- 1Non-Communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 2Student Research Committee, Alborz University of Medical Sciences, Karaj, Iran
- 3Chronic Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Introduction: Non-alcoholic fatty liver disease (NAFLD) results from an excessive accumulation of fat particles that causes liver inflammation, which ultimately causes liver damage. There is still considerable uncertainty about the effects of any nutritional supplements compared to no additional intervention. This review aimed to evaluate the efficacy of Allium sativum (A. sativum), known as garlic, in preventing and treating NAFLD.
Methods: A systematic search based on a search strategy consisting of two components of “NAFLD” and “Allium sativum” in databases including PubMed, Web of Science (WoS), and SCOPUS was conducted on papers evaluating the effects of A. sativum on NAFLD treatment and prevention. We obtained studies from inception until 20 September 2022, followed by study selection and data extraction based on our eligibility criteria. Consequently, qualitative and quantitative synthesis was conducted.
Results: Our qualitative analysis reveals that A. sativum consumption is linked to the prevention of NAFLD, especially in males, although qualitative data in this study regarding the therapeutic properties of NAFLD was controversial. Our meta-analysis showed that NAFLD patients treated with A. sativum have significantly declined aminotransferase levels. That is to say, our meta-analysis revealed a lower alanine transaminase (ALT) (SMD = −0.580, 95%CI = −0.822 to −0.338), and aspartate transaminase (AST(SMD = −0.526, 95%CI = −0.767 to −0.284) in NAFLD patients treated with A. sativum compared to the placebo group. Also, pooling data from case-control studies showed that A. sativum consumption decreases the odds of being diagnosed with NAFLD by 46% (OR = 0.538, 95%CI = 0.451–0.625).
Conclusion: A. sativum consumption is not merely associated with NAFLD prevention but also results in a considerable decline in blood aminotransferase levels in patients diagnosed with NAFLD. To put it simply, A. sativum is linked to a decline in AST and ALT, which are considered reliable biomarkers of NAFLD response to treatment. Nevertheless, A. sativum is insufficient to improve NAFLD independent of other dietary amendments and lifestyle modifications.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is an excessive accumulation of fat particles that causes liver inflammation, which ultimately causes liver damage (1). NAFLD has become increasingly common in parallel with the increasing prevalence of cardiometabolic risk factors such as obesity, dyslipidemia, type 2 diabetes, and other components of metabolic syndrome (2). Although this condition is mainly diagnosed by ultrasonography, which sometimes is required to be verified by biopsy, guidelines strongly recommend physicians measure aminotransferase enzymes levels, including alanine transaminase (ALT) and aspartate transaminase (AST) each 3-6 months to evaluate their patients’ response to treatment (3–5).
It should be noted that not merely NAFLD as a metabolic disturbance independently increases the risk for cardiovascular morbidity and mortality (6–8), but also its progression can consequently lead to hepatocellular carcinoma (HCC), cirrhosis, and liver fibrosis (9).
Although NAFLD can cause severe hepatic sequels, its pathogenesis mainly involves metabolic disturbances. Its progression is closely associated with dyslipidemia and type 2 diabetes. In other words, previous studies demonstrated that insulin resistance increased levels of triglyceride (TG), and decreased levels of high-density lipoprotein cholesterol (HDL-C) are linked to NAFLD development (10).
Based on its development mechanism, the primary method of NAFLD treatment is lifestyle modification, which includes considerable weight loss, nutritional interventions, frequent exercise, and calorie restriction (11, 12). Moreover, some studies proposed anti-diabetic agents such as Pioglitazone and sodium-glucose cotransporter-2 (SGLT2) inhibitors as potential agents to manage NAFLD patients (13, 14). In contrast, some other studies supported the theory that antioxidant therapy using vitamin E supplementation exerts therapeutic effects in NAFLD patients (15, 16). Nevertheless, no drug has received the necessary approval from FDA (Food and Drug Administration) for NAFLD treatment (9), and efforts to discover therapeutic agents are ongoing. Beside clinical recommendations regarding prescribing pioglitazone and vitamin E in NAFLD patients, drugs such as Dapagliflozin, Aramchol, Resmetirom, Semaglutide, and Lanifibranor have shown promising effects and have entered phase III clinical trials (17).
Meantime, to control NAFLD, many traditional herbal formulas with antioxidant effects are getting studied (18–21). Researchers are also evaluating the impact of herbal agents that have shown beneficial effects in metabolic disturbances to treat NAFLD (20).
Allium sativum (A. sativum), known as garlic, has been shown to have well-established therapeutic effects on metabolic disturbances such as dyslipidemia and diabetes (22, 23), as well as notable antioxidant activity (24). In other words, previous studies demonstrated that consuming A. sativum is attributed to an enhanced lipid profile, altered glucose metabolism, and lower blood pressure (25–27). Moreover, A. sativum has shown significant antioxidant activity, which may decrease inflammation in the liver and consequently decrease NAFLD incidence (28). Nonetheless, there is a controversy over the effects of A. sativum on NAFLD patients in clinical practice (29–33). This systematic review aims to evaluate the impact of A. sativum in the prevention and treatment of NAFLD.
2. Materials and methods
This systematic review study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (34).
2.1. Study questions
• Does treatment with A. sativum improve the follow-up results (including ALT and AST levels) in patients previously diagnosed with NAFLD?
• Is consuming more A. sativum decrease the odds of being diagnosed with NAFLD?
2.2. Information sources and search strategy
In the current study, two researchers (MQ and RK) independently conducted the systematic search from inception until September 20, 2022, through PubMed, Scopus, and Web of Science (WoS) databases. The study questions were considered to design the search strategy based on two roots of “NAFLD” and “Allium sativum.” Conflicts were solved by another researcher (PM). Supplementary Table 1 summarizes the search strategy.
2.3. Study selection
We performed the study selection process using EndNote reference management software (EndNote 20.1). After including papers based on the search strategy, we initially removed the duplicated papers. Afterward, the titles and abstracts of the documents were evaluated concerning the eligibility criteria, followed by the screening of the full texts of the documents. Two authors (PM and MQ) conducted the study selection.
