- 1The Fourth Clinical Medical College of Guangzhou University of Chinese Medicine, Shenzhen, China
- 2Department of Endocrinology, Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, China
- 3Shenzhen Traditional Chinese Medicine Hospital Affiliated to Nanjing University of Chinese Medicine, Shenzhen, China
Background: Previous studies have investigated the link between fatty acid intake and bone mineral density (BMD), but the results are controversial. This study aims to examine the relationship between fatty acid intake and BMD in adults aged 20–59.
Methods: The association between fatty acid consumption and BMD was analyzed using a weighted multiple linear regression model with National Health and Nutrition Examination Survey data from 2011 to 2018. The linearity relationship and saturation value of the connection between fatty acid consumption and BMD were assessed by fitting a smooth curve and a saturation effect analysis model.
Results: The study included 8,942 subjects. We found a significant positive correlation between the consumption of saturated fatty acids, monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids and BMD. In subgroup analyses that were stratified by gender and race, this association was still shown to be significant. Based on the smooth curve and saturation effect analysis, we found no saturation effect for the three fatty acids and total BMD. However, there was a turning point (20.52 g/d) between MUFAs intake and BMD, and only MUFAs intake >20.52 g/d showed a positive correlation between MUFAs and BMD.
Conclusion: We found that fatty acid intake is beneficial for bone density in adults. Therefore, according to our findings, it is recommended that adults consume moderate amounts of fatty acids to ensure adequate bone mass but not metabolic diseases.
1. Introduction
Osteoporosis (OP) is a degenerative disease of the bones that results in weakened bones, weakened microarchitecture, increased fragility, and increased fracture risk (1). The prevalence of OP has been increasing in recent years. According to a study done by the International Society for Clinical Densitometry and the International Foundation for Osteoporosis, more than 70 million Americans will have osteoporosis or bone loss by 2030 (2). With the expansion of human life expectancy and population aging, OP will become a more severe public health problem worldwide (3, 4). Therefore, early detection and intervention of osteoporosis have attracted more and more attention.
As a controllable factor, lifestyle seems to play an important role. Numerous nutrients, especially dietary fatty acid intake, have been shown to potentially effect on bone health (5–10). Studies have shown that the type of fatty acid is critical (8, 10, 11). Saturated fatty acids (SFAs) improve bone health by enhancing osteoclast survival (12), decreasing mesenchymal stem cell differentiation (13), promoting calcium absorption or excretion (14), and suppressing inflammatory gene expression (15). Polyunsaturated fatty acids (PUFAs) affect bone metabolism by combining with PPARγ to induce bone marrow adipocyte differentiation (16), regulate inflammatory response (17), and improve bone marrow microcirculation (18). Additionally, monounsaturated fatty acids (MUFAs) may have possible impacts on prostaglandin activity, influencing bone production and bone resorption (19, 20).
However, after reviewing a lot of literature, we discovered that the effects of PUFAs on bone health have received considerable attention; nevertheless, large-scale clinical investigations are absent. At the same time, we find it interesting that no researcher has focused on the connection between the different types of fatty acids and bone mineral density (BMD). Therefore, we decided to conduct a large-scale cross-sectional study using the National Health and Nutrition Examination Survey (NHANES) Database to investigate the connection between the different types of fatty acids and BMD. This was done to help guide therapeutic efforts.
2. Methods
2.1. Data source and study population
Data collected by NHANES from 2011 to 2018 was used for this cross-sectional analysis. NHANES adopts an innovative survey mode that combines face-to-face interviews and physical examinations to comprehensively assess the health and nutritional status of residents in the United States. Questions about demographics, socioeconomics, diet, and health were included in the interviews. The medical section addresses medical, dentistry, and physiological examinations as well as laboratory testing performed by qualified medical specialists. In general, these statistics are used to assess the prevalence of diseases and the related risk factors, formulate guidelines for the implementation of practical public health policy, devise health initiatives and services, cultivate fundamental health awareness, and improve the quality of life.
