- 1Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy
- 2Unit of Oncology, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy
- 3Department of Biomedical, Experimental and Clinical Sciences “Mario Serio”, University of Florence, Florence, Italy
- 4Don Tonino Bello Oncology, IRCCS Istituto Tumori Giovanni Paolo II Bari, Bari, Italy
- 5CyberKnife Center, Istituto Fiorentino di Cura ed Assistenza, Florence, Italy
- 6Medical Oncology Division and Breast Unit, Antonio Perrino Hospital, Brindisi, Italy
- 7Department of Image Diagnostics, Ospedale San Camillo Forlanini, Rome, Italy
- 8Department of Surgical Oncology, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari, Italy
Malnutrition is a common condition in cancer patients which is usually associated with functional limitations, as well as increased morbidity and mortality. Based on the support of the young sections of Italian Association of Medical Oncology (AIOM), Italian Association of Radiotherapy and Clinical Oncology (AIRO) and Italian Society of Surgical Oncology (SICO) merged into the NutriOnc Research Group, we performed a multidisciplinary national survey with the aim to define the awareness of nutritional issues among healthcare professionals delivering anticancer care. The questionnaire was organized in four sections, as follows: Knowledge and practices regarding Nutritional Management of cancer patients; Timing of screening and assessment of Nutritional Status; Nutritional Treatment and prescription criteria; Immunonutrition and educational topics. The modules focused on esophagogastric, hepato-bilio-pancreatic and colorectal malignancies. Overall, 215 physicians completed the survey. As regards the management of Nutritional Status of cancer patients, many responders adopted the ERAS program (49.3%), while a consistent number of professionals did not follow a specific validated nutritional care protocol (41.8%), mainly due to lack of educational courses (14.5%) and financial support (15.3%). Nearly all the included institutions had a multidisciplinary team (92%) to finalize the treatment decision-making. Cancer patients routinely underwent nutritional screening according to 57.2% of interviewed physicians. The timing of nutritional assessment was at diagnosis (37.8%), before surgery (25.9%), after surgery (16.7%), before radiochemotherapy (13.5%) and after radiochemotherapy (7%). Most of the responders reported that nutritional status was assessed throughout the duration of cancer treatments (55.6%). An important gap between current delivery and need of nutritional assessment persists. The development of specific and defined care protocols and the adherence to these tools may be the key to improving nutritional support management in clinical practice.
1. Introduction
In the era of precision medicine, the “one-size-fits-all” approach has been revolutionarily replaced by a patient-centric vision, which aims at minimizing adverse events while enhancing therapeutic impact of new treatments (1). Despite the current period of innovation in oncology, including big data, genomic sequencing, and minimally invasive surgery as part of standard treatment for some cancers, nutritional issues are still serious clinical challenges (2–7).
It has been estimated that up to 80% of cancer patients suffer from malnutrition (8–12), but less than 30% receive nutritional interventions (13). Anyway, the range of reported data is extremely wide due to the lack of fundamental consensus on malnutrition diagnosis (14–16), as well as differences among several types of solid tumors (17, 18). Therefore, based on a consensus process, the Global Leadership Initiative on Malnutrition (GLIM) has recently suggested a shared method, termed the GLIM-process, for diagnosing malnutrition (19). To this address, patients with digestive tract cancers have been found to have one of the highest rates of malnutrition due to its local effects on bowel function from obstruction and malabsorption, and on glandular secretion from dysfunction and atrophy (20–23). Prior studies suggest that the severity of weight loss is significantly associated with advanced cancer (24), but over 40% of patients are malnourished or at risk for malnutrition, even in early disease stages (11).
Malnutrition is correlated with a prolonged hospital stay, increased morbidity and readmission, as well as higher mortality rates (13, 25–27). Furthermore, malnourished cancer patients may experience a reduced tolerance to chemotherapy, lower quality of life, and reduced overall survival (17, 28–31). As a result, clinical nutrition (32), plays an active role in modern cancer surgery hindering all the adverse effects of cancer-associated malnutrition (33). However, the attitude toward this issue varies considerably among oncologists, surgeons, and radiotherapists due to the lack of structured collaboration between healthcare professionals (34, 35).
Despite the European Society for Clinical Nutrition and Metabolism (ESPEN) recently emphasized individualized plans focused on increasing nutritional intake (31), the European Society of Surgical Oncology (ESSO) and its Young Alumni Club (EYSAC) reported in a global survey that surgical units often lack a structured nutritional assessment, postulating a significant impact on the clinical practice by the involvement of nutritionist medical doctors into the multidisciplinary tumor board (36).
We provide, for the first time ever, an intersociety and multidisciplinary national survey, supported by young sections of Italian Association of Medical Oncology (AIOM), Italian Association of Radiotherapy and Clinical Oncology (AIRO) and Italian Society of Surgical Oncology (SICO) merged into the NutriOnc Research Group (Figure 1).

Figure 1. NutriOnc Research Group. email: bnV0cmlvbmNAZ21haWwuY29t; LinkedIn: NutriOnc Research Group; Twitter: @NutriOncRG.
Our survey aims at defining the awareness of nutritional issues in oncological setting among anticancer professionals, mainly focused on esophagogastric (EG) hepato-bilio-pancreatic (HPB) and colorectal (CR) cancers. Of note, in EG and HPB tumors, malnutrition is present in up to 70% of patients, and multimodal anticancer treatments are mandatory, laying the groundwork for a more horizontal and crosscutting shared multimodal nutritional interventions.
2. Methods
2.1. Survey design
In 2020, young sections of AIOM, SICO and AIRO planned common research goals and founded the NutriOnc Research Group, aimed at implementing multidisciplinary strategies to improve patient quality of life. A web-based survey consisting of a total of 15 multiple choice questions was conducted among young (≤40 years) medical oncologists, surgeons and radiotherapists or young trainees who were AIOM, SICO, AIRO members.
The questionnaire was organized in the following sections: 1. Knowledge and practices regarding Nutritional Management of cancer patients, 2. Timing of screening and assessment of Nutritional Status, 3. Nutritional Treatment and prescription criteria, and 4. Immunonutrition (IMN) and Educational topics.
• Section 1: Use of internal protocols and presence of nutritionists/dieticians as part of the multi-disciplinary team: medical nutritionist/physicians with clinical expertise in nutritional assessment, medical oncologist, surgeon, radiotherapist and trained nurse.
• Section 2: Pre-and In-hospital Nutritional Screening and Assessment, who oversees patients’ nutritional assessment, when nutritional assessment is conducted, and which questionnaires are administered.
• Section 3: Post-Hospital Nutritional Management, who oversees activating a home therapeutic plan, what kinds of nutritional therapy are planned, what are the main discomforts encountered by patients.
• Section 4: The level of knowledge of IMN or Immunoenhanced nutrition, suggestions for further discussion.
The modules were mainly focused on EG, HPB, and CR cancers.
2.2. Survey dissemination
All young (≤ 40 years) professionals were encouraged to participate by completing modules according to their practice. The questionnaire was distributed online on the AIOM, AIRO and SICO websites in a reserved section and was accessible only through a direct link sent by email on SurveyMonkey®. Overall, the survey could be accessed online from June 30th, 2021, to July 10th, 2021. Responses were anonymously evaluated and analyzed.
2.3. Statistical analysis
This survey considered the sample of participants who answered the questionnaire, and therefore no sample size was calculated for a specific hypothesis test. Summary statistical measures for continuous and categorical data were used to describe the sample of doctors’ characteristics and their answers to each specific item. Results were compared among different societies. Given the descriptive and exploratory intent of the analysis, no attempt to control for multiple tests was pursued.
3. Results
All of the 939 health care professionals representing the target population received the invitation, and 215 (21.5%) participated to the online survey. An overview of participants’ demographics and geographic distribution is provided in Table 1.
Among 215 respondents’ young health professionals, 101 (47%) were members of SICO, 56 (26%) of AIOM and 45 (21%) of AIRO, while 13 (6%) were not part of any society (Figure 2). The findings regarding the medical specialty were consistent with the results of the membership of SICO, AIOM and AIRO: surgeons (53%) medical oncologist (26.5%), and radiation oncologist (20.4%). Most of the participants were residents (38.1%) and physicians with less and equal to/more than 5 years of medical experience were 67 (31.2%) and 66 (30.7%), respectively.
