- 1Department of Ophthalmology, The Fourth Affiliated Hospital of China Medical University, Shenyang, China
- 2Department of Ophthalmology, Eye Hospital of China Medical University, Shenyang, China
- 3Key Lens Research Laboratory of Liaoning Province, Shenyang, China
Introduction: Cataract is one of the leading causes of blindness and visual impairment, about 16 million people around the world. Trace elements play an important role in a variety of the processes in human body. This study aimed to investigate the association between daily dietary intake of trace elements and age-related cataract incidence based on data from the National Health and Nutrition Examination Survey (NHANES) 2001–2008.
Methods: Iron, zinc, copper, and selenium were conducted in this study among subjects aged 50 years and older for African Americans and 55 and older in US adults. Multivariate logistic regression analysis was used in different models to investigate the association of trace elements intake and cataract.
Results: After screening, 7,525 subjects were ultimately included in this study. A significant negative association was found between selenium intake and cataract incidence in adjusted models using multivariate logistic regression analysis (model 1: OR = 0.998, 95% CI = 0.997–1.000; model 2: OR = 0.997, 95% CI = 0.995–1.000; and model 3: OR = 0.998, 95% CI = 0.995–1.000). After dividing selenium intake into quintiles, significant negative associations between selenium intake and cataract were observed in the first quintile of model 3, the fourth and fifth quintiles of all models. In subgroup analyses adjusted for age and sex, a significant negative association was observed only in women aged 65–74 years.
Discussion: Our study points out that maintaining daily dietary selenium intake at higher levels is helpful for cataract prevention, and that increasing daily dietary selenium intake in American women aged 65–74 years may contribute to the prevention of age-related cataract. The intakes of iron, zinc, copper may not be associated with age-related cataract.
1. Introduction
Cataract is usually defined as opacification of the lens. Cataract is one of the leading causes of blindness and visual impairment, about 16 million people around the world (1–5). More than 541,000 cataract extraction procedures are performed at a cost of more than $3.8 billion each year in the United States (6). This indicates that cataract has a serious burden on human health and socioeconomy. Age related cataract is usually defined as cataract occurring at age 50 and older (7). Oxidative stress mechanisms is considered to have a part in the pathological process of cataract formation: when oxidative damage in the lens continuously accumulates until its intrinsic antioxidant capacity is exceeded, it will lead to the aggregation of lens proteins and apoptosis of human lens epithelial cells (8, 9). Epidemiological studies have revealed several risk factors for age related cataract, such as age, obesity, diabetes, smoking, and low socioeconomic status (10–14).
Currently cataract treatment modalities for which efficacy has been affirmed are surgery only, and the most commonly used surgical strategy is phacoemulsification and lens replacement (15). Small incision cataract surgery has been widely used, which accelerates the post-operative recovery of patients and improves the quality of post-operative vision (4). However, because the complications associated with cataract surgery similarly affect the visual quality of patients, such as posterior capsular disruption, retinal detachment, progressive myopic traction maculopathy, etc. (16–18). Therefore, effective prevention of cataractogenesis is perhaps the best way to combat the visual damage brought about by cataracts.
Trace elements in the human body include copper, selenium, zinc, manganese, cobalt, chromium, and molybdenum, among others, which function as co-factors or as prosthetic groups located in the enzymes (19–23). The role of these trace elements in diseases is the focus of current research. Currently, researchers have observed that differences in trace element levels exist in the aqueous humor, lens, plasma of cataract patients (24, 25). This difference is similarly present in other age-related eye diseases (26). Shearer et al. found that only selenium was able to cause cataract alone, and that seven other trace elements prevented cataract induced by selenium, with mercury showing the strongest protective ability (27). Selenite also has a strong ability to induce cataract, and because of this property, a rat model of selenite induced cataract has been widely used in cataract related research (28–30). These studies were all able to indicate the close relationship between trace elements and cataract. However, recently Post et al. found that low serum selenium levels maybe a risk factor of age-related cataract (31). This is in contrast to previous findings, perhaps indicating that the effect of selenium on cataract remains to be explored in a deeper step. The effects of other trace elements on cataracts and the specific molecular mechanism are also urgent to be confirmed by further studies.
To further explore the relationship between trace elements and cataract, we carried out this study. This study used National Health and Nutrition Examination Survey (NHANES) database and aimed to analyze the association between trace element intake and incidence of cataract, which may provide a foundation for guiding trace element supplementation.
2. Materials and methods
2.1. Data source and subjects selection
This study is based on NHANES data from 2001 to 2008. NHANES is a national cross-sectional research program aimed to assess the physical status of ordinary Americans, performed by the National Center for Health Statistics (NCHS). The NHANES subjects were all U.S. non-institutionalized civilian participants, all of whom had accepted comprehensive measurements. Each cycle of NHANES is an independent cross-sectional survey. The NCHS research ethics review board approved the survey protocol for NHANES. All participants gave written informed consent (32).
Referred to previous studies, we set the inclusion criteria for age as 50 years or older for non-Hispanic blacks and 55 years or older for other races (33). In the study, a total of 28,332 subjects who underwent ophthalmic examination were identified, and 7,525 subjects were finally included. 20,807 subjects were excluded for the following reasons: (1) No valid data on cataract diagnosis; (2) No valid data on trace element intake; (3) Not meeting the above age inclusion criteria.
2.2. Cataract identification criteria
National Health and Nutrition Examination Survey asked participants aged 20 years and older if they had undergone ophthalmic surgery for cataracts prior to their ocular examination (34). If participants answered yes, they were defined as having cataract in this study. Participants with non-response or uncertain response were excluded. Because of the increased rate and lower threshold for cataract surgery in the United States (4, 35), self-reported cataract surgery may be able to represent clinically meaningful cataract. This defining criterion for cataract has also been used in previous studies (36, 37).
2.3. Determination of intake of various trace elements and daily energy intake
Dietary data were collected in the in-person interview using the Automated Multiple Pass Method (AMPM). Participants recalled all of the foods they had consumed on the previous day and told staff. The staff calculated the amount of various nutrients they ingested daily based on what the subjects said. The AMPM is a USDA′s dietary data collection instrument and a fully computerized recall method. The NHANES Mobile Examination Center (MEC) provided a set of measuring guides that facilitated participants to describe the amount of foods they had ingested (38).
Four trace elements of iron, copper, zinc, selenium were included in our study. Because in the NHANES dietary survey database, there are no dietary intake data for other trace elements except these four. Daily energy intake was used to represent the total amount of food the participants consumed on a daily basis.
