- 1Department of Geriatrics, Medical Center on Aging of Shanghai Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Nutrition, College of Health Science and Technology, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Geriatrics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5Department of Clinical Nutrition, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Some studies have shown that a pro-inflammatory diet may be associated with cognitive function, but their conclusions have varied considerably. We here present a meta-analysis of the current published literature on DII score and its association with cognitive health.
Methods: In this meta-analysis, the PubMed, Embase, Web of Science, and Cochrane databases were searched in September 2022. The reported indexes, specifically OR, RR, and β, were extracted and analyzed using R version 3.1.0.
Results: A total of 636 studies in databases were identified, and 12 were included in the meta-analysis. Higher DII was associated with an increased risk of AD and MCI (OR = 1.34; 95% CI = 1.21–1.49). Meanwhile, it may also cause global function impairment (categorical: OR = 1.63; 95% CI = 1.36–1.96) and verbal fluency impairment (continuous: OR = 0.18; 95% IC = 0.08–0.42). But there was no significant association between DII and executive function (categorical: OR = 1.12; 95% IC = 0.84–1.49; continuous: OR = 0.48; 95% IC = 0.19–1.21) or episodic memory (continuous: OR = 0.56; 95% IC = 0.30–1.03).
Conclusion: A pro-inflammatory diet is related to AD, MCI, and the functions of some cognitive domains (specifically global function and verbal fluency). However, the current evidence on the role of diet-induced inflammation in different cognitive domains should be supported by further studies in the future.
Introduction
The aging of today’s population has become a critical challenge worldwide. The proportion of the elderly population has been gradually increasing. By 2030, the proportion of people aged 60 and over may increase from 1 billion to 1.4 billion. By 2050, the world’s population aged 60 and older may double (2.1 billion) (1). Many issues come with this demographic shift, including increased rates of cognitive decline and dementia. According to WHO, there are 55 million people with dementia at present around the world, and nearly 10 million new cases annually (2). The most common form of dementia is Alzheimer’s disease, which may cause 60–80% of cases (3). Alzheimer’s disease and other types of dementia are reported to be the seventh leading cause of death among the elderly and some of the most common causes of disability worldwide (2). Mild cognitive impairment (MCI) is considered a precursor stage of dementia. One study found that 32% of patients with mild cognitive impairment progressed to dementia within 5 years (4), so that effective measures to prevent such cognitive impairment are essential.
Inflammation has been confirmed to be one possible mechanism underlying cognitive impairment (AD, dementia, and MCI) in recent studies (5, 6). Whether it is dietary patterns, dietary composition, or compounds in the diet, diet seems to be related to the level of inflammation in the body (7, 8). From this perspective, diet might affect cognition by modulating inflammation in the body. Some studies have investigated the association between diet and cognitive health, and their findings suggest that different diets increase (8) or decrease (9) the risk of cognitive impairment. Establishing a means of quantitatively measuring the inflammatory potential of diet would allow observation of a direct and dynamic link between the ability of diet to modulate levels of inflammation in vivo and cognitive health. This would make it possible to prevent or delay cognitive impairment with specific modifications to dietary factors within a flexible range.
In order to determine the inflammatory potential of a given diet, Shivappa et al. proposed a dietary inflammatory index (DII), which includes 36 anti-inflammatory parameters for fiber, alcohol, monounsaturated fatty acids, polyunsaturated fatty acids, omega 3, omega 6, niacin, thiamin, riboflavin, vitamin B6, B12, zinc, magnesium, selenium, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β carotene, anthocyanidins, flavan-3-ols, flavonols, flavanones, flavones, isoflavones, garlic, ginger, onions, thyme, oregano, saffron, turmeric, rosemary, eugenol, caffeine and tea, and 9 pro-inflammatory parameters for energy, carbohydrates, proteins, total fat, trans fat, cholesterol, vitamin B12, saturated fatty acids, and iron, for a total of 45 parameters. Individuals’ diets were classified along the DII according to inflammatory potential on a continuum from maximum pro-inflammatory to maximum anti-inflammatory. The higher the DII score, the greater the inflammatory potential of the given diet (10).
Existing epidemiological studies have explored the association between DII and cognitive impairment (MCI, dementia, and AD) and cognitive function (global cognition, episodic memory, executive function, verbal fluency, tested using mini-mental state examination (MMSE), or other common cognitive function tests such as the digit span-backward test or MoCA-score). All studies on the relationship between DII and the incidence of MCI or AD showed a positive relationship (11–15). Some studies showed a negative relationship between DII and global cognition and verbal fluency (12, 14, 16–20). Other studies searched for a relationship between DII and episodic memory and executive function and found none (19–21). Some systematic reviews have focused on the effects of DII on mental health (22, 23) and the influences of dietary factors on cognitive health (9, 24), but, as far as we know, no systematic review has investigated the effects of DII on cognitive disorders or cognitive function. Given this, we performed a meta-analysis to comprehensively examine the association between DII and cognitive health including cognitive disorders (impairment) and cognitive function.
Methods
This meta-analysis was performed in accordance with the PRISMA statement to ensure comprehensive and transparent reporting of methods and results.
Search strategy
The literature databases PubMed, EMBASE, Web of Science, and Cochrane were searched in September 2022 and the search terms used were “mild cognitive impairment” OR dementia OR “Alzheimer disease” OR “cognitive function” OR “cognitive decline” AND “dietary inflammatory index” OR “anti-inflammatory diet” OR “inflammatory diet.” Two authors independently screened each title and abstract and then read the full text to assess the article for compliance with the inclusion and exclusion criteria which will be mentioned later. Disagreements were resolved through consensus.
Selection criteria
Studies included in this meta-analysis met all the following inclusion criteria: (1) studies that focused on humans and were published in English; (2) investigated the association between the DII/E-DII and cognitive function (global cognition, episodic memory, executive function, verbal fluency) or the risk of cognitive disorders (MCI, dementia, AD); (3) the study design was case–control, cohort or cross-sectional study. (4) If multiple studies were published using the same cohort, only the most recent study was selected. If more than one article based on the same cohort was from a different population or reported different results, both were included. Studies were excluded if they were systematic reviews or narrative reviews, reports, letters, in vitro studies, animal studies or duplicate studies.