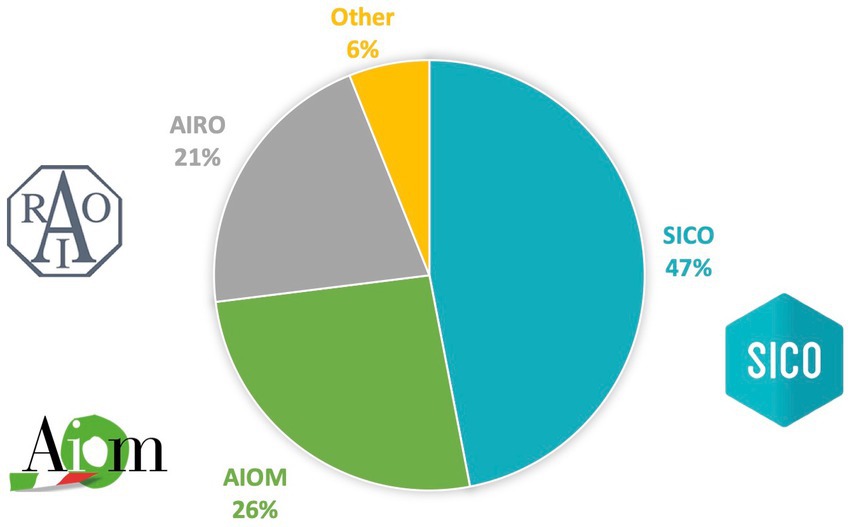
Figure 2. Affiliations of participants. Italian Association of Medical Oncology (AIOM), Italian Association of Radiotherapy and Clinical Oncology (AIRO) and Italian Society of Surgical Oncology (SICO).
Concerning the geographical distribution of the responders, 59 were from the Tuscany region, 29 were from Puglia, 20 from Lazio and 17 from Campania (Figure 3). In general, participants were equally distributed between north, center and south-insular Italy.
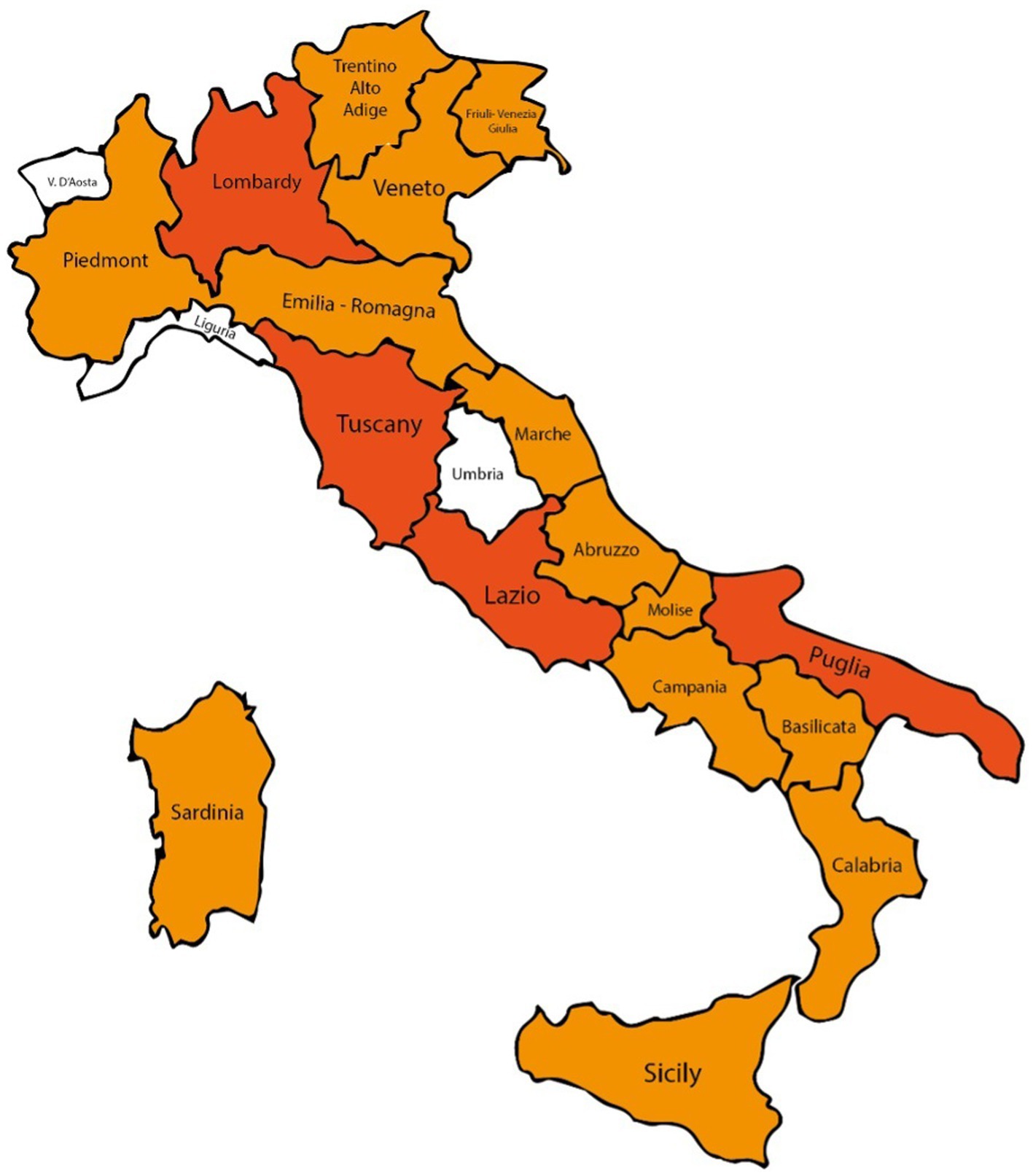
Figure 3. Italian map of survey participants: regions with >20 interviewees in dark orange, with 1–19 interviewees in orange, otherwise in white.
The physicians dealt with gastrointestinal cancers, and they were focused on CR (61%), EG (24.2%) and HPB (14.8%) cancers. Most of them (34.9%) defined the volume per year of locally advanced CR patients in their own institution as more than 100 patients. Sixty (27.9%) and 48 (22.3%) respondents treated more than 40 HPB and EG l cancer patients per year, respectively. Nearly half of the respondents adopted up-front surgery (42.5%) while 57.5% planned neoadjuvant treatments (radio/chemotherapy).
