198-008-0822-9
13. Piñar-Gutierrez A, García-Fontana C, García-Fontana B, Muñoz-Torres M. Obesity and bone health: a complex relationship. Int J Mol Sci. (2022) 23:13662. doi: 10.3390/ijms23158303
14. Dennard E, Kristjansson E, Tchangalova N, Totton S, Winham D, O’Connor A. Food insecurity among African Americans in the United States: a scoping review. PLoS One. (2022) 17:e0274434. doi: 10.1371/journal.pone.0274434
15. Ersoy B, Kizilay D, Yilmaz S, Taneli F, Gümüşer G. Bone mineral density, vitamin D status, and calcium intake in healthy female university students from different socioeconomic groups in Turkey. Arch Osteoporos. (2018) 13:135. doi: 10.1007/s11657-018-0482-0
17. Guh D, Zhang W, Bansback N, Amarsi Z, Birmingham C, Anis A. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. (2009) 9:88. doi: 10.1186/1471-2458-9-88
18. Xie R, Zhang Y. Is assessing the degree of hepatic steatosis and fibrosis based on index calculations the best choice for epidemiological studies? Environ Pollut. (2022) 317:120783. doi: 10.1016/j.envpol.2022.120783
19. Zhang Y, Xie R, Ou J. A U-shaped association between serum albumin with total triiodothyronine in adults. J Clin Lab Anal. (2022) 36:e24473. doi: 10.1002/jcla.24473
20. Ouyang Y, Quan Y, Guo C, Xie S, Liu C, Huang X, et al. Saturation effect of body mass index on bone mineral density in adolescents of different ages: a population-based study. Front Endocrinol (Lausanne). (2022) 13:922903. doi: 10.3389/fendo.2022.922903
21. Xie R, Zhang Y. Index-based calculation or Transient Elastography to assess the degree of hepatic steatosis and fibrosis. J Nutr. (2022).
22. Xie R, Huang X, Zhang Y, Liu Q, Liu M. High low-density lipoprotein cholesterol levels are associated with osteoporosis among adults 20-59 years of age. Int J Gen Med. (2022) 15:2261–70.
23. Pincus T, Callahan L, Burkhauser R. Most chronic diseases are reported more frequently by individuals with fewer than 12 years of formal education in the age 18-64 United States population. J Chronic Dis. (1987) 40:865–74. doi: 10.1016/0021-9681(87)90186-x
24. Mol G, van de Lisdonk E, Smits J, van den Hoogen J, Bor J, Westert G. A widening health gap in general practice? Socio-economic differences in morbidity between 1975 and 2000 in The Netherlands. Public Health. (2005) 119:616–25. doi: 10.1016/j.puhe.2004.08.023
25. La Vecchia C, Negri E, Pagano R, Decarli A. Education, prevalence of disease, and frequency of health care utilisation. The 1983 Italian National Health Survey. J Epidemiol Community Health. (1987) 41:161–5. doi: 10.1136/jech.41.2.161
26. Garn S, Clark D. Problems in the nutritional assessment of black individuals. Am J Public Health. (1976) 66:262–7.
27. Fehily A, Coles R, Evans W, Elwood P. Factors affecting bone density in young adults. Am J Clin Nutr. (1992) 56:579–86.
28. Du Y, Zhao L, Xu Q, Wu K, Deng H. Socioeconomic status and bone mineral density in adults by race/ethnicity and gender: the Louisiana osteoporosis study. Osteoporos Int. (2017) 28:1699–709. doi: 10.1007/s00198-017-3951-1
29. Brennan S, Pasco J, Urquhart D, Oldenburg B, Wang Y, Wluka A. Association between socioeconomic status and bone mineral density in adults: a systematic review. Osteoporos Int. (2011) 22:517–27.
30. Eicher-Miller H, Mason A, Weaver C, McCabe G, Boushey C. Food insecurity is associated with diet and bone mass disparities in early adolescent males but not females in the United States. J Nutr. (2011) 141:1738–45. doi: 10.3945/jn.111.142059
31. Stounbjerg N, Mølgaard C, Cashman K, Michaelsen K, Damsgaard C. Vitamin D status of 3-year-old children in Denmark: determinants and associations with bone mineralisation and blood lipids. Eur J Nutr. (2023) [Online ahead of print]. doi: 10.1007/s00394-023-03084-1
32. Luo J, Liu M, Zheng Z, Zhang Y, Xie R. Association of urinary caffeine and caffeine metabolites with bone mineral density in children and adolescents. Medicine. (2022) 101:e31984. doi: 10.1097/MD.0000000000031984
33. Xie R, Liu Y, Wang J, Zhang C, Xiao M, Liu M, et al. Race and gender differences in the associations between cadmium exposure and bone mineral density in US Adults. Biol Trace Elem Res. (2022) [Online ahead of print]. doi: 10.1007/s12011-022-03521-y
34. Coin A, Sergi G, Benincà P, Lupoli L, Cinti G, Ferrara L, et al. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos Int. (2000) 11:1043–50.
