- 1The ICAR Research Complex for North Eastern Hill Region (ICAR RC NEH), Umiam, India
- 2National Bureau of Plant Genetic Resources, Indian Council of Agricultural Research (ICAR), New Delhi, India
- 3Fazl Ali College, Mokokchung, Nagaland, India
- 4Indian Council of Agricultural Research (ICAR) - Indian Institute of Agricultural Biotechnology, Ranchi, India
Introduction
Perilla (Perilla frutescens L.) is an annual aromatic plant of the Lamiaceae family cultivated and widely consumed in most Asian countries. The cultivated tetraploid species P. frutescens L. Britton include var. frutescens, a regular vegetable crop and an oil crop, and var. crispa, known for both medicinal and nutritional value. It is commonly known as beefsteak plant, perilla mint, Chinese basil, or purple mint. Leaves are used as culinary herb, seedlings and seed oils are added to salads while seeds are used as garnish or condiment. Perilla is also integral to Chinese traditional medicine with proven cures and treatments (1).
Nutrition and bioactives content
Perilla seed oil is considered one of the best plant sources of omega fatty acids, with about 53–62% α-linolenic acid (ALA, 18:3, ω-3), 10–13% linoleic acid (LA, 18:2, ω-6) and 11–16% oleic acid (18:1, ω-9) and mean ω-6/ω-3 ratio of about 0.20 (2, 3). Moreover, it hosts adequate concentrations of minerals, vitamins, amino acids, flavonoids and polyphenols in different edible forms of seeds and leaves, making it a versatile nutraceutical crop (Table 1). Seeds contain significantly higher essential amino acids and mineral content compared to leaves. Seeds also provide higher Fe, Mg, Cr and comparable amounts of protein and P among commercial oilseeds, viz. mustard, linseed, groundnut, and sunflower (4). Fresh leaves have higher β-carotene and lutein content as compared to common carotenoids-rich vegetables like carrots, spinach, broccoli, lettuce, parsley and pumpkin leaves (5, 6). Drying perilla leaves effectively boosted the concentrations of flavonoids without compromising on flavor, and thereby emphasize the efficiency of bioactives in dried forms (1, 3, 7).
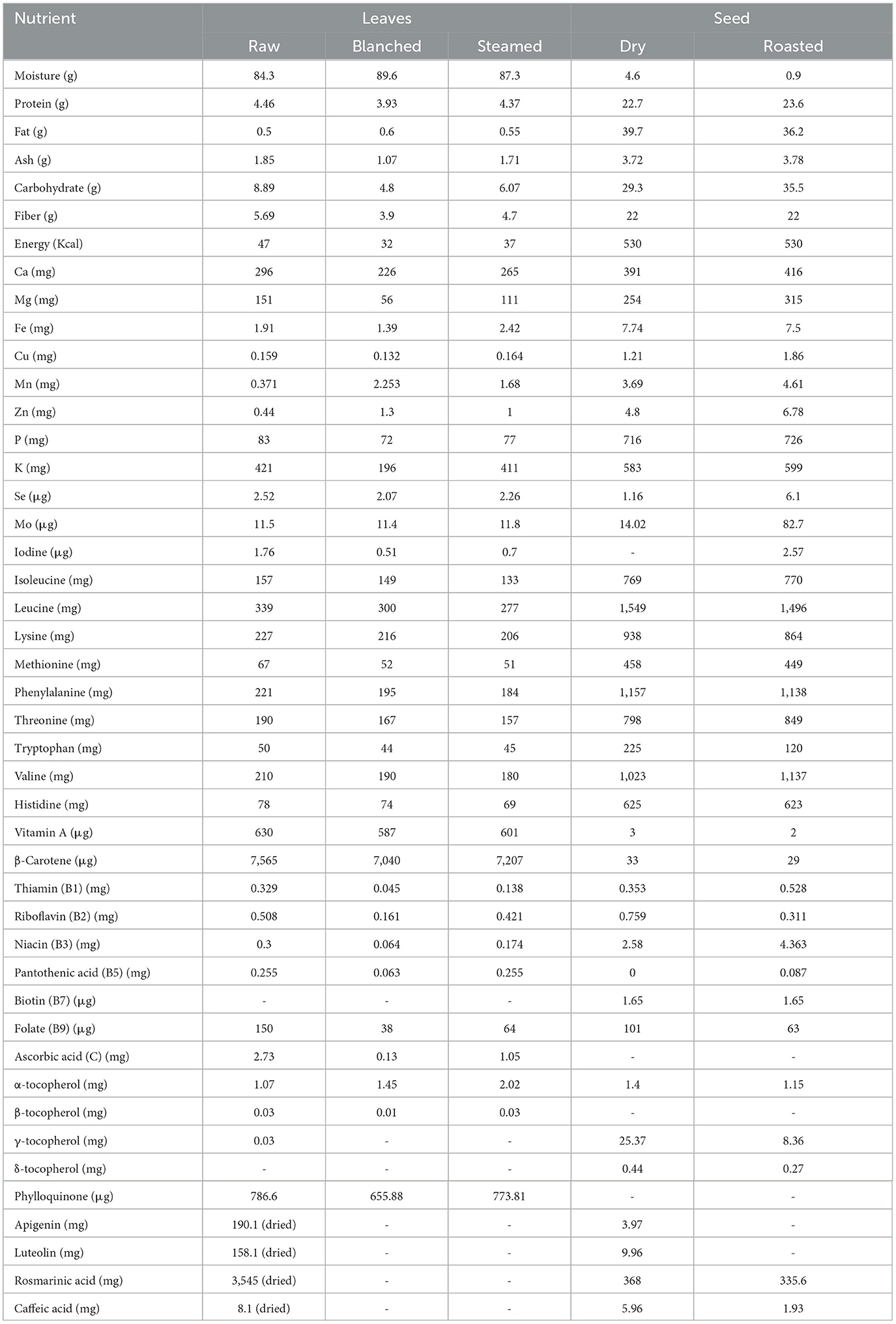
Table 1. Nutritional composition of Perilla in edible forms of leaves and seeds (per 100 g) (3).
Optimal factors
Perilla thrives in higher altitudes of semi-tropical environments with relatively lower mean annual temperature with suitable humidity. Transplanting seedlings during cooler spring season produced taller plants, higher leaf area index, and greater fresh weight of aerial parts, and harvesting of leaves 110 to 120 days after transplanting is considered optimal time to derive highest quantity of leaf perillaldehyde (8). Harvesting of vegetative crop at the end of the second month after sowing is recommended for an optimal compromise between yield and nutritional value (9).