3.1. Knowledge and practices regarding nutritional management of cancer patients (Table 2; Q1-Q4)
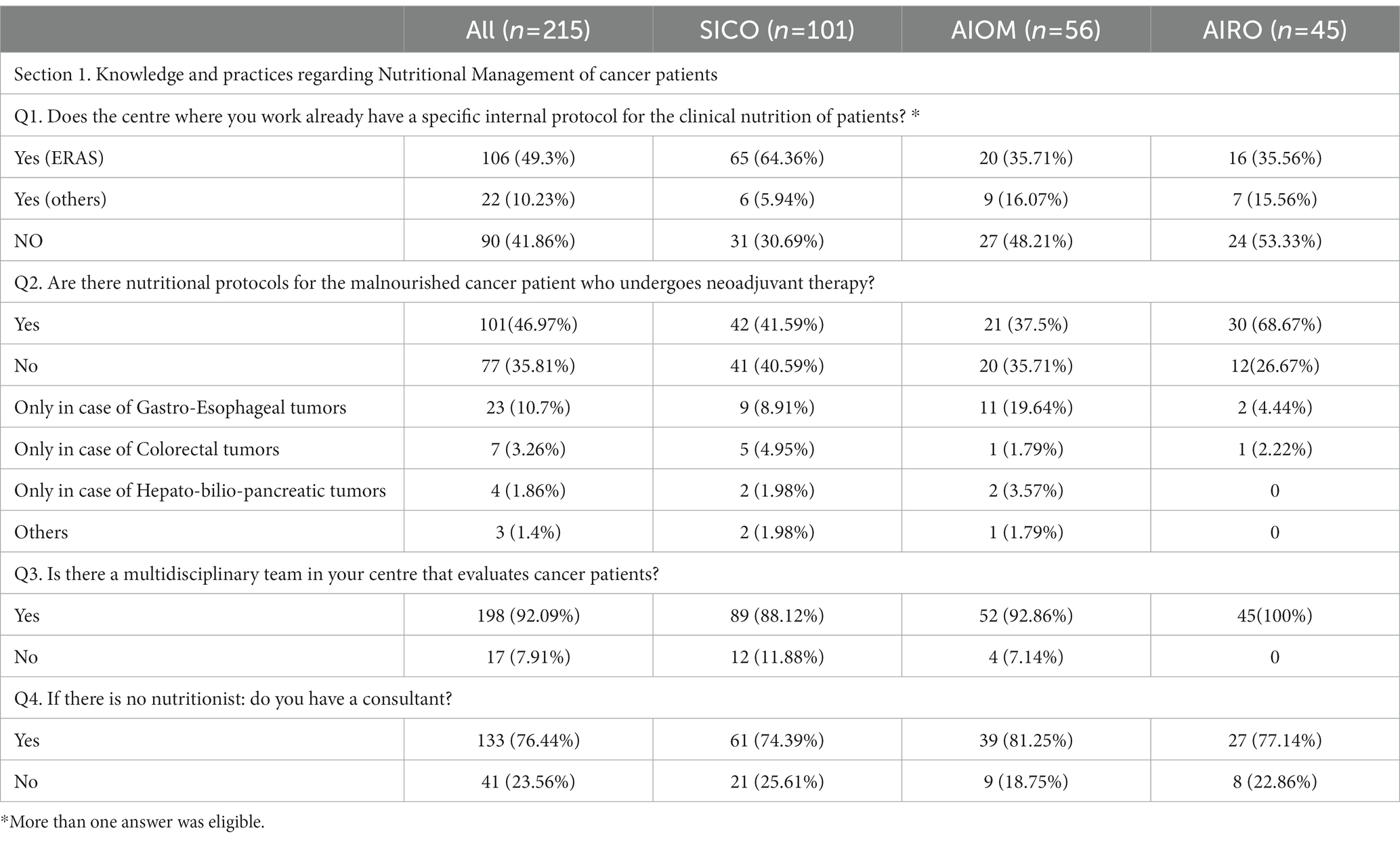
Table 2. Section 1: Knowledge and practices regarding nutritional management of cancer patients. Questionnaire answer distribution [n (%)].
Concerning the management of Nutritional Status of cancer patients, many respondents adopted the Enhanced Recovery After Surgery (ERAS) program (49.3%). Of note, a large number of specialists did not follow a specific validated nutritional care protocol (41.8%) due to a lack of educational courses (14.5%), financial support (15.3%) and understaffing (13%). Only 50.7% of health professionals were aware of specific protocols tailored to diagnose and treat malnutrition in cancer patients undergoing neoadjuvant treatment, because there is a practically lack of medical nutritional specialist in the institutions. Of note, nearly all the Institutions had a multidisciplinary team (92%) to finalize the treatment decision-making. In the absence of dietician, the nutritional assessment of patients performing oncological care was managed by physicians with clinical expertise in nutritional (76.4%).
3.2. Timing of screening and assessment of nutritional status (Table 3; Q5-Q8)
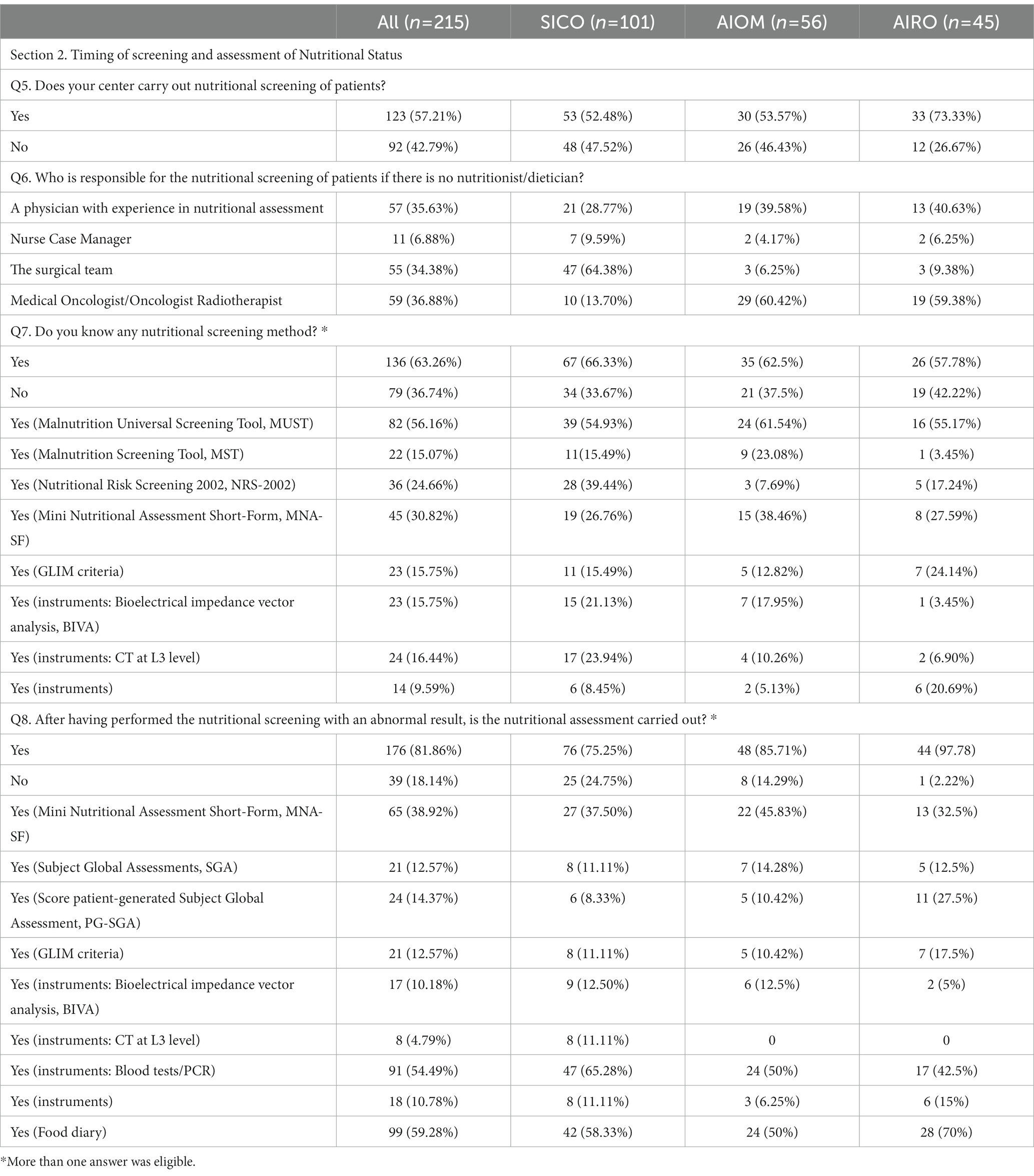
Table 3. Section 2: Timing of screening and assessment of Nutritional Status. Questionnaire answer distribution [n (%)].
