- 1College of Sports and Health, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Sports Coaching College, Beijing Sport University, Beijing, China
- 3China Institute of Sport and Health Science, Beijing Sport University, Beijing, China
- 4Hebrew SeniorLife Hinda and Arthur Marcus Institute for Aging Research, Harvard Medical School, Boston, MA, United States
- 5School of Strength and Conditioning Training, Beijing Sport University, Beijing, China
Background: Fatigue is oftentimes induced by high-intensity exercise potentially via the exceeded amount of reactive oxygen species, leading to diminished functions (e.g., aerobic capacity) and increased risk of injuries. Studies indicate that molecular hydrogen (H2), with antioxidant and anti-inflammatory properties, may be a promising strategy to alleviate fatigue and improve aerobic capacity. However, such effects have not been comprehensively characterized.
Objective: To systematically assess the effects of in taking H2 on fatigue and aerobic capacity in healthy adults.
Methods: The search was conducted in August 2022 in five databases. Studies with randomized controlled or crossover designs that investigated the rating of perceived exertion (RPE), maximal oxygen uptake (VO2max), peak oxygen uptake (VO2peak), and endurance performance were selected. The data (mean ± standard deviation and sample size) were extracted from the included studies and were converted into the standardized mean difference (SMD). Random-effects meta-analyses were performed. Subgroup analysis was used to analyze potential sources of heterogeneity due to intervention period, training status, and type of exercise.
Results: Seventeen publications (19 studies) consisting of 402 participants were included. The pooled effect sizes of H2 on RPE (SMDpooled = −0.38, 95%CI −0.65 to −0.11, p = 0.006, I2 = 33.6%, p = 0.149) and blood lactate (SMDpooled = −0.42, 95% CI −0.72 to −0.12, p = 0.006, I2 = 35.6%, p = 0.114) were small yet significant with low heterogeneity. The pooled effect sizes of H2 on VO2max and VO2peak (SMDpooled = 0.09, 95% CI −0.10 to 0.29, p = 0.333, I2 = 0%, p = 0.998) and endurance performance (SMDpooled = 0.01, 95% CI −0.23 to 0.25, p = 0.946, I2 = 0%, p > 0.999) were not significant and trivial without heterogeneity. Subgroup analysis revealed that the effects of H2 on fatigue were impacted significantly by the training status (i.e., untrained and trained), period of H2 implementation, and exercise types (i.e., continuous and intermittent exercises).
Conclusions: This meta-analysis provides moderate evidence that H2 supplementation alleviates fatigue but does not enhance aerobic capacity in healthy adults.
Systematic review registration: www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022351559.
1. Introduction
Aerobic capacity enables the performance of daily activities that require repetitive movements of the body for prolonged periods of time and/or against physical loads (e.g., exercise) (1). Fatigue induced by such activities is a significant contributor to reduced performance, as well as to exhaustion and weakness (2–5). Considerable effort has therefore been taken to develop safe strategies to effectively reduce fatigue and in turn improve aerobic capacity within both the sport and non-sport setting.
One mechanism of fatigue development appears to be that high-intensity exercise induces high amounts of reactive oxygen species (ROS) within mitochondria, which leads to dysregulation within human inflammatory and neuroendocrinological systems (6–10). Studies have thus emerged to implement antioxidant nutrients, such as vitamins and resveratrol, to alleviate fatigue and facilitate recovery from fatigue (11, 12). These studies have shown promise, but have also highlighted that the appropriate dosage of these nutrients is critical and that the intake of excessive amounts may induce side effects such as oxidative stress and the inhibition of exercise-induced physiological adaptations within skeletal muscle and the cardiovascular system (12–16).
Since Ohsawa's pioneering discovery of the selective antioxidant function of molecular hydrogen (H2), research suggests that the intake of H2 holds promise as a non-toxic strategy to alleviate exercise-induced oxidative stress and inflammation (17–19). Studies have reported that H2 molecules administered via inhaled gas or oral water, can penetrate cell membranes and diffuse rapidly into organelles (20), thus selectively reducing OH and ONOO− (21, 22). Recently, a number of relatively small studies have also demonstrated that the intake of H2 via hydrogen-rich water (HRW) or hydrogen-rich gas (HRG) may reduce fatigue and enhance aerobic capacity (23–27). However, results of these studies have been inconsistent. For example, one study observed that intake of 500 mL hydrogen-rich water within 10 min before exercise increased maximum oxygen uptake (VO2max) and reduced subjective fatigue during an incremental cycling exercise in healthy young adults (25). In contrast, another study reported that intake two doses of 290 mL hydrogen-rich water before an incremental treadmill running exercise could not induce such benefits in endurance athletes (28). These and other studies suggest that the impact of H2 supplementation may depend upon dosage, as well as other factors including training status of the individual and the type of exercise in question(23, 29, 30).
The purpose of this study was to examine the impact of H2 intake on fatigue and aerobic capacity by conducting a systematic review and meta-analysis of available peer-reviewed publications on the topic. Subgroup analyses were also completed in hopes of guiding future research by providing insight into whether dosage, training status, and exercise types potentially influences the impact of H2 supplementation. Our overarching hypothesis was that H2 supplementation prior to or during exercise would significantly reduce fatigue and increase aerobic capacity in healthy adults.
2. Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (31). This study was registered with PROSPERO (CRD42022351559).
2.1. Data sources, searches, and study selection
Two authors (KZ and ML) independently searched PubMed, Web of Science, Medline, Sport-Discus, and PsycINFO databases from inception to August 5, 2022, using a comprehensive search strategy (eTable 1). Manual searches of the reference lists in the related publications were also performed.
Studies were included if: (1) the participants were healthy adults with a mean age≥18 years and were free from any dietary supplements or medications; (2) the intervention was the intake of molecular hydrogen by the participants; (3) the control group with placebo; (4) the outcomes include at least one of RPE, blood lactate, VO2max, peak oxygen uptake (VO2peak), and performance of endurance exercises (i.e., cycling time to exhaustion, race time, etc.); (5) randomized controlled or crossover design.
Studies were excluded if they were: (1) animal trials; (2) written by non-English language, (3) without specific data; (4) review and conference articles; and (4) repeated publications.
2.2. Data extraction, outcomes, and risk of bias assessment
Two independent reviewers (ML and YW) extracted relevant data from each included study (32), including the authors, publication year, sample size, participant characteristics, H2 administration protocol, design of exercise, and outcome measures. Any disagreement between the two authors was discussed with JZ and DB until a consensus was achieved.
The primary outcome of fatigue was the RPE score and that of aerobic capacity was VO2max, or VO2peak (33, 34) when VO2max was not available. The secondary outcome of fatigue was blood lactate and that of aerobic capacity was performance of endurance exercises, including cycling time to exhaustion, and race time. The mean and standard deviation of each outcome in post-tests in each study were extracted. The post-test data of outcomes in each study were summarized in eTable 2.