35. Xie R, Zhang Y, Yan T, Huang X, Xie S, Liu C, et al. Relationship between nonalcoholic fatty liver disease and bone mineral density in adolescents. Medicine. (2022) 101:e31164.
36. Xie R, Liu M. Relationship between non-alcoholic fatty liver disease and degree of hepatic steatosis and bone mineral density. Front Endocrinol. (2022) 13:857110. doi: 10.3389/fendo.2022.857110
37. Khosla S, Atkinson E, Riggs B, Melton L III. Relationship between body composition and bone mass in women. J Bone Miner Res. (1996) 11:857–63.
38. Felson D, Zhang Y, Hannan M, Anderson J. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. (1993) 8:567–73. doi: 10.1002/jbmr.5650080507
39. Paganini-Hill A, Chao A, Ross R, Henderson B. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology. (1991) 2:16–25. doi: 10.1097/00001648-199101000-00004
40. Cummings S, Nevitt M, Browner W, Stone K, Fox K, Ensrud K, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. (1995) 332:767–73. doi: 10.1056/NEJM199503233321202
41. Venegas-Aviles Y, Rodríguez-Ramírez S, Monterrubio-Flores E, García-Guerra A. Sociodemographic factors associated with low intake of bioavailable iron in preschoolers: National Health and Nutrition Survey 2012, Mexico. Nutr J. (2020) 19:57. doi: 10.1186/s12937-020-00567-3
42. Zuercher M, Harvey D, Santiago-Torres M, Au L, Shivappa N, Shadyab A, et al. Dietary inflammatory index and cardiovascular disease risk in Hispanic women from the Women’s Health Initiative. Nutr J. (2023) 22:5. doi: 10.1186/s12937-023-00838-9
43. Wilson O, Smith M, Duncan S, Hinckson E, Mizdrak A, Richards J. Differences in physical activity participation among young adults in Aotearoa New Zealand. BMC Public Health. (2023) 23:150. doi: 10.1186/s12889-023-15063-6
44. Kim H, Rajbhandari A, Krile R, Lang I, Antonakos C, Colabianchi N. Body mass index trajectories among the healthy communities study children: racial/ethnic and socioeconomic disparities in Childhood Obesity. J Racial Ethn Health Disparities. (2023) doi: 10.1007/s40615-023-01511-x [Epub ahead of print].
45. Lim H, Park Y, Lee H, Kim T, Kim S. Comparison of calcium intake status by region and socioeconomic status in Korea: the 2011-2013 Korea National Health and Nutrition Examination Survey. J Bone Metab. (2015) 22:119–26. doi: 10.11005/jbm.2015.22.3.119
46. Richman A. Concurrent social disadvantages and chronic inflammation: the intersection of race and ethnicity, gender, and socioeconomic status. J Racial Ethn Health Disparities. (2018) 5:787–97. doi: 10.1007/s40615-017-0424-3
47. Tang Y, Peng B, Liu J, Liu Z, Xia Y, Geng B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018. Front Immunol. (2022) 13:975400. doi: 10.3389/fimmu.2022.975400
48. Fang Y, Zhu J, Fan J, Sun L, Cai S, Fan C, et al. Dietary Inflammatory Index in relation to bone mineral density, osteoporosis risk and fracture risk: a systematic review and meta-analysis. Osteoporos Int. (2021) 32:633–43. doi: 10.1007/s00198-020-05578-8
49. Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
Keywords: bone mineral density, mediation effect, NHANES, socioeconomic status, body mass index
Citation: Zhang Y, Tan C and Tan W (2023) BMI, socioeconomic status, and bone mineral density in U.S. adults: Mediation analysis in the NHANES. Front. Nutr. 10:1132234. doi: 10.3389/fnut.2023.1132234
Received: 27 December 2022; Accepted: 21 February 2023;
Published: 07 March 2023.
Edited by:
Paola De Luca, Ospedale Galeazzi S.p.A., ItalyCopyright © 2023 Zhang, Tan and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenfu Tan, dXNjdHdmQDE2My5jb20=; orcid.org/0000-0001-5975-3021
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.