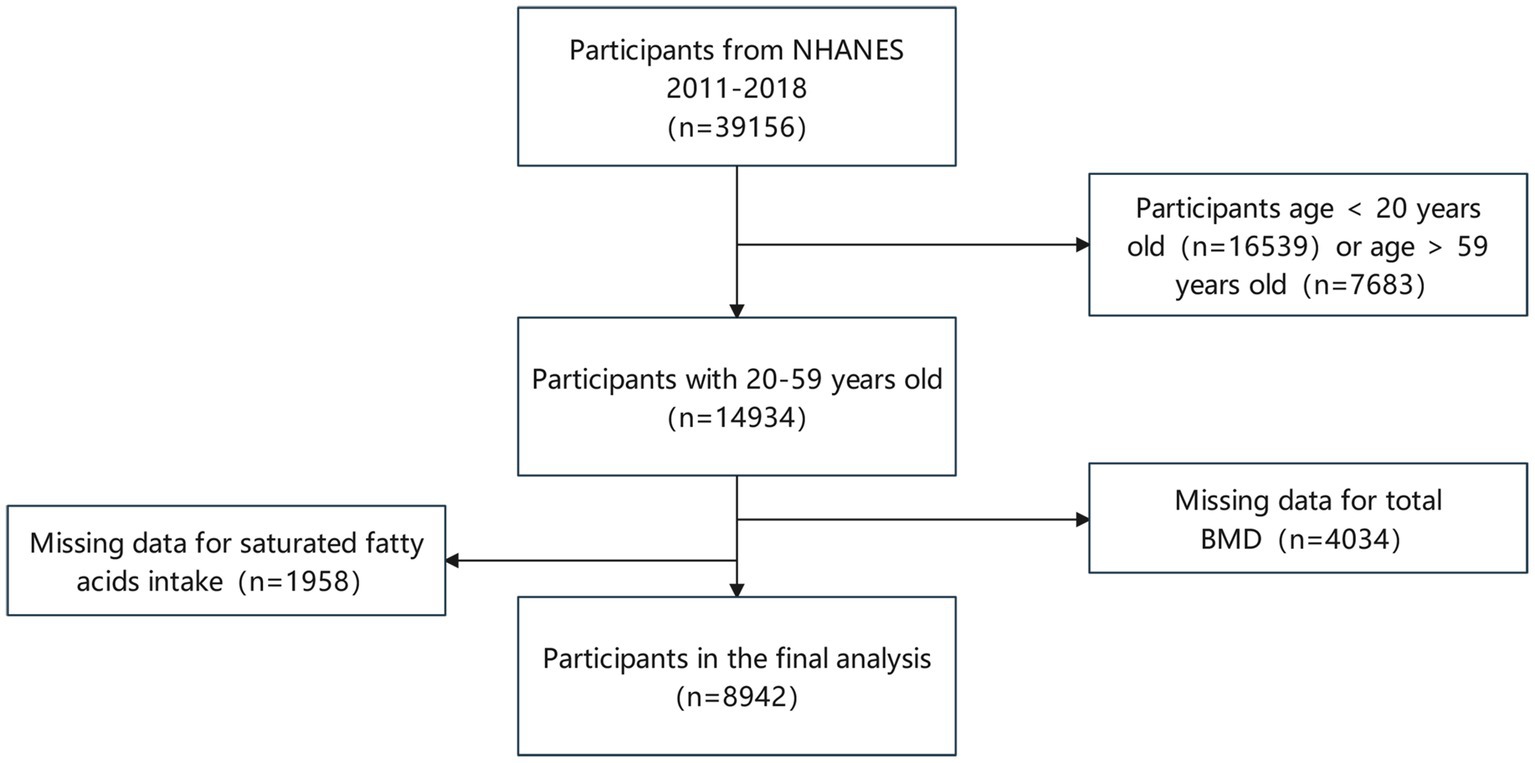
Our analysis comprised 39,156 participants from NHENAS 2011 to 2018, excluding participants under 20 (16,539 individuals) and those beyond 59 (7,683 individuals). Simultaneously, missing data on fatty acids intake (1,958 individuals) and total BMD (4,034 individuals) were excluded. Following the screening mentioned above, we included a total of 8,942 individuals (Figure 1).
2.2. Ethics statement
All survey participants were informed of the poll’s specifics and signed an informed consent form. The National Center for Health Statistics Ethics Review Board assessed and authorized the informed consent. Following the completion of official anonymization, all of the data is then made available to the public in order to make the most effective use of these resources. Anyone may access these statistics as long as they adhere to the NHENAS database regulations and are used exclusively for statistical analysis. All studies based on these data should adhere to applicable laws and legislation.
2.3. Covariates
The independent variable in our study was daily fatty acids consumption, which was determined through two 24-h food recall interviews. Interviews were conducted in person and over the phone, respectively. In the Mobile Examination Center (MEC), a small room was used to perform the first 24-h recall interviews. Each MEC dietary interview room had a set of uniform measuring parameters. These methods assist respondents in reporting the quantity and variety of food they consume. The information for the second food recall was gathered over the phone and was due 3 to 10 days later. After the participants had finished the in-person interviews, they were provided with measuring glasses, teaspoons, a ruler, and a food model guide to equip them with the tools necessary to record meal portions accurately during the phone interviews. All study participants were required to complete two in-person interviews, each performed by a professional dietary interviewer who spoke Spanish and English fluently. Some categorical variables, such as gender, education level, and moderate exercise, were also included in our study, as well as some continuous variables, including age, body mass index (BMI), the ratio of family income to poverty, alkaline phosphatase, blood calcium, blood phosphorus, blood uric acids, total cholesterol, triglyceride, glycohemoglobin, blood urea nitrogen, serum creatinine, urinary albumin creatinine ratio, total albumin, vitamin D intake, alcohol intake, total SFAs intake, total MUFAs intake, total PUFAs intake, and total BMD. For further details on gathering covariate data and 24-h dietary recall interviews, go to.1
2.4. Outcome variable
Dual-energy X-ray absorptiometry (DXA) is a clinically recognized method for measuring BMD. Its results can be used for osteoporosis fracture, fracture risk prediction, and drug efficacy evaluation (21). Total BMD, as determined by DXA whole-body scans, served as the outcome variable in our study. Because of their quickness, simplicity, and low radiation exposure are widely used to estimate body composition. In the NHANES, DXA scans of the participants have conducted on a Hologic Discovery Model A densitometer (Hologic, Inc., Bedford, Massachusetts), and data processing was carried out using Hologic APEX (version 4.0) software. Professional technicians who have received training and certificates do the operations above. The official NHANES website has a body composition manual with more information about how the DXA exam works.