Cancer patients underwent nutritional screening for 57.2% of respondents. In the absence of nutritional staff as described before, the nutritional screening was managed by physicians with clinical expertise in nutritional assessment (35.6%), by surgeons (34.3%), medical/radiation oncologists (36.8%) and by case-manager nurses (6.8%) (Figure 4A). The timing of nutritional assessment was at the first visit (37.8%), before surgery (25.9%), after surgery (16.7%), before radiochemotherapy (RT-CT) (13.5%) and after RT-CT (7%). Most of the respondents reported that nutritional status was assessed throughout the duration of cancer treatments (55.6%) (Figure 4B). Knowledge of nutritional screening tools was reported by 63.3% of physicians. Malnutrition Universal Screening Tool (MUST), Malnutrition Screening Tool (MST), Nutritional Risk Screening 2002 (NRS – 2002), Mini Nutritional Assessment Short-Form (MNA-SF) and Global Leadership Initiative on Malnutrition (GLIM) criteria were known by 56.1, 15, 30.8 and 15.7% of cases. Moreover, 23 (15.7%) respondents were aware of body composition analysis by means of bioimpedance analysis (Bioelectrical Impedance Vector Analysis, BIVA) and 24 (16.4%) of computed tomography (CT) scan of L3 vertebral body as nutritional screening tools (Figure 4C). Nutritional assessment has been performed in the 81.8% of cases reporting abnormal results at nutritional screening. The physicians reported the periodical assessment of the nutritional status of cancer patients during oncological care (60.5%). Concerning the timing, 31.3% conducted the nutritional assessment at diagnosis, 28.1% before surgery, 14.5% after surgery, 20.5% before RT-CT and 8.1% after RT-CT (Figure 4D). Haematochemical indices, such as serum albumin, pre-albumin, creatinine, iron levels, 3-methylhistidine, cholesterol and total lymphocyte (37) (54.4%), food diary (59.3%) and MNA-SF (38.9%) were the main methods adopted by the responders (Figure 4E).
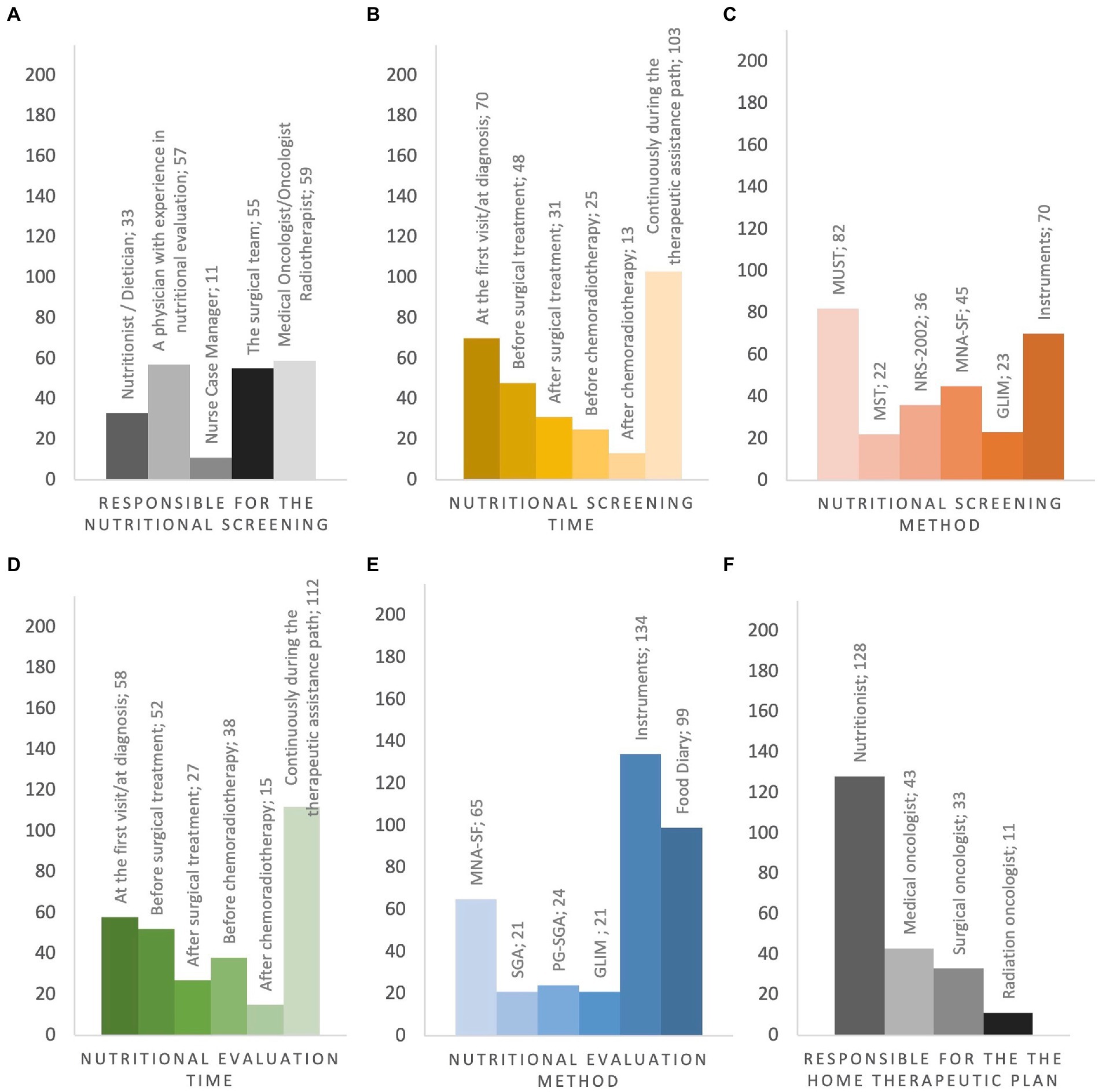
Figure 4. Pre-and In-hospital Nutritional Screening and Assessment, Post-Hospital Nutritional Management. (A) responsible for the nutritional screening; (B) when nutritional screening is performed; (C) type of questionnaire administered in nutritional screening; (D) when the severity of malnutrition is investigated; (E) type of questionnaire administered in nutritional grading assessment; (F) who is in charge of activating the home therapeutic plan. MUST Malnutrition Universal Screening Tool, MST Malnutrition Screening Tool, NRS-2002 Nutritional Risk Screening 2002, MNA-SF Mini Nutritional Assessment Short-Form, GLIM Global Leadership Initiative on Malnutrition, SGA Subject Global Assessments, PG-SGA Score patient-generated Subject Global Assessment, Instruments Bioelectrical impedance vector analysis, CT-scan at L3 level, Blood test.
3.3. Nutritional treatment and prescription criteria (Table 4; Q9-Q11)

Table 4. Section 3: Nutritional Treatment and prescription criteria. Questionnaire answer distribution [n (%)].
After discharge from the hospital, the prescription of nutritional support was provided by dieticians (59.5%), medical oncologists (20%), surgeons (15.3%), radiation oncologists (5.12%) (Figure 4F). The respondents were asked about the main issues regarding nutritional support facilities, available in their institutions. Most of them (44.2%) identified the difficulty of finding nutritional products in hospital pharmacies and for patients to have refundability as the main crucial factors. Seventy-seven (35.8%) specialists declared fatigue and loss of appetite as crucial factors concerning nutritional therapy. Similarly, respondents mentioned that nausea (29.3%), malabsorption (24.1%), dysphagia (21.8%), vomiting (18.6%) and mucositis (11.1%) negatively affected the management of nutritional therapy. Additionally, the absence of a dietician/nutritionist in the department (28.3%) as well as the lack of specific rules concerning the prescription criteria of nutritional support (13%) were described as further key issues as factor negatively affecting the optimal nutritional management. In cases of malnutrition (e.g., more than 5% of weight loss in the last 3 months) (38), the physician choices were nutritional counseling (34.8%), oral nutritional supplement (28.8%), enteral nutrition (2.8%), parenteral nutrition (3.3%), and no treatments (0.9%).