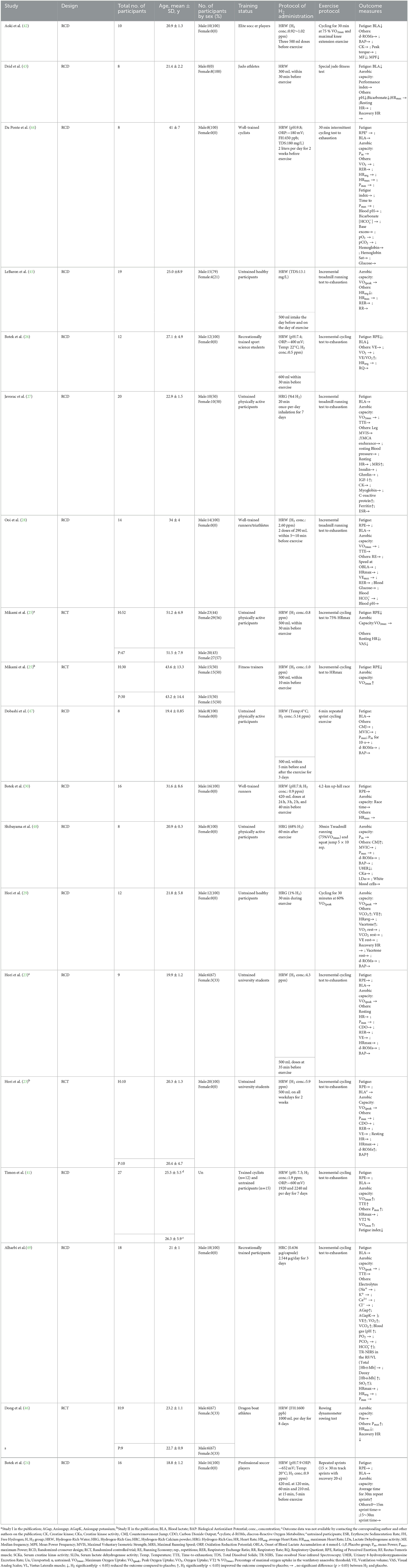
Two investigators (KZ and ML) independently assessed the risk of bias in the included studies using the Cochrane Collaboration's tool (35), containing the following criteria: (1) selection bias; (2) performance bias; (3) detection bias; (4) attrition bias; (5) reporting bias; (6) other sources of bias. Studies were defined as high risk of bias when ≥1 of these items were with a high risk of bias, and as low risk if all these items were with low risk of bias. In other situations, it was defined as moderate risk.
2.3. Statistical analysis and grading the evidence
To determine the effect size (ES) of the intervention, the standardized mean difference (SMD; Hedges' g) of the outcomes was calculated, with a 95% confidence interval (CI). ES was classified as trivial (<0.2), small (0.2~0.49), moderate (0.5~0.79), or large (>0.8) (36). Meta-analysis was performed in Stata v15.1 (STATA Corp., College Station, TX) using the inverse variance method. Heterogeneity was assessed by measuring the inconsistency (I2 statistic) of intervention effects among the trials. The level of heterogeneity was interpreted according to guidelines from the Cochrane Collaboration: trivial (<25%), low (25~50%), moderate (50~75%), and high (>75%) (37). A random-effects model was used to estimate the pooled effect in anticipation of heterogeneity across the studies due to differences in participants and intervention characteristics. The publication bias was assessed by the funnel plot and Egger's test. Subgroup analysis was used to analyze potential sources of heterogeneity due to intervention period, training status, and type of exercise. If a significant asymmetry was detected, we used the Trim and Fill method for sensitivity analysis of the results (38). All the statistical significance was set at p < 0.05.
Additionally, the quality of evidence for outcomes was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE), which characterizes the evidence on the study limitations, imprecision, inconsistency, indirectness, and publication bias (39, 40).
3. Results
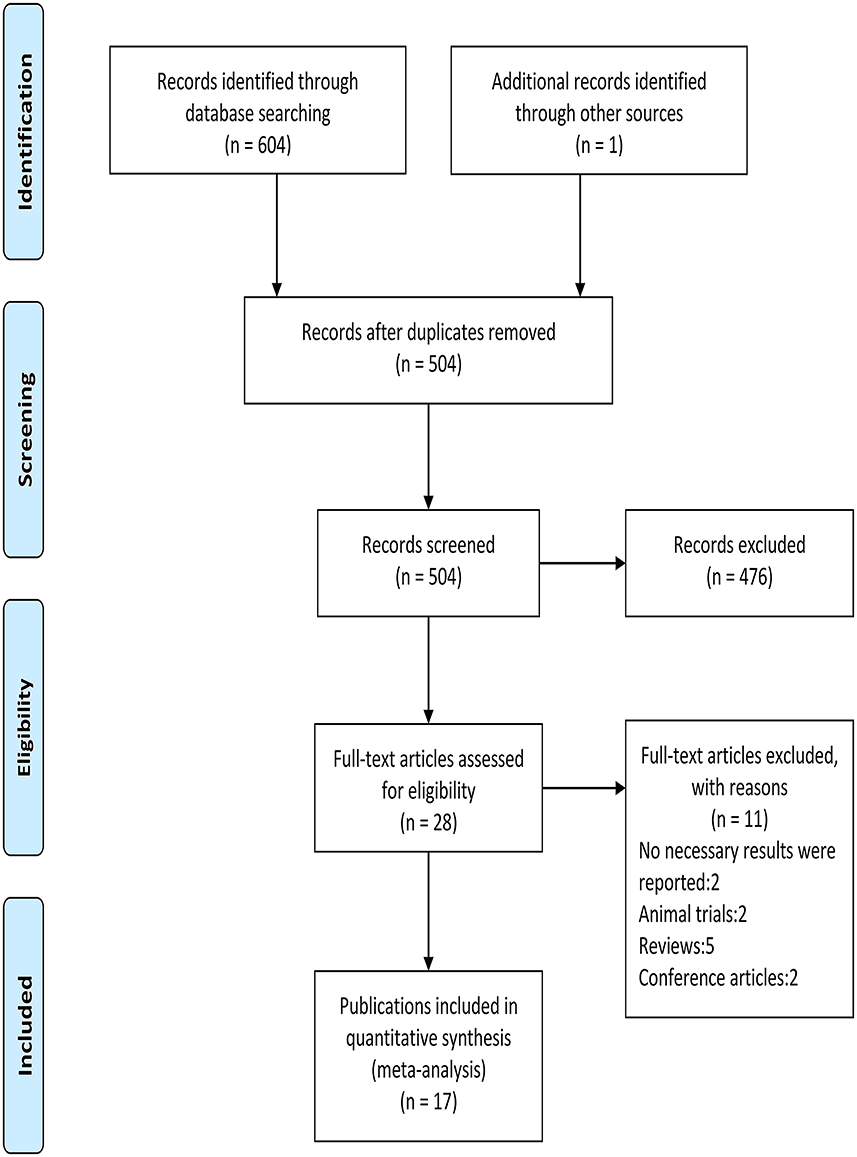
The flow diagram of screening is shown in Figure 1. A total of 605 relevant publications were retrieved (PubMed n = 50, Web of Science n = 305, Medline n = 210, Sport-Discus n = 36, PsycINFO n = 3, Manual search n = 1), and 577 publications were excluded after reviewing the titles and abstracts. After the evaluation of full texts, 11 of the 28 publications were removed, and thus 17 publications consisting of 19 studies (i.e., 15 randomized crossover designs and four randomized controlled trials) were included in the following analyses (Table 1). One publication (25) included two randomized controlled trials, and the other one (23) included a randomized crossover study and a randomized controlled trial.
