
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 25 January 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1102660
 Zhongmian Zhang1†
Zhongmian Zhang1† Lan Wang1†
Lan Wang1† Zili Lin1†
Zili Lin1† Weitian Yan2
Weitian Yan2 Jiaqin Chen1
Jiaqin Chen1 Xiyan Zhang1
Xiyan Zhang1 Wangyu Ye1
Wangyu Ye1 Jian Li3*
Jian Li3* Zhihong Li1*
Zhihong Li1*Background and aims: This study aims to investigate whether the Dietary Inflammatory Index (DII) is associated with non-alcoholic fatty liver disease (NAFLD) and advanced hepatic fibrosis (AHF) among non-institutionalized adults in the United States.
Methods: Utilizing data from the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2016, a total of 10,052 adults aged ≥18 years were included in the analysis. We used multivariable analysis, controlling for demographic variables, to evaluate the association between DII and NAFLD and AHF, a restricted cubic spline (RCS) was used to model the non-linear relationship between DII and NAFLD.
Results: For 10,052 participants, DII ranges from -4.63 to 5.47. Compared with quartile 1, higher DII group were associated with higher levels of female, separated/divorced, lower education level, heavy alcohol use, current smoke status, BMI, poverty income ratio, and waist circumference. DII also showed a significantly positive correlation with ALT, AST. In the fully adjusted multivariable model, DII was positively associated with the presence of NAFLD (OR 1.09, 1.06–1.13 CI, p trend <0.0001), and AHF (OR 1.15, 1.07–1.23 CI, p trend <0.001). The association remained statistically significant after stratified by gender in terms of NAFLD, but in case of AHF only in males (Q4 vs. Q1: OR 2.68, 1.63–4.41 CI, p trend <0.0001) was statistically significant. In the RCS models, the relation of DII and NAFLD started increase rapidly until around 1.80 and then started relatively flat afterward.
Conclusion: Higher pro-inflammatory level was associated with higher risk of NAFLD in males and females, and with higher risk of AHF in males but not in females. Therefore, strategies to promote an Zhang anti-inflammatory diet should be considered to prevent and ameliorate NAFLD and AHF in adults.
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease among adults in the United States, with a prevalence of 30–40% (1). The spectrum of NAFLD is ranging from variable degrees of simple steatosis to non-alcoholic steatohepatitis (NASH) (2) with varying amounts of fibrosis and cirrhosis (3, 4), And at least one third of patients with NAFLD will progress to NASH (5) which is characterized by hepatic fat accumulation coincidental with inflammation and potential of advanced hepatic fibrosis (AHF) (6). In patients with NAFLD and AHF (7), liver-related mortality is higher. It has been shown that NAFLD (8) and AHF are associated with hepatic and systemic inflammation.
Dietary modification is a significant impact on liver health (9) and is one of the main modulators of sub-clinical inflammation (10). The overall effect of diet on inflammatory potential can be quantified by Dietary Inflammatory Index (DII), a literature-derived dietary index that was developed to predict inflammation. DII can be used by any population that has dietary data, as it is standardized to global dietary intakes (11). Thus far, a few studies have revealed a correlation between DII and obesity (12), metabolic syndrome (13), cardiovascular diseases (14) and all-cause mortality (15). Moreover, it has been shown that there was an association between DII and hepatic health, Cantero et al. (16) showed putative anti-inflammatory components could specifically ameliorate NAFLD manifestations. However, there were also inconsistent conclusion, Ramírez-Vélez et al. (17) found that the transient elastography parameters, including liver stiffness measure (LSM) and controlled attenuation parameter (CAP), which are markers of hepatic fibrosis and steatosis, respectively, were not correlated with the anti-inflammatory diet profile. To data, there is no epidemiological evidence available about the association between DII and NAFLD or AHF.
Therefore, we aim to assess the cross-sectional relationships between DII and risk of NAFLD and AHF, and the difference between males and females using data from the National Health and Nutrition and Examination Surveys (NHANES). We hypothesized that increased consumption of a pro-inflammatory diet would associate higher NAFLD or AHF risk.
The NHANES is a complex, stratified, 4-stage survey design, and probability-cluster designed program conducted by the National Center for Health Statistics (NCHS) (18), which aimed to evaluate the health and nutritional status of adults and children in the US. The National Center for Health Statistics Institutional Review Board and Ethics Review Board has continuously approved the NHANES study since 1999. Using deidentified data to conduct the secondary analysis was officially classified as exempt by the Albert Einstein College of Medicine Institutional Review Board. There is no need to provide specific written consent for this secondary analysis of existing data. We drafted this report in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies (19).
