
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 08 March 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1113875
This article is part of the Research Topic Nutrition, Sarcopenia, and Sarcopenic Obesity View all 18 articles
 Jiahua Lyu1,2
Jiahua Lyu1,2 Ningjing Yang2
Ningjing Yang2 Ling Xiao1
Ling Xiao1 Xinyu Nie2
Xinyu Nie2 Jing Xiong2
Jing Xiong2 Yudi Liu1,2
Yudi Liu1,2 Min Zhang2
Min Zhang2 Hangyue Zhang2
Hangyue Zhang2 Cunhan Tang2
Cunhan Tang2 Shiyi Pan1
Shiyi Pan1 Long Liang2
Long Liang2 Hansong Bai2
Hansong Bai2 Churong Li2
Churong Li2 Hao Kuang2
Hao Kuang2 Tao Li1,2*
Tao Li1,2*Objectives: It remains controversial whether sarcopenia has any significant impact on the efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) or immune checkpoint inhibitors (ICIs) in patients with advanced non-small cell lung cancer (NSCLC). Therefore, in this study, we aimed to assess the association between sarcopenia and clinical outcomes in patients with advanced NSCLC receiving EGFR-TKIs or ICIs as a first-line therapy.
Methods: We retrospectively enrolled 131 patients with advanced NSCLC treated with first-line EGFR-TKIs or ICIs between 1 March 2019 and 31 March 2021. To estimate sarcopenia, we calculated skeletal muscle index (SMI) as the ratio of skeletal muscle area (cm2) to height squared (m2). Associations between sarcopenia and overall survival (OS) and progression-free survival (PFS) were evaluated using the Kaplan–Meier method and log-rank tests, respectively. A Cox proportional hazards regression model was used to assess the factors associated with OS and PFS. The Student’s t-test or Mann–Whitney U test was used to compare the SMI between patients with or without objective response and disease control. The chi-squared test was used to compare adverse events (AEs) between patients with and without sarcopenia.
Results: Among the 131 patients, 35 (26.7%) were diagnosed with sarcopenia. Sarcopenia was an independent predictor of poor OS and PFS (p < 0.05) overall and in the EGFR-TKI- and ICI-treated cohorts. Among all patients, those with sarcopenia showed significantly shorter OS and PFS than those without sarcopenia (median OS and PFS: 13.0 vs. 26.0 months and 6.4 vs. 15.1 months; both p < 0.001). These associations were consistent across the subtypes of most clinical characteristics. Statistically significant differences between the objective response (OR) and non-OR groups were also observed in the mean SMI (OR group, 43.89 ± 7.55 vs. non-OR group, 38.84 ± 7.11 cm2/m2; p < 0.001). In addition, we observed similar results with disease control (DC) and non-DC groups (DC group, 42.46 ± 7.64 vs. non-DCR group, 33.74 ± 4.31 cm2/m2; p < 0.001). The AEs did not differ significantly between the sarcopenia and non-sarcopenia groups.
Conclusion: Sarcopenia before treatment might be a significant predictor of poor clinical outcomes (shorter OS and PFS, fewer ORs, less DC) in patients with advanced NSCLC treated with EGFR-TKIs or ICIs as the first-line therapy.
Lung cancer is the second most common malignant tumor and the leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) comprises the majority (85%) of all lung cancers (1). Approximately, 25% of NSCLC patients present with an advanced stage at initial diagnosis (2), with a 5-year survival rate of less than 20% (3).
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and immune checkpoint inhibitors (ICIs) have been shown to significantly improve the survival of metastatic NSCLC patients with mutant and wild-type EGFR, respectively (4, 5). However, not all eligible patients can benefit equally from EGFR-TKIs or ICIs (6, 7). Although EGFR mutation and the PD-L1 expression level have been reported as potential predictors of the therapeutic efficacy for EGFR-TKIs and ICIs, it is essential to identify additional biomarkers that can help determine those patients most likely to benefit from these therapies.
Sarcopenia is characterized by progressive loss of skeletal muscle strength and mass and is associated with decreased muscle protein synthesis and increased protein degradation (8, 9). Sarcopenia has been reported in approximately 50% of lung cancer patients and is associated with a decrease in the efficacy of surgery or chemotherapy, toxicity, and a worse quality of life (10, 11). However, the impact of sarcopenia on the efficacy and toxicity of EGFR-TKIs and ICIs remains unclear.
Several articles have suggested that sarcopenia is correlated with poor clinical outcomes in patients receiving PD-1/PD-L1 inhibitors (12, 13), however, other researches have reached inconsistent conclusions (14–16). The same controversy also exists In NSCLC patients treated with EGFR-TKIs. A retrospective study showed that sarcopenia did not affect the response to gefitinib in patients with EGFR-mutated NSCLC (17). In contrast, another retrospective study enrolling 72 NSCLC patients treated with erlotinib found that sarcopenia was a negative biomarker that was significantly associated with response and survival outcomes (18).
In brief, there is no consensus as to whether sarcopenia is a prognostic biomarker for EGFR-TKI or ICI treatment of advanced NSCLC, especially when used as the first-line treatment. Consequently, we sought to investigate the potential predictive value of sarcopenia on the efficacy of EGFR-TKIs in NSCLC patients harboring EGFR mutations or of ICIs in patients with wild-type EGFR.
In this study, we retrospectively collected the data of patients with pathologically confirmed metastatic NSCLC who were treated with EGFR-TKIs or ICIs as the first-line therapy at Sichuan Cancer Hospital, China, from 16 January 2018 to 8 June 2021. Patients who met the inclusion criteria below were enrolled: (1) histologically confirmed stage IV metastatic NSCLC; (2) treated with EGFR-TKIs or ICIs as first-line therapies; and (3) underwent a chest/abdominal CT scan within 4 weeks prior to EGFR-TKI or ICI therapy. This study was approved by the Ethics Committee of Sichuan Cancer Hospital and carried out in strict accordance with the Declaration of Helsinki.
