- 1Senior Department of Cardiology, The Sixth Medical Center, Chinese PLA General Hospital, Beijing, China
- 2Chinese PLA Medical School, Beijing, China
- 3Department of Cardiology, Hainan Hospital, Chinese PLA General Hospital, Sanya, China
Background and objective: Nutritional status assessment in acute coronary syndrome (ACS) patients has been neglected for a long time. The geriatric nutritional risk index (GNRI) is a sensitive indicator for assessing the nutritional status of the elderly. This study aims to explore the association between GNRI and all-cause mortality in the oldest-old patients with ACS.
Methods: The patients who met the inclusion criteria were consecutively enrolled from January 2006 to December 2012. Clinical data were collected on admission, and all subjects were followed after being discharged. The nutritional status was evaluated using GNRI. The relationship between GNRI and all-cause mortality was assessed by using different analyses.
Results: A total of 662 patients with a mean age of 81.87 ± 2.14 years old were included in our study, and followed (median: 63 months, IQR 51–71). Patients whose GNRI ≤ 98 were reported as at risk of malnutrition (31.11%, n = 206). In multivariable analysis, we found that for each SD increase in GNRI, the risk of all-cause mortality lowered by 23%, and the HR for GNRI ≤ 98 was 1.39 (95% CI 1.04–1.86). After stratifying patients into three groups by tertiles of GNRI, we found that the HRs for tertile 2 and tertile 3 were 1.49 (95% CI 1.02–2.19) and 1.74 (95% CI 1.22–2.50), respectively. The trend test revealed a dose–response relationship between GNRI and all-cause mortality in the oldest-old with ACS. Lastly, in subgroup analyses, we found a reliable association between GNRI and all-cause mortality.
Conclusion: Malnutrition is common in the oldest-old patients with ACS, and GNRI could predict their long-term all-cause mortality in a dose-dependent manner. GNRI may be a prospective index for risk-stratification and secondary-prevention in the oldest-old patients with ACS.
1. Introduction
Acute coronary syndrome (ACS) is one of the leading causes of morbidity and mortality worldwide (1, 2). Aging is a vital risk factor for its prevalence and poor clinical outcomes. Almost a third of patients admitted for ACS and two-thirds of those dying from ACS are >75 years old (3, 4). Multi-comorbidities, complicated coronary artery lesions, and high prevalence of frailty in the oldest-old have increased the risk of re-infarcted, bleeding complications, and mortality when compared to younger patients (5–7).
Malnutrition is a common but under-recognized problem in hospitalized patients and caused detrimental and extensive impacts on clinical results with negative and far-reaching consequences for clinical outcomes (8, 9). As estimated, about 30–60% of hospitalized patients are malnourished. It not only leads to a high economic burden but is associated with longer hospital stays and higher mortality (10, 11). A higher prevalence of malnutrition has been found in the elderly (12). Recently, the side effects of malnutrition in cardiovascular diseases have come under the researchers’ spotlight. Clinical studies have demonstrated that malnutritional status has negative effects on people affected by cardiovascular diseases including ACS (13–16).
Geriatric nutritional risk index (GNRI) was first created by Bouillanne et al. to identify nutritional-related complications among the elderly (17–19). It has been used to assess the nutritional status of patients with heart failure (20), chronic kidney disease (CKD) (21), tumors (22), etc. And many studies have revealed that GNRI was significantly associated with vascular calcification, length of hospital stay, and mortality (23, 24). However, studies about the relationship between GNRI and the prognosis of ACS have seldom been conducted (25).
Herein our study aimed to explore the relationship between GNRI and all-cause mortality of the oldest-old ACS patients, investigate the predictive value of GNRI on patients; long-term prognosis, and assess the effectiveness of the risk-stratify for them.
2. Methods
2.1. Study design and population
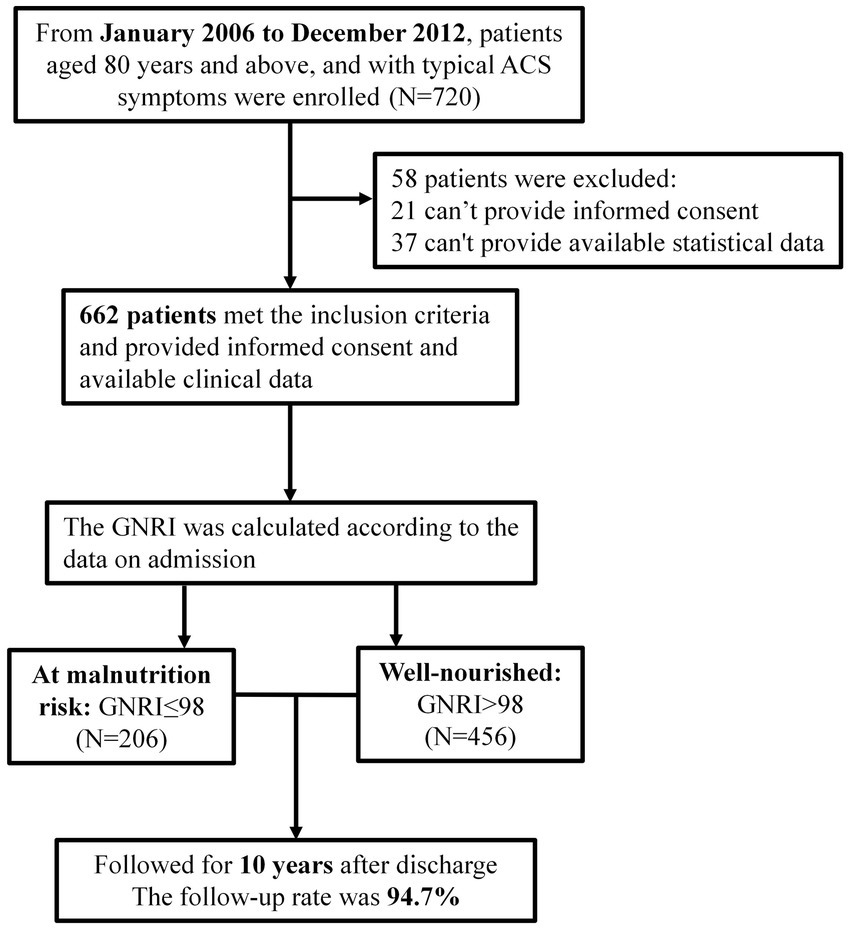
From January 2006 to December 2012, 720 patients aged ≥80 admitted to the cardiology department of the Chinese People’s Liberation Army (PLA) General Hospital for coronary angiography due to ACS symptoms, were enrolled (Figure 1). A total of 699 patients signed their informed consent. The exclusion criteria were: (1) patients with severe valvular heart disease, severe pulmonary hypertension, severe liver or renal insufficiency, rheumatoid arthritis, infectious diseases, and malignant tumors; (2) patients with familial hypertriglyceridemia (triglyceride ≥5.65 mmol/L); and (3) patients with neuropsychiatric problems that prevent them cooperating with the researchers. Most critically, our study followed the Declaration of Helsinki and was certified by the Chinese PLA General Hospital’s Ethics Service Center.
To confirm the diagnosis of coronary heart disease, the cardiac intervention center of the PLA General Hospital performed coronary intervention and perioperative treatment according to current guidelines. All of the angiography results were analyzed using the same image analysis tool. Loading doses of aspirin (300 mg) and clopidogrel (300 mg) were administered before the intervention. The degree of coronary stenosis was determined using the Gensini score (26), and two experienced experts trained the recorders. Individualized interventions, such as intensive medication therapy, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG), were administered based on coronary angiography results, and long-term follow-up was conducted after being discharged.