Data from six cycles of the NHANES that extracted during 2005–2016 were included in this analysis. All participants aged ≥18 years who completed the full 24 h dietary history were included in the cohort. Participants were excluded if they: (1) had unreliable dietary recall status; (2) missed information included fatty liver index (FLI) and NAFLD fibrosis score (NFS), could not define NAFLDa nd AHF; (3) had elevated alcohol intake (>21 standard drinks per week in males; or >14 standard drinks per week in females) (20); (4) had self-reported cancer; (5) were pregnant women; (6) had positive hepatitis B surface antigen, or hepatitis C virus RNA (21).
Dietary inflammatory index is a potential tool to evaluate the anti-inflammatory or pro-inflammatory tendency of an individual’s diet (22), which development and validation has been presented in detail elsewhere (11, 23). To eliminate the effects of different total energy intake, we used energy-standardized version of world database to adjust the intake of each food parameter by 1,000 kcal. The participants’ DII score was related to Z-score which was created to express an individual’s exposure relative to the “standard global mean.” Z-score is equal to the value of adjusted participant’s intake subtract the adjusted global daily mean intake, divided by its standard deviation, then converted Z-score to a percentile score and multiplied by two and subtracted from one, next multiplied the percentile score by the score of inflammatory effect of corresponding food parameter. Finally, all the specific DII scores of a food parameter are summed to create the overall DII score for an individual (11). Higher DII scores indicate more pro-inflammatory diets, and lower DII scores indicate more anti-inflammatory diets. We analyzed DII score as a continuous variable, dividing the total sample into quartiles to analysis.
Fatty liver index and NFS have been used for non-invasive diagnostic indexes for liver disease detection. FLI, a validated diagnostic index, was used to define NAFLD (24). FLI score ≥60 was assumed to have NAFLD. NFS had been proved to show higher predictive performance in NAFLD cohort (20, 25, 26) and in this study, we used NFS to define AHF. NFS score >0.676 in the presence of NAFLD was assumed to have AHF. Formulas of FLI and NFS are as follows (21, 24, 25):
NFS =
Here, diabetes weas defined as glycated hemoglobin ≥6.5%, or self- reported diagnosed diabetes, or current use of antidiabetic medication (27).
Several potential confounding variables were selected as covariates based on the literature (28–31). We extracted these covariates, including age, poverty income ratio, waist circumference, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyl transferase (GGT), triglyceride (TG), total cholesterol (TC), High Density Lipoprotein cholesterol (HDL), body mass index (BMI), were measured as continuous variables; sex, race, marital status, education level, drinking status, smoking status, were assessed as categorical variables. A five-category system was used to divide race, namely Non-Hispanic Black, Non-Hispanic White, Mexican American, Other Hispanic, and Other Racial. The marital status of the participants was classified as never married, married/cohabitant, separated/divorced, and widowed. Level of education was categorized into “less than high school,” “high school,” “some college” and “college graduate or above.” The drinking status (32) was categorized as never (never drank 12 or more drinks in lifetime), former (drank 12 or more drinks in 1 year and didn’t drink last year, or didn’t drink last year but drank 12 or more drinks in lifetime), current light/moderate drinker (drank 1 or less drink per day for women or drank 2 or less drink per day for men on average over the past year), or current heavier drinker (drank more than 1 drink per day for women or more than 2 drinks per day for men on average over the past year). Smoking status (33) was classified into three categories: never (smoked less than 100 cigarettes in lifetime), former (smoked more than 100 cigarettes in lifetime and smoke not at all now), now (smoked moth than 100 cigarettes in lifetime and smoke some days or every day).
In the present study, all analyses accounted for the complex survey design of the NHANES and weighting variables. MEC subsample weight (WTMEC2YR for 2005–2016) was used, due to DII was used as major indicators in this study. Chi-squared test was used for comparing categorical variables and presented as numbers (n) and percentage (%). As for continuous variables, weighted t-test was used for comparing mean ± standard. Logistic regression was applied to determine associations between the DII, NAFLD, and AHF. A multivariable logistic regression model which included three models with increasing degrees of adjustment was used to examine the relationship between DII and NAFLD and AHF risk. Model I was the crude model. Model II was minimally adjusted for only race, sex, poverty income ratio, marital status, and education level. Model III was fully adjusted for race, sex, poverty income ratio, marital status, and education level, smoke status, BMI, waist circumference, AST, ALT, and GGT. A restricted cubic spline (RCS) was used to model the non-linear relationship between DII and NAFLD, and we used RCS with three knots at the 10th, 50th, 90th centiles to flexibly model the association of DII with NAFLD, however, DII is linearly related to AHF, and we did not execute RCS. Two-tailed p-values <0.05 were considered statistically significant. Statistical analysis software R version 4.1.2. was used for statistical analyses.