2.4. Eligibility criteria
The following criteria for screening the papers
• Records should be a clinical trial, a cohort, or a case-control study that includes patients’ A. sativum consumption level and‘ their prescribed dosage.
• Records should include data on patients’ NAFLD profiles, including diagnostic or follow-up modalities’ results comprising ultrasonographic studies, histological studies, or aminotransferase enzymes level.
• Records should demonstrate a link between A. sativum and patients’ NAFLD-related indices.
• Records can be published in any language. No language restriction was applied.
2.5. Data collection process and data items
We designed data extraction forms that include author name, year, provenance, study design, sample size, participants’ age and gender, exposure (A. sativum) related indices, outcome (NAFLD-related) indices, the measure of association, confounders, and study findings. Two researchers have filled out extraction forms independently (RK And MQ). Conflicts were solved by another researcher (PM).
2.6. Quality assessment (QA)
The quality of cohort and case-control studies was assessed using the Newcastle-Ottawa scale (NOS) statement (35). Moreover, the QA of clinical trials was carried out using the CONsolidated Standards of Reporting Trials (CONSORT) guideline (36). Two researchers independently performed the QA based on the guidelines (RK and MQ).
2.7. Data synthesis
Results are presented as standard mean difference (SMD) and their 95% confidence interval (95% CI). STATA version 14 (StataCorp, College Station, TX, USA) software was used to conduct the meta-analysis. We performed a meta-analysis when two or more than two studies report the same measure that shows the association between A. sativum and patients’ NAFLD-related indices.
Standard mean differences and their 95%CI pooled estimate were measured using the fixed model on the data extracted from the included clinical trials. In contrast, for case-control studies, a random model meta-analysis was conducted.
The Chi-square-based Q test and I2 statistic were used to evaluate heterogeneity and, consequently, identified the model used for analysis. Lack of heterogeneity was defined when the p-value was more than 0.10. Regression and Galbraith analyses were used to identify the source of heterogeneity in studies. Publication bias assessment was carried out using Egger’s regression asymmetry test. Lack of publication bias was defined when the p-value was more than 0.10.
Although it is challenging to interpret and might lead to crucial methodological bias, to pool all studies showing the overall link between A. sativum and NAFLD, we transformed SMDs into ORs based on Cochran handbook 5-1 (part 2: general methods for Cochrane reviews) (37). This process was followed by pooling ORs.
3. Results
3.1. Systematic review results
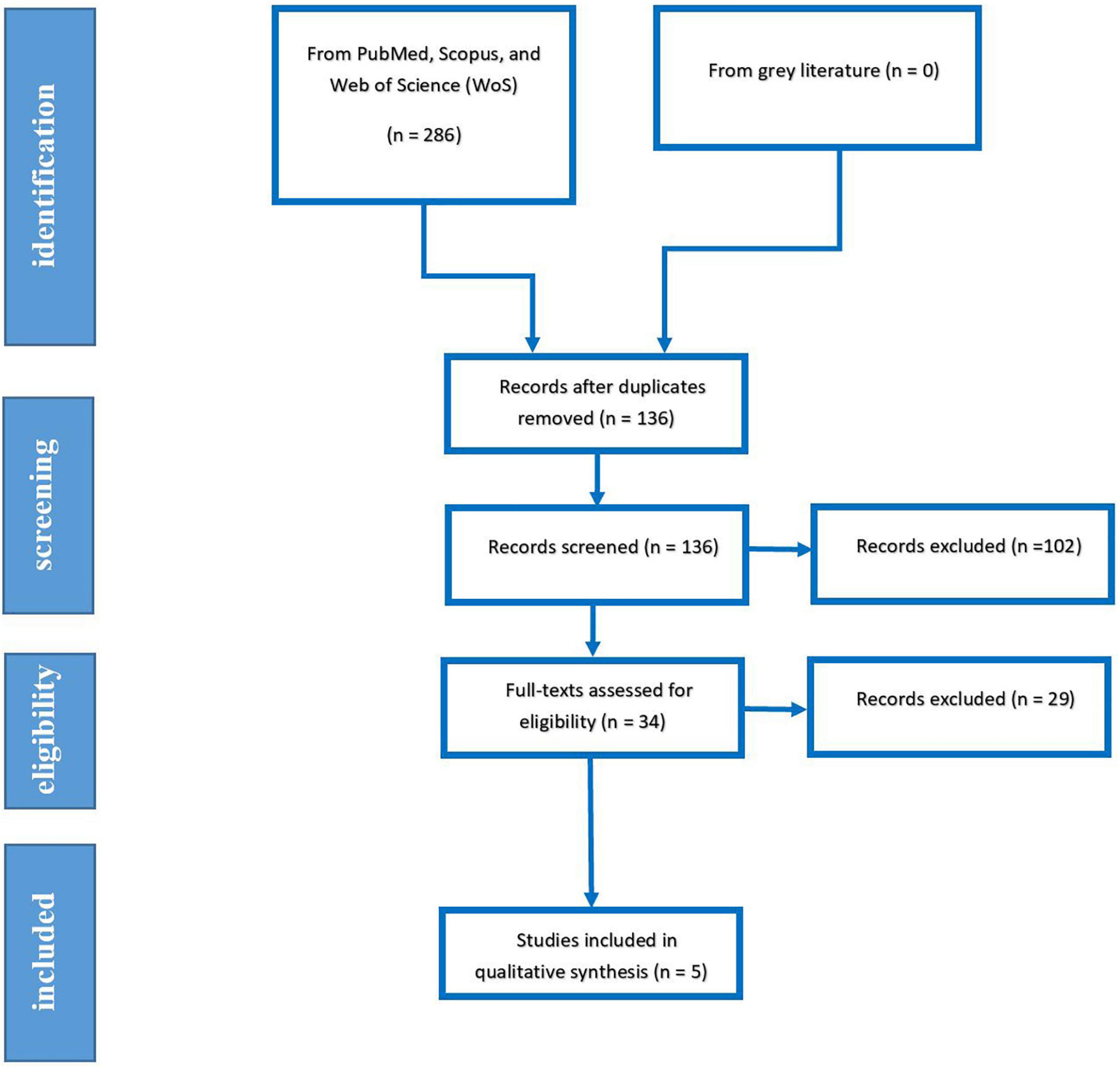
Our systematic search yielded 286 papers, which was eliminated to 136 papers after removing duplicated papers. After evaluating the papers’ title, abstract, and full text, 5 Records, including three RCTs and two case controls, were eventually included in qualitative and quantitative analysis. The PRISMA flow diagram of systematic search is demonstrated in Figure 1.