2.2. Data collection and index evaluation
We collected demographic data (age and gender), anthropometric data [height, body weight, BMI, heart rate, systolic blood pressure (SBP), and diastolic blood pressure (DBP)], laboratory data [total cholesterol (TC), triglycerides (TG), low-density lipid-cholesterol (LDL-C), high-density lipid-cholesterol (HDL-C), estimated glomerular filtration rate (eGFR), fasting plasma glucose (FPG), uric acid (UA), albumin], left ventricular ejection fraction (LVEF), comorbidity data [diabetes mellitus type 2 (T2DM), hypertension, stroke, prior myocardial infarction (MI), hyperlipidemia and chronic kidney disease (CKD)], smoking, drug-usage data [aspirin, clopidogrel, statins, β-blocker and angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB)], coronary lesions data[left anterior descending branch (LAD), left circumflex branch (LCX), right coronary artery (RCA), left main coronary artery (LM), multivessel lesions and Gensini score] and treatment data (intensive medications, PCI and CABG).
The body mass index (BMI) was calculated as follows:
According to WHO criteria in the Asian population, patients could be classified as overweight (BMI > 24.9 kg/m2), normal-weight (BMI 18.5–24.9 kg/m2), and under-weight (BMI < 18.5 kg/m2) (27).
The eGFR was calculated by the Chinese modified Modification of Diet in Renal Disease equation:
Standardized creatinine (Scr) was calculated by the calibration equation:
Chronic kidney disease was defined as eGFR <60 ml/min/1.73 m2.
The diagnostic criteria for diabetes mellitus type 2 (T2DM) were: FPG ≥ 7.0 mmol/L; and (or) random blood glucose (RBG) ≥ 11.1 mmol/L; blood glucose ≥11.1 mmol/L 2 h after oral glucose tolerance test (OGTT).
Hyperlipidemia was defined as the use of lipid-lowering drugs or total serum cholesterol ≥240 mg/dl.
Based on coronary angiography results, the multivessel lesion was defined as having more than 2 vessels with significant diameter stenosis of 50%.
2.3. Assessment of nutritional status
The geriatric nutritional risk index (GNRI) was used in this study to assess the nutritional status of the oldest-old patients with ACS. GNRI is calculated as follow (17):
The ideal weight was calculated as follows: 22 × square of height (m2) (18). It is worth noting that GNRI was created to identify and predict nutritional-related complications (17). The original GNRI cut-off values and grades of nutrition-related risk were: severe risk (GNRI <82), moderate risk (GNRI 82–92), low risk (GNRI 92–98), and no risk (GNRI >98). Patients were often considered as having a normal nutritional status if their GNRI >98 (19). Hence, we used 98 as the cut-off value in the present study. GNRI >98 was defined as well-nourished, while GNRI≤98 was defined as at malnutrition risk.
2.4. Endpoint and follow-up
The follow-up period lasted up to 10 years performed every 12 months after discharge via outpatient visits, telephone records, or medical records of outcomes. During the follow-up period, 37 patients were lost follow-up, leaving 662 (94.7%) patients enrolled in the final statistical analysis. All-cause mortality (cardiac and non-cardiac) was the ultimate endpoint of our research.
2.5. Statistical analysis
The baseline characteristics of the participants were shown according to GNRI >98 and GNRI ≤98. The measurement data of normal distribution were expressed as mean ± SD, and the T-test was used for homogeneity of variance. If the variance is not uniform, the rank-sum test was used. Non-normally distributed measurements were represented by the median and interquartile range (IQR). Statistical data were expressed by quantity, the chi-square test was used to evaluate differences between groups, and an analysis of variance was used to compare data between groups. Pearson correlation test was used to evaluate the correlation between GNRI and clinical parameters. Unadjusted survival curves were generated using log-rank tests in Kaplan–Meier plots. Univariate Cox regression analysis (HR, 95% CI) was used to identify the factors associated with all-cause mortality. p < 0.05 was considered statistically significant. The Cox proportional hazards model was used to estimate the association between GNRI and all-cause mortality. We built three regression models: model 1 is the unadjusted model, model 2 is the partially adjusted model (age and gender), and model 3 is the completely adjusted model (age, gender, diabetes, stroke, CKD, aspirin, eGFR, FPG, UA, EF, Gensini score, LM lesions, multivessel lesions, and HDL-C). The GNRI was transformed into three classification variables for the primary analysis. For the trend test, the new categorical variables were recorded as continuous variables and entered into the regression model. We also standardized the GNRI and then put it into a regression model to determine the relationship between the change in GNRI per SD and all-cause mortality. In addition, we performed a subgroup analysis to explore whether the relationship between GNRI and all-cause mortality could be modified by the following variables: gender, diabetes, hypertension, prior MI, hyperlipidemia, CKD, smoking, LM lesions, and multivessel lesions. Interactions between GNRI and the above variables were tested. Results were reported as HR and 95% CI. Two-sided p < 0.05 was considered statistically significant. All analyses were performed using the statistical software packages R (28) and Empower Stats (29).
3. Results
3.1. Baseline characteristics and malnutrition assessment
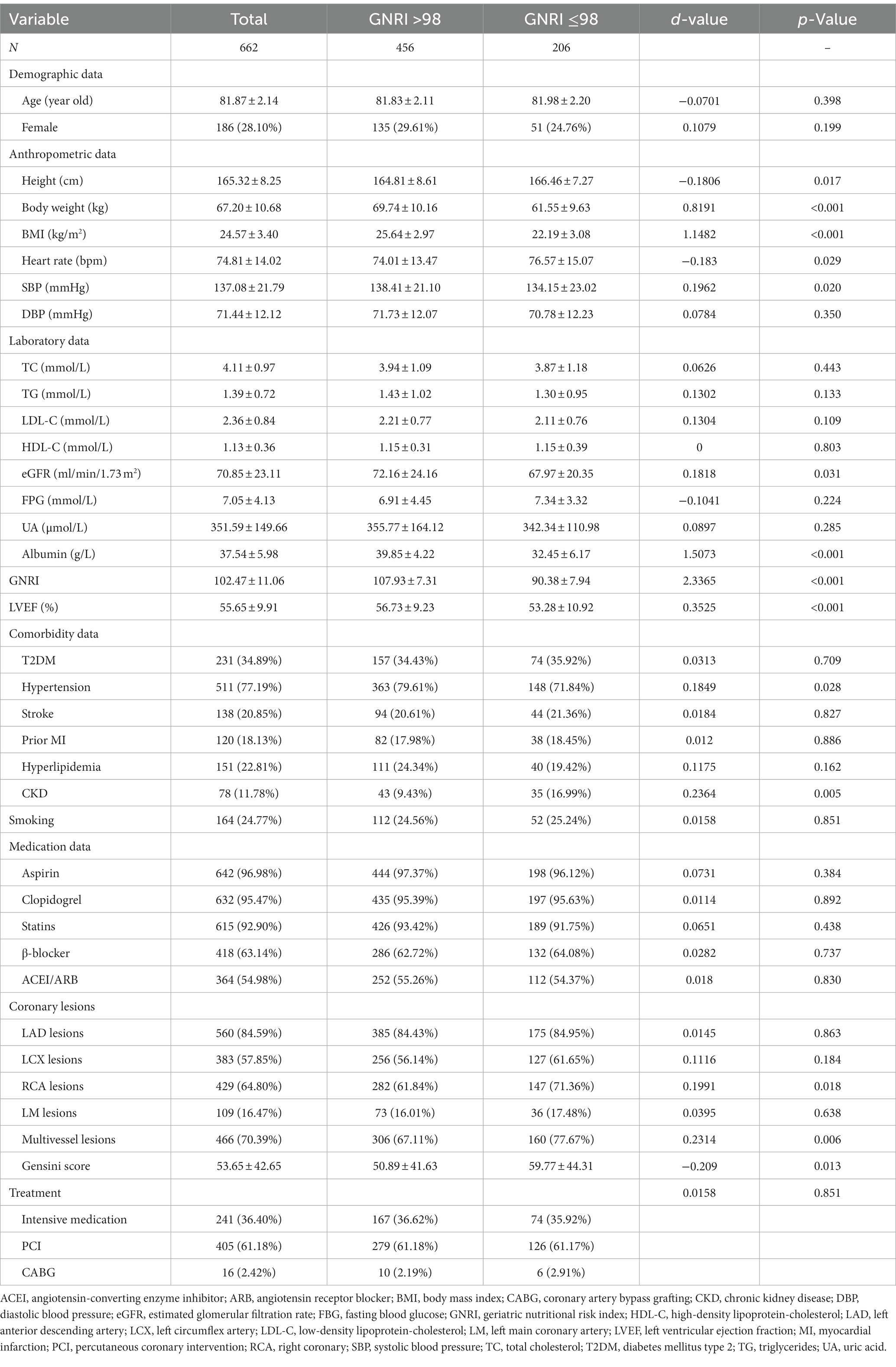
There were 662 oldest-old patients with ACS enrolled in our study, 71.9% (n = 476) of whom were males (Table 1). The mean age of the participants was 81.87 ± 2.14 years old (IQR 80–89), and the average GNRI at admission was 102.47 ± 11.06. According to GNRI, the patients were classified as well-nourished (GNRI >98, n = 456, 68.89%), and at malnutrition risk (GNRI ≤98, n = 206, 31.11%). Patients with low GNRI had lower body weight, BMI, SBP, LVEF, albumin, and eGFR, while they have higher height and heart rate. There were no statistical differences in TC, TG, LDL-C, HDL-C, FPG, and UA between the two groups. Patients at malnutrition risk had a higher proportion of CKD but a lower proportion of hypertension. There was no significant difference in the prevalence of other common complications such as diabetes, hyperlipidemia, or stroke. What is more, we noticed that patients with low GNRI were more likely to have RCA lesions and multivessel lesions, and got a higher Gensini score. In terms of smoking behavior, medication usage, and treatment manners, there was no significant difference between the two groups.