A total of 10,052 subjects were invited to participant in the NHANES. These participants represented a weighted population of 72,033,969 non-institutionalized US. Figure 1 shows flowchart of the study.
Table 1 lists the participants characteristics according to DII quartiles, with scores ranging from -4.63 (most anti-inflammatory) to +5.47 (most pro-inflammatory). More than 65% of the population was non-Hispanic White, and rate decreased with ascending DII quartiles. Compared with quartile 1, higher DII group were associated with higher levels of female, separated/divorced, lower education level, heavy alcohol use, current smoke status, BMI, poverty income ratio, and waist circumference. DII also showed a significantly positive correlation with ALT, AST. Without adjustment for confounders, DII was positively associated with the risk of NAFLD and AHF.
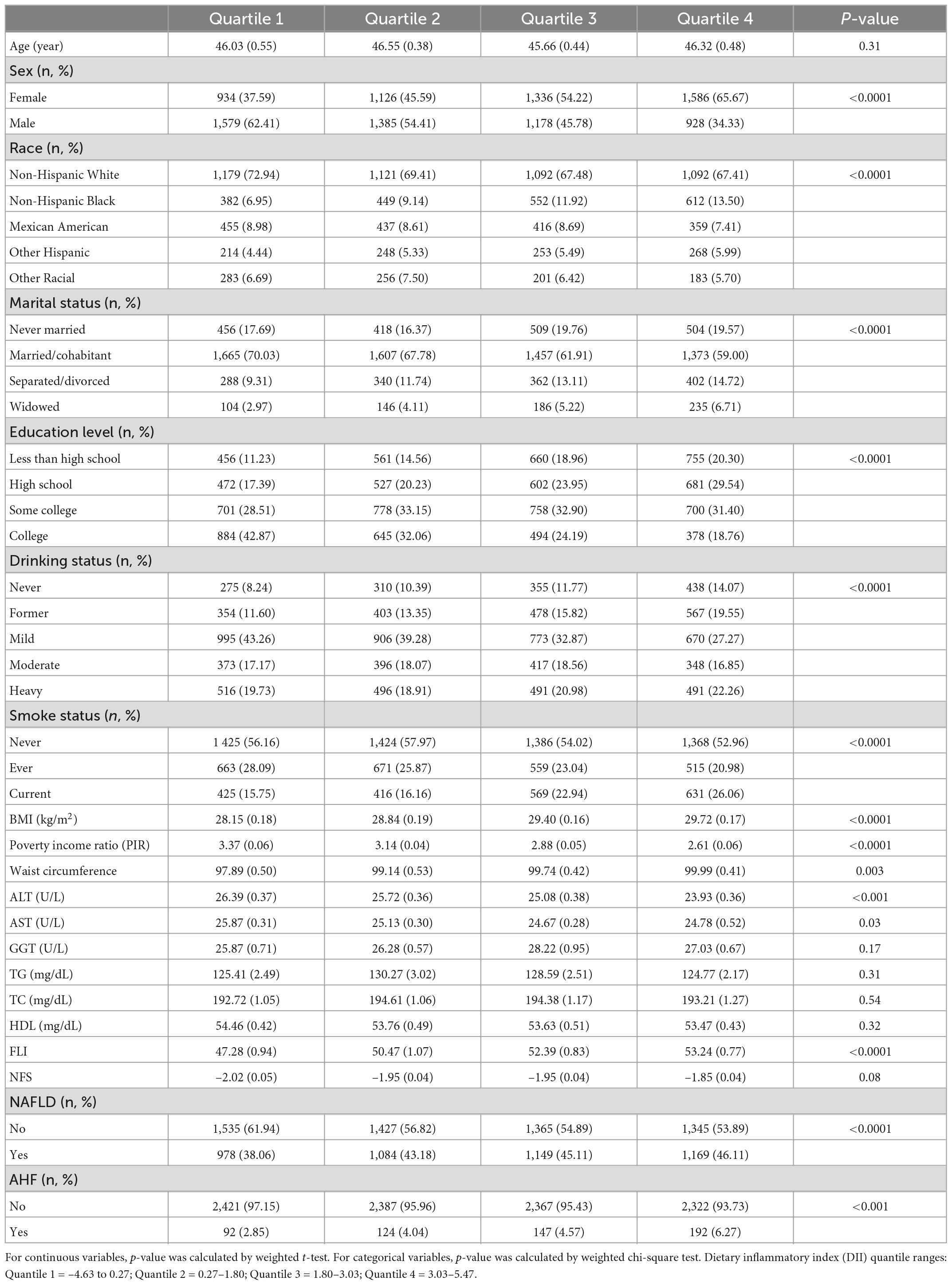
Table 1. Characteristics of participants in the 2005–2016 continuous National Health and Nutrition Examination Survey (NHANES).