3.2. Characteristics of included studies
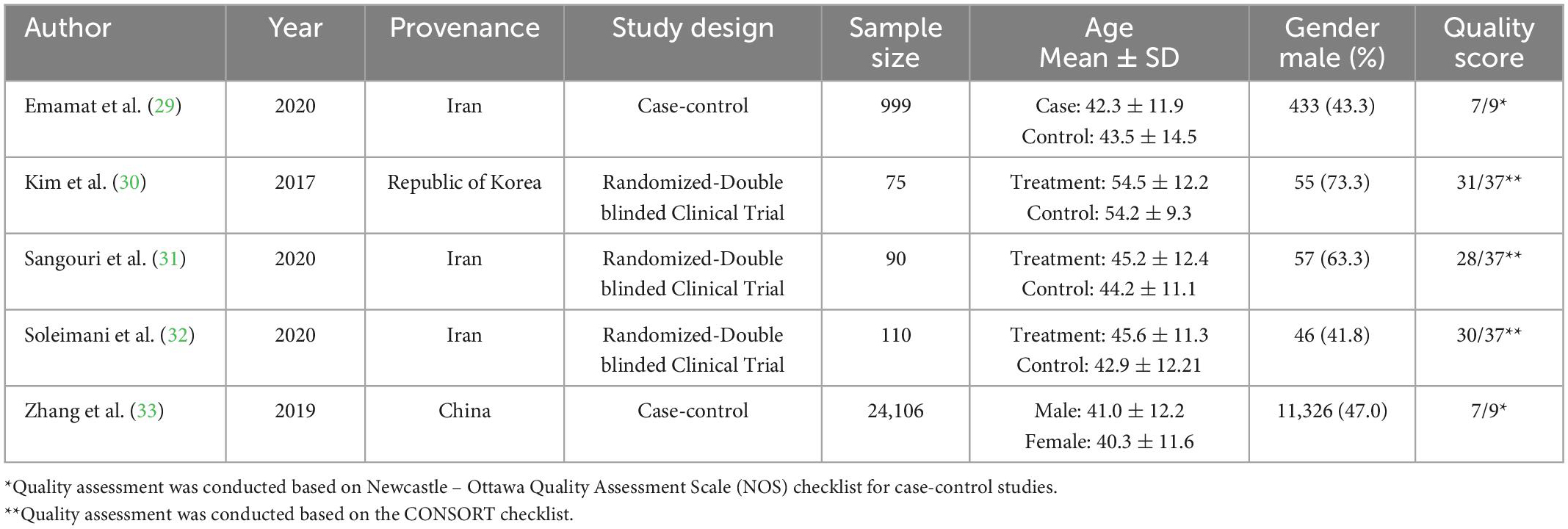
As previously mentioned, two case-control (29, 33) and three randomized controlled trial studies (30–32) were included in the meta-analysis separately. All studies were conducted in Asia, and three out of five included studies were conducted in Iran. Table 1 provides the characteristics of included studies. A total of 25380 participants (11917 males) were assessed in included records.
3.3. Qualitative analysis
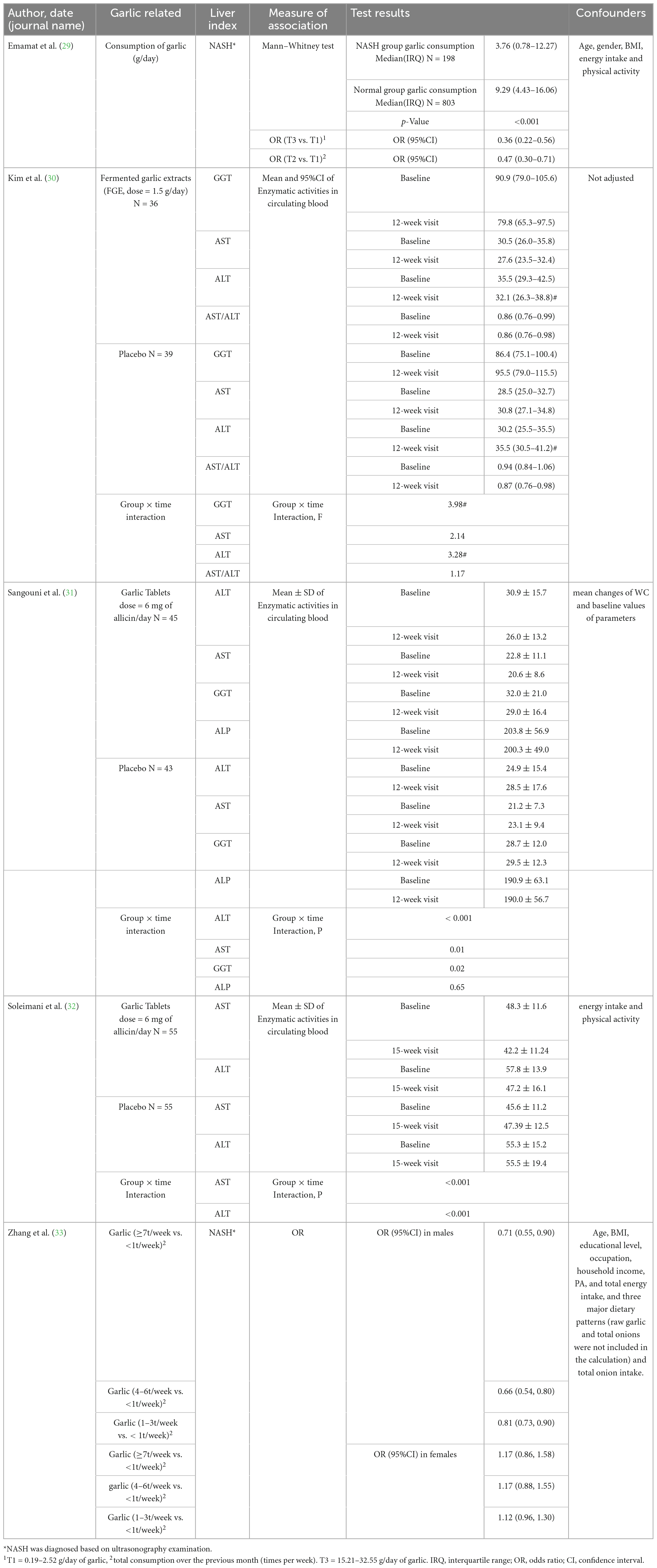
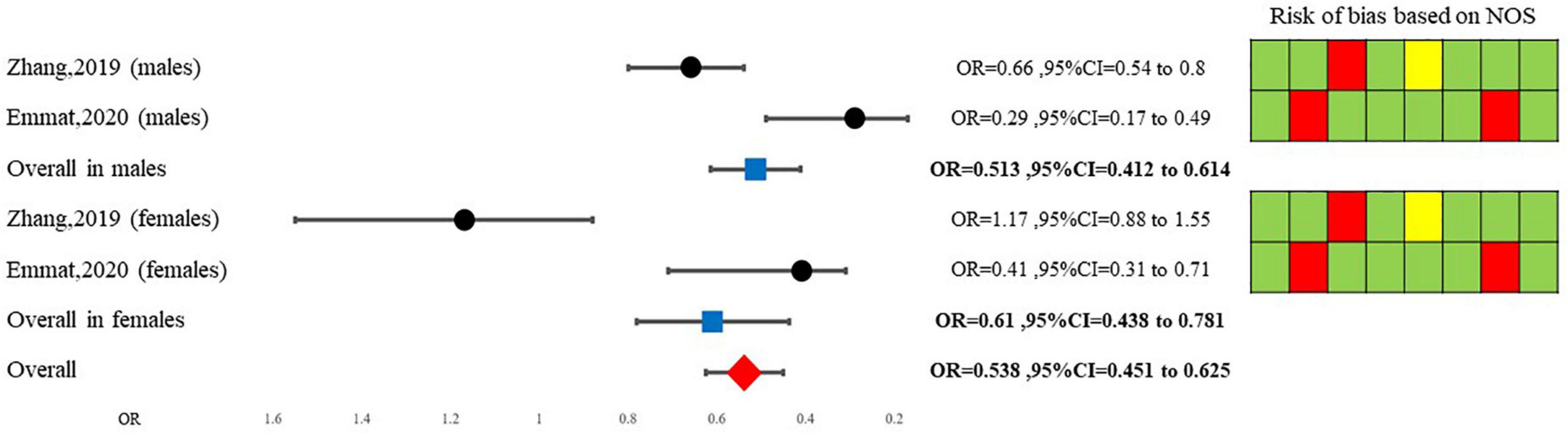
The qualitative analysis of this review comprises two sections; Emamat et al. revealed that patients consuming less A. sativum are at higher odds of being diagnosed with NAFLD based on the ultrasonographic method (29). In contrast, Zhang et al. study revealed that consuming raw garlic more than seven times a week only decreases the odds of NAFLD by 29 (OR = 0.71, 95%CI: 0.55–0.90) percent in males while leading to insignificant findings in females. Notably, their findings were insignificant in females (OR = 1.17, 95%CI: 0.86–1.58) (33). The second section assessed included trials. All randomized clinical trials revealed decreasing properties of A. sativum for ALT level (30–32), while two (31, 32) out of three included clinical trials demonstrated a significant decline in AST following treatment with A. sativum. It should be considered that data regarding GGT were not parallel Table 2.
3.4. Quantitative analysis
3.4.1. Clinical trials (therapeutic properties)
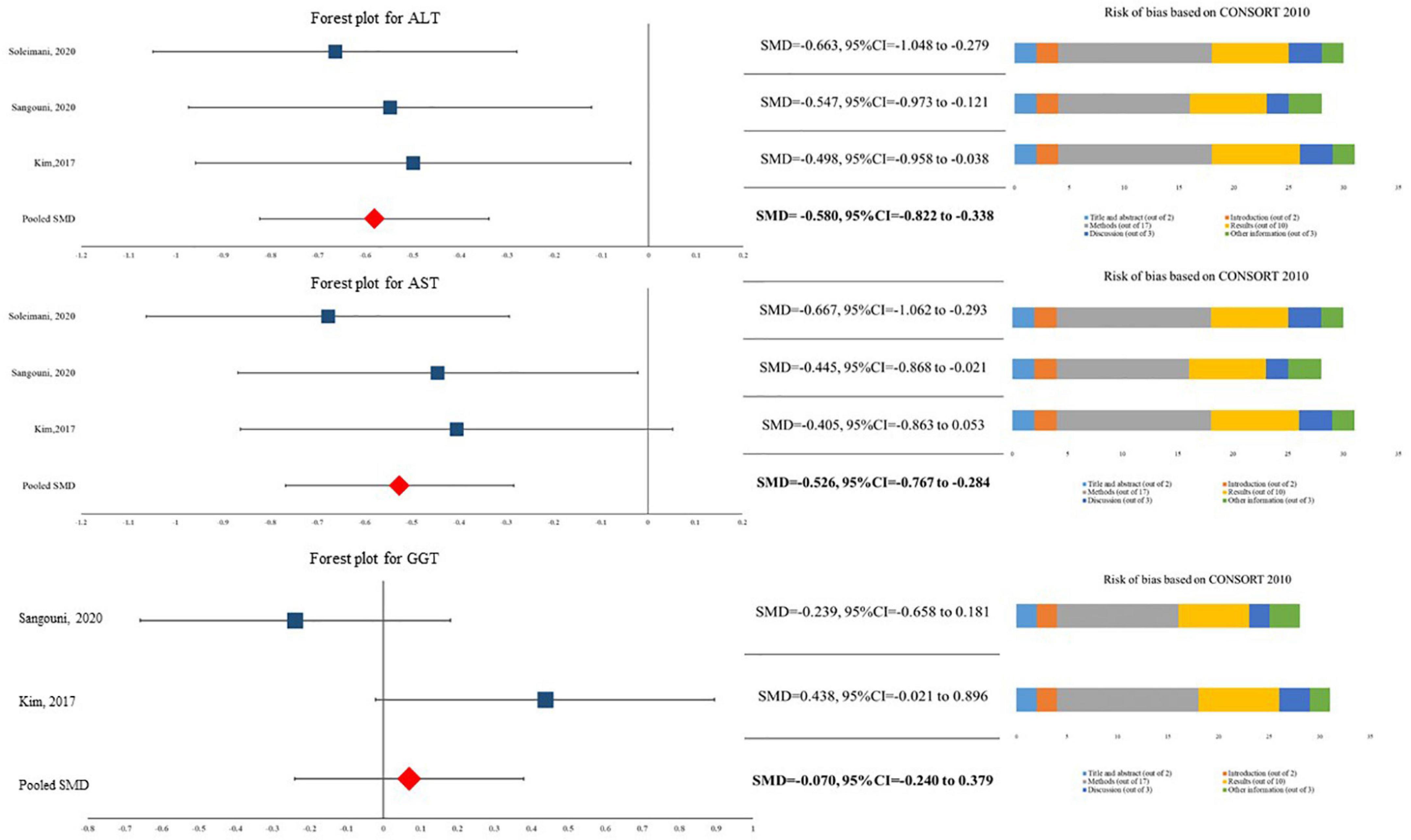
The current meta-analysis showed that patients in A. sativum have significantly 9.479 U/L (95%CI for MD = −13.350 U/L to −5.608 U/L) lower levels of ALT compared to their peers in the placebo group (SMD = −0.580, 95%CI = −0.822 to −0.338). Similarly, our data indicated that A. sativum results in significantly lower levels of AST (SMD = −0.526, 95%CI = −0.767 to −0.284). It should be noted that