
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 01 March 2023
Sec. Nutrition and Sustainable Diets
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1052086
This article is part of the Research TopicIndigenous Food Systems to Address Food Security and Nutritional StatusView all 6 articles
 Hammylliende Talang1†
Hammylliende Talang1† Aabon Yanthan2†
Aabon Yanthan2† Ranbir Singh Rathi3
Ranbir Singh Rathi3 Kanakasabapathi Pradheep3
Kanakasabapathi Pradheep3 Soyimchiten Longkumer3
Soyimchiten Longkumer3 Bendangla Imsong2
Bendangla Imsong2 Laishram Hemanta Singh4
Laishram Hemanta Singh4 Ruth S. Assumi1
Ruth S. Assumi1 M. Bilashini Devi1
M. Bilashini Devi1 Vanlalruati1
Vanlalruati1 Ashok Kumar3
Ashok Kumar3 Sudhir Pal Ahlawat3
Sudhir Pal Ahlawat3 Kailash C. Bhatt3*
Kailash C. Bhatt3* Rakesh Bhardwaj3*
Rakesh Bhardwaj3*Introduction: India’s north-eastern hill region (NEH) is one of the biodiversity hotspots, inhabited by several tribal communities still maintaining their traditional food habits. Much of their food resources are drawn from wild sources.
Materials and methods: Fourteen species of wild edible plants of high ethnic importance were collected from remote localities of Nagaland and Meghalaya states of the NEH region of India for nutritional profiling. Nutritional profiling of leaves of six species comprising Gynura cusimbua, Garcinia cowa, Herpetospermum operculatum, Plukenetia corniculata, Trichodesma khasianum, and Elatostemma sessile is conducted first time under present study. Samples were analyzed as per the Official Method of Analysis (AOAC) and standard methods.
Results and discussion: The range of variation in proximate composition was observed for moisture (72–92%), protein (1.71–6.66%), fat (0.22–1.36%), dietary fibre (5.16–14.58%), sugar (0.30–3.41%), and starch (0.07–2.14%). The highest protein content (6.66%) was recorded in Herpetospermum operculatum, followed by Trichodesma khasianum (5.89%) and Plukenetia corniculata (5.27%). Incidentally, two of these also have high iron (>7.0 mg/100 g) and high zinc (>2.0 mg/100 g) contents, except Trichodesma khasianum, which has low zinc content. High antioxidant activities in terms of gallic acid equivalent (GAE) by the cupric ion reducing antioxidant capacity (CUPRAC) method ranged from 1.10 to 8.40 mg/100 g, and by the Fluorescence recovery after photobleaching (FRAP) method ranged from 0.10 to 1.9 mg/100 g, while phenol content ranged between 0.30 and 6.00 mg/100 g. These wild vegetables have high potential because of their nutritional properties and are fully capable of enhancing sustainability and improving ecosystem services. Efforts were also initiated to mainstream these resources, mainly for widening the food basket of native peoples.
The north-eastern hill (NEH) region of India comprising the states of Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura, and Sikkim lies between 21°50’ and 29°34’N latitude and 85°34’ and 97°50’E longitude, is one of the biodiversity hotspots of the world. The region’s diverse topography, altitude, soil, and climate are favorable for the evolution and development of different wild species (1). About 800 different species of wild edible plants have been reported in India. Around 300 species are utilized by the tribal population of the NEH region for food and medicinal purposes (2–5). Generally, these plants grow in wild and semi-wild conditions, are less known to the mass population, and have lesser market demand.
Wild edible plants (WEPs’) are an essential component of ethnic foods. They are integrated with the culture and tradition of many indigenous communities worldwide, serving as a staple food for indigenous people and as a supplement to non-indigenous people’s diets (6). They are a rich source of micro-nutrients used in treating several diseases (7). When analyzed for nutritional value, many WEPs have more vitamins (A and C) and folic acid and exhibit remarkable antioxidant properties than common vegetables (8–10).
Inventory of such wild food resources, associated indigenous knowledge, mode of usage, and present status, coupled with nutritional evaluation, can establish an alternative to achieve food and nutritional security up to a large extent. Recently, the demand for healthy food and increasing health consciousness among the masses has created renewed global interest in utilizing wild food plants. Efforts were also initiated to mainstream these resources, mainly for widening the food basket of native peoples (8).
In NEH region, the inhabitants live in and around forest areas, and practice shifting cultivation, where several crops are cultivated. Besides, inhabitants gather wild edible plants from community forests and some are also grown in kitchen gardens/homesteads. These communities follow traditional processing and cooking methods to make them palatable and suitable for consumption by children, adults, pregnant and lactating women, and ill persons using traditional wisdom. The region is also known for its diverse food basket, which is also indicated in dietary diversity, an indicator of adequate micronutrients in food intake. In the national family health survey (NFHS-5) India survey 2019–2021, the criteria of feeding the child food from at least four food groups were considered as minimum dietary diversity (MDD). Results on MDD have shown contrasting values for the NEH region and the rest of India. NEH region of India has 38% of youngest children aged 6–23 months living with their mother meeting MDD, while the average value for India is 24.1%. This is also indicated in other health parameters particularly the prevalence of any anemia (hemoglobin < 11.0 g/dl) in children of 06–59 months where 67.1% are anemic in India. However, rich dietary diversity has contributed to lower incidences (50.6%) in the NEH region. Similarly, among women of age, 15–49 years country average is 57.0% whereas for the NEH region is 51.9 (11).
