
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Nutr. , 23 March 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1122102
 Chunrong Li1,2
Chunrong Li1,2 Yan Gao3
Yan Gao3 Tongyong Luo3
Tongyong Luo3 Shiji Qin1
Shiji Qin1 Xue Yao4
Xue Yao4 Ye Wen5
Ye Wen5 Xue Wang3
Xue Wang3 Jing Zhang1
Jing Zhang1 Qiong Zhong2
Qiong Zhong2 Hao Shi6
Hao Shi6 Jing Liu3*
Jing Liu3*Background: Increased post-prandial glycemic excursions contribute to the development of diabetes and have been observed in women with recent gestational diabetes mellitus (GDM) and with normal glucose tolerance at post-partum. As a convenient meal replacement, low-GI biscuits are helpful for improving glycemic excursions in patients with type 2 diabetes. However, it is unknown whether low-GI biscuits as pre-loads or mid-meal snacks have a better effect in diminishing post-prandial glycemic excursions from the individual level in women with recent GDM. Therefore, the aim of this trial is to tailor a better dietary strategy utilizing low-GI biscuits (Fitmeal) to improve post-prandial glycemic excursions through within-subject comparison in such a population and observe the long-term effect of a tailored dietary approach in glycemic control.
Methods: We have designed a two-phase trial including a randomized, crossover, non-blinded trial in the first phase, followed by a 4-week tailored intervention in the second phase. A total of 52 post-partum women with recent GDM will be allocated into four meal plans: (1) Fitmeal pre-load 30 min before standard lunch meal (P+L), (2) Fitmeal as a mid-meal snack 2 h before standard lunch meal (S+L), (3) isocaloric standard control with co-ingestion of Fitmeal and standard lunch meal (CL) at the same time, and (4) placebo control with 200 ml of water taken 30 min before standard lunch meal (W + L), on four consecutive days. Acute post-prandial glycemic response (PGR) measured by continuous glucose monitoring (CGM) will be compared among the four meals. In the second phase, all participants will receive a 4-week tailored intervention using Fitmeal as pre-loads or mid-meal snacks based on within-subject PGR results from the first phase. Glycemic metrics, dietary behaviors, and psychosocial factors (e.g., quality of life, self-efficacy, perceived stress, and depression) will be examined at baseline and end-point.
Discussion: This trial is expected to optimize the use of low-GI biscuits as pre-loads or mid-meal snacks in improving individual post-prandial glycemic excursions among women with recent GDM. Furthermore, the findings of this study will provide novel information on how to deliver an effective dietary intervention at the individual level and guide future clinical practice of medical nutrition therapy for diabetes prevention.
Trial registration number: Chinese clinical trial registry, ChiCTR2200060923.
Gestational diabetes mellitus (GDM) is rising in prevalence with maternal hyperglycemia currently affecting one in every six pregnancies worldwide (1) and 18.9% in China (2). Compared with their peers, women with a history of GDM have a 7- to 10-fold higher risk of developing type 2 diabetes (T2DM) in the years thereafter (3, 4) and a 2-fold higher risk of cardiovascular disease within the first decade after the index pregnancy (5). With the development of the continuous glucose monitoring (CGM) technique, several studies found that the glucose fluctuation and post-prandial glucose excursions were significantly higher in women diagnosed with GDM during pregnancy and with normal glucose tolerance by OGTT at post-partum follow-up, as compared to women with normal glucose metabolism during pregnancy (6, 7). Glucose fluctuations during post-prandial periods exhibited a more specific triggering effect on oxidative stress than chronic sustained hyperglycemia, promoting the development and progression of T2DM (8, 9). Thus, it is suggested that interventional trials in such a population should be initiated early after delivery, targeting not only HbA1c and mean glucose concentrations but also acute post-prandial glucose excursions (10).
Lifestyle interventions initiated within 3 years of GDM during pregnancy targeting lowering calorie intake and body weight have been reported to be effective in reducing the risk of post-partum diabetes as compared to controls (11), but recently, a large randomized clinical trial (n = 1,812) has reported inconsistent result that a 12-month lifestyle intervention did not prevent glycemic deterioration, including the development of diabetes, among South Asian women with recent GDM within 2 years of childbirth (12). The low glycemic index (GI) diet is recommended by national guidelines to improve post-prandial glucose levels for individuals with diabetes or GDM (13, 14). However, in post-partum women with recent GDM, 6-month interventions with a low-GI diet alone or integrated with intensive lifestyle management did not halt or reverse glycemic deterioration although the risk of developing diabetes was reduced in the intervention group compared with usual care (15, 16). The limited success of current interventions might be explained by several reasons. First, increasing studies have shown that differences in individual post-prandial glycemic responses occur even when consuming the same food (17–19), but current lifestyle or dietary interventions are mainly based on one-size-fits-all advice and do not consider such differences between individuals. Moreover, if participants are unable to get immediate feedback on whether dietary approaches are effective in improving post-prandial glucose levels, it is hard for them to comply with recommended advice (20). Finally, post-partum women with recent GDM are commonly confronted with some barriers including lack of time and energy, limited childcare and social supports, emotional stress, workload-related stress of post-partum returning to work, insufficient knowledge or comprehension about GDM, and body image concerns (21, 22) such as competing demands may have a great impact on mothers' participation in dietary interventions. Thus, for post-partum women with recent GDM, whether a dietary intervention could improve post-prandial glucose immediately in a convenient and feasible strategy is a big challenge for increasing compliance with a recommended meal plan and then achieving long-term benefits.