this quantitative synthesis did not demonstrate a significant difference between patients in the A. sativum group and placebo group in terms of GGT level (SMD = −0.070, 95%CI = −0.240 to −0.379) (Figure 2). As I2 was reported at 0.00% in all three analyses, we assumed heterogeneity was not considerable. Likewise, no evidence of publication bias was reported in Eggers’s test.
3.4.2. Case-controls (preventive properties)
Our random model meta-analysis showed that overall, A. sativum consumption decreases the odds of being diagnosed with NAFLD by 46% (OR = 0.538, 95%CI = 0.451–0.625). Due to high heterogeneity among studies, a Galbraith and a meta-regression analysis were conducted. Initially, Galbraith’s synthesis revealed that Zhang et al. study for females is an outlier study. After its exclusion, our fixed model decreased OR to 0.492 (95%CI = 0.402–0.582). Furthermore, meta-regression analysis showed that while the gender of participants may be a source of heterogeneity, other variables such as age, NAFLD diagnostic method, body mass index, diabetes diagnosis, or lipid profile may not be linked to heterogeneity among studies. Sub-group analysis based on gender is illustrated in Figure 3.
3.4.3. Overall
Current analysis showed a significant link between A. sativum and NAFLD (pooled OR = 0.495, 95%CI: 0.418–0.572).
4. Discussion
In this systematic review and meta-analysis, we analyzed studies that examined the effect of A. sativum consumption on NAFLD. Our systematic review and meta-analysis show that A. sativum extract prescription for NAFLD patients significantly reduces ALT and AST levels. In addition, this study shows that consuming more A. sativum is correlated with a lower risk of being diagnosed with NAFLD.