2.4. Covariates assessment
We selected demographic variables such as age, race/ethnicity, sex, and education level to be included as covariates in this study. These demographic data were obtained through computer-assisted face-to-face interviews (39). Social status and living status affect physical wellbeing. But these indicators could not be quantified, so we took the above demographic data to evaluate the social status and living status of participants.
Diabetes mellitus, smoking, obesity/overweight are all risk factors for age-related cataract, therefore (40–44), they were also included as covariates in this study. Diabetes status was defined by self-reported diagnosis (45). Smoking status was defined by serum cotinine levels to reflect both direct and indirect smoking quantity (46, 47). Obesity/overweight was reflected by body mass index (BMI). BMI was calculated by the weight in kilograms divided by the square of height in meters (kg/m2) (48).
2.5. Statistical analysis
All statistical analyses were performed using SAS 9.4. NHANES uses a stratified, multistage sampling method, so we incorporated sampling weights and strata, sampling units in our statistical analysis to account for the complex sampling design. For continuous variables, we used means and standard errors (SE) expressed with t-test to compare participants′ characteristic variables. For categorical variables, we expressed percentages and SE with the Rao Scott Chi-square test to compare participants′ characteristic variables. Logistic regression models were used to determine the association of various trace element intakes with the presence of cataracts. To better determine their association, we selected three models. Model 1 was adjusted by age, race, gender, and education level to correct the influence of demographic characteristics. Model 2 = Model 1 and adjusted by diabetes, BMI and daily energy intake to correct the influence of daily food intake, obesity, and diabetes. Model 3 = Model 2 and adjusted by serum cotinine to correct the influence of smoking. Since a significant association between selenium and cataract was observed, to better investigate the association between the two, we further performed quintile regression between selenium and cataract after dividing the intake levels of selenium into quintiles. Finally, because age and sex were the most prominent risk factors, we performed subgroup analyses for age and sex. Because of the setting of the inclusion criteria, participants 50–54 years of age were all black, and to avoid this selection bias, subgroup analyses were performed starting at age 55 in each decade as one group. Gender was grouped in males and females. All statistical analysis results with a two-tailed p value < 0.05 were considered significant.
3. Results
3.1. Description of baseline information of the study sample
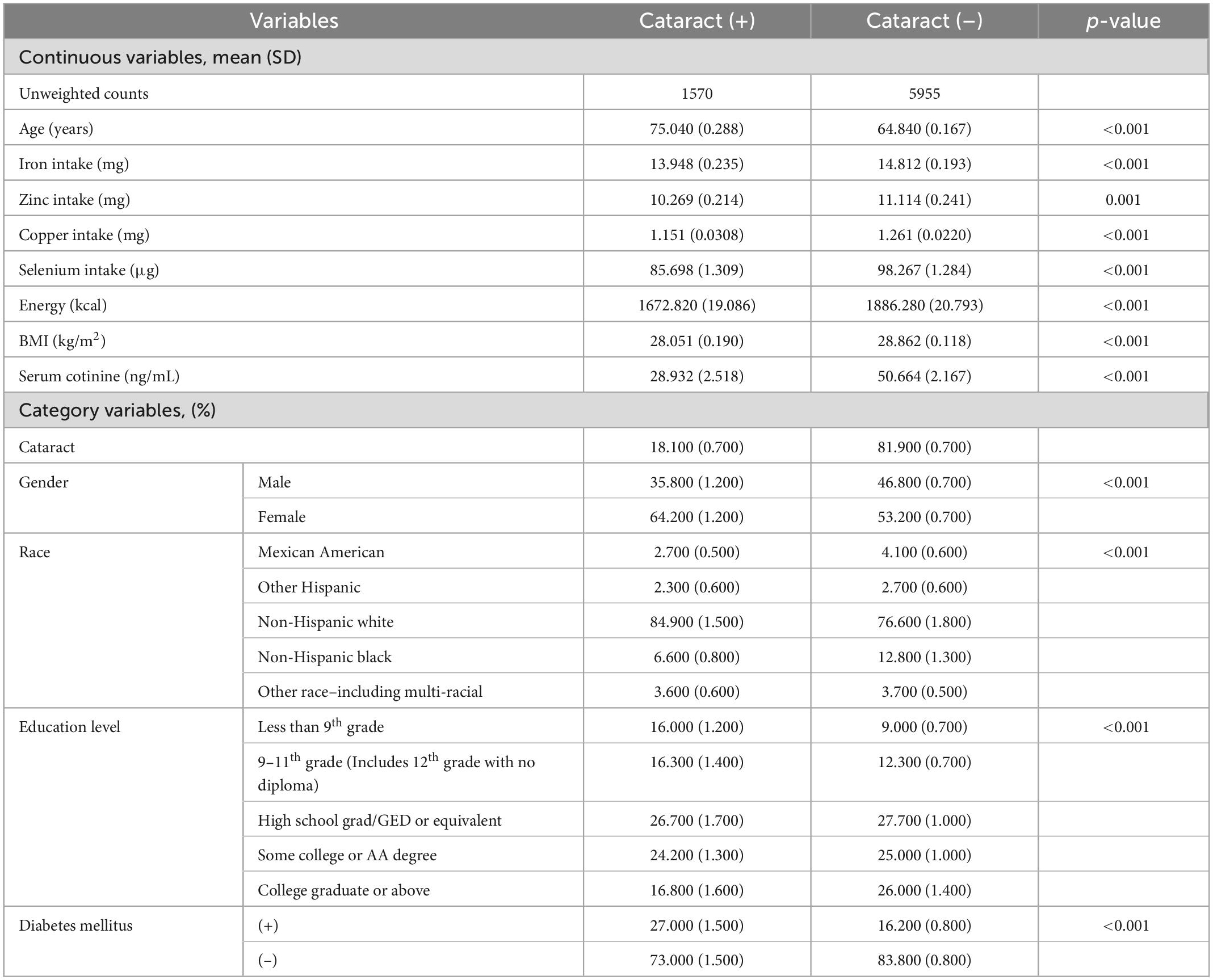
Table 1 shows the demographic data as well as other characteristic data of the participants with and without cataracts. Of all included participants, 1,570 had cataracts, 18.10% of the total after weighting, 5,955 did not have cataracts, and 81.90% of the total after weighting. Participants with cataracts all had significantly lower intakes of trace elements, including iron (13.948 vs. 14.812 mg), zinc (10.269 vs. 11.114 mg), copper (1.151 vs. 1.261 mg), selenium (85.698 vs. 98.267 μg). Significant differences were also observed in other covariates. Older age, female sex, non-Hispanic white race, and lower educational level groups were all more likely to have cataracts.