3.4. Immunonutrition and educational topics (Table 5; Q12-Q15)
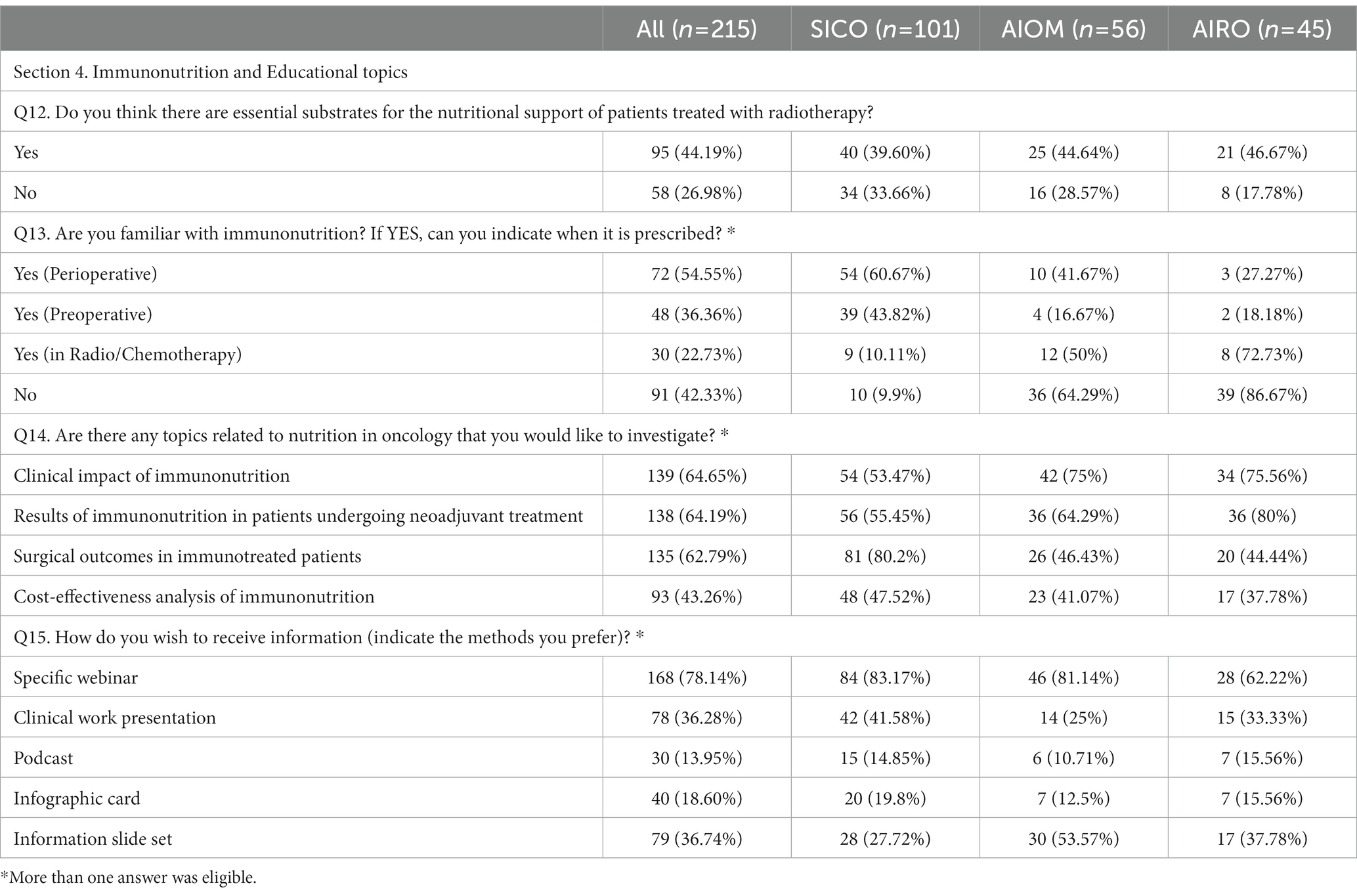
Table 5. Section 4: Immunonutrition and Educational topics. Questionnaire answer distribution [n (%)].
During radiotherapy, 95% of physicians declared that patients needed a supplementary nutrition. Arginine, Omega-3 fatty acids (omega-3 Fas), vitamins/antioxidants, Glutamine, Whey Protein (WP) and RNA were described as essential supplements during radiotherapy by 29.7, 39.5, 49.8, 23.3, 20.9 and 4.7% of respondents, respectively. Fifty-eight (27%) physicians stated that supplementation was not necessary during radiotherapy. Knowledge of IMN was reported by 58.1% of respondents. IMN was prescribed perioperatively (54.5%), before surgery (36.3%) and during neoadjuvant RT-CT (22.3%). One hundred physicians expressed interest in educational courses addressing nutritional issues as a whole. Webinar were mentioned as preferred modality for educational programs (78.1%).
4. Discussion
According to our results based on 215 physicians, almost half of the respondents declared to adopt the ERAS (Enhanced Recovery After Surgery) program (49.3%), while an important number of professionals did not follow a specific validated nutritional care protocol (41.8%) due several reasons, such as lack of educational courses (14.5%) and financial support (15.3%). In addition, nearly all the included centers had a multidisciplinary team (92%) and the timing of nutritional assessment was at diagnosis (37.8%), before surgery (25.9%), after surgery (16.7%), before radiochemotherapy (RT-CT) (13.5%) and after RT-CT (7%). Moreover, nutritional status was assessed throughout the duration of cancer treatments in 55.6% of cases.
Cancer-related malnutrition is very common among patients affected by solid malignancies, with a high percentage of patients experiencing malnutrition during the course of their disease due to several reasons, including the type of tumor, the disease stage and the type of treatment (e.g., surgery, chemotherapy, concomitant RT-CT, etc.) (39–41). Thus, it is mandatory to identify cancer patients at nutritional risk as a component of a proper, comprehensive care (42). Based on these premises, the delivering of optimal nutritional care is a crucial need for cancer patients. Early detection of nutritional status is essential to initiate nutritional treatment for preserving body composition and avoiding sarcopenia and cachexia, as the toxicity of anticancer therapies depends on body cell mass, and oncological clinical outcomes could be negatively related to malnutrition regardless of therapies (43–46).
In the current survey, we aimed to define the awareness of nutritional issues among young healthcare professionals delivering anticancer care, by providing an intersociety and multidisciplinary national survey supported by young sections of different associations. Despite a notable participation, nutritional screening was carried out at diagnosis by less than 40% of anticancer professionals. This is considered the first step in defining patients at risk of malnutrition or malnourished, followed by a nutritional assessment that may more deeply investigate and schedule the patients to a tailored nutritional support. As previously reported, only 50.7% of responders were aware of specific tools to diagnose timely malnutrition in cancer patients undergoing neoadjuvant treatment. An increasing awareness of nutritional pathway, especially in the surgical community (64.3%), is related to the wide adoption of ERAS protocol (47). Interestingly, no ERAS guidelines consider a specific nutritional protocol for patients scheduled to neoadjuvant treatment, but only perioperatively. Interestingly, this timeframe could represent a “window of opportunity” to define a specific and tailored protocol, to improve treatment compliance, quality of life, and to complete the specific neoadjuvant treatment. Fewer than 60% of the surveyed professionals report that nutritional assessment was carried out continuously throughout the therapeutic care pathway, which calls for its implementation and increased use. The current survey underlines the importance of proper nutritional screening and assessment by health professionals and highlights that an important gap between current delivery and the need for nutritional assessment and care persists. Some strengths and limitations of the current survey should be highlighted. Among the strengths of this study, it is worth noting that this survey included the young sections of three different anticancer Italian associations (AIOM, AIRO, and SICO, merged into the NutriOnc Research Group) and an overall high number of included professionals and Institutions while its limitations include the lack of data on clinical outcomes. Our findings are partially in line with previous experiences suggesting that a remarkable proportion of interviewed professionals does not follow a specific nutritional protocol in the daily clinical practice due to several reasons (e.g., lack of financial support, lack of educational courses, lack of human resources) (48). The value of using different screening tools has practical limitations: parameters should be easy, quick, useful in different populations, and carried out by both dieticians and physicians with clinical expertise in nutritional assessment. Our group would encourage the use of single, shared screening tools, such as MST, to screen all adults for malnutrition in all settings, according to the last position of the Academy of Nutrition and Dietetic (39).