Meal replacement is helpful for people with diabetes for being convenient and providing known level of calories and nutrients which facilitate meal planning and adherence to dietary recommendations (23), because of it being convenient and being able to provide known calorie amounts with specific macronutrient and micronutrient levels. In addition to replacing main meals and mid-meal snacks, meal replacement has been used as pre-load food given 15–30 min before a main meal, which is helpful to diminish post-prandial glycemic excursions, representing a novel dietary approach to control blood glucose (24–26). Recent studies reported that low-GI nutritional shakes pre-load given 30 min before each of three main meals reduced post-prandial 2 h blood glucose in patients with T2DM (26) and women with GDM (27). Our earlier study suggested that low-GI nutritional shakes given 20 min before each main meal significantly reduced post-prandial 2 h blood glucose and improved metabolic measurements in patients with obesity and metabolic syndrome in a 3-week before–after intervention study (28). As another form of meal replacement, low-GI biscuits with high protein and high fiber are more suitable to be applied as pre-load food compared with shakes using that biscuit is palatable, easy to carry, and can be consumed immediately without any pre-treatment as a mid-meal snack or as a part of main meal replacement. Several studies have shown that using low-GI biscuits as mid-meal snacks improved immediate post-prandial glucose levels (29), reduced post-prandial glucose levels 90 min after dinner (30), and improved satiety (31) in healthy subjects and individuals with T2DM. However, we are unaware of studies that have compared the benefits of using low-GI biscuits as pre-load foods and mid-meal snacks on the post-prandial glycemic response of the following main meal in a within-subject design, particularly in post-partum women with recent GDM.
A dietary strategy confirmed to be effective for reducing PGR individually is of paramount importance in increasing compliance with a recommended meal plan. Therefore, to optimize the use of low-GI biscuits in reducing individual PGR and provide valuable information in clinical practice of preventing T2DM for post-partum women with recent GDM, this proposed study will be conducted in two phases. The first phase (week 1) is designed with a randomized crossover trial with the main objective to compare the effect of low-GI biscuits consumed as pre-loads or as mid-meal snacks on individual PGR of the following main meal assessed by CGM. In the second phase (weeks 2–5), individualized intervention either with pre-loads or mid-meal snacks will be conducted depending on the results of personal PGR from the first phase. The objectives of the second phase are to observe before–after differences among all participants in their glycemic control using CGM metrics and glycated albumin (GA), dietary behavior, and psychosocial factors and compare those differences between the two dietary strategies. This article presents the development of the study design and procedure.
This is a randomized, crossover, and a non-blinded trial comparing the effects of four different test meals over four consecutive days on PGRs in women with recent GDM. The four meal plans are as follows: (1) Fitmeal pre-load 30 min before standard lunch meal (P + L), (2) Fitmeal as mid-meal snack 2 h before standard lunch meal (S + L), (3) co-ingestion of Fitmeal with standard lunch meal (CL) as isocaloric standard control, and (4) 200 ml of water taken 30 min before standard lunch meal (W + L) as a placebo control. All participants will complete four test meals during the trial period in a randomized counterbalanced order by Latin Square Design (Figure 1). Crossover randomization process is performed by the study biostatistician. Different orders for four test meals will be concealed in envelopes. Each subject is randomized by dispensing the next sequentially numbered subject code and opening the corresponding code envelope indicating the order for four test meals.
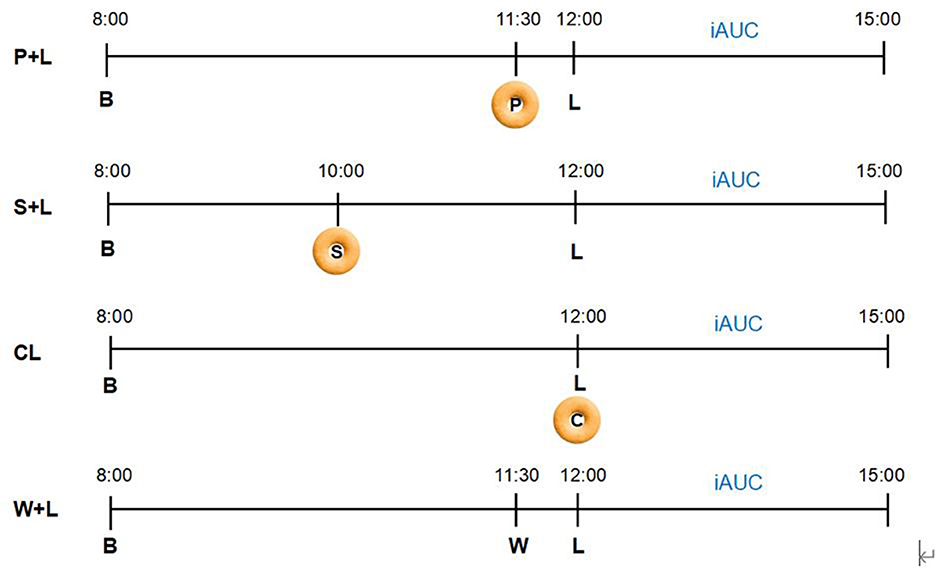
Figure 1. Randomized crossover design. Participants will complete four test meals over 4 consecutive days in a randomized counterbalanced order using Latin Square Design. Participants will be randomized to receive one of the four meal arrangement orders as follows: (1) P + L → S + L → W + L → CL; (2) S + L → CL → P + L → W + L; (3) CL → W + L → S + L → P + L; (4) W + L → P + L → CL → S + L. To diminish the glycemic impact from the last meal, breakfast, lunch, pre-load, and mid-meal snack foods are provided, with breakfast and lunch identical for 4 test days. 3h-iAUC after each lunch using continuous glucose monitoring (CGM) will be calculated as the primary outcome (P, Pre-load; S, Mid-meal snack; B, Breakfast; L, Lunch; W, Water; C, Co-ingestion; iAUC, Incremental areas under the curve of post-prandial glycemic response; P + L, Fitmeal pre-load 30 min before lunch; S + L, Fitmeal as mid-meal snack 2 h before lunch; CL, Co-ingestion of Fitmeal with lunch; W + L, Drinking water 30 min before lunch).