NFHS-5 findings are also an indicator of the role and nutritional potential of wild edible plants, however, that cannot be accounted for in dietary intake due to the lack of nutrient composition data for these resources. Thus an attempt has been made to record their edible value and estimate their nutritional properties. This study will help assess these WEPs contribution to nutrient intake and determine their potential to improve the socio-economic status of local communities in the NEH region.
This study provides robust data for important wild edible plants of the NEH region, which were collected thrice during 2016–2019 on edible proportion, proximate composition, total sugars, total starch, total phenols, antioxidant potential, and important minerals. However, in this study, we could not include the estimation of vitamins, oxalates, and profiles for polyphenols, amino acids, fatty acids, phytosterols, sugars, and oligosaccharides.
For gathering information on indigenous technical knowledge which includes local names, uses, parts used and methods of processing wild edible plants, experienced elderly persons were selected as respondents and in the presence of local guides, they were asked open-ended questions in the local dialect (12). Questions in the interview schedule include traditional knowledge (TK) about local edible plant species, the habitat of edible plant species, the economic value of species, and cultural and livelihood dimensions of the foods. Based on the responses from the respondents, indigenous traditional knowledge on wild edible plants available in the study areas was collected and documented.
A total of 10 field surveys were carried out in parts of Nagaland and Meghalaya states four each in 2016 and 2018 and two in 2019 in different seasons to identify, collect and document the local plant species used in the tribal foods of North Eastern Hill (NEH) region. Based on the inhabitants’ information and consulting regional floras and expert taxonomists based at NBPGR, New Delhi and Botanical Survey of India, Eastern Circle, Shillong, fourteen wild edible plants, namely, Gynura cusimbua, Centella asiatica, Diplazium esculentum, Garcinia cowa, Eryngium foetidum, Zanthoxylum rhetsa, Houttuynia cordata, Clerodendrum glandulosum, Herpetospermum operculatum, Plukenetia corniculata, Trichodesma khasianum, Piper pedicellatum, Litsea cubeba, Elatostemma sp., were identified and prioritized for their nutritional profiling.
The samples were washed initially with tap water to remove any adhering substances, followed by double distilled water, while surface water was removed by spreading evenly under a fan. Finally, the samples were evaluated in duplicate by following AOAC (13); moisture (934.01), ash (938.08), crude fat (920.58) by gravimetric method; total dietary fiber (985.29) enzymatic gravimetric method; total protein (2011.11) by kjeldahl method using jones factor 6.25, total starch (996.11) enzymatic colorimetric assay, and mineral profiles by atomic absorption spectrometry (AAS, 999.11). The total soluble sugars (14), total phenols (15), ferric-reducing antioxidant potential (16), and cupric-reducing antioxidant capacity (17) were analyzed as explained in Singh et al. (8), including method validation to ensure data quality. Results were analyzed for bivariate Pearson correlation using SPSS17.
Traditional communities of the NEH region have considerable dependence on wild edible plants and have valued them based on experiential learning for food and medicinal value. In this study, we have presented only those claims w.r.t. medicinal value which is part of common knowledge in multiple communities, confirmed by traditional healers, and supported through published literature. Wide variation was observed in the nutritional value of these resources such as in the content of protein 1.6–6.7%, iron 1.86–9.4 mg/100 g, total phenols, and antioxidant potential. Results from this study are discussed below.
Many indigenous communities worldwide rely on wild edible plants as part of their diet (18, 19), and as a potential source of bioactive compounds, colors, and flavors (20). Traditional knowledge on the recipe, food, and medicinal uses was recorded from the tribal people of Nagaland and Meghalaya. The uses and method of processing, botanical name, family, growth habit, habitat, flowering and fruiting season, and edible parts of selected plants under the study are presented in Supplementary Table 1. All fourteen plants are used locally for vegetables, salad, and medicinal purposes. The juice of the stem and leaves of Gynura cusimbua (D.Don) S.Moore is applied on fresh wounds to stop bleeding and fast healing; the leaf paste is also applied on the forehead to relieve headache and is also used for deworming and analgesic drug by the local people (21, 22). Eating of fresh and dry root of Diplazium esculentum (Retz.) Sw. cures dysentery (23). The sundried slices of fruits of Garcinia cowa Roxb. ex Choicy are used for garnishing vegetable-based curries (24). It is also the source of a natural diet ingredient hydroxycitric acid (HCA) which is an anti-obesity compound (25). Eryngium foetidum L. is an important spice as well as culinary herb used to garnish, marinate, flavor, and season different cuisines (26). Young leaves of Zanthoxylum rhetsa DC. are used as a vegetable. At the same time, fruit and stem bark are aromatic, stimulant, astringent, and prescribed for stomach aches, digestive disorders, urinary diseases, dyspepsia, diarrhea, and with honey in rheumatism (27). Local markets in the region sell Clerodendrum glandulosum Lindl. for various purposes, including household consumption as a vegetable stew (28); leaves are used as a therapeutic agent against diabetes, obesity, and hypertension (29–31). People from Mon district of Nagaland preferred Herpetospermum operculatum for soup preparation. Tender inflorescences of Trichodesma khasianum C.B. Clarke are cooked and chopped into pieces to make chutney with dried or fermented fish (32). Leaves and tender shoots of P. pedicellatum are used as vegetables by the people of Sikkim, Arunachal Pradesh, and the tribes of Manipur (33). Litsea cubeba Blume is used by the tribal people of the region as a spice and as medicine. Tender leaves of Elatostemma sessile are cooked as vegetables by the Monpa community of Arunachal Pradesh (34). There is considerable variability in the wild edible plants of the NEH region, which can be tapped for nutritional, socio-economic, ecological, and livelihood improvement of the tribal people of the region.