Value-added prospects
Perillaldehyde, the major essential oil of perilla, contributes to aroma and exudes its distinctive flavor in baked foods, pickled vegetables, sauces, salads, meats, puddings and beverages (10). Perillaldehyde has also been judged safe by FAO/ WHO Joint Expert Committee on Food Additives (JECFA) (11). Perilla-fortified products include meatballs from perilla seed meals as fat replacements, traditional pepper oil sauce with 10% replaced perilla oil, yogurt with 10% substituted perilla oil, muffins with freeze-dried perilla leaves, chewable tablets with 8% powdered blanched leaf with improved nutrition profiles (12–16). Premium quality perilla tea is prepared with roasted perilla leaves (180oC for 20 min), with optimal extraction of polyphenols and flavonoids and antioxidant functions (17). Perilla plant flowers when other blossoming plants are scarce, and this aspect of phenology is touted to provide alternative resources in achieving higher honey production with an autumn harvest. Moreover, this extends the egg-laying period of queen bee which thereby, lessen the extent of losses from the parasite Varroa destructor (Anderson & Trueman) (18). Intercropping of tea plants with aromatic plants perilla and basil is reported to improve tree crown formation and vigor of young leaves, and enhance catechins content, thereby improving the yield and quality of tea (19).
Bioavailability of dietary ω-fatty acids
ALA is an essential fatty acid due to the deficiency of human ω-3 fatty acid desaturase enzyme. The metabolic products of LA and ALA include long chains ω-6 PUFA arachidonic acid (C20:4) and ω-3 acids eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6), found abundantly in fish oil, respectively. The rate of synthesis of AA and EPA is directly dependent on the availability of substrate LA and ALA since the same enzymes catalyze the conversion reactions (20). Dietary ALA also undergoes mitochondrial β-oxidation, therefore, its use as a substrate for conversions to EPA and DHA becomes more limited. The consumption of healthy PUFA is paradoxical. The increased dietary consumption of LA-rich oils of soybean, safflower, sunflower and corn in the West accounts for up to 15 times higher intake of LA than ALA, shooting up the ω-6/ω-3 ratio to 16–20:1 against a dietary recommendation of ≤ 4:1, and has in turn increased disease risks (21). The intake of ALA-rich oils like perilla oil, therefore, becomes significant.
