
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 23 February 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1046749
This article is part of the Research TopicNutrition and Sustainable Development Goal 2: Zero HungerView all 24 articles
Background: Magnesium deficiency is related to an increased risk of anemia, but epidemiological evidence supporting this association remains scarce. The purpose of the present survey was to evaluate the relationship between dietary magnesium intake and the risk of anemia.
Methods: In total, 13,423 participants aged 20–80 years were enrolled using data from the National Health and Nutrition Examination Survey 2011–2016. Magnesium consumption was evaluated using 24 h dietary recalls. Multivariable generalized linear models were developed to demonstrate the association between dietary magnesium intake and the prevalence of anemia.
Results: An inverse association between dietary magnesium intake and the risk of anemia was detected based on a full adjustment model. We evaluated magnesium intake as a categorical variable (five quartiles). Compared with the lowest value, the highest multivariate adjusted odds ratio (95% confidence interval) for anemia was 0.64 (0.46–0.89). Stratified analyses revealed a reverse relationship between magnesium intake and anemia in women. However, no significant association was observed in men (pfor trend = 0.376). A similar reverse association was found among the older group (aged ≥60 years).
Conclusion: Magnesium deficiency is closely related to a higher rate of anemia occurrence, especially among women and older Americans. Further larger-scale prospective studies are required to confirm these conclusions.
Anemia threatens public health worldwide. The prevalence of anemia nearly doubled (4.0 to 7.1%) from 2003–2004 to 2011–2012 (1). Anemia is associated with an increased sequence of adverse effects, including cardiovascular disease, low quality of life, morbidity, and mortality (2–4). Anemia reflects the decreased oxygen-carrying capacity of the blood, which may lead to fatigue, cardiovascular complications, and impaired body function (5–7). Moreover, anemia has been shown to increase hospitalization rates, especially in older adults (8).
Magnesium plays a crucial role in the functioning and sustainment of the body (9). The imbalance of magnesium homeostasis can lead to modification of the cell membrane and increased oxidative stress (10). Magnesium deficiency often leads to inflammation through the activation of the nuclear factor kappa B (NF-kB) pathway in immune cells and in the pathogenesis of many chronic disorders, including congestive heart failure, type 2 diabetes and hypertension (11, 12). In recent decades, a few studies have indicated that magnesium is involved in the regulation of cell replication, differentiation, and apoptosis (13–15). Magnesium is important for the hematopoietic system (16). In the United States, dietary magnesium intake is often below the recommended dietary intake, and 28% of women develop anemia during pregnancy due to magnesium deficiency (17). A cross-sectional retrospective study by Zeynep et al. identified a positive relationship between magnesium deficiency and anemia among individuals with chronic kidney disease (18). Moreover, Cinar et al. reported that magnesium supplementation increases hemoglobin levels in athletes (19).
Although these studies have reported that magnesium deficiency has a potentially modifiable association with anemia, they have mostly focused on specific populations. Data on the relationship between dietary magnesium intake and anemia in the general population are limited. Therefore, we explored the association of magnesium intake with anemia in adults, as well as possible effects of age and sex, using data from the National Health and Nutrition Examination Survey (NHANES) database between 2011 and 2016.
Data were collected from three continuous survey cycles (between 2011 and 2016) of the NHANES, which was a nationally representative survey conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS) to estimate health and nutrition in the US population. The NHANES contains demographic, nutritional, and medical examination information of civilian noninstitutionalized people in the US. Written informed consent was provided by all participants, and the survey protocol was supported by the Institutional Review Board of the NCHS (20).
Data of 14,754 individuals aged 20–80 years from 2011 to 2016 were extracted. Pregnant or lactating females (n = 124) and participants with unreliable or missing magnesium intake data, hemoglobin data, and important confounders were further excluded (n = 1,207). Ultimately, 13,423 participants were included in the analysis (Figure 1).
Nutritional information including total dietary energy, vitamin D, calcium, magnesium, protein, and fiber intakes was collected via the first 24 h dietary recall interview, which was performed at the Mobile Examination Center (MEC). Data collection by 24-h recall interview is the most common method used to determine dietary intake in large-scale surveys and has been used in the NHANES for many years, based on expert consensus (21). Details of the dietary interview have been described in the Dietary Interviewers Procedure Manuals (22). The dietary interview information included food species, consumption frequency, duration, and quantity. Information on dietary intake is detailed in the NHANES.1
Anemia was described as a hemoglobin concentration < 120 g/L for women and < 130 g/L for men (23).