As previously reported, nutritional screening has been performed globally in the 57.2%, in low percentage by a nutritionist, while in high percentage by surgeons, oncologists and radiotherapists or physicians with “an experience in nutritional issue.” The nutritional assessment was performed by nurse case managers in only 6.8% of cases. On the one hand, these data suggest the poor inclusion of nutritionist medical specialists in several centers, while these figures should be considered of pivotal importance in this setting. On the other hand, given the lack of financial support and human resources, the disappointing data regarding nurse case managers poses some reflections. In our opinion, this health care figure should be more actively engaged in the nutritional pathway of cancer patients, ranging from the diagnosis to the follow-up of cancer. The central role of nurses has been also suggested by a study published by McHugh and colleagues, since the presence of specialized nurses has an impact in terms of better outcomes, reduced length of hospital stay, less readmissions, and even a decreased mortality rate (49).
At the same time, the current study underlines a higher awareness of nutritional assessment as a fundamental component of cancer patients care in younger health professionals compared with previous multicenter reports, including the study published by Caccialanza and colleagues (34). In this previous Italian experience, the authors observed that nutritional assessment was performed by less than 30% of oncologists at diagnosis, a percentage which is significantly lower compared to that observed in the current survey. According to 42% of oncologists included in this previous Italian study, nutritional assessment was carried out only after patients requested it, and almost 60% of patient affiliates were not aware of clinical referrals for home artificial nutrition management. At the same time, for almost all respondents, the nutritional status assessment was considered fundamental in predicting tolerance to anticancer treatment. In another German survey across certified oncologic care institutions, the authors reported that only around 30% of patients received nutritional counseling, regardless of their nutritional status and disease stage, and none of the included centers presented a systematic screening procedure and had paucity of dieticians available (50).
Another point to highlight includes the presence of a multidisciplinary team, which was reported in 92% of the Institutions included in the current survey. This is particularly important since measuring and monitoring metabolic and nutritional status should be reviewed as a defined, standardized part of each multidisciplinary team process, and the presence of specialist oncology dieticians should be encouraged as an essential component of team and involved in all phases of cancer patient management (51, 52). In fact, the high frequency of nutritional issues in each phase of cancer care, ranging from diagnosis to end of life, makes the integration of nutrition assessment within multidisciplinary teams a key topic.
At the same time, if malnutrition seems to be perceived as a severely limiting factor by both patients and anticancer professionals, adherence to recommendations and guidelines appears low. The development of specific and defined care protocols and the adherence to these shared tools may be one of the keys to improve nutritional support management. Increased awareness of the impact of malnutrition on clinical outcomes is crucial to allow for high-quality nutrition treatments before, during, and following anticancer therapies. In addition, the most commonly used tools are based on the items established in the ERAS protocol being efficient and with adequate specificity and sensitivity, something that should help a wider diffusion of nutritional assessment (25). Of note, through the application of ERAS items protocol IMN has gained more awareness, especially in the perioperative period. However, our survey demonstrated a wide variation among surgeons, oncologist, and radiotherapist in “familiarity” about IMN, while most of the responders consider IMN in a future and common area of interest represented by the impact on clinical outcome in patients undergoing neoadjuvant and surgical treatment and finally cost-effectiveness.
As previously stated, if the impact of early nutritional intervention on quality of life and clinical outcomes of cancer patients is widely recognized, some steps forward remain to be taken, and unfortunately, nutritional assessment is frequently neglected. Certainly, healthcare professionals delivering anticancer care have a long way to go to make nutrition care a standardized component of cancer management, but positive signals emanating from this intersociety and multidisciplinary national survey should encourage anticancer professionals to improve systematic nutritional screening and management of oncological patients.
5. Conclusion
To the best of the Authors’ knowledge, the current study represents the first survey in literature supported by young sections of three healthcare professionals delivering anticancer care organizations from Italy (AIOM, AIRO, and SICO). Although the results could be affected by a not wide response rate from the target population receiving the invitation, our findings could suggest the persistence of an important gap between current delivery and need of nutritional assessment due to several reasons, and nutritional screening was performed globally in the 57.2% of cases, and in low percentage by a nutritionist. Despite the importance of the assessment of nutritional status in cancer patients is widely recognized by anticancer professionals, some questions remain unanswered, and our results call for an implementation, development, and larger use of specific tools able to perform nutritional assessment in this setting, and to improve nutritional support management in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Group members of NutriOnc Research Group
NutriOnc Research Group Collaborators:
SICO: Silvia Sofia, Marco Milone, Benedetto Ielpo, Maria Teresa Mita, Silvia Ministrini, Mario Giuffrida, Roberta Tutino, Caterina Baldi, Giampaolo Perri, Anna Stella Lippolis, Chiara Marafante, Giusy Giannandrea, Marco Vito Marino, Letizia Laface, Salomone Di Saverio, Luca Aldrighetti, Nicola de’Angelis, Nick Salimian, Marco Caricato, Gianluca Pellino, Sara Vertaldi, Federica Cipriani, Gabriella Teresa Capolupo, Antonio Costanzo, Letizia Santandrea, Gaetano Gallo, Andrea Belli, Laura Mastrangelo, Fausto Rosa, Nicolò Pecorelli, Graziella Marino, Alessio Giordano, Nicola Cillara, Maria Lemma, Francesco Pata, Federico Cammillini, Gianmario Edoardo Poto, Giulia Grassi, Donato Francesco Altomare, Arcangelo Picciariello, Lorenzo Petagna, Luca Ippolito, Elio Treppiedi, Daniele Delogu, Abdallah Moukachar, Stefano Granieri, Giuseppe Cuticone, Osvaldo Carpineto Samorani, Daniela Rega, Leonardo Solaini, Stefano de Pascale, Francesca Ascari, Michele Manigrasso, Simona Badalucco, Salvatore Paiella, Sara Coppola, Roberta Iadarola, Giovanna Di Meo, Isacco Montroni, Fabio Vistoli, Valentina Ferraro, Edoardo Saladino, Federico Fazio, Roberta Rota, Francesco Orlando, Simone Famularo, Cinzia Bizzoca, Giorgio Dalmonte, Marco Inama, Luigi Verre, Leandro Siragusa, Casoni Pattacini Gianmaria, Michele Benedetti, Nicolò Tamini, Cristian Conti, Giorgio Ammerata, Serena Mantova, Vito Leonardo Pinto, Arianna Corvasce, Giorgio Micheletti, Teresa Perra, Marco Pellicciaro, Marco Materazzo, Michele Zuolo, Emanuele Doria, Antonio Brillantino, Luca Del Prete, Andrea Muratore, Claudio Luciani, Giulia Turri, Fabio Casciani, Giuliani Giuseppe, Graziana Barile, Gaia Oldrà, Valeria Restaino, Simona Deidda, Michele Ammendola, Andrea Fares Bucci, Patrizia Marsanic, Dario Cassetti, Luca Resca, Daniele Fusario, Eleonora Andreucci.
AIOM: Anna Michelotti, Brunella Amoruso, Isabella Franco, Laura Noto, Andrea Spallanzani, Raimondo Calogero Scalia, Teresa Del Giudice, Valeria Merz, Gianmarco Motta, Alessandro Parisi, Mikol Modesti, Antonella Argentiero, Debora Basile, Gianmarco Vannini, Carlotta Ottanelli, Salvatore Corallo, Eufemia Stefania Lutrino, Daniele Rossini, Federica Morano, Luigia Stefania Stucci, Costanza Winchler, Martina Catalano, Andrea Marini, Giuseppe Brisinda, Enrico Sammarco, Martina Carullo, Giandomenico Roviello, Mirko Barone, Maria Grazia Rodriquenz, Giuseppe Tirino, Alessia Amoruso, Anna Russo, Veronica Conca, Laura Orgiano, Sveva Macrini, Giulia Nazzicone, Maria Bensi, Martina Montesano, Emanuela Dell’Aquila, Andrea Sbrana, Beatrice Borelli, Lorenzo Fornaro, Lucrezia Raimondi, Valeria Zurlo, Mattia Garutti, Elena Ongaro, Arianna Pellegrino, Andrea lanese, Laura Bernardini, Alessandra Boccaccino, Patrizia Farina, Federica Buzzacchino, Angelica Petrillo, Ada Taravella, Vittorio Studiale, Paolo Ciracì.