3.1. Participant characteristics
A total of 402 healthy participants with mean ages ranging from 18.8 to 51.5 years were included (Table 1). For the training status, 210 of them were untrained, and 192 were trained. Sex information was missing in one study (41) (Table 1).
3.2. Protocol of H2 administration
The included studies implemented three types of hydrogen, that is, hydrogen-rich water (HRW) (n = 13) (23–26, 28, 30, 41–47), hydrogen-rich gas (HRG) (n = 3) (27, 29, 48), and hydrogen-rich calcium powder (HRC) (n = 1) (49). Hydrogen concentrations varied considerably (e.g., HRW: 0.5~5.9 ppm; HRG: 1 to 68%) across these studies (Table 1). Eight studies (23, 25, 26, 28, 30, 42, 43, 50) examined the effects of a single intake of H2 within 24 h before exercise. Seven studies (23, 27, 41, 44–46, 49) implemented the protocol of repeatedly intaking H2 from 2 to 14 days before exercise. One study (47) used the intake of HRW before and after exercise for 3 days, and another study (48) used a single 60-min inhalation of HRG immediately after exercise. The specific amount of H2 was presented in Table 1. Placebos used in these studies depended upon the type of supplement and drinking water, inhaling normal air, or swallowing capsules (i.e., capsules containing calcium powder).
3.3. Exercise protocol
Two exercise types (continuous and intermittent) were used to induce fatigue. Specifically, eight studies (23, 25–28, 41, 45, 49) used continuous load-incremental exercise (i.e., incremental treadmill running test, incremental cycling test); four studies (29, 42, 46, 48) used continuous fixed-load exercise; one study (30) used a 4.2-km up-hill race; three studies (24, 44, 47) used intermittent sprint exercises; and the other one (43) used an intermittent judo fitness test.
3.4. Outcome measurements
Nine of the studies (23–25, 28, 30, 41, 43, 44, 49) assessed both fatigue and aerobic capacity immediately after exercise. Three studies (26, 42, 47) assessed only fatigue immediately after exercise. Four studies (29, 45, 46, 48) assessed only aerobic capacity. In addition, other outcomes such as heart rate, explosive power, respiratory circulations, blood metabolites, and muscle functions were also assessed (Table 1).
3.5. Risk of bias
The results of the quality evaluation of the included 19 studies were shown in Figure 2. Four (23, 29, 44, 47) of them used a randomized, single-blinded and placebo-controlled design; and the others used a randomized, double-blinded and placebo-controlled design. One study (23) was evaluated as high risk of bias, 11 were as low risk, and the other seven (23, 25, 29, 44, 46, 47) were as moderate risk.
3.6. Meta-analysis
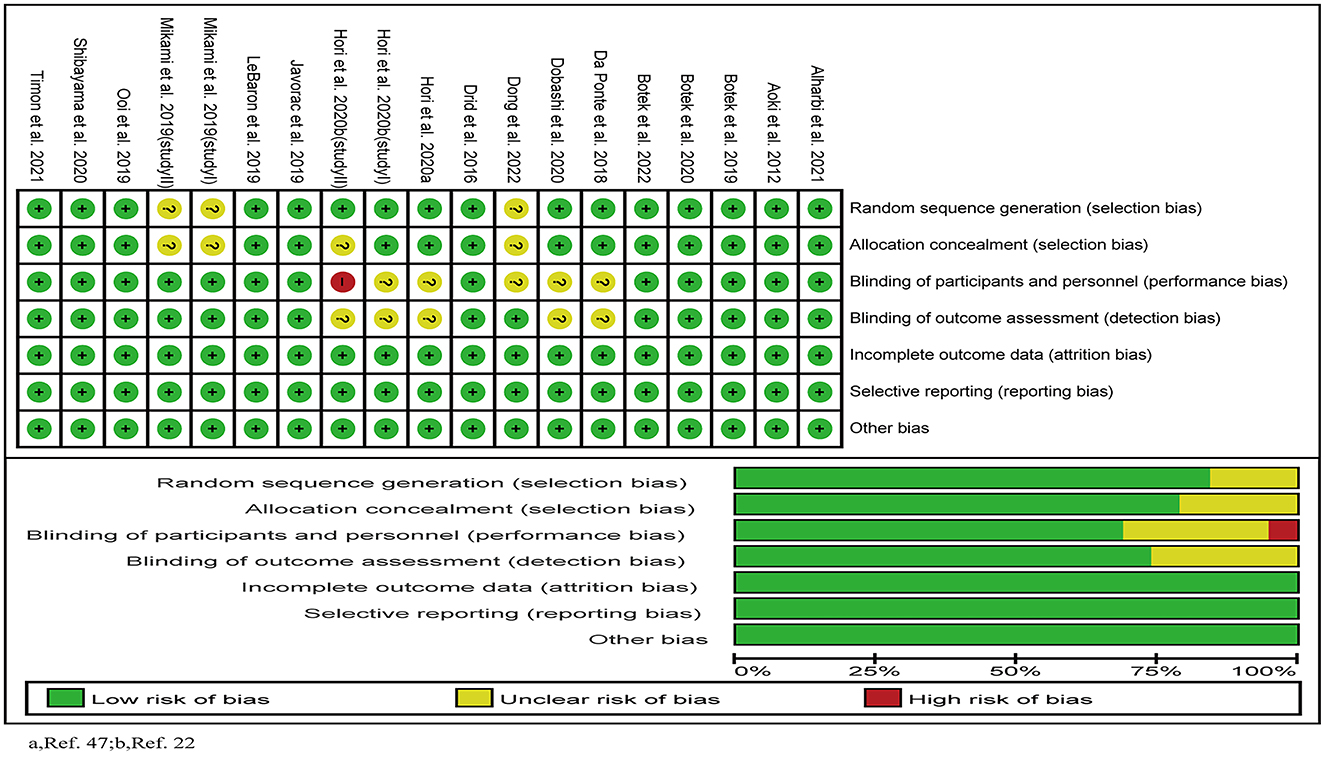
Based on the heterogeneity, we performed subgroup analyses of RPE and blood lactate by comparing between training status (i.e., untrained and trained), intervention period (i.e., a single time within 24 h and multiple days before exercise), and exercise modes (i.e., continuous and intermittent exercises) as variables (Table 2).