Although there was a controversy over the clinical effect of A. sativum on NAFLD patients, previous studies revealed possible biological justifications. Various medicinal properties have been reported for A. sativum, including lowering cholesterol and blood pressure, anti-platelet effects, antimicrobial activities, and preventing cancer (38–41). The hepatoprotective effects of A. sativum are due to its water-soluble and fat-soluble compounds. Water-soluble compounds comprise of g-glutamyl S-allyl-cysteine (SAC) group, including SAC and S- allyl-mercapto cysteine (SAMC) and lipid-soluble compounds include allyl sulfur compounds such as diallyl disulfide (DADS) and diallyl trisulfide (DATS) (42, 43). It is demonstrated that SAC induces the AMPK pathway by activating calcium/calmodulin-dependent kinase (CaMKK) and silent information regulator T1 (SIRT1), inhibiting hepatic lipotoxicity and lipogenesis.
Moreover, it can reduce cell death caused by lipid accumulation by suppressing free fatty acid (FFA)-induced reactive oxygen species production and caspase activation (44). The antifibrotic, anti-inflammatory and antioxidant effects of SAMC have been observed in a study by Xiao et al. SAMC alleviates fibrosis by reducing Transforming Growth Factor-β1 and α-Smooth Muscle Actin and inhibiting the activation of hepatic Kupffer cells and hepatic stellate cells (HSCs). SAMC exerts anti-inflammatory effects by lowering the nuclear transcription factors NF-κB and AP-1. SAMC also protects the liver from hepatic oxidative stress by inhibiting cytochrome P450 2E1 and elevating antioxidant enzymes glutathione peroxidase and catalase (45). Furthermore, upregulating the expression of lipolytic genes peroxisome proliferator-activated receptor α and carnitine palmitoyltransferase-1 and down-regulating the expression of lipogenic gene sterol regulatory element-binding protein-1c by DATS reported, which can prevent the progression of NAFLD (46). A recent study by Zhang et al. (43) revealed that DATS could mitigate the profibrogenic process and oxidative stress during NAFLD mediated by Hydrogen Sulfide production in HSCs. To sum up, the composition of A. sativum has played a crucial role in preventing and treating NAFLD.