Data extraction
Two authors (TD and MA) screened the papers and abstracted the data independently, and conflicts were resolved by a third reviewer (DB) who was blinded to the authors and institutions of the studies undergoing review. The following information was extracted from each study: name of the first author, year of publication, country, study design, sample size, study period, dietary assessment method, number of food parameters, cognitive assessment methods, cognitive domains, results, and covariates used for adjustment.
Statistical analysis
In this meta-analysis, all the reported effects, odds ratios (ORs), hazard ratios (HRs), risk ratios (RRs) and β with 95% confidence intervals and effect size were extracted. The heterogeneity test was assessed using Cochrane’s Q test and the I2statistic. Significance was defined as p < 0.05. The I2 statistic represents the amount of total variation that could be attributed to heterogeneity. I2 values ≤25%, ≤50%, ≤75%, and >75% indicated no, little, moderate, and high heterogeneity, respectively. For lower heterogeneity (I2 ≤ 50%), the fixed effect models were used, and random effect models were used for higher heterogeneity (I2 > 50%). A sensitivity analysis had been conducted by excluding one study at a time to identify potential sources of heterogeneity when the result had a high heterogeneity (I2 > 50%). The meta-analysis was performed using the “meta” package in R. Statistical analyses were performed using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Literature selection
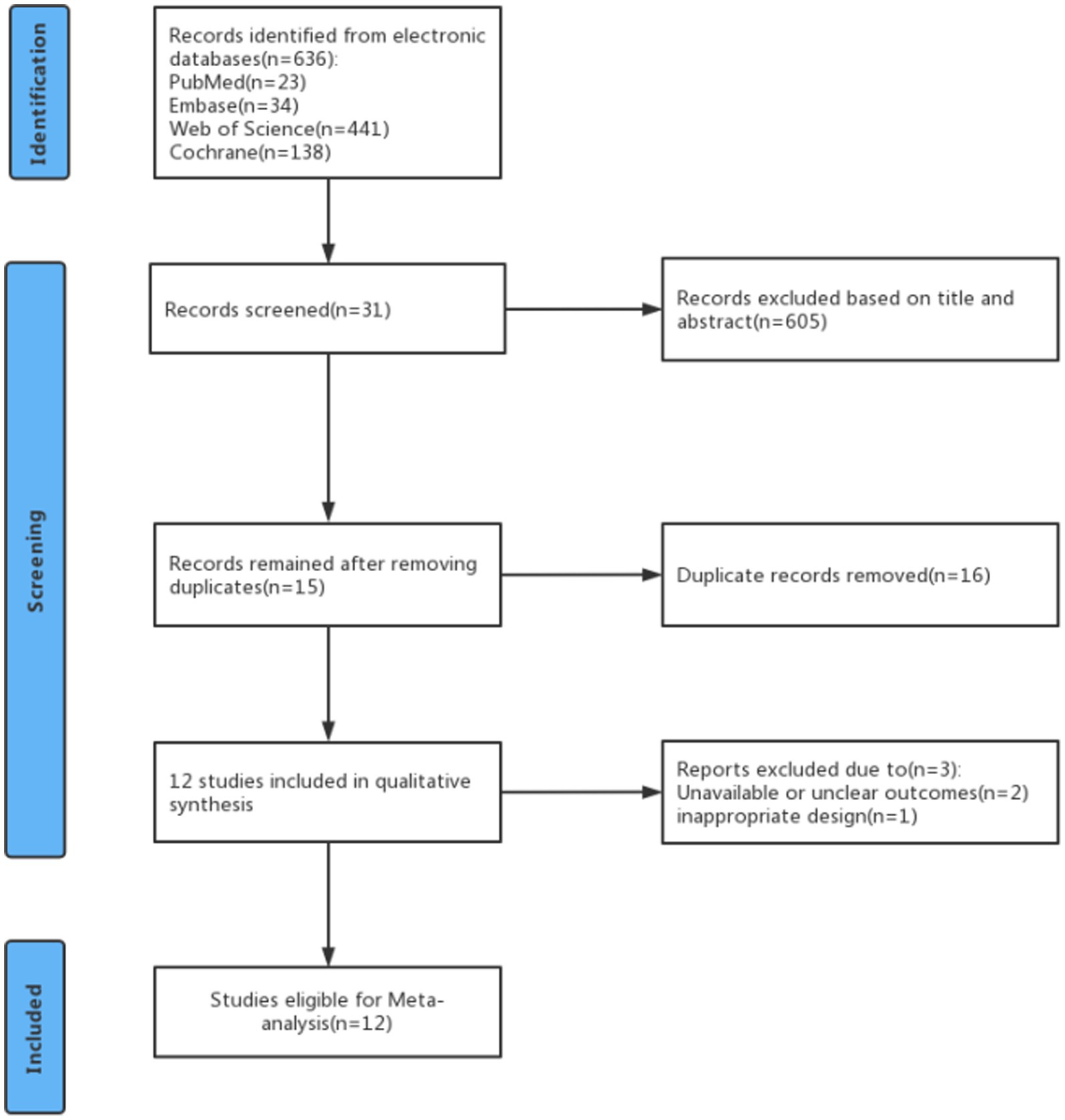
A total of 636 studies were identified in the PubMed, Embase, Web of Science, and Cochrane databases. Of these, 605 studies were excluded after reviewing their titles and abstracts to assess relevance and eligibility. Then 16 articles were excluded because they were duplicates. After careful perusal of the full texts of these articles, 3 were excluded because of unavailable or unclear outcomes and inappropriate design. Finally, 12 studies were included in the meta-analysis (Figure 1).