3.6.1. Effects of H2 on fatigue
3.6.1.1. RPE score
Two publications (25, 26) showed that H2 can significantly reduce RPE score as compared to the placebo; while another six publications (23, 24, 28, 30, 41, 44) showed that H2 cannot significantly reduce RPE score (Table 1).
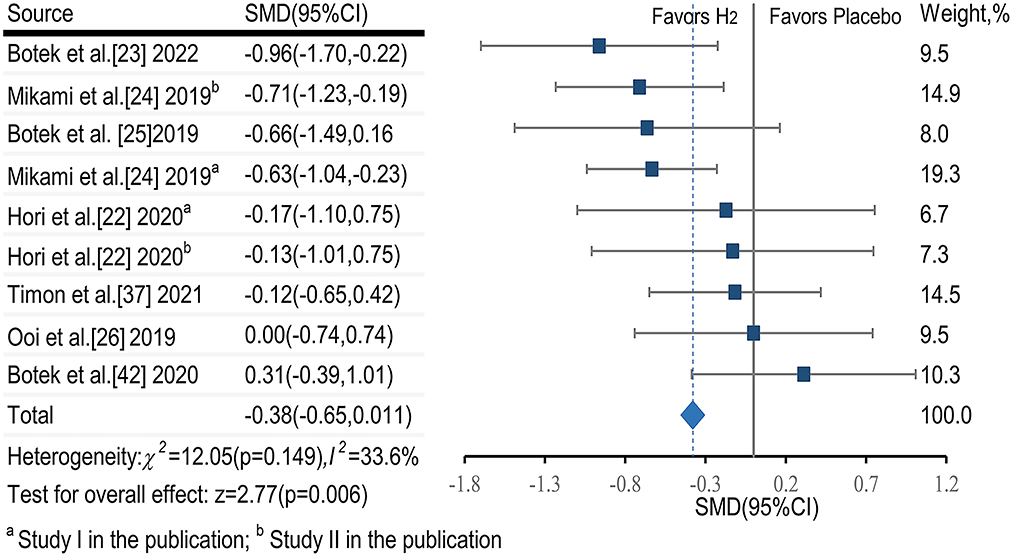
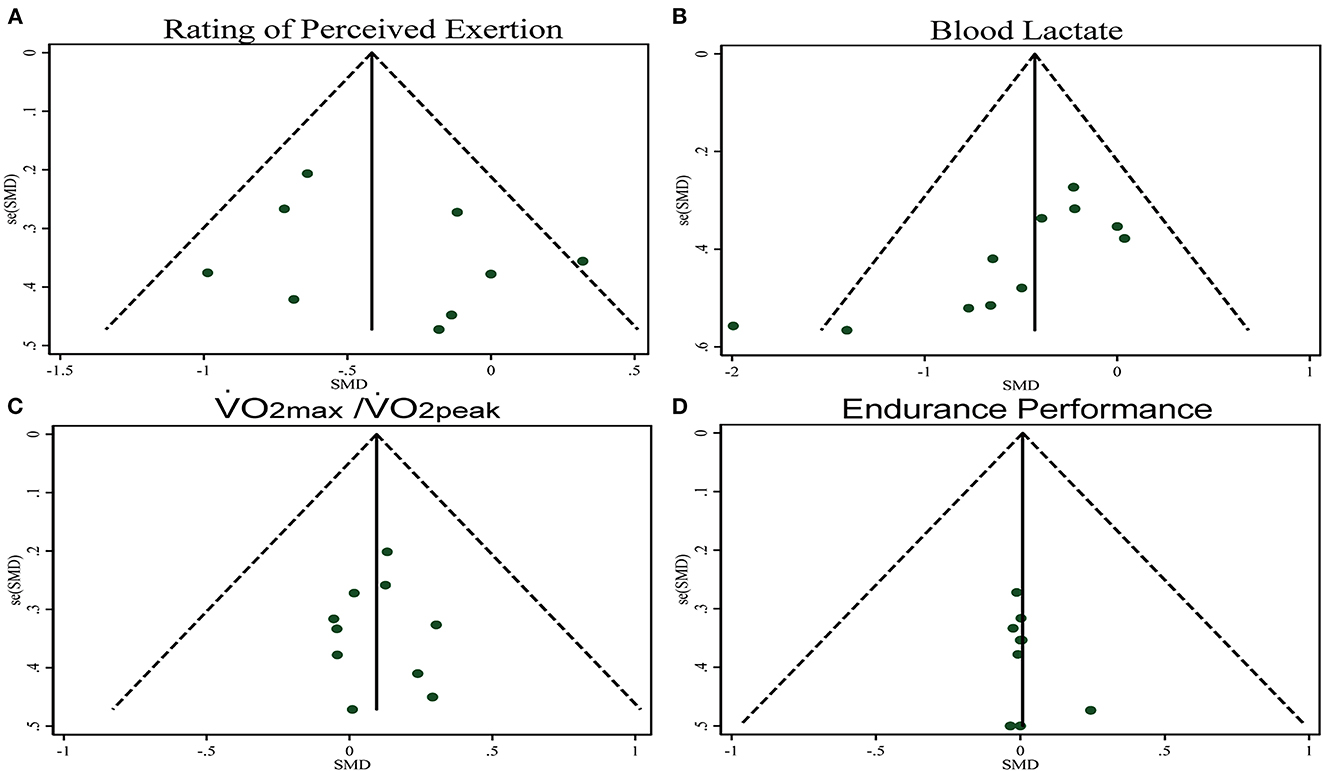
The pooled ES of RPE score was small and significant (SMDpooled = −0.38, 95% CI −0.65 to −0.11, p = 0.006, Figure 3) with low heterogeneity (I2 = 33.6%, p = 0.149). The funnel plot (Figure 7A) and Egger's test (t = 0.98, p = 0.358) indicated no publication bias.
Subgroup analysis showed that participant training status contributed significantly to the effects of H2. Specifically, the ES in untrained participants was significant and small (SMD = −0.47, 95% CI −0.78 to −0.16, p = 0.003). The ES in trained participants was not significant and small (SMD = −0.36, 95% CI −0.75 to 0.12, p = 0.161). A significant and small ES for single-dose H2 intake before exercise (SMD = −0.44, 95% CI −0.77 to −0.12, p = 0.007) was observed, while only trivial ES was observed in multiple-day intakes of H2 (SMD = −0.12, 95% CI −0.58 to 0.33, p = 0.606). With respect to exercise type, the ES was large for intermittent exercises (SMD = −0.96, 95% CI −1.70 to −0.22, p = 0.011), and was small and significant (SMD = −0.32, 95% CI −0.59 to −0.06, p = 0.017) for continuous exercises.