To illustrate the effectiveness of A. sativum in preventing and treating NAFLD, a comparison of its efficacy with routine and herbal treatments commonly used for NAFLD is vital. Currently, there are no FDA-approved therapies for the disease. However, lifestyle modifications, including dietary changes, weight loss, and increased physical activity, are generally recommended for these patients. Besides, vitamin E and pioglitazone are usually employed to treat patients.
The efficacy of weight loss in improving NAFLD has been shown in several studies. That is to say, in 97% of people who have lost more than 10% of their total body weight, the resolution of the disease occurs. It should be noted that only 10–20% of people can lose more than 10% of their weight in 1–2 years (47). In other words, the success rate of this method is significantly reduced due to its low participation rate. In this respect, treatment with A. sativum may be superior because it is more likely be better tolerated.
On the other hand, vitamin E, which has antioxidant properties, is one of the therapies suggested for fatty liver. Numerous studies have reported that vitamin E, similar to A. sativum, can modulate the aminotransferase level. Vitamin E also improves histological abnormalities; However, no significant effect of this vitamin on liver fibrosis has been observed (48). It should be noted that a cohort study revealed that a six-month course of vitamin E therapy, in contrast to A. sativum consumption, did not alter patients’ metabolic profile, body weight, and lipid and glucose levels (49).
In addition to routine treatments, several natural products have been suggested to control liver steatosis. Utilizing natural products as an elective approach to treating NAFLD has drawn developing attention among doctors. Consequently, researchers are using various pipelines to determine the effects of natural products to control NAFLD. Many of these products are being assessed in both animal and human subjects (17, 50).
Similar to the current study, Goodarzi et al. (51) demonstrated a significant decline in the serum concentrations of ALT and AST following turmeric/curcumin supplementation. Silymarin also significantly reduces levels of ALT and AST. However, it does not affect BMI (52). Although resveratrol administration in animal studies has reduced inflammation and liver steatosis (53), there is a controversy over its effects on human participants. While some studies have found Resveratrol to be effective in reducing liver enzymes as well as metabolic parameters (54), others have shown no significant effect on either aminotransferases or metabolic factors (55, 56).
Conversely, there are no such controversies over the effects of A. sativum on NAFLD. A recent study showed that baseline health status is a significant confounder in the outcome of green tea on liver enzyme and bilirubin levels. While green tea decreases liver enzymes level in NAFLD patients, it leads to a notable increase in liver enzymes in healthy individuals (57). Therefore, green tea, unlike A. sativum, could not be a suitable option for prevention.
In this systematic review, we included not merely the frequency of patients diagnosed with NAFLD but also their aminotransferase level (58). Although liver biopsy is the gold standard for diagnosing NAFLD, AST and ALT levels are suggested for follow-up of patients previously diagnosed (59). It is shown that the proportion of NAFLD patients with normal ALT is significantly lower than individuals with elevated ALT levels. However, the aminotransferase levels could not predict advanced fibrosis in NAFLD patients (60, 61). Nevertheless, the need for further studies to determine the effect of A. sativum on the fatty liver by evaluating more specific outcomes, such as biopsies, to confirm the current results is undeniable.
This study had several limitations; The number of included studies and, consequently, the overall sample size were limited in the current study. There was also a difference in the design of included studies. It should be considered that the duration of treatments in RCTs varied from 12 to 15 weeks. More importantly, different studies used different doses of A. sativum, which makes it challenging to mirror the findings of the current review to clinical practice.
Not merely consuming more A. sativum is attributed to lower odds of being diagnosed with NAFLD, but also consuming garlic significantly decreases AST and ALT levels in patients previously diagnosed with NAFLD. In other words, along with other dietary amendments and lifestyle modifications, A. sativum can be used in two clinical perspectives and approaches. First, it can be prescribed for people with NAFLD to slow down the progression of the disease. Second, taking A. sativum as prophylaxis in high-risk participants decreases the odds of being diagnosed with NAFLD.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
PM participated in drafting the manuscript, participated in the systematic search, and conducted the analysis. RK participated in the systematic search. RF participated in drafting the manuscript. MQ participated in a systematic search, revised the manuscript, and supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Alborz University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1059106/full#supplementary-material
References
1. Younossi Z, Tacke F, Arrese M, Sharma BC, Mostafa I, Bugianesi E, et al. Global perspectives on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Hepatology. (2019) 69:2672–82. doi: 10.1002/hep.30251
2. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Non-alcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. (2003) 37:917–23. doi: 10.1053/jhep.2003.50161
3. Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. (2013) 28:64–70. doi: 10.1111/jgh.12271
4. Rinella ME, Loomba R, Caldwell S, Kowdley K, Charlton M, Tetri B, et al. Controversies in the diagnosis and management of NAFLD and NASH. Gastroenterol Hepatol. (2014) 10:219.