Moreover, a before–after intervention of 4-week duration based on within-subject meal test results will be followed for all participants to compare changes in PGRs from the baseline. The study will be carried out at four maternal and child healthcare hospitals in Chengdu. The protocol (CWCCH 2021027) has been approved by the ethics committee of Chengdu Women's and Children's Central Hospital, and written informed consent has been obtained from all participants. The study is conducted in accordance with the principles expressed in the Declaration of Helsinki.
Women will be invited to participate in this study if they meet the following inclusion criteria: (1) those who are aged between 20 and 49 years; (2) those diagnosed with GDM during the latest pregnancy and <1 year post-partum at the beginning of the study; (3) those who have telephone access. Exclusion criteria are as follows: (1) those who are currently pregnant; (2) those who are still breastfeeding; (3) those who have been diagnosed with diabetes by OGTT test at post-partum follow-up or screening visit; (4) those who have been taking medicines that influence glucose metabolism; (5) those with severe heart, liver, and kidney dysfunction, psychiatric disorders, or other serious diseases that could reduce the ability to participate in the study; and (6) those who are unwilling to give informed consent.
The recruitment of potential participants will occur by reviewing electronic medical records of women who delivered babies during the past 1 year at the research site by trained research nurses. Potential participants who meet the inclusion criteria will then be approached by a trained research nurse through a phone call to further confirm eligibility and provide study documentation in advance. Women who wish to participate will be invited to attend the screening visit and fasting for at least 12 h overnight before the screening visit will be required.
The detailed medical eligibility questionnaire based on the abovementioned inclusion/exclusion criteria will be confirmed, and informed consent will be obtained at the screening visit. As part of the eligibility assessment, all potential participants will have OGTT testing and HbA1c to make sure the potential subjects do not have T2DM. If a woman has newly diagnosed T2DM (fasting glucose ≥7.0 mmol/l or 2-h glucose ≥11.1 mmol/l or HbA1c ≥6.5%), she will be excluded from this study and referred to a diabetes specialist for further T2DM diagnosis and treatment. If eligible, subjects will complete a self-administered questionnaire and undergo a physical examination at the same time, and continuous glucose monitoring devices will be fitted to each participant.
Fitmeal consists of soybean protein, whey protein, skimmed milk powder, whole egg, wheat powder, apple powder, stachyose, and L-arabinose. Each serving of Fitmeal (15 g) contains 7.6 g of protein, 1.8 g of fat, 1.9 g of fiber, and 5.2 g of carbohydrates, which provide 70 kCal of energy (Table 1, Tianyi Cuisine, Chengdu, China). GI value of low-GI biscuit (Fitmeal) is 43, according to test results by Peking Union Medical College Hospital and China National Research Institute of Food and Fermentation Industries following the ISO guidelines. The effect of Fitmeal on weight loss and blood glucose improvement has been described in a previous study (28). Standard test meals for breakfast and lunch for 4 consecutive test days will be provided to participants, and the composition and nutrient content of test meals are presented in Table 2.
Participants consumed the same test meals for 4 consecutive days according to a randomized crossover order: (1) Fitmeal pre-load 30 min before standard lunch meal (P + L), (2) Fitmeal as mid-meal snack 2 h before standard lunch meal (S + L), (3) co-ingestion of Fitmeal with standard lunch meal (CL) at the same time, containing 46.2 g of available carbohydrates where the Fitmeal contributes 8.1 g and standard meal provides 36.1 g, and (4) drinking water 30 min before standard lunch meal (W + L). To control the impact of different types of breakfast on the glycemic response of the next meal, the same breakfast will be provided to all participants on 4 test days. Participants are instructed to start breakfast, mid-meal snack, pre-load, and lunch at 8:00 a.m., 10:00 a.m., 11:30 a.m., and 12:00 p.m., respectively, during test days. Participants will be suggested to consume biscuits for 5 min and test meals for 10–15 min with 200 ml of water. During each test day, participants are required to have a 12-h fast, without strenuous exercise performed, and without alcohol consumed during the past 24 h before the breakfast meals. Extra foods and beverages are not permitted to consume from the time of starting breakfast to 3 h post-lunch during each test day, with specific reasons and benefits informed by the research nurse. In the event that participants consume extra food during the test period, they are required to record in the paper logbook to monitor compliance and report to the research nurse in time. Even if not reported, abnormal glucose excursions monitored by CGM will also provide objective information to the research team for evaluation.
Participants will wear CGM (FreeStyle Libre 1, FSL1, Abbott Diabetes Care, Inc., Alameda, CA) that measures interstitial glucose concentrations for the first week. On days 1 and 2 of CGM use, women are instructed to eat at their diet routine for monitoring baseline glucose profile. On days 3–6, women are instructed and reminded by the research nurse to consume the test foods provided according to their randomized order. On day 7, the CGM will be removed, and data from days 3 to 6 will be extracted for comparison of different test meals. From day 1 to 6, participants are required to record their food diary and the time to start each meal using the paper logbook, additional photos of each meal should be taken for a clear memory at later counseling.