Fatty acid biosynthesis and abiotic stress tolerance
Two important transcription factor binding sites, AP2 and B3, responsible for β-ketoacyl-acyl carrier protein synthase II (kasII) and fatty acid desaturase-3 (fad3) genes, respectively, in perilla seeds was highlighted; increase in these genes during oil accumulation phase is directly associated with ALA biosynthesis. Moreover, AP2 and B3 transcription factor families are also integral to abiotic stress such as drought and low temperature environments, where increase of kasII and fad3 genes was seen (22). One significant drought tolerance response is to incorporate newly synthesized PUFA into membrane lipids through gene action (23). This holds relevance for perilla crop which is a short-day plant, requiring longer nights for induction of flowering and low temperatures for PUFA production (24). Perilla crop, which is accustomed to mountainous or terrains of higher altitude is, therefore, seen to thrive in lower temperature and drier conditions during seed maturation. This linked duality of functions of perilla for both fatty acid production and abiotic stress tolerance gives impetus to its importance in both nutrition and abiotic stress response.
Advances in nutritional research
PrLeg, an 11S legumin-like storage protein isolated from developing seeds of perilla, and containing high levels of sulfur-containing amino acids when transformed into potato plant could produce transgenic potato lines that could accumulate high amount of PrLeg transcript with 3-fold increase in methionine content (25). Transgenic perilla lines was developed with overexpression of γ-tocopherol methyltransferase (γ-TMT) gene leading to efficient conversion of γ-tocopherol to α-tocopherol and dramatic increase in seed α-tocopherol (26). Using metabolomics studies, glycolic acid has been highlighted as potential biomarker for perilla seeds to distinguish between perilla from different geographical regions. While this pioneering study is restricted to Korea and Japan, the potential to trace and secure the regional authenticity of perilla sources to ensure global trade safety is promising (27). SSR markers associated with total fatty acid content, ALA and oleic acid have been deciphered (28). Development of such markers linked to target genes can be utilized in selecting superior germplasms and breeding for superior oil quality through marker-assisted selection. Besides, 43 genes involved in fatty acid and triacylglycerol synthesis in perilla seed oil was elucidated recently (29). These studies provide critical information not only for studies on mechanisms involved in ALA synthesis but also for biotechnological production of ALA in other oilseeds.
Advances in health research
In very active female athletes, daily intake of perilla oil as ω-3 fatty acid supplement improved their gut microbiota diversity including butyrate-producing bacteria and subdued the growth of Proteobacteria, besides supplying additional energy (30). Methanolic extracts of seed, leaves and stalk could exhibit antiproliferative activities against human non-small cell lung A549 cancer cells (31). Perilla anthocyanins could induce apoptosis in human cervix Adenocarcinoma Hela cells at 300 μg/mL (32). The addition of perilla oil to drug treatments (epirubicin combined with paclitaxel) in breast cancer patients could effectively improve the quality of life and reduce adverse reactions rates (33). Unlike PUFA-rich safflower and fish oils, perilla oil is reported to regulate brown and white adipose tissue metabolism and prevent the accumulation of body fat and regulation of glucose metabolism (34). Luteolin-rich perilla seed meal fractions (25–100 μg/mL) potentially induce anti-inflammatory action against spike glycoprotein S1 of SARS-CoV-2-induced inflammation in A549 lung cells during incidence of long-COVID by downregulating JAK1/STAT3-inflammasome-dependent inflammatory pathway (35). Perilla pomace extract in cosmetic formulations could exhibit collagenase inhibitory effects at 400 μg/mL and anti-melanogenic effect on B16F10 melanoma cells without inducing cell cytotoxicity, and with clinical improvements in skin elasticity and reduced hyperpigmentation (36).
Looking ahead
The potential of Perilla as a future food crop is immense. In non-Asian countries, studies on perilla or its dietary and culinary use is sporadic. Perilla was identified as new genus in Turkey in 2002, and in Bosnia and Herzegovina only in 2018 (37, 38). Reportedly perilla is considered an invasive plant in natural areas across the mid-Atlantic region of United States (39). The nutritional potential and the versatility of perilla crop is still to be realized in non-Asian countries.