Study characteristics
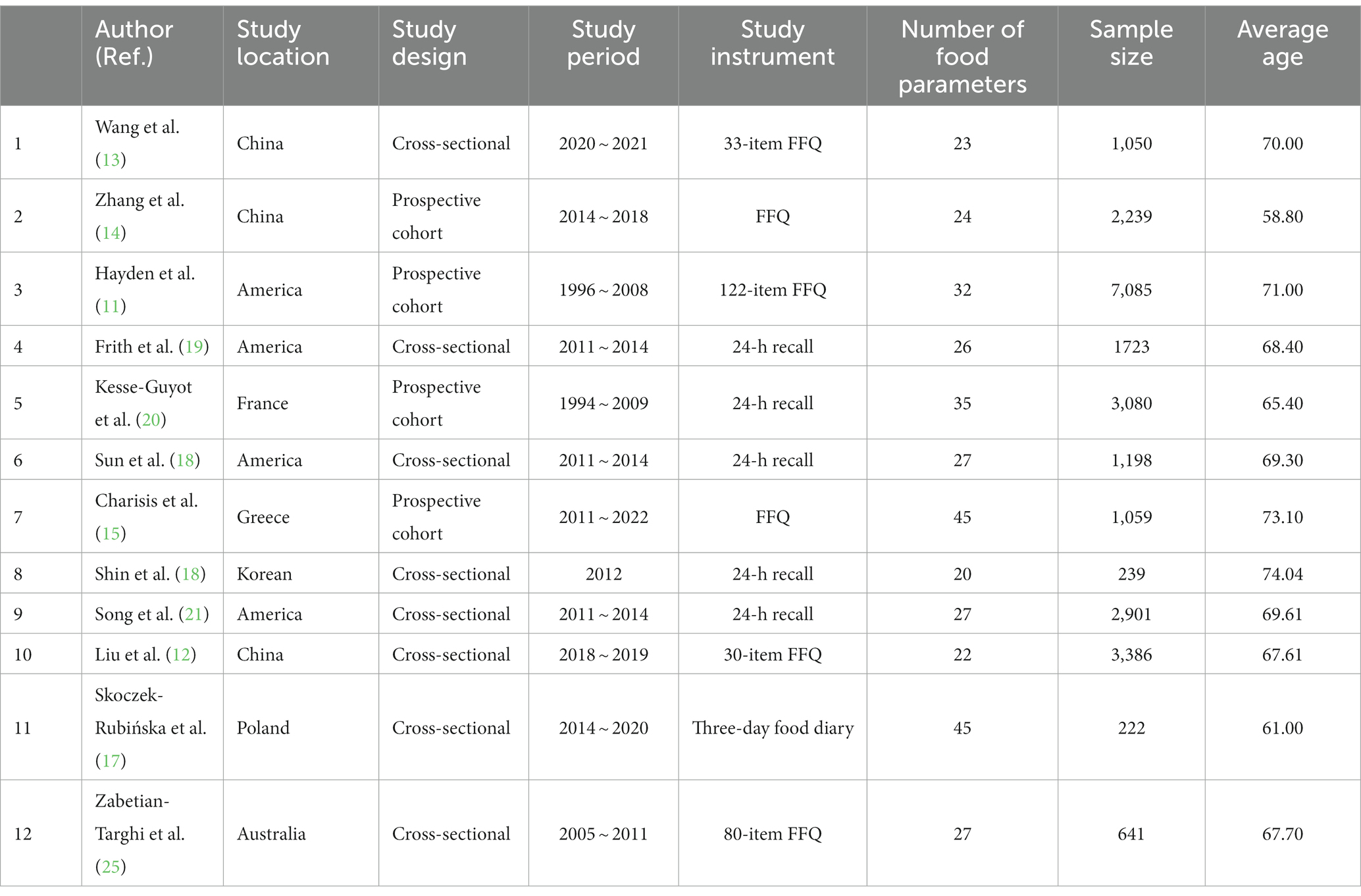
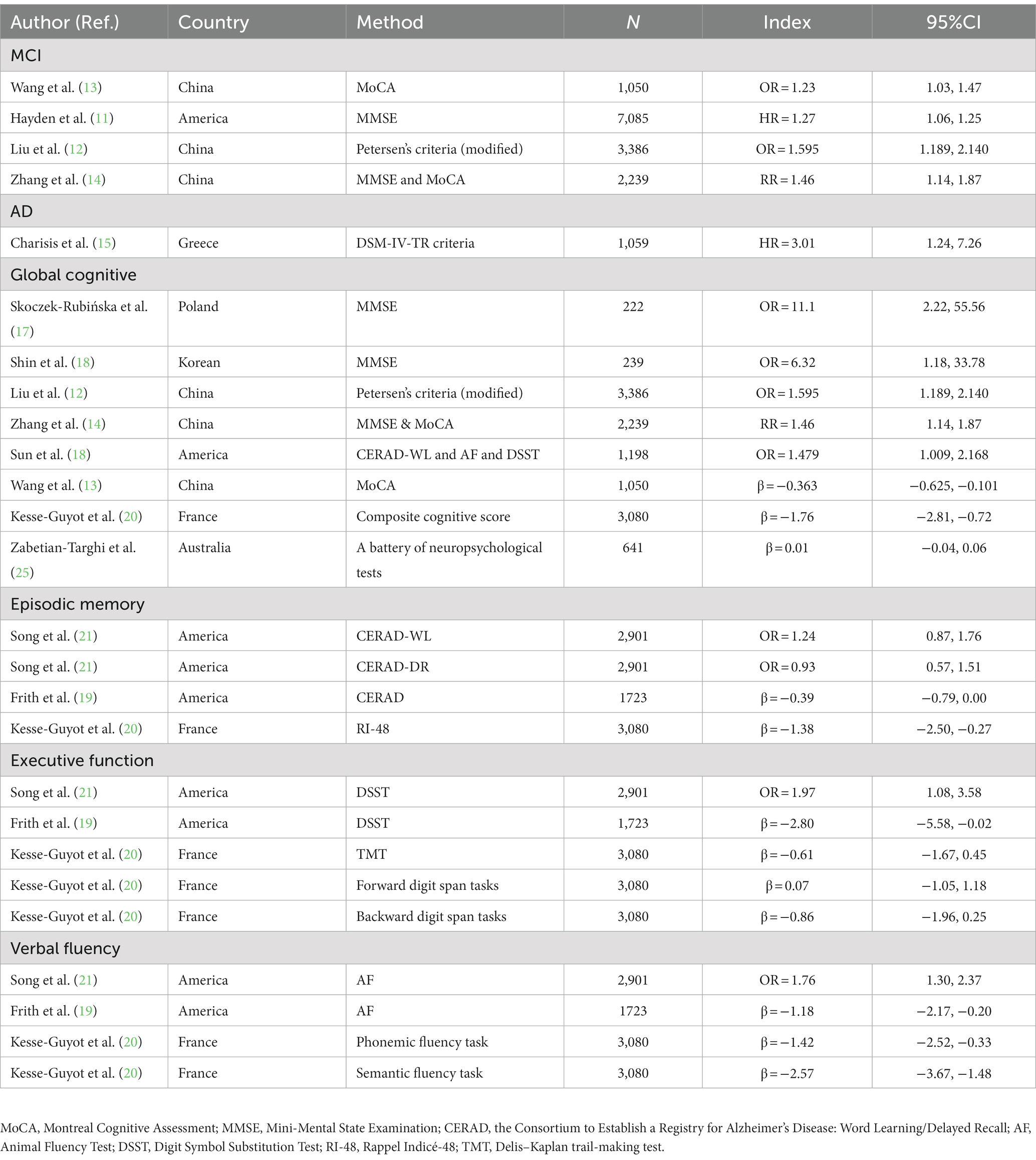
The characteristics of the included studies are summarized in Table 1. Of these studies (11–21, 25) were published between 2017 and 2022, and their sample sizes varied from 222 to 7,085 participants with a total number of 24,823 individuals from seven countries on four continents. Four studies were carried out in the United States, three studies (12–14) in China, and one each in France (20), Greece (15), Poland (17), Australia (25), and South Korea (16). The average age of participants varied from 61.00 to 74.04. In terms of dietary assessment, six studies (11–15, 25) used Food Frequency Questionnaire (FFQ), five studies (16, 18–21) used 24-h recall, and only one study (17) used three-day food diaries. Regarding the number of food parameters used to calculate DII score, two studies (15, 17) used all 45 parameters; three studies (18, 21, 25) used the E-DII score, which contains 27 parameters; and other studies varied between 22 and 35 parameters. More detailed summaries of each article