AIRO: Giovanna Lovino, Dora Di Cosmo, Sabrina Montrone, Fabiana Gregucci, Luca Dominici, Alba Fiorentino, Filippo Carannante, Giambattista Siepe, Giampaolo Montesi, Manuele Roghi, Michele Aquilano, Andrea Romei, Ilaria Bonaparte, Roberta Grassi, Emma D’Ippolito, Giulio Frosini, Giuseppina De Marco, Gennaro Giovine, Chiara Mattioli, Ilaria Morelli, Victoria Lorenzetti, Matteo Mariotti, Carolina Orsatti, Vincenzo Troncone, Lorenzo Livi, Antonio Angrisani, Marco Banini, Teresa Di Pietro, Giuseppe Carlo Iorio, Iacopo Cavallo, Cecilia Cerbai, Valerio Nardone, Francesca De Felice, Consuelo Rosa, Giulia Stocchi, Sara Lucidi, Michele Ganovelli, Damiano Dei, Chiara Cascone, Anna Peru, Luisa Caprara, Lucia Angelini, Luca Visani, Giulio Francolini, Beatrice Bettazzi.
Non-affiliated: Francesco Belia, Virginia Boccardi, Simone Serafini, Vincenzo Bottino, Luca Ferrario, Giuseppe Frazzetta, Felice Pirozzi, Gennaro Martines, Francesco Antonio Ciarleglio, Daniele Indiani, Giuliano Barugola, Luca Mazza, Chiara Guarini.
Funding
The study was received funding from Nestlè Health Science. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors thank Barbara Borlotti Amico (Nestlè Health Science) and Iside Morabito (Isi Events) for support, Mariateresa Cirillo for graphic design. The authors affiliated to the IRCCS Istituto Tumori “Giovanni Paolo II” in Bari are responsible for the views expressed in this article, which do not necessarily represent the Institute.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. De Maria, MR, Di Sante, G, Piro, G, Carbone, C, Tortora, G, Boldrini, L, et al. Translational research in the era of precision medicine: where we are and where we will go. J Pers Med. (2021) 11:216. doi: 10.3390/jpm11030216
2. Zhang, X, Tang, M, Zhang, Q, Zhang, K-P, Guo, Z-Q, Xu, H-X, et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin Nutr. (2021) 40:1224–32. doi: 10.1016/j.clnu.2020.08.004
3. Qin, L, Tian, Q, Zhu, W, and Wu, B. The validity of the GLIM criteria for malnutrition in hospitalized patients with gastric cancer. Nutr Cancer. (2021) 73:2732–9. doi: 10.1080/01635581.2020.1856894
4. Baracos, VE. Cancer-associated malnutrition. Eur J Clin Nutr. (2018) 72:1255–9. doi: 10.1038/s41430-018-0245-4
5. Garla, P, Waitzberg, DL, and Tesser, A. Nutritional therapy in gastrointestinal cancers. Gastroenterol Clin N Am. (2018) 47:231–42. doi: 10.1016/j.gtc.2017.09.009
6. Virizuela, JA, Camblor-Álvarez, M, Luengo-Pérez, LM, Grande, E, Álvarez-Hernández, J, Sendrós-Madroño, MJ, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Transl Oncol. (2018) 20:619–29. doi: 10.1007/s12094-017-1757-4
7. Castillo-Martínez, L, Castro-Eguiluz, D, Copca-Mendoza, ET, Pérez-Camargo, DA, Reyes-Torres, CA, Damasco-Ávila, EA, et al. Nutritional assessment tools for the identification of malnutrition and nutritional risk associated with cancer treatment. Rev Investig Clin. (2018) 70:121–5. doi: 10.24875/RIC.18002524
8. Lim, SL, Ong, KCB, Chan, YH, Loke, WC, Ferguson, M, and Daniels, L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. (2012) 31:345–50. doi: 10.1016/j.clnu.2011.11.001
9. Contreras-Bolívar, S-T, Ruiz-Vico, G-A, Barrios, P, and Alba, O. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer inpatients. Nutrients. (2019) 11:2043. doi: 10.3390/nu11092043
10. Kim, J-Y, Wie, G-A, Cho, Y-A, Kim, S-Y, Kim, S-M, Son, K-H, et al. Development and validation of a nutrition screening tool for hospitalized cancer patients. Clin Nutr. (2011) 30:724–9. doi: 10.1016/j.clnu.2011.06.001
11. Muscaritoli, M, Lucia, S, Farcomeni, A, Lorusso, V, Saracino, V, Barone, C, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. (2017) 8:79884–96. doi: 10.18632/oncotarget.20168
12. Beirer, A. Malnutrition and cancer, diagnosis and treatment. Eur Soc Med Oncol. (2021) 14:168–73. doi: 10.1007/s12254-020-00672-3
13. Song, C, Cao, J, Zhang, F, Wang, C, Guo, Z, Lin, Y, et al. Nutritional risk assessment by scored patient-generated subjective global assessment associated with demographic characteristics in 23,904 common malignant Tumors patients. Nutr Cancer. (2019) 71:50–60. doi: 10.1080/01635581.2019.1566478
14. Poulter, S, Steer, B, Baguley, B, Edbrooke, L, and Kiss, N. Comparison of the GLIM, ESPEN and ICD-10 criteria to diagnose malnutrition and predict 30-day outcomes: an observational study in an oncology population. Nutrients. (2021) 13:2602. doi: 10.3390/nu13082602
15. Zhang, Z, Wan, Z, Zhu, Y, Zhang, L, Zhang, L, and Wan, H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: a multi-center study. Nutrition. (2021) 83:111072. doi: 10.1016/j.nut.2020.111072
16. Gascón-Ruiz, M, Casas-Deza, D, Torres-Ramón, I, Zapata-García, M, Alonso, N, Sesma, A, et al. GLIM vs ESPEN criteria for the diagnosis of early malnutrition in oncological outpatients. Clin Nutr. (2021) 40:3741–7. doi: 10.1016/j.clnu.2021.04.025
17. Hébuterne, X, Lemarié, E, Michallet, M, de Montreuil, CB, Schneider, SM, and Goldwasser, F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. (2014) 38:196–204. doi: 10.1177/0148607113502674
18. Bozzetti, F, SCRINIO Working Group. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer. (2009) 17:279–84. doi: 10.1007/s00520-008-0476-3
19. Cederholm, T, Jensen, GL, Correia, MITD, Gonzalez, MC, Fukushima, R, Higashiguchi, T, et al. GLIM criteria for the diagnosis of malnutrition–a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
20. Hu, W-H, Cajas-Monson, LC, Eisenstein, S, Parry, L, Cosman, B, and Ramamoorthy, S. Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS-NSQIP. Nutr J. (2015) 14:91. doi: 10.1186/s12937-015-0081-5
21. Santos, I, Mendes, L, Mansinho, H, and Santos, CA. Nutritional status and functional status of the pancreatic cancer patients and the impact of adjacent symptoms. Clin Nutr. (2021) 40:5486–93. doi: 10.1016/j.clnu.2021.09.019
22. Gianotti, L, Fumagalli Romario, U, De Pascale, S, Weindelmayer, J, Mengardo, V, Sandini, M, et al. Association between compliance to an enhanced recovery protocol and outcome after elective surgery for gastric cancer. Results from a Western population-based prospective Multicenter study. World J Surg. (2019) 43:2490–8. doi: 10.1007/s00268-019-05068-x
23. Schwegler, I, von Holzen, A, Gutzwiller, J-P, Schlumpf, R, Mühlebach, S, and Stanga, Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. (2009) 97:92–7. doi: 10.1002/bjs.6805
24. Hopkinson, JB, Wright, DNM, McDonald, JW, and Corner, JL. The prevalence of concern about weight loss and change in eating habits in people with advanced cancer. J Pain Symptom Manag. (2006) 32:322–31. doi: 10.1016/j.jpainsymman.2006.05.012
25. Sandrucci, S, Beets, G, Braga, M, Dejong, K, and Demartines, N. Perioperative nutrition and enhanced recovery after surgery in gastrointestinal cancer patients. A position paper by the ESSO task force in collaboration with the ERAS society (ERAS coalition). Eur J Surg Oncol. (2018) 44:509–14. doi: 10.1016/j.ejso.2017.12.010