Table 2 shows the association between DII and risk of NAFLD and AHF by a multivariable logistic regression model. Our results revealed that higher DII was associated with increased risk of NAFLD (model I, OR 1.08, 1.05–1.11 CI; model II, OR 1.09, 1.06–1.13 CI). After full adjustment (model III), DII was positively associated with the presence of NAFLD (OR 1.09, 1.06–1.13 CI), and this association meet the level of statistical significance. The association remained statistically significant after DII was grouped as quartiles. Participants in DII quartile 4 had a significantly 52% higher risk of NAFLD than those in DII quartile 1 (model III, OR 1.52, 1.27–1.83 CI, p trend <0.0001). After adjustment for potential confounding factors (model III), DII exhibited a significant positive association with AHF risk (OR 1.15, 1.07–1.23 CI, p trend <0.001).
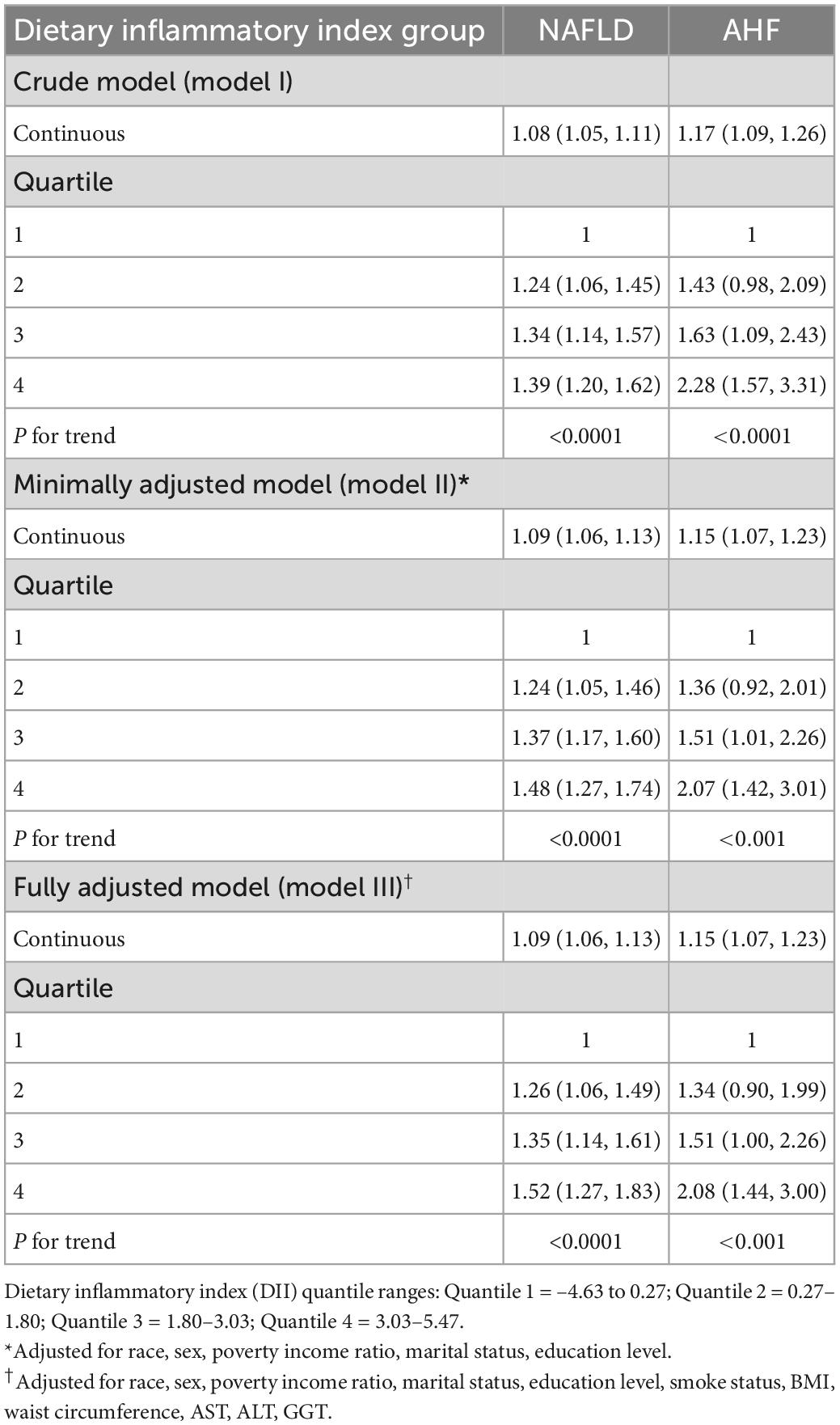
Table 2. Association between dietary inflammatory index (DII) and the presence of non-alcoholic fatty liver disease (NAFLD) and advanced hepatic fibrosis (AHF) in the 2005–2016 continuous National Health and Nutrition Examination Survey (NHANES).