are included in Table 2, which provides information regarding assessment instruments for cognitive function and results for each study. Cognitive function was assessed using a large number of tests to quantify the cognitive domains of global cognition, episodic memory, executive function, and verbal fluency. There were differences among individual studies. For example, studies used different assessment instruments, including MoCA, MMSE, Petersen’s Criteria, CERAD, DSM-IV-TR criteria, DSST, Animal Fluency Test, and RI-48.
Meta-analysis
The forest plots of the meta-analysis were shown in Figure 2 (categorical variables) and Figure 3 (continuous variables). The weight in each plot was calculated by the inverse-variance method (shown below), which meant that if a study had more participants, more events and a narrower confidence interval, it would have a higher weight.
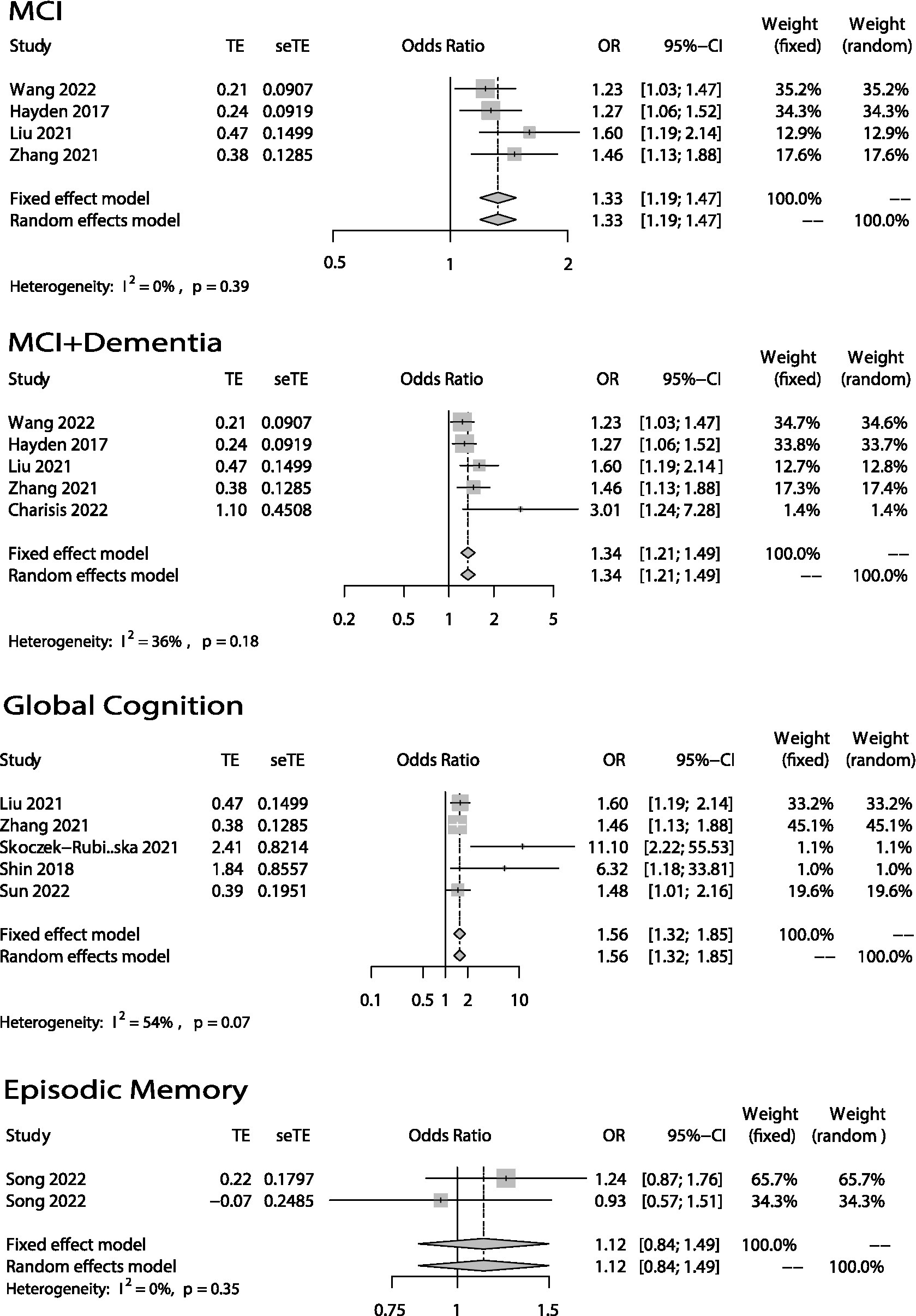
Figure 2. Odds ratios (ORs) of the highest versus lowest category of DII and cognitive health (MCI, MCI + Dementia, Global Cognition and Episodic Memory). Categorical variables.