Covariates included age, sex, race/ethnicity, educational experience, smoking status, physical activity level, and body mass index (BMI). Dietary information included total energy, protein, fiber, magnesium, calcium, and Vitamin D intakes. Race was classified into the following categories: Mexican American, non-Hispanic Black, non-Hispanic White, Other Hispanic, and Other Race. Educational background was categorized into “less than a high school diploma,” “graduated from high school,” or “education beyond high school” category. Poverty income ratio (PIR) was calculated as the federal poverty level divided by the family income and defined as a value <1 or ≥ 1. BMI was estimated as weight in kilograms divided by height in meters squared and categorized into <25.0, 25.0 to <30.0, and ≥ 30.0 kg/m2. The participants were classified as current smokers, former smokers, and never smokers according to their smoking status. Physical activity was categorized into three strata: light activity, moderate activity, and vigorous activity. Dietary data, including total dietary energy, vitamin D, calcium, magnesium, protein, and fiber intakes, were acquired from a 24-h dietary recall interview.
Statistical analysis was conducted using the statistical software package R2 and Empower Stats (X&Y Solutions, Inc., Boston, MA, United States).3 Categorical variables and continuous variables were evaluated using chi-square tests and t-tests, respectively. Generalized linear models were developed to explore the connection between magnesium intake and anemia. First, we used univariable logistic regression to identify factors linked to anemia. Second, multivariate logistic regression models were applied. In the crude model, we made no adjustments. Model 1 was adjusted for sex, age and race. Model 2 was adjusted for all confounders listed in Table 1, including age; sex; race; PIR value; educational level; BMI; smoking status; physical activity level; and dietary energy, protein, fiber, vitamin D, calcium, and magnesium intakes. Third, a series of sensitivity analyses were conducted to identify the robustness of the results. Magnesium intake was divided into five quartiles to test the p for trends, and the lowest quartile was considered the reference. Finally, to explore the robustness of our results, analyses were stratified by sex and age as shown in Tables 2, 3. The results were considered statistically significant at p < 0.05.
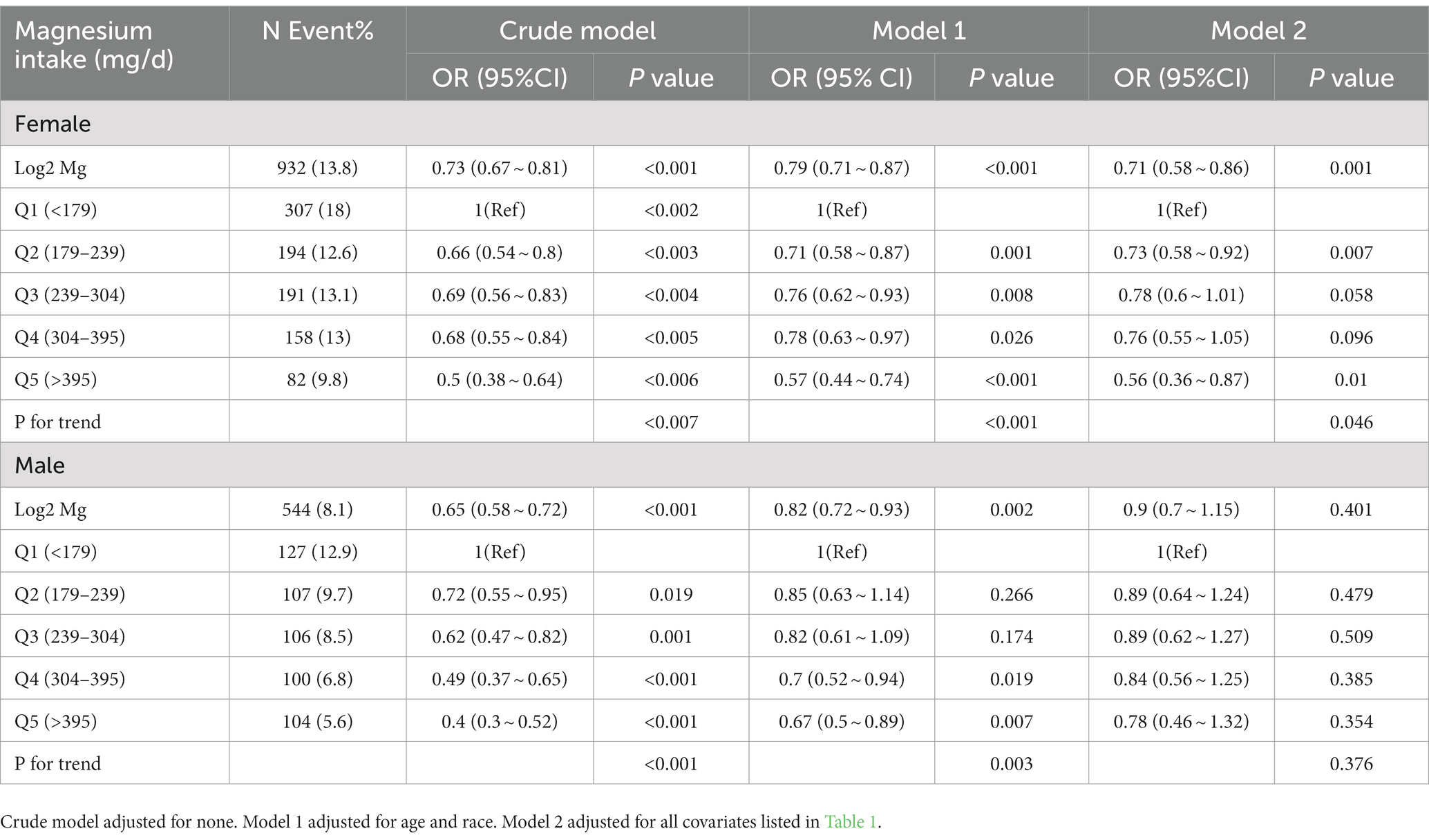
Table 2. Odds ratios (95% confidence intervals) of anemia across quartiles of dietary magnesium intake stratify by gender, NHANES 2011–2016.
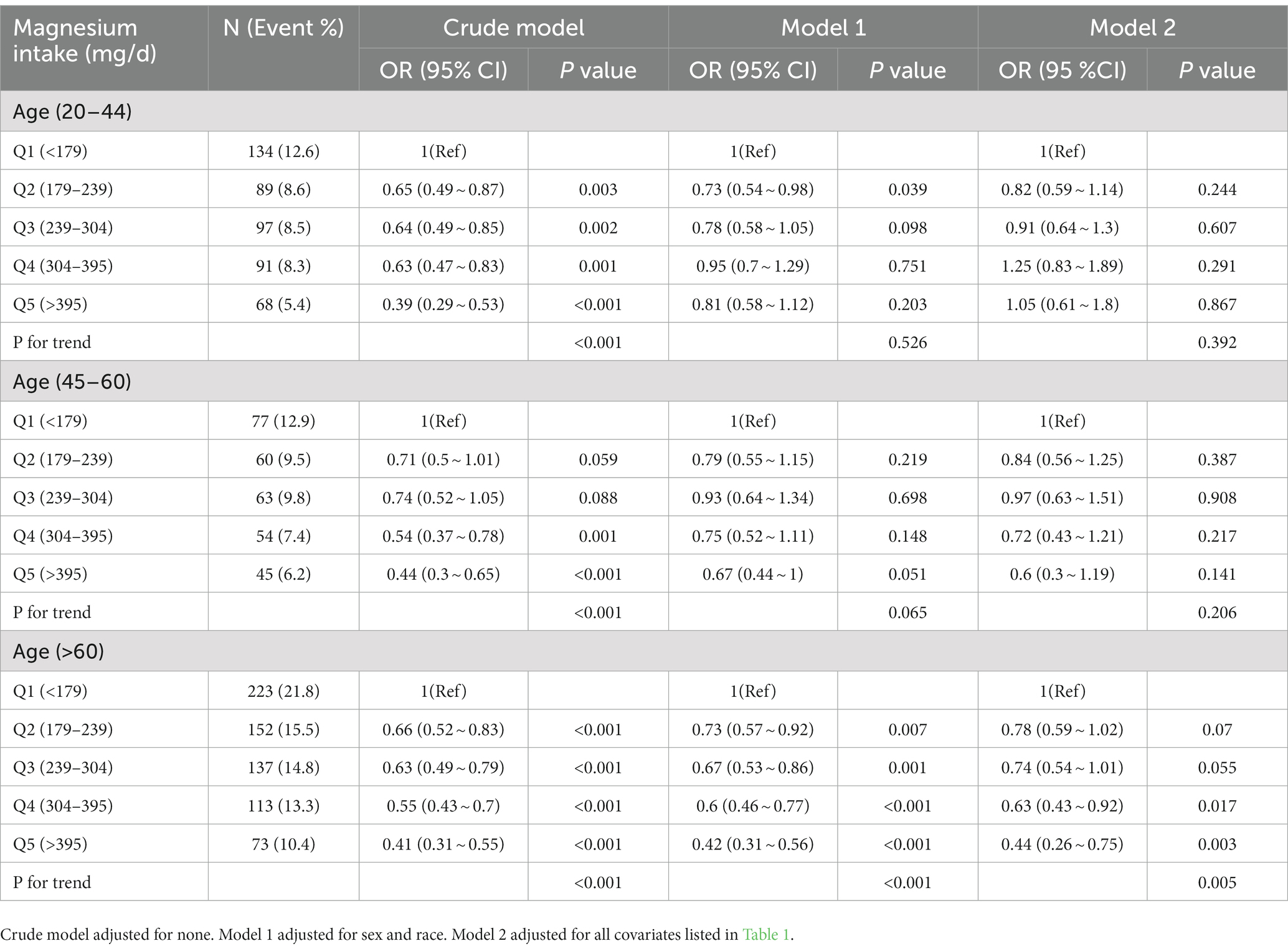
Table 3. Odds ratios (95% confidence intervals) of anemia across quartiles of dietary magnesium intake stratified by age NHANES 2011–2016.