Major perilla growing countries in Asia have standardized cultivation practices suited to their climatic conditions. In India, cultivation is generally at household level with no established cultivation practices. Released varieties are scanty in the absence of effective selection criteria. Several genotypes of Perilla still occur as cultivars in farmers' fields in several East Asian regions (40, 41). Integrating sporadic research data on perilla across different climatic zones and cultivation practices can help address certain challenges. Moreover, in many tribal pockets in India, perilla is restricted only to traditional culinary use; technologies are lacking to upscale perilla resources on a commercial scale.
Perilla is generally a long duration crop, and colder temperatures during flowering time reportedly affect seed setting, along with seed shattering around crop maturation (41). Seed shattering is an evolutionary trait quite common to weedy species. At the same time, the difficulty in distinguishing between Perilla cultivars and weedy species is a challenge. While morphological distinctions are described for cultivated varieties and their weedy forms, obscurity is still observed in large populations (42).
Core collections are lacking in Perilla. The first core collection with 44 accessions based on SSR markers and morphological characteristics is recently reported from South Korea, accounting for 11% of the Perilla collection (43). Unlike traditional crops, in a versatile crop like perilla, the nutritional quality and metabolic content become the most important phenotypic traits along with agromorphological characteristics for development of core collection, and molecular data ensures reliable genetic variations between germplasm. Development of integrated core collections, therefore, is essential for future breeding programs.
Genes coding various transcription factors like WRINKLED, FUSCA3, LEAFY COTYLEDON1, ABSCISIC ACID INSENSITIVE3, along with various enzymes for PUFA biosynthesis and acyl-related enzymes is identified in Perilla (44). Besides their functional validation, the identification of epigenetic control of FAs biosynthesis is also important to understand the vast network of FA biosynthesis in perilla. The complete chloroplast genome of P. frutescens (L.) Britton var. frutescens, 153 Kb in length, has been assembled with 127 annotated genes (45). A near-complete chromosome-level assembly (99.2%) of P. frutescens cultivar Hoko-3 (Pfru_yukari_1.0) is established with a genome size of 1.258 Gb and 72,983 functionally annotated genes (46). Advances in perilla genomics are unique resources for future genome editing studies, and metabolic engineering of perilla to enhance the biosynthesis of important metabolites.
While the therapeutic uses of perilla is documented, ample preclinical studies in vitro and in vivo and in animal models provide potential biological evidence for preventive therapy. More investigations on the therapeutic findings in clinical settings are required for validation of their efficacy for efficient product development and ethical use.
This ancient underutilized food crop requires contemporary interventions. Variations in the performance of perilla crop in terms of geographical influences, sowing time, sampling time has been established. New leads in the adaptation and performance of perilla under natural stress conditions like light fluctuations, salinity, acidic soils, waterlogging, high temperatures etc. are still lacking for climate-smart breeding programmes. New emerging pests and diseases also pose new threats. A new fungal disease of Perilla, stem blight caused by Corynespora cassiicola, reported from Korea affected greenhouse production (47). The shifts and extremities in the climate pattern can affect the phenology of crop and thereby crop and vegetative yield. Intercropping with phenolics-rich perilla crop requires attention given its potential to decrease disease index and incidence rate (48). The existence of various abiotic and biotic stressors in perilla crop calls for concurrent research and management strategies across different geographical and climatic factors. The potential of Perilla as a future food crop will be realized when the vagaries of a changing climate on the crop are also addressed.
Author contributions
CA planned, contributed, and revised the manuscript. K-uP contributed and provided technical guidance. AK, SA, SH, RS, LJ, TA, BB, and BI contributed to different subheadings. SJ and PS contributed and compiled the manuscript. AP and VM provided objective insights. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China Vol. I. Beijing: Chemical Industry Publishing House (2005). 53 p.
2. Sargi SC, Silva BC, Santos HMC, Montanher PF, Boeing JS, Santos OOS et al. Antioxidant capacity and chemical composition in seeds rich in omega-3 chia, flax and perilla. Food Sci Tech. (2013) 33:541–548. doi: 10.1590/S0101-20612013005000057
3. Korean Food Composition Table. National Rural Living Science Institute, Rural Development Administration, South Korea (2011).