26. Santarpia, L, Contaldo, F, and Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle. (2011) 2:27–35. doi: 10.1007/s13539-011-0022-x
27. Zhang, K-P, Tang, M, Fu, Z-M, Zhang, Q, Zhang, X, Guo, Z-Q, et al. Global leadership initiative on malnutrition criteria as a nutrition assessment tool for patients with cancer. Nutrition. (2021) 91–92:111379. doi: 10.1016/j.nut.2021.111379
28. Lim, H-S, Cho, G-S, Park, Y-H, and Kim, S-K. Comparison of quality of life and nutritional status in gastric cancer patients undergoing Gastrectomies. Clin Nutr Res. (2015) 4:153–9. doi: 10.7762/cnr.2015.4.3.153
29. Andreyev, HJN, Norman, AR, Oates, J, and Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. (1998) 34:503–9. doi: 10.1016/S0959-8049(97)10090-9
30. Seo, SH, Kim, S-E, Kang, Y-K, Ryoo, B-Y, Ryu, M-H, Jeong, JH, et al. Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer. (2016) 16:900. doi: 10.1186/s12885-016-2934-5
31. Arends, J, Baracos, V, Bertz, H, Bozzetti, F, Calder, PC, Deutz, NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. (2017) 36:1187–96. doi: 10.1016/j.clnu.2017.06.017
32. Cederholm, T, Barazzoni, R, Austin, P, Ballmer, P, Biolo, G, Bischoff, SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
33. Weimann, A, Braga, M, Carli, F, Higashiguchi, T, Hübner, M, Klek, S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. (2017) 36:623–50. doi: 10.1016/j.clnu.2017.02.013
34. Caccialanza, R, Lobascio, F, Cereda, E, Aprile, G, Farina, G, Traclò, F, et al. Cancer-related malnutrition management: a survey among Italian oncology units and patients’ associations. Curr Probl Cancer. (2020) 44:100554. doi: 10.1016/j.currproblcancer.2020.100554
35. Sonmez, O, Tezcanli, E, Bas, D, Kazanci, HB, Altinok, A, Demir, A, et al. Identifying knowledge and practices regarding cancer patient malnutrition: a survey study among oncologists. Nutr Cancer. (2022) 74:2392–9. doi: 10.1080/01635581.2021.2008987
36. Lorenzon, L, Brandl, A, Guiral, DC, Hoogwater, F, Lundon, D, Marano, L, et al. Nutritional assessment in surgical oncology: an ESSO-EYSAC global survey. Eur J Surg Oncol. (2020) 46:2074–82. doi: 10.1016/j.ejso.2020.08.028
37. Serón-Arbeloa, C, Labarta-Monzón, L, Puzo-Foncillas, J, Mallor-Bonet, T, Lafita-López, A, Bueno-Vidales, N, et al. Malnutrition screening and assessment. Nutrients. (2022) 14:2392. doi: 10.3390/nu14122392
38. Sieber, CC. Malnutrition and sarcopenia. Aging Clin Exp Res. (2019) 31:793–8. doi: 10.1007/s40520-019-01170-1
39. Reber, E, Schönenberger, KA, Vasiloglou, MF, and Stanga, Z. Nutritional risk screening in cancer patients: the first step toward better clinical outcome. Front Nutr. (2021) 8:603936. doi: 10.3389/fnut.2021.603936
40. Taylor, SR, Falcone, JN, Cantley, LC, and Goncalves, MD. Developing dietary interventions as therapy for cancer. Nat Rev Cancer. (2022) 22:452–66. doi: 10.1038/s41568-022-00485-y
41. Marano, L, Porfidia, R, Pezzella, M, Grassia, M, Petrillo, M, Esposito, G, et al. Clinical and immunological impact of early postoperative enteral Immunonutrition after Total Gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. (2013) 20:3912–8. doi: 10.1245/s10434-013-3088-1
42. Baracos, VE, Martin, L, Korc, M, Guttridge, DC, and Fearon, KCH. Cancer-associated cachexia. Nat Rev Dis Prim. (2018) 4:17105. doi: 10.1038/nrdp.2017.105
43. Sukkar, SG. The impact of clinical nutrition on cancer therapy: a frequently underestimated perspective. A complementary approach to cancer patients. Med J Nutr Metab. (2012) 5:75–9. doi: 10.1007/s12349-012-0105-z
44. Bozzetti, F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. (2017) 28:2107–18. doi: 10.1093/annonc/mdx271
45. Prado, CM, Purcell, SA, and Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. (2020) 11:366–80. doi: 10.1002/jcsm.12525
46. Bossi, P, Delrio, P, Mascheroni, A, and Zanetti, M. The Spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients. (2021) 13:1980. doi: 10.3390/nu13061980
47. Weimann, A. Is there a rationale for perioperative nutrition therapy in the times of ERAS? Innov Surg Sci. (2019) 4:152–7. doi: 10.1515/iss-2019-0012
48. Bozzetti, F, Gurizzan, C, Lal, S, Van Gossum, A, Wanten, G, Golusinski, W, et al. A European survey on the practice of nutritional interventions in head–neck cancer patients undergoing curative treatment with radio(chemo)therapy. Eur Arch Otorhinolaryngol. (2022) 279:1499–508. doi: 10.1007/s00405-021-06920-4
49. McHugh, MD, Aiken, LH, Sloane, DM, Windsor, C, Douglas, C, and Yates, P. Effects of nurse-to-patient ratio legislation on nurse staffing and patient mortality, readmissions, and length of stay: a prospective study in a panel of hospitals. Lancet. (2021) 397:1905–13. doi: 10.1016/S0140-6736(21)00768-6
50. Hauner, H, Kocsis, A, Jaeckel, B, Martignoni, M, Hauner, D, and Holzapfel, C. Häufigkeit eines risikos für mangelernährung bei patienten in onkologischen schwerpunktpraxen–eine querschnittserhebung. Dtsch Med Wochenschr. (2020) 145:e1–9. doi: 10.1055/a-1008-5702
51. Taberna, M, Gil Moncayo, F, Jané-Salas, E, Antonio, M, Arribas, L, Vilajosana, E, et al. The multidisciplinary team (MDT) approach and quality of care. Front Oncologia. (2020) 10:85. doi: 10.3389/fonc.2020.00085
52. Lin, J-X, Chen, X-W, Chen, Z-H, Huang, X-Y, Yang, J-J, Xing, Y-F, et al. A multidisciplinary team approach for nutritional interventions conducted by specialist nurses in patients with advanced colorectal cancer undergoing chemotherapy. Medicine (Baltimore). (2017) 96:e7373. doi: 10.1097/MD.0000000000007373
Keywords: clinical nutrition, cancer, malnutrition, nutritional assessment, survey
Citation: Marano L, Marmorino F, Desideri I, Carbone L, Rizzo A, Salvestrini V, Roviello F, Cinieri S, Donato V, De Luca R and NutriOnc Research Group (2023) Clinical nutrition in surgical oncology: Young AIOM-AIRO-SICO multidisciplinary national survey on behalf of NutriOnc research group. Front. Nutr. 10:1045022. doi: 10.3389/fnut.2023.1045022
Edited by:
Shanjun Tan, Zhongshan Hospital, Fudan University, ChinaReviewed by:
Samir Giuseppe Sukkar, San Martino Hospital (IRCCS), ItalyAlessandro Laviano, Sapienza University of Rome, Italy
Copyright © 2023 Marano, Marmorino, Desideri, Carbone, Rizzo, Salvestrini, Roviello, Cinieri, Donato, De Luca R and NutriOnc Research Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ludovico Carbone, bHVkb3ZpY29jYXJib25lMUBnbWFpbC5jb20=
†These authors have contributed equally to this work
‡ORCID: Ludovico Carbone, https://orcid.org/0000-0003-2862-6788
 Luigi Marano
Luigi Marano Federica Marmorino
Federica Marmorino Isacco Desideri3†
Isacco Desideri3† Ludovico Carbone
Ludovico Carbone Alessandro Rizzo
Alessandro Rizzo Viola Salvestrini
Viola Salvestrini Raffaele De Luca
Raffaele De Luca