5. Rinella ME, Lominadze Z, Loomba R, Charlton M, Neuschwander-Tetri B, Caldwell S, et al. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Ther Adv Gastroenterol. (2016) 9:4–12. doi: 10.1177/1756283X15611581
6. Satapathy SK, Jiang Y, Eason JD, Kedia SK, Wong E, Singal AK, et al. Cardiovascular mortality among liver transplant recipients with non-alcoholic steatohepatitis in the United States—a retrospective study. Transplant Int. (2017) 30:1051–60. doi: 10.1111/tri.13001
7. Sanyal AJ. NASH: a global health problem. Hepatol Res. (2011) 41:670–4. doi: 10.1111/j.1872-034X.2011.00824.x
8. Patil R, Sood GK. Non-alcoholic fatty liver disease and cardiovascular risk. World J Gastrointest Pathophysiol. (2017) 8:51–8. doi: 10.4291/wjgp.v8.i2.51
9. Rinella ME. Non-alcoholic fatty liver disease: a systematic review. JAMA. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
10. Amor AJ, Perea V. Dyslipidemia in non-alcoholic fatty liver disease. Curr Opin Endocrinol Diabetes Obes. (2019) 26:103–8. doi: 10.1097/MED.0000000000000464
11. Neuschwander-Tetri BA. Lifestyle modification as the primary treatment of NASH. Clin Liver Dis. (2009) 13:649–65. doi: 10.1016/j.cld.2009.07.006
12. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of non-alcoholic steatohepatitis. Gastroenterology. (2015) 149:367–78. doi: 10.1053/j.gastro.2015.04.005
13. Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. (2019) 1:312–28. doi: 10.1016/j.jhepr.2019.07.002
14. Rowe IA, Wong VW-S, Loomba R. Treatment candidacy for pharmacologic therapies for NASH. Clin Gastroenterol Hepatol. (2021) 20:1209–17. doi: 10.1016/j.cgh.2021.03.005
15. Ji H-F, Sun Y, Shen L. Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: results from a meta-analysis. Nutrition. (2014) 30:986–91. doi: 10.1016/j.nut.2014.01.016
16. Perumpail BJ, Li AA, John N, Sallam S, Shah ND, Kwong W, et al. The role of vitamin E in the treatment of NAFLD. Diseases. (2018) 6:86. doi: 10.3390/diseases6040086
17. Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for non-alcoholic fatty liver disease. Metabolism. (2022) 126:154925. doi: 10.1016/j.metabol.2021.154925
18. Yan T, Yan N, Wang P, Xia Y, Hao H, Wang G, et al. Herbal drug discovery for the treatment of non-alcoholic fatty liver disease. Acta Pharm Sin B. (2020) 10:3–18. doi: 10.1016/j.apsb.2019.11.017
19. Jadeja R, Devkar RV, Nammi S. Herbal medicines for the treatment of non-alcoholic steatohepatitis: current scenario and future prospects. Evid Based Complement Alternat Med. (2014) 2014:648308. doi: 10.1155/2014/648308
20. Xu Y, Guo W, Zhang C, Chen F, Tan HY, Li S, et al. Herbal medicine in the treatment of non-alcoholic fatty liver diseases-efficacy, action mechanism, and clinical application. Front Pharmacol. (2020) 11:601. doi: 10.3389/fphar.2020.00601
21. Sandhu N, Au J. Herbal medicines for the treatment of nonalcoholic steatohepatitis. Curr Hepatol Rep. (2021) 20:1–11. doi: 10.1007/s11901-020-00558-2
22. Chan W-JJ, McLachlan AJ, Luca EJ, Harnett JE. Garlic (Allium sativum L.) in the management of hypertension and dyslipidemia–A systematic review. J Herb Med. (2020) 19:100292. doi: 10.1016/j.hermed.2019.100292
23. Seo Y-J, Gweon O, Im J, Lee YM, Kang M, Kim J. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. Prev Nutr Food Sci. (2009) 14:1–7. doi: 10.3746/jfn.2009.14.1.001
24. Farhat Z, Hershberger PA, Freudenheim JL, Mammen MJ, Blair RH, Aga DS, et al. Types of garlic and their anticancer and antioxidant activity: a review of the epidemiologic and experimental evidence. Eur J Nutr. (2021) 60:3585–609. doi: 10.1007/s00394-021-02482-7
25. Sun Y-E, Wang W, Qin J. Anti-hyperlipidemia of garlic by reducing the level of total cholesterol and low-density lipoprotein: a meta-analysis. Medicine. (2018) 97:e0255–0255. doi: 10.1097/MD.0000000000010255
26. Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J Endocrinol Invest. (2015) 38:1147–57. doi: 10.1007/s40618-015-0313-8
27. Sangouni AA, Alizadeh M, Jamalzehi A, Parastouei K. Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: a randomized clinical trial. Phytother Res. (2021) 35:4433–41. doi: 10.1002/ptr.7146
28. Rahman M, Fazlic V, Saad N. Antioxidant properties of raw garlic (Allium sativum) extract. Int Food Res J. (2012) 19:589–91.
29. Emamat H, Farhadnejad H, Tangestani H, Saneei Totmaj A, Poustchi H, Hekmatdoost A. Association of allium vegetables intake and non-alcoholic fatty liver disease risk: a case-control study. Nutr Food Sci. (2020) 50:1075–83. doi: 10.1108/NFS-11-2019-0334
30. Kim H, Kang S, Roh YK, Choi M, Song S. Efficacy and safety of fermented garlic extract on hepatic function in adults with elevated serum gamma-glutamyl transpeptidase levels: a double-blind, randomized, placebo-controlled trial. Eur J Nutr. (2017) 56:1993–2002. doi: 10.1007/s00394-016-1318-6
31. Sangouni AA, Mohammad Hosseini Azar MR, Alizadeh M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: a double-blind randomised controlled clinical trial. Br J Nutr. (2020) 124:450–6. doi: 10.1017/S0007114520001403
32. Soleimani D, Paknahad Z, Rouhani MH. Therapeutic effects of garlic on hepatic steatosis in nonalcoholic fatty liver disease patients: a randomized clinical trial. Diabetes Metab Syndr Obes. (2020) 13:2389–97. doi: 10.2147/DMSO.S254555
33. Zhang S, Gu Y, Wang L, Zhang Q, Liu L, Lu M, et al. Association between dietary raw garlic intake and newly diagnosed non-alcoholic fatty liver disease: a population-based study. Eur J Endocrinol. (2019) 181:591–602. doi: 10.1530/EJE-19-0179
34. Moher D, Altman D, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. (2011) 22:128. doi: 10.1097/EDE.0b013e3181fe7825
35. Wells G, et al. Newcastle-Ottawa Quality Assessment Scale Cohort Studies. Ottawa, ON: University of Ottawa (2014).
36. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. (2010) 152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
38. National Library of Medicine (Us). Garlic, in Drugs and Lactation Database (LactMed). Bethesda, MD: National Library of Medicine (US) (2006).