We further analyzed GNRI in patients with different BMI and albumin levels (Figure 2). The high prevalence of malnutrition was found in patients with BMI <18.5 kg/m2 (94.12%), and patients with albumin<35 g/L (74.68%). What is more, there were substantial malnutritional patients in the normal-to over-weight groups.
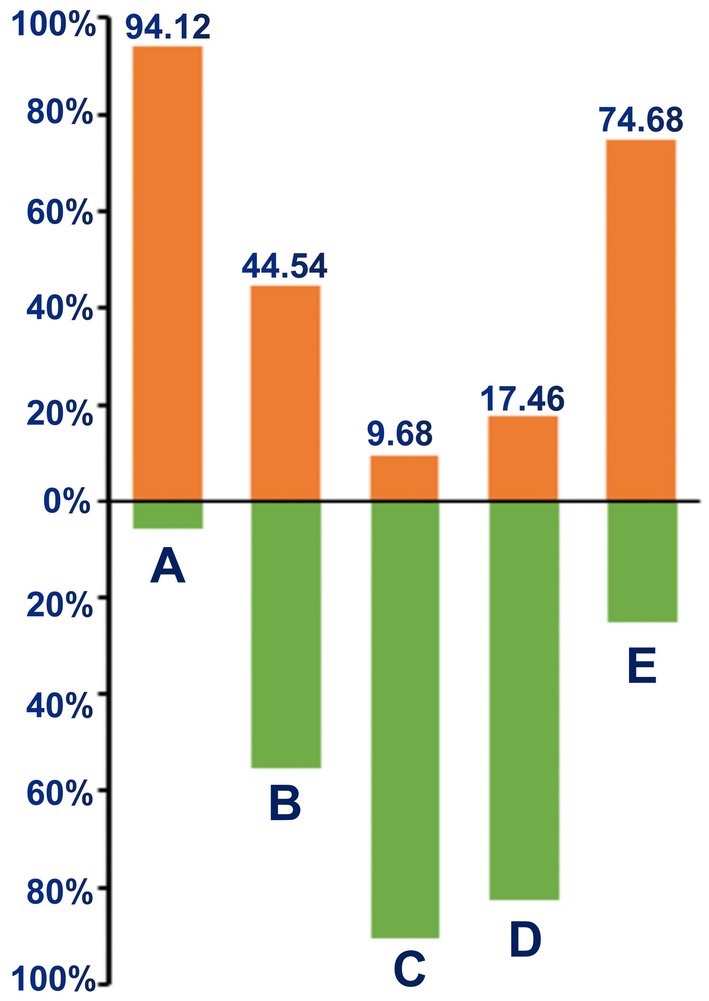
Figure 2. Percentage of malnutrition according to BMI and albumin. (A) Under-weight (BMI <18.5 kg/m2); (B) Normal-weight (BMI 18.5–24.9 kg/m2); (C) overweight (BMI >24.9 kg/m2); (D) normal-albumin (≥35 g/L); (E) hypo-albumin (<35 g/L).
3.2. Association between GNRI and all-cause mortality
The participants were followed for a median of 63 months (IQR 51–74). There were 201 endpoint-events during the follow-up, with 82 occurring in the low GNRI group and 119 in the other. As a nutritional screening index, GNRI was associated with many traditional cardiovascular risk factors (Supplementary Table 1). In univariate analysis (Table 2), a strong positive association was found between all-cause mortality and age, diabetes, stroke, CKD, FPG, Gensini scores, LM lesions, and multivessel lesions. Meanwhile, aspirin, HDL-C, eGFR, LVEF, albumin, and GNRI (HR = 0.97, 95% CI [0.96, 0.99]) were closely associated with a reduction in all-cause mortality. However, male, hypertension, BMI, TC, and LDL-C did not show a significant association with the outcome.