We consecutively enrolled 205 patients with advanced NSCLC treated with EGFR-TKIs or ICIs in our hospital. After selection according to the inclusion and exclusion criteria, 54 eligible patients receiving ICIs and 77 eligible patients receiving EGFR-TKIs were included (Supplementary Figure 1).
Subsequently, we obtained basic demographic and clinical data for all eligible patients, including age, sex, history of smoking and alcohol consumption, Karnofsky performance status (KPS) score, histopathology, height, weight, routine biochemical and hematological test results, CT images, EGFR mutation status, PD-L1 expression before EGFR-TKI or ICI initiation, treatment option, treatment response, and toxicity. The follow-up date ended at the date of the outcome event, the date of death, or the end of follow-up, whichever came first.
The chest/abdomen CT scan for sarcopenia evaluation was obtained within 4 weeks before the start of EGFR-TKIs or ICIs. The skeletal muscle area was measured at the L3 level by two experienced radiologists (NJY and XYN) using sliceOmatic (TomoVision 5.0, Magog, QC, Canada) with –29 to 150 Hounsfield unit (HU) (Supplementary Figure 2). The skeletal muscle index (SMI) was calculated using the formula (L3 muscle area in cm2)/(patient height in m2). Sarcopenia was defined as a low SMI as follows: (1) for women, SMI < 31.6 cm2/m2; (2) for men, SMI < 40.2 cm2/m2 (19).
Tumor response evaluations were performed based on chest CT according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (for patients receiving EGFR-TKIs) or iRECIST criteria (for patients receiving ICIs). PFS and overall survival (OS) were calculated from the date of initiation of EGFR-TKI or ICI treatment to the date of progression (for PFS) or patient death (for OS) or to the last follow-up. The incidence and severity of all adverse events (AEs) were monitored and evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE V5.0).
We used R software, version 4.0.2 (R Foundation for Statistical Computing), for statistical analysis. Multiple Cox regression analyses were conducted to evaluate the impact of sarcopenia and other candidate prognostic factors on the OS and PFS. The Kaplan–Meier method and log-rank test were used to compare OS and PFS between patients with and without sarcopenia. The association between the presence of sarcopenia and demographic, clinical, and laboratory parameters, treatment response, and occurrence of AEs was established using the exact Fisher test and χ2 test. Scatter plots were used to graphically represent the association between sarcopenia and hemoglobin, total protein, albumin, prealbumin, serum triglyceride, and serum cholesterol levels and BMI using Spearman’s correlation. All statistics were two-tailed, and p-values ≤ 0.05 were considered statistically significant.