3.2. Association between the intake of iron, zinc, copper, selenium, and the presence of cataract
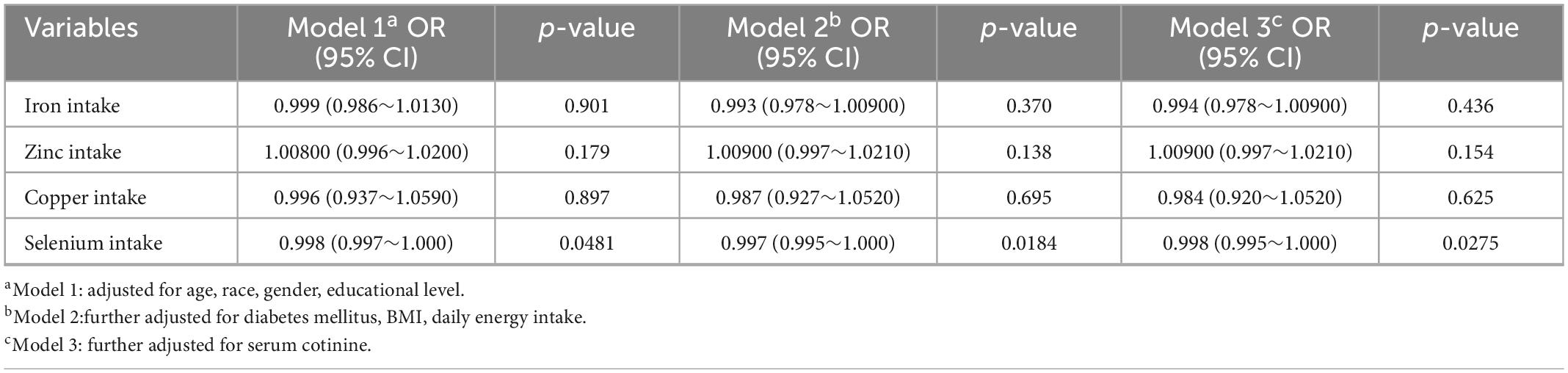
Table 2 shows the associations that existed between the intake of various trace elements and cataract as addressed by multivariate logistic regression models. A significant negative association between selenium intake and incident cataract was shown in all models (model 1: OR = 0.998, 95% CI = 0.997–1.000; model 2: OR = 0.997, 95% CI = 0.995–1.000; model 3: OR = 0.998, 95% CI = 0.995–1.000). No significant association with cataract was observed for the intakes of iron, zinc, copper.
3.3. Relationship of different quintiles of selenium with the presence of cataract
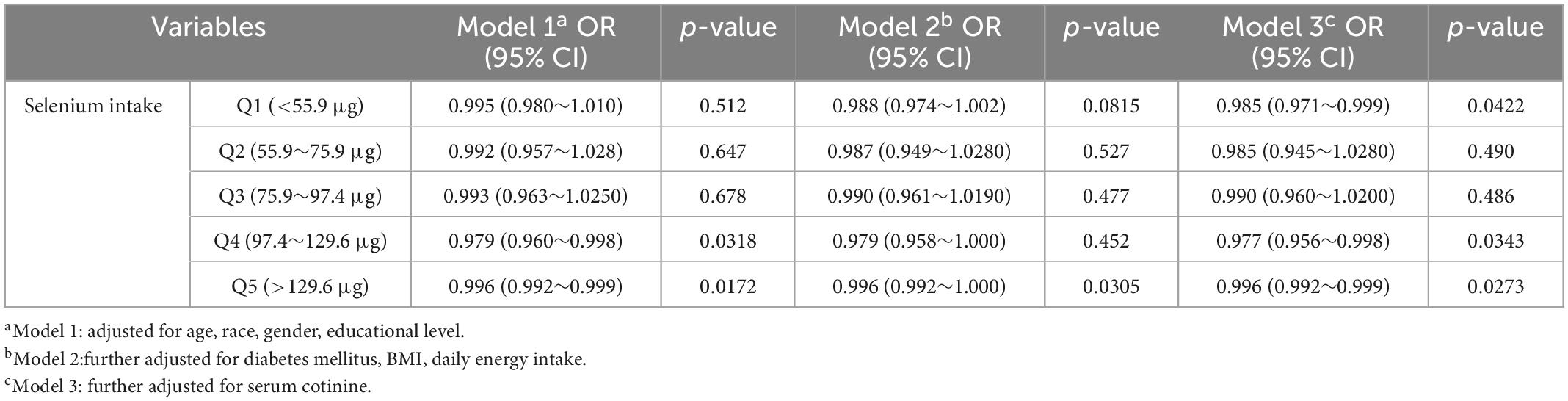
Table 3 demonstrates the analysis of the association of different grades of selenium intake with cataract after dividing selenium intake into quintiles. The quintiles of selenium intake levels were 55.9, 75.9, 97.4, and 129.6 μg. In the first quintile of model 3 (OR = 0.985, 95% CI = 0.971–0.999), and the fourth (model 1: OR = 0.979, 95% CI = 0.960–0.998; model 2: OR = 0.979, 95% CI = 0.958–1.000; model 3: OR = 0.977, 95% CI = 0.956–0.998) and fifth quintiles (model 1: OR = 0.996, 95% CI = 0.992–0.999; model 2: OR = 0.996, 95% CI = 0.992–1.000; model 3: OR = 0.996, 95% CI = 0.992–0.999) of all models, we observed a significant negative association between selenium intake and cataract.