After removing CGM, each participant will receive a 20-min face-to-face counseling from a trained healthcare provider in each research site, including the explanation of baseline CGM glucose patterns and a comparison of PGRs of four test meals in a visual and individualized manner. Dietary advice about healthy food choices will be given as is the routine recommendation in China Medical Nutrition Therapy Guideline for Diabetes (36): (1) an appropriate amount of fish, eggs, low-fat milk, lean meat, and reduction in fatty meat in the diet, (2) more fiber-rich food, such as whole grains, vegetables, and fruits, (3) avoidance of simple sugars and refined carbohydrates, and (4) appropriate energy intake. Each participant's current status of body weight, physical activity, and dietary behaviors collected at the baseline survey will be considered in providing dietary advice.
A dietary approach of pre-load (P + L) or mid-meal snack (S + L) with lower iAUC from test results will be recommended to follow during the next 4 weeks. Considering rush time in the morning before breakfast, pre-load or snacks will be suggested to consume before lunch and dinner. For participants who are suggested to follow S + L, three Fitmeal at each morning tea and afternoon tea are asked to consume, approximately at 10 a.m. and 4 p.m., 2 h before lunch and dinner. If suggested to follow P + L, participants are asked to consume three Fitmeal 30 min before lunch and dinner, ~11:30 a.m. and 5:30 p.m. Blood glucose meter and testing supplies will be provided to participants for self-monitoring of blood glucose (SMBG) with six times a day in a manner of paired pre-meal and 1 h-post-meal, at least 2 days per week.
From week 6, participants will be invited to the research site to complete an end-point questionnaire and physical examination, another CGM will be applied to monitor glucose profile for at least 72 h, and a food diary and the time to start each meal are required to record using the log book for 2 continuous days (midnight to midnight) from the second day of applying CGM. At the end of this trial, the comprehensive glucose profiles and the impact of diet on glucose levels will be reviewed and discussed with each participant in face-to-face counseling for facilitating their understanding and compliance with the dietary strategy to stabilize glucose levels. The study flow chart can be seen in Figure 2.
To make sure this proposed trial is implemented in each research site as a procedure, healthcare professionals and nurses working in the research sites will be recruited to take training provided by the research team. Their tasks are as follows: (1) to recruit and enroll the eligible participants; (2) to fit in CGM for participants and teach them how to use blood glucose meter for SMBG; (3) to set up automatic and individualized messages to remind each participant complying with each test meal and taking on-site measurements on schedule through an interactive WeChat Mini Program, in combination with telephone and WeChat for a double check; and (4) to provide dietary counseling and explaining CGM results to the participants. Moreover, dietitians and clinicians from the research team are available to provide advice and support about this trial implementation.
A baseline electronic questionnaire will be completed in a face-to-face interview with assistance from a research assistant at enrollment including sociodemographic data, medical history data, obstetric history, family history of diabetes, diet and physical activity behaviors during the last week, quality of life, stress, and depressive status. Sociodemographic, medical history data, and obstetric history of participants will be collected from the hospital medical records. In addition to the collection at enrollment, the 12-item Short Form Survey (SF-12) score (32) for the assessment of the quality of life, the Edinburgh Post-natal Depression Scale (EPDS) questionnaire (33) for the assessment of depressive symptoms, perceived stress (34), international physical activity questionnaire (IPAQ) (35) for evaluating physical activity level, and changes in diet behavior will also be completed at the moment of the end-point visit. Food diary and the time to start each meal will be required to record using a semi-structured paper logbook daily during the first week, at least 2 days from week 2 to week 5 paired with SMBG, and 2 consecutive days from the second day of CGM use at week 6. The electronic questionnaire at the end-point includes also questions concerning satisfaction with the intervention accepted.
Physical measurements (including weight, height, waist circumference, and body fat %) will be assessed at the enrollment and end-point visit. Participants will be measured barefoot and in light clothing. Weight will be recorded to the nearest 0.1 kg using a digital scale (HW-900B, He'Nan Lejia Electronic Technology co., Ltd, Zhengzhou). Height will be assessed to the nearest 0.1 cm with a calibrated wall-mounted stadiometer (HW-900B, He'Nan Lejia Electronic Technology Co., Ltd., Zhengzhou). Body mass index (BMI; in kg/m2) will be calculated as weight divided by square height. Waist circumference, defined as the midpoint between the lowest rib margin and the iliac crest, will be measured to the nearest 0.1 cm with a non-elastic tape. Measurements will be performed by a trained research nurse, following standard protocols.
Laboratory tests performed at baseline and end-point visits include fasting plasma glucose, 75 g 2-h oral glucose tolerance test (OGTT), glycated albumin (GA), triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol (Table 3).
Glycemic metrics will be calculated with values from CGM and SMBG. Participants who have signed the consent form will wear the CGM (FreeStyle Libre 1, FSL1, Abbott Diabetes Care, Inc., Alameda, CA) two times, monitoring for 7 days on week 1 and 3 days on week 6, respectively. The FSL1 is the most used biosensor for intermittent scanned CGM. All glucose data will be given each 15 min with a total of 96 readings every 24 h. From all these glucose measurements, several glucose metrics are calculated and reported on a 24-h basis average, including the percentage of time in a range from 3.9 to 10 mmol/L (TIR), percentage of time above range (TAR), percentage of time below range (TBR), a standard difference of mean glucose (SD), mean amplitude of glycemic excursions (MAGE), %CV, average glucose concentration (AG), incremental areas under the curve (iAUC), and post-prandial glycemic excursions (PE). Changes in glycemic metrics are calculated by subtracting the averaged values obtained on day 1 and 2 with CGM use at the baseline from the averaged values obtained on the second and third days of CGM use at the end-point. iAUCs will be calculated to represent the PGRs of four test meals from day 3 to 6, respectively. SMBG will be tested by glucose meter data (Qin Zhi, Sinocare, Inc., Changsha, China) six times a day in a paired test regimen at pre-meal and 1 h post-meal, at least 2 days per week from week 2 to week 5. SMBG frequency, average fasting, and pre-meal blood glucose concentrations, average 1 h post-prandial glucose concentrations (1hPG), and 1 h post-prandial excursions (1hPE) will be calculated. CGM and glucose meter data (An Wen/Qin Zhi, Sinocare, Inc. Changsha, China) will be uploaded automatically to the data platform and will be analyzed at the end of each monitoring period.