Sociodemographic characteristics and possible confounding factors grouped by anemia status are shown in Table 1. In total, 13,423 individuals were included in this sample, and 1,476 participants (11%) were defined as having anemia. Compared with participants without anemia, those with anemia were more likely to be female, older, non-Hispanic black, individuals with a lower daily dietary intake (magnesium, calcium, vitamin D, energy, fiber, and protein), those with a lower family income, and individuals with obesity.
The multivariate logistic regression analysis results are displayed in Table 4. We determined an inversely proportional association between dietary magnesium intake (log2 transformation) and the risk of anemia in the crude model (OR, 0.66; 95% CI, 0.61–0.7). In Model 1, we adjusted for age, sex, and race. The OR (95% CI) was 0.78 (0.72–0.84). After adjusting for all possible confounders, the result was compatible with that of Model 1 (OR, 0.78; 95% CI, 0.68–0.91). We divided magnesium intake into five quartiles. The p for trends was robust irrespective of the three different model analyses (crude model: p < 0.001, Model 1: p < 0.001, Model 2: p = 0.035).
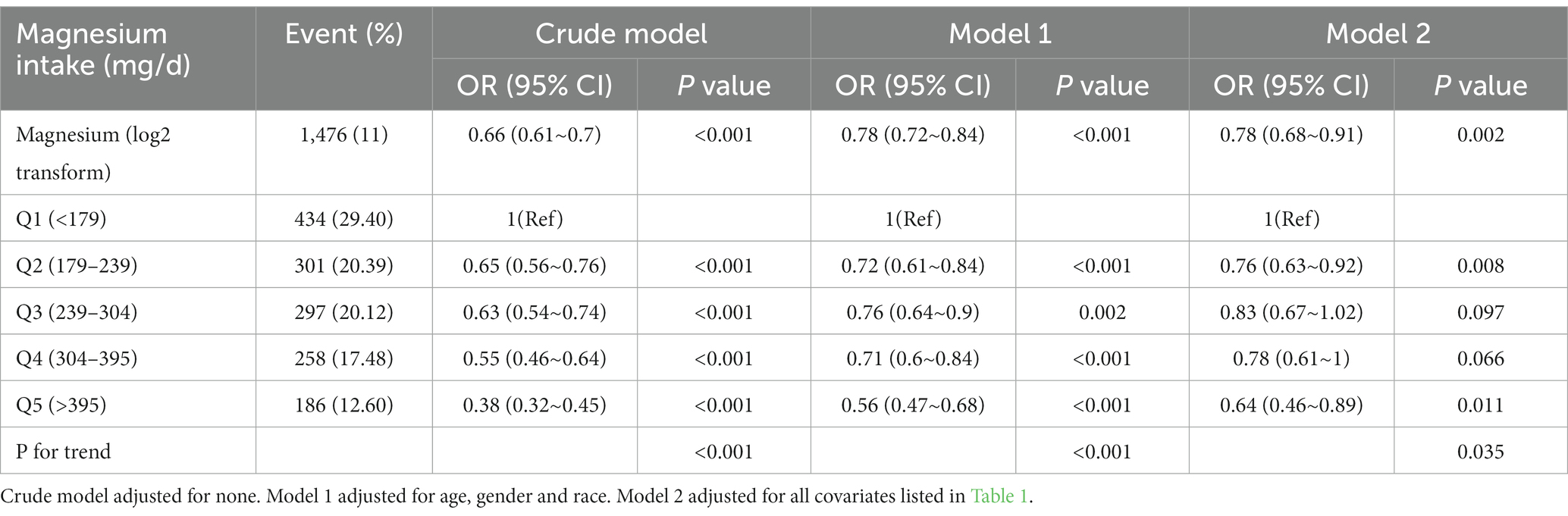
Table 4. Odds ratios (95% confidence intervals) of anemia across quartiles of dietary magnesium intake.