The baseline characteristics of the 131 enrolled patients are presented in Table 1. A total of 77 and 54 patients received first-line treatment with EGFR-TKIs or ICIs, respectively. Patients were categorized into two groups (sarcopenia and non-sarcopenia) according to the previously defined criteria for sarcopenia. Among all patients, 35 (26.7%) were diagnosed with sarcopenia, including 17 EGFR-TKI-treated and 18 ICI-treated patients. A full comparison of the baseline characteristics between the sarcopenia and non-sarcopenia groups is presented in Table 1. Sarcopenia was significantly more common in patients who had smoked (p = 0.038) or drunk (p = 0.041) regularly, or those who had a low KPS score (p = 0.002) or low albumin (p = 0.023). The baseline characteristics of the EGFR-TKI and ICI cohorts are listed in the Supplementary Tables 1, 2, respectively.
The relationship between nutritional status and L3 SMI is shown in Figure 1. The scatter plot shows a high correlation between nutritional status factors, including BMI (p < 0.001, Figure 1A), hemoglobin level (p = 0.014, Figure 1B), albumin level (p = 0.029, Figure 1D), prealbumin level (p = 0.018, Figure 1E), and cholesterol level (p = 0.016, Figure 1F), and L3 SMI, indicating that poor nutritional status is a risk factor for sarcopenia in NSCLC patients.
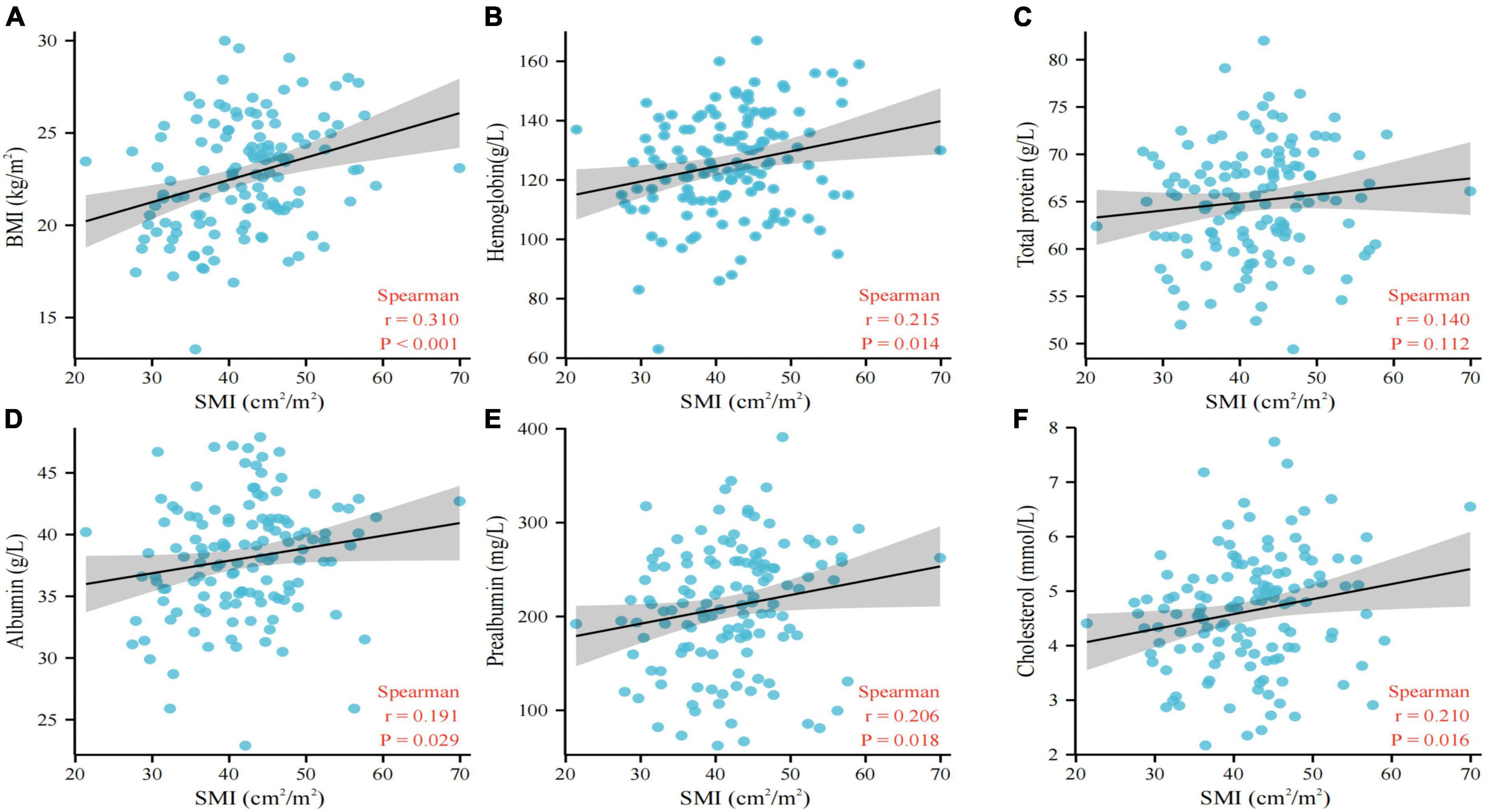
Figure 1. Association between BMI (A), hemoglobin (B), total protein (C), albumin (D), prealbumin (E), cholesterol (F), and L3 SMI. BMI, body mass index; SMI, skeletal muscle index.
The Kaplan–Meier curves for OS and PFS grouped by sarcopenia and non-sarcopenia are shown in Figure 2. Analysis of the entire patient cohort showed a significant difference in OS between patients with sarcopenia and those without sarcopenia, with a median OS of 13 and 26 months, respectively (p < 0.001; Figure 2A). Kaplan–Meier analysis also revealed that patients with sarcopenia had a significantly shorter PFS than those without sarcopenia (6.4 months vs. 15.1 months, p < 0.001; Figure 2D).
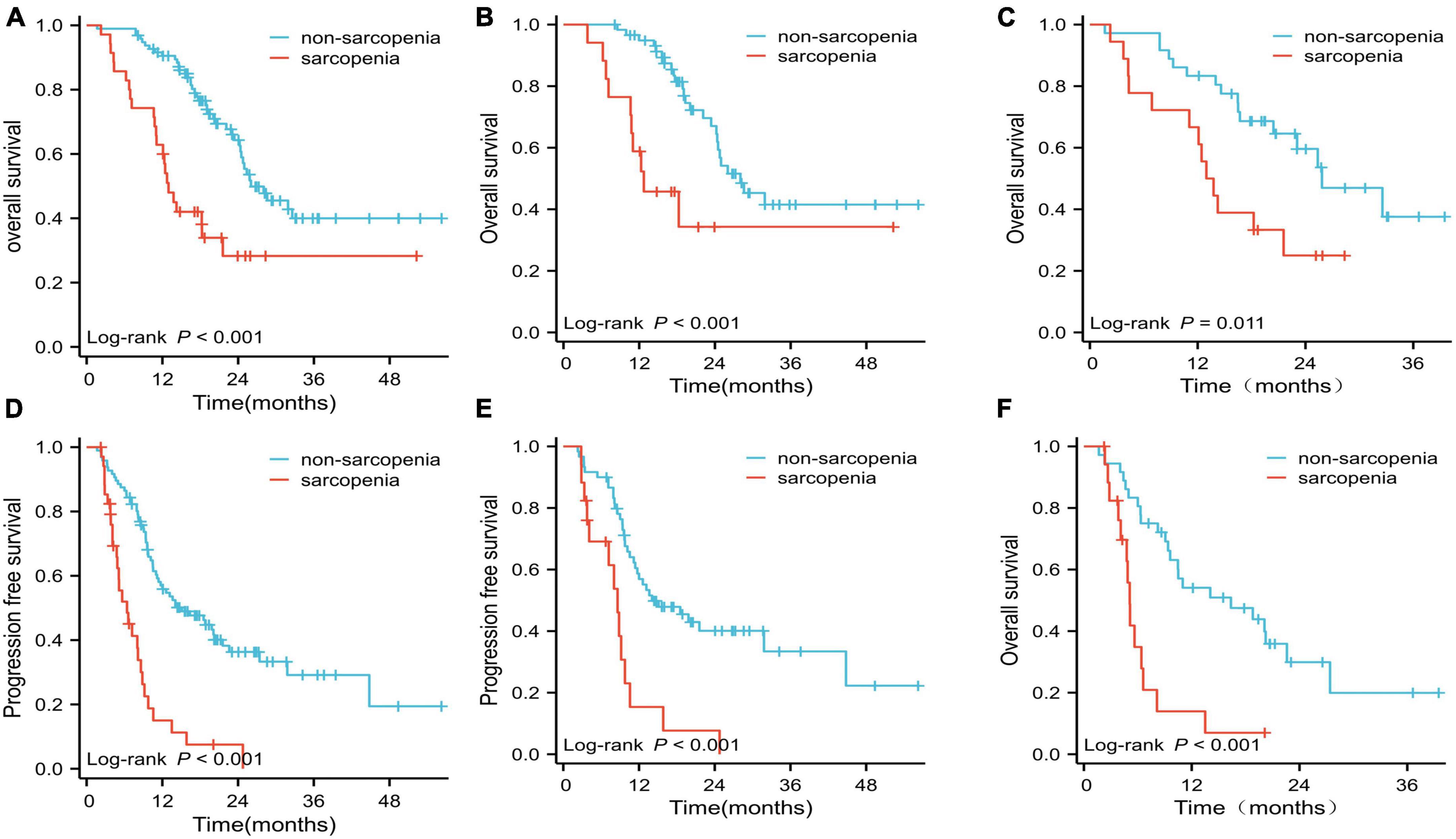
Figure 2. Overall survival (OS) and progression-free survival (PFS) curves. (A) OS for the two groups in the whole cohort; (B) OS for the two groups treated with EGFR-TKIs; (C) OS for the two groups treated with ICIs; (D) PFS for the sarcopenia and non-sarcopenia groups in the whole cohort; (E) PFS for the two groups treated with EGFR-TKIs; (F) PFS for the two groups treated with ICIs.