2.5. Statistical analysis
We used EmpowerStats2 and R (3.4.4 version) software for statistical analysis. Typically, we consider a statistical result to be meaningful if the p value is lower than 0.05. In this study, all sample sizes are weighted. Continuous variables are reported as Mean ± SD for the comparison of baseline data, and the p-value was determined using a weighted linear regression model. The chi-square test was used to figure out the p-value, and categorical variables were given as a percentage. Weighted multiple linear regression analyses were performed to assess the effect of intake of the three types of fatty acids on BMD. We established three models of SFAs, MUFAs, and PUFAs intake and BMD, with all confounders (age, gender, race, education level, the ratio of family income to poverty, moderate activity, body mass index, alkaline phosphatase, serum calcium, serum uric acid, total cholesterol, blood urea nitrogen, serum phosphorus, triglyceride, glycohemoglobin, urinary albumin creatinine ratio, and total protein, serum creatinine, vitamin D intake, total saturated fatty acids, total monounsaturated fatty acids, and total polyunsaturated fatty acids) corrected for each model. This was done to improve the reporting of epidemiological observational studies and get the most out of the data. Concurrently, fatty acids consumption was transformed into categorical group data using the quartile approach, and P for trend was computed. Segregated according to age, gender, and race, subgroup analysis was performed to assess the association between fatty acids consumption and BMD in varying ages, gender, and race differences. In addition, after controlling for all confounding factors, smooth curve fitting was done, and a saturation effect analysis model was constructed to assess the correlation between the consumption of different fatty acids types and BMD. Results were expressed using turn point, effect–β (95%Cl, p value), and the log-likelihood ratio test (LRT test).
3. Results
3.1. Characteristics of participants
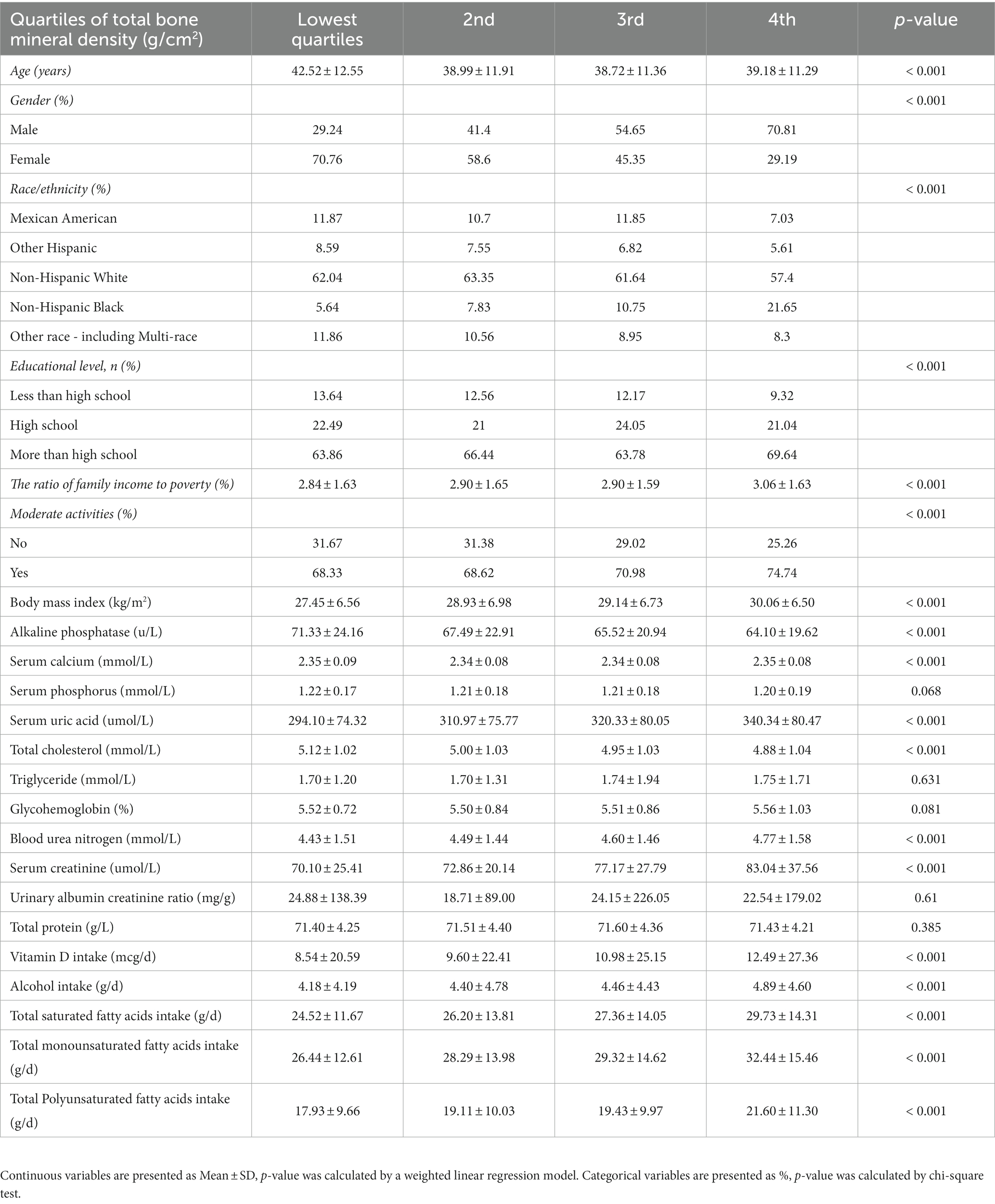
We stratified total bone mineral density by quartiles, Table 1 shows the study sample’s baseline data, including demographic information, physical examination data, laboratory test indicators, and dietary interview information for 8,942 subjects. There are significant differences in age, gender, race, education level, the ratio of family income to poverty, moderate activity, body mass index, alkaline phosphatase, serum calcium, serum uric acid, total cholesterol, blood urea nitrogen, serum creatinine, vitamin D intake, total saturated fatty acids, total monounsaturated fatty acids, and total polyunsaturated fatty acids. However, the difference was not significant in terms of serum phosphorus, triglyceride, glycohemoglobin, urinary albumin creatinine ratio, and total protein.