Significant variations were observed in selected wild edible plants’ nutritional quality. Results are presented (Table 1) as 3 years mean to account for temporal and spatial variations. The result of the analysis revealed that the edible part ranged between 54.1 and 98.9%, with the highest value in Centella asiatica (98.92%), followed by Litsea cubeba (83.4%) and Herpetospermum operculatum (82.4%). The moisture content ranged between 72.2 and 91.8%, with the highest value in Eryngium foetidum (91.8%) and gave significant negative correlation with most of the nutritional traits as moisture act as a diluent (Table 2). The high moisture content in leafy vegetables significantly impacts energy density (amount of energy in a given food weight). Water adds substantial weight to food without energy and provides better satiety (35). Different authors have reported similar moisture content in some wild edible plants, such as 60–68% in Litsea cubeba (36); 56.2, and 56.9% in Clerodendrum sp. and Zanthoxylum acanthopodium, respectively, (37); 79.3, 85.2, 82.2% in Clerodendrum glandulosum, Diplazium esculentum, and Zanthoxylum rhetsa, respectively, (38). An inorganic residue that reflects the entire amount of minerals in food is ash (39). A higher ash concentration predicts the existence of a variety of mineral components (40). Ash concentration ranged from 0.99 to 4.95%, with Elatostema sp. (4.95%) having the highest value and Garcinia cowa having the lowest (0.99%). The above-cited results are at par with the reported value of 6.08% ash content in Houttuynia cordata and 7.2% in Zanthoxylum acanthopodium (36). Similarly, Singh et al. (8) have also reported ash content of 0.87% in Eryngium foetidum, 1.91% in Diplazium esculentum, 2.45% in Gynura cusimbue, and 4.05% in Solanum spirale. Protein helps build and maintain whole-body tissue and forms an essential part of enzymes, fluids, and hormones. It also helps form antibodies to fight against the infection, supply energy, and build the body (41). Protein content ranged between 1.71 and 6.66%, with the highest value in Herpetospermum operculatum (6.66%) and the lowest in Elatostema sp. (1.71%). Other plants which have also depicted rich protein contents are Trichodesma khasianum (5.89%) and Plukenetia corniculata (5.27%). Protein was found to have a significant positive correlation with total phenols, antioxidant activity indicator CUPRAC, and FRAP, iron, and magnesium content (Table 2). Protein content in the range of about 6% in leaves on a fresh weight basis is also reported for Gynura nepalensis, Pouzolzia zeylanica and upto 9% in Sauropus androgynus (8). The crude protein content of 18.5 g/100 g in Diplazium esculentum on a dry weight basis (42). In leaves of Centella asiatica protein content is reported on a fresh weight basis in the range of 3–4% (43, 44). Fats represent the chemical energy and contain twice the calorific value equivalent to the molecular weight of sugar, which acts as a carrier for fat-soluble vitamins and antioxidants. The fat content as recorded in Plukenetia corniculata (1.36%), Litsea cubeba (1.18%), and Zanthoxylum rhetsa (0.50%) is comparable with the common vegetables like spinach (0.7%) and lettuce (0.20%) (45). The results conform with previous reports of 1.69% crude fat content in Clerodendrum colebrookianum and 2.91% in Gynura cusimbua (38). While in Eryngium foetidum fat content of 0.60–1.34% is reported (8, 46). Dietary fiber contributes to the bulk of food, provides satiety, reduces glycemic index, and maintains gut health. In the present analysis, dietary fiber content ranged between 5.16 and 16.0%, with the highest value in Litsea cubeba and the lowest in Houttuynia cordata (5.16%), which is almost at par with the dietary value of commercial fruits and vegetables like apple (3.2%), broad beans (8.9%), cabbage (2.8%), potato (1.7%), and spinach (2.5%) (45). The high crude fiber content (16–20%) in Litsea cubeba is very well reported (47). Sugar plays a vital role in carbohydrate metabolism (48). Hence, the sugar content level indicates the leaves’ supply capacity and grains’ ability to use as assimilates (49). The data of the present investigation revealed that starch content ranged between 0.27 and 2.14%, with the highest value in Litsea cubeba (2.14%) and the lowest in Elatostema sp. As per literature, several authors have reported carbohydrate and starch content in different wild edible plants, such as 3.81% carbohydrate in Centella asiatica (43), 5.45% in Diplazium esculentum (42), and 4.5% in Eryngium foetidum (46). Among commonly consumed green leafy vegetables amaranth and fenugreek are rich in ash, protein, fat, and dietary fiber (50), however, their value is far lower than results obtained for some wild edible plants such as Herpetospermum operculatum, Plukenetia corniculate and Trichodesma khasianum.