In the EGFR-TKI-treated cohort (n = 77), the median OS and PFS for patients with sarcopenia were significantly shorter than those for patients without sarcopenia (OS: 12.7 vs. 28.0 months; PFS: 8.6 vs. 14.1 months, respectively; both p < 0.001; Figures 2B, E). The median OS and PFS for patients with sarcopenia in the ICI-treated cohort were significantly shorter than those for patients without sarcopenia (OS: 13.4 vs. 25.8 months, p = 0.011; PFS: 5.1 vs. 16.4 months, p < 0.001; Figures 2C, F).
In the entire patient cohort, univariate Cox regression analysis revealed that BMI, sarcopenia, KPS score, levels of hemoglobin, hypersensitive C-reactive protein (hCRP) and albumin were significant prognostic factors for OS (all p < 0.05; Table 2). Multivariate analysis of all the above potential factors identified only sarcopenia [hazard ratio (HR): 2.187, 95% confidence interval (CI): 1.230–3.891, p = 0.008; Table 2] and albumin [hazard ratio (HR): 0.921, 95% confidence interval (CI): 0.860–0.987, p = 0.019; Table 2] as strong independent predictors of OS. Univariate and multivariate Cox regression analyses of both the EGFR-TKI-treated and ICI-treated cohorts confirmed that sarcopenia was an independent negative factor for OS (EGFR-TKI-treated group, HR: 2.806, 95% CI: 1.304–6.037, p = 0.008; Supplementary Table 3; ICI-treated group, HR: 2.155, 95% CI: 1.107–4.484, p = 0.028; Supplementary Table 4).
Univariate Cox regression analysis revealed that BMI, sarcopenia, KPS score, hCRP level, and albumin level were significant prognostic factors for PFS (all p < 0.05; Table 3). Multivariate analysis confirmed the independent prognostic relevance of BMI (HR, 0.883; 95% CI: 0.814–0.958, p = 0.003) and sarcopenia (HR, 2.830; 95% CI: 1.662–4.817, p < 0.001) for PFS (Table 3). Univariate and multivariate Cox regression analyses of both the EGFR-TKI and ICI cohorts confirmed that sarcopenia was an independent negative factor for PFS (EGFR-TKI cohort, HR: 2.946, 95% CI: 1.430–6.068, p = 0.003, Supplementary Table 5; ICI cohort, HR: 3.567, 95% CI: 1.647–7.724, p = 0.001, Supplementary Table 6).