The 3-h post-lunch incremental areas under the curve of post-prandial glycemic responses (iAUC) using CGM are compared between the low-GI biscuits as pre-load food (P + L) or mid-meal snack (S + L) and their isocaloric standard control of co-ingestion (CL) and placebo control (W + L) in women with recent GDM. Primary outcomes also include differences in CGM measures (AG, SD, %CV, MAGE, TIR, TAR, TBR, and PE) and GA after a 4-week intervention compared with those variables at the baseline.
Secondary outcomes are differences in weight, glucose tolerance, dietary behaviors, quality of life, self-efficacy, perceived stress, and depression after a 4-week intervention compared with those variables at the baseline, and those differences will also be compared between two dietary strategies.
The sample size of the trial is estimated using PASS 15 Power Analysis and Sample Size software (NCSS, Kaysville, UT, United States). Assuming that the standard deviation (SD) is 32.9 mmol·min/L, a sample size of n = 39 provides 90% power to detect a change of 20 mmol·min/L in iAUC (p < 0.05) with Bonferroni correction, based on the previous study (36). Anticipating a 20% dropout rate, 49 subjects should be enrolled to obtain a final sample size of 39. Considering participants enrolled in four hospitals, 52 subjects will be enrolled with 13 subjects for each center.
The incremental areas under the curve (iAUC) will be calculated by using the trapezoidal rule (37) for PGR. All areas below pre-prandial glucose are excluded from the calculations. Two-factor repeated-measures analysis of variance (ANOVA) is performed to analyze the effects of treatment × time on outcome variables measured over the crossover study period, including iAUC for PGR and post-prandial glucose excursion. When treatment and time interaction are statistically significant, one-factor ANOVA and Bonferroni's post-hoc tests for multiple comparisons are performed to investigate the effect of treatment on the aforementioned measurement of PGR. Comparison between baseline and end-points is assessed by paired Student's t-test. Values are presented as mean ± SEM unless otherwise indicated. P-values of <0.05 are considered statistically significant. All analyses are performed with SPSS software version 25 (SPSS Inc., Chicago, IL, USA).
With a high risk to develop T2DM, women with a history of GDM should receive appropriate intervention early after delivery (38). A clinically feasible intervention targeting post-partum women with recent GDM should be convenient to follow and easy to experience effectiveness in the shortest possible time, which would facilitate long-term compliance of participants. To the best of our knowledge, this proposed study will be the first to optimize the use of low-GI biscuits as a different dietary approach to diminishing post-prandial glycemic excursions and examine the efficacy of a tailored dietary intervention for post-partum women with recent GDM.
Current evidence suggested that lifestyle interventions within 3 years of GDM pregnancy reduced the risk of post-partum diabetes compared to controls, but traditional interventions targeting dietary energy limitation and weight control are challenging in evaluating whether the glycemic control is effective (15, 38, 39), in addition to difficulties in maintaining long-term compliance (11). Recent studies evaluating the effectiveness of diabetes prevention for women with prior GDM through a 6-month intensive lifestyle intervention showed inconsistent results in glycemic control, with a significant reduction in FBG and 2hPG of OGTT observed in the intervention group compared with controls, but HbA1c in two groups showed a clear trend of increase, and there is no difference between the two groups after 6-month intervention (15, 40). These inconsistent results suggest that it could be ignored about acute glucose fluctuations during post-prandial periods, which have been observed to be higher in women with recent GDM and with normal glucose tolerance by OGTT at post-partum follow-up measured by CGM, as compared to women with normal glucose metabolism during pregnancy (6, 7). Furthermore, increased acute post-prandial glucose contributes to the development of diabetes and is widespread in the Chinese population with diabetes and prediabetes. According to a national survey in China, more than 70% of the participants with prediabetes had isolated impaired glucose tolerance (41). Thus, a dietary intervention strategy for post-partum women with recent GDM should target on diminishing post-prandial glycemic excursions, which are sensitive to dietary adjustment, and the effect can be monitored in real-time.
In addition to commonly used mid-meal snacks (13, 42), pre-load represents a novel dietary approach to lower post-prandial glycemic excursions (14, 43). A variety of foods, such as olive oil, milk proteins, fruits, and low-GI nutritional shakes, have been demonstrated to improve post-prandial glucose levels (26, 27, 44–47). However, previous studies mainly examined the influence of pre-load on acute PGRs only (44–47) or assessed changes in 2hPG after pre-load intervention for 8–12 weeks in healthy population, women diagnosed with GDM, or individuals with T2DM (26, 27). Due to individual glycemic response differences occurring even when consuming the same food (17), it is necessary to integrate individualized PGR into a specific dietary approach, i.e., pre-load or mid-meal snack, with a continuing period of intervention based on the optimal approach to confirm the effect of specific dietary approach on improving glycemic excursions in real life. Being more convenient to consume without any pre-treatment compared with low-GI nutritional shakes, low-GI biscuits are more suitable to be applied as pre-load food for post-partum women with recent GDM. Based on the literature and our preliminary data, the proposed intervention will compare the impact of low-GI biscuits as pre-load food or commonly used mid-meal snacks upon post-prandial glycemic excursions measured by CGM. Furthermore, the present trial is intended to optimize the use of low-GI biscuits as a different dietary approach in improving post-prandial glycemic excursions, in combination with continuing intervention to evaluate whether the optimal approach would promote dietary self-management behavior and improve related psychosocial factors for post-partum women with recent GDM.