3.4. Subgroup analyses for age and sex
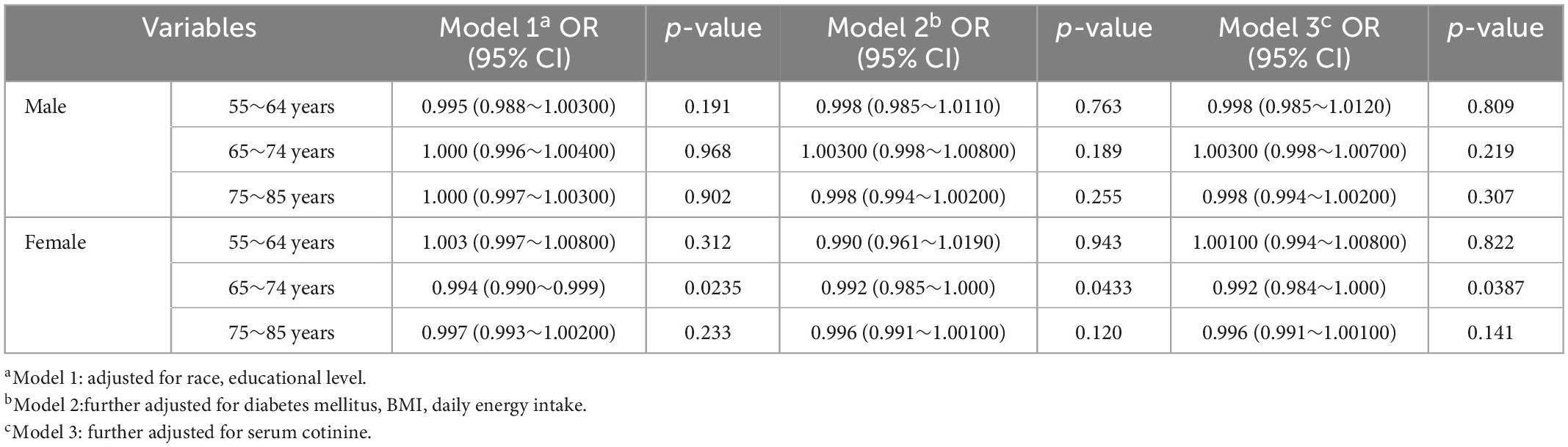
Table 4 presents the association of cataracts and selenium in male and female participants at different ages. In accordance with our results, we only observed a significant negative association in women aged 65–74 years in all models (model 1: OR = 0.994, 95% CI = 0.990–0.999; model 2: OR = 0.992, 95% CI = 0.985–1.000; model 3: OR = 0.992, 95% CI = 0.984–1.000). No significant association was observed at other ages for women and at all ages for men.
4. Discussion
Our study included large-scale cross-sectional data from four NHANES cycles. Logistic regression results showed a significant negative association between selenium intake and cataract. There was no significant associations between the intake of iron, copper, and zinc and cataract. Therefore, our study points out that increasing selenium intake in daily diet may decrease the risk of cataract. This notion was subsequently confirmed in the multivariable logistic regression model performed after dividing selenium intake into quintiles. Because significant negative associations were observed in the first quintile of model 3, the fourth, and fifth quintiles of all models, we hypothesized that maintaining daily dietary selenium intake at lower or higher levels is helpful for cataract prevention. In subgroup analyses adjusted for age and sex, the inverse association of selenium intake with cataract was observed only among US women 65–74 years of age. Four trace elements, iron, zinc, copper, and selenium, have a non-negligible role in the human body and they are involved in all aspects of human physiological activities (49–54), as well as in the visual system (55–57). Oxidative stress mechanism played an important role in the pathological process of cataract, in which iron, zinc, copper were involved (58–64). However, the relationship between these three trace elements and cataract could not be well confirmed in the current study (62, 65–71). This is consistent with our study, which perhaps indicated that the intake of these three trace elements had a negligible relationship with cataract.
In our results, selenium was the only significant variable, which deserves our high attention. All the time, rats with high-dose selenite intake have frequently been used to prepare animal models of cataract, which is able to laterally reflect the promoting effect of high-dose selenium on cataract (28, 72). Post et al.’s cross-sectional findings showed that low-serum selenium showed a positive association with age-related cataract only in the first quartile range of serum selenium levels (OR = 7.969, p < 0.01) (31). While the results of the SELECT Eye Endpoints (SEE) study conducted by Christen et al. indicated that an additional 200 μg/d of L-selenomethionine daily as a supplement source of selenium during an average 5.6 years of follow-up failed to observe a protect effect of selenium on age-related cataract (33). In the study of Xiangjia Zhu et al., after oral supplementation of different doses of selenium to rats with cataract induced by naphthalene solution, the slowing of the increase of lens density or the decrease of turbid density could be observed at all selenium intake doses. And they also observed an elevation of glutathione peroxidase (GPx) activity in the lens of Se supplemented group rats. This suggests that selenium supplementation is able to slow down the development of naphthalene induced cataract by slowing down oxidative stress (73). These above results suggest that the effect of selenium on cataract remains inconclusive, which is still a topic of investigation. Dietary intake is the main body′s access to selenium and selenium in food includes both organic and inorganic forms. The organic forms of selenium include selenomethionine and selenocysteine, and their bioavailability is high, up to 90–95%; the inorganic form of selenium includes selenite, selenides, etc., and its bioavailability is low, only 80–85% (54). The effects of these two forms of selenium on cataract are distinct. For the organic form of selenium, after the tRNA mediated incorporation of selenocysteine, these selenium can synthesize a variety of selenoproteins, including GPx and thioredoxin reductases (TrxR), among others, which have a powerful antioxidant capacity; organic forms of selenium or are able to prevent cataractogenesis by combating oxidative stress (54, 74–76). The inorganic form of selenium is in the oxidation state; this might aggravate oxidative stress and thus promote cataract (74). In addition, selenite is also able to promote cataracts through mechanisms such as altered epithelial metabolism, calcium accumulation, calpain induced proteolysis, crystallin precipitation, phase transition, and cytoskeletal loss (28). Notably, Huang et al. also found that the effect of selenite on the lens changed over time, with a 30% decrease in DNA replication in lens epithelial cells observed at 6–12 h after administration of the selenite into rats, but an 80% increase in DNA replication in lens epithelial cells was observed by 24 h. This suggests that selenite, after a period of action on the lens, has an effect that shifts from damage caused by oxidative stress to repair of the lens epithelium (77). Combined with our results, we speculate that the reason that selenium only has a protective effect on cataract at lower and higher doses may be the gap in bioavailability between the organic and inorganic forms of selenium and the different maintenance times of high concentrations of selenite within the lens resulting in different effects. Because the bioavailability of the organic form of selenium is greater than that of the inorganic form, at low dietary doses of selenium, the organic form of selenium absorbed by the body predominates, at which point the organic form of selenium exerts antioxidant effects through a series of biological metabolism, thereby playing a protective role against cataracts. But when selenium intake increases, the uptake of inorganic forms of selenium increases in the body, and a range of damaging effects on the lens begin to manifest. Since at this time the protective effect exerted by the organic form of selenium was similarly enhanced with increasing dose, it did not show an absolute cataract promoting negative effect. However, as selenium intake continues to rise to higher levels, it is sufficient within the lens to maintain higher selenite concentrations for an extended period of time at which point the reparative effects of selenite on lens cells begin to manifest and, together with selenoproteins, exert a protective effect on the lens, thereby against cataracts. The reason why selenium gradually exerted different effects on cataract at different intake levels is because the main aggregation sites of selenium in the human body are liver, muscle, kidney. Of the selenium ingested by humans, the dose of selenium able to aggregate into lens is low. Thus, only when a large change in selenium intake occurs can it lead to an influential change in the amount of selenium accumulated within the lens, resulting in a potent effect on the lens (78). However, although we found a protective role of selenium in cataract, because selenium is an essential nutrient for humans, prevention of cataract by reducing selenium intake seems an unwise regimen. Therefore, we more recommend that elderly people prevent cataract by moderately increasing their intake of selenium in their daily diet.