Phenolics are non-nutritive secondary metabolites found in plants that are beneficial in curing various diseases due to their antioxidant activity. Free radicals encourage the oxidation of proteins, DNA, and lipids, resulting in disturbances and functional loss of biological membranes and enzymes, ultimately producing toxins. Free radical scavenging is an important function of antioxidants (51–54). The wild edible plants selected for the study contain phenols ranging from 0.30 mg/g (Elatostema sp.) to 6.00 mg/g (Herpetospermum operculatum) (Table 3). The Cupric Reducing Antioxidant Capacity (CUPRAC) method is a simple and versatile antioxidant capacity assay useful for analyzing various secondary metabolites with antioxidant potential. A higher CUPRAC value indicates higher antioxidant activity. In the present study, the CUPRAC value ranged between 1.10 and 8.40 mg/g, with the highest (8.40 mg/g) recorded in Plukenetia corniculata, Herpetospermum operculatum and the lowest in Diplazium esculentum (1.10 mg/g). Ferric reducing antioxidant power (FRAP) assay was another method used to investigate the antioxidant activity of wild edible plants in this study. FRAP values ranged between 0.10 and 1.90 mg/g with the highest value in Herpetospermum operculatum (1.90 mg/g) and the lowest in Elatostema sp. (0.10 mg/g). High phenolic content (2.39 mg/g) is associated with high antioxidant activity (94.94%) inhibition of free radicals in DPPH assay (42) as tested in Diplazium esculentum. A highly positive correlation of phenols with CUPRAC (0.842) and FRAP (0.969) was observed in our samples, (Table 2) which is in conformity with facts and reports that phenols contribute maximum to antioxidant activity.
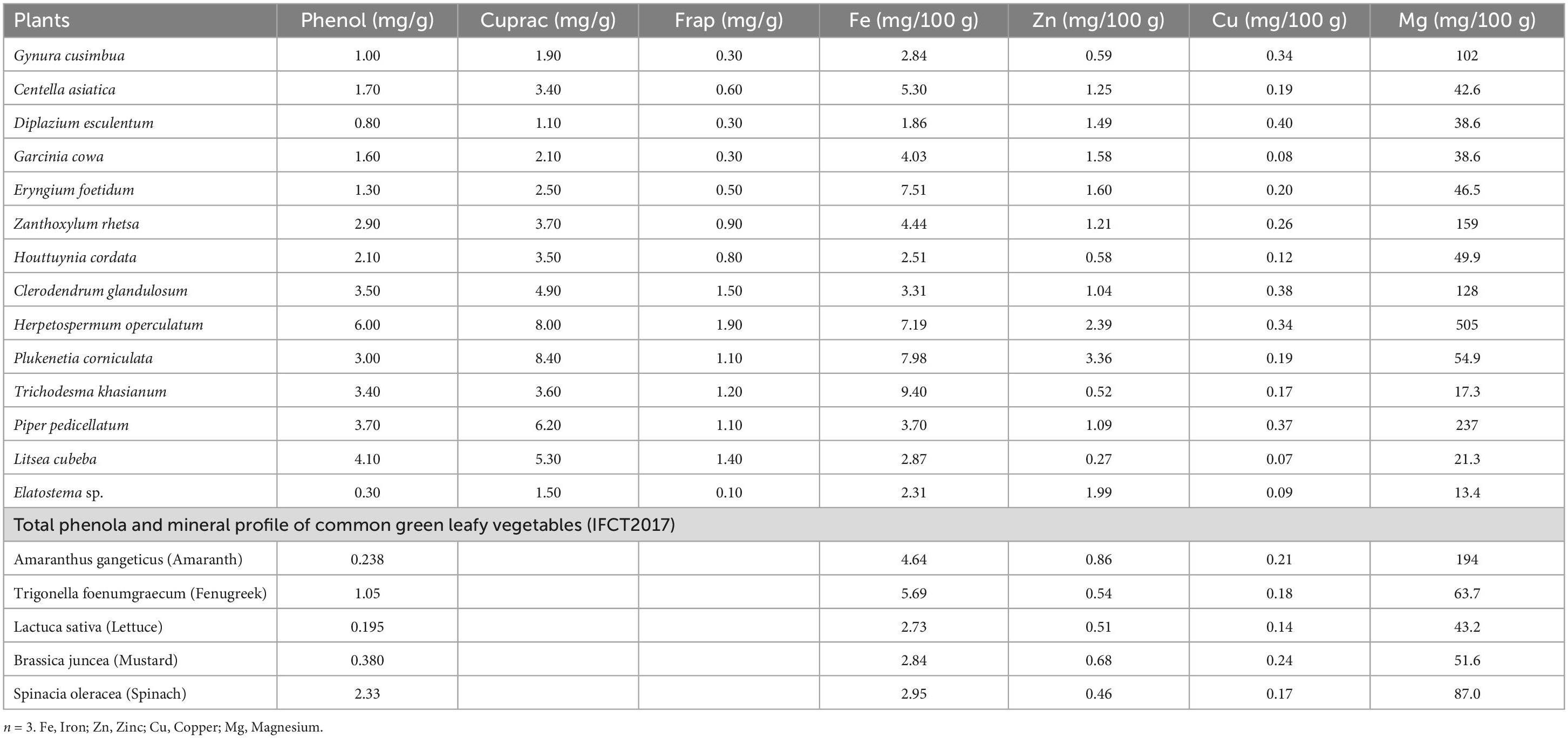
Table 3. Total antioxidant activity and mineral composition of wild edible plants of North Eastern region, India.