This proposed trial has some limitations. First, the free-living conditions of participants may raise non-compliance and bias as composed with in-site tests, although standard main meals for breakfast and lunch will be provided to participants to test acute PGRs by CGM. We will pre-set the automatic messages through WeChat Mini-Program to remind participants complying with each test meal, and the time to start each test meal will be confirmed in time by trained nurse. Second, CGM will be applied only two times at the baseline and after intervention rather than the whole period of intervention. It may not be possible to analyze the comprehensive glucose profile changes during the intervention, but a finger-prick blood glucose monitor will be provided to take daily blood glucose tests as a recommended regimen. Third, there is no washout period in-between 2 test days to minimize any carryover effects, but the same standard breakfast is provided to each participant to diminish the impact. Finally, the second stage of intervention is pragmatic and tailored based on optimal results from the first stage, it is impossible to implement randomization or to blind participants and investigators to provide robust evidence for confirming which dietary approach is better in improving post-prandial glycemic excursions with a 4-week intervention.
To minimize potential bias due to the study limitations, this study uses several approaches to reduce potential bias. First, to reduce the in-between differences in research sites, four tertiary hospitals in Chengdu city are selected for recruiting participants. Healthcare professionals and nurses working in the research sites are recruited to take training provided by the research team. Second, to improve data collection quality and reduce information bias, all electronic questionnaires in the proposed trial will be collected in a face-to-face interview by trained research assistants who are blinded to the treatment order and group assignments. All samples for laboratory tests are collected for centralized testing. Third, the study biostatistician, involved in data handling and analysis, will be blinded to participant study treatment and any potential identifiers.
At present, there is a shortage of evidence providing clinical practice and dietary management modes for post-partum women with recent GDM in China. We propose an innovative and pragmatic study that aims to assess the effectiveness of different dietary strategies in improving post-prandial glucose excursions in women with recent GDM. The proposed study will be a randomized crossover design, followed by a 4-week tailored intervention based on an optimum strategy that may facilitate compliance and self-efficacy in dietary self-management. We hope that the implementation of this protocol would help to provide a feasible and effective dietary management approach and behavioral strategy for improving post-prandial glucose excursions in the shortest possible time and contribute to reduce the incidence of T2DM in post-partum women with recent GDM in the long term.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Sichuan Jinxin Women and Children Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CL: study conception and manuscript writing. YG: study conception and study coordination. TL: manuscript writing and data analysis. SQ: manuscript writing. XY, YW, QZ, HS, JZ, and XW: data collection. JL: study conception, manuscript writing, and study coordination. All authors contributed to the article and approved the submitted version.
This work was supported by the Chengdu Science and Technology Bureau (Grant number: 2019-YF09-00240-SN).
The authors sincerely thank the support of Chengdu Tianyi Cuisine Nutritional Food Co., Ltd.
YW was employed by Chengdu Tianyi Cuisine Nutritional Food Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ANOVA, Analysis of variance; AG, Average glucose; B, Breakfast; BMI, Body mass index; CGM, Continuous glucose monitoring; C, Co-ingestion; CL, Co-ingestion of Fitmeal with lunch; CV, Coefficient of variation; EPDS, Edinburgh post-natal depression scale; FBG, Fasting blood glucose; GDM, Gestational diabetes mellitus; GI, Glycemic index; GA, Glycated albumin; HbA1c, Glycated hemoglobin; HDL, High-density lipoprotein; iAUC, The incremental areas under the curve; L, Lunch; LDL, Low-density lipoprotein; MAGE, Mean amplitude of glycemic excursion; OGTT, Oral glucose tolerance test; P, Pre-load; P + L, Fitmeal pre-load 30 min before lunch; PG, Post-prandial glucose; PE, Post-prandial excursion; PGR, Post-prandial glycemic response; S, Mid-meal snack; S + L, Fitmeal as mid-meal snack 2h before lunch; SMBG, Self-monitoring of blood glucose; SD, Standard deviation; SPSS, Statistical product service solutions; T2DM, Type 2 diabetes; TG, Triglyceride; TC, Total cholesterol; TIR, Time in range; TAR, Time above range; TBR, Time below range; W, Water; W + L, Drinking water 30 min before lunch.
1. International Diabetes Federation. IDF Diabetes Atlas. 10th ed. International Diabetes Federation (2019). Available online at: https://www.diabetesatlas.org/en (accessed September 01, 2022).