In the results of subgroup analysis, no significant association was observed in males of all ages. This is similar to the findings of Christen et al. (33). A significant negative association was observed in women aged 65–74 years, suggesting that increased selenium intake may be a protective factor for women in this age group of cataract. This result is presented, perhaps because of the role that estrogen withdrawal plays in cataracts (79). Another NHANES study showed that the mean age at menopause among US women enrolled in the study from 2001 to 2008 was 49.9 years (80). The course of cataracts is long, ranging from the onset of pathological changes to patients′ self-conscious symptoms for up to several years. And when visual symptoms occur, the time that patients go to the hospital and undergo cataract surgery is delayed because of personal psychological or socioeconomic factors. The results observed in our study should therefore be broadly consistent with the actual situation. Taken together, for women, taking selenium supplements at the beginning of menopause may help prevent cataract.
In the present study, since no direct data were available on the prevalence of cataracts in NHANES 2001–2008, we can only roughly estimate the prevalence of cataracts by cataract surgery status. This approach has been widely used in previous cataract studies using the NHANES database (36, 37, 81, 82). However, the plausibility of this approach has not been discussed in detail in previous studies, but we believe it is necessary. First, the study by Varma et al. estimated that the prevalence of cataract was 19.50% in the US population (83), and the meta-analysis by Hashemi et al. indicated that the worldwide cataract prevalence was approximately 17.20% (84). While in the present study, cataract surgery accounted for 18.10% of all participants, which was consistent with the above data. Second, in terms of cataract surgical coverage (CSC), CSC was defined as the percentage of cataract patients who underwent cataract surgery as a percentage of the total number of cataract patients (85). To the best of our knowledge, there are no studies conducted on CSCs for the US population, but based on the Rapid Assessment of Avoidable Blindness (RAAB) survey data, researchers have conducted extensive studies on CSCs for other countries, for example, Tabin et al.’s study indicated that CSCs can reach 50–70% in most developing countries (85), while Szabó et al. indicated that CSCs in Hungary, which is the same developed country as the US, are even higher up to 90% (86). Based on the above data, we can predict that the United States, as a developed country and with a high level of medical care, should also have CSCs at a high level, and most cataract patients are able to undergo cataract surgery. Finally the reduced cost of cataract surgery and the higher health gains for patients after surgery are also reasons for increased CSCs (87). Taken together, we believe that the cataract surgery data in this paper are broadly representative of the prevalence of cataract.
Although our study benefited from a reasonable sampling design to obtain a large sample size and nationally representative population, there are still certain limitations of this study that deserve to be explored. First, according to the NHANES method of ophthalmological examination, we can only know patients who have had cataract surgery and give a rough estimate of cataract occurrence in terms of cataract surgery status. The inability to detect those patients who had cataracts but remained unoperated would therefore make us underestimate the incidence of cataracts in our study. Second, cataracts are divided into subtypes, each of which has some differences in pathogenesis and risk factors that are not available in NHANES ophthalmological examination results. Third, dietary nutrient intake data were verbally ascertained through participants′ recall, and although based on the NHANES complete and rigorous measurement method, which enables accurate access to dietary nutrient data for a large proportion of participants, there may still be a small proportion of participants with varying degrees of recall bias. Fourth, because the contents of these trace elements in serum, aqueous humor, lens were not present in the results of the 2001–2008 NHANES study, these findings may be affected by other factors, such as different levels of subject digestive absorption, which could not be eliminated by adjustment. Finally, as a cross-sectional study, causality between observed variables cannot be directly concluded. However, our study also has a number of irreplaceable strengths. First, this is the first study to investigate the association between trace element intake and the risk of incident cataract through a nationally representative population-based survey. Second, to the best of our knowledge, this is the first study to report that high and low selenium intakes are protective against cataracts. Third, this is also the first study to report differential effects of selenium intake on cataracts in different genders. Fourth, it is also the first study that has explored the availability of data from cataract related studies in NHANES 2001–2008 in detail.
5. Conclusion
Our study points out that maintaining daily dietary selenium intake at higher levels is helpful for cataract prevention, and that increasing daily dietary selenium intake in American women aged 65–74 years may contribute to the prevention of age-related cataract. The intakes of iron, zinc, copper may not be associated with the incidence of age-related cataract.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
Approval for data collection was obtained from the NCHS Research Institutional/Ethics Review Board (IRB/ERB) (Protocol #98-12, Protocol #2005-06, Continuation of Protocol #2005-06). The patients/participants provided their written informed consent to participate in this study.
Author contributions
BX: conceptualization, software, investigation, resources, and data curation. BX and JZ: methodology. ZY and JZ: supervision and project administration. BX, ZY, and JZ: writing—original draft preparation and writing—review and editing. ZY: funding acquisition. BX and ZL: formal analysis. ZY and ZL: validation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Natural Science Foundation of China (NSFC 82000877).