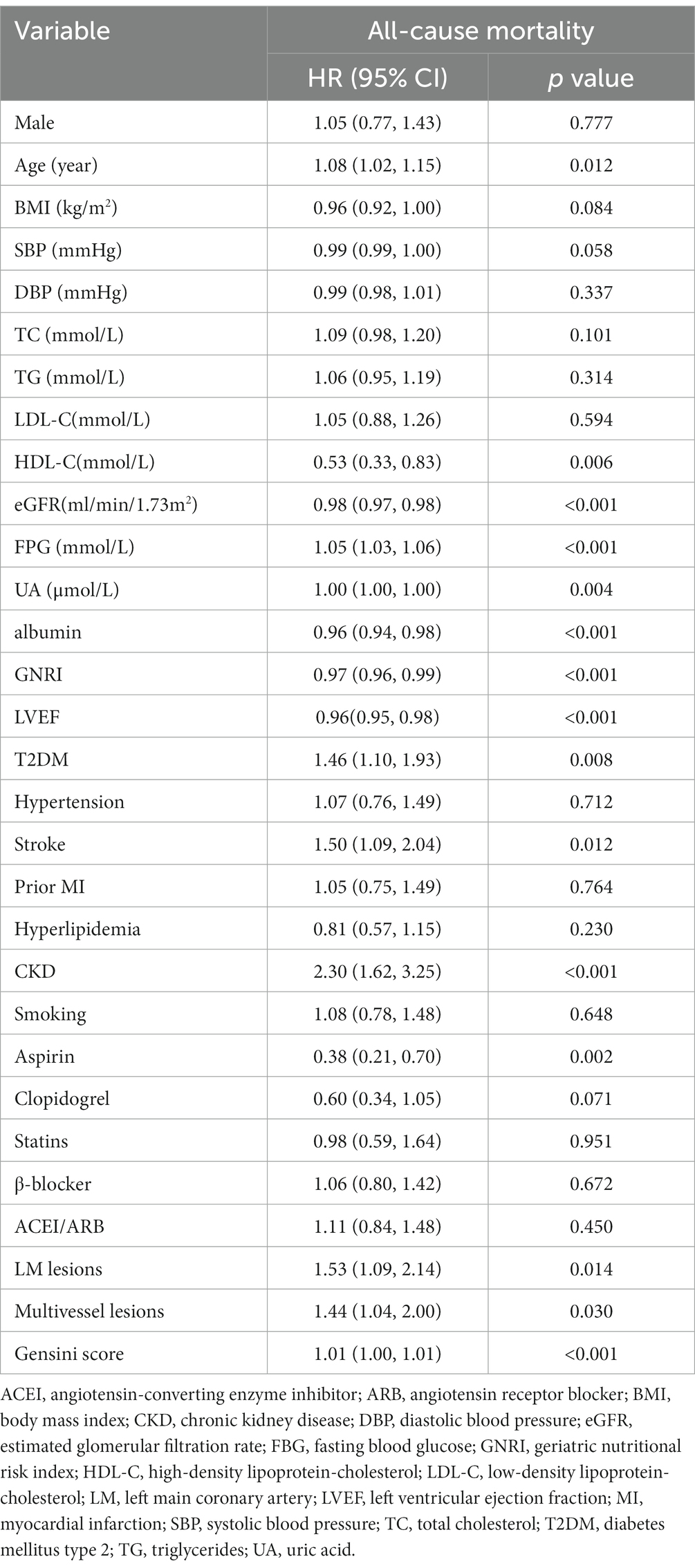
Table 2. Univariate Cox regression analyses for independent variables associated with all-cause mortality.
We further assessed the association between GNRI and all-cause mortality by multivariable Cox regression analysis (Table 3 and Figure 3). For each SD increase in GNRI, the risk of all-cause mortality was lowered by 23%. And when compared with the high GNRI group, the HR of all-cause mortality in the low GNRI group was 1.39 (95% CI [1.04, 1.86], p < 0.05). Then we divided the patients into three groups according to the tertiles of GNRI. Compared with tertile 1, the HRs of tertile 2 and tertile 3 were 1.49 (95% CI [1.02, 2.19], p < 0.05) and 1.74 (95% CI [1.22, 2.50], p < 0.01), respectively. Subsequently, the trend test revealed a dose–response between GNRI and all-cause mortality.
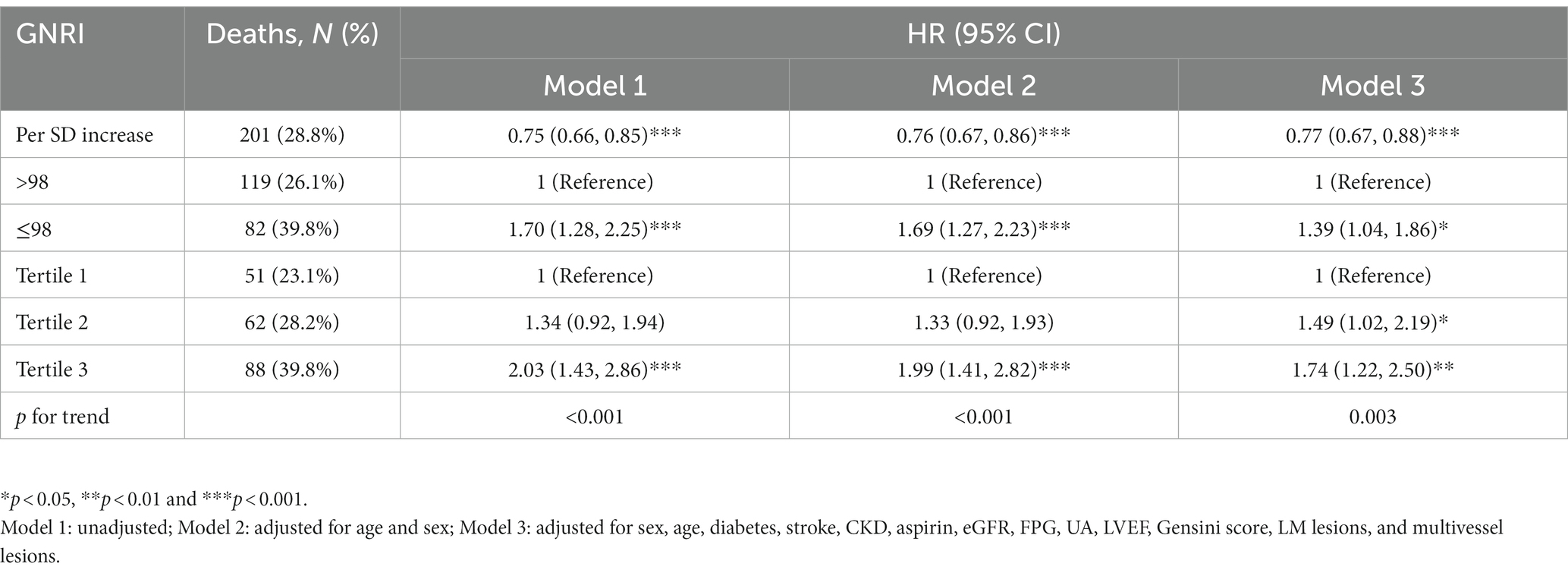
Table 3. Multivariable Cox regression analyses for the association between GNRI and all-cause mortality.
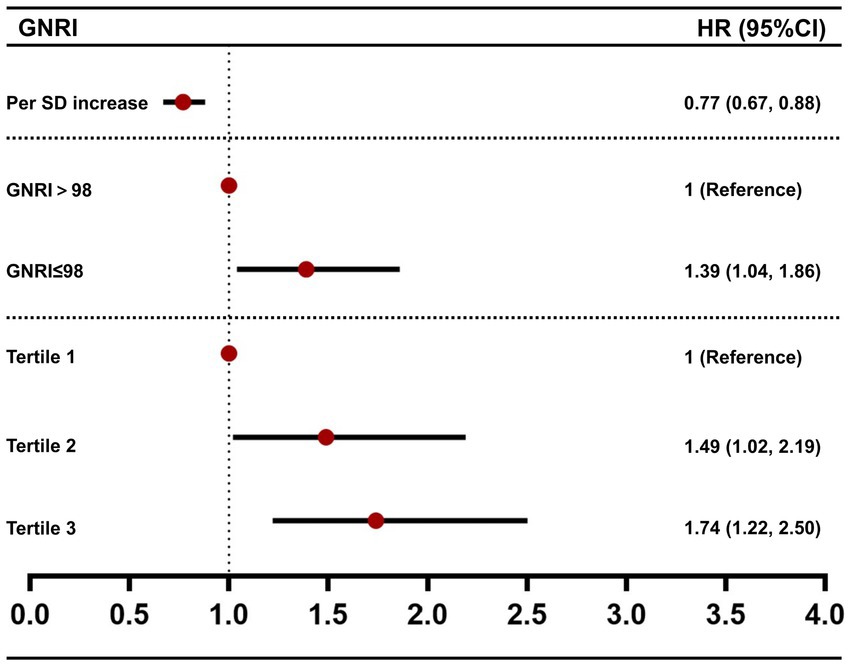
Figure 3. Forest plot of the association between GNRI and all-cause mortality. HR values were from model 3 of Table 3.