2. Wei Y, Yang H, Zhu W, Yang H, Li H, Yan J, et al. International Association of Diabetes and Pregnancy Study Group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chin Med J. (2014) 127:3553–6. doi: 10.3760/cma.j.issn.0366-6999.20140898
3. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes after gestational diabetes a systematic review and meta-analysis. Lancet. (2009) 373:1773–9. doi: 10.1016/S0140-6736(09)60731-5
4. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. (2020) 369:m1361. doi: 10.1136/bmj.m1361
5. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. (2019) 62:905–14. doi: 10.1007/s00125-019-4840-2
6. Wang YM, Zhao LH, Su JB, Qiao HF, Wang XH, Xu F, et al. Glycemic variability in normal glucose tolerance women with the previous gestational diabetes mellitus. Diabetol Metab Syndr. (2015) 7:82. doi: 10.1186/s13098-015-0077-5
7. Colatrella A, Framarino M, Toscano V, Bongiovanni M, Festa C, Mattei L, et al. Continuous glucose monitoring during breastfeeding in women with recent gestational diabetes mellitus. Diabetes Technol Ther. (2012) 14:576–82. doi: 10.1089/dia.2011.0266
8. Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. (2007) 30: 263–9. doi: 10.2337/dc06-1612
9. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. (2003) 26: 881–5. doi: 10.2337/diacare.26.3.881
10. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. (2006) 295: 1681–7. doi: 10.1001/jama.295.14.1681
11. Li N, Yang Y, Cui D, Li C, Ma RCW, Li J, et al. Effects of lifestyle intervention on long term risk of diabetes in women with prior gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. (2021) 22:e13122. doi: 10.1111/obr.13122
12. Tandon N, Gupta Y, Kapoor D, Lakshmi JK, Praveen D, Bhattacharya A, et al. Effects of a lifestyle intervention to prevent deterioration in glycemic status among South Asian women with recent gestational diabetes: a randomized clinical trial. JAMA Netw Open. (2022) 5:e220773. doi: 10.2139/ssrn.3905922
13. WS/T 601-−2018. Dietary Guide for Patients With Gestational Diabetes Mellitus. Beijing: China's National Health Commission (2018).
14. Nutrition and Metabolic Management Branch of China International Exchange and Promotive Association for Medical and Health Care, Clinical Nutrition Branch of Chinese Nutrition Society, Chinese Diabetes Society Chinese Society for Parenteral and Enteral Nutrition, Chinese Clinical Nutritionist Center of Chinese Medical Doctor Association. Chinese guidelines of medical nutrition therapy in diabetes. Chin J Diabetes Mellitus. (2022) 14:881–933. doi: 10.3760/cma.j.cn115791-20220704-00324
15. Guo J, Long Q, Yang J, Lin Q, Wiley J, Chen JL. The efficacy of an intensive lifestyle modification program on psychosocial outcomes among rural women with prior gestational diabetes mellitus: six months follow-up of a randomized controlled trial. Int J Environ Res Public Health. (2021) 18:1519. doi: 10.3390/ijerph18041519
16. Shyam S, Arshad F, Abdul Ghani R, Wahab NA, Safii NS, Nisak MY, et al. Low glycaemic index diets improve glucose tolerance and body weight in women with previous history of gestational diabetes: a six months randomized trial. Nutr J. (2013) 12:68. doi: 10.1186/1475-2891-12-68
17. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. (2015) 163:1079–94. doi: 10.1016/j.cell.2015.11.001
18. Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. (2020) 26:964–73. doi: 10.1038/s41591-020-0934-0
19. Ungersboeck M, Tang X, Neeff V, Steele D, Grimm P, Fenech M. Personalised nutritional recommendations based on individual post-prandial glycaemic responses improve glycaemic metrics and PROMs in Patients with type 2 diabetes: a real-world assessment. Nutrients. (2022) 14:2123. doi: 10.3390/nu14102123
20. Engler S, Fields S, Leach W, van Loon M. Real-time continuous glucose monitoring as a behavioral intervention tool for T2D: a systematic review. J Technol Behav Sci. (2022) 7:252–363. doi: 10.1007/s41347-022-00247-5
21. Nielson KK, Kapul A, Damm P, de Courten M, Bygbjerg IC. From screening to postpartum follow-up-the determinants and barriers for gestational diabetes mellitus services, a systematic review. BMC Pregn Childb. (2014) 14:41. doi: 10.1186/1471-2393-14-41
22. Bennett WL, Ennen CS, Carrese JA, Hill-Briggs F, Levine DM, Nicholson WK, et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: a qualitative study. J Womens Health. (2011) 20:239–45. doi: 10.1089/jwh.2010.2233
23. Mustad VA, Hegazi RA, Hustead DS, Budiman ES, Rueda R, Maki K, et al. Use of a diabetes-specific nutritional shake to replace a daily breakfast and afternoon snack improves glycemic responses assessed by continuous glucose monitoring in people with type 2 diabetes: a randomized clinical pilot study. BMJ Open Diabetes Res Care. (2020) 8:e001258. doi: 10.1136/bmjdrc-2020-001258
24. Clifton PM, Galbraith C, Coles L. Effect of a low dose whey/guar pre-load on glycemic control in people with type 2 diabetes–a randomised controlled trial. Nutr J. (2014) 13:103. doi: 10.1186/1475-2891-13-103
25. Watson LE, Phillips LK, Wu T, Bound MJ, Checklin HL, Grivell J, et al. A whey/guar “preload” improves postprandial glycaemia and HbA1c in type 2 diabetes-a 12-week, single-blind, randomized, placebo-controlled trial. Diabetes Obes Metab. (2019) 21:930–8. doi: 10.1111/dom.13604
26. Li CJ, Norstedt G, Hu ZG, Yu P, Li DQ, Li J, et al. Effects of a macro-nutrient preload on type 2 diabetic patients. Front Endocrinol. (2015) 6:139. doi: 10.3389/fendo.2015.00139
27. Li L, Xu J, Zhu W, Fan R, Bai Q, Huang C, et al. Effect of a macronutrient preload on blood glucose level and pregnancy outcome in gestational diabetes. J Clin Transl Endocrinol. (2016) 5:36–41. doi: 10.1016/j.jcte.2016.04.001
28. Tang Qi, Yang Huazhen, Wen Zhi, et al. Effect of low glycemic-load diet in patients with obesity and metabolic syndrome. Modern Prev Med. (2017) 44:2952–4.