3.2. Relationship between SFAs intake and BMD
Table 2 displays the weighted multiple linear regression model. The result showed that total intake of SFAs was positively linked with total BMD (p < 0.001). When the quartile of total SFAs intake was constructed, the lowest quartiles was used as a reference, the trend analysis was statistically significant (p for trend = 0.002), and the 4th quartile was significantly positively associated with BMD (p < 0.01). After controlling for all confounding variables, the association between total SFAs consumption and BMD was positive and statistically significant in subgroups stratified by age. However, in subgroups stratified by gender, total SFAs intake was statistically positively linked with BMD in male individuals but not in female subjects. In subgroups stratified by race, there was a positive correlation between total BMD and total saturated fatty acids intake among whites, blacks, and other race. These outcomes reach statistical significance. As shown in Figures 2A, B, we found no saturation effect between SFAs and BMD when we performed smooth curve fitting on the revised model.
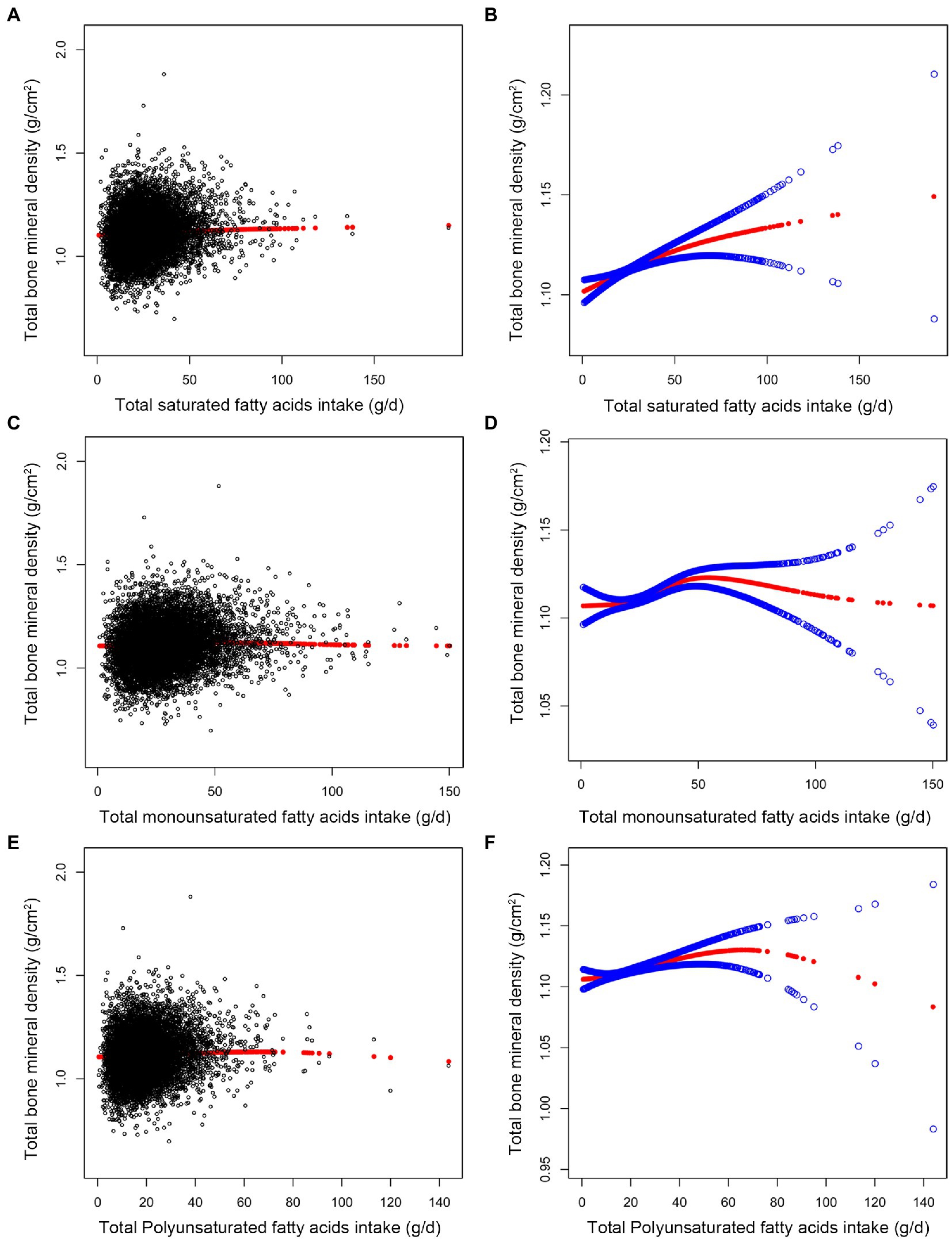
Figure 2. Association between fatty acids intake (g/d) and total bone mineral density (g/cm2). (A,C,E) Each black point represents a sample. (B,D,F) The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. All confounding factors were adjusted.
3.3. Relationship between MUFAs intake and BMD
As shown in Table 2, we found a significant positive connection between total MUFAs intake and BMD (p < 0.001) using the revised model. When quartiles of total MUFAs were quantified, the 2nd quartile was negatively connected with BMD (p < 0.05). In contrast, the 4th quartile was positively correlated with BMD (p < 0.01), and the trend analysis was statistically significant (p for trend = 0.006). There was a statistically significant correlation between total MUFAs intake and BMD across age groups in subgroups stratified by age and gender. BMD was linked to total MUFA intake in whites, blacks, and people of other race, these associations are statistically significant.