39. Adaki S, Adaki R, Shah K, Karagir A. Garlic: review of literature. Indian J Cancer. (2014) 51:577–81. doi: 10.4103/0019-509X.175383
40. Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. (1999) 1:125–9. doi: 10.1016/S1286-4579(99)80003-3
41. Asgharpour M, Khavandegar A, Balaei P, Enayati N, Mardi P, Alirezaei A, et al. Efficacy of oral administration of allium sativum powder “Garlic Extract” on Lipid profile, inflammation, and cardiovascular indices among hemodialysis patients. Evid Based Complement Alternat Med. (2021) 2021:6667453. doi: 10.1155/2021/6667453
42. Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets. (2003) 3:67–81. doi: 10.2174/1568009033333736
43. Zhang F, Jin H, Wu L, Shao J, Zhu X, Chen A, et al. Diallyl trisulfide suppresses oxidative stress-induced activation of hepatic stellate cells through production of hydrogen sulfide. Oxid Med Cell Longev. (2017) 2017:1406726. doi: 10.1155/2017/1406726
44. Hwang YP, Kim HG, Choi JH, Do MT, Chung YC, Jeong TC, et al. S-allyl cysteine attenuates free fatty acid-induced lipogenesis in human HepG2 cells through activation of the AMP-activated protein kinase-dependent pathway. J Nutr Biochem. (2013) 24:1469–78. doi: 10.1016/j.jnutbio.2012.12.006
45. Xiao J, Ching YP, Liong EC, Nanji AA, Fung ML, Tipoe GL. Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. Eur J Nutr. (2013) 52:179–91. doi: 10.1007/s00394-012-0301-0
46. Lai Y, Chen W, Ho C, Lu K, Lin S, Tseng H, et al. Garlic essential oil protects against obesity-triggered non-alcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J Agric Food Chem. (2014) 62:5897–906. doi: 10.1021/jf500803c
47. Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. (2019) 1:468–79. doi: 10.1016/j.jhepr.2019.10.008
48. Usman M, Bakhtawar N. Vitamin E as an adjuvant treatment for non-alcoholic fatty liver disease in adults: a systematic review of randomized controlled trials. Cureus. (2020) 12:e9018. doi: 10.7759/cureus.9018
49. Kim GH, Chung JW, Lee JH, Ok KS, Jang ES, Kim J, et al. Effect of vitamin E in non-alcoholic fatty liver disease with metabolic syndrome: a propensity score-matched cohort study. Clin Mol Hepatol. (2015) 21:379–86. doi: 10.3350/cmh.2015.21.4.379
50. Tarantino G, Balsano C, Santini S, Brienza G, Clemente I, Cosimini B, et al. It is high time physicians thought of natural products for alleviating NAFLD. Is there sufficient evidence to use them? Int J Mol Sci. (2021) 22:13424. doi: 10.3390/ijms222413424
51. Goodarzi R, Sabzian K, Shishehbor F, Mansoori A. Does turmeric/curcumin supplementation improve serum alanine aminotransferase and aspartate aminotransferase levels in patients with non-alcoholic fatty liver disease? A systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2019) 33:561–70. doi: 10.1002/ptr.6270
52. Kalopitas G, Antza C, Doundoulakis I, Siargkas A, Kouroumalis E, Germanidis G, et al. Impact of Silymarin in individuals with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Nutrition. (2021) 83:111092. doi: 10.1016/j.nut.2020.111092
53. Perumpail BJ, Li A, Iqbal U, Sallam S, Shah N, Kwong W, et al. Potential therapeutic benefits of herbs and supplements in patients with NAFLD. Diseases. (2018) 6:80. doi: 10.3390/diseases6030080
54. Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis. (2015) 47:226–32. doi: 10.1016/j.dld.2014.11.015
55. Wei S, Yu X. Efficacy of resveratrol supplementation on liver enzymes in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Complement Ther Med. (2021) 57:102635. doi: 10.1016/j.ctim.2020.102635
56. Elgebaly A, Radwan IA, AboElnas MM, Ibrahim HH, Eltoomy MF, Atta AA, et al. Resveratrol supplementation in patients with non-alcoholic fatty liver disease: systematic review and meta-analysis. J Gastrointestin Liver Dis. (2017) 26:59–67. doi: 10.15403/jgld.2014.1121.261.ely
57. Mahmoodi M, Hosseini R, Kazemi A, Ofori-Asenso R, Mazidi M, Mazloomi S. Effects of green tea or green tea catechin on liver enzymes in healthy individuals and people with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized clinical trials. Phytother Res. (2020) 34:1587–98. doi: 10.1002/ptr.6637
58. Tsai E, Lee TP. Diagnosis and evaluation of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, including noninvasive biomarkers and transient elastography. Clin Liver Dis. (2018) 22:73–92. doi: 10.1016/j.cld.2017.08.004
59. Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in non-alcoholic steatohepatitis. Am J Gastroenterol. (2001) 96:519–25. doi: 10.1111/j.1572-0241.2001.03553.x
60. Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. (2013) 33:1398–405. doi: 10.1111/liv.12226
Keywords: A. sativum, NAFLD, fatty liver, NASH, garlic (A. sativum)
Citation: Mardi P, Kargar R, Fazeli R and Qorbani M (2023) Allium sativum: A potential natural compound for NAFLD prevention and treatment. Front. Nutr. 10:1059106. doi: 10.3389/fnut.2023.1059106
Received: 16 October 2022; Accepted: 09 January 2023;
Published: 02 February 2023.
Edited by:
Domenico Sergi, University of Ferrara, ItalyReviewed by:
Krystyna Winiarczyk, Maria Curie-Skłodowska University, PolandGiovanni Tarantino, University of Naples Federico II, Italy
Copyright © 2023 Mardi, Kargar, Fazeli and Qorbani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mostafa Qorbani,  bXFvcmJhbmkxMzc5QHlhaG9vLmNvbQ==
bXFvcmJhbmkxMzc5QHlhaG9vLmNvbQ==
 Parham Mardi
Parham Mardi Reza Kargar
Reza Kargar Ramina Fazeli2
Ramina Fazeli2 Mostafa Qorbani
Mostafa Qorbani