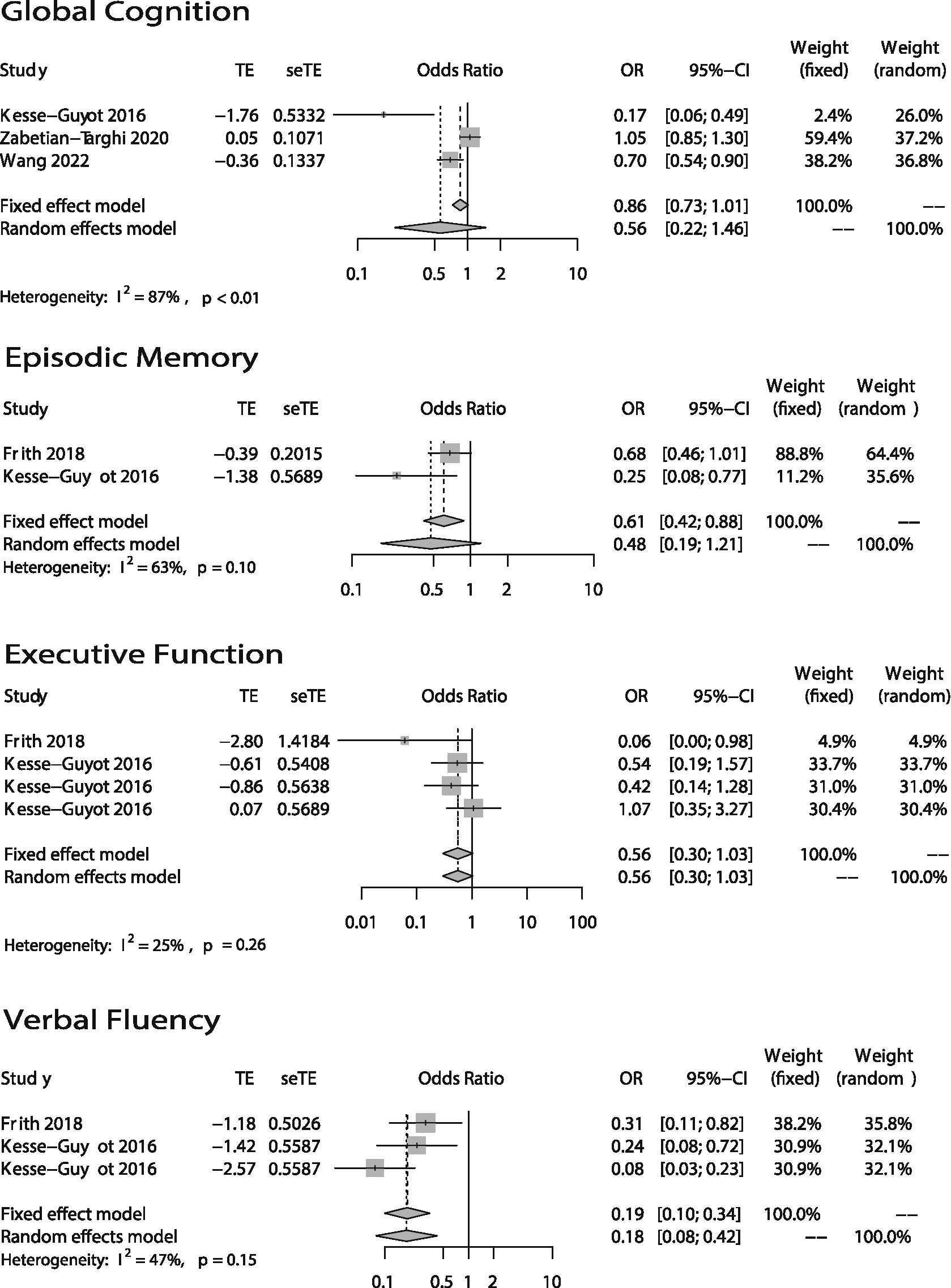
Figure 3. Odds ratios (ORs) of the highest versus lowest category of DII and cognitive health (Global Cognition, Episodic Memory, Executive Function, and Verbal Fluency). Continuous variables.
The association between DII and global cognition was shown in Figures 2, 3. There were eight (12–14, 16–18, 20, 25) studies. And the outcome variables of five of them (12, 14, 16–18) were categorical variables while the other three’s (13, 20, 25) were continuous variables. As to the five studies that used categorical variables, there is a negative association (OR = 1.56; 95% CI = 1.32–1.85) between DII and global cognition with no evidence of statistically significant heterogeneity between the studies (I2 = 54%, p = 0.07). The sensitivity analysis (Supplementary Figure S1) revealed that the result from Skoczek-Rubińska was the largest contributor to the heterogeneity and its exclusion reduced heterogeneity to 0% (p = 0.39) with substantially unchanged effect size (OR = 1.53; 95% CI = 1.29–1.81). However, when it comes to the other three studies which used continuous variables, the relationship between DII and global cognition is not significant (OR = 0.56; 95% CI = 0.22–1.46) and heterogeneity is high (I2 = 87%, p < 0.01). The sensitivity analysis (Supplementary Table S1) conducted by excluding one study at a time showed that the heterogeneities between every two articles were high.
Only three studies (19–21) evaluated the relationship between DII and episodic memory (Figures 2, 3). The study (21) that used categorical variables evaluated episodic memory via two parts from the same instrument. The results showed that there is no relationship between DII and episodic memory (categorical: OR = 1.12; 95% IC = 0.84–1.49; continuous: OR = 0.48; 95% CI = 0.19–1.21). Both heterogeneities were not high (categorical: I2 = 0%, p = 0.35; continuous: I2 = 63%, p = 0.10).
In terms of the association between DII and executive function, two articles (19, 20) used four methods to evaluate it (Figure 3). Both articles took continuous variables as their outcome variables. Results showed DII has no association with executive function (OR = 0.56; 95% CI = 0.30–1.03) and there was no evidence of statistically significant heterogeneity between the studies (I2 = 25%, p = 0.26).