We found a turning point between total MUFAs intake and BMD using a smooth curve fit inside a model that controlled all covariates Figures 2C, D. According to the saturation effect analysis model, the effect value for total MUFAs consumption was 20.52 g/d (Table 3). Taken together, the connection between MUFAs intake and total BMD showed an inverted U-shaped curve.
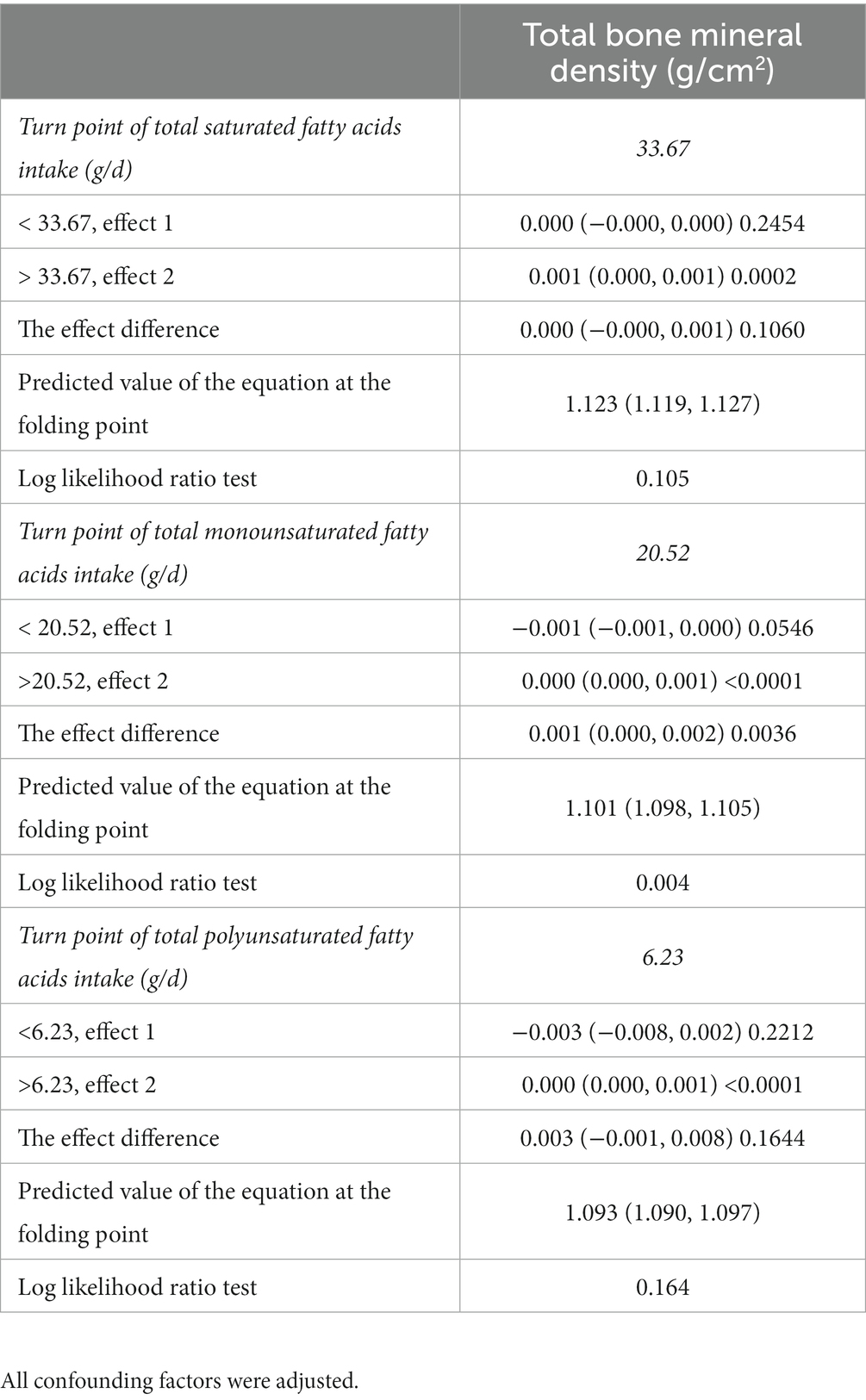
Table 3. Saturation effect analysis of fatty acids intake (g/d) and total bone mineral density (g/cm2).
3.4. Relationship between PUFAs intake and BMD
In the fully adjusted model, total PUFAs intake was also found to be positively associated with BMD (p < 0.001). When total PUFAs intake was analyzed by quartile, fatty acids intake was positively associated with total BMD in the 3rd group (p < 0.01) and the 4th group (p < 0.01), and the trend analysis was statistically significant (p for trend = 0.004) (Table 2). In subgroups stratified by age and gender, the positive association between total PUFAs intake and total BMD remained statistically significant. In subgroups stratified by race, we observed this positive association only in blacks and other genders. These outcomes possess statistical significance. As shown in Figures 2E, F, we found no saturation effect between PUFAs and BMD when we performed smooth curve fitting on the revised model.