3.6.1.2. Blood lactate
Three publications (26, 42, 43) showed that H2 can significantly reduce blood lactate levels as compared to the placebo; while another eight publications (23, 24, 27, 28, 41, 44, 47, 49) showed opposite results that H2 cannot significantly reduce blood lactate levels (Table 1).
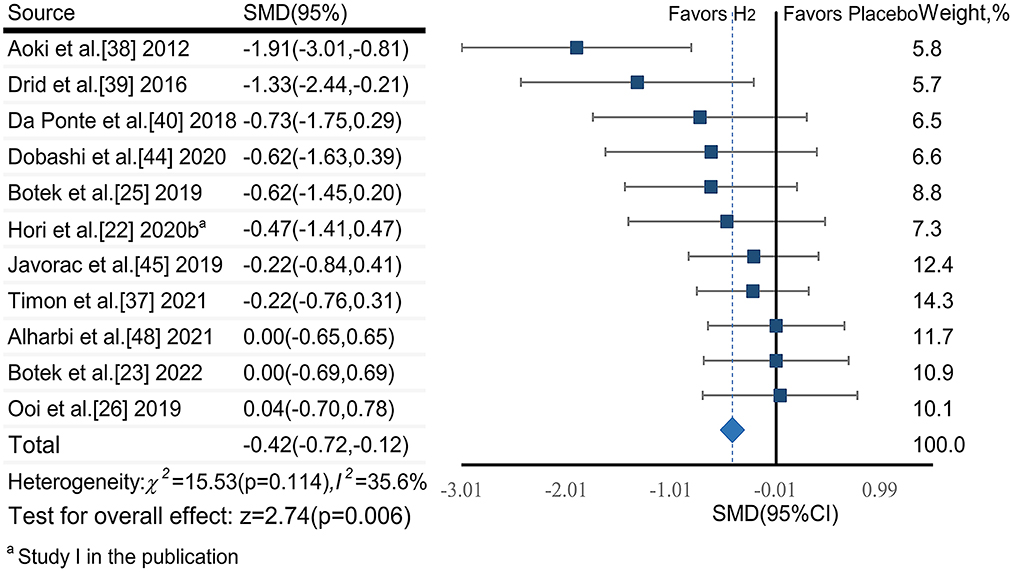
The pooled ES of blood lactate was significant and small (SMDpooled = −0.42, 95% CI −0.72 to −0.12, p = 0.006, Figure 4) with low heterogeneity (I2 = 35.6%, p = 0.114). The funnel plot (Figure 7B) and Egger's test (t = −3.64, p = 0.005) indicated a potential risk of publication bias; but the Trim and Fill method for sensitive analysis showed that the pooled ES (Fixed: SMDpooled = –.362, p = 0.002; Random: SMDpooled = −0.418, p = 0.006) was robust.
Subgroup analysis showed that participant training status contributed significantly to the effects of H2. Specifically, the ES in trained participants was significant and close to moderate (SMD = −0.49, 95% CI −0.92 to −0.06, p = 0.025), but was small and not significant in untrained participants (SMD = −0.30, 95% CI −0.69 to 0.09, p = 0.132). A significant and moderate ES for single-dose H2 intake before exercise (SMD = −0.62, 95% CI −1.19 to −0.05, p = 0.032) was observed, while only small ES was observed in multiple-day intakes of H2 (SMD = −0.26, 95% CI −0.57 to 0.06, p = 0.107). Regarding exercise type, the ES was moderate for intermittent exercises (SMD = −0.56, 95% CI −1.12 to 0.01, p = 0.053), and was small (SMD = −0.37, 95% CI −0.74 to 0.00, p = 0.052) for continuous exercises.
3.6.2 Effects of H2 on aerobic capacity
3.6.2.1. VO2max/VO2peak
Three publications (25, 29, 41) reported that H2 induced significant improvement in VO2max or VO2peak as compared to the placebo; while six publications (23, 25, 27, 28, 45, 49) reported no such effect (Table 1).
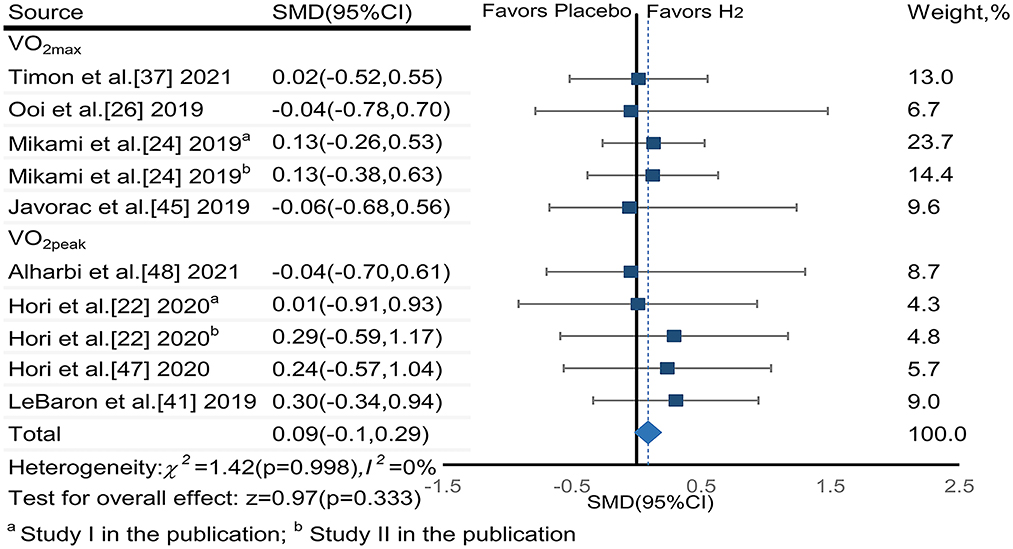
The pooled ES of VO2max and VO2peak was not significant and trivial (SMDpooled = 0.09,95% CI −0.10 to 0.29, p = 0.333, Figure 5) without heterogeneity (I2 = 0%, p = 0.996). The funnel plot (Figure 7C) and Egger's test (t = 0.01, p = 0.990) indicated no publication bias.
3.6.2.2. Endurance performance
Two publications (24, 41) reported that H2 significantly increased cycling to exhaustion time or multiple repeat sprint performance as compared to the placebo; while another eight publications (27, 28, 30, 43, 44, 46, 48, 49) reported no such effect (Table 1).