The association between DII and verbal fluency is shown in Figure 3. The two articles are the articles mentioned above which studied the association between DII and executive function. The results showed that DII score is associated with verbal fluency (OR = 0.18; 95% CI = 0.08–0.42) and the heterogeneity is medium (I2 = 47%, p = 0.15).
Discussion
This meta-analysis summarizes the evidence underlying associations between DII and cognitive function. High DII was associated with an increased risk of overall cognitive decline, including the morbidity rate of AD and MCI, decline of both global cognition and verbal fluency. However, there was hardly any association detected between DII and either executive function or episodic memory.
The dietary inflammation index (DII) (10) is a quantitative measure of pro-inflammatory diets and is associated with systemic inflammatory markers including tumor necrosis factor (TNF), interleukins (IL), and Interferon gamma (IFN-γ) (10, 26). It was created based on nearly 6,500 studies and has 45 dietary components, including common food items, micronutrients, and important bioactive polyphenols. All these components can affect an individual’s level of inflammation. It is generally acknowledged that neuroinflammation plays an important role in the pathogenesis of Alzheimer’s disease (27, 28) and there are more inflammatory markers (TNFα, IL-1β, IL-6, IL-10) in patients with AD and mild cognitive impairment (MCI) than in healthy controls (29–32). Consistently, previous findings in humans and animals have suggested that neuroinflammation is associated with cognitive decline, especially learning and memory (5, 33). Although we have yet to establish how these inflammatory markers damage our brains, there are some studies that have tried to explain this pathogenesis. One study conducted in Japan (34) suggested that TNFα competes with the cAMP signaling pathway, thereby inhibiting memory retrieval by blocking cAMP-PKA-GluA1 S845 signaling. In this way, TNFα could cause cognitive decline. Moreover, other studies focused on IL-1 found that IL-1β could act directly on hippocampal neurons to interfere with memory reconsolidation and the mice’s working memory impairment was prevented by global IL-1R1 knockout (35–37). Given these links, it is not surprising that we observed such a positive association between pro-inflammatory diets (higher DII) and AD, MCI, and poor global function in the present work.
Among all the forest plots in this article, only the global cognition plot (continuous variables) in Figure 3. Shows high heterogeneity (I2 = 87%, p < 0.01). And the source of heterogeneity could not be found by sensitivity analysis (Supplementary Table S1). This could have been due to differences in the global cognition scoring methods and dietary questionnaires used in the studies. Specifically, Kesse-Guyot (20) used such methods as RI-48, TMT, and forward and backward digit span tasks to create a composite cognitive score and then used this score to evaluate global cognition. Wang (13) only used MoCA. Zabetian-Targhi et al. (25) used the Rey-Osterrieth Complex Figure, a delayed reproduction after 20 min and other 7 tests. Global cognitive function was calculated as the standardized mean of all tests. In theory, comprehensive neuropsychological test batteries used by Kesse-Guyot and Zabetian-Targhi are more sensitive than cognitive decline screening tests such as the MoCA used in Wang’s research to capture subtle changes in cognition in individuals with no severe deterioration of cognitive functions and with no clinical dementia. In addition, the dietary assessment method is another reason for the differences between studies since the DII scores are calculated on them. At present, the most used method is the food frequency questionnaire, and the more items, the more accurate the results are. The 24-h recall used by Kesse-Guyot is relatively less accurate.
The global cognition plot (categorical variables) in Figure 2. Also has a relatively high heterogeneity (I2 = 54%, p = 0.07). After excluding the paper written by Skoczek-Rubińska, the heterogeneity was reduced to 0% (p = 0.39) with a substantially unchanged effect size (OR = 1.53; 95% CI = 1.29–1.81). Although the small sample size and the single assessment method may be one of the reasons for the larger contribution to heterogeneity, we believe that the greatest feature of this article is the study of postmenopausal women as a specific population. Depletion of estrogen due to menopausal transition is also associated with a decline in cognitive health in women. This happens since estrogen benefits hippocampal and prefrontal cortical function, potentially enhancing verbal memory and executive function (38, 39). Therefore, we believed that the difference in the study population is the main reason for the high heterogeneity. But all in all, either include or exclude this article, the conclusion that higher DII, poorer global cognition does not change.
Our study also showed that high DII scores are negatively associated with verbal fluency, but there was no significant association between DII and executive function or episodic memory. Only three articles (19–21) studied the association between DII and these three cognitive domains. Song used categorical variables, while Frith and Kesse-Guyot used continuous variables. As to the meta-analysis of episodic memory (categorical variables) in Figure 2, we can find that the two data are from the same article, the participants are the same, and the evaluation method is two different parts of CERAD, so there is probably a bias. And when it comes to the meta-analysis of episodic memory (continuous variables) in Figure 3, the heterogeneity was relatively high (I2 = 63%, p = 0.10). However, because only two articles were included in the meta-analysis, we were unable to perform a sensitivity analysis to identify the source of heterogeneity. We can only speculate that the possible reasons for the high heterogeneity are different study populations (one was American while another was French) and different episodic memory assessment methods (one used CERAD while another used RI-48). Although few papers took these three cognitive domains into account, we did find one paper published in 2020 that did so (40). Its authors found that there was no association between CRP and verbal fluency. To some extent, this is inconsistent with our own conclusion. However, the paper’s participants were not elderly, and it only took CRP into account. There are many inflammatory cytokines other than CRP, such as TNFα, IL-6, and IL-10, that may be related to cognitive function. This could be why its conclusion differed from ours. Regarding executive function and episodic memory, one study conducted in the United States (41) suggested that TNFα had no association with executive function or episodic memory. Its findings were consistent with our conclusion. Even so, the evidence of the connection between DII and verbal fluency, executive function, and episodic memory is limited.
Several limitations of this work should be addressed. Firstly, most of the included studies were cross-sectional studies. Although we found that DII was associated with MCI and AD, further prospective cohort studies with different ethnic or age groups are still needed to confirm the results. For example, prospective cohort studies that include more young and healthy groups for long-term follow-up. Furthermore, a recently developed plasma biomarker for imaging, cerebrospinal fluid, or plasma biomarkers of amyloid and tau pathology can be used to assist in the early screening and diagnosis of MCI and AD, as well as the use of cognitive-related scales. Secondly, although there is convincing evidence from observational studies, including the evidence presented in the present meta-analysis, that a dietary intervention with an anti-inflammatory diet might be beneficial to counteract the risk of developing dementia or cognitive decline, there are no clinical trials to definitively support this result. Therefore, it is important for future research to address this issue. A pro-inflammatory diet might be a good candidate for an easy-to-implement, relatively cheap, and safe intervention that can be studied in association with dementia and cognitive decline using an experimental design. Thirdly, when conducting subgroup analysis, such as episodic memory (continuous variables, heterogeneities were high level), because fewer than three studies were included, we were unable to conduct sensitivity analysis to further identify the source of heterogeneity. Meanwhile, although current evidence suggests that inflammation has influences on cognitive function to some extent, the mechanism is still not clear. We look forward to further studies to address this issue in the future.