4. Discussion
We analyzed the association of fatty acid intake with BMD using data on adults aged 20–59 years in the NHANES from 2011 to 2018. Three classes of fatty acids (SFAs, PUFAs, and MUFAs) were favorably linked with BMD in this cross-sectional study of 8,942 people. In this study, we analyzed fatty acid intake by quartile and found that higher fatty acid intake was associated with better bone health within a certain range of fatty acid intake. Furthermore, saturation effect model analysis and smooth curve fitting showed that total MUFAs had an inverted U-shaped relationship with BMD, with a turning point of 20.52 g/d, while other fatty acids had a linear relationship with BMD once confounding factors were taken into account. When total MUFAs were higher than 20.52 g/d, there was a beneficial association between MUFAs intake and BMD (p < 0.0001). Study (22) has shown that MUFAs activate peroxisome proliferators receptor-β/δ, regulating the RANKL signaling pathway, and inhibiting osteoclast formation.
Currently, PUFAs have received the most attention in bone health investigations, but the results are controversial. In the ORENTRA experiment, Jørgensen et al. (23) supplied omega-3 fatty acids to kidney transplant recipients and olive oil to the control group. After 44 weeks of treatment, omega-3 fatty acids had no significant effect on BMD. The researchers believe that it may be due to the threshold effect of n-3 PUFAs on BMD. In a retrospective study of 275 healthy women from Japan, Kuroda et al. (24). used multiple linear regression analysis to show that omega-3 fatty acid intake contributes to hip BMD. Results from this study showed that the connection between PUFAs intake and BMD was stronger in the 3rd and 4th quartiles of the distribution, but not in the 2nd quartile. We believe that PUFAs intake is positively correlated with BMD only when PUFAs intake reaches a certain threshold, which seems to be consistent with previous research. Notably, a 5-year longitudinal study (25) found that a higher intake of PUFAs and MUFAs was linked to lower BMD in the femoral neck, even after controlling for possible confounding factors, these associations are statistically significant. This contradictory conclusion may be owing to the limited sample size of the population included in this study. Similarly, in animal study (26), BMD was significantly reduced in rats fed an atherogenic diet, and monounsaturated fatty acids ameliorated these changes but remained lower than in controls. Little research has been conducted on the correlation between saturated fatty acid consumption and BMD to date, and there has been a dearth of large-scale investigations into the topic. Because of this, we conducted this extensive retrospective study and found that consuming saturated fatty acids actually improves BMD.
In addition to the regulation of bone metabolism, fatty acids have other biological effects, such as omega-3 PUFAs to protect cardiovascular (27) and nerve (28), anti-tumor (29), possibly by inhibiting inflammation, reducing oxidative stress, regulating cell apoptosis and other mechanisms. A meta-analysis with 23 literature showed that when SFAs intake >17 g/d, there will be a clear protective effect of type 2 diabetes (30). Data from the literature indicates that diabetes is a high risk factor for osteoporosis (31). According to a number of studies, those who suffer from diabetes have lower bone mineral density than those who do not suffer from the condition (32, 33). In addition, the ratio of PUFAs (e.g., omega-6, omega-3) also affects metabolism. Studies have shown that a high intake of omega-6 fatty acids or a high omega-6/omega-3 nutrient ratio is linked to an increased risk of obesity. Obesity is also a protective factor for bone density, which partially explain why PUFAs are good for BMD (34, 35). In addition, BMD has been shown to be higher in those with adequate fat intake than in those with insufficient fat intake (36).
We performed weighted multiple linear regression analysis and smooth curve fitting analysis with data from 8,942 participants and found that fatty acid intake in adults aged 20–59 was beneficial to bone mineral density, which is also associated with osteoporosis. Prevention provides dietary guidance. However, our study also has some flaws. Because this is a cross-sectional study, it cannot show that the association between fatty acid consumption and BMD is caused by one or the other. More prospective clinical studies and basic research are needed to back up these results. According to our findings, the positive correlation between total BMD and MUFAs intake occurs only when the MUFAs intake is >20.52 g/d. We have studied a sizable amount of literature. However, to our knowledge, the saturation effect and threshold between MUFAs and BMD are not supported by any pertinent data. The exact mechanism is yet unknown, and more studies are needed to confirm it. There is no literature on the saturation effect between SFAs, PUFAs, and BMD. Therefore, in the future, we suggest carrying out a larger prospective study on SFAs, PUFAs, and BMD to further understand the causal relationship between fatty acids and BMD. Since there are only total SFAs, PUFAs, and MUFAs intakes in the NHANES database, but no specific fatty acid intakes, such as the specific intakes of n-3 and n-6 PUFAs, we suggest that future studies should focus on the association between specific fatty acids and BMD.
In conclusion, SFAs, MUFAs, and PUFAs intake were positively associated with BMD, and the associations persisted in subgroups stratified by age, gender, and race in this study. Notably, when fatty acid intake was quartiled, MUFAs in the 2nd quartile were negatively correlated with BMD and those in the 4th quartile were positively correlated. Meanwhile, this research found that MUFAs were positively correlated with BMD, but there was a threshold. Therefore, according to our findings, it is recommended that adults consume moderate amounts of fatty acids to ensure adequate bone mass but not metabolic diseases.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
All survey participants were informed of the poll’s specifics and signed an informed consent form. The National Center for Health Statistics Ethics Review Board assessed and authorized the informed consent. Following the completion of official anonymization, all of the data is then made available to the public in order to make the most effective use of these resources. Anyone may access these statistics as long as they adhere to the NHENAS database regulations and are used exclusively for statistical analysis. All studies based on these data should adhere to applicable laws and legislation. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Z-BF and G-XW contributed equally to this study and made contributions to data collection, curation, statistical analysis, and manuscript writing and revision. G-ZC and P-XZ contributed to the statistical analysis. D-LL, S-FC, and H-XZ supervised the study and contributed to the polishing and reviewing of the manuscript. S-FC provided financial assistance for this research. H-LL supervised, wrote the review, and edited this study. All authors contributed to the article and approved the submitted version.