Table 3 shows the association between DII and risk of NAFLD and AHF stratified by gender. DII levels were significantly and positively associated with the odds of NAFLD in males (Q4 vs. Q1: OR 1.62, 1.24–2.13 CI, p trend <0.001) and in females (Q4 vs. Q1: OR 1.37, 1.04–1.79 CI, p trend = 0.022) after full adjustment in model III. DII levels were associated with higher odds of AHF only in males (Q4 vs. Q1: OR 2.68, 1.63–4.41 CI, p trend <0.0001), and AHF risk was not significantly increased among Q2–Q4 participants compared with Q1.

Table 3. Survey weighted odds ratio (95% CI) for association between dietary inflammatory index (DII) and the presence of non-alcoholic fatty liver disease (NAFLD) and advanced hepatic fibrosis (AHF) stratified by sex in the 2005–2016 continuous National Health and Nutrition Examination Survey (NHANES).
Figure 2 shows the association of DII and NAFLD among adults. we used restricted cubic spline to flexibly model and visualize the relation of DII and NAFLD among adults. The relationship started increase rapidly until around 1.80 and then started relatively flat afterward.
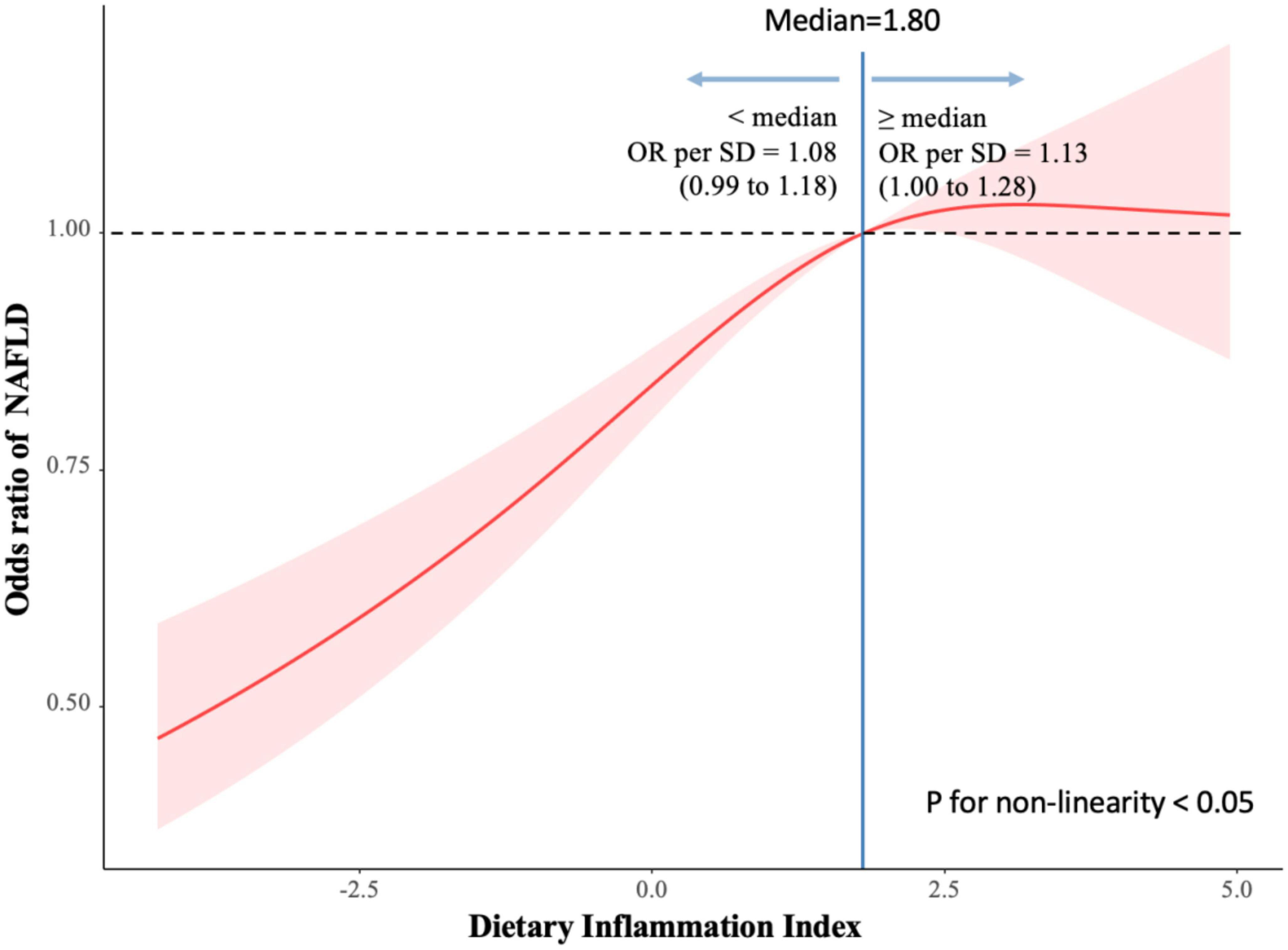
Figure 2. Association of dietary inflammatory index (DII) and non-alcoholic fatty liver disease (NAFLD) among adults. Reference point is median for NAFLD, with knots placed at 10th, 50th, 90th centiles of NAFLD distribution.