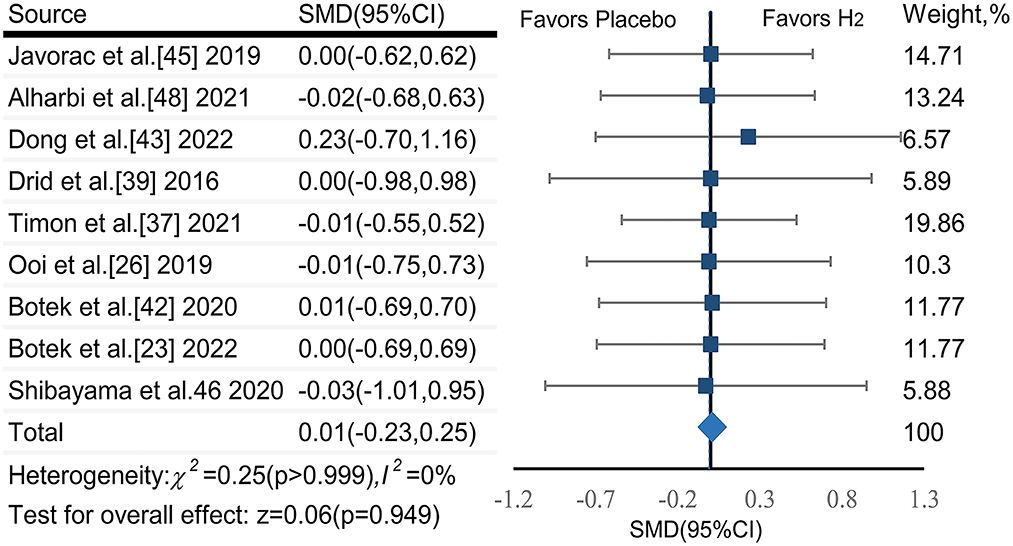
The pooled ES of endurance performance was not significant and trivial (SMDpooled = 0.01,95% CI −0.23 to 0.25, p = 0.949, Figure 6) without heterogeneity (I2 = 0%, p > 0.999). The funnel plot (Figure 7D) and Egger's test (t = 1.18, p = 0.278) indicated no publication bias.
3.7. GRADE assessment
The quality of evidence was determined to be moderate, and details for the evaluation of the GRADE framework are presented in eTable 3.
4. Discussion
The results of this systematic review and meta-analysis suggest that H2 supplementation is a promising strategy for alleviating subjective fatigue and clearing blood lactate as induced by high-intensity exercise. However, H2 supplementation does not appear to enhance aerobic capacity. The quality of available evidence to date was moderate. Subgroup analyses revealed that the training status, the period of H2 implementation, and the type of exercise may all influence the effects of H2 on fatigue and thus need to be carefully considered in the design of future research and practice.
While our results indicate that H2 can significantly reduce subjective fatigue and blood lactate after high-intensity exercise in healthy adults, they do not provide evidence for the underlying bio-neurophysiological mechanisms involved. One possible explanation is that H2 appears to be a neuroprotective agent that facilitates the restoration of neuronal oxidative damage by reducing oxidative stress and neuroinflammation (51–54). H2 intake has also been reported to induce positive effects on exercise acidosis and reduce blood lactate concentration (26), thus modulating intracellular and extracellular buffering capacity during high-intensity exercise (55). It is also possible that the effects of H2 intake may depend upon the resting redox state of the user (56). Future studies are thus warranted to further investigate these potential pathways, which will ultimately help the design of appropriate strategies for fatigue alleviation using H2.
Subgroup analyses reveal several important factors that likely contribute to the effects of H2 supplementation on fatigue. First, we observed that the effects were greater in untrained as compared to trained individuals. This may be due to the antioxidant capacity during high-intensity exercise is relatively lower in untrained participants compared to trained participants (57), which may thus interfere with the effects of H2 on fatigue (30), presenting relatively smaller effect size of H2 on fatigue in this cohort (42, 47). Second, longer period of H2 implementation, or multiple intakes of H2, was not associated with greater reduction of fatigue in healthy adults, as compared to the intake of a single dose immediately before exercise. This observation may be due to the low between-day retention rate of H2; for example, it was observed that over 59% of H2 can be exhaled within the first hour after the intake of HRW (25). Third, subgroup analyzes revealed that H2 supplementation may be more effective for fatigue induced by intermittent exercise as compared to continuous exercise (24, 28, 30). This may be because bouts of intermittent exercise are typically completed against greater external physical load, which may enable mitochondrial respiratory function to more efficiently intake H2 and/or increase the concentration level of ROS in muscles, boosting the redox procedure between ROS and molecular hydrogen (58–60).
Intriguingly, H2 supplementation did not appear to significantly improve aerobic capacity. This suggests that the observed impact of H2 intake on fatigue during high-intensity exercise was not sufficient to translate into improved aerobic capacity in healthy adults. Aerobic capacity depends upon multiple underlying bio-physiological procedures, including respiratory function, regulation of oxygen, and local muscle oxygen utilization (61, 62). Previous studies have reported that acute H2 supplementation does not substantially modulate these critical factors of aerobic capacity (23, 26–28, 49), which may at least in part underlie its lack of effect on this important function in humans.
4.1. Limitations
Several of the included studies were conducted on a small number of participants (n ≤ 10) (23, 43, 44, 47, 48), which may lead to potential bias. Most included studies focused on only younger and middle-aged men. As such, future studies are thus needed to examine whether the effects of H2 supplementation differs by age and/or sex. With respect to the latter, studies have reported that compared to men, the antioxidant protective function in women is greater due to estrogen (63, 64), which may potentially influence the effects of H2 on fatigue. Finally, considerable work is still needed to determine the dose-response relationship between H2 and fatigue and the impact of such supplementation on physiological adaptation to exercise and the risk of injury over time.
5. Conclusions
This analysis indicates that H2 supplementation can alleviate fatigue but cannot significantly enhance aerobic capacity in healthy adults. The knowledge obtained from this study, such as the appropriate protocols of H2 administration and selection of exercise type to induce fatigue, will ultimately help inform future studies to confirm and explicitly examine the benefits of H2 on athletes and untrained people with more rigorous design (e.g., matched number of men and women), helping optimize the protocols of fatigue recovery in the daily routines of professional athletes and untrained people.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KZ, ML, and DB had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: DB, KZ, and JZ. Acquisition, analysis, or interpretation of data: KZ, ML, YW, HL, and JZ. Drafting of the manuscript: KZ and ML. Critical revision of the manuscript for important intellectual content: KZ, ML, YW, HL, BM, DB, LZ, and JZ. Statistical analysis: YW, LZ, and HL. All authors contributed to the article and approved the submitted version.