Funding
H-LL was supported by the Shenzhen Municipal Science and Technology Innovation Council (JCYJ20170817094838619). S-FC was supported by the Natural Science Foundation of Guangdong Provincial (No. 2019A1515110108), National Natural Science Foundation of China (grant number. 82104759), and the Shenzhen Municipal Science and Technology Innovation Council (No. JCYJ20180302173821841). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Song, S, Guo, Y, Yang, Y, and Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. (2022) 237:108168. doi: 10.1016/j.pharmthera.2022.108168
2. Clynes, MA, Westbury, LD, Dennison, EM, Kanis, JA, Javaid, MK, Harvey, NC, et al. Bone densitometry worldwide: a global survey by the ISCD and IOF. Osteoporos Int. (2020) 31:1779–86. doi: 10.1007/s00198-020-05435-8
3. Hernlund, E, Svedbom, A, Ivergård, M, Compston, J, Cooper, C, Stenmark, J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the international osteoporosis foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. (2013) 8:136. doi: 10.1007/s11657-013-0136-1
4. Salari, N, Ghasemi, H, Mohammadi, L, Behzadi, MH, Rabieenia, E, Shohaimi, S, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. (2021) 16:609. doi: 10.1186/s13018-021-02772-0
5. Omer, M, Ali, H, Orlovskaya, N, Ballesteros, A, Cheong, VS, Martyniak, K, et al. Omega-9 modifies viscoelasticity and augments bone strength and architecture in a high-fat diet-fed murine model. Nutrients. (2022) 14:3165. doi: 10.3390/nu14153165
6. Feehan, O, Magee, PJ, Pourshahidi, LK, Armstrong, DJ, Slevin, MM, Allsopp, PJ, et al. Associations of long chain polyunsaturated fatty acids with bone mineral density and bone turnover in postmenopausal women. Eur J Nutr. (2022) 62:95–104. doi: 10.1007/s00394-022-02933-9
7. Bischoff-Ferrari, HA, Vellas, B, Rizzoli, R, Kressig, RW, Da, SJ, Blauth, M, et al. Effect of vitamin D supplementation, Omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. (2020) 324:1855–68. doi: 10.1001/jama.2020.16909
8. Saini, RK, and Keum, YS. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance - A review. Life Sci. (2018) 203:255–7. doi: 10.1016/j.lfs.2018.04.049
9. Pino, AM, and Rodríguez, JP. Is fatty acid composition of human bone marrow significant to bone health? Bone. (2019) 118:53–61. doi: 10.1016/j.bone.2017.12.014
10. Yang, L, Yang, C, Chu, C, Wan, M, Xu, D, Pan, D, et al. Beneficial effects of monounsaturated fatty acid-rich blended oils with an appropriate polyunsaturated/saturated fatty acid ratio and a low n-6/n-3 fatty acid ratio on the health of rats. J Sci Food Agric. (2022) 102:7172–85. doi: 10.1002/jsfa.12083
11. Al, SA, Myers, DE, Stupka, N, and Duque, G. 1,25(OH)(2)D(3) ameliorates palmitate-induced lipotoxicity in human primary osteoblasts leading to improved viability and function. Bone. (2020) 141:115672. doi: 10.1016/j.bone.2020.115672
12. Harasymowicz, NS, Dicks, A, Wu, CL, and Guilak, F. Physiologic and pathologic effects of dietary free fatty acids on cells of the joint. Ann N Y Acad Sci. (2019) 1440:36–53. doi: 10.1111/nyas.13999
13. Yaghooti, H, Mohammadtaghvaei, N, and Mahboobnia, K. Effects of palmitate and astaxanthin on cell viability and proinflammatory characteristics of mesenchymal stem cells. Int Immunopharmacol. (2019) 68:164–09. doi: 10.1016/j.intimp.2018.12.063
14. Wang, Y, Dellatore, P, Douard, V, Qin, L, Watford, M, Ferraris, RP, et al. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr Res. (2016) 36:742–09. doi: 10.1016/j.nutres.2016.03.002
15. Rogero, MM, and Calder, PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. (2018) 10:432. doi: 10.3390/nu10040432
16. Casado-Díaz, A, Ferreiro-Vera, C, Priego-Capote, F, Dorado, G, Luque-de-Castro, MD, and Quesada-Gómez, JM. Effects of arachidonic acid on the concentration of hydroxyeicosatetraenoic acids in culture media of mesenchymal stromal cells differentiating into adipocytes or osteoblasts. Genes Nutr. (2014) 9:375. doi: 10.1007/s12263-013-0375-1
17. Korbecki, J, Bobiński, R, and Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res. (2019) 68:443–8. doi: 10.1007/s00011-019-01231-1
18. Wang, K, Zha, Y, Lei, H, and Xu, X. MRI study on the changes of bone marrow microvascular permeability and fat content after Total-body X-ray irradiation. Radiat Res. (2018) 189:205–2. doi: 10.1667/RR14865.1
19. Müller, AK, Albrecht, F, Rohrer, C, Koeberle, A, Werz, O, Schlörmann, W, et al. Olive oil extracts and oleic acid attenuate the LPS-induced inflammatory response in murine RAW264.7 macrophages but induce the release of prostaglandin E2. Nutrients. (2021) 13:4437. doi: 10.3390/nu13124437