The present study found a pro-inflammatory diet as measured by the DII was associated with a deteriorating NAFLD and AHF risk profile in a nationally representative sample of US adults. In this cohort, the highest quartile of DII, the most pro-inflammatory diet, was positively associated with increased risk of NAFLD and AHF compared to the lowest quartile, the most anti-inflammatory diet. Additionally, in the subgroup analysis by sex basing on multiple logistic regression, DII exhibited a significant positive association with NAFLD, and AHF was not significantly in females.
Limited studies have investigated the link between the DII and NAFLD and AHF. Mazidi et al. (34) showed the significant association between the inflammatory potential of diet and FLI by resulting that individuals with the highest DII quartile had nearly a 6-fold higher likelihood of fatty liver (OR 5.97, 4.44–8.02 CI) compared with those with the lowest DII quartile. NFS is commonly used for detecting fibrosis among NAFLD patients and has been shown to be as accurate as a liver biopsy in stratifying patients at risk for liver-related morbidity and mortality (35). We used NFS to define AHF in this study. To date, the association between AHF and DII has not been previously investigated. We found that AHF were positively associated with DII scores. Highest DII quartile had higher odds than those in the lowest DII quartile after full adjustment (OR 2.08, 1.44–3.00 CI, p trend <0.001, Table 2). Then, subgroup analysis was performed for gender, DII was significantly correlated with higher risk of NAFLD in both genders, and with higher risk of AHF in males but not in females. Further studies in independent populations would be essential to support these findings.
Our results highlight that anti-inflammatory diets were associated with lower risks of NAFLD and the prevention of its progression to advanced fibrosis. Kenđel et al. (36) investigated the relation between energy-reduced anti-inflammatory diet and liver status using a two-arm randomized controlled trial, founding that FLI was 14.3% lower after 6 months energy-restricted anti-inflammatory diet and for FIB-4 (a validated diagnostic index, to estimating the liver fibrosis possibility) was 2.5% lower. Another study included (37) 8,520 adults in western Iran and found that more pro-inflammatory diet in participants was associated with higher FLI. Similarly, Li and Chen (38) suggested that anti-inflammatory diets might have hepatoprotective effects that reduce the risk of NAFLD.
The major highlights of this study are the relatively large and well-designed population-based sample, the results can be extrapolated to the entire population. Due to the strong standardization of the NHANES study procedures, the measurement and information biases are very low (39). Our findings suggest that a more specific focus on the DII level for NAFLD patients is required, and AHF patients, especially in males.
There were several limitations to this study as follows. First, the cross-sectional nature of NHANES severely constrains causal inferences. Second, in the absence of gold standard techniques to diagnose NAFLD and AHF, we used non-invasive diagnosis indexes (i.e., the FLI and NFS). Third, the lack of data on physical activity makes it impossible to predict whether it would be a potential predictor. However, combined with previous study (34), this predictor may not have a decisive effect on outcomes. Finally, a 24 h dietary recall is used to calculate DII, limiting the ability to accurately describe individuals’ habitual diets, and recall bias is inevitable. In addition, the non-availability of 18 food parameters for calculating the DII score may be a limitation of the study as well. Nevertheless, it has been shown in previous studies that the absence of these missing components is unlikely to have a major impact on DII scores since they are consumed infrequently in the US population (40). Thus, further studies needed to clarify the causal relationship and to confirm these findings.
In conclusion, data from this study suggesting a possible association between NAFLD and AHF risk with the DII. We suggest the anti-inflammatory diet can be a step of NAFLD and AHF management and be considered to prevent NAFLD and AHF in adults. However, further longitudinal studies, larger sample sizes and repeated measures are necessary to validate and verify the current evidence.