Stratified analyses were performed to clarify the relationship between sarcopenia and the HRs of OS and PFS in various patient subgroups (Figures 3, 4). Overall, sarcopenia was consistently associated with poor OS and PFS across most subgroups of patients.

Figure 3. The association between sarcopenia and hazard ratios of OS in various subgroups. BMI, body mass index; KPS, Karnofsky performance status; hCRP, hypersensitive C-reactive protein.
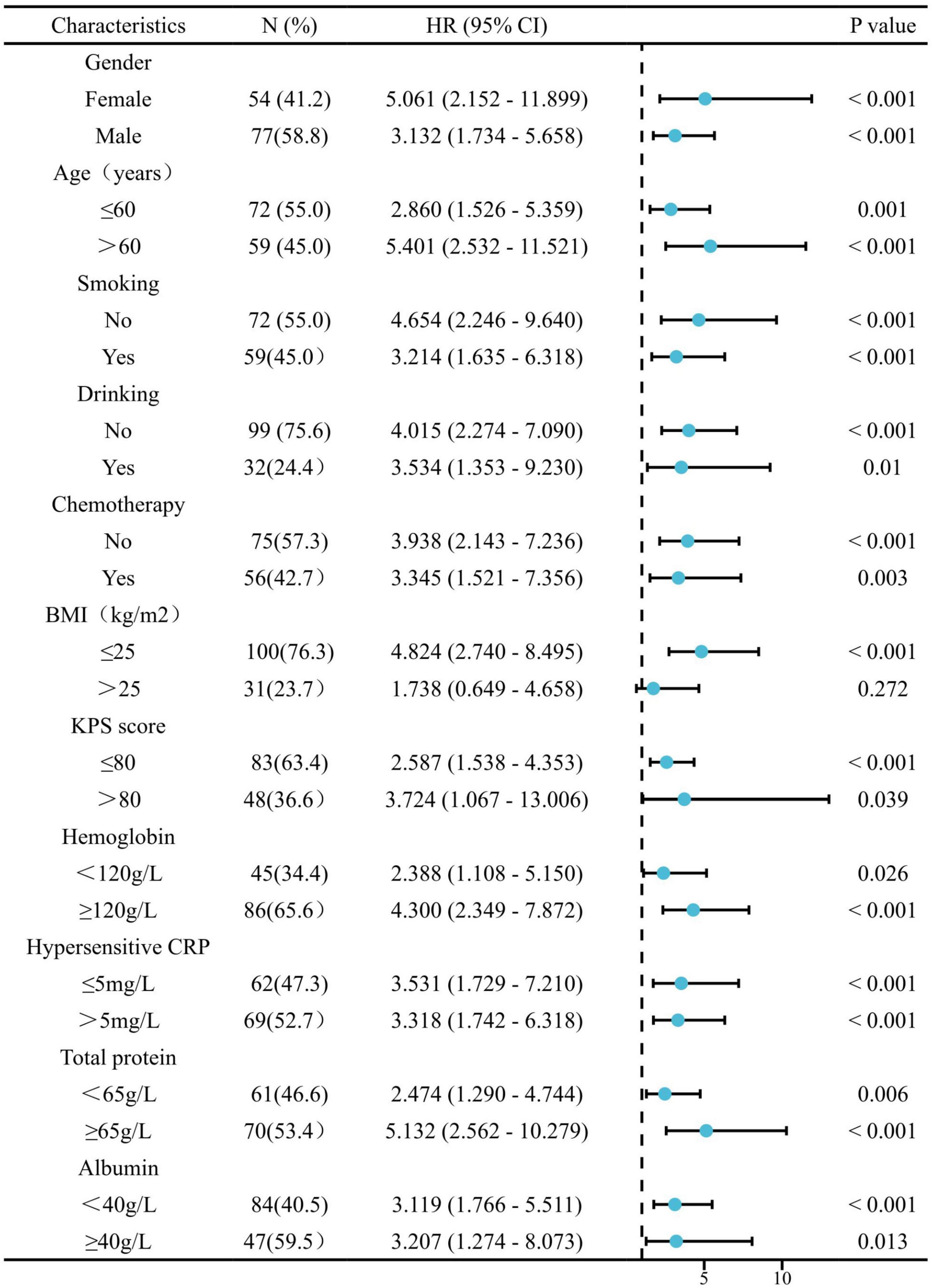
Figure 4. The association between sarcopenia and hazard ratios of PFS in various subgroups. BMI, body mass index; KPS, Karnofsky performance status; hCRP, hypersensitive C-reactive protein.
Of the 131 patients, 81 had an OR and 125 had DC. The mean SMI was significantly lower in the non-OR group than in the OR group, 38.84 ± 7.11 vs. 43.89 ± 7.55 cm2/m2, respectively (p < 0.001; Figure 5A). Similarly, a significant difference was also found in SMI between the DC group and non-DC group (42.46 ± 7.64 vs. 33.74 ± 4.31 cm2/m2, p = 0.002; Figure 5D). All analyses were repeated in the subgroups of patients treated with EGFR-TKIs or ICIs, and the findings were similar to those of the primary analysis (Figures 5B, C, E, F). The patients in the OR and DC groups had a significantly higher SMI than those in the non-OR and non-DC groups, regardless of whether the patients received EGFR-TKI (Figures 5B, E) or ICI (Figures 5C, F) treatment.
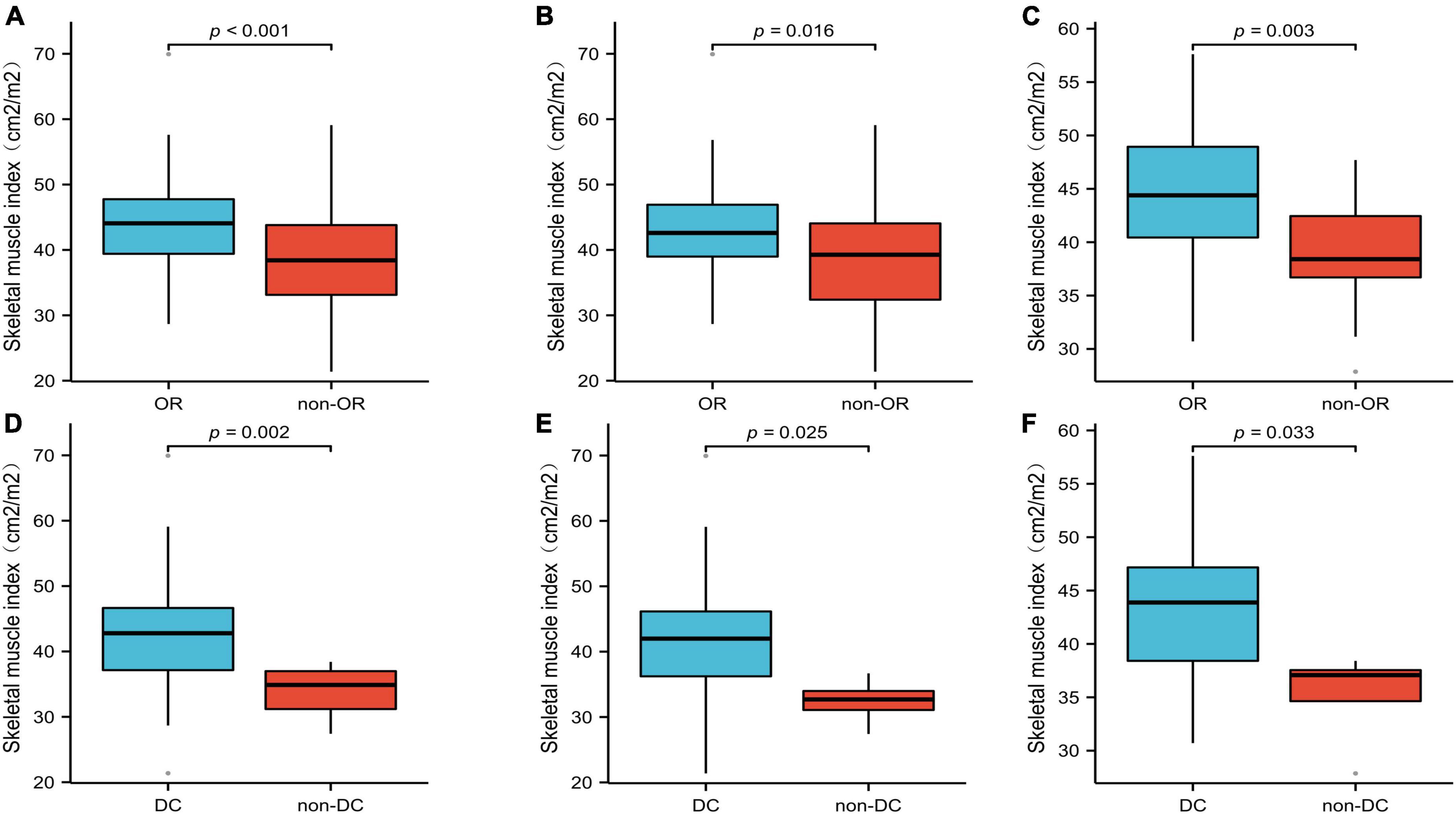
Figure 5. The mean SMI of the OR and DC groups in all patients (A,D), patients treated with EGFR-TKIs (B,E), and patients treated with ICIs (C,F). SMI, skeletal muscle index; OR, objective response; DC, disease control.