Stratified analyses for dietary magnesium intake by sex were conducted (Table 2). Among female participants, magnesium consumption was inversely associated with anemia (p for trend = 0.046). Nevertheless, this correlation did not show a significant difference in males (p for trend = 0.376).
We further conducted age-stratified analyses to evaluate the association of magnesium intake with anemia (Table 3). After adjusting for all confounders, an inverse relationship between dietary magnesium intake and the risk of anemia was statistically remarkable among the older group (age ≥ 60 years [p for trend = 0.005]). However, there was no statistically significance in the other two groups (age < 60 years). To ensure the robustness of our findings, a subgroup analysis was conducted to evaluate the potential interaction between magnesium intake and anemia (Table 5). After adjustment for age, sex, race, PIR value, educational level, BMI, smoking status, physical activity level, and dietary intake of energy, protein, fiber, vitamin D, and calcium, our smoking status had an interactive effect on the relationship between daily magnesium intake and anemia (p for interaction = 0.012). Non-interactive effect was calculated in other subgroup (p for interaction <0.05).
In the present cross-sectional survey, an inverse association between dietary magnesium intake and anemia was found among US adults, utilizing data from three continued NHANES cycles. In the sex-stratified analysis, an inverse association was found in females, whereas no significant difference was observed in males. Furthermore, we noticed a similar relationship between dietary magnesium intake and the risk of anemia among older participants (age ≥ 60 years). To the best of our knowledge, this is the first and largest sociodemographic investigation to reveal the relationship between magnesium intake and the prevalence of anemia in a general population.
The recommended daily allowance for magnesium intake in US adults is 420 mg for males and 320 mg for females. However, our data showed that daily magnesium intake value was 239 mg in the anemia group, which is significantly lower than recommended daily allowance. Inadequate magnesium intake has been a growing concern in recent years. Magnesium deficiency has been partially attributed to unhealthy dietary pattern such as the consumption of so-called “Western diet” (24, 25). Clinical magnesium deficiency or magnesium deficiency patients can be found in internal medicine. Magnesium deficiency has been associated with a number of diseases, including atherosclerosis (26), diabetes (27), hypertension (28), myocardial infarction (29), and calculi (30). Nuts, fresh vegetables, and integral grains are the major sources of magnesium. Moreover, with the exception of milk, the concentration of magnesium in dairy products is very low (24). Therefore, the consumption of foods rich in magnesium may decrease the risk of certain diseases.
A limited number of studies have quantified the relationship between magnesium intake and anemia. A similar study found an inverse correlation between magnesium intake and anemia depending on ferritin levels among 8,511 Chinese adults. However, this association was not significant with serum ferritin levels <15 ng/mL (25). A cross-sectional domestic review of 2,849 Chinese adults aged 20 years or older reported that sufficient magnesium and iron intakes were positively correlated with hemoglobin levels and negatively linked to the prevalence of anemia (31). Another similar study involving 2,401 individuals aged 60 years or older in China showed that adopting a modern dietary pattern (magnesium consumption) is an appropriate strategy for preventing anemia in older Chinese people (32). In addition, an investigation indicated that low levels of magnesium and serum ferritin were linked to a higher risk of anemia among 180 pregnant women from Khartoum, Sudan (33). Our study extended these findings in a much larger cohort (n = 13,423) and different subgroups.