3.3. Survival analyses of GNRI and all-cause mortality
According to the Kaplan–Meier survival analysis (Figure 4A), we found that patients with high GNRI had a longer survival time (p < 0.001). Then we further divided GNRI into the tertiles and analyzed them. As shown in Figure 4B, all-cause mortality in tertile 3 was significantly lower than in tertile 1 and tertile 2 (p < 0.001).
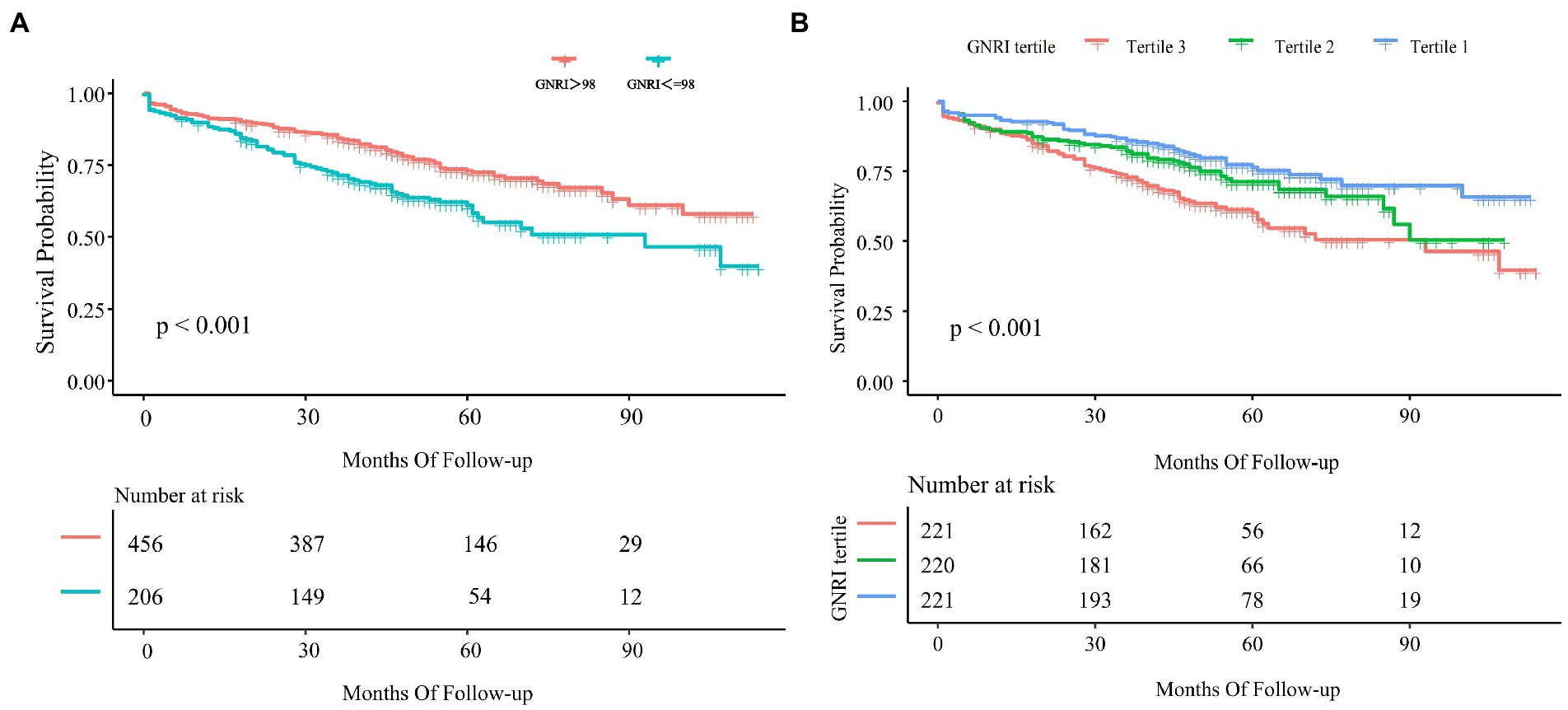
Figure 4. Kaplan–Meier curve of GNRI and all-cause mortality. (A) Kaplan–Meier survival curve for all-cause mortality by GNRI 98. (B) Kaplan–Meier curve for all-cause mortality by tertiles of GNRI. The result was shown as the Kaplan–Meier curve and p-value.
3.4. Subgroup analyses
In the subgroup analysis (Figure 5), we adjusted sex, age, diabetes, stroke, CKD, aspirin, eGFR, FPG, UA, LVEF, Gensini score, LM lesions, multivessel lesions, and HDL-C except for the stratified variables. The results showed that low GNRI was associated with increased all-cause mortality in those males, with diabetes mellitus, with previous MI, without multivessel lesions, and without LM lesions. But the values of p for interaction were all >0.05, suggesting the inverse association between low GNRI with all-cause mortality across all those subgroups.
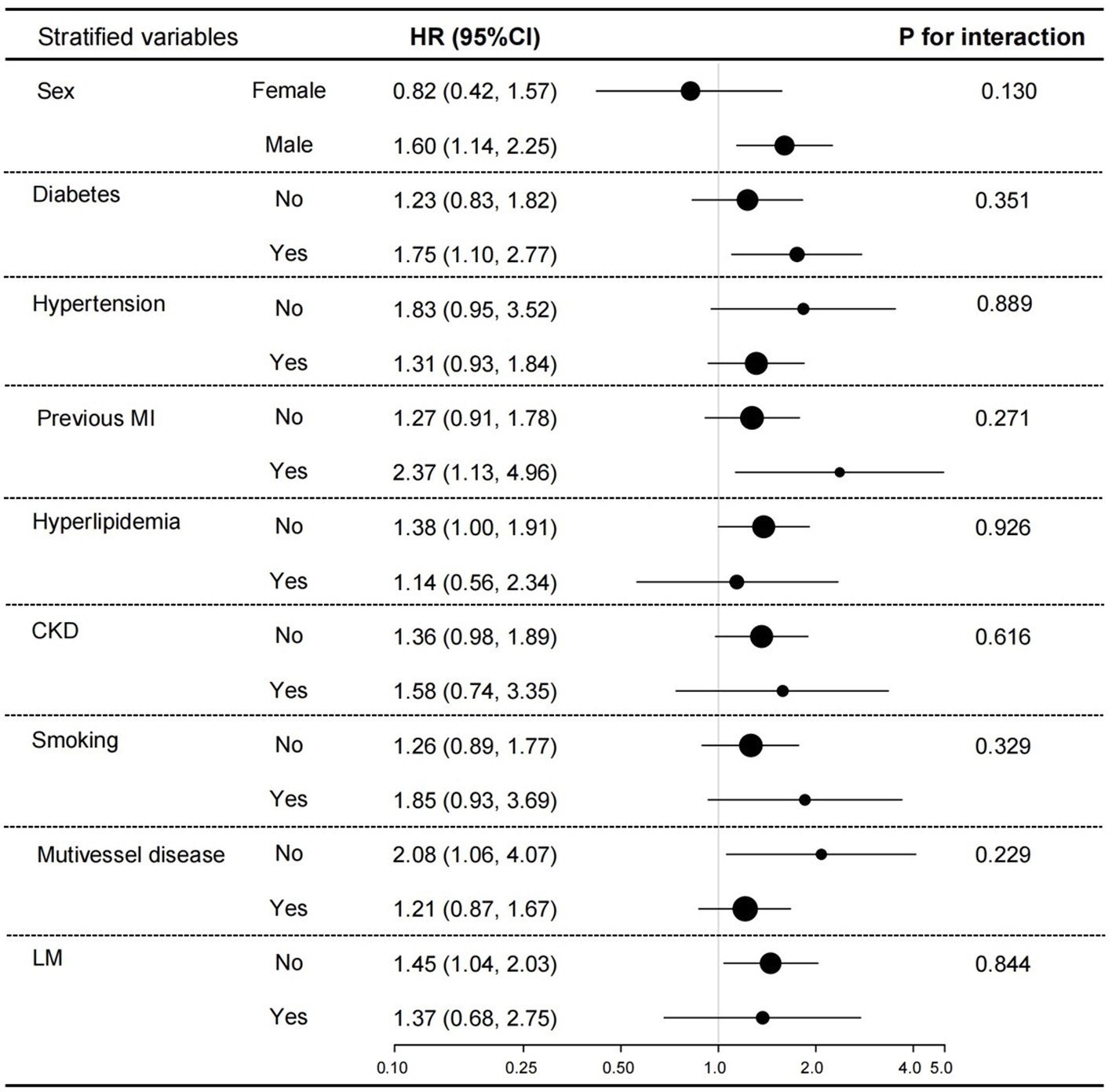
Figure 5. Subgroup analyses for the association between GNRI and all-cause mortality. Adjusted sex, age, diabetes, stroke, CKD, aspirin, eGFR, FPG, UA, LVEF, Gensini score, LM lesions, multivessel lesions, and HDL-C.