In our study, 51 patients (38.9%) experienced treatment-related AEs: 12 (12/35, 34.3%) in the sarcopenia group and 39 (39/96, 40.6%) in the non-sarcopenia group. There was no statistically significant difference between the two groups (p = 0.550). The most frequent AEs were hypothyroidism and skin rashes.
The present study presents considerable real-world data on sarcopenia as a prognostic marker in patients with advanced NSCLC receiving first-line EGFR-TKIs or ICIs. We confirmed that patients with sarcopenia had significantly shorter OS and PFS than those without sarcopenia in the entire patient, EGFR-TKI-treated, and ICI-treated cohorts. In addition, statistically significant differences were observed in mean SMI between the OR and non-OR groups and the DC and non-DC groups. Therefore, nutritional intervention and physical activity programs are recommended to patients with sarcopenia receiving immunotherapy or EGFR-TKI therapy to improve the therapy outcome.
Although there are many studies on the relationship between sarcopenia and immunotherapy in lung cancer, their conclusions are inconsistent. Most of these studies found that a low SMI or a sarcopenia diagnosis is associated with shortened survival in advanced NSCLC patients treated with PD1/PD-L1 checkpoint inhibitors (14, 16, 20–25). However, other studies showed no differences in OS and PFS between patients with and without sarcopenia (26–29). In these studies, ICIs were used in different treatment lines, which may have partially affected the results. The majority of patients included in these studies were treated with second-line or later immunotherapy, whereas only four studies enrolled patients who were receiving first-line immunotherapy, and the sample size in these was relatively small (16, 22, 23, 29). Currently, immunotherapy is increasingly used as the first-line treatment for advanced lung cancer; therefore, our study included advanced lung cancer patients receiving ICIs only as the first-line therapy. Our study included the largest sample size of patients receiving first-line immunotherapy reported to date and provided strong evidence of the negative impact of sarcopenia on the prognosis of lung cancer when using first-line ICIs.
Similarly, using univariate and multivariate analyses of EGFR-TKI subgroups, we found that patients without sarcopenia had significantly longer OS and PFS than those with sarcopenia. In the few studies evaluating the prognostic impact and predictive value of sarcopenia in NSCLC patients harboring EGFR mutations and treated with EGFR-TKIs, as with ICI therapies, the results are inconsistent; however, most studies agree that sarcopenia does not affect PFS and OS (30–32). In a retrospective study conducted by Sabrina et al., sarcopenia did not affect the response to gefitinib in patients with EGFR-mutated NSCLC, even though it was an indicator of poor prognosis in terms of OS (17). In contrast, Atakan et al. found that sarcopenia was an independent factor of poor prognosis for OS and PFS in NSCLC patients receiving EGFR-TKI-targeted therapy (18). These inconsistent results in different studies might come from different study design, different inclusion and exclusion criteria, different sample size and different way of measuring muscle area or definition of sarcopenia, etc.
Next, stratified analyses were performed to clarify the relationship between sarcopenia and the HRs of OS and PFS in various patient subgroups. Overall, sarcopenia was consistently associated with both poor OS and PFS across most subgroups of patients except for patients with BMI > 25kg/m2. The reason may be that, for cancer patients, body weight and body fat are also important indicators to reflect the nutritional status of patients and significantly affect the treatment outcome of patients (33). Therefore, for obese (BMI > 25kg/m2) cancer patients, a comprehensive body composition analysis may be a better prognostic indicator more than a single myopenia.
The underlying mechanisms by which sarcopenia affects the efficacy of ICIs and EGFR-TKIs are not yet fully understood. Previous studies have found that interleukin-15 is the most abundant cytokine expressed in the skeletal muscle that can regulate CD8+ T cells and promote T cell survival (34, 35), which is important for maintaining immune function. Serum interleukin-15 levels decrease in older adults with the loss of muscle mass, suggesting that muscle loss may lead to impaired immune function, which may have some relevance to sarcopenia. Additionally, CD4+FoxP3+ Tregs infiltrate damaged skeletal muscles, suggesting that sarcopenia may play an important role in tumor immune escape (36). Another possible mechanism for the poor prognosis of NSCLC patients with sarcopenia could be different drug clearance rates in cancer patients with or without sarcopenia, as there is a strong association between pembrolizumab clearance and OS. Patients with high ICI clearance rates had worse survival rates than those with low clearance rates. Some researchers believe the primary method of ICI elimination may be related to the development of cancer cachexia and sarcopenia. Procatabolic status can affect survival by leading to faster protein turnover through monoclonal antibody clearance (37).
The mechanism by which sarcopenia affects the efficacy of EGFR-TKIs is still unclear, but some of the reasons may be similar to those for immunotherapy drugs, such as drug clearance. Retrospective studies have shown that patients with the same body weight and BMI may have different skeletal muscle masses and adipose tissue levels, which could affect EGFR-TKI therapy outcomes (13). When administered, EGFR-TKIs, including gefitinib, are widely distributed in various tissues of the human body, and when bound to human serum albumin and α1-acid glycoprotein, they can have half-lives of up to 48 h. Researchers have demonstrated in animal models that gefitinib is present in lower concentrations in the skin and fat and in higher concentrations in highly perfused organs (38). In addition, studies have shown that gefitinib lasts for up to 96 h in muscle and for only 2 h in fat after oral consumption (39). Therefore, as the diffusion and disposition of drugs in fat are different from those in muscle, this could be one of the mechanisms by which sarcopenia affects the prognosis and toxicity of EGFR-TKIs.