Although the precise potential mechanism of this association between magnesium and anemia remains unclarified, several possible mechanisms may explain our results. Magnesium is considered an important coenzyme for glutathione peroxidase, which is involved in the synthesis of hemoglobin (34, 35). Furthermore, animal experiments have shown that magnesium deficiency can cause microcytic anemia, damage the membranes of red blood cells, and reduce the osmotic fragility of erythrocytes in rats (36–38). Magnesium deficiency reduces erythrocyte energy metabolism and hemoglobin synthesis, leading to anemia (25). Moreover, chronic magnesium deficiency may promote the release of inflammatory compounds (39). Moreover, a study reported that anemia is more prevalent in individuals with hemodialysis who suffer from decreasing erythropoietin (EPO) concentrations; nevertheless, increased serum magnesium level appears to reduce the risk of anemia by enhancing EPO response (40). In addition, higher concentrations of magnesium may promote the driving of HIF-1α (hypoxia-inducible factor) expression, which is mediated by reactive oxygen species (ROS) via the NF-κB signaling pathway, in which HIFs are considered an important factor in the process of hemoglobin production (41, 42). A supposed mechanism is that magnesium deficiency may alter macrophage and iron homeostasis through the NF-κB pathway, which may indirectly impair the membranes and accelerate the aging of and damage to RBCs (43).
This study had several advantages. (1) To our knowledge, this was the first study to investigate the relationship between dietary magnesium intake and anemia using a nationally representative sample of US adults. (2) This was the largest investigation exploring this association, which may ensure statistical efficiency. (3) We controlled and adjusted for more potential confounders. (4) A sensitivity analysis stratified by sex and age was performed to explore potential special populations. However, our study had certain limitations. First, in consideration of the characteristics of cross-sectional studies, the temporal sequence of this relationship could not be assessed. Second, biochemical parameters, serum magnesium, and the type of anemia information were not available in the database. In addition, multiple 24-h dietary recalls could not signify long-term magnesium status. Finally, our study was vulnerable to unmeasured confounders. Therefore, more large sample prospective studies are needed to further probe the mechanisms of the relationship between magnesium and anemia.
In conclusion, magnesium deficiency is positively associated with a higher rate of anemia occurrence, especially among females and older populations. Healthy and adequate dietary magnesium intake should be promoted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the NCHS. The patients/participants provided their written informed consent to participate in this study.
JH drafted the manuscript. JX and PY collected the clinical data. XX conceived the study. All authors read and approved the final manuscript.
We thank Zhang Jing from Shanghai Tongren Hospital in Shanghai, China, for providing help with data collection for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Le, CH. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003-2012). PLoS One. (2016) 11:e0166635. doi: 10.1371/journal.pone.0166635
2. Liu, ZY, Deng, W, Zhang, RY, Huang, JP, Wang, XF, Qian, DG, et al. Anemia, physical function, and mortality in long-lived individuals aged 95 and older: a population-based study. J Am Geriatr Soc. (2015) 63:2202–4. doi: 10.1111/jgs.13685
3. Dong, X, Mendes de Leon, C, Artz, A, Tang, Y, Shah, R, and Evans, D. A population-based study of hemoglobin, race, and mortality in elderly persons. J Gerontol A Biol Sci Med Sci. (2008) 63:873–8. doi: 10.1093/gerona/63.8.873
4. Penninx, BW, Pahor, M, Woodman, RC, and Guralnik, JM. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. (2006) 61:474–9. doi: 10.1093/gerona/61.5.474
5. Del Fabbro, P, Luthi, JC, Carrera, E, Michel, P, Burnier, M, and Burnand, B. Anemia and chronic kidney disease are potential risk factors for mortality in stroke patients: a historic cohort study. BMC Nephrol. (2010) 11:27. doi: 10.1186/1471-2369-11-27