Funding
DB was supported by the Key Research and Development Projects of the Ministry of Science and Technology (2018YFC2000602).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1094767/full#supplementary-material
References
1. Degens H, Veerkamp JH. Changes in oxidative capacity and fatigue resistance in skeletal muscle. Int J Biochem. (1994) 26:871–8. doi: 10.1016/0020-711X(94)90079-5
2. Martin K, Meeusen R, Thompson KG, Keegan R, Rattray B. Mental fatigue impairs endurance performance: a physiological explanation. Sports Med. (2018) 48:2041–51. doi: 10.1007/s40279-018-0946-9
3. Pageaux B, Lepers R. Fatigue induced by physical and mental exertion increases perception of effort and impairs subsequent endurance performance. Front Physiol. (2016) 7:587. doi: 10.3389/fphys.2016.00587
4. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. (2013) 80:409–16. doi: 10.1212/WNL.0b013e31827f07be
5. Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. (2014) 31:562–75. doi: 10.1177/1049909113494748
6. Huang WC, Wei CC, Huang CC, Chen WL, Huang HY. The beneficial effects of lactobacillus plantarum ps128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients. (2019) 11:353. doi: 10.3390/nu11020353
7. Slattery K, Bentley D, Coutts AJ. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. (2015) 45:453–71. doi: 10.1007/s40279-014-0282-7
8. Powers SK, Radak Z, Ji LL. Exercise-induced oxidative stress: past, present and future. J Physiol. (2016) 594:5081–92. doi: 10.1113/JP270646
9. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. (2008) 88:1243–76. doi: 10.1152/physrev.00031.2007
10. Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: friend or foe? J Sport Health Sci. (2020) 9:415–25. doi: 10.1016/j.jshs.2020.04.001
11. Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. (2011) 41:1043–69. doi: 10.2165/11594400-000000000-00000
12. Braakhuis AJ, Hopkins WG. Impact of dietary antioxidants on sport performance: a review. Sports Med. (2015) 45:939–55. doi: 10.1007/s40279-015-0323-x
13. Li S, Fasipe B, Laher I. Potential harms of supplementation with high doses of antioxidants in athletes. J Exerc Sci Fit. (2022) 20:269–75. doi: 10.1016/j.jesf.2022.06.001
14. Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, et al. Oral administration of vitamin c decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. (2008) 87:142–9. doi: 10.1093/ajcn/87.1.142
15. Close GL, Ashton T, Cable T, Doran D, Holloway C, McArdle F, et al. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. (2006) 95:976–81. doi: 10.1079/BJN20061732
16. Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J Physiol. (2014) 592:1887–901. doi: 10.1113/jphysiol.2013.267419
17. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. (2007) 13:688–94. doi: 10.1038/nm1577
18. Ostojic SM. Hydrogen gas as an exotic performance-enhancing agent: challenges and opportunities. Curr Pharm Des. (2021) 27:723–30. doi: 10.2174/1381612826666200922155242
19. LeBaron TW, Laher I, Kura B, Slezak J. Hydrogen gas: from clinical medicine to an emerging ergogenic molecule for sports athletes. Can J Physiol Pharmacol. (2019) 97:797–807. doi: 10.1139/cjpp-2019-0067
20. Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. (2014) 144:1–11. doi: 10.1016/j.pharmthera.2014.04.006
21. Hong Y, Chen S, Zhang JM. Hydrogen as a selective antioxidant: a review of clinical and experimental studies. J Int Med Res. (2010) 38:1893–903. doi: 10.1177/147323001003800602
22. Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. (2015) 36:273–9. doi: 10.1055/s-0034-1395509
23. Hori A, Sobue S, Kurokawa R, Hirano SI, Ichihara M, Hotta N. Two-week continuous supplementation of hydrogenrich water increases peak oxygen uptake during an incremental cycling exercise test in healthy humans: a randomized, single-blinded, placebo-controlled study. Med Gas Res. (2020) 10:163–9. doi: 10.4103/2045-9912.304223
24. Botek M, Khanna D, Krejčí J, Valenta M, McKune A, Sládečková B, et al. Molecular hydrogen mitigates performance decrement during repeated sprints in professional soccer players. Nutrients. (2022) 14:508. doi: 10.3390/nu14030508
25. Mikami T, Tano K, Lee H, Lee H, Ohta SJ. Pharmacology drinking hydrogen water enhances endurance and relieves psychometric fatigue: randomized, double-blind, placebo-controlled study. Can J Med. (2019) 97:857–62. doi: 10.1139/cjpp-2019-0059
26. Botek M, Krejčí J, McKune AJ, Sládečková B, Naumovski N. Hydrogen rich water improved ventilatory, perceptual and lactate responses to exercise. Int J Sports Med. (2019) 40:879–85. doi: 10.1055/a-0991-0268
27. Javorac D, Stajer V, Ratgeber L, Betlehem J, Ostojic S. Short-Term H inhalation improves running performance and torso strength in healthy adults. Biol Sport. (2019) 36:333–9. doi: 10.5114/biolsport.2019.88756
28. Ooi CH, Ng SK, Omar EA. Metabolism acute ingestion of hydrogen-rich water does not improve incremental treadmill running performance in endurance-trained athletes. Appl Nutr Metab. (2019) 45:513–19. doi: 10.1139/apnm-2019-0553
29. Hori A, Ichihara M, Kimura H, Ogata H, Kondo T, Hotta N. Inhalation of molecular hydrogen increases breath acetone excretion during submaximal exercise: a randomized, single-blinded, placebo-controlled study. Med Gas Res. (2020) 10:96–102. doi: 10.4103/2045-9912.296038
30. Botek M, Krejčí J, McKune AJ, Sládečková B. Hydrogen-rich water supplementation and up-hill running performance: effect of athlete performance level. Int J Sports Physiol Perform. (2020) 15:1–4. doi: 10.1123/ijspp.2019-0507
31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
32. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Intervention. 2nd Edn. London: John Wiley & Sons. (2019).
33. Baumgart JK, Brurok B, Sandbakk Ø. Comparison of peak oxygen uptake between upper-body exercise modes: a systematic literature review and meta-analysis. Front Physiol. (2020) 11:412. doi: 10.3389/fphys.2020.00412
34. Wallace A, Pietrusz A, Dewar E, Dudziec M, Jones K, Hennis P, et al. Community exercise is feasible for neuromuscular diseases and can improve aerobic capacity. Neurology. (2019) 92:e1773–e85. doi: 10.1212/WNL.0000000000007265
35. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
36. Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Lawrence Earlbaum Associates. (1988).
37. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
38. Duval S, Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
39. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. Grade guidelines: rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
40. Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A Checklist designed to aid consistency and reproducibility of grade assessments: development and pilot validation. Syst Rev. (2014) 3:82. doi: 10.1186/2046-4053-3-82
41. Timon R, Olcina G, Gonzalez-Custodio A, Camacho-Cardenosa M, Camacho-Cardenosa A, Martinez Guardado I. Effects of 7-day intake of hydrogen-rich water on physical performance of trained and untrained subjects. Biol Sport. (2021) 38:269–75. doi: 10.5114/biolsport.2020.98625
42. Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. Pilot study: effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. (2012) 2:12. doi: 10.1186/2045-9912-2-12
43. Drid P, Trivic T, Casals C, Trivic S, Stojanovic M, Ostojic SM. Is molecular hydrogen beneficial to enhance post-exercise recovery in female athletes? Sci Sports. (2016) 31:207–13. doi: 10.1016/j.scispo.2016.04.010
44. Da Ponte A, Giovanelli N, Nigris D, Lazzer S. Effects of hydrogen rich water on prolonged intermittent exercise. J Sports Med Phys Fitness. (2018) 58:612–21. doi: 10.23736/S0022-4707.17.06883-9
45. LeBaron TW, Larson AJ, Ohta S, Mikami T, Barlow J, Bulloch J, et al. Acute supplementation with molecular hydrogen benefits submaximal exercise indices. Randomized, double-blinded, placebo-controlled crossover pilot study. J Lifestyle Med. (2019) 9:36–43. doi: 10.15280/jlm.2019.9.1.36
46. Dong G, Fu J, Bao D, Zhou J. Short-term consumption of hydrogen-rich water enhances power performance and heart rate recovery in dragon boat athletes: evidence from a pilot study. Int J Env Res Public Health. (2022) 19:413. doi: 10.3390/ijerph19095413
47. Dobashi S, Takeuchi K, Koyama K. Hydrogen-rich water suppresses the reduction in blood total antioxidant capacity induced by 3 consecutive days of severe exercise in physically active males. Med Gas Res. (2020) 10:21–6. doi: 10.4103/2045-9912.279979
48. Shibayama Y, Dobashi S, Arisawa T, Fukuoka T, Koyama K. Impact of hydrogen-rich gas mixture inhalation through nasal cannula during post-exercise recovery period on subsequent oxidative stress, muscle damage, and exercise performances in men. Med Gas Res. (2020) 10:155–62. doi: 10.4103/2045-9912.304222
49. Alharbi AAD, Ebine N, Nakae S, Hojo T, Fukuoka Y. Application of molecular hydrogen as an antioxidant in responses to ventilatory and ergogenic adjustments during incremental exercise in humans. Nutrients. (2021) 13:459. doi: 10.3390/nu13020459
50. Botek M, Krejčí J, Valenta M, McKune A, Sládečková B, Konečný P, et al. Molecular hydrogen positively affects physical and respiratory function in acute post-COVID-19 patients: a new perspective in rehabilitation. Int J Env Res Pub Health. (2022) 19:1992. doi: 10.3390/ijerph19041992
51. Liu L, Xie K, Chen H, Dong X, Li Y, Yu Y, et al. Inhalation of hydrogen gas attenuates brain injury in mice with cecal ligation and puncture via inhibiting neuroinflammation, oxidative stress and neuronal apoptosis. Brain Res. (2014) 1589:78–92. doi: 10.1016/j.brainres.2014.09.030
52. Dohi K, Satoh K, Miyamoto K, Momma S, Fukuda K, Higuchi R, et al. Molecular hydrogen in the treatment of acute and chronic neurological conditions: mechanisms of protection and routes of administration. J Clin Biochem Nutr. (2017) 61:1–5. doi: 10.3164/jcbn.16-87
53. Iketani M, Ohsawa I. Molecular hydrogen as a neuroprotective agent. Curr Neuropharmacol. (2017) 15:324–31. doi: 10.2174/1570159X14666160607205417
54. Ishii A, Tanaka M, Watanabe Y. Neural mechanisms of mental fatigue. Rev Neurosci. (2014) 25:469–79. doi: 10.1515/revneuro-2014-0028
55. Lancha Junior AH, Painelli Vde S, Saunders B, Artioli GG. Nutritional strategies to modulate intracellular and extracellular buffering capacity during high-intensity exercise. Sports Med. (2015) 45 Suppl 1:S71–81. doi: 10.1007/s40279-015-0397-5
56. Margaritelis NV, Paschalis V, Theodorou AA, Kyparos A, Nikolaidis MG. Antioxidants in personalized nutrition and exercise. Adv Nutr. (2018) 9:813–23. doi: 10.1093/advances/nmy052
57. Koltai E, Bori Z, Osvath P, Ihasz F, Peter S, Toth G, et al. Master athletes have higher mir-7, sirt3 and sod2 expression in skeletal muscle than age-matched sedentary controls. Redox Biol. (2018) 19:46–51. doi: 10.1016/j.redox.2018.07.022
58. Combes A, Dekerle J, Bougault V, Daussin FN. Physiological comparison of intensity-controlled, isocaloric intermittent and continuous exercise. Eur J Sport Sci. (2018) 18:1368–75. doi: 10.1080/17461391.2018.1491627
59. Combes A, Dekerle J, Webborn N, Watt P, Bougault V, Daussin FN. Exercise-induced metabolic fluctuations influence AMPK, P38-MAPK and camkii phosphorylation in human skeletal muscle. Physiol Rep. (2015) 3:12462. doi: 10.14814/phy2.12462
60. Trewin AJ, Parker L, Shaw CS, Hiam DS, Garnham A, Levinger I, et al. Acute hiie elicits similar changes in human skeletal muscle mitochondrial HO release, respiration, and cell signaling as endurance exercise even with less work. Am J Physiol Regul Integr Comp Physiol. (2018) 315:R1003–r16. doi: 10.1152/ajpregu.00096.2018
61. Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. (2000) 32:70–84. doi: 10.1097/00005768-200001000-00012
62. Coyle EF. Integration of the physiological factors determining endurance performance ability. Exerc Sport Sci Rev. (1995) 23:25–63. doi: 10.1249/00003677-199500230-00004
63. Behl C, Holsboer F. The female sex hormone oestrogen as a neuroprotectant. Trends Pharmacol Sci. (1999) 20:441–4. doi: 10.1016/S0165-6147(99)01392-9
Keywords: molecular hydrogen, fatigue, aerobic capacity, rating of perceived exertion, maximal oxygen uptake
Citation: Zhou K, Liu M, Wang Y, Liu H, Manor B, Bao D, Zhang L and Zhou J (2023) Effects of molecular hydrogen supplementation on fatigue and aerobic capacity in healthy adults: A systematic review and meta-analysis. Front. Nutr. 10:1094767. doi: 10.3389/fnut.2023.1094767
Received: 10 November 2022; Accepted: 16 January 2023;
Published: 02 February 2023.
Edited by:
Marcin Maciejczyk, University of Physical Education in Krakow, PolandReviewed by:
Hassane Zouhal, University of Rennes 2 – Upper Brittany, FranceTakuji Kawamura, University of Physical Education (Budapest), Hungary
Copyright © 2023 Zhou, Liu, Wang, Liu, Manor, Bao, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dapeng Bao,  YmFvZHBAYnN1LmVkdS5jbg==; Luyu Zhang,
YmFvZHBAYnN1LmVkdS5jbg==; Luyu Zhang,  bHV5dV96aGFuZ0Bic3UuZWR1LmNu
bHV5dV96aGFuZ0Bic3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Kaixiang Zhou
Kaixiang Zhou Meng Liu
Meng Liu Yubo Wang
Yubo Wang Haoyang Liu
Haoyang Liu Brad Manor4
Brad Manor4 Dapeng Bao
Dapeng Bao Luyu Zhang
Luyu Zhang Junhong Zhou
Junhong Zhou