In conclusion, data from this study support an association between DII and increased risk of AD and MCI. A negative association was observed between DII and global cognition and verbal fluency. However, there was no significant association observed between DII and executive function or episodic memory. This study indicates that an anti-inflammatory diet can help prevent cognitive decline. However, current evidence on the role of diet-induced inflammation in different cognitive domains should be supplemented with further work in the future.
Author contributions
DB: conceptualization, formal analysis, resources, writing – review and editing, and funding acquisition. DB, LW, SC, TD, and MA: methodology and investigation. TD and MA: writing – original draft preparation. JS: visualization. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Medicine and Engineering Interdisciplinary Research Fund of Shanghai Jiao Tong University, grant number (YG2022QN011), Shanghai Municipal Health Commission, grant number (2020YJZX0139), Shanghai Municipal Science and Technology (22692191800) and National Key Research and Development Program of China (2021YFE0111800).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1104255/full#supplementary-material
References
1. WHO, Aging and Health. (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (Accessed October 15, 2022).
2. WHO, Dementia. (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/dementia (Accessed October 15, 2022).
3. 2022 Alzheimer's disease facts and figures. Alzheimers Dement. (2022) 18:700–89. doi: 10.1002/alz.12638
4. Ward, A, Tardiff, S, Dye, C, and Arrighi, HM. Rate of conversion from prodromal Alzheimer's disease to Alzheimer's dementia: a systematic review of the literature. Dement Geriatr Cognit Disord Extra. (2013) 3:320–32. doi: 10.1159/000354370
5. Heppner, FL, Ransohoff, RM, and Becher, B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. (2015) 16:358–72. doi: 10.1038/nrn3880
6. King, E, O'Brien, JT, Donaghy, P, Morris, C, Barnett, N, Olsen, K, et al. Peripheral inflammation in prodromal Alzheimer's and Lewy body dementias. J Neurol Neurosurg Psychiatry. (2018) 89:339–45. doi: 10.1136/jnnp-2017-317134
7. Calder, PC, Ahluwalia, N, Brouns, F, Buetler, T, Clement, K, Cunningham, K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. (2011) 106:S5–S78. doi: 10.1017/S0007114511005460
8. Więckowska-Gacek, A, Mietelska-Porowska, A, Wydrych, M, and Wojda, U. Western diet as a trigger of Alzheimer's disease: from metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. (2021) 70:101397. doi: 10.1016/j.arr.2021.101397
9. McGrattan, AM, McEvoy, CT, McGuinness, B, McKinley, MC, and Woodside, JV. Effect of dietary interventions in mild cognitive impairment: a systematic review. Br J Nutr. (2018) 120:1388–405. doi: 10.1017/S0007114518002945
10. Shivappa, N, Steck, SE, Hurley, TG, Hussey, JR, and Hébert, JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
11. Hayden, KM, Beavers, DP, Steck, SE, Hebert, JR, Tabung, FK, Shivappa, N, et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women: the Women's Health Initiative memory study. Alzheimers Dement. (2017) 13:1187–96. doi: 10.1016/j.jalz.2017.04.004
12. Liu, Q, Zhou, D, Duan, H, Zhu, Y, Du, Y, Sun, C, et al. Association of dietary inflammatory index and leukocyte telomere length with mild cognitive impairment in Chinese older adults. Nutr Neurosci. (2021) 26:50–9. doi: 10.1080/1028415X.2021.2017660
13. Wang, X, Li, T, Li, H, Li, D, Wang, X, Zhao, A, et al. Association of Dietary Inflammatory Potential with blood inflammation: the prospective markers on mild cognitive impairment. Nutrients. (2022) 14:2417. doi: 10.3390/nu14122417
14. Zhang, X, Wang, Y, Liu, W, Wang, T, Wang, L, Hao, L, et al. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am J Clin Nutr. (2021) 114:429–40. doi: 10.1093/ajcn/nqab078
15. Charisis, S, Ntanasi, E, Yannakoulia, M, Anastasiou, CA, Kosmidis, MH, Dardiotis, E, et al. Diet inflammatory index and dementia incidence: a population-based study. Neurology. (2021) 97:e2381–91. doi: 10.1212/WNL.0000000000012973
16. Shin, D, Kwon, SC, Kim, MH, Lee, KW, Choi, SY, Shivappa, N, et al. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition. (2018) 55-56:56–62. doi: 10.1016/j.nut.2018.02.026
17. Skoczek-Rubińska, A, Muzsik-Kazimierska, A, Chmurzynska, A, Jamka, M, Walkowiak, J, and Bajerska, J. Inflammatory potential of diet is associated with biomarkers levels of inflammation and cognitive function among postmenopausal women. Nutrients. (2021) 13:2323. doi: 10.3390/nu13072323
18. Sun, M, Wang, L, Guo, Y, Yan, S, Li, J, Wang, X, et al. The association among inflammatory diet, glycohemoglobin, and cognitive function impairment in the elderly: based on the NHANES 2011-2014. J Alzheimers Dis. (2022) 87:1713–23. doi: 10.3233/JAD-215688
19. Frith, E, Shivappa, N, Mann, JR, Hébert, JR, Wirth, MD, and Loprinzi, PD. Dietary inflammatory index and memory function: population-based national sample of elderly Americans. Br J Nutr. (2018) 119:552–8. doi: 10.1017/S0007114517003804
20. Kesse-Guyot, E, Assmann, KE, Andreeva, VA, Touvier, M, Neufcourt, L, Shivappa, N, et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr. (2017) 56:1647–55. doi: 10.1007/s00394-016-1211-3