Green leafy vegetables are generally considered a rich source of minerals, and the results of the present investigation also corroborate these findings w.r.t. wild leafy vegetables (Table 3). The highest concentration of iron (Fe) was recorded in Trichodesma khasianum (9.40 mg/100 g) and the lowest in Diplazium esculentum (1.86 mg/100 g). Among commonly consumed green leafy vegetables fenugreek leaves have the highest iron content of 5.69 mg/100 g, followed by amaranth leaves having 4.64 mg/100 g, which is nearly half of maximum content obtained in Trichodesma khasianum (50). These plants can fulfill the daily iron requirement and prevent iron deficiency (55). Zinc (Zn) is an important mineral considered a nucleic acid metabolizer, membrane stabilizer, and immune response stimulator (56). Its concentration was between 0.27 mg/100 g (Litsea cubeba) and 3.36 mg/100 g (Plukenetia corniculata). Amararanth and brassica leaves have high content of zinc 0.86 and 0.68 mg/100 g, respectively, (50), which is much lower than the content we found in most of the wild edible plants evaluated in this study. Copper (Cu) is a vital mineral for the human body, and it serves as a cofactor for several enzymes involved in oxidation-reduction reactions and hematopoiesis (56). A significant amount of Cu was also found in the plants analyzed in the study. The concentration ranged between 0.07 and 0.40 mg/100 g, with the highest value in Diplazium esculentum (0.40 mg/100 g), followed by Clerodendrum glandulosum (0.38 mg/100 g) and Piper pedicellatum (0.37 mg/100 g) which are nearly double of the reported value of copper content in commonly consumed GLV, namely, mustard and amaranth leaves (50) and our findings conform with (55). Magnesium (Mg) is essential to maintain normal nerve and muscle functions. Regularly consuming Mg-rich food resources can control blood glucose levels and support a healthy immune system (55). Mg concentrations ranged between 13.4 and 505 mg/100 g, with the highest amount in Herpetospermum operculatum which is more than double of amaranth leaves (50). However, a good amount of Mg was also present in Piper pedicellatum (237 mg/100 g) and Zanthoxylum rhetsa (159 mg/100 g). Magnesium content of the studied wild edible plants, as shown in Table 3, are at par with some common leafy vegetables. Consumption of these locally available plants meets nutritional needs and is part of traditional food habits and customs. Our findings are in conformity with (37) for iron (1.55 mg/g), zinc (0.18 mg/g), copper (0.017 mg/g) in Clerodendrum colebrokianum; zinc (0.09 mg/g), iron (0.96 mg/g), copper (0.008 mg/g) in Houttuynia cordata; and zinc (0.867 mg/g), iron (1.175 mg/g), copper (0.012 mg/g) in Zanthoxylum acanthopodium. Centella asiatica contains iron (18 mg/100 g), magnesium (271 mg/100 g), zinc (20 mg/100 g), copper (7 mg/100 g). Similar result were also obtained by Odhav et al. (43), Diplazium esculentum is reported to have copper (14.38 mg/100 g), magnesium (9.56 mg/100 g), zinc (0.10 mg/100 g) as reported by Chettri (44) while Eryngium foetidum contains magnesium (98.5 mg/100 g), iron (111.21 mg/100 g), zinc (4.5 mg/100 g), copper (441.4 mg/100 g) as reported (57, 58).