4. Discussion
In this study, we collected clinical data from 662 oldest-old patients with ACS who received coronary angiography and followed for 10 years. As far as we know, this’s the first study to investigate the association between GNRI and all-cause mortality in the oldest-old patients with ACS. Our key findings were the following: (1) the prevalence of malnutrition risk was high in the oldest-old patients with ACS; (2) as a reliable tool for assessing the nutritional status of the elderly, GNRI is associated with many traditional cardiovascular risk factors; (3) low GNRI is an independent risk factor of all-cause mortality in a dose-dependent manner; (4) GNRI has a stable predictive ability for all-cause mortality; and (5) GNRI is a prospective indicator to stratify the risk of all-cause mortality in the oldest-old patients with ACS.
Aging and diseases, especially cardiovascular diseases, are the risk factors for malnutrition (30–32). The oldest-old (elderly >80 years old) tend to have a higher malnutrition risk than those 65–80 years old (33). Despite its high prevalence and negative impact on short-and long-term prognosis, malnutrition remains underdiagnosed. One of the reasons is the lack of a broadly acknowledged definition and diagnostic criterion, and assessing patients’ nutritional status in an emergency like ACS is even more challenging. GNRI seems a promising index because that it can be readily calculated using three objective measurements: serum albumin concentration, height, and body weight. GNRI was strongly associated with poor outcomes in elderly emergency surgery patients, according to Jia et al. (34). Therefore, we picked GNRI as the screening tool for malnutrition in the oldest-old patients with ACS and further assess the association between GNRI and the prognosis of the oldest-old ACS patients.
Serum albumin and BMI are common nutrition indicators; however, they are affected by dehydration, heart failure, inflammation, and other factors (9, 35). In comparison, GNRI is more reliable. It is not simply an overlap of albumin and BMI. Adding GNRI to a baseline model of established risk factors increased the predictive effect of mortality beyond BMI or serum albumin (36, 37). GNRI performed much better than serum albumin level alone in predicting MACE in patients receiving the PCI with rotational atherectomy in the previous study (38). Lots of studies have been conducted to investigate the relationship between GNRI and the prognosis of some chronic diseases, such as chronic heart failure, CKD, and tumors. And the results revealed that low GNRI correlated with longer hospital stays and high mortality. However, few studies evaluated the association between GNRI and the prognosis of elderly patients with ACS, let alone the oldest-old patients. To our knowledge, this was the first time to confirm that a low GNRI was an independent predictor of long-term prognosis in the oldest-old patients with ACS.
Common nutrition screening tools include subjective global assessment (SGA), mini nutritional assessment (MNA), MNA-short form (MNA-SF), prognostic nutritional index (PNI), controlling nutritional status (CONUT), etc. (35, 39). These methods are sensitive to subjective biases and have limitations in terms of time, personnel, and the potential for overdiagnosis. Several studies have compared GNRI with them (40, 41). Wafaa et al. (25) demonstrated that GNRI had a stronger predictive value for describing and classifying nutritional status and nutritional-related problems in elderly hospitalized patients. The GNRI was created as a nutrition-related risk index to recognize and anticipate nutritional-related problems. In the present study, we noticed that a low GNRI at admission was a robust predictor for all-cause mortality in the oldest-old individuals with ACS.
In our study, the GNRI was found to have a positive correlation with BMI, eGFR, LVEF, and serum albumin concentration. The roles of abnormal glucose and lipid metabolism in the pathogenesis of ACS have been widely acknowledged (26). Surprisingly, we did not find an association like in other studies (36, 37, 42) between GNRI and FPG, TC, TG, LDL-C, or HDL-C. Our distinct subjects may explain the differences. As we all know, malnutrition is more common in the elderly due to decreased appetite, impaired digestion and absorption ability, and the impact of diseases (43). So, in our oldest-old patients, hyperglycemia and hyperlipidemia were relatively less prevalent. And our patients followed prescriptions more strictly (92.90% vs. 78.7%) so that maintained their superior blood glucose and cholesterol levels. CKD is a severe risk factor for coronary artery disease (CAD) (44, 45). Patients with CKD generated atherosclerotic plaque as a result of traditional (hyperlipidemia, hypertension, diabetes mellitus, smoking, and so on) and non-traditional (uremia-related cardiovascular disease risk factors such as inflammation, aberrant calcium-phosphorus metabolism, and so on) risk factors (46, 47). As eGFR declines, this progress gets even worse. Herein our study, we discovered the correlation between GNRI and eGFR which may further inspire researchers to explore the relationship between nutritional status and cardio-renal syndrome.
Obesity has been considered a traditional risk factor for cardiovascular diseases. Herein the present study, we found that there were substantial malnutritional patients in the normal-to over-weight patients who may be seen as relatively strong before, and our findings may provide evidence supporting the existence of the obesity paradox (48, 49). Inflammation is a crucial factor in the development of ACS, and it also makes a significant contribution to malnutrition. The malnutrition-inflammation-atherosclerosis (MIA) syndrome has already been proposed as a crucial component of geriatric syndrome (50–52). GNRI was strongly associated with the progression of atherosclerosis in elderly CAD patients (53). In the present study, we found that patients with a low GNRI were more prone to developing multivesicular lesions and got a higher Gensini score. This was the first to report an association between GNRI and the location and severity of the culprit coronary arteries.
There have been reported that effective nutritional interventions could significantly reduce the length of hospital stay and mortality of malnutritional patients (54, 55). By using GNRI, one can quickly recognize the risk of malnutrition and then take measures to improve nutritional status, and the prognosis gets ultimately enhanced. The GNRI was created to assess and forecast nutritionally associated problems. In multi-variate analysis and subgroup analysis, we demonstrated that GNRI stably predicts all-cause mortality of the oldest-old patients in a dose-manner. Based on these, we concluded that the GNRI could serve as a perspective index for risk-stratification and secondary prevention of the oldest-old patients with ACS.
4.1. Strengths and limitations
This was the first time enrolled oldest-old patients with ACS, assessed their nutritional status with GNRI on admission, and followed for 10 years. Then we evaluated the association between GNRI and long-term all-cause mortality. In the end, we demonstrated that GNRI is a nutrition assessing tool with prominent advantages, and it may be a hopeful tool to quickly and accurately evaluate nutritional status and predict their prognosis. The results of our study were reliable and practical for clinical use.