21. Song, W, Feng, Y, Gong, Z, and Tian, C. The association between dietary inflammatory index and cognitive performance in older adults aged 60 years and older. Front Nutr. (2022) 9:748000. doi: 10.3389/fnut.2022.748000
22. Lassale, C, Batty, GD, Baghdadli, A, Jacka, F, Sánchez-Villegas, A, Kivimäki, M, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. (2019) 24:965–86. doi: 10.1038/s41380-018-0237-8
23. Chen, GQ, Peng, CL, Lian, Y, Wang, BW, Chen, PY, and Wang, GP. Association between dietary inflammatory index and mental health: a systematic review and dose-response meta-analysis. Front Nutr. (2021) 8:662357. doi: 10.3389/fnut.2021.662357
24. Scarmeas, N, Anastasiou, CA, and Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. (2018) 17:1006–15. doi: 10.1016/S1474-4422(18)30338-7
25. Zabetian-Targhi, F, Srikanth, VK, Smith, KJ, Oddy PhD, WH, Beare, R, Moran, C, et al. Associations between the dietary inflammatory index, brain volume, small vessel disease, and global cognitive function. J Acad Nutr Diet. (2021) 121:915–924.e3. doi: 10.1016/j.jand.2020.11.004
26. Shivappa, N, Hebert, JR, Marcos, A, Diaz, L-E, Gomez, S, Nova, E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. (2017) 61. doi: 10.1002/mnfr.201600707
27. Scheltens, P, De Strooper, B, Kivipelto, M, Holstege, H, Chételat, G, Teunissen, CE, et al. Alzheimer's disease. Lancet (London, England). (2021) 397:1577–90. doi: 10.1016/S0140-6736(20)32205-4
28. Irwin, MR, and Vitiello, MV. Implications of sleep disturbance and inflammation for Alzheimer's disease dementia. Lancet Neurol. (2019) 18:296–306. doi: 10.1016/S1474-4422(18)30450-2
29. Grubman, A, Choo, XY, Chew, G, Ouyang, JF, Sun, G, Croft, NP, et al. Transcriptional signature in microglia associated with Aβ plaque phagocytosis. Nat Commun. (2021) 12:3015. doi: 10.1038/s41467-021-23111-1
30. Salter, MW, and Stevens, B. Microglia emerge as central players in brain disease. Nat Med. (2017) 23:1018–27. doi: 10.1038/nm.4397
31. Leng, K, Li, E, Eser, R, Piergies, A, Sit, R, Tan, M, et al. Molecular characterization of selectively vulnerable neurons in Alzheimer's disease. Nat Neurosci. (2021) 24:276–87. doi: 10.1038/s41593-020-00764-7
32. Parhizkar, S, Arzberger, T, Brendel, M, Kleinberger, G, Deussing, M, Focke, C, et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci. (2019) 22:191–204. doi: 10.1038/s41593-018-0296-9
33. Griffin, WST. Neuroinflammatory cytokine signaling and Alzheimer's disease. N Engl J Med. (2013) 368:770–1. doi: 10.1056/NEJMcibr1214546
34. Takahashi, S, Fukushima, H, Yu, Z, Tomita, H, and Kida, S. Tumor necrosis factor α negatively regulates the retrieval and reconsolidation of hippocampus-dependent memory. Brain Behav Immun. (2021) 94:79–88. doi: 10.1016/j.bbi.2021.02.033
35. DiSabato, DJ, Nemeth, DP, Liu, X, Witcher, KG, O'Neil, SM, Oliver, B, et al. Interleukin-1 receptor on hippocampal neurons drives social withdrawal and cognitive deficits after chronic social stress. Mol Psychiatry. (2021) 26:4770–82. doi: 10.1038/s41380-020-0788-3
36. Machado, I, González, P, Schiöth, HB, Lasaga, M, and Scimonelli, TN. α-Melanocyte-stimulating hormone (α-MSH) reverses impairment of memory reconsolidation induced by interleukin-1 beta (IL-1 beta) hippocampal infusions. Peptides. (2010) 31:2141–4. doi: 10.1016/j.peptides.2010.07.018
37. Machado, I, Schiöth, HB, Lasaga, M, and Scimonelli, T. IL-1β reduces GluA1 phosphorylation and its surface expression during memory reconsolidation and α-melanocyte-stimulating hormone can modulate these effects. Neuropharmacology. (2018) 128:314–23. doi: 10.1016/j.neuropharm.2017.09.041
38. Pertesi, S, Coughlan, G, Puthusseryppady, V, Morris, E, and Hornberger, M. Menopause, cognition and dementia - a review. Post Reprod Health. (2019) 25:200–6. doi: 10.1177/2053369119883485
39. Au, A, Feher, A, McPhee, L, Jessa, A, Oh, S, and Einstein, G. Estrogens, inflammation and cognition. Front Neuroendocrinol. (2016) 40:87–100. doi: 10.1016/j.yfrne.2016.01.002
40. John, A, Rusted, J, Richards, M, and Gaysina, D. Accumulation of affective symptoms and midlife cognitive function: the role of inflammation. Brain Behav Immun. (2020) 84:164–72. doi: 10.1016/j.bbi.2019.11.021
Keywords: cognitive health, mild cognitive impairment, dietary inflammatory index, cognitive impairment, pro-inflammatory diet
Citation: Ding T, Aimaiti M, Cui S, Shen J, Lu M, Wang L and Bian D (2023) Meta-analysis of the association between dietary inflammatory index and cognitive health. Front. Nutr. 10:1104255. doi: 10.3389/fnut.2023.1104255
Edited by:
Usune Etxeberria, Basque Culinary Center, SpainReviewed by:
Kioko Rubi Guzman-Ramos, Autonomous Metropolitan University, Lerma, MexicoSokratis Charisis, National and Kapodistrian University of Athens, Greece
Copyright © 2023 Ding, Aimaiti, Cui, Shen, Lu, Wang and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongsheng Bian, YmRzMDQxNTlAcmpoLmNvbS5jbg==; Lei Wang, d2wxMDc3OUByamguY29tLmNu
†These authors have contributed equally to this work
 Tianze Ding
Tianze Ding Maimaitiyusupu Aimaiti
Maimaitiyusupu Aimaiti Shishuang Cui3
Shishuang Cui3 Dongsheng Bian
Dongsheng Bian