The present article is an attempt to know the nutritional contribution, supplementary role, and market potential of wild edibles to boost the economy and nutritional security of traditional communities of the NEH region. NEH region being a hotspot of biodiversity, thorough investigation for validation of the nutritional potential of WEPs is utmost required. Given the conservation and popularization as future potential resources, a lot of issues about their domestication, selection of quality material, development of good cultivation practices, and value addition for wider acceptability are required to be addressed. Considerable variations existed in the nutritional value, total antioxidant activity, and mineral composition of wild edible plants selected for nutritional profiling under the present study. Most wild edible plants exhibited higher concentrations of nutrients such as ash, protein, fat, dietary fiber, sugar, starch, phenol, antioxidants, iron, zinc, copper, and magnesium than commercial crops. Herpetospermum operculatum, Plukenetia corniculata, Trichodesma khasianum, and Piper pedicellatum are the most nutritious wild leafy vegetables, with high proportions of protein, ash, sugar, starch, total phenols, antioxidant activity, iron, zinc, and magnesium, according to overall nutritional profiling. In Nagaland, Herpetospermum operculatum and Plukenetia corniculata are in high demand, and commercial production of these plants has begun in some areas to meet demand. Similarly, Clerodendron glandulosum and Spilanthes accmella are also much sought after locally for their food and medicinal value. As a result, it can be inferred that wild edible plants are nutrient-dense and can be used as an alternative source of nutrients to help alleviate poverty and achieve food and nutritional security. Domestication and promotion of commercial cultivation of these wild edible plants will generate substantial income for the poor farmers residing in the region’s remote areas. Moreover, immediate efforts for collecting their diverse germplasm for ex situ conservation are equally important at the national level. These wild leafy vegetables can be promoted in the region through their inclusion in the All India Coordinated Network Project on Potential Crops.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
HT, AY, RR, KP, SL, BI, LS, RA, MD, and V: collection of samples from Meghalaya, Nagaland. HT: preparation of draft manuscript. AY, HT, and RB: biochemical evaluation. SA and KB: documentation of traditional knowledge. AK: formal analysis. KB and RB: critical review. All authors contributed to the article and approved the submitted version.
This work was supported through funding from ICAR, New Delhi, under in-house research projects on Germplasm Exploration and Germplasm Evaluation.
The authors acknowledge the support from Director, ICAR-NBPGR in carrying out the exploration and evaluation of wild edible plants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1052086/full#supplementary-material
1. Champion H, Seth S. A Revised Survey of the Forest Types of India. New Delhi: Government of India (1968).
2. Sawian J, Jeeva S, Lyndem F, Mishra B, Laloo R. Wild edible plants of Meghalaya, North-east India. Nat Prod Radiance. (2007) 6:410–26.
3. Bhatt K, Pandey A, Dhariwal O, Panwar N, Bhandari D. ‘Tum-thang’ (Crotalaria tetragona Roxb. ex Andr.): a little known wild edible species in the north-eastern hill region of India. Genet Resour Crop Evol. (2009) 56:729–33. doi: 10.1007/s10722-009-9428-0
4. Narzary H, Brahma S, Basumatary S. Wild edible vegetables consumed by Bodo Tribe of Kokrajhar District (Assam), North-East India. Arch Appl Sci Res. (2013) 5:182–90.
5. Konsam S, Thongam B, Handique A. Assessment of wild leafy vegetables traditionally consumed by the ethnic communities of Manipur, northeast India. J Ethnobiol Ethnomed. (2016) 12:1–5. doi: 10.1186/s13002-016-0080-4
6. Shin T, Fujikawa K, Moe A, Uchiyama H. Traditional knowledge of wild edible plants with special emphasis on medicinal uses in Southern Shan State, Myanmar. J Ethnobiol Ethnomed. (2018) 14:1–3. doi: 10.1186/s13002-018-0248-1
7. Misra S, Maikhuri R, Kala C, Rao K, Saxena K. Wild leafy vegetables: a study of their subsistence dietetic support to the inhabitants of nanda devi biosphere reserve, India. J Ethnobiol Ethnomed. (2008) 4:1–9. doi: 10.1186/1746-4269-4-15
8. Singh R, Bhardwaj R, Singh A, Payum T, Rai A, Singh A, et al. Mainstreaming local food species for nutritional and livelihood security: insights from traditional food systems of adi community of Arunachal Pradesh, India. Front Nutr. (2021) 8:590978. doi: 10.3389/fnut.2021.590978
9. Sundriyal M, Sundriyal D. C. Wild edible plants of the Sikkim Himalaya: nutritive values of selected species. Econ Bot. (2001) 58:286–99.
10. Shukla A. Ethnic food culture of Chhattisgarh state of India. J Ethn Foods. (2021) 8:1–6. doi: 10.1186/s42779-021-00103-6
11. International Institute of Population sciences. India fact Sheet NFHS-5, 2019-21. Mumbai: India Report (2022).
12. Reyes-Garcia V, Byron E, Vadez V, Godoy R, Apaza L, Limache E, et al. Measuring culture as shared knowledge: do data collection formats matter? Cultural knowledge of plant uses among Tsimane’Amerindians, Bolivia. Field Methods. (2004) 15:1–22. doi: 10.1177/1525822X03262804