6. Milionis, H, Papavasileiou, V, Eskandari, A, D'Ambrogio-Remillard, S, Ntaios, G, and Michel, P. Anemia on admission predicts short-and long-term outcomes in patients with acute ischemic stroke. Int J Stroke. (2015) 10:224–30. doi: 10.1111/ijs.12397
7. Li, Z, Zhou, T, Li, Y, Chen, P, and Chen, L. Anemia increases the mortality risk in patients with stroke: a meta-analysis of cohort studies. Sci Rep. (2016) 6:26636. doi: 10.1038/srep26636
8. Culleton, BF, Manns, BJ, Zhang, J, Tonelli, M, Klarenbach, S, and Hemmelgarn, BR. Impact of anemia on hospitalization and mortality in older adults. Blood. (2006) 107:3841–6. doi: 10.1182/blood-2005-10-4308
9. Jahnen-Dechent, W, and Ketteler, M. Magnesium basics. Clin Kidney J. (2012) 5:i3–i14. doi: 10.1093/ndtplus/sfr163
10. Weglicki, WB, Mak, IT, Kramer, JH, Dickens, BF, Cassidy, MM, Stafford, RE, et al. Role of free radicals and substance P in magnesium deficiency. Cardiovasc Res. (1996) 31:677–82. doi: 10.1016/S0008-6363(95)00196-4
11. Nielsen, FH. Dietary magnesium and chronic disease. Adv Chronic Kidney Dis. (2018) 25:230–5. doi: 10.1053/j.ackd.2017.11.005
12. Aguilar, MV, Saavedra, P, Arrieta, FJ, Mateos, CJ, González, MJ, Meseguer, I, et al. Plasma mineral content in type-2 diabetic patients and their association with the metabolic syndrome. Ann Nutr Metab. (2007) 51:402–6. doi: 10.1159/000108108
13. Yoshizawa, S, Chaya, A, Verdelis, K, Bilodeau, EA, and Sfeir, C. An in vivo model to assess magnesium alloys and their biological effect on human bone marrow stromal cells. Acta Biomater. (2015) 28:234–9. doi: 10.1016/j.actbio.2015.08.037
14. Luthringer, BJ, and Willumeit-Römer, R. Effects of magnesium degradation products on mesenchymal stem cell fate and osteoblastogenesis. Gene. (2016) 575:9–20. doi: 10.1016/j.gene.2015.08.028
15. da Silva Lima, F, da Rocha Romero, AB, Hastreiter, A, Nogueira-Pedro, A, Makiyama, E, Colli, C, et al. An insight into the role of magnesium in the immunomodulatory properties of mesenchymal stem cells. J Nutr Biochem. (2018) 55:200–8. doi: 10.1016/j.jnutbio.2018.02.006
16. McCreary, PA, Battifora, HA, Hahneman, BM, Laing, GH, and Hass, GM. Leukocytosis, bone marrow hyperplasia and leukemia in chronic magnesium deficiency in the rat. Blood. (1967) 29:683–90. doi: 10.1182/blood.V29.4.683.683
17. Adams, JB, Sorenson, JC, Pollard, EL, Kirby, JK, and Audhya, T. Evidence-based recommendations for an optimal prenatal supplement for women in the U.S., part two: minerals. Nutrients. (2021) 13:1849. doi: 10.3390/nu13061849
18. Biyik, Z, Yavuz, YC, and Altintepe, L. Association between serum magnesium and anemia in patients with chronic kidney disease. Int Urol Nephrol. (2020) 52:1935–41. doi: 10.1007/s11255-020-02525-8
19. Cinar, V, Nizamlioglu, M, Mogulkoc, R, and Baltaci, AK. Effects of magnesium supplementation on blood parameters of athletes at rest and after exercise. Biol Trace Elem Res. (2007) 115:205–12. doi: 10.1007/bf02685995
20. Parsons, VL, Moriarity, C, Jonas, K, Moore, TF, Davis, KE, and Tompkins, L. Design and estimation for the national health interview survey, 2006-2015. Vital Health Stat 2. (2014):1–53.
21. Huang, J, Hu, L, and Yang, J. Dietary magnesium intake ameliorates the association between household pesticide exposure and type 2 diabetes: data from NHANES, 2007-2018. Front Nutr. (2022) 9:903493. doi: 10.3389/fnut.2022.903493
22. Ahluwalia, N, Dwyer, J, Terry, A, Moshfegh, A, and Johnson, C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutrit Bethesda. (2016) 7:121–34. doi: 10.3945/an.115.009258
23. Garcia-Casal, MN, Pasricha, SR, Sharma, AJ, and Peña-Rosas, JP. Use and interpretation of hemoglobin concentrations for assessing anemia status in individuals and populations: results from a WHO technical meeting. Ann N Y Acad Sci. (2019) 1450:5–14. doi: 10.1111/nyas.14090
24. Gröber, U, Schmidt, J, and Kisters, K. Magnesium in prevention and therapy. Nutrients. (2015) 7:8199–226. doi: 10.3390/nu7095388
25. Zhan, Y, Chen, R, Zheng, W, Guo, C, Lu, L, Ji, X, et al. Association between serum magnesium and anemia: China health and nutrition survey. Biol Trace Elem Res. (2014) 159:39–45. doi: 10.1007/s12011-014-9967-x