We analyzed publicly available datasets in this study. The datasets presented in this study can be found in online repositories. The names of the repositories can be found below: Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey (NHANES), https://wwwn.cdc.gov/nchs/nhanes/Default.aspx, 2005–2016. Using deidentified data to conduct the secondary analysis was officially classified as exempt by the Albert Einstein College of Medicine Institutional Review Board.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
ZhL and JL were responsible for the conception and design of the study. ZZ, LW, and ZiL wrote the first draft of the manuscript, interpreted the data, and wrote the final version. WeY, JC, XZ, WaY, and JL performed the statistical analysis and interpreted the analysis. ZhL, JL, JC, XZ, and WeY were critically revised the manuscript. ZhL obtained public funding. All authors critically revised the article for important intellectual content and approved the final version.
This project was supported by the National Natural Science Foundation of China (Grants Number: 82074187).
The authors thank Jing Zhang (Shanghai Tongren Hospital) for providing statistical methodology consultation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DII, dietary inflammatory index; NAFLD, non-alcoholic fatty liver disease; AHF, advanced hepatic fibrosis; NHANES, National Health and Nutrition Examination Survey; RCS, restricted cubic spline; NASH, non-alcoholic steatohepatitis; LSM, liver stiffness measure; CAP, controlled attenuation parameter; NCHS, National Center for Health Statistics; STROBE, strengthening the reporting of observational studies in epidemiology; FLI, fatty liver index; NFS, NAFLD fibrosis score; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, Γ-glutamyl transferase; TG, triglyceride; TC, total cholesterol; HDL, high density lipoprotein cholesterol; BMI, body mass index.
1. Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. (2011) 34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x
2. Siddiqui MS, Natta ML, Connelly MA, Vuppalanchi R, Neuschwander-Tetri BA, Tonascia J, et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. (2020) 72:25–33. doi: 10.1016/j.jhep.2019.10.006
3. Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med. (2018) 18:245–50. doi: 10.7861/clinmedicine.18-3-245
4. Rinella M. Nonalcoholic fatty liver disease: a systematic review. JAMA. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
5. European Association for the Study of the Liver [EASL]. European association for the study of diabetes (easd); european association for the study of obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. (2016) 59:1121–40. doi: 10.1007/s00125-016-3902-y
6. Neuschwander-Tetri B, Caldwell S. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. (2003) 37:1202–19. doi: 10.1053/jhep.2003.50193
7. Younossi Z. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. (2019) 70:531–44. doi: 10.1016/j.jhep.2018.10.033
8. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
9. Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. (2016) 63:2032–43. doi: 10.1002/hep.28392
10. Mazidi M, Shivappa N, Wirth MD, Hebert JR, Mikhailidis DP, Kengne AP, et al. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis. (2018) 276:23–7. doi: 10.1016/j.atherosclerosis.2018.02.020
11. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
12. Wang YB, Shivappa N, Hébert JR, Page AJ, Gill TK, Melaku YA. Association between dietary inflammatory index, dietary patterns, plant-based dietary index and the risk of obesity. Nutrients. (2021) 13:1536. doi: 10.3390/nu13051536
13. Yi Q, Li X, He Y, Xia W, Shao J, Ye Z, et al. Associations of dietary inflammatory index with metabolic syndrome and its components: a systematic review and meta-analysis. Public Health Nutr. (2021) 24:5463–70. doi: 10.1017/S1368980021000288
14. Namazi N, Larijani B, Azadbakht L. Dietary inflammatory index and its association with the risk of cardiovascular diseases, metabolic syndrome, and mortality: a systematic review and meta-analysis. Horm Metab Res. (2018) 50:345–58. doi: 10.1055/a-0596-8204
15. Garcia-Arellano A, Martínez-González MA, Ramallal R, Salas-Salvad J, Hébert JR, Corella D, et al. Dietary inflammatory index and all-cause mortality in large cohorts: the SUN and PREDIMED studies. Clin Nutr. (2019) 38:1221–31. doi: 10.1016/j.clnu.2018.05.003
16. Cantero I, Abete I, Babio N, Arós F, Corella D, Estruch R, et al. Dietary inflammatory index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin Nutr. (2018) 37:1736–43. doi: 10.1016/j.clnu.2017.06.027
17. Ramírez-Vélez R, García-Hermoso A, Izquierdo M, Correa-Rodríguez M. The dietary inflammatory index and hepatic health in the US adult population. J Hum Nutr Diet. (2022) 35:968–79. doi: 10.1111/jhn.12962
18. Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National health and nutrition examination survey, 2015-2018: sample design and estimation procedures. Vital Health Stat 2. (2020) 184:1–35.