20. Tsai, YW, Lu, CH, Chang, RC, Hsu, YP, Ho, LT, and Shih, KC. Palmitoleic acid ameliorates palmitic acid-induced proinflammation in J774A.1 macrophages via TLR4-dependent and TNF-α-independent signallings. Prostaglandins Leukot Essent Fatty Acids. (2021) 169:102270. doi: 10.1016/j.plefa.2021.102270
21. Hsieh, CI, Zheng, K, Lin, C, Mei, L, Lu, L, Li, W, et al. Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nat Commun. (2021) 12:5472. doi: 10.1038/s41467-021-25779-x
22. Kasonga, A, Kruger, MC, and Coetzee, M. Activation of PPARs modulates signalling pathways and expression of regulatory genes in osteoclasts derived from human CD14+ monocytes. Int J Mol Sci. (2019) 20:E1798. doi: 10.3390/ijms20071798
23. Jørgensen, HS, Eide, IA, Jenssen, T, Åsberg, A, Bollerslev, J, Godang, K, et al. Marine n-3 polyunsaturated fatty acids and bone mineral density in kidney transplant recipients: a randomized, placebo-controlled trial. Nutrients. (2021) 13:2361. doi: 10.3390/nu13072361
24. Kuroda, T, Ohta, H, Onoe, Y, Tsugawa, N, and Shiraki, M. Intake of omega-3 fatty acids contributes to bone mineral density at the hip in a younger Japanese female population. Osteoporos Int. (2017) 28:2887–91. doi: 10.1007/s00198-017-4128-7
25. Macdonald, HM, New, SA, Golden, MH, Campbell, MK, and Reid, DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. (2004) 79:155–5. doi: 10.1093/ajcn/79.1.155
26. Macri, EV, Lifshitz, F, Alsina, E, Juiz, N, Zago, V, Lezón, C, et al. Monounsaturated fatty acids-rich diets in hypercholesterolemic-growing rats. Int J Food Sci Nutr. (2015) 66:400–8. doi: 10.3109/09637486.2015.1025719
27. Bernasconi, AA, Wiest, MM, Lavie, CJ, Milani, RV, and Laukkanen, JA. Effect of Omega-3 dosage on cardiovascular outcomes: an updated meta-analysis and meta-regression of interventional trials. Mayo Clin Proc. (2021) 96:304–3. doi: 10.1016/j.mayocp.2020.08.034
28. Chen, X, Chen, C, Fan, S, Wu, S, Yang, F, Fang, Z, et al. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation. (2018) 15:116. doi: 10.1186/s12974-018-1151-3
29. Dierge, E, Debock, E, Guilbaud, C, Corbet, C, Mignolet, E, Mignard, L, et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. (2021) 33:1701–1715.e5. doi: 10.1016/j.cmet.2021.05.016
30. Neuenschwander, M, Barbaresko, J, Pischke, CR, Iser, N, Beckhaus, J, Schwingshackl, L, et al. Intake of dietary fats and fatty acids and the incidence of type 2 diabetes: a systematic review and dose-response meta-analysis of prospective observational studies. PLoS Med. (2020) 17:e1003347. doi: 10.1371/journal.pmed.1003347
31. Ebeling, PR, Nguyen, HH, Aleksova, J, Vincent, AJ, Wong, P, and Milat, F. Secondary osteoporosis. Endocr Rev. (2022) 43:240–3. doi: 10.1210/endrev/bnab028
32. Loxton, P, Narayan, K, Munns, CF, and Craig, ME. Bone mineral density and type 1 diabetes in children and adolescents: a meta-analysis. Diabetes Care. (2021) 44:1898–05. doi: 10.2337/dc20-3128
33. Pan, H, Wu, N, Yang, T, and He, W. Association between bone mineral density and type 1 diabetes mellitus: a meta-analysis of cross-sectional studies. Diabetes Metab Res Rev. (2014) 30:531–2. doi: 10.1002/dmrr.2508
34. Simopoulos, AP . An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients. (2016) 8:128. doi: 10.3390/nu8030128
35. Rinonapoli, G, Pace, V, Ruggiero, C, Ceccarini, P, Bisaccia, M, Meccariello, L, et al. Obesity and bone: a complex relationship. Int J Mol Sci. (2021) 22:13662. doi: 10.3390/ijms222413662
Keywords: nutrition, bone mineral density, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, NHANES
Citation: Fang Z-B, Wang G-X, Cai G-Z, Zhang P-X, Liu D-L, Chu S-F, Li H-L and Zhao H-X (2023) Association between fatty acids intake and bone mineral density in adults aged 20–59: NHANES 2011–2018. Front. Nutr. 10:1033195. doi: 10.3389/fnut.2023.1033195
Edited by:
Yulong Li, University of Nebraska Medical Center, United StatesReviewed by:
Marija Takic, Institute for Medical Research, University of Belgrade, SerbiaElena Planells, University of Granada, Spain
Copyright © 2023 Fang, Wang, Cai, Zhang, Liu, Chu, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Liang Liu, bGRsMjU4MEBnenVjbS5lZHUuY24=; Shu-Fang Chu, Y2h1c2h1ZmFuZ2d6aHRjbUAxNjMuY29t; Hui-Lin Li, c3p0Y21saGxAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Ze-Bin Fang
Ze-Bin Fang Gao-Xiang Wang
Gao-Xiang Wang Gui-Zhang Cai1,2
Gui-Zhang Cai1,2 Peng-Xiang Zhang
Peng-Xiang Zhang De-Liang Liu
De-Liang Liu Shu-Fang Chu
Shu-Fang Chu Hui-Lin Li
Hui-Lin Li Hing-Xia Zhao
Hing-Xia Zhao