13. AOAC. Official Method of Analysis. 18th ed. Washington DC: Association of Official Analytical Chemists (2010).
14. Roe J. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. (1955) 212:335–43. doi: 10.1016/S0021-9258(18)71120-4
15. Slinkard K, Singleton V. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. (1977) 28:49–55. doi: 10.5344/ajev.1974.28.1.49
16. Benzie I, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. (1996) 239:70–6. doi: 10.1006/abio.1996.0292
17. Apak R, Güçlü K, Özyürek M, Karademir S. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric Food Chem. (2004) 52:7970–81. doi: 10.1021/jf048741x
18. Orech F, Aagaard-Hansen J, Friis H. Ethnoecology of traditional leafy vegetables of the Luo people of Bondo district, western Kenya. Int J Food Sci Nutr. (2007) 58:522–30. doi: 10.1080/09637480701331163
19. Rankoana S. Indigenous plant foods of Dikgale community in South Africa. J Ethn Foods. (2021) 8:1–8. doi: 10.1186/s42779-021-00080-w
20. Sanchez-Mata M, Cabrera Loera R, Morales P, Fernandez-Ruiz V, Camara M, Diez Marques C, et al. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet Resour Crop Evol. (2012) 59:431–43. doi: 10.1007/s10722-011-9693-6
22. Panmei R, Gajurel P, Singh B. Ethnobotany of medicinal plants used by the Zeliangrong ethnic group of Manipur, northeast India. J Ethnopharmacol. (2019) 235:164–82. doi: 10.1016/j.jep.2019.02.009
24. Dutta S, Neog M, Saikia A. Shelf-life enhancement of cowa (Garcinia cowa Roxb.). Internat J Proc Post Harvest Technol. (2017) 8:50–5. doi: 10.15740/HAS/IJPPHT/8.1/50-55
25. Jena B, Jayaprakasha G, Sakariah K. Organic acids from leaves, fruits, and rinds of Garcinia cowa. J Agric Food Chem. (2002) 50:3431–4. doi: 10.1021/jf011627j
26. Singh B, Ramakrishna Y, Ngachan S. Spiny coriander (Eryngium foetidum L.): a commonly used, neglected spicing-culinary herb of Mizoram, India. Genet Resour Crop Evol. (2014) 61:1085–90. doi: 10.1007/s10722-014-0130-5
27. Rastogi R, Mehrotra B, Sinha S, Pant P, Seth R. Compendium of Indian Medicinal Plants: 1985-1989. New Delhi: Central Drug Research Institute and Publications & Information Directorate (1990).
28. Pradheep K, Pandey A, Bhatt K. Wild edible plants used by Konyak tribe in Mon district of Nagaland: survey and inventorisation. Indian J Nat Prod Resour. (2016) 7:74–81.
29. Kala C. Ethnomedicinal botany of the Apatani in the Eastern Himalayan region of India. J Ethnobiol Ethnomed. (2005) 1:1–8. doi: 10.1186/1746-4269-1-11
30. Deb S, Arunachalam A, Das A. Indigenous knowledge of Nyishi tribes on traditional agroforestry systems. Indian J Tradit Knowl. (2009) 8:41–6.
31. Jadeja R, Thounaojam M, Singh T, Devkar R, Ramachandran A. Traditional uses, phytochemistry and pharmacology of Clerodendron glandulosum Coleb–a review. Asian Pac J Trop Med. (2012) 5:1–6. doi: 10.1016/S1995-7645(11)60236-8
32. Pfoze N. Ethnobotanical studies and phytochemical analysis of selected medicinal plants of Senapati district, Manipur. Ph.D. thesis in botany. Shillong: North Eastern Hill University (2012).
33. Chanchal C, Thongam B, Handique P. Morphological diversity and characterization of some of the wild Piper species of North East India. Genet Resour Crop Evol. (2015) 62:303–13. doi: 10.1007/s10722-014-0172-8
34. Tsering J, Gogoi B, Hui P, Tam N, Tag H. Ethnobotanical appraisal on wild edible plants used by the Monpa community of Arunachal Pradesh. Indian J Tradit Knowl. (2017) 16:626–37.
35. Ng X, Chye F, Mohd Ismail A. Nutritional profile and antioxidative properties of selected tropical wild vegetables. Int Food Res J. (2012) 19:1487–96.
36. Kakati B, Kakati L, Chetia B. Seasonal variation of the foliar constituents of primary (Machilus bombycina) and secondary (Litsea citrata) food plants of Antherae assamensis Helfer (muga silkworm) in Nagaland. Bioscan. (2015) 10:1637–40.
37. Seal T. Determination of nutritive value, mineral contents and antioxidant activity of some wild edible plants from Meghalaya state, India. Asian J Appl Sci. (2011) 4:238–46. doi: 10.3923/ajaps.2011.238.246
38. Bhardwaj R, Singh R, Sureja A, Upadhyaya S, Devi M, Singh A. Nutritionally rich wild vegetables of tribal communities of Northeast India: gaining insights on valuable traditional biocultural resources. Int Symp Underutilized Plants Food Secur Nutr Income Sustain Dev. (2008) 806:243–8. doi: 10.17660/ActaHortic.2009.806.30
39. Marshall M. Ash analysis. In: SN Nielsen editor. Food Analysis. Boston MA: Springer (2010). p. 105–15. doi: 10.1007/978-1-4419-1478-1_7
40. Bazezew A, Emire S, Sisay M. Bioactive composition, free radical scavenging and fatty acid profile of Ximenia americana grown in Ethiopia. Heliyon. (2021) 7:e07187. doi: 10.1016/j.heliyon.2021.e07187
41. Who. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser. (2007) 1:1–265.
42. Saha J, Biswal A, Deka S. Chemical composition of some underutilized green leafy vegetables of Sonitpur district of Assam, India. Int Food Res J. (2015) 22:1466–73.