Whether sarcopenia is associated with treatment-related toxicity in lung cancer remains unclear. In this study, we found that the treatment-related toxicities in patients with sarcopenic and non-sarcopenic lung cancer were similar (31, 34). Nie et al. reported that treatment-related toxicity occurred more frequently in patients with sarcopenic lung cancer using afatinib (30). In contrast, Alessio et al. did not find a significant relationship between baseline SMI and AEs (14). The toxicities of EGFR-TKIs or ICIs are closely related to the duration of medication. As the survival times of non-sarcopenia patients were longer than those of sarcopenia patients, this may have affected the incidence of adverse reactions, resulting in the lack of a statistically significant difference between the two groups.
Our study has several strengths. It is the first to include both EGFR-TKIs and ICIs, and the targeted immunotherapy included in our study was a first-line treatment, which conforms to the current standard treatment regimen. In addition, compared with similar studies, ours has the largest number of cases, and there are few studies focusing on both OS and PFS in patients, as in our study.
Our study also has several limitations. First, this was a retrospective, single-center study. Second, sarcopenia was defined only according to SMI and was not based on muscle strength and function, such as grip strength.
In conclusion, sarcopenia before first-line EGFR-TKI or ICI therapy might be a significant predictor of poor clinical outcomes, leading to shortened OS and PFS and reduced OR and DC. Sarcopenia should be considered before using EGFR-TKIs or ICIs in clinical practice.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Sichuan Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
JL and TL were responsible for conceptualizing and designing this study, data collection, data interpretation, and manuscript drafting. NY, LX, and XN played a major role in body composition assessment and data analysis. JX, YL, MZ, HZ, CT, SP, LL, HB, CL, and HK participated in acquisition of clinical records, data analysis, and revision of the manuscript. All authors read and approved the final version of manuscript.
This work was supported by grants from the Project of Sichuan Science and Technology Department (grant number: 2021YJ0010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1113875/full#supplementary-material
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Blandin Knight S, Crosbie P, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. (2017) 7:170070. doi: 10.1098/rsob.170070
3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (concord-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
4. Rodríguez-Abreu D, Powell S, Hochmair M, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from keynote-189. Ann Oncol. (2021) 32:881–95. doi: 10.1016/j.annonc.2021.04.008
5. Soria J, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee K, et al. Osimertinib in untreated egfr-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
6. Wang J, Wang B, Chu H, Yao Y. Intrinsic resistance to egfr tyrosine kinase inhibitors in advanced non-small-cell lung cancer with activating egfr mutations. Onco Targets Ther. (2016) 9:3711–26. doi: 10.2147/OTT.S106399
7. Mok T, Wu Y, Kudaba I, Kowalski D, Cho B, Turna H, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (keynote-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
8. Fielding R, Vellas B, Evans W, Bhasin S, Morley J, Newman A, et al. Sarcopenia: an undiagnosed condition in older adults. current consensus definition: prevalence, etiology, and consequences. international working group on sarcopenia. J Am Med Dir Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
9. Cruz-Jentoft A, Baeyens J, Bauer J, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
10. Chen X, Hou L, Shen Y, Wu X, Dong B, Hao Q. The role of baseline sarcopenia index in predicting chemotherapy-induced undesirable effects and mortality in older people with stage III or IV non-small cell lung cancer. J Nutr Health Aging. (2021) 25:878–82. doi: 10.1007/s12603-021-1633-3
11. Takahashi Y, Suzuki S, Hamada K, Nakada T, Oya Y, Sakakura N, et al. Sarcopenia is poor risk for unfavorable short- and long-term outcomes in stage i non-small cell lung cancer. Ann Transl Med. (2021) 9:325. doi: 10.21037/atm-20-4380
12. Li S, Wang T, Tong G, Li X, You D, Cong M. Prognostic impact of sarcopenia on clinical outcomes in malignancies treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol. (2021) 11:726257. doi: 10.3389/fonc.2021.726257
13. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin M, McCargar L, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. (2013) 31:1539–47. doi: 10.1200/JCO.2012.45.2722
14. Cortellini A, Bozzetti F, Palumbo P, Brocco D, Di Marino P, Tinari N, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving pd-1/pd-l1 checkpoint inhibitors: a multicenter real-life study [Sci. rep.]. Sci Rep. (2020) 10:1456. doi: 10.1038/s41598-020-58498-2
15. Degens J, Dingemans A, Willemsen A, Gietema H, Hurkmans D, Aerts J, et al. The prognostic value of weight and body composition changes in patients with non-small-cell lung cancer treated with nivolumab. J Cachexia Sarcopenia Muscle. (2021) 12:657–64. doi: 10.1002/jcsm.12698
16. Li S, Liu Z, Ren Y, Liu J, Lv S, He P, et al. Sarcopenia was a poor prognostic predictor for patients with advanced lung cancer treated with immune checkpoint inhibitors. Front Nutr. (2022) 9:900823. doi: 10.3389/fnut.2022.900823
17. Rossi S, Di Noia V, Tonetti L, Strippoli A, Basso M, Schinzari G, et al. Does sarcopenia affect outcome in patients with non-small-cell lung cancer harboring egfr mutations? Future Oncol. (2018) 14:919–26. doi: 10.2217/fon-2017-0499