29. Jenkins AL, Jenkins DJ, Wolever TM, Rogovik AL, Jovanovski E, Bozikov V, et al. Comparable postprandial glucose reductions with viscous fiber blend enriched biscuits in healthy subjects and patients with diabetes mellitus: acute randomized controlled clinical trial. Croat Med J. (2008) 49:772–82. doi: 10.3325/cmj.2008.49.722
30. Skalkos S, Moschonis G, Thomas CJ, McMillan J, Kouris-Blazos A. Effect of lupin-enriched biscuits as substitute mid-meal snacks on post-prandial interstitial glucose excursions in post-surgical hospital patients with type 2 diabetes. Nutrients. (2020) 12:1239. doi: 10.3390/nu12051239
31. Papakonstantinou E, Orfanakos N, Farajian P, Kapetanakou AE, Makariti IP, Grivokostopoulos N, et al. Short-term effects of a low glycemic index carob-containing snack on energy intake, satiety, and glycemic response in normal-weight, healthy adults: results from two randomized trials. Nutrition. (2017) 42:12–9. doi: 10.1016/j.nut.2017.05.011
32. Ware JJ, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. (1996) 34:220–33. doi: 10.1097/00005650-199603000-00003
33. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
34. Yang YZ, Huang HT. An epidemiological study on stress among urban residents in social transition period. Chin J Epidemiol. (2003) 24:760–4. doi: 10.3760/j.issn:0254-6450.2003.09.004
35. Macfarlane DJ, Lee CC, Ho EY, Chan KL, Chan DT. Reliability and validity of the Chinese version of IPAQ (short, last 7 days). J Sci Med Sport. (2007) 10:45–51. doi: 10.1016/j.jsams.2006.05.003
36. Sun L, Goh HJ, Govindharajulu P, Leow MK, Henry CJ. Postprandial glucose, insulin and incretin responses differ by test meal macronutrient ingestion sequence (PATTERN study). Clin Nutr. (2020) 39:950–7. doi: 10.1016/j.clnu.2019.04.001
37. Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, et al. Glycaemic index methodology. Nutr Res Rev. (2005) 18:145–71. doi: 10.1079/NRR2005100
38. Fu J, Retnakaran R. The life course perspective of gestational diabetes: an opportunity for the prevention of diabetes and heart disease in women. E Clin Med. (2022) 45:101294. doi: 10.1016/j.eclinm.2022.101294
39. Hu G, Tian H, Zhang F, Liu H, Zhang C, Zhang S, et al. Tianjin gestational diabetes mellitus prevention program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract. (2012) 98:508–17. doi: 10.1016/j.diabres.2012.09.015
40. Chen Y, Zhong Q, Luo J, Tang Y, Li M, Lin Q, et al. The 6-month efficacy of an intensive lifestyle modification program on type 2 diabetes risk among rural women with prior gestational diabetes mellitus: a cluster randomized controlled trial. Prev Sci. (2022) 23:1156–68. doi: 10.1007/s11121-022-01392-2
41. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. (2010) 362:1090–101. doi: 10.1056/NEJMoa0908292
42. Schübert H, Müller UA, Kramer G, Müller N, Heller T, Kloos C, et al. Snacking is common in people with diabetes type 1 and type 2 with insulin therapy and is not associated with metabolic control or quality of life. Exp Clin Endocrinol Diab. (2019) 127:461–7. doi: 10.1055/a-0631-8813
43. Pham H, Holen IS, Phillips LK, Hatzinikolas S, Huynh LQ, Wu T, et al. The effects of a whey protein and guar gum-containing preload on gastric emptying, glycaemia, small intestinal absorption and blood pressure in healthy older subjects. Nutrients. (2019) 11:2666. doi: 10.3390/nu11112666
44. Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, et al. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab. (2006) 91:2062–7. doi: 10.1210/jc.2005-2644
45. Lu J, Zhao W, Wang L, Fan Z, Zhu R, Wu Y, et al. Apple preload halved the postprandial glycaemic response of rice meal in healthy subjects. Nutrients. (2019) 11: 2912. doi: 10.3390/nu11122912
46. Lu X, Lu J, Fan Z, Liu A, Zhao W, Wu Y, et al. Both isocarbohydrate and hypercarbohydrate fruit preloads curbed postprandial glycemic excursion in healthy subjects. Nutrients. (2021) 13:2470. doi: 10.3390/nu13072470
Keywords: gestational diabetes, pre-load, post-prandial glycaemic response, continuous glucose monitoring, glycaemic index
Citation: Li C, Gao Y, Luo T, Qin S, Yao X, Wen Y, Wang X, Zhang J, Zhong Q, Shi H and Liu J (2023) Effects of low-GI biscuits as pre-loads or mid-meal snacks on post-prandial glycemic excursions in women with recent gestational diabetes: A protocol for a randomized crossover trial and an extended tailored intervention. Front. Nutr. 10:1122102. doi: 10.3389/fnut.2023.1122102
Received: 12 December 2022; Accepted: 01 March 2023;
Published: 23 March 2023.
Edited by:
A. Seval Ozgu-Erdinc, Ankara City Hospital, TürkiyeReviewed by:
Stefan Kabisch, Charité Universitätsmedizin Berlin, GermanyCopyright © 2023 Li, Gao, Luo, Qin, Yao, Wen, Wang, Zhang, Zhong, Shi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu, bGl1anNwbWNoQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.