Acknowledgments
The authors would like to thank all reviewers for their valuable comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1042893/full#supplementary-material
References
1. Leske MC, Sperduto RD. The epidemiology of senile cataracts: a review. Am J Epidemiol. (1983). 118:152–65. doi: 10.1093/oxfordjournals.aje.a113625
2. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. (2017) 28:98–103. doi: 10.1097/ICU.0000000000000340
3. Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman S, Jonas J, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. (2015) 56:6762–9. doi: 10.1167/iovs.15-17201
4. Liu YC, Wilkins M, Kim T, Malyugin B, Mehta J. Cataracts. Lancet. (2017) 390:600–12. doi: 10.1016/S0140-6736(17)30544-5
5. Gbd 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. (2021) 9:e144–60. doi: 10.1016/S2214-109X(20)30489-7
6. Taylor A. Associations between nutrition and cataract. Nutr Rev. (1989) 47:225–34. doi: 10.1111/j.1753-4887.1989.tb02848.x
7. Shiels A, Hejtmancik JF. Mutations and mechanisms in congenital and age-related cataracts. Exp Eye Res. (2017) 156:95–102. doi: 10.1016/j.exer.2016.06.011
8. Liu X, Hao J, Xie T, Malik TH, Lu C, Liu C, et al. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell. (2017) 16:934–42. doi: 10.1111/acel.12645
9. Periyasamy P, Shinohara T. Age-related cataracts: role of unfolded protein response, Ca(2+) mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog Retin Eye Res. (2017) 60:1–19. doi: 10.1016/j.preteyeres.2017.08.003
10. Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: the Beaver Dam eye study. Am J Ophthalmol. (1998) 126:782–90. doi: 10.1016/s0002-9394(98)00280-3
11. West S, Munoz B, Emmett EA, Taylor HR. Cigarette smoking and risk of nuclear cataracts. Arch Ophthalmol. (1989) 107:1166–9. doi: 10.1001/archopht.1989.01070020232031
12. Glynn RJ, Christen WG, Manson JE, Bernheimer J, Hennekens CH. Body mass index. An independent predictor of cataract. Arch Ophthalmol. (1995) 113:1131–7. doi: 10.1001/archopht.1995.01100090057023
13. Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiol Rev. (1995) 17:336–46. doi: 10.1093/oxfordjournals.epirev.a036197
14. Miglior S, Bergamini F, Migliavacca L, Marighi P, Orzalesi N. Metabolic and social risk factors in a cataractous population. A case-control study. Dev Ophthalmol. (1989) 17:158–64. doi: 10.1159/000417021
16. Yao Y, Lu Q, Wei L, Cheng K, Lu Y, Zhu X. Efficacy and complications of cataract surgery in high myopia. J Cataract Refract Surg. (2021) 47:1473–80. doi: 10.1097/j.jcrs.0000000000000664
17. Chan E, Mahroo OA, Spalton DJ. Complications of cataract surgery. Clin Exp Optom. (2010) 93:379–89. doi: 10.1111/j.1444-0938.2010.00516.x
18. Goel R, Shah S, Malik KP, Sontakke R, Golhait P, Gaonker T. Complications of manual small-incision cataract surgery. Indian J Ophthalmol. (2022) 70:3803–11.
19. Sourkes TL. Influence of specific nutrients on catecholamine synthesis and metabolism. Pharmacol Rev. (1972) 24:349–59.
20. Baecker T, Mangus K, Pfaender S, Chhabra R, Boeckers TM, Grabrucker AM. Loss of COMMD1 and copper overload disrupt zinc homeostasis and influence an autism-associated pathway at glutamatergic synapses. Biometals. (2014) 27:715–30. doi: 10.1007/s10534-014-9764-1
21. Vogler NW, Betti VM, Goldberg JM, Tzounopoulos T. Mechanisms underlying long-term synaptic zinc plasticity at mouse dorsal cochlear nucleus glutamatergic synapses. J Neurosci. (2020) 40:4981–96. doi: 10.1523/JNEUROSCI.0175-20.2020
22. Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. (2009) 158:126–36. doi: 10.1016/j.neuroscience.2008.01.061
23. Ramirez-Bello V, Martinez-Seoane J, Fernández-Silva A, Amero C. Zinc and copper ions induce aggregation of human beta-crystallins. Molecules. (2022) 27:2970. doi: 10.3390/molecules27092970
24. Micun Z, Falkowska M, Młynarczyk M, Kochanowicz J, Socha K, Konopińska J. Levels of trace elements in the lens, aqueous humour, and plasma of cataractous patients-a narrative review. Int J Environ Res Public Health. (2022) 19:10376. doi: 10.3390/ijerph191610376
25. Dolar-Szczasny J, Świȩch A, Flieger J, Tatarczak-Michalewska M, Niedzielski P, Proch J, et al. Levels of trace elements in the aqueous humor of cataract patients measured by the inductively coupled plasma optical emission spectrometry. Molecules. (2019) 24:4127. doi: 10.3390/molecules24224127
26. Bede-Ojimadu O, Orish CN, Bocca B, Ruggieri F, Frazzoli C, Orisakwe OE. Trace elements exposure and risk in age-related eye diseases: a systematic review of epidemiological evidence. J Environ Sci Health C Toxicol Carcinog. (2021) 39:293–339. doi: 10.1080/26896583.2021.1916331
27. Shearer TR, Anderson RS, Britton JL. Influence of selenite and fourteen trace elements on cataractogenesis in the rat. Invest Ophthalmol Vis Sci. (1983) 24:417–23.
28. Shearer TR, Ma H, Fukiage C, Azuma M. Selenite nuclear cataract: review of the model. Mol Vis. (1997) 3:8.
29. Anderson RS, Trune DR, Shearer TR. Histologic changes in selenite cortical cataract. Invest Ophthalmol Vis Sci. (1988) 29:1418–27.
30. Bunce GE, Hess JL, Batra R. Lens calcium and selenite-induced cataract. Curr Eye Res. (1984) 3:315–20. doi: 10.3109/02713688408997215
31. Post M, Lubiński W, Lubiński J, Krzystolik K, Baszuk P, Muszyńska M, et al. Serum selenium levels are associated with age-related cataract. Ann Agric Environ Med. (2018) 25:443–8. doi: 10.26444/aaem/90886
32. Centers for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. Atlanta, GA: Centers for Disease Control and Prevention (2022).
33. Christen WG, Glynn RJ, Gaziano JM, Darke AK, Crowley JJ, Goodman PJ, et al. Age-related cataract in men in the selenium and vitamin e cancer prevention trial eye endpoints study: a randomized clinical trial. JAMA Ophthalmol. (2015) 133:17–24. doi: 10.1001/jamaophthalmol.2014.3478
34. National Center for Health Statistics. National Health and Nutrition Examination Survey 2007-2008 Data Documentation, Codebook, and Frequencies: Vision (VIQ_E). (2022). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/VIQ_E.htm (accessed October 18, 2022).