26. Altura, BM, and Altura, BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. (1995) 41:347–59.
27. Guerrero-Romero, F, Simental-Mendía, LE, Hernández-Ronquillo, G, and Rodriguez-Morán, M. Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: a double-blind placebo-controlled randomized trial. Diabetes Metab. (2015) 41:202–7. doi: 10.1016/j.diabet.2015.03.010
28. Kisters, K, and Gröber, U. Lowered magnesium in hypertension. Hypertension. (2013) 62:e19. doi: 10.1161/hypertensionaha.113.02060
29. Shah, NC, Shah, GJ, Li, Z, Jiang, XC, Altura, BT, and Altura, BM. Short-term magnesium deficiency downregulates telomerase, upregulates neutral sphingomyelinase and induces oxidative DNA damage in cardiovascular tissues: relevance to atherogenesis, cardiovascular diseases and aging. Int J Clin Exp Med. (2014) 7:497–514.
30. Geiger, H, and Wanner, C. Magnesium in disease. Clin Kidney J. (2012) 5:i25–38. doi: 10.1093/ndtplus/sfr165
31. Shi, Z, Hu, X, He, K, Yuan, B, and Garg, M. Joint association of magnesium and iron intake with anemia among Chinese adults. Nutrition. (2008) 24:977–84. doi: 10.1016/j.nut.2008.05.002
32. Xu, X, Hall, J, Byles, J, and Shi, Z. Dietary pattern, serum magnesium, ferritin, C-reactive protein and anaemia among older people. Clin Nutr. (2017) 36:444–51. doi: 10.1016/j.clnu.2015.12.015
33. Eltayeb, R, Rayis, DA, Sharif, ME, Ahmed, ABA, Elhardello, O, and Adam, I. The prevalence of serum magnesium and iron deficiency anaemia among Sudanese women in early pregnancy: a cross-sectional study. Trans R Soc Trop Med Hyg. (2019) 113:31–5. doi: 10.1093/trstmh/try109
35. Zieve, FJ, Freude, KA, and Zieve, L. Effects of magnesium deficiency on protein and nucleic acid synthesis in vivo. J Nutr. (1977) 107:2178–88. doi: 10.1093/jn/107.12.2178
36. Cohlan, SQ, Jansen, V, Dancis, J, and Piomelli, S. Microcytic anemia with erythroblastosis in offspring of magnesium-deprived rats. Blood. (1970) 36:500–6. doi: 10.1182/blood.V36.4.500.500
37. Piomelli, S, Jansen, V, and Dancis, J. The hemolytic anemia of magnesium deficiency in adult rats. Blood. (1973) 41:451–9. doi: 10.1182/blood.V41.3.451.451
38. Cosens, G, Diamond, I, Theriault, LL, and Hurley, LS. Magnesium deficiency anemia in the rat fetus. Pediatr Res. (1977) 11:758–64. doi: 10.1203/00006450-197706000-00013
39. Pelczyńska, M, Moszak, M, and Bogdański, P. The role of magnesium in the pathogenesis of metabolic disorders. Nutrients. (2022) 14:1714. doi: 10.3390/nu14091714
40. Yu, L, Song, J, Lu, X, Zu, Y, Li, H, and Wang, S. Association between serum magnesium and erythropoietin responsiveness in hemodialysis patients: a cross-sectional study. Kidney Blood Press Res. (2019) 44:354–61. doi: 10.1159/000500921
41. Torii, S, Kobayashi, K, Takahashi, M, Katahira, K, Goryo, K, Matsushita, N, et al. Magnesium deficiency causes loss of response to intermittent hypoxia in paraganglion cells. J Biol Chem. (2009) 284:19077–89. doi: 10.1074/jbc.M109.004424
42. Haase, VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. (2013) 27:41–53. doi: 10.1016/j.blre.2012.12.003
Keywords: magnesium intake, anemia, association, adult, risk
Citation: Huang J, Xu J, Ye P and Xin X (2023) Association between magnesium intake and the risk of anemia among adults in the United States. Front. Nutr. 10:1046749. doi: 10.3389/fnut.2023.1046749
Received: 19 September 2022; Accepted: 08 February 2023;
Published: 23 February 2023.
Edited by:
Aleyda Pérez Herrera, National Council of Science and Technology (CONACYT), MexicoReviewed by:
Joško Osredkar, University Medical Centre Ljubljana, SloveniaCopyright © 2023 Huang, Xu, Ye and Xin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqin Xin, ✉ d3d0MTA1NzI2QHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.