4. Longvah T, Deosthale YG. Chemical and nutritional studies on Hanshi (Perilla frutescens), a traditional oilseed from northeast India. J Am Oil Chem Soc. (1991) 68:781–4. doi: 10.1007/BF02662172
5. Müller-Waldeck, F. Sitzmann, J Schnitzler, WH Grassmann J. Determination of toxic perilla ketone, secondary plant metabolites and antioxidative capacity in five Perilla frutescens L varieties. Food Chem Toxicol. (2010) 4:264–70. doi: 10.1016/j.fct.2009.10.009
6. Yoon GA, Yeum KJ, Cho YS, Chen CY, Tang G, Blumberg JB et al. Carotenoids and total phenolic contents in plant foods commonly consumed in Korea. Nutr Res Pract. (2012) 6:481–90. doi: 10.4162/nrp.2012.6.6.481
7. Kagawa N, Iguchi H, Henzan M, Hanaoka M. Drying the leaves of Perilla frutescens increases their content of anticancer nutraceuticals. Food Sci Nut. (2019) 7:1494–501. doi: 10.1002/fsn3.993
8. Lee YJ, Yang CM. Seasonal changes of growth and leaf perillaldehyde in Perilla frutescens (L). Britton J Taiwan Agric Res. (2009) 58:114–24. doi: 10.6156/JTAR/2009.05802.05
9. Peiretti PG. Fatty acid content and chemical composition of vegetative parts of perilla (Perilla frutescens L.) after different growth lengths. Res J Med Plants. (2011) 5:72–8. doi: 10.3923/rjmp.2011.72.78
10. Malu TJ, Banerjee N, Singh AK, Kannadasan S, Ethiraj KR. A study of antioxidant potential of Perilladehyde. IOP Conf Ser: Mater Sci Eng. (2017) 263:022014. doi: 10.1088/1757-899X/263/2/022014
11. JECFA. Safety evaluation of certain food additives. In: Fifty-ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series. (2003) No. 50.
12. Ran M, Chen C, Li C, He L, Zeng X. Effects of replacing fat with Perilla seed on the characteristics of meatballs. Meat Sci. (2020) 161:107995. doi: 10.1016/j.meatsci.2019.107995
13. Kim HR, Sanjeev D, Kim ID, Park IJ. Physicochemical and sensory characteristics of pepper oil sauce prepared from perilla oil. Afr J Food Sci. (2016) 10:352–8. doi: 10.5897/AJFS2016.1500
14. Zheng S, He Z, He L, Li C, Tao H, Wang X, et al. Influence of adding Perilla seed oil on potato blueberry yogurt quality during storage at 4° C. LWT. (2022) 168:113921. doi: 10.1016/j.lwt.2022.113921
15. Yoon MH, Kim KH, Kim NY, Byun MW, Yook HS. Quality characteristics of muffin prepared with freeze dried-perilla leaves (Perilla frutescens var. japonica HARA) powder J Kor Soc Food Sci Nut. (2011) 40:581–5. doi: 10.3746/jkfn.2011.40.4.581
16. Wu J, Yang C, Rong Y, Wang Z. Preparation and nutritional characterization of perilla chewable tablet. Procedia Engineering. (2012) 37:202–7. doi: 10.1016/j.proeng.2012.04.227
17. Yun UJ, Yang SY, Lee HS, Hong CO, Lee KW. Optimum roasting conditions for maximizing the quality of tea leached from high functional Perilla frutescens leaves. Kor J Food Sci Tech. (2012) 44:34–40. doi: 10.9721/KJFST.2012.44.1.034
18. Barbieri C, Ferrazzi P. Perilla frutescens: Interesting new medicinal and melliferous plant in Italy. Nat Prod Commun. (2011) 6:1461–3. doi: 10.1177/1934578X1100601013
19. Zhang Z, Tian Y, Gao S, Xu Y, Huang X, Zhang L. Ecological effects of intercropping tea with aromatic plant basil and perilla in young tea plantation. J Tea Sci. (2016) 36:389–95.