However, there were some deficits inevitably. First, this was a single-center cohort study and all participants were Chinese and our was something old, so some selection bias existed. Second, we did not record dietary intake, physical activity, and other factors during the follow-up that may affect nutritional status. Finally, we took 98 as the cut-off value of GNRI, it may need further adjustment according to race or age, etc.
5. Conclusion
In this study, we confirmed that low GNRI was an independent predictive factor for all-cause death in the oldest-old patients with ACS, and the relationship between the two was dose-dependent. GNRI may be a perspective index for risk stratification in the oldest-old patients with ACS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Chinese PLA General Hospital’s Ethics Service Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL, JS, and XH: conducted research and wrote the paper. YL and YS: analyzed the data. YJ, JW, and HL: conducted research. ZF: designed research and had primary responsibility for final content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1129978/full#supplementary-material
References
1. Bergmark, BA, Mathenge, N, Merlini, PA, Lawrence-Wright, MB, and Giugliano, RP. Acute coronary syndromes. Lancet. (2022) 399:1347–58. doi: 10.1016/S0140-6736(21)02391-6
2. Lahnwong, S, Palee, S, Apaijai, N, Sriwichaiin, S, Kerdphoo, S, Jaiwongkam, T, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. (2020) 19:91. doi: 10.1186/s12933-020-01066-9
3. Kayani, WT, Khan, MR, Deshotels, MR, and Jneid, H. Challenges and controversies in the management of ACS in elderly patients. Curr Cardiol Rep. (2020) 22:51. doi: 10.1007/s11886-020-01298-x
4. Jiménez-Méndez, C, Díez-Villanueva, P, and Alfonso, F. Non-ST segment elevation myocardial infarction in the elderly. Rev Cardiovasc Med. (2021) 22:779–86. doi: 10.31083/j.rcm2203084
5. Madhavan, MV, Gersh, BJ, Alexander, KP, Granger, CB, and Stone, GW. Coronary artery disease in patients ≥80 years of age. J Am Coll Cardiol. (2018) 71:2015–40. doi: 10.1016/j.jacc.2017.12.068
6. Ariza-Solé, A, Guerrero, C, Formiga, F, Aboal, J, Abu-Assi, E, Marín, F, et al. Global geriatric assessment and in-hospital bleeding risk in elderly patients with acute coronary syndromes: insights from the LONGEVO-SCA registry. Thromb Haemost. (2018) 118:581–90. doi: 10.1055/s-0038-1623532
7. García-Blas, S, Cordero, A, Diez-Villanueva, P, Martinez-Avial, M, Ayesta, A, Ariza-Solé, A, et al. Acute coronary syndrome in the older patient. J Clin Med. (2021) 10:4132. doi: 10.3390/jcm10184132
8. Norman, K, Haß, U, and Pirlich, M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients. (2021) 13:2764. doi: 10.3390/nu13082764
9. Zhang, Z, Pereira, SL, Luo, M, and Matheson, EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients. (2017) 9:829. doi: 10.3390/nu9080829
10. Freeman, AM, Morris, PB, Barnard, N, Esselstyn, CB, Ros, E, Agatston, A, et al. Trending cardiovascular nutrition controversies. J Am Coll Cardiol. (2017) 69:1172–87. doi: 10.1016/j.jacc.2016.10.086
11. Correia, M, Perman, MI, and Waitzberg, DL. Hospital malnutrition in Latin America: a systematic review. Clin Nutr. (2017) 36:958–67. doi: 10.1016/j.clnu.2016.06.025
12. Tonet, E, Campana, R, Caglioni, S, Gibiino, F, Fiorio, A, Chiaranda, G, et al. Tools for the assessment of the malnutrition status and possible interventions in elderly with cardiovascular diseases. J Clin Med. (2021) 10:1508. doi: 10.3390/jcm10071508
13. Anzaki, K, Kanda, D, Ikeda, Y, Takumi, T, Tokushige, A, Ohmure, K, et al. Impact of malnutrition on prognosis and coronary artery calcification in patients with stable coronary artery disease. Curr Probl Cardiol. (2022):101185. doi: 10.1016/j.cpcardiol.2022.101185
14. Czapla, M, Karniej, P, Juárez-Vela, R, and Łokieć, K. The association between nutritional status and in-hospital mortality among patients with acute coronary syndrome-a result of the retrospective nutritional status heart study (NSHS). Nutrients. (2020) 12:3091. doi: 10.3390/nu12103091
15. Kang, SH, Song, HN, Moon, JY, Kim, SH, Sung, JH, Kim, IJ, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome treated with percutaneous coronary intervention. Medicine. (2022) 101:e30100. doi: 10.1097/MD.0000000000030100
16. Sacks, D, Baxter, B, Campbell, BCV, Carpenter, JS, Cognard, C, Dippel, D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. (2018) 13:612–32. doi: 10.1177/1747493018778713
17. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
18. Sze, S, Pellicori, P, Kazmi, S, Rigby, A, Cleland, JGF, Wong, K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. (2018) 6:476–86. doi: 10.1016/j.jchf.2018.02.018
19. Cereda, E, and Pedrolli, C. The geriatric nutritional risk index. Curr Opin Clin Nutr Metab Care. (2009) 12:1–7. doi: 10.1097/MCO.0b013e3283186f59
20. Minamisawa, M, Seidelmann, SB, Claggett, B, Hegde, SM, Shah, AM, Desai, AS, et al. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC Heart Fail. (2019) 7:664–75. doi: 10.1016/j.jchf.2019.04.020
21. Nakagawa, N, Maruyama, K, and Hasebe, N. Utility of geriatric nutritional risk index in patients with chronic kidney disease: a mini-review. Nutrients. (2021) 13:3688. doi: 10.3390/nu13113688
22. Ruan, GT, Zhang, Q, Zhang, X, Tang, M, Song, MM, Zhang, XW, et al. Geriatric nutrition risk index: prognostic factor related to inflammation in elderly patients with cancer cachexia. J Cachexia Sarcopenia Muscle. (2021) 12:1969–82. doi: 10.1002/jcsm.12800
23. Cereda, E, Klersy, C, Pedrolli, C, Cameletti, B, Bonardi, C, Quarleri, L, et al. The geriatric nutritional risk index predicts hospital length of stay and in-hospital weight loss in elderly patients. Clin Nutr. (2015) 34:74–8. doi: 10.1016/j.clnu.2014.01.017
24. Hao, X, Li, D, and Zhang, N. Geriatric nutritional risk index as a predictor for mortality: a meta-analysis of observational studies. Nutr Res. (2019) 71:8–20. doi: 10.1016/j.nutres.2019.07.005
25. Abd-El-Gawad, WM, Abou-Hashem, RM, El Maraghy, MO, and Amin, GE. The validity of geriatric nutrition risk index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with mini nutritional assessment. Clin Nutr. (2014) 33:1108–16. doi: 10.1016/j.clnu.2013.12.005
26. Jiao, Y, Su, Y, Shen, J, Hou, X, Li, Y, Wang, J, et al. Evaluation of the long-term prognostic ability of triglyceride-glucose index for elderly acute coronary syndrome patients: a cohort study. Cardiovasc Diabetol. (2022) 21:3. doi: 10.1186/s12933-021-01443-y
27. Raposeiras Roubín, S, Abu Assi, E, Cespón Fernandez, M, Barreiro Pardal, C, Lizancos Castro, A, Parada, JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. (2020) 76:828–40. doi: 10.1016/j.jacc.2020.06.058