35. Lundström M, Goh P, Henry Y, Salowi MA, Barry P, Manning S, et al. The changing pattern of cataract surgery indications: a 5-year study of 2 cataract surgery databases. Ophthalmology. (2015) 122:31–8. doi: 10.1016/j.ophtha.2014.07.047
36. Zhang X, Cotch M, Ryskulova A, Primo S, Nair P, Chou C, et al. Vision health disparities in the United States by race/ethnicity, education, and economic status: findings from two nationally representative surveys. Am J Ophthalmol. (2012) 154(Suppl. 6):S53–62. doi: 10.1016/j.ajo.2011.08.045
37. Wang W, Schaumberg DA, Park SK. Cadmium and lead exposure and risk of cataract surgery in U.S. adults. Int J Hyg Environ Health. (2016) 219:850–6. doi: 10.1016/j.ijheh.2016.07.012
38. National Center for Health Statistics. National Health and Nutrition Examination Survey 2007-2008 Data Documentation, Codebook, and Frequencies: Dietary Interview - Total Nutrient Intakes, First Day (DR1TOT_E). (2022). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/DR1TOT_E.htm (accessed October 18, 2022).
39. National Center for Health Statistics. National Health and Nutrition Examination Survey 2007-2008 Data Documentation, Codebook, and Frequencies: Demographic Variables & Sample Weights (DEMO_E). (2022). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/DEMO_E.htm (accessed October 18, 2022).
41. Kelly SP, Thornton J, Edwards R, Sahu A, Harrison R. Smoking and cataract: review of causal association. J Cataract Refract Surg. (2005) 31:2395–404. doi: 10.1016/j.jcrs.2005.06.039
42. Ye J, He J, Wang C, Wu H, Shi X, Zhang H, et al. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci. (2012) 53:3885–95. doi: 10.1167/iovs.12-9820
43. Janghorbani M, Amini M. Cataract in type 2 diabetes mellitus in Isfahan, Iran: incidence and risk factors. Ophthalmic Epidemiol. (2004) 11:347–58. doi: 10.1080/09286580490888753
44. Pan CW, Lin Y. Overweight, obesity, and age-related cataract: a meta-analysis. Optom Vis Sci. (2014) 91:478–83. doi: 10.1097/OPX.0000000000000243
45. National Center for Health Statistics. National Health and Nutrition Examination Survey 2007-2008 Data Documentation, Codebook, and Frequencies: Diabetes (DIQ_E). (2022). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/DIQ_E.htm (accessed October 18, 2022).
46. National Center for Health Statistics. National Health and Nutrition Examination Survey 2005-2006 Data Documentation, Codebook, and Frequencies: Cotinine - Serum (COT_D). (2022). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/COT_D.htm (accessed October 18, 2022).
47. National Center for Health Statistics. National Health and Nutrition Examination Survey 2007-2008 Data Documentation, Codebook, and Frequencies: Cotinine - Serum & Total NNAL - Urine (COTNAL_E). (2022). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/COTNAL_E.htm (accessed October 18, 2022).
48. National Center for Health Statistics. National Health and Nutrition Examination Survey 2007-2008 Data Documentation, Codebook, and Frequencies: Body Measures (BMX_E). (2022). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/BMX_E.htm (accessed October 18, 2022).
49. Garcia-Castineiras S. Iron, the retina and the lens: a focused review. Exp Eye Res. (2010) 90:664–78. doi: 10.1016/j.exer.2010.03.003
50. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. (2021) 31:107–25. doi: 10.1038/s41422-020-00441-1
51. Scheiber I, Dringen R, Mercer JF. Copper: effects of deficiency and overload. Met Ions Life Sci. (2013) 13:359–87. doi: 10.1007/978-94-007-7500-8_11
52. Kahlson MA, Dixon SJ. Copper-induced cell death. Science. (2022) 375:1231–2. doi: 10.1126/science.abo3959
54. Mojadadi A, Au A, Salah W, Witting P, Ahmad G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients. (2021) 13:3256. doi: 10.3390/nu13093256
55. Amemiya T. The eye and nutrition. Jpn J Ophthalmol. (2000) 44:320. doi: 10.1016/S0021-5155(00)00161-1
56. Kaminska A, Romano G, Rejdak R, Zweifel S, Fiedorowicz M, Rejdak M, et al. Influence of trace elements on neurodegenerative diseases of the eye-the glaucoma model. Int J Mol Sci. (2021) 22:4323. doi: 10.3390/ijms22094323
57. Pellegrini M, Senni C, Bernabei F, Cicero A, Vagge A, Maestri A, et al. The role of nutrition and nutritional supplements in ocular surface diseases. Nutrients. (2020) 12:952. doi: 10.3390/nu12040952
58. Zigler JJ, Huang QL, Du X. Oxidative modification of lens crystallins by H2O2 and chelated iron. Free Radic Biol Med. (1989) 7:499–505. doi: 10.1016/0891-5849(89)90025-7
59. Lee W, Park SY, Park TK, Kim HK, Ohn YH. Mature cataract and lens-induced glaucoma associated with an asymptomatic intralenticular foreign body. J Cataract Refract Surg. (2007) 33:550–2. doi: 10.1016/j.jcrs.2006.09.043
60. Loh A, Hadziahmetovic M, Dunaief JL. Iron homeostasis and eye disease. Biochim Biophys Acta. (2009) 1790:637–49. doi: 10.1016/j.bbagen.2008.11.001
61. Wei Z, Hao C, Huangfu J, Srinivasagan R, Zhang X, Fan X. Aging lens epithelium is susceptible to ferroptosis. Free Radic Biol Med. (2021) 167:94–108. doi: 10.1016/j.freeradbiomed.2021.02.010
62. Chakraborty I, Kunti S, Bandyopadhyay M, Dasgupta A, Chattopadhyay GD, Chakraborty S. Evaluation of serum zinc level and plasma SOD activity in senile cataract patients under oxidative stress. Indian J Clin Biochem. (2007) 22:109–13. doi: 10.1007/BF02913326
63. Olofsson EM, Marklund SL, Behndig A. Enhanced diabetes-induced cataract in copper-zinc superoxide dismutase-null mice. Invest Ophthalmol Vis Sci. (2009) 50:2913–8. doi: 10.1167/iovs.09-3510
64. Olofsson EM, Marklund SL, Karlsson K, Brännström T, Behndig A. In vitro glucose-induced cataract in copper-zinc superoxide dismutase null mice. Exp Eye Res. (2005) 81:639–46. doi: 10.1016/j.exer.2005.03.022
65. Talebnejad MR, Azimi A, Khalili MR, Meshksar A. The role of trace elements in pseudoexfoliation syndrome: a cross-sectional study. J Ophthalmic Vis Res. (2021) 16:165–70. doi: 10.18502/jovr.v16i2.9079
66. Dawczynski J, Blum M, Winnefeld K, Strobel J. Increased content of zinc and iron in human cataractous lenses. Biol Trace Elem Res. (2002) 90:15–23. doi: 10.1385/BTER:90:1-3:15
67. Cumurcu T, Mendil D, Etikan I. Levels of zinc, iron, and copper in patients with pseudoexfoliative cataract. Eur J Ophthalmol. (2006) 16:548–53. doi: 10.1177/112067210601600408
68. Yang W, Yu W, Li Z. [The study on superoxide dismutase and trace element in patients with senile cataract]. Yan Ke Xue Bao. (2000) 16:246–8.