20. Djuricic I, Calder PC. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An Update for 2021. Nutrients. (2021) 13:2421. doi: 10.3390/nu13072421
21. Simopoulos AP. Genetic variation, diet, inflammation, and the risk for COVID-19. Lifestyle Genomics. (2021) 14:37–42. doi: 10.1159/000513886
22. Chumphukam O, Pintha K, Khanaree C, Tantipaiboonwong P, Chaiwangyen W, Tipsuwan W et al. Alpha-linolenic acid content and expression of KASII and FAD3 in perilla seeds correlated cultivated areas of northern Thailand. Sci Asia. (2019) 45:408–18. doi: 10.2306/scienceasia1513-1874.2019.45.408
23. Gigon A, Matos A-R, Laffray D, Zuily-Fodil Y, Pham-Thi AT. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann Bot. (2004) 94:345–51. doi: 10.1093/aob/mch150
24. Khodakovskaya M, McAvoy R, Peters J, Wu H, Li Y. Enhanced cold tolerance in transgenic tobacco expressing a chloroplast ω-3 fatty acid desaturase gene under the control of a cold-inducible promoter. Planta. (2006) 223:1090–100. doi: 10.1007/s00425-005-0161-4
25. Goo Y-M, Kim T-W, Lee M-K, Lee S-W. Accumulation of PrLeg, a perilla legumin protein in potato tuber results in enhanced level of sulphur-containing amino acids. C R Biologies. (2013) 336:433–9. doi: 10.1016/j.crvi.2013.09.002
26. Lee B-K, Kim S-L, Kim K-H., Yu S-Hee, Lee S-C, Zhang Z, et al. Seed specific expression of perilla γ-tocopherol methyltransferase gene increases α-tocopherol content in transgenic perilla (Perilla frutescens). Plant Cell Tiss Organ Cult. (2008) 92:47–54. doi: 10.1007/s11240-007-9301-9
27. Kim TJ, Park JG, Kim HY, Ha SH, Lee B, Park SU et al. Metabolite profiling and chemometric study for the discrimination analyses of geographic origin of perilla (Perilla frutescens) and sesame (Sesamum indicum) seeds. Foods. (2020) 9:989. doi: 10.3390/foods9080989
28. Park H, Sa KJ, Hyun DY, Lee S, Lee JK. Identifying SSR markers related to seed fatty acid content in Perilla crop. (Perilla frutescens L) Plants. (2021) 10:1404. doi: 10.3390/plants10071404
29. Kim HU, Lee KR. Shim D, Lee JH, Chen GQ, Hwang S. Transcriptome analysis and identification of genes associated with ω-3 fatty acid biosynthesis in Perilla frutescens (L) var frutescens. BMC Gen. (2016) 17:474. doi: 10.1186/s12864-016-2805-0
30. Kawamura A, Nemoto K, Sugita M. Effect of 8-week intake of the n-3 fatty acid-rich perilla oil on the gut function and as a fuel source for female athletes: a randomised trial. Brit J Nut. (2022) 10:1–11. doi: 10.1017/S0007114522001805
31. Lin ES, Chou HJ, Kuo PL, Huang YC. Antioxidant and antiproliferative activities of methanolic extracts of Perilla frutescens. J Med Plants Res. (2010) 4:477–83. doi: 10.5897/JMPR10.035
32. He YK, Yao YY, Chang YN. Characterization of anthocyanins in Perilla frutescens var. acuta extract by advanced UPLC-ESI-IT-TOF-MSn method and their anticancer bioactivity. Molecules. (2015) 20:9155–69. doi: 10.3390/molecules20059155
33. Kumar K, Teotia D, Gamal A, Al-kaf A. Clinical application of perilla oil in breast cancer. Pharm Bioprocess. (2018) 6:59–63.
34. Takahashi Y, Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Brit J Nut. (2000) 84:175–84. doi: 10.1017/S0007114500001409
35. Dissook S, Umsumarng S, Mapoung S, Semmarath W, Arjsri P, Srisawad K et al. Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: a potential anti-inflammatory compound against inflammation-induced long-COVID. Front Med. (2023) 9:1072056. doi: 10.3389/fmed.2022.1072056
36. Mungmai L, Preedalikit W, Pintha K, Tantipaiboonwong P, Aunsri N. Collagenase and melanogenesis inhibitory effects of Perilla frutescens pomace extract and its efficacy in topical cosmetic formulations. Cosmetics. (2020) 7:69. doi: 10.3390/cosmetics7030069
38. Maslo S, Šemso Š, Sarajlić N. Perilla frutescens (L) Britton (Lamiaceae), a new alien species in the flora of Bosnia and Herzegovina. Glasnik Hrvatskog botaničkog društva. (2019) 7:62–5.