28. Foundation TR. Available at: http://www.R-project.org. (Accessed May 20, 2022).
29. X&Y Solutions I. Available at: http://www.empowerstats.com. (Accessed May 20, 2022).
30. Damião, R, Santos, ÁDS, Matijasevich, A, and Menezes, PR. Factors associated with risk of malnutrition in the elderly in South-Eastern Brazil. Rev Bras Epidemiol. (2017) 20:598–610. doi: 10.1590/1980-5497201700040004
31. Damayanthi, H, Moy, FM, Abdullah, KL, and Dharmaratne, SD. Prevalence of malnutrition and associated factors among community-dwelling older persons in Sri Lanka: a cross-sectional study. BMC Geriatr. (2018) 18:199. doi: 10.1186/s12877-018-0892-2
32. Gingrich, A, Volkert, D, Kiesswetter, E, Thomanek, M, Bach, S, Sieber, CC, et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. (2019) 19:120. doi: 10.1186/s12877-019-1115-1
33. Leij-Halfwerk, S, Verwijs, MH, van Houdt, S, Borkent, JW, Guaitoli, PR, Pelgrim, T, et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis. Maturitas. (2019) 126:80–9. doi: 10.1016/j.maturitas.2019.05.006
34. Jia, Z, El Moheb, M, Nordestgaard, A, Lee, JM, Meier, K, Kongkaewpaisan, N, et al. The geriatric nutritional risk index is a powerful predictor of adverse outcome in the elderly emergency surgery patient. J Trauma Acute Care Surg. (2020) 89:397–404. doi: 10.1097/TA.0000000000002741
35. Abd Aziz, NAS, Teng, N, Abdul Hamid, MR, and Ismail, NH. Assessing the nutritional status of hospitalized elderly. Clin Interv Aging. (2017) 12:1615–25. doi: 10.2147/CIA.S140859
36. Jia, Y, Gao, Y, Li, D, Cao, Y, Cheng, Y, Li, F, et al. Geriatric nutritional risk index score predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction. J Cardiovasc Nurs. (2020) 35:E44–52. doi: 10.1097/JCN.0000000000000674
37. Kunimura, A, Ishii, H, Uetani, T, Aoki, T, Harada, K, Hirayama, K, et al. Impact of geriatric nutritional risk index on cardiovascular outcomes in patients with stable coronary artery disease. J Cardiol. (2017) 69:383–8. doi: 10.1016/j.jjcc.2016.09.004
38. Katayama, T, Hioki, H, Kyono, H, Watanabe, Y, Yamamoto, H, and Kozuma, K. Predictive value of the geriatric nutritional risk index in percutaneous coronary intervention with rotational atherectomy. Heart Vessel. (2020) 35:887–93. doi: 10.1007/s00380-020-01558-4
39. Dent, E, Hoogendijk, EO, Visvanathan, R, and Wright, ORL. Malnutrition screening and assessment in hospitalised older people: a review. J Nutr Health Aging. (2019) 23:431–41. doi: 10.1007/s12603-019-1176-z
40. Yıldırım, A, Kucukosmanoglu, M, Koyunsever, NY, Cekici, Y, Belibagli, MC, and Kılıc, S. Combined effects of nutritional status on long-term mortality in patients with non-st segment elevation myocardial infarction undergoing percutaneous coronary intervention. Rev Assoc Med Bras. (1992) 67:235–42. doi: 10.1590/1806-9282.67.02.20200610
41. Kalkan, Ç, Kartal, A, Karakaya, F, Tüzün, A, and Soykan, I. Utility of three prognostic risk scores in predicting outcomes in elderly non-malignant patients after percutaneous gastrostomy. J Nutr Health Aging. (2017) 21:1344–8. doi: 10.1007/s12603-016-0853-4
42. Zhao, Q, Zhang, TY, Cheng, YJ, Ma, Y, Xu, YK, Yang, JQ, et al. Impacts of geriatric nutritional risk index on prognosis of patients with non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Nutr Metab Cardiovasc Dis. (2020) 30:1685–96. doi: 10.1016/j.numecd.2020.05.016
43. Collins, N. Dietary regulation of memory T cells. Int J Mol Sci. (2020) 21:4363. doi: 10.3390/ijms21124363
44. Jankowski, J, Floege, J, Fliser, D, Böhm, M, and Marx, N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. (2021) 143:1157–72. doi: 10.1161/CIRCULATIONAHA.120.050686
45. Brennan, E, Kantharidis, P, Cooper, ME, and Godson, C. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat Rev Nephrol. (2021) 17:725–39. doi: 10.1038/s41581-021-00454-y
46. Sarnak, MJ, Amann, K, Bangalore, S, Cavalcante, JL, Charytan, DM, Craig, JC, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 74:1823–38. doi: 10.1016/j.jacc.2019.08.1017
47. Rodin, R, and Chan, CT. Determinants and prevention of coronary disease in patients with chronic kidney disease. Can J Cardiol. (2019) 35:1181–7. doi: 10.1016/j.cjca.2019.05.025
48. Zhou, D, Li, Z, Shi, G, and Zhou, J. Obesity paradox for critically ill patients may be modified by age: a retrospective observational study from one large database. Crit Care. (2020) 24:425. doi: 10.1186/s13054-020-03157-1
49. Katta, N, Loethen, T, Lavie, CJ, and Alpert, MA. Obesity and coronary heart disease: epidemiology, pathology, and coronary artery imaging. Curr Probl Cardiol. (2021) 46:100655. doi: 10.1016/j.cpcardiol.2020.100655
50. Matsuo, Y, Kumakura, H, Kanai, H, Iwasaki, T, and Ichikawa, S. The geriatric nutritional risk index predicts long-term survival and cardiovascular or limb events in peripheral arterial disease. J Atheroscler Thromb. (2020) 27:134–43. doi: 10.5551/jat.49767
51. Arikawa, R, Kanda, D, Ikeda, Y, Tokushige, A, Sonoda, T, Anzaki, K, et al. Prognostic impact of malnutrition on cardiovascular events in coronary artery disease patients with myocardial damage. BMC Cardiovasc Disord. (2021) 21:479. doi: 10.1186/s12872-021-02296-9
52. Maraj, M, Hetwer, P, Kuśnierz-Cabala, B, Maziarz, B, Dumnicka, P, Kuźniewski, M, et al. α(1)-acid glycoprotein and dietary intake in end-stage renal disease patients. Nutrients. (2021) 13:3671. doi: 10.3390/nu13113671
53. Kawamiya, T, Suzuki, S, Ishii, H, Hirayama, K, Harada, K, Shibata, Y, et al. Correlations between geriatric nutritional risk index and peripheral artery disease in elderly coronary artery disease patients. Geriatr Gerontol Int. (2017) 17:1057–62. doi: 10.1111/ggi.12828
54. Meza-Valderrama, D, Marco, E, Dávalos-Yerovi, V, Muns, MD, Tejero-Sánchez, M, Duarte, E, et al. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. (2021) 13:761. doi: 10.3390/nu13030761
Keywords: oldest old, acute coronary syndrome, malnutrition, GNRI, mortality
Citation: Li Y, Shen J, Hou X, Su Y, Jiao Y, Wang J, Liu H and Fu Z (2023) Geriatric nutritional risk index predicts all-cause mortality in the oldest-old patients with acute coronary syndrome: A 10-year cohort study. Front. Nutr. 10:1129978. doi: 10.3389/fnut.2023.1129978
Edited by:
Gabriela Salim de Castro, University of São Paulo, BrazilReviewed by:
Michał Czapla, Wroclaw Medical University, PolandYue Ma, Peking University Third Hospital, China
Copyright © 2023 Li, Shen, Hou, Su, Jiao, Wang, Liu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhong Fu, ZnV6aGVuaEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Ying Li1
,2
†
Ying Li1
,2
†
 Yang Jiao
Yang Jiao Jihang Wang
Jihang Wang Zhenhong Fu
Zhenhong Fu