69. Chen CZ. [Analysis of 7 elements in the serum and lens of senile cataract patients]. Zhonghua Yan Ke Za Zhi. (1992) 28:355–7.
70. Soares FM, Nogueira N d, Marreiro D d, Carvalho CM, Monte SJ, Neto JM, et al. [Plasma and erythrocyte zinc concentrations in elderly patients with and without senile cataract in a tertiary eye care center at Teresina-Piaui]. Arq Bras Oftalmol. (2008) 71:674–8. doi: 10.1590/S0004-27492008000500012
71. Akyol N, Değer O, Keha EE, Kili S. Aqueous humour and serum zinc and copper concentrations of patients with glaucoma and cataract. Br J Ophthalmol. (1990) 74:661–2. doi: 10.1136/bjo.74.11.661
72. Shearer TR, Anderson RS, Britton JL, Palmer EA. Early development of selenium-induced cataract: slit lamp evaluation. Exp Eye Res. (1983) 36:781–8. doi: 10.1016/0014-4835(83)90032-5
73. Zhu X, Lu Y. Selenium supplementation can slow the development of naphthalene cataract. Curr Eye Res. (2012) 37:163–9. doi: 10.3109/02713683.2011.639123
74. Zeng H. Selenium as an essential micronutrient: roles in cell cycle and apoptosis. Molecules. (2009) 14:1263–78. doi: 10.3390/molecules14031263
75. Dai J, Liu H, Zhou J, Huang K. Selenoprotein R protects human lens epithelial cells against D-galactose-induced apoptosis by regulating oxidative stress and endoplasmic reticulum stress. Int J Mol Sci. (2016) 17:231. doi: 10.3390/ijms17020231
76. Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. (2004) 195:221–30. doi: 10.1016/j.tox.2003.10.012
77. Huang LL, Hess JL, Bunce GE. DNA damage, repair, and replication in selenite-induced cataract in rat lens. Curr Eye Res. (1990) 9:1041–50. doi: 10.3109/02713689008997578
78. Mehdi Y, Hornick J, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. (2013) 18:3292–311. doi: 10.3390/molecules18033292
79. Zetterberg M, Celojevic D. Gender and cataract–the role of estrogen. Curr Eye Res. (2015) 40:176–90. doi: 10.3109/02713683.2014.898774
80. Appiah D, Nwabuo CC, Ebong IA, Wellons MF, Winters SJ. Trends in age at natural menopause and reproductive life span among US women, 1959-2018. JAMA. (2021) 325:1328–30. doi: 10.1001/jama.2021.0278
81. De La Cruz N, Shabaneh O, Appiah D. The association of ideal cardiovascular health and ocular diseases among US adults. Am J Med. (2021) 134:252.e–9.e. doi: 10.1016/j.amjmed.2020.06.004
82. Zhu Z, Liao H, Wang W, Scheetz J, Zhang J, He M. Visual impairment and major eye diseases in chronic kidney disease: the national health and nutrition examination survey, 2005-2008. Am J Ophthalmol. (2020) 213:24–33. doi: 10.1016/j.ajo.2020.01.002
83. Varma R, Paz SH, Azen SP, Klein R, Globe D, Torres M, et al. The Los Angeles latino eye study: design, methods, and baseline data. Ophthalmology. (2004) 111:1121–31. doi: 10.1016/j.ophtha.2004.02.001
84. Hashemi H, Pakzad R, Yekta A, Aghamirsalim M, Pakbin M, Ramin S, et al. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye (Lond). (2020) 34:1357–70. doi: 10.1038/s41433-020-0806-3
85. Tabin G, Chen M, Espandar L. Cataract surgery for the developing world. Curr Opin Ophthalmol. (2008) 19:55–9. doi: 10.1097/ICU.0b013e3282f154bd
86. Szabó D, Sándor GL, Tóth G, Pék A, Lukács R, Szalai I, et al. Visual impairment and blindness in Hungary. Acta Ophthalmol. (2018) 96:168–73. doi: 10.1111/aos.13542
Keywords: cataract, trace elements, National Health and Nutrition Examination Survey (NHANES), selenium, cross-sectional study
Citation: Xu B, Liu Z, Zhao J and Yu Z (2023) Selenium intake help prevent age-related cataract formation: Evidence from NHANES 2001–2008. Front. Nutr. 10:1042893. doi: 10.3389/fnut.2023.1042893
Received: 13 September 2022; Accepted: 09 January 2023;
Published: 27 January 2023.
Edited by:
Daniela Caetano Gonçalves, Federal University of São Paulo, BrazilReviewed by:
Daniel Henrique Bandoni, Federal University of São Paulo, BrazilCarlos Amero, Universidad Autónoma del Estado de Morelos (UAEM), Mexico
Copyright © 2023 Xu, Liu, Zhao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangyue Zhao,  anl6aGFvQGNtdS5lZHUuY24=; Ziyan Yu,
anl6aGFvQGNtdS5lZHUuY24=; Ziyan Yu,  eXV6aXlhbkBob3RtYWlsLmNvbQ==
eXV6aXlhbkBob3RtYWlsLmNvbQ==
 Baiwei Xu
Baiwei Xu Zhongwei Liu1,2,3
Zhongwei Liu1,2,3 Jiangyue Zhao
Jiangyue Zhao Ziyan Yu
Ziyan Yu