39. Swearingen JM, Fulton JP. Plant Invaders of Mid-Atlantic Natural Areas, Field Guide, 6th Edn. London: Passiflora Press (2022). p. 79-80.
40. Lee BH Ryu SN, Kwak TS. Current status and prospects of quality evaluation in perilla. Korean J Crop Sci. (2002) 47:150–62.
41. Bahuguna A, Prasad B. Plant development and yield as prejudiced by perilla (Perilla frutescens L) germplasm lines in India hill condition. Res J Med Plants. (2014) 8:121–5. doi: 10.3923/rjmp.2014.121.125
42. Luitel BP, Ko, H-C, Hur O-S, Rhee J-H, Baek H-J, Ryu K-Y. Variation for morphological characters in cultivated and weedy types of Perilla frutescens Britt germplasm. Kor J Plant Res. (2017) 30:298–310. doi: 10.7732/kjpr.2017.30.3.298
43. Sa KJ, Kim DM, Oh JS, Park H, Hyun DY, Lee S et al. Construction of a core collection of native Perilla germplasm collected from South Korea based on SSR markers and morphological characteristics. Sci Rep. (2021) 11:23891. doi: 10.1038/s41598-021-03362-0
44. Bae SH, Zoclanclounon YAB, Kumar TS, Oh JH, Lee J, Kim TH, et al. Advances in understanding the genetic basis of fatty acids biosynthesis in perilla: An update. Plants. (2022) 11:1207. doi: 10.3390/plants11091207
45. Shen Q, Yang J, Lu C, Wang B, Song C. The complete chloroplast genome sequence of Perilla frutescens (L). Mitochon DNA Part A. (2016) 27:3306–7. doi: 10.3109/19401736.2015.1015015
46. Tamura K, Sakamoto M, Tanizawa Y, Mochizuki T, Matsushita S, Kato Y et al. A highly contiguous genome assembly of red perilla (Perilla frutescens) domesticated in Japan. DNA Res. (2023) 30:dsac044. doi: 10.1093/dnares/dsac044
47. Lee HB, Kim CJ, Mun HY. First report of stem blight on perilla (Perilla frutescens) caused by Corynespora cassiicola in Korea. Plant Dis. (2009) 93:550. doi: 10.1094/PDIS-93-5-0550A
Keywords: fatty acid biosynthesis, climate resilience, Perilla, nutrition, bioavailability
Citation: Aochen C, Kumar A, Jaiswal S, Puro K-u, Shimray PW, Hajong S, Sangma RHC, Aochen S, Iangrai B, Bhattacharjee B, Jamir L, Angami T, Pattanayak A and Mishra VK (2023) Perilla frutescens L.: a dynamic food crop worthy of future challenges. Front. Nutr. 10:1130927. doi: 10.3389/fnut.2023.1130927
Received: 23 December 2022; Accepted: 15 May 2023;
Published: 01 June 2023.
Edited by:
Salej Sood, Central Potato Research Institute (ICAR), IndiaReviewed by:
Jiban Mitra, Central Research Institute for Jute and Allied Fibres (ICAR), IndiaLiang Leng, China Academy of Chinese Medical Sciences, China
Copyright © 2023 Aochen, Kumar, Jaiswal, Puro, Shimray, Hajong, Sangma, Aochen, Iangrai, Bhattacharjee, Jamir, Angami, Pattanayak and Mishra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chubasenla Aochen, YWFvY2hlbkBnbWFpbC5jb20=
 Chubasenla Aochen
Chubasenla Aochen Amit Kumar
Amit Kumar Sandeep Jaiswal
Sandeep Jaiswal Kekungu-u Puro1
Kekungu-u Puro1 Philanim Wungmarong Shimray
Philanim Wungmarong Shimray Rumki Heloise Ch Sangma
Rumki Heloise Ch Sangma Banshanlang Iangrai
Banshanlang Iangrai Bijoya Bhattacharjee
Bijoya Bhattacharjee Lemnaro Jamir
Lemnaro Jamir Thejangulie Angami
Thejangulie Angami Arunava Pattanayak
Arunava Pattanayak