43. Odhav B, Beekrum S, Akula U, Baijnath H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Compos Anal. (2007) 20:430–5. doi: 10.1016/j.jfca.2006.04.015
44. Chettri S. Nutrient and elemental composition of wild edible ferns of the Himalaya. Am Fern J. (2018) 108:95–106. doi: 10.1640/0002-8444-108.3.95
45. Sundriyal M, Sundriyal R. C. Wild edible plants of the Sikkim Himalaya: nutritive values of selected species. Econ Bot. (2004) 58:286–99. doi: 10.1663/0013-0001(2004)058[0286:WEPOTS]2.0.CO;2
46. Booth S, Bressani R, Johns T. Nutrient content of selected indigenous leafy vegetables consumed by the Kekchi people of Alta Verapaz, Guatemala. J Food Compos Anal. (1992) 5:25–34. doi: 10.1016/0889-1575(92)90005-5
47. Kakati L, Chutia B, Kakati B. Seasonal variation of the foliar constituents of host plants of certain wild silkmoth in Nagaland. Indian J Seric. (2013) 52:122–30.
48. Wilcox JR. Sixty years of improvement in publicly developed elite soybean lines. Crop Sci. (2001) 41:1711–6. doi: 10.2135/cropsci2001.1711
49. Kumudini S, Hume D, Chu G. Genetic improvement in short season soybeans: I. Dry matter accumulation, partitioning, and leaf area duration. Crop Sci. (2001) 41:391–8. doi: 10.2135/cropsci2001.412391x
50. Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. (2017). Available online at: https://drive.google.com/file/d/1eqQ578gHiPoIaHaVYjQa_3sFe_LzGhm1/view (assesed January 18, 2023)
51. Ayoola G, Ipav S, Sofidiya M, Adepoju-Bello A, Coker H, Odugbemi T. Phytochemical screening and free radical scavenging activities of the fruits and leaves of Allanblackia floribunda Oliv (Guttiferae). Int J Health Res. (2008) 1:87–93. doi: 10.4314/ijhr.v1i2.47920
52. Egea I, Sanchez-Bel P, Romojaro F, Pretel M. Six edible wild fruits as potential antioxidant additives or nutritional supplements. Plant Foods Hum Nutr. (2010) 65:121–9. doi: 10.1007/s11130-010-0159-3
53. Awika J, Rooney L, Wu X, Prior R, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem. (2003) 51:6657–62. doi: 10.1021/jf034790i
54. Niki E. Antioxidant capacity: which capacity and how to assess it? J Berry Res. (2011) 1:169–76. doi: 10.3233/JBR-2011-018
55. Saikia P, Deka D. Mineral content of some wild green leafy vegetables of North-East India. J Chem Pharm Res. (2013) 5:117–21.
56. Ihedioha J, Okoye C. Nutritional evaluation of Mucuna flagellipes leaves: an underutilized legume in Eastern Nigeria. Am J Plant Nutr Fertil Tech. (2011) 1: 55–63. doi: 10.3923/ajpnft.2011.55.63
57. Singh S, Singh D, Salim K, Srivastava A, Singh L, Srivastava R. Estimation of proximate composition, micronutrients and phytochemical compounds in traditional vegetables from Andaman and Nicobar Islands. Int J Food Sci Nutr. (2011) 62:765–73. doi: 10.3109/09637486.2011.585961
Keywords: proximate composition, minerals, antioxidants, ethnic foods, biodiversity
Citation: Talang H, Yanthan A, Rathi RS, Pradheep K, Longkumer S, Imsong B, Singh LH, Assumi RS, Devi MB, Vanlalruati, Kumar A, Ahlawat SP, Bhatt KC and Bhardwaj R (2023) Nutritional evaluation of some potential wild edible plants of North Eastern region of India. Front. Nutr. 10:1052086. doi: 10.3389/fnut.2023.1052086
Received: 23 September 2022; Accepted: 30 January 2023;
Published: 01 March 2023.
Edited by:
Shauna Downs, Rutgers, The State University of New Jersey, United StatesReviewed by:
Emily Merchant, Rutgers, The State University of New Jersey, United StatesCopyright © 2023 Talang, Yanthan, Rathi, Pradheep, Longkumer, Imsong, Singh, Assumi, Devi, Vanlalruati, Kumar, Ahlawat, Bhatt and Bhardwaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakesh Bhardwaj, cmFrZXNoLmJoYXJkd2FqMUBpY2FyLmdvdi5pbg==; Kailash C. Bhatt, a2FpbGFzaC5iaGF0dEBpY2FyLmdvdi5pbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.