18. Topcu A, Ozturk A, Yurtsever I, Besiroglu M, Yasin A, Turk H, et al. The effect of sarcopenia on erlotinib therapy in patients with metastatic lung adenocarcinoma. Bosn J Basic Med Sci. (2022) 22:982–91. doi: 10.17305/bjbms.2022.7147
19. Kong M, Geng N, Zhou Y, Lin N, Song W, Xu M, et al. Defining reference values for low skeletal muscle index at the L3 vertebra level based on computed tomography in healthy adults: a multicentre study. Clin Nutr. (2022) 41:396–404. doi: 10.1016/j.clnu.2021.12.003
20. Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, et al. Association of sarcopenia with and efficacy of anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. J Clin Med. (2019) 8:450. doi: 10.3390/jcm8040450
21. Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with pd-1 inhibitors: a preliminary retrospective study. Sci Rep. (2019) 9:2447. doi: 10.1038/s41598-019-39120-6
22. Takada K, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, Wakasu S, et al. Clinical impact of skeletal muscle area in patients with non-small cell lung cancer treated with anti-pd-1 inhibitors. J Cancer Res Clin Oncol. (2020) 146:1217–25. doi: 10.1007/s00432-020-03146-5
23. Tenuta M, Gelibter A, Pandozzi C, Sirgiovanni G, Campolo F, Venneri M, et al. Impact of sarcopenia and inflammation on patients with advanced non-small cell lung cancer (ncscl) treated with immune checkpoint inhibitors (icis): a prospective study. Cancers (Basel). (2021) 13:6355. doi: 10.3390/cancers13246355
24. Tsukagoshi M, Yokobori T, Yajima T, Maeno T, Shimizu K, Mogi A, et al. Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer. Med (Baltim). (2020) 99:e19059. doi: 10.1097/MD.0000000000019059
25. Wang Y, Chen P, Huang J, Liu M, Peng D, Li Z, et al. Assessment of sarcopenia as a predictor of poor overall survival for advanced non-small-cell lung cancer patients receiving salvage anti-pd-1 immunotherapy. Ann Transl Med. (2021) 9:1801. doi: 10.21037/atm-21-6578
26. Haik L, Gonthier A, Quivy A, Gross-Goupil M, Veillon R, Frison E, et al. The impact of sarcopenia on the efficacy and safety of immune checkpoint inhibitors in patients with solid tumours. Acta Oncol. (2021) 60:1597–603. doi: 10.1080/0284186X.2021.1978540
27. Minami S, Ihara S, Tanaka T, Komuta K. Sarcopenia and visceral adiposity did not affect efficacy of immune-checkpoint inhibitor monotherapy for pretreated patients with advanced non-small cell lung cancer. World J Oncol. (2020) 11:9–22. doi: 10.14740/wjon1225
28. Nishioka N, Naito T, Notsu A, Mori K, Kodama H, Miyawaki E, et al. Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non-small cell lung cancer. Cancer Med. (2021) 10:247–56. doi: 10.1002/cam4.3631
29. Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures J, Pujol J, et al. Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer. (2020) 143:19–26. doi: 10.1016/j.lungcan.2020.03.003
30. Nie X, Zhang P, Gao J, Cheng G, Liu W, Li L. Sarcopenia as a predictor of initial administration dose of afatinib in patients with advanced non-small cell lung cancer. Thorac Cancer. (2021) 12:1824–30. doi: 10.1111/1759-7714.13934
31. Arrieta O, De la Torre-Vallejo M, López-Macías D, Orta D, Turcott J, Macedo-Pérez EO, et al. Nutritional status, body surface, and low lean body mass/body mass index are related to dose reduction and severe gastrointestinal toxicity induced by afatinib in patients with non-small cell lung cancer. Oncologist. (2015) 20:967–74. doi: 10.1634/theoncologist.2015-0058
32. Minami S, Ihara S, Nishimatsu K, Komuta K. Low body mass index is an independent prognostic factor in patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitor. World J Oncol. (2019) 10:187–98. doi: 10.14740/wjon1244
33. Oruc Z, Akbay A, Ali Kaplan M, Oruç Ý, Urakçı Z, Işıkdoğan A. A low body fat mass ratio predicts poor prognosis in patients with advanced non-small cell Lung Cancer. Nutr Cancer. (2022) 74:3284–91. doi: 10.1080/01635581.2022.2074064
34. Conlon K, Lugli E, Welles H, Rosenberg S, Fojo A, Morris J, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. (2015) 33:74–82. doi: 10.1200/JCO.2014.57.3329
35. Crane J, MacNeil L, Lally J, Ford R, Bujak A, Brar I, et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. (2015) 14:625–34. doi: 10.1111/acel.12341
36. Saini J, McPhee J, Al-Dabbagh S, Stewart C, Al-Shanti N. Regenerative function of immune system: modulation of muscle stem cells. Ageing Res Rev. (2016) 27:67–76. doi: 10.1016/j.arr.2016.03.006
37. Turner D, Kondic A, Anderson K, Robinson A, Garon E, Riess J, et al. Pembrolizumab exposure-response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res. (2018) 24:5841–9. doi: 10.1158/1078-0432.CCR-18-0415
38. Bi Y, Deng J, Murry D, An GA. whole-body physiologically based pharmacokinetic model of gefitinib in mice and scale-up to humans. AAPS J. (2016) 18:228–38. doi: 10.1208/s12248-015-9836-3
Keywords: sarcopenia, lung cancer, immune-checkpoint inhibitors, EGFR-TKIs, prognosis
Citation: Lyu J, Yang N, Xiao L, Nie X, Xiong J, Liu Y, Zhang M, Zhang H, Tang C, Pan S, Liang L, Bai H, Li C, Kuang H and Li T (2023) Prognostic value of sarcopenia in patients with lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors or immune checkpoint inhibitors. Front. Nutr. 10:1113875. doi: 10.3389/fnut.2023.1113875
Received: 01 December 2022; Accepted: 21 February 2023;
Published: 08 March 2023.
Edited by:
Wen Hu, Sichuan University, ChinaReviewed by:
Denisse Castro-Eguiluz, National Council of Science and Technology (CONACYT), MexicoCopyright © 2023 Lyu, Yang, Xiao, Nie, Xiong, Liu, Zhang, Zhang, Tang, Pan, Liang, Bai, Li, Kuang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Li, bGl0YW94bWZAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.