19. Elm Ev, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
20. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
21. Yang HH, Chen GC, Li DM, Lan L, Chen LH, Xu JY, et al. Serum iron and risk of nonalcoholic fatty liver disease and advanced hepatic fibrosis in US adults. Sci Rep. (2021) 11:10387. doi: 10.1038/s41598-021-89991-x
22. Ricordi C, Garcia-Contreras M, Farnetti S. Diet and inflammation: possible effects on immunity, chronic diseases, and life span. J Am Coll Nutr. (2015) 34(Suppl. 1):10–3. doi: 10.1080/07315724.2015.1080101
23. Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
24. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
25. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. (2007) 45:846–54. doi: 10.1002/hep.21496
26. Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. (2020) 14:889–919. doi: 10.1007/s12072-020-10094-2
27. Akinkugbe AA, Avery CL, Barritt AS, Cole SR, Lerch M, Mayerle J, et al. Do genetic markers of inflammation modify the relationship between periodontitis and nonalcoholic fatty liver disease? Findings from the SHIP study. J Dent Res. (2017) 96:1392–9. doi: 10.1177/0022034517720924
28. Huang T, Behary J, Zekry A. Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern Med J. (2020) 50:1038–47. doi: 10.1111/imj.14709
29. Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I. Non-alcoholic fatty liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int J Environ Res Public Health. (2021) 18:5227. doi: 10.3390/ijerph18105227
30. Huber Y, Schulz A, Schmidtmann I, Beutel M, Pfeiffer N, Münzel T, et al. Prevalence and risk factors of advanced liver fibrosis in a population-based study in Germany. Hepatol Commun. (2022) 6:1457–66. doi: 10.1002/hep4.1899
31. Flores YN, Yee HF Jr, Leng M, Escarce JJ, Bastani R, Salmerón J, et al. Risk factors for chronic liver disease in blacks, mexican americans, and whites in the United States: results from NHANES IV, 1999-2004. Am J Gastroenterol. (2008) 103:2231–8. doi: 10.1111/j.1572-0241.2008.02022.x
32. Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral neuropathy and all-cause and cardiovascular mortality in U.S. Adults: a prospective cohort study. Ann Intern Med. (2021) 174:167–74. doi: 10.7326/M20-1340
33. Zhang Z, Li Z, Zhang X, Ye W, Chen J, Wang L, et al. Association between secondhand smoke and cancers in adults in the US population. J Cancer Res Clin Oncol. (2022). [Epub ahead of print]. doi: 10.1007/s00432-022-04266-w
34. Mazidi M, Shivappa N, Wirth MD, Hebert JR, Kengne AP. Diet with greater inflammatory potential is associated with higher prevalence of fatty liver among US adults. Eur J Clin Nutr. (2019) 73:1653–6. doi: 10.1038/s41430-018-0364-y
35. Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, et al. Prognostic accuracy of FIB-4. NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review Liver Int. (2021) 41:261–70. doi: 10.1111/liv.14669
36. Kenđel JG, Mrakovcic-Sutic I, Pavičić Žeželj S, Benjak Horvat I, Šuša L, Rahelić D, et al. Metabolic and hepatic effects of energy-reduced anti-inflammatory diet in younger adults with obesity. Can J Gastroenterol Hepatol. (2021) 2021:6649142. doi: 10.1155/2021/6649142
37. Darbandi M, Hamzeh B, Ayenepour A, Rezaeian S, Najafi F, Shakiba E, et al. Anti-inflammatory diet consumption reduced fatty liver indices. Sci Rep. (2021) 11:22601. doi: 10.1038/s41598-021-98685-3
38. Li R, Chen Z. Title of manuscript: validation and comparison of two dietary indexes for predicting nonalcoholic fatty liver disease among US adults. J Nutr. (2022). [Epub ahead of print]. doi: 10.1093/jn/nxac230
39. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, et al. The US department of agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. (2008) 88:324–32. doi: 10.1093/ajcn/88.2.324
Keywords: dietary inflammatory index, non-alcoholic fatty liver disease, advanced hepatic fibrosis, NHANES, population-based study
Citation: Zhang Z, Wang L, Lin Z, Yan W, Chen J, Zhang X, Ye W, Li J and Li Z (2023) Dietary inflammatory index and risk of non-alcoholic fatty liver disease and advanced hepatic fibrosis in US adults. Front. Nutr. 10:1102660. doi: 10.3389/fnut.2023.1102660
Received: 19 November 2022; Accepted: 04 January 2023;
Published: 25 January 2023.
Edited by:
Carmine Gazzaruso, University of Milan, ItalyReviewed by:
Samantha Maurotti, Magna Græcia University, ItalyCopyright © 2023 Zhang, Wang, Lin, Yan, Chen, Zhang, Ye, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihong Li,  bGl6aGlob25nQGJ1Y20uZWR1LmNt; Jian Li,
bGl6aGlob25nQGJ1Y20uZWR1LmNt; Jian Li,  bGlqaWFuQGJ1Y20uZWR1LmNu
bGlqaWFuQGJ1Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.